- 1Department of Rheumatology and Immunology, Nanjing Drum Tower Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Graduate School of Peking Union Medical College, Nanjing, China
- 2People’s Hospital of Ningxiang City, Ningxiang, China
- 3Hunan University of Science and Technology, Xiangtan, China
- 4Key Laboratory of Hunan Province for Integrated Traditional Chinese and Western Medicine on Prevention and Treatment of Cardio-Cerebral Diseases, School of Integrated Chinese and Western Medicine, Hunan University of Chinese Medicine, Changsha, China
- 5Department of Nephrology, The Central Hospital of Shaoyang, Shaoyang, China
- 6The First Hospital of Hunan University of Chinese Medicine, Changsha, China
- 7Fudan University, Shanghai, China
- 8Department of Rehabilitation Medicine, Guangzhou Panyu Central Hospital, Guangzhou, China
- 9Department of Rheumatology and Immunology, The First Affiliated Hospital of Anhui Medical University, Anhui, China
Objective: To evaluate efficacy and safety of iguratimod (IGU) in the treatment of rheumatic and autoimmune diseases.
Methods: Databases such as Pubmed, Embase, Sinomed were searched (as of July 2022) to collect randomized controlled trials (RCTs) of IGU in the treatment of rheumatic and autoimmune diseases. Two researchers independently screened the literature, extracted data, assessed the risk of bias of the included literature, and performed meta-analysis using RevMan 5.4 software.
Results: A total of 84 RCTs and 4 types of rheumatic and autoimmune diseases [rheumatoid arthritis (RA), ankylosing spondylitis (AS), primary Sjögren’s syndrome (PSS) and Autoimmune disease with interstitial pneumonia]. Forty-three RCTs reported RA and showed that IGU + MTX therapy can improve ACR20 (RR 1.45 [1.14, 1.84], p = 0.003), ACR50 (RR 1.80 [1.43, 2.26], p < 0.0000), ACR70 (RR 1.84 [1.27, 2.67], p = 0.001), DAS28 (WMD −1.11 [−1.69, −0.52], p = 0.0002), reduce ESR (WMD −11.05 [−14.58, −7.51], p < 0.00001), CRP (SMD −1.52 [−2.02, −1.02], p < 0.00001), RF (SMD −1.65 [−2.48, −0.82], p < 0.0001), and have a lower incidence of adverse events (RR 0.84 [0.78, 0.91], p < 0.00001) than the control group. Nine RCTs reported AS and showed that IGU can decrease the BASDAI score (SMD −1.62 [−2.20, −1.05], p < 0.00001), BASFI score (WMD −1.07 [−1.39, −0.75], p < 0.00001), VAS (WMD −2.01 [−2.83, −1.19], p < 0.00001), inflammation levels (decreasing ESR, CRP and TNF-α). Thirty-two RCTs reported PSS and showed that IGU can reduce the ESSPRI score (IGU + other therapy group: WMD −1.71 [−2.44, −0.98], p < 0.00001; IGU only group: WMD −2.10 [−2.40, −1.81], p < 0.00001) and ESSDAI score (IGU + other therapy group: WMD −1.62 [−2.30, −0.94], p < 0.00001; IGU only group: WMD −1.51 [−1.65, −1.37], p < 0.00001), inhibit the inflammation factors (reduce ESR, CRP and RF) and increase Schirmer’s test score (IGU + other therapy group: WMD 2.18 [1.76, 2.59], p < 0.00001; IGU only group: WMD 1.55 [0.35, 2.75], p = 0.01); The incidence of adverse events in IGU group was also lower than that in control group (IGU only group: RR 0.66 [0.48, 0.98], p = 0.01). Three RCTs reported Autoimmune disease with interstitial pneumonia and showed that IGU may improve lung function.
Conclusion: Based on current evidence, IGU may be a safe and effective therapy for RA, AS, PSS and autoimmune diseases with interstitial pneumonia.
Systematic Review Registration: (CRD42021289489).
1 Introduction
The pathogenesis of rheumatic immune diseases is complex, and it is an inflammatory disease that may lead to impaired immune system due to various reasons (involving the musculoskeletal system, joints and their surrounding soft tissues, etc.) (Konig, 2020; Adelowo et al., 2021). In recent years, the prevalence of rheumatic immune diseases has been on the rise (Hyrich and Machado, 2021), among which rheumatoid arthritis (RA), systemic lupus erythematosus (SLE) and ankylosing spondylitis (AS) are more common and have certain disability (Charoenngam, 2021) ]. Meanwhile, with the progression of the disease, most patients may develop complications such as kidney, iris, skin, heart and other organ damage (van der Woude and van der Helm-van Mil, 2018; Dai et al., 2021). Especially in active disease, there may be radioactive progression, and severe cases may lead to joint deformity and even loss of self-care function in life (Otón and Carmona, 2019). Therefore, rheumatic immune diseases with high disease activity will generate a great economic burden for both society and patients (Otón and Carmona, 2019). The current treatments for rheumatic diseases and autoimmune diseases are precision medicine based on drugs (Aletaha, 2020; Radu and Bungau, 2021), with the aim of controlling the progression of inflammation and reducing inflammatory damage (Winthrop, 2017; Aletaha and Smolen, 2018). It mainly includes traditional synthetic DMARDs, biologics DMARDs and synthetic targeted DMARDs (Goodman, 2015). Among them, biological DMARDs can be divided into two categories: biological agents (bDMARDs) and synthetic targeted (tsDMARDs) (Akram et al., 2021). bDMARDs include the tumor necrosis factor inhibitor class of adalimumab, infliximab, etanercept, and the IL-6 antagonist tocilizumab. tsDMARDs include the Janus kinase (JAK) inhibitor tofacitinib (Winthrop, 2017). Although the efficacy of the above drugs has been proven, their high prices make it impossible for patients in developing countries, including China, to benefit (Drosos et al., 2020). Studies have shown that patients in developed countries are also becoming increasingly prominent due to poor compliance and high recurrence rates related to medication problems (Tanaka, 2016; Ghabri et al., 2020). Traditional DMARDs are widely used in clinic because of their acceptable side effects and reasonable price. For example, methotrexate (MTX) is the most widely used DMARDs for the treatment of RA (Wang W. et al., 2018). Because of its effectiveness, acceptable side effects, and reasonable price, ACR recommends it as the first-choice drug in the initial treatment regimen for RA patients (Cronstein and Aune, 2020). However, there are still about 30%–40% of patients who are insensitive to MTX treatment, have poor treatment effect, or fail to benefit from it because of side effects (Cronstein and Aune, 2020). Strand et al. reported that the ACR50 of MTX in RA was 46%, and the ACR70 was 23% (Strand et al., 1999). According to multiple clinical trials, the combined use of DMARDs is one of the effective ways to improve the efficacy (Kremer et al., 2002; Ichikawa et al., 2005; Capell et al., 2007).
Iguratimod (IGU) is a new type of small molecule DMARDs developed in Japan. As an immunomodulator, through immunomodulation, it reduces immune response, inhibits collagenous arthritis, and relieves the destruction of bone and cartilage tissue (Li et al., 2013; Mizutani et al., 2021). IGU can also inhibit the activity of nuclear factors, thereby inhibiting the production of inflammatory cytokines, IL-1, IL-6, IL-8, and TNF, and inhibiting the production of immunoglobulins to exert anti-inflammatory, anti-immune, and anti-inflammatory effects. (Li et al., 2013; Xie S. et al., 2020). Several studies have shown that IGU has good efficacy in rheumatic diseases and autoimmune diseases, such as improving RA, AS, systemic lupus erythematosus, IG4-RD, pulmonary interstitial disease, primary Sjögren’s syndrome (PSS), etc. (Harjacek, 2021; Pu et al., 2021; Zeng et al., 2022a). In clinical practice, more and more rheumatologists use IGU to treat rheumatic and autoimmune diseases, but its efficacy and safety are still uncertain. Therefore, we collected randomized controlled trials (RCTs) of IGU in the treatment of rheumatic and autoimmune diseases in order to conduct a systematic review and meta-analysis of its efficacy and safety.
2 Materials and methods
2.1 Protocol
This systematic review and meta-analysis were conducted strictly in accordance with the protocol registered in PROSPERO (CRD42021289489) and PRISMA-guidelines (see Supplementary Materials) (Page et al., 2021).
2.2 Search criteria
2.2.1 Study design
All RCTs on IGU for rheumatic and autoimmune diseases were included. There are no restrictions on publication year, publication language, publication journal, etc.
2.2.2 Participants
Patients were diagnosed with any rheumatic and autoimmune diseases by accepted criteria.
2.2.3 Intervention methods
The experimental group was treated with IGU, which was administered orally. The course of treatment and the dose were not limited, and it could be combined or not combined with other therapies. The control group is therapy that does not contain IGU, including but not limited to placebo, conventional therapy, etc.
2.2.4 Outcomes
Outcomes are the disease activity indices (such as BASDAI and ACR20), inflammatory factor indicators (such as ESR, CRP, RF) and adverse events.
2.2.5 Exclusion criteria
1) Duplicate publications; 2) Unable to obtain full text or incomplete data; 3) Reviews, case reports, animal experiments, etc.,; 4) Retracted studies; 5) observational studies.
2.3 Search strategy
Pubmed, Wanfang Database, Web of Science, China National Knowledge Infrastructure (CNKI), Sinomed, VIP Database, Medline Complete, Embase were searched for literature on IGU for the treatment of rheumatic and autoimmune diseases. The retrieval time is from inception to 1 July 2022. We also searched ClinicalTrials.gov and Cochrane Library. The search strategy was shown in Supplementary Table S1.
2.4 Data collection and analysis
2.4.1 Literature screening and data extraction
Two researchers independently screened the title and abstract of the articles revealed from the search. Then, they screened the full text of the relevant articles based on search criteria. Finally, the two researchers reconciled the results and negotiated inconsistencies through discussions with all researchers (Deeks et al., 2020a). Then two researchers independently extracted the basic information, medication regimen, course of treatment, and outcome indicators of eligible RCTs. For inconsistencies, the solution is the same as before.
2.4.2 Quality assessments
The risk of bias assessment of the included trials was independently performed by two investigators. The Cochrane Collaboration’s tool was used for assessing risk of bias (Deeks et al., 2020b). The content of the evaluation mainly includes: 1) Whether the method of random allocation is described; 2) Whether the allocation concealment is sufficient; 3) Whether the blind method is used; 4) Whether the withdrawal from the experiment and the loss to follow-up are completely described; 5) Whether the outcome indicators are selectively reported; 6) Whether there are other factors that may affect the quality of the trial. According to the Cochrane Handbook, the above items were judged as “Yes” (low risk of bias), “No” (high risk of bias), and “Unclear” (unclear risk of bias) (Deeks et al., 2020b).
2.5 Statistical analysis
Revman 5.4 software were utilized for meta-analysis (Deeks et al., 2020c). For dichotomous variables data, use the risk ratio (RR). For continuous variables data, when the results of different experiments are expressed in the same unit of measurement, the weighted mean difference (WMD) is used; when the results of the experiments are expressed in different units of measurement, the standard mean difference (SMD) is used. Effect sizes were expressed as 95% confidence intervals (CI). To analyze the heterogeneity between results, the chi-square test was employed. If heterogeneity was deemed small (p > 0.1, I2<50%), the fixed-effects model was utilized for analysis. Otherwise, the random-effects model was used. STATA 15 was used to detect publication bias with the Egger method (for continuous variables) and Harbord methods (for dichotomous variables) for outcomes with RCTs ≥4. p > 0.1 is considered indicative of no publication bias. The level of evidence of efficacy indicators (such as ACR and BASFI) and adverse events was evaluated by the GRADE tool (GRADEpro, 2015), following the GRADE handbook (Schünemann et al., 2013).
3 Results
3.1 Literature search results
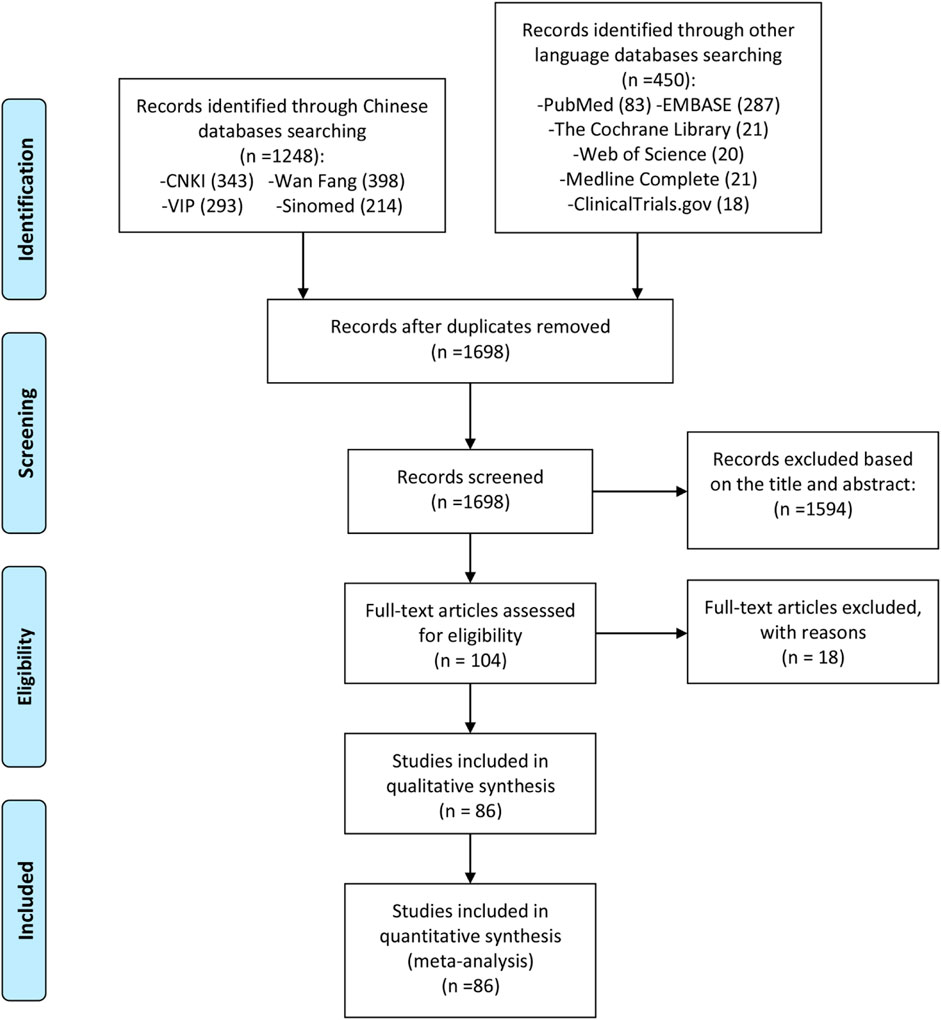
A total of 1,698 preliminary related literature were detected in this study, and a total of 1,594 literature that did not conform to the research type and content were excluded. After the primary screening, 104 records were obtained. According to the inclusion and exclusion criteria and the completeness of the literature information, 18 records were excluded from the second screening after reading the full text (GuifengLi, 2014; He et al., 2015; Okamura et al., 2015; Meng et al., 2016a; Lin, 2016; Yoshioka et al., 2016; Zhu et al., 2016; Wang, 2017; Wang et al., 2017; Luo Y. et al., 2018; Wang X. et al., 2018; Huang and Ma, 2018; Luo et al., 2019; Shang et al., 2019; Suto et al., 2019; Gu et al., 2020; ManXie, 2020; Xu et al., 2021), and 86 records [(GuifengLi, 2014; Lü et al., 2008; Tian and Tao, 2017; Qi et al., 2019; Hara et al., 2014; Ishiguro et al., 2013; Li L. et al., 2019; Hu, 2014; Xia et al., 2020; Xia et al., 2016; Lu, 2014; Zhao et al., 2016; Shi et al., 2015; Meng et al., 2015; Bi, 2019; Zhang, 2018; Li et al., 2016; Mo et al., 2018; Duan et al., 2015; Xiong and GengGuanghui, 2020; Shang, 2014; Mo and Ma, 2015; Tian et al., 2020; Xu B. et al., 2015; Xu LM. et al., 2017; Yan and Wang, 2018; Fan et al., 2020; Meng et al., 2016b; Wang L. et al., 2019; Meng et al., 2017; Ju et al., 2020; Zhao and Hao, 2018; Li and WH, 2020; Xu YM. et al., 2015; Chen et al., 2018; Zhao et al., 2017a; Deng, 2017; Xie et al., 2018; Rao et al., 2014; Wang et al., 2022; Dai et al., 2022; Sun and Li, 2022; Wu et al., 2022; Dong Zhang et al., 2019; Qiu et al., 2016; Yuan et al., 2020; Pang et al., 2020; Lin et al., 2019; Xu et al., 2019; Zeng et al., 2016; Li Y. et al., 2021; Bai et al., 2021; Li X. et al., 2021; Gu, 2020; Jiang et al., 2014; Zhao, 2019; Lu and Zhang, 2021; Li et al., 2020; Zhang, 2019; Jia, 2020; Yu, 2020; Shao et al., 2020; Chen et al., 2022; Donghui, 2019; Zhang and Shen, 2019; Jiang et al., 2016; Xie H. et al., 2020; Jiang et al., 2020; Bai and Jiao, 2019; Rao et al., 2022; Ding et al., 2022; Xu D. et al., 2017; Zhang et al., 2019; Luo Q. et al., 2018; Wang Y. et al., 2019; Zhao, 2020; Liang et al., 2021; Li et al., 2018; Jiang, 2021; Yi, 2018; Zhuang, 2020; Xia et al., 2017; Gu, 2022; Liu, 2022; Zhuang et al., 2021; Du et al., 2008; Lu et al., 2009) were finally included in the quantitative and qualitative analysis of the review. The literature screening process and results are shown in Figure 1.
3.2 Description of included trials
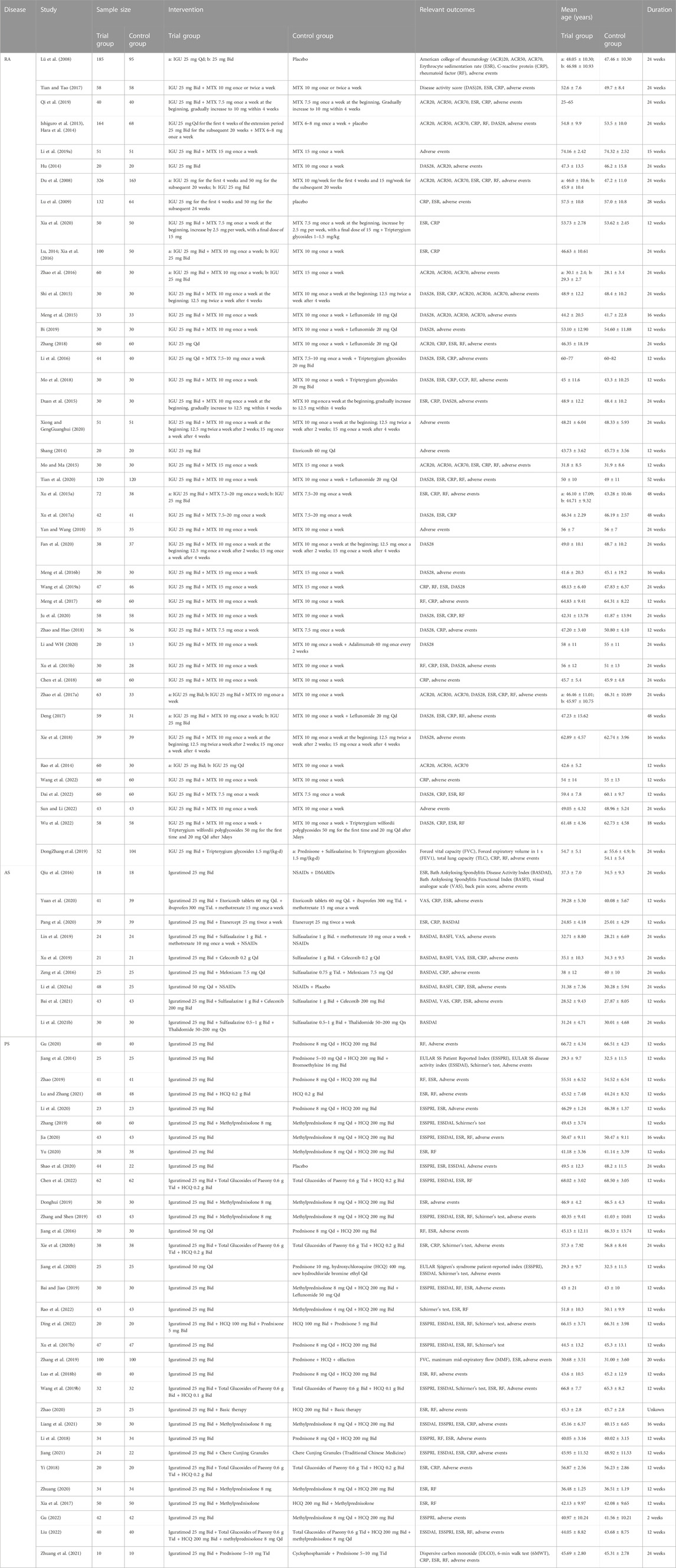
Two records (Ishiguro et al., 2013; Hara et al., 2014) came from the same RCT and were therefore recorded as Hara et al., 2014 (Ishiguro et al., 2013; Hara et al., 2014). Two records (Lu, 2014; Xia et al., 2016) came from the same RCT and were therefore recorded as Lu (2014); Xia et al. (2016). Therefore, 86 records actually involve 84 RCTs. In some RCTs, there were 2 experimental groups, and to match them, the control group was split into 2 equal parts with half the population each, and labeled as groups a and b (e.g., Xu et al., 2015a and Xu YM. et al., 2015). The included RCTs involved 4 rheumatic and autoimmune diseases (RA, AS, PSS and Autoimmune disease with interstitial pneumonia). The details of study characteristics are presented in Table 1.
