- 1Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2Department of Emergency, Weifang Hospital of Traditional Chinese Medicine, Weifang, China
Cardiovascular disease is a global health problem. Astragaloside IV (AS-IV) is a saponin compound extracted from the roots of the Chinese herb Astragalus. Over the past few decades, AS-IV has been shown to possess various pharmacological properties. It can protect the myocardium through antioxidative stress, anti-inflammatory effects, regulation of calcium homeostasis, improvement of myocardial energy metabolism, anti-apoptosis, anti-cardiomyocyte hypertrophy, anti-myocardial fibrosis, regulation of myocardial autophagy, and improvement of myocardial microcirculation. AS-IV exerts protective effects on blood vessels. For example, it can protect vascular endothelial cells through antioxidative stress and anti-inflammatory pathways, relax blood vessels, stabilize atherosclerotic plaques, and inhibit the proliferation and migration of vascular smooth muscle cells. Thus, the bioavailability of AS-IV is low. Toxicology indicates that AS-IV is safe, but should be used cautiously in pregnant women. In this paper, we review the mechanisms of AS-IV prevention and treatment of cardiovascular diseases in recent years to provide a reference for future research and drug development.
1 Introduction
Cardiovascular diseases (CVD) are a global health problem, causing the largest number of deaths worldwide, and their incidence is still increasing. It is estimated that approximately 20 million people died from CVDs in 2016, accounting for 31% of all deaths worldwide (Tan et al., 2020). There is no doubt that CVD has become a public health crisis. Tu Youyou’s discovery of artemisinin shows that Chinese herbal medicine is a great treasure. Over the past few decades, natural compounds derived from Chinese herbs have become important resources for drug research and development, particularly for treating CVD (Lin et al., 2022).
Astragalus membranaceus (known as Huangqi in China; HQ) is a plant of the genus Astragalus in the leguminous family, whose dried roots are used in traditional Chinese herbal medicine. HQ is one of the most widely used traditional Chinese medicines and was first described in Shennong’s Classic of Material Medical. It has the effect of tonifying qi and uplifting Yang, solidifying the surface, and acting as an antiperspirant, collecting sores, and generating muscles. Clinically, a variety of Chinese patent medicines and injections are made from or contain HQ, including Astragalus injection, Astragalus polysaccharide injection, Yupingfeng Powder, Xuefu Zhuyu Decoction, Huangqi Siwu Decoction, etc (Fu et al., 2011; Lu et al., 2011).
Astragaloside IV (AS-IV) is a purified small-molecule saponin that is one of the main active components of astragaloside (Ren et al., 2013). Previous studies have found that AS-IV has various activities, such as anti-oxidative stress (Ko et al., 2005), anti-inflammatory (Zhang and Frei, 2015), anti-apoptosis (Huang et al., 2012), calcium balance (Lu et al., 2018), diabetes (Lv et al., 2010) and anti-CVD (Han et al., 2011). Currently, studies on the AS-IV are limited. To further determine the protective mechanism of AS-IV against CVD, we reviewed recent research reports on AS-IV and provided a systematic summary (Figure 1).
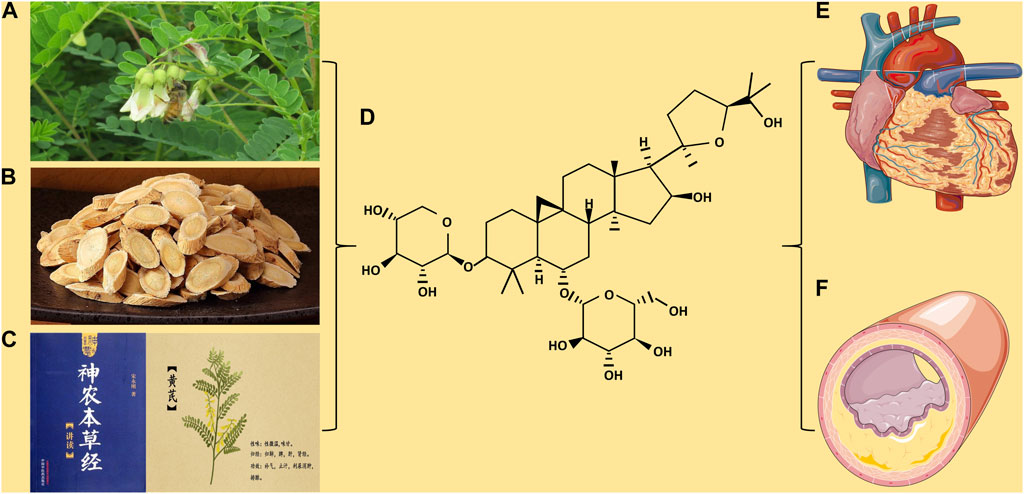
FIGURE 1. (A) Astragalus membranaceus. (B) Astragalus membranaceus. Decoction pieces. (C) Shennong’s Classic of Material Medical and picture of Astragalus membranaceus. (D) Chemical structure of Astragaloside IV. (E) The protection of Astragaloside IV on myocardial disease. (F) The protection of Astragaloside IV on blood vessels.
2 Cardiac protection
2.1 Alleviating oxidative stress
Myocardial Ischemia/reperfusion (I/R) injury is a significant clinical problem because of the significant explosion of free radicals during the first few minutes of I/R due to the reintroduction of oxygen, which, coupled with reduced antioxidant activity, makes the heart muscle extremely vulnerable (Moens et al., 2005). Energy metabolism provides substrates for reactive oxygen species (ROS) and regulating the utilization of myocardial metabolic substrates is a therapeutic strategy for mitigating ROS-related injuries. Succinic acid is an important metabolite of the tricarboxylic acid (TCA) cycle. It has previously been reported that succinic acid accumulates during myocardial ischemia and can be consumed in large quantities during reperfusion to produce ROS (Chouchani et al., 2014). Jiang et al. found that AS-IV (40 mg/kg) prevented the accumulation of succinic acid in the myocardium of Sprague Dawley (SD) rats after I/R, thus reducing the production of ROS. In addition, AS-IV activates the Nrf2/HO-1 signaling pathway and upregulates the expression of antioxidant enzymes, thereby protecting cardiomyocytes from I/R damage (Jiang et al., 2019). The mitochondrial permeability transition pore (mPTP) plays an important role in the pathogenesis of myocardial I/R injury (Bauer and Murphy, 2020). Glycogen synthase kinase 3β (GSK-3β) is a mature component that promotes apoptosis (Frame and Cohen, 2001). He et al. found that AS-IV (50 μM) phosphorylated GSK-3β through NO/cGMP/PKG pathway, thereby inhibiting oxidative stress-induced mPTP opening of H9C2 cells (He et al., 2012). In another study, AS-IV (5 and 10 mg/kg) alleviated myocardial I/R damage in SD rats by modulating the PI3K/Akt/GSK-3β signaling pathway (Wei et al., 2019).
Doxorubicin (DOX) is a classic antitumor chemotherapy drug that induces fatal cardiotoxicity. DOX causes oxidative stress by increasing the expression of NADPH oxidase 2 (NOX2) and NOX4 in rat hearts, leading to cardiomyopathy (McLaughlin et al., 2017). Lin et al. found that AS-IV (40 mg/kg) significantly reduced myocardial injury, myocardial cell apoptosis, cardiac fibrosis, and cardiac dysfunction in DOX-treated C57BL/6 mice by inhibiting the expression of NOX2 and NOX4 (Lin et al., 2019). Histone deacetylases (HDAC) work with histone acetylases to regulate the homeostasis of acetylated histones in vivo. Zhang et al. found that AS-IV (12.5 and 50 µM) could inhibit HDAC activity, thereby protecting HL-1 mouse cardiomyocytes from oxidized low-density lipoprotein (ox-LDL) oxidative damage (Zhang et al., 2021) (Figure 2).
