- 1Department of Oncology, Wuhan Asia General Hospital, Wuhan, China
- 2Department of Pharmacy, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Objective: Our study aimed to identify potential correlations between anti-tumor efficacy and immune-related adverse events (irAEs) in non-small-cell lung cancer (NSCLC).
Methods: We conducted a comprehensive search of online electronic databases up to March 2023 to identify any correlations between irAEs and immune checkpoint inhibitor (ICI) efficacy in NSCLC. We used meta-analysis RevMan 5.3 software to calculate pooled results.
Results: Our meta-analysis of 54 studies revealed that patients who experienced irAEs achieved a significantly higher objective response rate (p < 0.00001) and longer progression-free survival (PFS) (p < 0.00001) and overall survival (OS) (p < 0.00001) than those who did not experience irAEs. Additionally, patients with ≥2 irAEs had better PFS, whereas no significant difference was observed between patients with or without squamous cell carcinoma. Subgroup analysis of irAE types indicated that irAEs (thyroid dysfunction and gastrointestinal, skin, or endocrine irAEs) were associated with better PFS and OS. However, no significant differences were observed between patients with pneumonitis or hepatobiliary irAEs.
Conclusion: Our study showed that the occurrence of irAEs was a strong predictor of survival efficacy in patients with NSCLC treated with ICIs. Specifically, patients with ≥2 irAEs and those with thyroid dysfunction and gastrointestinal, skin, or endocrine irAEs achieved a better survival benefit.
Systematic Review Registration: Website: https://www.crd.york.ac.uk/prospero/, Identifier: CRD42023421690
Introduction
Immunotherapy, which targets the programmed death-1 (PD-1) and programmed death-ligand 1 (PD-L1) pathway, has emerged as a revolutionary and highly efficient treatment alternative for advanced-stage non-small-cell lung cancer (NSCLC) (Assi et al., 2018). Specifically, immune checkpoint inhibitors (ICIs), including those that target the PD-1/PD-L1 axis, enhance T-cell-mediated attack and exert anti-tumor effects (Wei et al., 2018).
In contrast to conventional chemotherapy, the use of ICIs can sometimes lead to a unique toxicity effect resembling autoimmune disorders. This “self-response” of the immune system is known as immune-related adverse events (irAEs) (Young et al., 2018).
Several retrospective studies have highlighted that the occurrence of irAEs in patients with melanoma is associated with improved survival outcomes (Freeman-Keller et al., 2016; Hua et al., 2016; Nakamura et al., 2017), suggesting that irAEs may be a predictive marker for the clinical benefit of ICIs. However, achieving the maximum therapeutic efficacy with ICIs requires a careful balance between anti-tumor immunity and autoimmunity. Conversely, studies on patients with advanced NSCLC have identified a correlation between adverse events and clinical outcomes of ICIs. Whether the occurrence of irAEs is associated with the clinical efficacy in NSCLC remains a subject of ongoing research. However, the results of current studies investigating the correlation between anti-tumor efficacy and the development of irAEs have been inconsistent (Kothari et al., 2017; Haratani et al., 2018; Cortellini et al., 2019).
With the aim to provide a systematical, up-to-date assessment of the potential predictive value of irAEs in NSCLC and gain a better understanding of the relationship between irAEs and clinical outcomes, we performed an update meta-analysis of the association between the occurrence of irAEs and anti-tumor efficacy.
Materials and methods
Search strategy
The meta-analysis was based on the Cochrane Manual of Intervention System Assessments and Guidelines for Systematic Reviews and Meta-Analyses. PubMed, Embase, and the Cochrane Library were searched for articles published up to March 2023. The process was conducted to find all relevant studies using the following keywords: “non-small cell lung cancer” AND “immune checkpoint inhibitors” OR “immunotherapy” AND “immune-related adverse events” AND “prognosis” terms, and the relevant Medical Subject Heading terms were used during the literature search process. The reference lists were also checked for retrieving additional relevant articles.
Eligibility criteria
Articles that met the following criteria were included: (1) patients had clinical diagnosis of NSCLC treated with an ICI; (2) trials focused on assessing the effectiveness of ICI in relation to the advent of irAEs; (3) outcomes of interest were effectiveness (overall survival [OS], progression-free survival [PFS], and tumor response) and selection of AE designation related to treatment; and (4) only full texts were included.
Quality assessment
Two authors (Li Lin and Yu Liu) separately justified the quality of the retrieved articles. Study quality was assessed using the Newcastle–Ottawa Quality Assessment Scale (Cook and Reed, 2015). The process was conducted by two researchers independently, and differences were resolved through discussion. The Newcastle–Ottawa Scale method uses three domains to assess the quality of cohort studies: the selection of patients with cancer, comparability between two groups, and assessment of outcomes. According to the NOS method, four, two, and three points were awarded to the three domains, respectively. Studies with ≥7 points were identified as having high quality, but those with ≤6 points were identified as having low quality. Publication bias was evaluated using funnel plots.
Data extraction
A data extraction form was used independently to retrieve the content containing the first author, publication year, ICI treatment regimen, region, number of patients, mean age, study design, follow-up period, and outcomes of interest. Two researchers (Li Lin and Yu Liu) independently evaluated the data. If there was a disagreement, a third researcher (Wei Li) resolved the disagreement through discussion.
Statistical analysis
Heterogeneity between studies was analyzed using the I2 statistic (Higgins and Thompson, 2002). A value of I2 > 50% implied a high degree of heterogeneity (Higgins et al., 2003). The random-effects model was used when there was high heterogeneity among the articles; otherwise, a fixed-effects model was used. Statistically significant differences were identified using a p-value of <0.05. Statistical analyses were conducted using the ReviewManager software package version 5.3 (RevMan; Cochrane Collaboration, Oxford, United Kingdom). Odds ratios (ORs) and 95% confidence intervals (CIs) were used for binary data and effect sizes in the meta-analysis. Forest plots were used to present the results of the study.
