
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Pharmacol. , 17 May 2023
Sec. Inflammation Pharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1199580
This article is part of the Research Topic Women in Inflammation Pharmacology: 2023 View all 6 articles
 Katherine N. Theken1*
Katherine N. Theken1* Mengxiang Chen1
Mengxiang Chen1 D. Lucas Wall1
D. Lucas Wall1 Truongan Pham1
Truongan Pham1 Stacey A. Secreto1
Stacey A. Secreto1 Thomas H. Yoo2
Thomas H. Yoo2 Allison N. Rascon2
Allison N. Rascon2 Yu-Cheng Chang2
Yu-Cheng Chang2 Jonathan M. Korostoff2
Jonathan M. Korostoff2 Claire H. Mitchell3
Claire H. Mitchell3 Elliot V. Hersh1
Elliot V. Hersh1Introduction: Post-surgical pain following dental implant placement surgery is typically managed with non-opioid analgesics, including non-steroidal anti-inflammatory drugs (NSAIDs) and acetaminophen. However, the comparative analgesic efficacy of over-the-counter doses of non-steroidal anti-inflammatory drugs and acetaminophen in implant patients is unknown. Therefore, we compared the analgesic and anti-inflammatory effects of naproxen sodium and acetaminophen after surgical placement of one or two dental implants.
Methods: Adult patients were treated with naproxen sodium (440 mg loading dose +220 mg q8h, n = 15) or acetaminophen (1,000 mg q6h—max daily dose 3,000 mg, n = 15) for 3 days after implant placement in a randomized, double-blind design. Pain was assessed on a 0–10 scale every 20 min for 6 h after study medication treatment. Tramadol (50 mg) was available as a rescue medication. Plasma and gingival crevicular fluid (GCF) were collected prior to the surgery and 0, 1, 2, 4, 6, 24, and 72 h after surgery for quantification of interleukin (IL)-6, IL-8, and IL-1β levels.
Results: Pain scores were significantly lower in patients treated with naproxen sodium compared to those treated with acetaminophen. Inflammatory mediator levels in plasma and gingival crevicular fluid increased after surgery and returned to near baseline levels by 72 h. Plasma IL-6 levels were significantly lower 6 h after surgery in patients treated with naproxen sodium compared to acetaminophen. No differences in inflammatory mediator concentrations in gingival crevicular fluid were observed between the treatment groups. The number of implants placed and body mass index (BMI) influenced inflammatory mediator concentrations in plasma and gingival crevicular fluid, respectively.
Discussion: Naproxen sodium was more effective than acetaminophen in reducing post-operative pain and systemic inflammation following surgical placement of one or two dental implants. Further studies are needed to determine whether these findings are applicable to more complex implant cases and how they affect clinical outcomes following implant placement.
Clinical Trial Registration: ClinicalTrials.gov, identifier NCT04694300
Placement of dental implants is a frequently performed outpatient surgical procedure, and the number of patients opting for this procedure continues to increase (Elani et al., 2018). Implants have become the gold standard for replacing missing teeth due to their high level of predictability and patient acceptance, with long-term success rates greater than 95% (Schmitt and Zarb, 1993; Zarb and Schmitt, 1993; Ali and Kay, 2019). The soft tissue and bony trauma associated with dental implant surgery upregulates inflammatory mediators (Pietruski et al., 2001; Li et al., 2015), leading to post-operative pain that typically persists for several days after surgery (Hashem et al., 2006; Al-Khabbaz et al., 2007; Alissa et al., 2009; Bockow et al., 2013; Samieirad et al., 2017).
Numerous placebo-controlled studies support the use of non-steroidal anti-inflammatory drugs (NSAIDs) as first-line agents in managing pain following outpatient dental procedures due to their effectiveness and lack of addictive potential (Moore and Hersh, 2013; Hersh et al., 2020). Most of these studies have been performed in patients undergoing surgical extraction of bony impacted mandibular third molars, but similar results have been observed in studies of implant placement (Melini et al., 2020; Khouly et al., 2021; Mattos-Pereira et al., 2021). Pre-emptive administration of dexketoprofen 25 mg (Sanchez-Perez et al., 2018), ibuprofen 600 mg (Pereira et al., 2020), or piroxicam 40 mg (Bhutani et al., 2019), 15–60 min prior to surgery, resulted in less post-operative pain compared to placebo. Dental implant patients are generally older with more comorbidities and concomitant medications than dental impaction patients (Elani et al., 2018; Aghaloo et al., 2019). Thus, there is greater concern for adverse events and drug interactions in this population. The pain intensity level following implant placement is generally less than dental impaction surgery (Al-Khabbaz et al., 2007; Al-Bayati et al., 2021), so over-the-counter (OTC) doses of NSAIDs and/or acetaminophen are an option for pain management in these patients. OTC dosing is more conservative than prescription dosing mainly for safety reasons (Hersh et al., 2007), and these lower dosages along with the shorter maximum durations of use (no more than 10-day) contribute to a side-effect profile no different from placebo (DeArmond et al., 1995; Kellstein et al., 1999). However, studies evaluating the comparative analgesic efficacy of OTC NSAIDs and acetaminophen in implant patients have not been performed.
