
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 22 June 2023
Sec. Pharmacology of Anti-Cancer Drugs
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1189910
Vascular endothelial growth factor (VEGF) contributes to angiogenesis and vasculogenesis. The occurrence and progression of tumors are accompanied by angiogenesis. Vascular endothelial growth factor inhibitors (VEGFI) have been used in anti-tumor treatment. However, aortic dissection (AD) is one of the VEGFI-associated adverse reactions with cute onset, rapid progression, and high case fatality rate. We collected case reports of VEGFI related to aortic dissection in PubMed and CNKI (China National Knowledge Infrastructure) from inception to 28 April 2022. Seventeen case reports were selected. The medication included sunitinib, sorafenib, pazopanib, axitinib, apatinib, anlotinib, bevacizumab, and ramucirumab. This review discusses the pathology, risk factors, diagnosis, and treatment of AD. Vascular endothelial growth factor inhibitors are related to aortic dissection. Although current literature lacks clear statistical evidence on the population, we offer points to encourage further confirmation of the best methods of care for these patients.
Tumor cells secrete vascular endothelial growth factor (VEGF) promoting neovascularization to provide the oxygen and nutrients for tumor growth. VEGF and its receptor signaling pathway are important in angiogenesis. Anti-angiogenesis drugs mainly target VEGF and VEGF receptors (VEGFR) by inhibiting their expression, which blocks the signal transduction pathway or exhausts the VEGF produced by tumor cells (Figure 1). Therefore, they inhibit the generation of neovascularization and suspend the blood supply for tumors, restraining tumor growth, development, and metastasis.
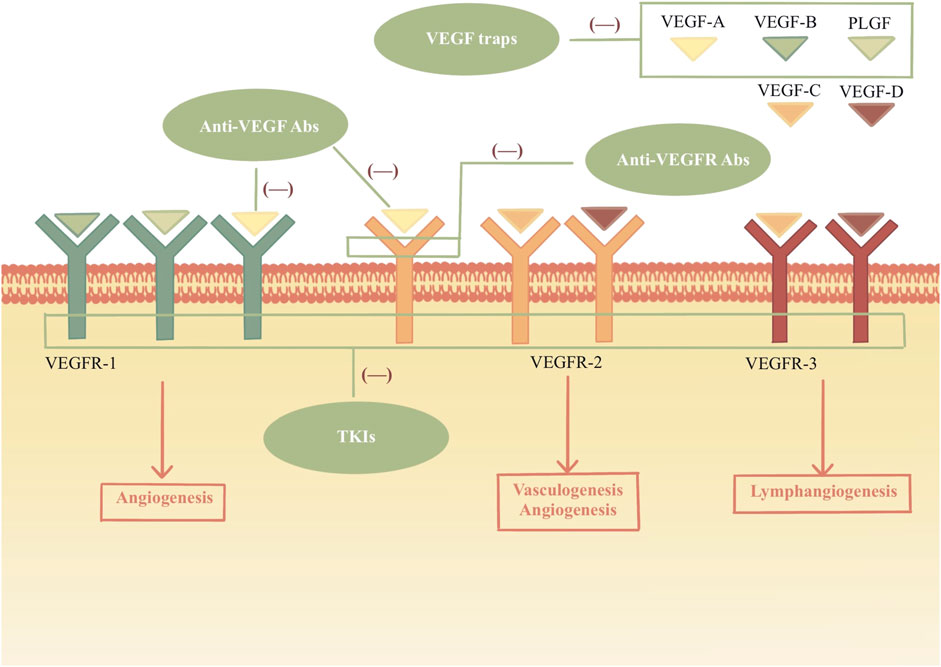
FIGURE 1. Mechanism of VEGF and inhibitors. VEGF family bind to the VEGF receptors and tyrosine kinases are activated, which leads to the angiogenesis, vasculogenesis and lymphangiogenesis. VEGFIs inhibit the VEGF signal pathways by acting different sites. Anti-VEGF Abs: anti-VEGF monoclonal antibodies; Anti-VEGFR Abs: anti-VEGFR monoclonal antibodies; TKIs: tyrosine kinase inhibitors.
Four classes of VEGF inhibitors (VEGFI) have been developed (Table 1): (a) anti-VEGF monoclonal antibodies; (b) anti-VEGFR monoclonal antibodies; (c) VEGF traps; (d) tyrosine kinase inhibitors (TKIs) (Dobbin et al., 2021).
