- Department of Orthopedics, The Affiliated Cancer Hospital of Zhengzhou University and Henan Cancer Hospital, Zhengzhou, China
Despite the low incidence of soft tissue sarcomas (STSs), hundreds of thousands of new STS cases are diagnosed annually worldwide, and approximately half of them eventually progress to advanced stages. Currently, chemotherapy is the first-line treatment for advanced STSs. There are difficulties in selecting appropriate drugs for multiline chemotherapy, or for combination treatment of different STS histological subtypes. In this study, we first comprehensively reviewed the efficacy of various chemotherapeutic drugs in the treatment of STSs, and then described the current status of sensitive drugs for different STS subtypes. anthracyclines are the most important systemic treatment for advanced STSs. Ifosfamide, trabectedin, gemcitabine, taxanes, dacarbazine, and eribulin exhibit certain activities in STSs. Vinca alkaloid agents (vindesine, vinblastine, vinorelbine, vincristine) have important therapeutic effects in specific STS subtypes, such as rhabdomyosarcoma and Ewing sarcoma family tumors, whereas their activity in other subtypes is weak. Other chemotherapeutic drugs (methotrexate, cisplatin, etoposide, pemetrexed) have weak efficacy in STSs and are rarely used. It is necessary to select specific second- or above-line chemotherapeutic drugs depending on the histological subtype. This review aims to provide a reference for the selection of chemotherapeutic drugs for multi-line therapy for patients with advanced STSs who have an increasingly long survival.
1 Introduction
Soft tissue sarcomas (STSs) are rare malignancies, accounting for only approximately 1% of all malignancies (Bhatt et al., 2016; Yang et al., 2019). There are more than 70 histological subtypes, and the clinical characteristics and prognoses of these subtypes greatly vary (Amadeo et al., 2020; Parikh et al., 2018; von Mehren et al., 2022; Tos et al., 2023). Despite the low incidence of STSs, hundreds of thousands of new STS cases are diagnosed annually worldwide, and approximately half of them eventually progress to advanced stages (Corey et al., 2014; Bhatt et al., 2016; Hung et al., 2019; Yang et al., 2019). Currently, chemotherapy is the first-line treatment for advanced STSs (Cojocaru et al., 2022; de Juan Ferre et al., 2021; von Mehren et al., 2020). Anthracyclines (mainly doxorubicin) were found to be effective against STSs in 1973 (Tan et al., 1973; Sritharan and Sivalingam, 2021). Since then, various clinical trials have been conducted to prolong survival or reduce adverse events in patients with STSs using intensive chemotherapy, non-anthracycline regimens, or alternative anthracycline drugs. These drugs include, but are not limited to, oxazaphosphorines, trabectedin, gemcitabine, taxanes, dacarbazine, eribulin, vinca alkaloid agents (vindesine, vinblastine, vinorelbine, vincristine), methotrexate, cisplatin, etoposide, and pemetrexed (Ratan and Patel, 2016; Hatcher et al., 2017; Smrke et al., 2020). The characteristics, efficacy, and safety of these drugs for STSs vary, and the responses of different STS subtypes to these chemotherapeutic drugs greatly vary. Although some drugs have shown good efficacy in individual subtypes, none have exceeded the efficacy and safety achieved by doxorubicin for STSs. To date, doxorubicin remains the first-line chemotherapeutic drug for STSs (Cojocaru et al., 2022; de Juan Ferre et al., 2021; von Mehren et al., 2020; Smrke et al., 2020; Smolle et al., 2020; Meyer and Seetharam, 2019; Yang et al., 2022; Gronchi et al., 2017). Selecting second- or higher-line drugs for advanced STS remains a challenge (Frezza et al., 2017; Kim et al., 2019; Haddox and Riedel, 2020; Younger et al., 2021; Kojima et al., 2022).
In the past decade, anti-vascular endothelial factor receptor multi-target tyrosine kinase inhibitors (TKIs), such as pazopanib, have been widely used in STS, which is a major breakthrough in the treatment of this type of malignancy, leading to significantly prolonged survival in patients with STS (Tang et al., 2021; Kyriazoglou et al., 2022; Thirasastr et al., 2022). Immunotherapeutic agents, such as programmed cell death protein 1 inhibitors, have also shown some therapeutic effects in some STS histological subtypes (Tang et al., 2021; Banks and D'Angelo, 2022; Tawbi et al., 2017). Furthermore, the combination of conventional chemotherapeutic drugs with targeted agents (TKIs or immunotherapeutic agents) is considered the next breakthrough in STS treatment (Tang et al., 2021; Patel et al., 2022; Principe et al., 2022; Tian and Yao, 2022). There are significant differences in the efficacy of different chemotherapeutic drugs combined with different targeted agents (Kyriazoglou et al., 2022; Principe et al., 2022; Fuchs et al., 2023). Based on the different STS subtypes, selecting potential chemotherapeutic drugs to combine with targeted drugs is important to achieve better efficacy (Principe et al., 2022; Tian and Yao, 2022). In recent years, few studies have systematically summarized the differences between the chemotherapeutic drugs used to treat STSs and the differences in the efficacy of these drugs in different STS subtypes. This leads to difficulties in selecting appropriate chemotherapeutic drugs for combination treatment of different STS subtypes.
In this study, we first comprehensively reviewed the efficacy of various chemotherapeutic drugs in the treatment of STSs, and then described the current status of sensitive drugs for different STS subtypes. We aim to provide a reference for the selection of chemotherapeutic drugs for multi-line therapy for patients with advanced STSs who have an increasingly long-survival.
2 Efficacy of different drugs in STSs
As a traditional method of cancer treatment, chemotherapy has been used in STSs for more than 50 years since the introduction of doxorubicin in the 1970s. Currently, chemotherapeutic drugs, such as anthracyclines, ifosfamide, trabectedin, gemcitabine, paclitaxel, dacarbazine, and eribulin, have therapeutic effects in STSs and are widely used in clinical treatment (Seddon, 2016; Bleloch et al., 2017; Frezza et al., 2017; Hatcher et al., 2017; Smrke et al., 2020). Here, we comprehensively reviewed the clinical trial results of various drugs for STSs to accurately describe the efficacy of them in STSs. To improve the reliability of this study, we attempted to use data from multicenter, prospective, phase II–IV clinical trials as much as possible. In the case of the absence of prospective clinical trial results, a multicenter retrospective study with large sample size conducted by multinational sarcoma organizations was included in the analysis. During our review, we found that the outcomes of different studies were presented using different measures, including the objective response rate (ORR), disease control rate, median progression-free survival (PFS), PFS rate, median overall survival (OS), and OS rate. We uniformly selected the most common measures, ORR, median PFS, and median OS, as comparative indicators.
2.1 Anthracyclines
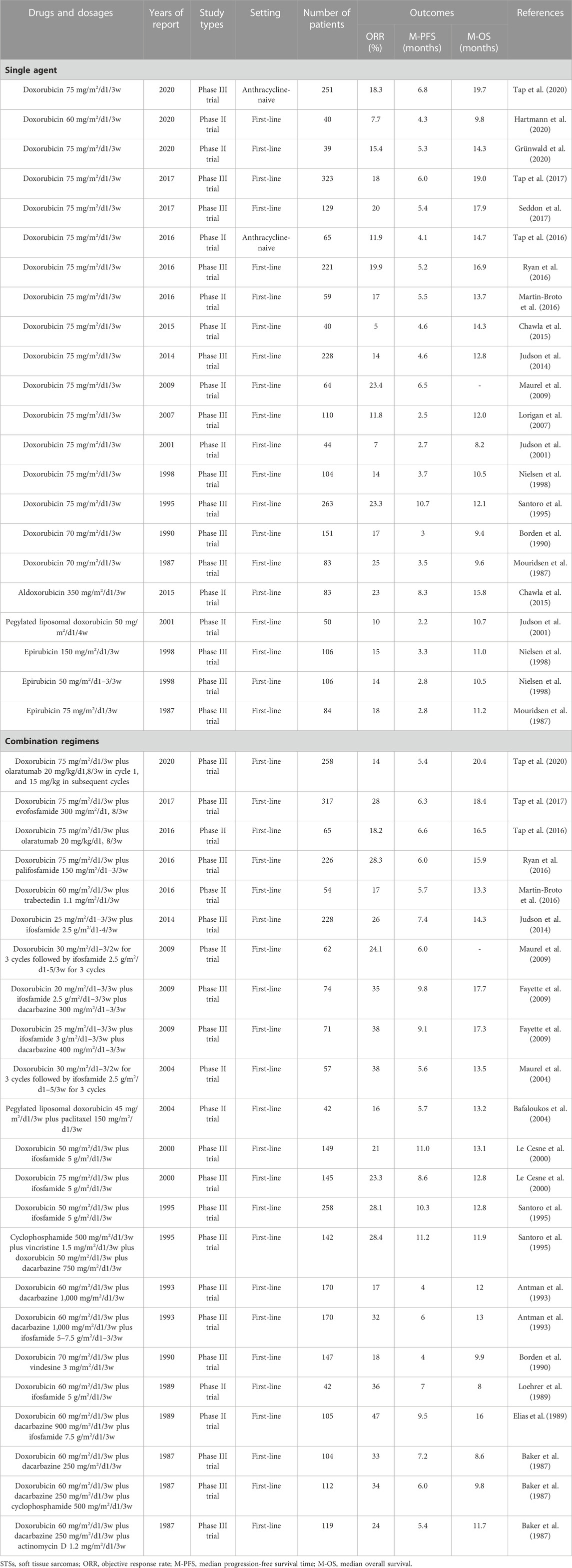
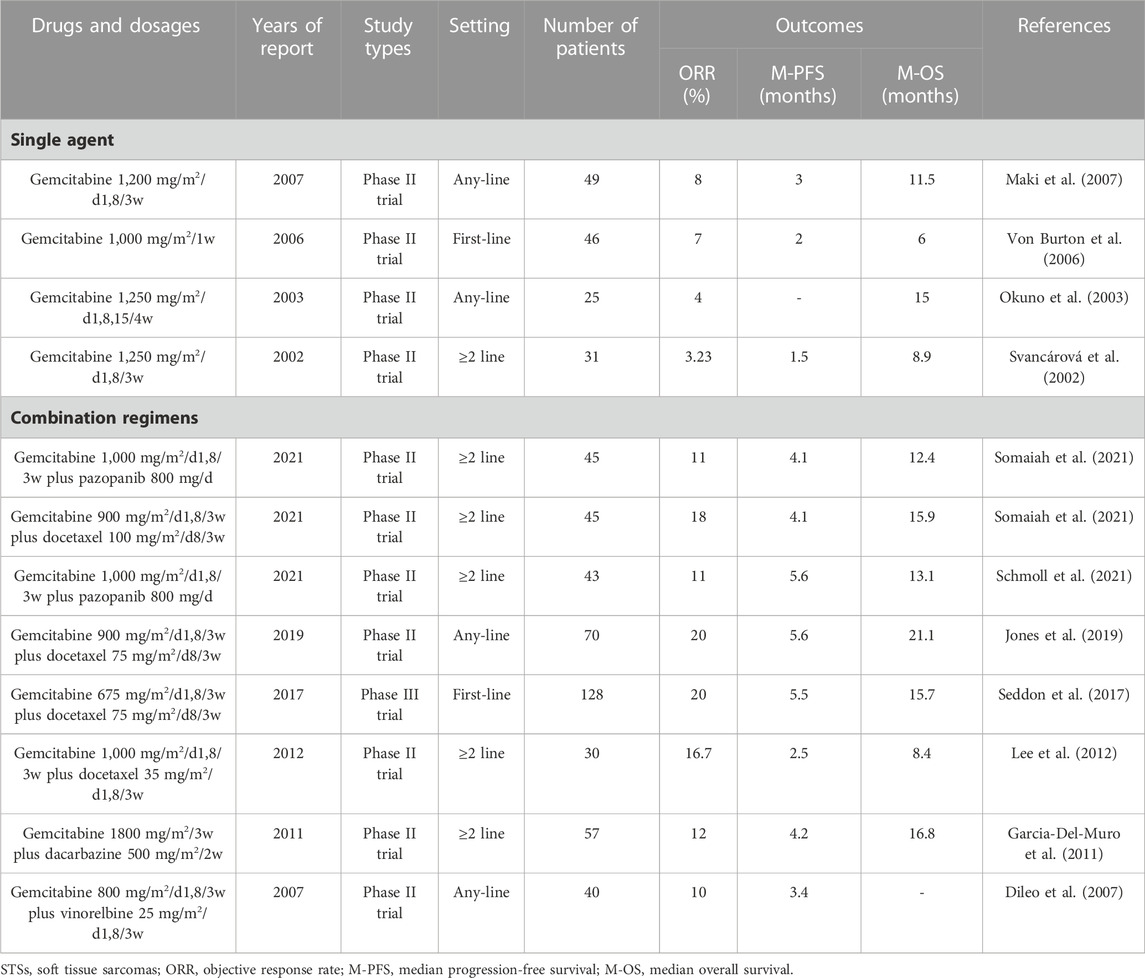
Anthracyclines are among the most effective chemotherapeutics for cancer. They are glycoside drugs comprising the amino sugar daunosamine linked to a hydroxyanthraquinone aglycone, and they induce cell death through multiple intracellular targets: reactive oxygen species generation, DNA-adduct formation, topoisomerase II inhibition, histone eviction, Ca2 + and iron hemostasis regulation, and ceramide overproduction (Rabbani et al., 2005; Jasra and Anampa, 2018; Martins-Teixeira and Carvalho, 2020). Doxorubicin is the most effective and widely used anthracycline for the treatment of STSs (Table 1). Several other anthracyclines, such as aldoxorubicin, epirubicin, and pegylated liposomal doxorubicin, have also been used for the clinical treatment of STSs (Table 1). However, none of the other anthracyclines exceed the efficacy of doxorubicin in STSs (Table 1) (Nielsen et al., 2000a; Judson et al., 2001; Chamberlain et al., 2019; Martins-Teixeira and Carvalho, 2020; Peter et al., 2022).
Doxorubicin (adriamycin) was isolated from Streptomyces suis and S. peucetius in the late 1960s (Tan et al., 1973; Peter et al., 2022). Since its Food and Drug Administration approval in 1974, doxorubicin alone or in combination with other drugs has been widely used as a first-line therapy for a myriad of cancers (Aubel-Sadron and Londos-Gagliardi, 1984; Sun et al., 2017). Doxorubicin induces cell death through multiple intracellular targets, including reactive oxygen species generation, DNA adduct formation, topoisomerase II inhibition, histone eviction, Ca2 + and iron hemostasis regulation, and ceramide overproduction. Moreover, doxorubicin-treated dying cells undergo cellular modifications that enable neighboring dendritic cell activation and enhance the presentation of tumor antigens. In addition, doxorubicin aids in the immune-mediated clearance of tumor cells (Carvalho et al., 2009; Sritharan and Sivalingam, 2021).
To date, Numerous clinical trials have demonstrated the efficacy of doxorubicin alone or doxorubicin-based chemotherapy for the treatment of STSs (Sritharan and Sivalingam, 2021; Peter et al., 2022). The results of the representative multicenter prospective clinical trials over the past 30 years are listed in Table 1. Due to various reasons such as the long-time interval between different clinical trials and errors caused by small sample sizes, the reported efficacy of doxorubicin monotherapy for STS varies significantly. However, clinical trials with sample sizes exceeding 100 in the past decade have shown that the ORR of doxorubicin monotherapy with a conventional dose (75 mg/m2/d1/3w) for STSs was 14%–20%, with a median PFS of 4.6–6.8 months (Table 1).
To further improve the efficacy of chemotherapy, doxorubicin in combination with other drugs has also been widely used (Table 1). The drug most commonly used in combination with doxorubicin is ifosfamide. The ORR of doxorubicin plus ifosfamide in treating STSs is 21%–38%, with a median PFS of 5.6–11 months (Table 1). Although the combination of doxorubicin and ifosfamide improves the ORR and median PFS compared with doxorubicin alone, it does not improve the median OS (Table 1) and instead increases hematological toxicity such as leucopenia and anemia (Maurel et al., 2009; Judson et al., 2014; Wang et al., 2021). Therefore, recently, this combined regimen is not recommended as a first-line chemotherapy for advanced STSs but is only recommended for preoperative neoadjuvant chemotherapy of high-risk STSs (Judson et al., 2014; Weiss et al., 2020). Furthermore, no combination regimen has been shown to significantly extend the median OS over doxorubicin monotherapy in patients with advanced STSs (Table 1). Notably, the doxorubicin plus ifosfamide plus dacarbazine achieves the highest ORR (38%) and median PFS (9.8 months) in patients with advanced STSs (Table 1). This combined regimen should also be tested in the setting of neoadjuvant therapy.
In summary, as the most recognized chemotherapeutic drug, doxorubicin is the cornerstone of advanced STS chemotherapy. The testing of new drugs in the field of STSs is always guided by doxorubicin. With the invention and testing of an increasing number of targeted and immunotherapeutic drugs, various doxorubicin-based combination therapies will be widely tested and applied for the treatment of STSs.