3.3 Risk of bias assessments
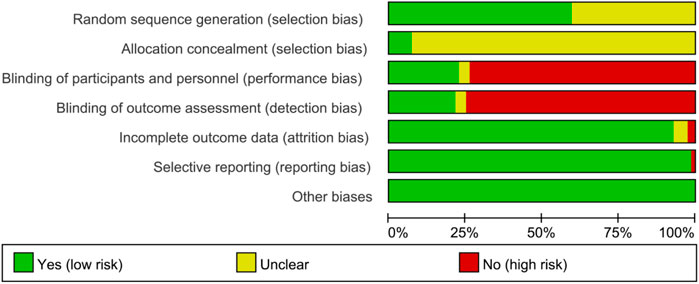
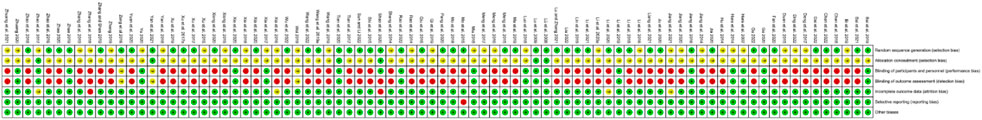
The summary and graph of risk of bias ware shown in Figures 2, 3.
3.3.1 Sequence generation and allocation concealment
Fifty RCTs described detailed random sequence generation methods and were therefore assessed as low risk of bias, whereas the remainder were assessed as unclear risk of bias. Lü et al. (2008), Du et al. (2008), Tian et al. (2020), Zhao et al. (2017a), Li Y. et al., 2021) and Shao et al. (2020) described methods of allocation concealment and was therefore assessed as low risk of bias, whereas the remainder were assessed as unclear risk of bias.
3.3.2 Blinding
Zeng et al. (2016), Li Y. et al. (2021), and Donghui (2019) reported the use of blinding in their RCTs, but did not provide sufficient details about the implementation process, resulting in an unclear risk of bias assessment. Of the total 84 RCTs, 19 reported blinding of participants, and 18 reported blinding of assessors, indicating a low risk of bias. The remaining RCTs were assessed as high risk of bias because blinding was not described and outcomes included subjectively assessed outcomes.
3.3.3 Incomplete outcome data and selective reporting
Zhang (2018) and SShao et al. (2020) had incomplete outcomes and were therefore assessed as high risk of bias. There was not enough evidence to prove whether there were incomplete outcomes in Lu (2014), Xia et al. (2016), Li and WH (2020), Zhao et al. (2017a) and Jiang (2021), so they were assessed as unknown risk of bias. The remaining RCTs did not have incomplete outcomes and were therefore assessed as low risk of bias.
Mo et al. (2018) did not report all data planned in the methodology and was therefore assessed as high risk of bias. The remaining RCTs did not have selective reports and were therefore assessed as low risk of bias.
3.3.4 Other potential bias
No other sources of bias were identified in any of the RCTs, indicating a low risk of bias from other sources.
3.4 IGU for RA
3.4.1 RA remission rate
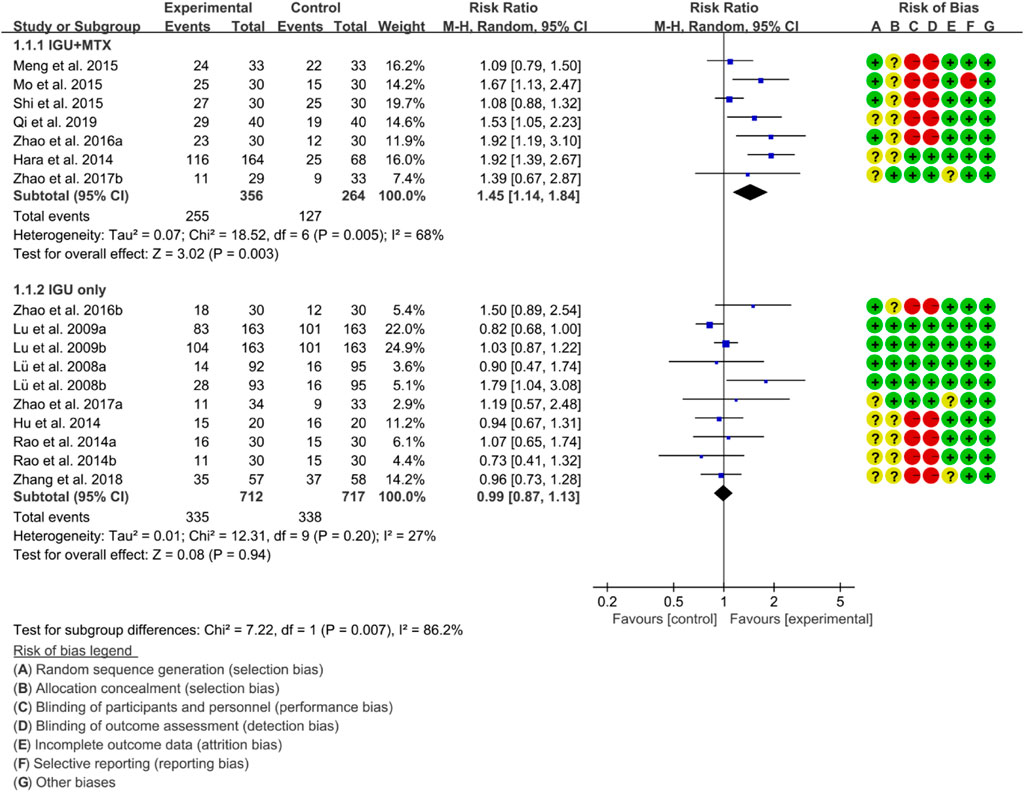
ACR20, ACR50 and ACR70 were used to represent RA remission rate. According to the medication of the IGU group, it is divided into IGU + MTX subgroup and IGU only subgroup.
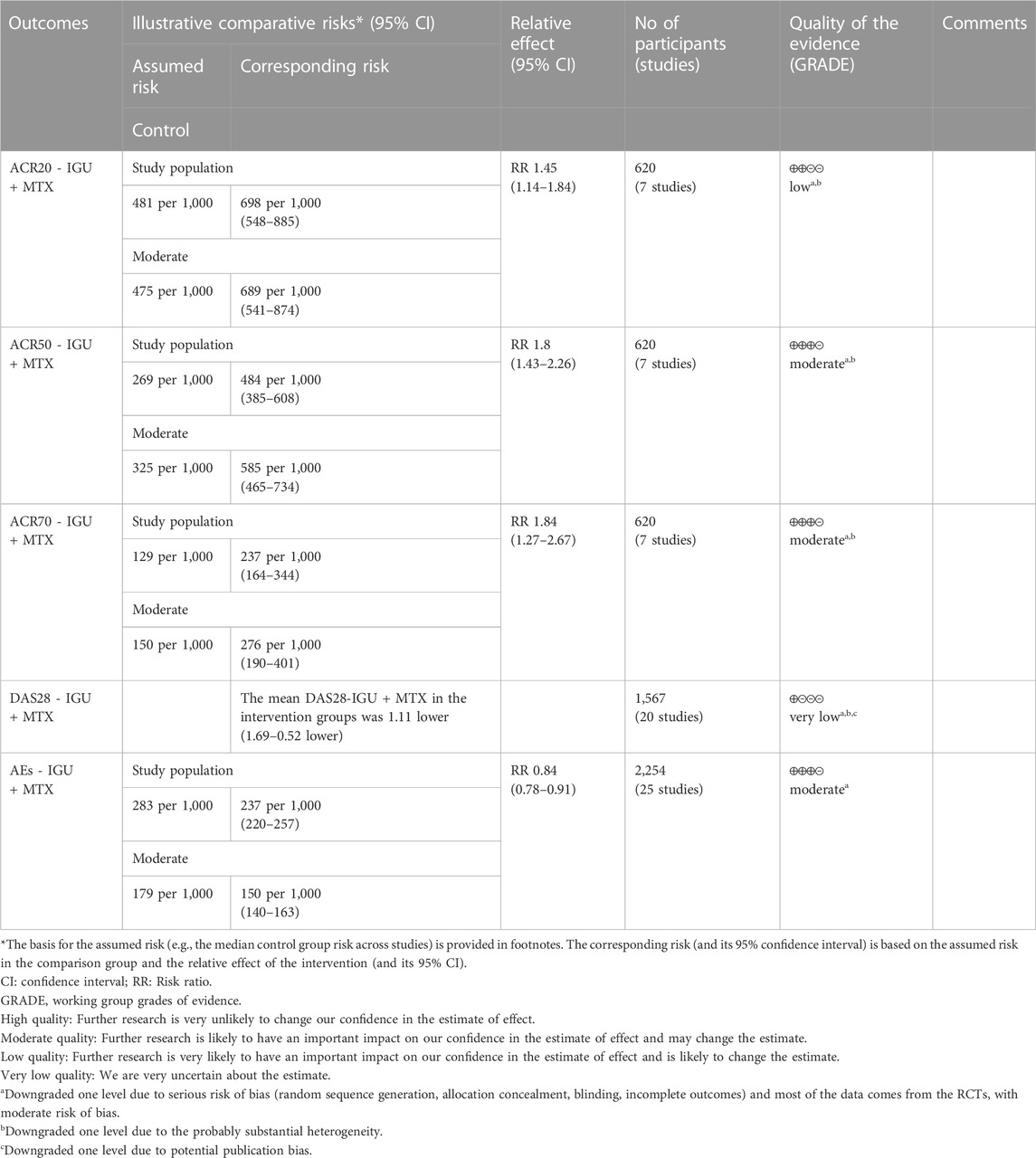
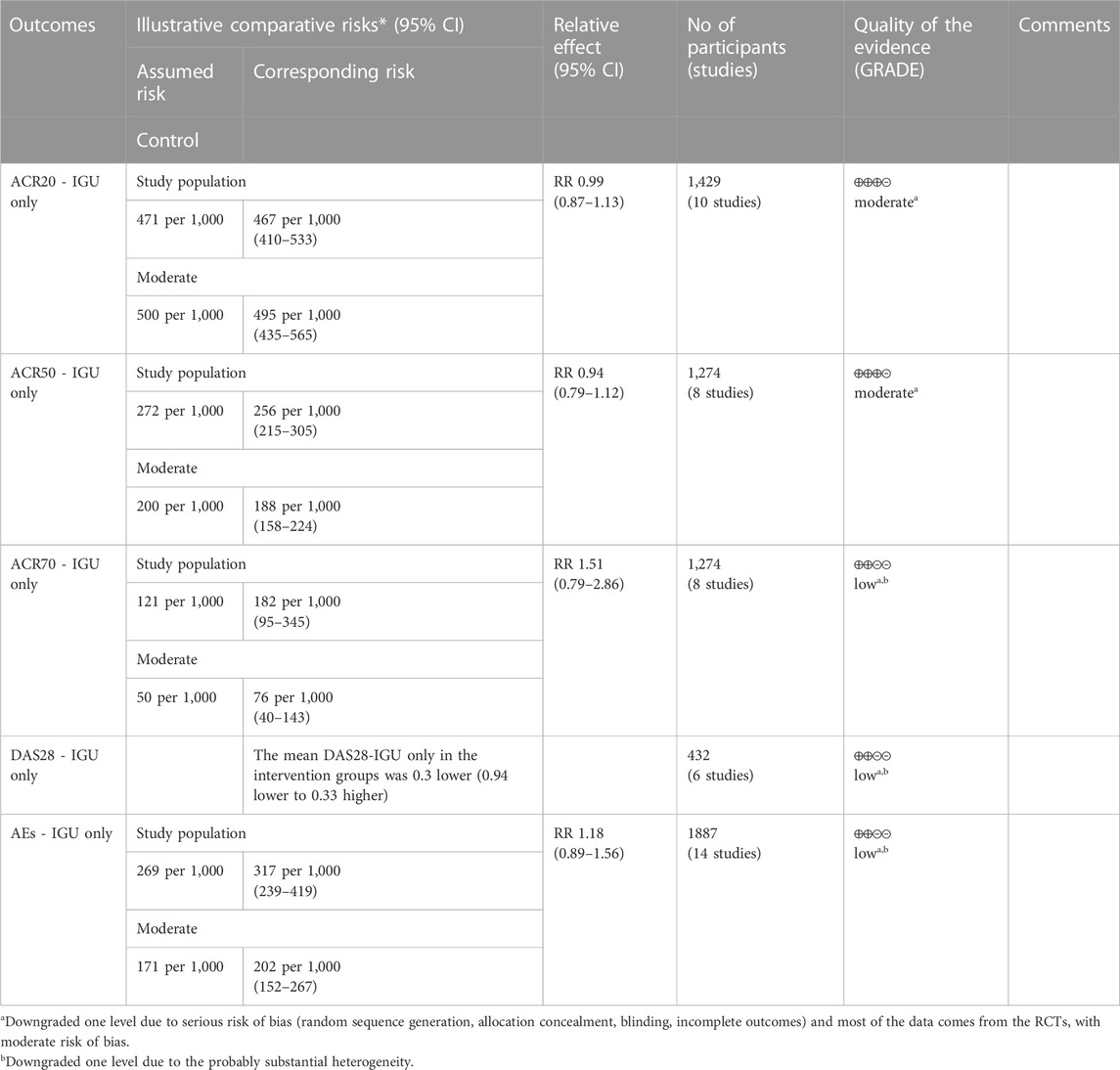
For ACR20, the heterogeneity test showed that some subgroups had high heterogeneity (IGU + MTX subgroup: p = 0.005, I2 = 68%; IGU only subgroup: p = 0.20, I2 = 27%), and a random effect model was used. The meta-analysis findings indicate that the IGU + MTX group had a significantly lower ACR20 compared to the control group (RR 1.45 [1.14, 1.84], p = 0.003; random-effect model). However, there was no significant difference in ACR20 between the IGU-only group and the control group (RR 0.99 [0.87, 1.13], p = 0.94; random-effect model) (Figure 4). The results of publication bias test showed that it was less likely to have publication bias in IGU + MTX subgroup (p = 0.313) and IGU only subgroup (p = 0.396).
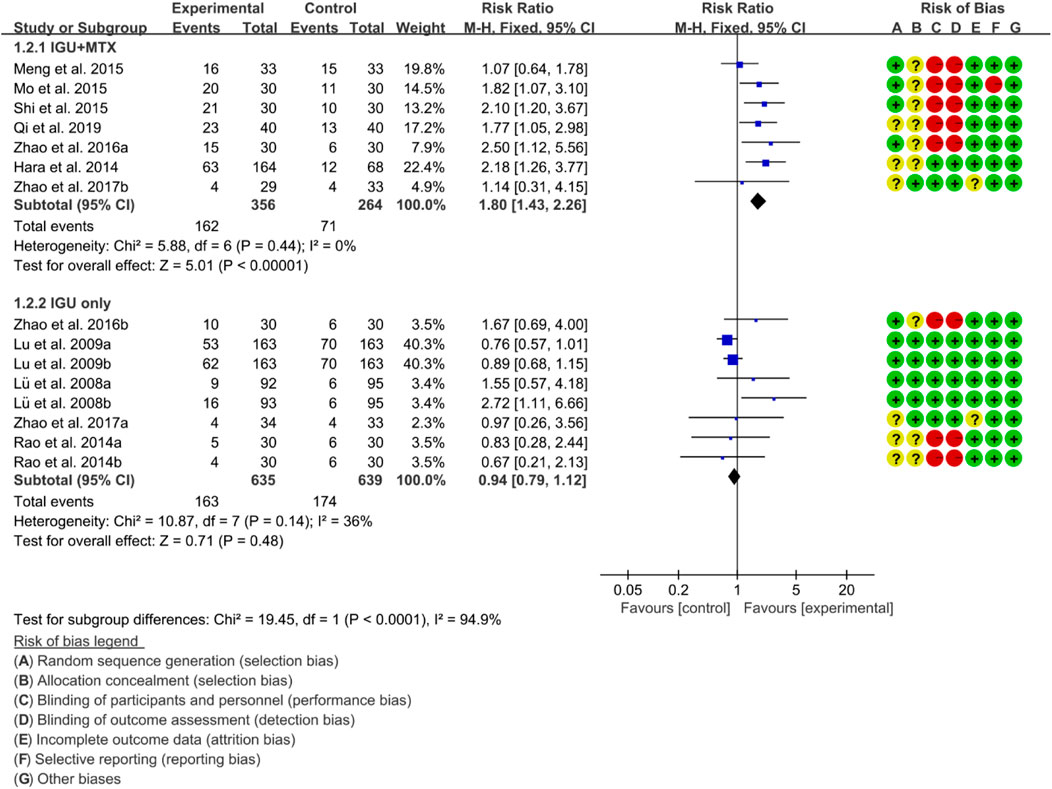
For ACR50, the heterogeneity test showed that the heterogeneity was low (IGU + MTX subgroup: p = 0.44, I2 = 0%; IGU only subgroup: p = 0.14, I2 = 36%), and a fixed effect model was used. The meta-analysis findings indicate that the IGU + MTX group had a lower ACR50 compared to the control group (RR 1.80 [1.43, 2.26], p < 0.00001; fixed-effect model). However, there was no significant difference between the IGU only group and the control group (RR 0.94 [0.79, 1.12], p = 0.48; fixed-effect model) (Figure 5). The results of publication bias test showed that it was less likely to have publication bias in IGU + MTX subgroup (p = 0.433) and IGU only subgroup (p = 0.245).
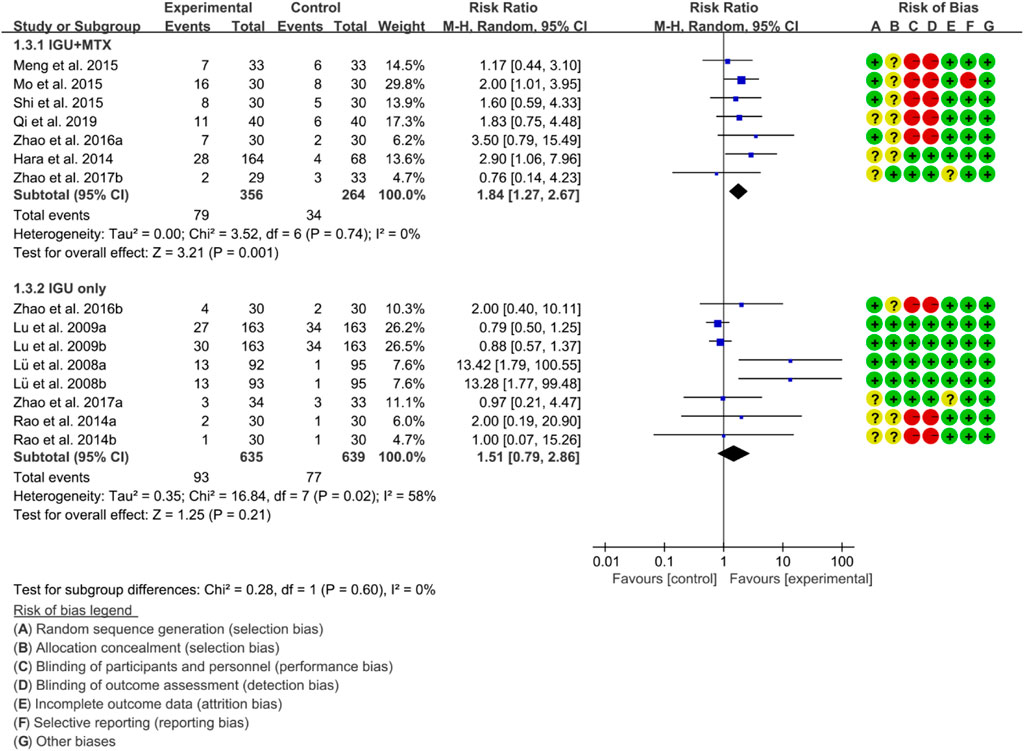
For ACR70, the heterogeneity test showed that some subgroups had high heterogeneity (IGU + MTX subgroup: p = 0.74, I2 = 0%; IGU only subgroup: p = 0.02, I2 = 58%), and a random effect model was used. The findings of the meta-analysis indicate that the IGU + MTX group had a lower ACR70 than the control group (RR 1.84 [1.27, 2.67], p = 0.001; random effect model), while the difference between the IGU only group and the control group did not reach statistical significance (RR 1.51 [0.79, 2.86], p = 0.21; random effect model) (Figure 6). The results of publication bias test showed that it was less likely to have publication bias in IGU + MTX subgroup (p = 0.193) and IGU only subgroup (p = 0.230).
