- 1Department of Gastroenterology, Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 2Institute of Digestive Diseases, Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 3Department of Intensive Care Unit, Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, China
Ulcerative colitis (UC) is a refractory inflammatory bowel disease, and the outcomes of conventional therapies of UC, including 5-aminosalicylic acid, glucocorticoids, immunosuppressants, and biological agents, are not satisfied with patients and physicians with regard to adverse reactions and financial burden. The abnormality of the intestinal mucosal barrier in the pathogenesis of UC was verified. Qingchang Suppository (QCS) is an herbal preparation and is effective in treating ulcerative proctitis. The mechanism of QCS and its active ingredients have not been concluded especially in mucosal healing. This review elucidated the potential mechanism of QCS from the intestinal mucosal barrier perspective to help exploring future QCS research directions.
1 Introduction
Ulcerative colitis (UC) is a chronic idiopathic inflammation of the colon and rectum (Eisenstein, 2018; Kobayashi et al., 2020). The World Health Organization (WHO) has recognized UC as one of the refractory diseases (Kobayashi et al., 2020). Several clinical studies demonstrated UC patients who achieve mucosal healing (MH) have significantly lower rates of clinical recurrence, hospitalization, surgery, colonic dysplasia, and tumors (Shah et al., 2016; Kobayashi et al., 2020). The American College of Gastroenterology (ACG) introduced MH as a new target for UC treatment in UC clinical guidelines in 2019 (Ungaro et al., 2019).MH is strongly associated with the intestinal mucosal barrier (Boal Carvalho and Cotter, 2017). Thus, repairing the intestinal mucosa is important for the regression of UC. Qingchang Suppository (QCS) is an herbal formula preparation. It is composed of Indigo Naturalis, purslane; Herba Portulacae, Radix Notoginseng; Panax notoginseng (Burk.) F. H. Chen Ex C. Chow, Gallnut; Galla Chinensis and borneol; and Borneolum Syntheticum in a ratio of 2:2:5:5:1 under the guidance of TCM theory (Dai et al., 2010; Xu et al., 2019; Zhou G. et al., 2021).
Based on clinical studies, QCS therapy shows good treatment results in clinical symptoms’ relief, patient satisfaction, and recurrent rate decrease. Clinical symptoms’ relief includes decrease in pain, hemorrhage, and diarrhea, a satisfactory patient outcome (Xu et al., 2019).
According to published experimental research articles (Lu and Xie, 2010; Han et al., 2016; Sun et al., 2018; Yu et al., 2021), QCS plays roles in repairing barrier function, suppressing colonic permeability, ameliorating colonic hypoxia, and decreasing colonic micro-vascular permeability (VP).
Mucoal healing is the goal of UC treatment. It has been reported that QCS could promote the colonic mucosa repair in the clinical trials and the animal experiments. This review tends to systematically elucidate the mechnisms of QCS and its ingredients on repairing the colonic mucosa.
2 Damaged intestinal barrier in UC
The intestinal barrier is an important interface between the body and the external environment for preventing the invasion of pathogenic antigens and plays a crucial role in maintaining internal homeostasis (Du et al., 2015). The intestinal physical barrier is composed by four sections: the chemical barrier, the mechanical barrier, the immune barrier, and the microbial barrier (Yao et al., 2021). The integrity of the intestinal epithelium is essential for the intestine to function as a barrier (Salvo Romero et al., 2015), and the destruction of tight junctions (TJs) of intestinal epithelial cells increased the intestinal permeability (Miner-Williams and Moughan, 2016; Xiao et al., 2018). TJs are the most important structure and composed of occludin, claudin, and ZOs proteins (Vancamelbeke and Vermeire, 2017), whose integrity is essential for the functioning of the intestinal mucosal barrier (Otani and Furuse, 2020). Several studies have proven that the disruption of the TJs can cause structural and functional damage of the intestinal mucosal barrier (Fukui, 2016; Li et al., 2020a; Li et al., 2020b).
It is now commonly accepted that the abnormality of the intestinal mucosal barrier is the basic pathogenesis initiating and promoting the development of UC (Ungaro et al., 2017; van der Post et al., 2019). The increased epithelial permeability (EP) can lead to intestinal mucosal barrier dysfunction. Intestinal infections and inflammatory factors can induce abnormal intestinal mucosal barrier function, leading to intestinal mucosal permeability increased, and a large number of bacteria and antigens are transported into the lamina propria, activating abnormal mucosal immune responses (Li et al., 2022), and intestinal mucosal barrier function is damaged (Mankertz and Schulzke, 2007; Al-Sadi et al., 2009).
3 Clinical research of QCS and its active ingredients
From 1990 to 2020, 11 controlled clinical studies were conducted (Li and June 2018). Especially, in 2020, active mild-to-moderate UC patients were enrolled in a prospective, randomized, positive drug parallel control trial. The study demonstrated that the efficacy, effective time, and course of clinical remission were not inferior to those of SASP suppository (Dai, 2020).
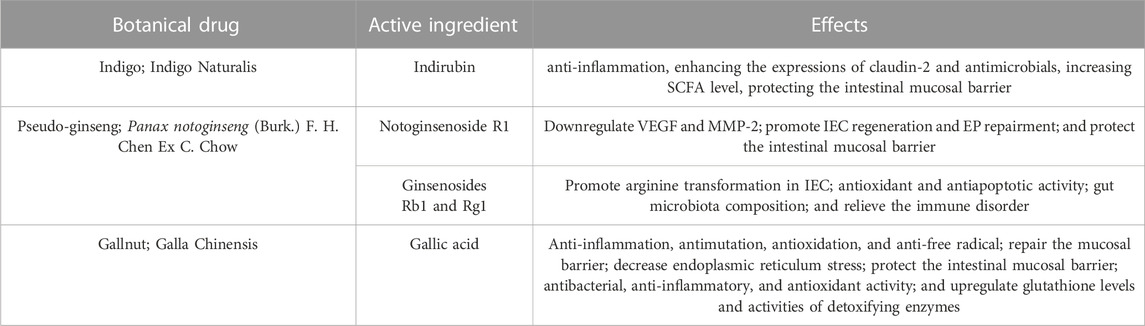
According to the results of HPLC-MC/MC analysis, QCS contains multiple bioactive compounds including indirubin, notoginsenoside R1, ginsenosides Rb1 and Rg1, and gallic acid α (Sun et al., 2018) (Table 1). Indirubin has a significant anti-inflammatory activity (Gao et al., 2016). Notoginsenoside R1, ginsenosides Rb1 (GRb1) and Rg1 are active ingredients in Radix notoginseng. Notoginsenoside R1 could down-regulate vascular endothelial growth factor (VEGF) and matrix metalloproteinase-2 (MMP-2) and promote the regeneration of endothelial cells (ECs) (Chen et al., 2004). GRb1 and Rg1 could induce the production of NO by motivating the PI3K/Akt/eNOS signal pathway to promote arginine transformation in endothelial cells, so as to increase the amount of vessels in rats (Pan et al., 2012). Our recent research found that Panax notoginseng could downregulate VEGFA and inhibit the Rap1GAP/TSP1 signaling pathway to promote EP repairment (Wang S. et al., 2018). Gallic acid (GA), an active ingredient of gallnut, could also ameliorate oxidative stress and inflammation and regulate proliferation and apoptosis of the colonic epithelia (Chang et al., 2015). Purslane possesses a wide range of pharmacological effects, such as antimicrobial, antioxidant, anti-inflammatory, antiulcerogenic, and anticancer activities (Zhou et al., 2015) (Table 2).