The VEGF family includes seven members: VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, and placenta-derived growth factor (PLGF). VEGF binds to VEGFRs (VEGFR-1 and VEGFR-2) in the signaling pathway. VEGFR-2 is the primary signaling receptor for VEGF binding. The VEGF-A/VEGFR signal axis induces the process of vasculogenesis and angiogenesis. VEGF-C and VEGF-D bind to VEGFR-3, a master regulator of lymphangiogenesis. It is a high affinity that VEGFR-1 can bind with VEGF-A, VEGF-B, and PLGF. As a kinase insert domain receptor, VEGFR-2 is encoded by the human KDR gene. It can bind with VEGF-A, VEGF-C and VEGF-D. (Ahmad and Nawaz, 2022).
The humanized anti-VEGF monoclonal antibody, bevacizumab, binds VEGF with an affinity very similar to that of the original antibody. Bevacizumab binds and neutralizes all human VEGF-A isoforms and bioactive proteolytic fragments, as does its mouse counterpart. (Ferrara et al., 2004). Ramucirumab effectively prevents VEGF ligands from binding and activating the receptor by binding to both soluble and cell surface VEGFR-2. (Sanchez-Gastaldo et al., 2016). Aflibercept, as a decoy receptor, targets VEGF-A, VEGF-B, and PLGF to inhibit angiogenesis. It has a higher affinity for VEGF-A than either VEGFR or bevacizumab. (Ciombor and Berlin, 2014).
Upregulation of alternative angiogenic signaling pathways, including PDGF and fibroblast growth factor (FGF), is a postulated mechanism by which resistance to anti-VEGF therapy may develop. TKIs that inhibit multiple angiogenic pathways may help overcome this resistance by blocking overlapping pathways. Axitinib, sorafenib, sunitinib, cediranib, tivozanib, cabozatinib, vatalanib, vandetanib, pazopanib, nintedanib, lenvatinib, ponatinib, and regorafenib inhibit VEGFR and other targets (Table 1). (Wood et al., 2000; Wedge et al., 2002; Mendel et al., 2003; Wilhelm et al., 2004; Wedge et al., 2005; Nakamura et al., 2006; Patyna et al., 2006; Polverino et al., 2006; Wilhelm et al., 2006; Kumar et al., 2007; Roskoski, 2007; Sonpavde and Hutson, 2007; Hilberg et al., 2008; Hu-Lowe et al., 2008; Wilhelm et al., 2011; Gozgit et al., 2013; King and Lee, 2013)
Since VEGF is widely distributed in other tissues and organs such as the heart, promoting the formation of new blood vessels and the growth of vascular endothelial cells, it also plays a key role in the blood vessels of non-tumor tissues. While acting on the tumor vascular system, VEGFI inevitably inhibits the blood vessels of non-tumor tissues, leading to cardiotoxicity. VEGFI-associated cardiovascular adverse reactions include hypertension, left ventricular systolic dysfunction, and QT interval prolongation, along with arterial and venous thromboembolism, heart failure, and arrhythmia (Dobbin et al., 2021). However, previous literature on the subject rarely discusses that VEGFI could induce aortic dissection (AD).
The aorta is composed of the inner, middle, and outer layers. The elastic muscle in the layer protects the wall against blood pressure. The existence of physiological conditions and use of VEGFI might weaken the media. A crevasse is commonly caused by blood pumping against the weakened section of the inner layer. Blood moves through the break in the inner wall, separating it from the middle. Consequently, there is a septum between the true aortic channel and the false channel, called aortic dissection. Due to the tear in the inner layer, the blood may break through the outer layer of the aortic wall, causing a life-threatening problem, or it may reenter the aorta through another tear in the inner layer. As time goes on, there may be a thrombus in the false channel. (Figure 2). There are the Stanford type and the Debakey type. The classification of AD is Stanford type A if the ascending aorta is involved (historically DeBakey type I and type II), and Stanford type B if the descending aorta is involved (historically DeBakey type IIIa or type IIIb). (Carrel et al., 2023). Debakey type I begins in the ascending aorta and extends distally throughout the remaining aorta. Debakey type II is limited to the ascending aorta. Debakey type IIIa describes a limited dissection to the thoracic aorta, whereas type IIIb describes more distal extension into the abdominal aorta. (Debakey et al., 1965).
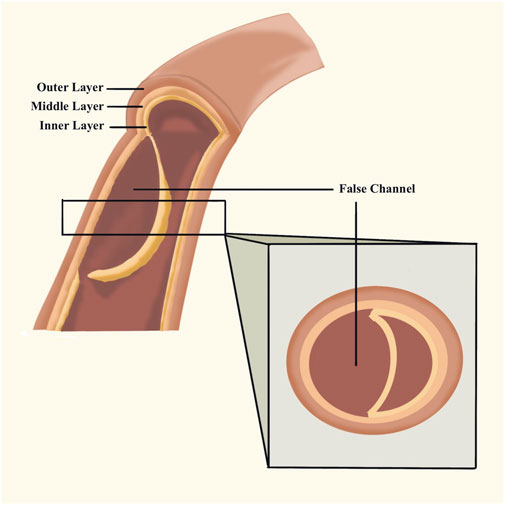
FIGURE 2. Aortic dissection. The blood stream reenters the aorta through another tear in the inner layer and forms thrombus the false channel.