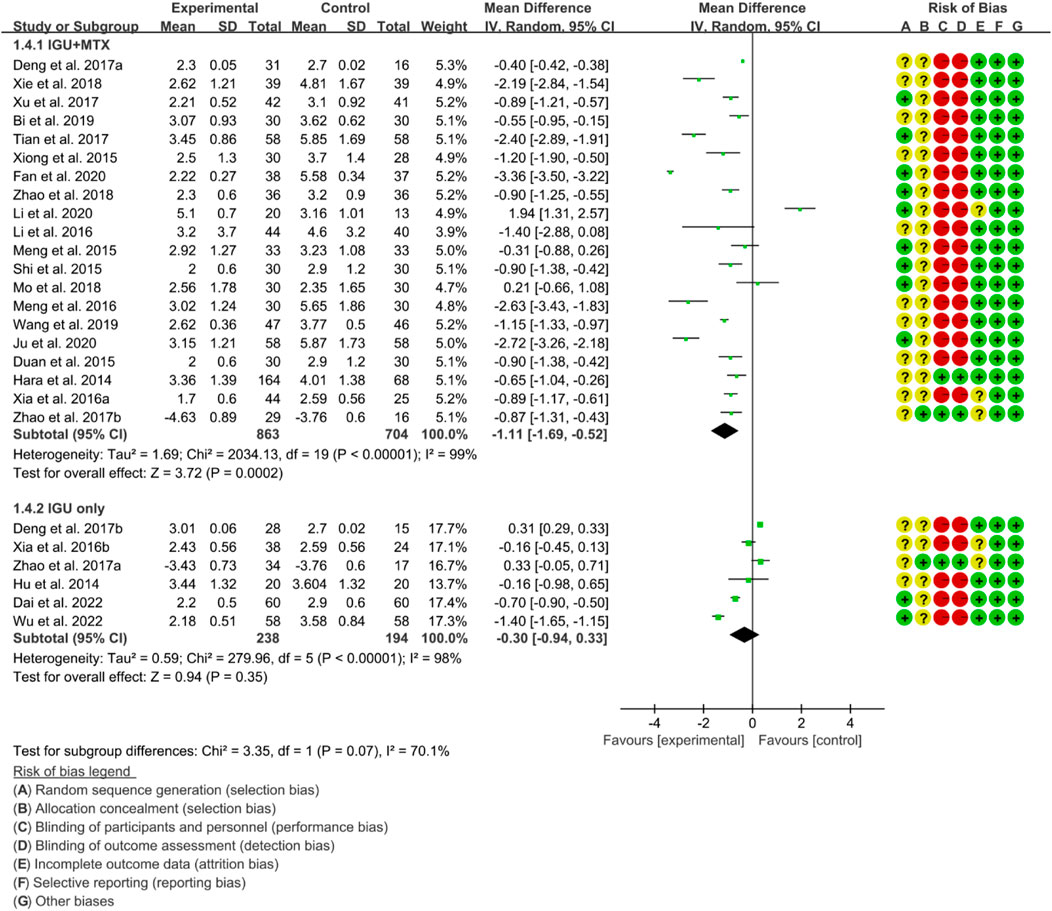
3.4.2 DAS28
According to the medication of the IGU group, it is divided into IGU + MTX subgroup and IGU only subgroup. The heterogeneity test showed that the heterogeneity was high (IGU + MTX subgroup: p < 0.00001, I2 = 99%; IGU only subgroup: p < 0.00001, I2 = 98%), and a random effect model was used. According to the meta-analysis results, the IGU + MTX group showed a significant decrease in DAS28 compared to the control group (WMD −1.11 [−1.69, −0.52], p = 0.0002; random effect model). However, the difference between the IGU only group and control group was not statistically significant (WMD −0.30 [−0.94, 0.33], p = 0.35; random effect model) (Figure 7). The results of publication bias test showed that it may be likely to have publication bias in IGU + MTX subgroup (p = 0.080); but was less likely in and IGU only subgroup (p = 0.122).
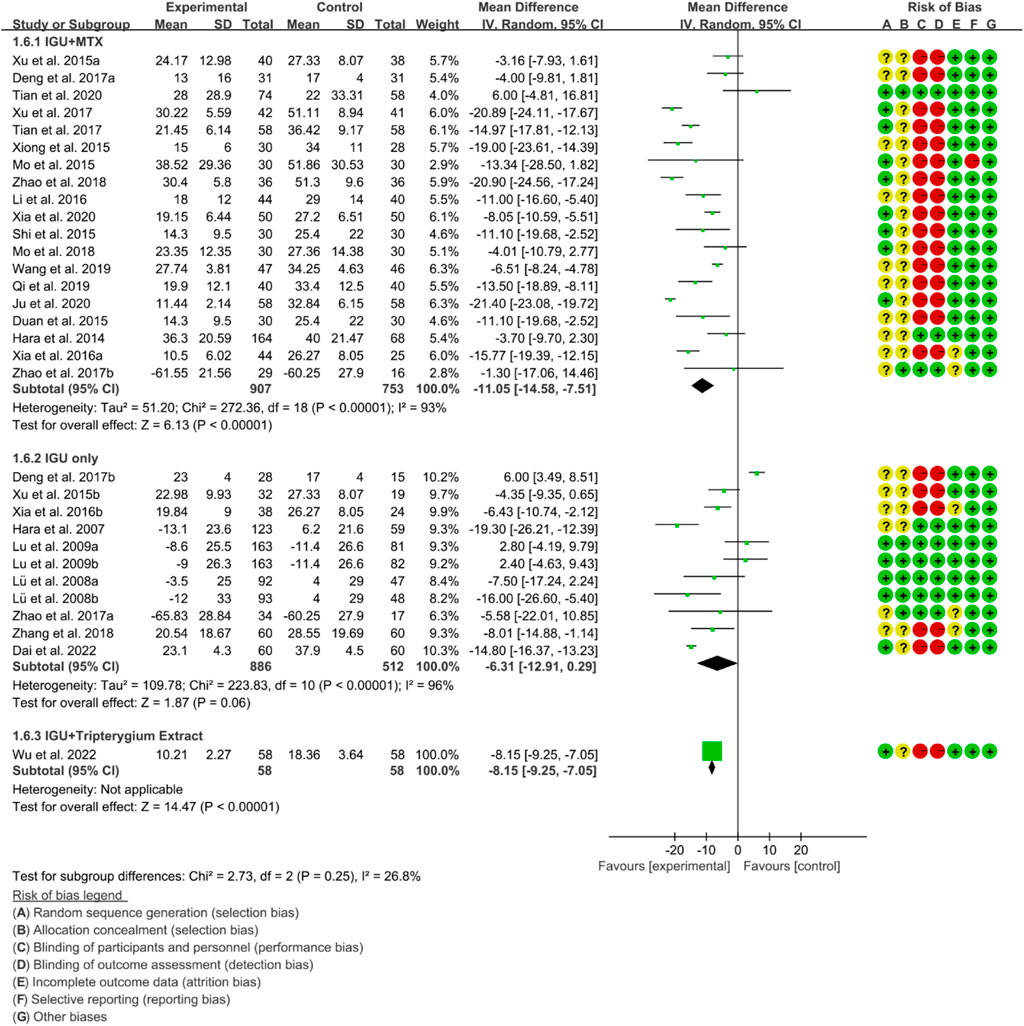
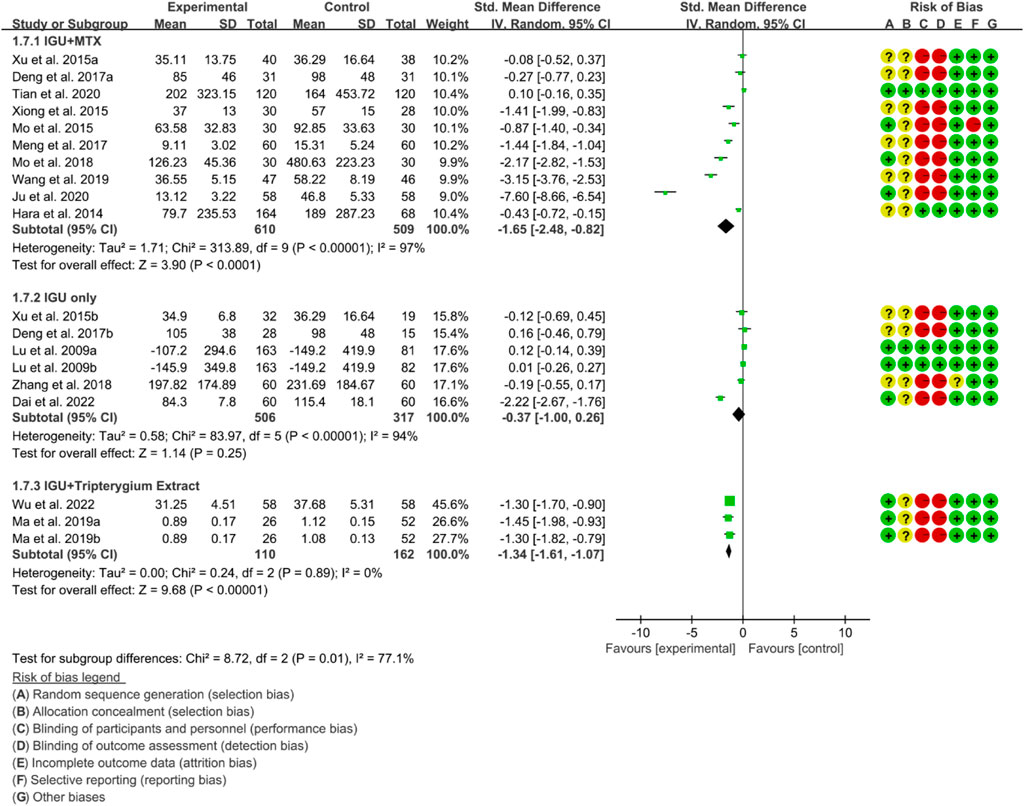
3.4.3 Inflammatory factor
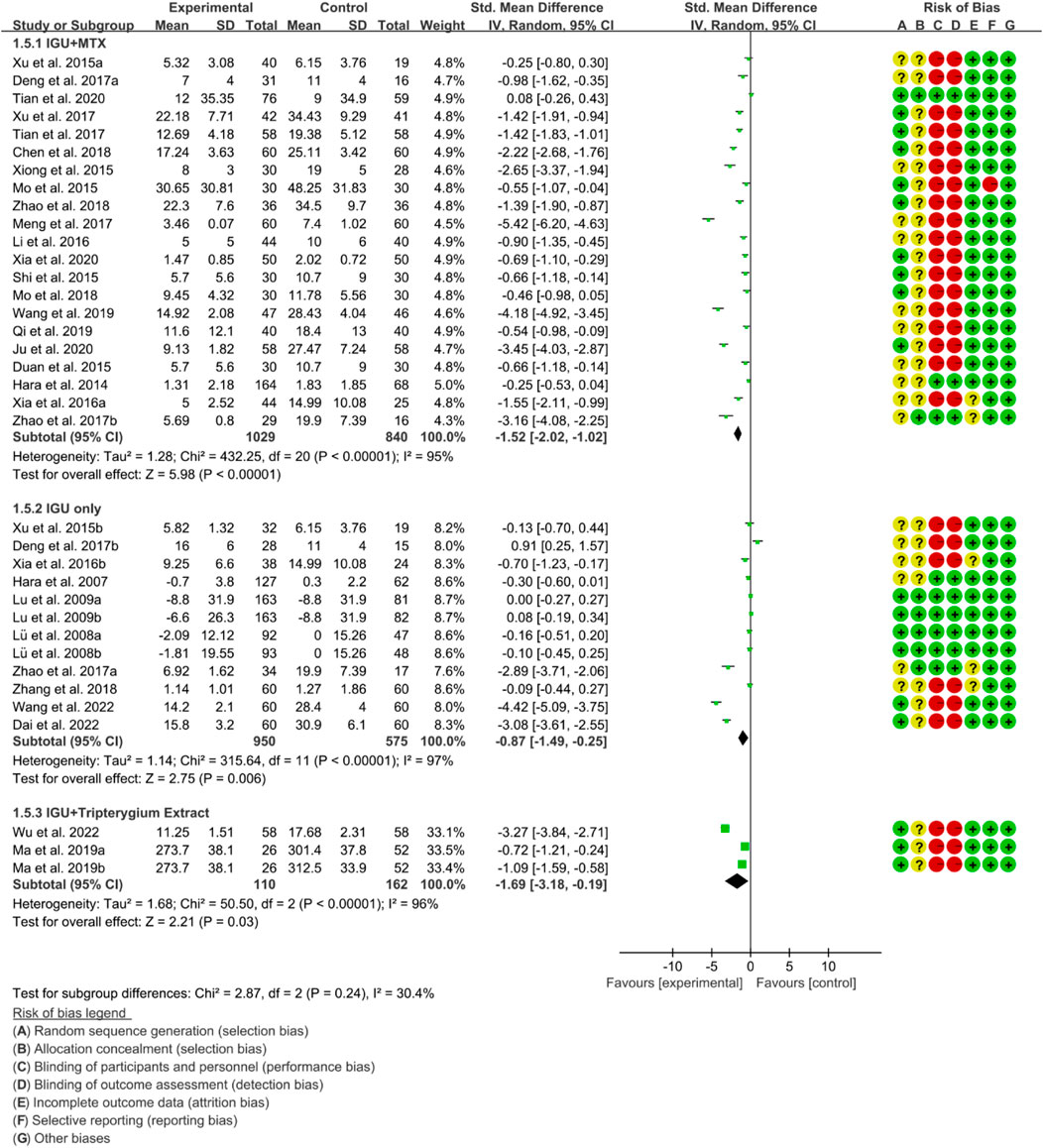
Inflammatory factors include CRP, ESR and RF. According to the medication of the IGU group, it is divided into IGU + MTX subgroup, IGU only subgroup and IGU + Tripterygium Extract subgroup.
For CRP, the heterogeneity test showed that the heterogeneity was high (IGU + MTX subgroup: p < 0.00001, I2 = 95%; IGU only subgroup: p < 0.00001, I2 = 96%; IGU + Tripterygium Extract subgroup: p < 0.00001, I2 = 96%), and a random effect model was used. The meta-analysis results show that compared with the control group, the CRP in the IGU + MTX group, IGU only subgroup and IGU + Tripterygium Extract subgroup was lower (Figure 8).
For ESR, the heterogeneity test showed that the heterogeneity was high (IGU + MTX subgroup: p < 0.00001, I2 = 93%; IGU only subgroup: p < 0.00001, I2 = 96%; IGU + Tripterygium Extract subgroup: p < 0.00001, I2 = 96%), and a random effect model was used. The meta-analysis results show that compared with the control group, the ESR in the IGU + MTX group (WMD −11.05 [−14.58, −7.51], p < 0.00001; random effect model) and IGU + Tripterygium Extract group was lower (WMD −8.15 [−9.25, −7.05], p < 0.00001; random effect model), while its difference between IGU only group and control group was of no statistical significance (WMD −6.31 [−12.91, 0.29], p = 0.06; random effect model) (Figure 9).
For RF, the heterogeneity test showed that the heterogeneity was high (IGU + MTX subgroup: p < 0.00001, I2 = 97%; IGU only subgroup: p < 0.00001, I2 = 94%; IGU + Tripterygium Extract subgroup: p = 0.89, I2 = 0%), and a random effect model was used. The meta-analysis results indicate that compared with the control group, the RF in the IGU + MTX group (SMD −1.65 [−2.48, −0.82], p < 0.0001; random effect model) and IGU + Tripterygium Extract group were significantly lower (SMD −1.34 [−1.61, −1.07], p < 0.00001; random effect model). However, there was no significant difference between the IGU only group and control group (SMD −0.37 [−1.00, 0.26], p = 0.25; random effect model) (Figure 10).
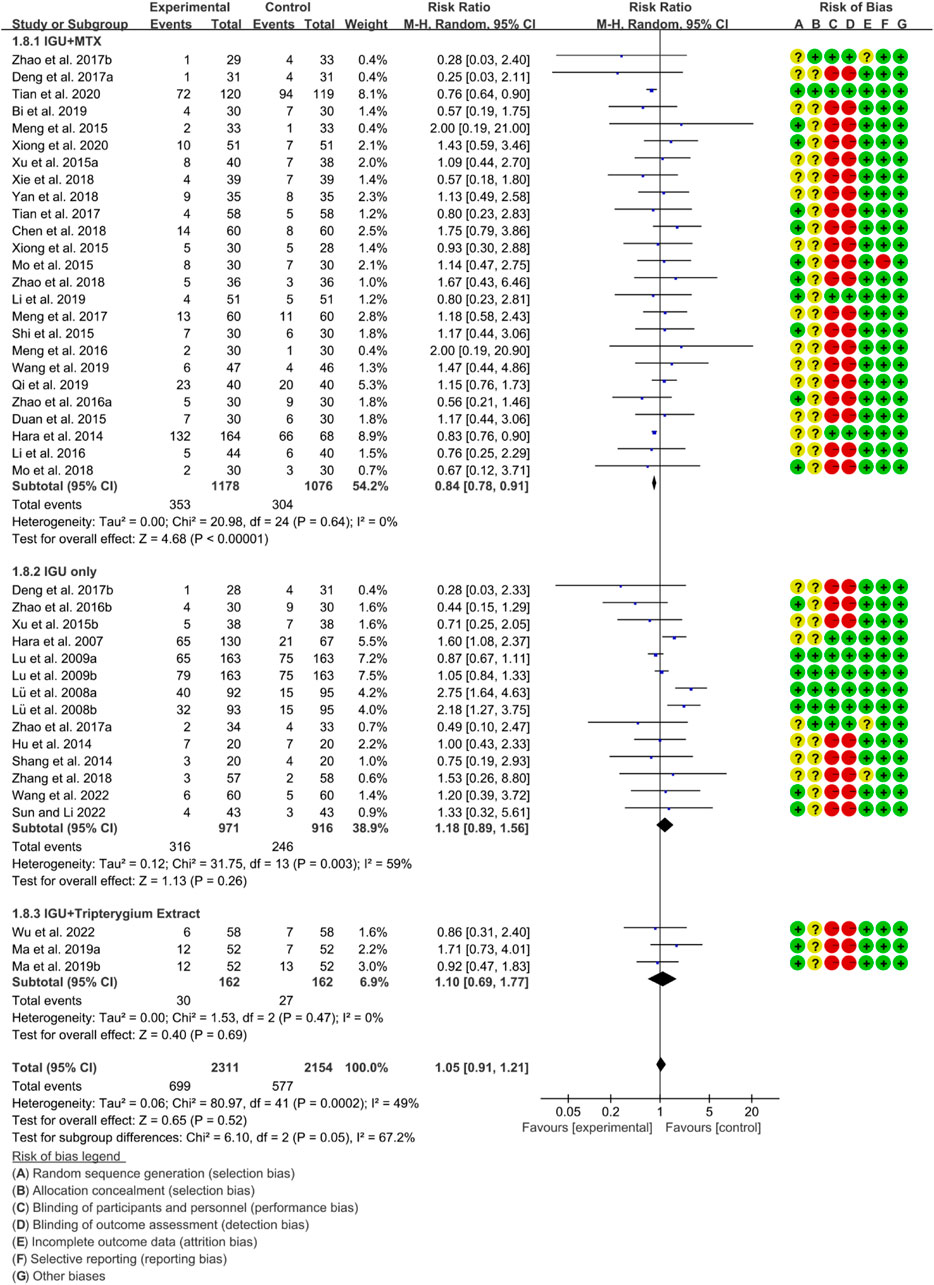
3.4.4 Adverse events
According to the medication of the IGU group, it is divided into IGU + MTX subgroup, IGU only subgroup and IGU + Tripterygium Extract subgroup. The heterogeneity test showed that the heterogeneity was high (IGU + MTX subgroup: p = 0.64, I2 = 0%; IGU only subgroup: p = 0.003, I2 = 59%; IGU + Tripterygium Extract subgroup: p = 0.47, I2 = 0%), and a random effect model was used. The meta-analysis results show that compared with the control group, the adverse events in the IGU + MTX group was lower (RR 0.84 [0.78, 0.91], p < 0.00001; random effect model), while its difference between IGU only group and control group (RR 1.18 [0.89, 1.56], p = 0.26; random effect model), and between IGU + Tripterygium Extract and control group was of no statistical significance (RR 1.10 [0.69, 1.77], p = 0.69; random effect model) (Figure 11). The results of publication bias test showed that it was less likely to have publication bias in IGU + MTX subgroup (p = 0.443) and in IGU only subgroup (p = 0.474).
3.4.5 Quality of evidence
Only IGU + MTX and IGU only subgroups met the requirements of publication bias detection and evidence quality assessments.
According to the GRADE handbook, the evidence of IGU + MTX subgroup was judged to be moderate to very low (Table 2). The evidence of IGU only subgroup was judged to be moderate to low (Table 3).
