- 1Department of Gastroenterology, Sir Run Run Shaw Hospital, College of Medicine Zhejiang University, Hangzhou, China
- 2Inflammatory Bowel Disease Center of Sir Run Run Shaw Hospital, College of Medicine Zhejiang University, Hangzhou, China
Introduction: The effectiveness and safety of vedolizumab (VDZ) against ulcerative colitis (UC) have been validated in several randomized controlled trials and real-world studies in Western countries. However, there are few studies on VDZ in Asia, and the follow-up period for these studies is generally short. Therefore, this study evaluates the long-term effectiveness and safety of VDZ in Chinese patients with UC.
Methods: This retrospective study included patients with moderate to severe UC treated with VDZ between September 2019 and April 2022 at Sir Run Run Shaw Hospital, College of Medicine Zhejiang University. Clinical response and remission were assessed using the patient reported outcomes and the partial Mayo Score, and mucosal remission and healing were assessed using the Mayo Endoscopy Score. The primary endpoint was defined as clinical remission at week 14, and secondary endpoints included clinical response and steroid-free clinical remission at week 14, clinical response, clinical remission, and steroid-free clinical remission at week 52, and mucosal remission and healing at weeks 14 ± 8 and 52 ± 8.
Results: Overall, 64 patients with moderate to severe UC were enrolled. The clinical response, clinical remission, and steroid-free clinical remission rates at week 14 were 73.4% (47/64), 65.6% (42/64), and 54.7% (35/64), respectively. Mucosal remission and healing rates at week 14 ± 8 were 64.7% (22/34) and 38.2% (13/34), respectively. A total of 48 patients were treated with VDZ for 52 weeks. Based on intention-to-treat analysis, the clinical response, clinical remission, and steroid-free clinical remission rates at week 52 were 68.8% (44/64), 64.1% (41/64), and 64.1% (41/64), respectively. Mucosal remission and healing rates at week 52 ± 8 were 70.6% (12/17) and 35.3% (6/17), respectively. During the follow-up period, the most common adverse event was skin rash (6/64). No cases of acute infusion reactions, delayed allergic reactions, new hepatitis B infections, active tuberculosis, or malignant tumors were reported.
Conclusion: In this single-center retrospective real-world study, the effectiveness of long-term use of VDZ for Chinese patients with UC was similar to the outcomes previously reported in other geographical regions and populations; no new safety signals were found compared with other registered studies.
1 Introduction
Ulcerative colitis (UC), a chronic inflammatory bowel disease (IBD), primarily affects the rectum and colon and is caused by environmental factors, genetic predisposition, immune dysregulation, and gut dysbiosis (Lee et al., 2018). UC often presents with persistent or recurrent episodes of diarrhea, mucopurulent stools, abdominal pain, rectal tenesmus, and varying degrees of systemic symptoms. The annual incidence of UC is increasing in Asia, especially in newly industrialized countries, placing a severe disease burden on patients and society (Park and Cheon, 2021).
Conventional therapeutic agents for UC include 5-aminosalicylates, immunomodulators, and corticosteroids. Biologics can be considered for patients with an inadequate response or intolerance to conventional therapies (Raine et al., 2022). Initially, only anti-tumor necrosis factor (TNF) agents (including infliximab [IFX], adalimumab [ADA], and golimumab) were approved for UC. However, up to a third of patients do not respond to anti-TNF agents (Argollo et al., 2020). Therefore, new biologics are urgently required for conversion therapy in these patients.
Vedolizumab (VDZ) is a humanized gut-specific monoclonal antibody that selectively binds to α4β7 integrin and inhibits its binding to mucosal addressin cell adhesion molecule-1, thereby reducing the migration of lymphocytes through the endothelium of the gut and reducing inflammation in intestinal tissues (Wyant et al., 2016). In the GEMINI randomized controlled trial (RCT), VDZ was superior to the placebo in inducing and maintaining clinical remission and mucosal healing and showed a favorable safety profile during follow-up (Feagan et al., 2013). Therefore, the US Food and Drug Administration and the European Medicines Agency approved VDZ for UC in May 2014. IFX and VDZ are the only biological agents that have been approved by the State Drug Administration of China for the treatment of UC; the latter was approved in March 2020. However, owing to explicit inclusion and exclusion criteria, patients participating in clinical trials may not adequately represent the entire patient population. Therefore, real-world studies (RWSs) have become as important as RCTs (Kim et al., 2018).
Although several RWSs have been conducted for VDZ (Kotze et al., 2018; Narula et al., 2018; White et al., 2020), they have primarily focused on Western populations. Meanwhile, a limited number of studies have been reported on the real-world effectiveness of VDZ among Asian patients with IBD; those that have been performed have only presented short-term results. Therefore, the current RWS was performed to evaluate the long-term effectiveness and safety of VDZ in Chinese patients with UC.
2 Materials and methods
2.1 Study design and population
This was a retrospective study conducted at the Inflammatory Bowel Disease Center of Sir Run Run Shaw Hospital, College of Medicine Zhejiang University. Patients with active UC at our center who were treated with VDZ between September 2019 and April 2022 were enrolled. The inclusion criteria were as follows: 1) ≥ 18 years of age, 2) diagnosed as having UC for ≥3 months, 3) moderate to severe disease activity (Mayo Score ≥6), and 4) had received at least three doses of intravenous VDZ. Ultimately, 64 patients were enrolled in this study.
VDZ was administered intravenously at a dose of 300 mg on weeks 0, 2, and 6 for the induction treatment and every 8 weeks thereafter for maintenance. Complete blood count, liver function tests, C-reactive protein (CRP) level, erythrocyte sedimentation rate, and other relevant laboratory tests were conducted before each infusion.
A combination of corticosteroids was permitted in the study. That is, prednisone was initially administered at a dose of 40 mg/day for 4 weeks. The dose was then reduced by 5 mg/day over 1 week intervals until reaching a dose of 20 mg/day (i.e., week 5: 35 mg/day, week 6: 30 mg/day, week 7: 25 mg/day, week 8: 20 mg/day), which was maintained for 4 weeks and then further reduced by 2.5 mg/day over 1 week intervals until prednisone was discontinued. The total treatment duration was 4–6 months.
