
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 03 October 2023
Sec. Ethnopharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1226008
Background: Astragaloside IV (ASIV) is the primary pharmacologically active compound found in Astragalus propinquus Schischkin, which has potential protective effects on cardiac function. However, there are almost no systematic evaluations of ASIV for the treatment of heart failure (HF).
Methods: Preclinical studies published before 27 December 2022, were retrieved from PubMed, Web of Science, MEDLINE, SinoMed, Chinese National Knowledge Infrastructure (CNKI), VIP information database, and Wanfang Data information site. The quality of included research was evaluated using SYRCLE’s RoB tool. Review Manager 5.4.1 was used to perform meta-analyses of the cardiac function parameters and other indicators. Regression analysis was conducted to observe the dose-efficacy relationship.
Results: Nineteen studies involving 489 animals were included. Results indicated that compared with the control group, ASIV could enhance cardiac function indicators, including left ventricular ejection fraction (LVEF), left ventricular fractional shortening (LVFS), left ventricular pressure change rate (±dp/dtmax), left ventricular end-diastolic pressure (LVEDP), left ventricular systolic pressure (LVSP), heart weight/body weight (HW/BW) and left ventricular weight/body weight (LVW/BW). Furthermore, the regression analysis showed that the treatment of HF with ASIV was dose-dependent.
Conclusion: Findings suggest that ASIV can inhibit cardiac hypertrophy by reducing cardiac preload and afterload, thereby protecting cardiac function.
Heart failure (HF) is a complex clinical syndrome that develops due to structural or functional damage of ventricular filling or ejection of blood, and it represents an advanced manifestation of various cardiovascular diseases (Heidenreich et al., 2022). The American Heart Association predicts that by 2030, HF will probably affect more than 8 million people over 18 years old in the United States (Heidenreich et al., 2013). In China, epidemiological surveys report a 4.1% ± 0.3% in-hospital mortality rate for HF (Zhang et al., 2017). Mortality and incidence rates increase with age (Huffman et al., 2013), which contributes to a rising economic burden from HF as the population ages (Cook et al., 2014). Despite significant progress in treatments that have improved the survival of HF patients, such as angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, β receptor blockers, coronary arterial blood revascularization, implantable cardioverter-defibrillators, and cardiac resynchronization therapy (Merlo et al., 2014; Vaduganathan et al., 2020), the 5-year mortality rate of HF remains high (Gerber et al., 2015).
Recent clinical studies suggest that natural medicine could significantly improve the prognosis of HF patients (Wang et al., 2018; Mao et al., 2020; Leung et al., 2021). Astragalus propinquus Schischkin, widely used in traditional Chinese clinical prescriptions, frequently features in prescriptions for treating HF (Guo et al., 2022). In a recent study, Huangqi injection (with active ingredients derived from Astragalus propinquus Schischkin) demonstrated the ability to improve cardiac function (Cao et al., 2022). Huangqi injection was also found to significantly improve various parameters of echocardiography in rats with heart failure, including LVEF and LVFS (Liu et al., 2018). Recent animal studies have shown that Astragaloside IV (ASIV) (Figure 1), the active ingredient of Astragalus propinquus Schischkin, can protect cardiovascular system (Dong et al., 2017; Liu et al., 2021). Accumulating evidence indicates that ASIV can promote angiogenesis (Wang et al., 2013), protect myocardial cells (Luo et al., 2019), and inhibit ventricular remodeling (Lu et al., 2017).
Preclinical systematic reviews can identify areas for testing in further animal experiments, preclude unnecessary study replication, refine animal experimentation, and lay the foundation for future clinical trials (Murphy and Murphy, 2010). Therefore, in this study, we conducted a systematic review and meta-analysis to evaluate the beneficial effects of ASIV on cardiac function in HF rat models. The results of our study could provide a reference for refining animal experimentation and designing clinical research, as well as identifying new therapeutic strategies for the treatment of HF.
This systematic review was registered (Invoice Number: CRD42023383485) in PROSPERO (https://www.crd.york.ac.uk/PROSPERO/) and has been reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
We conducted a comprehensive search of studies on the effect of ASIV in animal models of HF using various electronic databases, such as PubMed, Web of Science, MEDLINE, SinoMed, Chinese National Knowledge Infrastructure (CNKI), VIP information database, and Wanfang Data information site, from their inception to December 2022.
The following keywords combined with Medical Subject Headings (MeSH) terms were used for searching: (“Astragaloside IV” or “ASIV” or “astragaloside-A”) AND (“heart failure” or “HF” or “cardiac failure” or “Heart Decompensation” or “Myocardial Failure”).
To prevent bias, prespecified inclusion criteria were as follows:
(1) rat models of HF, without limiting specific modeling method;
(2) a controlled experiment;
(3) treatment group received the ASIV intervention merely, administration of ASIV at any dose or in any form is acceptable;
(4) control group received equivalent vehicle, saline or no treatment;
(5) the outcomes measured were parameters reflecting cardiac function, such as left ventricular ejection fraction (LVEF), and/or left ventricular fractional shortening (LVFS), and/or left ventricular pressure change rate (LV ± dp/dtmax), and/or left ventricular end-diastolic pressure (LVEDP) and/or left ventricular systolic pressure (LVSP) and/or heart weight/body weight (HW/BW) and/or left ventricular weight/body weight (LVW/BW).
Prespecified exclusion criteria met anyone of the following conditions:
(1) in vitro studies, case reports, and clinical trials;
(2) duplicate publications;
(3) Missing result data that can be obtained.
Two authors independently extracted data as follows: 1) the first author’s name and publication year; 2) the information of experimental animals such as number, species, sex, weight age; 3) the induction method of HF animal model; 4) the time of experimental drug intervention; 5) the information of treatment used in experimental group such as dose, method of administration, and duration of treatment; 6) the primary outcome measures. If there were multiple measurement results at different times, we recorded the last result. If the experimental animals received different doses of drug intervention, we recorded only the highest dose. The data were measured by the digital ruler software if the data was presented with graphs. For incomplete published data, we contacted the author for further information. For each comparison, we extracted the mean and standard deviation from the experimental and control groups of each study. Discrepancies were resolved after discussion between the two authors.
The data for LVEF and LVFS were obtained through echocardiography measurements. The data for LVEDP, LVSP, and ±dp/dtmax were obtained through hemodynamic monitoring. The data for HW/BW and LVW/BW were obtained through post-mortem measurements and calculations.
We evaluated the methodological quality of the included studies using the SYRCLE’s RoB tool (Hooijmans et al., 2014) with minor modification as follows: 1) randomization of sequence generation; 2) description of baseline characteristics; 3) allocation concealment; 4) animals randomly standardized housed; 5) feeding and intervention in blind; 6) criterion for the success of animal models; 7) random outcome assessment; 8) blinded assessment of outcomes; 9) incomplete outcome data; (10) Other sources of bias. We tried to quantify the evaluation results. Each study was given a total score of ten, with one point for each entry. Two authors independently evaluated the study quality, and disagreement was resolved through discussion or consultation.
We performed a meta-analysis using Review Manager 5.4.1. All the data of cardiac function were considered as continuous data, and then, we use mean deviation (MD) and random effect model (REM) to estimate the size of combined effects. Because of the heterogeneity between multiple studies must be considered, in this meta-analysis, we chose the REM to get the results. The χ2 test with a significance level of = 0.1 will be used as statistical measure of heterogeneity between the different studies. Moreover, the I2 statistic will be applied to quantifies inconsistency between studies, calculated as I2 = (Q - df)/Q*100%, where I2 statistic of 50% or more indicated a considerable heterogeneity, then additional subgroup and/or sensitivity analysis was performed. Probability values of 0.05 were considered significant. In addition, Origin 2021 was used for dosage-efficacy interval analyses, and regression analysis was used to test the reliability of the dosage-efficacy interval.
