- 1Department of Pharmacy, Xiangya Hospital, Central South University, Changsha, China
- 2National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China
- 3The Hunan Institute of Pharmacy Practice and Clinical Research, Changsha, China
- 4Department of General Surgery, Xiangya Hospital, Central South University, Changsha, China
- 5Department of Gynecology, Xiangya Hospital, Central South University, Changsha, China
- 6Gynecological Oncology Research and Engineering Center of Hunan Province, Changsha, China
Objective: Niraparib improved survival in platinum-sensitive recurrent ovarian cancer (PSROC) patients versus routine surveillance, accompanied by increased costs. Based on the NORA trial, we evaluated for the first time the cost-effectiveness of maintenance niraparib with individualized starting dosage (ISD) in China.
Methods: A Markov model was developed to simulate the costs and health outcomes of each strategy. The total costs, quality-adjusted life years (QALYs), and incremental cost-effectiveness ratios (ICERs) were measured. One-way and probabilistic sensitivity analysis were performed to estimate model robustness. Scenario analyses were also conducted.
Results: Compared to routine surveillance, niraparib additionally increased QALYs by 0.59 and 0.30 in populations with and without germline BRCA (gBRCA) mutations, with incremental costs of $10,860.79 and $12,098.54, respectively. The ICERs of niraparib over routine surveillance were $18,653.67/QALY and $39,212.99/QALY. At a willingness-to-pay (WTP) threshold of $37,488/QALY, the ISD enhanced the likelihood of cost-effectiveness from 9.35% to 30.73% in the gBRCA-mutated group and from 0.77% to 11.74% in the non-gBRCA mutated population. The probability of niraparib being cost-effective in the region with the highest per capita Gross Domestic Product (GDP) in China was 74.23% and 76.10% in the gBRCA-mutated and non-gBRCA mutated population, respectively. Niraparib was 100% cost-effective for National Basic Medical Insurance beneficiaries under the above WTP thresholds.
Conclusion: Compared to routine surveillance, the ISD of niraparib for maintenance treatment of PSROC is cost-effective in the gBRCA-mutated population and more effective but costly in the non-gBRCA mutated patients. The optimized niraparib price, economic status, and health insurance coverage may benefit the economic outcome.
1 Introduction
Ovarian cancer ranks eighth in incidence and mortality among female malignancies (Sung et al., 2021). According to the 2020 GLOBOCAN Global Cancer Data, there were 313,959 newly diagnosed ovarian cancer cases and 207,252 deaths worldwide. In China, ovarian cancer is the third most prevalent malignancy of the female reproductive system and a serious threat to women’s health (Zheng et al., 2022).
Ovarian cancer is characterized by an insidious onset, a high recurrence rate, a short patient survival period, and progressive resistance to multiline chemotherapy (Hanker et al., 2012; Zhang et al., 2022). Several factors, including stage, histological type, molecular characteristics, as well as treatment strategies, influence the prognosis of ovarian cancer (Liu et al., 2021). Approximately 90 percent of primary ovarian malignancies are epithelial, with high-grade serous carcinoma (HGSC) being the predominant histological type of epithelial ovarian cancer (EOC) (Cheung et al., 2022). HGSC is categorized as type II EOC that exhibits more aggressive, and harbors a defect in at least one DNA damage response (DDR) pathway (Pavlidis et al., 2021; Ovejero-Sanchez et al., 2023). It was not until the discovery of poly-ADP-ribose polymerase (PARP) inhibitors that the pattern of EOC treatment could be altered. PAPR inhibitors induce tumor cell death through a “synthetic lethal” mechanism, which is particularly effective in individuals with BRCA1 and/or 2 mutations or other homologous recombination deficiency (HRD) (Lau et al., 2022). It was identified that 23.8% of unselected Chinese patients with EOC carried BRCA1/2 mutations (20.3% germline and 4.1% systemic) (You et al., 2020). In addition, the synthetic lethal interaction may be exploited outside of germline BRCA mutations in the context of HRD, and research in this area is ongoing. In the absence of homologous recombination repair function, DNA double-strand breaks will be processed by alternative but error-prone repair pathways, such as non-homologous end joining repair (NHEJ), resulting in genomic instability and ultimately the death of cancer cells (Boussios et al., 2022).
PARP inhibitor maintenance therapy after initial chemotherapy or platinum-sensitive relapse therapy has been shown to enhance progression-free survival (PFS) to varying degrees, thus promoting prolonged survival in some patients (Tattersall et al., 2022). The Chinese National Medical Products Administration has approved olaparib, niraparib, and fluzoparib as maintenance treatments for recurrent ovarian cancer. Among them, niraparib is the only one with biomarker-independent and all-comer benefits. The first phase III trial of niraparib in China, namely, the NORA trial (NCT03705156), utilized an individualized starting dosage (ISD) of niraparib for platinum-sensitive recurrent ovarian cancer (PSROC) maintenance (Wu et al., 2021). Compared to placebo, maintenance niraparib prolonged the median PFS in patients with germline BRCA (gBRCA) mutations (5.5 months vs not reached) and without gBRCA mutations (3.9 vs 11.1 months) (Wu et al., 2021). Recently, ad hoc interim overall survival (OS) results demonstrated a certain degree of OS benefit in a gBRCA-mutated population (47.61 months vs not reached) and a non-gBRCA mutated group (38.41 vs 43.10 months) (Mirza et al., 2023). However, extended survival is accompanied by high drug costs and the expense associated with adverse event (AE) management.
