- 1Department of Pediatrics, Regional Hospital, St Johann in Tirol, Austria
- 2Department for Personalized Medicine, Privatklinik Maria Hilf, Klagenfurt, Austria
- 3PharmGenetix Gmbh, Niederalm, Austria
Metoclopramide is indicated for the management of gastroesophageal reflux, gastric stasis, nausea, and vomiting. Metoclopramide-induced acute dystonic reactions (MIADRs), along with repetitive involuntary protrusion of the tongue, are well-known phenomena in children and young adults that may appear after the first dose. The drug is primarily metabolized via oxidation by the cytochrome P450 enzyme CYP2D6 and to a lesser extent by CYP3A4 and CYP1A2. A recommendation to decrease metoclopramide dosing in patients with severely limited to no CYP2D6 activity (i.e., poor metabolizers, PMs) is included in the drug label. It is important to note, however, that a requirement or recommendation for pre-emptive testing for CYP2D6 metabolizer status is not included in the drug label. We present two cases of acute dystonia in two non-consanguineous male adolescents: one following metoclopramide and cimetidine administration in a 14-year-old to treat gastroesophageal reflux, and another following metoclopramide and pantoprazole administration in a 17-year-old with acute gastroenteritis. A retrospective pharmacogenetic analysis revealed both patients as CYP2D6 PMs.
Introduction
Metoclopramide is a dopamine receptor antagonist approved for the management of gastrointestinal distress (nausea, vomiting, etc.) with an i) increase in the lower esophageal sphincter pressure, ii) increase in the amplitude of both the esophageal and gastric antrum peristalsis, iii) relaxation of the pyloric sphincter, and iv) increase in the small-bowel transit via its significant modulation of the cholinergic nervous system (Schulze-Delrieu, 1981; Desmond and Watson, 1986). The drug also has a central effect on the chemoreceptor trigger zone and effects on the release of various hormones (Schulze-Delrieu, 1981; Desmond and Watson, 1986). It is rapidly absorbed into the gastrointestinal tract with a bioavailability varying between 35% and 100% depending on the extent of first-pass metabolism, the elimination half-life varies between 3 and 6 hours, and protein binding is low (Desmond and Watson, 1986). Metoclopramide metabolism involves oxidation (by the cytochrome P450 enzymes CYP2D6, CYP3A4, and CYP1A2) and, albeit to a lesser extent, glucuronide and sulfate conjugation by the UDP-glucuronosyltransferase (UGT) and sulfotransferase (SULT) (Argikar et al., 2010; Parkman et al., 2012; Livezey et al., 2014; Ge et al., 2020).
Metoclopramide-induced acute dystonic reactions (MIADRs), along with repetitive involuntary protrusion of the tongue, is a well-known phenomenon in children and young adults that may appear after the first dose, and includes involuntary movements of limbs, facial grimacing, torticollis, oculogyric crisis, bulbar type of speech, trismus, opisthotonus (tetanus-like reactions), and, rarely, stridor and dyspnea possibly due to laryngospasm (Bateman et al., 1989).
It is well known that underlying single nucleotide variants (SNVs) and other structural variations in CYP2D6 contribute to altered metabolizing capacities for this enzyme in some individuals (Nofziger et al., 2020). As such, oxidative metoclopramide metabolism could be reduced in patients without full CYP2D6 enzyme function. This is supported by the recommendation within the drug’s label that its dose should be reduced in CYP2D6 PMs, other case reports describing metoclopramide-induced adverse side effects in CYP2D6 PMs (van der Padt et al., 2006; Chua et al., 2019), and pharmacokinetic studies of metoclopramide and CYP2D6 (Bae et al., 2020).
Here, we describe additional cases of MIADR in adolescent CYP2D6 PMs.
Case description
Case 1
A 14-year-old, 62 kg male of Caucasian ancestry was admitted to the hospital with suspected seizures. He presented cooperative and fully conscious but with slurred speech and facial hypotonia with open mouth most of the time and repeated involuntary protrusion of the tongue (Figure 1). In addition, he complained of dystonic muscular spasms in the left side of the neck with intermittent retroflexion of the head. Because of the negation of any drug intake, the differential diagnostic spectrum included neurologic (paroxysmal dystonia) and psychiatric disease. A 3.4 mg oral dose of diazepam terminated the bizarre clinical episode. Then, 12 h later, after a calm night, the patient was well and without any clinical signs of disease. Only now the patient disclosed that 3 days before the hospital admission, he was started on metoclopramide (10 mg twice daily) and cimetidine (400 mg once daily) for gastroesophageal reflux. Exposure to metoclopramide prior to this case was not reported. A retrospective pharmacogenetic analysis was recommended to identify the reason for the suspected seizures and dystonia, and revealed a CYP2D6 diplotype of *68+*4/*5, which confers a PM phenotype (no residual function of CYP2D6).