According to our retrieval strategy, we identified 753 potentially relevant studies. There were 178 records after deleting duplicates. After reading the titles and abstracts, 138 studies were excluded because of non-animal study, clinical trial, review, other diseases, or compound prescription. The full text of the remaining 40 articles was read, and finally 21 articles were excluded for the following reasons: 1) non-rat model or non-heart failure model; 2) no control group; 3) treatment group was not intervened with ASIV; 4) the outcome measures did not include the cardiac function as previously mentioned. Ultimately, 19 randomized controlled animal experiments (Zhao et al., 2009; Cui et al., 2013; Zhang et al., 2015; Cheng et al., 2016; Jiang et al., 2016; Cheng, 2017; Ji et al., 2018; Lv, 2018; Tang et al., 2018; Zhao et al., 2018; Nie et al., 2019; Sui et al., 2020; Wang et al., 2020; Huang et al., 2021; Shi et al., 2021; Song et al., 2021; Zhang et al., 2021; Cui et al., 2022; Wang et al., 2022) were identified (Figure 2).
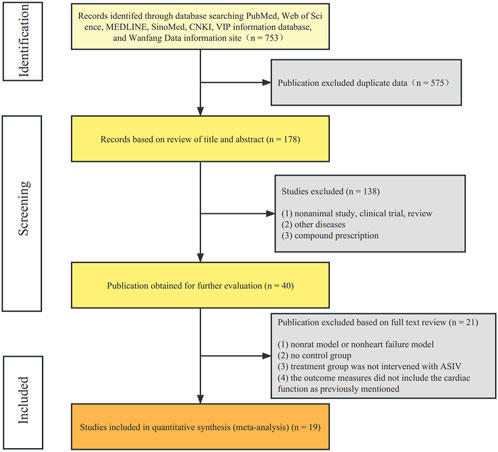
FIGURE 2. Flow diagram. The process of papers inclusion was divided into three steps: search, deduplication, and manual screening. Only literature that met the inclusion criteria were included.
A total of 489 animals were included in 19 studies. All studies were published in peer-reviewed journals. All studies were published between 2009 and 2022, including eight English studies (Zhao et al., 2009; Zhang et al., 2015; Cheng et al., 2016; Ji et al., 2018; Tang et al., 2018; Nie et al., 2019; Sui et al., 2020; Shi et al., 2021). Male Sprague Dawley rats were used in fifteen studies (Cui et al., 2013; Zhang et al., 2015; Cheng et al., 2016; Jiang et al., 2016; Cheng, 2017; Lv, 2018; Tang et al., 2018; Zhao et al., 2018; Sui et al., 2020; Wang et al., 2020; Huang et al., 2021; Shi et al., 2021; Song et al., 2021; Zhang et al., 2021; Wang et al., 2022), and male Wistar rats were used in two studies (Ji et al., 2018; Cui et al., 2022). In the remaining studies, one study (Zhao et al., 2009) used Wistar rats but did not report gender, and one study (Cui et al., 2022) did not report the type and gender of rats used. All studies reported animal weights. As for the rat model of HF, nine studies (Zhao et al., 2009; Cui et al., 2013; Cheng et al., 2016; Cheng, 2017; Ji et al., 2018; Sui et al., 2020; Wang et al., 2020; Shi et al., 2021; Wang et al., 2022) used the method of coronary artery ligation, six studies (Jiang et al., 2016; Lv, 2018; Tang et al., 2018; Zhao et al., 2018; Nie et al., 2019; Song et al., 2021) used abdominal aortic coarctation (AAC), and other methods included injection of isoproterenol (Zhang et al., 2015), injection of miRNA-1 lentivirus (Huang et al., 2021), injection of doxorubicin (Zhang et al., 2021), and high salt feeding (Cui et al., 2022). Thirteen studies (Zhao et al., 2009; Cui et al., 2013; Jiang et al., 2016; Cheng, 2017; Ji et al., 2018; Tang et al., 2018; Zhao et al., 2018; Nie et al., 2019; Sui et al., 2020; Shi et al., 2021; Song et al., 2021; Zhang et al., 2021; Wang et al., 2022) mentioned that the specific time of starting intervention was after modeling, and two studies (Zhang et al., 2015; Huang et al., 2021) mentioned that intervention was before modeling, the other four studies (Cheng et al., 2016; Lv, 2018; Wang et al., 2020; Cui et al., 2022) did not indicate the specific time of intervention. The administration methods include intravenous injection and gastric perfusion, and only one study (Zhao et al., 2009) used intravenous injection. The dose was not exactly the same, including the following: 80 mg/kg/d in four studies (Zhang et al., 2015; Nie et al., 2019; Huang et al., 2021; Shi et al., 2021); 70 mg/kg/d in two studies (Cheng, 2017; Lv, 2018); 60 mg/kg/d in four studies (Jiang et al., 2016; Tang et al., 2018; Zhao et al., 2018; Zhang et al., 2021); 50 mg/kg/d in two studies (Cheng et al., 2016; Song et al., 2021); 40 mg/kg/d in one study (Cui et al., 2022); 30 mg/kg/d in one study (Wang et al., 2022); 20 mg/kg/d in one study (Ji et al., 2018); 10 mg/kg/d in one study (Cui et al., 2013); 2 mg/kg/d in one study (Wang et al., 2020); 1 mg/kg/d in two studies (Zhao et al., 2009; Sui et al., 2020). The durations of administration time are diverse, including 56 days in eight studies (Cheng, 2017; Lv, 2018; Tang et al., 2018; Nie et al., 2019; Wang et al., 2020; Song et al., 2021; Zhang et al., 2021; Cui et al., 2022); 28 days in six studies (Zhang et al., 2015; Jiang et al., 2016; Ji et al., 2018; Zhao et al., 2018; Shi et al., 2021; Wang et al., 2022); 14 days in two studies (Zhao et al., 2009; Cheng et al., 2016); the remaining three studies different from each other.
Eleven studies (Cheng et al., 2016; Tang et al., 2018; Zhao et al., 2018; Nie et al., 2019; Sui et al., 2020; Wang et al., 2020; Huang et al., 2021; Song et al., 2021; Zhang et al., 2021; Cui et al., 2022; Wang et al., 2022) reported LVEF; eight studies (Zhao et al., 2009; Zhang et al., 2015; Cheng et al., 2016; Tang et al., 2018; Nie et al., 2019; Sui et al., 2020; Wang et al., 2020; Wang et al., 2022) reported LVFS; nine studies (Zhao et al., 2009; Cui et al., 2013; Zhang et al., 2015; Jiang et al., 2016; Cheng, 2017; Tang et al., 2018; Zhao et al., 2018; Shi et al., 2021; Song et al., 2021) reported LV + dp/dt; ten studies (Zhao et al., 2009; Cui et al., 2013; Zhang et al., 2015; Jiang et al., 2016; Cheng, 2017; Ji et al., 2018; Tang et al., 2018; Zhao et al., 2018; Shi et al., 2021; Song et al., 2021) reported LV-dp/dt; eight studies (Zhao et al., 2009; Cui et al., 2013; Zhang et al., 2015; Jiang et al., 2016; Cheng, 2017; Lv, 2018; Zhao et al., 2018; Shi et al., 2021) reported LVSP; twelve studies (Zhao et al., 2009; Cui et al., 2013; Zhang et al., 2015; Jiang et al., 2016; Cheng, 2017; Ji et al., 2018; Lv, 2018; Tang et al., 2018; Zhao et al., 2018; Wang et al., 2020; Shi et al., 2021; Song et al., 2021) reported LVEDP; five studies (Cheng et al., 2016; Jiang et al., 2016; Zhao et al., 2018; Nie et al., 2019; Wang et al., 2022) reported HW/BW; four studies (Jiang et al., 2016; Zhao et al., 2018; Song et al., 2021; Wang et al., 2022) reported LVW/BW. The main characteristics of the 19 studies are summarized in Table 1.