2.2 Variables
Electronic medical records of the included patients were reviewed. Information including sex, age, body mass index, smoking history, duration of UC, extent of UC, disease activity, time of initiation and withdrawal from VDZ treatment, laboratory tests, endoscopic examinations, prior and concomitant drug use, extraintestinal manifestations, and adverse events was obtained.
2.3 Outcomes and definitions
The extent of UC was defined according to disease site: disease involving the rectum only (E1), disease distal to the splenic flexure (E2), and disease extending proximal to the splenic flexure (E3). Disease activity was classified as mild (3–5 points), moderate (6–10 points), or severe (11–12 points), according to the Mayo Score. During follow-up, patient-reported outcomes (PRO2, rectal bleeding, and stool frequency) and the partial Mayo Score (rectal bleeding, stool frequency, and physician’s global assessment) were used to assess the degree of disease control; higher scores indicated more active disease. The Mayo Endoscopic Score (MES) was used to assess the degree of mucosal lesions and was scored 0–3 according to severity.
The primary endpoint of this study was clinical remission at week 14. The secondary endpoints included clinical response and steroid-free clinical remission at week 14 and week 52, respectively, and mucosal remission and mucosal healing at weeks 14 ± 8 and 52 ± 8, respectively, during the follow-up. Clinical response was defined as a decrease of at least 50% in PRO2, and clinical remission as PRO2 (rectal bleeding = 0, stool frequency = 0) or a partial Mayo Score <3 with no subscore >1. Mucosal remission was defined as an MES ≤1, and mucosal healing as an MES = 0.
All patients were followed up from the time of VDZ infusion until either one of the following occurred: death, surgery, treatment discontinuation, or termination of the follow-up period. All scoring procedures were performed by the same physician to reduce unnecessary bias. All adverse events related to VDZ, including acute infusion reactions, delayed allergic reactions, acute and chronic infections, and malignancies, were recorded during treatment.
2.4 Statistical analysis
SPSS 26.0 was used for statistical analysis, and p < 0.05 was considered to indicate statistical significance. Normally distributed continuous variables were expressed as mean ± standard deviation, and t-tests were used for comparison between groups. Non-normally distributed continuous variables were expressed as median and interquartile range (IQR), and the Mann–Whitney U test was used for comparison between groups. Categorical variables were expressed as numbers and percentages (%), and the chi-square or Fisher’s exact test was used for comparison between groups. Per protocol analysis and intention-to-treat analysis were used to analyze the clinical response and remission rates at Week 52.
2.5 Ethical considerations
This study was approved by the ethical review committee of the Sir Run Run Shaw Hospital, College of Medicine Zhejiang University. All patients provided written informed consent.
3 Results
3.1 Baseline characteristics
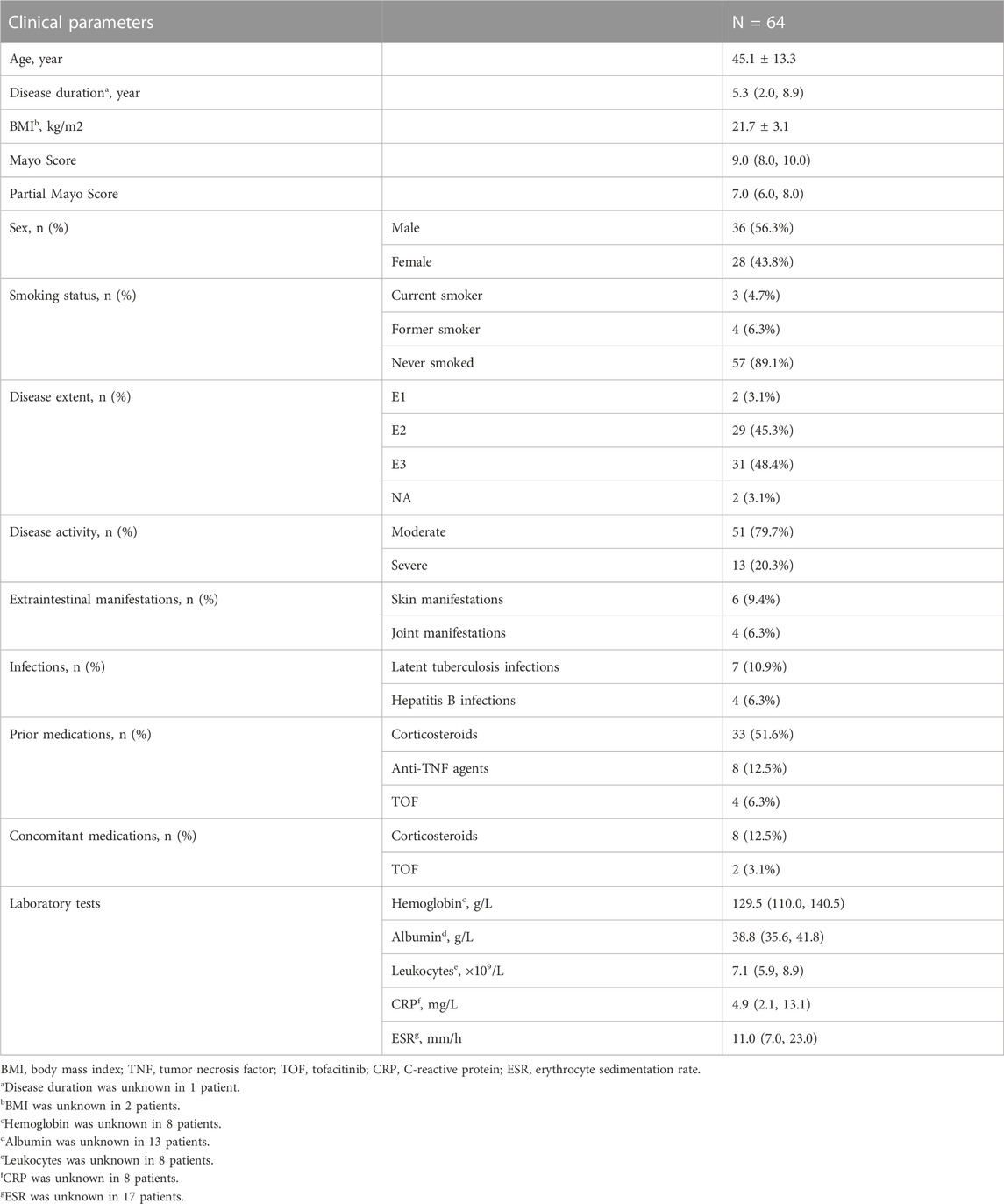
Between September 2019 and April 2022, 64 patients with moderate to severe UC met the inclusion criteria (Figure 1). The mean age of the patients was 45.1 ± 13.3 years, and 56.3% were males. The median disease duration was 5.3 (2.0, 8.9) years, and the median Mayo Score at baseline was 9.0 (8.0–10.0). Most patients had extensive (48.4%) and moderate (79.7%) disease activity. Before receiving VDZ, 33 (51.6%) patients had previously received corticosteroids, and 8 were still receiving corticosteroids at baseline. Additionally, 8 (12.5%) patients had received anti-TNF agents, all of which were eventually discontinued due to loss of response. An additional 4 (6.3%) patients had received tofacitinib (TOF); 2 were still receiving TOF at the start of VDZ treatment. At baseline, the mean albumin level was 38.8 (35.6, 41.8) g/L, while the median leukocyte count, hemoglobin and CRP levels, and erythrocyte sedimentation rate were within the normal range. Patient characteristics are shown in Table 1.
