- 1Department of Pediatrics, Faculty of Medicine, Saga University, Saga, Japan
In this study, we compared the success rate of eradicating Helicobacter pylori (H. pylori) in adults and children using vonoprazan (VPZ)-based H. pylori regimens to that of proton pump inhibitors (PPIs). In Japan, the success rate of a VPZ-based regimen as first-line therapy was lower in children than in adults. Compared with adults, children around puberty have higher CYP2C19 and CYP3A4 enzymatic activity to metabolize PPIs and VPZ. Further, children generally have shorter intestinal transit times than adults and may absorb antibiotics to a lesser extent. When comparing success rates of pediatric and adult eradication therapy using VPZ, it is very important to maintain a higher intragastric pH with sufficient gastric acid suppression to maintain H. pylori in a replicating state and amoxicillin and clarithromycin in the intestinal tract for as long as possible by reducing diarrhea as a side effect. Based on the above, it is reasonable that VPZ, which can suppress stomach acids more strongly than PPI, is a more relevant H. pylori eradication therapy.
1 Introduction
Previous research suggested that vonoprazan (VPZ)-based Helicobacter pylori (H. pylori) regimens for eradication therapy had a superior eradication efficacy than frequently used proton pump inhibitor (PPI)-based regimens in adults (Murakami et al., 2016; Lyu et al., 2019; Sun et al., 2022). VPZ is a new, potent acid-inhibitory drug, which competitively inhibits potassium binding to the hydrogen-potassium ATPase in gastric parietal cells more efficiently than PPIs (Abdel-Aziz et al., 2021). Therefore, VPZ-based regimens facilitate a shorter eradication therapy and potentially provide significantly higher eradication rates of clarithromycin-resistant strains. To examine why VPZ-based H. pylori regimens for eradication therapy were more effective in eradicating H. pylori than PPI regimens, we compared its success rate for H. pylori eradication in adults and children.
2 Helicobacter pylori eradication success rate between adults and children
Table 1 shows that the first-line H. pylori eradication therapy’s success rate by VPZ, amoxicillin (AMPC) and clarithromycin (CAM) for 7 days in Japan, and divided it into adults and children. All these data were previously reported. Considering regional H. pylori genotype, I intentionally limited the reports from Japan and omitted the results using second-line H. pylori eradication therapy, VPZ dual therapy, and metronidazole. VPZ-based regimen as first-line therapy’s success rate in children was lower than those in adults.
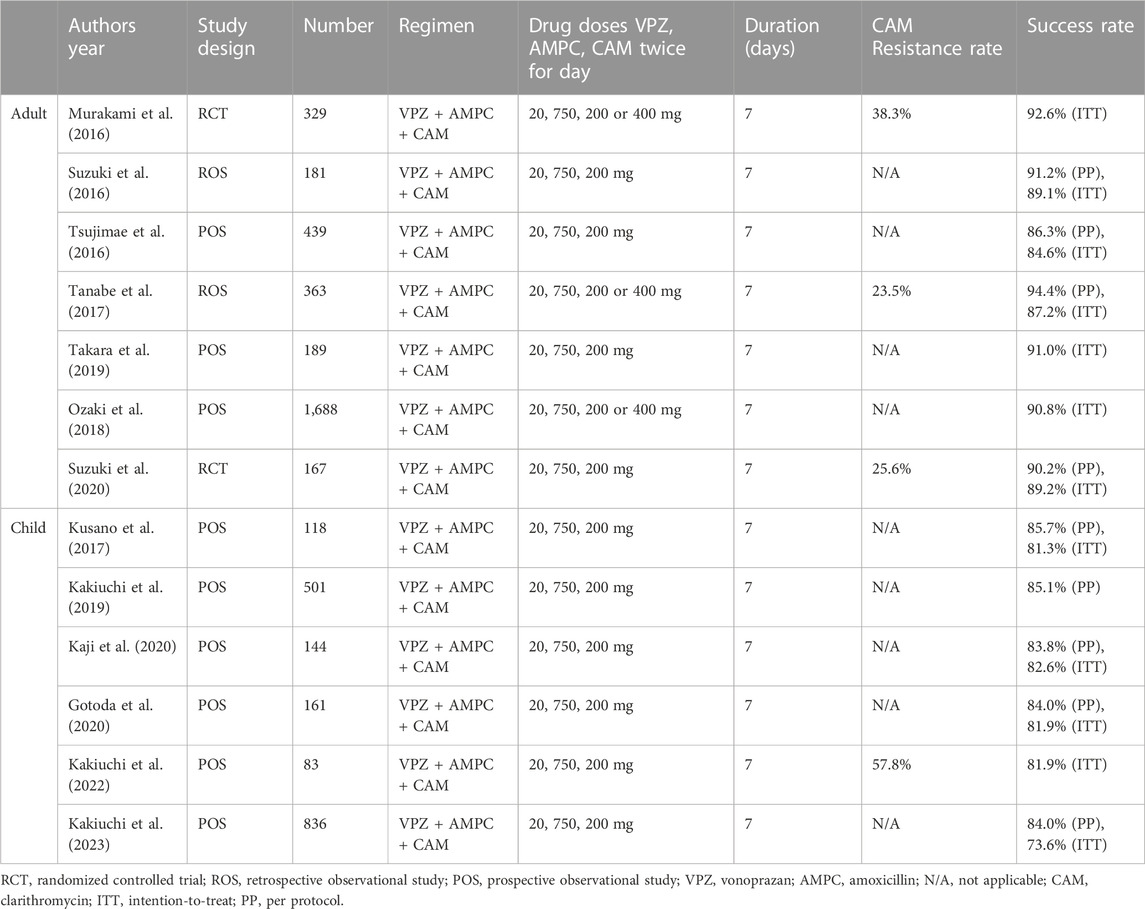
TABLE 1. List of manuscripts on Helicobacter pylori eradication therapy success rate using vonoprazan as first-line agent in Japan.