Despite its significant clinical benefits, the economic burden of maintenance niraparib on both patients and society is a prominent concern. In this study, we examined for the first time the cost-effectiveness of maintenance niraparib ISD versus routine surveillance in PSROC patients classified by gBRCA status from the perspective of the Chinese healthcare system.
2 Methods
2.1 Model overview
Our model simulation study used data from a published trial. No human participants were involved in this study, and no institutional review board approval by an ethics committee was needed. Economic evaluations were based on the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) (Husereau et al., 2022).
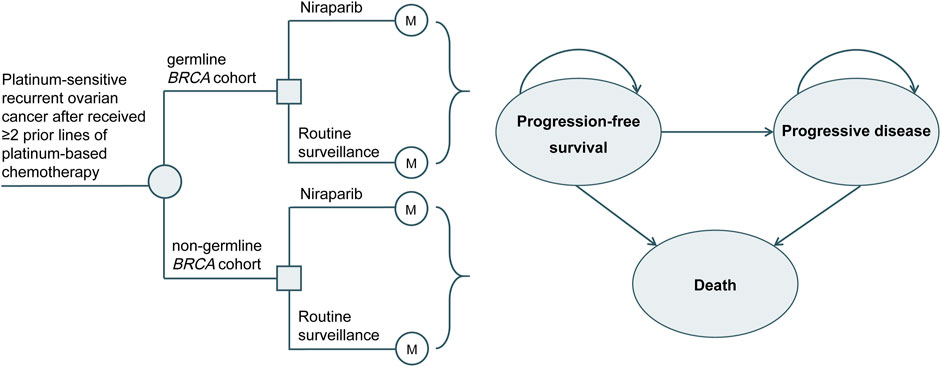
In this study, TreeAge Pro 2021 (TreeAge Software, Williamstown, MA, United States) was applied to develop a 3-state Markov model to simulate the costs and health outcomes of maintenance niraparib or routine surveillance for PSROC (Figure 1). The primary outcomes included life years (LYs), quality-adjusted life years (QALYs), total costs, and incremental cost-effectiveness ratios (ICERs). Based on the World Health Organization (WHO) recommendations, the willingness-to-pay (WTP) threshold in this study was equal to 3 times China’s gross domestic product (GDP) per capita in 2021 ($37,488) (World Health Organization, 2021).
The cycle length was 4 weeks, consistent with the dosing cycle in the NORA trial. The time horizon was 21.3 years, during which more than 99.99% of patients died in both arms. The characteristics of the simulated population were consistent with those of the NORA trial population (Wu et al., 2021). Patients received either maintenance treatment with niraparib (300 mg orally once daily for those with a body weight ≥77 kg and platelet count ≥150×103/µL, otherwise 200 mg orally once daily) or routine surveillance until disease progression or intolerable toxicity. Upon progression, patients in both arms were permitted to receive subsequent treatment until death. Due to the unavailability of subsequent therapy in both arms of the NORA trial, the choice of follow-up treatment was assumed from the published literature (Poveda et al., 2021) combined with clinical experience (Supplementary Table S1). Intervention discontinuation associated with AEs was 4% and 5.70% in the niraparib and routine surveillance arms, respectively (Wu et al., 2021). We applied a half-cycle correction, and a discount rate of 5% per year to the cost and effect outcomes (Chinese Pharmaceutical Association, 2020).
2.2 Model probabilities
All patients entered the research in the PFS state, and the subsequently observed states were PFS, progressing disease (PD), or death. The probabilities of PD and death from platinum-sensitive recurrent state for the niraparib and routine surveillance arms in the gBRCA-mutated and non-gBRCA mutated populations were calculated based on the Kaplan-Meier PFS and OS curves from the NORA study (Wu et al., 2021; Mirza et al., 2023). The individual patient data were recreated using the method of Hoyle and Henley (2011). We implemented GetData Graph Digitizer software to extract data points for the PFS and OS curves, and then the recreated PFS/OS curves were fitted to the following parametric survival functions: exponential, Weibull, log-logistic, lognormal, generalized gamma, gamma, and Gompertz distributions. The best-fit distribution for each curve was chosen according to the lowest value of the Akaike information criterion and the Bayesian information criterion, combined with a visual inspection. Details of the survival models of niraparib and routine surveillance in the gBRCA-mutated and non-gBRCA mutated cohorts with PSROC are shown in Supplementary Table S2, Supplementary Figure S1, Supplementary Figure S2.
2.3 Cost and health state utility values
Only direct medical costs, such as drug costs, intravenous chemotherapy, serious adverse event (SAE) management, gBRCA mutation tests, follow-up, and terminal care, were examined in this study (Supplementary Table S1). Drug prices were collected from the payment standards in China’s 2021 national insurance drug list and the winning bid prices in the drug procurement system (The State Council the People’s Republic of China, 2021; Pharmaceutical Classified Procurement System of Hunan Province, 2022). The expenses of SAE management and follow-up were estimated from the clinical experience. The costs of chemotherapy intravenous infusion and gBRCA mutation testing were based on current local pricing, while the remaining costs were sourced from prior cost-effectiveness studies (Li et al., 2020). Using China’s consumer price index, we adjusted all costs to their equal value in U.S. dollars for the year 2021 (1 US dollar equates to 6.45 Chinese yuan) (National Bureau of Statistics of China, 2022).