Results
Overview of the literature search
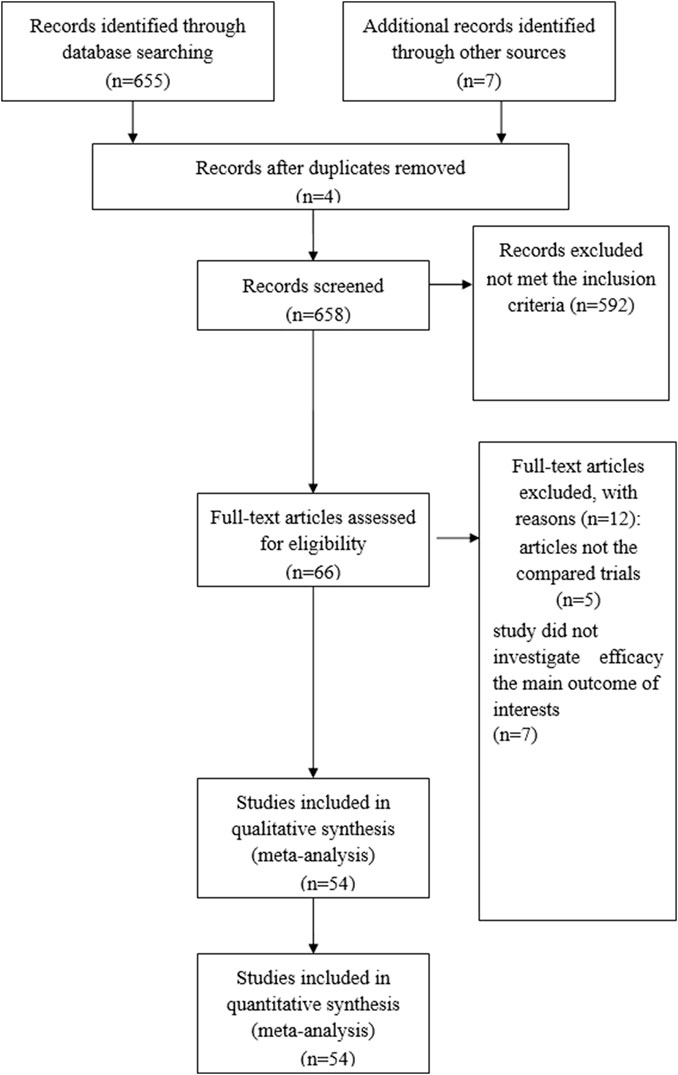
A total of 658 articles were identified as potentially eligible for inclusion. Using the criteria outlined in the Methods section, 66 publications were evaluated by browsing the full set of studies; however, some did not provide sufficient outcome data from the two approaches. Finally, 54 articles (Ahn et al., 2019; Akamatsu et al., 2020; Aso et al., 2020; Baldini et al., 2020; Berner et al., 2019; Bjørnhart et al., 2019; Bouhlel et al., 2020; Campredon et al., 2019; Cortellini et al., 2019; Chen et al., 2021; Chmielewska et al., 2021; Cui et al., 2020; Cortijo-Cascajares et al., 2023; Daniello et al., 2021; Dupont et al., 2019; Fujimoto et al., 2018; Fukihara et al., 2019; Grangeon et al., 2019; Guezour et al., 2022; Haratani et al., 2018; Imai et al., 2020; Isono et al., 2021; Kim et al., 2017; Kobayashi et al., 2020; Ksienski et al., 2019; Kubo et al., 2020; Lesueur et al., 2018; Lisberg et al., 2018; Morimoto et al., 2021; Naqash et al., 2020; Noguchi et al., 2020; Osorio et al., 2017; Owen et al., 2018; Park et al., 2021; Peiró et al., 2019; Ricciuti et al., 2019; Rose et al., 2021; Rubino et al., 2021; Sato et al., 2018; Sayer et al., 2023; Schweizer et al., 2020; Shukla et al., 2021; Sonehara et al., 2021; Socinski et al., 2023; Sugano et al., 2020; Suh et al., 2018; Tambo et al., 2020; Teraoka et al., 2017; Thuillier et al., 2021; Toi et al., 2018; Wang et al., 2022; Yamaguchi et al., 2020; Zhang et al., 2021; Zhou et al., 2021) were assessed for eligibility in the meta-analysis. Figure 1 illustrates the search process. A brief description of the 54 studies is provided in Table 1.
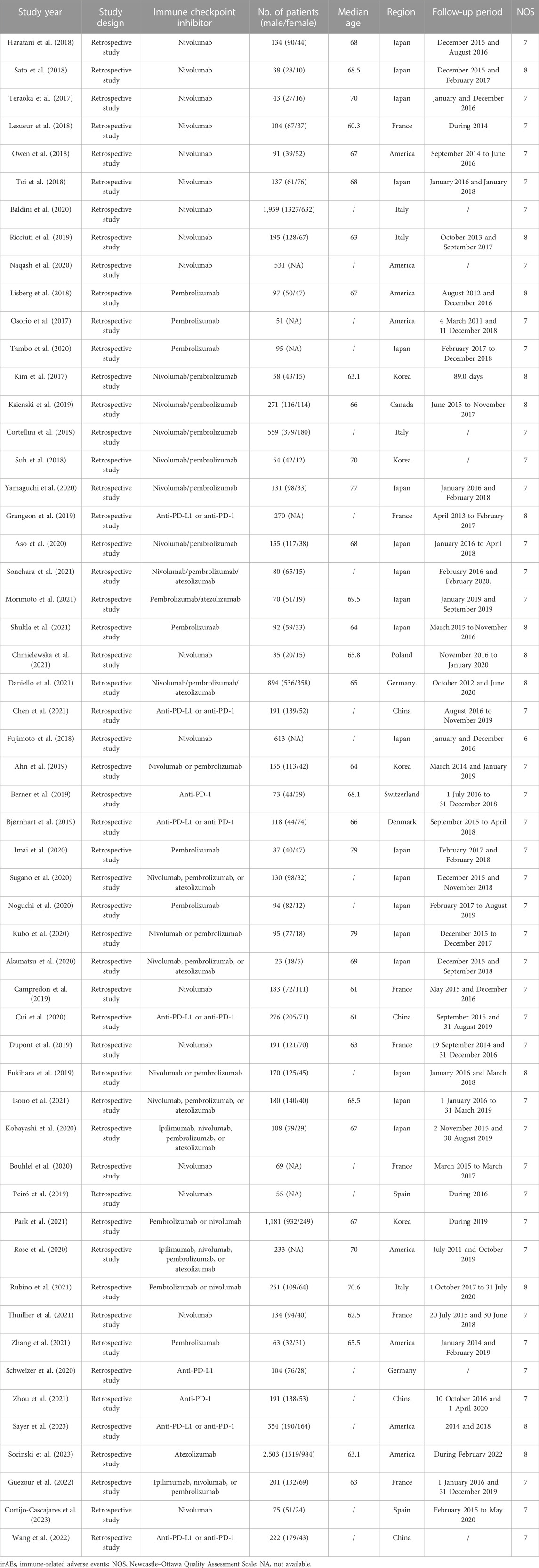
A total of 54 studies had high methodological quality. Table 1 summarizes the quality appraisal process.
Outcomes and synthesis of results
Pooled analysis of the OS between IrAEs and ICI efficacy
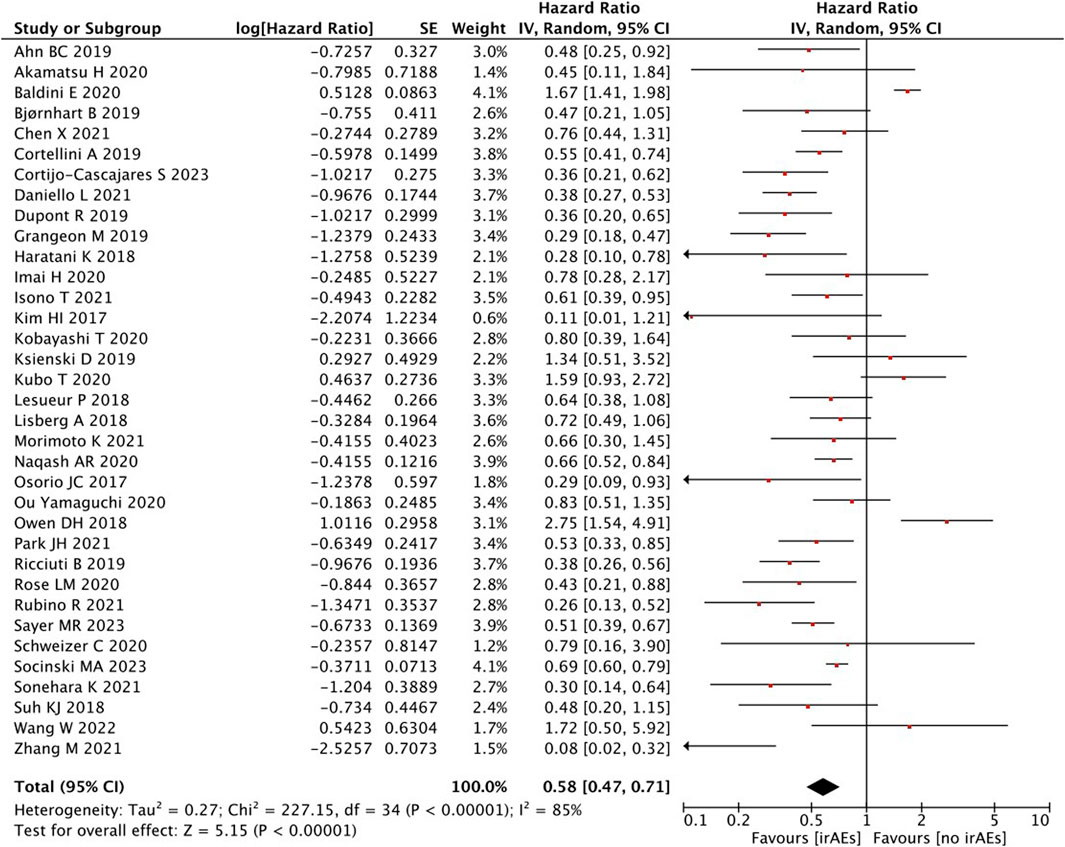
A high statistical between-study heterogeneity was found in the ORs of the studies (I2 = 85%), and a random-effects model was used for merging. As shown in Figure 2, the pooled effect size estimates showed that there was a statistically significant difference in OS when comparing the irAEs with the no-irAE group (HR = 0.58, 95% CI = 0.47–0.71, p < 0.00001).