3 Discussion
There were three possible reasons why the eradication rate of adults using VPZ was higher than that of children. First, adolescents tend to have a higher rate of H. pylori CAM resistance. It has been reported that the H. pylori CAM resistance rate is high among young people in Japan (Okamura et al., 2014), which is believed to be a result of the frequent CAM prescription use for childhood respiratory tract infections in this generation (Tsuboi and Iinuma, 2021). Among CAM-resistant strains, VPZ has a significantly higher eradication success rate than PPI (Murakami et al., 2016; Okubo et al., 2020), suggesting that it is important to suppress acid and make AMPC, not CAM, more effective. In addition, sufficient acid suppression is crucial for effectiveness because, bacteria enter their replicative state and become susceptible to AMPC and CAM at high pH. Second, children around puberty have higher CYP2C19 and CYP3A4 enzyme activity to metabolize PPIs and VAP (Anderson and Lynn, 2009). As a result, children metabolize VAP more rapidly than adults and may be attenuated at a higher rate than adults. Third, it has been reported that the ionized agent absorption (many antibacterial agents) that are poorly lipophilic is affected by the small bowel transit time (Martinez and Amidon, 2002). Children generally have shorter intestinal transit times than adults and may absorb less antibiotics than adults. This may be supported by our report (Kakiuchi et al., 2021) that diarrhea during eradication therapy reduces H. pylori eradication success rate. The former two suggests that sufficient gastric acid suppression is important for H. pylori eradication. The last reason suggests the importance of sufficient antibiotic absorption for H. pylori eradication. Graham (Graham DY, 2023) demonstrated that H. pylori eradication was successfully achieved by high intragastric concentrations of antibiotics or an intragastric pH at which amoxicillin is active.
In contrast, the efficacy of a VPZ-based regimen in a Western adult population was not higher compared with that in an Asian population (Chey WD et al., 2022). VPZ is mainly metabolized by CYP3A4 enzyme. Given the lack of clinically significant differences in VPZ pharmacokinetics between Asian and non-Asian populations, the differences between studies conducted among Western adults and Japanese populations are most likely not due to race alone. Different treatment compliance rates may also have contributed to outcome differences. With respect to children, there is no data regarding the eradication of H. pylori using VPZ in Western countries; therefore, we could not compare its eradication success rate between populations of Asian, including Japan, and Western countries.