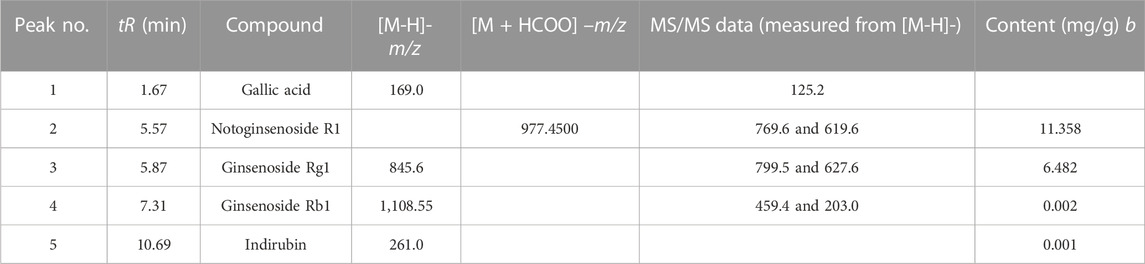
TABLE 1. Characterization and quantification of the major biochemical components from Qingchang Suppository by LC-ESI-MS/MS.
4 Effect and mechanisms of QCS on repairing the intestinal barrier
4.1 Effect of QCS against the increased colonic permeability
The most commonly used method to assess intestinal mucosal barrier function is the measurement of intestinal mucosal permeability with fluorescein-dextran 4,000 (FITC-dextran 4,000, FD-4), D-lactate, or diamine oxidase (DAO). FD-4 is an organic fluorescent pigment commonly used in immunofluorescence and flow cytometry to bind to different antibodies with the help of isothiocyanate reactive groups. D-lactate is a metabolite of many bacteria in the gastrointestinal tract. Mammals lack D-lactate dehydrogenase and cannot metabolize it rapidly, so the amount of D-lactate in the body under normal condition is small and relatively stable. When the intestinal mucosal barrier is disrupted, more D-lactate enters the circulation through the damaged intestinal mucosa and the blood D-lactate level increases significantly.
DAO is an intracellular enzyme in mammalian intestinal mucosal cells. When the mucosal cells are damaged, the intracellular DAO is released into the blood and the serum DAO is increased. The higher serum DAO, the severer mucosal damage as well as higher permeability of the intestinal mucosa (Xun et al., 2015). In addition, it has been reported that the level of endotoxin in circulation is positively associated with the degree of permeability of the intestinal mucosa (Tornai et al., 2017).
Lu et al. reported that FD4 in the colon of the colitis rats were increased as six times as that of normal rats and QCS could effectively decrease FD4 in the colon and promote ulcer healing of the colitis rats (Lu and Xie, 2010). Previous studies showed that the levels of DAO in serum were increased in the intestinal ischemia/reperfusion (IIR) rats, suggesting IIR could damage the mucosal integrity (Peng et al., 2018). GRb1 has antioxidant and antiapoptotic effects (Zheng et al., 2017). Chen et al. occluded the superior mesenteric artery for 75 min and re-perfused for 3 h to establish an IIR-intestinal epithelial injury model. Compared with the model group, GRb1 treatment group and pretreatment group could decrease the serum DAO and the expression of Akt and p-Akt. The protection of GRb1 could be eliminated by PI3K inhibitor, suggesting that GRb1 could decrease the intestinal permeability via PI3K/Akt pathway (Chen et al., 2019) (Figure 1).
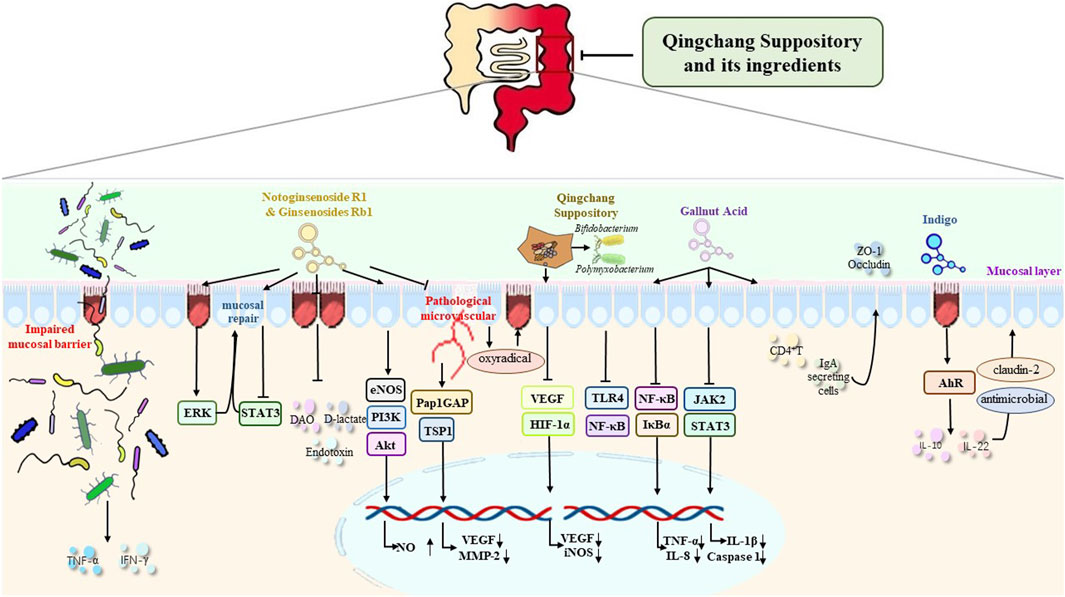
FIGURE 1. Mechanism of QCS and its ingredients in the repair of the intestinal mucosal barrier and in the improvement of the damaged intestinal barrier, the expression of TJs, epithelial cell survival and migration, mucosal blood vessels, intestinal microbiota, and mucosal inflammation and immunity.