3.5 IGU for AS
3.5.1 BASDAI
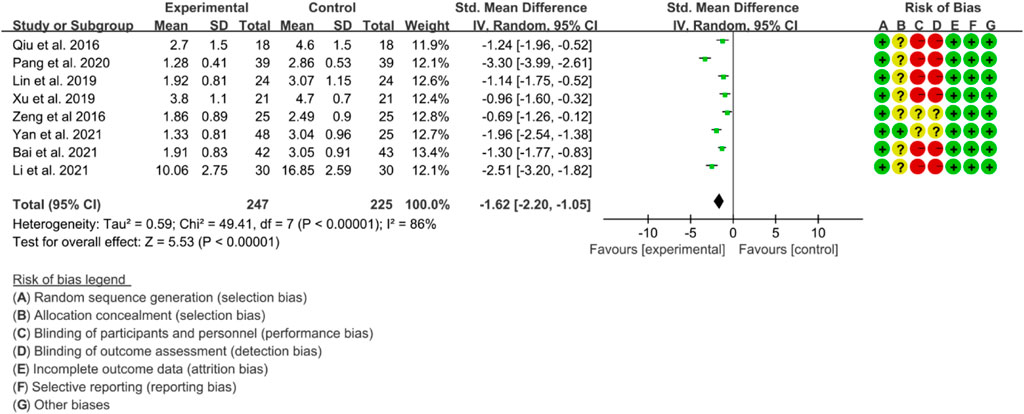
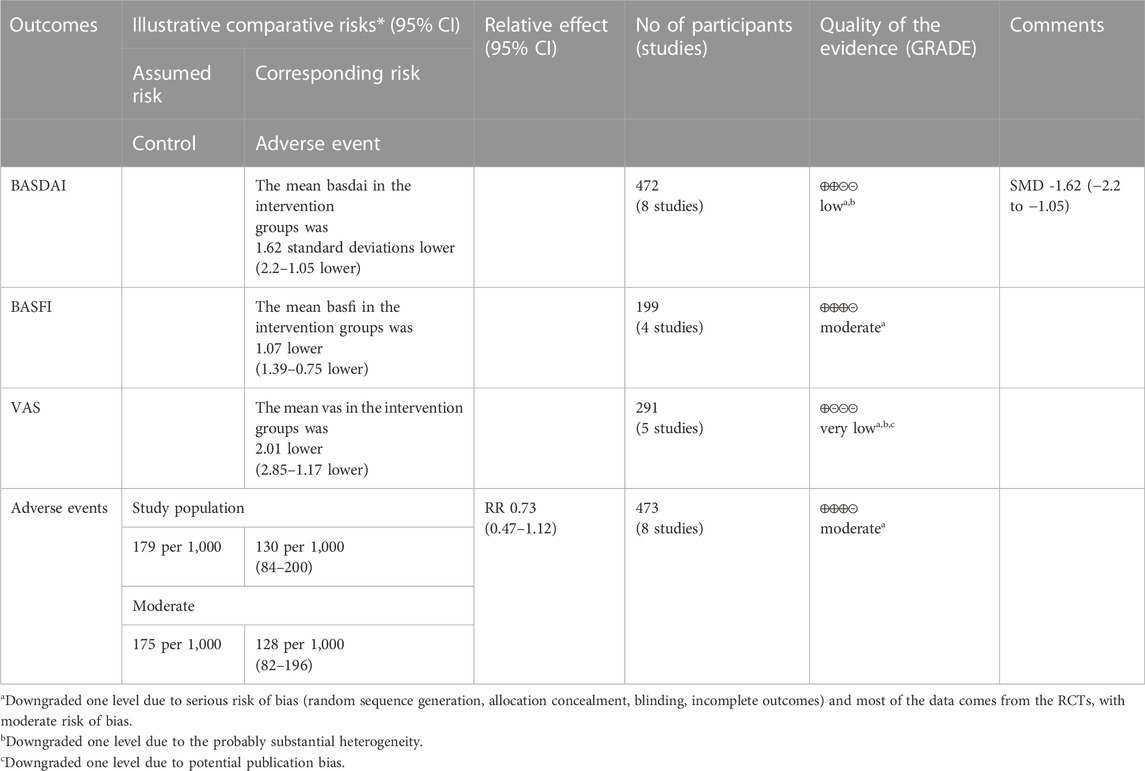
Eight RCTs used BASDAI as an assessment tool to evaluate the effectiveness of IGU in improving AS. The included studies showed high heterogeneity, with p < 0.00001 and I2 = 86%, and thus a random effects model was used for analysis. The meta-analysis results showed that the IGU group had a significantly lower BASDAI score compared to the control group (SMD −1.62 [−2.20, −1.05], p < 0.00001; random effect model) (Figure 12). The results of publication bias test showed that it was less likely to have publication bias (p = 0.302).
3.5.2 BASFI
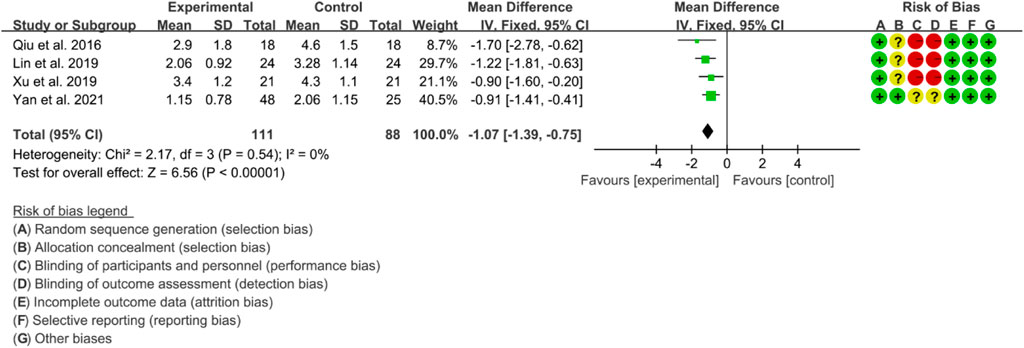
Four RCTs were included in the meta-analysis, all of whom were assessed using BASFI to evaluate the improvement of AS. The heterogeneity test showed low heterogeneity, with p = 0.54 and I2 = 0%, indicating that a fixed effects model was appropriate for analysis. The results of the meta-analysis indicated that the IGU group had a significantly lower BASFI score compared to the control group (WMD −1.07 [−1.39, −0.75], p < 0.00001; fixed effect model) (Figure 13). The results of publication bias test showed that it was less likely to have publication bias (p = 0.254).
3.5.3 VAS
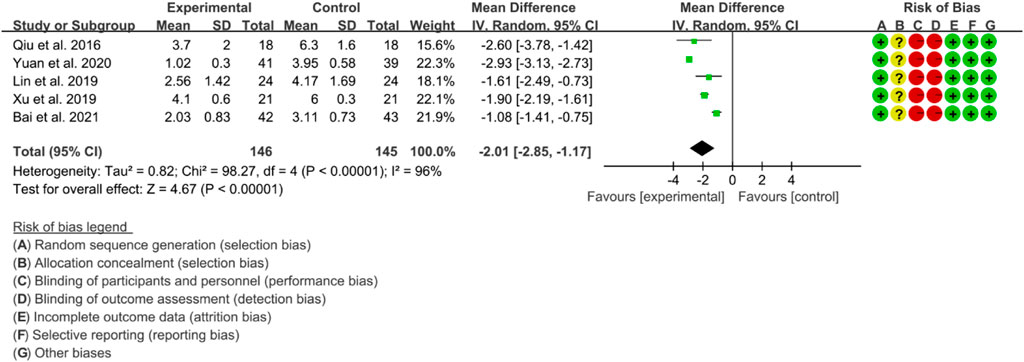
Four RCTs were used to evaluate the effect of IGU on the improvement of AS through VAS, with a total of 137 patients in the IGU group and 135 patients in the control group. The heterogeneity test showed significant heterogeneity with p < 0.00001 and I2 = 95%, indicating the use of a random effects model for analysis. The meta-analysis results indicated a significant reduction in the VAS score for the IGU group compared to the control group (WMD −2.01 [−2.83, −1.19], p < 0.00001; random effects model) (Figure 14). The results of publication bias test showed that it may be likely to have publication bias (p = 0.071).
3.5.4 Inflammatory factor
3.5.4.1 Inflammatory factors include ESR, CRP and TNF-α.
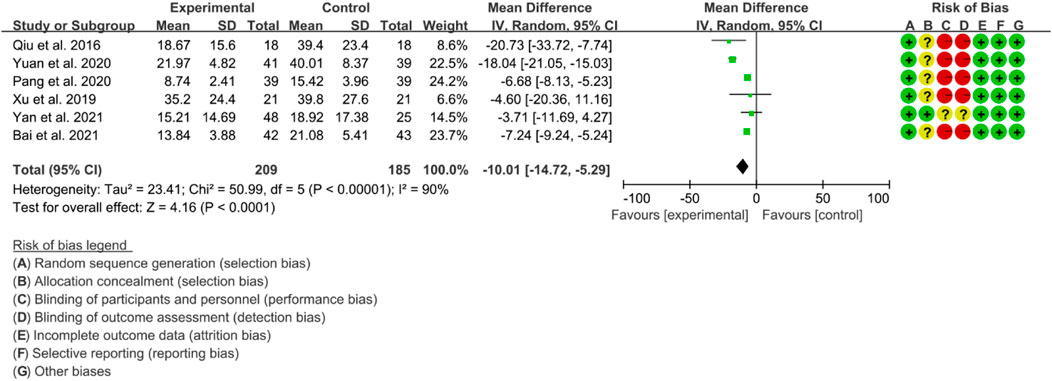
Six RCTs were included in the meta-analysis to evaluate the improvement of AS using ESR. High heterogeneity was observed (p < 0.00001, I2 = 90%), and therefore, a random effects model was used for the analysis. The results of the meta-analysis showed that the IGU group had a significantly lower ESR compared to the control group (WMD −10.01 [−14.72, −5.29], p < 0.0001; random effect model) (Figure 15).
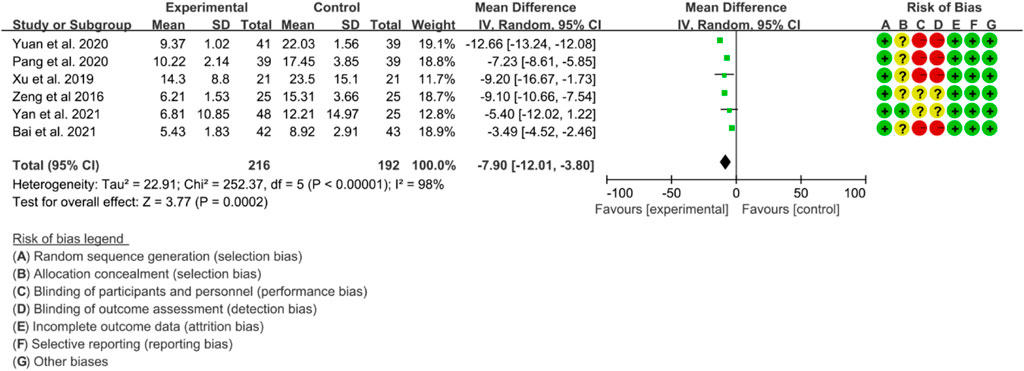
Six RCTs were included in the analysis of CRP to evaluate the improvement of AS. The heterogeneity test indicated high heterogeneity (p < 0.00001, I2 = 98%), thus a random effects model was utilized for the analysis. The results of the meta-analysis demonstrated that IGU significantly decreased CRP levels compared to the control group (WMD −7.90 [−12.01, −3.80], p < 0.00001; random effect model) (Figure 16).
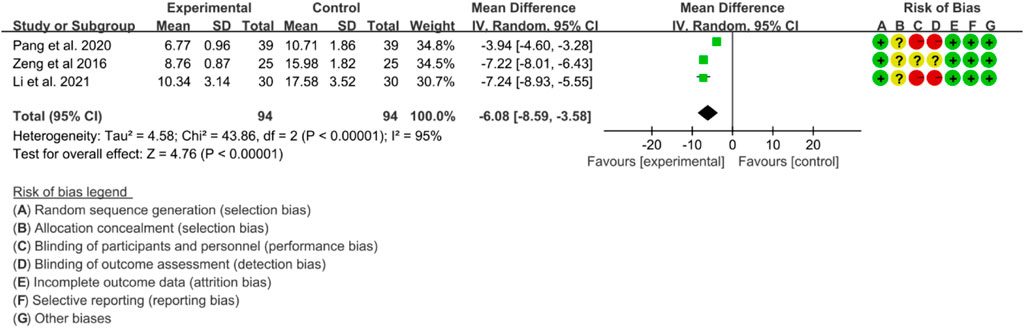
Three RCTs evaluated the effects of IGU on TNF-α levels in the treatment of AS. Significant heterogeneity was detected by the heterogeneity test (p < 0.00001, I2 = 95%), and a random effects model was applied for analysis. The results of the meta-analysis indicated that TNF-α levels were significantly lower in the IGU group compared to the control group (WMD -6.08 [-8.59, −3.58], p < 0.00001; random effects model) (Figure 17).
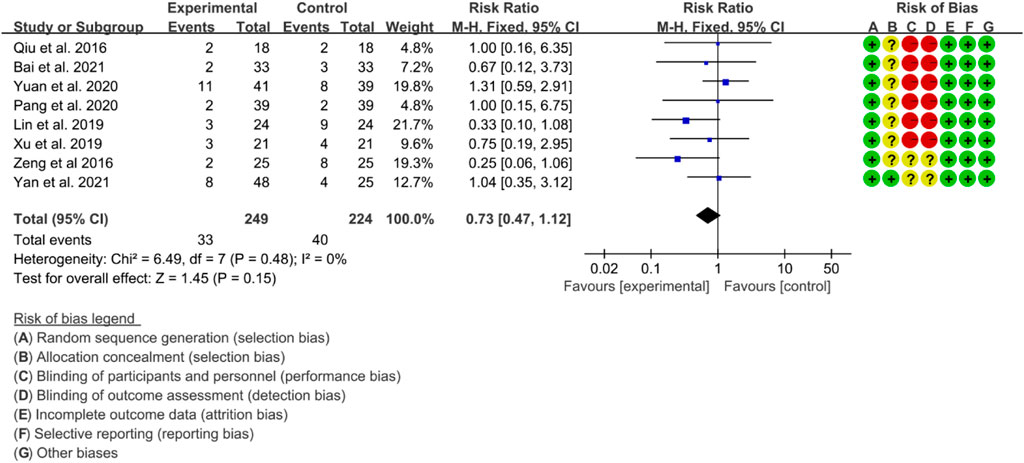
3.5.5 Adverse events
A total of eight RCTs provided data on adverse events. The heterogeneity test indicated low heterogeneity with p = 0.48 and I2 = 0%, suggesting that a fixed effects model was appropriate for analysis. The meta-analysis indicated that there was no significant difference in adverse events between the IGU and control groups (RR 0.72 [0.47, 1.12], p = 0.15; fixed effect model) (Figure 18). The results of publication bias test showed that it was less likely to have publication bias (p = 0.766).
3.5.6 Quality of evidence
According to the GRADE handbook, the evidence was judged to be moderate to very low (Table 4).
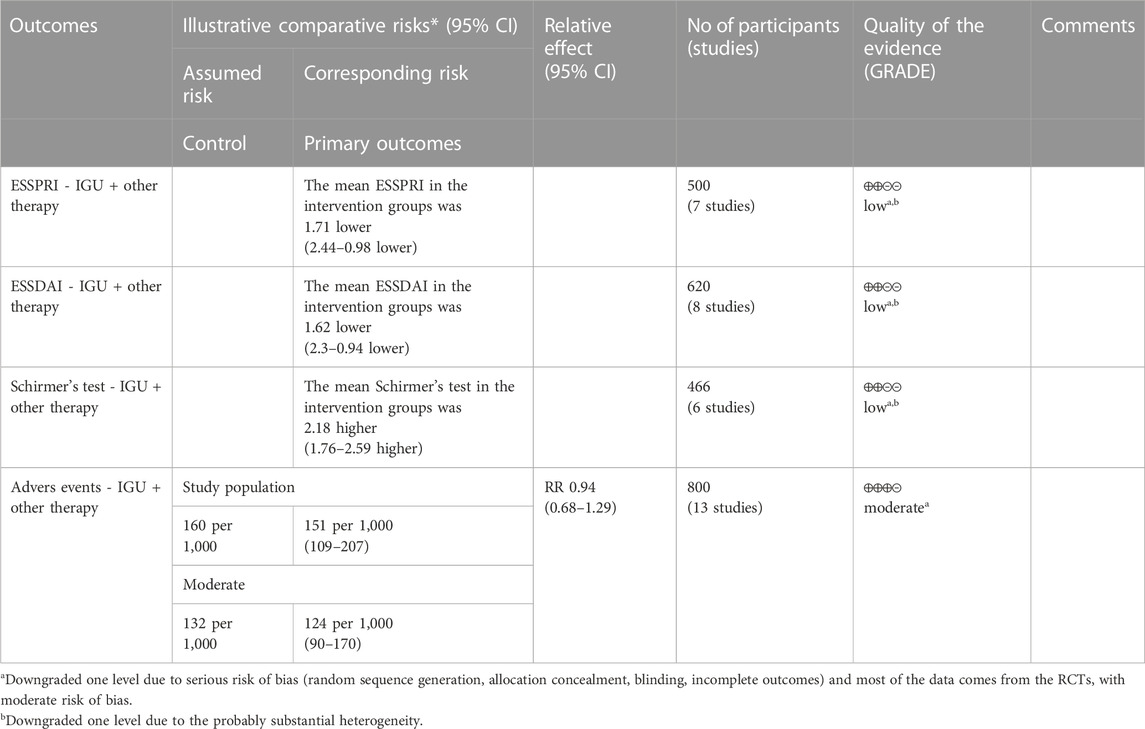
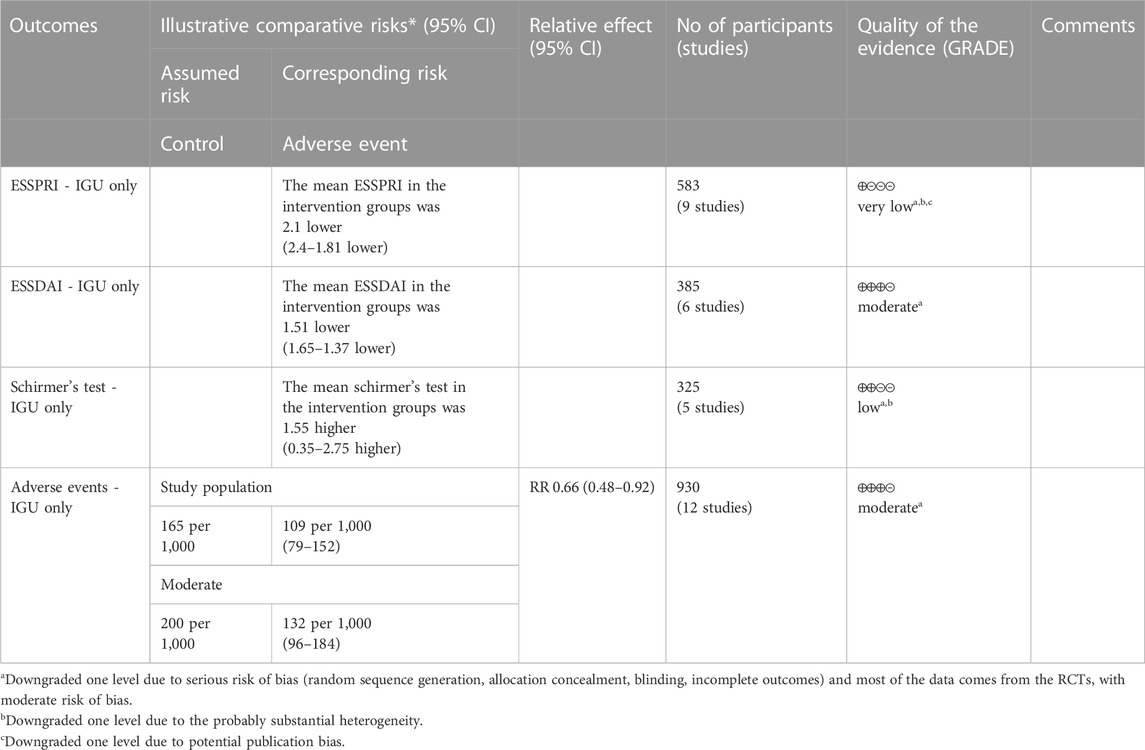
3.6 IGU for PSS
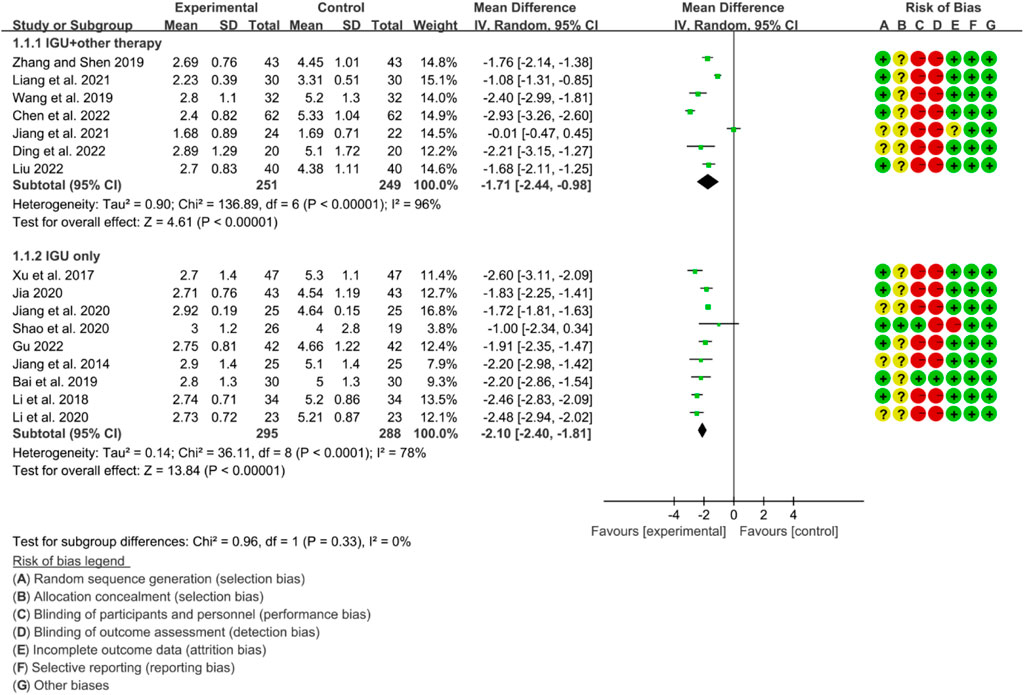
3.6.1 ESSPRI
The heterogeneity test showed that some subgroups had high heterogeneity (IGU + other therapy subgroup: p < 0.00001, I2 = 96%; IGU only subgroup: p < 0.0001, I2 = 78%), and a random effect model was used. The meta-analysis results show that compared with the control group, the ESSPRI in the IGU + other therapy group (WMD −1.71 [−2.44, −0.98], p < 0.00001; random effect model) and IGU only group (WMD −2.10 [−2.40, −1.81], p < 0.00001; random effect model) was lower (Figure 19). The results of publication bias test showed that it was less likely to have publication bias in IGU + other therapy subgroup (p = 0.667), while the publication bias test showed that it was likely to have publication bias in IGU only subgroup (p = 0.066).
