- 1Xin-Huangpu Joint Innovation Institute of Chinese Medicine, Guangzhou, China
- 2Institute of Basic Theory for Chinese Medicine, China Academy of Chinese Medicine Science, Beijing, China
- 3China Science and Technology Development Center of Chinese Medicine, Beijing, China
- 4Institute of Traditional Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin, China
With the introduction of various subjects, such as clinical epidemiology and evidence-based medicine, the qualities and levels of Traditional Chinese Herbal Medicine (TCHM) in China improved substantially, and the processes of internationalization of Traditional Chinese Medicine (TCM) are further accelerated. Since, a variety of drug products in China have been approved for marketing in other countries, and approximately 10 products have submitted the IND application to FDA of United States, of which various Chinese herbal preparations such as compound Danshen dripping pills, Xingling granules, and HMPL-004 have been approved to be investigated in phase III clinical trials. In general, multi-center studies of TCHM are increasing with years, but most of the studies are performed in some certain country, and the actual international multi-center clinical trials are very rare. Number of SCI literatures on multi-center clinical trials of TCHM that published in the recent decade also showed increasing tendency with years, despite the evident reduction in the past 2 years due to the influence of COVID-19 pandemic. Of the multi-center clinical trials of TCHM that performed by mainland China and other oversees regions, except for Taiwan, China, nearly 70% were focused on classic Chinese medicinal formulae and Chinese patent medicine, while the other 30% were on dietary supplements and plant extracts. Facing the future, the “human experience” has attracted close attentions from researchers throughout the world. Effectively utilizing the historic “human experience” is an important method to vitalize potential of original scientific and technological resources of TCHM. Performing multi-center clinical trials with high qualities is still an essential method for TCHM in accessing the mainstream medicine market. In addition, it is also required to further improve the evaluation techniques and methods that not only meet the international standards but also meet the characteristics of TCHM. Furthermore, we should also focus on the TCHM specific clinical values and scientific reports.
1 Introduction
The unique theories of Traditional Chinese Medicine (TCM) and complexity of Chinese herbal compounds have made it quite difficult to evaluate the treatment efficacies of Traditional Chinese Herbal Medicine (TCHM) in studies, and the various indicators that difficult to be qualified also influenced the objective and accurate assessment of safety and effectiveness of TCHM (Shen et al., 2013). Clinical trial of drugs is an important process in drug development, and the quality could directly influence the safety and effectiveness of drugs after marketing. In this globally rampant pandemic of COVID-19, TCHM has been extensively used throughout the anti-pandemic processes, various TCHM that represented by Qing-Fei-Pai-Du Decoction, Huashibaidu Formula, Xuanfeibaidu Formula, Jin-Hua-Qing-Gan Granule, Lian-Hua-Qing-Wen Capsule, and Xuebijing injection have become important foreign-aid materials (Zhang et al., 2020), and have been approbated by various countries. In the past 4 decades, the introduction of subjects such as clinical epidemiology and evidence-based medicine has substantially improved the qualities and levels of clinical studies on TCHM, and a lot of high-quality high-level clinical studies on TCHM [including Investigational New Drugs (IND) not aiming for registration] have been performed successively, and a large group of TCHM have been registered for marketing in other countries, which have promoted the advancement of TCHM industry and the internationalization of TCHM.
2 Registration, marketing, and clinical trials of Chinese domestic TCHM overseas
According to the statistics of World Health Organization (WHO), TCM has been acknowledged by 29 countries and regions, including Australia, Canada, Austria, Singapore, Vietnam, by legislations, and 18 countries and regions have included TCHM in medical insurance. A lot of TCHM have been registered successively in the European Union (EU), Russia, Singapore, Cuba, and Vietnam.
The current 2004/24/EC decree on traditional herbal medicinal product (THMP) that issued by EU in 2004 provided a simplified registration procedure of THMP that had been used traditionally for long term but lacked evidence from modern studies, of which the “non-clinical” and “clinical” studies were derated from the application files of the Common Technical Document (CTD) of the products, while only literatures, evidence from experts, and reviews and reports on safety were required to demonstrate that the product had sufficient traditional use and safety, after which the product could be registered for marketing. Such measurements substantially reduced the difficulties in registration. This decree also encouraged the approval of THMP, including compound products of TCHM, to obtain EU market admittance for drugs (Qu et al., 2021). Currently, several varieties including Di’ao Xinxuekang capsule produced by the Chengdu Di’ao Pharmaceutical Group (2013, Netherland) (Wang, 2012), Danshen capsule produced by the Tasly Pharmaceutical Group Co. LTD. (2016, Netherland) (Zhang et al., 2017), Yufengningxin tablets produced by the Tong Ren Tang Group (2019, Netherland) (The Medicines Evaluation Board, 2019), and Banlangen granule produced by the Xiangxue-Cambridge International Research Center of Traditional Chinese Medicine (United Kingdom, 2015) (Medicines & Healthcare products Regulatory Agency, 2022) have been registered for marketing in EU.