4.2 QCS repairs the damaged intestinal barrier by elevating the expression of TJs
The disruption of intestinal TJs play an important role in the pathogenesis of UC (Tan et al., 2019). The decreased expression of ZO-1 and occludin proteins in UC patients leads to increased intestinal permeability and abundant pro-inflammatory factors invading the lamina propria of the colon, which triggers an intestinal immune response (Kim et al., 2018). Zhou et al. found QCS could increase the expressions of ZO-1 and occludin which were inhibited in the colitis mice induced by DSS (Zhou G. et al., 2021). Cai et al. found that LPS could suppress the expression of IPEC-J2 cells while this suppression could be reversed when the cells were pretreated by GA, an ingredient of gallnut (Liu et al., 2019; Cai et al., 2022).
4.3 Active ingredients of QCS repair the damaged intestinal barrier by regulating the epithelial cell survival and migration
Zhang et al. found purslane extract could not only decrease p-ERK, p-eIF2 α, Beclin1, and LC3II protein expression in IL-10−/− model mice. But also reduce the damage of IECs by decreasing endoplasmic reticulum stress through the pERK-eIF2α/Beclin1-LC3II pathway (Zhang et al., 2022). Ginsenosides were found to significantly enhance colonic mucosal repair and promote intestinal mucosal healing in a TNBS-induced rat colitis model by activating the ERK and Rho-dependent pathways (Toyokawa et al., 2019). Indigo could increase IL-10 and IL-22 expression in isolated LP monocytes from colitis rats induced by TNBS and DSS. But this effect was not present in AhR−/− mice. Moreover, indigo and indirubin could increase IL-10-producing CD4+ T cells and IL-22-producing CD3+−RORγt cells instead of CD4+Foxp3+Treg cells (Dudakov et al., 2015; Kawai et al., 2017; Wang et al., 2017).
Makoto et al. carried out a multicenter randomized controlled trial to investigate the safety and efficacy of IN in UC patients. A total of 86 patients were enrolled and given IN 0.5, 1.0, and 2.0 g or placebo for 8 weeks. The rates of mucosal healing were 13.6% in the placebo group, 56.5% in the 0.5 g IN group, 60.0% in the 1.0 g IN group, and 47.6% in the 2.0 g IN group (p = 0.0278 compared with placebo) (Naganuma et al., 2018). The post hoc analysis of this trial also demonstrated that the MH rate of IN was significantly higher than that of the placebo in the patients with steroid-dependent disease (p = 0.009) (Naganuma et al., 2020).
4.4 QCS repairs the damaged intestinal barrier by regulating the mucosal blood vessels
Intestinal EP is determined by the epithelia as well as the mucosal micro-vascular endothelia. The pathological micro-vascular endothelia cannot supply sufficient oxygen to the intestinal epithelia, destroy vasular function and accelerate the infiltration of inflammatory cells (Thornton and Solomon, 2002). Increased mucosal VP and decreased and slow blood flow also could lead to epithelia hypoxia (Taylor and Colgan, 2007), which induces increased pro-inflammatory cytokines resulting in intestinal epithelial cell injury and dissolution of TJs between goblet cells (Rezaie et al., 2007). Therefore, VP elevation may be the initial episode, earlier than increased EP, in UC occurrence and recurrence (Tolstanova et al., 2012), and VEGF inhibition can decrease VP and treat UC (Tolstanova et al., 2009). Wang S. et al. (2018) intervened DSS-colitis mice with QCS and found that QCS could improve colonic hypoxia and reduce colonic VP via VEGF/HIF-1a signaling pathway to improve vascular endothelial barrier function.
4.5 QCS repairs the damaged intestinal barrier by regulating intestinal microbiota
Intestinal microbiota is a complex community (Franzosa et al., 2019). It is also a crucial factor in intestinal mucosal injury (Kostic et al., 2014). The importance of intestinal flora in the occurrence and recurrence of UC has drawn increasing attention from the global scientific community. Increasing evidence has proven that the alteration of intestinal flora is a common feature in UC patients and mice colitis (Wlodarska et al., 2015). It has been generally accepted that UC is related to lower diversity of bacteria (Machiels et al., 2014; Prosberg et al., 2016; Knoll et al., 2017; Lopez-Siles et al., 2017) and decreased abundance of bacteria (Yao et al., 2016; Ricciuto et al., 2020). The damaged intestinal mucosal defense allows a large number of bacteria and their metabolites to enter the blood circulation and triggers a systemic immune response, which plays an extremely important role in the pathogenesis of UC (Kato et al., 2014; Liu et al., 2016). Therefore, the maintenance of intestinal microbial homeostasis is important to prevent the occurrence and recurrence of UC. Wen et al. observed whether QCS could treat UC via regulating colonic microbiota. They divided SD rats into 4 groups: normal group, DSS-induced colitis model group(M), M1 group and M2 group. The rats in M1 group were treated with DSS and filtrate of faeces of colitis rats. The rats in M2 group were treated with DSS and QCS-treated filtrate of faeces of colitis rats. They found the DAI score and the expression of TLR4 and NF-κB of M2 group were significantly lower than those of M group and M1 group. Additionally, QCS were found to improve the proliferation of B. bifidum and B. thetaiotaomicron. These results suggested that QCS could promote colon mucosa repair by improving the growth of Bifidobacterium bifidum and Bacteroides thetaiotaomicron and inhibiting TLR4/NF-kB signaling pathway (Wen et al., 2021). Harmful bacteria like Bacteroidetes and Proteobacteria producing TNF-α, IL-6, and IL-8 were found to increase, while beneficial bacterium like Firmicutes secreting IL-10 decrease in DSS-induced colitis mice (Ma et al., 2018; Liang et al., 2019). 16S rDNA sequence analysis proved that IN could adjust the composition of intestinal flora in colitis mice, especially related to the anaerobic Gram-positive bacteria of Turicibacter and Peptococcus (Liang et al., 2019; Yang et al., 2021). IN treatment could decrease the percentage of the harmful bacteria in colitis mice and restore the microbiota composition, which proves that IN could repair the DSS-induced gut microbiota imbalance (Liang et al., 2019). Indigo might exert its protective effects by increasing butyrate of the microbiota (Sun et al., 2020). The interaction between indigo and portulaca oleracea polysaccharide could promote short-chain fatty acid (SCFA) generation and metabolism (Fu et al., 2022). Wang et al. divided metronidazole-disposed-C57BL/6 mice into control, DSS, and DSS + ginseng pretreatment (28 mg/d/kg) groups. They found that the levels of three fecal endogenous metabolites including lactate, linoleic acid and malic acid of ginseng pretreatment group were significantly lower than those of DSS group, suggesting ginseng might repair the damaged intestinal barrier by regulating the intestinal microbiota (Wang CZ. et al., 2018).