For administration dosage, we used a standard AUC of 5 mg/mL/min, with the assumption that an average female weighs 61 kg and has a body surface area of 1.64 m2 (Wu et al., 2021). SAEs occurring in greater than 10% of patients in either strategy and with an occurrence difference of more than 5% between the groups were assessed in this study, including anemia, thrombocytopenia, and neutropenia. It was supposed that all SAEs were experienced in the first cycle of the model.
For the niraparib and routine surveillance groups, the PFS state utility values were 0.849 and 0.820, respectively, and the PD state utility values were 0.793 and 0.775, respectively, based on literature assumptions (Guy et al., 2019). According to the published literature, neutropenia, anemia, and thrombocytopenia do not cause a significant decrease in quality of life, so disutility values for AEs were not considered in this study (Oza et al., 2018).
2.4 Sensitivity and scenario analyses
We explore the influence of model parameters on the robustness of the results through sensitivity analysis, including one-way sensitivity analysis and probabilistic sensitivity analysis. The parameter range in the one-way sensitivity analysis was determined by either the reported 95% confidence interval or a ±20% change from the baseline value, except for the drug price parameter, for which the range was based on market fluctuations (Supplementary Table S1).
Ten thousand Monte Carlo simulations were used to perform probabilistic sensitivity analyses, in which critical model parameters were simultaneously varied in a specific type of distribution. Cost parameters were assumed to obey a gamma distribution, and probability parameters and utility values were assumed to obey a beta distribution.
Furthermore, we conducted scenario analyses to investigate the effect of fixed dosing, economic development level, and enrollment in the National Basic Medical Insurance program on the cost-effectiveness of niraparib. Scenario 1: We assumed that all patients received a fixed starting dosage (FSD) of 300 mg/day regardless of body weight and platelet count. Scenario 2: Due to the large wealth gap between regions in China, we added a scenario analysis at WTP thresholds of $19,002/QALY (Gansu, the province with the lowest GDP in China) and $85,176/QALY (Beijing, the city with the highest GDP in China). Scenario 3: Considering that most cancer treatment drugs are included in China’s 2021 national insurance drug list released by the National Healthcare Security Administration (The State Council the People’s Republic of China, 2021), we included the out-of-pocket drug prices after a certain reimbursement percentage.
3 Results
3.1 Base-case analysis
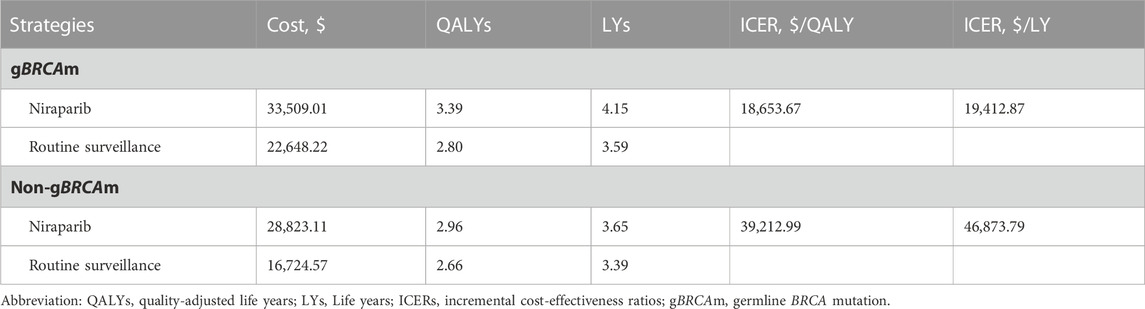
For the gBRCA-mutated population, an additional 0.59 QALY (0.56LY) was obtained with maintenance niraparib compared to routine surveillance, accompanied by an incremental cost of $10,860.79 and an ICER of $18,653.67/QALY ($19,412.87/LY). For the non-gBRCA mutated cohort, the incremental cost of maintenance niraparib was $12,098.54 compared to routine surveillance, with an incremental effect of 0.30 QALY (0.26LY) and an ICER of $39,212.99/QALY ($46873.79/LY) (Table 1).
3.2 Sensitivity analysis
The tornado diagram demonstrated the outcomes of the one-way sensitivity analysis (Figure 2). For the gBRCA-mutated population, the expense of niraparib had the dominant impact on the ICER, followed by the proportion of patients receiving niraparib at 200 mg per day and the discount rate. Regardless of the change in any parameter within the given range, the ICER was always under the WTP threshold ($37,488/QALY). In the non-gBRCA mutated cohort, the most influential modeling variable was also the niraparib cost, while the other sensitive parameters were the proportion of patients subsequently receiving platinum plus bevacizumab in the niraparib group, the utility for PD states in the routine surveillance group and the niraparib group, and the proportion of patients receiving niraparib at a daily dose of 200 mg. Maintenance therapy was found to be cost-effective when the price of niraparib was below $0.2320/mg.