In conclusion, a comparison of pediatric and adult eradication therapy success rates using VPZ could reinforce the following conclusions: with regard to H. pylori eradication therapy, it is very important to maintain a higher intragastric pH with sufficient gastric acid suppression to keep H. pylori in a replicating state and to keep AMPC and CAM in the intestinal tract as long as possible by reducing diarrhea as a side effect. It is reasonable that VPZ, which is more potent in stomach acid suppression than PPI, should gain a dominant position in H. pylori eradication therapy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
TK searched the literature. TK conceived and wrote the manuscript. TK contributed to the manuscript and approved the submitted version.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Aziz, Y., Metz, D. C., and Howden, C. W. (2021). Review article: Potassium-competitive acid blockers for the treatment of acid-related disorders. Alimentary Pharmacol. Ther. 53 (7), 794–809. doi:10.1111/apt.16295
Anderson, G. D., and Lynn, A. M. (2009). Optimizing pediatric dosing: A developmental pharmacologic approach. Pharmacotherapy 29 (6), 680–690. doi:10.1592/phco.29.6.680
Chey, W. D., Mégraud, F., Laine, L., López, L. J., Hunt, B. J., and Howden, C. W. (2022). Vonoprazan triple and dual therapy for helicobacter pylori infection in the United States and europe: Randomized clinical trial. Gastroenterology 163 (3), 608–619. doi:10.1053/j.gastro.2022.05.055
Gotoda, T., Kusano, C., Suzuki, S., Horii, T., Ichijima, R., and Ikehara, H. (2020). Clinical impact of vonoprazan-based dual therapy with amoxicillin for H. pylori infection in a treatment-naïve cohort of junior high school students in Japan. J. gastroenterology 55 (10), 969–976. doi:10.1007/s00535-020-01709-4
Graham, D. Y. (2023). Why the vonoprazan helicobacter pylori therapies in the US-European trial produced unacceptable cure rates. Dig. Dis. Sci. 68 (5), 1691–1697. doi:10.1007/s10620-023-07886-5
Kaji, E., Yoden, A., Otani, M., Okuhira, T., Aomatsu, T., Tamai, H., et al. (2020). Helicobacter pylori test-and-treat strategy for second-year junior high school students aimed at the prevention of gastric cancer in Takatsuki City. Helicobacter 25, e12696. doi:10.1111/hel.12696
Kakiuchi, T., Matsuo, M., Endo, H., Nakayama, A., Sato, K., Takamori, A., et al. (2019). A Helicobacter pylori screening and treatment program to eliminate gastric cancer among junior high school students in saga prefecture: A preliminary report. J. gastroenterology 54 (8), 699–707. doi:10.1007/s00535-019-01559-9
Kakiuchi, T., Matsuo, M., Endo, H., Sakata, Y., Esaki, M., Noda, T., et al. (2023). Efficacy and safety of vonoprazan-based regimen for Helicobacter pylori eradication therapy in Japanese adolescents: A prospective multicenter study. J. gastroenterology 58 (3), 196–204. doi:10.1007/s00535-022-01942-z
Kakiuchi, T., Matsuo, M., Endo, H., Sakata, Y., Esaki, M., Noda, T., et al. (2021). Gastrointestinal adverse reactions reduce the success rate of Helicobacter pylori eradication therapy: A multicenter prospective cohort study. Helicobacter 26 (2), e12776. doi:10.1111/hel.12776
Kakiuchi, T., Okuda, M., Matsuo, M., and Fujimoto, K. (2022). Smart Gene™ as an effective non-invasive point-of-care test to detect Helicobacter pylori clarithromycin-resistant mutation. J. Gastroenterol. Hepatol. 37 (9), 1719–1725. doi:10.1111/jgh.15887
Kusano, C., Gotoda, T., Ishikawa, H., and Moriyama, M. (2017). The administrative project of Helicobacter pylori infection screening among junior high school students in an area of Japan with a high incidence of gastric cancer. Gastric Cancer 20 (Suppl. 1), 16–19. doi:10.1007/s10120-017-0688-7
Lyu, Q. J., Pu, Q. H., Zhong, X. F., and Zhang, J. (2019). Efficacy and safety of vonoprazan-based versus proton pump inhibitor-based triple therapy for Helicobacter pylori eradication: A meta-analysis of randomized clinical trials. Biomed. Res. Int. 2019, 9781212. doi:10.1155/2019/9781212