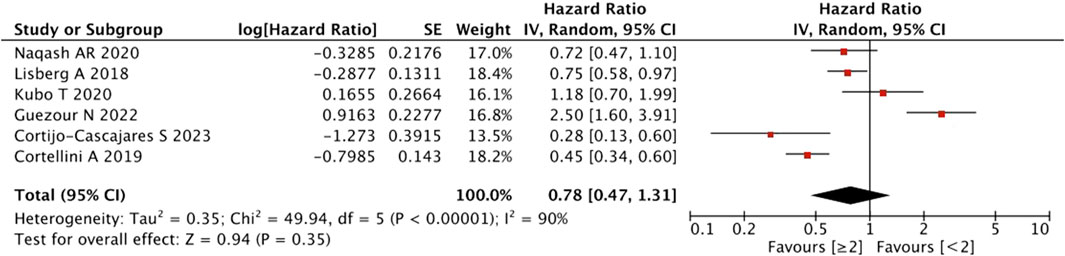
With regard to the number of irAEs developed, no significant difference was found in patients with ≥2 irAEs than those with <2 irAEs (HR = 0.78, 95% CI = 0.47–1.31, p = 0.35) (Figure 3).
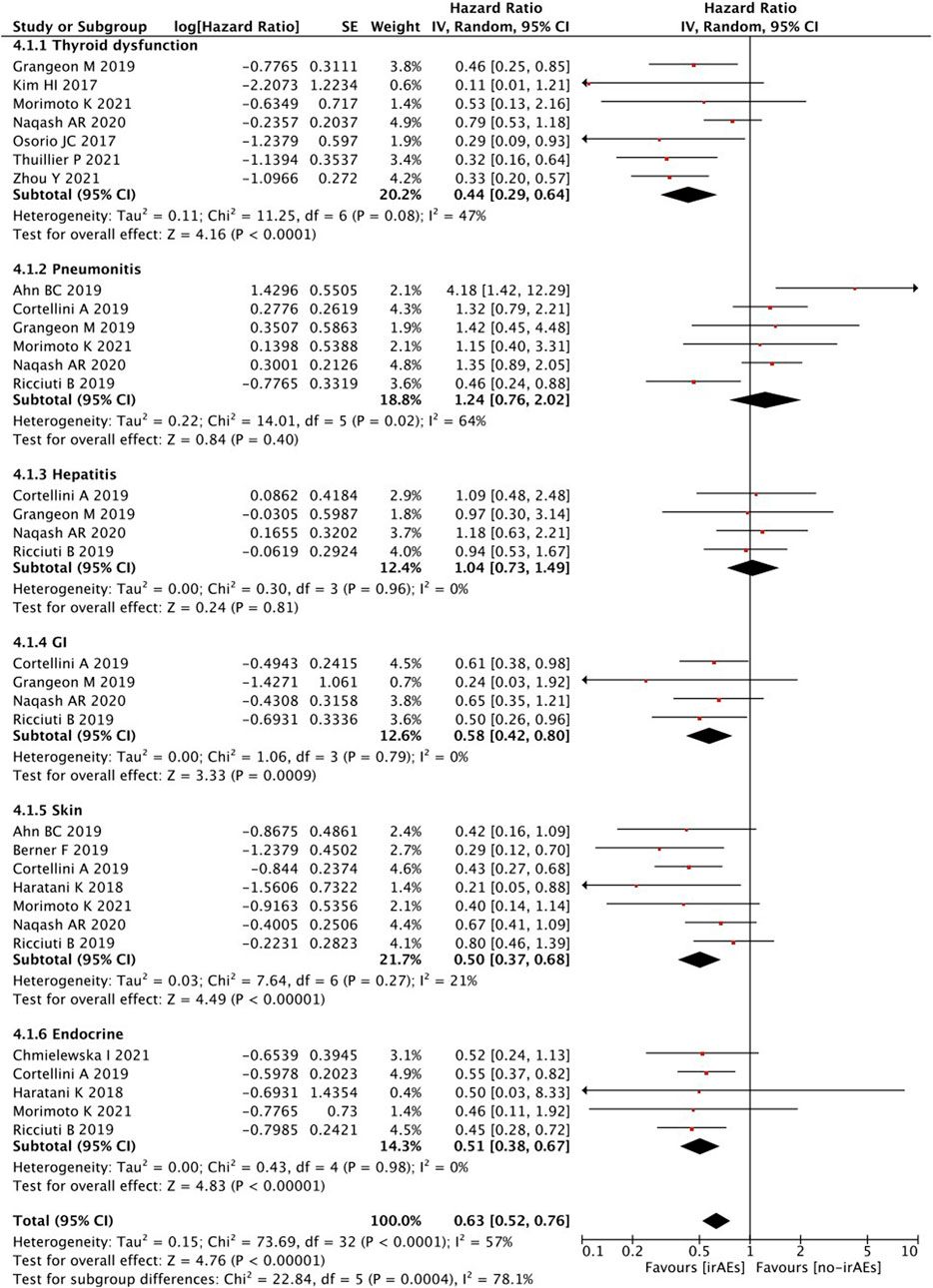
When survival outcomes were analyzed according to the types of irAEs, patients who developed any irAEs (p < 0.00001), thyroid dysfunction (p < 0.00001), and gastrointestinal (p = 0.0009), skin (p < 0.00001), or endocrine (p < 0.00001) irAEs experienced a significantly longer OS, whereas there was no significant change observed in patients with pneumonitis (p = 0.40) and hepatobiliary (p = 0.81) irAEs (Figure 4).
In terms of the pathological subtype, no differences were observed between the presence and absence of squamous cell carcinoma (HR = 1.08, 95% CI = 0.96–1.23, p = 0.21) (Figure 5).
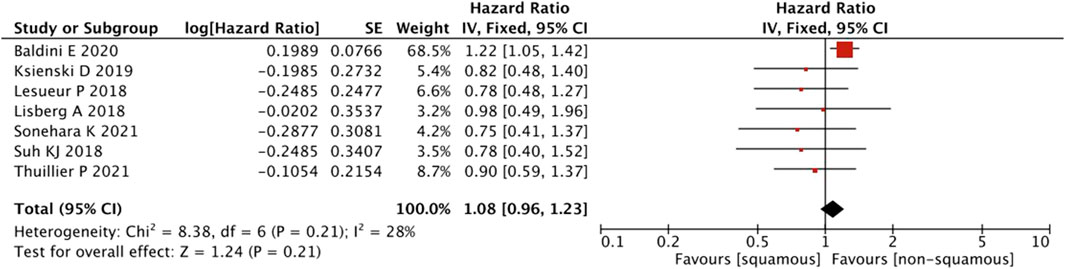
FIGURE 5. Sub-group analysis of OS between IrAEs and ICI efficacy with pathological subtypes of irAEs.
