- 1Key Laboratory of Ethnomedicine of Ministry of Education, School of Pharmacy, Center on Translational Neuroscience, Minzu University of China, Beijing, China
- 2Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences, Beijing, China
- 3College of Life and Environmental Sciences, Minzu University of China, Beijing, China
- 4Institute of National Security, Minzu University of China, Beijing, China
Background: Stroke survivors are at significantly increased risk of cognitive impairment, which affects patients’ independence of activities of daily living (ADLs), social engagement, and neurological function deficit. Many studies have been done to evaluate the efficacy and safety of post-stroke cognitive impairment (PSCI) treatment, and due to the largely inconsistent clinical data, there is a need to summarize and analyze the published clinical research data in this area.
Objective: An umbrella review was performed to evaluate the efficacy and safety of PSCI therapies.
Methods: Three independent authors searched for meta-analyses and systematic reviews on PubMed, the Cochrane Library, and the Web of Science to address this issue. We examined ADL and Barthel index (BI), Montreal Cognitive Assessment (MoCA), neurological function deficit as efficacy endpoints, and the incidence of adverse events as safety profiles.
Results: In all, 312 studies from 19 eligible publications were included in the umbrella review. The results showed that angiotensin-converting enzyme inhibitors (ACEI) and N-methyl-D-aspartate (NMDA) antagonists, cell therapies, acupuncture, and EGB76 can improve the MoCA and ADL, and the adverse effects were mild for the treatment of PSCI. Moreover, Vinpocetine, Oxiracetam, Citicoline, thrombolytic therapy, Actovegin, DL-3-n-Butylphthalide, and Nimodipine showed adverse events or low article quality in patients with PSCI. However, the research evidence is not exact and further research is needed.
Conclusion: Our study demonstrated that ACEI inhibitors (Donepezil) and NMDA antagonists (Memantine), EGB761, and acupuncture are the ADL and BI, MoCA, and neurological function deficit medication/therapy, respectively, for patients with PSCI.
Clinical Trial Registration: https://inplasy.com/inplasy-2022-11-0139/; Identifier: INPLASY2022110139.
Introduction
Ischemic stroke seriously threatens human health and life, and is a common cardiovascular and cerebrovascular disease (Saini et al., 2021). According to the World Health Organization (WHO), the incidence of stroke has been increasing in recent years, and disability rates are very high. PSCI is a frequent complication after stroke, with a prevalence of 50%–70%, and effective treatment is needed to improve the prognosis of patients (Mijajlovic et al., 2017). Epidemiological studies have shown that stroke is the second leading cause of death in the world and the first fatal and disabling disease among the Chinese population. PSCI, including mild cognitive impairment and dementia, not only affects patients’ ability to do daily living but also hinders rehabilitation and exercise, increasing the economic and mental burden of family care. In addition, as little is known about the efficacy and safety of PSCI treatment in the recovery phase after stroke, the critical challenge in PSCI treatment is to determine the most effective way of current interventions.
There is some evidence supporting the notion that neurological deficits can be greatly improved by the use of recombinant tissue plasminogen activator (tPA) thrombolysis, ACEI inhibitors, and NMDA antagonists (Donepezil and galantamine) recommended by national guidelines for the treatment of PSCI (Ebihara et al., 2007; Liraz-Zaltsman et al., 2018; Yang et al., 2022). In addition, the neuroprotection, statins, and control of high-risk factors are recommended as secondary prevention of PSCI (Mijajlovic et al., 2017). Additionally, memantine and B1 and B2 bradykinin receptor agonists do not lead to significant improvement in PSCI cognition but provide overall functional benefits (Glass et al., 2020) (Martins, 2012). In addition, many clinical studies have shown that many other neuroprotective drugs improve cognitive impairment and are safe and effective (Zhang et al., 2014).
Much research has attempted to study the commonly used PSCI treatment methods. However, the results of these studies are still biased and contradictory (Wu et al., 2007). It is necessary to review the latest literature, delete duplicate or problematic studies, and then conduct a meta-analysis to obtain a pooled prevalence. Therefore, to draw a definitive conclusion and determine which commercially available therapies for PSCI patients are effective and safe, we have performed an umbrella review of the systematic reviews and meta-analyses of PSCI therapies through a comprehensive and updated literature search.
Materials and methods
Our study conforms with the standard guidelines of Preferred Reporting Items for Systematic Reviews and Meta-analysis (Moher et al., 2009). The protocol for this review has been registered at INPLASY PROTOCOL (INPLASY2022110139).
Search strategy and quality assessment
A systematic search of published peer-reviewed English-language literature was conducted using PubMed, Web of Science, and the Cochrane Library up until October 2022. The database search terms were as follows: (Post-stroke cognitive impairment/Post-stroke dementia) and (systematic review or meta-analysis) and clinical trial. We included meta-analyses and systematic reviews that determined the efficacy and safety of treatments in patients with PSCI. Inclusion criteria were: 1) articles written in English; 2) published systematic reviews or meta-analyses; 3) articles including any evaluation of clinical assessment scales for PSCI; and 4) articles published in peer-reviewed journals. Studies were excluded if 1) they were unpublished studies; 2) there were no necessary sample data; 3) patients were diagnosed with other PSCI; 4) the study reported insufficient details and other outcomes; and 5) there was a presence of risk of bias/study limitations.
We used the AMSTAR2 tool to evaluate systematic reviews and meta-analyses (De Santis et al., 2022). The methodological quality of the studies was determined by the percentage of the AMSTAR2 score. The percentage of the AMSTAR2 score was classified into 0%–15.8%, 15.8%–21.05%, and 21.05%–100%, indicating low quality, medium quality, and high quality, respectively.
We used keywords and filtered titles searching for related articles, and two review authors independently screened articles. These downloaded articles were screened by inclusion/exclusion criteria, and any irrelevant or duplicate articles were removed. Thereafter, we manually searched the reference lists from the selected literature for any other relevant studies that were not identified in the initial search. Finally, a full-text search was conducted to extract and analyze article data.
Data extraction
According to the following criteria, three investigators (Yongbiao Li, Ruyi Cui, and Shaobao Liu) independently selected the trials that met the inclusion criteria. The main characteristics of the selected study were extracted and displayed in a table, including year of publication, study design, number of studies, and regimens for the treatment. We included results evaluating the efficacy of drugs in patients with at least one of the clinical assessment scales: 1) baseline mini-mental state examination (MMSE) scores; 2) the primary outcomes included global neurological deficit scores such as the National Institutes of Health Stroke Scale (NIHSS) score ≤1 and MoCA; 3) Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog); 4) dependence assessed by Clinical Global Impression of Change (CIBIC-plus or CGIC); 5) activities of ADL; 6) clinical effect, defined according to the nationally approved criteria, was divided into essentially recovered, significant improvement, improvement, no change, deterioration, and death (the first three categories were judged to be effective); 7) the secondary outcomes included: abilities of daily living (evaluated by BI), related hemorheology and lipid metabolism outcomes, and quality of life; and 8) incidence of adverse events (AE). The selection of assessments was extracted on study size, sample size, mean difference (Fixed, 95% CI) or odds ratio (Fixed, 95% CI), and heterogeneity (I2). A percentage of 0%–25% was classified as mild, 26%–50% was classified as moderate, and 51%–75% was classified as significant between-study heterogeneity. If I2 > 50%, a random-effects model was used for the analysis, or the data was analyzed on a fixed-effects model (Wang et al., 2016).