The quality of the study included 19 studies with quality scores ranging from 2 to 6, with an average of 3.8. Five studies (Cui et al., 2013; Tang et al., 2018; Zhao et al., 2018; Nie et al., 2019; Wang et al., 2020) adopted random number table method to the groups, and the other two studies (Jiang et al., 2016; Cheng, 2017) did not mention randomization. No research has described the method and process of randomization in detail. One study (Tang et al., 2018) detailed the baseline of animal characteristics. No study described allocation concealment and random placement of animals. Seven studies (Cui et al., 2013; Cheng et al., 2016; Jiang et al., 2016; Cheng, 2017; Ji et al., 2018; Lv, 2018; Huang et al., 2021) did not mention specific environments regarding animal feeding. Nonetheless, it is a pity that none of these studies reported blinding of feeding and intervention. Twelve studies (Zhao et al., 2009; Jiang et al., 2016; Cheng, 2017; Ji et al., 2018; Lv, 2018; Tang et al., 2018; Zhao et al., 2018; Nie et al., 2019; Huang et al., 2021; Song et al., 2021; Zhang et al., 2021; Cui et al., 2022) used the results of hemodynamics and/or ultrasonic cardiogram as the standard for evaluating HF models; six studies (Cui et al., 2013; Cheng et al., 2016; Sui et al., 2020; Wang et al., 2020; Shi et al., 2021; Wang et al., 2022) used results of ECG; one study (Zhang et al., 2015) did not report evaluation methods. One study (Nie et al., 2019) mentioned blind evaluation of results, but none of the studies assessed the outcomes randomly. One study (Sui et al., 2020) did not include all animals in the final data. As anesthetic, six studies (Cui et al., 2013; Cheng et al., 2016; Cheng, 2017; Sui et al., 2020; Song et al., 2021; Wang et al., 2022) used pentobarbital sodium, three studies (Tang et al., 2018; Nie et al., 2019; Shi et al., 2021) used isoflurane, five studies (Jiang et al., 2016; Ji et al., 2018; Zhao et al., 2018; Wang et al., 2020; Huang et al., 2021) used chloral hydrate, one study (Zhao et al., 2009) used ether, one study (Zhang et al., 2015) used urethane, and three studies (Lv, 2018; Zhang et al., 2021; Cui et al., 2022) did not report the use of drugs. No study reports conflicts of interest. The methodological quality of each study is summarized in Table 2.
Eleven studies (Cheng et al., 2016; Tang et al., 2018; Zhao et al., 2018; Nie et al., 2019; Sui et al., 2020; Wang et al., 2020; Huang et al., 2021; Song et al., 2021; Zhang et al., 2021; Cui et al., 2022; Wang et al., 2022) reported LVEF, and the results of meta-analysis showed that ASIV had a significant effect on improving LVEF compared with the control group (n = 268, MD 17.04, 95% CI: 11.01∼23.08, p < 0.01; heterogeneity Chi2 = 1114.52, p < 0.01, I2 = 99%). Due to significant statistical heterogeneity, we considered using subgroup analysis to explore the sources of heterogeneity. We noticed that the LVEF results of the control group were significantly different in the included studies. Among the sham group results of these studies, there are five studies (Cheng et al., 2016; Tang et al., 2018; Nie et al., 2019; Cui et al., 2022; Wang et al., 2022) for 70% < LVEF <90%, four studies (Zhao et al., 2018; Wang et al., 2020; Song et al., 2021; Zhang et al., 2021) for LVEF <70%, and two studies (Sui et al., 2020; Huang et al., 2021) for LVEF >90%. Subgroup analysis showed that, in sham group (LVEF <70%) (Zhao et al., 2018; Wang et al., 2020; Song et al., 2021; Zhang et al., 2021) (n = 123, MD 23.78, 95% CI: 20.28∼27.28, p < 0.01; heterogeneity Chi2 = 27.92, p < 0.01, I2 = 89%), sham group (70% < LVEF <90%) (Cheng et al., 2016; Tang et al., 2018; Nie et al., 2019; Cui et al., 2022; Wang et al., 2022) (n = 107, MD 8.84, 95% CI: 7.20∼10.48, p < 0.01; heterogeneity Chi2 = 8.17, p = 0.09, I2 = 51%) and sham group (LVEF >90%) (Sui et al., 2020; Huang et al., 2021) (n = 38, MD 25.88, 95% CI: 0.23∼51.52, p = 0.05; heterogeneity Chi2 = 108.07, p < 0.01, I2 = 99%), ASIV improved LVEF more than control group. It suggests that the baseline characteristics of animals may be the potential cause of heterogeneity. Next, considering that the difference in total drug dose is huge (mean = 1935.82, standard deviation = 1395.01), we removed three studies (Nie et al., 2019; Sui et al., 2020; Wang et al., 2020) (dose >4000 mg/kg or <500 mg/kg). Subgroup analysis showed that, in sham group (LVEF <70%) (Zhao et al., 2018; Song et al., 2021; Zhang et al., 2021) (n = 83, MD 22.41, 95% CI: 19.77∼25.06, p < 0.01; heterogeneity Chi2 = 4.64, p = 0.10, I2 = 57%) and sham group (70 < LVEF <90) (Cheng et al., 2016; Tang et al., 2018; Cui et al., 2022; Wang et al., 2022) (n = 87, MD 7.89, 95% CI: 6.19∼9.60, p < 0.01; heterogeneity Chi2 = 1.83, p = 0.61, I2 = 0%), ASIV improved LVEF more than control group (Figure 3A). An absence of heterogeneity test in sham group (LVEF >90%) because only a single study was included. Therefore, the drug dose may also be the potential cause of heterogeneity. Additionally, due to the different initiation times of drug administration, we conducted subgroup analyses based on another classification. The analyses were performed separately for “prophylactic administration,” “acute phase administration,” “chronic phase administration,” and “not mentioned.” The results revealed a high level of heterogeneity (Figure 3B). The differences in drug administration timing may not explain the source of heterogeneity. The symmetrical shape of the funnel plot suggests a relatively balanced inclusion of studies, implying minimal publication bias (Figure 3C).
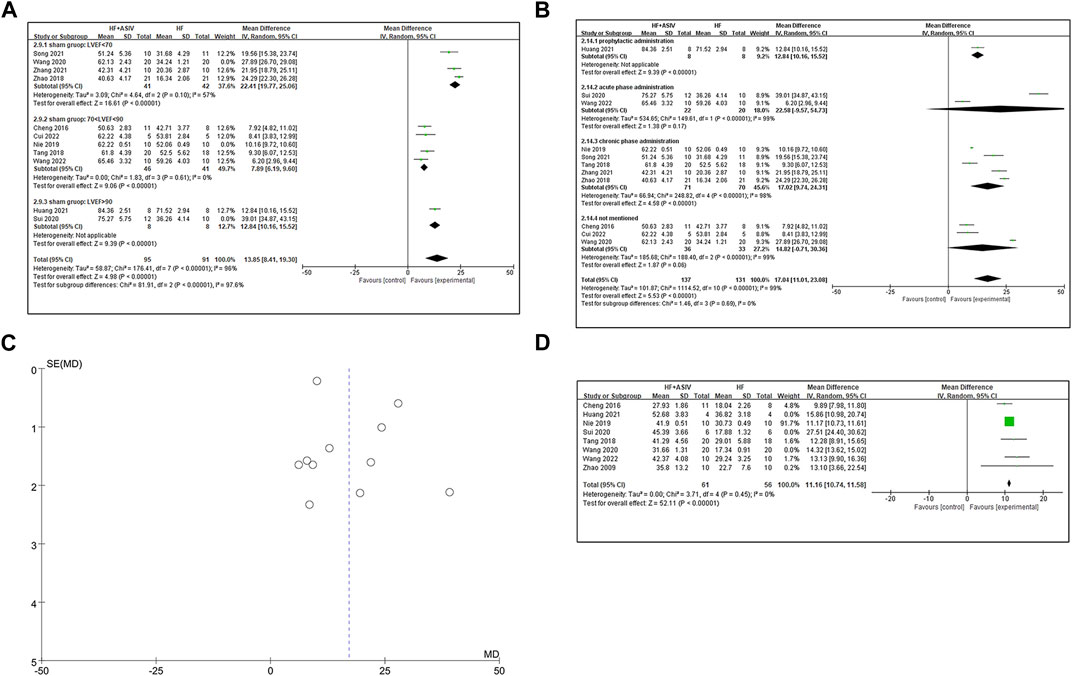
FIGURE 3. (A) The forest plot: subgroup analysis of ASIV in sham group (LVEF <70%), sham group (70% < LVEF <90%) and sham group (LVEF >90%) for improving LVEF compared with the control group. (B) The forest plot: subgroup analysis of ASIV in prophylactic administration group, acute phase administration group, chronic phase administration group and not mentioned group for improving LVEF compared with the control group. (C) Funnel plot indicating a predominantly symmetrical distribution of the 11 included studies assessing the outcome of LVEF. (D) The forest plot: effects of ASIV for increasing LVFS compared with the control group.