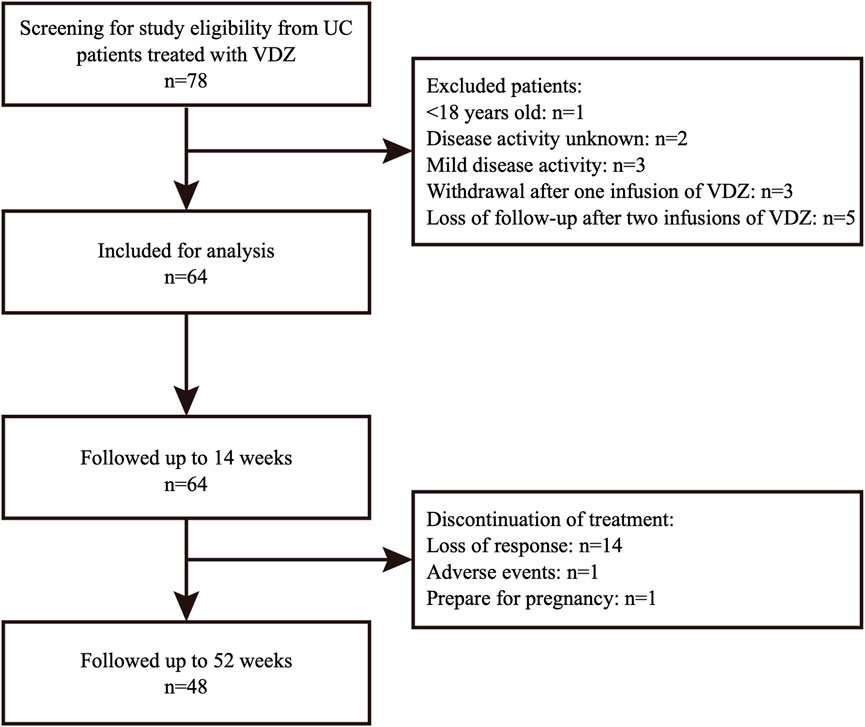
FIGURE 1. Study flowchart of patients included in analysis. UC, ulcerative colitis; VDZ, vedolizumab.
Prior to VDZ treatment, four patients had postoperative malignant tumors, including two lung cancers, one thyroid cancer, and one breast cancer. Four patients had hepatitis B infection at baseline and were treated with antiviral drugs (two with entecavir and two with tenofovir disoproxil fumarate). Seven patients had latent tuberculosis infections, three of which were prophylactically treated with antituberculosis drugs, while the remaining four did not routinely receive antituberculosis prophylaxis. As extraintestinal manifestations, four patients had arthropathy involving the knee and hand joints, with magnetic resonance imaging showing joint effusion. Six patients had dermatological extraintestinal manifestations, including urticaria and facial acne.
3.2 Assessment at week 14
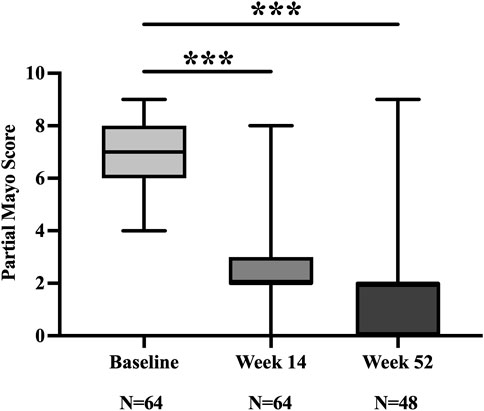
At week 14, 73.4% (47/64) and 65.6% (42/64) of patients achieved clinical response and remission, respectively. The partial Mayo Score decreased from 7.0 (6.0, 8.0) at baseline to 2.0 (2.0, 3.0) (Figure 2). Of the 42 patients who achieved clinical remission, 7 remained on concomitant corticosteroids, yielding a steroid-free clinical remission rate of 54.7% (35/64).
Thirty-four patients underwent follow-up endoscopy at week 14 ± 8, of which 64.7% (22/34) achieved mucosal remission, and 38.2% (13/34) achieved mucosal healing (Figure 3).

FIGURE 3. Clinical response, clinical remission, and steroid-free clinical remission at weeks 14 and 52 (A). Mucosal remission and mucosal healing at weeks 14 ± 8 and 52 ± 8 (B).