2.2 Oxazaphosphorines
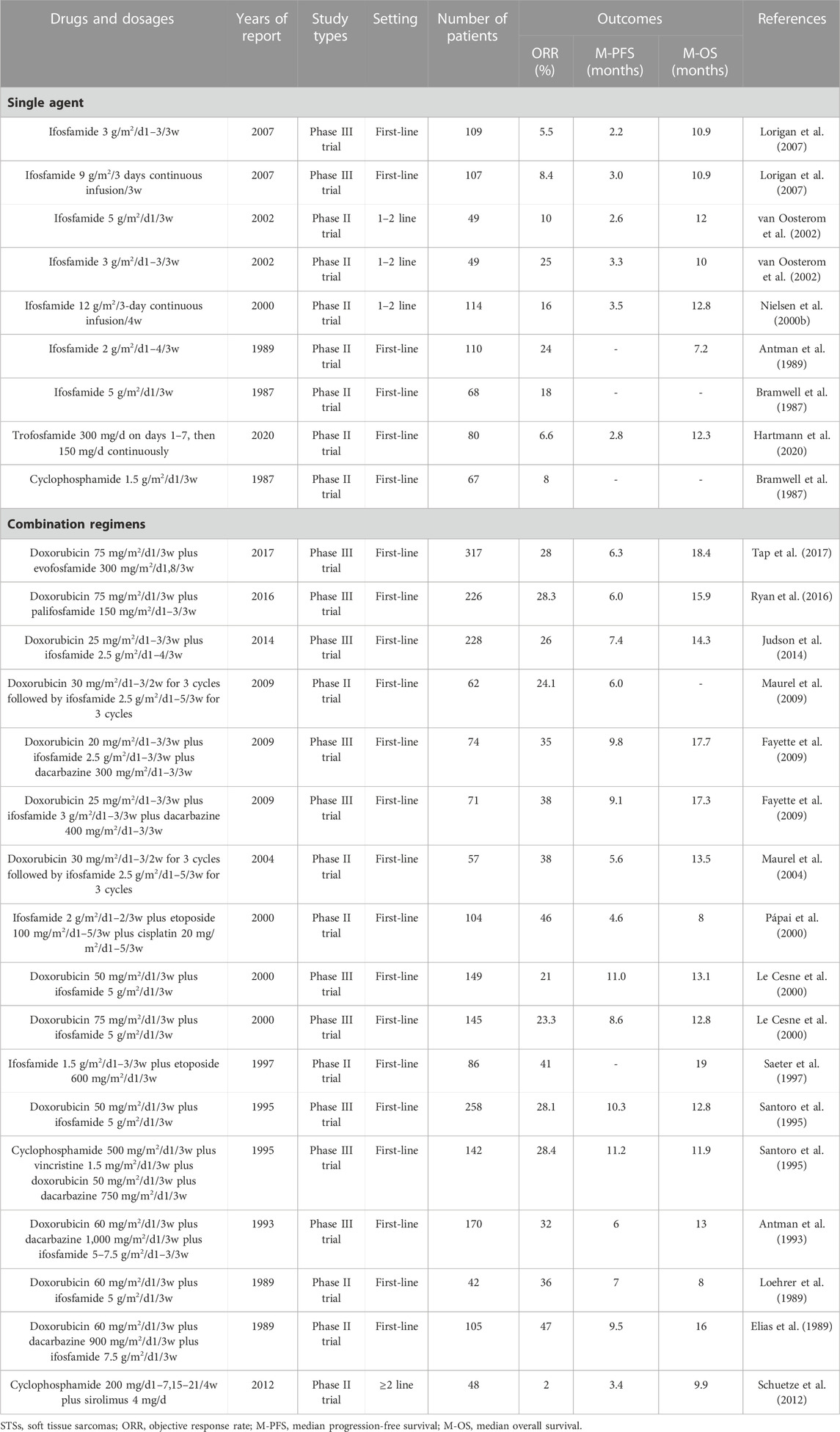
Oxazaphosphorines are a class of bifunctional alkylating agents that have been extensively investigated over the past 50 years and have a wide spectrum of anticancer and immune-regulating activities (Giraud et al., 2010). Most oxazaphosphorines are designed as prodrugs that require cytochrome P450 enzyme-mediated bioactivation to generate highly reactive alkylating nitrogen mustards, which exert their chemotherapeutic effects by attacking specific nucleophilic groups of DNA molecules in target cancer cells (Misiura, 2006; Liang et al., 2007; Wang and Wang, 2012). In STS chemotherapy, ifosfamide is the most widely used oxazaphophorine with definite efficacy (Table 2). Other oxazaphosphorines, such as cyclophosphamide, trofosfamide, evofosfamide, and palifosfamide, have also been used for the treatment of STSs (Table 2). However, to date, none of the other oxazaphosphorines have exceeded the efficacy of ifosfamide in STSs (Table 2) (Giraud et al., 2010; Mulder et al., 2015; Tap et al., 2017; Hartmann et al., 2020).
Among the chemotherapeutic drugs for STSs, ifosfamide is the second most effective after doxorubicin (Tascilar et al., 2007). Ifosfamide was first synthesized in the 1960s. It was introduced as a chemical modification of cyclophosphamide with a different position of its two chloroethyl groups on the central ring, providing a structure with greater water solubility and antitumor activity and a better toxicity profile (Kerbusch et al., 2001; Misiura, 2006; Tascilar et al., 2007). Numerous clinical trials and retrospective studies have demonstrated the efficacy of ifosfamide alone or ifosfamide-based chemotherapy for the treatment of STSs. The results of representative multicenter prospective clinical trials are presented in Table 2. Because there are no cardiotoxicity concerns, ifosfamide can be administered at significantly higher doses than doxorubicin. Current evidence indicates that the ORR of using large doses of ifosfamide to treat STSs is significantly higher than that of using low doses (Table 1). The efficacy of ifosfamide in the treatment of STSs is slightly lower than that of doxorubicin (ORR, 5.5%–25% vs. 5%–25%; median PFS, 2.2–3.5 vs. 2.5–10.7 months; median OS, 7.2–12.8 vs. 8.2–19.7 months, respectively) (Table 1; Table 2). Therefore, doxorubicin is still considered the first choice of chemotherapy for advanced STSs (Lorigan et al., 2007). However, ifosfamide may be superior to doxorubicin in synovial sarcoma (Nielsen et al., 2000b; Carter et al., 2020).
In the real world, ifosfamide is most commonly used in combination with doxorubicin (Table 1; Table 2). The ORR of the doxorubicin plus ifosfamide in treating STS is 21%–38%, and the median PFS is 5.6–11 months (Table 1; Table 2). Compared with doxorubicin or ifosfamide alone, the combination of doxorubicin and ifosfamide increases the ORR and median PFS but does not improve the median OS in patients with advanced STSs (Table 1; Table 2) (Maurel et al., 2009; Judson et al., 2014; Wang et al., 2021). Currently, this combined regimen is recommended for preoperative neoadjuvant chemotherapy in high-risk STSs (Judson et al., 2014; Weiss et al., 2020). Other drugs that are commonly used in combination with ifosfamide include dacarbazine and etoposide (Table 2). Notably, the doxorubicin plus ifosfamide plus dacarbazine has the highest ORR (32%–47%) and median PFS (6–9.8 months) in patients with advanced STSs (Table 2). The combination of these three drugs has not received sufficient attention in the era of targeted therapy and immunotherapy for STSs.
In summary, as a chemotherapeutic agent that is as well-known as doxorubicin, ifosfamide has an important effect on the chemotherapy of STSs. Ifosfamide is also worthy of further testing for the treatment of synovial sarcoma. However, the other oxazaphosphorines have not exceeded the role of ifosfamide in STSs.
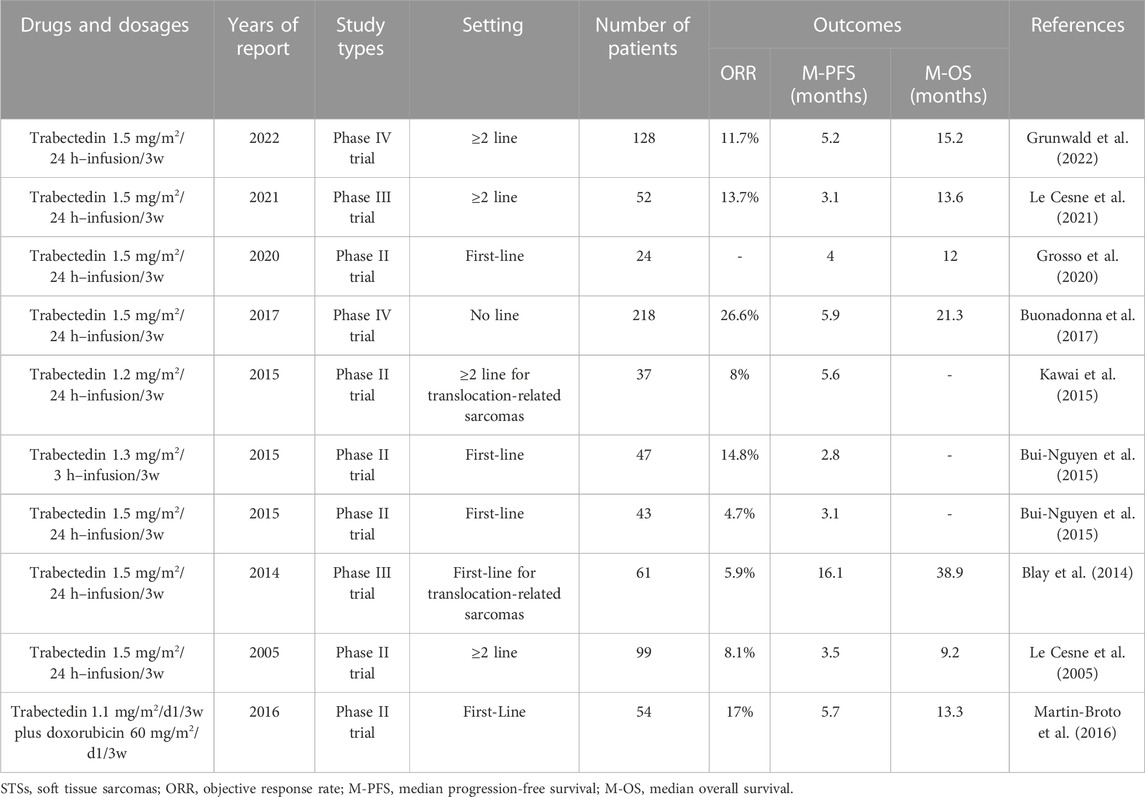
2.3 Trabectedin
Trabectedin is a natural compound initially isolated from the marine ascidian Ecteinascidia turbinata and can be obtained by high-purity chemical synthesis (Trabectedin, 2003; Cuevas and Francesch, 2009; Ganjoo and Patel, 2009). It has a unique structure with three-fused tetrahydroisoquinoline rings, which allow it to inhibit cancer cells by causing single- and double-strand DNA breaks, and several other key cellular biological processes and tumor microenvironments (Trabectedin, 2003; Cuevas and Francesch, 2009; Gordon et al., 2016; Larsen et al., 2016; Ratan and Patel, 2017; Wang et al., 2022). Trabectedin was approved in Europe in 2007 for the treatment of advanced STSs with previous anthracycline treatment failure and in the United States in 2015 for the treatment of patients with advanced leiomyosarcoma and liposarcoma with previous anthracycline treatment failure (Nakamura and Sudo, 2022). It is the most studied and widely used chemotherapeutic drug for STSs, in addition to doxorubicin and ifosfamide (Rastogi and Bakhshi, 2016; Dang et al., 2021; Le Cesne, 2022; Nakamura and Sudo, 2022; Wang et al., 2022). The ORR of trabectedin monotherapy for STS is 4.7%–14.8%, the median PFS is 2.8–5.9 months, and the median OS is 9.2–21.3 months (Table 3). Although these data are similar to those of doxorubicin or ifosfamide monotherapy, recent randomized controlled studies have demonstrated that trabectedin cannot replace doxorubicin as a first-line treatment for advanced STSs (Bui-Nguyen et al., 2015; Martin-Broto et al., 2016). In addition, several studies have demonstrated that the efficacy of trabectedin in the treatment of leiomyosarcoma and liposarcoma at the second- or above-line setting is significantly higher than in other STS subtypes (median PFS 5.1 versus 1.4 months, respectively) (Rastogi and Bakhshi, 2016; Schuetze, 2021; Vincenzi et al., 2023). Therefore, it is necessary to conduct randomized controlled clinical trials in a first-line setting to compare the activity of trabectedin and doxorubicin in these histological subtypes (Blay et al., 2014; Dang et al., 2021).
2.4 Taxanes
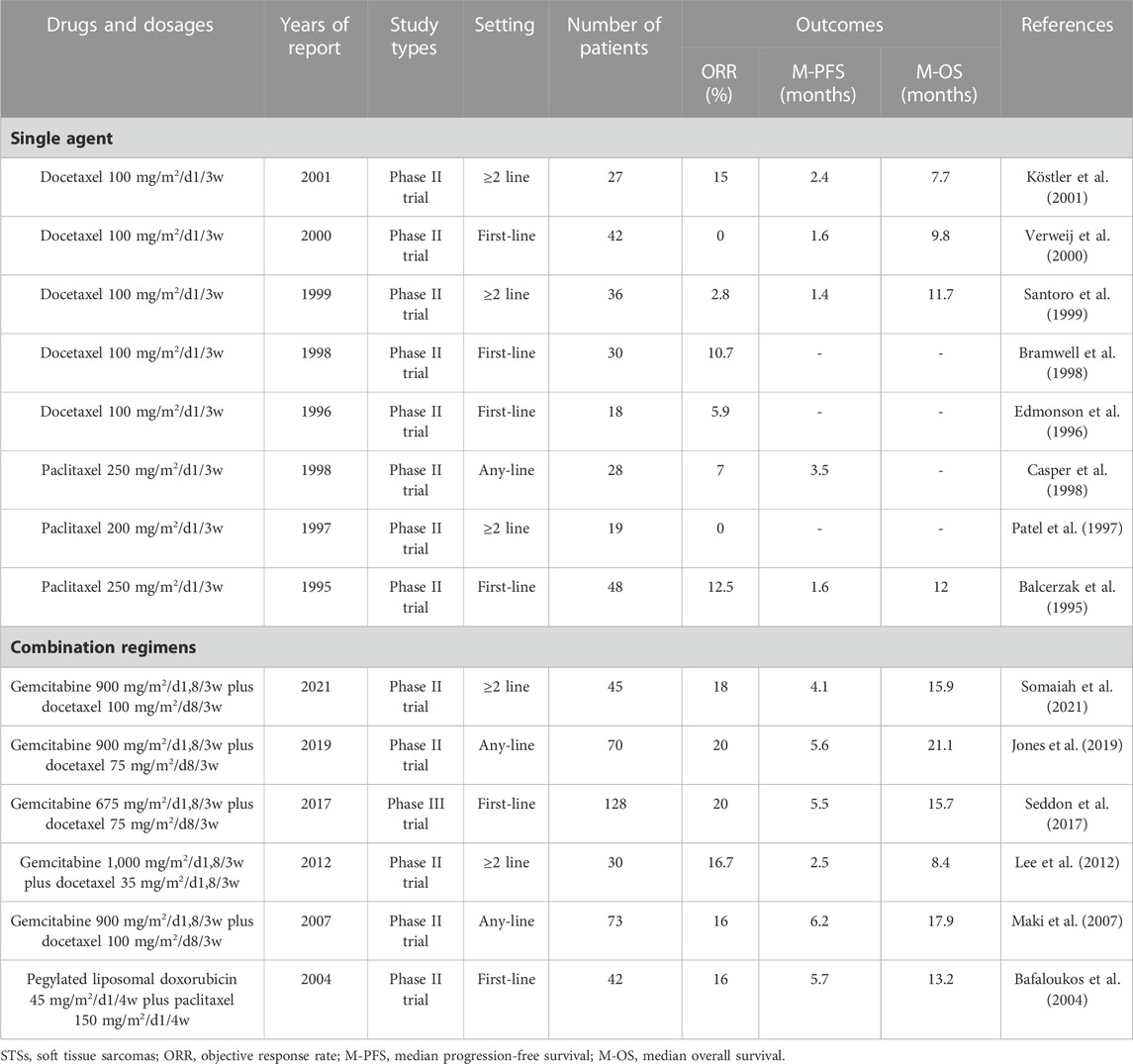
Taxanes are an important class of antitumor drugs that can interfere with the function of microtubules in cells, leading to chromosomal non-aggregation in multipolar spindles, mitotic failure, and ultimately cell death induction (Yared and Tkaczuk, 2012; Weaver, 2014). They include paclitaxel and docetaxel and various analogs or processes thereof.
Paclitaxel was originally extracted from Pacific yew trees with a minimal yield. After its synthesis, paclitaxel has been widely used for the treatment of many cancers with significant therapeutic effects (Mekhail and Markman, 2002). However, the efficacy of paclitaxel monotherapy in the treatment of the majority of STSs is poor (Casper et al., 1998). Currently, paclitaxel alone is the recommended treatment for angiosarcoma (Skubitz and Haddad, 2005; Bui et al., 2018; Pink et al., 2021). A 2004 study demonstrated that a combination of paclitaxel and liposomal doxorubicin achieved appropriate efficacy in the treatment of STSs (Bafaloukos et al., 2004). However, because this chemotherapy regimen has no significant advantages over other regimens, it is rarely mentioned.
Docetaxel is a reprocessed taxol-like substance produced by the needles of Taxus chinensis. The chemical structures between docetaxel and paclitaxel differ in two ways (Ojima et al., 2016). These small changes make docetaxel different from paclitaxel in terms of water solubility, cellular effects, and pharmacology (Zhang et al., 2019). However, docetaxel monotherapy for STSs has also been proven ineffective (Santoro et al., 1999; Verweij et al., 2000). The efficacy of the combination of docetaxel and gemcitabine in the treatment of STSs is significantly higher than that of docetaxel alone or gemcitabine alone (Maki et al., 2007). Moreover, the efficacy of docetaxel plus gemcitabine is comparable to that of doxorubicin alone (Table 4). However, docetaxel plus gemcitabine is cumbersome, costly, and toxic than doxorubicin monotherapy; therefore, it is not recommended as a first-line treatment for advanced STSs (Seddon et al., 2017). Notably, docetaxel plus gemcitabine is deemed more effective in patients with leiomyosarcoma than in patients with other histological subtypes (Bay et al., 2006; Maki, 2007; Pautier et al., 2012; Choi et al., 2018).
In summary, single-drug taxane is not recommended for the treatment of STSs. However, the docetaxel plus gemcitabine is considered second only to doxorubicin-based chemotherapy for STSs.