Eight studies (Zhao et al., 2009; Tang et al., 2018; Nie et al., 2019; Sui et al., 2020; Wang et al., 2020; Huang et al., 2021; Shi et al., 2021; Wang et al., 2022) reported LVFS, and the results of meta-analysis showed that ASIV had a significant effect on improving LVEF compared with the control group (n = 177, MD 14.55, 95% CI: 12.05∼17.06, p < 0.01; heterogeneity Chi2 = 157.54, p < 0.01, I2 = 96%). Because of the high heterogeneity, we conducted sensitivity analysis to find the source of heterogeneity. Considering the previously mentioned baseline, after excluding three studies (Sui et al., 2020; Wang et al., 2020; Huang et al., 2021) with LVEF >90% or LVEF <70% in the sham group, the results of the remaining five studies (Zhao et al., 2009; Cheng et al., 2016; Tang et al., 2018; Nie et al., 2019; Wang et al., 2022) showed that ASIV could significantly improve LVFS (n = 117, MD 11.16, 95% CI: 10.74∼11.58, p < 0.01; heterogeneity Chi2 = 3.71, p = 0.45, I2 = 0%) (Figure 3D).
Nine studies (Zhao et al., 2009; Cui et al., 2013; Zhang et al., 2015; Jiang et al., 2016; Cheng, 2017; Tang et al., 2018; Zhao et al., 2018; Shi et al., 2021; Song et al., 2021) reported LV + dp/dtmax, and the results of meta-analysis showed that ASIV had a significant effect on improving LV + dp/dtmax compared with the control group (n = 235, MD 1.19, 95% CI: 0.89∼1.49, p < 0.01; heterogeneity Chi2 = 58.01, p < 0.01, I2 = 86%). Furthermore, we established a subgroup analysis based on induction method of animal model due to the remarkable heterogeneity among the various studies. Four studies (Zhao et al., 2009; Cui et al., 2013; Cheng, 2017; Shi et al., 2021) used coronary artery ligation, four studies (Zhao et al., 2018; Shi et al., 2021; Song et al., 2021; Wang et al., 2022) used AAC, and one study (Zhang et al., 2015) used injection of Iso. Meta-analysis of four studies (Zhao et al., 2009; Cui et al., 2013; Cheng, 2017; Shi et al., 2021) using coronary ligation showed that ASIV could significantly improve LV + dp/dtmax (n = 91, MD 1.14, 95% CI: 0.91∼1.37, p < 0.01; heterogeneity Chi2 = 1.82, p = 0.61, I2 = 0%) (Figure 4A). In the four studies using abdominal aortic constriction, the heterogeneity improvement was poor (n = 124, MD 1.23, 95% CI: 0.62∼1.84, p < 0.01; heterogeneity Chi2 = 46.41, p < 0.01, I2 = 94%). Ten studies (Zhao et al., 2009; Cui et al., 2013; Zhang et al., 2015; Jiang et al., 2016; Cheng, 2017; Ji et al., 2018; Tang et al., 2018; Zhao et al., 2018; Shi et al., 2021; Song et al., 2021) reported LV - dp/dtmax, and the results of meta-analysis showed that ASIV had a significant effect on improving LV - dp/dtmax compared with the control group (n = 251, MD 1.28, 95% CI: 1.04∼1.51, p < 0.01; heterogeneity Chi2 = 35.25, p < 0.01, I2 = 74%). Sensitivity analysis is used to explore the source of heterogeneity. As an anesthetic, after excluding one study on the use of urethane and three studies on the use of chloral hydrate, the meta-analysis of six studies (Zhao et al., 2009; Cui et al., 2013; Cheng, 2017; Tang et al., 2018; Shi et al., 2021; Song et al., 2021) on the use of other anesthetics (ether, isoflurane or pentobarbital sodium) showed that ASIV could significantly improve LV - dp/dtmax (n = 150, MD 1.27, 95% CI: 1.04∼1.50, p < 0.01; heterogeneity Chi2 = 8.96, p < 0.11, I2 = 44%) (Figure 4B). It suggests that anesthetics may be the potential cause of heterogeneity.
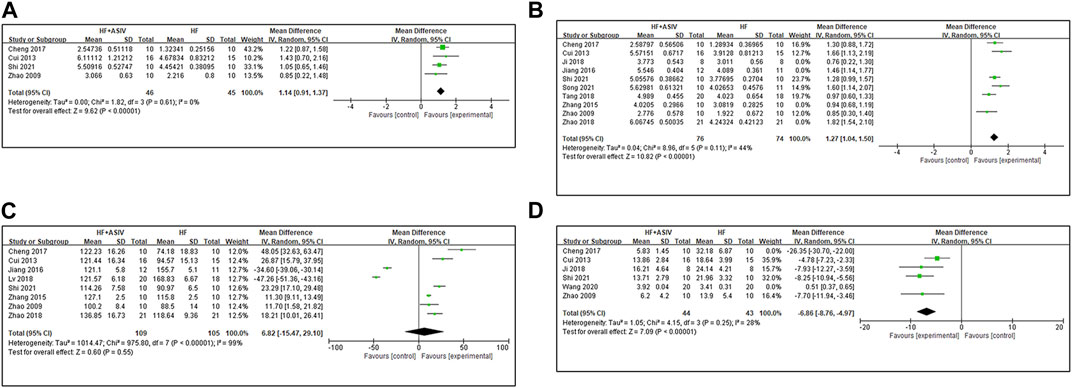
FIGURE 4. (A) The forest plot: effects of ASIV for increasing LV + dp/dt compared with the control group. (B) The forest plot: effects of ASIV for increasing LV - dp/dt compared with the control group. (C) The forest plot: effects of ASIV for increasing LVSP compared with the control group. (D) The forest plot: effects of ASIV for increasing LVEDP compared with the control group.
Eight studies (Zhao et al., 2009; Cui et al., 2013; Zhang et al., 2015; Jiang et al., 2016; Cheng, 2017; Lv, 2018; Zhao et al., 2018; Shi et al., 2021) reported LVSP, and the results of meta-analysis showed that ASIV could not be considered to increase LVSP (n = 214, MD 6.82, 95% CI: −15.47∼29.10, p = 0.55; heterogeneity Chi2 = 975.80, p < 0.01, I2 = 99%) (Figure 4C). High heterogeneity may be due to different methods of modeling or different anesthetics. Because of high heterogeneity and subgroup analysis and sensitivity analysis cannot reasonably explain the source of heterogeneity, we consider qualitative analysis. Two studies (Jiang et al., 2016; Lv, 2018) reported that ASIV decreased LVSP compared with the control group (p < 0.01). LVSP decreases in HF (Walley, 2016). We noticed that in these two studies, LVSP in the HF model group was higher than that in the sham group. The author did not explain or analyze this in the results. It may be the compensatory increase caused by AAC (Katz et al., 2019). Hence, we consider that it is inappropriate to combine the results of these two studies with other studies After excluding these two studies, the other six studies (Zhao et al., 2009; Cui et al., 2013; Zhang et al., 2015; Cheng, 2017; Zhao et al., 2018; Shi et al., 2021) reported that ASIV had a positive effect on reducing LVSP compared with the control group (p < 0.01 or p < 0.05).