Analysis of the laboratory test results showed that anemia and hypoproteinemia were significantly corrected at week 14 among these patients. Levels of inflammatory markers, such as leukocytes and CRP, also decreased (Figure 4).
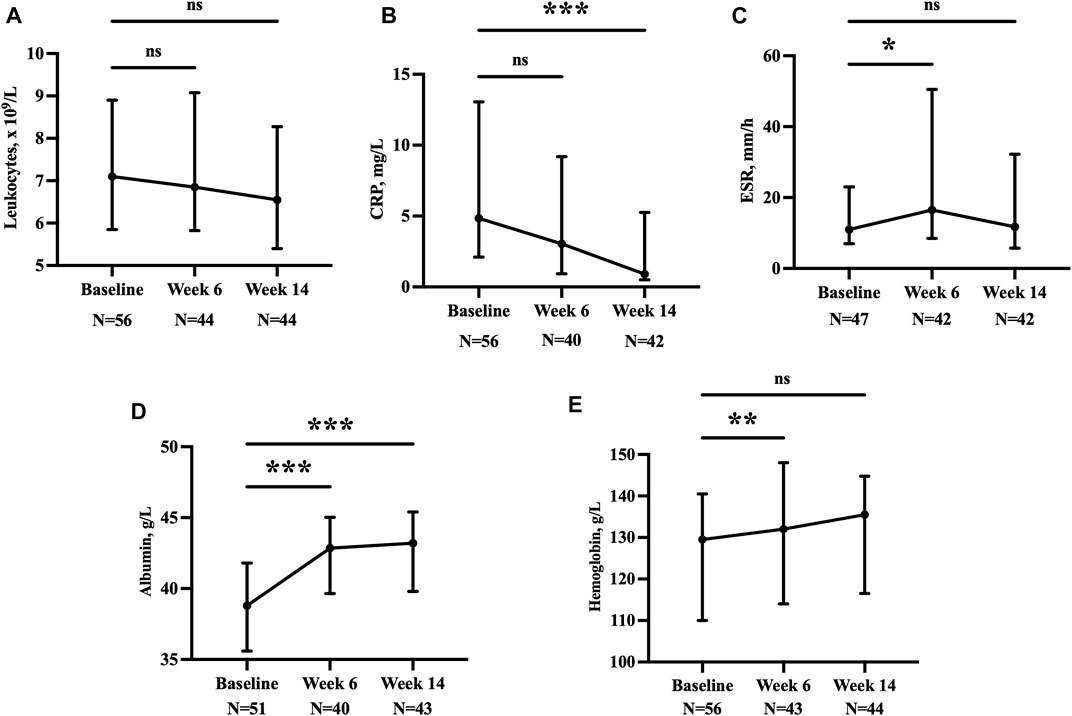
FIGURE 4. Leukocytes (A), CRP (B), ESR (C), albumin (D), and hemoglobin (E) at baseline, week 6, and week 14. *p < 0.05; **p < 0.01; ***p < 0.001; ns, p > 0.05. CRP, C-reactive protein; ESR, erythrocyte sedimentation rate.
For the 17 patients who did not achieve clinical response at week 14, their partial Mayo Score also demonstrated improvement from 7.0 (5.0, 7.5) to 5.0 (4.0, 7.0) (p = 0.002).
3.3 Assessment at week 52
Of the 47 patients showing clinical response at week 14, 46 continued VDZ treatment, with 43 having been followed up to 52 weeks. Three patients discontinued treatment due to loss of response (one discontinued after five infusions and two after seven infusions), of which one was switched to IFX, one to TOF, and one to corticosteroids. One patient did not enter the maintenance phase due to pregnancy preparation.
Of the 17 patients who did not achieve clinical response at week 14, 2 patients discontinued treatment: one was switched to IFX, and one (history of lung cancer) began chemotherapy due to an enlarged pulmonary nodule. Overall, 15 patients showed improvement in their symptoms. At Week 52, ten patients had discontinued treatment due to loss of response, including six patients after four infusions, three after six infusions, and one after seven infusions. Of the patients who discontinued treatment, two were switched to anti-TNF agents (one was administered IFX and one was administered ADA), two to TOF, two to mesalazine, two to herbal treatment, one to corticosteroids, and one to a risankizumab clinical trial.
Overall, 48 patients completed 52 weeks of treatment. Based on per protocol analysis, 91.7% (44/48) achieved clinical response, 85.4% (41/48) achieved clinical remission, and 85.4% (41/48) achieved steroid-free clinical remission. Meanwhile, intention-to-treat analysis revealed that the clinical response, clinical remission, and steroid-free clinical remission rates were 68.8% (44/64), 64.1% (41/64), and 64.1% (41/64), respectively. Seventeen patients underwent follow-up endoscopy at week 52 ± 8; 70.6% (12/17) achieved mucosal remission, and 35.3% (6/17) achieved mucosal healing (Figure 3).
3.4 Assessment at the end of follow-up
Among the patients who were followed up for 52 weeks, 43 continued VDZ treatment to the end of the follow-up period, of which 1 did not achieve clinical response, and concomitant use of mesalazine was initiated for symptom control. Five patients discontinued treatment, four due to loss of response and conversion to IFX, and one due to clinical remission and discontinuing VDZ on his own.
During the follow-up period, one patient underwent surgery. The patient was unresponsive to mesalazine and corticosteroids and was switched to IFX after five infusions of VDZ; the MES was 3. After two IFX infusions, the patient’s symptoms did not significantly improve, and the MES remained at 3. Finally, ileal pouch–anal anastomosis was performed.