Pooled analysis of the PFS between the IrAEs and ICI efficacy
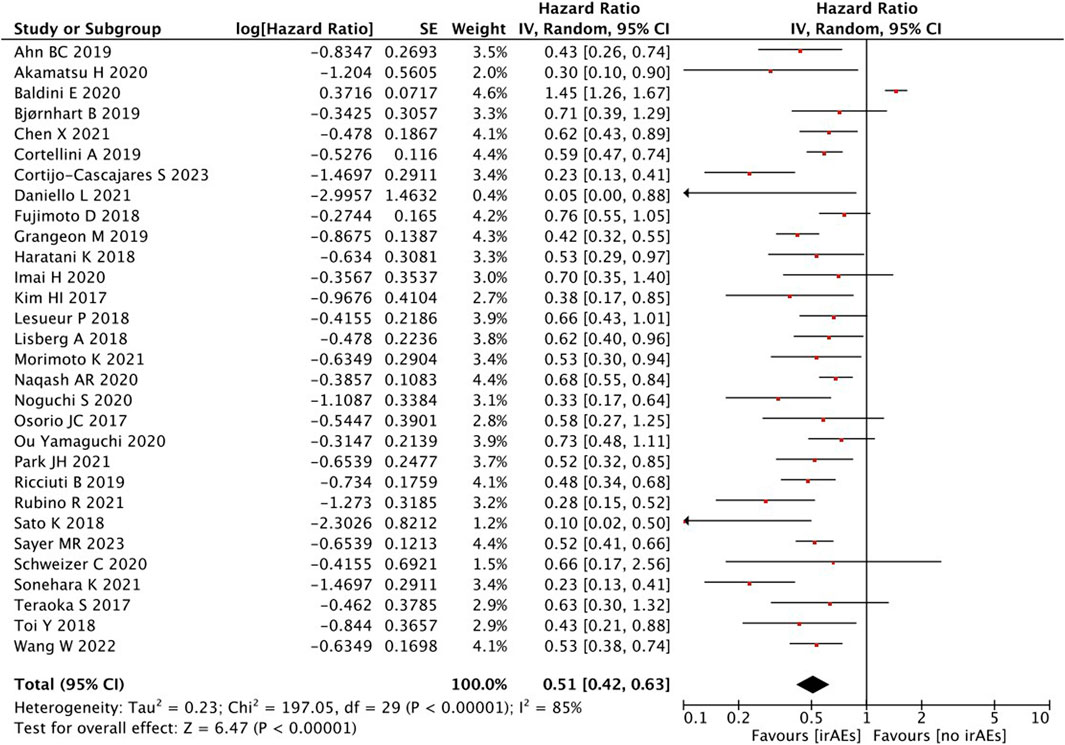
A statistical between-study heterogeneity was found in the OR of studies (I2 = 85%); therefore, a random-effects model was used for merging. When the PFS was pooled, it was found that patients with irAEs were associated with a better PFS than those without irAEs (HR = 0.51, 95% CI = 0.42–0.63, p < 0.00001) (Figure 6).
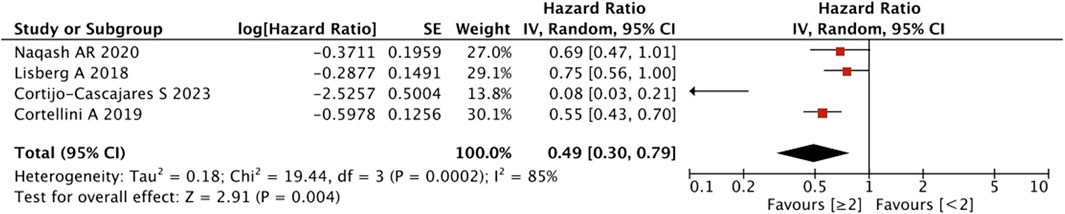
As shown in Figure 7, the pooled effect size estimates showed a significant statistical difference in PFS for patients with an increasing number of irAEs compared with those with <2 irAEs (HR = 0.49, 95% CI = 0.30–0.79, p = 0.004).
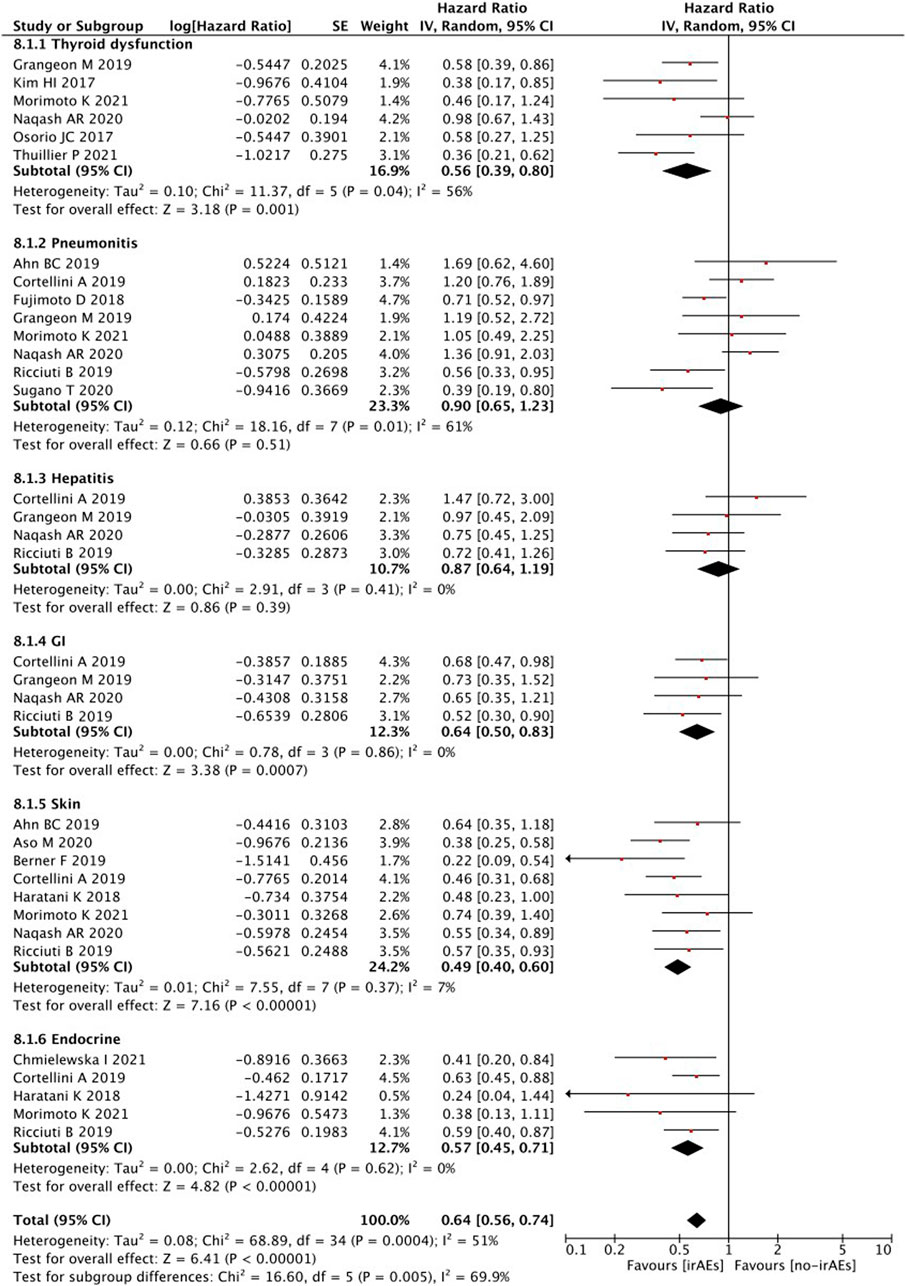
Subgroup analyses of treatment-related AEs revealed that PFS was significantly better in patients with any irAEs (p < 0.00001), thyroid dysfunction (p = 0.001), and gastrointestinal (p = 0.0007), skin (p < 0.00001), or endocrine (p < 0.0001) irAEs (Figure 8). However, no statistically significant differences were detected in the PFS of patients with irAEs associated with pneumonitis (p = 0.51) or hepatobiliary disease (p = 0.39).