Twelve studies (Zhao et al., 2009; Cui et al., 2013; Zhang et al., 2015; Jiang et al., 2016; Cheng, 2017; Ji et al., 2018; Lv, 2018; Tang et al., 2018; Zhao et al., 2018; Wang et al., 2020; Shi et al., 2021; Song et al., 2021) reported LVEDP, and the results of meta-analysis showed that ASIV had a significant effect on improving LVEDP compared with the control group (n = 329, MD -11.59, 95% CI: −17.35∼−5.84, p < 0.01; heterogeneity Chi2 = 5572.36, p < 0.01, I2 = 100%). In order to explore the source of heterogeneity, we conducted subgroup analysis. Six studies (Zhao et al., 2009; Cui et al., 2013; Cheng, 2017; Ji et al., 2018; Wang et al., 2020; Shi et al., 2021) of coronary artery ligation modeling were included in the meta-analysis, and the results showed that ASIV had a significant effect on improving LVEDP compared with the control group (n = 147, MD -8.92, 95% CI: −15.46∼-2.38, p = 0.008; heterogeneity Chi2 = 232.55, p < 0.01, I2 = 98%). Then, we conducted sensitivity analysis to further explore the source of heterogeneity. LVEDP >15 is a common standard to judge HF (Bøkenes et al., 2008). Therefore, we excluded one study (Wang et al., 2020) with LVEDP much lower than 15 in the control group. After continuing to exclude a study (Cheng, 2017) with a large total dosage (3920 mg/kg), the results of four studies (Zhao et al., 2009; Cui et al., 2013; Ji et al., 2018; Shi et al., 2021) showed that ASIV had a significant effect on improving LVEDP compared with the control group (n = 87, MD -6.86, 95% CI: −8.76∼-4.97, p < 0.01; heterogeneity Chi2 = 4.15, p = 0.25, I2 = 28%) (Figure 4D).
Five studies (Cheng et al., 2016; Jiang et al., 2016; Zhao et al., 2018; Nie et al., 2019; Wang et al., 2022) reported HW/BW, and the results of meta-analysis showed that the effect of ASIV on reducing HW/BW was not statistically significant compared with the control group (n = 124, MD -1.08, 95% CI: −2.32∼0.17, p = 0.9; heterogeneity Chi2 = 2050.18, p < 0.01, I2 = 100%). After excluding a study (Nie et al., 2019) with the largest total dose (4480 mg/kg), the results of meta-analysis of four studies (Cheng et al., 2016; Jiang et al., 2016; Zhao et al., 2018; Wang et al., 2022) showed that ASIV could significantly reduce HW/BW compared with the control group (n = 104, MD -0.60, 95% CI: −0.72∼−0.48, p < 0.01; heterogeneity Chi2 = 5.56, p = 0.14, I2 = 46%) (Figure 5A).
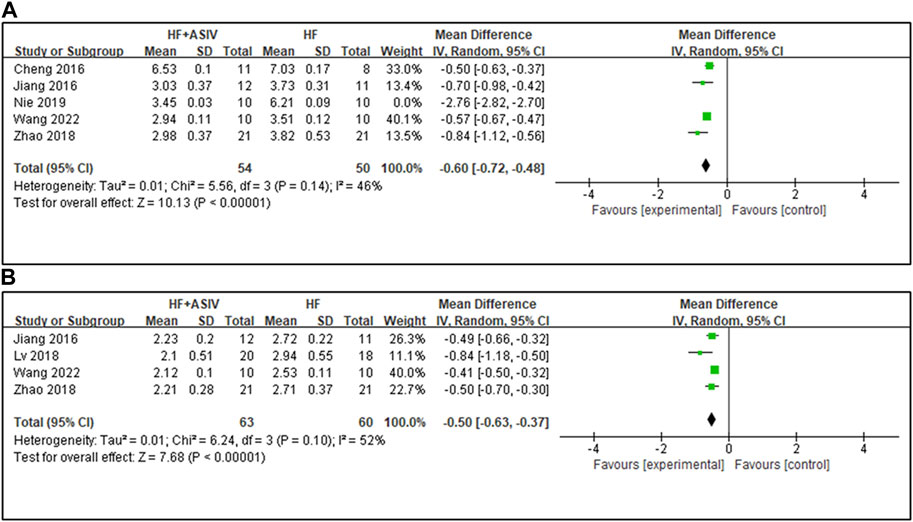
FIGURE 5. (A) The forest plot: effects of ASIV for reducing HW/BW compared with the control group. (B) The forest plot: effects of ASIV for reducing LVW/BW compared with the control group.
Four studies (Jiang et al., 2016; Lv, 2018; Zhao et al., 2018; Wang et al., 2022) reported LVW/BW, and the results showed that ASIV had a significant effect on improving LVW/BW compared with the control group (n = 123, MD -0.50, 95% CI: −0.63∼−0.37, p < 0.01; heterogeneity Chi2 = 6.24, p = 0.10, I2 = 52%) (Figure 5B). The reason for the high heterogeneity may be the differences in the total dose.
We explored whether the total dose of ASIV would affect the improvement of cardiac function. For this reason, we selected three main indexes (LVEF, LVEDP and LVW/BW) to evaluate cardiac function and analyzed the dosage-efficacy relationship. First, we excluded the study with extremely low dose (<5 mg/kg/d). For the index of LVEDP, when the total dose of ASIV ranged from 400 mg/kg to 3920 mg/kg, the dosage-efficacy relationship shows a significant positive correlation (Significance F < 0.01, p < 0.01). However, it should be noted that for the index of LVEF, the dosage-efficacy relationship did not show a positive correlation at the dose of 700 mg/kg - 3360 mg/kg (Significance F > 0.05, p > 0.05). For the index of LVW/BW (mg/g), when the total dose of ASIV ranged from 840 mg/kg to 3920 mg/kg, the dosage-efficacy relationship shows a significant positive correlation (Significance F < 0.01, p < 0.01) (Figure 6). These results may be affected by the mode of model establishment, the drug intervention starting time, the duration of intervention and other factors. Therefore, we consider carefully that in the range of ASIV dosage from 10 mg/kg/d to 80 mg/kg/d, the effect of treating HF may be dose-dependent and/or time-dependent, but this relationship might be nonlinear.
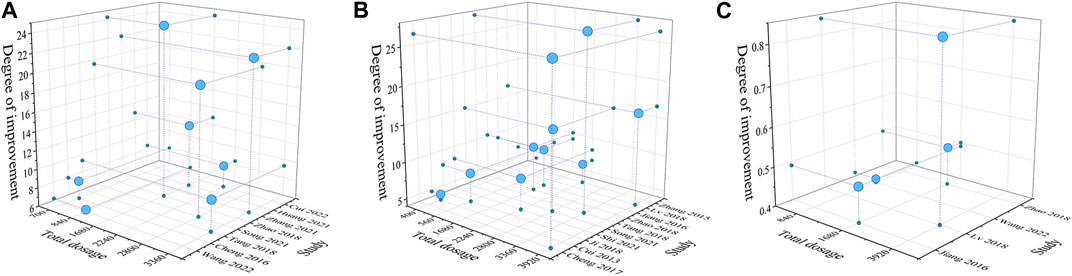
FIGURE 6. Three-dimensional images based on dosage - efficacy interval analyses [(A): LVEF; (B) LVEDP; (C) LVW/BW]. The effects of ASIV on improving LVEF was not significant with the increase of dosage. The effects of ASIV on improving LVEDP and LVW/BW were enhanced with the increase of dosage.
Our meta-analysis comprised 19 studies, encompassing a total of 489 animals. Our meta-analysis demonstrates that ASIV exerts cardioprotective effects in HF, as evidenced by increased LVEF, LVFS, and LV ± dp/dtmax, as well as decreased LVSP, LVEDP, HW/BW and LVW/BW. ASIV has been shown to enhance cardiac function post myocardial infarction by inhibiting myocardial fibrosis (Zhang et al., 2022) and promoting angiogenesis (Cheng et al., 2019). Our findings also support this conclusion as evidenced by the changes in the HW/BW and LVW/BW. The dosage-efficacy relationship of ASIV is positively correlated in a range of 10–80 mg/kg/d, indicating that higher doses and longer intervention times may be more effective in treating HF with ASIV, but this relationship may not increase linearly.
This meta-analysis and systematic review evaluated the latest research on the therapeutic effects of ASIV in ameliorating heart function decline caused by HF. In the past 3 years from 2020 to 2022, eight related animal experimental studies have been published. However, there have been no recent studies reviewing and discussing animal experiments, hence our work is timely and necessary. Our study focused on targeted analyses of multiple measurements to assess the positive effects of ASIV on reducing cardiac preload and afterload while inhibiting cardiac hypertrophy under conditions of HF. The dosage-efficacy interval analyses provide valuable information for future animal experiments to determine appropriate treatment times and doses. Additionally, this study contributes to reducing duplicate animal studies, improving animal research design, and provides reference evidence for converting preclinical experimental results into clinical use.