AD is a critical emergency disease in cardiovascular disease, with acute onset, rapid progression, high mortality rate, and easy to be misdiagnosed and missed. Although analysis of the relation between AD and VEGFI based on the databases has been reported (Oshima et al., 2017; Cheng et al., 2021; Dörks et al., 2021; Wang et al., 2021), the current literature lacks a comprehensive review of containing wilder population in which aortic dissection occurs. To provide general insight into VEGFI and AD, we reviewed the case reports and discuss the pathophysiology, risk factors, diagnosis, treatment, and future directions of innovation.
The search was conducted in PubMed and CNKI (China National Knowledge Infrastructure) from inception to 28 April 2022. The terms for the search strategy were “aortic dissection” and “antiangiogenic”, “angiogenesis inhibitors”, “vascular endothelial growth factor”, “VEGF”, “VEGFR”, “sunitinib”, “axitinib”, “sorafenib”, “anlotinib”, “apatinib”, “lenvatinib”, “vandetanib”, “pazopanib”, “cabozantinib”, “nintedanib”, “ponatinib”, “regorafenib”, “vatalanib”, “bevacizumab”, “ramucirumab”, “aflibercept”. The resulting articles were screened for case reports.
In total, seventeen articles were selected for this study. Information was individually extracted from each article, including gender, age, type of tumors, VEGFI drug and duration, types of AD, history, adverse reaction, treatment for AD, and outcome. Findings are summarized in Table 2 and Figures 3–5.
The review of the literature identified seventeen eligible case reports with VEGFI related to AD (Aragon-Ching et al., 2008; Edeline et al., 2009; Carles, 2010; Formiga and Fanelli, 2014; Kurtz et al., 2014; Niwa et al., 2015; Koda et al., 2016; Hatem et al., 2017; Xu et al., 2017; Atsunori et al., 2018; Takada et al., 2018; Toru et al., 2018; Fubo and Gong, 2019; Zenoni et al., 2019; Jiang et al., 2020; Nikai et al., 2020; Ting and Lo, 2021). Thirteen of the seventeen patients are male, and four patients are female. Of the seventeen case reports included in our analysis, seven were conducted in patients with renal cell carcinoma; four were in patients with gastrointestinal tumor (two cases in gastrointestinal stromal tumor [Hatem et al., 2017; Toru et al., 2018), one case in colon cancer (Koda et al., 2016), one case in signet-ring cell carcinoma (Zenoni et al., 2019)]; three results related to patients with hepatocellular carcinoma; one for patients with esophagogastric junction cancer; one was in patients with lung squamous cell carcinoma; one was in patients with prostate cancer.
The patients using VEGFI including sunitinib, sorafenib, pazopanib, axitinib, apatinib, anlotinib, bevacizumab, and ramucirumab are from 48 to 70 s years old. The occurrence of aortic dissection ranges from 1 day to 6 years after the application of VEGFI, which shows the possibility of aortic dissection at any time with VEGFI.
Among the screened cases, eleven case reports (Table 2) described adverse reactions and most of them showed increased blood pressure, besides proteinuria, hypopigmentation of the skin, thyroid dysfunction, stomatitis, thrombocytopenia, palmar-plantar erythrodysesthesia, aortoesophageal fistula rupture, cephalalgia, hot flashes, nausea, oedema, liver dysfunction, cardiac dysfunction, cardiomyopathy, fatigue, and hypertriglyceridemia. The patients were administered antihypertensive drugs, besides sedative hypnotics, analgesics, and anticoagulants.
The five patients that reported AD had pre-existing hypertension before VEGFI medication, while two had no hypertension. Of the seventeen cases, seven cases were Stanford type A, four in Stanford type B, one in DeBakey type III [equivalent to Stanford type B (Surgeon, 2017)], and five were not mentioned in the article. Among the non-mentioned cases, it was inferred from the reports that three (Aragon-Ching et al., 2008; Carles, 2010; Xu et al., 2017) were Stanford type B (Ince and Nienaber, 2007).
