- Department of Hematology and Oncology, China-Japan Union Hospital of Jilin University, Changchun, China
To demonstrate the efficacy of fruquintinib administration after local radiotherapy in a patient with metastatic colon cancer with high microsatellite instability and the KRAS exon 2 p. G12D mutation. The patient was administered four cycles of pembrolizumab intravenous infusion and achieved stable disease as the best outcome. He was then underwent follow-up concurrent radiochemical therapy (local DT4600cGy/23f/32d radiotherapy, and S-1 to increase sensitivity to radiotherapy), but this had little efficacy. Following this, he was administered fruquintinib and achieved sustained partial remission. At the time of last follow-up, the patient was in continuous remission for 30 months. Administration of fruquintinib after local radiotherapy may be an effective treatment for specific populations with metastatic colorectal cancer.
Introduction
Colorectal cancer (CRC) is the third leading cause of cancer-related death in the United States (Siegel et al., 2021). Approximately 20% of patients have metastatic disease at the time of diagnosis, and 40% experience recurrence after initial treatment (Biller and Schrag, 2021). Metastatic CRC (mCRC) remains incurable. The outcome of these patients is often dismal, with a median overall survival (OS) of 15.4 months and a 5-year survival rate of <20% (Goldstein et al., 2014). Systemic treatments, including chemotherapy, antiangiogenic drugs, immune checkpoint inhibitors (ICIs), local treatment, and their combinations, are recommended for mCRC (Benson et al., 2021). Although first-line and second-line treatments can delay disease progression, most patients require third-line treatment (Marshall, 2021). Strategies to improve the survival benefits of such patients by 3 months or longer remain limited (Grothey et al., 2013; Mayer et al., 2015; Li et al., 2018). Here, we present a case of recurrent colon cancer with high microsatellite instability (MSI-H) and the KRAS G12D mutation presenting as a large abdominal mass. The patient did not respond to ICIs but has been in continuous remission for 30 months after receiving third-line sequential treatment with local radiotherapy and fruquintinib.
Case presentation
A 53-year-old man diagnosed with sigmoid colon cancer 1 year ago underwent radical resection for colon cancer. He presented with abdominal pain and difficulty in constipation and was admitted to the hospital on December 2018. Preoperative abdominal CT revealed space occupying lesions from the sigmoid colon to the lower part of the descending colon, with multiple peripheral lymph node enlargement. Tumor markers (including carcinoembryonic antigen, carbohydrate antigen 199) were within the normal range. Due to the lack of standardized experimental procedures, circulating tumor DNA (ctDNA) analysis is generally not carried out routinely at our hospital. The postoperative pathological report showed invasion of the gastric serosa with a moderately differentiated adenocarcinoma and cancer cell infiltration of vessels without invasion. Peri-intestinal lymph node metastasis (2/12) near the lesion in the sigmoid colon and a cancer nodule were also detected. His postoperative pathological stage was pT4aN2a.m.0 IIIC. Immunohistochemical markers were as follows: Ki-67 (+70%), MLH1 (+80%), PMS2 (+80%), MSH2 (−), MSH6 (−), and p53 (+70%), indicating the MSI-H type. His relatives did not have lynch syndrome-associated cancer. Two weeks after the operation, the patient received standard adjuvant chemotherapy of sufficient duration and dosage. Eight cycles of CAPOX chemotherapy (oxaliplatin 130 mg/m2 was administered on day 1 and capecitabine was administered orally at a dose of 2000 mg/m2/day, divided into two split daily doses for 14 days followed by 7 days of rest) were administered. The patient tolerated chemotherapy well and only exhibited grade 2 hand foot syndrome. After chemotherapy, he was followed-up regularly every 3 months.
In December 2019, he developed abdominal pain and constipation again. F-fluorodeoxyglucose positron emission tomography computed tomography detected a large mass with elevated glucose metabolism near the anastomosis (Figures 1A–D), as well as multiple retroperitoneal lymph node metastases. The mass was diagnosed as moderately differentiated adenocarcinoma by biopsy pathology and was considered to originate from the intestine (Figure 1E). Immunohistochemical staining showed that cells were positive for MLH1 (Figure 1F) and negative for MSH2 (Figure 1G) and MSH6 (Figure 1H). CEA and CA 19–9 were within the normal reference value range. Next-generation sequencing (NGS) of the recurrence lesion was performed to detect clinically relevant KRAS, TP53, PIK3CA, BRAF, NRAS, FGFR1, FGFR3, FGFR2 and HER-2 mutations. Only KRAS exon 2 p. G12D mutation was detected, and the mutation allele frequency was 32.21%. The MSI score of the tumor tissue was 0.6087 (cutoff value, 0.2).