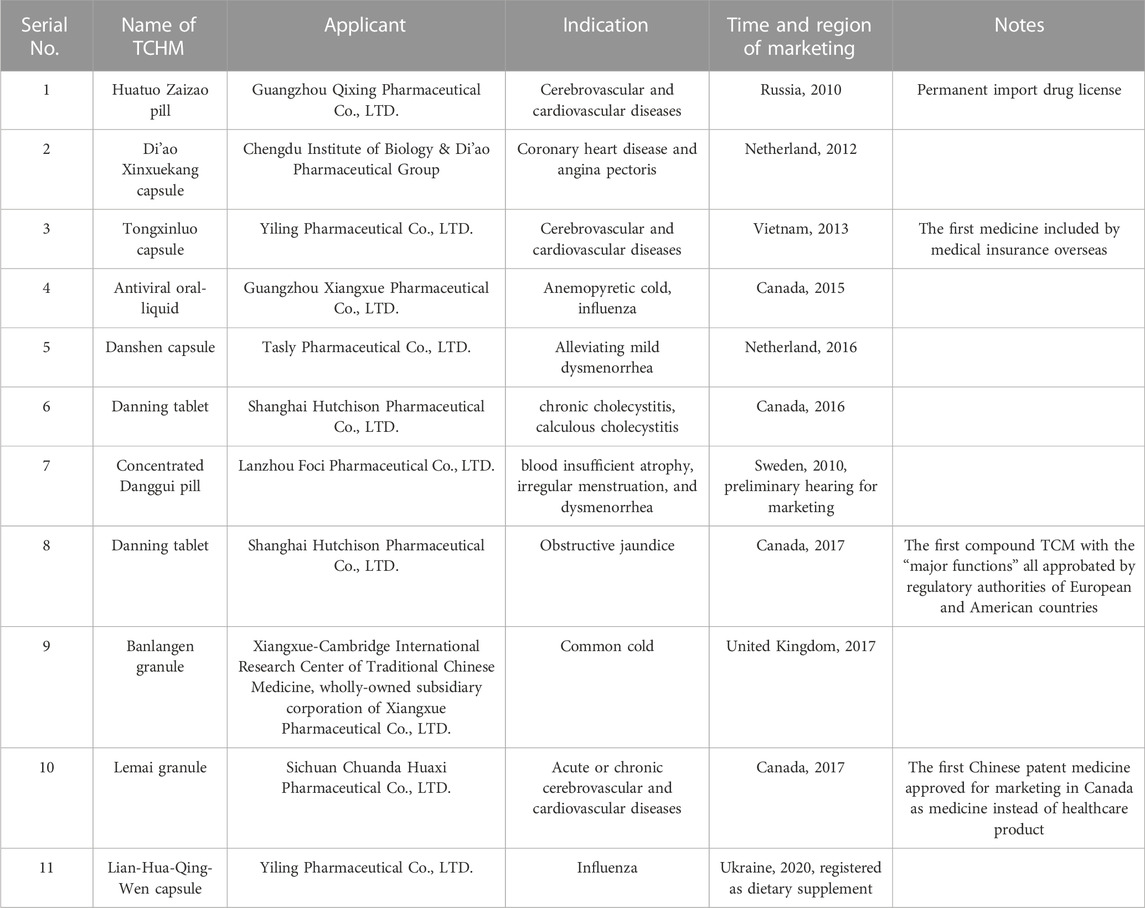
TCHM could be registered as natural health products (NHPs) in Canada through the “traditional-application (also known as traditional efficacy application)” processes, for which the TCHM products of the formula, preparation processes, and indications being used for more than 50 years. Regarding the preparation of files for application, the files on treatment efficacies of the products are relatively flexible, while the safety and quality of the products are highly concerned. Since 2010, several TCHM products have acquired the marking qualification in countries other than China (Table 1). In 2008, the Fufang Danshen dripping pill and Chaihu dripping pill produced by the Tasly Group passed all the review and approval procedures by Health Canada as a Traditional Drug (Zhao et al., 2009). In 2016, the Danning tablet produced by the Shanghai Hutchison Pharmaceutical Co., LTD. was approved for marketing by the Natural and Non-prescription Health Products Directorate of Health Canada (Huang et al., 2018). In addition, the Juhong Tan Ke liquid produced by the Xiangxue Pharmaceutical Group (2012) and Antiviral Oral-Liquid (2016) were also approved by Health Canada (Xiangxue Pharmaceutical, 2021).
The Huatuo Zaizao pill produced by the Guangzhou Baiyunshan Qixing Pharmaceutical Co., LTD. is one of the confidential products in China, which has accessed 27 countries and regions including Russia, Canada, Australia, and Vietnam, and has been included in the medical insurance of Vietnam and Essential Medicine List of Russia. Huatuo Zaizao pill has already acquired the permanent drug registration certificate in Russia in 2010. In May 2012, the Qixing Pharmaceutical Group signed an “evidence-based clinical study agreement for treating cerebral stroke in rehabilitation stage by Huatuo Zaizao pill” with the Belarus National Scientific Practice Center of Cardiology, and jointly initiated the evidence-based clinical study of “Huotuo Zaizao pill.” In 2005, Xuezhikang was registered in Taiwan, China and Singapore as a prescription drug, and the clinical study results were published in Norway in 2006. In 2011, the clinical findings of Xuezhikang were included in the European Guidelines for Blood Lipid Management. Furthermore, the Juhong Tan Ke liquid produced by the Xiangxue Pharmaceutical Group was approved in Kenya (2013), and Xiaoerhuashi oral-liquid was approved for marketing by the Singapore Health Sciences Authority (2017). Lian-Hua-Qing-Wen Capsule was approved for marketing as “Chinese patent medicine,” “medicine,” “botanical medicine,” “natural health product,” “dietary supplement,” “modern botanical medicine,” and “natural medicine” in Hong Kong and Macao, China, Brazil, Indonesia, Canada, Mozambique, Romania, Thailand, Ecuador, Singapore, Laos, Kyrgyzstan, Philippines, Kuwait, Mauritius, Uganda, and Russia. The registration of Lian-Hua-Qing-Wen Capsule has also been initiated in the Middle East and Africa.
