
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 19 October 2023
Sec. Translational Pharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1207141
This article is part of the Research TopicIdentifying Novel Drug Delivery Systems and Treatments for Hearing Loss and Related Ear Disorders, Volume IIView all 7 articles
Despite significant advances in the development of therapeutics for hearing loss, drug delivery to the middle and inner ear remains a challenge. As conventional oral or intravascular administration are ineffective due to poor bioavailability and impermeability of the blood-labyrinth-barrier, localized delivery is becoming a preferable approach for certain drugs. Even then, localized delivery to the ear precludes continual drug delivery due to the invasive and potentially traumatic procedures required to access the middle and inner ear. To address this, the preclinical development of controlled release therapeutics and drug delivery devices have greatly advanced, with some now showing promise clinically. This review will discuss the existing challenges in drug development for treating the most prevalent and damaging hearing disorders, in particular otitis media, perforation of the tympanic membrane, cholesteatoma and sensorineural hearing loss. We will then address novel developments in drug delivery that address these including novel controlled release therapeutics such as hydrogel and nanotechnology and finally, novel device delivery approaches such as microfluidic systems and cochlear prosthesis-mediated delivery. The aim of this review is to investigate how drugs can reach the middle and inner ear more efficiently and how recent innovations could be applied in aiding drug delivery in certain pathologic contexts.
Hearing loss is one of the most common sensory disabilities, with the World Health Organization estimating over 1.5 billion people worldwide to currently experience some form of hearing impairment (Chadha et al., 2021). The ear is a highly compartmentalized organ with anatomical barriers that preclude conventional drug delivery both locally to affected sites or systemically due to the blood labyrinth barrier (BLB; Figure 1). Systemically administered drugs additionally risk being poorly bioavailable or having unwanted effects in other organs. Local administration is preferred to solve both issues, however the procedures involved are significantly more invasive. This presents a clinical challenge for the administration of multiple doses and some newer technologies such as gene therapy, which shows great promise in the treatment of hereditary conditions and the regeneration of hair cells (Nyberg et al., 2019; Szeto et al., 2019; French et al., 2020). We will briefly discuss the etiology of otitis media, perforation of the tympanic membrane and cholesteatoma of the middle ear and sensorineural hearing loss of the cochlea; then discuss current and emerging treatments and recent progress in improving drug delivery to the middle and inner ear for these diseases.
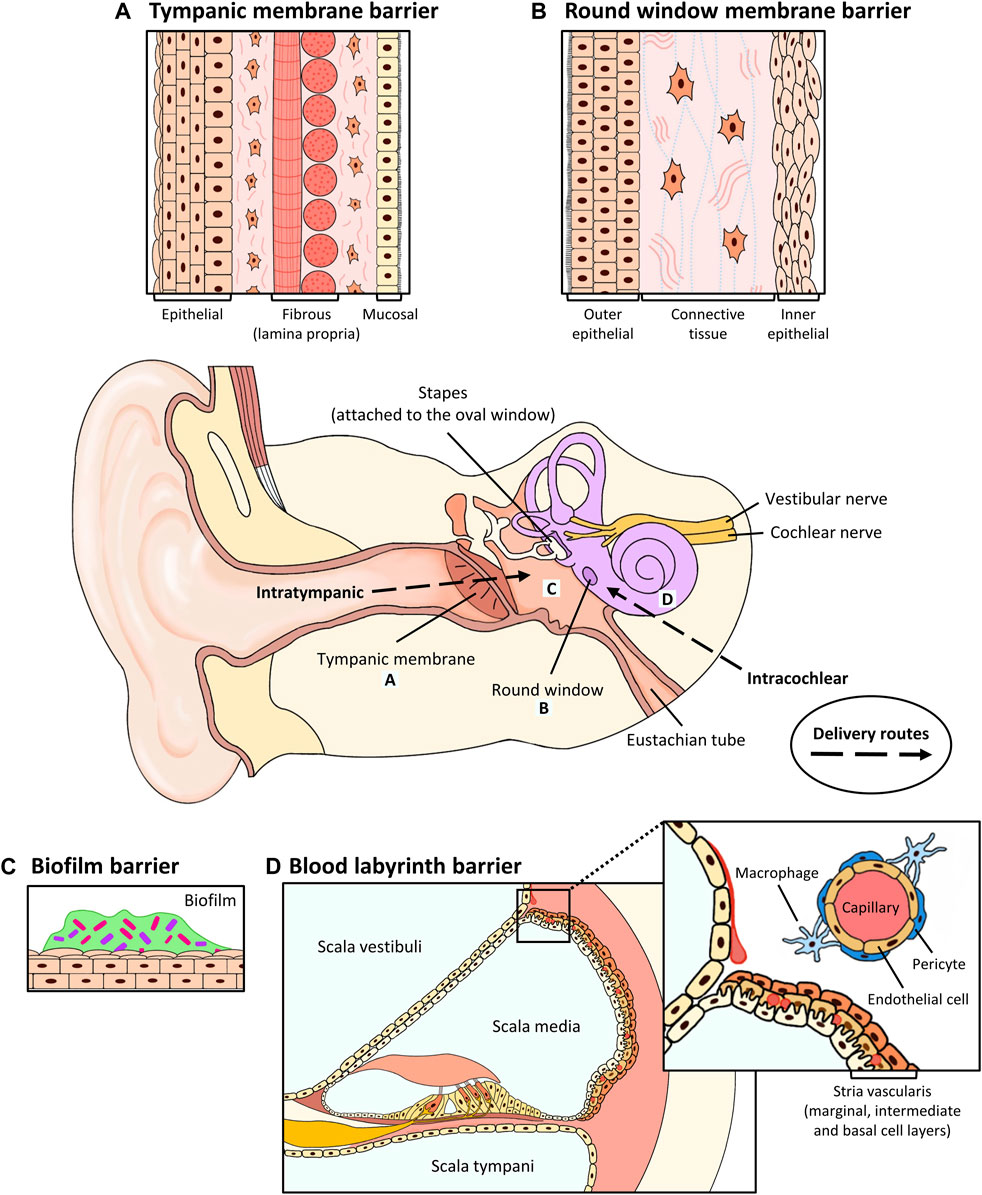
FIGURE 1. Anatomical barriers to drug penetration in the middle and inner ear, and routes of local delivery. (A) Tympanic membrane. The TM consists of a stratified outer epithelium, a lamina propria (radial and circular fibrous) containing fibroblasts and a thin layer of mucosal epithelium. Intratympanic and topical drugs are administered here. (B) Round window membrane. The RWM consists of an outer epithelium, a connective tissue layer containing fibroblasts, collagen and elastic fibers and an inner epithelium. Intratympanic drugs are administered here. (C) Biofilm. Biofilm forms an encapsulated layer of extracellular proteins to protect pathogenic microorganisms against antibiotics. (D) Blood labyrinth barrier. The stria vascularis contains 3 cell layers: the marginal, intermediate and basal cell layers. The BLB consists of pericytes, endothelial cells and macrophages and surrounds the capillaries that are embedded within the intermediate cell layers of the stria vascularis. As shown in the inset in (D), an enlarged view is shown to illustrate the cellular component of the BLB system. Systemically administered drugs would need to cross the BLB to access the inner ear.
Otitis media (OM), perforation of the tympanic membrane (TM), and cholesteatoma are the most commonly reported middle ear diseases in clinical practice. Common signs and symptoms such as pain, fever, sudden hearing loss, and the sensation of fullness in the ear may help identify middle ear diseases, but additional approaches such as otoscopy and magnetic resonance imaging (MRI) are often required to confirm the diagnosis (Trojanowska et al., 2012; Lo and Nemec, 2015).
OM represents a spectrum of middle ear inflammatory diseases which can be classified into acute otitis media (AOM), otitis media with effusion (OME, also known as glue ear), and chronic suppurative otitis media (CSOM), which is one of the most severe middle ear diseases. The common symptoms for AOM and OME include earache, hearing loss, swelling and bulging of the TM, and the accumulation of fluid behind the TM. Meanwhile, CSOM is characterized by chronic inflammation and recurrent purulent discharge through a perforated TM (Leach et al., 2021).
OM is one of the leading causes of permanent hearing loss in children and a significant disease burden in developing countries and several OM-prone populations such as Australian Aboriginals (Li et al., 2015; DeAntonio et al., 2016; DeLacy et al., 2020). Children and adolescents are particularly susceptible to OM, and two of the most accepted theories are an immature Eustachian tube (structural and functional) that is prone to blockage; and underdeveloped innate and adaptive immune systems (Bluestone, 2008; Pichichero, 2020).
Bacterial pathogens such as Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis and Pseudomonas aeruginosa are the most common cause of OM, but it can also be caused by Influenza viruses or fungal species such as Aspergillus (Mittal et al., 2015; Bhutta et al., 2017; Rowe et al., 2019). There is increasing evidence that biofilm, a microhabitat of microorganisms entrapped in self-produced extracellular proteins, contributes to the pathogenesis of OM. Biofilms are difficult to eradicate as they are resistant to antibiotics and firmly adhered to the middle ear epithelium, ossicles and tympanostomy tube (Mittal et al., 2015; Mittal et al., 2018; Silva and Sillankorva, 2019).
Perforations can occur due to OM or trauma to the TM, such as the piercing of the TM by a sharp object or a sudden change in pressure (barotrauma) (Spandow et al., 1996; Wang et al., 2014a). Most traumatic or acute TM perforations heal spontaneously within 1–2 weeks, but those that fail to heal within 3 months are considered chronic TM perforations which require surgical intervention (Tan et al., 2016). The mechanism that underlies the development of chronic TM perforation is unknown, but studies have identified risk factors that are linked to delayed healing of TM perforations, including perforation size and location (Lou et al., 2011; Tan et al., 2016), Eustachian tube dysfunction (Varsak and Santa Maria, 2016; Paltura et al., 2017) and long-term tympanostomy tube usage (Kay et al., 2001; Alrwisan et al., 2016; Knutsson et al., 2018).
Cholesteatoma is described as an inflammatory, non-malignant (and pearl-like) epithelial lesion in the middle ear cavity that can lead to conductive hearing loss, erosion of the ossicles, and other intracranial complications. Congenital cholesteatoma is rare and contributes to 1%–5% of all cholesteatoma cases; and is often detected within the first decade of life (Mansour et al., 2018; Jenks et al., 2022). The development of congenital cholesteatoma is thought to be due to the entrapment of epithelial remnants during embryogenesis, but the actual mechanism that drives this process remains under debate (Gilberto et al., 2020). Meanwhile, the more common acquired cholesteatoma is categorized into either primary acquired, which is caused by a retraction pocket in the TM; or secondary acquired, which is thought to be caused by an abnormal growth of the TM epithelial layer in the middle ear through a perforation (Cho et al., 2016; Clark et al., 2016). The pathogenesis of acquired cholesteatoma can be grouped into four theories: retraction pocket or invagination theory; epithelial invasion theory; squamous metaplasia theory and basal cell hyperplasia theory (Kuo et al., 2015; Hamed et al., 2016). More recently, the dysregulation of cell signaling and immunological pathways such as EGF; IL-6/JAK/STAT3, MicroRNA-21, Notch and TNF signaling have been identified as additional potential contributors (Hilton et al., 2011; Liu et al., 2014; Chen et al., 2016; Xie et al., 2016; Fukuda et al., 2021).
The most common type of hearing impairment is sensorineural hearing loss (SNHL). Patients with SNHL perceive sound as muted and distorted when compared to other forms of hearing loss, and it is caused by the degeneration or malfunction of cochlear hair cells or spiral ganglion nerves of the inner ear. This can be caused by a wide variety of environmental insults such as excessive noise, which is the second largest cause of SNHL, the administration of ototoxic drugs, exposure to ototoxic chemicals including certain solvents and metals, intrauterine infections including toxoplasmosis, cytomegalovirus and human immunodeficiency virus, birth asphyxia and hyperbilirubinemia. Genetics also play a role in more sporadic, yet highly severe forms of SNHL where aspects of cochlear development or structure are crippled by mutations to certain genes. These mutations can be inherited or, less commonly, arise de novo in the developing cochlea. In the following subsections, we summarize the etiology of the more characterized common or severe forms of SNHL: hereditary, noise-induced, age-related, drug-induced and idiopathic.
Hereditary SNHL is caused by the inheritance of one or more gene mutations from biological parents that carry defective alleles, and affects about 1 per 1,000 newborns with profound deafness (Gettelfinger and Dahl, 2018). Mutations cause the translation of malfunctional proteins that can no longer fulfil their native function and cohesively work with other components of the auditory system. Most hereditary-related deafness is monogenic, with approximately 80% of cases being autosomal recessive, although other modes of transmission such as autosomal dominant and X-linked can also occur (Schrujver, 2004). There have been 124 deafness-associated genes identified to date and some of the most prevalent include MYO7A and USH2A which are associated with Usher syndrome, PAX3 and SOX10 which are associated with Waardenburg syndrome, and PDS which is associated with Pendred syndrome (Nishio et al., 2015; Van Camp and Smith, 2023).
Noise-induced hearing loss (NIHL) is caused by progressive over-exposure to noise. NIHL affects all ages, however there is an increasing prevalence of NIHL occurring in younger populations from recreational noise exposure, for example from listening to music using headphones for prolonged periods, with over 50% of those aged 12–35 being estimated to risk developing NIHL (Chadha et al., 2021). Occupational NIHL from jobs involving prolonged exposure to loud noise, including from power tools, automotive, gunfire and music, has been attributed to 16% of worldwide severe hearing loss, with men shown to be more susceptible than women (Nelson et al., 2005). Long-term noise exposure can risk damage to the auditory structures which deteriorates cochlear hair cells and spiral ganglion nerves. Moreover, certain genetic variants have been identified clinically and experimentally to increase susceptibility to NIHL (Beaulac et al., 2021; Jiang et al., 2021). The early signs of NIHL are the loss of high frequency hearing and decreased ability to distinguish speech over background noises, and is often accompanied by tinnitus (Le et al., 2017).
Age-related hearing loss (ARHL), or presbycusis, affects the elderly as they gradually lose hearing in both ears and over 65% of adults over 60 years of age have been shown to be affected (Cunningham and Tucci, 2017; Chadha et al., 2021). ARHL usually has a complex etiology which encompasses lifestyle aspects such as noise, but does have some other defined risk factors such as smoking, ototoxic drug use and hypertension (Gates and Mills, 2005; Bowl and Dawson, 2019). Moreover, ARHL has been shown to cluster in families with prevalent cases (Gates et al., 1999). In terms of pathological features, four classifications of ARHL have been proposed from post-mortem human tissue examination, including ARHL from progressive hair cell loss, sensory and neural degeneration, loss of stria vascularis integrity and stiffening of the basilar membrane (Schuknecht, 1964; Schuknecht and Gacek, 1993). Additional etiologies have been proposed based on post-mortem human tissue analyses and animal models, involving oxidative stress, chronic inflammation, and loss of lateral wall fibrocytes (Ohlemiller and Gagnon, 2004; Suzuki et al., 2006; Tawfik et al., 2020), but it is likely that the clinical presentation will involve more than one of these causes.
Drug-induced hearing loss occurs when patients take drugs that have ototoxic side effects. Aminoglycoside and macrolide antibiotics in particular, such as gentamicin and azithromycin, are commonly used in the treatment of Meniere’s disease and in developing countries for other diseases, however they are highly ototoxic (Rauch et al., 2011; Rybak et al., 2021). Aminoglycosides can permeate through the BLB or round/oval window (when administered intratympanically for Meniere’s disease) due to their small molecular size, and accumulate in the apical side of hair cells through mechanoelectrical transduction (MET) channels or by endocytosis (Nyberg et al., 2019). When MET channels are stimulated by sound, antibiotics can enter them and exert cytotoxicity on hair cells through a yet unclear mechanism (Alharazneh et al., 2011; Zimmerman and Lahav, 2013). For ototoxic chemotherapies, cisplatin is perhaps the most widely prescribed example (Greene et al., 2015; Frisina et al., 2016). Cisplatin increases the generation of reactive oxygen species by inhibiting peroxidase enzymes which progressively damages hair cells over time, leading to SNHL (Sheth et al., 2017). Other ototoxic drugs include the antimalarial drug quinine and loop diuretics such as furosemide and acetylsalicylic acid (Tange et al., 1997; Ding et al., 2016). Furthermore, ototoxicity can be exacerbated by interactions with certain drugs, genetic polymorphisms and cochlear inflammation (Barbieri et al., 2019; Coffin et al., 2021).
Sudden sensorineural hearing loss (SSNHL) is characterized by the presentation of patients with at least 30 dB hearing loss occurring within 72 h. The incidence in the United States was reported to be 5 to 20 per 100,000 people with the vast majority of cases being unilateral and having an unknown etiology, being termed idiopathic (Kuhn et al., 2011). Bilateral SSNHL is less prevalent, comprising an estimated 4.9% of SSNHL cases, however is not usually idiopathic and is secondary to other complications including cancer, vascular disorders and autoimmune disease (Sara et al., 2013). Idiopathic SSNHL does spontaneously resolve in 45%–65% of patients, however controversy remains on potential etiologies and best course of treatment (Conlin and Parnes, 2007; Kuhn et al., 2011).