Statistical analysis
Four clinical assessment scales were calculated using sample sizes and mean differences. The NIHSS/BI/SCORE was used to assess neurological status, and the patient’s behavioral symptoms were calculated using ADL/MMSE/ADAS. The clinical effects we focused on were divided into basic recovery, significant improvement, no change, and deterioration, as well as cognitive function scores and quality of life as an activity of daily living. Graphpad Prim 8 software was used for all clinical data analysis. Results are expressed as MD±SD (standard deviation). The incidence of adverse events was assessed and ORs were calculated. Therefore, the mean difference or odds ratio with 95% CI and p values were used to assess the effectiveness and safety of the study treatments.
Results
Through the initial search, 970 records were retrieved from PubMed, Web of Science, and the Corevchrane Library. Then, 50 studies were selected for further full-text scrutiny after titles and abstracts were examined. In all, 31 studies were excluded due to the following reasons: samples overlap with other studies (n = 7), no necessary sample data (n = 10), other outcomes (n = 4), other PSCI (n = 3), not written in English (n = 4), and no placebo group (n = 3) (Figure 1). Thus, 19 studies were included in the umbrella review: Kim and Kang, (2020), Guekht et al. (2017), Kwon (2019), Huang et al. (2021), Tan (2015), Jin and Liu (2019), Shi et al. (2022), Lopez-Arrieta and Birks (2002), Fan (2021), Yi et al. (2020), Alvarez-Sabín. (2013), Huahui and Hehua (2015), Malykh and Sadaie (2010), Ming (2016), NanZhu et al. (2018), Wei (2020), You et al. (2019), Szatmári and Whitehouse (2009), Szatmari and Whitehouse (2003).
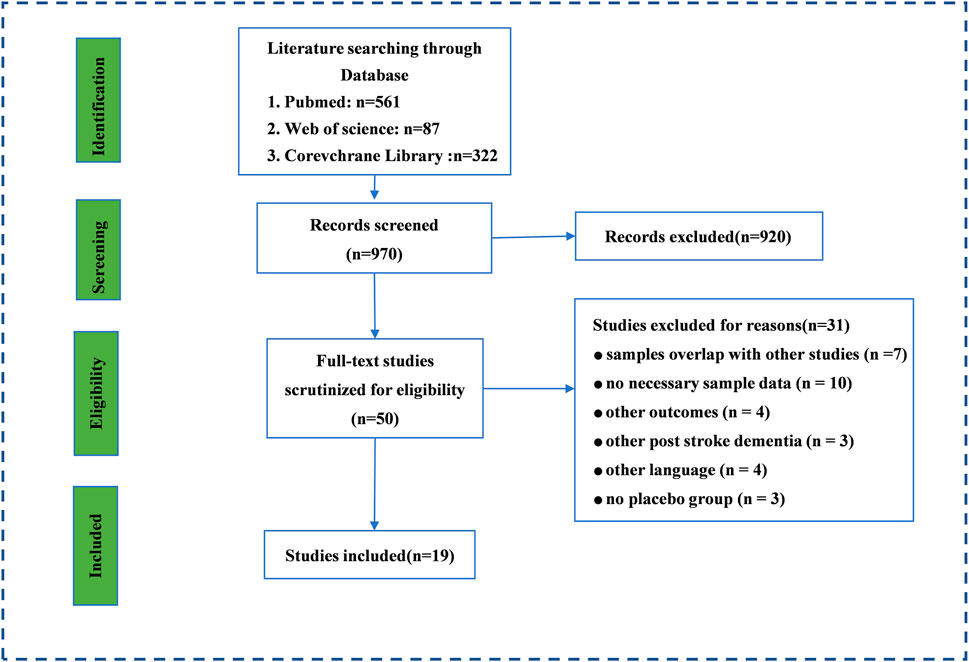
FIGURE 1. The search and screening process. Literature search and study selection. Through the initial search, we retrieved a total of 3,808 records from PubMed, Web of Science, and the Corevchrane Library. After examining the titles and abstracts, Through the initial search, 970 records was retrieved from PubMed, Web of science and Corevchrane Library. 50 studies were selected for further full-text scrutiny after examining the titles and abstracts. In all, 31 studies were excluded due to the following reasons: samples overlap with other studies (n = 7), no necessary sample data (n = 10), other outcomes (n = 4), other PSCI (n =3), other language (n = 4), no placebo group (n = 3).