Substantially different from EU and other regions, TCHM could only apply for marketing in the United States through the new drug application (NDA) procedures, according to the Guidance for Industry Botanical Drug Products that issued by the Food and Drug Administration (FDA) of USA. In recent years, approximately 10 TCHM products submitted IND application to FDA (Table 2). The compound Danshen dripping pills produced by the Tasly Pharmaceutical Group Co., LTD., Xingling granules produced by the Xingling Sci-tech Pharmaceutical Co., LTD., and HMPL-004 produced by the Hutchison Whampoa Co., LTD. have been approved to be investigated in phase III clinical trials.
3 Advances in international multi-center clinical trials on TCHM
In general, international multi-center studies on TCHM are increasing with years in the recent decade, despite the fact that the total number is still limited. When using “Herbal medicine” as the keyword to search the United States Clinical Trial Registry, 212 clinical trials on TCHM were found registered in European or American countries (From January 1989 to April 2022). One hundred and fifty-six (73.58%) of the clinical trials were mainly from East Asia (including 104 from mainland China, 27 from Taiwan, China, and 15 from Hong Kong, China), 22 of the clinical trials were from North America (including 8 from New York, United States; 7 from California, United States; 3 from Connecticut, United States; and 3 from Maryland, United States), 4 of the clinical trials were from Southeast Asia, and 3 of the clinical trials were from Europe (Figure 1). Unfortunately, most of these studies were performed in some single countries, while the real international multi-center clinical trials were very rare. From the aspect of study status, 67 studies have completed as planned (31.60% completed), 39 studies are recruiting subjects (18.40% recruiting), 24 studies have not started the subject recruitment (11.32% Not yet recruiting), 6 studies terminated prematurely and subjects were not further treated or examined (2.83% Terminated), 3 studies are active and on-going (1.42% Active, not recruiting), 2 studies terminated prematurely but may be re-started again (0.94% Suspended), and 2 studies terminated before the recruitment of first subject (0.94% Withdrawn). From the phases of studies, 86 studies (40.57%) were phase II clinical trials, 34 studies (16.04%) were phase III clinical trials, 21 studies (9.91%) were phase I clinical trials, 21 studies (9.91%) were phase IV clinical trials, and 9 studies (4.25%) were early phase explorative trials.
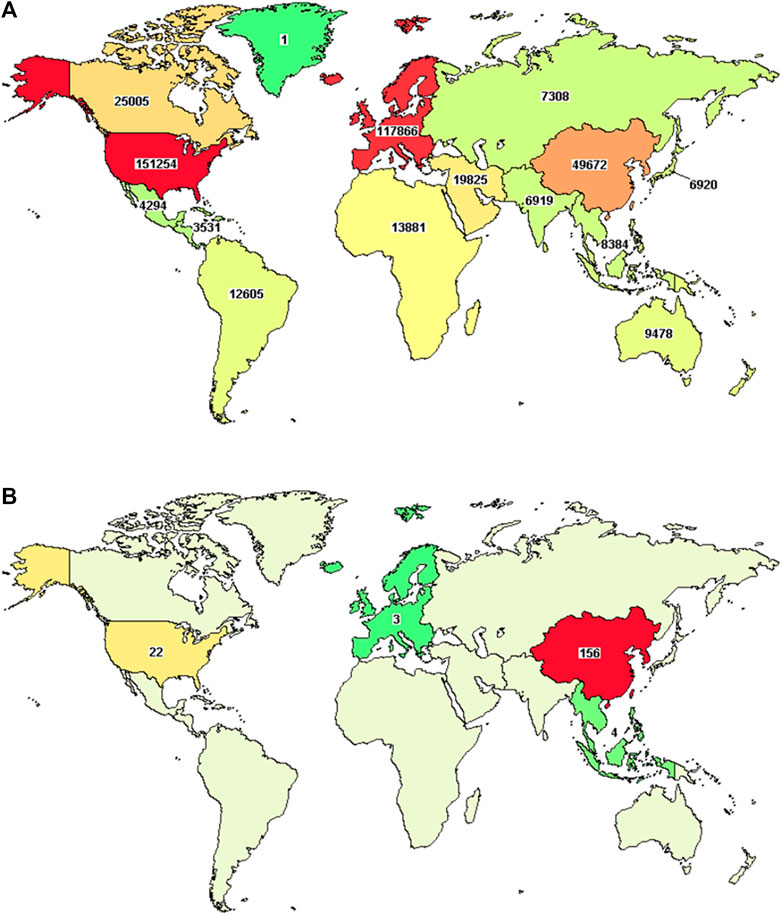
FIGURE 1. Distribution map of clinical trials that registered in United States clinical trial registry. [(A), distribution of all clinical trials; (B), distribution of clinical trials on TCHM]. The images are from publicly available data, the url: https://www.clinicaltrials.gov/ct2/results/map?term=Chinese+herbal+medicine&map=.