Implant placement surgery also promotes a local and systemic inflammatory response. Elevated levels of interleukin (IL)-6, IL-8, and macrophage inflammatory protein (MIP)-1β have been observed in gingival crevicular fluid (GCF) from the implant site and the adjacent teeth 1 week after surgery and diminish over 12 weeks with subsequent healing (Emecen-Huja et al., 2013). Dental implant surgery also increases tumor necrosis factor (TNF)-α and IL-6 concentrations in plasma, indicative of a systemic inflammatory response (Pietruski et al., 2001; Li et al., 2015). Peak blood levels of these inflammatory mediators corresponded to peak pain intensity scores of 3–4 (mild to moderate pain) on a 0-10 visual analog scale in patients receiving an average of 2.5 implants (Li et al., 2015). However, the effects of OTC NSAIDs or acetaminophen on local or systemic levels of inflammatory mediators after implant placement have not been studied. Therefore, we sought to compare the analgesic and anti-inflammatory effects of OTC regimens of naproxen sodium and acetaminophen in patients following placement of one or two dental implants.
This was a randomized, double-blind, parallel-group pilot study in adult patients receiving one or two dental implants. Patients were recruited from the Graduate Periodontics and Penn Family Practice Clinics at the University of Pennsylvania School of Dental Medicine (PDM) enrolled between April 2021 and August 2022. Subjects having either maxillary or mandibular implant surgery, including those requiring bone grafting on the day of surgery, were included. The study protocol and informed consent document were approved by the University of Pennsylvania Institutional Review Board (IRB protocol #844440, ClinicalTrials.gov: NCT04694300). All patients provided informed consent prior to any study-related procedure, and the study was conducted in accordance with United States Federal Policy for the Protection of Human Subjects.
Patients were excluded from the study if they presented with advanced periodontal disease (>20% Clinical Attachment Loss + >20% radiographic bone loss); poor oral hygiene; smoked or used recreational drugs; were pregnant or nursing a child; had a history of bisphosphonate usage or systemic steroid use for longer than 2 weeks in the past 2 years; had a history of diabetes mellitus, substance use disorder, chronic pain, or inflammatory/autoimmune disease; or had contraindications to any of the study medications (naproxen sodium, acetaminophen, tramadol) including any scheduled or recent cardiac procedures (within 6 months), a history of asthma, urticaria or hypersensitivity reactions after taking NSAIDs, a history of, or active gastrointestinal perforation, ulcer or bleeding, severe heart failure, anticoagulant use due to increased bleeding risk with concomitant naproxen sodium therapy, or antidepressant therapy due to risk of serotonin syndrome with concomitant tramadol use.
Baseline blood and GCF samples were collected prior to surgery. GCF samples were collected by inserting paper filter strips (Periopaper, Proflow, Amitville NY) into the gingival crevice of a tooth adjacent to the implant site (Aboyoussef et al., 1998). Surgical implant placement was performed according to standard of care in the PDM Graduate Periodontics and Penn Family Practice Clinics. Local anesthesia was achieved with lidocaine plus 1:100,000 epinephrine (1:50,000 for hemostasis when necessary), and/or 3% mepivacaine plain. Nitrous oxide sedation was allowed. The use of glucocorticoids or the long-acting local anesthetic 0.5% bupivacaine plus 1:200,000 epinephrine was not permitted.
Patients were randomized in a ratio of 1:1 to receive either naproxen sodium (440 mg loading dose followed by 220 mg q8h; maximum daily dose 660 mg) or acetaminophen (1,000 mg q6h; maximum daily dose 3,000 mg). These regimens were chosen to align with OTC dosing recommendations from the product labeling (Aleve, 2019; Tylenol, 2022). The allocation sequence was generated by simple randomization, and the investigators and participants were blinded to allocation. The investigational pharmacist, who was not involved in the analysis of the study, maintained the unblinded allocation sequence and assigned participants to interventions sequentially. Drug blinding was performed by the University of Pennsylvania Investigational Drug Services employing an over-encapsulation technique.
After surgery, blood and GCF samples were collected (T = 0), and patients received their first dose of blinded study medication (naproxen sodium 440 mg or acetaminophen 1,000 mg) according to their randomization assignment. Patients then reported their pain intensity every 20 min for 6 h after taking the first dose of blinded study medication or until rescue medication was requested using the 0–10 Numeric Rating Scale, where 0 = no pain and 10 = worst pain imaginable. Blood and GCF samples were collected at 1, 2, 4, and 6 h post-study drug administration. Rescue analgesic (tramadol 50 mg) was allowed upon request. Following the 6 h sample collection, patients were asked to rate the effectiveness of the medication in managing their pain (completely or mostly effective, somewhat effective, somewhat ineffective, completely or mostly ineffective).
Patients were then discharged with a blister pack containing blinded study medication, as well as rescue medication (tramadol 50 mg) in case of insufficient pain relief. Due to the differences in dosing interval between naproxen sodium and acetaminophen, the blister pack contained placebo capsules in addition to active medication to maintain blinding of treatment allocation (Supplementary Table S1). Patients were given a diary to record outpatient study medication use and pain intensity scores at the time each dose was taken. They returned to clinic at 24 and 72 h for blood and GCF collections. Adherence to study medication was verified by pill counts and review of pain diary entries at the 24 and 72 h follow-up visits. A global assessment of study medication (poor, fair, good, very good or excellent) occurred at 72 h.
Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Pennsylvania Perelman School of Medicine (Harris et al., 2009; Harris et al., 2019). REDCap (Research Electronic Data Capture) is a secure, web-based software platform designed to support data capture for research studies.
Cyclooxygenase (COX)-1 activity was evaluated ex vivo by quantifying serum thromboxane B2 levels, as previously described (Patrignani et al., 1982). Briefly, whole blood was collected into vacuum tubes containing clot activator and incubated at 37°C for 1 h. Serum was separated by centrifugation and stored at −80°C until analysis. Thromboxane B2 levels were quantified using the Thromboxane B2 Express Monoclonal ELISA kit (Cayman Chemical, Ann Arbor, MI).
COX-2 activity was evaluated ex vivo by quantifying plasma prostaglandin (PG)E2 levels following lipopolysaccharide (LPS) stimulation in whole blood, as previously described (Panara et al., 1999). Briefly, heparinized whole blood was treated with aspirin (1 mM) and incubated at room temperature for 15 min. LPS (Escherichia coli, serotype O111:B4, 10 μg/ml whole blood) was added, and the sample was incubated at 37°C for 24 h. Plasma was separated by centrifugation and stored at −80°C until analysis. PGE2 levels were quantified using the PGE2 Monoclonal ELISA kit (Cayman Chemical, Ann Arbor, MI).
Paper strips containing GCF were placed in 100 μl phosphate buffered saline (PBS), incubated at 4°C for 2 h and stored at −80°C until analysis. IL-6, IL-8, and IL- 1β levels in plasma and GCF were quantified using Human Quantikine ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturers’ instructions. Levels of IL-8 and IL-1β in plasma and IL-6 in GCF were below the limit of detection, so further statistical analyses were not performed.
The primary endpoint for analgesic effects was a comparison of pain intensity scores between the naproxen sodium and acetaminophen groups. The primary endpoint for anti-inflammatory effects was a comparison of inflammatory mediator levels in plasma and GCF between the naproxen sodium and acetaminophen groups. A sample size of 30 patients (n = 15/treatment) provided approximately 80% power to detect a 2-fold difference in these endpoints at α = 0.05. For plasma IL-6, the percent change from baseline for each patient was calculated by dividing the plasma IL-6 concentration at 6 h by the plasma IL-6 concentration at baseline prior to surgery. Data are reported as mean ± standard deviation or median [interquartile range (IQR)]. Baseline characteristics and biochemical measurements were compared by t-test or Mann-Whitney test, as appropriate. Pain scores and biochemical measurements over time were analyzed by mixed effect modeling, including time and treatment as main effects. All tests were two-sided and p < 0.05 was considered statistically significant.
The CONSORT flow diagram (Supplementary Figure S1) is included in the Supplementary Information.
The study cohort included 30 adults (12 men and 18 women) with a mean age of 46.2 ± 14.3 years. After implant placement surgery, 15 patients received naproxen sodium and 15 patients received acetaminophen. No significant differences in demographic or clinical factors were observed between the treatment groups (Table 1). All patients completed the inpatient sample collections and pain assessments. In the naproxen sodium group, one patient missed both follow-up visits and one patient missed the 72-h follow-up. Both patients reported outpatient medication use and pain assessments, but global assessments were not provided.
The study treatments were well-tolerated, with no serious adverse events observed throughout the study. During the inpatient period, one patient in the acetaminophen group reported agitation, nausea, and emesis following treatment with tramadol. During the outpatient period, one patient in the acetaminophen group reported an episode of emesis and one patient in the naproxen group reported a headache. All adverse events were mild and resolved without intervention.
Most patients reported no or mild pain after implant placement, with a median maximum pain score of 2 (IQR: 0-3.25) during the inpatient phase. Maximum pain scores did not differ between patients receiving one (median: 2; IQR: 0.25-3) or two implants (median: 2.5; IQR: 0-6.25; p = 0.82). Naproxen sodium was significantly more effective than acetaminophen in controlling pain during both the inpatient and outpatient periods (Figure 1). Similar results were observed when analysis was restricted only to patients receiving one implant (data not shown). During the inpatient period, patients treated with acetaminophen reported a significantly greater maximum pain score (median: 3; IQR: 2-7) compared to patients who received naproxen (median: 1; IQR: 0-2; p < 0.01). The inpatient effectiveness ratings and global evaluations (Supplementary Table S2) favored naproxen sodium and were consistent with the pain intensity scores, but these differences were not statistically significant.
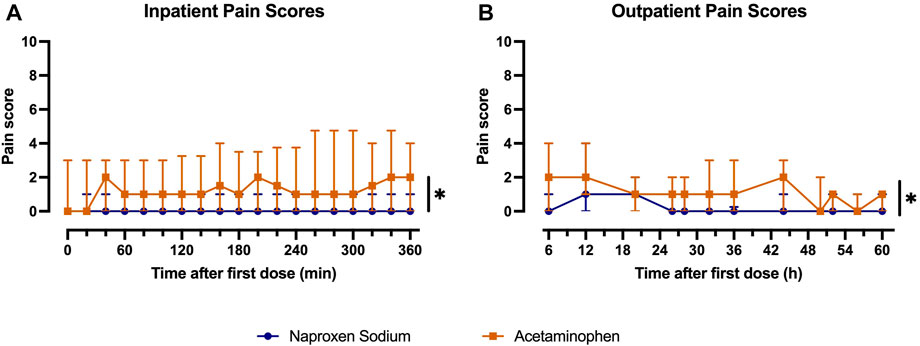
FIGURE 1. Comparison of median pain scores at each pain assessment during (A) inpatient and (B) outpatient periods between patients treated with naproxen sodium (blue) and acetaminophen (orange). Error bars indicate interquartile range (*p < 0.05 for treatment).