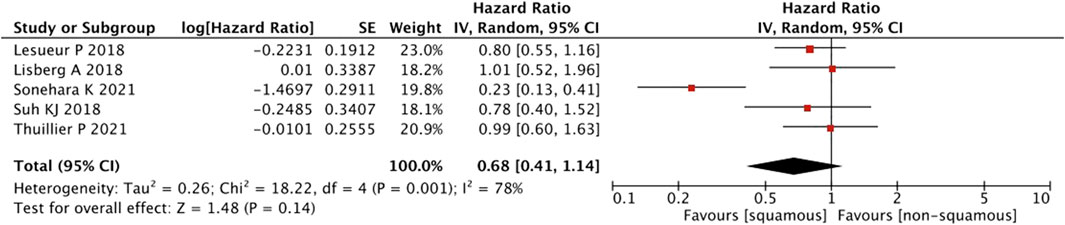
As shown in Figure 9, a pooled estimates of effect size showed no significant statistical difference in patients with or without squamous cell carcinoma (HR = 0.68, 95% CI = 0.41–1.14, p = 0.14).
Pooled analysis of the objective response rate between IrAEs and ICI efficacy
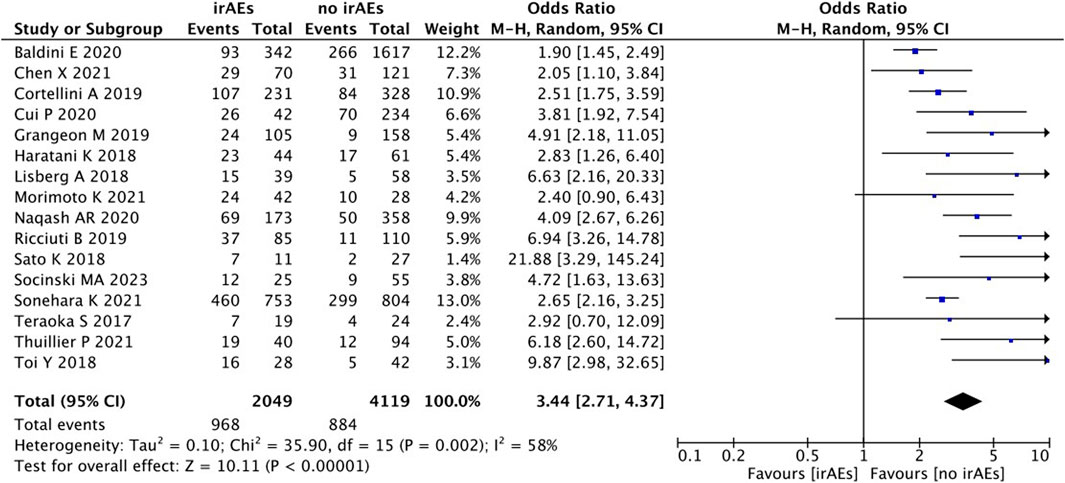
A statistical between-study heterogeneity was found in the OR of the studies (I2 = 58%), and a random-effects model was used for merging. As shown in Figure 10, ORR (OR 3.44, 95% CI = 2.71–4.37), p < 0.00001) was longer for patients with irAEs than those without irAEs.
Publication Bias
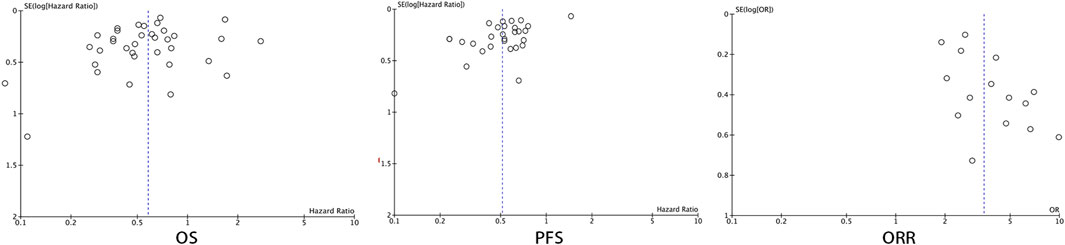
Forest plots were used to present the publication bias. The Figure 11 has shown the funnel plots of the OS, PFS, and ORR.
Discussion
ICIs are believed to initiate irAEs as a consequence of the body’s anti-tumor response (Naidoo et al., 2015; Champiat et al., 2016). Evidence suggests that patients who experienced irAEs are more likely to derive survival benefits from ICIs (Downey et al., 2007; Weber et al., 2012). However, a growing body of evidence has supported this hypothesis. The relationship between irAEs and their impact on clinical efficacy in NSCLC remains poorly defined owing to conflicting data.
In our meta-analysis, we demonstrated significant differences in both PFS/OS and ORR between patients who did and did not experience irAEs, indicating a strong correlation between treatment-related irAEs and their clinical benefits. However, the underlying mechanisms remain unclear. This may be explained by the fact that there may be an abnormal presentation of the molecular mimicry of antigens shared between tumor and normal tissues, which leads to simultaneous T-cell- and B-cell-mediated cross-reactions (Rose and Bona, 1993). This increases the possibility that latent “tissue-specific” autoimmunity may be associated with not only therapy but also healthy tissue. Thus, irAEs are more likely to exacerbate host immune function.
To further evaluate the independent prognostic value of the relationship between treatment-related irAEs and improved clinical outcomes, we performed subgroup analyses to incorporate various clinicopathological covariates. Our results further support the conclusion that this relationship is mediated by confounding factors, such as the number, pathologic subtypes, and various types of irAEs experienced. In our study, no significant difference in OS was found in patients who developed ≥2 irAEs than those who developed <2 irAEs, but PFS showed significant difference. These data suggest that irAEs can serve as a positive predictor of response to therapy, with the balance of its advantages and disadvantages depending on the severity of the irAE itself. In addition, no differences were observed in OS or PFS in terms of pathologic subtypes. This finding indicates that the pathologic subtype had no association with irAEs and survival. IrAEs affecting the skin, gastrointestinal tract, and endocrine system (including thyroid dysfunction) tend to be more manageable than those affecting the lungs and liver. The present study found that patients with these types of irAEs are more likely to experience improved survival benefits. The result may be explained by the toxicities of these irAE subtypes, which could usually be resolved completely with appropriate treatments. Though glucocorticoid is known to be a key element to treat patients with irAEs of pneumonitis and hepatobiliary disease, the effect of steroids still serves as a double-edged sword. The adverse effect of the use of steroids might lead to inferior survival tendency of lung- and liver-related irAEs. In terms of treatment efficacy, further investigation of immune checkpoint therapies based on specific molecular subtypes and genomic alterations may help make informed treatment decisions while maintaining a manageable safety profile.
There are some limitations to our study. First, the retrospective nature and various investigators’ irAE-reporting profiles of all the included studies resulted in an imbalance between the two groups. Additional randomized clinical trials are warranted to address these issues. Second, our study did not include potential confounders, such as the duration of response, discontinuation of therapy, different grades of irAEs, and different ICIs, owing to the limited covariate data available for analysis. Therefore, there is a strong need for high-quality research using additional data to clarify this issue.