2.5 Gemcitabine
Gemcitabine is a cytotoxic nucleoside analog widely used in the treatment of malignant tumors. The metabolites of gemcitabine in cells can inhibit DNA synthesis via the inhibition of ribonucleotide reductase and compete with the nucleoside deoxycytidine as a fraudulent base, thereby producing antitumor effects (Barton-Burke, 1999; Wong et al., 2009). Although gemcitabine is widely used in other cancers, its efficacy alone in STSs is poor, with an ORR of 3%–8% and a median PFS of 1.5–3 months (Table 5). However, gemcitabine monotherapy has better efficacy in leiomyosarcoma and angiosarcoma (Pautier et al., 2012; Stacchiotti et al., 2012; Ducoulombier et al., 2016; Watson et al., 2022). Fortunately, gemcitabine combined with other drugs (such as docetaxel plus gemcitabine described above) can achieve better efficiency in STSs (Table 4; Table 5). Moreover, docetaxel plus gemcitabine has an efficacy comparable to that of doxorubicin-based chemotherapy in leiomyosarcoma and epithelioid sarcoma (Ducoulombier et al., 2016; Choi et al., 2018; Frezza et al., 2018). The efficacy of gemcitabine in combination with other drugs for STSs is inferior to that of docetaxel plus gemcitabine (Table 5). In addition, gemcitabine in combination with emerging drugs, such as pazopanib and eribulin, has also been tested for the treatment of STSs (Somaiah et al., 2021; Lopez-Alvarez et al., 2022). In summary, gemcitabine and docetaxel have similar efficacy and status in the treatment of STSs. The efficacy of their single-drug treatment is relatively low, whereas the gemcitabine plus docetaxel has comparable efficacy to first-line chemotherapy for STSs.
2.6 Dacarbazine
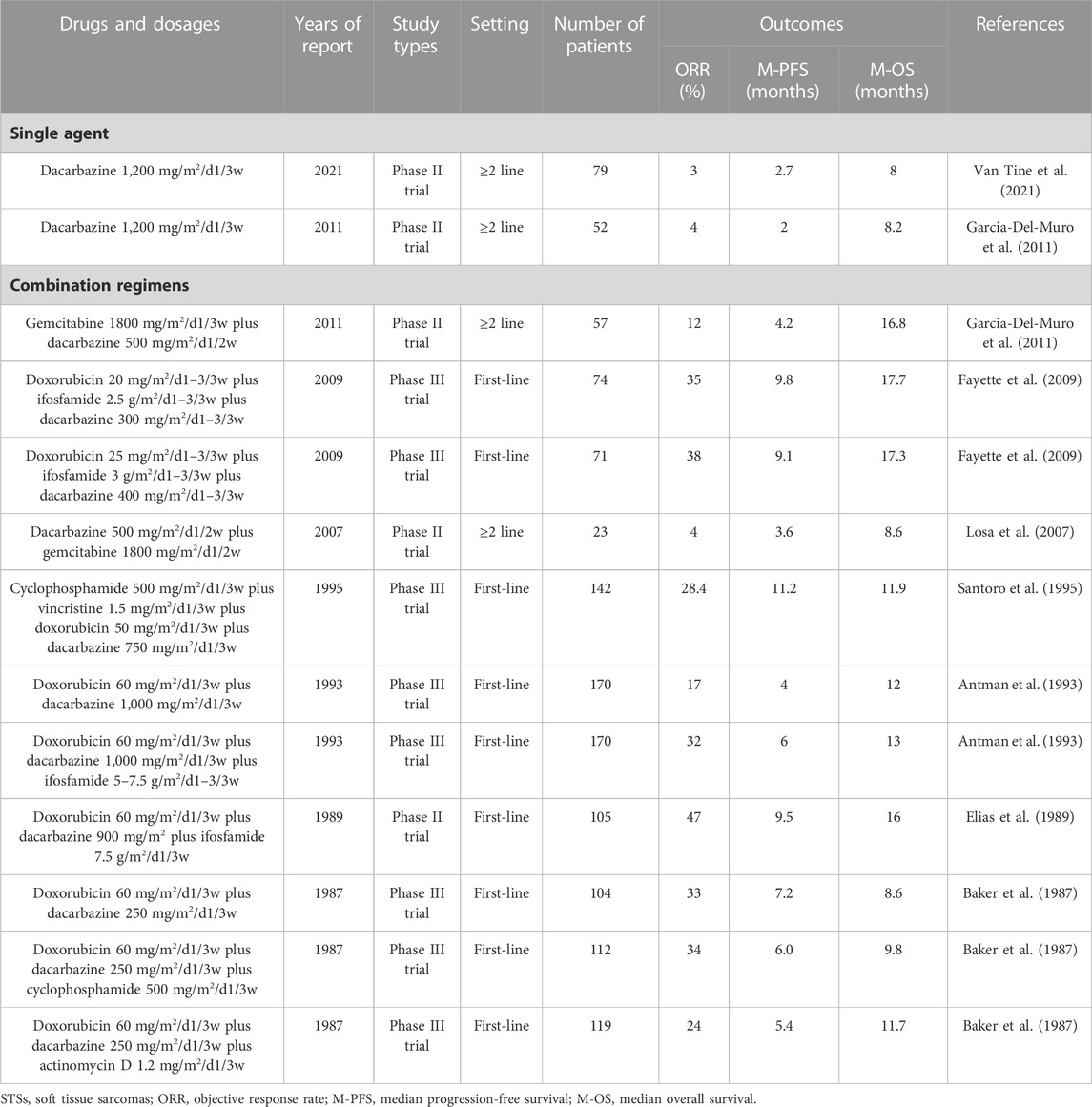
Dacarbazine is an alkylating agent, similar to oxazaphosphorine, which binds to DNA through metabolites in the body and establishes cross connections between the two strands, causing DNA replication to stop and ultimately leading to cell death (Huitema et al., 2000; Kantrowitz-Gordon et al., 2018; Karati et al., 2022). Additionally, dacarbazine exerts immune-stimulatory effects (Ugurel et al., 2013). Dacarbazine has a long history of application in STSs, with only mild activity, an ORR of 3%–4%, and a median PFS of 2–2.7 months (Table 6). Therefore, it is often used as a control drug in clinical trials of new drugs for second- or above-line treatment of STS (Demetri et al., 2016; Schoffski et al., 2016). In terms of combined use, dacarbazine is most commonly used in combination with doxorubicin and ifosfamide, and the efficacy is significant (Tables 1, 2, and 6). The combined regimen of dacarbazine and gemcitabine also has some efficacy in STSs (Table 6), but it is rarely used. In summary, as a veteran drug for the treatment of STSs, it is worthwhile to use dacarbazine in patients with STSs who have failed multiline treatment. In addition, a combined regimen of dacarbazine and other new drugs (such as trabectedin, eribulin, TKIs) is worth studying.
2.7 Eribulin
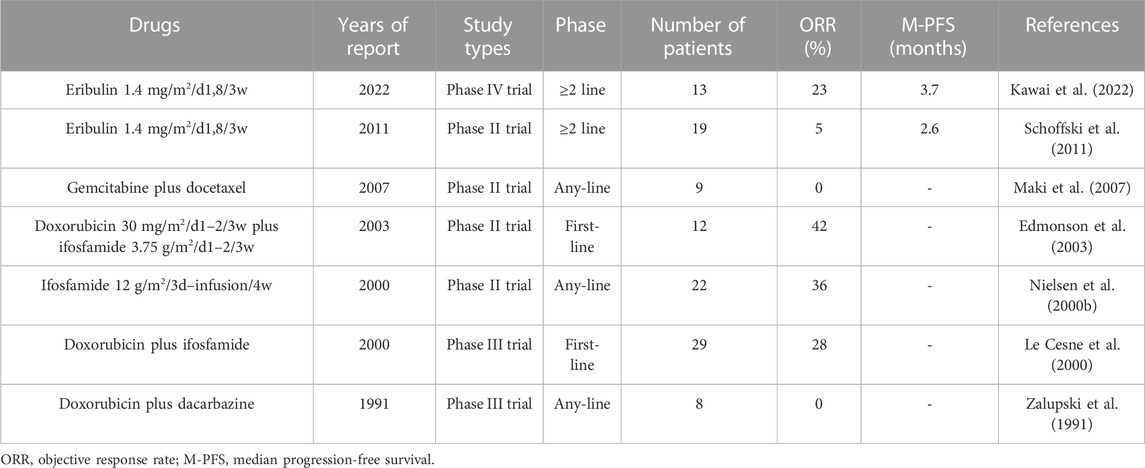
Similar to taxanes, eribulin inhibits microtubule polymerization. Similar to trabectedin, it is an anticancer drug found in marine organisms (Ratan and Patel, 2017). Eribulin is a synthetic analog of the naturally occurring anticancer agent halichondrin B in marine sponges (Shetty and Gupta, 2014). It exerts anticancer effects via multiple pathways. These pathways include the normalization of tumor blood vessels, inhibition of microtubule growth, isolation of microtubule proteins, reduction of microtubule supply, and reversal of the transition from mesenchymal to epithelial cells (Young and Woll, 2016; Phillips et al., 2022). In addition, eribulin has an important effect on the tumor immune microenvironment (Phillips et al., 2022). Although eribulin has various antitumor mechanisms, single-drug chemotherapy has limited efficacy in STSs (ORR, 0%–8%; median PFS, 2–4 months) (Table 7). However, eribulin alone has better efficacy in leiomyosarcoma and liposarcoma, especially in liposarcoma (Kawai et al., 2022). Owing to the short time since eribulin was approved for the treatment of STSs, there have been no clinical trials on eribulin-based combined chemotherapy for STSs. Eribulin also has therapeutic effects on angiosarcoma, pleomorphic sarcoma, synovial sarcoma, rhabdomyosarcomas, and myxofibrosarcoma (Phillips et al., 2022). Therefore, it is necessary to continue studying the activity of various eribulin-based combination regimens in STSs.
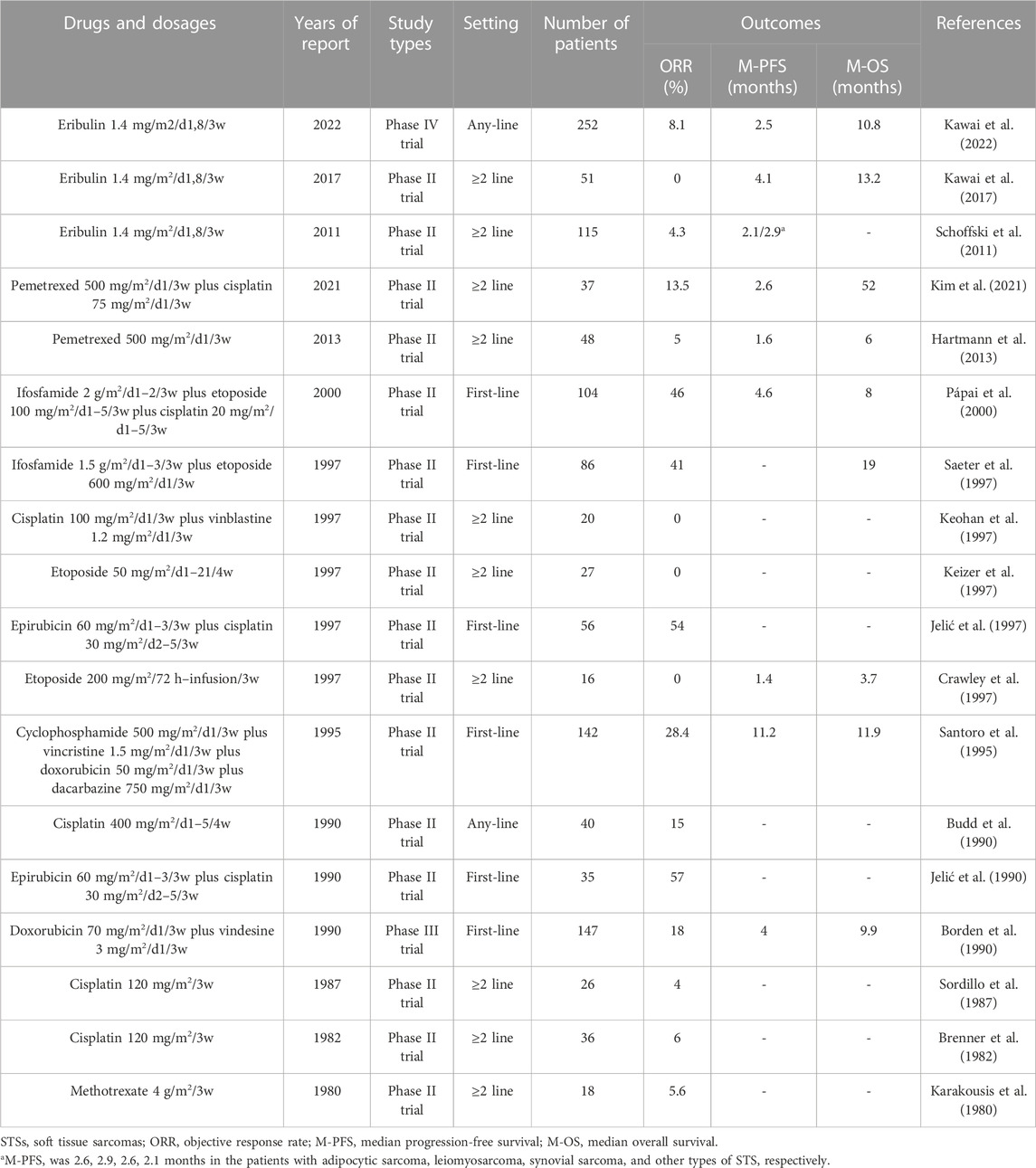
TABLE 7. Outcomes of representative clinical trials of eribulin and other drugs in nonspecific STSs.
2.8 Other chemotherapeutic drugs
In addition to the abovementioned drugs for treating STSs, many other drugs have been tested for the treatment of STSs. Vinca alkaloids (vindesine, vinblastine, vinorelbine, vincristine) have been widely tested in STSs, ultimately proving that they have important therapeutic effects in specific histological subtypes of STSs, such as rhabdomyosarcomas, whereas their activity in other subtypes is weak (Table 7). Methotrexate is one of the main drugs for the treatment of osteosarcomas (Belayneh et al., 2021). However, it is not involved in STS activity (Table 7) (Karakousis et al., 1980). Similarly, cisplatin is also one of the main drugs used for the treatment of osteosarcomas (Belayneh et al., 2021), with only slight activity in STSs (Table 7) (Brenner et al., 1982; Sordillo et al., 1987; Budd et al., 1990). The combined regimen of cisplatin with vinblastine or pemetrexed shows poor efficacy in STSs (Table 7) (Keohan et al., 1997; Kim et al., 2021). Cisplatin plus epirubicin has some activity in STSs (Table 7) (Jelić et al., 1990; Jelić et al., 1997), but this regimen is rarely used in the real world due to its high toxicity (Leahy et al., 2012; Nagar et al., 2018; Kim et al., 2019). As a widely used anticancer drug, etoposide has been tested repeatedly in STSs (Belani et al., 1994). However, whether administered orally or intravenously, the activity of etoposide alone in the STS is weak (Table 7) (Licht et al., 1994; Crawley et al., 1997; Keizer et al., 1997; Kebudi et al., 2004). Although ifosfamide plus etoposide shows some activity in STSs (Table 7) (Saeter et al., 1997; Yalçin et al., 1998; Pápai et al., 2000), this combined regimen is rarely used in the real world (Leahy et al., 2012; Nagar et al., 2018; Kim et al., 2019). In addition, researchers tested the activity of pemetrexed in STSs, and the results were disappointing (Table 7) (Hartmann et al., 2013; Kim et al., 2021).
In summary, anthracyclines, ifosfamide, trabectedin, gemcitabine, taxanes, dacarbazine and eribulin have certain activities in STSs. Vinca alkaloid agents (vindesine, vinblastine, vinorelbine, vincristine) have important therapeutic effects in specific STS subtypes, such as rhabdomyosarcomas, whereas their activity in other histological subtypes is weak. Other chemotherapeutic drugs (methotrexate, cisplatin, etoposide, pemetrexed) have weak efficacy in STSs and are rarely used.
3 Efficacy of different drugs in different STS histological subtypes
The high heterogeneity of STSs leads to a wide variety of histological subtypes. The rarity of STSs limits the development of large-scale, histologically specific clinical trials. Owing to the differences in the histological subtypes of STSs enrolled in clinical trials, there are differences in the efficacy of the same drug in various clinical trials. In addition, different STS subtypes respond differently to the same drugs. Therefore, the results of most clinical trials on advanced STSs are not applicable to all histological subtypes. To accurately describe the sensitivity of each STS subtype to different chemotherapeutic drugs, we analyzed the results of the prospective, multicenter clinical trials mentioned earlier in this review and recorded the efficacy of various drugs in different STS subtypes. Because most studies have not reported the remission results for each STS subtype in detail, the available results for some STS subtypes are sparse and limited. Therefore, we supplemented the results of some multicenter retrospective studies on some subtypes.