4.6 QCS repairs the damaged intestinal barrier function by regulating mucosal inflammation and immunity
A fundamental experiment demonstrated that the inhibition of JAK/STAT pathway could benefit the integrity of the intestinal mucosa barrier (Chu et al., 2018; Soendergaard et al., 2018). Tofacitinib can inhibit JAK and is used to treat UC (De Vries et al., 2019). Researchers stimulated murine peritoneal macrophages with lipopolysaccharide (LPS), and they found the inhibition of JAK/STAT could decrease the secretion of inflammatory factors like IL-1β and IL-18 (Montoya et al., 2018). QCS and its ingredients, including indigo, ginsenoside R1, and GA, could exert the same effect as the inhibitor of JAK/STAT to alleviate inflammation and protect the intestinal mucosal barrier (Yu et al., 2021). In addition, GA has shown its antibacterial, anti-inflammatory, and antioxidant activity in vitro (Phonsatta et al., 2017; Cai et al., 2022). IgA produced by B cells, is a strong immunoglobulin on goblet, and protect the intestinal mucosal barrier from microorganism invasion (Pabst, 2012). IgA synthesis and secretion is the most recognized characteristic in mucosal immunity (Lycke and Bemark, 2017). Ginsenoside relieves the immune disorder in three ways: in the spleen, it can reverse proinflammatory and anti-inflammatory lymphocyte subsets ratio; in the intestine, it can stimulate CD4+ T cells to produce mucosal beneficial cytokines; and on the surface of goblet, it can assist B cells to secrete IgA to help mucin expression and the expression of TJs (Zhou R. et al., 2021). The decreased secretion of mucin by goblet cells weakens the intestinal mucus barrier and further impairs the function of the intestinal barrier (Zheng et al., 2019). GA could ameliorate dimethylhydrazine (DMH)-induced colonic inflammation, mucin depletion and intestinal epithelial cells’ oxidative stress, proliferation, and apoptosis disintegration in Wistar rats by upregulating the glutathione levels and activities of detoxifying enzymes (Shree et al., 2020).
5 Summary and prospect
The studies of mucosal healing, have developed rapidly in the past 10 years. The new perspectives of mucosal healing include new diagnostic tools like endomicroscopy, new etiology learning of intestinal inflammation like autoimmune response, and new biomarkers to predict mucosal healing like molecular markers. Based on the studies of QCS and its active ingredients, the mechanisms of QCS on repairing the intestinal mucosal barrier include suppression of colonic permeability, up-regulation of TJs, epithelial cell survival and migration regulation, mucosal blood vessels regulation, intestinal microbiota regulation, and mucosal inflammation and immunity regulation. Some of the above mechanisms and clinical results were concluded from the studies on the active ingredients of QCS instead of QCS. Whether the effects of the active ingredients could reflect the effect of the whole formula has not been clear. Therefore, it is necessary to study the mechanisms and the efficacy of the whole formula in the future. Additionally, the available clinical results of QCS are all from short term clinical trials. The long-term clinical effectiveness and safety of QCS are unknown. It is essential to conduct long-term clinical trial to provide substantial evidence for treating UC with QCS.
Author contributions
JS, SL, and HL were involved in the bibliography and literature search, and JS completed the draft. JY and JL provided thoughts on the framework and critically revised the draft. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82104743), the Shanghai Municipal Health Commission and Shanghai Municipal Administrator of Traditional Chinese Medicine (ZY(2021-2023)-0207-01) and Longhua Hospital affiliated to Shanghai University of Traditional Chinese Medicine, Longhua Hospital Innovation Project (KY2056).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Al-Sadi, R., Boivin, M., and Ma, T. (2009). Mechanism of cytokine modulation of epithelial tight junction barrier. Front. Biosci. (Landmark Ed. 14 (7), 2765–2778. doi:10.2741/3413
Boal Carvalho, P., and Cotter, J. (2017). Mucosal healing in ulcerative colitis: a comprehensive review. Drugs 77 (2), 159–173. doi:10.1007/s40265-016-0676-y
Cai, L., Wei, Z., Zhao, X., Li, Y., Li, X., and Jiang, X. (2022). Gallic acid mitigates LPS-induced inflammatory response via suppressing NF-κB signalling pathway in IPEC-J2 cells. J. animal physiology animal Nutr. 106 (5), 1000–1008. doi:10.1111/jpn.13612
Chang, Y. J., Hsu, S. L., Liu, Y. T., Lin, Y. H., Lin, M. H., Huang, S. J., et al. (2015). Gallic acid induces necroptosis via TNF-α signaling pathway in activated hepatic stellate cells. PloS one 10 (3), e0120713. doi:10.1371/journal.pone.0120713
Chen, S., Li, X., Wang, Y., Mu, P., Chen, C., Huang, P., et al. (2019). Ginsenoside Rb1 attenuates intestinal ischemia/reperfusion-induced inflammation and oxidative stress via activation of the PI3K/Akt/Nrf2 signaling pathway. Mol. Med. Rep. 19 (5), 3633–3641. doi:10.3892/mmr.2019.10018
Chen, S. W., Li, X. H., Ye, K. H., Jiang, Z. F., and Ren, X. D. (2004). Total saponins of Panax notoginseng protected rabbit iliac artery against balloon endothelial denudation injury. Acta Pharmacol. Sin. 25 (9), 1151–1156.