3.5 Subgroup analysis
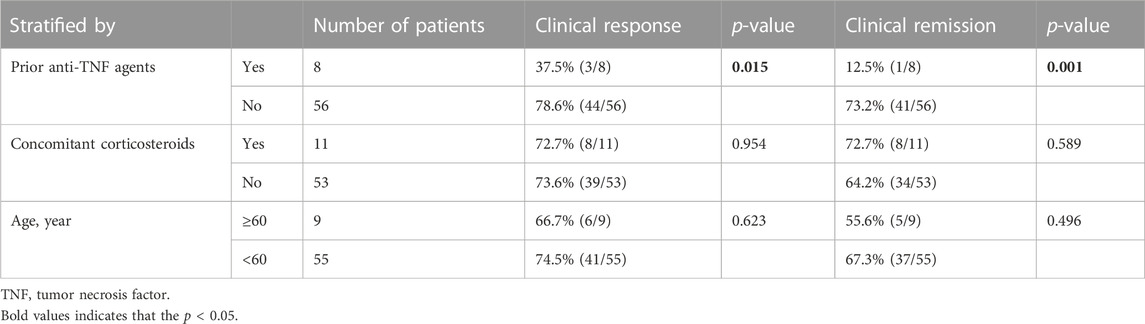
Subgroup analysis showed that the clinical response and remission rates were 78.6% and 73.2% in patients who never received anti-TNF agents, which were both higher than those in patients who had. As for treatment combined with corticosteroids, 11 patients received corticosteroids at week 14, and the clinical response and remission rates for these patients were both 72.7%; these values were not significantly different compared with those in patients treated without concomitant corticosteroids. Further analysis revealed that the clinical response and remission rates were 66.7% and 55.6% in patients aged ≥60 years, respectively, and neither value differed significantly from those in patients aged <60 years (Table 2).
3.6 Safety
At the end of the final follow-up visits, the proportion of patients who experienced ≥1 adverse event (AE) was 29.7% (19/64), most of which were mild. The most common AEs were skin rashes (6/64). Arthralgia, fatigue, and elevated alanine transaminase were relatively common. No cases of acute infusion reactions, delayed allergic reactions, newly onset hepatitis B or tuberculosis infection, or new malignant tumors were reported. The incidence of serious adverse events was 1.6% (1/64). The patient discontinued VDZ due to an enlarged pulmonary nodule.
At baseline, 55 patients underwent chest imaging, of whom 35 had pulmonary nodules. During the follow-up, 15 patients underwent chest imaging again. Seven patients showed no significant changes on imaging. Pulmonary nodules disappeared or decreased among four patients. Three patients had an increase in the size of the pulmonary nodule. One patient developed a new pulmonary nodule, which was diagnosed postoperatively as lung cancer. The nodule was found at week 32 after VDZ treatment, with imaging suggesting a 4-mm nodule in the upper lobe of the right lung.
4 Discussion
In this single-center retrospective study, analysis of data from 69 patients with UC revealed that the clinical response and remission rates after VDZ treatment were 73.4% and 65.6% at week 14% and 68.8% and 64.1% at week 52, respectively.
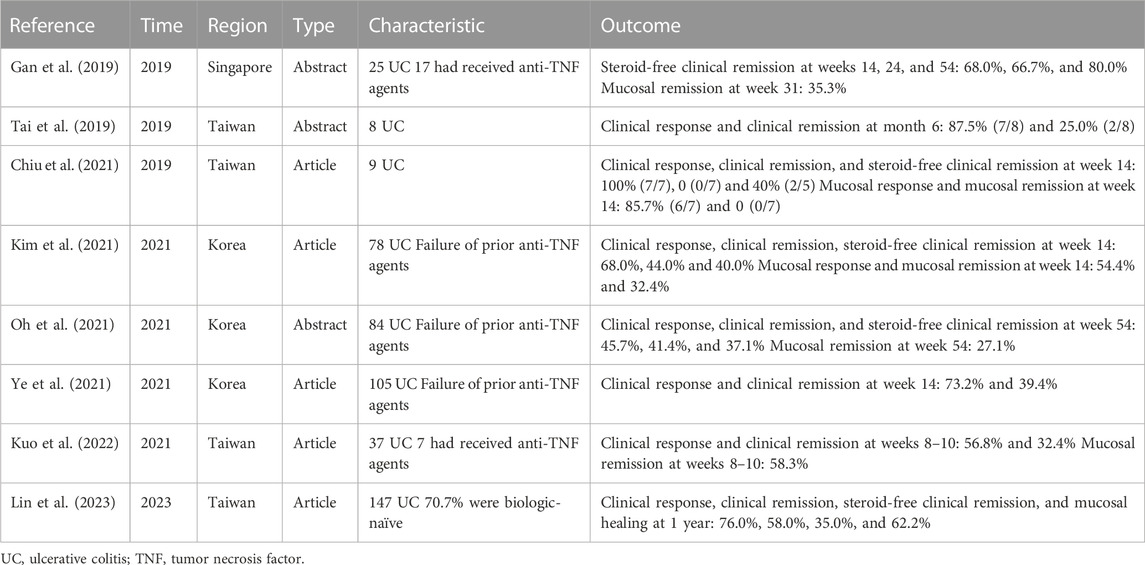
In Western countries, the effectiveness of VDZ for UC has been demonstrated in RCTs. For instance, the GEMINI 1 RCT showed clinical remission rates of 16.9% and 41.8% at weeks 6 and 52, respectively, significantly higher than those of the placebo (Feagan et al., 2013). By further analyzing 58 Asian participants, the findings were found to be consistent with the overall population (Ooi et al., 2021). In terms of long-term efficacy, the GEMINI LTS trial showed a clinical remission rate of 88% in patients who responded to induction therapy after a 2-year follow-up period (Loftus et al., 2017). In addition to RCTs, several RWSs from the United States, Canada, and Australia have evaluated the effectiveness of VDZ for UC. Data from one study that included nine RWSs showed that the effectiveness of VDZ for UC was similar to that reported in RCTs, with clinical remission rates of 32% and 39% at weeks 14 and 52, respectively (Engel et al., 2018). For Asian populations, few RWSs have been conducted, with most performed in Korea and Taiwan (Table 3). In the VIOLET study conducted in Taiwan, the clinical response, clinical remission, and mucosal healing rates at the 1-year follow-up were 76.0%, 58.0%, and 62.2%, respectively (Lin et al., 2023). In comparison, the clinical response and remission rates were higher in our study, which may be attributed to the larger number of patients who had not previously received anti-TNF agents (84.1%).