Few patients required an opioid rescue analgesic. During the inpatient period, 3 patients who received acetaminophen used tramadol, while no patients who received naproxen sodium requested tramadol. During the outpatient period, 5 patients in the acetaminophen group and 2 patients in the naproxen sodium group used tramadol. Of the patients who used tramadol during the outpatient period, 4 patients (2 naproxen sodium vs. 2 acetaminophen) used only one dose, 2 patients (acetaminophen) used 2 doses, and 1 patient (acetaminophen) used 3 doses.
Naproxen sodium inhibited both COX-1 and COX-2 activity, while acetaminophen inhibited only COX-2 activity (Supplementary Figure S2). COX-1 activity ex vivo was inhibited by >90% in naproxen sodium-treated subjects during the inpatient period. Naproxen sodium tended to inhibit COX-2 activity ex vivo to a greater degree than acetaminophen, but this difference was not statistically significant (p = 0.097 for treatment effect).
Implant placement surgery increased plasma IL-6 concentrations, with maximum levels observed 6 h after surgery (Figure 2A; p < 0.05 for time effect). Naproxen sodium treatment blunted the increase in plasma IL-6 over time compared to acetaminophen (Figure 2A; p < 0.05 for time × treatment interaction). At 6 h, the median percent change in plasma IL-6 levels relative to baseline in patients treated with naproxen sodium was 276.9% (IQR: 181.3%–348.4%) compared to 519.1% (IQR: 262.6%–1091%) in patients treated with acetaminophen (Figure 2B; p < 0.05).
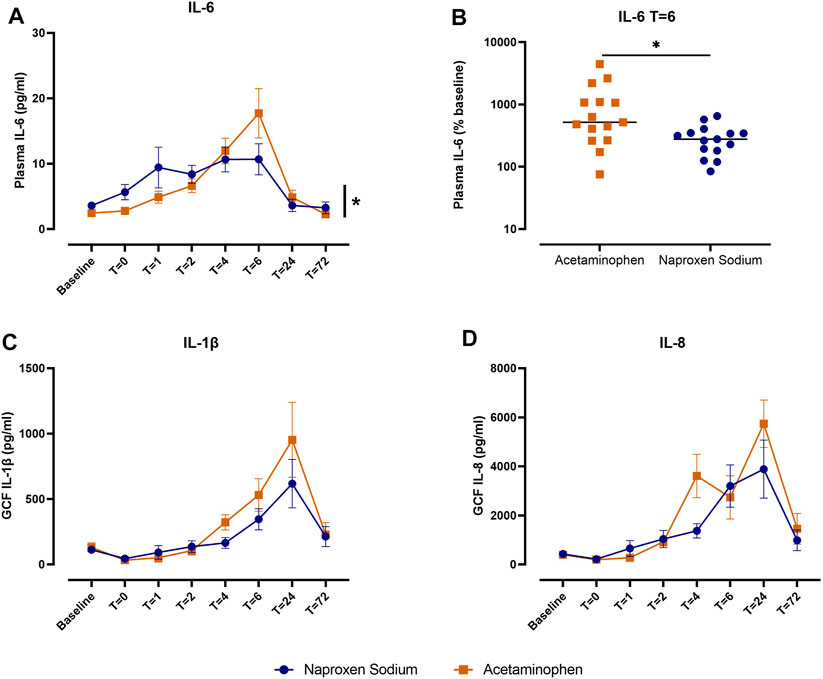
FIGURE 2. Comparison of systemic and local inflammatory mediators between patients treated with naproxen sodium (blue) and acetaminophen (orange). (A) Plasma IL-6 concentrations over time by treatment. Data are shown as mean ± SEM (*p < 0.05 for time × treatment interaction). (B) Plasma IL-6 levels at T = 6 h expressed as percent change from baseline. Crossbars indicate median, and data are plotted on a log10 scale (*p < 0.05; Mann-Whitney test). (C) IL-1β concentrations in gingival crevicular fluid (GCF) over time by treatment. Data are shown as mean ± SEM. (D) IL-8 concentrations in gingival crevicular fluid (GCF) over time by treatment. Data are shown as mean ± SEM.
Implant placement surgery also increased IL-1β and IL-8 concentrations in GCF (Figures 2C, D; p < 0.05 for time effect). IL-1β levels peaked at 24 h and returned to near baseline levels by 72 h. IL-8 levels peaked at 24 h and remained elevated at 72 h relative to baseline. No significant differences were observed between treatment groups for either IL-1β or IL-8 in GCF. After implant placement surgery, 70%–85% of GCF samples were contaminated with blood during the inpatient period. For the 24 and 72 h follow-up visits, approximately 40% of GCF samples were contaminated with blood.