3.6.2 ESSDAI
The heterogeneity test showed that some subgroups had high heterogeneity (IGU + other therapy subgroup: p < 0.00001, I2 = 90%; IGU only subgroup: p = 0.80, I2 = 0%), and a random effect model was used. The meta-analysis results show that compared with the control group, the ESSDAI in the IGU + other therapy group (WMD −1.62 [−2.30, −0.94], p < 0.00001; random effect model) and IGU only group (WMD −1.51 [−1.65, −1.37], p < 0.00001; random effect model) was lower (Figure 20). The results of publication bias test showed that it was less likely to have publication bias in IGU + other therapy (p = 0.691) and IGU only subgroup (p = 0.659).
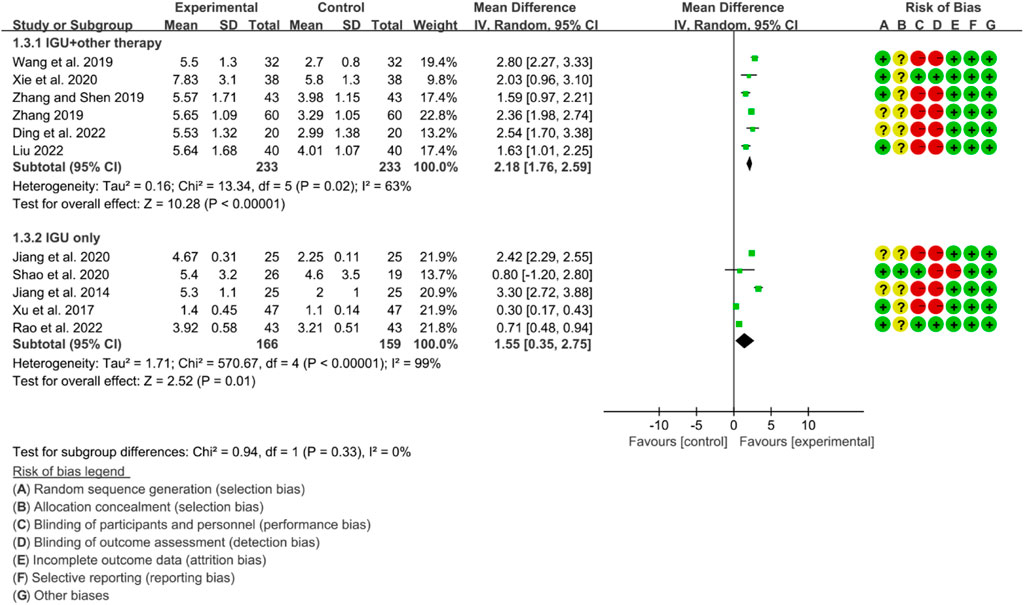
3.6.3 Schirmer’s test
The heterogeneity test showed that some subgroups had high heterogeneity (IGU + other therapy subgroup: p = 0.02, I2 = 63%; IGU only subgroup: p < 0.00001, I2 = 99%), and a random effect model was used. The meta-analysis results show that compared with the control group, the schirmer’s test in the IGU + other therapy group (WMD 2.18 [1.76, 2.59], p < 0.00001; random effect model) and IGU only group (WMD 1.55 [0.35, 2.75], p = 0.01; random effect model) was higher (Figure 21). The results of publication bias test showed that it was less likely to have publication bias in IGU + other therapy (p = 0.612) and IGU only subgroup (p = 0.934).
3.6.4 Inflammation factors
Inflammation factors include ESR, CRP and RF.
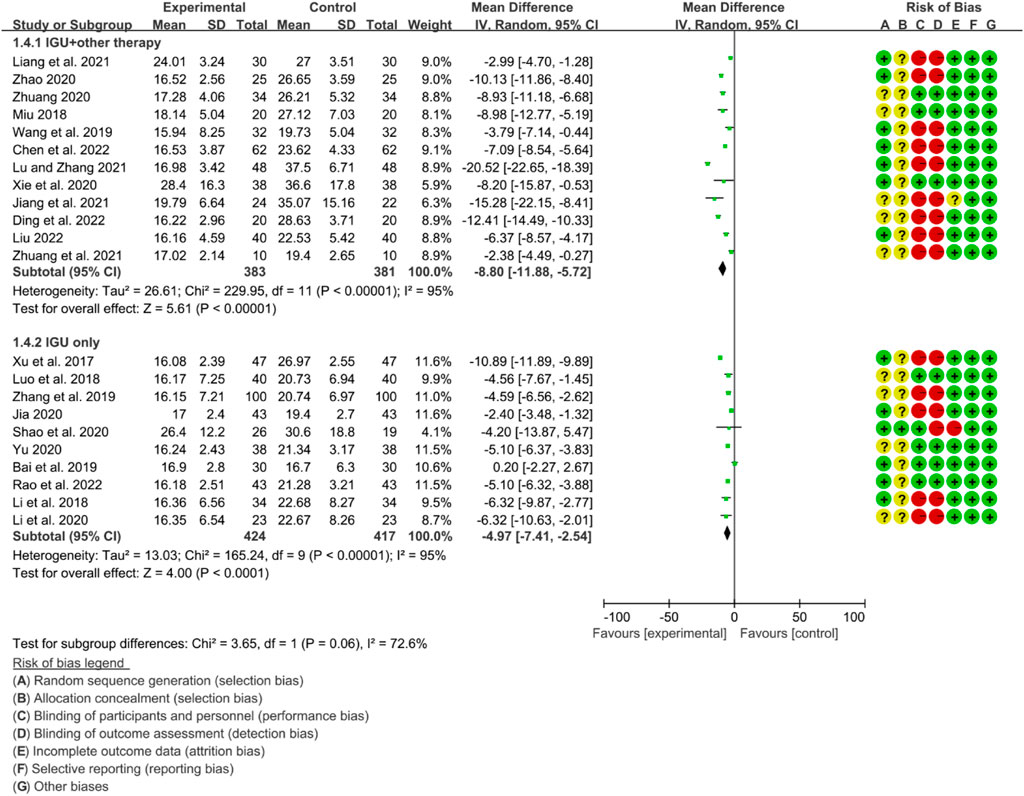
For ESR, the heterogeneity test showed that some subgroups had high heterogeneity (IGU + other therapy subgroup: p < 0.00001, I2 = 95%; IGU only subgroup: p < 0.00001, I2 = 95%), and a random effect model was used. The meta-analysis results show that compared with the control group, the ESR in the IGU + other therapy group (WMD −8.80 [−11.88, −5.72], p < 0.00001; random effect model) and IGU only group (WMD −4.97 [−7.41, −2.54], p < 0.0001; random effect model) was lower (Figure 22).
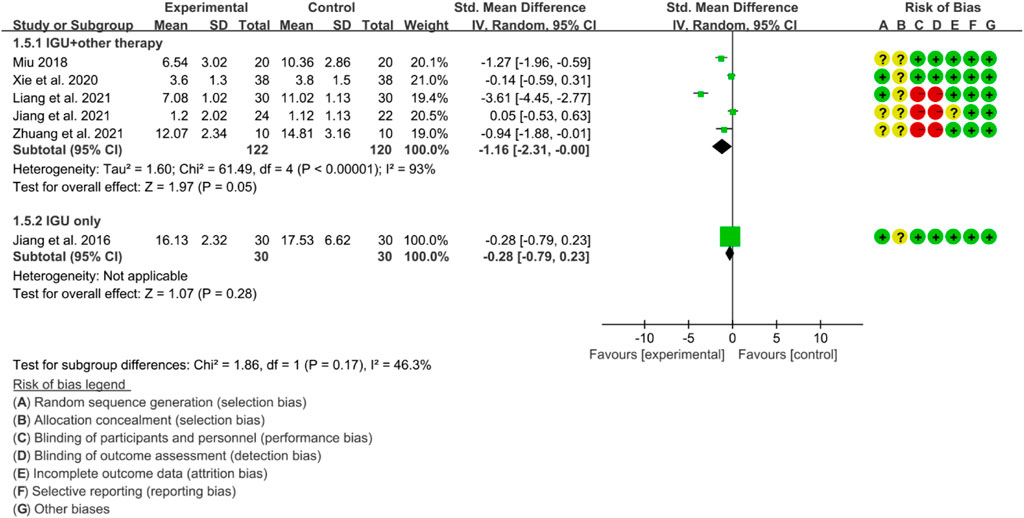
For CRP, the heterogeneity test showed that some subgroups had high heterogeneity (IGU + other therapy subgroup: p < 0.00001, I2 = 93%; IGU only subgroup: not applicable), and a random effect model was used. The meta-analysis results show that compared with the control group, the CRP in the IGU + other therapy group was lower (SMD −1.16 [−2.31, −0.00], p = 0.05; random effect model) (Figure 23).
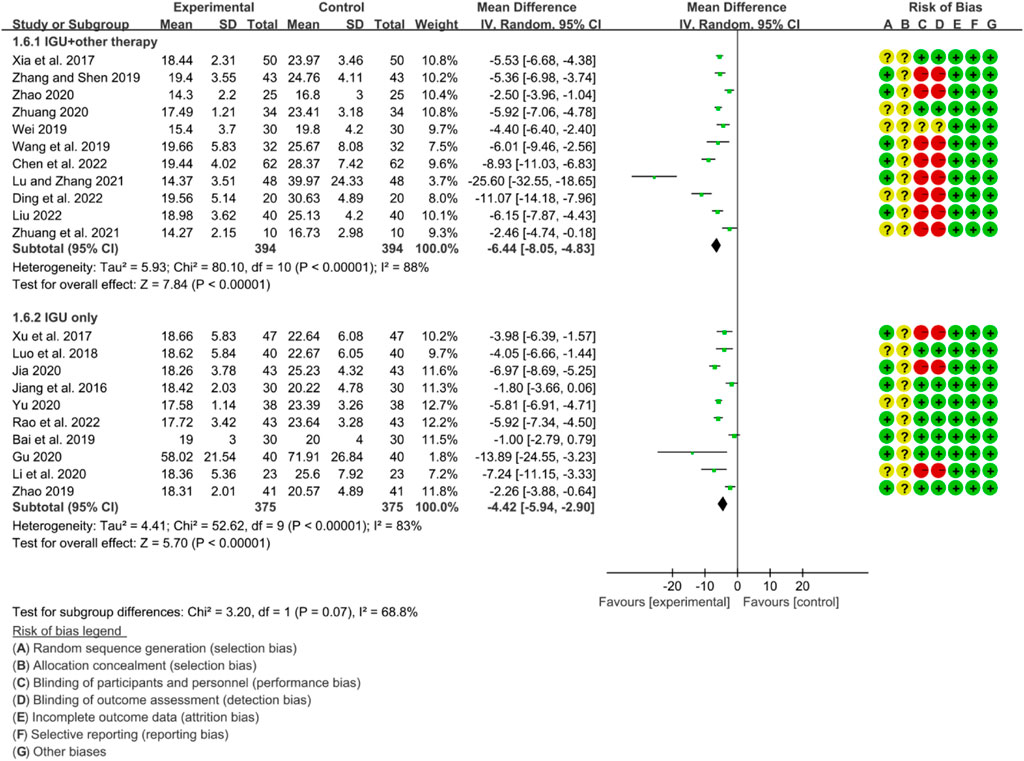
For RF, the heterogeneity test showed that some subgroups had high heterogeneity (IGU + other therapy subgroup: p < 0.00001, I2 = 88%; IGU only subgroup: p < 0.00001, I2 = 83%), and a random effect model was used. The meta-analysis results show that compared with the control group, the RF in the IGU + other therapy group (WMD −6.44 [−8.05, −4.83], p < 0.00001; random effect model) and IGU only group (WMD −4.42 [−5.94, −2.90], p < 0.0001; random effect model) was lower (Figure 24).
3.6.5 Adverse events
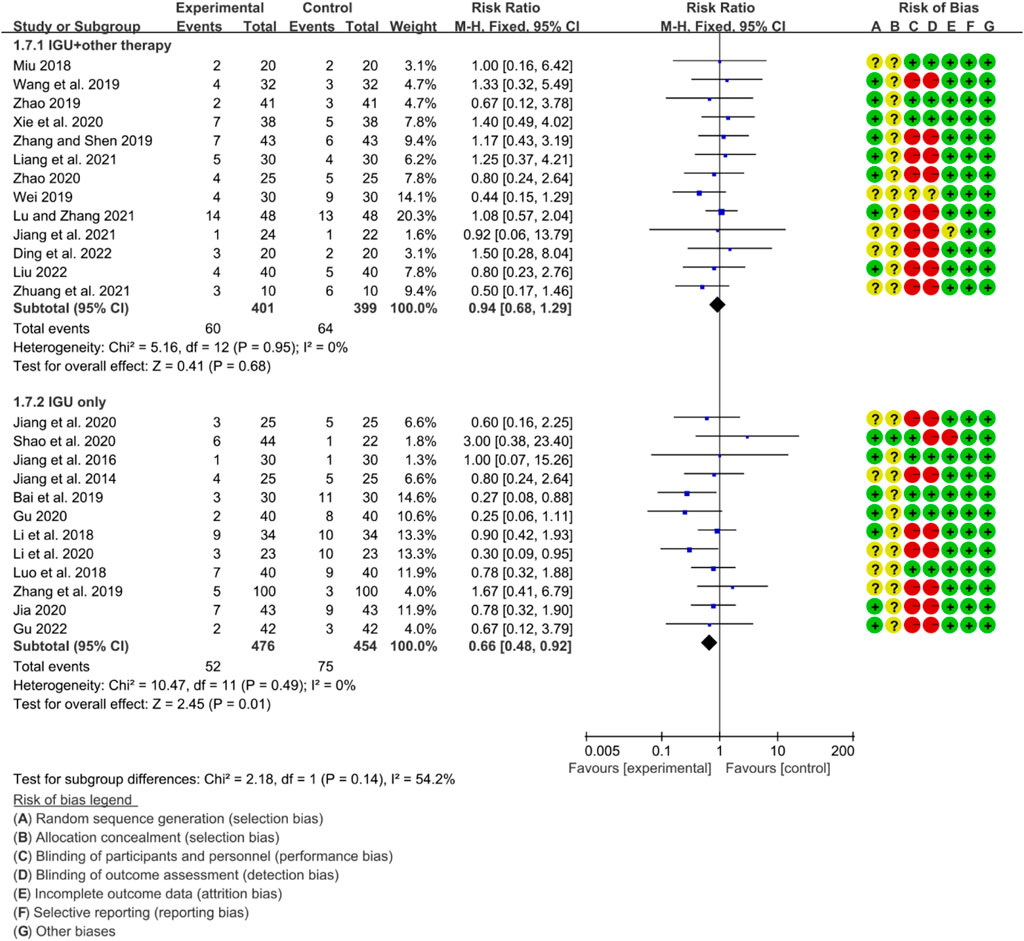
The heterogeneity test showed that some subgroups had high heterogeneity (IGU + other therapy subgroup: p = 0.95, I2 = 0%; IGU only subgroup: p = 0.49, I2 = 0%), and a fixed effect model was used. The meta-analysis results show that compared with the control group, the incidence of adverse events in the IGU only group (RR 0.66 [0.48, 0.98], p = 0.01; fixed effect model) was lower, while the difference of the incidence of adverse events between IGU + other therapy group and control grouo was of no statistical significance (RR 0.94 [0.68, 1.29], p = 0.68; fixed effect model) was lower (Figure 25). The results of publication bias test showed that it was less likely to have publication bias in IGU + other therapy (p = 0.777) and IGU only subgroup (p = 0.501).
3.6.6 Quality of evidence
According to the GRADE handbook, the evidence of IGU + other therapy subgroup was judged to be moderate to low (Table 5). The evidence of IGU only subgroup was judged to be moderate to very low (Table 6).
3.7 IGU for autoimmune disease with interstitial pneumonia
Zhuang et al. (2021) and Zhang et al. (2019) reported the treatment of PSS with interstitial pneumonia. DongZhang et al. (2019) reported the treatment of RA with interstitial pneumonia. Zhang et al. (2019) and DongZhang et al. (2019) reported FVC; they found that IGU may improve FVC.
Meanwhile, Zhuang et al. (2021) showed that both DLCO and 6MWT improved in both groups after treatment, and the degree of improvement in 6MWT in the IGU group was due to that in the control group. Zhang et al. (2019) reported that MMF was also improved after treatment, and the improvement was greater in the IGU group than in the control group. DongZhang et al. (2019) showed that compared with the control group, both FEV1 and TLC were improved after IGU treatment (p < 0.05).
4 Discussion
4.1 IGU for RA
IGU was approved for the treatment of RA in China and Japan in 2012, and in the RA guidelines of the Asia Pacific Association of Rheumatology (APLAR) meeting in 2014. It is recommended as an effective option for intensive treatment of refractory RA (Li et al., 2013; Li J. et al., 2019). It is now widely used to treat autoimmune diseases and improve related inflammation, such as PSS, IgG4-related diseases, lupus nephritis, etc. (Nozaki, 2021). Studies have shown that compared with other traditional DMARDs drugs, IGU can not only inhibit the production of immunoglobulin and various inflammatory cytokines (IL-1, IL-6, IL-8 and TNF), promote the differentiation of bone cells, inhibit the generation of osteoclasts, reduce bone resorption and joint destruction, but also reduce the expression of matrix metalloproteinases by inhibiting the production of MMP-1 and MMP-3, thereby playing an anti-inflammatory role (Liu et al., 2021a; Mizutani et al., 2021; Mu et al., 2021; Tanaka, 2021). In addition, IGU can also inhibit COX-2 and reduce the short-term synergistic effect of pain and inflammation (Mu et al., 2021; Tanaka, 2021).
This meta-analysis found that IGU + MTX therapy can improve ACR20, ACR50, ACR70, DAS28, reduce ESR, CRP, RF, and have a lower incidence of adverse events than the control group. However, IGU alone only significantly improved CRP. IGU + Tripterygium Extract can also improve ESR, CRP and RF. This suggests that IGU + MTX may be a better combination of IGU in the treatment of RA, because it has obvious efficacy, can reduce inflammatory factors, and has a lower incidence of adverse events than the control group therapy (mainly MTX). There is heterogeneity in most outcomes, which is considered to be related to the following points: 1) the dose and duration of IGU and MTX are different; 2) the degree of disease activity of patients at baseline is not the same. Since the extent of disease activity in patients at baseline was not clearly stated in each study, further analysis was not performed. In addition, the dose of IGU in all RCTs was 25–50 mg (25 mg Bid for most RCTs; and 25 Qd or 50 mg Qd for a few RCTs), suggesting that IGU at this dose had a good effect on RA without increasing the incidence of adverse events.
A recent 52-week randomized, double-blind, parallel-controlled, multicenter study by Bao et al. showed that IGU (Use alone) was more effective than MTX in the treatment of RA (Du F. et al., 2021). In terms of efficacy, the ACR20 response rate of IGU was 77.44%, which was significantly better than that of MTX (65.87%). In the direction of imaging improvement, the results showed that the proportion of patients with no imaging progression in IGU or combined therapy for 1 year was higher than that in MTX therapy, indicating that IGU therapy was significantly better than MTX therapy. The efficacy of IGU + MTX is similar to that of IGU only, suggesting that patients with early RA can consider IGU alone, and only when the single drug is not effective, combined with other drugs such as biological agents. They also found that IGU or combination therapy can delay the imaging progress of RA patients, which provides an important reference for clinical medication. Another important factor for RA patients and doctors when choosing a drug is the efficacy, safety and cost of the drug. Jie et al. reported data from a real-world pharmacoeconomics study on IGU and other drugs in RA at the 2022 EULAR meeting. Their results show that IGU combined with MTX in the treatment of RA is both safe and effective, and the price is moderate, providing a treatment plan for RA patients that takes into account efficacy, safety and economic cost.
4.2 IGU for AS
The current study shows that IGU, as a new type of DMARD, mainly acts through anti-inflammatory and immune regulation. For example, IGU can inhibit the production of inflammatory cytokines (such as IL-1 and TNF-α), block the IL-17 signaling pathway and inhibit cyclooxygenase, and regulate the balance of osteoclasts (Liu et al., 2021b; Harjacek, 2021), so it may be effective against AS/SpA in mechanism. Therefore, a number of exploratory RCTs have previously applied IGU to AS/SpA (Qiu et al., 2016; Zeng et al., 2016; Lin et al., 2019; Xu et al., 2019; Pang et al., 2020; Yuan et al., 2020; Li Y. et al., 2021; Bai et al., 2021; Li X. et al., 2021).
The meta-analysis findings revealed that IGU was effective in reducing the BASDAI score, BASFI score, and VAS. Additionally, IGU was able to lower inflammation levels by decreasing ESR, CRP, and TNF-α. However, there was considerable heterogeneity in the results, especially in VAS, ESR, CRP, and TNF-α. This could be attributed to the fact that BASDAI and VAS are subjective measures, and the experiences of patients across different RCTs may differ. Moreover, ESR, CRP, and TNF-α are individual biochemical indicators, and variations in patients’ conditions across different RCTs may also contribute to the heterogeneity. All RCTs reported adverse events, but no patient deaths were recorded. Compared to the control group, the IGU group did not experience any statistically significant difference in adverse events. Therefore, IGU does not appear to increase the risk of adverse events. Notably, the IGU dose was 50 mg in all RCTs (25 mg Bid in most RCTs and 50 mg Qd in a few RCTs), indicating that this dose had a beneficial effect on AS without raising the incidence of adverse events.