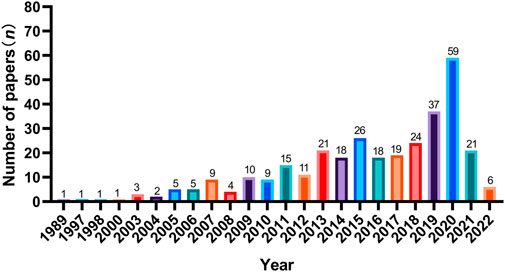
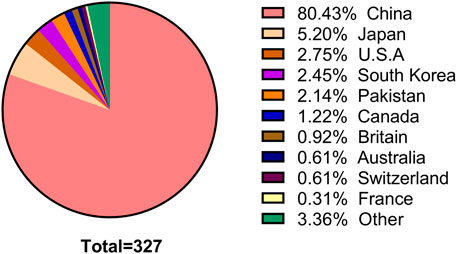
The number of SCI articles of multi-center clinical trials on TCHM is also increasing with years, and most of the studies were published in the recent 10 years. However, the number of studies was substantially lower in the recent 2 years, due to the influences of COVID-19 pandemic. When using “herbal medicine AND Randomized control AND multicenter” as the keywords to search the NewPubmed literature analysis system (pubmedplus.cn), 327 literatures were retrieved (From January 1989 to April 2022). Specifically, 4 studies (1.2%) were published in 1989–2000, 47 studies (14.4%) were published in 2001–2010, and 275 studies (84.1%) were published in 2011–2022. The number of studies published in 2020 was the highest (n = 59), which reduced to 21 in 2021, and further reduced to 6 in 2022 (Figure 2). China is the country with the most multi-center clinical trials on TCHM performed and most relevant SCI articles published in the world. The total number of SCI articles on multi-center clinical trials on TCHM in China was 263 (80.4%), followed by 5.2% in Japan, 2.8% in United States, 2.5% in Korea, and 2.1% in Pakistan. The five cities participated in most multi-center clinical trials on TCHM in China and overseas were Beijing, Shanghai, Guangzhou, Tianjin, and Chengdu, and Tokyo (Japan), Karachi (Pakistan), Fukuoka (Japan), Kyoto (Japan), Chiba (Japan), respectively. The subjects in these trials mainly with tumor, cardiovascular diseases, respiratory diseases, and gastrointestinal diseases.
Nearly 70% of multi-center clinical trials of TCHM performed in regions other than mainland China and Taiwan, China are on classic Chinese medicinal formulae and Chinese patent medicines, and the other 30% are mainly on dietary supplements or plant extracts. For instance, the following multi-center clinical trials were performed in Japan: Buzhong Yiqi decoction, Gegen decoction, and Xiaochaihu decoction for prevention and treatment of COVID-19; Wulingsan for treatment of glossalgia and prevention of recurrence of chronic subdural hematoma; Dahuang Mudan decoction for treatment of acute diverticulitis; Liujunzi decoction for the treatment of cervical carcinoma or endometrial carcinoma; Daikenchuto for the treatment of intestinal dysfunction after liver transplantation, as well as paralytic ileus after resection of colorectal carcinoma (CRC) or pancreatic carcinoma; Banxia Xiexin decoction for the treatment of CRC; Fangfeng Tongsheng granule for the treatment of obesity hypertension; Jinkui Shenqi pill for the treatment of chemotherapy induced peripheral neuropathy (neurotoxicity); and Goshuyuto for the treatment of headache. Furthermore, trial of Food Allergy Herbal Formula (FAHF-2) for the treatment of food allergy was performed in United States, and trials of Palm (Zonglv) pill for the treatment of chronic waist pain, as well as Ojeok-san (Wujisan) and Danggui Sini decoction for the treatment of cold hypersensitivity in the hands and feet were performed in Korea (Table 3; Figure 3).
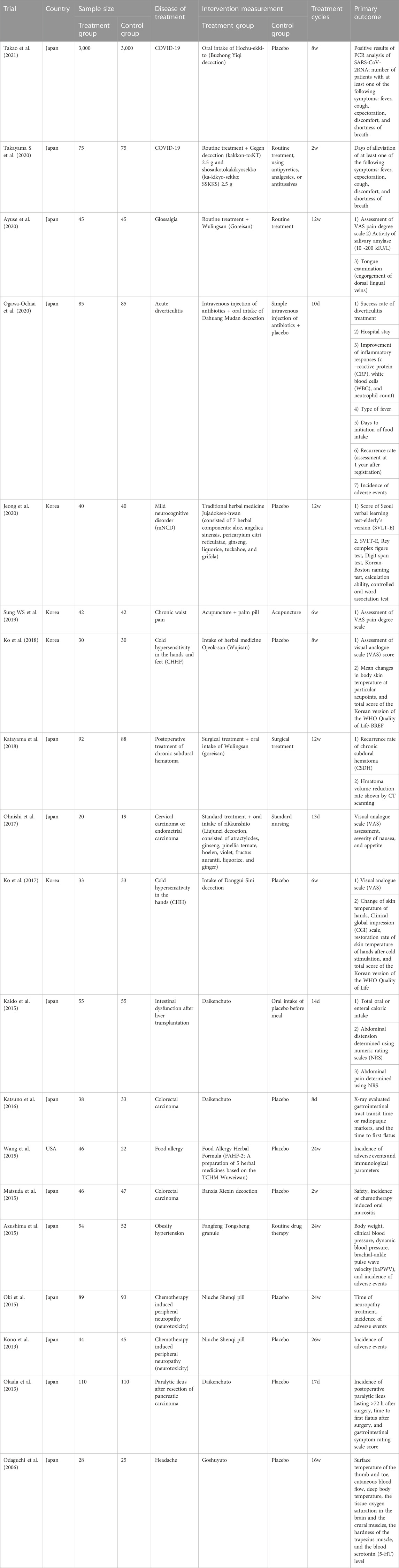
TABLE 3. General characteristics of multi-center clinical trials on TCHM performed overseas (in regions other than mainland China and Taiwan, China) in the recent 15 years.