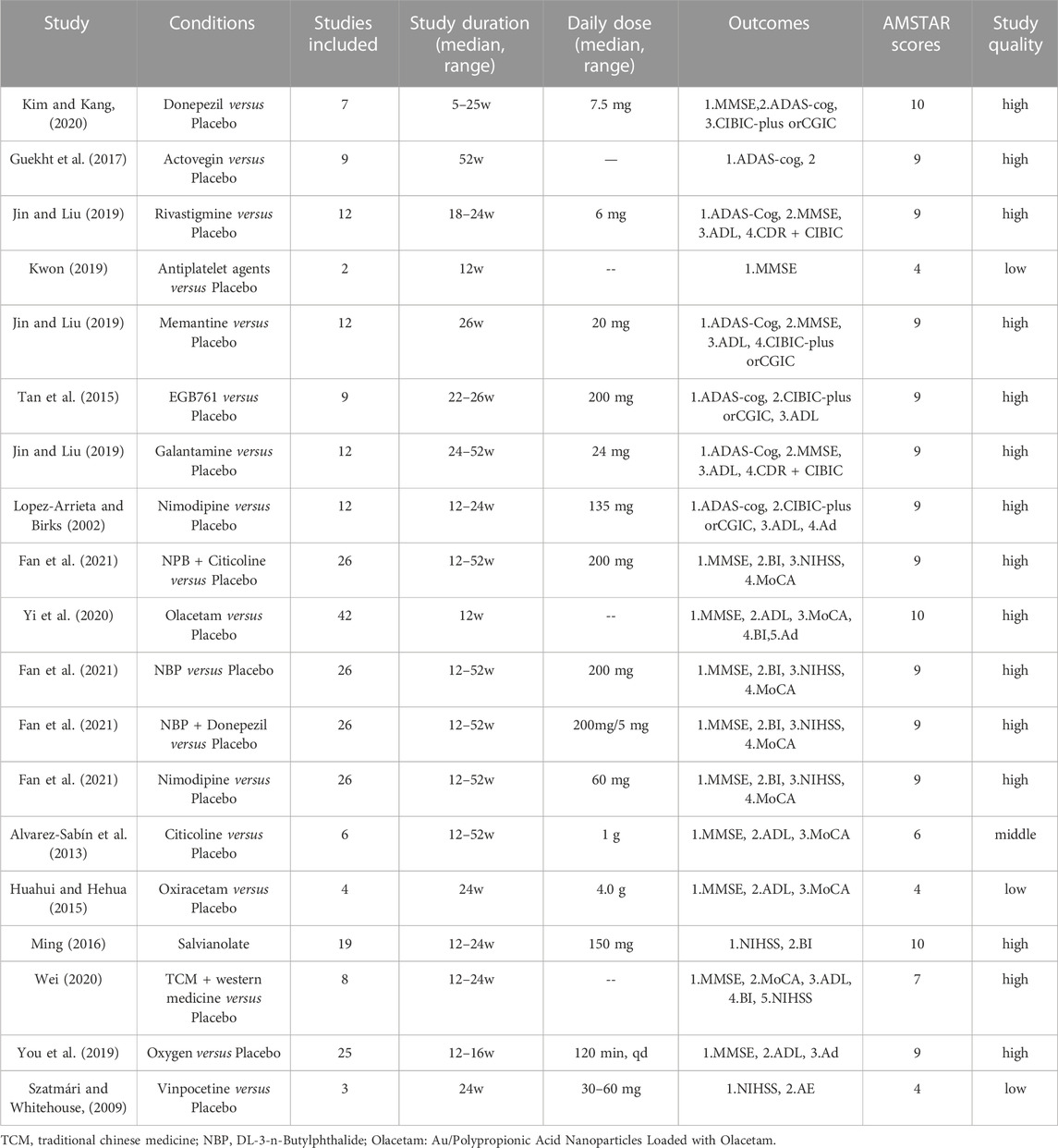
As shown in Table 1, a total of 312 clinical trials were included, with 19 drugs or drug combination therapies in the treatment groups. All studies were randomized controlled clinical trials, and the treatment duration ranged from 1 to 52 weeks. Among the included literature, there were 17 that were considered of high quality, 1 that was of moderate quality, and 1 that was of low quality.
MMSE score
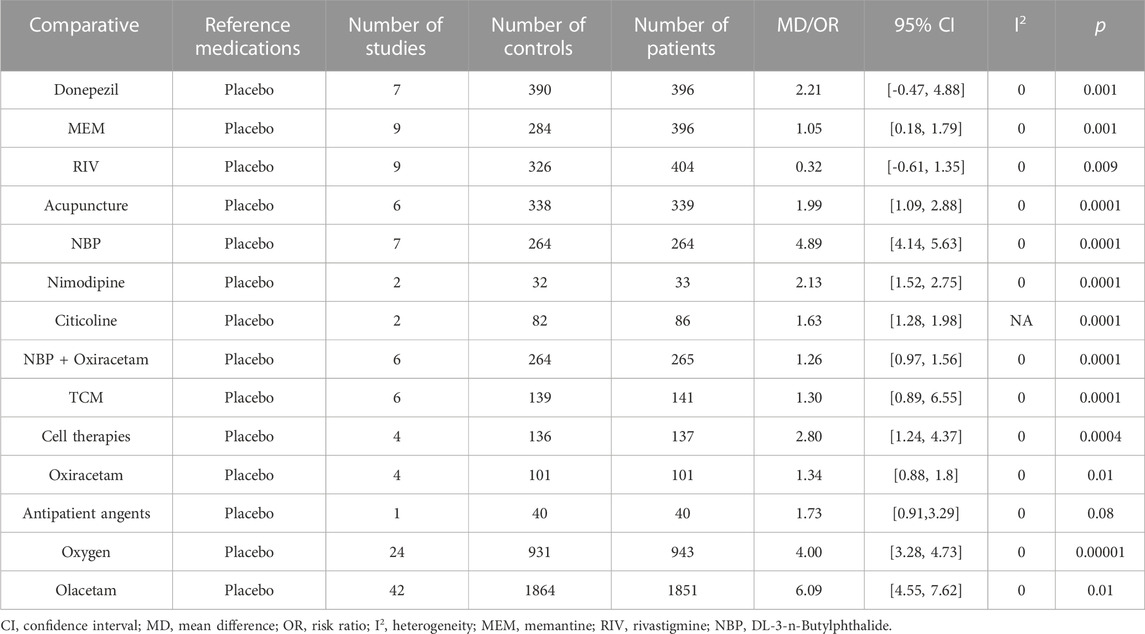
For our search, the mini-mental state examination (MMSE) score was used to assess the effects of the medications on clinical change (Table 2). A total of 15 studies (79.0%) including Donepezil (MD: 2.21, 95% CI: −0.466 to 4.882, p < 0.001), MEM (MD: 1.05, 95% CI: 0.18 to 1.79, p < 0.001), RIV (MD: 0.32, 95% CI: −0.61 to 1.35, p < 0.009), Acupuncture (MD: 1.99, 95% CI: 1.09 to 2.88, p < 0.0001), NBP (MD: 4.89, 95% CI: 4.14 to 5.63, p < 0.0001), NBP + Nimodipine (MD: 2.13, 95% CI: 1.52 to 2.75, p < 0.0001), Citicoline (MD: 1.63, 95% CI: 1.28 to 1.98, p < 0.0001), NBP + Oxiracetam (MD: 1.26, 95% CI: 0.97 to 1.56, p < 0.0001), traditional Chinese medicine (TCM) + Western medicine (MD: 3.72, 95% CI: 0.45 to 2.15, p < 0.003), Oxiracetam (MD: 1.34, 95% CI: 0.88, 1.8, p < 0.01), oxygen (MD: 4.0, 95% CI: 3.28 to 4.73, p < 0.00001), and Olacetam (MD = 6.09, 95% CI: 4.55 to 7.62, p < 0.01) showed better outcomes for MMSE score compared to placebo. The other treatments “anti-patient agents (MD = 1.73, 95% CI: 0.91 to 3.29, p = 0.08)” indicated no significant difference in effectiveness as compared to placebo.