We performed exploratory analyses to evaluate whether clinical or demographic factors influenced peak levels of inflammatory mediators in plasma (T = 6 h) or GCF (T = 24 h). Plasma IL-6 levels trended toward being higher 6 h after surgery in individuals who received two implants (n = 6), compared to those who received one implant (n = 24; p = 0.10; Supplementary Table S3). Naproxen sodium appeared to have an anti-inflammatory effect compared to acetaminophen in both groups (Figure 3), but the study was underpowered to formally evaluate this interaction. In contrast, the number of implants did not impact IL-1β or IL-8 levels in GCF at 24 h. Levels of both IL-1β and IL-8 in GCF at 24 h were significantly higher after implant placement in overweight and obese individuals (n = 18; mean BMI: 28.6 ± 2.1 kg/m2), compared to individuals in the healthy weight range (n = 12; mean BMI: 22.0 ± 2.5 kg/m2; Figure 4). No significant differences were observed between men and women for either plasma IL-6 or GCF IL-1β or IL-8 levels.
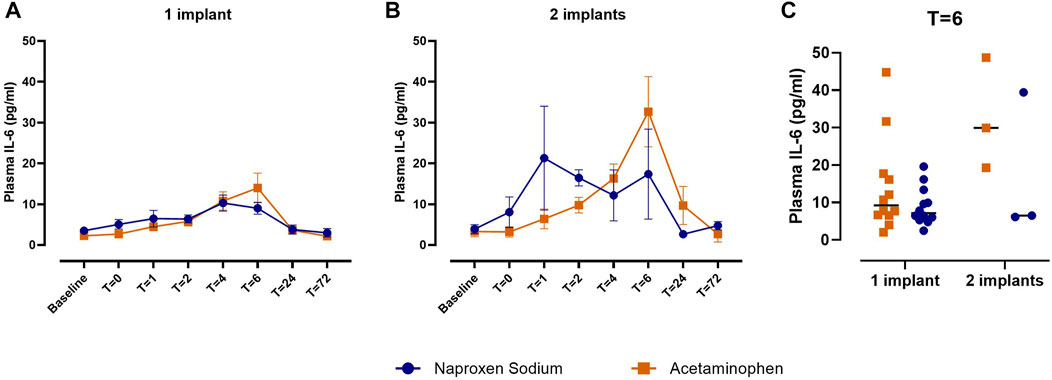
FIGURE 3. Comparison of plasma IL-6 levels between patients treated with naproxen sodium (blue) and acetaminophen (orange) by number of implants. (A) Plasma IL-6 concentrations over time in patients receiving one implant. Data are shown as mean ± SEM. (B) Plasma IL-6 concentrations over time in patients receiving two implants. Data are shown as mean ± SEM. (C) Plasma IL-6 concentrations at T = 6 h by treatment and number of implants. Crossbars indicate median.
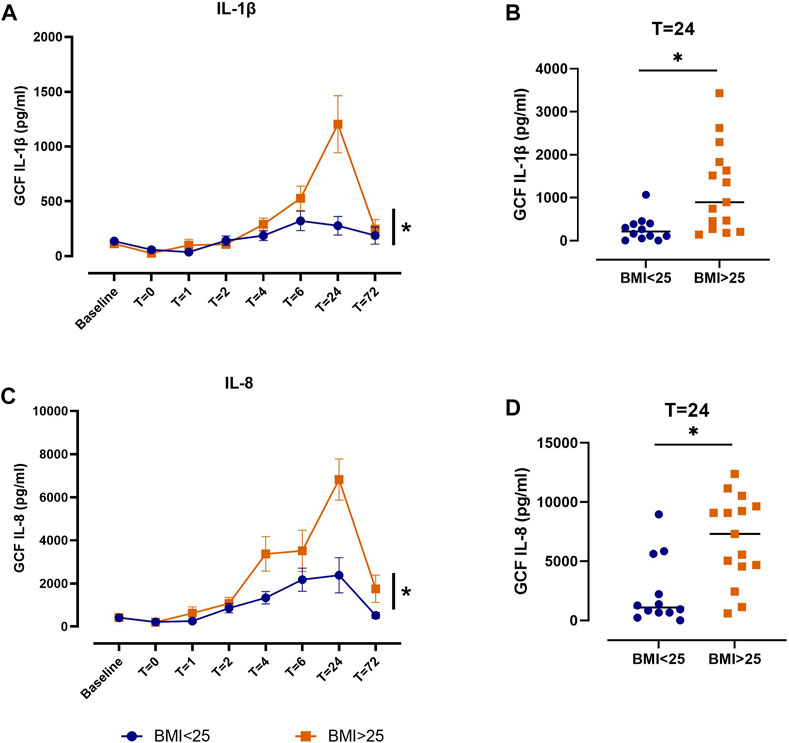
FIGURE 4. Comparison of IL-1β and IL-8 levels in gingival crevicular fluid (GCF) between patients with body mass index (BMI) < 25 kg/m2 (blue) and BMI > 25 kg/m2 (orange). (A) IL-1β concentrations in GCF over time. Data are shown as mean ± SEM (*p < 0.05 for BMI). (B) IL-1β concentrations in GCF at T = 24 h. Crossbars indicate median (*p < 0.05; Mann-Whitney test). (C) IL-8 concentrations in GCF over time. Data are shown as mean ± SEM (*p < 0.05 for BMI). (D) IL-8 concentrations in GCF at T = 24 h. Crossbars indicate median (*p < 0.05; Mann-Whitney test).