4 Thoughts and perspectives of international multi-center clinical trials on TCHM
The “human experience” of TCHM has attracted close attentions from researchers throughout the world, and effectively utilizing the historic “human experience” is an important method to vitalize potential of original scientific and technological resources of TCHM. In recent years, several countries and regions issued new drug development policies and laws with human use histories regarding the surveillance of traditional herbal medicine. Taking FAD as an example, the Botanical Drug Development Guidance for Industry was issued in December 2016 to provide guiding for pre-marketing reviewing of botanical drugs. The applicants are required to provide data of human experience when applying for phase I or phase II clinical trial, which could help providing surveillance requirements of pre-clinical and clinical studies. Countries or regions such as EU, Japan, and Korea also have special policies on reviewing, approving, and surveillance of traditional herbal medicines, Chinese prescription medicines, and prescriptions from Classic medicine books. “Human experience” is the summary of repeatable experience of treatments by TCHM that has certain regularities from long-term clinical practices. The historic human experience is mainly documented in ancient medical books, while the experience of using Chinese Patent Medicines is mainly from the use of preparations of medical settings and expert empirical prescriptions from prestigious physicians of TCM. It is not difficult to find that adhere to the guiding of clinical values, and establish the “three combinations” evidence system of TCHM reviewing based on TCM theories, human experience, and clinical trials will be the important innovation method for the advancement of TCHM to higher qualities and internationalization. How to summarize the involved scientific law and dig the implied scientific values of TCHM based on the thousand years’ historic human experience have profound strategic importance and important practical significances for improving the quality and efficiency of TCHM and promoting the processes of internationalization of TCHM. Therefore, we should not improperly belittle ourselves.
Performing multi-center clinical trials with high qualities is still the essential method for TCHM to access the mainstream international medicine markets. Since the implement of GCP, the overall quality of clinical trials of new drugs in China has improved substantially, despite that there are still several limitations (Yuan et al., 2021). Such as non-standard collection of human safety data, insufficient basis for researchers to judge the relationship between adverse events and trial drugs, and the inability to trace the main efficacy indicators such as scale in effectiveness data. To address these problems, targeted quality control measures should be formulated and principle of blind law should be strictly abide by. Encourage the use of modern technology, such as TCM equipment and other advanced tools for evaluation. The 14th Five Year Development Program for Pharmaceutical Industry in Interpretation 2021 described supporting clinical study settings in China to actively participate and organize international multi-center clinical trials, and improve the internationalization levels of clinical trials (http://www.gov.cn/zhengce/2022-02/01/content_5671569.htm). Therefore, developing specific quality control measurements according to the characteristics of multi-center clinical trials when obeying the current GCP and ICH-GCP in China has important strategic significances for improving the development of new TCHM in China, as well as accessing the international mainstream medicine markets. The quality of TCM clinical trials depends on the design, especially the method of statistical data analysis. Therefore, in the process of trial design, considerations should be taken into account in how to collect research data, control test standardization and statistical analysis, so as to improve the quality of clinical trials. Besides, the clinical evaluation of TCM is a complex evaluation system. If we want to accurately screen the clinical trial indications of modern disease classification in early clinical research, and identify and evaluate the safety risk information number, we should reasonably use the information of the toxic action mechanism and target organs of TCM based on computer prediction. Network pharmacology in recent years has been widely used in the field of traditional Chinese medicine. The research methods based on network pharmacology can quickly identify the key medicinal components and targets of TCM, providing an important basis for the development and screening of TCM compounds in clinical practice. At the same time, it provides clearer guidance and direction for key safety signal monitoring in later large-scale clinical research.
It is necessary to further develop clinical evaluation techniques and methods not only meet the international standards but also meet the characteristics of TCHM. In recent years, the new techniques and methods of international clinical trials on drugs have been gradually applied in clinical trials of new drugs of TCHM, which showed the following advantages: 1) broke through the adaptive design of choosing the frequency-based statistical methods that has been long used in design and analysis in clinical trials; 2) enrichment strategy was developed to improve the accuracy of subject inclusion in clinical trials, and reduce the confounding factors (Yan et al., 2017); 3) master protocols and other high-efficient clinical trial design strategy, such as basket trial and umbrella trial (Guo et al., 2021), were used to improve the efficiency of clinical trials, shorten the duration of clinical trials, reduced and decrease of the costs of clinical trials. In 2015, China issued the formal announcement of the implement of ICH E17 guiding principle (National Medical Products Administration (NMPA), 2015).
Identifying the clinical position of drugs for treatment diseases that meet the characteristics of TCHM effects, and capable of leading to evident clinical values and benefits, and seeking, investigating, establishing, and designing scientific clinical efficacy evaluation tools and methods that been acknowledged by consensus to meet the clinical objectives and clinical positions will be an important study direction in the methodological field of clinical evaluation of new drugs of TCHM. Quality marker (Q-marker) was proposed by Liu C. X. et al. (2016) in response to the problem of TCM quality research. Since then, quality studies of TCM, in particular Q-marker studies were performed as to establish new research pattern. It will be of great significance to include Q-marker in TCM clinical trials in the future (Zhang T et al., 2018).