The three patients who died due to AD were all Stanford type A, and treated with ramucirumab (two cases) and pazopanib (one case). The age distribution of patients who died was the 40 s, 50 s, and 70 s, indicating that age was not a risk factor for death. Among them were two male and one female patient. Notably, two patients who died had a history of hypertension. We supposed that the physical condition with high blood pressure made the patients’ recovery difficult.
Among the patients with AD, there were significantly more men than women. According to the age analysis of the patients in these case reports, although the ages ranged from 48 to 77 years old, the majority (n = 14, 82.4%) of patients were concentrated in the 58 to 67-year-old range.
Overall, across the case reports, the treatment for AD included reconstruction and replacement of the aorta, endovascular chest stent grafting, and drug therapy.
The etiology and mechanism of aortic dissection have been the subject of research and some progress has been made, but the specific pathogenesis of aortic dissection has not yet been outlined conclusively. Abnormal phenotypic transformation and apoptosis of vascular smooth muscle cells, abnormal degradation of extracellular matrix, endothelial dysfunction, and immune cell infiltration have been implicated in the pathogenesis of aortic dissection (Yin et al., 2022).
In vasa vasorum, the VEGF signal pathway is related to the activation of the phosphatidylinositol-3-kinase-AKT signaling pathway. Inhibition of the pathway might result in overexpression of matrix metalloproteinase 9 (MMP9), which leads to extracellular matrix degradation. (Takada et al., 2018). Besides the previous point on the overexpression of MMP9, the inhibition of the VEGF signal pathway might induce the increase of NO, which leads to SMC apoptosis.
The driving mechanism of aortic dissection is aortic intima tear and cystic medial necrosis. First, the existence of some underlying diseases increases blood cholesterol and glucose levels, which leads to atherosclerosis. These changes negatively affect collagen formation and connective tissue strength, leading to increased vascular fragility and increased blood pressure. (Kurtz et al., 2014). Meanwhile, VEGFI may cause increased stiffness in arteries, making them more subject to dissection. Then, VEGFI such as sunitinib (Edeline et al., 2009), sorafenib (Carles, 2010), and pazopanib (Kurtz et al., 2014) target VEGF and platelet-derived growth factor receptors, interfering with the normal function of these factors. The damaged vascular endothelium cannot regenerate and heal normally, and AD is formed eventually.
VEGFI also impairs vasodilation and increases vasoconstriction. It reduces NO and prostacyclin to weaken vasodilation and increases the secretion of endothelin-1 from vascular endothelial cells to enhance vasoconstriction. Another proposed mechanism is that VEGF helps regulate the sympathetic innervation of blood vessels (Hatem et al., 2017).
Aortic dissection generally occurs in patients who have gotten hypertension, atherosclerosis, diabetes, and Marfan syndrome. It is considered that hypertension is the most common predisposing factor for aortic dissection (Ince and Nienaber, 2007). However, in two cases, hypertension had not been observed before VEGFI therapy (Hatem et al., 2017; Fubo and Gong, 2019).
Oshima et al. (Oshima et al., 2017) have outlined that the use of VEGFI had a comparatively higher adjusted odds ratio than hypertension. However, Dörks (Dörks et al., 2021) suggested that the dose-rising of VEGFI was related to increased blood pressure. Pre-existing hypertension is not an inevitable factor for VEGFI causing AD. The increase in blood pressure after the medication is more critical. Together, the combination of pre-existing hypertension and increased blood pressure after VEGFI causes the risk of AD.
One case (Toru et al., 2018) had a history of vascular calcification. It could be considered that AD was caused by two reasons: (a) hypertension caused by sunitinib; and (b) vascular fragility caused by vascular calcification and sunitinib.
The main parallel between Cheng et al. (Cheng et al., 2021) and our studies indicate that the most common underlying risk factor reported in the case series is hypertension. Other cases have also been reported without preexisting hypertension. Both case series reported axitinib, bevacizumab, pazopanib, ramucirumab, sorafenib, and sunitinib related to AD. In the study by Cheng et al. 44% (105/240) of cases were reported in female patients. Cabozantinib, lenvatinib, ponatinib, regorafenib, vandetanib, and ziv-aflibercept were reported. Among our screened cases, thirteen of the seventeen patients were male, and four patients were female. Apatinib and anlotinib were reported.
The main complaint of AD patients is tear-like or knife-like persistent and unbearable sharp pain. The location of the pain is related to the rupture and progression of AD. Stanford type A presents with chest pain or back pain, and Stanford type B presents with back pain or abdominal pain. The two pain areas may also overlap. The possibility of AD should be suspected in patients with severe chest and back pain with high-risk medical history and signs. Other than that, migrating pain may indicate the progression of dissection. If the patient has lower extremity pain, it may indicate that the dissection may involve the iliac or femoral arteries. However, some patients may also have no pain symptoms (Surgeon, 2017). Among the patients mentioned in this review, a patient (Kurtz et al., 2014) with Stanford type A complained of no chest pain. In addition to pain, AD could lead to cardiac complications and other adverse effects of organ perfusion. Hypotension in some patients may be related to cardiac tamponade or dissection involving the brachiocephalic artery.