Middle ear diseases such AOM and OME are most commonly managed with supportive care such as painkillers or oral antibiotics such as Amoxicillin, but vaccination against common middle ear pathogens such as S. pneunomiae and H. influenzae has become standard practice in some countries (Martinovich et al., 2021; Clark et al., 2022; Izurieta et al., 2022). The treatment of CSOM remains a clinical challenge and often requires the combination of oral antibiotics, eardrops, and surgery due to the presence of antibiotic-resistant bacteria and bacterial biofilm (Mittal et al., 2018; Leach et al., 2021). Moreover, recurrence of the infection can occur from the presence of persister cells, a phenotype of bacteria within biofilms that exhibit low metabolic activity (Tolker-Nielsen, 2014; Fisher et al., 2017). Persister cells have been shown to escape the initial course of antibiotics and resume growth and metabolism once the administration is halted (Khomtchouk et al., 2020; Santa Maria et al., 2021).
Acute or traumatic TM perforations are most often treated with supportive care as with OM, to minimize pain and prevent infection (Orji and Agu, 2008; Lieberthal et al., 2013). Surgery is only indicated for chronic TM perforations (over 3 months) and this procedure is called myringoplasty (or tympanoplasty if ossicles are involved), which involves the placement of an autologous graft (typically temporalis fascia or auricular cartilage) over or under the defect to encourage wound healing and restore hearing. The success rate for such surgery is approximately 87% (Tan et al., 2016), but the harvesting of autologous grafts is associated with donor site morbidity (infection and pain) and higher operation cost (Villar-Fernandez and Lopez-Escamez, 2015; Sainsbury et al., 2022).
For cholesteatoma, surgical excision remains the only curative approach and this is often performed in conjunction with reconstruction surgeries (e.g., mastoid wall reconstruction) to repair damage caused by the lesion and surgical procedure (Mansour et al., 2018). Unfortunately, the recurrence rate for cholesteatoma is high with 10%–40% of patients requiring repeated surgery (Britze et al., 2017; Angeli et al., 2020; Bächinger et al., 2021). The recurrence of cholesteatoma may be affected by the type of surgical procedure and is thought to vary between congenital and acquired disease (Edfeldt et al., 2012; Morita et al., 2017; van der Toom et al., 2021; Adriaansens et al., 2022).
Bacteriophage or viral-based treatment has emerged as an alternative strategy to counteract the increasing threat of antibiotic resistance (Gordillo Altamirano and Barr, 2019). Wright et al. (2009) conducted the first clinical trial on the safety of a bacteriophage preparation against P. aeruginosa in patients with chronic OM. Overall, the treatment did not cause any severe side effects, significantly lowered the bacteria count (CFU per gram) and improved the clinical scores of patients. In a later clinical trial, bacteriophage therapy was conducted in burns patients infected with P. aeruginosa (Jault et al., 2019). Unlike the previous trial, a daily topical application of a cocktail of 12 bacteriophages suspended in saline solution was compared to the standard of care (1% sulfadiazine silver emulsion cream) over 7 days. Overall, the treatment was well tolerated, but there was no significant clinical improvement or reduction in bacterial load when compared to standard of care. This study also highlighted a few challenges associated with bacteriophage treatment, including the stability and titer of bacteriophage after manufacturing and concerns about bacterial resistance to bacteriophages. Overall, bacteriophage-based treatment remains an exciting and promising therapeutic approach for the treatment of OM and warrants further investigation.
There has been an increased interest in the development of effective anti-biofilm technology as a preventative and treatment approach. One of the most promising treatment targets is Type IV pili (Tfp), a type of proteinous appendage on the surface of most bacterial species, which plays a critical role in mechanosensing, motility, and the formation of biofilm (Lee et al., 2018; Horna et al., 2019). Novotny and colleagues have successfully developed a transcutaneous immunization strategy against Tfp and showed both in vitro and in animal studies the efficacy of anti-Tfp antibodies in destabilization of H. Influenzae biofilm and the eradication of the pathogen (Novotny et al., 2013; Novotny et al., 2015; Novotny et al., 2016; Novotny et al., 2021). Anti-Tfp treatment has also been shown to be effective against other middle ear pathogens such as M. catarrhalis (Mokrzan et al., 2018). More importantly, the antibody-mediated release bacteria (from the biofilm) were found to be more susceptible to antibiotic killing as compared to their planktonic (free-living) counterparts (Mokrzan et al., 2018; Mokrzan et al., 2020). Other promising strategies to tackle problems associated with biofilm include antibody treatment against bacterial DNABII protein, a key structural component of the biofilm (Barron et al., 2020; Novotny et al., 2021). Dornase alfa, a recombinant human deoxyribonuclease that breaks down DNA, has also been shown to reduce the development of otorrhea in pediatric patients undergoing tympanostomy (Thornton et al., 2013; Chan et al., 2018).
For the repair of TM, much research has been focused on developing biocompatible scaffolds using natural or synthetic materials such as collagen, chitosan, decellularized tissue and polylactic acid (PLA) (Hussain and Pei, 2021; Sainsbury et al., 2022). Some of the current commercially available scaffolding materials indicated for myringoplasty are EpiFilm® (Medtronic, Ireland), which is a hyaluronic acid-derived implantable device, and acellular dermal allografts such as Alloderm (Allergan, Ireland) (Vos et al., 2005; Sayin et al., 2013).
Otherwise, adjuvant therapies using topical application of biomolecules are becoming popular in addition to myringoplasty to enhance the healing of perforations. The most commonly used biomolecules are growth factors such as epidermal growth factor (EGF), basic fibroblast growth factor (bFGF) (Hong et al., 2013; Sainsbury et al., 2022). The efficacy of EGF on the closure of TM perforations has been investigated predominantly in acute/traumatic perforations. (Lou et al., 2016; Lou and Lou, 2017; Lou and Lou, 2018b). Despite the positive outcomes, the benefits of EGF on the repair of chronic TM perforation remain unclear due to the lack of well-controlled clinical studies.
Multiple clinical trials have been conducted to study the effects of bFGF on both acute and chronic TM perforations. For acute TM perforations, topical drops administration is the most commonly used approach and is associated with improved healing outcomes when compared to untreated controls (Huang et al., 2020). For chronic perforations, the most widely used approach for bFGF therapy was developed by Kanemaru and colleagues in which a bFGF-impregnated scaffold (Gelfoam) is applied over the perforation, which is then sealed with fibrin glue (Kanemaru et al., 2011). Clinical trials conducted in Japan have reported closure rates of 62%–100% in chronic perforations treated with bFGF (Hakuba et al., 2003; Hakuba et al., 2010; Hakuba et al., 2013; Hakuba et al., 2015). In contrast, a recent Phase 2 clinical trial comparing chronic TM perforations treated with bFGF or placebo (water) reported no statistical difference in closure rates between the two treatment groups (Santos et al., 2020). Besides that, there have been concerns about the safety of bFGF as it was found to lead to other middle ear disorders such as myringitis and cholesteatoma (Hakuba et al., 2013; Lou and Lou, 2018a).
Platelet-rich-plasma (PRP), a type of blood product that is rich in platelets and bioactive molecules, has been shown to be a promising alternative treatment option for tissue repair and regeneration (Amable et al., 2013; Everts et al., 2020; Huang et al., 2021). PRP has also emerged as a promising treatment for chronic TM perforations with 8 clinical trials conducted in the past 10 years (El-Anwar et al., 2015; Fawzy et al., 2018; Fouad et al., 2018; Yadav et al., 2018; Mandour et al., 2019; Anwar et al., 2020; Ersözlü and Gultekin, 2020; Taneja, 2020). The closure rate ranged from 85.7% to 100% in the PRP treatment group while a 55%–92% closure rate was reported in the control group. It is noteworthy that PRP was used in conjunction with a scaffolding material including a fat graft, temporalis fascia, or conchal perichondrium. Therefore, the real therapeutic benefit of PRP as a stand-alone treatment remains unclear.
Overall, adjuvant therapy using biomolecules with or without myringoplasty remains an attractive avenue for the management of TM perforations with research expanded beyond the traditional growth factors (Shen et al., 2014; Araujo et al., 2016). More recently, a Phase 1 clinical trial investigating the effect of a recombinant heparin-binding epidermal growth factor-like growth factor (HB-EGF) (dubbed ASP0598 Otic Solution) on the healing of chronic TM perforations has started patient recruitment in multiple locations in the United States (NCT04305184, clinicaltrials.gov).
Auditory rehabilitation with hearing devices or cochlear implants remains the primary treatment approach for SNHL. These technologies can significantly aid in communication, yet they are still unable to mimic the quality of natural hearing and, more importantly, they do not treat the underlying cause of the hearing loss (Beck and Le Goff, 2018; Solheim et al., 2018).
Hearing aid technology has advanced greatly over the past decade, with innovations allowing customization of every device to fit the unique hearing needs of each patient. These devices are designed to amplify sounds and restore hearing, however no currently available devices can provide sound quality comparable to that of a healthy cochlea. Some hearing aids are capable of selectively reducing background noise while maintaining access to all distinct speech sounds, but this typically requires the user to select the voice they wish to focus on and are usually ineffective in group settings. Moreover, hearing aids still rely on functional hair cells for sound transduction (Geleoc and Holt, 2014).
In contrast, cochlear implants have proven to be a successful therapeutic approach for patients, even for those with abnormal hair cells and severe hearing impairment. There is clear evidence showing rapid development in oral communication and auditory skills in infants and children with SNHL with cochlear implants (Mok et al., 2010; Dettman et al., 2016; Hoff et al., 2019; Dettman et al., 2021). Moreover, adult patients with cochlear implants were shown to have improved speech outcomes and quality of life (Santa Maria et al., 2013; Völter et al., 2020). However, evidence in patients and animal models show the surgical installation procedure risks chronic cochlear inflammation or fibrosis, which reduces the effectiveness of the implant over time (Wilk et al., 2016; Foggia et al., 2019).
There is currently a wide spectrum of drug types undergoing clinical trials for different types of SNHL, which has been recently comprehensively summarized by Isherwood et al. (2022). In recent years, strategies aiming to manipulate hair cell differentiation pathways have garnered significant interest. For example, hair cell differentiation is shown to be accompanied by ATOH1 gene expression, crucial for progenitor cells residing in the organ of Corti to be directed to differentiate into a non-sensory or sensory lineage (Cai et al., 2013; Driver et al., 2013). Currently, the CGF166 adenoviral vector contains ATOH1 cDNA transcript to replace absent hair cells has completed Phase 2 investigation (NCT02132130, clinicaltrials.gov). However, most recently reported novel otoprotective, regenerative and gene replacement treatments remain too early in their stage of development to be applied clinically.
For cisplatin-induced hearing loss, sodium thiosulfate has recently been approved by the US Food and Drug Administration as an otoprotective drug. As mentioned, cisplatin is a highly ototoxic drug, with children and adolescents undergoing cisplatin treatment being particularly vulnerable to cisplatin-induced hearing loss (Orgel et al., 2022). Sodium thiosulfate has been demonstrated in animal models and clinical trials to abrogate cisplatin-induced ototoxicity, thought to occur by directly chelating cisplatin (Videhult et al., 2006; Brock et al., 2018). However, as sodium thiosulfate directly binds cisplatin and is currently intravenously injected, this may risk decreasing the efficacy of the chemotherapy on the cancer itself. Methods of locally administering sodium thiosulfate to the inner ear are currently being investigated to reduce this risk and will be discussed in later sections.
Another developing approach to treating inner ear disorders is the application of exosomes. Exosomes are a subset of extracellular vesicle derived from the budding of endosomes from the plasma membrane, incorporating transmembrane proteins and lipids, and range from ∼30 to 160 nm in diameter. The precise physiological role of exosomes is unclear, however they have been demonstrated to have pleiotropic roles in cell-cell communication, signaling, proliferation, angiogenesis, immune response and metabolism and have been shown to carry proteins, nucleic acids and metabolites (Kalluri and LeBleu, 2020; Warnecke et al., 2022). Exosomes have been recently identified, isolated and characterized from the perilymph of adult patients with various forms of SNHL or mixed hearing loss, with the study suggesting a hair cell origin of the exosomes by their expression of MYO7A (Zhuang et al., 2021). On the therapeutic side, a study from Athanasia Warnecke and colleagues demonstrated protective effects of bone marrow mesenchymal stromal cell-derived extracellular vesicles on a mouse model of NIHL, which additionally promoted neurite outgrowth in vitro (Warnecke et al., 2020). This was followed up by a study demonstrating the safety of implanted extracellular vesicles in a Meniere’s disease patient that had undergone cochlear implantation 24 months post-operation (Warnecke et al., 2021). Exosomes thus represent an interesting potential avenue of biological, yet cell-free treatment of inner ear disorders.
Oral or intravenous delivery of antibiotics are the standard approach for treating middle ear disorders, but this has been shown to risk poor bioavailability and side effects such as diarrhea (Chee et al., 2016). Topical treatments such as ciprofloxacin/dexamethasone ear drops or Otiprio® are efficacious for OM, however they are indicated exclusively for OM with perforated TM (e.g., post tympanostomy or CSOM) (van Dongen et al., 2014). While animal and human cadaveric studies have showed that intact TMs are readily permeable to ciprofloxacin (Yang et al., 2016; Yang et al., 2018b; Early et al., 2021), it remains unclear whether such results can be achieved in a clinical setting. Apart from this physical barrier, bacterial biofilm formed during OM creates a second physical-chemical barrier that hinders treatment (Silva and Sillankorva, 2019).
Similarly, the cochlea is among the most difficult organs to deliver drugs to by conventional systemic routes. It is encased by the petrous bone, which is the densest bone in the human body, and is only accessible to the circulation via the BLB. As with the treatment of OM, drugs administered systemically generally accumulate poorly in the inner ear and can exert unwanted effects elsewhere due to the aforementioned barriers (Rauch et al., 2011). Therefore, to effectively deliver drugs to the cochlea, more invasive localized delivery procedures are required to ensure bioavailability and avoid unwanted systemic effects. These are injections by intratympanic and intracochlear routes (Figure 1). While being more invasive and potentially traumatic, these injections allow more direct administration of drug to the inner ear. Intratympanic injection generally relies on the permeation of the therapeutic agent across the round window (RWM) or oval window membrane, which are more permeable than the BLB or TM. However, it still presents a delivery challenge as most drugs remain poorly bioavailable in the inner ear due to clearance across the BLB or Eustachian canal (Salt and Plontke, 2018). Non-specific binding or aggregation can also occur due to the positively-charged perilymph if the molecule is negatively charged, for example DNA or RNA. A recent study comparing intratympanic and intracochlear delivery of a novel supraparticle encapsulated neurotrophin-3 has showed the latter to be a superior approach, but may not be practical for drugs that require multiple doses due to the risk of scarring or inner ear fibrosis (Gunewardene et al., 2022). Another alternative injection method that has become more prevalent is postauricular or retroauricular injection which delivers drug to the postauricular region of the ear without perforating the tympanic membrane. This has the advantage of avoiding potential side effects of intratympanic injection such as persistent pain, otitis media or acute or chronic tympanic perforation. (Li et al., 2021; Chen et al., 2022c; Xie et al., 2023). However, it is currently not performed worldwide and it remains unclear how drugs reach the inner ear from the postauricular region (Qiu et al., 2022).
To counteract these physiological challenges, there has recently been considerable progress in the use of biomaterials such as hydrogels and nanotechnology. Formulations involving these biomaterials are aimed at enhancing drug bioavailability and providing controlled and sustained release. Sustained release can be triggered either upon temperature change, phase change, implantation, pH changes or administration of a secondary agent (You and Auguste, 2009; Lajud et al., 2015; Li and Mooney, 2016; Dai et al., 2018). The following sections will address how hydrogels and nanotechnology are being employed to aid drug delivery and facilitate the delivery of novel and repurposed therapeutics into the middle and inner ear. We additionally have summarized examples of biomaterial-based drug delivery platforms in Table 1.