Additional attentions should be paid on TCHM specific clinical values and the scientific reports (Hu, 2021). In recent years, the relevant laws and regulations in China highly highlighted the TCHM specific clinical values. What are TCHM specific clinical values, and how to scientifically express them are very critical questions. It can be said that this is one of the questions related to the “life-gate” of high-quality development of TCHM industry. When facing the requirement for TCHM caused by deep aging, health issues caused by chronic non-communicable diseases, and threats from infectious diseases such as COVID-19, everyone are anticipating solutions from TCHM. However, comparing with the heated extolling of TCHM, the TCHM industry showed falling tendency in recent years, of which one important reason is that the specific clinical value has not been clearly described. TCHM has specific characteristics; however, acknowledged expression methods are required to describe the TCHM specific clinical values, which could help the mass to better understand. The clarification and description of clinical efficacy are the keys, instead of the mechanisms of effects. Artemisinin has already won the Nobel Prize, while the mechanisms of effects are still under investigation. The scientific evidence of clinical efficacy could be various, and the description of clinical values of TCHM should not only based on the results of randomized controlled trials, but also from other sources, such as case report, classic literatures, and records of clinical experience. The founder of evidence-based medicine, Gordon Guyatt, said that any empirical observations could be served as potential evidence, regardless of the systemic collection of it (Guyatt G et al., 2008). The focus of the innovation in the new TCHM reviewing and approval is the “three combinations” of clinical trials, human experience, and TCM theories. We speculated that systemically digging and scientifically evaluating the multidimensional evidence reaching the same study conclusion are worth encouraging. However, we need to further free our minds on how to promote the implement of relevant policies, which could free us from the limitations of randomized clinical trials.
In recent years, China has increased the investment on TCHM, and the internationalization of TCHM are now embracing the all-round, tridimensional, multi-level development, as a result of the promotion of “Belt and Road Initiative” construction and accelerated overseas layout of leading enterprises of TCHM. Despite the various challenges of integration of standards, policy barriers, and protection of intellectual property, TCHM internationalization has much to do and a long way to go, but still has a bright future.
Author contributions
JH contributed to the study’s conception and design, data acquisition, and drafting of the manuscript. SW and CW wrote the manuscript. DB wrote the discussion. NC conducted the data analysis. JZ read and made suggestions for amendment and approved the final manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This research was a part of the project titled “TCM Modernization Research” special project “Innovative study on etiology, pathogenesis, diagnosis and treatment of phlegm-stasis interaction in coronary heart disease” (Project No. 2019YFC1708500) funded by the National Classification of Project and The Fundamental Research Funds for the Central public welfare research institutes (Project No. ZZ15-YQ-069).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CTD, Common Technical Document; CGI, Clinical Global Impression; FDA, Food and Drug Administration; GCP, Good Clinic Practice; ICH-GCP, Guideline for Good Clinical Practice of the International Conference on Harmonisation; ICH E17, ICH guideline E17 on general principles for planning and design of multi-regional clinical trials; IND, Investigational New Drug; NHPs, Natural Health Products; NDA, New Drug Application; TCM, Traditional Chinese Medicine; TCHM, Traditional Chinese Herbal Medicine; THMP, Traditional Herbal Medicinal P; WHO, World Health Organization.
References
Ayuse, T., Okayasu, I., Tachi-Yoshida, M., Sato, J., Saisu, H., Shimada, M., et al. (2020). Examination of pain relief effect of Goreisan for glossodynia. Med. Baltim. 99 (33), e2153. doi:10.1097/MD.0000000000021536
Azushima, K., Tamura, K., Haku, S., Wakui, H., Kanaoka, T., Ohsawa, M., et al. (2015). Effects of the oriental herbal medicine bofu-tsusho-san in obesity hypertension: A multicenter, randomized, parallel-group controlled trial (ATH-D-14-01021.R2). Atherosclerosis 240 (1), 297–304. doi:10.1016/j.atherosclerosis.2015.01.025
Guo, X., Huang, Q., Xie, Y., and Wen, Z. H. (2021). Application of umbrella design and basket design in evidence-based research for traditional Chinese medicine [J]. Zhong Guo Zhong Yao Za Zhi 46 (08), 2010–2015. doi:10.19540/j.cnki.cjcmm.20210204.501
Guyatt, G., Rennie, G., and Meade, M. (2008). Users’guides to the medical literature: A manual for evidence-based clinical practice[M]. 2nd. New York: McGraw-Hill Professional.
Hu, J. (2021). A new situation in the development of new Chinese medicines. Beijing: Peking University Medical Press.
Huang, X., Zhou, W., and Zhan, C. (2018). International path exploration experience of Daning tablets registered in Canada [J]. Shanghai Pharm. 39 (11), 67–70. doi:10.3969/j.issn.1006-1533.2018.11.021
Interpretation (2022). Interpretation of 14th five year development Program for pharmaceutical industry. Available at: http://www.gov.cn/zhengce/2022-02/01/content_5671569.htm, 2022-04-01 Accessed on Feb 09, 2022).