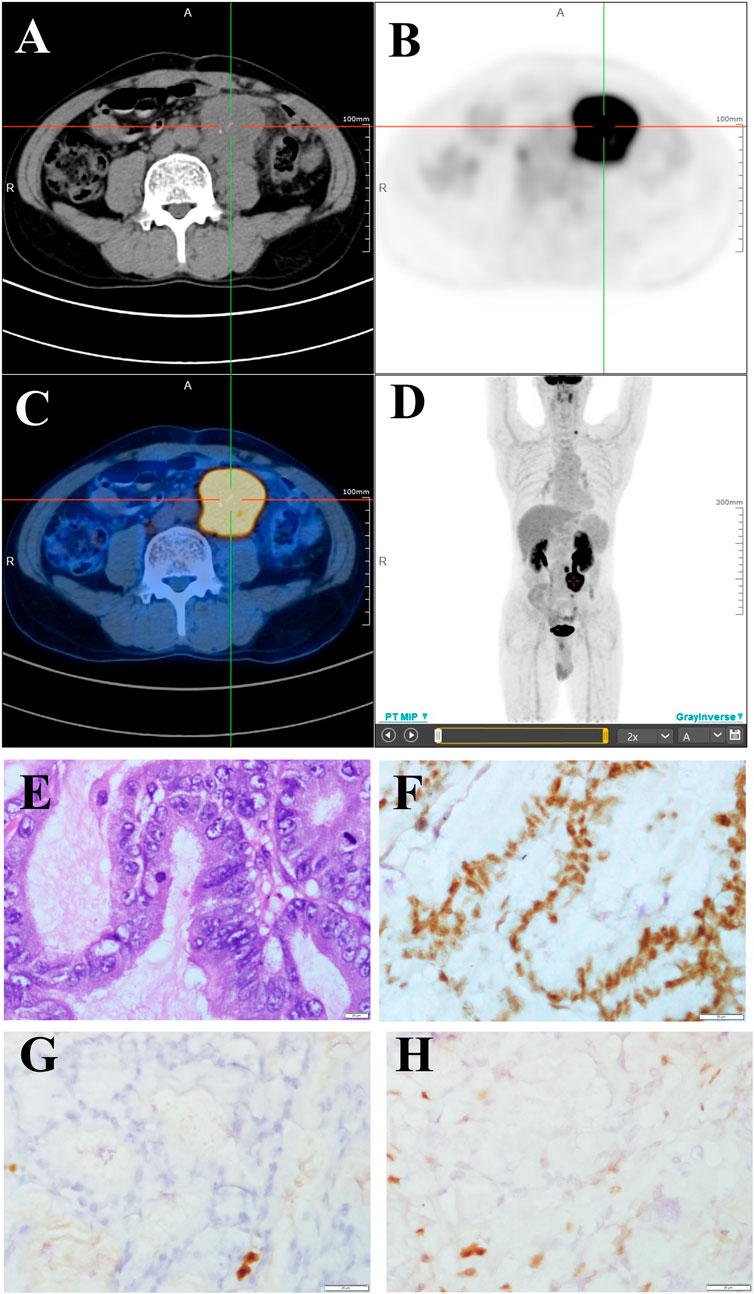
FIGURE 1. FDG PET/CT and pathological changes (A–C) PET/CT cross sectional view. A mass shadow with increased glucose metabolism can be seen near the anastomotic site. Its maximum SUV value is 18.18 and its size is about 49.5 × 42.5 mm. It is nodular with strip-shaped calcification and the boundary with the left ureter is unclear. (D) 18F-FDG PET/CT maximum density projection (MIP). Hypermetabolic nodules are visible in the left neck and lumpy lesions with increased glucose metabolism are visible in the abdomen. (E) Moderately differentiated adenocarcinoma (H&E staining, 400×). (F–H) Immunohistochemical staining showed that cells were positive for MLH1 (F) and negative for MSH2 (G) and MSH6 (H) (400×).
Abdominal metastatic colon cancer with MSI-H was confirmed and considered unresectable after surgical consultation. The patient received four cycles of pembrolizumab (200 mg every 3 weeks by intravenous infusion). However, the patient experienced a grade III gastrointestinal reaction after 15 weeks of immunotherapy, which manifested as loss of appetite, fatigue, and repeated diarrhea, and refused further immunotherapy. The patient refused colonoscopy for the detection of immune related enteritis. The response to treatment, as evaluated by imaging, was stable disease (Figure 2A). Because of the risk of intestinal obstruction and the ineligibility for surgical resection, the patient received local DT4600cGy/23f/32d radiotherapy. S-1, an oral fluoropyrimidine, was administered at 60 mg/m2 per day, orally, on days 1–14 and 29–42 in combination with radiotherapy to increase the sensitivity to radiotherapy. After radiotherapy, the abdominal pain was slightly relieved, and no serious treatment-related adverse events were observed. However, imaging follow-up showed no changes in the abdominal mass (Figure 2B). Considering that the previous efficacy was not ideal, the imaging revealed incomplete intestinal obstruction, and the patient’s general state was poor, we decided not to proceed with chemotherapy. The patient was then treated with the third-line drug fruquintinib, a small molecule multi-target tyrosine kinase inhibitor, at 5 mg orally for a 28-day treatment cycle of 3 weeks followed by 1 week off starting in May 2020.