4.3 IGU for PSS
The pathogenesis of PSS is complex and has not yet been clearly studied. At present, it is believed that it may be related to various factors such as genetics, environment, endocrine, and immune abnormalities (Fasano et al., 2020a; Huang et al., 2021). Among them, the excessive activation of B cells produces a variety of autoantibodies and hyperimmunoglobulinemia plays an important role in the development of pSS. In this process, T cells also participate in the maturation and differentiation of B cells by secreting a variety of cytokines (Rivière et al., 2020). More than 80% of patients with Sjögren’s syndrome will experience symptoms of dryness, fatigue and joint pain, which will affect the patient’s work efficiency and reduce the patient’s quality of life (Marshall and Stevens, 2018). However, there is currently no specific drug for the treatment of pSS. Therefore, exploratory research on PSS therapeutic drugs is currently underway (Carsons et al., 2017; Vehof et al., 2020). As a new type of DMARD, IGU’s main mechanism of action is highly compatible with the complex pathogenesis of SS, and has therapeutic potential. A number of clinical studies have shown that IGU can effectively improve the disease activity (such as ESSDAI), various serum indicators (IgG, IgM, IgA, ESR, RF) and lacrimal gland secretion function (detected by Schirmer I test) in patients with pSS.
This meta-analysis also showed that IGU can reduce the ESSPRI score and ESSDAI score, inhibit the inflammation factors (reduce ESR, CRP and RF) and increase Schirmer’s test score. The incidence of adverse events in IGU group was also lower than that in control group, indicating that the addition of IGU may be an effective and safe treatment plan. In addition, the dose of IGU in all RCTs was 50 mg (25 mg Bid for most RCTs and 50 mg Qd for a few RCTs), suggesting that IGU at this dose had a good effect on PSS without increasing the incidence of adverse events. B cell hyperactivity is a key pathogenic factor in pSS, which is mainly characterized by the formation of ectopic germinal centers in the lacrimal and salivary glands (Carsons et al., 2017; Fasano et al., 2020b; Du W. et al., 2021). Therefore, reducing B cell activity and suppressing immunoglobulin production have become the key to treatment. Studies have shown that IGU not only inhibits the proliferation of T cells, but also inhibits the differentiation of antibody secreting cells (ASCs) in RA patients by activating the PKC/EGR1 pathway, thereby regulating the immune response of B cell differentiation and relieving clinical symptoms (Ye et al., 2019a). However, whether IGU can play a role in the treatment of pSS patients by inhibiting the activity of B cells has not yet been determined.
4.4 IGU for interstitial pneumonia
Early symptoms of RA-interstitial pneumonia (RA-ILD) are often atypical and easy to miss (Chernau et al., 2019; Graney and Fischer, 2019). At present, there is no targeted treatment for RA-ILD, and two clinical strategies are mainly used: anti-inflammatory and anti-fibrosis. In terms of anti-inflammatory, the dosage and treatment time of hormones and immunosuppressants are difficult to grasp. Excessive immunosuppression can also lead to secondary infection aggravating the disease. Therefore, clinical studies are still searching for safe and effective therapeutic drugs for RA-ILD (Wells and Denton, 2014; Santhanam et al., 2020). The current study shows that the potential mechanisms of IGU treatment of pulmonary fibrosis include: inhibition of inflammation and epithelial-mesenchymal transition (EMT) process (Luppi et al., 2020). For example, Luo et al. found that inflammatory cell infiltration, inflammatory factor and chemokine expression in the lung tissue of mice treated with IGU treated mice with idiopathic pulmonary fibrosis decreased in a dose-dependent manner. This suggests that IGU can inhibit the pulmonary inflammatory response that accompanies the process of pulmonary fibrosis (Yoo et al., 2020). Zhao et al. found that high doses of IGU and methylprednisolone had inhibitory effects on alveolitis and pulmonary fibrosis in a bleomycin-induced mouse model of pulmonary fibrosis (England and Hershberger, 2020). Zhu et al. found that IGU can inhibit TGF-β1-mediated human lung fibroblast activation and collagen secretion through the Smad3/p300 pathway, and it may be an effective anti-fibrotic drug to delay the progression of PF (Kadura and Raghu, 2021).
In this systematic review and meta-analysis, Zhuang et al. (2021) and Zhang et al. (2019) reported the treatment of PSS with interstitial pneumonia. DongZhang et al. (2019) reported the treatment of RA with interstitial pneumonia. The meta-analysis results showed that FVC increased after IGU treatment. Meanwhile, Zhuang et al. (2021) showed that both DLCO and 6MWT improved in both groups after treatment, and the degree of improvement in 6MWT in the IGU group was due to that in the control group. Zhang et al. (2019) reported that MMF was also improved after treatment, and the improvement was greater in the IGU group than in the control group. DongZhang et al. (2019) showed that compared with the control group, both FEV1 and TLC were improved after IGU treatment. These all suggest the therapeutic effect of IGU on autoimmune diseases complicated with interstitial pneumonia. In terms of economics and drug insurance policy, IGU is a relatively inexpensive drug that is available in most countries. A real-world study retrospectively analyzed the population characteristics, efficacy and influencing factors of RA patients who received IGU treatment for at least 6 months between July 2015 and October 2020 and had more than 3 follow-up records. The results showed that IGU was well tolerated and an effective treatment drug, which is a treatment option for RA patients with interstitial lung disease.
4.5 IGU for other rheumatic and autoimmune diseases
SLE is an autoimmune inflammatory disease that affects multiple organs and connective tissues. It is more common in young women and is seeing an increase in early, mild, and atypical cases (Luo et al., 2015; Shao et al., 2021). Within 5 years, most SLE patients will develop LN, which remains a significant cause of morbidity and mortality (Zhao et al., 2017b). While several drugs have demonstrated efficacy in treating the disease, 20%–35% of LN patients experience relapse or treatment failure, and drug intolerance is a frequent issue (Fu et al., 2021). In preclinical studies with lupus, IGU prevented autoimmune nephritis, reduced proteinuria, and decreased immune complex deposition in MRL/lpr mice (Anders et al., 2020). As the most critical pathogenic cells in the progression and development of systemic lupus erythematosus, B cells are closely related to the systemic damage and antibody secretion of SLE (Gasparotto et al., 2020; Ayoub and Nachman, 2021). The earliest study on the mechanism of IGU on B cell differentiation found that it can inhibit the production of immunoglobulin by B cells (Mahajan et al., 2020). In a phase III clinical trial in RA, IGU reduced serum immunoglobulin concentrations (Yan et al., 2014; Canny and Jackson, 2021). In animal models of RA and lupus, IGU reduced autoantibody titers, including anti-collagen antibodies (Tanaka et al., 2003; Ma et al., 2019) and anti-double-stranded (dsDNA) antibodies [198]. Interestingly, IGU has been reported to reduce peripheral plasma cell counts without affecting the total B cell population in MRL/lpr mice (Anders et al., 2020). Further studies have shown that in RA patients receiving IGU only, IGU regulates key transcription factors affecting plasma cell differentiation through the PKC/Egr1 axis, especially Blimp-1 (Hara et al., 2007). A recent observational study found that more than 90% of patients with refractory LN responded to IGU within 24 weeks without the need to increase steroid dosage or add any other drugs during follow-up (Lu et al., 2009). Yan et al. are currently conducting a multicenter, randomized, 52-week parallel active drug-controlled study (Du et al., 2008). The study aims to investigate the efficacy of iguratimod as first-line treatment for patients with LN. Patients with biopsy-proven active lupus nephritis from six study sites in China were randomly assigned to the experimental or control group. During the first 24 weeks, IGU was compared to cyclophosphamide as induction therapy, while during the second 24 weeks, IGU was compared to azathioprine as maintenance therapy. The primary outcome was the rate of renal response, including complete and partial response at week 52, which will be analyzed using a noninferiority hypothesis test. This ongoing trial will determine whether iguratimod can be used as an alternative induction or maintenance therapy for lupus nephritis patients (Du et al., 2008).
In summary, the mechanism of IGU treatment of rheumatic and autoimmune diseases is summarized in Figure 26.
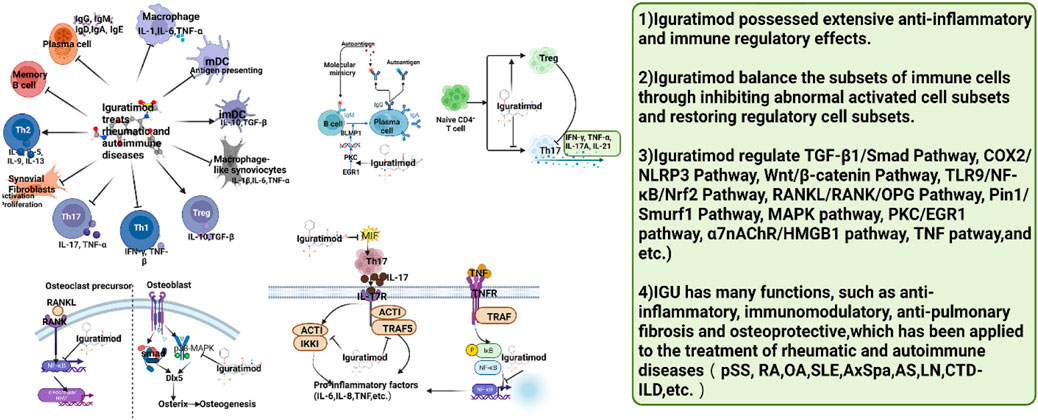
FIGURE 26. Summary of the mechanism of IGU treatment of rheumatic and autoimmune diseases (IGU may regulate immune cell function and activity to balance immune cell subsets, thereby further reducing inflammation and tissue damage. pSS, primary Sjögren’s syndrome; RA, rheumatoid arthritis; OA, Osteoarthritis; SLE, systemic lupus erythematosus; AS, ankylosing spondylitis; LN, lupus nephritis; CTD-ILD, connective tissue disease associated interstitial lung disease).
4.6 Strengths and limitations
Compared with previous systematic reviews and meta-analyses, the strengths of this study are: 1) Compared with previous studies on PSS (Luo et al., 2013; Pu et al., 2021), this study included newer and more RCTs (32, 5 of which were published in 2022), and the quality of evidence was assessed. 2) Compared with previous studies on RA (Ye et al., 2019b; Kang et al., 2020; Shrestha et al., 2020; Hu et al., 2021; Shrestha et al., 2021; Yan et al., 2021; Zeng et al., 2022a; Zeng et al., 2022b; Long et al., 2023), this study also included newer and more RCTs (43, 4 of which were published in 2022); and the intervention in the IGU group is IGU alone or IGU combined with other drugs, not limited to IGU + MTX, and further found that the combination of IGU + MTX may reduce the occurrence of adverse events, while IGU combined with other drugs only does not increase adverse events. 3) Compared with previous studies on AS (Chen et al., 2021; Liu B. et al., 2021; Deng et al., 2022; Ouyang et al., 2022; Long et al., 2023), this research employed a more rigorous screening process for RCTs. Moreover, this systematic review and meta-analysis integrated findings from various rheumatic and autoimmune diseases. As a result, the efficacy of IGU treatment for AS can be cross-compared with the outcomes of IGU treatment for other rheumatic and autoimmune diseases. 4) This study also evaluated the efficacy and safety of IGU in the treatment of autoimmune disease with interstitial pneumonia for the first time. 5) This study performed a thorough search of different databases and included Chinese databases.
The limitations include: 1) Although there is no language restriction, most of the included RCTs are in Chinese and English, and no literature in other languages has been found, so there may be publication bias. 2) The basic treatment, course of treatment, and observation time of the indicators are also different, and the clinical heterogeneity among the subgroups is high, which leads to a decrease in the accuracy and implementability of the results. 3) Although 84 RCTs were included, only 4 types of diseases (RA, AS, PSS and Autoimmune disease with interstitial pneumonia) were involved, and RCTs of IGU for other rheumatic and autoimmune diseases were not retrieved. 4) Since RCTs did not report on patients’ disease conditions in detail (such as naive RA and MTX-resistant RA), subgroup analysis of patients’ disease conditions could not be performed. 5) The RCTs included in this study are all in English or Chinese, and there are no literature in other languages (such as Japanese) for the time being, which may lead to potential bias. 6) The quality of evidence for most outcomes was assessed as low to very low, which may affect the generalization of conclusions.
Based on these shortcomings, more IGUs are needed in the future for RCTs of other rheumatic and autoimmune diseases (such as SLE). Furthermore, future RCTs are expected to report more detailed patient medication information to facilitate subgroup analysis and reduce clinical heterogeneity.
5 Conclusion
Based on current evidence, IGU may be a safe and effective for the treatment of RA, AS, PSS and autoimmune diseases with interstitial pneumonia. The quality of evidence was very low to moderate. The recommended dose is 25–50 mg. However, more RCTs about other type of rheumatic and autoimmune diseases are still needed.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
LZ and KY are responsible for the study concept and design. LZ, QH, YD, YL, JC, YL, AG, KY, XZ, ZL, and LS are responsible for the data collection, data analysis and interpretation; LZ and KY drafted the paper; LS supervised the study. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1189142/full#supplementary-material
References
Adelowo, O., Mody, G. M., Tikly, M., Oyoo, O., and Slimani, S. (2021). Rheumatic diseases in africa. Nat. Rev. Rheumatol. 17 (6), 363–374. doi:10.1038/s41584-021-00603-4
Akram, M., Daniyal, M., Sultana, S., Owais, A., Akhtar, N., Zahid, R., et al. (2021). Traditional and modern management strategies for rheumatoid arthritis. Clin. Chim. Acta 512, 142–155. doi:10.1016/j.cca.2020.11.003
Aletaha, D. (2020). Precision medicine and management of rheumatoid arthritis. J. Autoimmun. 110, 102405. doi:10.1016/j.jaut.2020.102405
Aletaha, D., and Smolen, J. S. (2018). Diagnosis and management of rheumatoid arthritis: a review. JAMA 320 (13), 1360–1372. doi:10.1001/jama.2018.13103
Anders, H. J., Saxena, R., Zhao, M. H., Parodis, I., Salmon, J. E., and Mohan, C. (2020). Lupus nephritis. Lupus nephritis. Nat. Rev. Dis. Prim. 6 (1), 7. doi:10.1038/s41572-019-0141-9
Ayoub, I., and Nachman, P. H. (2021). Advances in ANCA-associated vasculitis and lupus nephritis. Nat. Rev. Nephrol. 17 (2), 89–90. doi:10.1038/s41581-020-00388-x
Bai, J., and Jiao, Y. (2019). Observation on the clinical effect of Iramod in the treatment of primary Sjogren’s syndrome. Shanxi Med. J. 48 (14), 1724–1726. (in chinese).
Bai, Y. J., Wang, X. Y., and Yao, Y. J. (2021). Observation on the clinical effect of iguratimod in the treatment of axial spondyloarthritis. Chin. Med. Innov. 18 (2), 44–47. (in chinese). doi:10.3969/j.issn.1674-4985.2021.02.011
Bi, W. H. (2019). The effect of Iguratimod combined with methotrexate on serum VEGF levels in patients with rheumatoid arthritis and evaluation of the efficacy[D]. Inner Mongolia Medical University. (inchinese).
Canny, S. P., and Jackson, S. W. (2021). B cells in systemic lupus erythematosus: from disease mechanisms to targeted therapies. Rheum. Dis. Clin. North Am. 47 (3), 395–413. doi:10.1016/j.rdc.2021.04.006
Capell, H. A., Madhok, R., Porter, D. R., Munro, R. A., McInnes, I. B., Hunter, J. A., et al. (2007). Combination therapy with sulfasalazine and methotrexate is more effective than either drug alone in patients with rheumatoid arthritis with a suboptimal response to sulfasalazine: results from the double-blind placebo-controlled MASCOT study. Ann. Rheum. Dis. 66 (2), 235–241. doi:10.1136/ard.2006.057133
Carsons, S. E., Vivino, F. B., Parke, A., Carteron, N., Sankar, V., Brasington, R., et al. (2017). Treatment guidelines for rheumatologic manifestations of Sjögren's syndrome: use of biologic agents, management of fatigue, and inflammatory musculoskeletal pain. Arthritis Care Res. Hob. 69 (4), 517–527. doi:10.1002/acr.22968
Charoenngam, N. (2021). Vitamin D and rheumatic diseases: a review of clinical evidence. Int. J. Mol. Sci. 22 (19), 10659. doi:10.3390/ijms221910659
Chen, J., Ding, Z. H., Liu, J., Huang, X., Guo, X., et al. (2018). Enhanced poly(propylene carbonate) with thermoplastic networks: a one-pot synthesis from carbon dioxide, propylene oxide, and a carboxylic dianhydride. Zhejiang J. Integr. Traditional Chin. West. Med. 28 (1), 552–555. (in chinese). doi:10.3390/polym10050552
Chen, L. J., Zhou, Y. J., Wen, Z. H., Tian, F., and Li, J. Y. (2021). Efficacy and safety of iguratimod combined with methotrexate vs. methotrexate alone in rheumatoid arthritis: a systematic review and meta-analysis of randomized controlled trials. Z Rheumatol. 80 (5), 432–446. doi:10.1007/s00393-020-00944-7
Chen, Y., Shen, P., and Sun, D. (2022). Analysis of curative effect of iguratimod in the treatment of elderly patients with pSS and its effect on B cell activity and secretion level of immunoglobulin G. Med. Theory Pract. 35 (02), 244–246. (in chinese). doi:10.19381/j.issn.1001-7585.2022.02.025
Chernau, A. K., Leone, P. M., and Swigris, J. J. (2019). Interstitial pneumonia with autoimmune features and undifferentiated connective tissue disease. Semin. Respir. Crit. Care Med. 40 (2), 271–277. doi:10.1055/s-0039-1684007
Cronstein, B. N., and Aune, T. M. (2020). Methotrexate and its mechanisms of action in inflammatory arthritis. Nat. Rev. Rheumatol. 16 (3), 145–154. doi:10.1038/s41584-020-0373-9
Dai, Y., Wang, W., Yu, Y., and Hu, S. (2021). Rheumatoid arthritis-associated interstitial lung disease: an overview of epidemiology, pathogenesis and management. Clin. Rheumatol. 40 (4), 1211–1220. doi:10.1007/s10067-020-05320-z
Dai, Y., Deng, S., Zou, S., and Dou, T. (2022). Observation on the therapeutic effect of methotrexate combined with Ailamod in the treatment of rheumatoid arthritis. Mod. Pract. Med. 34 (01), 114–116. (in chinese).
Deeks, J. J., Higgins, J. P., and Altman, D. G. (2020b). “Chapter 8: assessing risk of bias in included studies,” in Cochrane handbook or systematic reviews of interventions version 6.1.0. Editors J. P. Higgins,, and S. Green (UK: The Cochrane Collaboration).
Deeks, J. J., Higgins, J. P., and Altman, D. G. (2020c). “Chapter 9: analyzing data and undertaking meta-analyses,” in Cochrane handbook for systematic reviews of interventions. Editors J. P. Higgins,, and S. Green (UK: The Cochrane Collaboration).
Deeks, J. J., Higgins, J. P., and Altman, D. G. (2020a). “Chapter 16: special topics in statistics,” in Cochrane handbook for systematic reviews of interventions. Editors J. P. Higgins,, and S. Green (UK: The Cochrane Collaboration).
Deng, J. X. (2017). The effect of Iguratimod on the proliferation and migration of fibroblast-like synovial cells in rheumatoid arthritis and the clinical observation[D]. Southern Medical University. (in chinese).
Deng, L., Yao, F., Tian, F., Luo, X., Yu, S., and Wen, Z. (2022). Influence of iguratimod on bone metabolism in patients with rheumatoid arthritis: a meta-analysis. Int. J. Clin. Pract. 2022, 5684293. doi:10.1155/2022/5684293
Ding, L., He, S., Wang, M., Zou, C., and Wang, M. (2022). The effect of Ailamod in the treatment of senile primary Sjögren's syndrome and its influence on the SSDAI and ESSPRI scores of patients. Clin. Med. Res. Pract. 7 (17), 78–80. (in chinese). doi:10.19347/j.cnki.2096-1413.202217020
Dong, M., Zhang, Z., and Wang, D. (2019). Efficacy of Tripterygium wilfordii polyglycosides combined with iguratimod in the treatment of RA complicated with interstitial lung disease and its effect on serum HIF-1α and IL-22 levels. J. Guangxi Med. Univ. 36 (1), 45–48. (in chinese). doi:10.16190/j.cnki.45-1211/r.2019.01.011
Donghui, W. (2019). Efficacy and safety of iguratimod and hydroxychloroquine in the treatment of Sjögren'ssyndrome. DOCTOR 11, 146–147. (in chinese).
Drosos, A. A., Pelechas, E., Kaltsonoudis, E., and Voulgari, P. V. (2020). Therapeutic options and cost-effectiveness for rheumatoid arthritis treatment. Curr. Rheumatol. Rep. 22 (8), 44. doi:10.1007/s11926-020-00921-8
Du, F., Lü, L. J., Fu, Q., Dai, M., Teng, J. L., Fan, W., et al. (2008). T-614, a novel immunomodulator, attenuates joint inflammation and articular damage in collagen-induced arthritis. Arthritis Res. Ther. 10 (6), R136. doi:10.1186/ar2554
Du, F., Xu, J., Li, X., Li, Z., and Zuo, X. (2021a). POS0664 A multicenter randomized study in rheumatoid arthritis to compare iguratimod, methotrexate, or combination: 52 week efficacy and safety results of the smile trial. Rheum. Dis. 80, 574–575. doi:10.1136/annrheumdis-2021-eular.1486
Du, W., Han, M., Zhu, X., Xiao, F., Huang, E., Che, N., et al. (2021b). The multiple roles of B cells in the pathogenesis of Sjögren's syndrome. Front. Immunol. 12, 684999. doi:10.3389/fimmu.2021.684999
Duan, X. W., Zhang, X. L., Mao, S. Y., Shang, J. J., and Shi, X. D. (2015). Efficacy and safety evaluation of a combination of iguratimod and methotrexate therapy for active rheumatoid arthritis patients: a randomized controlled trial. Clin. Rheumatol. 34 (2), 1513–1519. doi:10.1007/s10067-015-2999-6
England, B. R., and Hershberger, D. (2020). Management issues in rheumatoid arthritis-associated interstitial lung disease. CurrOpinRheumatol 32 (3), 255–263. doi:10.1097/BOR.0000000000000703
Fan, Z. X., Wu, P. C., and Song, M. H. (2020). The clinical efficacy of iguratimod combined with methotrexate in the treatment of rheumatoid arthritis. J. Clin. Ration. Use 13 (2), 81–83. (in chinese).