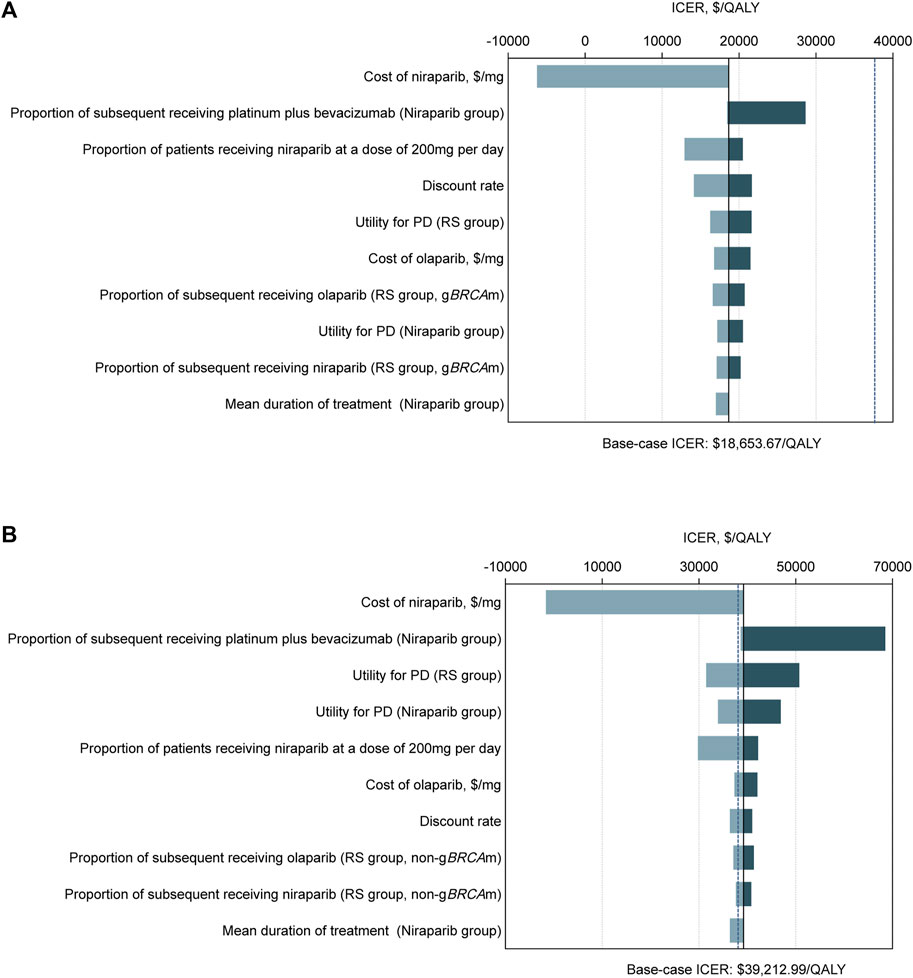
FIGURE 2. Tornado diagrams of one-way sensitivity analyses with greatest influence variables. The diagram shows the association of variables with the ICER of niraparib versus routine surveillance for platinum-sensitive recurrent ovarian cancer in (A) the gBRCAm cohort and (B) the non-gBRCAm cohort. The black vertical line represents the base-case result of $18,653.67 per QALY and $39,212.99 per QALY in the gBRCAm cohort and non-gBRCAm cohort, respectively. The blue vertical dotted line represents the WTP threshold of $37,488 per QALY. Abbreviation: ICER, incremental cost-effectiveness ratios; QALY, quality-adjusted life years; PD, progressed disease; RS, routine surveillance; gBRCAm, germline BRCA mutation.
In the probabilistic sensitivity analysis, the acceptability curves indicated that in the gBRCA-mutated population, the possibility of having cost-effectiveness in the niraparib group was 30.73% at a WTP threshold of $37,488 per QALY, and the likelihood was over 50% that the niraparib group would be cost-effective at a WTP threshold greater than $55,820 per QALY. For the non-gBRCA mutated cohort, when the WTP threshold reached $37,488/QALY, the possibility of niraparib being cost-effective was 11.74%, and this value reached 50% at $63,622/QALY (Figure 3).
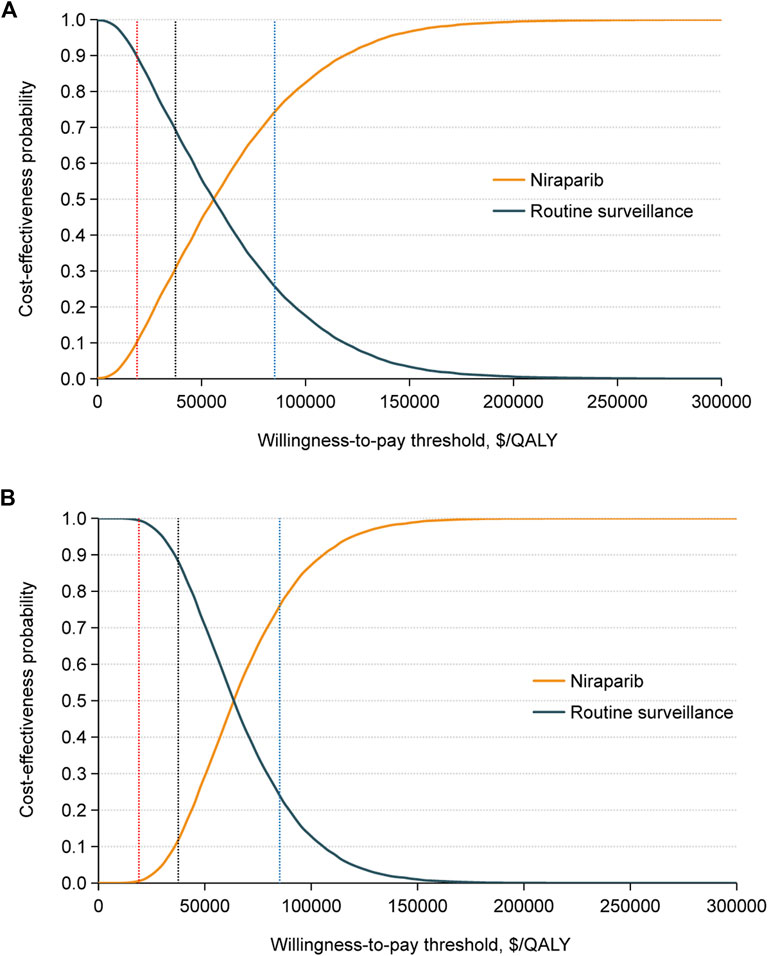
FIGURE 3. Cost-effectiveness acceptability curves for the niraparib and routine surveillance groups in (A) the gBRCAm cohort and (B) the non-gBRCAm cohort generated from the probabilistic sensitivity analysis (10,000 iterations). The red, black, and blue vertical dotted lines represent the $19,002, $37,488, and $85,176 per QALY. Abbreviation: QALY, quality-adjusted life years.