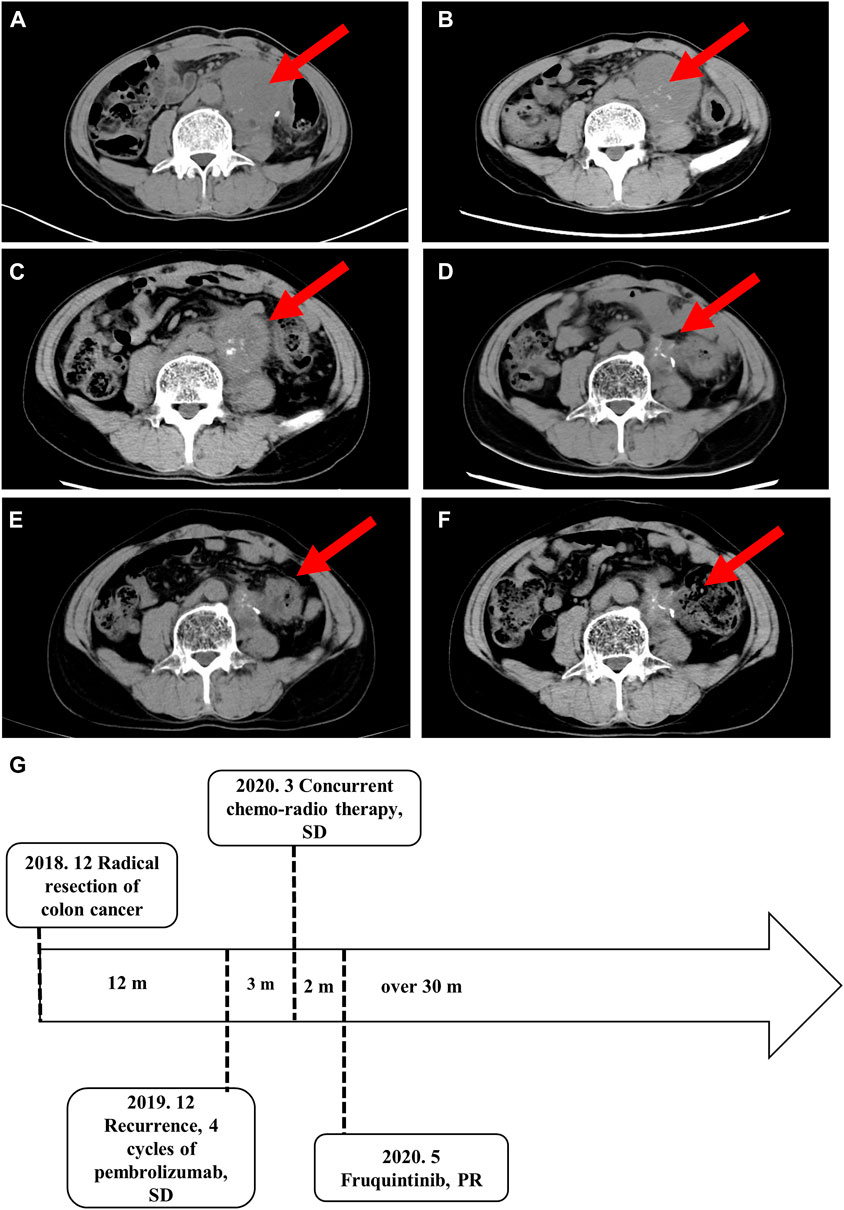
FIGURE 2. Imaging studies and the treatment time axis. Follow-up imaging of the abdominal mass. (A) After four cycles of pembrolizumab. (B) After local DT4600cGy/23f/32d radiotherapy. (C) Three months (D) 12 months (E)18 months, and (F) 30 months after the start of fruquintinib administration. (G) The treatment time axis. SD, stable disease; PR, partial response.
The abdominal pain and defecation difficulties improved significantly after treatment. The abdominal mass showed continuous shrinkage in the imaging follow-up starting on the third month of fruquintinib treatment (Figures 2C–E). After 14 months of oral administration of fruquintinib, serum creatinine increased to 110.2 μmol/L and the 24-h urinary protein was 4.8 g. Therefore, the fruquintinib treatment was interrupted for 2 weeks. After renal function and urinary protein returned to normal, oral administration of fruquintinib was restarted at a reduced dose of 3 mg per day. The last imaging follow-up was at 30 months, and the focus remains obtaining a continuous partial response, as determined using the RECIST1.1 guidelines (Figures 2F, G).
Discussion
DNA mismatch repair deficiency (MMR) was considered a biomarker of poor prognosis in the era before the advent of immune checkpoint inhibitors. MMR is present in 3%–5% of patients with metastatic colorectal cancer and is associated with tumors with a high tumor mutation burden (TMB) and MSI (Koopman et al., 2009; Venderbosch et al., 2014). The median progression free survival (PFS) and OS are significantly worse in patients with deficient DNA mismatch repair (dMMR) than in those with proficient MMR (pMMR) tumors (PFS: 6.2 vs 7.6 months, p = 0.001; OS: 13.6 vs 16.8 months, p = 0.001). The large proportion of mutant neoantigens in dMMR cancers make them sensitive to immune checkpoint blockade (Table 1). ICIs, namely, pembrolizumab or nivolumab, have shown durable antitumor activity and few treatment-related adverse events compared with chemotherapy in patients with MSI-H or dMMR mCRC in recent clinical trials (Overman et al., 2017; Diaz et al., 2022). This resulted in the US Food and Drug Administration approval of these agents for this patient population (Smith and Desai, 2018; Marcus et al., 2019). The objective remission rate (ORR) in CRC patients with MSI-H is 35.8%–45% (Overman et al., 2017; Diaz et al., 2022), indicating that more than half of the cases are at risk of stable or even disease progression. The present case did not benefit from programmed death-1 (PD-1) blocking therapy, which may be attributed to tumor intrinsic and extrinsic factors (Schreiber et al., 2011). Firstly, tumor immunity is a double-edged sword. After cancer immunoediting, existing tumor cells survive through immune escape. Secondly, the immunosuppressive state of the tumor microenvironment (TME) may provide a shelter for tumor growth. In addition, severe gastrointestinal reactions and diarrhea may cause an imbalance of the gastrointestinal flora, trigger negative immune regulation mechanisms, and could result in interruption of treatment, leading to loss of response to ICIs (Pitt et al., 2016). These factors may explain why this patient did not benefit from pembrolizumab. Another consideration is that the patient also had the KRAS exon 2 p. G12D mutation.