Notably, 84.1% of patients in our study had not previously received anti-TNF agents, which is higher than studies such as GEMINI 1 (59.0%) and VIOLET (70.7%). However, we included consecutive UC patients who used VDZ at our center strictly according to the inclusion criteria and did not exclude patients who had previously received anti-TNF agents. A possible reason for this discrepancy at baseline is the consensus of Chinese experts that VDZ may be used as first-line treatment against moderate to severe UC, especially for those with early onset, severe disease, rapid progression, and poor prognosis. In addition, hepatitis B and tuberculosis infections are prevalent in many Asian countries, including China (Banerjee et al., 2020); VDZ has a favorable safety profile and has not been reported to increase the risk of reactivation of these diseases (Ng et al., 2018). Finally, the inclusion of VDZ in the Chinese national basic medical insurance in March 2021 might have led to more patients choosing this drug as first-line therapy.
In this study, VDZ showed satisfactory safety profiles. During the follow-up period, no patient reported acute infusion reactions, delayed allergic reactions, or infections. Progressive multifocal leukoencephalopathy was not reported, consistent with the results of the GEMINI LTS trial (Loftus et al., 2020). Analysis of the 4-year post-marketing data using the VDZ Global Safety Database revealed that the most frequently reported adverse events were gastrointestinal events, and <1% of the patients reported malignancies (Cohen et al., 2020b). Overall, the frequency of adverse events was low, and most were non-serious. However, after treatment with VDZ, some patients in our study developed skin and joint manifestations, which must be considered by physicians. An RWS based on the OBSERV-IBD cohort also found that inflammatory arthropathy was observed in 34 (13.8%) of the 247 patients treated with VDZ (Tadbiri et al., 2018). Another study that included 112 patients also observed joint manifestations in 17 patients (15.2%) (Dupré et al., 2020).
Patients with different baseline characteristics may have different outcomes. In a meta-analysis that included 79 clinical trials, patients who did not receive biologics were more likely to achieve clinical remission at week 52 than those who had previously received biologics (relative risk [RR] = 1.32, 95% CI 1.14–1.53). Additionally, patients who did not receive biologics had a lower risk for serious adverse events (RR = 0.29, 95% CI 0.09–0.95) (Attauabi et al., 2022). Our study found that patients who had not previously received anti-TNF agents were more likely to achieve clinical response and remission at week 14 than those who had, demonstrating the advantages of VDZ as first-line biological therapy. However, the result should be interpreted with caution. First, subgroup analyses are post hoc analyses that cannot maintain randomization within the subgroup; second, the small sample size of patients who had not previously received anti-TNF agents may lead to false positives. Therefore, the results must be validated in subsequent clinical trials.
There is also a growing interest in the effectiveness and safety of VDZ in the elderly. Cohen et al. conducted a multicenter retrospective cohort study and found that patients with UC aged <40 and >60 years had similar clinical and endoscopic responses after a year of VDZ treatment (Cohen et al., 2020a). In another case-control study, similar findings were obtained, with no significant differences in mucosal healing between patients with UC aged ≥65 and <65 years (Shashi et al., 2020). The 2021 update of the AGA clinical guidelines states that VDZ has similar efficacy in older and younger patients, and the incidence of adverse events is not significantly correlated with age (Ananthakrishnan et al., 2021). Besides, VDZ tends to be used more frequently than other biological agents in older patients who are more likely to develop complications. Although there were only 11 elderly patients in the subgroup analysis in our study, nearly two-thirds achieved clinical remission at week 14, suggesting that VDZ is effective in elderly patients.
Currently, the combination of VDZ with small-molecule drugs is an option for patients who are unresponsive to first-line biological therapy. Goessens et al. reported 12 patients who received a combination of VDZ and TOF, eight of whom (67%) achieved endoscopic response after 11 months (Goessens et al., 2021). A case report by Bonastre et al. also showed improvement in the levels of fecal calprotectin, CRP, and other parameters with the combination of VDZ and TOF (Taberner Bonastre et al., 2021). In our study, two patients were treated with a combination of VDZ and TOF, but the results were unsatisfactory. The patients discontinued VDZ after four and seven infusions, respectively, due to loss of response. A recent meta-analysis suggested that dual biological or small-molecule therapy may be effective in patients with IBD. However, the study integrated various combinations of biologics and did not analyze the results of combinatorial VDZ and TOF alone (Ahmed et al., 2022). In fact, no large clinical trial has explored outcomes and prognosis while considering the combination of these two drugs.
Our study has certain limitations. First, data were obtained retrospectively and may have been subject to bias. Second, the small sample size did not allow for the analysis of risk factors affecting effectiveness, and we may have missed recording adverse events with a low probability of occurrence. Third, not all patients underwent follow-up endoscopy at the end of the induction phase and after 1 year, and not all patients who underwent follow-up endoscopy were concentrated on these two periods. Therefore, the time frame for the endoscopic endpoint follow-up was expanded in our study.
In summary, our findings indicate that the effectiveness of long-term use of vedolizumab for Chinese patients with UC was generally similar to that previously reported in other regions and populations. Patients who had not previously received anti-TNF agents may have better outcomes than those who had in the induction phase. More studies are warranted to explore the effectiveness and safety of VDZ in patients with different baseline characteristics and to investigate the combination of VDZ with other biological agents, which will help physicians to make better treatment decisions for patients with complex IBD.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the ethical review committee of the Sir Run Run Shaw Hospital, College of Medicine Zhejiang University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
KH, JL, WX, CT, LY, QC, and HC contributed to the conception and design of the study. WX and CT contributed to the acquisition of data. LY performed the statistical analysis. KH and JL wrote the first draft of the manuscript. KH, QC, and HC critically revised the manuscript. All authors contributed to the article, and read and approved the submitted version.