Non-addictive analgesics, including NSAIDs and acetaminophen, are recommended as first-line agents in the management of pain following outpatient dental procedures (Hersh et al., 2020). Prior studies have demonstrated that prescription doses of NSAIDs are superior to placebo in reducing post-operative pain and swelling following implant placement surgery (Melini et al., 2020; Khouly et al., 2021; Mattos-Pereira et al., 2021). However, to our knowledge, this is the first study to compare the analgesic and anti-inflammatory effects of OTC doses of an NSAID and acetaminophen following implant placement surgery.
We observed that naproxen sodium was more effective than acetaminophen at reducing post-operative pain, despite most patients reporting only mild pain intensity. This is consistent with prior studies performed in patients following third molar extraction. A single dose of naproxen sodium 440 mg was superior to acetaminophen 1,000 mg in peak analgesic effects and duration of action (Kiersch et al., 1994). Naproxen sodium 440 mg also produced analgesic effects at least equivalent to and for some endpoints superior to optimal doses of acetaminophen 650 mg plus hydrocodone 10 mg following third molar extraction (Cooper et al., 2021).
Implant placement surgery promoted a systemic inflammatory response in the acute post-surgical period, as indicated by induction of plasma IL-6. Similarly, Pietruski and colleagues observed that serum concentrations of IL-6 and IL-8, but not IL-1, increased the day after implant placement compared to baseline in a small cohort (N = 10) (Pietruski et al., 2001). Another study reported increases in plasma TNF-α and IL-6 concentrations 4 h after implant placement, which were significantly lower in patients who received dexmedetomidine during surgery compared to those who received midazolam (Li et al., 2015). We observed that patients treated with naproxen sodium had lower plasma IL-6 levels compared to acetaminophen-treated patients 6 h after surgery, consistent with a systemic anti-inflammatory effect of NSAID treatment. The analgesic and anti-inflammatory effects of naproxen sodium can be attributed to the greater degree of COX inhibition ex vivo, compared to acetaminophen. Plasma IL-6 levels decreased after 6 h in both treatment groups, likely reflecting the natural resolution of the systemic inflammatory response over time.
We also observed induction of IL-1β and IL-8 in GCF following implant placement surgery, which peaked at 24 h after surgery. Few studies have measured inflammatory mediator concentrations in GCF in the acute post-surgical period. Blood contamination was present in the majority of GCF samples collected after surgery. However, neither IL-1β nor IL-8 was detected in plasma; thus, it is likely that the level of blood contamination had minimal effects on our results. Interestingly, we observed no differences in either IL-1β or IL-8 levels in GCF between the treatment groups. Rather, the levels of these inflammatory mediators differed based on body weight. Notably, these effects were apparent only after surgery, and no differences in IL-1β and IL-8 levels in GCF between healthy weight and overweight/obese patients were observed at baseline. One study in men who had an implant for at least 12 months reported that obese patients had higher peri-implant bleeding on probing, peri-implant probing depth, peri-implant marginal bone loss and levels of whole salivary IL-1β and IL-6, compared to patients with a healthy weight. However, all obese patients had a history of periodontitis, compared to no patients in the control group, so these observations may be confounded by disease status (Abduljabbar et al., 2016). It is well-established that obesity can promote inflammation via multiple mechanisms, including metabolic dysregulation, adipokine signaling, and dysbiosis (Gregor and Hotamisligil, 2011). Future studies will be necessary to determine whether these mechanisms contribute to the elevated levels of IL-1β and IL-8 in GCF after surgery in overweight and obese patients, as well as determine the effect of body weight on clinical outcomes.
There are limitations to our study. This was a pilot study with only 30 participants. Thus, our analysis was not powered to comprehensively evaluate the clinical and demographic factors that influence the local and systemic inflammatory response to implant placement surgery or drug response. In addition, we excluded smokers and patients with diabetes, autoimmune diseases, or other comorbidities that might influence the inflammatory response to implant placement surgery. Although this limits potential confounding, it precludes interrogation of the influence of these factors on response to naproxen sodium or acetaminophen. The post-surgical GCF samples were taken from teeth adjacent to the surgical site, and the sulci may have been damaged due to the incision. Therefore, we cannot ensure that the samples were truly GCF, rather than an inflammatory exudate. Finally, we followed patients for only 72 h after surgery and did not collect data regarding clinical outcomes like peri-implantitis or implant failure.
In conclusion, our study demonstrates that naproxen sodium is more effective than acetaminophen in reducing post-operative pain and systemic inflammation following surgical placement of one or two dental implants. These findings lay a foundation for future studies to evaluate how clinical and demographic factors impact the response to analgesic therapy following implant placement surgery.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the University of Pennsylvania Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
KT and EH designed the study, interpreted the data, and drafted the manuscript. KT performed the statistical analyses. KT, MC, DW, TP, and CM conducted the laboratory analyses. SS coordinated the study visits and collected study data. TY, AR, Y-CC, and JK recruited participants and performed implant placement surgeries. All authors contributed to the article and approved the submitted version.
This study was supported by an investigator-initiated research grant from Bayer Healthcare, LLC to Dr. Theken and Dr. Hersh (IIR-21753).