3.3 Scenario analyses
Scenario 1: When administered at an FSD of 300 mg/day, the ICER for niraparib versus placebo in the gBRCA-mutated population was $33,009.06/QALY. This was cost-effective at the current WTP ($37,488/QALY) but significantly more costly than individualized dosing ($19,218.97 vs $10,860.79) (Supplementary Table S3). Probabilistic sensitivity analysis showed an economic-benefit probability of 9.35%, which was much lower than the ISD probability of 30.73% (Supplementary Figure S3). The fixed dosage of niraparib was not cost-effective for the non-gBRCA mutated population (ICER of $62,763.81/QALY) when administered at 300 mg/day (Supplementary Table S2). The probability sensitivity analysis revealed that the likelihood of cost-effectiveness was only 0.77% (Supplementary Figure S3).
Scenario 2: When the WTP threshold was $19,002 per QALY, the possibility of maintenance niraparib proving cost-effective was 10.25% for the gBRCA-mutated population and 0.55% for the non-gBRCA mutated population. When the WTP threshold was set at $85,176 per QALY, the likelihood of maintenance niraparib having cost-effectiveness was 74.23% in the gBRCA-mutated population and 76.10% in the non-gBRCA mutated cohort (Figure 3).
Scenario 3: After medical insurance coverage, the out-of-pocket payments for niraparib, paclitaxel, carboplatin, bevacizumab, albumin-bound paclitaxel, olaparib, and letrozole would be discounted to 20%, 0%, 0%, 30%, 20%, 30%, and 5%, respectively. When considering the out-of-pocket price after medical insurance reimbursement, the ICER of niraparib compared to routine surveillance was $3,372.19/QALY in the gBRCA-mutated population and $5,643.96/QALY in the non-gBRCA mutated population (Supplementary Table S4). Probabilistic sensitivity analysis suggested that maintenance niraparib is totally cost-effective relative to routine monitoring, regardless of the gBRCA mutation status (Supplementary Figure S4).
4 Discussion
The promising results of the NORA study, which is the only second-line maintenance treatment based on ISD for Chinese PSROC patients, indicate considerable therapeutic benefits (Wu et al., 2021). Additionally, the high economic burden on patients and society is a growing issue for Chinese authorities.
This study demonstrated that compared to routine surveillance, maintenance niraparib was cost-effective for patients with gBRCA mutations. In contrast, niraparib was only cost-effective for the non-gBRCA mutated population if it cost less than $0.232 per milligram. Probabilistic sensitivity analysis revealed that at the current WTP threshold of $37,488/QALY, niraparib was cost-effective for 30.73% of the gBRCA-mutated population and 11.74% of the non-gBRCA mutated group.
Several model-based economic studies have investigated the cost-effectiveness of niraparib as second-line maintenance treatment for ovarian cancer. Four of them were based on the healthcare system (Zhong et al., 2018), society (Dottino et al., 2019), or payer (Guy et al., 2019) perspective in the United States, one on the single-payer perspective of Taiwan China (Leung et al., 2022), and one on the mainland Chinese healthcare system perspective (Nie et al., 2022). Due to differences in modeling approaches, population characteristics, drug pricing, WTP thresholds, national conditions and so on, the results of pharmacoeconomic studies in one country or region cannot be simply replicated and applied to others (Drummond et al., 2009). The study conducted in Taiwan, China, revealed that maintenance niraparib was cost effective in patients with gBRCA mutations but not in non-gBRCA mutated patients (Leung et al., 2022). The study by Nie J et al. included only a gBRCA-mutated population and showed that compared to routine surveillance, niraparib was cost-effective from the perspective of the Chinese healthcare system (Nie et al., 2022). The previous two studies, however, were based on clinical data from the NOVA trial using a fixed dosage of niraparib in a non-Chinese population, which could bias the results. Additionally, the model time horizon for these two studies was just 2 and 5 years, respectively.
To our knowledge, this is the first study to assess the cost-effectiveness of maintenance niraparib with an ISD for PSROC in the gBRCA-mutated and non-gBRCA mutated populations from the perspective of the mainland Chinese healthcare system. The population characteristics and clinical outcomes in this study were from the NORA trial, the only Chinese population-based randomized controlled trial of niraparib. This made it more applicable to the Chinese population and might decrease population bias. Meanwhile, we conducted a scenario analysis for the first time to investigate the effect of FSD, economic development level and enrollment in the National Basic Medical Insurance program on the cost-effectiveness of niraparib.