3.1 Leiomyosarcoma
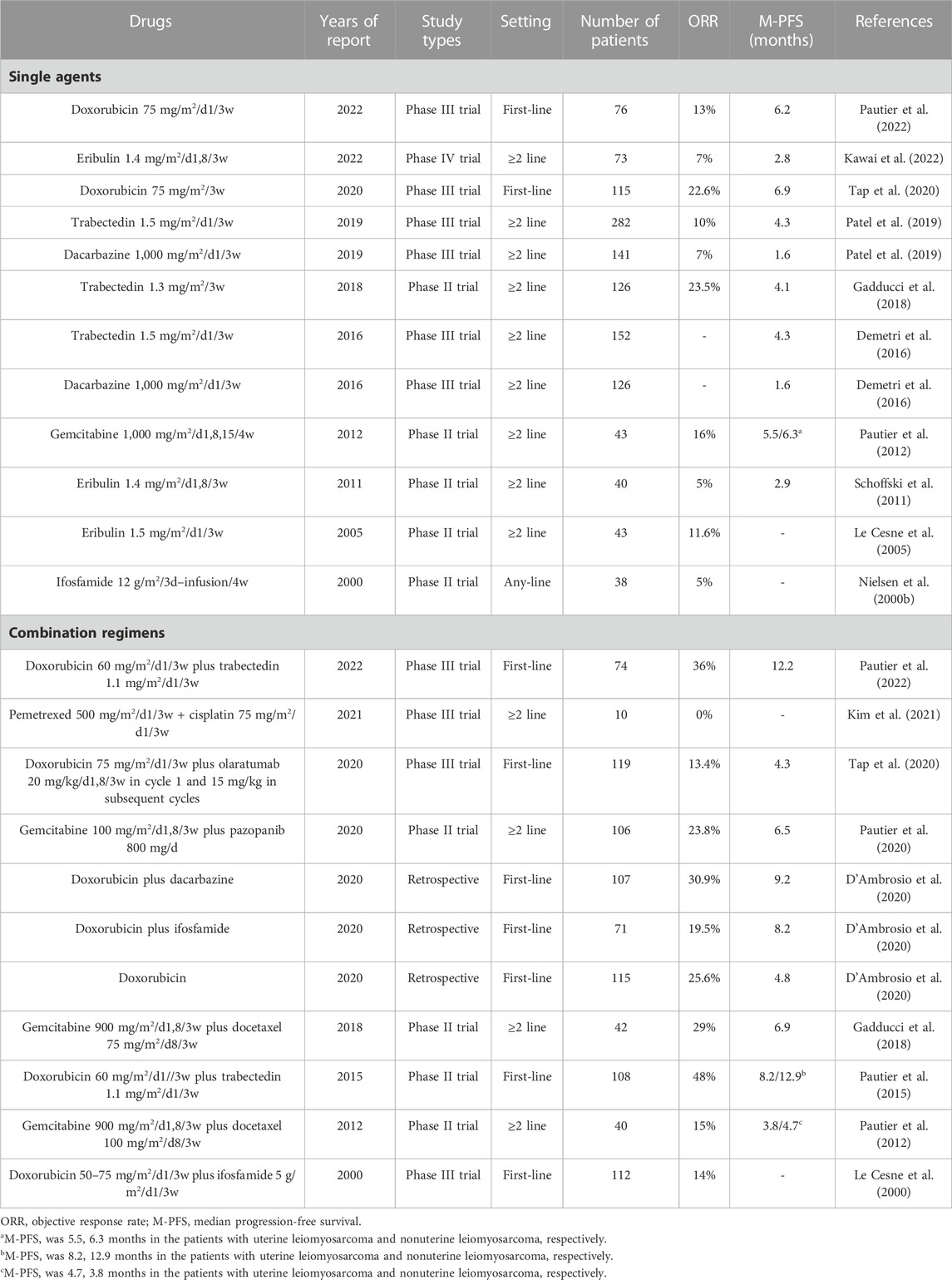
Leiomyosarcoma can be divided into those with uterine and non-uterine sources. The clinical characteristics of the two types of leiomyosarcoma are slightly different, and currently, the treatment options for leiomyosarcoma from both sources are the same (Pautier et al., 2022). Leiomyosarcoma is the most prevalent STS histotype, with an incidence of 0.5–1/100,000 (Hung et al., 2019; Kim et al., 2019; Gronchi et al., 2020). Thus, there has been a significant inclusion of leiomyosarcoma in most trials of STSs. According to the obtained data, the most effective agent for single-drug chemotherapy of leiomyosarcoma is doxorubicin, with an ORR of 13%–22.6% and a median PFS of 6.2–6.9 months (Table 8) (Tap et al., 2020; Pautier et al., 2022). The second is trabectedin or gemcitabine alone, which can also result in a median PFS of >4 months (Table 8) (Pautier et al., 2012; Demetri et al., 2016; Gadducci et al., 2018). Ifosfamide, dacarbazine, and eribulin alone show mild efficacy against leiomyosarcoma (Table 8). Currently, doxorubicin plus trabectedin is the most effective chemotherapy regimen for the treatment of leiomyosarcoma, with an ORR >36% and a median PFS of >12 months (Pautier et al., 2015). Gemcitabine-based combination chemotherapy also results in a median PFS of >6 months (Table 8). In addition, a retrospective study suggested that doxorubicin plus dacarbazine and doxorubicin plus ifosfamide also achieved better efficacy in advanced leiomyosarcoma (D'Ambrosio et al., 2020).
In summary, doxorubicin-based chemotherapy remains the first-line treatment for advanced leiomyosarcoma, with doxorubicin plus trabectedin achieving the longest median PFS. Gemcitabine-based chemotherapy also has good efficacy in leiomyosarcoma. However, other chemotherapeutic drugs show lower activity against leiomyosarcoma.
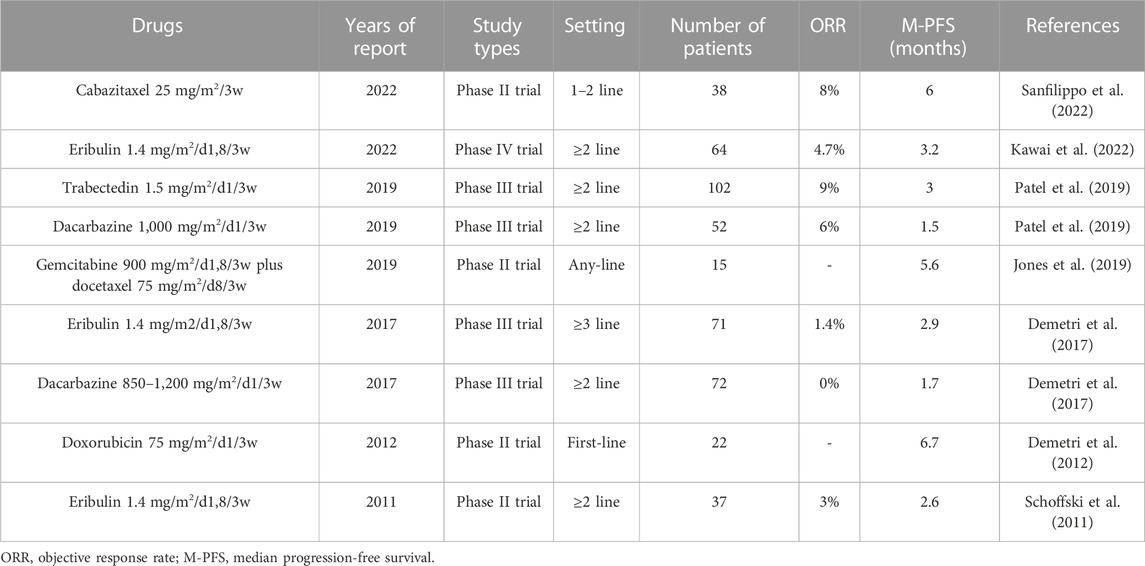
3.2 Liposarcomas
Liposarcomas are divided into well differentiated, dedifferentiated, myxoid, round cell, and pleomorphic subtypes. Each histological subtype has a unique clinical presentation and therapeutic response (Lee et al., 2018). Therefore, differences in the histological subtypes of the recruited patients may lead to large differences in the outcomes of different clinical trials. However, owing to the rarity of various histological subtypes of liposarcoma, most clinical trials have not differentiated responses between different subtypes of liposarcoma. To date, the most effective chemotherapeutic drug for treating advanced liposarcomas is doxorubicin, with a median PFS of 6.7 months (Table 9) (Demetri et al., 2012). Gemcitabine plus docetaxel also shows good efficacy, with a median PFS of 5.6 months (Table 9) (Jones et al., 2019). Trabectedin and eribulin, which have high activity in liposarcoma, achieve a median PFS of only approximately 3 months in clinical trials related to liposarcoma (Table 9), which is significantly lower than that of doxorubicin-based chemotherapy. This may be related to the fact that almost all clinical trials of trabectedin and eribulin in liposarcoma are set at second- or above-line setting. In addition, dacarbazine can only achieve a median PFS of <2 months in liposarcoma (Table 9).
In summary, doxorubicin-based chemotherapy or gemcitabine plus docetaxel is the first recommended option for advanced liposarcomas. In addition, it is worth testing the activity of trabectedin or eribulin alone or in combination with other drugs for advanced liposarcomas in a first-line setting.
3.3 Synovial sarcoma
Synovial sarcoma is a rare histotype of STSs, with an incidence of 0.1–0.5/100,000 (Wibmer et al., 2010; Hung et al., 2019; Aytekin et al., 2020). Clinical trials specifically targeting synovial sarcoma are rare. Extracting detailed treatment data for patients with synovial sarcoma from most clinical trials of STSs is also difficult. Nevertheless, important information can still be obtained. In prospective clinical trials, the currently proven drug with the best efficacy for the treatment of synovial sarcoma is ifosfamide (Table 10) (Nielsen et al., 2000b; Tap et al., 2017). Doxorubicin and eribulin also exhibit certain activities in synovial sarcoma (Table 10). However, gemcitabine, docetaxel, and dacarbazine show only weak activity against synovial sarcoma (Table 10). Retrospective studies confirmed these conclusions (Ferrari et al., 2015; Sanfilippo et al., 2015; Desar et al., 2018; Pender et al., 2018; Stacchiotti and Van Tine, 2018; Carter et al., 2020; Kogushi et al., 2020). A retrospective study demonstrated that trabectedin had activity in synovial sarcoma (Sanfilippo et al., 2015).
3.4 Other STS histological subtypes
The ORR of doxorubicin plus ifosfamide for undifferentiated pleomorphic sarcoma (UPS) is 29% (Le Cesne et al., 2000), that of doxorubicin plus dacarbazine is 26% (Zalupski et al., 1991), that of gemcitabine plus docetaxel is 11%–36% (Maki et al., 2007; Choi et al., 2018; Jones et al., 2019), and that of eribulin is 11% (Kawai et al., 2022).
Clinical trials of chemotherapy for angiosarcoma are rare. The only clinical trial has demonstrated an ORR of 45% for paclitaxel plus bevacizumab for the treatment of advanced angiosarcoma (Bui et al., 2018). Numerous other retrospective studies have demonstrated that doxorubicin- and gemcitabine-based chemotherapies can also achieve efficacy similar to that of paclitaxel in angiosarcoma (Skubitz and Haddad, 2005; Schlemmer et al., 2008; Penel et al., 2012; Stacchiotti et al., 2012; Young et al., 2014; Choi et al., 2018; Watson et al., 2022). In addition, eribulin is believed to exert activity in angiosarcoma (Kawai et al., 2022).
Doxorubicin plus ifosfamide is the most effective chemotherapy for treating malignant peripheral nerve tumors (MPNTs) (with an ORR of >20%) (Kroep et al., 2011). Doxorubicin and dacarbazine also have therapeutic effects (Zalupski et al., 1991). Gemcitabine, docetaxel, and eribulin are also ineffective against MPNT (Maki et al., 2007; Choi et al., 2018; Kawai et al., 2022).
Doxorubicin plus ifosfamide has a similar efficacy to gemcitabine plus docetaxel in epithelioid sarcoma, and both have moderate activity (Choi et al., 2018; Frezza et al., 2018; Touati et al., 2018).
Gemcitabine plus docetaxel chemotherapy has mild activity in clear cell sarcoma (Cojocaru et al., 2020).
3.5 Specific STS histological subtypes
In terms of chemotherapy, the main specific STS histological subtypes include the rhabdomyosarcoma family and the Ewing sarcoma family of tumors (Granowetter et al., 2009; Gallego et al., 2021; Agaram, 2022). They are more sensitive to chemotherapeutic drugs than other STSs (Chen et al., 2019; Bisogno and Hawkins, 2020; Gallego et al., 2021; Riggi et al., 2021; Setty et al., 2023). However, the sensitive drugs of these specific STS histological subtypes are significantly different from non-specific STS subtypes. Rhabdomyosarcomas can be divided into several subtypes, and the first-line chemotherapy drugs varies among different subtypes (Agaram, 2022; Sparber-Sauer, 2022; Bisogno et al., 2023). The first-line chemotherapy drug for pleomorphic rhabdomyosarcoma and adult spindle cell rhabdomyosarcoma is usually doxorubicin (Gallego et al., 2021; Gronchi et al., 2021), and for other subtypes include ifosfamide, vincristine, actinomycin D, doxorubicin, cyclophosphamide, and vinorelbine (Walterhouse et al., 2014; Walterhouse et al., 2017; Bisogno et al., 2019; Schoot et al., 2022; Bisogno et al., 2023). Ewing sarcoma family of tumors are considered main members of small round cell sarcomas (Rajwanshi et al., 2009; Marino-Enriquez and Fletcher, 2014; Domanski, 2022; Gajdzis et al., 2022). Vincristine, doxorubicin, cyclophosphamide, ifosfamide, and etoposide are the first-line drugs recommended for Ewing sarcomas (Brennan et al., 2022). Vincristine, irinotecan, and temozolomide are the recommended drugs for patients with rhabdomyosarcoma or Ewing sarcoma with recurrent or frontline chemotherapy failure (Defachelles et al., 2021; Xu et al., 2023). Trabectedin, gemcitabine, taxanes, dacarbazine and eribulin show ineffective or uncertain efficacy against rhabdomyosarcomas or Ewing sarcomas (Etcubanas et al., 1985; Baruchel et al., 2012; Mora et al., 2017; Oesterheld et al., 2020; Kawai et al., 2022).
In addition to the STS subtypes described above, dozens of other STS subtypes require chemotherapy in advanced stages. However, no specific prospective clinical trial has confirmed the chemotherapeutic efficacy of these STS subtypes. Generally, most silent STS subtypes are treated based on data from nonspecific STS clinical trials (Tables 1–7).
4 Discussion
We conducted this review to provide a reference for the selection of chemotherapeutic drugs for advanced STSs. In this study, we comprehensively reviewed the results of representative clinical trials related to chemotherapy for STS over the past 30 years and supplemented with some retrospective studies. Numerous clinical trial results have shown that doxorubicin is the most effective drug and remains the mainstay of chemotherapy for advanced STSs. In addition, ifosfamide, trabectedin, gemcitabine, taxanes, dacarbazine, and eribulin have certain activities in STSs and are commonly used in the real world. Vinca alkaloid agents (vindesine, vinblastine, vinorelbine, vincristine) have important therapeutic effects in special STS subtypes, such as rhabdomyosarcomas and Ewing sarcomas, whereas their activity in other histological subtypes is weak. Other chemotherapeutic drugs (methotrexate, cisplatin, etoposide, pemetrexed) have weak efficacy against STSs and are rarely used. Sensitive chemotherapeutic drugs vary for each STS histotype. Doxorubicin-based chemotherapy is the most effective treatment for leiomyosarcoma, liposarcoma, UPS, angiosarcoma, MPNT, and epithelioid sarcoma. Ifosfamide is the most effective chemotherapeutic drug for synovial sarcoma. Gemcitabine plus docetaxel shows good efficacy against many STS subtypes. However, except for the few histological subtypes mentioned above, other STS subtypes have become the silent majority, and few large sample size studies have focused on and reported the chemotherapeutic efficacy of these STSs in detail.
With an increase in drugs used for second- or above-line treatment, a better understanding of histotype-oriented therapy, and improved supportive care in oncology, the survival period of patients with advanced STSs has increased over the past decade (Kollar et al., 2019; Smrke et al., 2020; Stricker et al., 2023). The number of treatment lines for these patients is increasing, as is the demand for sensitive chemotherapeutic drugs. This study has important reference value for drug selection in multiline therapy of STSs. In addition, with the widespread and in-depth application of targeted drugs in STSs, the selection of specific chemotherapeutic drugs based on different histological subtypes in combination with targeted drugs is inevitable to achieve better therapeutic effects. This study provides important reference value for the selection of chemotherapeutic drugs for these combined regimens.
STSs are characterized by a low incidence rate and high heterogeneity compared with other cancers. The low incidence rate has led to a considerable number of STS histological subtypes not being studied in depth and has also led to a delay in the research and development of chemotherapeutic drugs related to STSs. Almost no important chemotherapeutic drugs for STSs have emerged in the last decade. The high heterogeneity of STSs has led to significant differences in the outcomes of clinical trials of chemotherapy in different STSs, leading to an inability to accurately compare the efficacy of different drugs in STSs. For example, although many studies have confirmed that different histological subtypes of STSs have different sensitivities to chemotherapy, the results of an important clinical trial showed that in a population of patients with high-risk STSs, there was no benefit of neoadjuvant histotype-tailored chemotherapy regimens over the standard doxorubicin plus ifosfamide chemotherapy (Gronchi et al., 2017; Gronchi et al., 2020). To eliminate the influence of the low incidence rate and high heterogeneity of STSs on judging the efficacy of chemotherapeutic drugs, significant work needs to be carried out, including the following: 1) Clinical trials targeting different STS subtypes should be conducted as much as possible, whereas clinical trials targeting nonselective STSs should be conducted to reduce the effects of high heterogeneity. 2) Detailed histotype data should be reported for clinical trials of STSs. We found that many clinical trials did not report the histotype outcomes, leading to difficulties in histotype studies. Reporting detailed histotype data is a fundamental requirement for these clinical trials. 3) The evaluation criteria are unified. Early evaluation of the chemotherapeutic efficacy of STSs often uses ORR while ignoring other indicators. The ORR does not represent the median PFS and OS. Therefore, the reference value for early clinical trials is limited. Currently, the number of drugs and lines for advanced STSs has increased significantly, and there is a significant error in using the median OS as the main evaluation index. We recommend using the median PFS and 3- or 6-month PFS rates as the main evaluation indicators.
We conducted extensive searches and reviews to include all the relevant studies. However, our approach does not represent a complete review of the literature. For example, some studies may have been omitted because we only included studies published in English and excluded most of the retrospective studies. However, efforts have been made to ensure the inclusion of key studies on the treatment of advanced STSs.