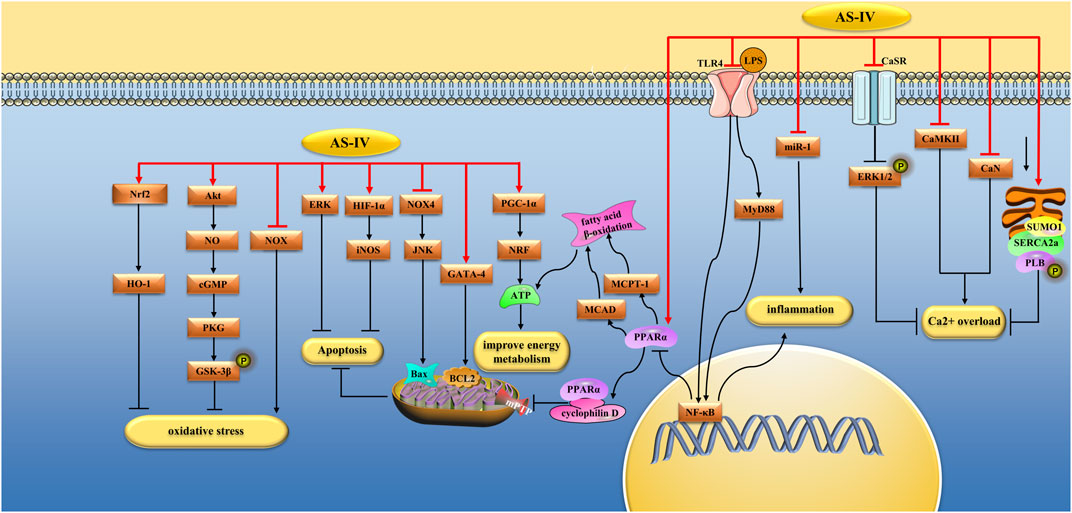
FIGURE 2. Pharmacological effects of AS-IV on myocardium. AS-IV protects myocardium by anti-oxidative stress, anti-inflammatory, regulating calcium balance, improving energy metabolism, and anti-apoptosis pathways.
2.2 Anti-inflammatory
Sepsis is a systemic inflammatory response that occurs after infection. Sepsis-induced cardiovascular dysfunction is the leading cause of death in critically ill patients (Bone et al., 1992). Lipopolysaccharide (LPS) produced by bacterial endotoxins is considered the main factor leading to cardiac dysfunction in sepsis, and its mechanism may be related to the release of many inflammatory cytokines (Zanotti-Cavazzoni and Hollenberg, 2009). Toll-like receptor 4 (TLR4) is a major LPS receptor and a key mediator of pro-inflammatory responses. Zhang et al. found that AS-IV (20, 40, and 80 mg/kg) significantly improved cardiac function, myocardial cell viability, and pathological changes in lipopolysaccharide-induced C57BL/6J mice. It also reduces the level of interleukin-1β (IL-1β), IL-6, and tumor necrosis factor α (TNF-α) in myocardial tissue. In addition, AS-IV increased the ratio of adenosine triphosphate (ATP)/adenosine monophosphate (AMP) in myocardial tissue and promoted the expression of ATP synthase. Its mechanism was related to the inhibition of the TLR4/NF-κB pathway and upregulation of proliferator-activated receptor α(PPARα) (Zhang et al., 2019a). Zhao et al. found that AS-IV (20 mg/kg) significantly reduced cardiac dysfunction and inflammatory mediator production in LPS-induced male C57BL/6 mice. This mechanism is related to the inhibition of NF-κB and the activation of the PI3K/Akt signaling pathway (Zhao et al., 2015a). Su et al. evaluated the effect of AS-IV on cardiac dysfunction of SD rats induced by cecal ligation and puncture (CLP). They found that AS-IV (30 mg/kg/d) improved the survival rate of rats after CLP surgery, improved cardiac function, and inhibited the secretion of pro-inflammatory cytokines. In vitro studies, AS-IV (100 μmol/mL) mitigated LPS (40 μg/mL) damage to H9C2 cells. This mechanism is related to the inhibition of the NOX4/JNK/Bax signaling pathway (Su et al., 2022). Yang et al. injected isoproterenol (5 mg/kg/d) peritoneally into SD rats to make myocardial hypertrophy models, and they found that AS-IV (20,40 and 80 mg/kg) inhibited myocardial hypertrophy and serum levels of TNF-α and IL-6, the mechanism of which was related to inhibition of TLR4/NF-κB signaling pathway (Yang et al., 2013). Shi et al. found that AS-IV (80 mg/kg/day) reduced myocardial inflammation in SD rats with acute myocardial infarction (AMI) by inhibiting the TLR4/MyD88/NF-κB pathway (Shi et al., 2021). Huang et al. found that AS-IV (40 mg/kg/d) reduced myocardial cell apoptosis in SD rats after CLP operation and reduced serum inflammatory cytokines IL-6, IL-10, and high mobility group protein B1 (HMGB-1), the mechanism of which is related to the inhibition of IKK/NF-κB pathway (Huang et al., 2021a).
MicroRNAs (MiRNAs) regulate gene expression after transcription. miR-1 has been identified as a muscle-specific miRNA that is upregulated in rats with myocardial infarction (MI) (Shan et al., 2009). Wang et al. found that AS-IV (80 mg/kg) alleviated LPS (10 mg/kg) induced cardiac dysfunction in SD rats by inhibiting miR-1-mediated inflammation and autophagy. In vitro, AS-IV (10 g/mL) alleviated LPS-induced H9C2 cell damage (10 g/mL) via the same mechanism (Wang et al., 2022). In another study by the same team, AS-IV (80 mg/kg) alleviated LPS-induced cardiac dysfunction in SD rats by inhibiting miR-1-mediated apoptosis. Mitochondrial energy metabolism and calcium signaling pathways are also involved in cardiac insufficiency. In this study, AS-IV (80 mg/kg) decreased calcium/calmodulin-dependent protein kinase II (CaMKII) expression, improved ryanodine receptor 2 (RyR2) and sarcoplasmic reticulum Ca2+-ATPase2a (SERCA2a) protein expression to inhibit myocardial cell damage caused by calcium overload. Peroxisome proliferator-activated receptor-gamma coactivator-1α (PGC-1α), and mitochondrial transcription factor A were involved in mitochondrial energy metabolism, and AS-IV (80 mg/kg) increased the expression of these two types of proteins (Wang et al., 2021a) (Figure 2).