Compared to the FSD of niraparib, the ISD reduced the prevalence of adverse events and treatment discontinuation or interruption due to adverse reactions without compromising efficacy (Mirza et al., 2016). In addition, it lowered drug costs and AE management expenses, with drug costs having the greatest impact on the economic model in our study. This could explain the scenario analysis results that there was a lower probability of cost-effectiveness with fixed dosing of niraparib compared to individualized dosing, regardless of the BRCA mutation status.
As one of the world’s largest developing countries, China’s economic development varies by region. The highest per capita GDP in China was $28,392 in Beijing, while the lowest was $6,334 in Gansu Province. In this study, the WTP threshold was set at 3 times the national GDP per capita, as recommended by the WHO(World Health Organization, 2021). This may not accurately reflect the acceptability of each region nationwide. Thus, we included a WTP threshold analysis with a range from 3 times Gansu Province’s GDP per capita to 3 times Beijing’s ($19,002/QALY to $85,176/QALY). The results suggested that the probability of niraparib having an economic advantage may be greatly improved for patients in regions with higher levels of economic development in China, independent of BRCA status. In regions with lower levels of economic development, niraparib has a lower probability of being cost-effective in the gBRCA-mutated population and is almost not cost-effective in the non-gBRCA mutated population.
One effective way to improve cost-effectiveness may be to reduce the cost of antineoplastic agents by negotiating trade-offs in drug expenditure and coverage. Currently, niraparib is significantly less expensive in China than in developed countries such as the United States (Chan et al., 2022). This stems from our unique national health insurance negotiation system. In 2015, China introduced a pricing negotiation approach for patented and exclusive medicine involving pharmaceutical corporations and other stakeholders (The State Council the People’s Republic of China, 2015). The Interim Measures for the Administration of the National Basic Medical Insurance Drugs, applied in 2020, require an economic evaluation for drugs admitted to or removed from the medical insurance list as well as broadening of the spectrum of limited payment (The State Council the People’s Republic of China, 2020). In 2021, the National Health Security Administration negotiated with manufacturers to decrease the cost of niraparib by 81%, from $1.29/mg to $0.24/mg. This has drastically reduced the economic burden for Chinese patients with ovarian cancer. In addition, almost 95% of the Chinese population has access to the National Basic Medical Insurance, which covers approximately 1.4 billion people (National Healthcare Security Administration, 2021). Depending on the type of health insurance, the National Basic Medical Insurance program provides 20%–80% savings off the $0.24/mg cost of niraparib. Our study demonstrates that when only out-of-pocket prescription expenses are considered, the probability of niraparib being cost-effective increases to 100%. This will significantly expand the number of patients who benefit from maintenance niraparib.
Additionally, we must note the limitations of this study. First, as the final OS results were not yet available, we utilized the newly published ad hoc interim OS results from the NORA trial, and the maturity of OS curves was less than 50% (Mirza et al., 2023). The use of final OS results would be preferred because it would reduce the uncertainty of the model’s predicted outcomes. Second, since data on subsequent treatment after progression with niraparib were not yet available from the NORA trial, the subsequent treatment regimens and ratios in this paper were referenced from the published literature and the practical experience of clinical experts, which may cause bias. Third, based on the NORA trial, PARP inhibitors were used in 54% of the gBRCA-mutated population and 36% of the non-gBRCA mutated population in this study after disease progression in the routine surveillance group, which may lead to an overestimation of survival and effect of the routine surveillance group. In the real world, patients in the routine surveillance group frequently receive PARP inhibitors after disease progression. Fourth, Due to the lack of data, we explored the cost-effectiveness of FSD of niraparib in scenario 1 analysis using data from the NORA study, assuming the same baseline characteristics of niraparib FSD and ISD administration. Indeed, patients administered with FSD and ISD may differ in the incidence of adverse events, treatment interruption or discontinuation, and even prognosis, which may have biased the results. To verify model stability, we conducted one-way sensitivity analyses.
5 Conclusion
Our study demonstrated that compared with routine surveillance, maintenance niraparib with an ISD is cost-effective for patients with gBRCA mutations in China. For the non-gBRCA mutated population, niraparib is more effective but costly than routine surveillance, and the price reduction will benefit its cost-effectiveness. Economic outcomes could be further improved for patients receiving ISD, for those in regions with higher per capita GDP in China, or for those covered by the National Basic Medical Insurance program, independent of BRCA status.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
YS: formal analysis, funding acquisition, software, writing–original draft, and writing–review and editing. DX: data curation, formal analysis, writing–original draft, and writing–review and editing. SsL: data curation, methodology, and visualization. SL: conceptualization, and supervision. YZ: conceptualization, supervision, and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
We thank the National Natural Science Foundation of China (No. 82073323) and the Natural Science Foundation of Hunan Province (No. 2021JJ31106 and No. 2022JJ70071) for their financial support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1198585/full#supplementary-material
References
Boussios, S., Rassy, E., Moschetta, M., Ghose, A., Adeleke, S., Sanchez, E., et al. (2022). BRCA mutations in ovarian and prostate cancer: Bench to bedside. Cancers (Basel) 14 (16), 3888. doi:10.3390/cancers14163888
Chan, V. K. Y., Yang, R., Wong, I. C. K., and Li, X. (2022). Cost-effectiveness of poly ADP-ribose polymerase inhibitors in cancer treatment: A systematic review. Front. Pharmacol. 13, 891149. doi:10.3389/fphar.2022.891149
Cheung, A., Shah, S., Parker, J., Soor, P., Limbu, A., Sheriff, M., et al. (2022). Non-epithelial ovarian cancers: How much do we really know? Int. J. Environ. Res. Public Health 19 (3), 1106. doi:10.3390/ijerph19031106
Chinese Pharmaceutical Association (2020). China guidelines for pharmcoeconomic evaluation [online]. Available at: https://www.ispor.org/heor-resources/more-heor-resources/pharmacoeconomic-quidelines/pe-quideline-detail/china-mainland (Accessed April 22, 2022).