NIHSS score
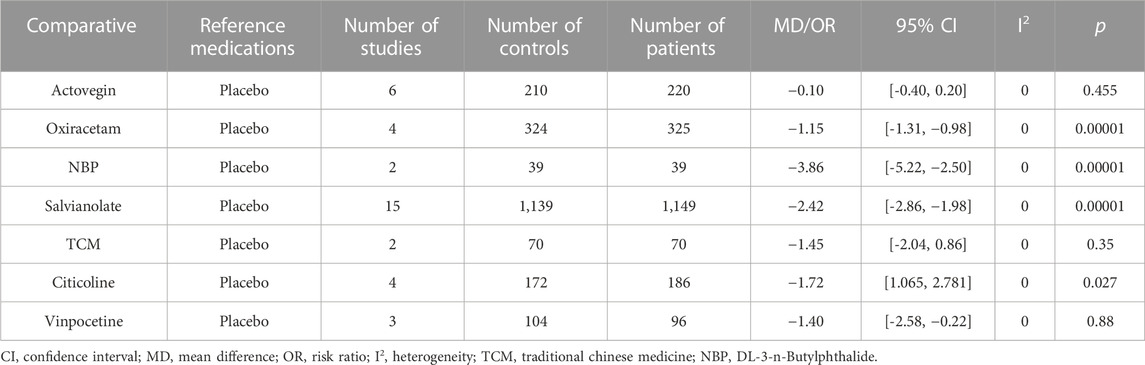
The National Institutes of Health Stroke scale was used to assess the effects of the medications on clinical change (Table 3). Seven studies (36.8%) showed that Citicoline (MD: −1.82, 95% CI: −2.25 to −1.40, p < 00001), Oxiracetam (MD: −1.15, 95% CI: −1.31, −0.98, p < 00001), NBP (MD: −3.86, 95% CI: 5.22 to −2.50, p < 0.00001), and salvianolate (MD: −2.42, 95% CI: −2.86 to −1.98, p < 00001) were significantly different compared with placebo. In contrast, Actovegin (MD: −0.1, 95% CI: −0.4 to 0.2, p < 0.455), TCM (MD: −1.45, 95% CI, −2.04 to −0.86, p < 0.35), and Citicoline (MD: 1.721, 95% CI: 1.065 to 2.781, p < 0.27) showed no change or deterioration.
Barthel index score
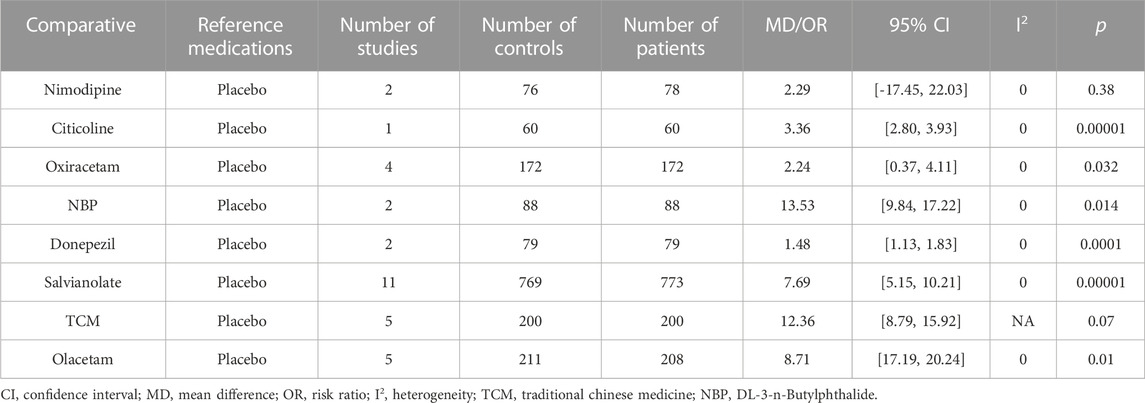
The Barthel Index (BI) score was used to assess the effects of the medications on clinical change (Table 4). Eight studies (42.1%) showed that Citicoline (MD: 3.36, 95% CI, 2.80, 3.93, p < 0.00001), Oxiracetam (MD: 2.24, 95% CI, 0.37, 4.11, p < 0.032), NBP (MD: 13.53, 95% CI: 9.84, 17.22, p < 0.014), Donepezil (MD: 1.48, 95% CI, 1.13 to 1.83, p < 0.00001), salvianolate (MD: 7.68, 95% CI: 5.15∼10.21, p < 0.00001), TMS (MD: 9.72, 95% CI: 6.78 to 12.66, p < 0.00001), and Olacetam (MD = 8.71, 95% CI (17.19, 20.24), p <0.01) were significantly different compared with placebo. In contrast, TCM (MD: 12.36, 95% CI: 8.79 to 15.92, p = 0.07) and Nimodipine (MD: 2.29, HKSJ 95% CI, −17.45 to 22.03, p = 0.380), showed no difference compared to placebo.
ADL score
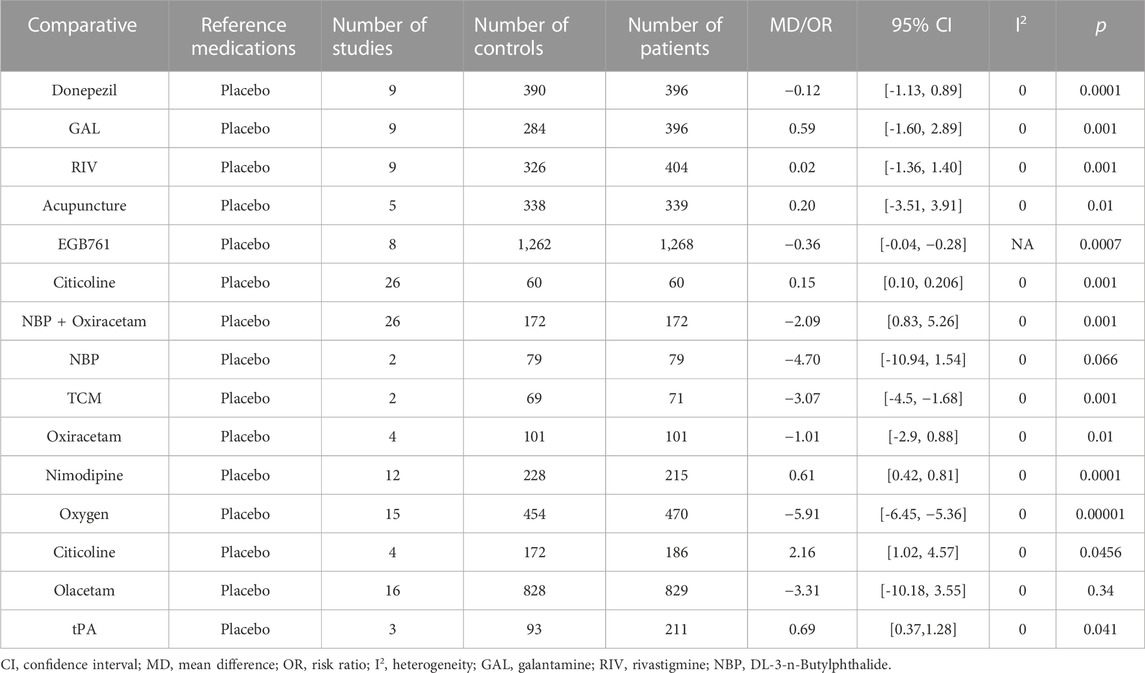
Table 5 presents the results of the comparisons of behavioral symptoms; a total of 15 studies were assessed by ADL scores. Patients treated with comparative Donepezil (MD: −0.12, 95% CI: −1.13 to 0.89, p < 0.0001), GAL (MD: 0.59, 95% CI: −1.60 to 2.89, p < 0.001), RIV (MD: 0.02, 95% CI: −1.36 to 1.40, p < 0.001), acupuncture (MD: 0.20, 95% CI: −3.51 to 3.91, p < 0.01), EGB761 (MD: −0.36, 95% CI: −0.04 to −0.28, p < 0.0007), Citicoline (MD: 0.15, 95% CI: 0.1 to 0.206, p < 0.001), NBP + Oxiracetam (MD: −2.09, 95% CI: 0.83 to 5.26, p < 0.001), TCM (MD: −3.07, 95% CI: −4.5 to −1.68, p < 0.001), Oxiracetam (MD: −1.01, 95% CI: −2.9 to 0.88, p < 0.01), Nimodipine (MD 0.61, 95% CI0.42 to 0.81, p < 0.00001), oxygen (MD: −5.91; 95% CI = −6.45, −5.36, p < 0.00001), tPA (OR = 0.69, 95% CI: 0.37 to 1.28, p < 0.041), and Citicoline (OR = 2.155, 95% CI: 1.017 to 4.566, p < 0.045) showed better behavioral symptoms than those administered a placebo (p < 0.05). Moreover, EGB761 use also improved the activities of daily living and functional outcomes (MD: 9.52; 4.66 to 14.33, p < 0.001). Subgroup analysis results suggest that the injectable formulation of EGB761 has more impact compared to the oral formulation. The other treatments indicated that there is no significant difference in effectiveness compared to placebo (p > 0.05), NBP (MD: −4.70, 95% CI: −10.94 to 1.54, p = 0.066), and Olacetam (MD = −3.31, 95% CI: −10.18 to 3.55, p = 0.34).