2.3 Regulating calcium balance
Dysregulation of intracellular calcium homeostasis plays an important role in myocardial cell injury induced by I/R or hypoxia/reoxygenation (H/R). An excessive increase in intracellular Ca2+ concentration leads to Ca2+ overload, leading to myocardial cell injury (Zucchi et al., 2001). The calcium-sensing receptor (CaSR) is a member of the G protein-coupled receptor (GPCRs) superfamily involved in systemic calcium homeostasis. Overactivation of CaSR increases intracellular Ca2+ levels, thereby inducing cardiomyocyte apoptosis (Lu et al., 2013). Yin et al. (Yin et al., 2019) found that AS-IV (80 mg/kg/day) significantly reduced MI size, serum cardiac troponin (cTnI) levels, and myocardial cell apoptosis in SD rats with myocardial I/R injury. In vitro, AS-IV (60 mol/L) pretreatment significantly increased cardiomyocyte viability, decreased lactate dehydrogenase (LDH) release and intracellular Ca2+ levels, and alleviated cardiomyocyte apoptosis. This mechanism is related to the downregulation of CaSR expression in cardiomyocytes and the upregulation of extracellular signal-regulated kinases1/2 (ERK1/2) phosphorylation (Yin et al., 2019). Lu et al. found that AS-IV treatment (80 mg/kg/day) inhibited the activation of CaSR, CaMKII, and Ca2+/Calcineurin (CaN) in the myocardium of isoproterenol-induced SD rats and inhibited the nuclear translocation of NFAT3, thereby alleviating myocardial hyperplasia and cardiomyocyte apoptosis (Lu et al., 2018). SERCA2a is another important regulatory protein that is responsible for Ca2+ homeostasis in cardiomyocytes. Xu et al. found that AS-IV (30 mol/L) inhibited H/R-induced Ca2+ overload and prevented the reduction in SERCA2a enzyme activity and SERCA2a mRNA and protein levels (Xu et al., 2008). Phospholamban (PLB), a small protein in the sarcoplasmic reticulum (SR), is a key regulator of SERCA2a. The phosphorylation of PLB enhances SERCA2a function by increasing its Ca2+ affinity (Koss and Kranias, 1996). In another study, Xu et al. found that AS-IV (5 and 10 mg/kg/day) reduced isoproterenol-induced myocardial injury in SD rats through a mechanism related to increased phosphorylation of PLB and SERCA2a protein expression (Xu et al., 2007). Decreased expression and activity of SERCA2a protein can lead to impaired calcium homeostasis in cardiomyocytes. Mitochondria-sourced ATP drives SERCA2a-mediated Ca2+ uptake into the SR. In addition, activation of small ubiquitin-like modifier1 (SUMO1) enhanced the binding affinity of SERCA2a to ATP. Dong et al. found that AS-IV could promote the expression of SERCA2a and the activation of SUMO1 (Dong et al., 2017) (Figure 2).
2.4 Improve myocardial energy metabolism
During fetal life, cardiomyocytes obtain energy mainly through glycolysis, which is converted to fatty acid β-oxidation after birth. However, in patients with heart failure (HF), the primary energy source for cardiomyocytes changes from fatty acid β-oxidation to glycolysis. Fatty acid oxidation provides more energy than glucose, and restoring cardiomyocytes to fatty acid use may be an effective treatment for HF. It has been shown that the expression of PPARα can stimulate the β-oxidation of fatty acids (Minnich et al., 2001). Dong et al. (Dong et al., 2017) found that AS-IV (0.3 and 1 mg/kg/d) can improve systolic myocardial function in mice with HF induced by transverse aortic contraction (TAC), and the mechanism is related to the activation of PPARα. In vitro studies, AS-IV not only inhibited the expression of hypertrophic marker genes in hypertrophic cardiomyocytes but also significantly inhibited the reduction of ATP, the mechanism of which is related to the inhibition of glycolysis and the acceleration of fatty acid β oxidation. Several studies have shown that mitochondrial Ca2+ uptake promotes ATP production to a certain extent (Di Benedetto et al., 2013). Cyclophilin D is a major regulator for the formation of mPTP. PPARα is involved in the opening of the mPTP by binding to cyclophilin D. Dong et al. (Dong et al., 2017) AS-IV could inhibit the significant decrease in the interaction between PPARα and cyclophilin D in the TAC group and the decrease in Ca2+ accumulation in the mitochondrial matrix.
PGC-1α is responsible for the metabolic transition from fatty acid oxidation to glucose oxidation. In addition, PGC-1α also regulates the expression and activity of ATP synthase subunit ATP5D, thus regulating energy biosynthesis. Zhang et al. found that AS-IV (80 mg/kg/day) had a protective effect against isoproterenol-induced myocardial hypertrophy in SD rats, increased the ATP/AMP ratio, reduced free fatty acid (FFA) content, and increased ATP5D expression. In vitro studies, AS-IV (100 μM) has the same protective effect on neonatal rat ventricular myocytes (NRVM) treated with isoproterenol, and the mechanism is related to the regulation of the NF-κB/PGC-1α signaling pathway (Zhang et al., 2015). Medium-chain acyl-CoA dehydrogenase (MCAD) and muscle carnitine palmitoyl transferase −1 (MCPT-1) are key enzymes involved in FFA oxidation, and their expression is controlled by PPARα (Sankaralingam and Lopaschuk, 2015). Tang et al. found that AS-IV (30 and 60 mg/kg/d) improved the cardiac function and structure, increased the expression of PPARα, MCAD, and MCPT1, and improved the utilization of FFA in rats with HF induced by abdominal aortic contraction (AAC) (Tang et al., 2018). The pathogenesis of diabetic cardiomyopathy is closely associated with metabolic disorders. PGC-1α stimulates the nuclear transcription factor nuclear respiratory factor (NRF), regulates cellular energy metabolism, and provides ATP to cardiac tissue (Koh et al., 2017). Zhang et al. induced diabetes in SD rats with streptozotocin (STZ) and found that AS-IV (10, 20, and 40 mg/kg/d) regulated energy metabolism by up-regulating PGC-1α and NRF, saving the abnormal energy metabolism caused by diabetes and thereby alleviating myocardial damage (Zhang et al., 2019b). In another study, Tu et al. found that AS-IV (10 mg/kg) mitigated I/R-induced cardiac insufficiency in SD rats and inhibited the reduction in ATP/AMP and ATP 5D proteins (Tu et al., 2013) (Figure 2).
2.5 Anti-apoptosis
Myocardial cell apoptosis is closely associated with HF. Studies have found that Patients with MI exhibit a certain degree of cardiomyocyte apoptosis (Saraste et al., 1997). The mitogen-activated protein kinase (MAPK) family comprises three kinases: c-Jun NH2-terminal kinases (JNKs), ERKs, and p38 enzymes (p38 MAPKs). The MAPK signaling pathway is involved in apoptosis. For example, inhibition of p38 and JNK can prevent myocardial cell apoptosis (Mackay and Mochly-Rosen, 1999; Mizukami et al., 2001). Sun et al. found that AS-IV (10 or 50 ng/mL) prevented H9C2 cell apoptosis induced by high glucose, fat (HG/HF), and hypoxia. Its mechanism is related to inhibiting the activation of the JNK and p38 signaling pathways and promoting the activation of the EKR signaling pathways. In animal experiments, AS-IV (10 and 50 mg/kg/day) protected the cardiac function of STZ-induced C57BL/6 mice and inhibited myocardial fibrosis and inflammation by regulating the MAPK signaling pathway (Sun et al., 2021a). Calpain-1 is a subtype of the cysteine protease family with Ca2+ and phospholipid-binding domains. When the intracellular free Ca2+ concentration reaches a trigger point, free Ca2+ binds to the Ca2+-binding domain, truncates downstream proteins, and induces associated reactions, including apoptosis (Momeni, 2011). Previous studies have shown that calpain-1 promotes apoptosis during myocardial I/R and pressure/overload (Suryakumar et al., 2010; Chen et al., 2011a). Mei et al. found that AS-IV (80 mg/kg/day) reduced isoproterenol-induced apoptosis of hypertrophic cardiomyocytes in SD rats by inhibiting calpain-1 and antioxidant stress (Mei et al., 2015). The transcription factor GATA-4 regulates the survival and adaptation response of cardiomyocytes under stress conditions, and the knockout of GATA-4 leads to cardiomyocyte apoptosis (Oka et al., 2006). Yang et al. found that AS-IV (0.5–300 μg/mL) could promote the expression of Bcl-2 by stimulating the overexpression of GATA-4, thereby reducing H/R injury-induced apoptosis in H9c2 cells (Yang et al., 2020). Hypoxia-inducible factor-1α (HIF-1α) is a key regulator of molecular hypoxia response, which can increase oxygen delivery or promote metabolic adaptation to hypoxia by activating gene transcription related to energy metabolism, angiogenesis, and apoptosis (Semenza, 2012). Si et al. found that AS-IV (50 μM) increased the strength of rat neonatal cardiac myocytes (RNCM) after ischemia and decreased the apoptotic index and LDH release by up-regulating HIF-lα/iNOS signaling pathway. In addition, AS-IV (20 μM) post-ischemic therapy protects the cardiac function of isolated I/R-injured hearts by reducing the infarct area, apoptotic index, and LDH release (Si et al., 2014) (Figure 2).