Funding
This research was funded by Zhejiang Provincial Natural Science Foundation of China (LQ21H030010).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmed, W., Galati, J., Kumar, A., Christos, P. J., Longman, R., Lukin, D. J., et al. (2022). Dual biologic or small molecule therapy for treatment of inflammatory bowel disease: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 20 (3), e361–e379. doi:10.1016/j.cgh.2021.03.034
Ananthakrishnan, A. N., Nguyen, G. C., and Bernstein, C. N. (2021). AGA clinical practice update on management of inflammatory bowel disease in elderly patients: Expert review. Gastroenterology 160 (1), 445–451. doi:10.1053/j.gastro.2020.08.060
Argollo, M., Kotze, P. G., Kakkadasam, P., and D'Haens, G. (2020). Optimizing biologic therapy in IBD: How essential is therapeutic drug monitoring? Nat. Rev. Gastroenterol. Hepatol. 17 (11), 702–710. doi:10.1038/s41575-020-0352-2
Attauabi, M., Madsen, G. R., Bendtsen, F., Seidelin, J. B., and Burisch, J. (2022). Vedolizumab as the first line of biologic therapy for ulcerative colitis and Crohn's disease - a systematic review with meta-analysis. Dig. Liver Dis. 54 (9), 1168–1178. doi:10.1016/j.dld.2021.11.014
Banerjee, R., Ali, R. A. R., Wei, S. C., and Adsul, S. (2020). Biologics for the management of inflammatory bowel disease: A review in tuberculosis-endemic countries. Gut Liver 14 (6), 685–698. doi:10.5009/gnl19209
Chiu, Y.-C., Chen, C.-C., Ko, C.-W., Liao, S.-C., Yeh, H.-Z., and Chang, C.-H. (2021). Real-world efficacy and safety of vedolizumab among patients with inflammatory bowel disease: A single tertiary medical center experience in central taiwan. Adv. Dig. Med. 8 (1), 40–46. doi:10.1002/aid2.13188
Cohen, N. A., Bhayat, F., Blake, A., and Travis, S. (2020b). The safety profile of vedolizumab in ulcerative colitis and crohn's disease: 4 Years of global post-marketing data. J. Crohns Colitis 14 (2), 192–204. doi:10.1093/ecco-jcc/jjz137
Cohen, N. A., Plevris, N., Kopylov, U., Grinman, A., Ungar, B., Yanai, H., et al. (2020a). Vedolizumab is effective and safe in elderly inflammatory bowel disease patients: A binational, multicenter, retrospective cohort study. United Eur. Gastroenterol. J. 8 (9), 1076–1085. doi:10.1177/2050640620951400
Dupré, A., Collins, M., Nocturne, G., Carbonnel, F., Mariette, X., and Seror, R. (2020). Articular manifestations in patients with inflammatory bowel disease treated with vedolizumab. Rheumatol. Oxf. 59 (11), 3275–3283. doi:10.1093/rheumatology/keaa107
Engel, T., Ungar, B., Yung, D. E., Ben-Horin, S., Eliakim, R., and Kopylov, U. (2018). Vedolizumab in IBD-lessons from real-world experience; A systematic review and pooled analysis. J. Crohns Colitis 12 (2), 245–257. doi:10.1093/ecco-jcc/jjx143
Feagan, B. G., Rutgeerts, P., Sands, B. E., Hanauer, S., Colombel, J. F., Sandborn, W. J., et al. (2013). Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 369 (8), 699–710. doi:10.1056/NEJMoa1215734
Gan, A. T. M., Chan, W. P. W., Ling, K. L., Hartono, L. J., Ong, D. E., Gowans, M., et al. (2019). P634 real-world data on the efficacy and safety of vedolizumab therapy in patients with inflammatory bowel disease: A retrospective nation-wide cohort study in Singapore. J. Crohns Colitis 13, S434–S435. doi:10.1093/ecco-jcc/jjy222.758
Goessens, L., Colombel, J. F., Outtier, A., Ferrante, M., Sabino, J., Judge, C., et al. (2021). Safety and efficacy of combining biologics or small molecules for inflammatory bowel disease or immune-mediated inflammatory diseases: A European retrospective observational study. United Eur. Gastroenterol. J. 9 (10), 1136–1147. doi:10.1002/ueg2.12170
Kim, H. S., Lee, S., and Kim, J. H. (2018). Real-world evidence versus randomized controlled trial: Clinical research based on electronic medical records. J. Korean Med. Sci. 33 (34), e213. doi:10.3346/jkms.2018.33.e213
Kim, J., Yoon, H., Kim, N., Lee, K.-M., Jung, S.-A., Choi, C. H., et al. (2021). Clinical outcomes and response predictors of vedolizumab induction treatment for Korean patients with inflammatory bowel diseases who failed anti-TNF therapy: A kasid prospective multicenter cohort study. Inflamm. bowel Dis. 27 (12), 1931–1941. doi:10.1093/ibd/izaa361
Kotze, P. G., Ma, C., Almutairdi, A., Al-Darmaki, A., Devlin, S. M., Kaplan, G. G., et al. (2018). Real-world clinical, endoscopic and radiographic efficacy of vedolizumab for the treatment of inflammatory bowel disease. Aliment. Pharmacol. Ther. 48 (6), 626–637. doi:10.1111/apt.14919
Kuo, C.-J., Le, P.-H., Tai, W. C., Wu, K.-L., Yen, H.-H., Yen, C.-W., et al. (2022). The effectiveness and safety of vedolizumab induction for moderate to severe ulcerative colitis for Asia patient: A real practice observational study. J. Formos. Med. Assoc. 121 (9), 1689–1695. doi:10.1016/j.jfma.2021.11.012
Lee, S. H., Kwon, J. E., and Cho, M. L. (2018). Immunological pathogenesis of inflammatory bowel disease. Intest. Res. 16 (1), 26–42. doi:10.5217/ir.2018.16.1.26