CIBIC-plus or CGIC score
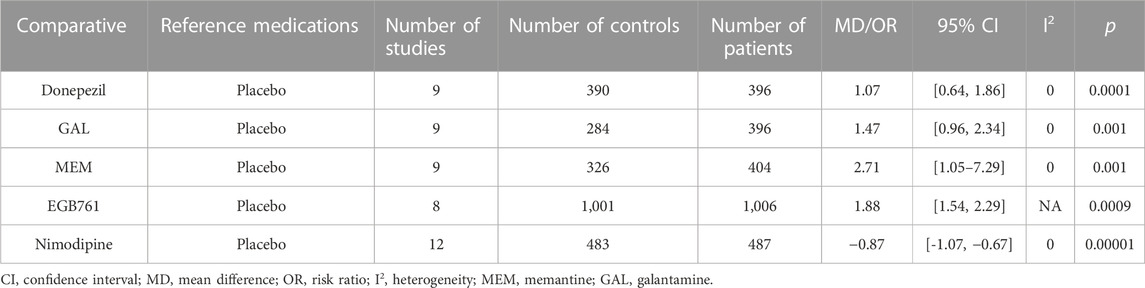
The CIBIC-plus or CGIC score from the administration of other treatments was mild, whereas the CIBIC-plus or CGIC scores were significantly different between placebo groups and the following groups: Donepezil (MD: 1.07, 95% CI: 0.64 to 1.86, p < 0.0001), GAL (MD: 1.47, 95% CI: 0.96 to 2.34, p < 0.001), MEM (MD: 2.78, 95% CI: 1.05–7.29, p < 0.001), EGB761 (MD: 1.88, 95% CI: 1.54 to 2.29, p < 0.0009), and Nimodipine (MD: −0.87, 95% CI: −1.07 to −0.67, p < 0.00001) (Table 6).
ADAS-cog score
For our search, the ADAS-cog score was used to assess the effects of the medications on clinical change. Seven studies (36.8%) including Donepezil (MD: −0.76, 95% CI: −2.104, 0.578, p < 0.001), Actovegin (MD: −3.70, 95% CI: −5.5 to −1.9, p < 0.001), GAL (MD: −1.67, 95% CI: −3.36 to −0.06, p < 0.0001), MEM (MD: −2,17, 95% CI: −3.91 to −0.53, p < 0.0001), RIV (MD: −0.28, 95% CI: −1.89 to 1.82, p < 0.0001), EGB761 (MD: −2.86, 95% CI: −3.18 to −2.54, p < 0.00001), and Nimodipine (MD: −7.59, 95% CI: −9.87 to −5.31, p < 0.0001) showed better outcomes for ADAS-cog score compared to placebo treatment (Table 7).
MoCA-cog score
MoCA-cog score was observed in 18 studies. Detailed information on included studies is listed in Table 2. The clinical effect of Actovegin (MD: 1.0, 95% CI: 0.3 to 1.7, p < 0.03), NBP (MD: 1.05, 95% CI: 0.69 to 1.42, p < 0.00001), Nimodipine (MD: 0.90, 95% CI: 0.46, 1.33, p < 0.0001), Donepezil (MD: 1.04, 95% CI: 0.71 to 1.38, p < 0.00001), Oxiracetam (MD: 0.81, 95% CI: 0.62 to 1.01, p < 0.00001), and Oxiracetam (MD: −1.01, 95% CI: −2.9 to 0.88, p < 0.01) was significantly better compared with placebo treatment. Moreover, the combination use of TCM and olanzapine (MD = 4.32, 95% CI: 2.03–6.61, p <0.01) showed a significant increase in the overall clinical efficacy rate compared to TCM use alone (Table 8).
Adverse events
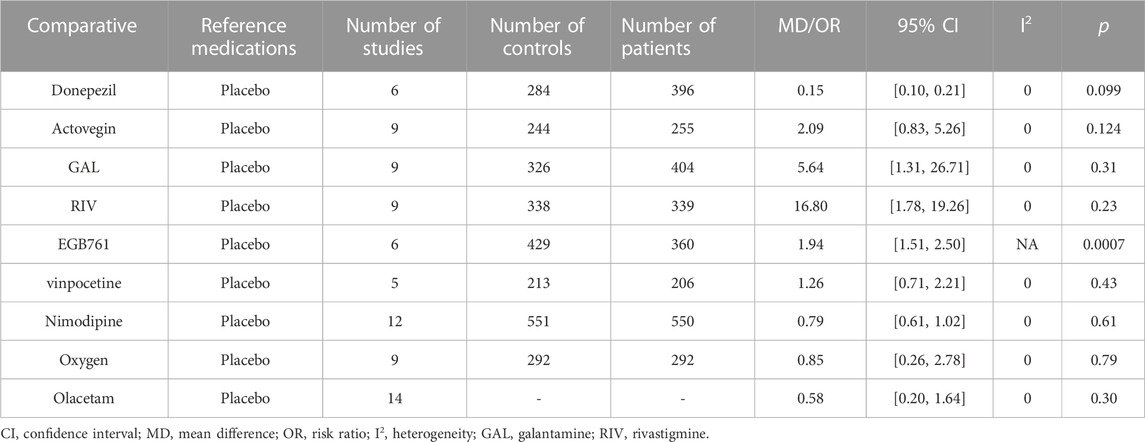
The meta-analysis of Donepezil (MD: 0.15, 95% CI: 0.100 to 0.206, p < 0.099), Actovegin (MD: 2.09, 95% CI: 0.83 to 5.26, p < 0.124), GAL (MD: 5.64, 95% CI: 1.31 to 26.71, p < 0.31), RIV (MD: 16.8, 95% CI: 1.78 to 19.26, p < 0.23) vinpocetine (MD: 1.26, 95% CI: 0.71 to 2.21, p < 0.43), Nimodipine (MD: 0.79, 95% CI: 0.61 to 1.02, p < 0.61), oxygen (MD: 0.85, 95% CI: 0.26 to 2.78, p < 0.79), and Olacetam (OR = 0.58, 95% CI: 0.20 to 1.64, p = 0.30) were no significant differences in adverse events between these groups and placebo groups (p > 0.05) (Table 9). Among all of the trials, in the EGB761 (MD: 1.94, 95% CI: 1.51 to 2.50, p < 0.0007) groups, six cases of hypotension, four cases of fever, two cases of flushing, two cases of vomiting, one case of headache, one case of arrhythmia, and one case of pruritus were reported. In addition, no deaths and two serious adverse events were reported in the Actovegin group.