In summary, our study validated that patients with NSCLC undergoing ICI treatment may experience irAEs, which may have a persistent response to ICI therapy that can be achieved using irAE as a valuable predictive/prognostic factor. Studies focusing on the molecular mechanisms underlying this correlation may contribute to improved clinical outcomes with ICIs and effective management of their side effects.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Author contributions
WL and AW designed the study; LL and YL extracted the data; YL and WL analyzed the data; LL drafted the manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahn, B. C., Pyo, K. H., Xin, C. F., Jung, D., Shim, H. S., Lee, C. Y., et al. (2019). Comprehensive analysis of the characteristics and treatment outcomes of patients with non-small cell lung cancer treated with anti-PD-1 therapy in real-world practice. J. cancer Res. Clin. Oncol. 145 (6), 1613–1623. doi:10.1007/s00432-019-02899-y
Akamatsu, H., Murakami, E., Oyanagi, J., Shibaki, R., Kaki, T., Takase, E., et al. (2020). Immune-related adverse events by immune checkpoint inhibitors significantly predict durable efficacy even in responders with advanced non-small cell lung cancer. Oncol. 25 (4), e679–e683. doi:10.1634/theoncologist.2019-0299
Aso, M., Toi, Y., Sugisaka, J., Aiba, T., Kawana, S., Saito, R., et al. (2020). Association between skin reaction and clinical benefit in patients treated with anti-programmed cell death 1 monotherapy for advanced non-small cell lung cancer. Oncol. 25 (3), e536–e544. doi:10.1634/theoncologist.2019-0550
Assi, H. I., Kamphorst, A. O., Moukalled, N. M., and Ramalingam, S. S. (2018). Immune checkpoint inhibitors in advanced non-small cell lung cancer. Cancer 124 (2), 248–261. doi:10.1002/cncr.31105
Baldini, E., Lunghi, A., Cortesi, E., Turci, D., Signorelli, D., Stati, V., et al. (2020). Immune-related adverse events correlate with clinical outcomes in NSCLC patients treated with nivolumab: The Italian NSCLC expanded access program. Lung cancer 140, 59–64. doi:10.1016/j.lungcan.2019.12.014
Berner, F., Bomze, D., Diem, S., Ali, O. H., Fässler, M., Ring, S., et al. (2019). Association of checkpoint inhibitor-induced toxic effects with shared cancer and tissue antigens in non-small cell lung cancer. JAMA Oncol. 5 (7), 1043–1047. doi:10.1001/jamaoncol.2019.0402
Bjørnhart, B., Hansen, K. H., Jørgensen, T. L., Herrstedt, J., and Schytte, T. (2019). Efficacy and safety of immune checkpoint inhibitors in a Danish real life non-small cell lung cancer population: A retrospective cohort study. Acta Oncol. Stockh. Swed. 58 (7), 953–961. doi:10.1080/0284186X.2019.1615636
Bouhlel, L., Doyen, J., Chamorey, E., Poudenx, M., Ilie, M., Gal, J., et al. (2020). Occurrence and number of immune-related adverse events are independently associated with survival in advanced non-small-cell lung cancer treated by nivolumab. Bull. Du. cancer 107 (9), 946–958. doi:10.1016/j.bulcan.2020.04.019
Campredon, P., Mouly, C., Lusque, A., Bigay-Game, L., Bousquet, E., Mazières, J., et al. (2019). Incidence of thyroid dysfunctions during treatment with nivolumab for non-small cell lung cancer: Retrospective study of 105 patients. Presse medicale 48 (4), e199–e207. doi:10.1016/j.lpm.2018.10.019
Champiat, S., Lambotte, O., Barreau, E., Belkhir, R., Berdelou, A., Carbonnel, F., et al. (2016). Management of immune checkpoint blockade dysimmune toxicities: A collaborative position paper. Ann. Oncol. J. Eur. Soc. Med. Oncol. 27 (4), 559–574. doi:10.1093/annonc/mdv623
Chen, X., Nie, J., Dai, L., Hu, W., Zhang, J., Han, J., et al. (2021). Immune-related adverse events and their association with the effectiveness of PD-1/PD-L1 inhibitors in non-small cell lung cancer: A real-world study from China. Front. Oncol. 11, 607531. doi:10.3389/fonc.2021.607531
Chmielewska, I., Dudzińska, M., Szczyrek, M., Świrska, J., Wojas-Krawczyk, K., and Zwolak, A. (2021). Do endocrine adverse events predict longer progression-free survival among patients with non-small-cell lung cancer receiving nivolumab? PloS one 16 (9), e0257484. doi:10.1371/journal.pone.0257484
Cook, D. A., and Reed, D. A. (2015). Appraising the quality of medical education research methods: The medical education research study quality instrument and the newcastle-ottawa scale-education. Acad. Med. J. Assoc. Am. Med. Coll. 90 (8), 1067–1076. doi:10.1097/ACM.0000000000000786
Cortellini, A., Chiari, R., Ricciuti, B., Metro, G., Perrone, F., Tiseo, M., et al. (2019). Correlations between the immune-related adverse events spectrum and efficacy of anti-PD1 immunotherapy in NSCLC patients. Clin. lung cancer 20 (4), 237–247. doi:10.1016/j.cllc.2019.02.006
Cortijo-Cascajares, S., Cercós-Lletí, A. C., Ortiz-Pérez, S., Caro-Teller, J. M., and Ferrari-Piquero, J. M. (2023). Analysis of immune-mediated reactions in patients with non-small cell lung cancer treated with nivolumab and its association with effectiveness. J. Oncol. Pharm. Pract. official Publ. Int. Soc. Oncol. Pharm. Pract. 29 (2), 290–298. doi:10.1177/10781552211067429
Cui, P., Huang, D., Wu, Z., Tao, H., Zhang, S., Ma, J., et al. (2020). Association of immune-related pneumonitis with the efficacy of PD-1/PD-L1 inhibitors in non-small cell lung cancer. Ther. Adv. Med. Oncol. 12, 1758835920922033. doi:10.1177/1758835920922033
Daniello, L., Elshiaty, M., Bozorgmehr, F., Kuon, J., Kazdal, D., Schindler, H., et al. (2021). Therapeutic and prognostic implications of immune-related adverse events in advanced non-small-cell lung cancer. Front. Oncol. 11, 703893. doi:10.3389/fonc.2021.703893
Downey, S. G., Klapper, J. A., Smith, F. O., Yang, J. C., Sherry, R. M., Royal, R. E., et al. (2007). Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin. cancer Res. official J. Am. Assoc. Cancer Res. 13 (22), 6681–6688. doi:10.1158/1078-0432.CCR-07-0187
Dupont, R., Bérard, E., Puisset, F., Comont, T., Delord, J. P., Guimbaud, R., et al. (2019). The prognostic impact of immune-related adverse events during anti-PD1 treatment in melanoma and non-small-cell lung cancer: A real-life retrospective study. Oncoimmunology 9 (1), 1682383. doi:10.1080/2162402X.2019.1682383
Freeman-Keller, M., Kim, Y., Cronin, H., Richards, A., Gibney, G., and Weber, J. S. (2016). Nivolumab in resected and unresectable metastatic melanoma: Characteristics of immune-related adverse events and association with outcomes. Clin. cancer Res. official J. Am. Assoc. Cancer Res. 22 (4), 886–894. doi:10.1158/1078-0432.CCR-15-1136
Fujimoto, D., Yoshioka, H., Kataoka, Y., Morimoto, T., Kim, Y. H., Tomii, K., et al. (2018). Efficacy and safety of nivolumab in previously treated patients with non-small cell lung cancer: A multicenter retrospective cohort study. Lung cancer 119, 14–20. doi:10.1016/j.lungcan.2018.02.017
Fukihara, J., Sakamoto, K., Koyama, J., Ito, T., Iwano, S., Morise, M., et al. (2019). Prognostic impact and risk factors of immune-related pneumonitis in patients with non-small-cell lung cancer who received programmed death 1 inhibitors. Clin. lung cancer 20 (6), 442–450. doi:10.1016/j.cllc.2019.07.006