Chu, X. Q., Wang, J., Chen, G. X., Zhang, G. Q., Zhang, D. Y., and Cai, Y. Y. (2018). Overexpression of microRNA-495 improves the intestinal mucosal barrier function by targeting STAT3 via inhibition of the JAK/STAT3 signaling pathway in a mouse model of ulcerative colitis. Pathology, Res. Pract. 214 (1), 151–162. doi:10.1016/j.prp.2017.10.003
Dai, X. L. (2020). “Randomized controlled clinical trial of relieving active mild to moderate ulcerative colitis induced by Qingchang Suppository [D],” (China: Shanghai University of Traditional Chinese Medicine). MFA thesis.
Dai, Y. C., Tang, Z. P., Ma, G. T., Gong, Y. P., Liu, W., and Zhang, Y. L. (2010). A review of Qingchang Shuan for treatment of ulcerative colitis. J. traditional Chin. Med. = Chung i tsa chih ying wen pan 30 (3), 237–240. doi:10.1016/s0254-6272(10)60049-0
De Vries, L. C. S., Duarte, J. M., De Krijger, M., Welting, O., Van Hamersveld, P. H. P., Van Leeuwen-Hilbers, F. W. M., et al. (2019). A JAK1 selective kinase inhibitor and Tofacitinib affect macrophage activation and function. Inflamm. Bowel Dis. 25 (4), 647–660. doi:10.1093/ibd/izy364
Du, J., Chen, Y., Shi, Y., Liu, T., Cao, Y., Tang, Y., et al. (2015). 1,25-Dihydroxyvitamin D protects intestinal epithelial barrier by regulating the myosin light chain kinase signaling pathway. Inflamm. Bowel Dis. 21 (11), 2495–2506. doi:10.1097/MIB.0000000000000526
Dudakov, J. A., Hanash, A. M., and van den Brink, M. R. (2015). Interleukin-22: immunobiology and pathology. Annu. Rev. Immunol. 33, 747–785. doi:10.1146/annurev-immunol-032414-112123
Eisenstein, M. (2018). Ulcerative colitis: towards remission. Nature 563 (7730), S33. doi:10.1038/d41586-018-07276-2
Franzosa, E. A., Sirota-Madi, A., Avila-Pacheco, J., Fornelos, N., Haiser, H. J., Reinker, S., et al. (2019). Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 4 (2), 293–305. doi:10.1038/s41564-018-0306-4
Fu, Q., Zhou, S., Yu, M., Lu, Y., He, G., Huang, X., et al. (2022). Portulaca oleracea polysaccharides modulate intestinal microflora in aged rats in vitro. Front. Microbiol. 13, 841397. doi:10.3389/fmicb.2022.841397
Fukui, H. (2016). Increased intestinal permeability and decreased barrier function: does it really influence the risk of inflammation? Inflamm. Intest. Dis. 1 (3), 135–145. doi:10.1159/000447252
Gao, W., Guo, Y., Wang, C., Lin, Y., Yu, L., Sheng, T., et al. (2016). Indirubin ameliorates dextran sulfate sodium-induced ulcerative colitis in mice through the inhibition of inflammation and the induction of Foxp3-expressing regulatory T cells. Acta Histochem. 118 (6), 606–614. doi:10.1016/j.acthis.2016.06.004
Han, Z., Cui, B., Lu, J., An, R., Xu, L., and Wang, X. (2016). Quality standard for Qingchang suppository. Chin. Tradit. Pat. Med. 38 (08), 1749–1753. (Chinese). doi:10.3969/j.issn.1001-1528.2016.08.019
Kato, L. M., Kawamoto, S., Maruya, M., and Fagarasan, S. (2014). The role of the adaptive immune system in regulation of gut microbiota. Immunol. Rev. 260 (1), 67–75. doi:10.1111/imr.12185
Kawai, S., Iijima, H., Shinzaki, S., Hiyama, S., Yamaguchi, T., Araki, M., et al. (2017). Indigo Naturalis ameliorates murine dextran sodium sulfate-induced colitis via aryl hydrocarbon receptor activation. J. gastroenterology 52 (8), 904–919. doi:10.1007/s00535-016-1292-z
Kim, M. W., Choi, S., Kim, S. Y., Yoon, Y. S., Kang, J. H., and Oh, S. H. (2018). Allyl isothiocyanate ameliorates dextran sodium sulfate-induced colitis in mouse by enhancing tight junction and mucin expression. Int. J. Mol. Sci. 19 (7), 2025. doi:10.3390/ijms19072025
Knoll, R. L., Forslund, K., Kultima, J. R., Meyer, C. U., Kullmer, U., Sunagawa, S., et al. (2017). Gut microbiota differs between children with inflammatory bowel disease and healthy siblings in taxonomic and functional composition: a metagenomic analysis. Am. J. physiology Gastrointest. liver physiology 312 (4), G327–g339. doi:10.1152/ajpgi.00293.2016
Kobayashi, T., Siegmund, B., Le Berre, C., Wei, S. C., Ferrante, M., Shen, B., et al. (2020). Ulcerative colitis. Nat. Rev. Dis. Prim. 6 (1), 74. doi:10.1038/s41572-020-0205-x
Kostic, A. D., Xavier, R. J., and Gevers, D. (2014). The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology 146 (6), 1489–1499. doi:10.1053/j.gastro.2014.02.009
Li, B., Wu, Z., and Yuan, C. (2020a). Protective effect of protectin D1 inhibitor on intestinal mucosal injury in mice with severe acute pancreatitis. Parenter. Enter. Nutr. 27 (04), 205–210. (Chinese). doi:10.16151/j.1007-810x.2020.04.004
Li, B., Yuan, C., Dong, J., Zhang, N., Yan, J. Y., and Zou, H. T. (2020b). Whole genome sequencing and comparative genomic analysis of oleaginous red yeast Sporobolomyces pararoseus NGR identifies candidate genes for biotechnological potential and ballistospores-shooting. Parenter. Enter. Nutr. 27 (03), 181–186. (Chinese). doi:10.1186/s12864-020-6593-1
Li, C. N., and Jun, Y. P. (2018). Progress of clinical and experimental research on the treatment of ulcerative colitis with Qingchang suppository. Electron. J. Clin. Med. literature 2018, 181–183+185. doi:10.16281/j.cnki.jocml.2018.09.098