In conclusion, anthracyclines are the most important systemic treatment for advanced STSs. Ifosfamide, trabectedin, gemcitabine, taxanes, dacarbazine, and eribulin exhibit certain activities in STSs. Other chemotherapeutic drugs have weak efficacy against STSs and are rarely used. Depending on the histological subtypes, it is necessary to select specific second- or above-line chemotherapeutic drugs.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agaram, N. P. (2022). Evolving classification of rhabdomyosarcoma. Histopathology 80 (1), 98–108. doi:10.1111/his.14449
Amadeo, B., Penel, N., Coindre, J. M., Ray-Coquard, I., Ligier, K., Delafosse, P., et al. (2020). Incidence and time trends of sarcoma (2000-2013): results from the French network of cancer registries (FRANCIM). BMC Cancer 20 (1), 190. doi:10.1186/s12885-020-6683-0
Antman, K., Crowley, J., Balcerzak, S. P., Rivkin, S. E., Weiss, G. R., Elias, A., et al. (1993). An intergroup phase III randomized study of doxorubicin and dacarbazine with or without ifosfamide and mesna in advanced soft tissue and bone sarcomas. J. Clin. Oncol. 11 (7), 1276–1285. doi:10.1200/jco.1993.11.7.1276
Antman, K. H., Ryan, L., Elias, A., Sherman, D., and Grier, H. E. (1989). Response to ifosfamide and mesna: 124 previously treated patients with metastatic or unresectable sarcoma. J. Clin. Oncol. 7 (1), 126–131. doi:10.1200/jco.1989.7.1.126
Aubel-Sadron, G., and Londos-Gagliardi, D. (1984). Daunorubicin and doxorubicin, anthracycline antibiotics, a physicochemical and biological review. Biochimie 66 (5), 333–352. doi:10.1016/0300-9084(84)90018-x
Aytekin, M. N., Ozturk, R., Amer, K., and Yapar, A. (2020). Epidemiology, incidence, and survival of synovial sarcoma subtypes: SEER database analysis. J. Orthop. Surg. Hong. Kong) 28 (2), 2309499020936009. doi:10.1177/2309499020936009
Bafaloukos, D., Papadimitriou, C., Linardou, H., Aravantinos, G., Papakostas, P., Skarlos, D., et al. (2004). Combination of pegylated liposomal doxorubicin (PLD) and paclitaxel in patients with advanced soft tissue sarcoma: a phase II study of the hellenic cooperative oncology group. Br. J. Cancer 91 (9), 1639–1644. doi:10.1038/sj.bjc.6602148
Baker, L. H., Frank, J., Fine, G., Balcerzak, S. P., Stephens, R. L., Stuckey, W. J., et al. (1987). Combination chemotherapy using adriamycin, dtic, cyclophosphamide, and actinomycin D for advanced soft tissue sarcomas: a randomized comparative trial. A phase III, southwest oncology group study (7613). J. Clin. Oncol. 5 (6), 851–861. doi:10.1200/jco.1987.5.6.851
Balcerzak, S. P., Benedetti, J., Weiss, G. R., and Natale, R. B. (1995). A phase II trial of paclitaxel in patients with advanced soft tissue sarcomas. A Southwest Oncology Group study. Cancer-am cancer Soc. 76 (11), 2248–2252. doi:10.1002/1097-0142(19951201)76:11<2248:aid-cncr2820761111>3.0.co;2-y
Banks, L. B., and D'Angelo, S. P. (2022). The role of immunotherapy in the management of soft tissue sarcomas: current landscape and future outlook. J. Natl. Compr. Canc Netw. 20 (7), 834–844. doi:10.6004/jnccn.2022.7027
Barton-Burke, M. (1999). Gemcitabine: a pharmacologic and clinical overview. Cancer Nurs. 22 (2), 176–183. doi:10.1097/00002820-199904000-00011
Baruchel, S., Pappo, A., Krailo, M., Baker, K. S., Wu, B., Villaluna, D., et al. (2012). A phase 2 trial of trabectedin in children with recurrent rhabdomyosarcoma, ewing sarcoma and non-rhabdomyosarcoma soft tissue sarcomas: A report from the children's oncology group. Eur. J. Cancer 48 (4), 579–585. doi:10.1016/j.ejca.2011.09.027
Bay, J. O., Ray-Coquard, I., Fayette, J., Leyvraz, S., Cherix, S., Piperno-Neumann, S., et al. (2006). Docetaxel and gemcitabine combination in 133 advanced soft-tissue sarcomas: a retrospective analysis. Int. J. Cancer 119 (3), 706–711. doi:10.1002/ijc.21867
Belani, C. P., Doyle, L. A., and Aisner, J. (1994). Etoposide: current status and future perspectives in the management of malignant neoplasms. Cancer chemoth Pharm. 34, S118–S126. doi:10.1007/BF00684875
Belayneh, R., Fourman, M. S., Bhogal, S., and Weiss, K. R. (2021). Update on osteosarcoma. Curr. Oncol. Rep. 23 (6), 71. doi:10.1007/s11912-021-01053-7
Bhatt, N., Deady, S., Gillis, A., Bertuzzi, A., Fabre, A., Heffernan, E., et al. (2016). Epidemiological study of soft-tissue sarcomas in Ireland. Cancer Med. 5 (1), 129–135. doi:10.1002/cam4.547
Bisogno, G., De Salvo, G. L., Bergeron, C., Gallego Melcon, S., Merks, J. H., Kelsey, A., et al. (2019). Vinorelbine and continuous low-dose cyclophosphamide as maintenance chemotherapy in patients with high-risk rhabdomyosarcoma (RMS 2005): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 20 (11), 1566–1575. doi:10.1016/S1470-2045(19)30617-5
Bisogno, G., and Hawkins, D. S. (2020). An unresolved issue in rhabdomyosarcoma treatment: the duration of chemotherapy. Pediatr. Blood Cancer 67 (5), e28174. doi:10.1002/pbc.28174
Bisogno, G., Minard-Colin, V., Zanetti, I., Ferrari, A., Gallego, S., Dávila Fajardo, R., et al. (2023). Nonmetastatic rhabdomyosarcoma in children and adolescents: overall results of the European pediatric soft tissue sarcoma study group RMS2005 study. J. Clin. Oncol. 41, 2342–2349. doi:10.1200/jco.22.02093
Blay, J. Y., Leahy, M. G., Nguyen, B. B., Patel, S. R., Hohenberger, P., Santoro, A., et al. (2014). Randomised phase III trial of trabectedin versus doxorubicin-based chemotherapy as first-line therapy in translocation-related sarcomas. Eur. J. Cancer 50 (6), 1137–1147. doi:10.1016/j.ejca.2014.01.012
Bleloch, J. S., Ballim, R. D., Kimani, S., Parkes, J., Panieri, E., Willmer, T., et al. (2017). Managing sarcoma: where have we come from and where are we going? Ther. Adv. Med. Oncol. 9 (10), 637–659. doi:10.1177/1758834017728927
Borden, E. C., Amato, D. A., Edmonson, J. H., Ritch, P. S., and Shiraki, M. (1990). Randomized comparison of doxorubicin and vindesine to doxorubicin for patients with metastatic soft-tissue sarcomas. Cancer-am cancer Soc. 66 (5), 862–867. doi:10.1002/1097-0142(19900901)66:5<862:aid-cncr2820660509>3.0.co;2-r
Bramwell, V., Blackstein, M., Belanger, K., Verma, S., Beare, S., and Eisenhauer, E. (1998). A phase II study of docetaxel in chemotherapy-naïve patients with recurrent or metastatic adult soft tissue sarcoma. Sarcoma 2 (1), 29–33. doi:10.1080/13577149878136
Bramwell, V. H., Mouridsen, H. T., Santoro, A., Blackledge, G., Somers, R., Verwey, J., et al. (1987). Cyclophosphamide versus ifosfamide: final report of a randomized phase II trial in adult soft tissue sarcomas. Eur. J. Cancer Clin. Oncol. 23 (3), 311–321. doi:10.1016/0277-5379(87)90075-7
Brennan, B., Kirton, L., Marec-Berard, P., Gaspar, N., Laurence, V., Martin-Broto, J., et al. (2022). Comparison of two chemotherapy regimens in patients with newly diagnosed ewing sarcoma (EE2012): an open-label, randomised, phase 3 trial. Lancet 400 (10362), 1513–1521. doi:10.1016/S0140-6736(22)01790-1
Brenner, J., Magill, G. B., Sordillo, P. P., Cheng, E. W., and Yagoda, A. (1982). Phase II trial of cisplatin (CPDD) in previously treated patients with advanced soft tissue sarcoma. Cancer-am cancer Soc. 50 (10), 2031–2033. doi:10.1002/1097-0142(19821115)50:10<2031:aid-cncr2820501010>3.0.co;2-z
Budd, G. T., Metch, B., Balcerzak, S. P., Fletcher, W. S., Baker, L. H., and Mortimer, J. E. (1990). High-dose cisplatin for metastatic soft tissue sarcoma. Cancer-am cancer Soc. 65 (4), 866–869. doi:10.1002/1097-0142(19900215)65:4<866:aid-cncr2820650406>3.0.co;2-#
Bui, N., Kamat, N., Ravi, V., Chawla, S., Lohman, M., and Ganjoo, K. N. (2018). A multicenter phase II study of Q3 week or weekly paclitaxel in combination with bevacizumab for the treatment of metastatic or unresectable angiosarcoma. Rare tumors 10, 2036361318771771. doi:10.1177/2036361318771771
Bui-Nguyen, B., Butrynski, J. E., Penel, N., Blay, J. Y., Isambert, N., Milhem, M., et al. (2015). A phase IIb multicentre study comparing the efficacy of trabectedin to doxorubicin in patients with advanced or metastatic untreated soft tissue sarcoma: the TRUSTS trial. Eur. J. Cancer 51 (10), 1312–1320. doi:10.1016/j.ejca.2015.03.023
Buonadonna, A., Benson, C., Casanova, J., Kasper, B., Lopez Pousa, A., Mazzeo, F., et al. (2017). A noninterventional, multicenter, prospective phase IV study of trabectedin in patients with advanced soft tissue sarcoma. Anticancer Drugs 28 (10), 1157–1165. doi:10.1097/CAD.0000000000000560
Carter, T. J., Milic, M., McDerra, J., McTiernan, A., Ahmed, M., Karavasilis, V., et al. (2020). Continuous 14 Day infusional ifosfamide for management of soft-tissue and bone sarcoma: a single centre retrospective cohort analysis. Cancers (Basel) 12 (11), 3408. doi:10.3390/cancers12113408
Carvalho, C., Santos, R. X., Cardoso, S., Correia, S., Oliveira, P. J., Santos, M. S., et al. (2009). Doxorubicin: the good, the bad and the ugly effect. Curr. Med. Chem. 16 (25), 3267–3285. doi:10.2174/092986709788803312
Casper, E. S., Waltzman, R. J., Schwartz, G. K., Sugarman, A., Pfister, D., Ilson, D., et al. (1998). Phase II trial of paclitaxel in patients with soft-tissue sarcoma. Cancer Invest. 16 (7), 442–446. doi:10.3109/07357909809011697
Chamberlain, F. E., Jones, R. L., and Chawla, S. P. (2019). Aldoxorubicin in soft tissue sarcomas. Future Oncol. 15 (13), 1429–1435. doi:10.2217/fon-2018-0922
Chawla, S. P., Papai, Z., Mukhametshina, G., Sankhala, K., Vasylyev, L., Fedenko, A., et al. (2015). First-line aldoxorubicin vs doxorubicin in metastatic or locally advanced unresectable soft-tissue sarcoma: a phase 2b randomized clinical trial. JAMA Oncol. 1 (9), 1272–1280. doi:10.1001/jamaoncol.2015.3101
Chen, C., Dorado Garcia, H., Scheer, M., and Henssen, A. G. (2019). Current and future treatment strategies for rhabdomyosarcoma. Front. Oncol. 9, 1458. doi:10.3389/fonc.2019.01458
Choi, Y., Yun, M. S., Lim, S. H., Lee, J., Ahn, J. H., Kim, Y. J., et al. (2018). Gemcitabine and docetaxel combination for advanced soft tissue sarcoma: a nationwide retrospective study. Cancer Res. Treat. 50 (1), 175–182. doi:10.4143/crt.2016.535
Cojocaru, E., Napolitano, A., Fisher, C., Huang, P., Jones, R. L., and Thway, K. (2022). What's the latest with investigational drugs for soft tissue sarcoma? Expert Opin. Investig. Drugs 31 (11), 1239–1253. doi:10.1080/13543784.2022.2152324
Cojocaru, E., Thway, K., Fisher, C., Messiou, C., Zaidi, S., Miah, A. B., et al. (2020). Efficacy of gemcitabine-based chemotherapy in clear cell sarcoma of soft tissue. Anticancer Res. 40 (12), 7003–7007. doi:10.21873/anticanres.14725
Corey, R. M., Swett, K., and Ward, W. G. (2014). Epidemiology and survivorship of soft tissue sarcomas in adults: a national cancer database report. Cancer Med. 3 (5), 1404–1415. doi:10.1002/cam4.288
Crawley, C. R., Judson, I. R., Verrill, M., Hill, C., and Raynaud, F. I. (1997). A phase I/II study of a 72-h continuous infusion of etoposide in advanced soft tissue sarcoma. Sarcoma 1 (3-4), 149–154. doi:10.1080/13577149778236
Cuevas, C., and Francesch, A. (2009). Development of Yondelis (trabectedin, ET-743). A semisynthetic process solves the supply problem. Nat. Prod. Rep. 26 (3), 322–337. doi:10.1039/b808331m
D'Ambrosio, L., Touati, N., Blay, J. Y., Grignani, G., Flippot, R., Czarnecka, A. M., et al. (2020). Doxorubicin plus dacarbazine, doxorubicin plus ifosfamide, or doxorubicin alone as a first-line treatment for advanced leiomyosarcoma: a propensity score matching analysis from the European organization for research and treatment of cancer soft tissue and bone sarcoma group. Cancer 126 (11), 2637–2647. doi:10.1002/cncr.32795
Dang, J., Fu, J., Zhang, Z., Liu, D., Cheng, D., and Fan, H. (2021). Comparison between trabectedin and doxorubicin in soft-tissue sarcomas: a systematic review and meta-analysis. Ann. Transl. Med. 9 (24), 1764. doi:10.21037/atm-21-6033
de Juan Ferre, A., Alvarez Alvarez, R., Casado Herraez, A., Cruz Jurado, J., Estival Gonzalez, A., Martin-Broto, J., et al. (2021). SEOM Clinical Guideline of management of soft-tissue sarcoma (2020). Clin. Transl. Oncol. 23 (5), 922–930. doi:10.1007/s12094-020-02534-0
Defachelles, A. S., Bogart, E., Casanova, M., Merks, J. H. M., Bisogno, G., Calareso, G., et al. (2021). Randomized phase II trial of vincristine-irinotecan with or without temozolomide, in children and adults with relapsed or refractory rhabdomyosarcoma: a European paediatric soft tissue sarcoma study group and innovative therapies for children with cancer trial. J. Clin. Oncol. 39 (27), 2979–2990. doi:10.1200/jco.21.00124
Demetri, G. D., Le Cesne, A., Chawla, S. P., Brodowicz, T., Maki, R. G., Bach, B. A., et al. (2012). First-line treatment of metastatic or locally advanced unresectable soft tissue sarcomas with conatumumab in combination with doxorubicin or doxorubicin alone: a phase I/II open-label and double-blind study. Eur. J. Cancer 48 (4), 547–563. doi:10.1016/j.ejca.2011.12.008
Demetri, G. D., Schöffski, P., Grignani, G., Blay, J. Y., Maki, R. G., Van Tine, B. A., et al. (2017). Activity of eribulin in patients with advanced liposarcoma demonstrated in a subgroup analysis from a randomized phase III study of eribulin versus dacarbazine. J. Clin. Oncol. 35 (30), 3433–3439. doi:10.1200/jco.2016.71.6605
Demetri, G. D., von Mehren, M., Jones, R. L., Hensley, M. L., Schuetze, S. M., Staddon, A., et al. (2016). Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. J. Clin. Oncol. 34 (8), 786–793. doi:10.1200/JCO.2015.62.4734
Desar, I. M. E., Fleuren, E. D. G., and van der Graaf, W. T. A. (2018). Systemic treatment for adults with synovial sarcoma. Curr. Treat. Options Oncol. 19 (2), 13. doi:10.1007/s11864-018-0525-1
Dileo, P., Morgan, J. A., Zahrieh, D., Desai, J., Salesi, J. M., Harmon, D. C., et al. (2007). Gemcitabine and vinorelbine combination chemotherapy for patients with advanced soft tissue sarcomas: results of a phase II trial. Cancer 109 (9), 1863–1869. doi:10.1002/cncr.22609
Domanski, H. A. (2022). The small round cell sarcomas complexities and desmoplastic presentation. Acta Cytol. 66 (4), 279–294. doi:10.1159/000524260
Ducoulombier, A., Cousin, S., Kotecki, N., and Penel, N. (2016). Gemcitabine-based chemotherapy in sarcomas: a systematic review of published trials. Crit. Rev. Oncol. Hematol. 98, 73–80. doi:10.1016/j.critrevonc.2015.10.020
Edmonson, J. H., Ebbert, L. P., Nascimento, A. G., Jung, S. H., McGaw, H., and Gerstner, J. B. (1996). Phase II study of docetaxel in advanced soft tissue sarcomas. Am. J. Clin. oncol-canc 19 (6), 574–576. doi:10.1097/00000421-199612000-00008
Edmonson, J. H., Ryan, L. M., Falkson, C. I., Hicks, D. G., and Blum, R. H. (2003). Phase II study of Ifosfamide+Doxorubicin in patients with advanced synovial sarcomas (E1793): a trial of the eastern cooperative oncology group. Sarcoma 7 (1), 9–11. doi:10.1080/1357714031000114156
Elias, A., Ryan, L., Sulkes, A., Collins, J., Aisner, J., and Antman, K. H. (1989). Response to mesna, doxorubicin, ifosfamide, and dacarbazine in 108 patients with metastatic or unresectable sarcoma and no prior chemotherapy. J. Clin. Oncol. 7 (9), 1208–1216. doi:10.1200/JCO.1989.7.9.1208
Etcubanas, E., Horowitz, M., and Vogel, R. (1985). Combination of dacarbazine and doxorubicin in the treatment of childhood rhabdomyosarcoma. Cancer Treat. Rep. 69 (9), 999–1000.