2.6 Anti-hypertrophy
Cardiac hypertrophy is an adaptive response of the heart against cardiac overloading, but continuous cardiac hypertrophy could accelerate cardiac remodeling and lead to HF in the future (Higashikuni et al., 2013). The suppressor of IKKϵ(SIKE) is a kind of ΙKKϵ and TANK-binding kinase 1 (TBK1) inhibiting factor. TBK1, also known as NF-κB activated kinase, promotes the translocation of NF-κB and causes inflammation (Shostak and Chariot, 2015). Liu et al. found that AS-IV (10 and 20 mg/kg/day) inhibited myocardial hypertrophy, inflammatory response, and myocardial cell apoptosis induced by aortic banding in C57BL6 mice, and the mechanism was related to the upregulation of SIKE and the inhibition of TBK1/PI3K/Akt activity. In vitro studies, AS-IV (50 μM) alleviated cardiomyocyte hypertrophy induced by angiotensin II (Ang II) by inhibiting these pathways (Liu et al., 2018). CaN is a serine/threonine protein phosphatase activated by calcium ions. Activated CaN binds to NFAT-3 transcription factors in the cytoplasm and dephosphorylates them. Dephosphorylated NFAT-3 is transferred to the nucleus to further interact with GATA-4 transcription factors to form complexes that participate in the development of myocardial hypertrophy. LPS is an antigenic component of the cell walls of Gram-negative bacteria that can cause cardiac hypertrophy (Liu et al., 2008). Lu et al. found that AS-IV (16, 32, and 64 µM) could reduce LPS-induced myocardial hypertrophy, and its mechanism was related to the inhibition of the CaN/NFAT-3/GATA-4 signaling pathway (Lu et al., 2014). The transcription factor nuclear factor-erythroid 2-related factor 2 (Nrf2) is a key regulator of cellular antioxidant defense. Nie et al. found that AS-IV (40 and 80 mg/kg/day) inhibited the degree of myocardial hypertrophy induced by AAC in rats and that this mechanism was related to the upregulation of the Nrf2/HO-1 signaling pathway (Nie et al., 2019a) (Figure 3).
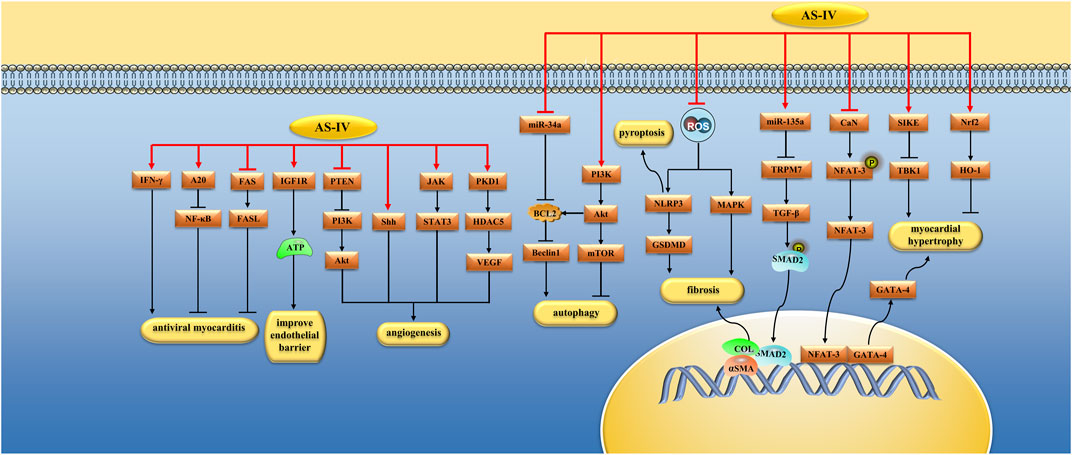
FIGURE 3. Pharmacological effects of AS-IV on myocardium. AS-IV protects myocardium by anti-cardiomyocyte hypertrophy, anti-fibrosis, regulation of autophagy, improvement of microcirculation, antiviral myocarditis.
2.7 Antifibrosis
Under pathological conditions such as inflammation and oxidative stress, cardiac fibroblasts (CF) can differentiate into myofibroblasts, accompanied by excessive accumulation of extracellular matrix (ECM) (Kania et al., 2009). Myocardial fibrosis is an important pathological change during the progression of HF (Yu and Xu, 2015). Transient receptor potential melastatin 7 (TRPM7) is a key mediator of fibrosis. The transforming growth factor-β (TGF-β)/Smad signaling pathway is the main pathway leading to myocardial fibrosis (Friedman et al., 2013). Wei et al. established a myocardial fibrosis model by subcutaneously injecting isoproterenol into SD rats and found that AS-IV (10 mg/kg/d) inhibited the increase of fibrosis-related proteins such as α smooth muscle actin (α-SMA) and collagen I. In vitro, AS-IV (10 μM) significantly inhibited isoproterenol-induced proliferation of neonatal CF in rats. Its mechanism is related to the upregulation of miR-135a and, thus inhibition of TRPM7/TGF-β/Smads signaling pathway (Wei et al., 2020). Oxidative stress is closely associated with myocardial fibrosis. Dai et al. found that AS-IV (100 µM) inhibited isoproterenol-induced cardiac fibrosis by inhibiting ROS-mediated MAPK activation (Dai et al., 2017).
Previous studies have shown that inflammation plays an important role in the progression of cardiac fibrosis (Biernacka et al., 2011). The NLRP3 inflammasome plays an important role in the inflammatory response by promoting the maturation and secretion of various pro-inflammatory cytokines, including IL-18 and IL-1β (Abderrazak et al., 2015). Wan et al. found that AS-IV (200 mg/kg/day) reduced isoproterenol-induced cardiac fibrosis in BALB/c mice by inhibiting the NLRP3 inflammasome pathway (Wan et al., 2018). Pyroptosis is programmed cell death mediated mainly by caspase-1 and is accompanied by the release of many pro-inflammatory cytokines (Liu et al., 2016). Inhibition of NLRP3 inflammasome protects cardiac function and reduces myocardial pyroptosis (Takahashi, 2014). Gasdermins (GSDMs) are a key effector molecule involved in pyroptosis. Six human GSDM genes have been identified: GSDMA, GSDMB, GSDMC, GSDMD, GSDME, and DFNB59. The mechanism by which GSDMD induces the classical pathway of pyroptosis is quite clear. Zhang et al. found that AS-IV (40 mg/kg/day) alleviated myocardial fibrosis and hypertrophy in C57BL/6 J mice with AMI. Its mechanism was related to reduced pyroptosis by inhibiting the ROS/NLRP3/GSDMD signaling pathway. In vitro, AS-IV (100 µM) alleviated LPS-induced pyroptosis in bone marrow-derived macrophages (BMDMs) by inhibiting the ROS/NLRP3/GSDMD signaling pathway (Zhang et al., 2022a).
The intestinal flora and its metabolites play important roles in host heart health (Miele et al., 2015). Studies have shown that gut microbes are crucial in heart fibrosis (Karbach et al., 2016). Du et al. found that AS-IV (200 mg/kg/day) alleviated isoproterenol-induced cardiac fibrosis in C57BL-6J mice by increasing the richness of Akkermansia, Defluviitaleaceae_UCG-011, and Rikenella and regulating amino acid metabolism (Du et al., 2022). Ferroptosis is a recently discovered iron-dependent cell death pathway. Luo et al. found that AS-IV (10 mg/kg/d) inhibited ferroptosis by upregulating the Nrf2 signaling pathway, thus alleviating DOX-induced myocardial fibrosis (Luo et al., 2021a) (Figure 3).