Li, X., Li, Q., Xiong, B., Chen, H., Wang, X., and Zhang, D. (2022). Discoidin domain receptor 1(DDR1) promote intestinal barrier disruption in Ulcerative Colitis through tight junction proteins degradation and epithelium apoptosis. Pharmacol. Res. 183, 106368. doi:10.1016/j.phrs.2022.106368
Liang, Y. N., Yu, J. G., Zhang, D. B., Zhang, Z., Ren, L. L., Li, L. H., et al. (2019). Indigo naturalis ameliorates dextran sulfate sodium-induced colitis in mice by modulating the intestinal microbiota community. Mol. (Basel, Switz. 24 (22), 4086. doi:10.3390/molecules24224086
Liu, B., Jiang, X., Cai, L., Zhao, X., Dai, Z., Wu, G., et al. (2019). Putrescine mitigates intestinal atrophy through suppressing inflammatory response in weanling piglets. J. animal Sci. Biotechnol. 10, 69. doi:10.1186/s40104-019-0379-9
Liu, Z., Wang, X., and Li, T. (2016). The clinical significance of intestinal flora changes in patients with ulcerative colitis. Chin. J. Gastroenterol. Hepatol. 25 (05), 554–556. (Chinese). doi:10.3969/j.issn.1006-5709.2016.05.018
Lopez-Siles, M., Duncan, S. H., Garcia-Gil, L. J., and Martinez-Medina, M. (2017). Faecalibacterium prausnitzii: from microbiology to diagnostics and prognostics. ISME J. 11 (4), 841–852. doi:10.1038/ismej.2016.176
Lu, L., and Xie, J-Q. (2010). Effect of Qingchang suppository on intestinal permeability in rats with ulcerative colitis. Chin. J. Integr. Traditional West. Med. 30, 1087–1090. (Chinese).
Lycke, N. Y., and Bemark, M. (2017). The regulation of gut mucosal IgA B-cell responses: recent developments. Mucosal Immunol. 10 (6), 1361–1374. doi:10.1038/mi.2017.62
Ma, X., Hu, Y., Li, X., Zheng, X., Wang, Y., Zhang, J., et al. (2018). Periplaneta americana ameliorates dextran sulfate sodium-induced ulcerative colitis in rats by keap1/nrf-2 activation, intestinal barrier function, and gut microbiota regulation. Front. Pharmacol. 9, 944. doi:10.3389/fphar.2018.00944
Machiels, K., Joossens, M., Sabino, J., De Preter, V., Arijs, I., Eeckhaut, V., et al. (2014). A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 63 (8), 1275–1283. doi:10.1136/gutjnl-2013-304833
Mankertz, J., and Schulzke, J. D. (2007). Altered permeability in inflammatory bowel disease: pathophysiology and clinical implications. Curr. Opin. gastroenterology 23 (4), 379–383. doi:10.1097/MOG.0b013e32816aa392
Miner-Williams, W. M., and Moughan, P. J. (2016). Intestinal barrier dysfunction: implications for chronic inflammatory conditions of the bowel. Nutr. Res. Rev. 29 (1), 40–59. doi:10.1017/S0954422416000019
Montoya, T., Aparicio-Soto, M., Castejón, M. L., Rosillo, M. Á., Sánchez-Hidalgo, M., Begines, P., et al. (2018). Peracetylated hydroxytyrosol, a new hydroxytyrosol derivate, attenuates LPS-induced inflammatory response in murine peritoneal macrophages via regulation of non-canonical inflammasome, Nrf2/HO1 and JAK/STAT signaling pathways. J. Nutr. Biochem. 57, 110–120. doi:10.1016/j.jnutbio.2018.03.014
Naganuma, M., Sugimoto, S., Fukuda, T., Mitsuyama, K., Kobayashi, T., Yoshimura, N., et al. (2020). Indigo naturalis is effective even in treatment-refractory patients with ulcerative colitis: a post hoc analysis from the INDIGO study. J. gastroenterology 55 (2), 169–180. doi:10.1007/s00535-019-01625-2
Naganuma, M., Sugimoto, S., Mitsuyama, K., Kobayashi, T., Yoshimura, N., Ohi, H., et al. (2018). Efficacy of indigo naturalis in a multicenter randomized controlled trial of patients with ulcerative colitis. Gastroenterology 154 (4), 935–947. doi:10.1053/j.gastro.2017.11.024
Otani, T., and Furuse, M. (2020). Tight junction structure and function revisited. Trends Cell Biol. 30 (10), 805–817. doi:10.1016/j.tcb.2020.08.004
Pabst, O. (2012). New concepts in the generation and functions of IgA. Nat. Rev. Immunol. 12 (12), 821–832. doi:10.1038/nri3322
Pan, C., Huo, Y., An, X., Singh, G., Chen, M., Yang, Z., et al. (2012). Panax notoginseng and its components decreased hypertension via stimulation of endothelial-dependent vessel dilatation. Vasc. Pharmacol. 56 (3-4), 150–158. doi:10.1016/j.vph.2011.12.006
Peng, Y., Li, S. N., Pei, X., and Hao, K. (2018). The multivariate regression statistics strategy to investigate content-effect correlation of multiple components in traditional Chinese medicine based on a partial least squares method. Mol. (Basel, Switz. 23 (3), 545. doi:10.3390/molecules23030545
Phonsatta, N., Deetae, P., Luangpituksa, P., Grajeda-Iglesias, C., Figueroa-Espinoza, M. C., Le Comte, J., et al. (2017). Comparison of antioxidant evaluation assays for investigating antioxidative activity of gallic acid and its alkyl esters in different food matrices. J. Agric. food Chem. 65 (34), 7509–7518. doi:10.1021/acs.jafc.7b02503
Prosberg, M., Bendtsen, F., Vind, I., Petersen, A. M., and Gluud, L. L. (2016). The association between the gut microbiota and the inflammatory bowel disease activity: a systematic review and meta-analysis. Scand. J. gastroenterology 51 (12), 1407–1415. doi:10.1080/00365521.2016.1216587