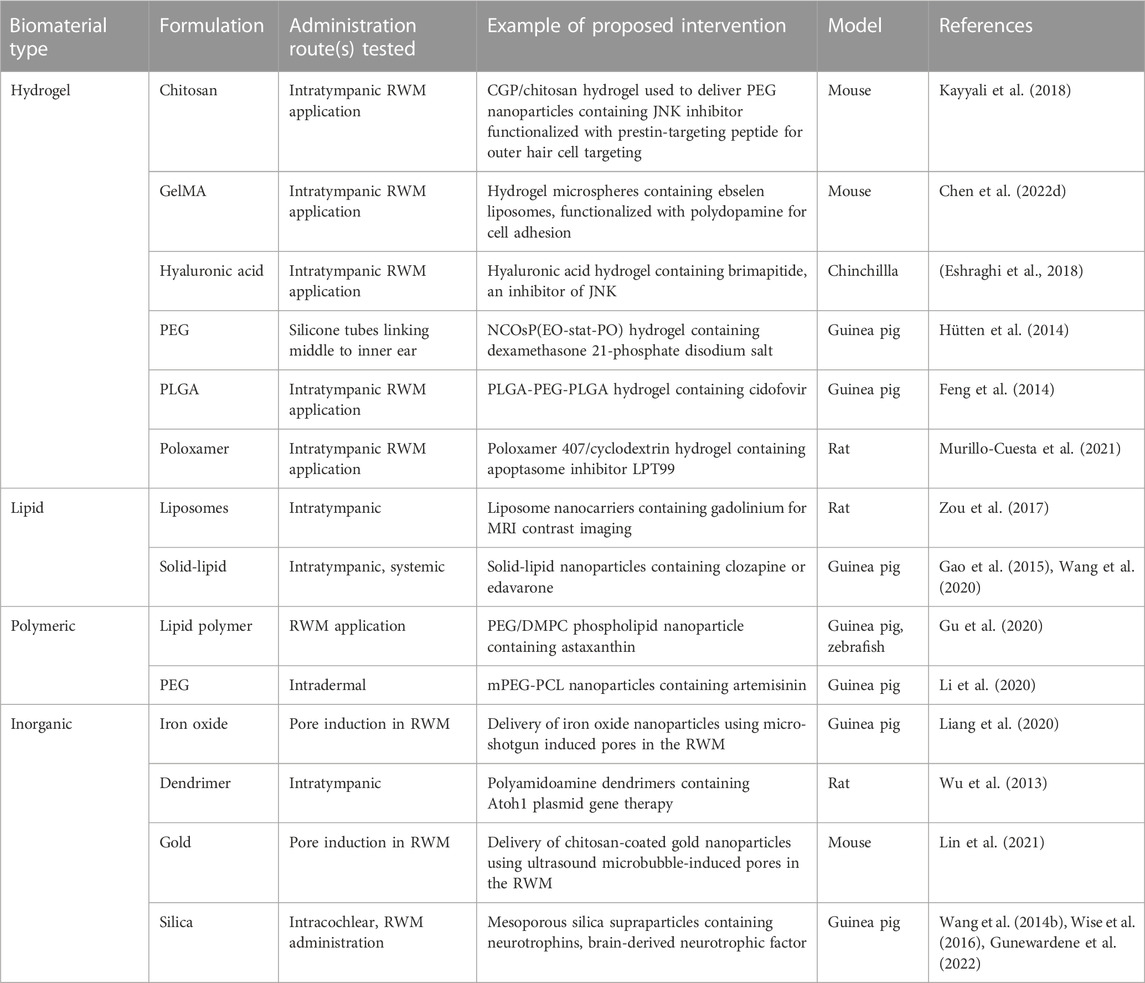
TABLE 1. Examples of proposed biomaterial-based therapies for sensorineural hearing loss undergoing preclinical studies.
Hydrogel delivery systems allow for the controlled release of a variety of therapeutic compounds. They can be manipulated into virtually any shape or size to facilitate controlled drug release and delivery to various locations in the body (Li and Mooney, 2016; Rathnam et al., 2019). For hearing-based therapies, the most common form tested preclinically is direct application of a liquid in situ gelling hydrogel. The hydrogel is administered to the TM or round window niche via intratympanic injection, which solidifies upon contact with the RWM (Rathnam et al., 2019). This allows for the continual and controlled release of drugs which diffuse across the RWM to the inner ear, without a need for highly invasive surgery (Paulson et al., 2008; Hütten et al., 2014; Lajud et al., 2015; Chen et al., 2022d).
Most hydrogel systems are advantageous due to their biocompatibility. For example, gelatin methacryloyl (GelMA) is highly biocompatible and has been extensively studied in the laboratory as a substrate for three-dimensional mammalian cell culture due to its inert nature, tunability, and higher batch-to-batch reproducibility over other commonly used substrates (Zhu et al., 2019). In an interesting recent approach for clinical application, GelMA hydrospheres were conjugated with polydopamine (PDA) for cell adhesion. The delivery of ebselen via these GelMA hydrospheres recovered hearing loss at some frequencies in a mouse model of NIHL (Chen et al., 2022d). Another biocompatible hydrogel composed of modified polyethylene glycol (PEG) was used to deliver a hydrophilic form of dexamethasone to a guinea pig cochlear trauma model, resulting in sustained drug delivery over several weeks, causing the recovery of hearing loss and reduced fibrosis (Hütten et al., 2014).
Additional classes of hydrogel include poloxamers, with a recent approach using the commercially available Poloxamer 407 to deliver a small molecule inhibitor of the apoptosome, LPT99. This approach was shown to preserve cell viability and auditory function in cisplatin-treated rats (Murillo-Cuesta et al., 2021). Finally, polylactic-co-glycolic acid (PLGA) hydrogels have shown promise due to their biodegradable nature and have potential to deliver a wide variety of drugs, including large molecules such as proteins (Dai et al., 2018; Kim et al., 2021). However high doses of a PLGA-PEG-PLGA copolymer were observed to affect hearing in guinea pigs (Feng et al., 2014).
Otiprio® is an FDA-approved ciprofloxacin solution suspended in a thermosensitive Poloxamer 407 gel and has been indicated primarily for the treatment of pediatric otitis externa and OME associated with tympanostomy tube placement (Edmunds, 2017). Otiprio® is thought to be superior to conventional ciprofloxacin eardrops (which usually requires three drops thrice a day) as it has been shown to reduce the occurrence of OM after a single intratympanic injection (Renukananda et al., 2014; Dohar et al., 2018). While Otriprio® is not currently approved for the treatment of CSOM, it is a promising “off-label” treatment due to the similarity of the two disease subtypes.
There are several hydrogel-based therapies for SNHL that are currently undergoing or have completed clinical trials. One of the earliest was developed by Otonomy termed “OTO-104”, a poloxamer gel injected intratympanically to deliver dexamethasone across the RWM to the inner ear (Piu et al., 2011). This therapy underwent Phase 2 and 3 clinical trials for vertigo in cisplatin-induced hearing loss and Meniere’s disease respectively, however both were terminated once no significant difference was observed compared to placebo for the former trial. This has been postulated to be due to accelerated clearance of the drug, as dexamethasone can readily cross the BLB and may be eliminated prior to affecting the more apical regions of the cochlea (Salt and Plontke, 2018). More recent developments include DB-020 and FX-322 from Decibel Therapeutics and Frequency Therapeutics respectively. DB-020 is a hyaluronic acid-based gel containing sodium thiosulfate for the inactivation of cisplatin, an ototoxic chemotherapy. Phase 1 clinical trials showed DB-020 was well tolerated and that 13 of 17 patients treated displayed partial or complete otoprotection (Viglietta et al., 2020). The progression and further studies of DB-020 will be useful to determine if the hydrogel confers additional otoprotection or higher tolerability in patients than the intravenously injected formulation recently approved by the U.S. Food and Drug Administration (Brock et al., 2018). FX-322 is a poloxamer-based gel containing the glycogen synthase kinase inhibitor, CHIR99021 and the histone deacetylase inhibitor, valproic acid. Both have been shown to promote the differentiation of stem cells into an otic lineage (Koehler et al., 2017). FX-322 showed a significant improvement in patients with chronic SNHL compared to the placebo in Phase 1b clinical trials (McLean et al., 2021). Unfortunately, Frequency Therapeutics reported that FX-322 showed no statistically significant difference from placebo at day 90 of a Phase 2b trial in February 2023 and development of the drug was halted.
Hydrogels have great potential to facilitate controlled delivery of a wide variety of drugs, including nanoparticles which will be discussed in the next section, and are an appealing avenue based on their ever-expanding tunability and composition. Despite these advantages, some precluding factors remain in their progression to more prevalent clinical usage. Namely, sterilization and storage could be challenging given their hydrated nature (Li and Mooney, 2016). Nevertheless, the degree of controlled release afforded by hydrogel drug delivery remains a promising factor in their applicability to treat hearing loss.
Due to their small size and theoretically endless possibilities for customization, nanoparticles have become an attractive option for drug delivery (Table 1). The ability to customize nanoparticles is advantageous as size, charge, lipid solubility and membrane thickness strictly govern molecular passage across the RWM and these properties cannot be readily modified in most drugs (Goycoolea and Lundman, 1997; Valente et al., 2017; Xu et al., 2021a; Xu et al., 2021b). Nanoparticles of sizes between 10 and 640 nm have been demonstrated to diffuse across the RWM, however most studies concur that sizes below 200 nm display the greatest permeation (Valente et al., 2017; Xu et al., 2021a; Chester et al., 2021).
Regarding charge, positively-charged nanoparticles display higher diffusion and greater distribution in the apical region of the cochlea, which is likely due to the ability of more positively charged nanoparticles to cross the lipid membranes and plasma membranes into cells (Liu et al., 2013; Yang et al., 2018a). A study using nanoparticles coated with a positively-charged arginine 8 peptide shell exploited this to deliver connexin 26 siRNA and plasmids containing green fluorescent protein or brain-derived neurotrophic factor (BDNF), along with dexamethasone, which induced nuclear pore opening (Yoon et al., 2016). Another study demonstrated greater distribution of cationic lipid nanoparticles in an in vitro RWM model, over neutrally charged or anionic nanoparticles of the same type. This nanoparticle also mitigated against an induced inflammatory reaction in vivo (Yang et al., 2018b).
Moreover, like hydrogels, nanoparticles can provide a means of controlled drug release in the middle and inner ear. This could decrease the need for surgical intervention for routine application of therapeutics and decrease potential systemic toxicity. Thus, nanotechnology has become of great recent interest in drug delivery to the inner ear.
Lipid nanoparticles are generally composed of amphipathic lipids, such as phospholipids. Phospholipids confer biocompatibility by allowing incorporation of the lipid nanoparticle into the cell membranes via endocytosis, micropinocytosis or degradation (Zou et al., 2017; Xu et al., 2021b; Chester et al., 2021; Lu et al., 2021). Furthermore, except for micelles, most lipid nanoparticle types can simultaneously encapsulate hydrophilic and lipophilic drugs in their aqueous region and lipid tail regions, respectively.
One of the original types of lipid nanoparticle are liposomes, which comprise an amphipathic lipid bilayer encapsulating a therapeutic payload. Liposomes were one of the first lipid nanoparticles to be clinically tested, however this class of nanoparticle has only been tested in the inner ear relatively recently in preclinical models. In a recent example by Zou et al. (2017), the biocompatibility of a liposome nanoparticle in rats was assessed through gadolinium MRI imaging which revealed no structural change to the inner ear and histology, which showed no cell death or inflammation, aside from a slight increase in the secretion of the extracellular matrix protein, hyaluronan. Another pre-clinical application of liposomes, delivered CRISPR Cas9-guide RNA complexes to target the pathogenic allele in the Bth mouse model of DFNA36 autosomal dominant deafness, using the commercially available Lipofectamine 2000 (Gao et al., 2018a). Lipofectamine 2000 is a liposome comprised of cationic lipids, but is more commonly used as a transfection reagent in laboratory settings (Dalby et al., 2004). Moreover it is slightly cytotoxic, so ideally a more biocompatible vector would be suitable for CRISPR-Cas or gene delivery (György et al., 2019). Nevertheless, this demonstrates the applicability of lipid nanoparticles for the delivery of a wide variety of therapeutics.
Other types of lipid nanoparticles include nanoemulsions, lipid core nanoparticles which have a lipid core enclosed by a shell of biocompatible polymers for stability, and solid-lipid nanoparticles (Figure 2). Solid-lipid nanoparticles (SLN) are composed of solid lipids, emulsifiers and water, and are of the most recently investigated for use in hearing loss. SLNs offer many advantages over other nanoparticle types. These include controlled drug release, stability at room temperature, higher payload, low toxicity and the ability to be functionalized and sterilized (Mehnert and Mäder, 2012). Recently, steric acid-based SLNs containing dexamethasone or hydrocortisone were shown to internalize in HEI-OC1 cells without compromising cell viability and additionally were otoprotective against cisplatin treatment (Cervantes et al., 2019). Other preclinical reports of SLN-mediated cochlear protection include the delivery of the antioxidant edaravone (Gao et al., 2015) and the antipsychotic clozapine (Wang et al., 2020), where both were shown to protect against NIHL in guinea pigs.
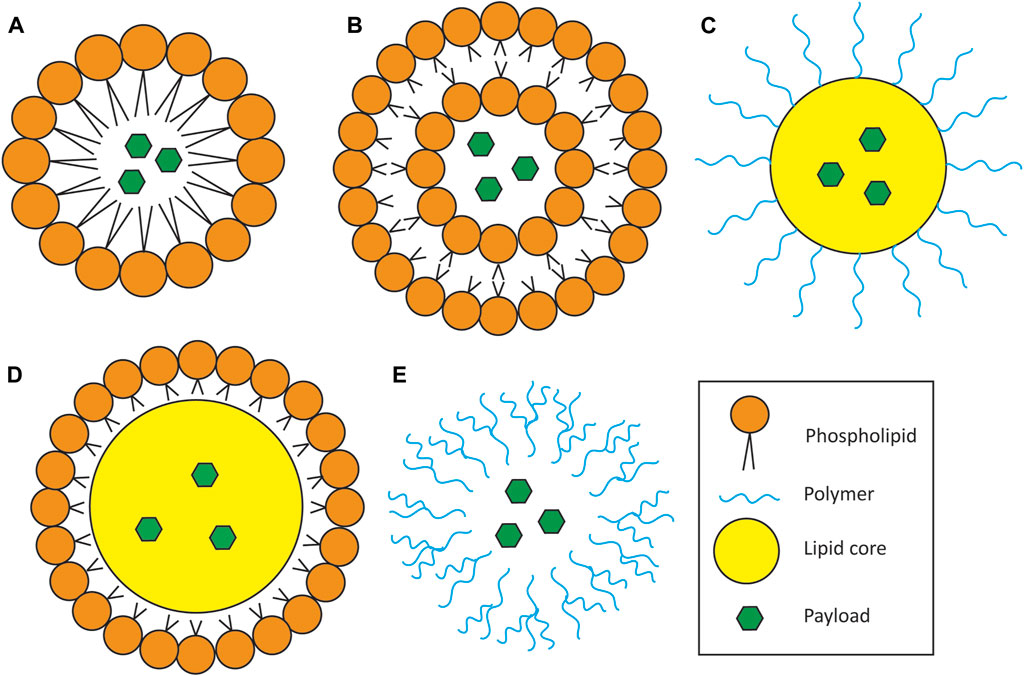
FIGURE 2. Schematic of nanoparticle types for drug delivery. (A) Micelles. Micelles consist of a single layer of amphipathic lipids surrounding a lipophilic payload. (B) Liposomes. Liposomes consist of an amphipathic lipid bilayer encapsulating either or both a hydrophilic payload in the core and/or a lipophilic payload inside the lipid bilayer (latter not depicted in cartoon). (C,D) Lipid core nanoparticle. Characterized by an outer shell of polymers (C) or other lipids (D) surrounding a lipid droplet core containing the therapeutic payload. (E). Polymeric nanoparticle. Made entirely of polymers and can carry both hydrophilic and lipophilic payloads depending on the polymer used. This particular depiction represents dendrimers, which consist of long, branched chains of polymers encapsulating the therapeutic payload.
There is much larger variety in polymeric nanoparticles, which are composed of long and branched or linear networks. These can be manufactured structurally similar to lipid nanoparticles, allowing for the encapsulation of both hydrophilic and lipophilic payloads. Polymeric nanoparticles have also been more widely investigated in inner ear therapy, offering better reproducibility and easier surface functionalization than most lipid nanoparticles. However, the large molecular weight of their polymer components carries higher risks in immunogenicity and clearance, and may decrease the amount of payload that can be delivered (Xu et al., 2021b; Lu et al., 2021).
Many hydrogel components can also constitute polymeric nanoparticles and in some instances, the terms “nanoparticle” and “nanogel” are interchangeable (Rathnam et al., 2019). For example, nanoparticles comprised of PLGA were shown to have increased RWM diffusion and cochlear penetration when polymerized with Poloxamer 407, PEG or chitosan, all of which are well-characterized hydrogel components (Cai et al., 2017). Moreover, PEG-based nanoparticles, loaded with artemisinin as an antioxidant, were shown to provide some protection against gentamicin-induced ototoxicity (Li et al., 2020). Another recent approach involved the polymerization of PEG and the commercially available DMPC phospholipid. The resulting lipid polymer nanoparticle was used to deliver the antioxidant astaxanthin, which alleviated the toxic effects of cisplatin on HEI-OC1 cells, as well as guinea pig and zebrafish outer hair cells (Gu et al., 2020). Finally, another PEG nanoparticle-based strategy used a bifunctionalized nanoparticle incorporating poly (propylene sulfide)120, a scavenger of reactive oxygen species, and an outer hair cell targeting peptide to deliver the Chinese herbal medicine berberine into a guinea pig model of NIHL (Zhao et al., 2021).