Fayette, J., Penel, N., Chevreau, C., Blay, J. Y., Cupissol, D., Thyss, A., et al. (2009). Phase III trial of standard versus dose-intensified doxorubicin, ifosfamide and dacarbazine (MAID) in the first-line treatment of metastatic and locally advanced soft tissue sarcoma. Invest. New Drugs 27 (5), 482–489. doi:10.1007/s10637-008-9217-1
Ferrari, A., De Salvo, G. L., Brennan, B., van Noesel, M. M., De Paoli, A., Casanova, M., et al. (2015). Synovial sarcoma in children and adolescents: the European pediatric soft tissue sarcoma study group prospective trial (EpSSG NRSTS 2005). Ann. Oncol. 26 (3), 567–572. doi:10.1093/annonc/mdu562
Frezza, A. M., Jones, R. L., Lo Vullo, S., Asano, N., Lucibello, F., Ben-Ami, E., et al. (2018). Anthracycline, gemcitabine, and pazopanib in epithelioid sarcoma: a multi-institutional case series. Jama Oncol. 4 (9), e180219. doi:10.1001/jamaoncol.2018.0219
Frezza, A. M., Stacchiotti, S., and Gronchi, A. (2017). Systemic treatment in advanced soft tissue sarcoma: what is standard, what is new. BMC Med. 15 (1), 109. doi:10.1186/s12916-017-0872-y
Fuchs, J. W., Schulte, B. C., Fuchs, J. R., and Agulnik, M. (2023). Targeted therapies for the treatment of soft tissue sarcoma. Front. Oncol. 13, 1122508. doi:10.3389/fonc.2023.1122508
Gadducci, A., Grosso, F., Scambia, G., Raspagliesi, F., Colombo, N., Grignani, G., et al. (2018). A phase II randomised (calibrated design) study on the activity of the single-agent trabectedin in metastatic or locally relapsed uterine leiomyosarcoma. Br. J. Cancer 119 (5), 565–571. doi:10.1038/s41416-018-0190-y
Gajdzis, P., Pierron, G., and Klijanienko, J. (2022). Cytology of undifferentiated round-cell sarcomas of bone and soft tissue: ewing sarcoma or not ewing sarcoma, that is the question. Acta Cytol. 66 (4), 295–306. doi:10.1159/000518146
Gallego, S., Bernabeu, D., Garrido-Pontnou, M., Guillen, G., Hindi, N., Juan-Ribelles, A., et al. (2021). GEIS-SEHOP clinical practice guidelines for the treatment of rhabdomyosarcoma. Clin. Transl. Oncol. 23 (12), 2460–2473. doi:10.1007/s12094-021-02654-1
Ganjoo, K. N., and Patel, S. R. (2009). Trabectedin: an anticancer drug from the sea. Expert Opin. Pharm. 10 (16), 2735–2743. doi:10.1517/14656560903277236
Garcia-Del-Muro, X., Lopez-Pousa, A., Maurel, J., Martin, J., Martinez-Trufero, J., Casado, A., et al. (2011). Randomized phase II study comparing gemcitabine plus dacarbazine versus dacarbazine alone in patients with previously treated soft tissue sarcoma: a Spanish group for research on sarcomas study. J. Clin. Oncol. 29 (18), 2528–2533. doi:10.1200/JCO.2010.33.6107
Giraud, B., Hebert, G., Deroussent, A., Veal, G. J., Vassal, G., and Paci, A. (2010). Oxazaphosphorines: new therapeutic strategies for an old class of drugs. Expert opin drug6 (8), 919–938. doi:10.1517/17425255.2010.487861
Gordon, E. M., Sankhala, K. K., Chawla, N., and Chawla, S. P. (2016). Trabectedin for soft tissue sarcoma: current status and future perspectives. Adv. Ther. 33 (7), 1055–1071. doi:10.1007/s12325-016-0344-3
Granowetter, L., Womer, R., Devidas, M., Krailo, M., Wang, C., Bernstein, M., et al. (2009). Dose-intensified compared with standard chemotherapy for nonmetastatic ewing sarcoma family of tumors: A children's oncology group study. J. Clin. Oncol. 27 (15), 2536–2541. doi:10.1200/JCO.2008.19.1478
Gronchi, A., Ferrari, S., Quagliuolo, V., Broto, J. M., Pousa, A. L., Grignani, G., et al. (2017). Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): an international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol. 18 (6), 812–822. doi:10.1016/S1470-2045(17)30334-0
Gronchi, A., Miah, A. B., Dei Tos, A. P., Abecassis, N., Bajpai, J., Bauer, S., et al. (2021). Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 32 (11), 1348–1365. doi:10.1016/j.annonc.2021.07.006
Gronchi, A., Palmerini, E., Quagliuolo, V., Martin Broto, J., Lopez Pousa, A., Grignani, G., et al. (2020). Neoadjuvant chemotherapy in high-risk soft tissue sarcomas: final results of a randomized trial from Italian (ISG), Spanish (GEIS), French (FSG), and polish (PSG) sarcoma groups. J. Clin. Oncol. 38 (19), 2178–2186. doi:10.1200/JCO.19.03289
Grosso, F., D'Ambrosio, L., Zucchetti, M., Ibrahim, T., Tamberi, S., Matteo, C., et al. (2020). Pharmacokinetics, safety, and activity of trabectedin as first-line treatment in elderly patients who are affected by advanced sarcoma and are unfit to receive standard chemotherapy: a phase 2 study (TR1US study) from the Italian sarcoma group. Cancer 126 (21), 4726–4734. doi:10.1002/cncr.33120
Grünwald, V., Karch, A., Schuler, M., Schöffski, P., Kopp, H. G., Bauer, S., et al. (2020). Randomized comparison of pazopanib and doxorubicin as first-line treatment in patients with metastatic soft tissue sarcoma age 60 Years or older: results of a German intergroup study. J. Clin. Oncol. 38 (30), 3555–3564. doi:10.1200/jco.20.00714
Grunwald, V., Pink, D., Egerer, G., Schalk, E., Augustin, M., Deinzer, C. K. W., et al. (2022). Trabectedin for patients with advanced soft tissue sarcoma: a non-interventional, prospective, multicenter, phase IV trial. Cancers (Basel) 14 (21), 5234. doi:10.3390/cancers14215234
Haddox, C. L., and Riedel, R. F. (2020). Individualizing systemic therapy for advanced soft tissue sarcomas based on tumor histology and biology. Expert Rev. Anticancer Ther. 20 (1), 5–8. doi:10.1080/14737140.2020.1708198
Hartmann, J. T., Bauer, S., Egerer, G., Horger, M. S., Kopp, H. G., Grunwald, V., et al. (2013). Pemetrexed in patients with refractory soft tissue sarcoma: a non-comparative multicenter phase II study of the German sarcoma group AIO-STS 005. Invest. New Drugs 31 (1), 167–174. doi:10.1007/s10637-012-9840-8
Hartmann, J. T., Kopp, H. G., Gruenwald, V., Piperno-Neumann, S., Kunitz, A., Hofheinz, R., et al. (2020). German Sarcoma Group within the Working Group Medical Oncology of the German Cancer Society/Aio-Sts: randomised phase II trial of trofosfamide vs. doxorubicin in elderly patients with untreated metastatic soft-tissue sarcoma. Eur. J. Cancer 124, 152–160. doi:10.1016/j.ejca.2019.10.016
Hatcher, H., Benson, C., and Ajithkumar, T. (2017). Systemic treatments in soft tissue sarcomas. Clin. Oncol. R. Coll. Radiol. 29 (8), 507–515. doi:10.1016/j.clon.2017.05.002
Huitema, A. D., Smits, K. D., Mathôt, R. A., Schellens, J. H., Rodenhuis, S., and Beijnen, J. H. (2000). The clinical pharmacology of alkylating agents in high-dose chemotherapy. Anti-cancer drug 11 (7), 515–533. doi:10.1097/00001813-200008000-00002
Hung, G. Y., Horng, J. L., Chen, P. C., Lin, L. Y., Chen, J. Y., Chuang, P. H., et al. (2019). Incidence of soft tissue sarcoma in taiwan: A nationwide population-based study (2007-2013). Cancer Epidemiol. 60, 185–192. doi:10.1016/j.canep.2019.04.007
Jasra, S., and Anampa, J. (2018). Anthracycline use for early stage breast cancer in the modern era: a review. Curr. Treat. Options Oncol. 19 (6), 30. doi:10.1007/s11864-018-0547-8
Jelić, S., Kovcin, V., Milanović, N., Babović, N., Kreacić, M., Ristović, Z., et al. (1997). Randomised study of high-dose epirubicin versus high-dose epirubicin-cisplatin chemotherapy for advanced soft tissue sarcoma. Eur. J. cancer 33 (2), 220–225. doi:10.1016/s0959-8049(96)00297-3
Jelić, S., Vuletić, L., Milanović, N., Tomasević, Z., and Kovcin, V. (1990). High-dose epirubicin-cisplatin chemotherapy for advanced soft tissue sarcoma. Tumori J. 76 (5), 467–471. doi:10.1177/030089169007600510
Jones, R. L., Chawla, S. P., Attia, S., Schoffski, P., Gelderblom, H., Chmielowski, B., et al. (2019). A phase 1 and randomized controlled phase 2 trial of the safety and efficacy of the combination of gemcitabine and docetaxel with ontuxizumab (MORAb-004) in metastatic soft-tissue sarcomas. Cancer 125 (14), 2445–2454. doi:10.1002/cncr.32084
Judson, I., Radford, J. A., Harris, M., Blay, J. Y., van Hoesel, Q., le Cesne, A., et al. (2001). Randomised phase II trial of pegylated liposomal doxorubicin (DOXIL/CAELYX) versus doxorubicin in the treatment of advanced or metastatic soft tissue sarcoma: A study by the EORTC soft tissue and bone sarcoma group. Eur. J. cancer 37 (7), 870–877. doi:10.1016/s0959-8049(01)00050-8
Judson, I., Verweij, J., Gelderblom, H., Hartmann, J. T., Schoffski, P., Blay, J. Y., et al. (2014). Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol. 15 (4), 415–423. doi:10.1016/S1470-2045(14)70063-4
Kantrowitz-Gordon, I., Hays, K., Kayode, O., Kumar, A. R., Kaplan, H. G., Reid, J. M., et al. (2018). Pharmacokinetics of dacarbazine (DTIC) in pregnancy. Cancer Chemother. Pharmacol. 81 (3), 455–460. doi:10.1007/s00280-017-3511-6
Karakousis, C. P., Rao, U., and Carlson, M. (1980). High-dose methotrexate as secondary chemotherapy in metastatic soft-tissue sarcomas. Cancer-am cancer Soc. 46 (6), 1345–1348. doi:10.1002/1097-0142(19800915)46:6<1345:aid-cncr2820460608>3.0.co;2-r
Karati, D., Mahadik, K. R., Trivedi, P., and Kumar, D. (2022). Alkylating agents, the road less traversed, changing anticancer therapy. Anticancer Agents Med. Chem. 22 (8), 1478–1495. doi:10.2174/1871520621666210811105344
Kawai, A., Araki, N., Naito, Y., Ozaki, T., Sugiura, H., Yazawa, Y., et al. (2017). Phase 2 study of eribulin in patients with previously treated advanced or metastatic soft tissue sarcoma. Jpn. J. Clin. Oncol. 47 (2), 137–144. doi:10.1093/jjco/hyw175
Kawai, A., Araki, N., Sugiura, H., Ueda, T., Yonemoto, T., Takahashi, M., et al. (2015). Trabectedin monotherapy after standard chemotherapy versus best supportive care in patients with advanced, translocation-related sarcoma: A randomised, open-label, phase 2 study. Lancet Oncol. 16 (4), 406–416. doi:10.1016/S1470-2045(15)70098-7
Kawai, A., Narahara, H., Takahashi, S., Nakamura, T., Kobayashi, H., Megumi, Y., et al. (2022). Safety and effectiveness of eribulin in Japanese patients with soft tissue sarcoma including rare subtypes: A post-marketing observational study. BMC Cancer 22 (1), 528. doi:10.1186/s12885-022-09527-y
Kebudi, R., Gorgun, O., and Ayan, I. (2004). Oral etoposide for recurrent/progressive sarcomas of childhood. Pediatr. Blood Cancer 42 (4), 320–324. doi:10.1002/pbc.10393
Keizer, H. J., Crowther, D., Nielsen, O. S., Oosterom, A. T., Muguiro, J. H., Pottelberghe, C. V., et al. (1997). EORTC group phase II study of oral etoposide for pretreated soft tissue sarcoma. Sarcoma 1 (2), 99–101. doi:10.1080/13577149778371
Keohan, M. L., Grever, M. R., Balcerzak, S. P., and Antman, K. (1997). A phase II Southwest Oncology Group study of cisplatin and continuous infusion vinblastine in the treatment of advanced soft tissue sarcoma. Invest. new drug 15 (3), 255–256. doi:10.1023/a:1005803921371
Kerbusch, T., de Kraker, J., Keizer, H. J., van Putten, J. W., Groen, H. J., Jansen, R. L., et al. (2001). Clinical pharmacokinetics and pharmacodynamics of ifosfamide and its metabolites. Clin. Pharmacokinet. 40 (1), 41–62. doi:10.2165/00003088-200140010-00004
Kim, H. S., Nam, C. M., Jang, S. Y., Choi, S. K., Han, M., Kim, S., et al. (2019). Characteristics and treatment patterns of patients with advanced soft tissue sarcoma in korea. Cancer Res. Treat. 51 (4), 1380–1391. doi:10.4143/crt.2018.476
Kim, J. H., Kim, S. H., Jeon, M. K., Kim, J. E., Kim, K. H., Yun, K. H., et al. (2021). Pemetrexed plus cisplatin in patients with previously treated advanced sarcoma: a multicenter, single-arm, phase II trial. ESMO Open 6 (5), 100249. doi:10.1016/j.esmoop.2021.100249
Kogushi, K., LoPresti, M., and Ikeda, S. (2020). Systematic literature review of clinical outcomes in adults with metastatic or advanced synovial sarcoma. Future Oncol. 16 (35), 2997–3013. doi:10.2217/fon-2020-0575
Kojima, Y., Shimoi, T., Seo, T., Yazaki, S., Okuya, T., Ohtake, Y., et al. (2022). Poor treatment outcomes with second-line chemotherapy in advanced synovial sarcoma. Oncology 100 (7), 370–375. doi:10.1159/000524500
Kollar, A., Rothermundt, C., Klenke, F., Bode, B., Baumhoer, D., Arndt, V., et al. (2019). Incidence, mortality, and survival trends of soft tissue and bone sarcoma in Switzerland between 1996 and 2015. Cancer Epidemiol. 63, 101596. doi:10.1016/j.canep.2019.101596
Köstler, W. J., Brodowicz, T., Attems, Y., Hejna, M., Tomek, S., Amann, G., et al. (2001). Docetaxel as rescue medication in anthracycline- and ifosfamide-resistant locally advanced or metastatic soft tissue sarcoma: results of a phase II trial. Ann. Oncol. 12 (9), 1281–1288. doi:10.1023/a:1012272007146
Kroep, J. R., Ouali, M., Gelderblom, H., Le Cesne, A., Dekker, T. J. A., Van Glabbeke, M., et al. (2011). First-line chemotherapy for malignant peripheral nerve sheath tumor (MPNST) versus other histological soft tissue sarcoma subtypes and as a prognostic factor for MPNST: an EORTC soft tissue and bone sarcoma group study. Ann. Oncol. 22 (1), 207–214. doi:10.1093/annonc/mdq338
Kyriazoglou, A., Gkaralea, L. E., Kotsantis, I., Anastasiou, M., Pantazopoulos, A., Prevezanou, M., et al. (2022). Tyrosine kinase inhibitors in sarcoma treatment. Oncol. Lett. 23 (6), 183. doi:10.3892/ol.2022.13303
Larsen, A. K., Galmarini, C. M., and D'Incalci, M. (2016). Unique features of trabectedin mechanism of action. Cancer chemoth Pharm. 77 (4), 663–671. doi:10.1007/s00280-015-2918-1
Le Cesne, A., Blay, J. Y., Cupissol, D., Italiano, A., Delcambre, C., Penel, N., et al. (2021). A randomized phase III trial comparing trabectedin to best supportive care in patients with pre-treated soft tissue sarcoma: T-SAR, a French sarcoma group trial. Ann. Oncol. 32 (8), 1034–1044. doi:10.1016/j.annonc.2021.04.014
Le Cesne, A., Blay, J. Y., Judson, I., Van Oosterom, A., Verweij, J., Radford, J., et al. (2005). Phase II study of ET-743 in advanced soft tissue sarcomas: a European organisation for the research and treatment of cancer (EORTC) soft tissue and bone sarcoma group trial. J. Clin. Oncol. 23 (3), 576–584. doi:10.1200/jco.2005.01.180
Le Cesne, A., Judson, I., Crowther, D., Rodenhuis, S., Keizer, H. J., Van Hoesel, Q., et al. (2000). Randomized phase III study comparing conventional-dose doxorubicin plus ifosfamide versus high-dose doxorubicin plus ifosfamide plus recombinant human granulocyte-macrophage colony-stimulating factor in advanced soft tissue sarcomas: A trial of the European organization for research and treatment of cancer/soft tissue and bone sarcoma group. J. Clin. Oncol. 18 (14), 2676–2684. doi:10.1200/JCO.2000.18.14.2676
Le Cesne, A. (2022). The role of trabectedin as second-line treatment of advanced soft tissue sarcoma: update of key efficacy and safety data. Future Oncol. 18 (30), 1–3. doi:10.2217/fon-2022-0516
Leahy, M., Garcia Del Muro, X., Reichardt, P., Judson, I., Staddon, A., Verweij, J., et al. (2012). Chemotherapy treatment patterns and clinical outcomes in patients with metastatic soft tissue sarcoma. The SArcoma treatment and Burden of Illness in North America and Europe (SABINE) study. Ann. Oncol. 23 (10), 2763–2770. doi:10.1093/annonc/mds070
Lee, A. T. J., Thway, K., Huang, P. H., and Jones, R. L. (2018). Clinical and molecular spectrum of liposarcoma. J. Clin. Oncol. 36 (2), 151–159. doi:10.1200/JCO.2017.74.9598
Lee, E. M., Rha, S. Y., Lee, J., Park, K. H., and Ahn, J. H. (2012). Phase II study of weekly docetaxel and fixed dose rate gemcitabine in patients with previously treated advanced soft tissue and bone sarcoma. Cancer Chemother. Pharmacol. 69 (3), 635–642. doi:10.1007/s00280-011-1742-5