The initial examination usually uses imaging, which aims to comprehensively evaluate the whole aorta, including the scope and shape of AD involvement, the diameter of the aorta in different parts, the involvement of the aortic valve and its branches, the relationship with surrounding tissues, and other AD relevant manifestations such as pericardial effusion, pleural effusion, and organ ischemia (Surgeon, 2017). Computed tomography angiography (CTA) is the first-choice imaging method for the diagnosis of AD, and magnetic resonance imaging (MRI) can be used when CTA cannot be performed. Although the diagnostic accuracy of echocardiography for AD is slightly lower than that of CT and MRI, it can be used for preoperative, intraoperative, and postoperative evaluation of patients with various conditions because of its strong portability.
In addition, the D-dimer test is used as the diagnosis and differential diagnosis of AD. Routine laboratory inspection items cover blood routine and blood type, urine routine, biochemical complete set, blood gas analysis, hepatitis B and other infectious disease screening, myocardial enzymes, and myocardial markers, myoglobin, coagulation five items inspection. In the acute phase, leukocytosis with an increased proportion of neutrophils and an increased erythrocyte sedimentation rate is common. Another biomarker is plasma C-reactive protein, which can reflect the inflammatory activity.
AD needs to be differentiated from acute myocardial infarction, acute abdomen, acute pulmonary embolism, acute pericarditis, and pneumothorax to avoid misdiagnosis and delay the disease. The doctors who initially treat patients should improve understanding of the AD and correctly analyze the condition. Patients with abdominal pain symptoms and previous abdominal diseases should not be missed diagnosis. The clinical manifestations of AD are complex and lack specificity, and chest pain, abdominal pain, and back pain are common. It is especially important to choose the correct auxiliary examination, such as CTA. Blood pressure monitoring and control should be routinely performed (Fubo and Gong, 2019).
Pharmacotherapy, surgery, and endoluminal intervention are mainly used in the treatment of AD. The general purpose of drug therapy is to relieve pain and reduce the rate of ejection from the left ventricle and systolic blood pressure. Morphine is commonly used in analgesics. Drugs controlling blood pressure are A (angiotensin converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB)), B (β-receptor blockers), C (calcium channel clockers), and D (diuretic). B is recommended in AD, which includes metoprolol and labetalol. While there are contraindications to B, C is used. During the course of treatment, adjuvant medication including sedative-hypnotics and anticoagulants help improve patients’ compliance and prognosis.
Stanford type A is generally considered surgery which is artificial vascular grafting, replacement, and reconstruction, removing the aortic segment where the intimal tear is located. Age is not a contraindication to surgery, but for patients of advanced age, the condition of other organs should be comprehensively evaluated, such as type A in a persistent coma that is not suitable for surgery.
Patients with Stanford type B are treated with endovascular intervention, known as endovascular repair of thoracic aortic dissection (TEVAR). This treatment method inserts a stent graft into the true lumen and blocks the primary rupture of the dissection, so that the blood in the false lumen loses communication, effectively reducing the pressure of the false lumen and reducing the risk of aortic rupture.
This review provides doctors with a working direction for the long-term recording of cardiac function in cancer patients and suggests the key points of diagnosis and treatment of AD. Patients using VEGFI must be aware of symptoms relating to pain and the fluctuation of blood pressure.
Due to the low probability of AD in patients, there are only a few case reports and no randomized clinical trials to provide a statistical basis. Case reports can also have limitations in terms of under-reporting and non-reporting adverse reactions. Thus, our results only indicate an increased risk, which must be confirmed in prospective controlled trials.
Vascular endothelial growth factor inhibitors are related to aortic dissection. AD must be diagnosed early and treated aggressively in good time, or else it can be fatal. Although current literature lacks clear statistical evidence on the population, we offer these points to encourage further confirmation of the care for these patients.