Dendrimers have been of recent interest for delivery of non-viral gene therapy in many areas, including SNHL. Dendrimers are nanoparticles characterized by long, branched polymer chains and are readily customizable in terms of their molecular architecture, which confers the ability to carry a wider variety of drugs and allows surface functionalization, for example for conjugating permeation or targeting moieties (Kheiriabad et al., 2021). One study demonstrated greater gene transfection efficiency of a polyamidoamine dendrimer over the commercially available transfection reagent, Lipofectamine 2000, a lipid nanoparticle (Gao et al., 2018b). Another polyamindoamine-based dendrimer was used to deliver an ATOH1-containing plasmid to the rat inner ear. The dendrimer was shown to permeate across the RWM and transfer the plasmid to hair cells (Wu et al., 2013). Moreover, dendrimers have shown promise in delivering CRISPR-Cas systems in other applications, demonstrating their future potential in gene therapy for the inner ear (Kretzmann et al., 2019).
Nanoparticles have additionally shown promise for the treatment of chronic middle ear disorders. Two recent studies using gold nanoclusters has shown specific targeting of antibiotic-resistant persister cells. The first used the nanoclusters as an adjuvant therapy with ofloxacin. The combination therapy sterilized in vitro biofilms and significantly reduced P. aeruginosa numbers in a mouse model of CSOM without inducing ofloxacin resistance (Cao et al., 2022). The second study functionalized the gold nanoclusters with adenosine triphosphate and showed significant lethal effect on metabolically inactive bacteria (Bekale et al., 2023). Both approaches showed the nanoclusters induced cell death through disruption of outer membrane integrity by various mechanisms and were well tolerated in vivo.
One interesting approach for cholesteatoma used indocyanine green nanocapsules to target cholesteatoma-derived keratinocytes. This approach is designed as an adjuvant therapy to eradicate residual cells left behind after surgery. The nanocapsules were coated with an antibody against epidermal growth factor receptor (EGFR) to selectively target the keratinocytes. In vitro results showed selective targeting and killing of keratinocytes, but not mucosal cells after activation of the nanocapsules with infrared light (Gluth et al., 2015).
While nanoparticles address controlled release of drugs in the inner ear, ultimately they must themselves be delivered to the inner ear. We have addressed a subset of nanoparticles that have demonstrated ability to cross the RWM, however there are many other classes of nanoparticle that have shown therapeutic potential for the treatment of hearing loss. These are usually larger nanoparticles with larger payloads or those that can be externally directed once implanted.
Nanoparticles can be engineered with inherently magnetic properties. Superparamagnetic iron oxide nanoparticles are already used in the clinic, with many applications in imaging, such as MRI (Dadfar et al., 2020). While it may be difficult to precisely deliver magnetic nanoparticles to the inner ear via the circulation, magnetic approaches have been proposed to deliver drugs to the inner ear locally. This is an advantageous approach as the drugs would be actively delivered into the inner ear, rather than through passive diffusion. One study demonstrated this by showing penetration of superparamagnetic nanoparticles into the scala tympani and apex of the cochlea, when magnetically directed after application onto the RWM in a hyaluronic acid gel (Leterme et al., 2019). Another approach used magnetic nanoparticles to deliver prednisolone into the cochlea of cisplatin-treated mice, which showed significant otoprotection compared to control or intratympanic injection (Ramaswamy et al., 2017).
As previously discussed, limitations on drugs and nanoparticles administered on the RWM include size, charge, lipophilicity and membrane thickness. However, as nanoparticles can allow for controlled release of drugs once injected, invasive surgeries such as intracochlear injection, would not be repeatedly required once the nanoparticle is delivered. Intracochlear injection would additionally allow the delivery of larger drugs, including large nanoparticles, microparticles and therapeutic peptides.
An example of intracochlear injection for large therapeutic delivery involves the administration of mesoporous silica supraparticles (MS-SP) containing neurotrophins. These MS-SPs are comprised of MS nanoparticles which self-assemble into larger SPs capable of delivering larger payloads, with pore sizes of 15–30 nm for neurotrophins (Wang et al., 2014b). Intracochlear injections of MS-SPs containing BDNF (Wise et al., 2016) or neurotrophin-3 (Gunewardene et al., 2022) were shown to recover damage to auditory nerves and the organ of Corti in deafened guinea pigs, while eliciting minimal inflammatory response. Furthermore, the use of MS-SPs achieved controlled release of the neurotrophins up to a month post-injection, though the authors noted some loss of hearing at higher frequencies caused by intracochlear injection when compared with RWM administration (Gunewardene et al., 2022). These studies highlight the advantages and risks associated with intracochlear injection.
The delivery of nanoparticles can additionally be enhanced through hydrogel-mediated release. As discussed previously, drugs contained in hydrogels that are implanted on the RWM reach the inner ear by passive diffusion through the membrane. Nano-encapsulation could provide an extra layer of controlled release and ensure these drugs are not compromised after RWM diffusion. For example, PEG-based nanoparticles containing a JNK inhibitor were delivered using a chitosan/glycero-2-phosphate (CGP) hydrogel implanted through intratympanic injection. These nanoparticles were functionalized by adding a peptide targeting prestin, a protein exclusively expressed by outer hair cells, and was shown to provide protection against NIHL (Kayyali et al., 2018). A previous study by this group showed the ability to abrogate drug delivery of the hydrogel upon treatment with chitosanase, which digests the CGP hydrogel (Lajud et al., 2013).
Some creative methods of introducing transient pores in the RWM have been designed for the delivery of nanoparticles into the inner ear. A “micro-shotgun” was developed to transport iron oxide and chitosan-based nanoparticles through the RWM. Briefly, this was constructed by loading nanoparticles and sodium carbonate/citric acid “fuel” into a microtube which was guided using a magnetic field and iron oxide nanoparticles in the microtube. The addition of water to the loaded tube causes a reaction generating a large amount of carbon dioxide, which launches the nanoparticle. Pores in the guinea pig RWM created by the launch closed 24 h after with no injury to the stria vascularis, organ of Corti or spiral ganglion neurons observed (Liang et al., 2020). Another vastly different approach used ultrasound microbubbles to deliver chitosan-coated gold nanoparticles. These microbubbles caused a transient disruption of tight junctions in the RWM upon light sonication, which facilitated the entry of chitosan-coated gold nanoparticles through the RWM (Lin et al., 2021).
Nanoparticle-based treatment is an emerging area with a multitude of new preclinical studies being reported. Nanoparticles address many challenges in therapeutic delivery to the inner ear, with some formulations having the ability to traverse the RWM and effectively distribute along the cochlea. However, key areas need be addressed, including drug release kinetics and clearance, with many studies cited in this review focusing on penetration into the inner ear without the nanoparticle necessarily carrying a therapeutic payload. There are additionally some cell-protective mechanisms by which nanoparticles can be prevented from reaching their therapeutic target, most notably endosomal escape (Smith et al., 2019). Furthermore, challenges in large scale production and sterilization remain depending on the type of nanoparticle. Nevertheless, advances in nanoparticle technology have created the possibility of the transport of drugs that cannot penetrate the RWM, such as proteins and nucleic acid-based therapies for hearing loss. The field continues to rapidly expand, increasing our understanding of how to deliver nanoparticles and new forms of drugs into the inner ear.
Homing peptides are widely used to deliver therapeutic payloads to tumors, diseased blood vessels and to treat neurological disorders. These peptides use “molecular zip codes” or cell/extracellular matrix recognition motifs to selectively identify cell surface markers and extracellular matrix, which permit accumulation of the peptide in not only specific tissues, but specific cells (Ruoslahti, 2022). Homing peptides additionally can be conjugated to a therapeutic cargo to guide the payload to the site of pathology (Mann et al., 2016; Yeow et al., 2019). They are commonly generated from ex vivo/in vivo phage display screening, where bacteriophages are incubated with cells from target tissue, generating a library of potential homing peptides on the phage surface. These peptides are generally small, usually up to 20–30 amino acids in length, which allows greater permeation across membranes than larger targeting moieties such as antibodies.
Recently, Allen Ryan’s group at the University of California, San Diego used this technique to identify homing peptides that could cross the TM. The ex vivo study used TM from rats with induced OM as the substrate for peptide identification, which produced candidate peptides which were able to transport the phage across the TM in vivo (Kurabi et al., 2018a). A follow up ex vivo study showed the homing peptides were able to cross human TM both as isolated peptides and connected to phage at the same rate (Kurabi et al., 2018b). The mechanism of transport was eventually implicated to be via transcytosis (Kurabi et al., 2022). Based on the relatively large size of bacteriophages (900 nm-1 µm in length), these would make homing peptides a highly promising approach for the delivery of larger drugs and nanoparticles to the middle and potentially inner ear.
The aforementioned intratympanic approach generally relies on passive diffusion for the drug to cross the RWM and reach its target. This may not be sufficient to achieve an adequate therapeutic concentration for some drugs and approaches designed to allow reloading of drug to a surgically-implanted device are under development. These devices would be relatively easy to access or “reload” from the outside and be directly connected to a compartment within the middle or inner ear. One of the earliest examples of such a device in clinical use is the Silverstein MicroWick, which was developed by Herbert Silverstein and colleagues. The MicroWick is a polyvinyl acetate catheter threaded through a ventilation tube which is surgically-installed in the TM. The catheter contacts the RWM and drugs such as gentamicin and dexamethasone can be self-administered as eardrops up to three times daily for treatment of Meniere’s disease and sudden deafness respectively (Silverstein et al., 2004). More complex solutions involving continual delivery have since been envisioned to cater for a greater variety of therapeutics, as the MicroWick must be removed after 4 weeks and may not be able to deliver larger or more sensitive drugs, such as therapeutic peptides or gene therapies.
Miniaturized perfusion systems have been trialed in animal models as a possible delivery device for drugs. Directed intracochlear delivery overcomes many limitations of intratympanic RWM administration. Larger and more unstable drugs such as RNA therapeutics or proteins can be continuously delivered and multiple doses can be accounted for with the continual delivery. However, introducing more fluid must be precise and delivered in small volumes to not risk increasing intracochlear pressure and damaging sensory tissue. Moreover, the design of the preclinical systems thus far suggests a direct translation of a wearable or implanted pump for human use. Such systems would necessitate the highest practical degree of miniaturization to avoid inconveniencing the patient and reduce any possible stigma if the device is visible, as those with hearing aids may experience (Wallhagen, 2009).
Jeffrey Borenstein’s group has been extensively involved in the design of micropumps for inner ear delivery. In 2015, they reported the design of a head-mounted micropump (using guinea pigs for demonstration), with infuse-withdraw capabilities. This functioned through the infusion-withdrawal of perilymph so as to not disturb intracochlear pressure and was used to deliver the glutamate receptor antagonist DNQX (Tandon et al., 2015). The group also created a device with a reciprocating pump and drug reservoir to ensure no net volume change in the cochlea (Kim et al., 2014). Another group was able to recently create an implantable micropump for mice (Forouzandeh et al., 2019).
Cochleostomy is a highly invasive process, which itself may risk affecting hearing. This procedure is usually employed to install cochlear implants and there is growing in vivo evidence in animal models and humans of a fibro-inflammatory response to implants (Wilk et al., 2016; Foggia et al., 2019). The resulting fibrosis interferes with the implant electrode array, and is thought to contribute to impedance, which hampers the effectiveness of the cochlear implant over time. To counteract the proliferation of fibrotic tissue, anti-inflammatory drugs, commonly dexamethasone, incorporated into the implant electrode array have shown effectiveness at preventing fibrosis and additionally trauma-induced hair cell and neural damage (Chang et al., 2009; Eastwood et al., 2010; Bas et al., 2016; Wilk et al., 2016; Plontke et al., 2017; Qnouch et al., 2021). Coating the surface of the implant with less adsorbing and immunogenic biomaterials has also shown benefit in inhibiting the inflammatory response (Chen et al., 2022a).
As discussed in previous sections however, certain drugs, especially dexamethasone and other glucocorticoids, risk being cleared prior to achieving sufficient bioavailability (Salt and Plontke, 2018). A study by Liebau et al. (2020) demonstrated this by implanting silicone dummy rods with different concentrations of dexamethasone, showing a dose-dependent relationship between drug loading amount and perilymph concentration over 7 weeks in guinea pigs. To ensure continual release of drugs, some compelling controlled release strategies have been developed. One study used Pluronic-coated gold nanoparticles to deliver dexamethasone in the round window niche after cochleostomy and demonstrated modest improvement in ABR threshold at 8 kHz, but no difference at other ranges (Blebea et al., 2022). Another study from Chen et al. (2022b) presented an interesting strategy using mesoporous silica nanoparticles containing siRNA against the pro-fibrotic cytokine, TGFβ, which were adsorbed onto the surface of the electrode array. In vivo results from animal studies from this group are highly anticipated.
We have reviewed existing clinical problems and recent innovations in drug delivery to the middle and inner ear. While drug delivery to the middle and inner ear remains a significant clinical problem, great advances have been made recently. As the anatomical barriers to the middle and inner ears preclude straightforward local delivery, it will likely require a combinatorial approach of the techniques mentioned to ensure effectiveness of these novel technologies. For example, intratympanic hydrogel administration of many drugs, including corticosteroids by themselves, is insufficient due to rapid clearance from the cochlea (Salt and Plontke, 2018). However, a dual-delivery system that utilizes a hydrogel and nanoencapsulation of drugs could counteract the rapid clearance rates in the middle and inner ear. Also targeting moieties, such as homing peptides, could ensure the administered drug reaches its cellular or molecular target once administered in the relatively static cochlear fluids (Kayyali et al., 2018; Ruoslahti, 2022).
An additional challenge for the field is how the development of drug delivery systems keep up with the development of novel treatment types. Many gene therapies for example, are relatively unstable molecules and must be kept in a medium whereby they remain viable once they reach the inner ear. Conventional gene therapies, for example the delivery of whole genes, may present a problem based on molecular size. This is common and reasonably expected for those replacing the large multidomain proteins of the cochlear stereocilia bundles, and already challenges the limited capacity of the more conventional gene delivery system of adeno-associated viruses (French et al., 2020). This presents an opportunity for non-viral gene delivery systems such as nanoparticles, however nanoparticles themselves are also constrained by their own size when considering intratympanic RWM delivery and passage (Mehnert and Mäder, 2012). Furthermore, technical considerations such as sterility and dosage consistency between nanocapsules of these more sensitive therapies need to be considered.
The emergence of biomaterial-based delivery systems, particularly hydrogels, in mid-late stage clinical trials demonstrates the potential for these novel systems to become mainstream options for local middle and inner ear drug delivery. Future studies should continue to address drug release and pharmacokinetics of encapsulated drugs in animal models of hearing loss in terms of tissue penetration and clearance. Moreover, the development of reliable in vitro RWM models would additionally be useful for large scale assessment of drug penetration. These types of studies would ensure the maintenance of drug safety and efficacy as other promising delivery systems are developed.
Conceptualization and design: DD and EW; writing: DD, LL, JL, and EW; figures: DD and JL; editing: all authors; funding: MA and EW. All authors contributed to the article and approved the submitted version.
This research is supported by the Australian government National Health and Medical Research Council (NHMRC) Medical Research Future Fund (MRFF) Stem Cell Therapies Mission Grant, Western Australian (WA) Future Health Research and Innovation Fund (FHRIF) Near-miss Awards: Ideas Grants (WANMA), Commonwealth Scientific and Industrial Research Organisation (CSIRO) ON Prime Innovation Program, Passe and Williams Foundation, Stan Perron Charitable Foundation and Channel 7 Telethon Trust and and WA Department of Jobs, Tourism, Science and Innovative (JTSI) Industries Fund 2023 Innovation Booster Grant.