Discussion
The data used in our umbrella review was from patients undergoing treatment for cognitive impairment after stroke and was used to assess the relative effectiveness and safety of these treatments. The data from published systematic reviews and meta-analyses were summarized to determine the treatment that was the most beneficial and effective for patients. Our study showed that ACEI inhibitors and NMDA antagonists, stem cell-based therapies, EGB761, and acupuncture can improve neurological deficits and activities of daily living in patients with PSCI. Antiplatelet agents (aspirin and clopidogrel), thrombolytic therapy (tPA), Oxiracetam, Citicoline, Vinpocetine, Actovegin, DL-3-n-Butylphthalide, and Nimodipine have little effect or no difference on neurological deficits or daily activities. In addition, there were no serious adverse events during treatments by ACEI inhibitors and NMDA antagonists, EGB761, and acupuncture. Interpretation of the study results requires caution to determine the best treatment strategy for patients with PSCI.
Cholinergic and neurotransmitters are vulnerable to vascular damage, which leads to cognitive impairment (Damodaran T et al., 2019). It is known that acetylcholinesterase inhibitors compensate for cerebral cholinergic neurotransmitter deficiency by inhibiting acetylcholine hydrolysis to regulate cognitive function, and that it is an effective treatment for PSCI and vascular dementia patients. The effects of ACEI inhibitors and NMDA antagonists may be considerable and there is no cure for current treatment, but other drugs that may slow the progression of PSCI patients are worth exploring. Previous studies showed that ACEI inhibitors and NMDA antagonists are beneficial for PSCI (Jongstra et al., 2016; Saini et al., 2021). In addition, one study showed that ACEI inhibitors-Donepezil showed the best performance (Kim and Kang, 2020). It is suggested by our results that neurological dysfunction and activities of daily living in people with PSCI can be improved by all ACEI inhibitors and NMDA antagonists compared to placebo treatment. Studies have shown that NMDA antagonists have led to the best-observed effects on PSCI. In our study, it was observed that NMDA antagonists treatment improved clinical effect significantly compared to placebo treatment. It is demonstrated that acupuncture has shown remarkable efficacy in PSCI (Huang et al., 2021). Our review mainly selected clinical studies to demonstrate short-term efficacy on neurological function, while PSCI is a progressive disease. Long-term clinical trials are ethically questionable, and high-quality clinical trials are critical to reveal differences in the treatment of PCSI by different treatments.
Behavioral symptoms in patients with PSCI are usually assessed by MMSE ADL/NIHSS/BI/MoCA, which assesses the severity and frequency of neuropsychiatric symptoms. Patients with PSCI progressively worsen with degrees of other disease, and the pooled data results may be affected by this. Therefore, previous meta-analysis has reported that the efficacy of stem cell-based therapies may be related to the severity of PSCI. In addition, ACEI inhibitors and NMDA antagonists and acupuncture can improve neurological dysfunction and activities of daily living in patients with PSCI. TCM was only moderate therapeutic effect on PSCI (Tan et al., 2015; Gou et al., 2020; Wen-Yue et al., 2020). In our study, Actovegin was more effective in the rate of neurological improvement compared to a placebo. However, the lack of placebo controls in NIHSS/BI score studies may result in a reduction in validity. Moreover, nimodipine can improve clinical outcomes to some extent, but it does not significantly reduce the incidence rate of adverse reactions. In addition, Donepezil affected MMSE/MoCA/ADL. Moreover, we considered treatment that showed better clinical efficacy and safety. Antiplatelet agents (aspirin and clopidogrel), thrombolytic therapy (tPA), Oxiracetam, Citicoline, Actovegin, Nimodipine, and NBP did not affect neurological deficits and daily activities due to a lack of statistical significance of the results.
Previous meta-analyses have shown that patients who were treated with cell therapies received a modest and better improvement in clinical effect. In addition, the results of both short-term and long-term analysis suggest that a combination of drugs shows a statistically significant advantage over placebo. The effect of TCM use only is not ideal (Birks and Grimley Evans, 2009; Gou et al., 2020; Kim and Kang, 2020; Wen-Yue et al., 2020), however, it is better when used in combination with Western medicine (Gou et al., 2020; Wen-Yue et al., 2020). Furthermore, antiplatelet agents (aspirin and clopidogrel), thrombolytic therapy (tPA), Oxiracetam, and NBP may play an important role in increasing the neurological function or daily activities of patients with PSCI. In this study, the AMSTAR2 scores were low for antiplatelet agents, vinpocetine, Oxiracetaman, and Citicoline in the systematic reviews analyzed, indicating that these might not be of importance to neurological function or daily activities. Further analysis is needed to elucidate the factors associated with the placebo effect increasing over time in global clinical trials.