All authors listed have made substantial, direct, and intellectual contributions to the work and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ahmad, A., and Nawaz, M. I. (2022). Molecular mechanism of VEGF and its role in pathological angiogenesis. J. Cell. Biochem. 123 (12), 1938–1965. doi:10.1002/jcb.30344
Aragon-Ching, J. B., Ning, Y. M., and Dahut, W. L. (2008). Acute aortic dissection in a hypertensive patient with prostate cancer undergoing chemotherapy containing bevacizumab. Acta Oncol. 47 (8), 1600–1601. doi:10.1080/02841860801978905
Atsunori, T., Masahiro, O., Yusuke, W., Naruhiro, K., Kazunao, H., Takeshi, S., et al. (2018). Successful treatment of aortic dissection during sorafenib therapy for hepatocellular carcinoma. Clin. Case Rep. 6 (8), 1643–1644. doi:10.1002/ccr3.1674
Carles, J., Suárez, C., and Andreu, J. (2010). Acute aortic dissection during sorafenib-containing therapy. Ann. Oncol. Official J. Eur. Soc. Med. Oncol. 21 (1), 181–182. doi:10.1093/annonc/mdp468
Carrel, T., Sundt, T. M., von Kodolitsch, Y., and Czerny, M. (2023). Acute aortic dissection. Lancet (London, Engl. 401 (10378), 773–788. doi:10.1016/S0140-6736(22)01970-5
Cheng, C., Nguyen, M. N., Nayernama, A., Jones, S. C., Brave, M., Agrawal, S., et al. (2021). Arterial aneurysm and dissection with systemic vascular endothelial growth factor inhibitors: A review of cases reported to the fda adverse event reporting system and published in the literature. Vasc. Med. Lond. Engl. 26 (5), 526–534. doi:10.1177/1358863X211006470
Ciombor, K. K., and Berlin, J. (2014). Aflibercept--a decoy VEGF receptor. Curr. Oncol. Rep. 16 (2), 368. doi:10.1007/s11912-013-0368-7
Debakey, M. E., Henly, W. S., Cooley, D. A., Morris, G. C., Crawford, E. S., and Beall, A. C. (1965). Surgical management of dissecting aneurysms of the aorta. J. Thorac. Cardiovasc. Surg. 49, 130–149. doi:10.1016/s0022-5223(19)33323-9
Dobbin, S. J. H., Petrie, M. C., Myles, R. C., Touyz, R. M., and Lang, N. N. (2021). Cardiotoxic effects of angiogenesis inhibitors. Clin. Sci. (Lond) 135 (1), 71–100. doi:10.1042/CS20200305
Dörks, M., Jobski, K., Herget-Rosenthal, S., Hoffmann, F., and Douros, A. (2021). Tyrosine kinase inhibitors targeting vascular endothelial growth factor and the risk of aortic dissection-A pharmacovigilance analysis. Pharmacol. Res. Perspect. 9 (1), e00707. doi:10.1002/prp2.707
Edeline, J., Laguerre, B., Rolland, Y., and Patard, J. J. (2009). Aortic dissection in a patient treated by sunitinib for metastatic renal cell carcinoma. Ann. Oncol. 21 (1), 186–187. doi:10.1093/annonc/mdp480
Ferrara, N., Hillan, K. J., Gerber, H.-P., and Novotny, W. (2004). Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat. Rev. Drug Discov. 3 (5), 391–400. doi:10.1038/nrd1381
Formiga, M., and Fanelli, M. F. (2014). Aortic dissection during antiangiogenic therapy with sunitinib. A case report. Sao Paulo Med. J. 133 (3), 275–277. doi:10.1590/1516-3180.2013.7380002
Fubo, L. I., and Gong, G. (2019). Aortic dissection in apatinib treatment of advanced hepatocellular carcinoma: A case report. Cancer Res. Prev. Treat. 46 (07), 685–660. doi:10.3971/j.issn.1000-8578.2019.18.1957
Gozgit, J. M., Squillace, R. M., Wongchenko, M. J., Miller, D., Wardwell, S., Mohemmad, Q., et al. (2013). Combined targeting of FGFR2 and mTOR by ponatinib and ridaforolimus results in synergistic antitumor activity in FGFR2 mutant endometrial cancer models. Cancer Chemother. Pharmacol. 71 (5), 1315–1323. doi:10.1007/s00280-013-2131-z
Hatem, R., Bebawi, E., and Schampaert, E. (2017). Potential sunitinib-induced coronary artery and aortic dissections. Can. J. Cardiol. 33 (6), e17–e830. doi:10.1016/j.cjca.2017.03.002
Hilberg, F., Roth, G. J., Krssak, M., Kautschitsch, S., Sommergruber, W., Tontsch-Grunt, U., et al. (2008). BIBF 1120: Triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res. 68 (12), 4774–4782. doi:10.1158/0008-5472.CAN-07-6307
Hu-Lowe, D. D., Zou, H. Y., Grazzini, M. L., Hallin, M. E., Wickman, G. R., Amundson, K., et al. (2008). Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin. Cancer Res. 14 (22), 7272–7283. doi:10.1158/1078-0432.CCR-08-0652
Ince, H., and Nienaber, C. A. (2007). Diagnosis and management of patients with aortic dissection. Heart (British Card. Soc. 93 (2), 266–270. doi:10.1136/hrt.2005.078550
Jiang, B., Li, J., Chen, J., Xiang, X., and Deng, J. (2020). Aortic dissection in a patient treated with anlotinib for metastatic lung squamous cell carcinoma. Thorac. Cancer 11 (2), 461–464. doi:10.1111/1759-7714.13288
King, J. W., and Lee, S.-M. (2013). Axitinib for the treatment of advanced non-small-cell lung cancer. Expert Opin. Investigational Drugs 22 (6), 765–773. doi:10.1517/13543784.2013.775243
Koda, T., Koike, J., Masuhara, H., Kurihara, A., Kaneko, H., Ushigome, M., et al. (2016). A case of aortoesophageal fistula rupture due to descending thoracic aortic dissection with recurrent colon cancer during chemotherapy containing bevacizumab. Gan Kagaku Ryoho 43 (12), 1815–1817.