This study was supported by an investigator-initiated research grant from Bayer Healthcare, LLC to KT and EH. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1199580/full#supplementary-material
Abduljabbar, T., Al-Sahaly, F., Kellesarian, S. V., Kellesarian, T. V., Al-Anazi, M., Al-Khathami, M., et al. (2016). Comparison of peri-implant clinical and radiographic inflammatory parameters and whole salivary destructive inflammatory cytokine profile among obese and non-obese men. Cytokine 88, 51–56. doi:10.1016/j.cyto.2016.08.017
Aboyoussef, H., Carter, C., Jandinski, J. J., and Panagakos, F. S. (1998). Detection of prostaglandin E2 and matrix metalloproteinases in implant crevicular fluid. Int. J. Oral Maxillofac. Implants 13, 689–696.
Aghaloo, T., Pi-Anfruns, J., Moshaverinia, A., Sim, D., Grogan, T., and Hadaya, D. (2019). The effects of systemic diseases and medications on implant osseointegration: A systematic review. Int. J. Oral Maxillofac. Implants 34, s35–s49. doi:10.11607/jomi.19suppl.g3
Al-Bayati, O., Font, K., Soldatos, N., Carlson, E., Parsons, J., and Powell, C. A. (2021). Evaluation of the need to prescribe opioid medication to control post-surgical pain of different periodontal/oral surgeries. J. Periodontol. 92, 1030–1035. doi:10.1002/JPER.20-0315
Al-Khabbaz, A. K., Griffin, T. J., and Al-Shammari, K. F. (2007). Assessment of pain associated with the surgical placement of dental implants. J. Periodontol. 78, 239–246. doi:10.1902/jop.2007.060032
Aleve, (2019). Product information. [Online]. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020204Orig1s052lbl.pdf [Accessed 12/30/2019].
Ali, K., and Kay, E. J. (2019). What are the long-term survival and complication rates of complete-arch fixed implant rehabilitation in edentulous patients? Evid. Based Dent. 20, 97–98. doi:10.1038/s41432-019-0052-3
Alissa, R., Sakka, S., Oliver, R., Horner, K., Esposito, M., Worthington, H. V., et al. (2009). Influence of ibuprofen on bone healing around dental implants: A randomised double-blind placebo-controlled clinical study. Eur. J. Oral Implantol. 2, 185–199.
Bhutani, N., Sangolikar, D., Bhutani, S., Tapashetti, R., and Pushpalatha, H. (2019). Sublingual piroxicam as preemptive analgesia in single implant surgery. J. Contemp. Dent. Pract. 20, 750–753. doi:10.5005/jp-journals-10024-2591
Bockow, R., Korostoff, J., Pinto, A., Hutcheson, M., Secreto, S. A., Bodner, L., et al. (2013). Characterization and treatment of postsurgical dental implant pain employing intranasal ketorolac. Compend Contin. Educ. Dent. 34, 570–576.
Cooper, S. A., Desjardins, P. J., Bertoch, T., Paredes-Diaz, A., Troullos, E., Tajaddini, A., et al. (2021). Analgesic efficacy of naproxen sodium versus hydrocodone/acetaminophen in acute postsurgical dental pain: A randomized, double-blind, placebo-controlled trial. Postgrad. Med. 134, 463–470. doi:10.1080/00325481.2021.2008180
Dearmond, B., Francisco, C. A., Lin, J. S., Huang, F. Y., Halladay, S., Bartziek, R. D., et al. (1995). Safety profile of over-the-counter naproxen sodium. Clin. Ther. 17, 587–601. discussion 586. doi:10.1016/0149-2918(95)80036-0
Elani, H. W., Starr, J. R., Da Silva, J. D., and Gallucci, G. O. (2018). Trends in dental implant use in the U.S., 1999-2016, and projections to 2026. J. Dent. Res. 97, 1424–1430. doi:10.1177/0022034518792567
Emecen-Huja, P., Eubank, T. D., Shapiro, V., Yildiz, V., Tatakis, D. N., and Leblebicioglu, B. (2013). Peri-implant versus periodontal wound healing. J. Clin. Periodontol. 40, 816–824. doi:10.1111/jcpe.12127
Gregor, M. F., and Hotamisligil, G. S. (2011). Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 29, 415–445. doi:10.1146/annurev-immunol-031210-101322
Harris, P. A., Taylor, R., Minor, B. L., Elliott, V., Fernandez, M., O'Neal, L., et al. (2019). The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inf. 95, 103208. doi:10.1016/j.jbi.2019.103208
Harris, P. A., Taylor, R., Thielke, R., Payne, J., Gonzalez, N., and Conde, J. G. (2009). Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 42, 377–381. doi:10.1016/j.jbi.2008.08.010
Hashem, A. A., Claffey, N. M., and O'Connell, B. (2006). Pain and anxiety following the placement of dental implants. Int. J. Oral Maxillofac. Implants 21, 943–950.
Hersh, E. V., Moore, P. A., Grosser, T., Polomano, R. C., Farrar, J. T., Saraghi, M., et al. (2020). Nonsteroidal anti-inflammatory drugs and opioids in postsurgical dental pain. J. Dent. Res. 99, 777–786. doi:10.1177/0022034520914254
Hersh, E. V., Pinto, A., and Moore, P. A. (2007). Adverse drug interactions involving common prescription and over-the-counter analgesic agents. Clin. Ther. 29, 2477–2497. doi:10.1016/j.clinthera.2007.12.003
Kellstein, D. E., Waksman, J. A., Furey, S. A., Binstok, G., and Cooper, S. A. (1999). The safety profile of nonprescription ibuprofen in multiple-dose use: A meta-analysis. J. Clin. Pharmacol. 39, 520–532. doi:10.1177/009127009903900513
Khouly, I., Braun, R. S., Ordway, M., Alrajhi, M., Fatima, S., Kiran, B., et al. (2021). Post-operative pain management in dental implant surgery: A systematic review and meta-analysis of randomized clinical trials. Clin. Oral Investig. 25, 2511–2536. doi:10.1007/s00784-021-03859-y
Kiersch, T. A., Halladay, S. C., and Hormel, P. C. (1994). A single-dose, double-blind comparison of naproxen sodium, acetaminophen, and placebo in postoperative dental pain. Clin. Ther. 16, 394–404.