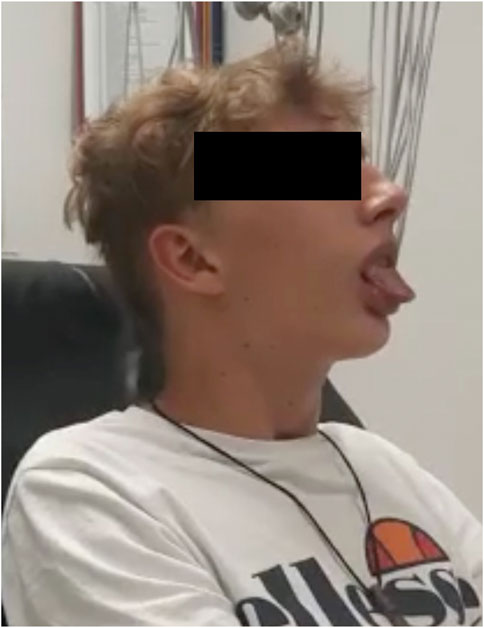
FIGURE 1. Visual example of facial hypotonia (with open mouth most of the time and repeated involuntary protrusion of the tongue) from Case 1.
Case 2
A 17-year-old, 63 kg male of Caucasian ancestry with acute gastroenteritis was administered metoclopramide OTC from the pharmacy. Immediately following the first 10 mg dose, the patient vomited. The consulted physician prescribed three doses of 10 mg metoclopramide, which were taken 4 and 5 h apart, respectively, together with 40 mg pantoprazole once daily. After the second dose of metoclopramide, mild torticollis was recognized by the patient’s father. In the evening of the same day, 2 hours after the third metoclopramide dose, the patient presented at an outpatient emergency center with a stiff neck and cervical muscle spasms, abnormal eye movements, and bilateral blepharospasm. Metoclopramide-induced acute dystonia with facial grimacing, torticollis, muscle spasms, and oculogyric crisis was diagnosed. A 3.4 mg oral dose of diazepam terminated the extrapyramidal movement disorder. Exposure to metoclopramide prior to this case was not reported. A retrospective pharmacogenetic analysis revealed that this patient was also a CYP2D6 PM, with a diplotype of *4/*68+*4.
Discussion
MIADRs are well-known adverse drug reactions (ADRs) in children and young adults. The respective summary of product characteristics (SmPCs) in different regulatory environments (United States of America, EU, etc.) mentions the important role of metoclopramide metabolism for the occurrence of ADRs. Metoclopramide is predominantly metabolized by the liver enzyme CYP2D6 (Livezey et al., 2014). As such, oxidative metoclopramide metabolism is expected to be reduced in patients without CYP2D6 enzyme function (i.e., PMs), which is underscored by the recommendation from the FDA that metoclopramide dosing should be reduced in CYP2D6 PMs, and should not be co-administered with strong CYP2D6 inhibitors (U.S. Food and Drug Administration, 2017). In 2014, the European Medicines Agency (EMA) issued a press release restricting the use of metoclopramide to short-term only (up to 5 days) due to “…well-known risks of neurological effects such as short-term extrapyramidal disorders … ” and recommended that the drug should not be used in children below 1 year of age and that in older children it should be used only as a second choice treatment for chemotherapy-induced emesis (European Medicines Agency, 2013). Interestingly, the press release made no mention of dosing guidance with respect to the CYP2D6 metabolizing capacity.