Jeong, J. .H., Lee, J. .Y., Kim, J. .Y., Seo, Y. K., Kang, W. C., Kang, H. W., et al. (2020). Safety and efficacy of jujadokseo-hwan for memory deficit (amnesia) in mild neurocognitive disorder: A protocol for randomized, double-blind, placebo-controlled, parallel-group, multicenter clinical trial. Med. Baltim. 99 (8), e19231. doi:10.1097/MD.0000000000019231
Kaido, T., Shimamura, T., Sugawara, Y., Sadamori, H., Shirabe, K., Yamamoto, M., et al. (2015). Multicentre, randomised, placebo-controlled trial of extract of Japanese herbal medicine Daikenchuto to prevent bowel dysfunction after adult liver transplantation (DKB 14 Study BMJ Open 5 (9), e008356. doi:10.1136/bmjopen-2015-008356
Katayama, K., Matsuda, N., Kakuta, K., Naraoka, M., Takemura, A., Hasegawa, S., et al. (2018). The effect of goreisan on the prevention of chronic subdural hematoma recurrence: Multi-center randomized controlled study. J. Neurotrauma 35 (13), 1537–1542. doi:10.1089/neu.2017.5407
Katsuno, H., Maeda, K., Ohya, M., Yoshioka, K., Tsunoda, A., Koda, K., et al. (2016). Clinical pharmacology of daikenchuto assessed by transit analysis using radiopaque markers in patients with colon cancer undergoing open surgery: A multicenter double-blind randomized placebo-controlled study (JFMC39-0902 additional study). J. Gastroenterol. 51 (3), 222–229. doi:10.1007/s00535-015-1100-1
Ko, Y., Go, H. .Y., Cho, Y. .Y., Shin, J. H., Kim, T. H., Choi, D. J., et al. (2017). The efficacy and safety of danggui-sayuk-Ga-Osuyu-Saenggang-tang on Korean patients with cold hypersensitivity in the hands: Study protocol for a pilot, double-blind, randomized, placebo-controlled, parallel-group clinical trial. J. Trials. 18 (1), 268. doi:10.1186/s13063-017-2002-8
Ko, Y., Go, H. .Y., Han, I. .S., Lee, K. Y., Kim, T. H., Lee, J. M., et al. (2018). Efficacy and safety of ojeok-san in Korean female patients with cold hypersensitivity in the hands and feet: Study protocol for a randomized, double-blinded, placebo-controlled, multicenter pilot study. J. trials. 19 (1), 662. doi:10.1186/s13063-018-3013-9
Kono, T., Hata, T., Morita, S., Munemoto, Y., Matsui, T., Kojima, H., et al. (2013). Goshajinkigan oxaliplatin neurotoxicity evaluation (GONE): A phase 2, multicenter, randomized, double‑blind, placebo‑controlled trial of goshajinkigan to prevent oxaliplatin‑induced neuropathy. Cancer Chemother. Pharmacol. 72 (6), 1283–1290. doi:10.1007/s00280-013-2306-7
Liu, C. X., Cheng, Y. Y., Guo, D. A., et al. (2016). A new concept on quality marker for quality assessment and process control of Chinese medicines. Chin. Traditional Herb. Drugs 9 (1), 11. doi:10.1016/S1674-6384(17)60070-4
Matsuda, C., Munemoto, Y., Mishima, H., Nagata, N., Oshiro, M., Kataoka, M., et al. (2015). Double-blind, placebo-controlled, randomized phase II study of TJ-14 (Hangeshashinto) for infusional fluorinated-pyrimidine-based colorectal cancer chemotherapy-induced oral mucositis. Cancer Chemother. Pharmacol. 76 (1), 97–103. doi:10.1007/s00280-015-2767-y
Medicines & Healthcare products Regulatory Agency (2022). Herbal medicines granted a traditional herbal registration. Available at: https://www.gov.uk/government/publications/herbal-medicines-granted-a-traditional-herbal-registration-thr/herbal-medicines-granted-a-traditional-herbal-registration Accessed on 15 03, 2022.
National Medical Products Administration (NMPA) (2015). Announcement of issuing the guidelines (trial) for international multi-center clinical trials on drugs issued by National Medical Products Adminitration. Available at: https://www.nmpa.gov.cn/xxgk/ggtg/qtggtg/20150130120001641.html, 2022-04-01.
Odaguchi, H., Wakasugi, A., Ito, H., Shoda, H., Gono, Y., Sakai, F., et al. (2006). The efficacy of goshuyuto, a typical Kampo (Japanese herbal medicine) formula, in preventing episodes of headache. Curr. Med. Res. Opin. 22 (8), 1587–1597. doi:10.1185/030079906X112769
Ogawa-Ochiai, K., Yoshimura, K., Shirai, A., Sakai, S., Moriyama, H., Nakamura, K., et al. (2020). Study protocol for daiobotanpito combined with antibiotic therapy for treatment of acute diverticulitis: A study protocol for a randomized controlled trial. Trials. 21 (1), 531. doi:10.1186/s13063-020-04370-7
Ohnishi, S., Watari, H., Kanno, M., Ohba, Y., Takeuchi, S., Miyaji, T., et al. (2017). Additive effect of rikkunshito, an herbal medicine, on chemotherapy-induced nausea, vomiting, and anorexia in uterine cervical or corpus cancer patients treated with cisplatin and paclitaxel: Results of a randomized phase II study (JORTC KMP-02). J. Gynecol. Oncol. 28 (5), e44. doi:10.3802/jgo.2017.28.e44
Okada, K., Kawai, M., Uesaka, K., Kodera, Y., Nagano, H., Murakami, Y., et al. (2013). Effect of daikenchuto (TJ-100) on postoperative bowel motility and on prevention of paralytic ileus after pancreaticoduodenectomy: A multicenter, randomized, placebo-controlled phase II trial (the Japan-pd study). Jpn. J. Clin. Oncol. 43 (4), 436–438. doi:10.1093/jjco/hyt005
Oki, E., Emi, Y., Kojima, H., Higashijima, J., Kato, T., Miyake, Y., et al. (2015). Preventive effect of goshajinkigan on peripheral neurotoxicity of FOLFOX therapy (GENIUS trial): A placebo-controlled, double-blind, randomized phase III study. Int. J. Clin. Oncol. 20 (4), 767–775. doi:10.1007/s10147-015-0784-9
Qu, L., Wang, M., and Zou, W. (2021). Current situation of registration management of herbal medicines in EU and EU registration strategy of traditional Chinese medicine [J]. Chin. Herb. Med. 552 (20), 6135–6143. doi:10.7501/j.issn.0253-2670.2021.20.001
Shen, Y., Peng, Z., and Zhang, K. (2013). Analysis of the present situation and countermeasures of clinical trials of traditional Chinese medicine [J]. Chin. J. New drugs 22 (20), 2365–2368.