Rezaie, A., Parker, R. D., and Abdollahi, M. (2007). Oxidative stress and pathogenesis of inflammatory bowel disease: an epiphenomenon or the cause? Dig. Dis. Sci. 52 (9), 2015–2021. doi:10.1007/s10620-006-9622-2
Ricciuto, A., Sherman, P. M., and Laxer, R. M. (2020). Gut microbiota in chronic inflammatory disorders: a focus on pediatric inflammatory bowel diseases and juvenile idiopathic arthritis. Clin. Immunol. Orl. Fla) 215, 108415. doi:10.1016/j.clim.2020.108415
Salvo Romero, E., Alonso Cotoner, C., Pardo Camacho, C., Casado Bedmar, M., and Vicario, M. (2015). The intestinal barrier function and its involvement in digestive disease. Rev. espanola enfermedades Dig. 107 (11), 686–696. doi:10.17235/reed.2015.3846/2015
Shah, S. C., Colombel, J. F., Sands, B. E., and Narula, N. (2016). Mucosal healing is associated with improved long-term outcomes of patients with ulcerative colitis: a systematic review and meta-analysis. Clin. gastroenterology hepatology 14 (9), 1245–1255. doi:10.1016/j.cgh.2016.01.015
Shree, A., Islam, J., Vafa, A., Mohammad Afzal, S., and Sultana, S. (2020). Gallic acid prevents 1, 2-Dimethylhydrazine induced colon inflammation, toxicity, mucin depletion, and goblet cell disintegration. Environ. Toxicol. 35 (6), 652–664. doi:10.1002/tox.22900
Soendergaard, C., Bergenheim, F. H., Bjerrum, J. T., and Nielsen, O. H. (2018). Targeting JAK-STAT signal transduction in IBD. Pharmacol. Ther. 192, 100–111. doi:10.1016/j.pharmthera.2018.07.003
Sun, B., Yuan, J., Wang, S., Lin, J., Zhang, W., Shao, J., et al. (2018). Qingchang suppository ameliorates colonic vascular permeability in dextran-sulfate-sodium-induced colitis. Front. Pharmacol. 9, 1235. doi:10.3389/fphar.2018.01235
Sun, Z., Li, J., Dai, Y., Wang, W., Shi, R., Wang, Z., et al. (2020). Indigo naturalis alleviates dextran sulfate sodium-induced colitis in rats via altering gut microbiota. Front. Microbiol. 11, 731. doi:10.3389/fmicb.2020.00731
Tan, Y., Guan, Y., Sun, Y., and Zheng, C. (2019). Correlation of intestinal mucosal healing and tight junction protein expression in ulcerative colitis patients. Am. J. Med. Sci. 357 (3), 195–204. doi:10.1016/j.amjms.2018.11.011
Taylor, C. T., and Colgan, S. P. (2007). Hypoxia and gastrointestinal disease. J. Mol. Med. 85 (12), 1295–1300. doi:10.1007/s00109-007-0277-z
Thornton, M., and Solomon, M. J. (2002). Crohn's disease: in defense of a microvascular aetiology. Int. J. Colorectal Dis. 17 (5), 287–297. doi:10.1007/s00384-002-0408-5
Tolstanova, G., Deng, X., French, S. W., Lungo, W., Paunovic, B., Khomenko, T., et al. (2012). Early endothelial damage and increased colonic vascular permeability in the development of experimental ulcerative colitis in rats and mice. Laboratory investigation; a J. Tech. methods pathology 92 (1), 9–21. doi:10.1038/labinvest.2011.122
Tolstanova, G., Khomenko, T., Deng, X., Chen, L., Tarnawski, A., Ahluwalia, A., et al. (2009). Neutralizing anti-vascular endothelial growth factor (VEGF) antibody reduces severity of experimental ulcerative colitis in rats: direct evidence for the pathogenic role of VEGF. J. Pharmacol. Exp. Ther. 328 (3), 749–757. doi:10.1124/jpet.108.145128
Tornai, T., Palyu, E., Vitalis, Z., Tornai, I., Tornai, D., Antal-Szalmas, P., et al. (2017). Gut barrier failure biomarkers are associated with poor disease outcome in patients with primary sclerosing cholangitis. World J. Gastroenterol. 23 (29), 5412–5421. doi:10.3748/wjg.v23.i29.5412
Toyokawa, Y., Takagi, T., Uchiyama, K., Mizushima, K., Inoue, K., Ushiroda, C., et al. (2019). Ginsenoside Rb1 promotes intestinal epithelial wound healing through extracellular signal-regulated kinase and Rho signaling. J. gastroenterology hepatology 34 (7), 1193–1200. doi:10.1111/jgh.14532
Ungaro, R., Colombel, J. F., Lissoos, T., and Peyrin-Biroulet, L. (2019). A treat-to-target update in ulcerative colitis: a systematic review. Am. J. Gastroenterol. 114 (6), 874–883. doi:10.14309/ajg.0000000000000183
Ungaro, R., Mehandru, S., Allen, P. B., Peyrin-Biroulet, L., and Colombel, J. F. (2017). Ulcerative colitis. Lancet (London, Engl. 389 (10080), 1756–1770. doi:10.1016/S0140-6736(16)32126-2
van der Post, S., Jabbar, K. S., Birchenough, G., Arike, L., Akhtar, N., Sjovall, H., et al. (2019). Structural weakening of the colonic mucus barrier is an early event in ulcerative colitis pathogenesis. Gut 68 (12), 2142–2151. doi:10.1136/gutjnl-2018-317571
Vancamelbeke, M., and Vermeire, S. (2017). The intestinal barrier: a fundamental role in health and disease. Expert Rev. gastroenterology hepatology 11 (9), 821–834. doi:10.1080/17474124.2017.1343143
Wang, C. Z., Zhang, C. F., Zhang, Q. H., Hesse-Fong, J., Lager, M., Du, W., et al. (2018b). Fecal metabolomic dataset of American ginseng-treated DSS mice: correlation between ginseng enteric inflammation inhibition and its biological signatures. Data brief 21, 1403–1408. doi:10.1016/j.dib.2018.10.131
Wang, S., Tao, P., Zhao, L., Zhang, W., Hu, H., and Lin, J. (2018a). Panax notoginseng promotes repair of colonic microvascular injury in sprague-dawley rats with experimental colitis. Evid. Based Complement. Altern. Med. 2018, 4386571. doi:10.1155/2018/4386571