In the treatment of PSCI, a critical issue is the safety of the treatments on a long-term basis. We extracted at least one adverse effect, such as diarrhea, nausea, gastrointestinal, cardiovascular, and other disorders. Previous meta-analyses have suggested that patioents with PSCI receiving 10 mg of donepezil (odds ratio (OR) = 3.04, 95% CI: 1.86–5.41) are at a higher risk of adverse events than those under a placebo treatment. Galantamine (OR = 5.64, 95% CI: 1.31–26.71) was associated with an increased risk of nausea. Rivastigmine (OR = 16.80, 95% CI: 1.78–319.26) was associated with an increased risk of vomiting. Moderate-certainty evidence showed that fewer people taking Memantine experienced agitation as an adverse event: RR 0.81 (95% CI 0.66–0.99) (25 fewer people per 1,000, 95% CI 1 to 44 fewer). There is also moderate-certainty evidence suggesting that Memantine is not beneficial as a treatment for agitation from three additional studies (e.g., Cohen Mansfield Agitation Inventory: clinical benefit of 0.50 CMAI points, 95% CI −3.71–4.71) (Gou et al., 2020). Moreover, it was found that statins were effective in the prevention of PSCI by actively lowering cholesterol in the study. Thrombolytic use of statins improves the overall situation, despite an increased risk of bleeding conversion. Recent studies have also linked statins to atrial fibrillation. In addition, neuroprotective drugs that promote collateral circulation may be related to the induction of vascular endothelial NO synthesis and angiogenesis (Fan et al., 2021). In addition, the rate of discontinuation due to adverse events tended to be higher in the salvianolic acid, tPA, NBP, and Nimodipine treatment groups than in placebo groups. Our study summarized that ACEI inhibitors and NMDA antagonists, stem cell-based therapies, acupuncture, and TCM plus Western medicine show no serious adverse events in patients with PSCI.
In general, the treatment for patients with PSCI is aimed at promoting independence, maintaining function, and treating symptoms. Previous meta-analyses and reviews have focused on the possible effectiveness and safety of AChEIs and NMDA antagonists (memantine) (Ebihara et al., 2007; Cao et al., 2013; Tan et al., 2015; You et al., 2019; Gou et al., 2020; Kim and Kang, 2020; Wen-Yue et al., 2020; Saini et al., 2021). As a result, we need to identify an efficacious and safe treatment paradigm for patients with PSCI. Studies have shown that ACEI inhibitors and NMDA antagonists, cell therapies, acupuncture, and Western medicine plus EGB761 improved neurological deficits and activities of daily living, and the adverse effects were mild for the treatment of PSCI. However, a larger sample size and long-term follow-up are needed to find the reliability of this treatment. Due to the efficacy of Donepezil, memantine, cell therapies, and Western medicine plus TCM in improving neurological deficits and activities of daily living, we suggest that Donepezil, memantine, and Donepezil plus TCM can be employed as first-line treatment.
Limitations
The limitations of this study should be acknowledged. First, direct comparative evidence of treatments for patients with PSCI in our included studies was limited. Second, other factors, such as the duration and quality of studies, may have led to inconsistencies in the umbrella review. Furthermore, a considerable number of studies could not be included as they did not have the abovementioned data.
Conclusion
Our study demonstrated that ACEI inhibitors (Donepezil) and NMDA antagonists (Memantine), EGB761, and acupuncture are the ADL and BI, MoCA, and neurological function deficit medication/therapy, respectively, for patients with PSCI. In the future, the combination of well-tolerated agents and other significant beneficial treatments should be used for patients with PSCI, which will contribute to the successful construction of a similar study.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Author contributions
YL, RC, and SL collected the data. YL, WS, ZQ, and RC analyzed the data and prepared tables. YL and RC wrote the manuscript. QL and YC designed the research. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (82071676 and 82174085) and the High-Level Hospital Development Program for Foshan “Climbing” Project (BZKY2022054).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alvarez-Sabin, J., Ortega, G., Jacas, C., Santamarina, E., Maisterra, O., Ribo, M., et al. (2013). Long-term treatment with citicoline may improve poststroke vascular cognitive impairment. Cerebrovasc. Dis. 35 (2), 146–154. doi:10.1159/000346602
Birks, J., and Grimley Evans, J. (2009). Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst. Rev. 1, CD003120. doi:10.1002/14651858.CD003120.pub2
Cao, H., Wang, Y., Chang, D., Zhou, L., and Liu, J. (2013). Acupuncture for vascular mild cognitive impairment: a systematic review of randomised controlled trials. Acupunct. Med. 31 (4), 368–374. doi:10.1136/acupmed-2013-010363
Damodaran, T., Müller, C. P., and Hassan, Z. (2019). Chronic cerebral hypoperfusion-induced memory impairment and hippocampal long-term potentiation deficits are improved by cholinergic stimulation in rats. Pharmacol. Rep. Jun 71 (3), 443–448. doi:10.1016/j.pharep.2019.01.012
De Santis, K. K., Lorenz, R. C., Lakeberg, M., and Matthias, K. (2022). The application of AMSTAR2 in 32 overviews of systematic reviews of interventions for mental and behavioural disorders: a cross-sectional study. Res. Synth. Methods 13 (4), 424–433. doi:10.1002/jrsm.1532
Ebihara, T., Ohrui, T., Ebihara, S., Tsuji, I., Sasaki, H., and Arai, H. (2007). The benefit of angiotensin converting enzyme inhibitor for geriatric syndrome in the elderly]. Nihon Ronen Igakkai Zasshi 44 (4), 448–451. doi:10.3143/geriatrics.44.448
Fan, X., Shen, W., Wang, L., and Zhang, Y. (2021). Efficacy and safety of DL-3-n-butylphthalide in the treatment of poststroke cognitive impairment: a systematic review and meta-analysis. Front. Pharmacol. 12, 810297. doi:10.3389/fphar.2021.810297
Glass, O. M., Hermida, A. P., Hershenberg, R., and Schwartz, A. C. (2020). Considerations and current trends in the management of the geriatric patient on a consultation-liaison service. Curr. Psychiatry Rep. 22 (5), 21. doi:10.1007/s11920-020-01147-2
Gou, J., Yang, H. X., Yu, Y., and Guo, R. J. (2020). Systematic review and Meta-analysis of efficacy and safety of Compound Congrong Yizhi Capsules on vascular cognitive impairment]. Zhongguo Zhong Yao Za Zhi 45 (8), 1924–1932. doi:10.19540/j.cnki.cjcmm.20190902.502