Dottino, J. A., Moss, H. A., Lu, K. H., Secord, A. A., and Havrilesky, L. J. (2019). U.S. Food and drug administration-approved poly (ADP-Ribose) polymerase inhibitor maintenance therapy for recurrent ovarian cancer: A cost-effectiveness analysis. Obstet. Gynecol. 133 (4), 795–802. doi:10.1097/aog.0000000000003171
Drummond, M., Barbieri, M., Cook, J., Glick, H. A., Lis, J., Malik, F., et al. (2009). Transferability of economic evaluations across jurisdictions: ISPOR good research practices task force report. Value Health 12 (4), 409–418. doi:10.1111/j.1524-4733.2008.00489.x
Guy, H., Walder, L., and Fisher, M. (2019). Cost-effectiveness of niraparib versus routine surveillance, olaparib and rucaparib for the maintenance treatment of patients with ovarian cancer in the United States. Pharmacoeconomics 37 (3), 391–405. doi:10.1007/s40273-018-0745-z
Hanker, L. C., Loibl, S., Burchardi, N., Pfisterer, J., Meier, W., Pujade-Lauraine, E., et al. (2012). The impact of second to sixth line therapy on survival of relapsed ovarian cancer after primary taxane/platinum-based therapy. Ann. Oncol. 23 (10), 2605–2612. doi:10.1093/annonc/mds203
Hoyle, M. W., and Henley, W. (2011). Improved curve fits to summary survival data: Application to economic evaluation of health technologies. BMC Med. Res. Methodol. 11, 139. doi:10.1186/1471-2288-11-139
Husereau, D., Drummond, M., Augustovski, F., de Bekker-Grob, E., Briggs, A. H., Carswell, C., et al. (2022). Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: Updated reporting guidance for health economic evaluations. Value Health 25 (1), 3–9. doi:10.1016/j.jval.2021.11.1351
Lau, C. H., Seow, K. M., and Chen, K. H. (2022). The molecular mechanisms of actions, effects, and clinical implications of PARP inhibitors in epithelial ovarian cancers: A systematic review. Int. J. Mol. Sci. 23 (15), 8125. doi:10.3390/ijms23158125
Leung, J. H., Lang, H. C., Wang, S. Y., Lo, H. F., and Chan, A. L. (2022). Cost-effectiveness analysis of olaparib and niraparib as maintenance therapy for women with recurrent platinum-sensitive ovarian cancer. Expert Rev. Pharmacoecon Outcomes Res. 22 (3), 489–496. doi:10.1080/14737167.2021.1954506
Li, H., Lai, L., and Wu, B. (2020). Cost effectiveness of ceritinib and alectinib versus crizotinib in first-line anaplastic lymphoma kinase-positive advanced non-small-cell lung cancer. Clin. Drug Investig. 40 (2), 183–189. doi:10.1007/s40261-019-00880-8
Liu, S., Wu, M., and Wang, F. (2021). Research progress in prognostic factors and biomarkers of ovarian cancer. J. Cancer 12 (13), 3976–3996. doi:10.7150/jca.47695
Mirza, M. R., Monk, B. J., Herrstedt, J., Oza, A. M., Mahner, S., Redondo, A., et al. (2016). Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N. Engl. J. Med. 375 (22), 2154–2164. doi:10.1056/NEJMoa1611310
Mirza, M. R., Wu, X., Zhu, J., Yin, R., Yang, J., Liu, J., et al. (2023). VP7-2022: An ad-hoc interim overall survival results of niraparib with individualized starting dose as maintenance therapy in patients with platinum-sensitive recurrent ovarian cancer (NORA): A double-blind, randomized, placebo-controlled, phase III trial. Ann. Oncol. 34 (1), 124–125. doi:10.1016/j.annonc.2022.11.007
National Bureau of Statistics of China (2022). Health care and personal articles of consumer price indices. Available at: http://data.stats.gov.cn/search.htm?s=CPI (Accessed July 9, 2021).
National Healthcare Security Administration (2021). The 14th Five-Year Plan for universal medical insurance [Online]. Available at: http://www.nhsa.gov.cn/art/2021/9/29/art_104_6545.html (Accessed December 15, 2022).