We would like to thank Dr. David Sly and Mr. Peter Millington for additional review and proof reading of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adriaansens, C., Bekkers, S., and Aarts, M. C. J. (2022). Determinants influencing cholesteatoma recurrence in daily practice: a retrospective analysis. J. Laryngology Otology 136 (2), 119–124. doi:10.1017/S0022215121003546
Alharazneh, A., Luk, L., Huth, M., Monfared, A., Steyger, P. S., Cheng, A. G., et al. (2011). Functional hair cell mechanotransducer channels are required for aminoglycoside ototoxicity. PLoS One 6 (7), e22347. doi:10.1371/journal.pone.0022347
Alrwisan, A., Winterstein, A. G., and Antonelli, P. J. (2016). Epidemiology of persistent tympanic membrane perforations subsequent to tympanostomy tubes assessed with real world data. Otology Neurotol. 37 (9), 1376–1380. doi:10.1097/mao.0000000000001195
Amable, P. R., Carias, R. B. V., Teixeira, M. V. T., da Cruz Pacheco, Í., Corrêa do Amaral, R. J. F., Granjeiro, J. M., et al. (2013). Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res. Ther. 4 (3), 67–13. doi:10.1186/scrt218
Angeli, S., Shahal, D., Brown, C. S., and Herman, B. (2020). Predicting recidivism for acquired cholesteatoma: evaluation of a current staging system. Otology Neurotol. 41 (10), 1391–1396. doi:10.1097/MAO.0000000000002823
Anwar, F., Shenoy, V., Kamath, P., Sreedharan, S., Deviprasad, D., and Domah, H. (2020). Study on use of platelet-rich plasma in myringoplasty. Indian J. Otology 26 (2), 71–74. doi:10.4103/indianjotol.INDIANJOTOL_103_18
Araujo, M. M., Murashima, A. A. B., Alves, V. M., Jamur, M. C., and Hyppolito, M. A. (2016). The topical use of insulin accelerates the healing of traumatic tympanic membrane perforations. Laryngoscope 126 (1), 156–162. doi:10.1002/lary.25300
Bächinger, D., Rrahmani, A., Weiss, N. M., Mlynski, R., Huber, A., and Röösli, C. (2021). Evaluating hearing outcome, recidivism and complications in cholesteatoma surgery using the ChOLE classification system. Eur. Archives Oto-Rhino-Laryngology 278 (5), 1365–1371. doi:10.1007/s00405-020-06208-z
Barbieri, M. A., Cicala, G., Cutroneo, P. M., Mocciaro, E., Sottosanti, L., Freni, F., et al. (2019). Ototoxic adverse drug reactions: a disproportionality analysis using the Italian spontaneous reporting database. Front. Pharmacol. 10, 1161. doi:10.3389/fphar.2019.01161
Barron, C. L., Kamel-Abusalha, L. B., Sethia, R., Goodman, S. D., Elmaraghy, C. A., and Bakaletz, L. O. (2020). Identification of essential biofilm proteins in middle ear fluids of otitis media with effusion patients. Laryngoscope 130 (3), 806–811. doi:10.1002/lary.28011
Bas, E., Bohorquez, J., Goncalves, S., Perez, E., Dinh, C. T., Garnham, C., et al. (2016). Electrode array-eluted dexamethasone protects against electrode insertion trauma induced hearing and hair cell losses, damage to neural elements, increases in impedance and fibrosis: a dose response study. Hear. Res. 337, 12–24. doi:10.1016/j.heares.2016.02.003
Beaulac, H. J., Gilels, F., Zhang, J., Jeoung, S., and White, P. M. (2021). Primed to die: an investigation of the genetic mechanisms underlying noise-induced hearing loss and cochlear damage in homozygous Foxo3-knockout mice. Cell Death Dis. 12 (7), 682. doi:10.1038/s41419-021-03972-6
Beck, D., and Le Goff, N. (2018). Contemporary hearing aid amplification: issues and outcomes in 2018. J. Otolaryngology-ENT Res. 10 (1), 00303. doi:10.15406/joentr.2018/10/00303
Bekale, L. A., Sharma, D., Bacacao, B., Chen, J., and Maria, P. L. S. (2023). Eradication of bacterial persister cells by leveraging their low metabolic activity using adenosine triphosphate coated gold nanoclusters. Nano Today 51, 101895. doi:10.1016/j.nantod.2023.101895
Bhutta, M. F., Thornton, R. B., Kirkham, L.-A. S., Kerschner, J. E., and Cheeseman, M. T. (2017). Understanding the aetiology and resolution of chronic otitis media from animal and human studies. Dis. Models Mech. 10 (11), 1289–1300. doi:10.1242/dmm.029983
Blebea, C. M., Necula, V., Potara, M., Dindelegan, M. G., Ujvary, L. P., Botan, E. C., et al. (2022). The effect of pluronic-coated gold nanoparticles in hearing preservation following cochlear implantation-pilot study. Audiology Res. 12 (5), 466–475. doi:10.3390/audiolres12050047
Bluestone, C. D. (2008). Impact of evolution on the eustachian tube. Laryngoscope 118 (3), 522–527. doi:10.1097/MLG.0b013e31815ddaa0
Bowl, M. R., and Dawson, S. J. (2019). Age-related hearing loss. Cold Spring Harb. Perspect. Med. 9 (8), a033217. doi:10.1101/cshperspect.a033217
Britze, A., Møller, M., and Ovesen, T. (2017). Incidence, 10-year recidivism rate and prognostic factors for cholesteatoma. J. Laryngology Otology 131 (4), 319–328. doi:10.1017/S0022215117000299
Brock, P. R., Maibach, R., Childs, M., Rajput, K., Roebuck, D., Sullivan, M. J., et al. (2018). Sodium thiosulfate for protection from cisplatin-induced hearing loss. N. Engl. J. Med. 378 (25), 2376–2385. doi:10.1056/NEJMoa1801109
Cai, H., Liang, Z., Huang, W., Wen, L., and Chen, G. (2017). Engineering PLGA nano-based systems through understanding the influence of nanoparticle properties and cell-penetrating peptides for cochlear drug delivery. Int. J. Pharm. 532 (1), 55–65. doi:10.1016/j.ijpharm.2017.08.084
Cai, T., Seymour, M. L., Zhang, H., Pereira, F. A., and Groves, A. K. (2013). Conditional deletion of Atoh1 reveals distinct critical periods for survival and function of hair cells in the organ of Corti. J. Neurosci. 33 (24), 10110–10122. doi:10.1523/JNEUROSCI.5606-12.2013
Cao, Z., Chen, X., Chen, J., Xia, A., Bacacao, B., Tran, J., et al. (2022). Gold nanocluster adjuvant enables the eradication of persister cells by antibiotics and abolishes the emergence of resistance. Nanoscale 14 (28), 10016–10032. doi:10.1039/D2NR01003H
Cervantes, B., Arana, L., Murillo-Cuesta, S., Bruno, M., Alkorta, I., and Varela-Nieto, I. (2019). Solid lipid nanoparticles loaded with glucocorticoids protect auditory cells from cisplatin-induced ototoxicity. J. Clin. Med. 8 (9), 1464. doi:10.3390/jcm8091464
Chadha, S., Kamenov, K., and Cieza, A. (2021). The world report on hearing, 2021. Bull. World Health Organ 99 (4), 242–242A. doi:10.2471/BLT.21.285643
Chan, K. H., Allen, G. C., Kelley, P. E., Streubel, S.-O., Friedman, N. R., Yoon, P., et al. (2018). Dornase alfa ototoxic effects in animals and efficacy in the treatment of clogged tympanostomy tubes in children: a preclinical study and a randomized clinical trial. JAMA Otolaryngology–Head Neck Surg. 144 (9), 776–780. doi:10.1001/jamaoto.2018.1101
Chang, A., Eastwood, H., Sly, D., James, D., Richardson, R., and O’Leary, S. (2009). Factors influencing the efficacy of round window dexamethasone protection of residual hearing post-cochlear implant surgery. Hear. Res. 255 (1), 67–72. doi:10.1016/j.heares.2009.05.010
Chee, J., Pang, K. W., Yong, J. M., Ho, R.C.-M., and Ngo, R. (2016). Topical versus oral antibiotics, with or without corticosteroids, in the treatment of tympanostomy tube otorrhea. Int. J. Pediatr. Otorhinolaryngology 86, 183–188. doi:10.1016/j.ijporl.2016.05.008
Chen, A., Chen, D., Lv, K., Li, G., Pan, J., Ma, D., et al. (2022a). Zwitterionic polymer/polydopamine coating of electrode arrays reduces fibrosis and residual hearing loss after cochlear implantation. Adv. Healthc. Mater. n/a, 2200807. doi:10.1002/adhm.202200807
Chen, A., Chen, Y., Liu, S., Ma, D., Tang, J., and Zhang, H. (2022b). Mesoporous silica nanoparticle-modified electrode arrays of cochlear implants for delivery of siRNA-TGFβ1 into the inner ear. Colloids Surfaces B Biointerfaces 218, 112753. doi:10.1016/j.colsurfb.2022.112753
Chen, A., Liu, W., Xu, L., Hou, Z., Fan, Z., Wang, H., et al. (2022c). Comparison of the pathway to the inner ear between postauricular and intramuscular injection of dexamethasone in Guinea pigs. Front. Neurology 13, 811626. doi:10.3389/fneur.2022.811626
Chen, K., Wang, F., Ding, R., Cai, Z., Zou, T., Zhang, A., et al. (2022d). Adhesive and injectable hydrogel microspheres for inner ear treatment. Small. doi:10.1002/smll.202106591
Chen, X., Li, X., and Qin, Z. (2016). MicroRNA-21 promotes the proliferation and invasion of cholesteatoma keratinocytes. Acta Oto-Laryngologica 136 (12), 1261–1266. doi:10.1080/00016489.2016.1202447
Chester, J., Johnston, E., Walker, D., Jones, M., Ionescu, C. M., Wagle, S. R., et al. (2021). A review on recent advancement on age-related hearing loss: the applications of nanotechnology, drug Pharmacology, and biotechnology. Pharmaceutics 13 (7), 1041. doi:10.3390/pharmaceutics13071041
Cho, H. S., Kim, H. G., Jung, D. J., Jang, J. H., Lee, S. H., and Lee, K. Y. (2016). Clinical aspects and surgical outcomes of congenital cholesteatoma in 93 children: increasing trends of congenital cholesteatoma from 1997 through 2012. J. Audiology Otology 20 (3), 168–173. doi:10.7874/jao.2016.20.3.168
Clark, J. H., Feng, A., Harun, A., Brown, G., and Francis, H. W. (2016). Secondary acquired cholesteatoma: presentation and tympanoplasty outcomes. Otology Neurotol. 37 (7), 902–907. doi:10.1097/MAO.0000000000001100
Clark, S. L., Seppanen, E. J., Kirkham, L.-A. S., Novotny, L. A., Bakaletz, L. O., Cripps, A. W., et al. (2022). Australian aboriginal otitis-prone children produce high-quality serum IgG to putative nontypeable Haemophilus influenzae vaccine antigens at lower titres compared to non-aboriginal children. Front. Cell. Infect. Microbiol. 333. doi:10.3389/fcimb.2022.767083
Coffin, A. B., Boney, R., Hill, J., Tian, C., and Steyger, P. S. (2021). Detecting novel ototoxins and potentiation of ototoxicity by disease settings. Front. Neurology 12, 725566. doi:10.3389/fneur.2021.725566
Conlin, A. E., and Parnes, L. S. (2007). Treatment of sudden sensorineural hearing loss: I. A systematic review. Archives Otolaryngology–Head Neck Surg. 133 (6), 573–581. doi:10.1001/archotol.133.6.573
Cunningham, L. L., and Tucci, D. L. (2017). Hearing loss in adults. N. Engl. J. Med. 377 (25), 2465–2473. doi:10.1056/NEJMra1616601
Dadfar, S. M., Camozzi, D., Darguzyte, M., Roemhild, K., Varvarà, P., Metselaar, J., et al. (2020). Size-isolation of superparamagnetic iron oxide nanoparticles improves MRI, MPI and hyperthermia performance. J. Nanobiotechnology 18 (1), 22. doi:10.1186/s12951-020-0580-1
Dai, J., Long, W., Liang, Z., Wen, L., Yang, F., and Chen, G. (2018). A novel vehicle for local protein delivery to the inner ear: injectable and biodegradable thermosensitive hydrogel loaded with PLGA nanoparticles. Drug Dev. Industrial Pharm. 44 (1), 89–98. doi:10.1080/03639045.2017.1373803
Dalby, B., Cates, S., Harris, A., Ohki, E. C., Tilkins, M. L., Price, P. J., et al. (2004). Advanced transfection with Lipofectamine 2000 reagent: primary neurons, siRNA, and high-throughput applications. Methods 33 (2), 95–103. doi:10.1016/j.ymeth.2003.11.023
DeAntonio, R., Yarzabal, J.-P., Cruz, J. P., Schmidt, J. E., and Kleijnen, J. (2016). Epidemiology of otitis media in children from developing countries: a systematic review. Int. J. Pediatr. Otorhinolaryngology 85, 65–74. doi:10.1016/j.ijporl.2016.03.032
DeLacy, J., Dune, T., and Macdonald, J. J. (2020). The social determinants of otitis media in Aboriginal children in Australia: are we addressing the primary causes? A systematic content review. BMC Public Health 20 (1), 492–499. doi:10.1186/s12889-020-08570-3
Dettman, S., Choo, D., Au, A., Luu, A., and Dowell, R. (2021). Speech perception and language outcomes for infants receiving cochlear implants before or after 9 Months of age: use of category-based aggregation of data in an unselected pediatric cohort. J. Speech, Lang. Hear. Res. 64(3), 1023–1039. doi:10.1044/2020_JSLHR-20-00228
Dettman, S. J., Dowell, R. C., Choo, D., Arnott, W., Abrahams, Y., Davis, A., et al. (2016). Long-term communication outcomes for children receiving cochlear implants younger than 12 Months: a multicenter study. Otology Neurotol. 37 (2), e82–e95. doi:10.1097/mao.0000000000000915
Ding, D., Liu, H., Qi, W., Jiang, H., Li, Y., Wu, X., et al. (2016). Ototoxic effects and mechanisms of loop diuretics. J. Otology 11 (4), 145–156. doi:10.1016/j.joto.2016.10.001
Dohar, J. E., Don, D., Koempel, J., Lu, C. H., Hakanson, D., and Chan, K. H. (2018). Safety and efficacy of intratympanic ciprofloxacin otic suspension post-tubes in a real-world pediatric population. Am. J. Otolaryngology 39 (2), 101–106. doi:10.1016/j.amjoto.2017.12.016
Driver, E. C., Sillers, L., Coate, T. M., Rose, M. F., and Kelley, M. W. (2013). The Atoh1-lineage gives rise to hair cells and supporting cells within the mammalian cochlea. Dev. Biol. 376 (1), 86–98. doi:10.1016/j.ydbio.2013.01.005
Early, S., Yang, R., Li, X., Zhang, Z., van der Valk, J. C., Ma, X., et al. (2021). Initial method for characterization of tympanic membrane drug permeability in human temporal bones in situ. Front. Neurology 12, 580392. doi:10.3389/fneur.2021.580392
Eastwood, H., Chang, A., Kel, G., Sly, D., Richardson, R., and O’Leary, S. J. (2010). Round window delivery of dexamethasone ameliorates local and remote hearing loss produced by cochlear implantation into the second turn of the Guinea pig cochlea. Hear. Res. 265 (1), 25–29. doi:10.1016/j.heares.2010.03.006
Edfeldt, L., Kinnefors, A., Strömbäck, K., Köbler, S., and Rask-Andersen, H. (2012). Surgical treatment of paediatric cholesteatoma: long-term follow up in comparison with adults. Int. J. Pediatr. Otorhinolaryngology 76 (8), 1091–1097. doi:10.1016/j.ijporl.2012.04.006
Edmunds, A. L. (2017). Otiprio: an FDA-approved ciprofloxacin suspension gel for pediatric otitis media with effusion. Pharm. Ther. 42 (5), 307–311.