It may also be important to note that the role of UGTs and SULTs, both important for the metabolism of various xenobiotics (i.e., paracetamol, anticancer drugs, etc.), in metoclopramide-related toxicities was highlighted as the main detoxifying metabolic pathways of the drug, followed by renal elimination of the more soluble metabolites (including 20% of the unchanged drug) (Ge et al., 2020). However, recent data, including that presented here from patients with no function of CYP2D6 (i.e., CYP2D6 PMs), underline the importance of CYP2D6 function for the development of metoclopramide-induced ADRs and is further substantiated by a recent case report describing metoclopramide-associated dystonia in a patient with an intermediate CYP2D6-metabolizing phenotype (Wong and Fogel, 2021), as well as a separate study showing increased metoclopramide plasma concentrations in CYP2D6 PMs and IMs compared to normal metabolizers (Bae et al., 2020). The observed frequency of CYP2D6 PMs in Europeans is as high as 8.45%, and the predicted frequency of CYP2D6 IMs in Europeans is as high as 35.3% (Gaedigk et al., 2017). However, 2 years after this publication, the method for calculating activity scores for CYP2D6 IMs changed, and the activity value of the relatively common CYP2D6*10 allele was lowered from 0.5 to 0.25 (Caudle et al., 2020). Therefore, the frequency of CYP2D6 IMs in Europeans reported by Gaedigk et al. (2017) may be underestimated.
The PM status of CYP2D6 in the aforementioned patients was likely the primary driver of the observed metoclopramide-induced acute dystonic episodes. Co-administration of cytochrome P450 inhibitors may transform NMs into IMs or PMs (phenoconversion) (Klomp et al., 2020). In both patients, the co-administration of cimetidine (inhibitor of CYP2D6, CYP3A4, and CYP1A2) and pantoprazole (inhibitor of CYP3A4), in case 1 and 2, respectively, may have further augmented the MIADR by decreasing the ability of the patients to metabolize metoclopramide (Martinez et al., 1999; Li et al., 2004).
Conclusion
With such a high percentage of the European population expected to have significant deficiencies in CYP2D6 function, the risk for developing ADRs after taking metoclopramide is notable, especially in the vulnerable population of children. Therefore, it seems warranted that the European regulatory environment adopts respective specific information regarding the importance of CYP2D6 function for the risk of development of metoclopramide-associated ADRs.
A pharmacogenetic test for CYP2D6 phenotype prediction enables the physician to adopt the prescription of drugs which are likely not well-tolerated. Based on this genetic information, dose reduction or the choice of an alternative drug might have prevented the ADRs described in this case report. In addition, whenever two or more drugs are administered simultaneously, the risk of phenoconversion should be considered.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
All authors made substantial contributions to the interpretation of the data. CN, MP, and F-MF wrote the manuscript. MB and PH treated the patients and provided clinical data. All authors contributed to the article and approved the submitted version.
Funding
PharmGenetix GmbH thanks Österreichische Forschungsförderungsgesellschaft GmbH (FFG) for support via the PGx-Next Generation Analytics Part 2 grant (FO0999891633/42175800).
Conflict of interest
CN was employed by PharmGenetix GmbH, a private laboratory providing PGx testing, reporting, and interpretation services.
The authors declare that this study received funding from the Österreichische Forschungsförderungsgesellschaft GmbH (FFG). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Argikar, U. A., Gomez, J., Ung, D., Parkman, H. P., and Nagar, S. (2010). Identification of novel metoclopramide metabolites in humans: In vitro and in vivo studies. Drug Metab. Dispos. 38 (8), 1295–1307. doi:10.1124/dmd.110.033357
Bae, J. W., Oh, K. Y., Yoon, S. J., Shin, H. B., Jung, E. H., Cho, C. K., et al. (2020). Effects of CYP2D6 genetic polymorphism on the pharmacokinetics of metoclopramide. Arch. Pharm. Res. 43 (11), 1207–1213. doi:10.1007/s12272-020-01293-4
Bateman, D. N., Darling, W. M., Boys, R., and Rawlins, M. D. (1989). Extrapyramidal reactions to metoclopramide and prochlorperazine. Q. J. Med. 71 (264), 307–311.
Caudle, K. E., Sangkuhl, K., Whirl-Carrillo, M., Swen, J. J., Haidar, C. E., Klein, T. E., et al. (2020). Standardizing CYP2D6 genotype to phenotype translation: Consensus recommendations from the clinical pharmacogenetics implementation consortium and Dutch pharmacogenetics working group. Clin. Transl. Sci. 13 (1), 116–124. doi:10.1111/cts.12692
Chua, E. W., Harger, S. P., and Kennedy, M. A. (2019). Metoclopramide-induced acute dystonic reactions may Be associated with the CYP2D6 poor metabolizer status and pregnancy-related hormonal changes. Front. Pharmacol. 10, 931. doi:10.3389/fphar.2019.00931
Desmond, P. V., and Watson, K. J. (1986). Metoclopramide--a review. Med. J. Aust. 144 (7), 366–369. doi:10.5694/j.1326-5377.1986.tb115923.x
European Medicines Agency (2013). European Medicines Agency recommends changes to the use of metoclopramide. Press release Available at: https://www.ema.europa.eu/en/news/european-medicines-agency-recommends-changes-use-metoclopramide.