Grangeon, M., Tomasini, P., Chaleat, S., Jeanson, A., Souquet-Bressand, M., Khobta, N., et al. (2019). Association between immune-related adverse events and efficacy of immune checkpoint inhibitors in non-small-cell lung cancer. Clin. lung cancer 20 (3), 201–207. doi:10.1016/j.cllc.2018.10.002
Guezour, N., Soussi, G., Brosseau, S., Abbar, B., Naltet, C., Vauchier, C., et al. (2022). Grade 3-4 immune-related adverse events induced by immune checkpoint inhibitors in non-small-cell lung cancer (NSCLC) patients are correlated with better outcome: A real-life observational study. Cancers 14 (16), 3878. doi:10.3390/cancers14163878
Haratani, K., Hayashi, H., Chiba, Y., Kudo, K., Yonesaka, K., Kato, R., et al. (2018). Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 4 (3), 374–378. doi:10.1001/jamaoncol.2017.2925
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ Clin. Res. ed) 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Higgins, J. P., and Thompson, S. G. (2002). Quantifying heterogeneity in a meta-analysis. Statistics Med. 21 (11), 1539–1558. doi:10.1002/sim.1186
Hua, C., Boussemart, L., Mateus, C., Routier, E., Boutros, C., Cazenave, H., et al. (2016). Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA dermatol. 152 (1), 45–51. doi:10.1001/jamadermatol.2015.2707
Imai, H., Wasamoto, S., Yamaguchi, O., Suzuki, K., Sugiyama, T., Uchino, J., et al. (2020). Efficacy and safety of first-line pembrolizumab monotherapy in elderly patients (aged ≥ 75 years) with non-small cell lung cancer. J. cancer Res. Clin. Oncol. 146 (2), 457–466. doi:10.1007/s00432-019-03072-1
Isono, T., Kagiyama, N., Takano, K., Hosoda, C., Nishida, T., Kawate, E., et al. (2021). Outcome and risk factor of immune-related adverse events and pneumonitis in patients with advanced or postoperative recurrent non-small cell lung cancer treated with immune checkpoint inhibitors. Thorac. cancer 12 (2), 153–164. doi:10.1111/1759-7714.13736
Kim, H. I., Kim, M., Lee, S. H., Park, S. Y., Kim, Y. N., et al. (2017). Development of thyroid dysfunction is associated with clinical response to PD-1 blockade treatment in patients with advanced non-small cell lung cancer. Oncoimmunology 7 (1), e1375642. doi:10.1080/2162402X.2017.1375642
Kobayashi, T., Iwama, S., Yasuda, Y., Okada, N., Okuji, T., Ito, M., et al. (2020). Pituitary dysfunction induced by immune checkpoint inhibitors is associated with better overall survival in both malignant melanoma and non-small cell lung carcinoma: A prospective study. J. Immunother. cancer 8 (2), e000779. doi:10.1136/jitc-2020-000779
Kothari, S., Bagley, S., Aggarwal, C., Bauml, J., Alley, E., Evans, T., et al. (2017). P3.02c-029 immune-related adverse events and their effect on outcomes in patients (pts) with non-small cell lung cancer (nsclc) treated with nivolumab. J. Thorac. Oncol. 12 (1), S1290. doi:10.1016/j.jtho.2016.11.1824
Ksienski, D., Wai, E. S., Croteau, N., Fiorino, L., Brooks, E., Poonja, Z., et al. (2019). Efficacy of nivolumab and pembrolizumab in patients with advanced non-small-cell lung cancer needing treatment interruption because of adverse events: A retrospective multicenter analysis. Clin. lung cancer 20 (1), e97–e106. doi:10.1016/j.cllc.2018.09.005
Kubo, T., Watanabe, H., Ninomiya, K., Kudo, K., Minami, D., Murakami, E., et al. (2020). Immune checkpoint inhibitor efficacy and safety in older non-small cell lung cancer patients. Jpn. J. Clin. Oncol. 50 (12), 1447–1453. doi:10.1093/jjco/hyaa152
Lesueur, P., Escande, A., Thariat, J., Vauléon, E., Monnet, I., Cortot, A., et al. (2018). Safety of combined PD-1 pathway inhibition and radiation therapy for non-small-cell lung cancer: A multicentric retrospective study from the gfpc. Cancer Med. 7 (11), 5505–5513. doi:10.1002/cam4.1825
Lisberg, A., Tucker, D. A., Goldman, J. W., Wolf, B., Carroll, J., Hardy, A., et al. (2018). Treatment-related adverse events predict improved clinical outcome in NSCLC patients on KEYNOTE-001 at a single center. Cancer Immunol. Res. 6 (3), 288–294. doi:10.1158/2326-6066.CIR-17-0063
Morimoto, K., Yamada, T., Takumi, C., Ogura, Y., Takeda, T., Onoi, K., et al. (2021). Immune-related adverse events are associated with clinical benefit in patients with non-small-cell lung cancer treated with immunotherapy plus chemotherapy: A retrospective study. Front. Oncol. 11, 630136. doi:10.3389/fonc.2021.630136
Naidoo, J., Page, D. B., Li, B. T., Connell, L. C., Schindler, K., Lacouture, M. E., et al. (2015). Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol. official J. Eur. Soc. Med. Oncol. 26 (12), 2375–2391. doi:10.1093/annonc/mdv383
Nakamura, Y., Tanaka, R., Asami, Y., Teramoto, Y., Imamura, T., Sato, S., et al. (2017). Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: A multi-institutional retrospective study. J. dermatology 44 (2), 117–122. doi:10.1111/1346-8138.13520
Naqash, A. R., Ricciuti, B., Owen, D. H., Florou, V., Toi, Y., Cherry, C., et al. (2020). Outcomes associated with immune-related adverse events in metastatic non-small cell lung cancer treated with nivolumab: A pooled exploratory analysis from a global cohort. Cancer Immunol. Immunother. CII 69 (7), 1177–1187. doi:10.1007/s00262-020-02536-5
Noguchi, S., Suminaga, K., Kaki, T., Kawachi, H., Fukao, A., Terashita, S., et al. (2020). Correlation of immune-related adverse events and effects of pembrolizumab monotherapy in patients with non-small cell lung cancer. Lung CancerAuckl. N Z) 11, 53–57. doi:10.2147/LCTT.S254146
Osorio, J. C., Ni, A., Chaft, J. E., Pollina, R., Kasler, M. K., Stephens, D., et al. (2017). Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann. Oncol. official J. Eur. Soc. Med. Oncol. 28 (3), 583–589. doi:10.1093/annonc/mdw640
Owen, D. H., Wei, L., Bertino, E. M., Edd, T., Villalona-Calero, M. A., He, K., et al. (2018). Incidence, risk factors, and effect on survival of immune-related adverse events in patients with non-small-cell lung cancer. Clin. lung cancer 19 (6), e893–e900. doi:10.1016/j.cllc.2018.08.008
Park, J. H., You, G. L., Ahn, M. J., Kim, S. W., Hong, M. H., Han, J. Y., et al. (2021). Real-world outcomes of anti-PD1 antibodies in platinum-refractory, PD-L1-positive recurrent and/or metastatic non-small cell lung cancer, and its potential practical predictors: First report from Korean cancer study group LU19-05. J. cancer Res. Clin. Oncol. 147 (8), 2459–2469. doi:10.1007/s00432-021-03527-4
Peiró, I., Palmero, R., Iglesias, P., Díez, J. J., Simó-Servat, A., Marín, J. A., et al. (2019). Thyroid dysfunction induced by nivolumab: Searching for disease patterns and outcomes. Endocrine 64 (3), 605–613. doi:10.1007/s12020-019-01871-7
Ricciuti, B., Genova, C., De Giglio, A., Bassanelli, M., Dal Bello, M. G., Metro, G., et al. (2019). Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: Long-term outcomes from a multi-institutional analysis. J. cancer Res. Clin. Oncol. 145 (2), 479–485. doi:10.1007/s00432-018-2805-3