2.8 Regulating autophagy
Autophagy is a lysosome-dependent pathway that degrades cytoplasmic material and damaged organelles (de la Ballina et al., 2020). Low levels of autophagy can phagocytize damaged organelles to maintain normal heart function; however, in chronic diseases such as diabetic cardiomyopathy, long-term autophagy may adversely affect cardiomyocytes (Guan et al., 2019). Under physiological conditions, B-cell lymphoma-2(Bcl2) protein binds to Beclin1; however, under starvation or oxidative stress, Bcl2 separates from Beclin1 and releases Beclin1 to induce autophagy. The mammalian target of the rapamycin (mTOR) signaling pathway is a classical pathway that regulates autophagy. The PI3K/Akt signaling pathway activates mTOR, thereby inhibiting autophagy (Kubli and Gustafsson, 2015). Zhu et al. found that AS-IV (100 µM) may inhibit HG-induced autophagy in H9C2 cells by regulating the miR-34a/Bcl2/(LC3II/LC3I) and pAkt/Bcl2 signaling pathways (Zhu et al., 2019a). Previous studies have shown that DOX induces myocardial injury by increasing cardiac autophagy (Luo et al., 2018). Luo et al. found that AS-IV (100 µM) inhibits autophagy and alleviates DOX damage in H9C2 cells by activating the PI3K/Akt/mTOR signaling pathway (Luo et al., 2021b). Myocardial I/R injury increases the production of reactive oxygen species (ROS), leading to autophagosome accumulation (Ma et al., 2012). Studies have shown that ischemia-induced autophagy is beneficial, whereas autophagy during reperfusion is destructive (Matsui et al., 2007). Huang et al. found that ASIV (50 μM) can protect myocardial I/R injury in C57BL/6 mice, and its mechanism is related to increasing superoxide dismutase (SOD) level to reduce the accumulation of superoxide radical O2•- and thus reduce autophagy (Huang et al., 2021b) (Figure 3).
2.9 Improve microcirculation and promote angiogenesis
Microvascular obstruction independently predicts adverse cardiovascular events after AMI (Padro et al., 2020). Therefore, microvascular protection and promoting microvascular regeneration are important methods for reducing adverse cardiovascular events. Protein kinase D1 (PKD1), a member of the serine/threonine protein kinase family, promotes angiogenesis in vitro and in vivo (di Blasio et al., 2010). PKD1 may upregulate the expression of vascular endothelial growth factors (VEGF) by binding to the downstream target protein, histone deacetylase 5 (HDAC5) (Matthews et al., 2006). Yang et al. found that AS-IV (40 mg/kg/day) promoted angiogenesis in the myocardial tissue of AMI rats by upregulating the PKD1/HDAC5/VEGF signaling pathway (Yang et al., 2019). The signal transducer and activator of transcription 3 (STAT3) is a widely studied nuclear factor that plays an important role in angiogenesis in cardiac pathogenesis (Osugi et al., 2002). Sui et al. found that AS-IV (0.1, 0.3, and 1 mg/kg/day) promotes angiogenesis by upregulating the JAK/STAT3 pathway and alleviating HF in a left coronary artery ligation rat model (Sui et al., 2019). Sonic hedgehog (Shh) is required to maintain coronary blood vessels and is an angiogenic factor in ischemic diseases (Pola et al., 2001; Lavine et al., 2008). Wang et al. found that AS-IV (20 mg/kg/d) improved left ventricular remodeling and cardiac function in SD rats after myocardial infarction. Its mechanism was related to the upregulation of the Shh pathway to promote angiogenesis (Wang et al., 2017). The phosphatase and tensin homolog (PTEN) was originally thought to inhibit human cancer. However, PTEN affects the pathogenesis of cancer; for example, downregulation of PTEN can increase the expression of VEGF and promote angiogenesis (Jiang et al., 2000). Cheng et al. found that AS-IV (20 and 50 mg/kg/day) promoted the formation of capillaries in the boundary area of the infarcted myocardium in AMI rats. In vitro experiments, AS-IV (80 μmol/L) promoted the proliferation and formation of human umbilical vein endothelial cells (HUVECs). This mechanism is related to the inhibition of PTEN expression and activation of the PI3K/Akt signaling pathway (Cheng et al., 2019).
Reperfusion therapy for AMI is often accompanied by microvascular injury, manifested as microvascular leakage (MVL), which can aggravate tissue inflammation and edema and increase the occurrence of irreversible myocardial injury. The endothelial barrier is mainly regulated by junctions between adjacent endothelial cells, which are composed of transmembrane proteins, such as claudins, zonula occludens (ZO), occludins, vascular endothelial (VE)-cadherin and Catenins (Weis, 2008). He et al. (He et al., 2022) found that AS-IV (10 mg/kg/day) could reduce interstitial edema and inflammatory cell infiltration after myocardial I/R injury in SD rats and reduce coronary albumin leakage, the mechanism of which is related to the inhibition of the downregulation of junction proteins, such as Claudin-5, Occludin, ZO-1 and VE-cadherin. Previous studies have shown that ATP depletion can affect connexins and lead to the breakdown of the endothelial barrier. Previous studies have shown that downregulation of the insulin-like growth factor 1 receptor (IGF1R) signaling pathway can reduce ATP synthesis in the heart, and ATP depletion can affect connexins, leading to endothelial barrier breakdown (Li et al., 2014; Huang et al., 2019). In vitro studies, He et al. (He et al., 2022) found that AS-IV (10–4 M) increased ATP synthesis by upregulating the IGF1R signaling pathway in human cardiac microvascular endothelial cells (HCMECs), thereby preserving connexins and improving endothelial barrier function (Figure 3).
2.10 Antiviral myocarditis
Viral myocarditis (VM) is a major cause of HF and sudden death in healthy individuals. Many studies have shown that myocardial cell apoptosis plays an important role in the pathogenesis of VM. Liu et al. found that AS-IV (100 mg/kg/day) improved coxsackievirus B3 (CVB3)-induced myocardial cell apoptosis in mice, and its mechanism was related to the inhibition of FAS/FASL signaling pathways (Liu et al., 2019). A20, also known as TNF-α induced protein 3 (TNFAIP3), can inhibit CVB3-induced IKK complex activation and phosphorylation of IκBα. Gui et al. found that AS-IV (40 mg/kg/d) can inhibit NF-κB signaling pathway by increasing the expression of A20, reduce the severity of myocarditis induced by CVB3 in BALB/c rats, and reduce myocardial inflammation (Gui et al., 2015). Myocardial fibrosis plays an important role in CVB3-induced dilated cardiomyopathy (DCM). Chen et al. found that AS-IV could reduce myocardial fibrosis in DCM induced by CVB3 and improve ventricular dilation by down-regulating TGF-β1/Smad signaling pathway (Chen et al., 2011b). In addition, Zhang et al. found that AS-IV (60 and 120 mg/kg/d) can play an antiviral role in BALB/c mice infected with CVB3 by up-regulating the expression of interferon-γ (IFN-γ) mRNA (Zhang et al., 2006a) (Figure 3).
3 Vascular protection
3.1 Anti-vascular endothelial cell inflammation
Vascular endothelial cells (VECs) are present in the circulatory system and play an important role in maintaining vascular structure and function. The inflammatory response is a risk factor for endothelial dysfunction and an increased risk of cardiovascular events (Garcia et al., 2010). The NLRP3 inflammasome is a multiprotein pro-inflammatory complex that activates caspase-1 and cleaves pro-IL-1β and pro-IL-18 into mature IL-1β and IL-18 (Ozaki et al., 2015). Leng et al. found that AS-IV (40 and 80 mg/kg/d) reduced the activation of NLRP3 inflammasomes and the secretion of pro-inflammatory cytokines in the aorta of STZ-induced diabetic rats (Leng et al., 2019). In another study, Leng et al. found that AS-IV (100 μM) reduced the expression of intercellular cell adhesion molecule-1(ICAM-1), vascular cell adhesion molecule-1(VCAM-1), IL-6, and TNF-α in STZ-induced diabetic rat aortas by inhibiting the TLR4/NF-κB signaling pathway (Leng et al., 2018). Calpain is a calcium-activated cysteine protease located in the cytoplasm and mitochondria that plays a critical role in activating the NLRP3 inflammasome (Zhang et al., 2018a). Sun et al. found that AS-IV (50 and 100 μmol/L) inhibited the expression of NLRP3, caspase-1, IL-18, and IL-1βμ in human pulmonary artery endothelial cells (PAECs) induced by monocrotaline pyrrole (MCTP) through the NLRP3/calpain-1 pathway (Sun et al., 2021b).