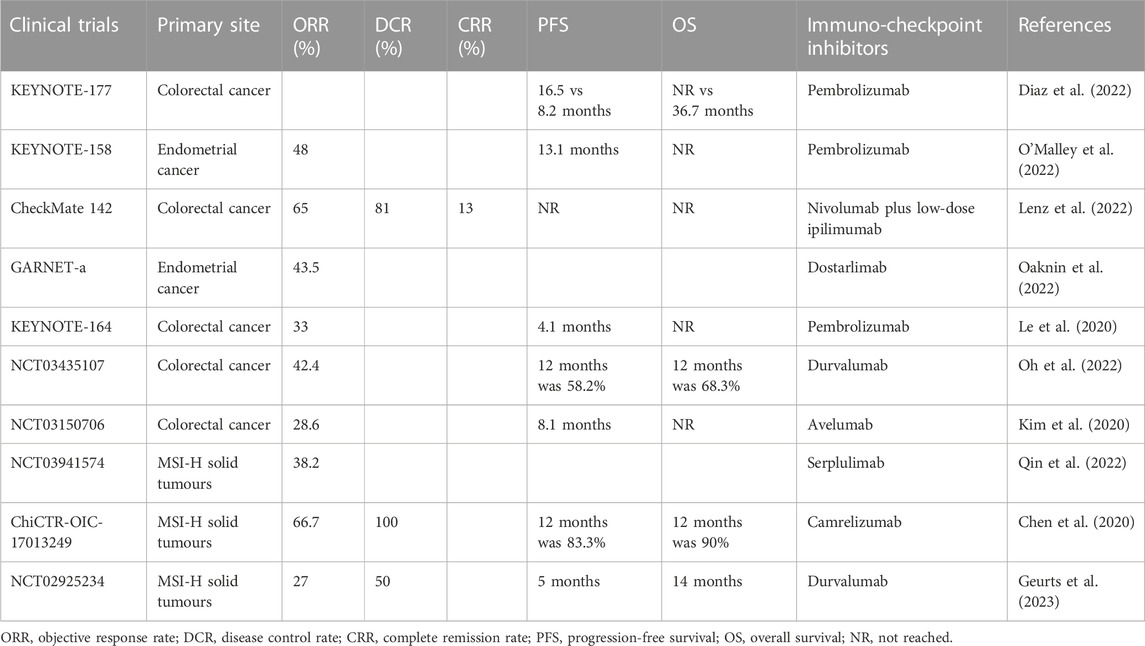
TABLE 1. Clinical trials of immuno-checkpoint inhibitors for DNA mismatch repair-deficient or microsatellite instability-high tumors.
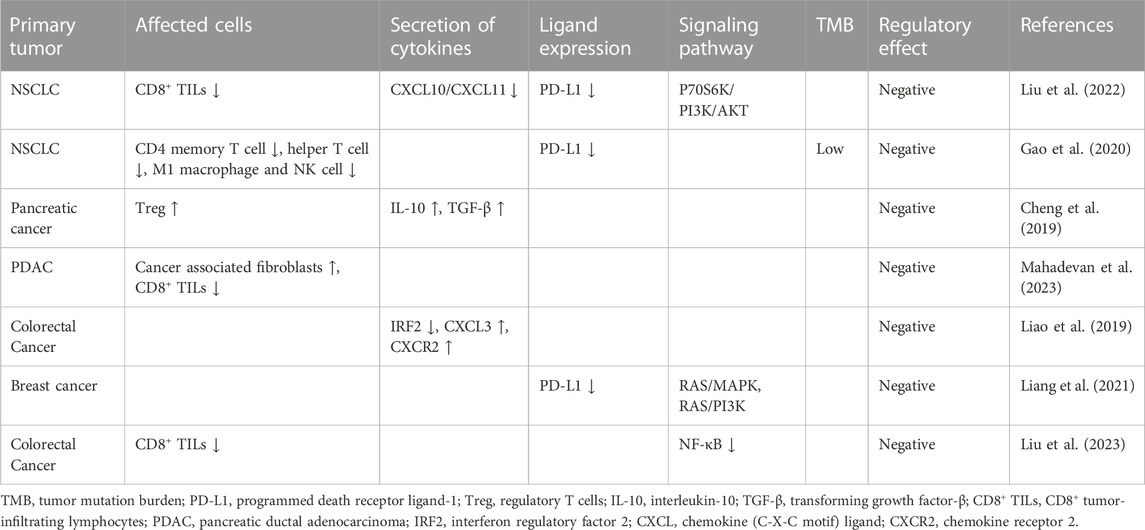
KRAS exon 2 mutations are detected in 40% of patients with CRC (Berlin, 2013). KRAS exon 2 p. G12D mutation plays a critical role in the conversion of regulatory T cells (Tregs) and promotes an immunosuppressive TME (Table 2) (Cheng et al., 2019; Liao et al., 2019). The KRAS G12D mutation decreases the TMB, downregulates programmed death receptor ligand-1 (PD-L1), and reduces immune cell infiltration (Gao et al., 2020). Therefore, the benefit from immunotherapy may be reduced in patients with KRAS mutations. Subgroup analysis in the KEYNOTE-177 trial showed that in KRAS mutated mCRC patients with MSI-H, the survival benefit of pembrolizumab was not superior to that of chemotherapy (Diaz et al., 2022). This may explain the loss of response to ICIs in the present case.