Fasano, S., Mauro, D., Macaluso, F., Xiao, F., Zhao, Y., Lu, L., et al. (2020a). Pathogenesis of primary Sjögren's syndrome beyond B lymphocytes. Clin. Exp. Rheumatol. 38 (4), 315–323.
Fasano, S., Mauro, D., Macaluso, F., Xiao, F., Zhao, Y., Lu, L., et al. (2020b). Pathogenesis of primary Sjögren's syndrome beyond B lymphocytes. Clin. Exp. Rheumatol. 38 (4), 315–323.
Fu, Z., Yuan, F., Lin, H., Zhang, G., and Zhang, X. (2021). Iramod inhibits transforming growth factor β_1-mediated activation and collagen secretion of human lung fibroblasts via Smad3/p300 pathway. Chin. J. Rheumatology 25 (11), 721–726. (in chinese).
Gasparotto, M., Gatto, M., Binda, V., Doria, A., and Moroni, G. (2020). Lupus nephritis: clinical presentations and outcomes in the 21st century. Rheumatol. Oxf. 59 (S5), v39–v51. doi:10.1093/rheumatology/keaa381
Ghabri, S., Lam, L., Bocquet, F., and Spath, H. M. (2020). Systematic literature review of economic evaluations of biological treatment sequences for patients with moderate to severe rheumatoid arthritis previously treated with disease-modifying anti-rheumatic drugs. Pharmacoeconomics 38 (5), 459–471. doi:10.1007/s40273-020-00887-6
Goodman, S. M. (2015). Rheumatoid arthritis: perioperative management of biologics and DMARDs. Semin. Arthritis Rheum. 44 (6), 627–632. doi:10.1016/j.semarthrit.2015.01.008
Gradepro, G. D. T. (2015). GRADEpro guideline development tool [software]. McMaster University. 2015 (developed by Evidence Prime, Inc.). Available from gradepro.org.
Graney, B. A., and Fischer, A. (2019). Interstitial pneumonia with autoimmune features. Ann. Am. Thorac. Soc. 16 (5), 525–533. doi:10.1513/AnnalsATS.201808-565CME
Gu, J. (2022). Clinical effect of methylprednisolone combined with iguratimod in the treatment of primary Sjögren's syndrome and its influence on the level of immunoglobulin. Contemp. Med. 28 (08), 158–160. (in chinese).
Gu, J. (2020). Clinical effect of methylprednisolone combined with Ilamod in the treatment of elderly patients with primary Sjogren’s syndrome. Chin. Natl. Health Med. 32 (18), 1–2+5. (in chinese).
Gu, X. J., Chen, H., Li, R. P., Gan, F. Y., and Guo, D. B. (2020). The clinical efficacy of Ilamud combined with methotrexate in the treatment of early rheumatoid arthritis (RA). Contemp. Med. 26, 138–139. (inchinese).
Guifeng, H., and Li, Y. (2014). Observation on the short-term clinical effect of Ilamod on rheumatoid arthritis complicated with chronic interstitial pneumonia. China Mod. Appl. Pharm. 31 (10), 1275–1278. (in chinese). doi:10.13748/j.cnki.issn1007-7693.2014.10.029
Hara, M., Abe, T., Sugawara, S., Mizushima, Y., Hoshi, K., Irimajiri, S., et al. (2007). Efficacy and safety of iguratimod compared with placebo and salazosulfapyridine in active rheumatoid arthritis: a controlled, multicenter, double-blind, parallel-group study. Mod. Rheumatol. Jpn. Rheum. Assoc. 17 (1), 1–9. doi:10.1007/s10165-006-0542-y
Hara, M., Ishiguro, N., Katayama, K., Kondo, M., Sumida, T., Mimori, T., et al. (2014). Safety and efficacy of combination therapy of iguratimod with methotrexate for patients with active rheumatoid arthritis with an inadequate response to methotrexate: an open-label extension of a randomized, double-blind, placebo-controlled trial. Mod. Rheumatol. 24 (3), 410–418. doi:10.3109/14397595.2013.843756
Harjacek, M. (2021). Immunopathophysiology of juvenile spondyloarthritis (jSpA): the "out of the box" view on epigenetics, neuroendocrine pathways and role of the macrophage migration inhibitory factor (MIF). Front. Med. (Lausanne) 8, 700982. doi:10.3389/fmed.2021.700982
He, Y., Yang, G., Zheng, Y., Xu, X., Pan, X., and Wu, H. (2015). Clinical observation of Ilamod in the treatment of rheumatoid arthritis complicated with interstitial lung disease. J. Pract. Clin. Med. 19 (23), 152–159. (in chinese).
Hu, C. J., Zhang, L., Zhou, S., Jiang, N., Zhao, J. L., Wang, Q., et al. (2021). Effectiveness of iguratimod as monotherapy or combined therapy in patients with rheumatoid arthritis: a systematic review and meta-analysis of RCTs. J. Orthop. Surg. Res. 16 (1), 457. doi:10.1186/s13018-021-02603-2
Hu, H. (2014). Efficacy and safety observation of Ellamod in the treatment of rheumatoid arthritis[D]. Soochow University. (in chinese).
Huang, B. J., and Ma, J. X. (2018). Observation on the short-term curative effect of Iramod in the treatment of ankylosing spondylitis. Chin. Community Physician 34 (13), 92–93.
Huang, H., Xie, W., Geng, Y., Fan, Y., and Zhang, Z. (2021). Mortality in patients with primary Sjögren's syndrome: a systematic review and meta-analysis. Rheumatol. Oxf. 60 (9), 4029–4038. doi:10.1093/rheumatology/keab364
Hyrich, K. L., and Machado, P. M. (2021). Rheumatic disease and COVID-19: epidemiology and outcomes. Nat. Rev. Rheumatol. 17 (2), 71–72. doi:10.1038/s41584-020-00562-2
Ichikawa, Y., Saito, T., Yamanaka, H., Akizuki, M., Kondo, H., Kobayashi, S., et al. (2005). Therapeutic effects of the combination of methotrexate and bucillamine in early rheumatoid arthritis: a multicenter, double-blind, randomized controlled study. Mod. Rheumatol. 15 (5), 323–328. doi:10.1007/s10165-005-0420-z
Ishiguro, N., Yamamoto, K., Katayama, K., Kondo, M., Sumida, T., Mimori, T., et al. (2013). Concomitant iguratimod therapy in patients with active rheumatoid arthritis despite stable doses of methotrexate: a randomized, double-blind, placebo-controlled trial. Mod. Rheumatol. 23 (3), 430–439. doi:10.1007/s10165-012-0724-8
Jia, X. (2020). Analysis of the effect of iguratimod combined with hydroxychloroquine in the treatment of Sjögren'ssyndrome. Gansu Sci. Technol. 36 (18), 106–108. (inchinese).
Jiang, D., Bai, Y., Zhao, L., Zhang, Y., and Chen, Z. (2016). Observation on the clinical effect of Iramod combined treatment of primary Sjogren’s syndrome. Clin. Misdiagnosis Mistreatment 29 (08), 90–93. (in chinese).
Jiang, H. (2021). Clinical observation of Shure Cunjin granules combined with iguratimod in the treatment of primary Sjögren's syndrome with high IgG[D]. Hunan Univ. Traditional Chin. Med. (in chinese). doi:10.27138/d.cnki.ghuzc.2021.000018
Jiang, W., Zhang, L., Zhao, Y., He, X., Hu, C., and Liu, Y. (2020). The efficacy and mechanism for action of iguratimod in primary Sjögren's syndrome patients. Int. Ophthalmol. 40 (11), 3059–3065. (in chinese). doi:10.1007/s10792-020-01490-6
Jiang, W., Zhao, Y., Lin, H., Liu, Y., and Jin, C. (2014). Observation on the curative effect of Iramod in the treatment of primary Sjogren’s syndrome. West. Med. 26 (6), 719–721. (in chinese).
Ju, Y. J., Guo, D. B., and Chen, H. (2020). Evaluation of the clinical efficacy of methotrexate and ilamod in the treatment of refractory rheumatoid arthritis. China Mod. Dr. 58 (1), 106–109. (in chinese).
Kadura, S., and Raghu, G. (2021). Rheumatoid arthritis-interstitial lung disease: manifestations and current concepts in pathogenesis and management. Eur. Respir. Rev. 30 (160), 210011. doi:10.1183/16000617.0011-2021
Kang, Y., Yan, Q., Fu, Q., Wang, R., Dai, M., Du, F., et al. (2020). Iguratimod as an alternative induction therapy for refractory lupus nephritis: a preliminary investigational study. Arthritis Res. Ther. 22 (1), 65. doi:10.1186/s13075-020-02154-7
Konig, M. F. (2020). The microbiome in autoimmune rheumatic disease. Best. Pract. Res. Clin. Rheumatol. 34 (1), 101473. doi:10.1016/j.berh.2019.101473
Kremer, J. M., Genovese, M. C., Cannon, G. W., Caldwell, J. R., Cush, J. J., Furst, D. E., et al. (2002). Concomitant leflunomide therapy in patients with active rheumatoid arthritis despite stable doses of methotrexate. A randomized, double-blind, placebo-controlled trial. Ann. Intern Med. 137 (9), 726–733. doi:10.7326/0003-4819-137-9-200211050-00007
Man, L., and Xie, Y. (2020). Clinical efficacy of islamod in the treatment of inflammatory myopathy complicated with interstitial pneumonia. J. Clin. Ration. Med. 13 (21), 12–14. (in chinese). doi:10.15887/j.cnki.13-1389/r.2020.21.004
Li, C., Li, R., Liu, H., Cheng, C., and Zhao, T. (2018). Efficacy of methylprednisolone combined with ilamod in the treatment of primary Sjogren’s syndrome and its effect on immunoglobulin levels. China Pharm. 27 (14), 35–37. (in chinese).
Li, C. H., and Wh, H. (2020). The effect of irammod combined with methotrexate in the treatment of rheumatoid arthritis with peripheral blood nuclear factor kappa B receptor activator ligand and bone protective factor. Chin. Med. Clin. 20 (1), 2981–2983. (in chinese).
Li, J., Bao, J., Zeng, J., Yan, A., Zhao, C., and Shu, Q. (2019b). Iguratimod: a valuable remedy from the Asia pacific region for ameliorating autoimmune diseases and protecting bone physiology. Bone Res. 7, 27. doi:10.1038/s41413-019-0067-6
Li, J., Chen, Q. P., Liu, J. Y., and Wang, Y. (2016). Retraction notice to "ß-elemene against human lung cancer via up-regulation of P53 protein expression to promote the release of exosome": lung 86/2 (2014) 144-150. Shaanxi Med. J. 45 (1), 120–121. (in chinese). doi:10.1016/j.lungcan.2016.03.003
Li, J., Mao, H., Liang, Y., Lu, Y., Chen, S., Yang, N., et al. (2013). Efficacy and safety of iguratimod for the treatment of rheumatoid arthritis. Clin. Dev. Immunol. 2013, 310628. doi:10.1155/2013/310628
Li, L., Wang, J., and Li, X. (2019a). An integrated reconciliation framework for domain, gene, and species level evolution. China Pharm. 28, 63–76. (inchinese). doi:10.1109/TCBB.2018.2846253
Li, R., Long, H., and Zhou, C. (2020). Memory traces diminished by exercise affect new learning as proactive facilitation. World Complex Med. 6 (5), 189–191. (in chinese). doi:10.3389/fnins.2020.00189
Li, X., Pan, T., Chen, M. P., Zhuang, H. R., Gao, S. Y., and Zhao, T. L. (2021b). LncRNA TUG1 exhibits pro-fibrosis activity in hypertrophic scar through TAK1/YAP/TAZ pathway via miR-27b-3p. J Med Theor Prac 34 (17), 3009–3020. (in chinese). doi:10.1007/s11010-021-04142-0
Li, Y., Li, K., Zhao, Z., Wang, Y., Jin, J., Guo, J., et al. (2021a). Randomised, double-blind, placebo-controlled study of iguratimod in the treatment of active spondyloarthritis. Front. Med. (Lausanne). 8, 678864. doi:10.3389/fmed.2021.678864
Liang, Z., Feng, W., Ouyang, C., and Feng, M. (2021). Effects of iguratimod on the levels of ESR, CRP and immunoglobulin in patients with primary Sjögren'ssyndrome. Shanghai Med. 42 (01), 32–35. (inchinese).
Lin, Q. (2016). A clinical study on the treatment of rheumatoid arthritis complicated with pulmonary interstitium by iguratimod. North. Pharm. 13 (08), 124–125. (in chinese).
Lin, Y. P., Liu, H., and Gao, J. T. (2019). Preliminary observation on the treatment of ankylosing spondylitis with Iramod. J. Clin. Ration. Use 12 (14), 9–13.
Liu, B., Meng, X., Ma, Y., Li, H., Liu, Y., Shi, N., et al. (2021c). Clinical safety of total glucosides of paeony adjuvant therapy for rheumatoid arthritis treatment: a systematic review and meta-analysis. BMC Complement. Med. Ther. 21 (1), 102. doi:10.1186/s12906-021-03252-y
Liu, B. (2022). Observation on the effect of Ailamod in the treatment of Sjögren's syndrome. Chin. Community Physician 38 (13), 57–59. (in chinese).
Liu, S., Cui, Y., and Zhang, X. (2021b). Molecular mechanisms and clinical studies of iguratimod for the treatment of ankylosing spondylitis. Clin. Rheumatol. 40 (1), 25–32. doi:10.1007/s10067-020-05207-z
Liu, S., Song, L. P., Li, R. B., Feng, L. H., and Zhu, H. (2021a). Iguratimod promotes transformation of mononuclear macrophages in elderly patients with rheumatoid arthritis by nuclear factor-κB pathway. World J. Clin. Cases 9 (10), 2181–2191. doi:10.12998/wjcc.v9.i10.2181
Long, Z., Deng, Y., He, Q., Yang, K., Zeng, L., Hao, W., et al. (2023). Efficacy and safety of iguratimod in the treatment of ankylosing spondylitis: a systematic review and meta-analysis of randomized controlled trials. Front. Immunol. 14, 993860. doi:10.3389/fimmu.2023.993860
Lu, J. (2014). The therapeutic effect of combined application of iguratimod and methotrexate on active rheumatoid arthritis[D]. Shandong University. (in chinese).
Lu, L. J., Bao, C. D., Dai, M., Teng, J. L., Fan, W., Du, F., et al. (2009). Multicenter, randomized, double-blind, controlled trial of treatment of active rheumatoid arthritis with T-614 compared with methotrexate. Arthritis Rheum. 61 (7), 979–987. doi:10.1002/art.24643
Lü, L. J., Teng, J. L., Bao, C. D., Han, X. H., Sun, L. Y., Xu, J. H., et al. (2008). Safety and efficacy of T-614 in the treatment of patients with active rheumatoid arthritis: a double blind, randomized, placebo-controlled and multicenter trial. Chin. Med. J. Engl. 121 (7), 615–619. doi:10.1097/00029330-200804010-00008
Lu, T., and Zhang, W. (2021). Clinical efficacy of iguratimod combined with hydroxychloroquine sulfate in the treatment of primary Sjögren'ssyndrome. J. Clin. Ration. Med. 14 (31), 55–57. (inchinese). doi:10.15887/j.cnki.13-1389/r.2021.31.018
Luo, C., Shi, Y., Chen, X., and Wu, L. (2019). Clinical analysis of 46 cases of PrimarySjogren’s syndrome treated by ilamud. Chin. J. Pract. Diagn. Ther. 33 (12), 1232–1235. doi:10.13507/j.issn.1674-3474.2019.12.024
Luo, Q., Sun, Y., Liu, W., Qian, C., Jin, B., Tao, F., et al. (2013). A novel disease-modifying antirheumatic drug, iguratimod, ameliorates murine arthritis by blocking IL-17 signaling, distinct from methotrexate and leflunomide. J. Immunol. 191 (10), 4969–4978. doi:10.4049/jimmunol.1300832
Luo, Q., Sun, Y., and Xu, Q. (2015). Study on the immunological mechanism of Ilarmod in improving idiopathic pulmonary fibrosis in mice. Proc. 10th Natl. Congr. Immunol., 2015, 323–324. [Publisher unknown](in chinese).
Luo, Q., Guo, D., Yu, Y., and Lin, J. (2018b). Revisiting the enzymatic kinetics of pepsin using isothermal titration calorimetry. Clin. Res. Traditional Chin. Med. 10 (24), 94–100. (inchinese). doi:10.1016/j.foodchem.2018.06.042
Luo, Y., Zheng, N., and Wu, R. (2018a). Is iguratimod effective in refractory axial spondyloarthritis? Scand. J. Rheumatol. 47 (6), 518–520. doi:10.1080/03009742.2017.1390150
Luppi, F., Sebastiani, M., Silva, M., Sverzellati, N., Cavazza, A., Salvarani, C., et al. (2020). Interstitial lung disease in Sjögren's syndrome: a clinical review. Clin. Exp. Rheumatol. 38 (4), 291–300.
Ma, K., Du, W., Wang, X., Yuan, S., Cai, X., Liu, D., et al. (2019). Multiple functions of B cells in the pathogenesis of systemic lupus erythematosus. Int. J. Mol. Sci. 20 (23), 6021. doi:10.3390/ijms20236021
Mahajan, A., Amelio, J., Gairy, K., Kaur, G., Levy, R. A., Roth, D., et al. (2020). Systemic lupus erythematosus, lupus nephritis and end-stage renal disease: a pragmatic review mapping disease severity and progression. Lupus 29 (9), 1011–1020. doi:10.1177/0961203320932219
Marshall, L. L., and Stevens, G. A. (2018). Management of primary Sjögren's syndrome. Consult Pharm. 33 (12), 691–701. doi:10.4140/TCP.n.2018.691
Meng, D. Y., Pan, W. Y., Li, J., Li, H., Li, F., Liu, S. S., et al. (2016b). The effect of methotrexate combined with Ilamud in the treatment of refractory rheumatoid arthritis. China Med. Her. 13, 137–141. (in chinese).
Meng, D. Y., Pan, W. Y., Liu, Y., Jiang, Z., Li, J., Li, H., et al. (2016a). Iguratimod combined with methotrexate on angiogenesis-related cytokines in patients with refractory rheumatoid arthritis the influence of. Med. Her. 35, 148–151. (inchinese).
Meng, D. Y., Wang, G. R., and Pan, W. Y. (2015). Short-term clinical efficacy of methotrexate combined with Iguratimod on refractory rheumatoid arthritis. Chin. J. Clin. Res. 28, 40–42. (inchinese).
Meng, Y., Li, M. Y., Rode, M., Zhang, X. Y., and Luo, L. (2017). Clinical study on the treatment of senile rheumatoid arthritis with Iguratimod tablets combined with methotrexate tablets. Chin. J. Clin. Pharmacol. 33 (2), 1098–1101.
Mizutani, S., Kodera, H., Sato, Y., Nanki, T., Yoshida, S., and Yasuoka, H. (2021). Clinical effectiveness of iguratimod based on real-world data of patients with rheumatoid arthritis. Clin. Rheumatol. 40 (1), 123–132. doi:10.1007/s10067-020-05208-y
Mo, H., and Ma, S. B. (2015). Clinical study on the treatment of active rheumatoid arthritis with Iguratimod combined with methotrexate. Intern. Med. 10 (1), 156–159. (in chinese).
Mo, M. L., Tang, D. X., and Zhang, J. (2018). A randomized controlled trial of methotrexate combined with Iguratimod in the treatment of active rheumatoid arthritis. J. Fujian Med. Univ. 52 (2), 40–43. (in chinese).
Mu, R., Li, C., Li, X., Ke, Y., Zhao, L., Chen, L., et al. (2021). Effectiveness and safety of iguratimod treatment in patients with active rheumatoid arthritis in Chinese: a nationwide, prospective real-world study. Lancet Reg. Health West Pac 10, 100128. doi:10.1016/j.lanwpc.2021.100128
Nozaki, Y. (2021). Iguratimod: novel molecular insights and a new csDMARD for rheumatoid arthritis, from Japan to the world. Life (Basel) 11 (5), 457. doi:10.3390/life11050457
Okamura, K., Yonemoto, Y., Suto, T., Okura, C., and Takagishi, K. (2015). Efficacy at 52 weeks of daily clinical use of iguratimod in patients with rheumatoid arthritis. Mod. Rheumatol. 25, 534–539. doi:10.3109/14397595.2014.998361
Otón, T., and Carmona, L. (2019). The epidemiology of established rheumatoid arthritis. Best. Pract. Res. Clin. Rheumatol. 33 (5), 101477. doi:10.1016/j.berh.2019.101477
Ouyang, D., Ma, Y. Z., Zou, J., Wang, Y. L., Chen, Z., Yang, Y. Y., et al. (2022). Effectiveness and safety of iguratimod monotherapy or combined with methotrexate in treating rheumatoid arthritis: a systematic review and meta-analysis. Front. Pharmacol. 13, 911810. doi:10.3389/fphar.2022.911810
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Pang, L. X., Zheng, Z. H., and Li, Z. Q. (2020). Efficacy of Iramud combined with Etanercept in the treatment of ankylosing spondylitis. J. Trop. Med. 20 (4), 118–121.