El-Anwar, M. W., El-Ahl, M. A., Zidan, A. A., and Yacoup, M. A. (2015). Topical use of autologous platelet rich plasma in myringoplasty. Auris Nasus Larynx 42 (5), 365–368. doi:10.1016/j.anl.2015.02.016
Ersözlü, T., and Gultekin, E. (2020). A comparison of the autologous platelet-rich plasma gel fat graft myringoplasty and the fat graft myringoplasty for the closure of different sizes of tympanic membrane perforations. Ear, Nose, Throat J. 99 (5), 331–336. doi:10.1177/0145561319900388
Eshraghi, A. A., Aranke, M., Salvi, R., Ding, D., Coleman Jr, J. M. K., Ocak, E., et al. (2018). Preclinical and clinical otoprotective applications of cell-penetrating peptide D-JNKI-1 (AM-111). Hear. Res. 368, 86–91. doi:10.1016/j.heares.2018.03.003
Everts, P., Onishi, K., Jayaram, P., Lana, J. F., and Mautner, K. (2020). Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int. J. Mol. Sci. 21 (20), 7794. doi:10.3390/ijms21207794
Fawzy, T., Hussein, M., Eid, S., and Guindi, S. (2018). Effect of adding platelet-rich plasma to fat grafts in myringoplasty. Egypt. J. Otolaryngology 34 (4), 224–228. doi:10.4103/ejo.ejo_53_18
Feng, L., Ward, J. A., Li, S. K., Tolia, G., Hao, J., and Choo, D. I. (2014). Assessment of PLGA-PEG-PLGA copolymer hydrogel for sustained drug delivery in the ear. Curr. Drug Deliv. 11 (2), 279–286. doi:10.2174/1567201811666140118224616
Fisher, R. A., Gollan, B., and Helaine, S. (2017). Persistent bacterial infections and persister cells. Nat. Rev. Microbiol. 15 (8), 453–464. doi:10.1038/nrmicro.2017.42
Foggia, M. J., Quevedo, R. V., and Hansen, M. R. (2019). Intracochlear fibrosis and the foreign body response to cochlear implant biomaterials. Laryngoscope Investig. Otolaryngol. 4 (6), 678–683. doi:10.1002/lio2.329
Forouzandeh, F., Zhu, X., Alfadhel, A., Ding, B., Walton, J. P., Cormier, D., et al. (2019). A nanoliter resolution implantable micropump for murine inner ear drug delivery. J. Control. Release 298, 27–37. doi:10.1016/j.jconrel.2019.01.032
Fouad, Y. A., Abdelhady, M., El-Anwar, M., and Merwad, E. (2018). Topical platelet rich plasma versus hyaluronic acid during fat graft myringoplasty. Am. J. Otolaryngology 39 (6), 741–745. doi:10.1016/j.amjoto.2018.08.004
French, L. S., Mellough, C. B., Chen, F. K., and Carvalho, L. S. (2020). A review of gene, drug and cell-based therapies for usher syndrome. Front. Cell. Neurosci. 14, 183. doi:10.3389/fncel.2020.00183
Frisina, R. D., Wheeler, H. E., Fossa, S. D., Kerns, S. L., Fung, C., Sesso, H. D., et al. (2016). Comprehensive audiometric analysis of hearing impairment and tinnitus after cisplatin-based chemotherapy in survivors of adult-onset cancer. J. Clin. Oncol. 34 (23), 2712–2720. doi:10.1200/jco.2016.66.8822
Fukuda, A., Kano, S., Nakamaru, Y., Morita, S., Hoshino, K., Fujiwara, K., et al. (2021). Notch signaling in acquired middle ear cholesteatoma. Otology Neurotol. 42 (9), e1389–e1395. doi:10.1097/mao.0000000000003245
Gao, G., Liu, Y., Zhou, C.-H., Jiang, P., and Sun, J.-J. (2015). Solid lipid nanoparticles loaded with edaravone for inner ear protection after noise exposure. Chin. Med. J. 128 (2), 203–209. doi:10.4103/0366-6999.149202
Gao, X., Tao, Y., Lamas, V., Huang, M., Yeh, W.-H., Pan, B., et al. (2018a). Treatment of autosomal dominant hearing loss by in vivo delivery of genome editing agents. Nature 553 (7687), 217–221. doi:10.1038/nature25164
Gao, Y.-G., Lin, X., Dang, K., Jiang, S.-F., Tian, Y., Liu, F.-L., et al. (2018b). Structure–activity relationship of novel low-generation dendrimers for gene delivery. Org. Biomol. Chem. 16 (42), 7833–7842. doi:10.1039/C8OB01767K
Gates, G. A., Couropmitree, N. N., and Myers, R. H. (1999). Genetic associations in age-related hearing thresholds. Archives Otolaryngology–Head Neck Surg. 125 (6), 654–659. doi:10.1001/archotol.125.6.654
Gates, G. A., and Mills, J. H. (2005). Presbycusis. Lancet 366 (9491), 1111–1120. doi:10.1016/S0140-6736(05)67423-5
Geleoc, G. S., and Holt, J. R. (2014). Sound strategies for hearing restoration. Science 344 (6184), 1241062. doi:10.1126/science.1241062
Gettelfinger, J. D., and Dahl, J. P. (2018). Syndromic hearing loss: a brief review of common presentations and genetics. J. Pediatr. Genet. 7 (1), 1–8. doi:10.1055/s-0037-1617454
Gilberto, N., Custódio, S., Colaço, T., Santos, R., Sousa, P., and Escada, P. (2020). Middle ear congenital cholesteatoma: systematic review, meta-analysis and insights on its pathogenesis. Eur. Archives Oto-Rhino-Laryngology 277 (4), 987–998. doi:10.1007/s00405-020-05792-4
Gluth, M. B., Kaufmann, Y. C., Dornhoffer, J. L., and Ferguson, S. (2015). Immunotargeted photodynamic therapy for cholesteatoma: in vitro results with anti-EGFR–coated indocyanine green nanocapsules. Otology Neurotol. 36 (1), 76–81. doi:10.1097/mao.0000000000000590
Gordillo Altamirano, F. L., and Barr, J. J. (2019). Phage therapy in the postantibiotic era. Clin. Microbiol. Rev. 32 (2), e00066-18. doi:10.1128/cmr.00066-18
Goycoolea, M. V., and Lundman, L. (1997). Round window membrane. Structure function and permeability: a review. Microsc. Res. Tech. 36, 201–211. doi:10.1002/(SICI)1097-0029(19970201)36:3<201:AID-JEMT8>3.0.CO;2-R
Greene, J. B., Standring, R., Siddiqui, F., and Ahsan, S. F. (2015). Incidence of cisplatin induced ototoxicity in adults with head and neck cancer. Adv. Otolaryngology 2015, 1–4. doi:10.1155/2015/245613
Gu, J., Chen, Y., Tong, L., Wang, X., Yu, D., and Wu, H. (2020). Astaxanthin-loaded polymer-lipid hybrid nanoparticles (ATX-LPN): assessment of potential otoprotective effects. J. Nanobiotechnology 18 (1), 53. doi:10.1186/s12951-020-00600-x
Gunewardene, N., Lam, P., Ma, Y., Caruso, F., Wagstaff, S., Richardson, R. T., et al. (2022). Pharmacokinetics and biodistribution of supraparticle-delivered neurotrophin 3 in the Guinea pig cochlea. J. Control. Release 342, 295–307. doi:10.1016/j.jconrel.2021.12.037
György, B., Nist-Lund, C., Pan, B., Asai, Y., Karavitaki, K. D., Kleinstiver, B. P., et al. (2019). Allele-specific gene editing prevents deafness in a model of dominant progressive hearing loss. Nat. Med. 25 (7), 1123–1130. doi:10.1038/s41591-019-0500-9
Hakuba, N., Hato, N., Okada, M., Mise, K., and Gyo, K. (2015). Preoperative factors affecting tympanic membrane regeneration therapy using an atelocollagen and basic fibroblast growth factor. JAMA Otolaryngology–Head Neck Surg. 141 (1), 60–66. doi:10.1001/jamaoto.2014.2613
Hakuba, N., Hato, N., Omotehara, Y., Okada, M., and Gyo, K. (2013). Epithelial pearl formation following tympanic membrane regeneration therapy using an atelocollagen/silicone membrane and basic fibroblast growth factor: our experience from a retrospective study of one hundred sixteen patients. Clin. Otolaryngol. 38 (5), 394–397. doi:10.1111/coa.12164
Hakuba, N., Iwanaga, M., Tanaka, S., Hiratsuka, Y., Kumabe, Y., Konishi, M., et al. (2010). Basic fibroblast growth factor combined with atelocollagen for closing chronic tympanic membrane perforations in 87 patients. Otology Neurotol. 31 (1), 118–121. doi:10.1097/mao.0b013e3181c34f01
Hakuba, N., Taniguchi, M., Shimizu, Y., Sugimoto, A., Shinomori, Y., and Gyo, K. (2003). A new method for closing tympanic membrane perforations using basic fibroblast growth factor. Laryngoscope 113 (8), 1352–1355. doi:10.1097/00005537-200308000-00016
Hamed, M. A., Nakata, S., Sayed, R. H., Ueda, H., Badawy, B. S., Nishimura, Y., et al. (2016). Pathogenesis and bone resorption in acquired cholesteatoma: current knowledge and future prospectives. Clin. Exp. Otorhinolaryngology 9 (4), 298–308. doi:10.21053/ceo.2015.01662
Hilton, C. W., Ondrey, F. G., Wuertz, B. R., and Levine, S. C. (2011). Interleukin-8 production in response to tumor necrosis factor-alpha by cholesteatoma keratinocytes in cell culture. Laryngoscope 121 (2), 372–374. doi:10.1002/lary.21352
Hoff, S., Ryan, M., Thomas, D., Tournis, E., Kenny, H., Hajduk, J., et al. (2019). Safety and effectiveness of cochlear implantation of young children, including those with complicating conditions. Otol. Neurotol. 40 (4), 454–463. doi:10.1097/MAO.0000000000002156
Hong, P., Bance, M., and Gratzer, P. F. (2013). Repair of tympanic membrane perforation using novel adjuvant therapies: a contemporary review of experimental and tissue engineering studies. Int. J. Pediatr. Otorhinolaryngology 77 (1), 3–12. doi:10.1016/j.ijporl.2012.09.022
Horna, G., Quezada, K., Ramos, S., Mosqueda, N., Rubio, M., Guerra, H., et al. (2019). Specific type IV pili groups in clinical isolates of Pseudomonas aeruginosa. Int. Microbiol. 22 (1), 131–141. doi:10.1007/s10123-018-00035-3
Huang, J., Shi, Y., Wu, L., Lv, C., Hu, Y., and Shen, Y. (2021). Comparative efficacy of platelet-rich plasma applied in myringoplasty: a systematic review and meta-analysis. PLoS ONE 16 (1), e0245968. doi:10.1371/journal.pone.0245968
Huang, J., Teh, B. M., Eikelboom, R. H., Han, L., Xu, G., Yao, X., et al. (2020). The effectiveness of bFGF in the treatment of tympanic membrane perforations: a systematic review and meta-analysis. Otology Neurotol. 41 (6), 782–790. doi:10.1097/mao.0000000000002628
Hussain, Z., and Pei, R. (2021). Necessities, opportunities, and challenges for tympanic membrane perforation scaffolding-based bioengineering. Biomed. Mater. 16 (3), 032004. doi:10.1088/1748-605x/abcf5d
Hütten, M., Dhanasingh, A., Hessler, R., Stöver, T., Esser, K.-H., Möller, M., et al. (2014). In vitro and in vivo evaluation of a hydrogel reservoir as a continuous drug delivery system for inner ear treatment. PLoS ONE 9 (8), e104564. doi:10.1371/journal.pone.0104564
Isherwood, B., Gonçalves, A. C., Cousins, R., and Holme, R. (2022). The global hearing therapeutic pipeline: 2021. Drug Discov. Today 27 (3), 912–922. doi:10.1016/j.drudis.2021.11.009
Izurieta, P., Scherbakov, M., Nieto Guevara, J., Vetter, V., and Soumahoro, L. (2022). Systematic review of the efficacy, effectiveness and impact of high-valency pneumococcal conjugate vaccines on otitis media. Hum. Vaccines Immunother. 18 (1), 2013693. doi:10.1080/21645515.2021.2013693
Jault, P., Leclerc, T., Jennes, S., Pirnay, J. P., Que, Y. A., Resch, G., et al. (2019). Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): a randomised, controlled, double-blind phase 1/2 trial. Lancet. Infect. Dis. 19 (1), 35–45. doi:10.1016/s1473-3099(18)30482-1
Jenks, C. M., Purcell, P. L., Federici, G., Villari, D., Presutti, L., James, A. L., et al. (2022). Transcanal endoscopic ear surgery for congenital cholesteatoma: a multi-institutional series. Otolaryngology–Head Neck Surg. 167 (3), 537–544. doi:10.1177/01945998211067502
Jiang, Z., Fa, B., Zhang, X., Wang, J., Feng, Y., Shi, H., et al. (2021). Identifying genetic risk variants associated with noise-induced hearing loss based on a novel strategy for evaluating individual susceptibility. Hear. Res. 407, 108281. doi:10.1016/j.heares.2021.108281
Kalluri, R., and LeBleu, V. S. (2020). The biology, function, and biomedical applications of exosomes. Science 367 (6478), eaau6977. doi:10.1126/science.aau6977
Kanemaru, S., Umeda, H., Kitani, Y., Nakamura, T., Hirano, S., and Ito, J. (2011). Regenerative treatment for tympanic membrane perforation. Otology Neurotol. 32 (8), 1218–1223. doi:10.1097/mao.0b013e31822e0e53
Kay, D. J., Nelson, M., and Rosenfeld, R. M. (2001). Meta-analysis of tympanostomy tube sequelae. Otolaryngology-Head Neck Surg. 124 (4), 374–380. doi:10.1067/mhn.2001.113941
Kayyali, M. N., Wooltorton, J. R. A., Ramsey, A. J., Lin, M., Chao, T. N., Tsourkas, A., et al. (2018). A novel nanoparticle delivery system for targeted therapy of noise-induced hearing loss. J. Control. Release 279, 243–250. doi:10.1016/j.jconrel.2018.04.028
Kheiriabad, S., Dolatabadi, J. E. N., and Hamblin, M. R. (2021). “Chapter 16 - dendrimers for gene therapy,” in Dendrimer-based Nanotherapeutics. Editor P. Kesharwani (Academic Press), 285–309.
Khomtchouk, K. M., Kouhi, A., Xia, A., Bekale, L. A., Massa, S. M., Sweere, J. M., et al. (2020). A novel mouse model of chronic suppurative otitis media and its use in preclinical antibiotic evaluation. Sci. Adv. 6(33), eabc1828. doi:10.1126/sciadv.abc1828
Kim, D.-H., Nguyen, T. N., Han, Y.-M., Tran, P., Rho, J., Lee, J.-Y., et al. (2021). Local drug delivery using poly(lactic-co-glycolic acid) nanoparticles in thermosensitive gels for inner ear disease treatment. Drug Deliv. 28 (1), 2268–2277. doi:10.1080/10717544.2021.1992041
Kim, E. S., Gustenhoven, E., Mescher, M. J., Leary Pararas, E. E., Smith, K. A., Spencer, A. J., et al. (2014). A microfluidic reciprocating intracochlear drug delivery system with reservoir and active dose control. Lab a Chip 14 (4), 710–721. doi:10.1039/C3LC51105G
Knutsson, J., Priwin, C., Hessen-Soderman, A. C., Rosenblad, A., and von Unge, M. (2018). A randomized study of four different types of tympanostomy ventilation tubes - full-term follow-up. Int. J. Pediatr. Otorhinolaryngology 107, 140–144. doi:10.1016/j.ijporl.2018.02.012
Koehler, K. R., Nie, J., Longworth-Mills, E., Liu, X. P., Lee, J., Holt, J. R., et al. (2017). Generation of inner ear organoids containing functional hair cells from human pluripotent stem cells. Nat. Biotechnol. 35 (6), 583–589. doi:10.1038/nbt.3840
Kretzmann, J. A., Evans, C. W., Moses, C., Sorolla, A., Kretzmann, A. L., Wang, E., et al. (2019). Tumour suppression by targeted intravenous non-viral CRISPRa using dendritic polymers. Chem. Sci. 10 (33), 7718–7727. doi:10.1039/C9SC01432B
Kuhn, M., Heman-Ackah, S. E., Shaikh, J. A., and Roehm, P. C. (2011). Sudden sensorineural hearing loss: a review of diagnosis, treatment, and prognosis. Trends Amplif. 15 (3), 91–105. doi:10.1177/1084713811408349
Kuo, C. L., Shiao, A. S., Yung, M., Sakagami, M., Sudhoff, H., Wang, C. H., et al. (2015). Updates and knowledge gaps in cholesteatoma research. BioMed Res. Int. 2015, 854024, doi:10.1155/2015/854024
Kurabi, A., Pak, K., Chavez, E., Doan, J., and Ryan, A. F. (2022). A transcytotic transport mechanism across the tympanic membrane. Sci. Rep. 12 (1), 984. doi:10.1038/s41598-021-04748-w
Kurabi, A., Schaerer, D., Chang, L., Pak, K., and Ryan, A. F. (2018a). Optimisation of peptides that actively cross the tympanic membrane by random amino acid extension: a phage display study. J. Drug Target. 26 (2), 127–134. doi:10.1080/1061186X.2017.1347791
Kurabi, A., Schaerer, D., Noack, V., Bernhardt, M., Pak, K., Alexander, T., et al. (2018b). Active transport of peptides across the intact human tympanic membrane. Sci. Rep. 8 (1), 11815. doi:10.1038/s41598-018-30031-6
Lajud, S. A., Han, Z., Chi, F.-L., Gu, R., Nagda, D. A., Bezpalko, O., et al. (2013). A regulated delivery system for inner ear drug application. J. Control. Release 166 (3), 268–276. doi:10.1016/j.jconrel.2012.12.031
Lajud, S. A., Nagda, D. A., Qiao, P., Tanaka, N., Civantos, A., Gu, R., et al. (2015). A novel chitosan-hydrogel-based nanoparticle delivery system for local inner ear application. Otology Neurotol. 36 (2), 341–347. doi:10.1097/mao.0000000000000445
Le, T. N., Straatman, L. V., Lea, J., and Westerberg, B. (2017). Current insights in noise-induced hearing loss: a literature review of the underlying mechanism, pathophysiology, asymmetry, and management options. J. Otolaryngology - Head Neck Surg. 46 (1), 41. doi:10.1186/s40463-017-0219-x
Leach, A. J., Morris, P. S., Coates, H. L., Nelson, S., O'Leary, S. J., Richmond, P. C., et al. (2021). Otitis media guidelines for Australian Aboriginal and Torres Strait Islander children: summary of recommendations. Med. J. Aust. 214 (5), 228–233. doi:10.5694/mja2.50953
Lee, C. K., de Anda, J., Baker, A. E., Bennett, R. R., Luo, Y., Lee, E. Y., et al. (2018). Multigenerational memory and adaptive adhesion in early bacterial biofilm communities. Proc. Natl. Acad. Sci. 115(17), 4471–4476. doi:10.1073/pnas.1720071115
Leterme, G., Guigou, C., Oudot, A., Collin, B., Boudon, J., Millot, N., et al. (2019). Superparamagnetic nanoparticle delivery to the cochlea through round window by external magnetic field: feasibility and toxicity. Surg. Innov. 26 (6), 646–655. doi:10.1177/1553350619867217
Li, J., and Mooney, D. J. (2016). Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 1 (12), 16071. doi:10.1038/natrevmats.2016.71
Li, M. G., Hotez, P. J., Vrabec, J. T., and Donovan, D. T. (2015). Is chronic suppurative otitis media a neglected tropical disease? PLoS Neglected Trop. Dis. 9 (3), e0003485. doi:10.1371/journal.pntd.0003485
Li, X., Wang, Y., Xu, F., Zhang, F., Xu, Y., Tang, L., et al. (2020). Artemisinin loaded mPEG-PCL nanoparticle based photosensitive gelatin methacrylate hydrogels for the treatment of gentamicin induced hearing loss. Int. J. Nanomedicine 15, 4591–4606. doi:10.2147/IJN.S245188
Li, Y., Liang, J., Chiang, H.-J., and Liu, Y. (2021). Postauricular injection in the treatment of all-frequency and high frequency descending sudden hearing loss: a protocol for systematic review and meta-analysis. Medicine 100 (3), e23847. doi:10.1097/md.0000000000023847
Liang, Z., Yu, H., Lai, J., Wen, L., and Chen, G. (2020). An easy-to-prepare microshotgun for efficient transmembrane delivery by powering nanoparticles. J. Control. Release 321, 119–131. doi:10.1016/j.jconrel.2020.02.016
Liebau, A., Schilp, S., Mugridge, K., Schön, I., Kather, M., Kammerer, B., et al. (2020). Long-term in vivo release profile of dexamethasone-loaded silicone rods implanted into the cochlea of Guinea pigs. Front. Neurology 10, 1377. doi:10.3389/fneur.2019.01377
Lieberthal, A. S., Carroll, A. E., Chonmaitree, T., Ganiats, T. G., Hoberman, A., Jackson, M. A., et al. (2013). The diagnosis and management of acute otitis media. Pediatrics 131 (3), e964–e999. doi:10.1542/peds.2012-3488
Lin, Y.-C., Shih, C.-P., Chen, H.-C., Chou, Y.-L., Sytwu, H.-K., Fang, M.-C., et al. (2021). Ultrasound microbubble–facilitated inner ear delivery of gold nanoparticles involves transient disruption of the tight junction barrier in the round window membrane. Front. Pharmacol. 12, 689032. doi:10.3389/fphar.2021.689032
Liu, H., Chen, S., Zhou, Y., Che, X., Bao, Z., Li, S., et al. (2013). The effect of surface charge of glycerol monooleate-based nanoparticles on the round window membrane permeability and cochlear distribution. J. Drug Target. 21 (9), 846–854. doi:10.3109/1061186X.2013.829075
Liu, W., Xie, S., Chen, X., Rao, X., Ren, H., Hu, B., et al. (2014). Activation of the IL-6/JAK/STAT3 signaling pathway in human middle ear cholesteatoma epithelium. Int. J. Clin. Exp. Pathology 7 (2), 709–715.