Endoplasmic reticulum stress (ERS) is an adaptive response to unfolded protein accumulation and is associated with inflammation and apoptosis via the TXNIP/NLRP3 inflammasome pathway (Oslowski et al., 2012). Cycloastragenol is a microbial conversion of AS-IV, and both are present in the blood after the oral administration of astragalus (Zhou et al., 2012). Zhao et al. found that AS-IV combined with cycloastragenol reduced ERS induced by palmitate (PA) in EA.hy926 cells, and the mechanism was related to the inhibition of TXNIP/NLRP3 pathway activation (Zhao et al., 2015b). JNK is a serine/threonine protein kinase that is closely associated with cell differentiation, apoptosis, and stress response (Ip and Davis, 1998). You et al. found that AS-IV (50 μM) could reduce HG-induced apoptosis and inflammatory response in HUVECs by inhibiting JNK signaling pathway (You et al., 2019) (Figure 4).
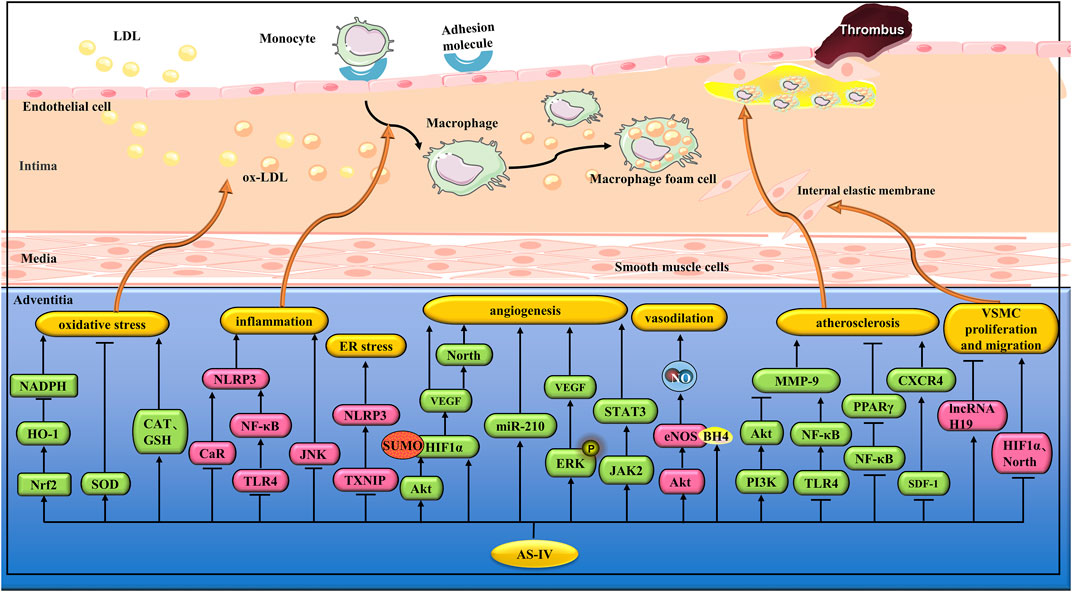
FIGURE 4. Pharmacological effects of AS-IV on blood vessels. AS-IV protects vascular endothelial cells by anti-inflammatory and antioxidant stress; AS-IV relaxes blood vessels, promotes angiogenesis, stabilizes atherosclerotic plaque, and inhibits vascular smooth muscle cell proliferation and migration.
3.2 Anti-oxidative stress of vascular endothelial cells
Endothelial dysfunction is associated with increased ROS production (Scioli et al., 2020). An imbalance between the production and elimination of intracellular oxides leads to the accumulation of intracellular ROS, which triggers oxidative stress. Zhu et al. found that AS-IV (10, 20, and 50 μM) enhanced the motility and migration of HUVECs induced by ox-LDL and inhibited ROS and NOX generation. This mechanism is related to the activation of the Nrf2/HO-1 signaling pathway (Zhu et al., 2019b). Hyperhomocysteinemia (HCY) is a risk factor for atherosclerosis (AS). Qiu et al. found that AS-IV (50 and 100 mg/mL) significantly improved HCY-induced HUVECs dysfunction caused by HCY by inhibiting ROS accumulation and increasing SOD activity (Qiu et al., 2010).
Studies have shown that chronic hyperglycemia can lead to endothelial dysfunction. Diabetic retinopathy is one of the main microvascular complications of diabetes, and hyperglycemia-induced oxidative stress plays an important role in diabetic retinopathy (Evans et al., 2002). Qiao et al. found that AS-IV (10 μM) significantly reduced HG-induced oxidative stress in retinal capillary endothelial cells (RCECs) by increasing SOD, catalase (CAT), and glutathione peroxidase (GSH-px) (Qiao et al., 2017). In another study, Nie et al. found that AS-IV (40 or 80 mg/kg/d) reduced ROS levels and increased SOD and GSH-Px activities in the aorta of diabetic rats (Nie et al., 2019b) (Figure 4).
3.3 Promote angiogenesis
Angiogenesis is an important pathological event in various chronic ischemic diseases, and remodeling angiogenesis around the ischemic area may be the most important strategy to improve the prognosis of ischemic diseases (Iwasaki et al., 2011). HIF-1α is a transcription factor that regulates the expression of several genes, including VEGF (Li et al., 2018a). Under normal oxygen levels, the HIF-1α protein is degraded by ubiquitination-mediated proteolysis, whereas under hypoxia conditions, HIF-1α preferentially undergoes SUMO modification, and the ubiquitination degradation pathway is inhibited (Wang et al., 2018). Wang et al. found that AS-IV (5 ng/mL) could stabilize HIF-1α protein by stimulating the production of SUMO1, thereby increasing the generation of VEGF and promoting angiogenesis under hypoxia conditions (Wang et al., 2021b). In another study, Zhang et al. found that AS-IV (0.25 μM) promoted tube formation in HUVECs and that its mechanism was related to the activation of the PI3K/Akt pathway, thereby promoting HIF-1α protein expression (Zhang et al., 2011). Liang et al. found that AS-IV (40 mg/kg/d) reduced cerebral infarction size and promoted angiogenesis in SD rats with middle cerebral artery occlusion. Its mechanism was related to the upregulation of miRNA-210 and the activation of the HIF/VEGF/Notch signaling pathway (Liang et al., 2020).
ERK1/2 play an important role in angiogenesis. Wang et al. found that AS-IV (10, 40, and 120 μM) could enhance the proliferation, migration, and tube formation of HUVECs by up-regulating the phosphorylation of ERK1/2 and activating the JAK2/STAT3 pathway (Wang et al., 2013). In another study, Wang et al. found that AS-IV (100 and 120 mg/L) upregulated VEGF synthesis by activating the ERK1/2 pathway, thus promoting angiogenesis in EA.hy926 cells (Wang et al., 2015). In addition, Zhang et al. found that AS-IV (10 μg/mL and 100 μg/mL) can promote tube formation of HUVECs by activating the Akt pathway (Zhang et al., 2012). Connexins (Cx) are a family of structurally dependent transmembrane proteins that form gap junctions. Among these, Cx37, Cx40, and Cx43 are closely associated with angiogenesis (Simon and McWhorter, 2003; Alonso et al., 2016; Leybaert et al., 2017). Li et al. found that AS-IV (0.2 μg/mL) can enhance the gap junctional intercellular communication function by up-regulating the expression of Cx37, Cx40, and Cx43, thus promoting the angiogenesis of endothelial cells (Li et al., 2018b) (Figure 4).