In the present case, because the mass in the abdominal cavity was large and could not be removed surgically, local radiotherapy was selected as the second-line treatment. Radiotherapy can regulate the TME via multiple mechanisms, including promoting immunogenic cell death through the “in situ” vaccination effect to increase immune cell trafficking and fighting against immune evasion mechanisms orchestrated by stromal cells (Menon et al., 2019). On the other hand, radiotherapy-induced tumor tissue hypoxia promotes the release of vascular endothelial growth factor receptor 2 (VEGFR2) and tumor neovascularization (Jarosz-Biej et al., 2019). This is the theoretical basis behind the combination of small molecule anti-vascular targeted drugs with radiotherapy. A prior study showed that regorafenib increases the anti-CRC efficacy of radiotherapy by inducing apoptosis and decreasing nuclear factor kappa B signaling (Liu et al., 2020).
Third-line treatment options for metastatic colorectal cancer are still confusing. Clinical research data have shown that the survival benefit of third-line treatment is still limited. Treatment plans need to weigh potential efficacy, treatment-related adverse events, patient tolerance, and subjective acceptance. In our case, our first choice was to use ICIs. However, the patient experienced disease progression during the previous four cycles of pembrolizumab administration. The disease progression revealed by imaging follow-up may be because the treatment course was not sufficient or lymphocyte infiltration caused local edema of the tumor focus. According to the data of clinical trial KEYNOTE-177, patients with MSI-H/dMMR mCRCs who received pembrolizumab do not show significant survival benefits in the initial 6 months. After 6 months of treatment, the ICIs continued to be effective with a significant upward trend in survival benefit. This study indicates that ICIs may have a slow but long-lasting effect. Unfortunately, our case developed a degree III gastrointestinal reaction that manifested as a loss of appetite, fatigue, and repeated diarrhea. The above clinical manifestations do not exclude immune related adverse events (IRAEs). Previous studies have shown that ICIs induce diarrhea and colitis, which should be identified and treated as soon as possible; otherwise, septic shock or intestinal perforation, or even death can ensue. Therefore, rechallenge with ICIs should only proceed after weighing the clinical benefits and the risks of this treatment.
Secondly, targeting the KRAS G12D mutation seems to be an ideal choice. KRAS G12D is an important mutation in a variety of cancers but is also a challenging target. Unfortunately, most drugs targeting KRAS G12C are not effective in patients with KRAS G12D. Preclinical trials of MRTX1133, a noncovalent and highly selective G12D inhibitor, are under way. MRTX1133 treatment might have been a very promising option in a later line of treatment for our patient.
Thirdly, is not clear whether it is worth attempting chemotherapy in combination with VEGFR antibody or tyrosine kinase inhibitors (TKIs). It is worth noting that during first-line and second-line treatment, our patient was emaciated, exhibited poor nutritional status, and had a very low physical strength score. More importantly, he did not want to receive chemotherapy and wanted a chemotherapy-free regimen instead. So far, TKIs appear to be one of the few therapeutic options.
Angiogenesis plays a vital role in the development of mCRC (Hanahan and Weinberg, 2000). Fruquintinib is a small molecule inhibitor with high selectivity against VEGFR-1, -2, and -3, and it inhibits VEGF-induced phosphorylation, endothelial cell proliferation, and tubule formation (Sun et al., 2014; Deng and Li, 2019). It was first approved in China in 2018 for patients with recurrent and metastatic CRC experiencing failure of more than two lines of systematic treatment (Shirley, 2018). However, the benefits of third-line and later line therapies for mCRCs remain limited. The FRESCO study showed that, compared with placebo, fruquintinib only prolonged median OS and PFS by 2.73 and 1.87 months, respectively (Li et al., 2018).