Lin, W. C., Tai, W. C., Chang, C. H., Tu, C. H., Feng, I. C., Shieh, M. J., et al. (2023). Real-world evidence of effectiveness and safety of vedolizumab for inflammatory bowel disease in taiwan: A prospective nationwide registry (VIOLET) study. Inflamm. Bowel Dis. 2023, izac269. doi:10.1093/ibd/izac269
Loftus, E. V., Colombel, J. F., Feagan, B. G., Vermeire, S., Sandborn, W. J., Sands, B. E., et al. (2017). Long-term efficacy of vedolizumab for ulcerative colitis. J. Crohns Colitis 11 (4), 400–411. doi:10.1093/ecco-jcc/jjw177
Loftus, E. V., Feagan, B. G., Panaccione, R., Colombel, J. F., Sandborn, W. J., Sands, B. E., et al. (2020). Long-term safety of vedolizumab for inflammatory bowel disease. Aliment. Pharmacol. Ther. 52 (8), 1353–1365. doi:10.1111/apt.16060
Narula, N., Peerani, F., Meserve, J., Kochhar, G., Chaudrey, K., Hartke, J., et al. (2018). Vedolizumab for ulcerative colitis: Treatment outcomes from the VICTORY consortium. Am. J. Gastroenterol. 113 (9), 1345. doi:10.1038/s41395-018-0162-0
Ng, S. C., Hilmi, I. N., Blake, A., Bhayat, F., Adsul, S., Khan, Q. R., et al. (2018). Low frequency of opportunistic infections in patients receiving vedolizumab in clinical trials and post-marketing setting. Inflamm. Bowel Dis. 24 (11), 2431–2441. doi:10.1093/ibd/izy153
Oh, K., Kim, J., Kim, N., Yoon, H., Lee, K. M., Park, D. I., et al. (2021). P560 clinical outcomes of vedolizumab maintenance treatment for Korean patients with inflammatory bowel disease who failed anti-TNF therapy: A kasid prospective multicenter cohort study. J. Crohns Colitis 15, S523–S524. doi:10.1093/ecco-jcc/jjab076.681
Ooi, C. J., Hilmi, I. N., Kim, H. J., Jalihal, U., Wu, D. C., Demuth, D., et al. (2021). Efficacy and safety of vedolizumab in ulcerative colitis in patients from Asian countries in the GEMINI 1 study. Intest. Res. 19 (1), 71–82. doi:10.5217/ir.2019.09159
Park, J., and Cheon, J. H. (2021). Incidence and prevalence of inflammatory bowel disease across Asia. Yonsei Med. J. 62 (2), 99–108. doi:10.3349/ymj.2021.62.2.99
Raine, T., Bonovas, S., Burisch, J., Kucharzik, T., Adamina, M., Annese, V., et al. (2022). ECCO guidelines on therapeutics in ulcerative colitis: Medical treatment. J. Crohns Colitis 16 (1), 2–17. doi:10.1093/ecco-jcc/jjab178
Shashi, P., Gopalakrishnan, D., Parikh, M. P., Shen, B., and Kochhar, G. (2020). Efficacy and safety of vedolizumab in elderly patients with inflammatory bowel disease: A matched case-control study. Gastroenterol. Rep. (Oxf) 8 (4), 306–311. doi:10.1093/gastro/goz041
Taberner Bonastre, P., Torres Vicente, G., Cano-Marron, M., Sese Abizanda, E., Volta Pardo, T. D., and Schoenenberger-Arnaiz, J. A. (2021). A patient with ulcerative colitis treated with a combination of vedolizumab and tofacitinib. Eur. J. Hosp. Pharm. 28 (6), 353–355. doi:10.1136/ejhpharm-2020-002437
Tadbiri, S., Peyrin-Biroulet, L., Serrero, M., Filippi, J., Pariente, B., Roblin, X., et al. (2018). Impact of vedolizumab therapy on extra-intestinal manifestations in patients with inflammatory bowel disease: A multicentre cohort study nested in the OBSERV-IBD cohort. Aliment. Pharmacol. Ther. 47 (4), 485–493. doi:10.1111/apt.14419
Tai, W. C., Tu, C. H., Chou, J. W., Feng, I. C., Chung, C. S., Shieh, M. J., et al. (2019). Treatment persistence and clinical outcomes of vedolizumab in inflammatory bowel disease patients in taiwan: Real-world evidence from the TSIBD registry. J. Gastroenterology Hepatology 34, 305.
White, J. R., Din, S., Ingram, R. J. M., Foley, S., Alam, M. A., Robinson, R., et al. (2020). Experiences of using vedolizumab in the treatment of inflammatory bowel disease in the East Midlands UK - a retrospective observational study. Scand. J. Gastroenterol. 55 (8), 907–916. doi:10.1080/00365521.2020.1790647
Wyant, T., Fedyk, E., and Abhyankar, B. (2016). An overview of the mechanism of action of the monoclonal antibody vedolizumab. J. Crohns Colitis 10 (12), 1437–1444. doi:10.1093/ecco-jcc/jjw092
Keywords: ulcerative colitis, vedolizumab, effectiveness, drug safety, real-world study, China
Citation: Huang K, Liu J, Xia W, Tian C, Yao L, Cao Q and Chen H (2023) Effectiveness and safety of vedolizumab for ulcerative colitis: a single-center retrospective real-world study in China. Front. Pharmacol. 14:1188751. doi: 10.3389/fphar.2023.1188751
Received: 17 March 2023; Accepted: 24 April 2023;
Published: 04 May 2023.
Edited by:
Grigorios L. Kyriakopoulos, National Technical University of Athens, GreeceReviewed by:
Toshiyuki Matsui, Fukuoka University, JapanMarc Henri De Longueville, UCB Pharma, Belgium
Copyright © 2023 Huang, Liu, Xia, Tian, Yao, Cao and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qian Cao, Y2FvcUB6anUuZWR1LmNu; Haotian Chen, MzQxNDI4OEB6anUuZWR1LmNu
 Kaituo Huang
Kaituo Huang Jing Liu
Jing Liu Wenhao Xia
Wenhao Xia Chuwen Tian
Chuwen Tian Lingya Yao
Lingya Yao Qian Cao
Qian Cao Haotian Chen1,2*
Haotian Chen1,2*