Li, S., Yang, Y., Yu, C., Yao, Y., Wu, Y., Qian, L., et al. (2015). Dexmedetomidine analgesia effects in patients undergoing dental implant surgery and its impact on postoperative inflammatory and oxidative stress. Oxid. Med. Cell Longev. 2015, 186736. doi:10.1155/2015/186736
Mattos-Pereira, G. H., Martins, C. C., Esteves-Lima, R. P., Alvarenga-Brant, R., Cota, L. O., and Costa, F. O. (2021). Preemptive analgesia in dental implant surgery: A systematic review and meta-analysis of randomized controlled trials. Med. Oral Patol. Oral Cir. Bucal 26, e632–e641. doi:10.4317/medoral.24639
Melini, M., Forni, A., Cavallin, F., Parotto, M., and Zanette, G. (2020). Analgesics for dental implants: A systematic review. Front. Pharmacol. 11, 634963. doi:10.3389/fphar.2020.634963
Moore, P. A., and Hersh, E. V. (2013). Combining ibuprofen and acetaminophen for acute pain management after third-molar extractions: Translating clinical research to dental practice. J. Am. Dent. Assoc. 144, 898–908. doi:10.14219/jada.archive.2013.0207
Panara, M. R., Renda, G., Sciulli, M. G., Santini, G., Di Giamberardino, M., Rotondo, M. T., et al. (1999). Dose-dependent inhibition of platelet cyclooxygenase-1 and monocyte cyclooxygenase-2 by meloxicam in healthy subjects. J. Pharmacol. Exp. Ther. 290, 276–280.
Patrignani, P., Filabozzi, P., and Patrono, C. (1982). Selective cumulative inhibition of platelet thromboxane production by low-dose aspirin in healthy subjects. J. Clin. Invest 69, 1366–1372. doi:10.1172/jci110576
Pereira, G. M., Cota, L. O., Lima, R. P., and Costa, F. O. (2020). Effect of preemptive analgesia with ibuprofen in the control of postoperative pain in dental implant surgeries: A randomized, triple-blind controlled clinical trial. J. Clin. Exp. Dent. 12, e71–e78. doi:10.4317/medoral.56171
Pietruski, J. K., Pietruska, M. D., Stokowska, W., and Pattarelli, G. M. (2001). Serum levels of interleukin-1 (IL-1), interleukin-6 (IL-6) and interleukin-8 (IL-8) in patients treated with dental implants. Rocz. Akad. Med. Bialymst 46, 28–37.
Samieirad, S., Afrasiabi, H., Tohidi, E., Qolizade, M., Shaban, B., Hashemipour, M. A., et al. (2017). Evaluation of caffeine versus codeine for pain and swelling management after implant surgeries: A triple blind clinical trial. J. Craniomaxillofac Surg. 45, 1614–1621. doi:10.1016/j.jcms.2017.06.014
Sanchez-Perez, A., Munoz-Penalver, J., Moya-Villaescusa, M. J., and Sanchez-Matas, C. (2018). Effects of the preoperative administration of dexketoprofen trometamol on pain and swelling after implant surgery: A randomized, double-blind controlled trial. J. Oral Implantol. 44, 122–129. doi:10.1563/aaid-joi-D-17-00185
Schmitt, A., and Zarb, G. A. (1993). The longitudinal clinical effectiveness of osseointegrated dental implants for single-tooth replacement. Int. J. Prosthodont 6, 197–202.
Tylenol, (2022). Tylenol® for health professionals. [Online]. Available at: https://www.tylenolprofessional.com/adult-dosage [Accessed 2/21/2022].
Keywords: dental implant, analgesia, post-operative (post op) pain, inflammation, non-steroidal anti-inflammatory drugs, cytokine, prostaglandin, non-prescription drugs
Citation: Theken KN, Chen M, Wall DL, Pham T, Secreto SA, Yoo TH, Rascon AN, Chang Y-C, Korostoff JM, Mitchell CH and Hersh EV (2023) A randomized, double-blind pilot study of analgesic and anti-inflammatory effects of naproxen sodium and acetaminophen following dental implant placement surgery. Front. Pharmacol. 14:1199580. doi: 10.3389/fphar.2023.1199580
Received: 03 April 2023; Accepted: 05 May 2023;
Published: 17 May 2023.
Edited by:
Giorgia Colombo, University of Eastern Piedmont, ItalyReviewed by:
Samir Ayoub, University of East London, United KingdomCopyright © 2023 Theken, Chen, Wall, Pham, Secreto, Yoo, Rascon, Chang, Korostoff, Mitchell and Hersh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katherine N. Theken, a3RoZWtlbkBwZW5ubWVkaWNpbmUudXBlbm4uZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.