In the present case, the PFS after treatment with fruquintinib was >30 months, which is considerably longer than that reported in clinical trials and longer than we expected. A patient with KRAS codon 12 mutation and oligometastatic CRC received radiotherapy (RT) combined with regorafenib and achieved PFS for up to 36 months (Roberto et al., 2017). Presumably, RT and anti-vascular targeted drugs may have synergistic antitumor effects. KRAS exon 2 p. G12D mutation seems to play a key role in suppressing the tumor’s response to immunotherapy. Multi-target anti-vascular tyrosine kinase inhibitors may inhibit tumor angiogenesis and improve the TME by blocking the Raf/MEK/ERK signaling pathway downstream of KRAS (Wang et al., 2015; Li et al., 2021). Only a few studies have reported using sequentially administered TKIs and RT or TKIs combined with RT. A clinical trial named REGINA aims to evaluate the survival benefits of neoadjuvant regorafenib in combination with nivolumab and short-course RT for stage II and III rectal cancer, the results of which are highly anticipated (Bregni et al., 2021). A previous case showed that a patient with metastatic hepatocellular carcinoma achieved long-term survival of over 71 months after receiving a combination of regorafenib and RT (Kim et al., 2023). RT may improve the tumor microenvironment, promote the release of tumor antigen, and synergistically increase the effects of TKIs and ICIs. This may explain the long-term sustained remission achieved in the present case. In the future, we intend to initiate clinical trials of combinations of RT and TKIs for the treatment of metastatic colorectal cancer.
In conclusion, despite a high degree of MSI, KRAS exon 2 p. G12D mutation played a critical role in the lack of response to anti-PD-1 immunotherapy in the present case. Sequential administration of local RT and fruquitinib may be an ideal treatment modality for patients with KRAS exon 2 p. G12D oligometastatic CRC. In future studies, we will establish relevant animal models to explore the synergistic mechanism and optimal administration mode of small molecule tyrosine kinase inhibitors with other therapies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics committee of China-Japan Union hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the participant/patient(s) for the publication of this case report
Author contributions
RW and DC wrote the manuscript. YB were responsible for data collection and revision of the manuscript. WZ conceived the study and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the Science and Technology Development Project of Jilin Province (20210401174YY and YDZJ202201ZYTS092, to WZ).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Benson, A. B., Venook, A. P., Al-Hawary, M. M., Arain, M. A., Chen, Y. J., Ciombor, K. K., et al. (2021). Colon cancer, version 2.2021, NCCN clinical practice guidelines in Oncology. J. Natl. Compr. Canc Netw. 19, 329–359. doi:10.6004/jnccn.2021.0012
Berlin, J. (2013). Beyond exon 2-the developing story of RAS mutations in colorectal cancer. N. Engl. J. Med. 369, 1059–1060. doi:10.1056/NEJMe1307992
Biller, L. H., and Schrag, D. (2021). Diagnosis and treatment of metastatic colorectal cancer: A Review. Jama 325, 669–685. doi:10.1001/jama.2021.0106
Bregni, G., Vandeputte, C., Pretta, A., Senti, C., Trevisi, E., Acedo Reina, E., et al. (2021). Rationale and design of REGINA, a phase II trial of neoadjuvant regorafenib, nivolumab, and short-course radiotherapy in stage II and III rectal cancer. Acta Oncol. 60, 549–553. doi:10.1080/0284186X.2020.1871067
Chen, J., Quan, M., Chen, Z., Zeng, T., Li, Y., Zhou, Y., et al. (2020). Camrelizumab in advanced or metastatic solid tumour patients with DNA mismatch repair deficient or microsatellite instability high: An open-label prospective pivotal trial. J. Cancer Res. Clin. Oncol. 146, 2651–2657. doi:10.1007/s00432-020-03251-5
Cheng, H., Fan, K., Luo, G., Fan, Z., Yang, C., Huang, Q., et al. (2019). Kras(G12D) mutation contributes to regulatory T cell conversion through activation of the MEK/ERK pathway in pancreatic cancer. Cancer Lett. 446, 103–111. doi:10.1016/j.canlet.2019.01.013
Deng, Y., and Li, X. (2019). Fruquintinib and its use in the treatment of metastatic colorectal cancer. Future Oncol. 15, 2571–2576. doi:10.2217/fon-2018-0454
Diaz, L. A., Shiu, K. K., Kim, T. W., Jensen, B. V., Jensen, L. H., Punt, C., et al. (2022). Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): Final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 23, 659–670. doi:10.1016/s1470-2045(22)00197-8
Gao, G., Liao, W., Ma, Q., Zhang, B., Chen, Y., and Wang, Y. (2020). KRAS G12D mutation predicts lower TMB and drives immune suppression in lung adenocarcinoma. Lung Cancer 149, 41–45. doi:10.1016/j.lungcan.2020.09.004