Kumar, R., Knick, V. B., Rudolph, S. K., Johnson, J. H., Crosby, R. M., Crouthamel, M.-C., et al. (2007). Pharmacokinetic-pharmacodynamic correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity. Mol. Cancer Ther. 6 (7), 2012–2021. doi:10.1158/1535-7163.MCT-07-0193
Kurtz, V., Seidel, G., Dierks, M. L., Ando, Y., Matsukawa, Y., and Gotoh, M. (2014). Acute aortic dissection in a patient receiving multiple tyrosine kinase inhibitors for 5 years. Aktuelle Urol. 45 (02), 132–134. doi:10.1055/s-0033-1363274
Mendel, D. B., Laird, A. D., Xin, X., Louie, S. G., Christensen, J. G., Li, G., et al. (2003). In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: Determination of a pharmacokinetic/pharmacodynamic relationship. Clin. Cancer Res. 9 (1), 327–337.
Nakamura, K., Taguchi, E., Miura, T., Yamamoto, A., Takahashi, K., Bichat, F., et al. (2006). KRN951, a highly potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, has antitumor activities and affects functional vascular properties. Cancer Res. 66 (18), 9134–9142. doi:10.1158/0008-5472.CAN-05-4290
Nikai, H., Akiyama, Y., Fujisawa, R., Endo, F., Sasaki, A., Koeda, K., et al. (2020). A thoracic aortic dissection case during treatment with ramucirumab plus nab-paclitaxel. Gan kagaku ryoho 47 (6), 981–983.
Niwa, N., Nishiyama, T., Ozu, C., Yagi, Y., and Saito, S. (2015). Acute aortic dissection in a patient with metastatic renal cell carcinoma treated with axitinib. Acta Oncol. 54 (4), 561–562. doi:10.3109/0284186X.2014.963887
Oshima, Y., Tanimoto, T., Yuji, K., and Tojo, A. (2017). Association between aortic dissection and systemic exposure of vascular endothelial growth factor pathway inhibitors in the Japanese adverse drug event report database. Circulation 135 (8), 815–817. doi:10.1161/CIRCULATIONAHA.116.025144
Patyna, S., Laird, A. D., Mendel, D. B., O'Farrell, A.-M., Liang, C., Guan, H., et al. (2006). SU14813: A novel multiple receptor tyrosine kinase inhibitor with potent antiangiogenic and antitumor activity. Mol. Cancer Ther. 5 (7), 1774–1782. doi:10.1158/1535-7163.MCT-05-0333
Polverino, A., Coxon, A., Starnes, C., Diaz, Z., DeMelfi, T., Wang, L., et al. (2006). AMG 706, an oral, multikinase inhibitor that selectively targets vascular endothelial growth factor, platelet-derived growth factor, and kit receptors, potently inhibits angiogenesis and induces regression in tumor xenografts. Cancer Res. 66 (17), 8715–8721. doi:10.1158/0008-5472.CAN-05-4665
Roskoski, R. (2007). Sunitinib: A VEGF and PDGF receptor protein kinase and angiogenesis inhibitor. Biochem. Biophysical Res. Commun. 356 (2), 323–328. doi:10.1016/j.bbrc.2007.02.156
Sanchez-Gastaldo, A., Gonzalez-Exposito, R., and Garcia-Carbonero, R. (2016). Ramucirumab clinical development: An emerging role in gastrointestinal tumors. Target. Oncol. 11 (4), 479–487. doi:10.1007/s11523-016-0419-8
Sonpavde, G., and Hutson, T. E. (2007). Pazopanib: A novel multitargeted tyrosine kinase inhibitor. Curr. Oncol. Rep. 9 (2), 115–119. doi:10.1007/s11912-007-0007-2
Surgeon, T. (2017). Chinese experts’ consensus of standardized diagnosis and treatment for aortic dissection. Chin. J. Thorac. Cardiovasc Surg. 33 (11), 641–654. doi:10.3760/cma.j.issn.1001-4497.2017.11.001
Takada, M., Yasui, T., Oka, T., Shioyama, W., Kuroda, T., Nakai, Y., et al. (2018). Aortic dissection and cardiac dysfunction emerged coincidentally during the long-term treatment with angiogenesis inhibitors for metastatic renal cell carcinoma. Int. Heart J. 59 (5), 1174–1179. doi:10.1536/ihj.17-461