Gaedigk, A., Sangkuhl, K., Whirl-Carrillo, M., Klein, T., and Leeder, J. S. (2017). Prediction of CYP2D6 phenotype from genotype across world populations. Genet. Med. 19 (1), 69–76. doi:10.1038/gim.2016.80
Ge, S., Mendley, S. R., Gerhart, J. G., Melloni, C., Hornik, C. P., Sullivan, J. E., et al. (2020). Population pharmacokinetics of metoclopramide in infants, children, and adolescents. Clin. Transl. Sci. 13 (6), 1189–1198. doi:10.1111/cts.12803
Klomp, S. D., Manson, M. L., Guchelaar, H. J., and Swen, J. J. (2020). Phenoconversion of cytochrome P450 metabolism: a systematic review. J. Clin. Med. 9 (9). doi:10.3390/jcm9092890
Li, X. Q, Andersson, T. B., Ahlstrom, M., and Weidolf, L. (2004). Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab. Dispos. 32 (8), 821–827. doi:10.1124/dmd.32.8.821
Livezey, M. R., Briggs, E. D., Bolles, A. K., Nagy, L. D., Fujiwara, R., and Furge, L. L. (2014). Metoclopramide is metabolized by CYP2D6 and is a reversible inhibitor, but not inactivator, of CYP2D6. Xenobiotica 44 (4), 309–319. doi:10.3109/00498254.2013.835885
Martinez, C., Albet, C., Agundez, J. A., Herrero, E., Carrillo, J. A., Marquez, M., et al. (1999). Comparative in vitro and in vivo inhibition of cytochrome P450 CYP1A2, CYP2D6, and CYP3A by H2-receptor antagonists. Clin. Pharmacol. Ther. 65 (4), 369–376. doi:10.1016/S0009-9236(99)70129-3
Nofziger, C., Turner, A. J., Sangkuhl, K., Whirl-Carrillo, M., Agúndez, J. A. G., Black, J. L., et al. (2020). PharmVar GeneFocus: CYP2D6. Clin. Pharmacol. Ther. 107 (1), 154–170. doi:10.1002/cpt.1643
Parkman, H. P., Mishra, A., Jacobs, M., Pathikonda, M., Sachdeva, P., Gaughan, J., et al. (2012). Clinical response and side effects of metoclopramide: Associations with clinical, demographic, and pharmacogenetic parameters. J. Clin. Gastroenterol. 46 (6), 494–503. doi:10.1097/MCG.0b013e3182522624
Schulze-Delrieu, K. (1981). Drug therapy. Metoclopramide. N. Engl. J. Med. 305 (1), 28–33. doi:10.1056/NEJM198107023050106
U.S. Food and Drug Administration (2017). Reglan (metoclopramide) [package insert]. U.S. Food and Drug Administration Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/017854s062lbl.pdf (Accessed March 30, 2023).
van der Padt, A., van Schaik, R. H., and Sonneveld, P. (2006). Acute dystonic reaction to metoclopramide in patients carrying homozygous cytochrome P450 2D6 genetic polymorphisms. Neth J. Med. 64 (5), 160–162.
Keywords: CYP2D6, poor metabolizer, metoclopramide, acute dystonia, metoclopramide-induced acute dystonic reactions, pharmacogenetics, pharmacogenomics
Citation: Fink F-M, Bognar M, Hengl P, Paulmichl M and Nofziger C (2023) Case report: metoclopramide induced acute dystonic reaction in adolescent CYP2D6 poor metabolizers. Front. Pharmacol. 14:1201566. doi: 10.3389/fphar.2023.1201566
Received: 06 April 2023; Accepted: 20 June 2023;
Published: 11 July 2023.
Edited by:
Elena García-Martín, University of Extremadura, SpainReviewed by:
Scott Mosley, University of Southern California, United StatesChad A. Bousman, University of Calgary, Canada
Copyright © 2023 Fink, Bognar, Hengl, Paulmichl and Nofziger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Charity Nofziger, Y2hhcml0eS5ub2Z6aWdlckBwaGFybWdlbmV0aXguY29t
†These authors have contributed equally to this work
 Franz-Martin Fink
Franz-Martin Fink Marta Bognar1†
Marta Bognar1† Petra Hengl
Petra Hengl Markus Paulmichl
Markus Paulmichl Charity Nofziger
Charity Nofziger