Geurts, B. S., Battaglia, T. W., Van Berge Henegouwen, J. M., Zeverijn, L. J., De Wit, G. F., Hoes, L. R., et al. (2023). Efficacy, safety and biomarker analysis of durvalumab in patients with mismatch-repair deficient or microsatellite instability-high solid tumours. BMC Cancer 23, 205. doi:10.1186/s12885-023-10663-2
Goldstein, J., Tran, B., Ensor, J., Gibbs, P., Wong, H. L., Wong, S. F., et al. (2014). Multicenter retrospective analysis of metastatic colorectal cancer (CRC) with high-level microsatellite instability (MSI-H). Ann. Oncol. 25, 1032–1038. doi:10.1093/annonc/mdu100
Grothey, A., Van Cutsem, E., Sobrero, A., Siena, S., Falcone, A., Ychou, M., et al. (2013). Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381, 303–312. doi:10.1016/s0140-6736(12)61900-x
Hanahan, D., and Weinberg, R. A. (2000). The hallmarks of cancer. Cell 100, 57–70. doi:10.1016/s0092-8674(00)81683-9
Jarosz-Biej, M., Smolarczyk, R., Cichoń, T., and Kułach, N. (2019). Tumor microenvironment as A "game changer" in cancer radiotherapy. Int. J. Mol. Sci. 20, 3212. doi:10.3390/ijms20133212
Kim, J. H., Kim, S. Y., Baek, J. Y., Cha, Y. J., Ahn, J. B., Kim, H. S., et al. (2020). A phase II study of avelumab monotherapy in patients with mismatch repair-deficient/microsatellite instability-high or POLE-mutated metastatic or unresectable colorectal cancer. Cancer Res. Treat. 52, 1135–1144. doi:10.4143/crt.2020.218
Kim, J. Y., Yi, N. J., Kim, Y. J., Chie, E. K., Kim, J., Choi, H. H., et al. (2023). Posttransplant sequential adrenal and spine metastasis of hepatocellular carcinoma responsive to combined regorafenib and radiotherapy: A case report. Korean J. Transpl. 37, 69–75. doi:10.4285/kjt.22.0054
Koopman, M., Kortman, G. A., Mekenkamp, L., Ligtenberg, M. J., Hoogerbrugge, N., Antonini, N. F., et al. (2009). Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br. J. Cancer 100, 266–273. doi:10.1038/sj.bjc.6604867
Le, D. T., Kim, T. W., Van Cutsem, E., Geva, R., Jäger, D., Hara, H., et al. (2020). Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J. Clin. Oncol. 38, 11–19. doi:10.1200/jco.19.02107
Lenz, H. J., Van Cutsem, E., Luisa Limon, M., Wong, K. Y. M., Hendlisz, A., Aglietta, M., et al. (2022). First-line nivolumab plus low-dose ipilimumab for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: The phase II CheckMate 142 study. J. Clin. Oncol. 40, 161–170. doi:10.1200/jco.21.01015
Li, J., Cai, Y., and Deng, Y. (2021). Selection of oral therapeutics in China for the treatment of colorectal cancer. Curr. Treat. Options Oncol. 22, 55. doi:10.1007/s11864-021-00852-1
Li, J., Qin, S., Xu, R. H., Shen, L., Xu, J., Bai, Y., et al. (2018). Effect of fruquintinib vs placebo on overall survival in patients with previously treated metastatic colorectal cancer: The FRESCO randomized clinical trial. Jama 319, 2486–2496. doi:10.1001/jama.2018.7855
Liang, H., Zhou, G., Lv, L., Lu, J., and Peng, J. (2021). KRAS expression is a prognostic indicator and associated with immune infiltration in breast cancer. Breast Cancer 28, 379–386. doi:10.1007/s12282-020-01170-4
Liao, W., Overman, M. J., Boutin, A. T., Shang, X., Zhao, D., Dey, P., et al. (2019). KRAS-IRF2 Axis drives immune suppression and immune therapy resistance in colorectal cancer. Cancer Cell 35, 559–572.e7. doi:10.1016/j.ccell.2019.02.008
Liu, C., Zheng, S., Wang, Z., Wang, S., Wang, X., Yang, L., et al. (2022). KRAS-G12D mutation drives immune suppression and the primary resistance of anti-PD-1/PD-L1 immunotherapy in non-small cell lung cancer. Cancer Commun. (Lond) 42, 828–847. doi:10.1002/cac2.12327
Liu, H., Liang, Z., Cheng, S., Huang, L., Li, W., Zhou, C., et al. (2023). Mutant KRAS drives immune evasion by sensitizing cytotoxic T-cells to activation-induced cell death in colorectal cancer. Adv. Sci. (Weinh) 10, e2203757. doi:10.1002/advs.202203757
Liu, Y. C., Chiang, I. T., Chung, J. G., Hsieh, J. H., Chiang, C. H., Weng, M. C., et al. (2020). Therapeutic efficacy and inhibitory mechanism of regorafenib combined with radiation in colorectal cancer. Vivo 34, 3217–3224. doi:10.21873/invivo.12157
Mahadevan, K. K., Mcandrews, K. M., Lebleu, V. S., Yang, S., Lyu, H., Li, B., et al. (2023). Oncogenic Kras (G12D) specific non-covalent inhibitor reprograms tumor microenvironment to prevent and reverse early pre-neoplastic pancreatic lesions and in combination with immunotherapy regresses advanced PDAC in a CD8 (+) T cells dependent manner. bioRxiv. doi:10.1101/2023.02.15.528757
Marcus, L., Lemery, S. J., Keegan, P., and Pazdur, R. (2019). FDA approval summary: Pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin. Cancer Res. 25, 3753–3758. doi:10.1158/1078-0432.ccr-18-4070
Marshall, J. L. (2021). Metastatic colorectal cancer: Strategies for third-line treatment. Clin. Adv. Hematol. Oncol. 19 (Suppl. 3), 7–15.