Liang, J., Huang, M., Duan, W., Yu, X. Q., and Zhou, S. (2007). Design of new oxazaphosphorine anticancer drugs. Curr. Pharm. Des. 13 (9), 963–978. doi:10.2174/138161207780414296
Licht, J. D., Mazanet, R., Loehrer, P. J., Gonin, R., and Antman, K. H. (1994). Phase IV trial of daily oral etoposide in the treatment of advanced soft-tissue sarcoma. Cancer chemoth Pharm. 34 (1), 79–80. doi:10.1007/BF00686117
Loehrer, P. J., Sledge, G. W., Nicaise, C., Usakewicz, J., Hainsworth, J. D., Martelo, O. J., et al. (1989). Ifosfamide plus doxorubicin in metastatic adult sarcomas: a multi-institutional phase II trial. J. Clin. Oncol. 7 (11), 1655–1659. doi:10.1200/jco.1989.7.11.1655
Lopez-Alvarez, M., Gonzalez-Aguilera, C., Moura, D. S., Sanchez-Bustos, P., Mondaza-Hernandez, J. L., Martin-Ruiz, M., et al. (2022). Efficacy of eribulin plus gemcitabine combination in L-sarcomas. Int. J. Mol. Sci. 24 (1), 680. doi:10.3390/ijms24010680
Lorigan, P., Verweij, J., Papai, Z., Rodenhuis, S., Le Cesne, A., Leahy, M. G., et al. (2007). Phase III trial of two investigational schedules of ifosfamide compared with standard-dose doxorubicin in advanced or metastatic soft tissue sarcoma: a European organisation for research and treatment of cancer soft tissue and bone sarcoma group study. J. Clin. Oncol. 25 (21), 3144–3150. doi:10.1200/JCO.2006.09.7717
Losa, R., Fra, J., Lopez-Pousa, A., Sierra, M., Goitia, A., Una, E., et al. (2007). Phase II study with the combination of gemcitabine and DTIC in patients with advanced soft tissue sarcomas. Cancer Chemother. Pharmacol. 59 (2), 251–259. doi:10.1007/s00280-006-0263-0
Maki, R. G. (2007). Gemcitabine and docetaxel in metastatic sarcoma: past, present, and future. Oncologist 12 (8), 999–1006. doi:10.1634/theoncologist.12-8-999
Maki, R. G., Wathen, J. K., Patel, S. R., Priebat, D. A., Okuno, S. H., Samuels, B., et al. (2007). Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J. Clin. Oncol. 25 (19), 2755–2763. doi:10.1200/JCO.2006.10.4117
Marino-Enriquez, A., and Fletcher, C. D. (2014). Round cell sarcomas - biologically important refinements in subclassification. Int. J. Biochem. Cell Biol. 53, 493–504. doi:10.1016/j.biocel.2014.04.022
Martin-Broto, J., Pousa, A. L., de Las Penas, R., Garcia Del Muro, X., Gutierrez, A., Martinez-Trufero, J., et al. (2016). Randomized phase II study of trabectedin and doxorubicin compared with doxorubicin alone as first-line treatment in patients with advanced soft tissue sarcomas: a Spanish group for research on sarcoma study. J. Clin. Oncol. 34 (19), 2294–2302. doi:10.1200/JCO.2015.65.3329
Martins-Teixeira, M. B., and Carvalho, I. (2020). Antitumour anthracyclines: progress and perspectives. Chemmedchem 15 (11), 933–948. doi:10.1002/cmdc.202000131
Maurel, J., Fra, J., Lopez-Pousa, A., Garcia del Muro, X., Balana, C., Casado, A., et al. (2004). Sequential dose-dense doxorubicin and ifosfamide for advanced soft tissue sarcomas: A phase II trial by the Spanish group for research on sarcomas (GEIS). Cancer 100 (7), 1498–1506. doi:10.1002/cncr.20115
Maurel, J., Lopez-Pousa, A., de Las Penas, R., Fra, J., Martin, J., Cruz, J., et al. (2009). Efficacy of sequential high-dose doxorubicin and ifosfamide compared with standard-dose doxorubicin in patients with advanced soft tissue sarcoma: an open-label randomized phase II study of the Spanish group for research on sarcomas. J. Clin. Oncol. 27 (11), 1893–1898. doi:10.1200/JCO.2008.19.2930
Mekhail, T. M., and Markman, M. (2002). Paclitaxel in cancer therapy. Expert Opin. Pharm. 3 (6), 755–766. doi:10.1517/14656566.3.6.755
Meyer, M., and Seetharam, M. (2019). First-line therapy for metastatic soft tissue sarcoma. Curr. Treat. Options Oncol. 20 (1), 6. doi:10.1007/s11864-019-0606-9
Misiura, K. (2006). Ifosfamide. Metabolic studies, new therapeutic approaches and new analogs. Mini-rev Med. Chem. 6 (4), 395–400. doi:10.2174/138955706776361385
Mora, J., Castaneda, A., Perez-Jaume, S., Lopez-Pousa, A., Maradiegue, E., Valverde, C., et al. (2017). GEIS-21: a multicentric phase II study of intensive chemotherapy including gemcitabine and docetaxel for the treatment of ewing sarcoma of children and adults: A report from the Spanish sarcoma group (GEIS). Br. J. Cancer 117 (6), 767–774. doi:10.1038/bjc.2017.252
Mouridsen, H. T., Bastholt, L., Somers, R., Santoro, A., Bramwell, V., Mulder, J. H., et al. (1987). Adriamycin versus epirubicin in advanced soft tissue sarcomas. A randomized phase II/phase III study of the EORTC Soft Tissue and Bone Sarcoma Group. Eur. J. Cancer Clin. Oncol. 23 (10), 1477–1483. doi:10.1016/0277-5379(87)90089-7
Mulder, R. L., Paulides, M., Langer, T., Kremer, L. C., and van Dalen, E. C. (2015). Cyclophosphamide versus ifosfamide for paediatric and young adult bone and soft tissue sarcoma patients. Cochrane Database Syst. Rev. 2015 (9), CD006300. doi:10.1002/14651858.CD006300.pub4
Nagar, S. P., Mytelka, D. S., Candrilli, S. D., D'Yachkova, Y., Lorenzo, M., Kasper, B., et al. (2018). Treatment patterns and survival among adult patients with advanced soft tissue sarcoma: a retrospective medical record review in the United Kingdom, Spain, Germany, and France. Sarcoma 2018, 5467057. doi:10.1155/2018/5467057
Nakamura, T., and Sudo, A. (2022). The role of trabectedin in soft tissue sarcoma. Front. Pharmacol. 13, 777872. doi:10.3389/fphar.2022.777872
Nielsen, O. S., Dombernowsky, P., Mouridsen, H., Crowther, D., Verweij, J., Buesa, J., et al. (1998). High-dose epirubicin is not an alternative to standard-dose doxorubicin in the treatment of advanced soft tissue sarcomas. A study of the EORTC soft tissue and bone sarcoma group. Brit J. cancer 78 (12), 1634–1639. doi:10.1038/bjc.1998.735
Nielsen, O. S., Dombernowsky, P., Mouridsen, H., Daugaard, S., Van Glabbeke, M., Kirkpatrick, A., et al. (2000). Epirubicin is not superior to doxorubicin in the treatment of advanced soft tissue sarcomas the experience of the EORTC soft tissue and bone sarcoma group. Sarcoma 4 (1-2), 31–35. doi:10.1155/s1357714x00000062
Nielsen, O. S., Judson, I., van Hoesel, Q., le Cesne, A., Keizer, H. J., Blay, J. Y., et al. (2000). Effect of high-dose ifosfamide in advanced soft tissue sarcomas. A multicentre phase II study of the EORTC Soft Tissue and Bone Sarcoma Group. Eur. J. cancer 36 (1), 61–67. doi:10.1016/s0959-8049(99)00240-3
Oesterheld, J. E., Reed, D. R., Setty, B. A., Isakoff, M. S., Thompson, P., Yin, H., et al. (2020). Phase II trial of gemcitabine and nab-paclitaxel in patients with recurrent ewing sarcoma: a report from the national pediatric cancer foundation. Pediatr. Blood Cancer 67 (7), e28370. doi:10.1002/pbc.28370
Ojima, I., Lichtenthal, B., Lee, S., Wang, C., and Wang, X. (2016). Taxane anticancer agents: a patent perspective. Expert Opin. Ther. Pat. 26 (1), 1–20. doi:10.1517/13543776.2016.1111872
Okuno, S., Ryan, L. M., Edmonson, J. H., Priebat, D. A., and Blum, R. H. (2003). Phase II trial of gemcitabine in patients with advanced sarcomas (E1797): a trial of the eastern cooperative oncology group. Cancer 97 (8), 1969–1973. doi:10.1002/cncr.11290
Pápai, Z., Bodoky, G., Szántó, J., Poller, I., Rahóty, P., Eckhardt, S., et al. (2000). The efficacy of a combination of etoposide, ifosfamide, and cisplatin in the treatment of patients with soft tissue sarcoma. Cancer-am cancer Soc. 89 (1), 177–180. doi:10.1002/1097-0142(20000701)89:1<177:aid-cncr23>3.0.co;2-3
Parikh, R. C., Lorenzo, M., Hess, L. M., Candrilli, S. D., Nicol, S., and Kaye, J. A. (2018). Treatment patterns and survival among older adults in the United States with advanced soft-tissue sarcomas. Clin. Sarcoma Res. 8, 8. doi:10.1186/s13569-018-0094-x
Patel, S. A., Nilsson, M. B., Le, X., Cascone, T., Jain, R. K., and Heymach, J. V. (2022). Molecular mechanisms and future implications of VEGF/VEGFR in cancer therapy. Clin. cancer Res. 29 (1), 30–39. doi:10.1158/1078-0432.CCR-22-1366
Patel, S. R., Linke, K. A., Burgess, M. A., Papadopoulos, N. E., Plager, C., Jenkins, J., et al. (1997). Phase II study of paclitaxel in patients with soft tissue sarcomas. Sarcoma 1 (2), 95–97. doi:10.1080/13577149778362
Patel, S., von Mehren, M., Reed, D. R., Kaiser, P., Charlson, J., Ryan, C. W., et al. (2019). Overall survival and histology-specific subgroup analyses from a phase 3, randomized controlled study of trabectedin or dacarbazine in patients with advanced liposarcoma or leiomyosarcoma. Cancer 125 (15), 2610–2620. doi:10.1002/cncr.32117
Pautier, P., Floquet, A., Chevreau, C., Penel, N., Guillemet, C., Delcambre, C., et al. (2015). Trabectedin in combination with doxorubicin for first-line treatment of advanced uterine or soft-tissue leiomyosarcoma (LMS-02): a non-randomised, multicentre, phase 2 trial. Lancet Oncol. 16 (4), 457–464. doi:10.1016/S1470-2045(15)70070-7
Pautier, P., Floquet, A., Penel, N., Piperno-Neumann, S., Isambert, N., Rey, A., et al. (2012). Randomized multicenter and stratified phase II study of gemcitabine alone versus gemcitabine and docetaxel in patients with metastatic or relapsed leiomyosarcomas: a federation nationale des centres de Lutte contre le Cancer (FNCLCC) French sarcoma group study (TAXOGEM study). Oncologist 17 (9), 1213–1220. doi:10.1634/theoncologist.2011-0467
Pautier, P., Italiano, A., Piperno-Neumann, S., Chevreau, C., Penel, N., Firmin, N., et al. (2022). Doxorubicin alone versus doxorubicin with trabectedin followed by trabectedin alone as first-line therapy for metastatic or unresectable leiomyosarcoma (LMS-04): a randomised, multicentre, open-label phase 3 trial. Lancet Oncol. 23 (8), 1044–1054. doi:10.1016/S1470-2045(22)00380-1
Pautier, P., Penel, N., Ray-Coquard, I., Italiano, A., Bompas, E., Delcambre, C., et al. (2020). A phase II of gemcitabine combined with pazopanib followed by pazopanib maintenance, as second-line treatment in patients with advanced leiomyosarcomas: a unicancer French sarcoma group study (LMS03 study). Eur. J. Cancer 125, 31–37. doi:10.1016/j.ejca.2019.10.028
Pender, A., Davis, E. J., Chauhan, D., Messiou, C., Al-Muderis, O., Thway, K., et al. (2018). Poor treatment outcomes with palliative gemcitabine and docetaxel chemotherapy in advanced and metastatic synovial sarcoma. Med. Oncol. 35 (10), 131. doi:10.1007/s12032-018-1193-5
Penel, N., Italiano, A., Ray-Coquard, I., Chaigneau, L., Delcambre, C., Robin, Y. M., et al. (2012). Metastatic angiosarcomas: doxorubicin-based regimens, weekly paclitaxel and metastasectomy significantly improve the outcome. Ann. Oncol. 23 (2), 517–523. doi:10.1093/annonc/mdr138
Peter, S., Alven, S., Maseko, R. B., and Aderibigbe, B. A. (2022). Doxorubicin-based hybrid compounds as potential anticancer agents: a review. Molecules 27 (14), 4478. doi:10.3390/molecules27144478
Phillips, E., Jones, R. L., Huang, P., and Digklia, A. (2022). Efficacy of eribulin in soft tissue sarcomas. Front. Pharmacol. 13, 869754. doi:10.3389/fphar.2022.869754
Pink, D., Andreou, D., Bauer, S., Brodowicz, T., Kasper, B., Reichardt, P., et al. (2021). Treatment of angiosarcoma with pazopanib and paclitaxel: results of the EVA (evaluation of Votrient® in angiosarcoma) phase II trial of the German interdisciplinary sarcoma group (GISG-06). Cancers (Basel) 13 (6), 1223. doi:10.3390/cancers13061223
Principe, D. R., Kamath, S. D., Korc, M., and Munshi, H. G. (2022). The immune modifying effects of chemotherapy and advances in chemo-immunotherapy. Pharmacol. Ther. 236, 108111. doi:10.1016/j.pharmthera.2022.108111
Rabbani, A., Finn, R. M., and Ausio, J. (2005). The anthracycline antibiotics: antitumor drugs that alter chromatin structure. Bioessays 27 (1), 50–56. doi:10.1002/bies.20160
Rajwanshi, A., Srinivas, R., and Upasana, G. (2009). Malignant small round cell tumors. J. Cytol. 26 (1), 1–10. doi:10.4103/0970-9371.54861
Rastogi, S., and Bakhshi, S. (2016). Trabectedin in soft tissue sarcoma: have we hit the bull's-eye? J. Clin. Oncol. 34 (29), 3582–3583. doi:10.1200/JCO.2015.65.7130
Ratan, R., and Patel, S. R. (2016). Chemotherapy for soft tissue sarcoma. Cancer 122 (19), 2952–2960. doi:10.1002/cncr.30191
Ratan, R., and Patel, S. R. (2017). Trabectedin and eribulin: where do they fit in the management of soft tissue sarcoma? Curr. Treat. Options Oncol. 18 (6), 34. doi:10.1007/s11864-017-0477-x
Riggi, N., Suva, M. L., and Stamenkovic, I. (2021). Ewing's sarcoma. N. Engl. J. Med. 384 (2), 154–164. doi:10.1056/NEJMra2028910
Ryan, C. W., Merimsky, O., Agulnik, M., Blay, J. Y., Schuetze, S. M., Van Tine, B. A., et al. (2016). Picasso III: a phase III, placebo-controlled study of doxorubicin with or without palifosfamide in patients with metastatic soft tissue sarcoma. J. Clin. Oncol. 34 (32), 3898–3905. doi:10.1200/JCO.2016.67.6684
Saeter, G., Alvegård, T. A., Monge, O. R., Strander, H., Turesson, I., Klepp, R., et al. (1997). Ifosfamide and continuous infusion etoposide in advanced adult soft tissue sarcoma. A Scandinavian Sarcoma Group Phase II Study. Eur. J. cancer 33 (10), 1551–1558. doi:10.1016/s0959-8049(97)00102-0
Sanfilippo, R., Dileo, P., Blay, J. Y., Constantinidou, A., Le Cesne, A., Benson, C., et al. (2015). Trabectedin in advanced synovial sarcomas: a multicenter retrospective study from four European institutions and the Italian rare cancer network. Anticancer Drugs 26 (6), 678–681. doi:10.1097/CAD.0000000000000228
Sanfilippo, R., Hayward, R. L., Musoro, J., Benson, C., Leahy, M. G., Brunello, A., et al. (2022). Activity of cabazitaxel in metastatic or inoperable locally advanced dedifferentiated liposarcoma: a phase 2 study of the eortc soft tissue and bone sarcoma group (stbsg). JAMA Oncol. 8 (10), 1420–1425. doi:10.1001/jamaoncol.2022.3218
Santoro, A., Romanini, A., Rosso, A., Frustaci, S., Comandone, A., Apice, G., et al. (1999). Lack of activity of docetaxel in soft tissue sarcomas: results of a phase II study of the Italian group on rare tumors. Sarcoma 3 (3-4), 177–181. doi:10.1080/13577149977613
Santoro, A., Tursz, T., Mouridsen, H., Verweij, J., Steward, W., Somers, R., et al. (1995). Doxorubicin versus CYVADIC versus doxorubicin plus ifosfamide in first-line treatment of advanced soft tissue sarcomas: a randomized study of the European organization for research and treatment of cancer soft tissue and bone sarcoma group. J. Clin. Oncol. 13 (7), 1537–1545. doi:10.1200/JCO.1995.13.7.1537
Schlemmer, M., Reichardt, P., Verweij, J., Hartmann, J. T., Judson, I., Thyss, A., et al. (2008). Paclitaxel in patients with advanced angiosarcomas of soft tissue: a retrospective study of the EORTC soft tissue and bone sarcoma group. Eur. J. Cancer 44 (16), 2433–2436. doi:10.1016/j.ejca.2008.07.037
Schmoll, H. J., Lindner, L. H., Reichardt, P., Heissner, K., Kopp, H. G., Kessler, T., et al. (2021). Efficacy of pazopanib with or without gemcitabine in patients with anthracycline- and/or ifosfamide-refractory soft tissue sarcoma: final results of the PAPAGEMO phase 2 randomized clinical trial. JAMA Oncol. 7 (2), 255–262. doi:10.1001/jamaoncol.2020.6564
Schoffski, P., Chawla, S., Maki, R. G., Italiano, A., Gelderblom, H., Choy, E., et al. (2016). Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet 387 (10028), 1629–1637. doi:10.1016/S0140-6736(15)01283-0
Schoffski, P., Ray-Coquard, I. L., Cioffi, A., Bui, N. B., Bauer, S., Hartmann, J. T., et al. (2011). Activity of eribulin mesylate in patients with soft-tissue sarcoma: a phase 2 study in four independent histological subtypes. Lancet Oncol. 12 (11), 1045–1052. doi:10.1016/S1470-2045(11)70230-3