Pu, J., Wang, X., Riaz, F., Zhang, T., Gao, R., Pan, S., et al. (2021). Effectiveness and safety of iguratimod in treating primary Sjögren's syndrome: a systematic review and meta-analysis. Front. Pharmacol. 12, 621208. doi:10.3389/fphar.2021.621208
Qi, D. X., Liu, Y., and Huang, D. H. (2019). Study on the efficacy and safety of methotrexate and isilamod in the treatment of rheumatoid arthritis. Chin. J. Drug Eval. 36, 217–220. (in chinese).
Qiu, Y. Y., Tang, Y., Rui, J. B., and Li, J. (2016). Observation on the clinical effect of Iramod in the treatment of refractory ankylosing spondylitis. J. Jiangsu Univ. Med. Ed. 26 (03), 235–239.
Radu, A. F., and Bungau, S. G. (2021). Management of rheumatoid arthritis: an overview. Cells 10 (11), 2857. doi:10.3390/cells10112857
Rao, Y., Tan, J., and Wu, D. (2014). Observation on the clinical efficacy of Ailamod in the treatment of rheumatoid arthritis. Chin. Med. Guide 12 (29), 41–42. (in chinese). doi:10.15912/j.cnki.gocm.2014.29.027
Rao, Y., Zhang, W., Lu, T., and Xu, J. (2022). Efficacy of iguratimod in the treatment of Sjögren's syndrome patients and its influence on immune function. J. Ningxia Med. Univ. 44 (02), 152–156. (in chinese). doi:10.16050/j.cnki.issn1674-6309.2022.02.008
Rivière, E., Pascaud, J., Tchitchek, N., Boudaoud, S., Paoletti, A., Ly, B., et al. (2020). Salivary gland epithelial cells from patients with Sjögren's syndrome induce B-lymphocyte survival and activation. Ann. Rheum. Dis. 79 (11), 1468–1477. doi:10.1136/annrheumdis-2019-216588
Santhanam, S., Mohanasundaram, K., and Krishnan, S. (2020). Interstitial pneumonia with autoimmune features. J. R. Coll. Physicians Edinb 50 (3), 247–255. doi:10.4997/JRCPE.2020.307
Schünemann, H., Brożek, J., Guyatt, G., and Oxman, A. (Editors) (2013). GRADE handbook for grading quality of evidence and strength of recommendations (The GRADE Working Group). Available from guidelinedevelopment.org/handbook.
Shang, Ke. (2014). Efficacy and safety analysis of Ailamod in the treatment of rheumatoid arthritis. Clin. Med. Eng. 21 (12), 1561–1562. (in chinese).
Shang, K., Jiang, L., and Wang, Y. (2019). Efficacy of Ailamod tablet monotherapy in the treatment of active rheumatoid arthritis and its effect on bone metabolism. Jiangxi Med. 54 (06), 593–595+619. (in chinese).
Shao, Q., Wang, S., Jiang, H., and Liu, L. (2020). Efficacy and safety of iguratimod on patients with primary Sjögren's syndrome: a randomized, placebo-controlled clinical trial. Scand. J. Rheumatol. 29, 143–152. doi:10.1080/03009742.2020.1809701
Shao, S., Qu, Z., Liang, Y., Xu, Y., Zhou, D., Li, D., et al. (2021). Iguratimod decreases bleomycin-induced pulmonary fibrosis in association with inhibition of TNF-α in mice. Int. Immunopharmacol. 99, 107936. doi:10.1016/j.intimp.2021.107936
Shi, X. D., Zhang, X. L., and Duan, X. W. (2015). Efficacy and safety of methotrexate combined with iguratimod in the treatment of active rheumatoid arthritis. J. Nanchang Univ. Med. Ed. 55 (2), 33–36+47. (in chinese).
Shrestha, S., Zhao, J., Yang, C., and Zhang, J. (2021). Iguratimod combination therapy compared with methotrexate monotherapy for the treatment of rheumatoid arthritis: a systematic review and meta-analysis. Clin. Rheumatol. 40 (10), 4007–4017. doi:10.1007/s10067-021-05746-z
Shrestha, S., Zhao, J., Yang, C., and Zhang, J. (2020). Relative efficacy and safety of iguratimod monotherapy for the treatment of patients with rheumatoid arthritis: a systematic review and meta-analysis. Clin. Rheumatol. 39 (7), 2139–2150. doi:10.1007/s10067-020-04986-9
Strand, V., Cohen, S., Schiff, M., Weaver, A., Fleischmann, R., Cannon, G., et al. (1999). Treatment of active rheumatoid arthritis with leflunomide compared with placebo and methotrexate. Leflunomide Rheumatoid Arthritis Investigators Group. Arch. Intern Med. 159 (21), 2542–2550. doi:10.1001/archinte.159.21.2542
Sun, P., and Li, R. (2022). Clinical efficacy of islamod combined with methotrexate in the treatment of rheumatoid arthritis and its effect on bone metabolism in patients. Clin. Med. Eng. 29 (1), 41–42. (in chinese). doi:10.3969/j.issn.1674-4659.2022.01.0041
Suto, T., Yonemoto, Y., Okamura, K., Sakane, H., Takeuchi, K., Tamura, Y., et al. (2019). The three-year efficacy of iguratimod in clinical daily practice in patients with rheumatoid arthritis. Mod. Rheumatol. 29, 775–781. doi:10.1080/14397595.2018.1510879
Tanaka, K., Yamamoto, T., Aikawa, Y., Kizawa, K., Muramoto, K., Matsuno, H., et al. (2003). Inhibitory effects of an anti-rheumatic agent T-614 on immunoglobulin production by cultured B cells and rheumatoid synovial tissues engrafted into SCID mice. Rheumatol. Oxf. Engl. 42 (11), 1365–1371. doi:10.1093/rheumatology/keg381
Tanaka, M. (2021). Conflict between efficacy and economy in rheumatoid arthritis treatment: iguratimod is found at a compromise. Lancet Reg. Health West Pac 10, 100144. doi:10.1016/j.lanwpc.2021.100144
Tanaka, Y. (2016). Stopping tumour necrosis factor-targeted biological DMARDs in rheumatoid arthritis. Rheumatol. Oxf. 55 (2), ii15–ii22. doi:10.1093/rheumatology/kew352
Tian, J. W., and Tao, P. F. (2017). The effect of Iguratimod combined with methotrexate on serum M-CSF, IL-6, IL-8 and bone metabolism in patients with rheumatoid arthritis. Hainan Med. 28, 391–394. (in chinese).
Tian, X. P., Liu, S. Y., Li, Q., Bi, L. Q., Kong, X. D., Zhao, D. B., et al. (2020). Efficacy and safety of ellamod or leflunomide combined with methotrexate in the treatment of active rheumatoid arthritis sexual comparison: a multicenter randomized double-blind double-simulation controlled clinical study. Chin. J. Rheumatology 24 (3), 148–158. (in chinese). doi:10.3760/cma.j.issn.1007-7480.2020.03.002
van der Woude, D., and van der Helm-van Mil, A. H. M. (2018). Update on the epidemiology, risk factors, and disease outcomes of rheumatoid arthritis. Best. Pract. Res. Clin. Rheumatol. 32 (2), 174–187. doi:10.1016/j.berh.2018.10.005
Vehof, J., Utheim, T. P., Bootsma, H., and Hammond, C. J. (2020). Advances, limitations and future perspectives in the diagnosis and management of dry eye in Sjögren's syndrome. Clin. Exp. Rheumatol. 38 (4), 301–309.
Wang, L., Liu, S., and Rong, C. (2019a). Clinical study on the treatment of rheumatoid arthritis with methotrexate tablets combined with Iguratimod tablets. Chin. J. Clin. Pharmacol. 35 (2), 231–234. (in chinese).
Wang, W., Zhou, H., and Liu, L. (2018a). Side effects of methotrexate therapy for rheumatoid arthritis: a systematic review. Eur. J. Med. Chem. 158, 502–516. doi:10.1016/j.ejmech.2018.09.027
Wang, X., Ma, C., Li, P., Zhao, F., and Bi, L. (2017). Effects of iguratimod on the levels of circulating regulators of bone remodeling and bone remodeling markers in patients with rheumatoid arthritis. Clin. Rheumatol. 36, 1369–1377. doi:10.1007/s10067-017-3668-8
Wang, X., Yuan, X., Wang, Q., Zhou, Y., Li, X., Wang, G., et al. (2018b). Clinical study ofEffectiveness and safety of iguratimod in treating primary Sjogren’sSyndrome. Chin. J. Dis. Control Prev. 22 (1), 75–78. doi:10.16462/j.cnki.zhjbkz.2018.01.017
Wang, X. T. (2017). Study on the effect of iguratimod on bone metabolism in patients with rheumatoid arthritis[D]. Jilin, China: Jilin University. (in chinese).
Wang, Y., Chen, C., Gao, W., and Guo, F. (2022). Evaluation of the efficacy of high-frequency ultrasound in the treatment of rheumatoid arthritis. Med. Rev. 28 (10), 2055–2059. (in chinese).
Wang, Y., Zhao, F., Ai, X., Liu, Y., Zhu, Z., and Li, Q. (2019b). Comparison of mammography and ultrasonography for tumor size of DCIS of breast cancer. Geriatrics Health Care 25 (02), 209–213. doi:10.2174/1573405614666180131163321
Wells, A. U., and Denton, C. P. (2014). Interstitial lung disease in connective tissue disease-mechanisms and management. Nat. Rev. Rheumatol. 10 (12), 728–739. doi:10.1038/nrrheum.2014.149
Winthrop, K. L. (2017). The emerging safety profile of JAK inhibitors in rheumatic disease. Nat. Rev. Rheumatol. 13 (4), 234–243. doi:10.1038/nrrheum.2017.23
Wu, J., Liang, Y., Li, M., Hu, S., Wang, C., Wang, L., et al. (2022). Repair effect and safety of Ellamod combined with methotrexate and tripterygium wilfordii on articular cartilage cell damage in rheumatoid arthritis Analysis. Anhui Med. 26 (01), 183–187. (in chinese).
Xia, N. N., Chen, Z. F., and Zhang, W. F. (2020). Observation on the effects of Iguratimod and Tripterygium Glycosides in the treatment of rheumatoid arthritis. Pract. Integr. Traditional Chin. West. Med. 20 (1), 76–77. (inchinese).
Xia, Z., Lyu, J., Hou, N., Song, L., Li, X., and Liu, H. (2016). Iguratimod in combination with methotrexate in active rheumatoid arthritis: therapeutic effects. Z Rheumatol. 75 (8), 828–833. English. doi:10.1007/s00393-015-1641-y
Xia, Z., Liu, Y., and Meng, F. (2017). Analysis of clinical effect of iguratimod combined treatment on primary Sjögren'ssyndrome. China Health Nutr. 27 (34), 263. (in chinese).
Xie, H., Liu, Y., Wang, J., Zeng, C., and Zhou, Y. (2020b). Hydroxychloroquine sulfate combined with total glucosides of paeony and ilamod in the treatment of primary Sjogren’s syndrome. West. Med. 32 (09), 1358–1362. (in chinese).
Xie, L., Zou, Q. H., Shi, Y., Cheng, X., and Fang, Y. F. (2018). The effect of Iguratimod combined with MTX on IL-1, serum TNF-α and VEGF levels in patients with refractory rheumatoid arthritis. Guizhou Med. 42 (1), 831–832. (in chinese).
Xie, S., Li, S., Tian, J., and Li, F. (2020a). Iguratimod as a new drug for rheumatoid arthritis: current landscape. Front. Pharmacol. 11, 73. doi:10.3389/fphar.2020.00073
Xiong, M., and GengGuanghui, Z. (2020). The clinical efficacy of methotrexate combined with iguratimod on active rheumatoid arthritis. Henan Med. Res. 29 (2), 93–95. (in chinese).
Xu, B. J., Mo, S. Q., Xue, X. Q., and Wu, Y. (2019). Study on the efficacy and safety of Iramod in the treatment of ankylosing spondylitis. New Med. 50 (12), 915–918.
Xu, B., Mo, S., and Xue, X. (2015a). Clinical study of methotrexate combined with Ilamud in the treatment of rheumatoid arthritis. J. Clin. Med. Pract. 19 (1), 120–122. (in chinese).
Xu, D., Lv, X., Cui, P., and Ma, S. (2017b). Comparison of the efficacy and safety of iguratimod and hydroxychloroquine in the treatment of patients with Sjögren'ssyndrome. J. Difficult Difficult Dis. 16 (09), 915–918. (in chinese).
Xu, L. M., Yuan, M., Liu, Y. F., Sun, H. X., Liu, L. N., Shi, Y. J., et al. (2017a). Clinical observation on the treatment of rheumatoid arthritis with Iguratimod combined with methotrexate. Guide Chin. Med. 15 (1), 47–48. (in chinese).
Xu, Y. M., Fan, S. N., and Zou, L. (2015b). Study on the effect of iguratimod combined with methotrexate treatment on anti-cyclic guanidine peptide antibodies and other indicators in patients with rheumatoid arthritis. J. Clin. Intern. Med. 32 (1), 833–835. (in chinese).
Xu, Y. W., Tao, Y. L., Zhang, H., and Dai, S. M. (2021). Observation on the clinical efficacy of iguratimod in the treatment of axial spondyloarthritis. Shanghai Med. J. 44 (6), 421–424. doi:10.19842/j.cnki.issn.0253-9934.2021.06.013
Yan, Q., Du, F., Huang, X., Fu, Q., Chen, S., Dai, D., et al. (2014). Prevention of immune nephritis by the small molecular weight immunomodulator iguratimod in MRL/lpr mice. PLoS One 9 (10), e108273. doi:10.1371/journal.pone.0108273
Yan, Q., Du, F., Kang, Y., Ye, P., Wang, X., Xu, J., et al. (2021). Comparison of iguratimod and conventional cyclophosphamide with sequential azathioprine as treatment of active lupus nephritis: study protocol for a multi-center, randomized, controlled clinical trial (iGeLU study). Trials 22 (1), 530. doi:10.1186/s13063-021-05475-3
Yan, X. Z., and Wang, F. L. (2018). Effect of Iguratimod combined with methotrexate on serum-related cytokines and bone metabolism in patients with rheumatoid arthritis. J. Changchun Univ. Traditional Chin. Med. 34 (2), 369–372. (in chinese).
Ye, Y., Liu, M., Tang, L., Du, F., Liu, Y., Hao, P., et al. (2019b). Iguratimod represses B cell terminal differentiation linked with the inhibition of PKC/EGR1 axis. Arthritis Res. Ther. 21 (1), 92. doi:10.1186/s13075-019-1874-2
Ye, Y., Liu, M., Tang, L., Du, F., and Hao, P. (2019a). Iguratimod represses B cell terminal differentiation linked with the inhibition of PKC/EGR1 axis. Arthritis Res. Ther. 21 (1), 92. doi:10.1186/s13075-019-1874-2
Yi, M. (2018). Observation of the changes of ESR, CRP, IgG, IgA, IgM levels in patients with primary Sjogren’s syndrome by Iramod. Electron. J. Clin. Med. Literature 5 (95), 147+149. (in chinese).
Yoo, H., Hino, T., Han, J., Franks, T. J., Im, Y., Hatabu, H., et al. (2020). Connective tissue disease-related interstitial lung disease (CTD-ILD) and interstitial lung abnormality (ILA): evolving concept of CT findings, pathology and management. Eur. J. Radiol. Open 8, 100311. doi:10.1016/j.ejro.2020.100311
Yoshioka, Y., Takahashi, N., Kaneko, A., Hirano, Y., Kanayama, Y., Kanda, H., et al. (2016). Disease activity early in treatment as a predictor of future low disease activity in RA patients treated with iguratimod. Mod. Rheumatol. 26, 169–174. doi:10.3109/14397595.2015.1069475
Yu, W. (2020). Clinical analysis of Iramod in the treatment of primary Sjogren’s syndrome. Guide Chin. Med. 18 (06), 150. (in chinese).
Yuan, F. F., Chen, Y. H., Lin, J. X., and Luo, J. (2020). Efficacy of islammod combined with methotrexate in the treatment of refractory ankylosing spondylitis and its effect on serum SOD and CTX-I levels in patients. J. Pharm. Epidemiol. 29 (3), 163–165+205.
Zeng, H. Q., Kong, W. H., Zhuang, P., Dong, H. J., Yin, Z. H., Chen, X., et al. (2016). Observation on the efficacy of Iramod in the treatment of ankylosing spondylitis. Hainan Med. 27 (1), 118–120.
Zeng, L., He, Q., Yang, K., Hao, W., Yu, G., and Chen, H. (2022b). A systematic review and meta-analysis of 19 randomized controlled trials of iguratimod combined with other therapies for sjogren's syndrome. Front. Immunol. 13, 924730. doi:10.3389/fimmu.2022.924730
Zeng, L., Yu, G., Yang, K., Hao, W., and Chen, H. (2022a). The effect and safety of iguratimod combined with methotrexate on rheumatoid arthritis: a systematic review and meta-analysis based on a randomized controlled trial. Front. Pharmacol. 12, 780154. doi:10.3389/fphar.2021.780154
Zhang, C. (2018). Clinical observation of Ailamod in the treatment of active rheumatoid arthritis. Gansu Med. 37 (6), 502–504. (in chinese). doi:10.15975/j.cnki.gsyy.2018.06.010
Zhang, J., and Shen, S. (2019). Efficacy and mechanism of iguratimod in the treatment of Sjögren'ssyndrome. Shaanxi Med. J. 48 (4), 452–455. (in chinese).
Zhang, L., Zhong, M., Qiao, L., and Guo, X. (2019). Efficacy evaluation of iguratimod in the treatment of Sjögren's syndrome complicated with interstitial pulmonary disease. China Mod. Med. Appl. 13 (20), 1–3. (in chinese). doi:10.14164/j.cnki.cn11-5581/r.2019.20.001
Zhang, X. (2019). Comparative observation of iguratimod and hydroxychloroquine in the treatment of patients with Sjögren'ssyndrome. Chin. Med. Guide 17 (32), 103. (in chinese). doi:10.15912/j.cnki.gocm.2019.32.081
Zhao, H. N., and Hao, X. J. (2018). The clinical effect of Iguratimod combined with methotrexate in the treatment of rheumatoid arthritis. Clin. Med. Res. Pract. 3 (1), 44–45. (in chinese).
Zhao, J. (2020). Comparison of the efficacy and safety of iguratimod and hydroxychloroquine in the treatment of Sjögren'ssyndrome. Chin. Med. Clin. Med. 20 (21), 3627–3629. (in chinese).
Zhao, L., Jiang, Z., and Zhang, O. (2017a). Analysis of efficacy and safety of treatment of active rheumatoid arthritis with iguratimod and methotrexate. Biomed. Res. 28 (1), 2353–2359.
Zhao, L. (2019). Observation on the effect of methylprednisolone combined with Iramud in the treatment of primary Sjogren’s syndrome. Chin. Natl. Health Med. 31 (17), 35–36. (in chinese).
Zhao, L., Zhou, R., Mu, B., and Huang, C. (2017b). The effect of iguratimod on the mouse model of bleomycin-induced pulmonary interstitial fibrosis. Chin. J. Rheumatology 21 (06), 370–374 +433. (in chinese).
Zhao, W. M., Yao, D. Y., Huo, H. S., Qin, C. M., Wei, Q. J., and Sun, K. (2016). Clinical study of Iguratimod in the treatment of active rheumatoid arthritis. Chin. J. Postgraduates Med. 39 (3), 450–452. (in chinese).
Zhu, Q., Song, J., Xu, Y., Liu, H., Miao, Y., Fan, Y., et al. (2016). A study on the clinical efficacy of Ailamod on rheumatoid arthritis and the regulation of T helper cell subsets. Chin. Rheumatism J. Sci. 20 (2), 93–99. (in chinese).
Zhuang, Y., Chen, Y., Wang, Z., and Lv, Z. (2021). Efficacy observation of Ailamod in the treatment of Sjögren's syndrome complicated with interstitial pneumonia. Chin. Prescr. Med. 19 (10), 104–105. (in chinese).
Keywords: autoimmune disease, iguratimod, rheumatoid arthritis, ankylosing spondylitis, primary Sjögren’s syndrome, autoimmune disease with interstitial pneumonia, systematic review, meta-analysis
Citation: Zeng L, He Q, Deng Y, Li Y, Chen J, Yang K, Luo Y, Ge A, Zhu X, Long Z and Sun L (2023) Efficacy and safety of iguratimod in the treatment of rheumatic and autoimmune diseases: a meta-analysis and systematic review of 84 randomized controlled trials. Front. Pharmacol. 14:1189142. doi: 10.3389/fphar.2023.1189142
Received: 18 March 2023; Accepted: 21 August 2023;
Published: 07 December 2023.
Edited by:
Mohammad Movahedi, University of Toronto, CanadaReviewed by:
Dazhi Fan, Foshan Women and Children Hospital, ChinaAbir Mokbel, Cairo University, Egypt
Copyright © 2023 Zeng, He, Deng, Li, Chen, Yang, Luo, Ge, Zhu, Long and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liuting Zeng, emx0YWIyMDE2QGhvdG1haWwuY29t; Lingyun Sun, bGxpbmd5dW5zdW5Abmp1LmVkdS5jbg==
†These authors share first authorship
 Liuting Zeng
Liuting Zeng Qi He
Qi He Ying Deng2†
Ying Deng2† Kailin Yang
Kailin Yang