Lo, A., and Nemec, S. (2015). Opacification of the middle ear and mastoid: imaging findings and clues to differential diagnosis. Clin. Radiol. 70 (5), e1–e13. doi:10.1016/j.crad.2014.11.014
Lou, Z.-C., and Lou, Z.-H. (2017). A comparative study to evaluate the efficacy of EGF, FGF-2, and 0.3% (w/v) ofloxacin drops on eardrum regeneration. Medicine 96 (30), e7654. doi:10.1097/md.0000000000007654
Lou, Z.-C., Lou, Z.-H., and Tang, Y.-M. (2016). Comparative study on the effects of EGF and bFGF on the healing of human large traumatic perforations of the tympanic membrane. Laryngoscope 126 (1), E23–E28. doi:10.1002/lary.25715
Lou, Z.-C., and Lou, Z.-H. (2018a). The short-and long-term adverse effects of FGF-2 on tympanic membrane perforations. Acta Otorhinolaryngol. Ital. 38 (3), 264–272. doi:10.14639/0392-100x-1480
Lou, Z. C., and Lou, Z. (2018b). Efficacy of EGF and gelatin sponge for traumatic tympanic membrane perforations: a randomized controlled study. Otolaryngology–Head Neck Surg. 159 (6), 1028–1036. doi:10.1177/0194599818792019
Lou, Z. C., Tang, Y. M., and Yang, J. (2011). A prospective study evaluating spontaneous healing of aetiology, size and type-different groups of traumatic tympanic membrane perforation. Clin. Otolaryngol. 36 (5), 450–460. doi:10.1111/j.1749-4486.2011.02387.x
Lu, H., Zhang, S., Wang, J., and Chen, Q. (2021). A review on polymer and lipid-based nanocarriers and its application to nano-pharmaceutical and food-based systems. Front. Nutr. 8, 783831. doi:10.3389/fnut.2021.783831
Mandour, M. F., Elsheikh, M. N., and Khalil, M. F. (2019). Platelet-rich plasma fat graft versus cartilage perichondrium for repair of medium-size tympanic membrane perforations. Otolaryngology-Head Neck Surg. 160 (1), 116–121. doi:10.1177/0194599818789146
Mann, A. P., Scodeller, P., Hussain, S., Joo, J., Kwon, E., Braun, G. B., et al. (2016). A peptide for targeted, systemic delivery of imaging and therapeutic compounds into acute brain injuries. Nat. Commun. 7 (1), 11980. doi:10.1038/ncomms11980
Mansour, S., Magnan, J., Nicolas, K., and Haidar, H. (2018). Cholesteatoma. Cham: Springer International Publishing.
Martinovich, K. M., Rahman, T., de Gier, C., Seppanen, E. J., Orami, T., Granland, C. M., et al. (2021). Differences in pneumococcal and Haemophilus influenzae natural antibody development in Papua New Guinean children in the first year of life. Front. Immunol. 12, 3249. doi:10.3389/fimmu.2021.725244
McLean, W. J., Hinton, A. S., Herby, J. T. J., Salt, A. N., Hartsock, J. J., Wilson, S., et al. (2021). Improved speech intelligibility in subjects with stable sensorineural hearing loss following intratympanic dosing of FX-322 in a phase 1b study. Otology Neurotol. 42 (7), e849–e857. doi:10.1097/mao.0000000000003120
Mehnert, W., and Mäder, K. (2012). Solid lipid nanoparticles: production, characterization and applications. Adv. Drug Deliv. Rev. 64, 83–101. doi:10.1016/j.addr.2012.09.021
Mittal, R., Lisi, C. V., Gerring, R., Mittal, J., Mathee, K., Narasimhan, G., et al. (2015). Current concepts in the pathogenesis and treatment of chronic suppurative otitis media. J. Med. Microbiol. 64 (10), 1103–1116. doi:10.1099/jmm.0.000155
Mittal, R., Parrish, J. M., Soni, M., Mittal, J., and Mathee, K. (2018). Microbial otitis media: recent advancements in treatment, current challenges and opportunities. J. Med. Microbiol. 67 (10), 1417–1425. doi:10.1099/jmm.0.000810
Mok, M., Galvin, K. L., Dowell, R. C., and McKay, C. M. (2010). Speech perception benefit for children with a cochlear implant and a hearing aid in opposite ears and children with bilateral cochlear implants. Audiology Neurotol. 15 (1), 44–56. doi:10.1159/000219487
Mokrzan, E. M., Ahearn, C. P., Buzzo, J. R., Novotny, L. A., Zhang, Y., Goodman, S. D., et al. (2020). Nontypeable Haemophilus influenzae newly released (NRel) from biofilms by antibody-mediated dispersal versus antibody-mediated disruption are phenotypically distinct. Biofilm 2, 100039. doi:10.1016/j.bioflm.2020.100039
Mokrzan, E. M., Novotny, L. A., Brockman, K. L., and Bakaletz, L. O. (2018). Antibodies against the majority subunit (PilA) of the type IV pilus of nontypeable Haemophilus influenzae disperse Moraxella catarrhalis from a dual-species biofilm. mBio 9 (6), e02423-18–e02418. doi:10.1128/mBio.02423-18
Morita, Y., Takahashi, K., Izumi, S., Kubota, Y., Ohshima, S., Yamamoto, Y., et al. (2017). Risk factors of recurrence in pediatric congenital cholesteatoma. Otology Neurotol. 38 (10), 1463–1469. doi:10.1097/MAO.0000000000001587
Murillo-Cuesta, S., Celaya, A. M., Cervantes, B., Bermúdez-Muñoz, J. M., Rodríguez-de la Rosa, L., Contreras, J., et al. (2021). Therapeutic efficiency of the APAF-1 antagonist LPT99 in a rat model of cisplatin-induced hearing loss. Clin. Transl. Med. 11 (4), e363. doi:10.1002/ctm2.363
Nelson, D. I., Nelson, R. Y., Concha-Barrientos, M., and Fingerhut, M. (2005). The global burden of occupational noise-induced hearing loss. Am. J. Industrial Med. 48 (6), 446–458. doi:10.1002/ajim.20223
Nishio, S.-y., Hattori, M., Moteki, H., Tsukada, K., Miyagawa, M., Naito, T., et al. (2015). Gene expression profiles of the cochlea and vestibular endorgans: localization and function of genes causing deafness. Ann. Otology, Rhinology Laryngology 124 (1), 6S-48S–48S. doi:10.1177/0003489415575549
Novotny, L. A., Chiang, T., Goodman, S. D., Elmaraghy, C. A., and Bakaletz, L. O. (2021). Humanized anti-DNABII fab fragments plus ofloxacin eradicated biofilms in experimental otitis media. Laryngoscope 131 (10), E2698–E2704. doi:10.1002/lary.29497
Novotny, L. A., Clements, J. D., and Bakaletz, L. O. (2013). Kinetic analysis and evaluation of the mechanisms involved in the resolution of experimental nontypeable Haemophilus influenzae-induced otitis media after transcutaneous immunization. Vaccine 31 (34), 3417–3426. doi:10.1016/j.vaccine.2012.10.033
Novotny, L. A., Jurcisek, J. A., Goodman, S. D., and Bakaletz, L. O. (2016). Monoclonal antibodies against DNA-binding tips of DNABII proteins disrupt biofilms in vitro and induce bacterial clearance in vivo. EBioMedicine 10, 33–44. doi:10.1016/j.ebiom.2016.06.022
Novotny, L. A., Jurcisek, J. A., Ward, M. O., Jordan, Z. B., Goodman, S. D., and Bakaletz, L. O. (2015). Antibodies against the majority subunit of type IV pili disperse nontypeable H aemophilus influenzae biofilms in a LuxS-dependent manner and confer therapeutic resolution of experimental otitis media. Mol. Microbiol. 96 (2), 276–292. doi:10.1111/mmi.12934
Nyberg, S., Abbott, N. J., Shi, X., Steyger, P. S., and Dabdoub, A. (2019). Delivery of therapeutics to the inner ear: the challenge of the blood-labyrinth barrier. Sci. Transl. Med. 11 (482), eaao0935. doi:10.1126/scitranslmed.aao0935
Ohlemiller, K. K., and Gagnon, P. M. (2004). Cellular correlates of progressive hearing loss in 129S6/SvEv mice. J. Comp. Neurology 469 (3), 377–390. doi:10.1002/cne.11011
Orgel, E., Villaluna, D., Krailo, M. D., Esbenshade, A., Sung, L., and Freyer, D. R. (2022). Sodium thiosulfate for prevention of cisplatin-induced hearing loss: updated survival from ACCL0431. Lancet Oncol. 23 (5), 570–572. doi:10.1016/S1470-2045(22)00155-3
Orji, F. T., and Agu, C. C. (2008). Determinants of spontaneous healing in traumatic perforations of the tympanic membrane. Clin. Otolaryngol. 33 (5), 420–426. doi:10.1111/j.1749-4486.2008.01764.x
Paltura, C., Can, T. S., Yilmaz, B. K., Dinç, M. E., Develioğlu, Ö. N., and Külekçi, M. (2017). Eustachian tube diameter: is it associated with chronic otitis media development? Am. J. Otolaryngology 38 (4), 414–416. doi:10.1016/j.amjoto.2017.03.012
Paulson, D. P., Abuzeid, W., Jiang, H., Oe, T., O'Malley, B. W., and Li, D. (2008). A novel controlled local drug delivery system for inner ear disease. Laryngoscope 118 (4), 706–711. doi:10.1097/MLG.0b013e31815f8e41
Pichichero, M. E. (2020). Immunologic dysfunction contributes to the otitis prone condition. J. Infect. 80 (6), 614–622. doi:10.1016/j.jinf.2020.03.017
Piu, F., Wang, X., Fernandez, R., Dellamary, L., Harrop, A., Ye, Q., et al. (2011). OTO-104: a sustained-release dexamethasone hydrogel for the treatment of otic disorders. Otology Neurotol. 32 (1), 171–179. doi:10.1097/MAO.0b013e3182009d29
Plontke, S. K., Götze, G., Rahne, T., and Liebau, A. (2017). Intracochlear drug delivery in combination with cochlear implants: current aspects. HNO 65 (1), 19–28. doi:10.1007/s00106-016-0285-9
Qiu, K., Mao, M., Deng, D., Jiang, C., Li, L., Zheng, Y., et al. (2022). Is postauricular injection a systemic or a topical route for inner ear drug delivery? Hear. Res. 422, 108570. doi:10.1016/j.heares.2022.108570
Qnouch, A., Solarczyk, V., Verin, J., Tourrel, G., Stahl, P., Danede, F., et al. (2021). Dexamethasone-loaded cochlear implants: how to provide a desired “burst release”. Int. J. Pharm. X 3, 100088. doi:10.1016/j.ijpx.2021.100088
Ramaswamy, B., Roy, S., Apolo, A. B., Shapiro, B., and Depireux, D. A. (2017). Magnetic nanoparticle mediated steroid delivery mitigates cisplatin induced hearing loss. Front. Cell. Neurosci. 11, 268. doi:10.3389/fncel.2017.00268
Rathnam, C., Chueng, S.-T. D., Ying, Y.-L. M., Lee, K.-B., and Kwan, K. (2019). Developments in bio-inspired nanomaterials for therapeutic delivery to treat hearing loss. Front. Cell. Neurosci. 13, 493. doi:10.3389/fncel.2019.00493
Rauch, S. D., Halpin, C. F., Antonelli, P. J., Babu, S., Carey, J. P., Gantz, B. J., et al. (2011). Oral vs intratympanic corticosteroid therapy for idiopathic sudden sensorineural hearing loss: a randomized trial. JAMA 305 (20), 2071–2079. doi:10.1001/jama.2011.679
Renukananda, G., Santosh, U., and George, N. M. (2014). Topical vs combination ciprofloxacin in the management of discharging chronic suppurative otitis media. J. Clin. Diagnostic Res. JCDR 8 (6), KC01–KC04. doi:10.7860/JCDR/2014/8038.4421
Rowe, H. M., Meliopoulos, V. A., Iverson, A., Bomme, P., Schultz-Cherry, S., and Rosch, J. W. (2019). Direct interactions with influenza promote bacterial adherence during respiratory infections. Nat. Microbiol. 4 (8), 1328–1336. doi:10.1038/s41564-019-0447-0
Ruoslahti, E. (2022). Molecular ZIP codes in targeted drug delivery. Proc. Natl. Acad. Sci. U. S. A. 119(28), e2200183119. doi:10.1073/pnas.2200183119
Rybak, L. P., Ramkumar, V., and Mukherjea, D. (2021). Ototoxicity of non-aminoglycoside antibiotics. Front. Neurology 12, 652674. doi:10.3389/fneur.2021.652674
Sainsbury, E., Amaral, R. d., Blayney, A. W., Walsh, R. M., O'Brien, F. J., and O'Leary, C. (2022). Tissue engineering and regenerative medicine strategies for the repair of tympanic membrane perforations. Biomaterials Biosyst. 6, 100046. doi:10.1016/j.bbiosy.2022.100046
Salt, A. N., and Plontke, S. K. (2018). Pharmacokinetic principles in the inner ear: influence of drug properties on intratympanic applications. Hear. Res. 368, 28–40. doi:10.1016/j.heares.2018.03.002
Santa Maria, P. L., Domville-Lewis, C., Sucher, C. M., Chester-Browne, R., and Atlas, M. D. (2013). Hearing preservation surgery for cochlear implantation—hearing and quality of life after 2 years. Otology Neurotol. 34 (3), 526–531. doi:10.1097/MAO.0b013e318281e0c9
Santa Maria, P. L., Kaufman, A. C., Bacacao, B., Thai, A., Chen, X., Xia, A., et al. (2021). Topical therapy failure in chronic suppurative otitis media is due to persister cells in biofilms. Otology Neurotol. 42 (9), e1263–e1272. doi:10.1097/mao.0000000000003222
Santos, F., Shu, E., Lee, D. J., Jung, D. H., Quesnel, A. M., Stankovic, K. M., et al. (2020). Topical fibroblast growth factor-2 for treatment of chronic tympanic membrane perforations. Laryngoscope Investig. Otolaryngol. 5 (4), 657–664. doi:10.1002/lio2.395
Sara, S. A., Teh, B. M., and Friedland, P. (2013). Bilateral sudden sensorineural hearing loss: review. J. Laryngology Otology 128 (S1), S8–S15. doi:10.1017/S002221511300306X
Sayin, I., Kaya, K. H., Ekizoğlu, O., Erdim, İ., and Kayhan, F. T. (2013). A prospective controlled trial comparing spontaneous closure and Epifilm® patching in traumatic tympanic membrane perforations. Eur. Archives Oto-Rhino-Laryngology 270 (11), 2857–2863. doi:10.1007/s00405-012-2331-x
Schrujver, I. (2004). Hereditary non-syndromic sensorineural hearing loss: transforming silence to sound. J. Mol. Diagn 6 (4), 275–284. doi:10.1016/S1525-1578(10)60522-3
Schuknecht, H. F. (1964). Further observations on the pathology of presbycusis. Archives Otolaryngology 80, 369–382. doi:10.1001/archotol.1964.00750040381003
Schuknecht, H. F., and Gacek, M. R. (1993). Cochlear pathology in presbycusis. Ann. Otology, Rhinology Laryngology 102 (1), 1–16. doi:10.1177/00034894931020s101
Shen, Y., Redmond, S. L., Papadimitriou, J. M., Teh, B. M., Yan, S., Wang, Y., et al. (2014). The biocompatibility of silk fibroin and acellular collagen scaffolds for tissue engineering in the ear. Biomed. Mater. 9 (1), 015015. doi:10.1088/1748-6041/9/1/015015