3.4 Vasodilation
Nitric oxide (NO) is a major endodermal relaxant that causes the relaxation of vascular smooth muscle cells (VSMCs) (Bian et al., 2008). Lin et al. found that AS-IV(10–4,10–3 and 10–2 M) dilates the rat aorta. In vitro studies, AS-IV increased the amount of NO in rat aorta endothelial cells (RAECs), which is related to the activation of the PI3K/Akt/eNOS pathway (Lin et al., 2018). Tetrahydrobiopterin (BH4) is a key cofactor in NO synthesis from eNOS. The absence of BH4 leads to the uncoupling of eNOS and the production of the superoxide radical O2•- instead of NO (Landmesser et al., 2003). Xu et al. found that AS-IV (50 mg/kg/d) alleviated the vasoconstriction response induced by isoproterenol and inhibited the production of the superoxide radical O2•- in the aorta of SD rats; the mechanism was related to the enhancement of BH4 levels and the inhibition of eNOS uncoupling (Xu et al., 2016a).
ROS can cause NO to form toxic peroxynitrite (OONO−), leading to the uncoupling of eNOS. Under normal physiological conditions, the NO produced by eNOS is sufficient to remove the superoxide-free radical O2•- and protect cells from oxidative stress (Tesauro et al., 2006). However, when the level of the eNOS cofactor BH4 is not ideal, it leads to the uncoupling of eNOS, thus reducing the production of NO (Varadharaj et al., 2015). Xu et al. found that AS-IV (20, 50, 100 μmol/L) increased the generation of NO in HUVECs induced by hydrogen peroxide (H2O2), and the mechanism was related to the inhibition of the ROS/NF-κB pathway to reduce eNOS unconjugation (Xu et al., 2016b). In another study, Nie et al. found that AS-IV (40 or 80 mg/kg/day) increased NO production and eNOS expression in the thoracic aorta of diabetic rats by inhibiting calpain-1 (Nie et al., 2019b) (Figure 4).
3.5 Effects on atherosclerotic plaque
The destruction of atherosclerotic plaque stability is an important cause of cardiovascular and cerebrovascular diseases (Rosenfeld et al., 2000). Wang et al. found that AS-IV (40 mg/kg) reduced the lipid area of atherosclerotic plaques and increased collagen content and fiber cap thickness in ApoE−/− mice fed a high-fat diet (HFD). Its mechanism is related to the regulation of PI3K/Akt and TLR4/NF-κB pathway, inhibition of MMP-9 expression, and anti-inflammatory. In vitro studies, AS-IV (5 mol/L) significantly reduced the ox-LDL-induced accumulation of cytoplasmic lipid droplets in RAW 264.7 macrophages induced by ox-LDL (Wang et al., 2020). In another study, AS-IV (10 mg/kg) reduced HFD-induced LDLR−/− mice lipid levels, plaque area, and plaque stability by inhibiting the MAPK/NF-κB signaling pathway (Zhang et al., 2022b).
The formation and stability of atherosclerotic plaques are closely associated with inflammation. Studies have shown that peroxisome proliferator-activated receptor γ (PPAR-γ) is closely related to the production of inflammatory cytokines in macrophages (Oh et al., 2015). Sun et al. found that AS-IV (20 mg/kg/d) could upregulate PPAR-γ by inhibiting NF-κB, and reduce ox-LDL, TNF-α, IL-6, and IL-18 in the serum of SD rats, thus inhibiting the development of AS (Sun et al., 2018). OxLDL is also an AS promoter. In another study, Shao et al. established an AS cell model by exposing human umbilical HUVECs to ox-LDL. AS-IV (100 μM) reduced ox-LDL damage in HUVECs by regulating the circ_0000231/miR-135a-5p/CLIC4 pathway (Shao et al., 2021). Recent studies have shown that AS is associated with the stromal cell-derived factor-1 (SDF-1)/CXC chemokine receptor 4 (CXCR4) pathway. After platelet activation, SDF-1 is expressed in large quantities and binds to CXCR4, inducing the differentiation of CD34+ stem cells into macrophages and foam cells. Qin et al. found that AS-IV (40 mg/kg/day) reduced the degree of AS in the apoE−/− mouse aorta by regulating the SDF-1/CXCR4 pathway (Qin et al., 2015) (Figure 4).
3.6 Anti-vascular smooth muscle cell proliferation and migration
Abnormal proliferation and migration of VSMCs are closely related to the occurrence and development of CVD, such as AS and restenosis. Platelet-derived growth factor (PDGF) stimulates VSMC proliferation and migration of VSMCs (Donovan et al., 2013). Chen et al. found that AS-IV (10 µM) could significantly weaken the effect of PDGF-BB on the proliferation of human dermal VSMCs (HDVSMCs) and inhibit the transformation of HDVSMCs from differentiation phenotype to proliferative phenotype. In addition, AS-IV can inhibit the expression of MMP2 protein and the migration of HDVSMCs by inhibiting the p38 MAPK signaling pathway (Chen et al., 2014). Cyclin-dependent kinases (CDKs) play a key role in the cell cycle. Among them, CDK2 can switch the cell cycle from G1 to S phase and regulate the G2 phase, thus promoting cell proliferation (De Boer et al., 2008). Zhang et al. found that AS-IV (10 µM) inhibited Ang II-induced proliferation of A10 cells (adult rat VSMCs) by decreasing CDK2 activity (Zhang et al., 2018b).
Pulmonary arterial hypertension (PAH) is a chronic progressive disease with high mortality rates. Inhibiting the inflammatory response of PAEC and reducing the abnormal proliferation of pulmonary artery smooth muscle cells (PASMC) are effective methods for PAH treatment. Jin et al. found that AS-IV (10 and 30 mg/kg/day) reduced MCT-induced pulmonary artery pressure increases and inhibited pulmonary artery remodeling in SD rats. In vitro studies, AS-IV (10–80 µM) inhibited hypoxia-induced proliferation and anti-apoptosis of human PASMCs and inhibited HIF-1α and p-ERK1/2 protein expression enhancement in human PASMCs (Jin et al., 2021). In another study, AS-IV (2 mg/kg) was shown to mitigate pulmonary artery remodeling in rats with PAH. In vitro studies, AS-IV (20 μmol/L) inhibited hypoxia-induced PASMC proliferation, and the mechanism was related to Notch signaling inhibition (Yao et al., 2021). Zhang et al. found that AS-IV (10 and 50 mg/kg/d) has a relaxing effect on the pulmonary artery of PAH model rats and reduces the serum levels of ET-1, Ang II, TNF-α, and IL-6 in PAH model rats. In vitro studies, AS-IV (50 μM) inhibited PASMC proliferation under hypoxic conditions, which is consistent with the conclusions of these two studies (Zhang et al., 2018c).
Mitochondria are the powerhouses of cells and are believed to be key regulators of cell death. Lu et al. found that AS-IV (50 μg/mL) reversed the decline in the production of ATP in Ang II-induced VSMCs. In addition, AS-IV prevented Ang II-induced ROS overproduction and increased SOD activity (Lu et al., 2015). In another study, Song et al. found that AS-IV (50 μg/mL) inhibited autophagy and mineralization of VSMC by up-regulating lncRNA H19 and inhibiting dual-specificity phosphatase 5 (DUSP5) (Song et al., 2019) (Figure 4).