Guekht, A., Skoog, I., Edmundson, S., Zakharov, V., and Korczyn, A. D. (2017). ARTEMIDA trial (A randomized trial of efficacy, 12 Months international double-blind Actovegin): a randomized controlled trial to assess the efficacy of Actovegin in poststroke cognitive impairment. Stroke 48 (5), 1262–1270. doi:10.1161/STROKEAHA.116.014321
Huahui , , and Hehua, W. (2015). Analysis on clinical efficacy and safety of oxiracetam for the treatment of cognitive dysfunction after stroke LE. Jiangxi Medical Journal 50 (7). doi:10.3969/j.issn.1006-2238.2015.07.012
Huang, L. C., Hsieh, S. W., Tsai, C. C., Chen, C. H., and Yang, Y. H. (2021). The role of cilostazol and inflammation in cognitive impairment after ischemic stroke. Am. J. Alzheimers Dis. Other Demen 36, 15333175211016185. doi:10.1177/15333175211016185
Jin, B. R., and Liu, H. Y. (2019). Comparative efficacy and safety of cognitive enhancers for treating vascular cognitive impairment: systematic review and Bayesian network meta-analysis. Neural Regen Res. 14 (5), 805–816. doi:10.4103/1673-5374.249228
Jongstra, S., Harrison, J. K., Quinn, T. J., and Richard, E. (2016). Antihypertensive withdrawal for the prevention of cognitive decline. Cochrane Database Syst. Rev. 11, CD011971. doi:10.1002/14651858.CD011971.pub2
Kim, T. H., and Kang, J. W. (2020). Herbal medicine for vascular dementia: an overview of systematic reviews. Curr. Vasc. Pharmacol. 18 (4), 394–409. doi:10.2174/1570161117666190618164443
Kwon, H. S., Lee, D., Lee, M. H., Yu, S., Lim, J. S., and Yu, K. H. (2020). Post-stroke cognitive impairment as an independent predictor of ischemic stroke recurrence: PICASSO sub-study. J Neurol. 267 (3), 688–693. doi:10.1007/s00415-019-09630-4
Liraz-Zaltsman, S., Slusher, B., Atrakchi-Baranes, D., Rosenblatt, K., Friedman Levi, Y., Kesner, E., et al. (2018). Enhancement of brain d-serine mediates recovery of cognitive function after traumatic brain injury. J. Neurotrauma 35 (14), 1667–1680. doi:10.1089/neu.2017.5561
Lopez-Arrieta, J. M., and Birks, J. (2002). Nimodipine for primary degenerative, mixed and vascular dementia. Cochrane Database Syst. Rev. 3, CD000147. doi:10.1002/14651858.CD000147
Malykh, A. G., and Sadaie, M. R. (2010). Piracetam and piracetam-like drugs: from basic science to novel clinical applications to CNS disorders. Drugs 70 (3), 287–312. doi:10.2165/11319230-000000000-00000
Mijajlovic, M. D., Pavlovic, A., Brainin, M., Heiss, W. D., Quinn, T. J., Ihle-Hansen, H. B., et al. (2017). Post-stroke dementia - a comprehensive review. BMC Med. 15 (1), 11. doi:10.1186/s12916-017-0779-7
Ming, Z., Jiaming, B., and Guoxiang, T. (2016). The effect of salvianolate on motor and cognitive function of patients with acute cerebral infarction: a systematic review. Chin. J. Evid. Based Cardiovasc. Med. 8 (11). doi:10.3969/j.issn.1674-4055.2016.11.06
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., and Group, P. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern Med. 151 (4), 264–269. doi:10.7326/0003-4819-151-4-200908180-00135
NanZhu, Y., AiChun, J., Xin, L., and XiangHua, Y. (2018). Salvianolate injection in the treatment of acute cerebral infarction: a systematic review and a meta-analysis. Med. Baltim. 97 (47), e12374. doi:10.1097/MD.0000000000012374
Saini, V., Guada, L., and Yavagal, D. R. (2021). Global epidemiology of stroke and access to acute ischemic stroke interventions. Neurology 97 (Suppl. 2), S6–S16. doi:10.1212/WNL.0000000000012781
Shi, X., Ren, G., Cui, Y., and Xu, Z. (2022). Comparative efficacy and acceptability of cholinesterase inhibitors and memantine based on dosage in patients with vascular cognitive impairment: a network meta-analysis. Curr. Alzheimer Res. 19 (2), 133–145. doi:10.2174/1567205019666220120112301
Szatmari, S. Z., and Whitehouse, P. J. (2003). Vinpocetine for cognitive impairment and dementia. Cochrane Database Syst. Rev. 1, CD003119. doi:10.1002/14651858.CD003119
Tan, M. S., Yu, J. T., Tan, C. C., Wang, H. F., Meng, X. F., Wang, C., et al. (2015). Efficacy and adverse effects of ginkgo biloba for cognitive impairment and dementia: a systematic review and meta-analysis. J. Alzheimers Dis. 43 (2), 589–603. doi:10.3233/JAD-140837
Wang, Z., Wei, X., Yang, J., Suo, J., Chen, J., Liu, X., et al. (2016). Chronic exposure to aluminum and risk of alzheimer's disease: a meta-analysis. Neurosci. Lett. 610, 200–206. doi:10.1016/j.neulet.2015.11.014
Wei, S., Zixiu, Z., and Xianglan, J. (2016). Systematic evaluation of clinical efficacy and safety of traditional Chinese medicine for post stroke cognitive impairment. Chinese Journal of Experimental Traditional Medical Formulae 26 (11). doi:10.13422/j.cnki.syfjx.20201151
Wen-Yue, H. U., Zhen-Yun, H., Hua-Ping, M. A., Jing-Feng, L., and Ze, C. (2020). Systematic evaluation and Meta-analysis of efficacy and safety of Tianzhi Granules in treatment of vascular cognitive impairment]. Zhongguo Zhong Yao Za Zhi 45 (19), 4766–4775. doi:10.19540/j.cnki.cjcmm.20200309.501
Yang, J. L., Lin, C. M., and Hsu, Y. L. (2022). Long-term functionality prediction for first time ischemic middle cerebral artery stroke patients receiving conventional medical treatment. Neuropsychiatr. Dis. Treat. 18, 275–288. doi:10.2147/NDT.S350266
Yi, Z., Liang, W., Ruan, W., Hua, W., and Lin, X. (2020). A meta-analysis of Au/polypropionic acid Nanoparticles loaded with Olacetam for the treatment of vascular cognitive impairment. J. Nanosci. Nanotechnol. 20 (12), 7433–7438. doi:10.1166/jnn.2020.18863
You, Q., Li, L., Xiong, S. Q., Yan, Y. F., Li, D., Yan, N. N., et al. (2019). Meta-analysis on the efficacy and safety of hyperbaric oxygen as adjunctive therapy for vascular dementia. Front. Aging Neurosci. 11, 86. doi:10.3389/fnagi.2019.00086
Keywords: post-stroke cognitive impairment, clinical trial, systematic review, umbrella review, neurological functional
Citation: Li Y, Cui R, Liu S, Qin Z, Sun W, Cheng Y and Liu Q (2023) The efficacy and safety of post-stroke cognitive impairment therapies: an umbrella review. Front. Pharmacol. 14:1207075. doi: 10.3389/fphar.2023.1207075
Received: 17 April 2023; Accepted: 25 July 2023;
Published: 24 August 2023.
Edited by:
Jiansong Fang, Guangzhou University of Chinese Medicine, ChinaReviewed by:
Sen Li, China Academy of Chinese Medical Science, ChinaJinlong Cui, Shanxi University, China
Copyright © 2023 Li, Cui, Liu, Qin, Sun, Cheng and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingshan Liu, bmxxc2hAMTYzLmNvbQ==; Yong Cheng, eW9uZ2NoZW5nQG11Yy5lZHUuY24=
†These authors have contributed equally to this work
 Yongbiao Li
Yongbiao Li Ruyi Cui
Ruyi Cui Shaobo Liu1
Shaobo Liu1 Yong Cheng
Yong Cheng Qingshan Liu
Qingshan Liu