Sheth, S., Mukherjea, D., Rybak, L. P., and Ramkumar, V. (2017). Mechanisms of cisplatin-induced ototoxicity and otoprotection. Front. Cell. Neurosci. 11, 338. doi:10.3389/fncel.2017.00338
Silva, M. D., and Sillankorva, S. (2019). Otitis media pathogens–A life entrapped in biofilm communities. Crit. Rev. Microbiol. 45 (5-6), 595–612. doi:10.1080/1040841X.2019.1660616
Silverstein, H., Thompson, J., Rosenberg, S. I., Brown, N., and Light, J. (2004). Silverstein MicroWick. Otolaryngologic Clin. N. Am. 37 (5), 1019–1034. doi:10.1016/j.otc.2004.04.002
Smith, S. A., Selby, L. I., Johnston, A. P. R., and Such, G. K. (2019). The endosomal escape of nanoparticles: toward more efficient cellular delivery. Bioconjugate Chem. 30 (2), 263–272. doi:10.1021/acs.bioconjchem.8b00732
Solheim, J., Gay, C., and Hickson, L. (2018). Older adults’ experiences and issues with hearing aids in the first six months after hearing aid fitting. Int. J. Audiology 57 (1), 31–39. doi:10.1080/14992027.2017.1380849
Spandow, O., Hellstrom, S., and Dahlstrom, M. (1996). Structural characterization of persistent tympanic membrane perforations in man. Laryngoscope 106 (3), 346–352. doi:10.1097/00005537-199603000-00020
Suzuki, T., Nomoto, Y., Nakagawa, T., Kuwahata, N., Ogawa, H., Suzuki, Y., et al. (2006). Age-dependent degeneration of the stria vascularis in human cochleae. Laryngoscope 116 (10), 1846–1850. doi:10.1097/01.mlg.0000234940.33569.39
Szeto, B., Chiang, H., Valentini, C., Yu, M., Kysar, J. W., and Lalwani, A. K. (2019). Inner ear delivery: challenges and opportunities. Laryngoscope Investig. Otolaryngol. 5 (1), 122–131. doi:10.1002/lio2.336
Tan, H. E., Santa Maria, P. L., Eikelboom, R. H., Anandacoomaraswamy, K. S., and Atlas, M. D. (2016). Type I tympanoplasty meta-analysis: a single variable analysis. Otology Neurotol. 37 (7), 838–846. doi:10.1097/mao.0000000000001099
Tandon, V., Kang, W. S., Spencer, A. J., Kim, E. S., Pararas, E. E. L., McKenna, M. J., et al. (2015). Microfabricated infuse-withdraw micropump component for an integrated inner-ear drug-delivery platform. Biomed. Microdevices 17 (2), 37. doi:10.1007/s10544-014-9923-8
Taneja, M. (2020). Role of platelet rich plasma in tympanoplasty. Indian J. Otolaryngology Head Neck Surg. 72, 247–250. doi:10.1007/s12070-020-01815-y
Tange, R. A., Dreschler, W. A., Claessen, F. A. P., and Perenboom, R. M. (1997). Ototoxic reactions of quinine in healthy persons and patients with Plasmodium falciparum infection. Auris Nasus Larynx 24 (2), 131–136. doi:10.1016/S0385-8146(96)00031-4
Tawfik, K. O., Klepper, K., Saliba, J., and Friedman, R. A. (2020). Advances in understanding of presbycusis. J. Neurosci. Res. 98 (9), 1685–1697. doi:10.1002/jnr.24426
Thornton, R. B., Wiertsema, S. P., Kirkham, L.-A. S., Rigby, P. J., Vijayasekaran, S., Coates, H. L., et al. (2013). Neutrophil extracellular traps and bacterial biofilms in middle ear effusion of children with recurrent acute otitis media – a potential treatment target. PLoS ONE 8 (2), e53837. doi:10.1371/journal.pone.0053837
Tolker-Nielsen, T. (2014). Pseudomonas aeruginosa biofilm infections: from molecular biofilm biology to new treatment possibilities. J. Pathology, Microbiol. Immunol. - APMIS J. 122 (138), 1–51. doi:10.1111/apm.12335
Trojanowska, A., Drop, A., Trojanowski, P., Rosińska-Bogusiewicz, K., Klatka, J., and Bobek-Billewicz, B. (2012). External and middle ear diseases: radiological diagnosis based on clinical signs and symptoms. Insights Into Imaging 3 (1), 33–48. doi:10.1007/s13244-011-0126-z
Valente, F., Astolfi, L., Simoni, E., Danti, S., Franceschini, V., Chicca, M., et al. (2017). Nanoparticle drug delivery systems for inner ear therapy: an overview. J. Drug Deliv. Sci. Technol. 39, 28–35. doi:10.1016/j.jddst.2017.03.003
Van Camp, G., and Smith, R. J. H. (2023). Hereditary hearing loss homepage. [Online]. Available at: https://hereditaryhearingloss.org (Accessed may 22, 2023).
van der Toom, H. F., van der Schroeff, M. P., Metselaar, M., van Linge, A., Vroegop, J. L., and Pauw, R. J. (2021). Treatment outcome of the bony obliteration tympanoplasty versus nonobliterative techniques in cholesteatoma surgery: a retrospective analysis. Otology Neurotol. 42 (9), 1366–1374. doi:10.1097/MAO.0000000000003246
van Dongen, T. M. A., van der Heijden, G. J. M. G., Venekamp, R. P., Rovers, M. M., and Schilder, A. G. M. (2014). A trial of treatment for acute otorrhea in children with tympanostomy tubes. N. Engl. J. Med. 370 (8), 723–733. doi:10.1056/NEJMoa1301630
Varsak, Y. K., and Santa Maria, P. L. (2016). Mouse model of experimental Eustachian tube occlusion: a surgical technique. Acta Oto-Laryngologica 136 (1), 12–17. doi:10.3109/00016489.2015.1082191
Videhult, P., Laurell, G., Wallin, I., and Ehrsson, H. (2006). Kinetics of cisplatin and its monohydrated complex with sulfur-containing compounds designed for local otoprotective administration. Exp. Biol. Med. 231 (10), 1638–1645. doi:10.1177/153537020623101009
Viglietta, V., Shi, F., Hu, Q. -Y., Ren, Y., Keilty, J., Wolff, H., et al. (2020). Phase 1 study to evaluate safety, tolerability and pharmacokinetics of a novel intra-tympanic administered thiosulfate to prevent cisplatin-induced hearing loss in cancer patients. Invest. New Drugs 38 (5), 1463–1471. doi:10.1007/s10637-020-00918-1
Villar-Fernandez, M. A., and Lopez-Escamez, J. A. (2015). Outlook for tissue engineering of the tympanic membrane. Audiology Res. 5 (1), 117–119. doi:10.4081/audiores.2015.117
Völter, C., Götze, L., Haubitz, I., Dazert, S., and Thomas, J. P. (2020). Benefits of cochlear implantation in middle-aged and older adults. Clin. Interventions Aging 15, 1555–1568. doi:10.2147/cia.s255363
Vos, J. D., Latev, M. D., Labadie, R. F., Cohen, S. M., Werkhaven, J. A., and Haynes, D. S. (2005). Use of AlloDerm in type I tympanoplasty: a comparison with native tissue grafts. Laryngoscope 115 (9), 1599–1602. doi:10.1097/01.mlg.0000172042.73024.ad
Wallhagen, M. I. (2009). The stigma of hearing loss. Gerontologist 50 (1), 66–75. doi:10.1093/geront/gnp107
Wang, A. Y., Shen, Y., Wang, J. T., Eikelboom, R. H., and Dilley, R. J. (2014a). Animal models of chronic tympanic membrane perforation: in response to plasminogen initiates and potentiates the healing of acute and chronic tympanic membrane perforations in mice. Clin. Transl. Med. 3 (1), 5. doi:10.1186/2001-1326-3-5
Wang, N., Gao, X., Li, M., Li, Y., and Sun, M. (2020). Use of solid lipid nanoparticles for the treatment of acute acoustic stress-induced cochlea damage. J. Nanosci. Nanotechnol. 20 (12), 7412–7418. doi:10.1166/jnn.2020.18522
Wang, Y., Wise, A. K., Tan, J., Maina, J. W., Shepherd, R. K., and Caruso, F. (2014b). Mesoporous silica supraparticles for sustained inner-ear drug delivery. Small 10 (21), 4244–4248. doi:10.1002/smll.201401767
Warnecke, A., Harre, J., Staecker, H., Prenzler, N., Strunk, D., Couillard-Despres, S., et al. (2020). Extracellular vesicles from human multipotent stromal cells protect against hearing loss after noise trauma in vivo. Clin. Transl. Med. 10 (8), e262. doi:10.1002/ctm2.262
Warnecke, A., Prenzler, N., Harre, J., Köhl, U., Gärtner, L., Lenarz, T., et al. (2021). First-in-human intracochlear application of human stromal cell-derived extracellular vesicles. J. Extracell. Vesicles 10 (8), e12094. doi:10.1002/jev2.12094
Warnecke, A., Staecker, H., Rohde, E., Gimona, M., Giesemann, A., Szczepek, A. J., et al. (2022). Extracellular vesicles in inner ear therapies—pathophysiological, manufacturing, and clinical considerations. J. Clin. Med. 11 (24), 7455. doi:10.3390/jcm11247455
Wilk, M., Hessler, R., Mugridge, K., Jolly, C., Fehr, M., Lenarz, T., et al. (2016). Impedance changes and fibrous tissue growth after cochlear implantation are correlated and can Be reduced using a dexamethasone eluting electrode. PLOS ONE 11 (2), e0147552. doi:10.1371/journal.pone.0147552
Wise, A. K., Tan, J., Wang, Y., Caruso, F., and Shepherd, R. K. (2016). Improved auditory nerve survival with nanoengineered supraparticles for neurotrophin delivery into the deafened cochlea. PLoS ONE 11 (10), e0164867. doi:10.1371/journal.pone.0164867
Wright, A., Hawkins, C. H., Änggård, E. E., and Harper, D. R. (2009). A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clin. Otolaryngol. 34 (4), 349–357. doi:10.1111/j.1749-4486.2009.01973.x
Wu, N., Li, M., Chen, Z.-T., Zhang, X.-B., Liu, H.-Z., Li, Z., et al. (2013). In vivo delivery of Atoh1 gene to rat cochlea using a dendrimer-based nanocarrier. J. Biomed. Nanotechnol. 9 (10), 1736–1745. doi:10.1166/jbn.2013.1684
Xie, S., Xiang, Y., Wang, X., Ren, H., Yin, T., Ren, J., et al. (2016). Acquired cholesteatoma epithelial hyperproliferation: roles of cell proliferation signal pathways. Laryngoscope 126 (8), 1923–1930. doi:10.1002/lary.25834
Xie, W., Karpeta, N., Liu, J., Peng, H., Li, C., Zhang, Z., et al. (2023). Efficacy of intratympanic or postauricular subperiosteal corticosteroid injection combined with systemic corticosteroid in the treatment of sudden sensorineural hearing loss: a prospective randomized study. Front. Neurology 14, 1138354. doi:10.3389/fneur.2023.1138354
Xu, X., Wang, S., Shu, Y., and Zhang, H. (2021a). Nanoparticle-based inner ear delivery systems for the treatment of hearing loss. Smart Mater. Med. 2, 350–353. doi:10.1016/j.smaim.2021.10.002
Xu, X., Zheng, J., He, Y., Lin, K., Li, S., Zhang, Y., et al. (2021b). Nanocarriers for inner ear disease therapy. Front. Cell. Neurosci. 15, 791573. doi:10.3389/fncel.2021.791573
Yadav, S. P. S., Malik, J. S., Malik, P., Sehgal, P. K., Gulia, J. S., and Ranga, R. K. (2018). Studying the result of underlay myringoplasty using platelet-rich plasma. J. Laryngology Otology 132 (11), 990–994. doi:10.1017/S0022215118001846
Yang, K.-J., Son, J., Jung, S. Y., Yi, G., Yoo, J., Kim, D.-K., et al. (2018a). Optimized phospholipid-based nanoparticles for inner ear drug delivery and therapy. Biomaterials 171, 133–143. doi:10.1016/j.biomaterials.2018.04.038
Yang, R., Sabharwal, V., Okonkwo, O. S., Shlykova, N., Tong, R., Lin, L. Y., et al. (2016). Treatment of otitis media by transtympanic delivery of antibiotics. Sci. Transl. Med. 8 (356), 356ra120. doi:10.1126/scitranslmed.aaf4363
Yang, R., Sabharwal, V., Shlykova, N., Okonkwo, O. S., Pelton, S. I., and Kohane, D. S. (2018b). Treatment of Streptococcus pneumoniae otitis media in a chinchilla model by transtympanic delivery of antibiotics. JCI Insight 3 (19), e123415. doi:10.1172/jci.insight.123415
Yeow, Y. L., Kotamraju, V. R., Wang, X., Chopra, M., Azme, N., Wu, J., et al. (2019). Immune-mediated ECM depletion improves tumour perfusion and payload delivery. EMBO Mol. Med. 11 (12), e10923. doi:10.15252/emmm.201910923
Yoon, J. Y., Yang, K.-J., Park, S.-N., Kim, D.-K., and Kim, J.-D. (2016). The effect of dexamethasone/cell-penetrating peptide nanoparticles on gene delivery for inner ear therapy. Int. J. Nanomedicine 11, 6123–6134. doi:10.2147/ijn.s114241
You, J.-O., and Auguste, D. T. (2009). Nanocarrier cross-linking density and pH sensitivity regulate intracellular gene transfer. Nano Lett. 9 (12), 4467–4473. doi:10.1021/nl902789s
Zhao, Z., Han, Z., Naveena, K., Lei, G., Qiu, S., Li, X., et al. (2021). ROS-responsive nanoparticle as a berberine carrier for OHC-targeted therapy of noise-induced hearing loss. ACS Appl. Mater. Interfaces 13 (6), 7102–7114. doi:10.1021/acsami.0c21151
Zhu, M., Wang, Y., Ferracci, G., Zheng, J., Cho, N.-J., and Lee, B. H. (2019). Gelatin methacryloyl and its hydrogels with an exceptional degree of controllability and batch-to-batch consistency. Sci. Rep. 9 (1), 6863. doi:10.1038/s41598-019-42186-x
Zhuang, P., Phung, S., Warnecke, A., Arambula, A., St. Peter, M., He, M., et al. (2021). Isolation of sensory hair cell specific exosomes in human perilymph. Neurosci. Lett. 764, 136282. doi:10.1016/j.neulet.2021.136282
Zimmerman, E., and Lahav, A. (2013). Ototoxicity in preterm infants: effects of genetics, aminoglycosides, and loud environmental noise. J. Perinatol. 33 (1), 3–8. doi:10.1038/jp.2012.105
Keywords: drug delivery, inner ear, middle ear, hydrogel, nanoparticle, biomaterials, microdevices
Citation: Delaney DS, Liew LJ, Lye J, Atlas MD and Wong EYM (2023) Overcoming barriers: a review on innovations in drug delivery to the middle and inner ear. Front. Pharmacol. 14:1207141. doi: 10.3389/fphar.2023.1207141
Received: 18 April 2023; Accepted: 02 October 2023;
Published: 19 October 2023.
Edited by:
Myung-Whan Suh, Seoul National University Hospital, Republic of KoreaReviewed by:
Helge Rask-Andersen, Uppsala University, SwedenCopyright © 2023 Delaney, Liew, Lye, Atlas and Wong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elaine Y. M. Wong, ZWxhaW5lLndvbmdAZWFyc2NpZW5jZS5vcmcuYXU=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.