Wang, Y., Mumm, J. B., Herbst, R., Kolbeck, R., and Wang, Y. (2017). IL-22 increases permeability of intestinal epithelial tight junctions by enhancing claudin-2 expression. J. Immunol. 199 (9), 3316–3325. doi:10.4049/jimmunol.1700152
Wen, H., Dai, X., and Jiang, L. (2021). Action mechanism of Qingchang suppository in treating UC based on intestinal flora. Acta Chin. Med. Pharmacol. 49 (11), 38–42. (Chinese). doi:10.19664/j.cnki.1002-2392.210259
Wlodarska, M., Kostic, A. D., and Xavier, R. J. (2015). An integrative view of microbiome-host interactions in inflammatory bowel diseases. Cell host microbe 17 (5), 577–591. doi:10.1016/j.chom.2015.04.008
Xiao, Z., Liu, L., Tao, W., Pei, X., Wang, G., and Wang, M. (2018). Clostridium tyrobutyricum protect intestinal barrier function from LPS-induced apoptosis via P38/JNK signaling pathway in IPEC-J2 cells. Cell. physiology Biochem. 46 (5), 1779–1792. doi:10.1159/000489364
Xu, L., Zhou, X., Yang, M., Wang, K., and Yu, T. (2019). Special review of inpatient prescriptions for Qingchang suppository at Longhua Hospital. Chin. Tradit. Pat. Med. 41 (11), 2821–2824. (Chinese). doi:10.3969/j.issn.1001-1528.2019.11.056
Xun, W., Shi, L., Zhou, H., Hou, G., Cao, T., and Zhao, C. (2015). Effects of curcumin on growth performance, jejunal mucosal membrane integrity, morphology and immune status in weaned piglets challenged with enterotoxigenic Escherichia coli. Int. Immunopharmacol. 27 (1), 46–52. doi:10.1016/j.intimp.2015.04.038
Yang, Q. Y., Ma, L. L., Zhang, C., Lin, J. Z., and He, Y. N. (2021). Exploring the mechanism of indigo naturalis in the treatment of ulcerative colitis based on TLR4/MyD88/NF-κB signaling pathway and gut microbiota. Front. Pharmacol. 12, 674416. doi:10.3389/fphar.2021.674416
Yao, D., Dai, W., Dong, M., Dai, C., and Wu, S. (2021). MUC2 and related bacterial factors: therapeutic targets for ulcerative colitis. EBioMedicine 74, 103751. doi:10.1016/j.ebiom.2021.103751
Yao, P., Cui, M., Wang, H., Gao, H., Wang, L., Yang, T., et al. (2016). Quantitative analysis of intestinal flora of uygur and han ethnic Chinese patients with ulcerative colitis. Gastroenterology Res. Pract. 2016, 9186232. doi:10.1155/2016/9186232
Yu, T., Li, Z., Xu, L., Yang, M., and Zhou, X. (2021). Anti-inflammation effect of Qingchang suppository in ulcerative colitis through JAK2/STAT3 signaling pathway in vitro and in vivo. J. Ethnopharmacol. 266, 113442. doi:10.1016/j.jep.2020.113442
Zhang, Z., Qiao, D., Zhang, Y., Chen, Q., Chen, Y., Tang, Y., et al. (2022). Portulaca oleracea L. Extract ameliorates intestinal inflammation by regulating endoplasmic reticulum stress and autophagy. Mol. Nutr. Food Res. 66 (5), e2100791. doi:10.1002/mnfr.202100791
Zheng, H., Gao, J., Man, S., Zhang, J., Jin, Z., and Gao, W. (2019). The protective effects of Aquilariae Lignum Resinatum extract on 5-Fuorouracil-induced intestinal mucositis in mice. Phytomedicine Int. J. phytotherapy Phytopharm. 54, 308–317. doi:10.1016/j.phymed.2018.07.006
Zheng, Q., Bao, X. Y., Zhu, P. C., Tong, Q., Zheng, G. Q., and Wang, Y. (2017). Ginsenoside Rb1 for myocardial ischemia/reperfusion injury: preclinical evidence and possible mechanisms. Oxidative Med. Cell. Longev. 2017, 6313625. doi:10.1155/2017/6313625
Zhou, G., Kong, W. S., Li, Z. C., Xie, R. F., Yu, T. Y., and Zhou, X. (2021a). Effects of qing chang suppository powder and its ingredients on IL-17 signal pathway in HT-29 cells and DSS-induced mice. Phytomedicine Int. J. phytotherapy Phytopharm. 87, 153573. doi:10.1016/j.phymed.2021.153573
Zhou, R., He, D., Xie, J., Zhou, Q., Zeng, H., Li, H., et al. (2021b). The synergistic effects of polysaccharides and ginsenosides from American ginseng (Panax quinquefolius L) ameliorating cyclophosphamide-induced intestinal immune disorders and gut barrier dysfunctions based on microbiome-metabolomics analysis. Front. Immunol. 12, 665901. doi:10.3389/fimmu.2021.665901
Keywords: ulcerative colitis, Qingchang Suppository, active ingredients, intestinal mucosal barrier, mechanism
Citation: Shan J, Liu S, Liu H, Yuan J and Lin J (2023) Mechanism of Qingchang Suppository on repairing the intestinal mucosal barrier in ulcerative colitis. Front. Pharmacol. 14:1221849. doi: 10.3389/fphar.2023.1221849
Received: 13 May 2023; Accepted: 07 August 2023;
Published: 22 August 2023.
Edited by:
Lixin Zhu, The Sixth Affiliated Hospital of Sun Yat-sen University, ChinaReviewed by:
Qihong Liu, Fujian University of Traditional Chinese Medicine, ChinaHaomeng Wu, Guangzhou University of Chinese Medicine, China
Copyright © 2023 Shan, Liu, Liu, Yuan and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianye Yuan, eXVhbmppYW55ZUBzaHV0Y20uZWR1LmNu; Jiang Lin, bGluamlhbmdAbG9uZ2h1YS5uZXQ=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share senior authorship
 Jingyi Shan1,2†
Jingyi Shan1,2† Suxian Liu
Suxian Liu Haoyue Liu
Haoyue Liu Jianye Yuan
Jianye Yuan Jiang Lin
Jiang Lin