Schoot, R. A., Chisholm, J. C., Casanova, M., Minard-Colin, V., Geoerger, B., Cameron, A. L., et al. (2022). Metastatic rhabdomyosarcoma: results of the European paediatric soft tissue sarcoma study group MTS 2008 study and pooled analysis with the concurrent BERNIE study. J. Clin. Oncol. 40 (32), 3730–3740. doi:10.1200/jco.21.02981
Schuetze, S. M., Zhao, L., Chugh, R., Thomas, D. G., Lucas, D. R., Metko, G., et al. (2012). Results of a phase II study of sirolimus and cyclophosphamide in patients with advanced sarcoma. Eur. J. Cancer 48 (9), 1347–1353. doi:10.1016/j.ejca.2012.03.022
Schuetze, S. (2021). Trabectedin: useful in leiomyosarcoma and liposarcoma, but less so in other soft tissue sarcomas. Ann. Oncol. 32 (8), 957–958. doi:10.1016/j.annonc.2021.04.025
Seddon, B. (2016). First-line treatment in advanced or metastatic disease: one size fits all or adapted to specific histiotypes? Curr. Opin. Oncol. 28 (4), 323–330. doi:10.1097/CCO.0000000000000301
Seddon, B., Strauss, S. J., Whelan, J., Leahy, M., Woll, P. J., Cowie, F., et al. (2017). Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): a randomised controlled phase 3 trial. Lancet Oncol. 18 (10), 1397–1410. doi:10.1016/S1470-2045(17)30622-8
Setty, B. A., Gikandi, A., and DuBois, S. G. (2023). Ewing sarcoma drug therapy: current standard of care and emerging agents. Paediatr. Drugs 25, 389–397. doi:10.1007/s40272-023-00568-9
Shetty, N., and Gupta, S. (2014). Eribulin drug review. South Asian J. Cancer 3 (1), 57–59. doi:10.4103/2278-330X.126527
Skubitz, K. M., and Haddad, P. A. (2005). Paclitaxel and pegylated-liposomal doxorubicin are both active in angiosarcoma. Cancer 104 (2), 361–366. doi:10.1002/cncr.21140
Smolle, M. A., Szkandera, J., Andreou, D., Palmerini, E., Bergovec, M., and Leithner, A. (2020). Treatment options in unresectable soft tissue and bone sarcoma of the extremities and pelvis - a systematic literature review. EFORT Open Rev. 5 (11), 799–814. doi:10.1302/2058-5241.5.200069
Smrke, A., Wang, Y., and Simmons, C. (2020). Update on systemic therapy for advanced soft-tissue sarcoma. Curr. Oncol. 27 (1), 25–33. doi:10.3747/co.27.5475
Somaiah, N., Van Tine, B. A., Wahlquist, A. E., Milhem, M. M., Hill, E. G., Garrett-Mayer, E., et al. (2021). A randomized, open-label, phase 2, multicenter trial of gemcitabine with pazopanib or gemcitabine with docetaxel in patients with advanced soft-tissue sarcoma. Cancer 127 (6), 894–904. doi:10.1002/cncr.33216
Sordillo, P. P., Magill, G. B., Brenner, J., Cheng, E. W., Dosik, M., and Yagoda, A. (1987). Cisplatin. A phase II evaluation in previously untreated patients with soft tissue sarcomas. Cancer-am cancer Soc. 59 (5), 884–886. doi:10.1002/1097-0142(19870301)59:5<884:aid-cncr2820590504>3.0.co;2-k
Sparber-Sauer, M. (2022). The future of rhabdomyosarcoma trials. Lancet Child. Adolesc. Health 6 (8), 510–511. doi:10.1016/S2352-4642(22)00132-8
Sritharan, S., and Sivalingam, N. (2021). A comprehensive review on time-tested anticancer drug doxorubicin. Life Sci. 278, 119527. doi:10.1016/j.lfs.2021.119527
Stacchiotti, S., Palassini, E., Sanfilippo, R., Vincenzi, B., Arena, M. G., Bochicchio, A. M., et al. (2012). Gemcitabine in advanced angiosarcoma: a retrospective case series analysis from the Italian rare cancer network. Ann. Oncol. 23 (2), 501–508. doi:10.1093/annonc/mdr066
Stacchiotti, S., and Van Tine, B. A. (2018). Synovial sarcoma: current concepts and future perspectives. J. Clin. Oncol. 36 (2), 180–187. doi:10.1200/JCO.2017.75.1941
Stricker, E., Reed, D. R., Schabath, M. B., Sok, P., Scheurer, M. E., and Lupo, P. J. (2023). Trends in overall survival among patients treated for sarcoma at a large tertiary cancer center between 1986 and 2014. Cancers (Basel) 15 (2), 514. doi:10.3390/cancers15020514
Sun, J., Wei, Q., Zhou, Y., Wang, J., Liu, Q., and Xu, H. (2017). A systematic analysis of FDA-approved anticancer drugs. BMC Syst. Biol. 11 (5), 87. doi:10.1186/s12918-017-0464-7
Svancárová, L., Blay, J. Y., Judson, I. R., van Hoesel, Q. G., van Oosterom, A. T., le Cesne, A., et al. (2002). Gemcitabine in advanced adult soft-tissue sarcomas. A phase II study of the EORTC Soft Tissue and Bone Sarcoma Group. Eur. J. cancer 38 (4), 556–559. doi:10.1016/s0959-8049(01)00408-7
Tan, C., Etcubanas, E., Wollner, N., Rosen, G., Gilladoga, A., Showel, J., et al. (1973). Adriamycin-an antitumor antibiotic in the treatment of neoplastic diseases. Cancer-am cancer Soc. 32 (1), 9–17. doi:10.1002/1097-0142(197307)32:1<9:aid-cncr2820320102>3.0.co;2-6
Tang, F., Tie, Y., Wei, Y. Q., Tu, C. Q., and Wei, X. W. (2021). Targeted and immuno-based therapies in sarcoma: mechanisms and advances in clinical trials. Biochim. Biophys. Acta Rev. Cancer 1876 (2), 188606. doi:10.1016/j.bbcan.2021.188606
Tap, W. D., Jones, R. L., Van Tine, B. A., Chmielowski, B., Elias, A. D., Adkins, D., et al. (2016). Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet 388 (10043), 488–497. doi:10.1016/S0140-6736(16)30587-6
Tap, W. D., Papai, Z., Van Tine, B. A., Attia, S., Ganjoo, K. N., Jones, R. L., et al. (2017). Doxorubicin plus evofosfamide versus doxorubicin alone in locally advanced, unresectable or metastatic soft-tissue sarcoma (TH CR-406/sarc021): an international, multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 18 (8), 1089–1103. doi:10.1016/S1470-2045(17)30381-9
Tap, W. D., Wagner, A. J., Schoffski, P., Martin-Broto, J., Krarup-Hansen, A., Ganjoo, K. N., et al. (2020). Effect of doxorubicin plus olaratumab vs doxorubicin plus placebo on survival in patients with advanced soft tissue sarcomas: the ANNOUNCE randomized clinical trial. JAMA 323 (13), 1266–1276. doi:10.1001/jama.2020.1707
Tascilar, M., Loos, W. J., Seynaeve, C., Verweij, J., and Sleijfer, S. (2007). The pharmacologic basis of ifosfamide use in adult patients with advanced soft tissue sarcomas. Oncologist 12 (11), 1351–1360. doi:10.1634/theoncologist.12-11-1351
Tawbi, H. A., Burgess, M., Bolejack, V., Van Tine, B. A., Schuetze, S. M., Hu, J., et al. (2017). Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 18 (11), 1493–1501. doi:10.1016/S1470-2045(17)30624-1
Thirasastr, P., Brahmi, M., Dufresne, A., Somaiah, N., and Blay, J. Y. (2022). New drug approvals for sarcoma in the last 5 years. Surg. Oncol. Clin. N. Am. 31 (3), 361–380. doi:10.1016/j.soc.2022.03.003
Tian, Z., and Yao, W. (2022). PD-1/L1 inhibitor plus chemotherapy in the treatment of sarcomas. Front. Immunol. 13, 898255. doi:10.3389/fimmu.2022.898255
Tos, A. P. D., Webster, F., Agaimy, A., Bovee, J., Dickson, B., Doyle, L., et al. (2023). Datasets for reporting of soft-tissue sarcoma: recommendations from the international collaboration on cancer reporting (ICCR). Histopathology 82, 745–754. doi:10.1111/his.14862
Touati, N., Schoffski, P., Litiere, S., Judson, I., Sleijfer, S., van der Graaf, W. T., et al. (2018). European organisation for research and treatment of cancer soft tissue and bone sarcoma group experience with advanced/metastatic epithelioid sarcoma patients treated in prospective trials: clinical profile and response to systemic therapy. Clin. Oncol. R. Coll. Radiol. 30 (7), 448–454. doi:10.1016/j.clon.2018.02.065
Trabectedin (2003). Trabectedin: ET 743, ecteinascidin 743, yondelis. Drugs 4 (1), 75–81. doi:10.2165/00126839-200304010-00016
Ugurel, S., Paschen, A., and Becker, J. C. (2013). Dacarbazine in melanoma: from a chemotherapeutic drug to an immunomodulating agent. J. Invest. Dermatol 133 (2), 289–292. doi:10.1038/jid.2012.341
van Oosterom, A. T., Mouridsen, H. T., Nielsen, O. S., Dombernowsky, P., Krzemieniecki, K., Judson, I., et al. (2002). Results of randomised studies of the EORTC Soft Tissue and Bone Sarcoma Group (STBSG) with two different ifosfamide regimens in first- and second-line chemotherapy in advanced soft tissue sarcoma patients. Eur. J. cancer 38 (18), 2397–2406. doi:10.1016/s0959-8049(02)00491-4
Van Tine, B. A., Weiss, M. C., Hirbe, A. C., Oppelt, P. J., Abaricia, S., Trinkaus, K., et al. (2021). Phase II study of dacarbazine given with modern prophylactic anti-emetics and growth factor support to patients with metastatic, resistant soft tissue, and bone sarcoma. Rare tumors 13, 20363613211052498. doi:10.1177/20363613211052498
Verweij, J., Lee, S. M., Ruka, W., Buesa, J., Coleman, R., van Hoessel, R., et al. (2000). Randomized phase II study of docetaxel versus doxorubicin in first- and second-line chemotherapy for locally advanced or metastatic soft tissue sarcomas in adults: A study of the european organization for research and treatment of cancer soft tissue and bone sarcoma group. J. Clin. Oncol. 18 (10), 2081–2086. doi:10.1200/JCO.2000.18.10.2081
Vincenzi, B., Napolitano, A., Comandone, A., Sanfilippo, R., Celant, S., Olimpieri, P. P., et al. (2023). Trabectedin use in soft-tissue sarcoma patients in a real-world setting: data from an Italian national drug-access registry. Int. J. Cancer 152 (4), 761–768. doi:10.1002/ijc.34309
Von Burton, G., Rankin, C., Zalupski, M. M., Mills, G. M., Borden, E. C., and Karen, A. (2006). Phase II trial of gemcitabine as first line chemotherapy in patients with metastatic or unresectable soft tissue sarcoma. Am. J. Clin. Oncol. 29 (1), 59–61. doi:10.1097/01.coc.0000195088.28956.dd
von Mehren, M., Kane, J. M., Agulnik, M., Bui, M. M., Carr-Ascher, J., Choy, E., et al. (2022). Soft tissue sarcoma, version 2.2022, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc Netw. 20 (7), 815–833. doi:10.6004/jnccn.2022.0035
von Mehren, M., Kane, J. M., Bui, M. M., Choy, E., Connelly, M., Dry, S., et al. (2020). NCCN guidelines insights: soft tissue sarcoma, version 1.2021. J. Natl. Compr. Canc Netw. 18 (12), 1604–1612. doi:10.6004/jnccn.2020.0058
Walterhouse, D. O., Pappo, A. S., Meza, J. L., Breneman, J. C., Hayes-Jordan, A. A., Parham, D. M., et al. (2014). Shorter-duration therapy using vincristine, dactinomycin, and lower-dose cyclophosphamide with or without radiotherapy for patients with newly diagnosed low-risk rhabdomyosarcoma: a report from the soft tissue sarcoma committee of the children's oncology group. J. Clin. Oncol. 32 (31), 3547–3552. doi:10.1200/JCO.2014.55.6787
Walterhouse, D. O., Pappo, A. S., Meza, J. L., Breneman, J. C., Hayes-Jordan, A., Parham, D. M., et al. (2017). Reduction of cyclophosphamide dose for patients with subset 2 low-risk rhabdomyosarcoma is associated with an increased risk of recurrence: a report from the soft tissue sarcoma committee of the children's oncology group. Cancer 123 (12), 2368–2375. doi:10.1002/cncr.30613
Wang, B. C., Kuang, B. H., Xiao, B. Y., and Lin, G. H. (2021). Doxorubicin/adriamycin monotherapy or plus ifosfamide in first-line treatment for advanced soft tissue sarcoma: a pooled analysis of randomized trials. Front. Oncol. 11, 762288. doi:10.3389/fonc.2021.762288
Wang, D., and Wang, H. (2012). Oxazaphosphorine bioactivation and detoxification the role of xenobiotic receptors. Acta Pharm. Sin. B 2 (2), 107–117. doi:10.1016/j.apsb.2012.02.004
Wang, J., Wang, P., Zeng, Z., Lin, C., Lin, Y., Cao, D., et al. (2022). Trabectedin in cancers: mechanisms and clinical applications. Curr. Pharm. Des. 28 (24), 1949–1965. doi:10.2174/1381612828666220526125806
Watson, S., Verret, B., Ropert, S., Adam, J., Bahleda, R., Briand, S., et al. (2022). Single-agent gemcitabine in patients with advanced, pre-treated angiosarcoma: a multicenter, retrospective study. Cancer Med. 12, 3160–3166. doi:10.1002/cam4.5147
Weaver, B. A. (2014). How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 25 (18), 2677–2681. doi:10.1091/mbc.E14-04-0916
Weiss, A. R., Chen, Y. L., Scharschmidt, T. J., Chi, Y. Y., Tian, J., Black, J. O., et al. (2020). Pathological response in children and adults with large unresected intermediate-grade or high-grade soft tissue sarcoma receiving preoperative chemoradiotherapy with or without pazopanib (ARST1321): a multicentre, randomised, open-label, phase 2 trial. Lancet Oncol. 21 (8), 1110–1122. doi:10.1016/S1470-2045(20)30325-9
Wibmer, C., Leithner, A., Zielonke, N., Sperl, M., and Windhager, R. (2010). Increasing incidence rates of soft tissue sarcomas? A population-based epidemiologic study and literature review. Ann. Oncol. 21 (5), 1106–1111. doi:10.1093/annonc/mdp415
Wong, A., Soo, R. A., Yong, W. P., and Innocenti, F. (2009). Clinical pharmacology and pharmacogenetics of gemcitabine. Drug Metab. Rev. 41 (2), 77–88. doi:10.1080/03602530902741828
Xu, J., Xie, L., Sun, X., Liu, K., Liang, X., Cai, Z., et al. (2023). Longer versus shorter schedules of vincristine, irinotecan, and temozolomide (vit) for relapsed or refractory ewing sarcoma: a randomized controlled phase 2 trial. Clin. Cancer Res. 29 (6), 1040–1046. doi:10.1158/1078-0432.CCR-22-3546
Yalçin, S., Güllü, I., Barişta, I., Tekuzman, G., Ozişik, Y., Celik, I., et al. (1998). Treatment of advanced refractory sarcomas with ifosfamide and etoposide combination chemotherapy. Cancer Invest. 16 (5), 297–302. doi:10.3109/07357909809084647
Yang, J., Xu, Y., Chen, Y., Li, T., Zhang, X., Hu, T., et al. (2022). Therapeutic perspectives for adult soft tissue sarcoma-updates from the 2022 ASCO annual meeting. Cancer Biol. Med. 19 (10), 1496–1502. doi:10.20892/j.issn.2095-3941.2022.0394
Yang, Z., Zheng, R., Zhang, S., Zeng, H., Li, H., and Chen, W. (2019). Incidence, distribution of histological subtypes and primary sites of soft tissue sarcoma in China. Cancer Biol. Med. 16 (3), 565–574. doi:10.20892/j.issn.2095-3941.2019.0041
Yared, J. A., and Tkaczuk, K. H. (2012). Update on taxane development: new analogs and new formulations. Drug Des. Devel Ther. 6, 371–384. doi:10.2147/DDDT.S28997
Young, R. J., Natukunda, A., Litiere, S., Woll, P. J., Wardelmann, E., and van der Graaf, W. T. (2014). First-line anthracycline-based chemotherapy for angiosarcoma and other soft tissue sarcoma subtypes: pooled analysis of eleven European organisation for research and treatment of cancer soft tissue and bone sarcoma group trials. Eur. J. Cancer 50 (18), 3178–3186. doi:10.1016/j.ejca.2014.10.004
Young, R. J., and Woll, P. J. (2016). Eribulin in soft-tissue sarcoma. Lancet 387 (10028), 1594–1596. doi:10.1016/S0140-6736(16)00162-8
Younger, E., Jones, R. L., den Hollander, D., Soomers, V., Desar, I. M. E., Benson, C., et al. (2021). Priorities and preferences of advanced soft tissue sarcoma patients starting palliative chemotherapy: baseline results from the HOLISTIC study. ESMO Open 6 (5), 100258. doi:10.1016/j.esmoop.2021.100258
Zalupski, M., Metch, B., Balcerzak, S., Fletcher, W. S., Chapman, R., Bonnet, J. D., et al. (1991). Phase III comparison of doxorubicin and dacarbazine given by bolus versus infusion in patients with soft-tissue sarcomas: a southwest oncology group study. Jnci-j Natl. cancer i 83 (13), 926–932. doi:10.1093/jnci/83.13.926
Keywords: chemotherapeutic drugs, sarcoma, review, chemotherapy, efficacy
Citation: Tian Z and Yao W (2023) Chemotherapeutic drugs for soft tissue sarcomas: a review. Front. Pharmacol. 14:1199292. doi: 10.3389/fphar.2023.1199292
Received: 03 April 2023; Accepted: 03 August 2023;
Published: 11 August 2023.
Edited by:
Wei Xu, University of Toronto, CanadaReviewed by:
Francesca Maffei, University of Bologna, ItalySalvatore Lorenzo Renne, Humanitas Research Hospital, Italy
Copyright © 2023 Tian and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weitao Yao, eXd0d2htQDE2My5jb20=
 Zhichao Tian
Zhichao Tian Weitao Yao*
Weitao Yao*