Nie, J., Wu, H., Sun, L., Ding, Y., Luan, Y., and Wu, J. (2022). Cost-effectiveness of fuzuloparib compared to routine surveillance, niraparib and olaparib for maintenance treatment of patients with germline BRCA1/2 mutation and platinum-sensitive recurrent ovarian carcinoma in China. Front. Pharmacol. 13, 987337. doi:10.3389/fphar.2022.987337
Ovejero-Sanchez, M., Gonzalez-Sarmiento, R., and Herrero, A. B. (2023). DNA damage response alterations in ovarian cancer: From molecular mechanisms to therapeutic opportunities. Cancers (Basel) 15 (2), 448. doi:10.3390/cancers15020448
Oza, A. M., Matulonis, U. A., Malander, S., Hudgens, S., Sehouli, J., Del Campo, J. M., et al. (2018). Quality of life in patients with recurrent ovarian cancer treated with niraparib versus placebo (ENGOT-OV16/NOVA): Results from a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 19 (8), 1117–1125. doi:10.1016/S1470-2045(18)30333-4
Pavlidis, N., Rassy, E., Vermorken, J. B., Assi, T., Kattan, J., Boussios, S., et al. (2021). The outcome of patients with serous papillary peritoneal cancer, fallopian tube cancer, and epithelial ovarian cancer by treatment eras: 27 years data from the SEER registry. Cancer Epidemiol. 75, 102045. doi:10.1016/j.canep.2021.102045
Pharmaceutical Classified Procurement System of Hunan Province (2022). Pharmaceutical classified procurement system of hunan province. Available at: https://jyjy.hnsggzy.com/(Accessed July 15, 2022).
Poveda, A., Floquet, A., Ledermann, J. A., Asher, R., Penson, R. T., Oza, A. M., et al. (2021). Olaparib tablets as maintenance therapy in patients with platinum-sensitive relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A final analysis of a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 22 (5), 620–631. doi:10.1016/S1470-2045(21)00073-5
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Tattersall, A., Ryan, N., Wiggans, A. J., Rogozińska, E., and Morrison, J. (2022). Poly(ADP-ribose) polymerase (PARP) inhibitors for the treatment of ovarian cancer. Cochrane Database Syst. Rev. 2 (2), CD007929. doi:10.1002/14651858.CD007929.pub4
The State Council the People's Republic of China (2015). Guiding opinions on improving the drug centralized bidding and purchasing system in public hospitals [Online]. Available at: http://www.gov.cn/zhengce/content/2015-02/28/content_9502.htm (Accessed December 15, 2022).
The State Council the People's Republic of China (2021). National drug catalogue for basic medical insurance, work-related injury insurance and maternity insurance [online]. Available at: http://www.gov.cn/zhengce/zhengceku/2021-12/03/content_5655651.htm (Accessed July 15, 2022).
The State Council the People's Republic of China (2020). The interim Measures for the administration of drugs under basic medical insurance [online]. Available at: http://www.gov.cn/zhengce/zhengceku/2020-08/04/content_5532409.htm (Accessed December 15, 2022).
World Health Organization (2021). Choosing interventions that are cost effective (WHO-choice), tables of costs and prices used in WHO-choice analysis [online]. Available at: https://www.who.int/entity/choice/costs/en/(Accessed October 4, 2022).
Wu, X. H., Zhu, J. Q., Yin, R. T., Yang, J. X., Liu, J. H., Wang, J., et al. (2021). Niraparib maintenance therapy in patients with platinum-sensitive recurrent ovarian cancer using an individualized starting dose (NORA): A randomized, double-blind, placebo-controlled phase III trial(☆). Ann. Oncol. 32 (4), 512–521. doi:10.1016/j.annonc.2020.12.018
You, Y., Li, L., Lu, J., Wu, H., Wang, J., Gao, J., et al. (2020). Germline and somatic BRCA1/2 mutations in 172 Chinese women with epithelial ovarian cancer. Front. Oncol. 10, 295. doi:10.3389/fonc.2020.00295
Zhang, S., Cheng, C., Lin, Z., Xiao, L., Su, X., Zheng, L., et al. (2022). The global burden and associated factors of ovarian cancer in 1990-2019: Findings from the global burden of disease study 2019. BMC Public Health 22 (1), 1455. doi:10.1186/s12889-022-13861-y
Zheng, R., Zhang, S., Zeng, H., Wang, S., Sun, K., Chen, R., et al. (2022). Cancer incidence and mortality in China, 2016. J. Natl. Cancer Cent. 2 (1), 1–9. doi:10.1016/j.jncc.2022.02.002
Keywords: niraparib, routine surveillance, ovarian cancer, maintenance therapy, cost-effectiveness
Citation: Shi Y, Xiao D, Li S, Liu S and Zhang Y (2023) Cost-effectiveness of maintenance niraparib with an individualized starting dosage in patients with platinum-sensitive recurrent ovarian cancer in China. Front. Pharmacol. 14:1198585. doi: 10.3389/fphar.2023.1198585
Received: 01 April 2023; Accepted: 18 July 2023;
Published: 27 July 2023.
Edited by:
Anindita Chakrabarty, Shiv Nadar University, IndiaReviewed by:
Stergios Boussios, King’s College London, United KingdomQingLei Gao, Huazhong University of Science and Technology, China
Copyright © 2023 Shi, Xiao, Li, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Zhang, eHl6aGFuZ3l1QGNzdS5lZHUuY24=; Shao Liu, bGl1c2hhbzk5OUBjc3UuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Yin Shi
Yin Shi Di Xiao
Di Xiao Shuishi Li2,4
Shuishi Li2,4 Yu Zhang
Yu Zhang