Ting, J., and Lo, Z. J. (2021). Vascular endothelial growth factor tyrosine kinase inhibitor targeted therapy: A potential cause of an acute aortic dissection lesser known to the emergency physician. BMJ Case Rep. 14 (10), e245653. doi:10.1136/bcr-2021-245653
Toru, A., Akira, S., Daisuke, H., and Kazutaka, A. (2018). Acute aortic dissection with sporadic aortic calcifications during chemotherapy with sunitinib. J. Vasc. Surg. Cases Innovative Tech. 4 (2), 147. doi:10.1016/j.jvscit.2018.02.003
Wang, S., Chen, M., Zhang, X., Zhang, L., Jia, M., Shen, Z., et al. (2021). Aneurysm and artery dissection following the use of vascular endothelial growth factor inhibitor: A real-world analysis using a spontaneous reporting system. J. Am. Heart Assoc. 10 (23), e020844. doi:10.1161/JAHA.121.020844
Wedge, S. R., Kendrew, J., Hennequin, L. F., Valentine, P. J., Barry, S. T., Brave, S. R., et al. (2005). AZD2171: A highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res. 65 (10), 4389–4400. doi:10.1158/0008-5472.CAN-04-4409
Wedge, S. R., Ogilvie, D. J., Dukes, M., Kendrew, J., Chester, R., Jackson, J. A., et al. (2002). ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration. Cancer Res. 62 (16), 4645–4655.
Wilhelm, S., Carter, C., Lynch, M., Lowinger, T., Dumas, J., Smith, R. A., et al. (2006). Discovery and development of sorafenib: A multikinase inhibitor for treating cancer. Nat. Rev. Drug Discov. 5 (10), 835–844. doi:10.1038/nrd2130
Wilhelm, S. M., Carter, C., Tang, L., Wilkie, D., McNabola, A., Rong, H., et al. (2004). BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 64 (19), 7099–7109. doi:10.1158/0008-5472.CAN-04-1443
Wilhelm, S. M., Dumas, J., Adnane, L., Lynch, M., Carter, C. A., Schütz, G., et al. (2011). Regorafenib (BAY 73-4506): A new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int. J. Cancer 129 (1), 245–255. doi:10.1002/ijc.25864
Wood, J. M., Bold, G., Buchdunger, E., Cozens, R., Ferrari, S., Frei, J., et al. (2000). PTK787/ZK 222584, a novel and potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor-induced responses and tumor growth after oral administration. Cancer Res. 60 (8), 2178–2189.
Xu, L., Wang, B., and Ding, W. (2017). Abdominal aortic dissection during sorafenib therapy for hepatocellular carcinoma. Clin. Res. Hepatology Gastroenterology 41 (2), e24–e25. doi:10.1016/j.clinre.2016.12.005
Yin, Z.-Q., Han, H., Yan, X., and Zheng, Q.-J. (2022). Research progress on the pathogenesis of aortic dissection. Curr. Problems Cardiol. 48, 101249. doi:10.1016/j.cpcardiol.2022.101249
Keywords: vascular endothelial growth factor inhibitors, adverse reaction, aortic dissection, hypertension, drug safety
Citation: Dai S, Zhong Y, Cui H, Zhao J and Li S (2023) Aortic dissection induced by vascular endothelial growth factor inhibitors. Front. Pharmacol. 14:1189910. doi: 10.3389/fphar.2023.1189910
Received: 20 March 2023; Accepted: 12 June 2023;
Published: 22 June 2023.
Edited by:
Peter Kruzliak, Masaryk University, CzechiaReviewed by:
Yu Zhang, Johns Hopkins University, United StatesCopyright © 2023 Dai, Zhong, Cui, Zhao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Su Li, bGlzdUBjYW5jZXJob3NwLWxuLWNtdS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.