Some limitations of the study are listed as follows. The methodological quality of the included studies is generally poor. All studies lacked descriptions of allocation concealment and random placement of animals, and there were no reports of blinding with regard to feeding or intervention. Poor methodological quality is an inherent limitation that can impact accuracy (Landis et al., 2012). Furthermore, eight studies employed chloral hydrate as an anesthetic agent. Intraperitoneal administration of chloral hydrate in rats can induce non-mechanical intestinal obstruction, peritonitis, gastric ulcers, and intraperitoneal hemorrhage, which raises ethical concerns in animal research (Silverman and Muir, 1993; Baxter et al., 2009; Percie du Sert et al., 2020). Furthermore, chloral hydrate may elicit intricate effects on the cardiovascular system, thereby compromising the reliability of the results (Laurent et al., 2006; Han et al., 2011; Grissinger, 2019). Therefore, due to the imperfections in some experimental designs, we should treat the present positive results with caution. Given that ASIV’s effect on treating HF may be multi-targeted, additional research is necessary to analyze potential mechanisms of action. Moreover, because of the small sample size, the dosage-efficacy relationship of ASIV in treating HF requires further investigation with larger sample sizes and higher-quality evidence.
Numerous studies have demonstrated the crucial role of high-quality animal experiments as a reference point for drugs in preclinical research prior to clinical trials. However, given the vast differences between animal models and clinical practice, meticulous attention must be paid to the experimental design in preclinical research. This systematic review highlights key considerations for researchers, including the necessity of providing detailed descriptions of baseline characteristics before and after establishing animal models, as well as the use of standardized assessments, such as the SYRCLE Risk of Bias tool and the ten-item scale, to promote methodological quality. In particular, randomization and blinding techniques should be fully employed throughout the experimental process, including during model induction and outcome assessment. In the majority of relevant in vivo investigations, SD rats or Wistar rats are commonly employed as animal models. However, the utilization of genetically modified mice holds paramount significance in elucidating the underlying mechanisms, thereby warranting the recommendation for a more diversified selection of genetically edited mice to explore potential mechanisms. Exploring various administration methods assumes critical importance in attaining a comprehensive understanding of drug delivery efficacy and variations, consequently enriching our overall comprehension of experimental outcomes. In light of this, we recommend including research on different administration routes to address this knowledge gap. Meanwhile, taking into account the ethics of animal experiments and the impact of anesthesia on cardiovascular indicators, we recommend the use of isoflurane or pentobarbital sodium as anesthetic agents. HF typically presents in elderly patients with underlying conditions such as hypertension. Therefore, the use of relevant animal models can enhance the meaningfulness of the results. In the treatment of HF, long-term interventions and therapies are of paramount importance (Arrigo et al., 2020). Therefore, it is equally crucial to enhance our understanding of the enduring impact of ASIV on overall prognosis by increasing relevant research, thus further investigating the clinical prospects of ASIV’s application in HF management. We stress the importance of conducting studies with a wider dose range, including grouping doses, to determine the optimal dosing regimen. Such studies are essential to improving the clinical relevance and translatability of experimental results (Singh et al., 2022).
ASIV, a promising natural compound, has garnered significant attention due to its anti-inflammatory, antioxidant stress, neuroprotective, and other beneficial effects (Liang et al., 2023). It has been extensively investigated for its potential therapeutic applications in cardiovascular and cerebrovascular diseases, hepatitis, cancer, and other conditions (Chen et al., 2021; Li et al., 2022). Part of the pharmacological effects of ASIV can be attributed to its hydrolyzed active metabolite, Cycloastragenol (Yu et al., 2018). Regarding pharmacokinetics, ASIV exhibits relatively low bioavailability and absorption rates in the gastrointestinal tract of rats, with an absolute bioavailability of 2.2% (Gu et al., 2004). The elimination half-life of AS-IV in rats ranges from 34.0 to 131.6 min (Zhang et al., 2006). Following intravenous administration, ASIV is rapidly absorbed and widely distributed in various tissues. The kidneys and liver show the highest concentrations of ASIV, followed by the lungs, heart, and spleen. However, ASIV has limited distribution in the brain, likely due to its poor ability to cross the blood-brain barrier (Chang et al., 2012). It is important to note that there is limited research on the drug metabolism and safety of ASIV, and the quality of existing studies is not optimal. This poses a challenge for further exploration of the clinical therapeutic effects of ASIV. Most studies have utilized relatively low dosages and short administration durations, which may not be sufficient to observe acute and chronic toxicity. Therefore, more comprehensive investigations are needed to fully understand the potential benefits and safety profile of ASIV.
Additionally, the lack of high-quality meta-analyses and systematic reviews contributes to a limited understanding of the preclinical research efficacy of ASIV. Our study presents initial preclinical evidence supporting ASIV as a promising drug candidate for HF therapy. ASIV shows potential to safeguard cardiac function by decreasing cardiac preload and afterload, as well as inhibiting myocardial hypertrophy. Notably, our dose-effect analysis indicates that ASIV’s therapeutic effects range from 10 mg/kg to 80 mg/kg daily dosage, with a possible non-linear positive relationship between the dose and the efficacy.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
ZZ and MZ is the main contributor to this manuscript. ZZ and CL performed the comprehensive and systematic sorting and analyses of the literature. YX and ML processed the images and tables in the manuscript. YX, ML, and LZ provided constructive suggestions for the improvement of the research. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (No. 82004276) and Natural Science Foundation of Shandong Province (No. 2019WS553).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1226008/full#supplementary-material
ASIV, Astragaloside IV; HF, Heart failure; CNKI, Chinese National Knowledge Infrastructure; CI, confidence interval; LVEF, left ventricular ejection fraction; LVFS, left ventricular fractional shortening; ± dp/dtmax, left ventricular pressure change rate; LVEDP, left ventricular end-diastolic pressure; LVSP, left ventricular systolic pressure; HW/BW, heart weight/body weight; LVW/BW, left ventricular weight/body weight; PRISMA, Preferred reporting items for systematic reviews and meta-analyses; MeSH, Medical Subject Headings; MD, mean deviation; REM, and random effect model.
Arrigo, M., Jessup, M., Mullens, W., Reza, N., Shah, A. M., Sliwa, K., et al. (2020). Acute heart failure. Nat. Rev. Dis. Prim. 6 (1), 16. doi:10.1038/s41572-020-0151-7
Baxter, M. G., Murphy, K. L., Taylor, P. M., and Wolfensohn, S. E. (2009). Chloral hydrate is not acceptable for anesthesia or euthanasia of small animals. Anesthesiology 111 (1), 209–210. author reply 209-10. doi:10.1097/ALN.0b013e3181a8617e
Bøkenes, J., Aronsen, J. M., Birkeland, J. A., Henriksen, U. L., Louch, W. E., Sjaastad, I., et al. (2008). Slow contractions characterize failing rat hearts. Basic Res. Cardiol. 103 (4), 328–344. doi:10.1007/s00395-008-0719-y
Cao, X., Liu, H., Zhou, M., Chen, X., and Long, D. (2022). Comparative efficacy of five Chinese medicine injections for treating dilated cardiomyopathy with heart failure: A bayesian network meta-analysis. J. Ethnopharmacol. 282, 114604. doi:10.1016/j.jep.2021.114604
Chang, Y. X., Sun, Y. G., Li, J., Zhang, Q. H., Guo, X. R., Zhang, B. L., et al. (2012). The experimental study of Astragalus membranaceus on meridian tropsim: the distribution study of astragaloside IV in rat tissues. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 911, 71–75. doi:10.1016/j.jchromb.2012.10.024
Chen, T., Yang, P., and Jia, Y. (2021). Molecular mechanisms of astragaloside-IV in cancer therapy (Review). Int. J. Mol. Med. 47 (3), 13. doi:10.3892/ijmm.2021.4846
Cheng, J., Liu, B., Farjat, A. E., and Routh, J. (2017). The public health resource utilization impact of airway foreign bodies in children. J. Xiangtan Univ. Nat. Sci. Ed. 39 (04), 68–71. doi:10.1016/j.ijporl.2017.03.009
Cheng, S., Yu, P., Yang, L., Shi, H., He, A., Chen, H., et al. (2016). Astragaloside IV enhances cardioprotection of remote ischemic conditioning after acute myocardial infarction in rats. Am. J. Transl. Res. 8 (11), 4657–4669.