Sung, W. .S., Jeon, S. .R., Hong, Y. .J., Kim, T. H., Shin, S., Lee, H. J., et al. (2019). Efficacy, safety, and cost-effectiveness analysis of adjuvant herbal medicine treatment, palmijihwang-hwan, for chronic low back pain: A study protocol for randomized, controlled, assessor-blinded, multicenter clinical trial. J. trials. 20 (1), 778. doi:10.1186/s13063-019-3776-7
Takao, N., Shin, T., Arita, R., Ishii, T., Kainuma, M., Makino, T., et al. (2021). A structured summary of a study protocol for a multi-center, randomized controlled trial (RCT) of COVID-19 prevention with kampo medicines (integrative management in Japan for epidemic disease by prophylactic study: IMJEDI P1 study). Trials 22 (1), 23. doi:10.1186/s13063-020-04939-2
Takayama, S., Namiki, T., Ito, T., Arita, R., Nakae, H., Kobayashi, S., et al. (2020). A multi-center, randomized controlled trial by the integrative management in Japan for epidemic disease (IMJEDI study-RCT) on the use of kampo medicine, kakkonto with shosaikotokakikyosekko, in mild-to-moderate COVID-19 patients for symptomatic relief and prevention of severe stage: A structured summary of a study protocol for a randomized controlled trial. Trials. 21 (1), 827. doi:10.1186/s13063-020-04746-9
The Medicines Evaluation Board (MEB) (2019). Summary of product characteristics of Yufeng Ningxin tablet [EB/OL]. Available at: https://www.geneesmiddeleninformatiebank.nl/ords/f?p=111:3::SEARCH Accessed on 02 05, 2019).
Wang, J., Jones, S. .M., Pongracic, J. .A., Song, Y., Yang, N., Sicherer, S. H., et al. (2015). Safety, clinical, and immunologic efficacy of a Chinese herbal medicine (Food Allergy Herbal Formula-2) for food allergy J. Allergy Clin. Immunol. 136 (4), 962–970. doi:10.1016/j.jaci.2015.04.029
Wang, X. (2012). Tense sprint for 6 years, achieving 4 "first" Diao Xinxuekang capsule leading the revolution in the international development of traditional Chinese medicine [J]. Chin. Community Physicians 28 (40), 4.
Xiangxue Pharmaceutical (2022). Xiangxue Pharmaceutical was selected as the "Leading project of brand power" and recognized by the industry again. Available at: https://www.xphcn.com/about/info_16.aspx?itemid=10988 Accessed on 01 22, 2021).
Yan, S., Wang, B., and Ma, L. (2017). Enrichment design method and Chinese medicine clinical research [J]. ZhongYi Za Zhi 58 (04), 307–310. doi:10.13288/j.11-2166/r.2017.04.010
Yuan, W., Tang, J., Gao, R., Hu, S. Y., Zhao, Y. L., Zou, C., et al. (2021). Expert consensus on key issues of quality control in clinical trials of new drugs of traditional Chinese medicine [J]. Zhong Guo Zhong Yao Za Zhi 46 (07), 1701–1705. doi:10.19540/j.cnki.cjcmm.20210219.501
Zhang, S., Chen, Q., and Liu, P. (2017). Study and practice on readability test of Danshen capsule in EU drug registration [J]. Chin. Herb. Med. 48 (04), 843–846. doi:10.7501/j.issn.0253-2670.2017.04.032
Zhang, T., Bai, G., Han, Y., Xu, J., Gong, S., Li, Y., et al. (2018). The method of quality marker research and quality evaluation of traditional Chinese medicine based on drug properties and effect characteristics. Phytomedicine 44, 204–211. doi:10.1016/j.phymed.2018.02.009
Zhang, X., Liu, S., and Sun, Y. (2020). Discussion on the concept, R&D ideas and strategies of new drugs of traditional Chinese medicine from the perspective of "three drugs and three prescriptions" [J]. Chin. J. New drugs 29 (16), 1818–1821.
Keywords: traditional Chinese herbal medicine, multi-center, clinical trial, clinical value, clinical epidemiology
Citation: Wu S, Wang C, Bai D, Chen N, Hu J and Zhang J (2023) Perspectives of international multi-center clinical trials on traditional Chinese herbal medicine. Front. Pharmacol. 14:1195364. doi: 10.3389/fphar.2023.1195364
Received: 28 March 2023; Accepted: 02 May 2023;
Published: 18 May 2023.
Edited by:
Daniela Calina, University of Medicine and Pharmacy of Craiova, RomaniaReviewed by:
Hee Geun Jo, Gachon University, Republic of KoreaAdina Turcu-Stiolica, University of Medicine and Pharmacy of Craiova, Romania
Eda Sönmez Gürer, Cumhuriyet University, Türkiye
Copyright © 2023 Wu, Wang, Bai, Chen, Hu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingqing Hu, Z2NwMzA2QDEyNi5jb20=; Junhua Zhang, empodGNtQGZveG1haWwuY29t
†These authors have contributed equally to this work
 Shan Wu
Shan Wu Chuanchi Wang1,3†
Chuanchi Wang1,3† Dong Bai
Dong Bai Jingqing Hu
Jingqing Hu Junhua Zhang
Junhua Zhang