Martinez, M. N., and Amidon, G. L. (2002). A mechanistic approach to understanding the factors affecting drug absorption: A review of fundamentals. J. Clin. Pharmacol. 42 (6), 620–643. doi:10.1177/00970002042006005
Murakami, K., Sakurai, Y., Shiino, M., Funao, N., Nishimura, A., and Asaka, M. (2016). Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: A phase III, randomised, double-blind study. Gut 65 (9), 1439–1446. doi:10.1136/gutjnl-2015-311304
Okamura, T., Suga, T., Nagaya, T., Arakura, N., Matsumoto, T., Nakayama, Y., et al. (2014). Antimicrobial resistance and characteristics of eradication therapy of Helicobacter pylori in Japan: A multi-generational comparison. Helicobacter 19 (3), 214–220. doi:10.1111/hel.12124
Okubo, H., Akiyama, J., Kobayakawa, M., Kawazoe, M., Mishima, S., Takasaki, Y., et al. (2020). Vonoprazan-based triple therapy is effective for Helicobacter pylori eradication irrespective of clarithromycin susceptibility. J. gastroenterology 55 (11), 1054–1061. doi:10.1007/s00535-020-01723-6
Ozaki, H., Harada, S., Takeuchi, T., Kawaguchi, S., Takahashi, Y., Kojima, Y., et al. (2018). Vonoprazan, a novel potassium-competitive acid blocker, should be used for the helicobacter pylori eradication therapy as first choice: A large sample study of vonoprazan in real world compared with our randomized control trial using second-generation proton pump inhibitors for helicobacter pylori eradication therapy. Digestion 97 (3), 212–218. doi:10.1159/000485097
Sun, Y., Yue, L., and Hu, W. (2022). Effectiveness and safety of vonoprazan-based regimens compared with those of proton pump inhibitor (PPI)-based regimens as first-line agents for Helicobacter pylori: A meta-analysis of randomized clinical trials. Eur. J. Clin. Pharmacol. 79 (2), 279–288. doi:10.1007/s00228-022-03430-y
Suzuki, S., Gotoda, T., Kusano, C., Ikehara, H., Ichijima, R., Ohyauchi, M., et al. (2020). Seven-day vonoprazan and low-dose amoxicillin dual therapy as first-line Helicobacter pylori treatment: A multicentre randomised trial in Japan. Gut 69 (6), 1019–1026. doi:10.1136/gutjnl-2019-319954
Suzuki, S., Gotoda, T., Kusano, C., Iwatsuka, K., and Moriyama, M. (2016). The efficacy and tolerability of a triple therapy containing a potassium-competitive acid blocker compared with a 7-day PPI-based low-dose clarithromycin triple therapy. Am. J. gastroenterology 111 (7), 949–956. doi:10.1038/ajg.2016.182
Takara, Y., Endo, H., Nakano, R., Kawachi, K., Hidaka, H., Matsunaga, T., et al. (2019). Smoking and drinking did not increase the failure of therapeutic helicobacter pylori eradication by vonoprazan, clarithromycin, and amoxicillin. Digestion 99 (2), 172–178. doi:10.1159/000490889
Tanabe, H., Ando, K., Sato, K., Ito, T., Goto, M., Sato, T., et al. (2017). Efficacy of vonoprazan-based triple therapy for helicobacter pylori eradication: A multicenter study and a review of the literature. Dig. Dis. Sci. 62 (11), 3069–3076. doi:10.1007/s10620-017-4664-1
Tsuboi, I., and Iinuma, K. (2021). Immunochromatography-application example and POCT type genetic testing. Chem. Pharm. Bull. 69 (10), 984–988. doi:10.1248/cpb.c21-00164
Keywords: Helicobacter pylori, vonoprazan, proton pump inhibitor, child, adult
Citation: Kakiuchi T (2023) Effectiveness of vonoprazan-based regimens compared with proton pump inhibitor-based regimens as first-line Helicobacter pylori agents. Front. Pharmacol. 14:1216433. doi: 10.3389/fphar.2023.1216433
Received: 03 May 2023; Accepted: 12 July 2023;
Published: 19 July 2023.
Edited by:
Karunakaran Kalesh, Teesside University, United KingdomReviewed by:
Mitsushige Sugimoto, Tokyo Medical University Hospital, JapanCopyright © 2023 Kakiuchi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Toshihiko Kakiuchi, a2FraXVjaHRAY2Muc2FnYS11LmFjLmpw
 Toshihiko Kakiuchi
Toshihiko Kakiuchi