Mayer, R. J., Van Cutsem, E., Falcone, A., Yoshino, T., Garcia-Carbonero, R., Mizunuma, N., et al. (2015). Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N. Engl. J. Med. 372, 1909–1919. doi:10.1056/NEJMoa1414325
Menon, H., Ramapriyan, R., Cushman, T. R., Verma, V., Kim, H. H., Schoenhals, J. E., et al. (2019). Role of radiation therapy in modulation of the tumor stroma and microenvironment. Front. Immunol. 10, 193. doi:10.3389/fimmu.2019.00193
O'malley, D. M., Bariani, G. M., Cassier, P. A., Marabelle, A., Hansen, A. R., De Jesus Acosta, A., et al. (2022). Pembrolizumab in patients with microsatellite instability-high advanced endometrial cancer: Results from the KEYNOTE-158 study. J. Clin. Oncol. 40, 752–761. doi:10.1200/jco.21.01874
Oaknin, A., Gilbert, L., Tinker, A. V., Brown, J., Mathews, C., Press, J., et al. (2022). Safety and antitumor activity of dostarlimab in patients with advanced or recurrent DNA mismatch repair deficient/microsatellite instability-high (dMMR/MSI-H) or proficient/stable (MMRp/MSS) endometrial cancer: Interim results from GARNET-a phase I, single-arm study. J. Immunother. Cancer 10, e003777. doi:10.1136/jitc-2021-003777
Oh, C. R., Kim, J. E., Hong, Y. S., Kim, S. Y., Ahn, J. B., Baek, J. Y., et al. (2022). Phase II study of durvalumab monotherapy in patients with previously treated microsatellite instability-high/mismatch repair-deficient or POLE-mutated metastatic or unresectable colorectal cancer. Int. J. Cancer 150, 2038–2045. doi:10.1002/ijc.33966
Overman, M. J., Mcdermott, R., Leach, J. L., Lonardi, S., Lenz, H. J., Morse, M. A., et al. (2017). Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 18, 1182–1191. doi:10.1016/s1470-2045(17)30422-9
Pitt, J. M., Vétizou, M., Daillère, R., Roberti, M. P., Yamazaki, T., Routy, B., et al. (2016). Resistance mechanisms to immune-checkpoint blockade in cancer: Tumor-intrinsic and -extrinsic factors. Immunity 44, 1255–1269. doi:10.1016/j.immuni.2016.06.001
Qin, S., Li, J., Zhong, H., Jin, C., Chen, L., Yuan, X., et al. (2022). Serplulimab, a novel anti-PD-1 antibody, in patients with microsatellite instability-high solid tumours: An open-label, single-arm, multicentre, phase II trial. Br. J. Cancer 127, 2241–2248. doi:10.1038/s41416-022-02001-3
Roberto, M., Falcone, R., Mazzuca, F., Archibugi, L., Castaldi, N., Botticelli, A., et al. (2017). The role of stereotactic body radiation therapy in oligometastatic colorectal cancer: Clinical case report of a long-responder patient treated with regorafenib beyond progression. Med. Baltim. 96, e9023. doi:10.1097/md.0000000000009023
Schreiber, R. D., Old, L. J., and Smyth, M. J. (2011). Cancer immunoediting: Integrating immunity's roles in cancer suppression and promotion. Science 331, 1565–1570. doi:10.1126/science.1203486
Shirley, M. (2018). Fruquintinib: First global approval. Drugs 78, 1757–1761. doi:10.1007/s40265-018-0998-z
Siegel, R. L., Miller, K. D., Fuchs, H. E., and Jemal, A. (2021). Cancer statistics, 2021. CA Cancer J. Clin. 71, 7–33. doi:10.3322/caac.21654
Smith, K. M., and Desai, J. (2018). Nivolumab for the treatment of colorectal cancer. Expert Rev. Anticancer Ther. 18, 611–618. doi:10.1080/14737140.2018.1480942
Sun, Q., Zhou, J., Zhang, Z., Guo, M., Liang, J., Zhou, F., et al. (2014). Discovery of fruquintinib, a potent and highly selective small molecule inhibitor of VEGFR 1, 2, 3 tyrosine kinases for cancer therapy. Cancer Biol. Ther. 15, 1635–1645. doi:10.4161/15384047.2014.964087
Venderbosch, S., Nagtegaal, I. D., Maughan, T. S., Smith, C. G., Cheadle, J. P., Fisher, D., et al. (2014). Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: A pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin. Cancer Res. 20, 5322–5330. doi:10.1158/1078-0432.ccr-14-0332
Keywords: metastatic colon cancer, microsatellite instability, KRAS exon 2 p.G12D mutation, fruquintinib, radiotherapy
Citation: Wang R, Cong D, Bai Y and Zhang W (2023) Case report: long-term sustained remission in a case of metastatic colon cancer with high microsatellite instability and KRAS exon 2 p.G12D mutation treated with fruquintinib after local radiotherapy: a case report and literature review. Front. Pharmacol. 14:1207369. doi: 10.3389/fphar.2023.1207369
Received: 17 April 2023; Accepted: 19 June 2023;
Published: 28 June 2023.
Edited by:
Léo Aubert, Université catholique de Louvain, BelgiumReviewed by:
Eleonora Lai, University Hospital and University of Cagliari, ItalyChaoyuan Kuang, Albert Einstein College of Medicine, United States
Copyright © 2023 Wang, Cong, Bai and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenlong Zhang, d2VueHVAamx1LmVkdS5jbg==
 Ruiqi Wang
Ruiqi Wang Yuansong Bai
Yuansong Bai Wenlong Zhang
Wenlong Zhang