Cheng, S., Zhang, X., Feng, Q., Chen, J., Shen, L., Yu, P., et al. (2019). Astragaloside IV exerts angiogenesis and cardioprotection after myocardial infarction via regulating PTEN/PI3K/Akt signaling pathway. Life Sci. 227, 82–93. doi:10.1016/j.lfs.2019.04.040
Cook, C., Cole, G., Asaria, P., Jabbour, R., and Francis, D. P. (2014). The annual global economic burden of heart failure. Int. J. Cardiol. 171 (3), 368–376. doi:10.1016/j.ijcard.2013.12.028
Cui, D., Chen, Y., Liu, D., Meng, K., and Zhu, H. (2013). Effect of astragalus sapson on hemodynamics and neuroendocrine of rats with heart failure following myocardial infarction. J. Navy Med. 34 (01), 18–20.
Cui, L., Ma, J., Zhang, X., Xue, S., Wang, F., Xu, H., et al. (2022). Astragaloside IV protects cardiac function in rats with congestive heart failure with preserved ejection fraction and its influence on myocardial microvascular inflammatory factors. J. Emerg. Traditional Chin. Med. 31 (01), 53–57.
Dong, Z., Zhao, P., Xu, M., Zhang, C., Guo, W., Chen, H., et al. (2017). Astragaloside IV alleviates heart failure via activating PPARα to switch glycolysis to fatty acid β-oxidation. Sci. Rep. 7 (1), 2691. doi:10.1038/s41598-017-02360-5
Gerber, Y., Weston, S. A., Redfield, M. M., Chamberlain, A. M., Manemann, S. M., Jiang, R., et al. (2015). A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med. 175 (6), 996–1004. doi:10.1001/jamainternmed.2015.0924
Grissinger, M. (2019). Chloral hydrate: Is it still being used? Are there safer alternatives? P t 44 (8), 444–459.
Gu, Y., Wang, G., Pan, G., Fawcett, J. P., Jiye, A, and Sun, J. (2004). Transport and bioavailability studies of astragaloside IV, an active ingredient in Radix Astragali. Basic Clin. Pharmacol. Toxicol. 95 (6), 295–298. doi:10.1111/j.1742-7843.2004.t01-1-pto950508.x
Guo, H. X., Wang, J. R., Peng, G. C., Li, P., and Zhu, M. J. (2022). A data mining-based study on medication rules of Chinese herbs to treat heart failure with preserved ejection fraction. Chin. J. Integr. Med. 28 (9), 847–854. doi:10.1007/s11655-022-2892-5
Han, P., Song, H., Yang, P., Xie, H., and Kang, Y. J. (2011). Cardiac arrhythmias induced by chloral hydrate in rhesus monkeys. Cardiovasc Toxicol. 11 (2), 128–133. doi:10.1007/s12012-011-9106-2
Heidenreich, P. A., Albert, N. M., Allen, L. A., Bluemke, D. A., Butler, J., Fonarow, G. C., et al. (2013). Forecasting the impact of heart failure in the United States: A policy statement from the American heart association. Circ. Heart Fail 6 (3), 606–619. doi:10.1161/HHF.0b013e318291329a
Heidenreich, P. A., Bozkurt, B., Aguilar, D., Allen, L. A., Byun, J. J., Colvin, M. M., et al. (2022). 2022 AHA/ACC/HFSA guideline for the management of heart failure: executive summary: A report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation 145 (18), e876–e894. doi:10.1161/cir.0000000000001062
Hooijmans, C. R., Rovers, M. M., de Vries, R. B., Leenaars, M., Ritskes-Hoitinga, M., and Langendam, M. W. (2014). SYRCLE's risk of bias tool for animal studies. BMC Med. Res. Methodol. 14, 43. doi:10.1186/1471-2288-14-43
Huang, Y., Wang, Q., Yang, X., Tao, G., and Wang, L. (2021). Metformin triggers apoptosis and induction of the G0/G1 switch 2 gene in macrophages. J. Jilin Univ. Med. Ed. 47 (06), 1437–1445. doi:10.3390/genes12091437
Huffman, M. D., Berry, J. D., Ning, H., Dyer, A. R., Garside, D. B., Cai, X., et al. (2013). Lifetime risk for heart failure among white and black Americans: cardiovascular lifetime risk pooling project. J. Am. Coll. Cardiol. 61 (14), 1510–1517. doi:10.1016/j.jacc.2013.01.022
Ji, Y., Wang, T., Zhang, X., Li, L., Li, L., Guo, Y., et al. (2018). Astragalosides increase the cardiac diastolic function and regulate the "Calcium sensing receptor-protein kinase C-protein phosphatase 1" pathway in rats with heart failure. Biomed. Pharmacother. 103, 838–843. doi:10.1016/j.biopha.2018.04.111
Jiang, H., Zhang, J., Tan, H., and Wei, X. (2016). Safety and efficacy of thulium laser prostatectomy versus transurethral resection of prostate for treatment of benign prostate hyperplasia: A meta-analysis. Chin. Circulation J. 31 (02), 165–170. doi:10.1111/luts.12092
Katz, M. G., Fargnoli, A. S., Gubara, S. M., Chepurko, E., Bridges, C. R., and Hajjar, R. J. (2019). Surgical and physiological challenges in the development of left and right heart failure in rat models. Heart Fail Rev. 24 (5), 759–777. doi:10.1007/s10741-019-09783-4
Landis, S. C., Amara, S. G., Asadullah, K., Austin, C. P., Blumenstein, R., Bradley, E. W., et al. (2012). A call for transparent reporting to optimize the predictive value of preclinical research. Nature 490 (7419), 187–191. doi:10.1038/nature11556
Laurent, Y., Wallemacq, P., Haufroid, V., Renkin, J., Liolios, A., and Hantson, P. (2006). Electrocardiographic changes with segmental akinesia after chloral hydrate overdose. J. Emerg. Med. 30 (2), 179–182. doi:10.1016/j.jemermed.2005.05.019
Leung, A. Y. L., Chen, H., Jia, Z., Li, X., and Shen, J. (2021). Study protocol: traditional Chinese Medicine (TCM) syndrome differentiation for heart failure patients and its implication for long-term therapeutic outcomes of the Qiliqiangxin capsules. Chin. Med. 16 (1), 103. doi:10.1186/s13020-021-00515-1
Li, M., Han, B., Zhao, H., Xu, C., Xu, D., Sieniawska, E., et al. (2022). Biological active ingredients of Astragali Radix and its mechanisms in treating cardiovascular and cerebrovascular diseases. Phytomedicine 98, 153918. doi:10.1016/j.phymed.2021.153918
Liang, Y., Chen, B., Liang, D., Quan, X., Gu, R., Meng, Z., et al. (2023). Pharmacological effects of astragaloside IV: A review. Molecules 28 (16), 6118. doi:10.3390/molecules28166118
Liu, J., Li, Y., Bian, X., Xue, N., Yu, J., Dai, S., et al. (2021). Astragaloside IV alleviates heart failure by regulating SUMO-specific protease 1. Exp. Ther. Med. 22 (4), 1076. doi:10.3892/etm.2021.10510
Liu, Y., Xu, W., Xiong, Y., Du, G., and Qin, X. (2018). Evaluations of the effect of HuangQi against heart failure based on comprehensive echocardiography index and metabonomics. Phytomedicine 50, 205–212. doi:10.1016/j.phymed.2018.04.027
Lu, J., Wang, Q. Y., Zhou, Y., Lu, X. C., Liu, Y. H., Wu, Y., et al. (2017). AstragalosideⅣ against cardiac fibrosis by inhibiting TRPM7 channel. Phytomedicine 30, 10–17. doi:10.1016/j.phymed.2017.04.002
Luo, Y., Wan, Q., Xu, M., Zhou, Q., Chen, X., Yin, D., et al. (2019). Nutritional preconditioning induced by astragaloside Ⅳ on isolated hearts and cardiomyocytes against myocardial ischemia injury via improving Bcl-2-mediated mitochondrial function. Chem. Biol. Interact. 309, 108723. doi:10.1016/j.cbi.2019.06.036
Lv, Q. (2018). The effects of Astragaloside on cardiac myocytes in rats with heart failure. J. North Pharm. 15 (03), 156–158.