Rose, L. M., DeBerg, H. A., Vishnu, P., Frankel, J. K., Manjunath, A. B., Flores, J. P. E., et al. (2021). Incidence of skin and respiratory immune-related adverse events correlates with specific tumor types in patients treated with checkpoint inhibitors. Front. Oncol. 10, 570752. doi:10.3389/fonc.2020.570752
Rose, N. R., and Bona, C. (1993). Defining criteria for autoimmune diseases (Witebsky's postulates revisited). Immunol. today 14 (9), 426–430. doi:10.1016/0167-5699(93)90244-F
Rubino, R., Marini, A., Roviello, G., Presotto, E. M., Desideri, I., Ciardetti, I., et al. (2021). Endocrine-related adverse events in a large series of cancer patients treated with anti-PD1 therapy. Endocrine 74 (1), 172–179. doi:10.1007/s12020-021-02750-w
Sato, K., Akamatsu, H., Murakami, E., Sasaki, S., Kanai, K., Hayata, A., et al. (2018). Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung cancer 115, 71–74. doi:10.1016/j.lungcan.2017.11.019
Sayer, M. R., Mambetsariev, I., Lu, K. H., Wong, C. W., Duche, A., Beuttler, R., et al. (2023). Predicting survival of NSCLC patients treated with immune checkpoint inhibitors: Impact and timing of immune-related adverse events and prior tyrosine kinase inhibitor therapy. Front. Oncol. 13, 1064169. doi:10.3389/fonc.2023.1064169
Schweizer, C., Schubert, P., Rutzner, S., Eckstein, M., Haderlein, M., Lettmaier, S., et al. (2020). Prospective evaluation of the prognostic value of immune-related adverse events in patients with non-melanoma solid tumour treated with PD-1/PD-L1 inhibitors alone and in combination with radiotherapy. Eur. J. cancer 140, 55–62. doi:10.1016/j.ejca.2020.09.001
Shukla, N. A., Althouse, S., Meyer, Z., Hanna, N., and Durm, G. (2021). Association of immune-related adverse events and efficacy outcomes with consolidation pembrolizumab after chemoradiation in patients with inoperable stage III non-small-cell lung cancer. Clin. lung cancer 22 (4), 274–281. doi:10.1016/j.cllc.2020.12.014
Socinski, M. A., Jotte, R. M., Cappuzzo, F., Nishio, M., Mok, T. S. K., Reck, M., et al. (2023). Association of immune-related adverse events with efficacy of atezolizumab in patients with non-small cell lung cancer: Pooled analyses of the phase 3 IMpower130, IMpower132, and IMpower150 randomized clinical trials. JAMA Oncol. 9 (4), 527–535. doi:10.1001/jamaoncol.2022.7711
Sonehara, K., Tateishi, K., Araki, T., Komatsu, M., Yamamoto, H., Koizumi, T., et al. (2021). The role of immune-related adverse events in prognosis and efficacy prediction for patients with non-small cell lung cancer treated with immunotherapy: A retrospective clinical analysis. Oncology 99 (5), 271–279. doi:10.1159/000511999
Sugano, T., Seike, M., Saito, Y., Kashiwada, T., Terasaki, Y., Takano, N., et al. (2020). Immune checkpoint inhibitor-associated interstitial lung diseases correlate with better prognosis in patients with advanced non-small-cell lung cancer. Thorac. cancer 11 (4), 1052–1060. doi:10.1111/1759-7714.13364
Suh, K. J., Kim, S. H., Kim, Y. J., Kim, M., Keam, B., Kim, T. M., et al. (2018). Post-treatment neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients with advanced non-small cell lung cancers treated with anti-PD-1 antibody. CII 67 (3), 459–470. doi:10.1007/s00262-017-2092-x
Tambo, Y., Sone, T., Shibata, K., Nishi, K., Shirasaki, H., Yoneda, T., et al. (2020). Real-World efficacy of first-line pembrolizumab in patients with advanced or recurrent non-small-cell lung cancer and high PD-L1 tumor expression. Clin. lung cancer 21 (5), e366–e379. doi:10.1016/j.cllc.2020.02.017
Teraoka, S., Fujimoto, D., Morimoto, T., Kawachi, H., Ito, M., Sato, Y., et al. (2017). Early immune-related adverse events and association with outcome in advanced non-small cell lung cancer patients treated with nivolumab: A prospective cohort study. J. Thorac. Oncol. official Publ. Int. Assoc. Study Lung Cancer 12 (12), 1798–1805. doi:10.1016/j.jtho.2017.08.022
Thuillier, P., Joly, C., Alavi, Z., Crouzeix, G., Descourt, R., Quere, G., et al. (2021). Thyroid dysfunction induced by immune checkpoint inhibitors is associated with a better progression-free survival and overall survival in non-small cell lung cancer: An original cohort study. Cancer Immunol. Immunother. CII 70 (7), 2023–2033. doi:10.1007/s00262-020-02802-6
Toi, Y., Sugawara, S., Kawashima, Y., Aiba, T., Kawana, S., Saito, R., et al. (2018). Association of immune-related adverse events with clinical benefit in patients with advanced non-small-cell lung cancer treated with nivolumab. Oncol. 23 (11), 1358–1365. doi:10.1634/theoncologist.2017-0384
Wang, W., Gu, X., Wang, L., Pu, X., Feng, H., Xu, C., et al. (2022). The prognostic impact of mild and severe immune-related adverse events in non-small cell lung cancer treated with immune checkpoint inhibitors: A multicenter retrospective study. Cancer Immunol. Immunother. CII 71 (7), 1693–1703. doi:10.1007/s00262-021-03115-y
Weber, J. S., Kähler, K. C., and Hauschild, A. (2012). Management of immune-related adverse events and kinetics of response with ipilimumab. J. Clin. Oncol. official J. Am. Soc. Clin. Oncol. 30 (21), 2691–2697. doi:10.1200/JCO.2012.41.6750
Wei, S. C., Duffy, C. R., and Allison, J. P. (2018). Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 8 (9), 1069–1086. doi:10.1158/2159-8290.CD-18-0367
Yamaguchi, O., Imai, H., Minemura, H., Suzuki, K., Wasamoto, S., Umeda, Y., et al. (2020). Efficacy and safety of immune checkpoint inhibitor monotherapy in pretreated elderly patients with non-small cell lung cancer. Cancer Chemother. Pharmacol. 85 (4), 761–771. doi:10.1007/s00280-020-04055-7
Young, A., Quandt, Z., and Bluestone, J. A. (2018). The balancing act between cancer immunity and autoimmunity in response to immunotherapy. Cancer Immunol. Res. 6 (12), 1445–1452. doi:10.1158/2326-6066.CIR-18-0487
Zhang, M., Rodrigues, A. J., Pollom, E. L., Gibbs, I. C., Soltys, S. G., Hancock, S. L., et al. (2021). Improved survival and disease control following pembrolizumab-induced immune-related adverse events in high PD-L1 expressing non-small cell lung cancer with brain metastases. J. neuro-oncology 152 (1), 125–134. doi:10.1007/s11060-020-03686-3
Keywords: immune-related adverse events, clinical efficacy, immune checkpoint inhibitors, meta-analysis, non-small-cell lung cancer
Citation: Lin L, Liu Y, Chen C, Wei A and Li W (2023) Association between immune-related adverse events and immunotherapy efficacy in non-small-cell lung cancer: a meta-analysis. Front. Pharmacol. 14:1190001. doi: 10.3389/fphar.2023.1190001
Received: 20 March 2023; Accepted: 04 May 2023;
Published: 22 May 2023.
Edited by:
Deqiang Dou, Liaoning University of Traditional Chinese Medicine, ChinaReviewed by:
Zhili Xu, Liaoning University of Traditional Chinese Medicine, ChinaNa Zhang, Xuzhou Medical University, China
Copyright © 2023 Lin, Liu, Chen, Wei and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anhua Wei, ahwei0716@163.com; Wei Li, isliwei_tiao@163.com
†These authors have contributed equally to this work
 Li Lin1†
Li Lin1† Chen Chen
Chen Chen Anhua Wei
Anhua Wei Wei Li
Wei Li