Mao, J., Zhang, J., Lam, C. S. P., Zhu, M., Yao, C., Chen, S., et al. (2020). Qishen yiqi dripping pills for chronic ischaemic heart failure: results of the CACT-IHF randomized clinical trial. Esc. Heart Fail 7 (6), 3881–3890. doi:10.1002/ehf2.12980
Merlo, M., Pivetta, A., Pinamonti, B., Stolfo, D., Zecchin, M., Barbati, G., et al. (2014). Long-term prognostic impact of therapeutic strategies in patients with idiopathic dilated cardiomyopathy: changing mortality over the last 30 years. Eur. J. Heart Fail 16 (3), 317–324. doi:10.1002/ejhf.16
Murphy, S. P., and Murphy, A. N. (2010). Pre-clinical systematic review. J. Neurochem. 115 (4), 805. doi:10.1111/j.1471-4159.2010.06998.x
Nie, P., Meng, F., Zhang, J., Wei, X., and Shen, C. (2019). Astragaloside IV exerts a myocardial protective effect against cardiac hypertrophy in rats, partially via activating the Nrf2/HO-1 signaling pathway. Oxidative Med. Cell. Longev. 2019, 4625912. doi:10.1155/2019/4625912
Percie du Sert, N., Ahluwalia, A., Alam, S., Avey, M. T., Baker, M., Browne, W. J., et al. (2020). Reporting animal research: explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 18 (7), e3000411. doi:10.1371/journal.pbio.3000411
Shi, H., Zhou, P., Gao, G., Liu, P.-P., Wang, S.-S., Song, R., et al. (2021). Astragaloside IV prevents acute myocardial infarction by inhibiting the TLR4/MyD88/NF-κB signaling pathway. J. food Biochem. 45 (7), e13757. doi:10.1111/jfbc.13757
Silverman, J., and Muir, W. W. (1993). A review of laboratory animal anesthesia with chloral hydrate and chloralose. Lab. Anim. Sci. 43 (3), 210–216.
Singh, D., Wasan, H., and Reeta, K. H. (2022). Preclinical stroke research and translational failure: A bird's eye view on preventable variables. Cell Mol. Neurobiol. 42 (7), 2003–2017. doi:10.1007/s10571-021-01083-6
Song, A., Wang, L., and Zhang, Y. (2021). Effect and mechanism of astragaloside iv on cardiomyocyte apoptosis in rats with chronic heart failure via tgf-β1/smad3 signaling pathway. Chin. J. Integr. Med. Cardio-Cerebrovascular Dis. 19 (10).
Sui, Y.-B., Zhang, K.-K., Ren, Y.-K., Liu, L., and Liu, Y. (2020). The role of Nrf2 in astragaloside IV-mediated antioxidative protection on heart failure. Pharm. Biol. 58 (1), 1192–1198. doi:10.1080/13880209.2020.1849319
Tang, B., Zhang, J.-G., Tan, H.-Y., and Wei, X.-Q. (2018). Astragaloside IV inhibits ventricular remodeling and improves fatty acid utilization in rats with chronic heart failure. Biosci. Rep. 38 (3). doi:10.1042/bsr20171036
Vaduganathan, M., Claggett, B. L., Jhund, P. S., Cunningham, J. W., Pedro Ferreira, J., Zannad, F., et al. (2020). Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: A comparative analysis of three randomised controlled trials. Lancet 396 (10244), 121–128. doi:10.1016/s0140-6736(20)30748-0
Walley, K. R. (2016). Left ventricular function: time-varying elastance and left ventricular aortic coupling. Crit. Care 20 (1), 270. doi:10.1186/s13054-016-1439-6
Wang, J., Yang, R., Zhang, F., Jia, C., Wang, P., Liu, J., et al. (2018). The effect of Chinese herbal medicine on quality of life and exercise tolerance in heart failure with preserved ejection fraction: A systematic review and meta-analysis of randomized controlled trials. Front. Physiol. 9, 1420. doi:10.3389/fphys.2018.01420
Wang, S., Chen, Y., Wang, N., Du, L., Li, Z., and Li, T. (2020). Experimental study on the effect of astragaloside IV on serum inflammatory factors and myocardial remodeling in rats with heart failure. World J. Integr. Traditional West. Med. 15 (09), 1661–1665. doi:10.13935/j.cnki.sjzx.200920
Wang, S. G., Xu, Y., Chen, J. D., Yang, C. H., and Chen, X. H. (2013). Astragaloside IV stimulates angiogenesis and increases nitric oxide accumulation via JAK2/STAT3 and ERK1/2 pathway. Molecules 18 (10), 12809–12819. doi:10.3390/molecules181012809
Wang, T., Ma, D., Liu, Y., Lv, F., and Wang, C. (2022). Effects of Astragaloside IV on myocardial mitochondrial energy metabolism in acute heart failure rats. J. Guangzhou Univ. Traditional Chin. Med. 39 (04), 884–891. doi:10.13359/j.cnki.gzxbtcm.2022.04.026
Yu, Y., Zhou, L., Yang, Y., and Liu, Y. (2018). Cycloastragenol: an exciting novel candidate for age-associated diseases. Exp. Ther. Med. 16 (3), 2175–2182. doi:10.3892/etm.2018.6501
Zhang, M., Quan, Y., Guo, M., Ganpan, P., Liu, C., and Huang, F. (2021). Effect of Astragaloside IV on ventricular remodeling and myocardial energy metabolism in Doxorubicin induced heart failure rat model. Chin. J. Gerontology 41 (07), 1494–1497.
Zhang, S. P., Tang, F. T., Yang, Y. H., Lu, M. L., Luan, A. N., Zhang, J., et al. (2015). Astragaloside IV protects against isoproterenol-induced cardiac hypertrophy by regulating NF-kappa B/PGC-1 alpha signaling mediated energy biosynthesis. Plos One 10 (3), e0118759. doi:10.1371/journal.pone.0118759
Zhang, W. D., Zhang, C., Liu, R. H., Li, H. L., Zhang, J. T., Mao, C., et al. (2006). Preclinical pharmacokinetics and tissue distribution of a natural cardioprotective agent astragaloside IV in rats and dogs. Life Sci. 79 (8), 808–815. doi:10.1016/j.lfs.2006.02.032
Zhang, X., Qu, H., Yang, T., Liu, Q., and Zhou, H. (2022). Astragaloside IV attenuate MI-induced myocardial fibrosis and cardiac remodeling by inhibiting ROS/caspase-1/GSDMD signaling pathway. Cell Cycle 21 (21), 2309–2322. doi:10.1080/15384101.2022.2093598
Zhang, Y., Zhang, J., Butler, J., Yang, X., Xie, P., Guo, D., et al. (2017). Contemporary epidemiology, management, and outcomes of patients hospitalized for heart failure in China: results from the China heart failure (China-HF) registry. J. Card. Fail 23 (12), 868–875. doi:10.1016/j.cardfail.2017.09.014
Zhao, Y., Yang, D., and Yu, S. (2018). Effect of Astragaloside IV on myocardial apoptosis and expression of P-Cx43 in rats with chronic heart failure. J. Clin. Exp. Med. 17 (20), 2143–2147.
Keywords: Astragaloside IV, heart failure, cardiac function, preclinical studies, meta-analysis
Citation: Zhang Z, Zhang M, Xu Y, Lu M, Zhang L and Li C (2023) Effect of Astragaloside IV on improving cardiac function in rats with heart failure: a preclinical systematic review and meta-analysis. Front. Pharmacol. 14:1226008. doi: 10.3389/fphar.2023.1226008
Received: 20 May 2023; Accepted: 21 September 2023;
Published: 03 October 2023.
Edited by:
Michał Tomczyk, Medical University of Bialystok, PolandReviewed by:
Jin-Wen Xu, Shanghai University of Traditional Chinese Medicine, ChinaCopyright © 2023 Zhang, Zhang, Xu, Lu, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Li, bGljaGFvNzE3OTVAaG90bWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.