4 Pharmacokinetics and toxicology of AS-IV
Most current reports on the pharmacokinetics and toxicology of AS-IV have been published in Chinese literature. Zhang et al. (Zhang et al., 2006b) found that more than 83% of AS-IV bound to plasma proteins, and the binding relationship with plasma proteins was linear in the concentration range of 250–1,000 ng/mL. The elimination half-lives of AS-IV (0.75, 1.5, and 3.0 mg/kg) were 98.1, 67.2, and 71.8 min in male SD rats, and 34.0, 66.9, and 131.6 min in female SD rats, respectively. The systemic clearance rate of AS-IV did not differ significantly, suggesting that it may have linear pharmacokinetic characteristics within the experimental dose range in rats. AS-IV also showed linear pharmacokinetic characteristics in beagles, with elimination half-lives of 51.9, 60.0, and 68.8 min in males and 62.9, 67.2, and 50.2 min in females at doses of 0.25, 0.5, and 1 mg/kg. The concentration of AS-IV was highest in the lungs and liver of rats, whereas its distribution in the brain was limited, possibly because of the difficulty of AS-IV penetrating the blood-brain barrier. In addition, only trace amounts of AS-IV were detected in the brain and testes of male rats (<0.10 μg/g), and lower levels were detected in the brain and ovaries of female rats (<0.26 μg/g). Zhang et al. (Zhang et al., 2006b) also studied the excretion of urine and feces in rats after intravenous administration of 1.5 mg/kg AS-IV and found that the total amounts of urine and feces excreted in male rats were 45.03% and 53.61%, respectively, indicating that nearly 50% of AS-IV could be metabolized in vivo. AS-IV is slowly cleared through the liver, with a systemic clearance (CL) of approximately 0.004 L/kg/min. In addition, the distribution of AS-IV in male and female dogs ranged from 0.23 to 0.36 L/kg (less than the overall level of 0.6 L/kg in vivo), suggesting that AS-IV may have limited distribution in the extravascular compartment.
Gu et al. (Gu et al., 2004) evaluated the transport and bioavailability of AS-IV. They found that the absolute bioavailability of AS-IV after oral administration was only 2.2% in a rat intestinal perfusion model. This low bioavailability may be attributable to its high molecular weight and poor solubility in water and lipids. Zhang et al. (Zhang et al., 2007) reported that the absolute bioavailability of AS-IV in beagle dogs was approximately 7.4%. The binding rate of AS-IV plasma protein was approximately 90% in the concentration range of 250–1,000 ng/mL. However, the low bioavailability of AS-IV limits its oral use.
Yu et al. (Yu et al., 2007) observed the subchronic toxicity of Radix Astragali extract (RAE) in SD rats and beagle dogs. The authors found that RAE was safe and did not cause significant toxic side effects. The safe dose range of astragaloside IV is 5.7–39.9 g/kg in SD rats and 2.85–19.95 g/kg in beagle dogs, which is 70 or 35 times of that in humans. Zhu et al. (Jiangbo et al., 2009) studied the effects of AS-IV on embryonic development in rats and New Zealand White rabbits. 0.25, 0.5, and 1.0 mg/kg/d of AS-IV were administered intravenously during 6–15 days of gestation in SD rats, and 0.5, 1.0, and 2.0 mg/kg/d of AS-IV were administered intravenously during 6–18 days of gestation in New Zealand White rabbits. The results showed that the fetal mortality of SD rats was significantly higher than that of the control group after the intravenous injection of 0.5 and 1.0 mg/kg/d. In a New Zealand White rabbit study, the stillbirth rate was significantly higher in each treatment group than in the control group. This suggests that AS-IV is fatally toxic at doses greater than 0.5 mg/kg/d. No major visceral abnormalities or skeletal malformations were observed in any group of rats or New Zealand White rabbits. Wan et al. (Xuying et al., 2010) evaluated the effects of AS-IV on perinatal reproductive toxicity in SD rats. They assessed rat fertility and early embryonic developmental toxicity by intravenous injections of 0.25, 0.5, and 1.0 mg/kg/d AS-IV 4 weeks before mating, throughout mating, and on day 6 of female gestation. They found no clinical signs of toxicity associated with AS-IV in male or female F0 rats before mating, during mating, or during pregnancy. No differences in the liver, kidney, or reproductive organs were observed. In addition, there were no significant changes in sperm count, percentage of motile sperm, or proportion of total abnormal sperm in male rats, but 1.0 mg/kg/d AS-IV reduced the mating index of rats. Next, we investigated the effects of AS-IV on physiological and reflex development in F1 rats. A maternal dose of 1.0 mg/kg/d of AS-IV resulted in significant delays in fur appearance, eye-opening, motor activity, and cliff avoidance reflex time. However, in the memory and learning tests, there were no significant differences between the two groups. As the expected clinical dose in humans is 10 mg/60 kg/day, which is comparable to the dose studied above, AS-IV should be used with caution in the treatment of cardiovascular and cerebrovascular diseases in perinatal women.
5 Discussion
In recent years, significant progress has been made in the research and development of therapeutic methods and drugs for CVD. However, CVD morbidity and mortality remain high, and the search for highly effective drugs with fewer adverse effects remains a priority. Astragalus, a commonly used Chinese herbal medicine, exerts protective effects on the cardiovascular system. Astragalus injection, in which AS-IV content is up to 11.30 mg/mL, has achieved good efficacy in the treatment of coronary heart disease, angina pectoris, viral myocarditis, and other CVDs. The protective pathway of AS-IV in CVDs is complex, and its protective mechanism is networked. For example, AS-IV (100 µM) inhibits isoproterenol-induced myocardial fibrosis by inhibiting ROS-mediated MAPK activation (Dai et al., 2017), suggesting that AS-IV improves myocardial fibrosis by inhibiting oxidative stress. AS-IV (200 mg/kg/day) alleviated isoproterenol-induced cardiac fibrosis in BALB/c mice by inhibiting the NLRP3 inflammasome pathway (Wan et al., 2018), indicating that AS-IV improves myocardial fibrosis by inhibiting inflammation. AS-IV (40 and 80 mg/kg/day) inhibited the degree of myocardial hypertrophy induced by AAC through upregulation of the Nrf2/HO-1 signaling pathway (Nie et al., 2019a), suggesting that AS-IV improved myocardial hypertrophy through antioxidants. Currently, studies on the pharmacological action and mechanism of AS-IV are not in-depth, and a regulatory relationship is lacking. Previous studies on the cardiovascular protective pathway of AS-IV may only be part of the downstream signaling pathway after the action of AS-IV on specific molecular targets. Therefore, in-depth studies of the specific molecular targets of AS-IV using systems biology approaches are urgently required. For example, a photoactive probe was used to label metformin, and PEN2 was identified as a direct target of metformin (Ma et al., 2022).
It should be noted that the results of different studies are not entirely consistent. Liu et al. (Liu et al., 2018) found that AS-IV (10 and 20 mg/kg/day) reduced myocardial hypertrophy, inflammatory response, and cardiomyocyte apoptosis induced by the aortic band in C57BL6 mice by inhibiting the TBK1/PI3K/Akt pathway. However, Wei et al. (Wei et al., 2019) found that AS-IV (5 and 10 mg/kg) alleviates myocardial I/R injury in SD rats by activating the PI3K/Akt signaling pathway. Zhao et al. (Zhao et al., 2015a) found that AS-IV (20 mg/kg) alleviates lipopolysaccharide (LPS)-induced cardiac dysfunction and inflammatory mediator production in male C57BL/6 mice by activating the PI3K/Akt signaling pathway. The regulation of AS-IV on PI3K/Akt has been inconsistent in different studies, which may be related to the accuracy of the experiments. GATA-4 regulates the survival and adaptive response of cardiomyocytes under stress conditions. Yang et al. (Yang et al., 2020) found that AS-IV (0.5–300 μg/mL) can promote the expression of Bcl-2 by stimulating the overexpression of GATA-4, thus reducing the apoptosis of H9c2 cells induced by H/R damage. However, Lu et al. (Lu et al., 2014) found that AS-IV reduces LPS-induced myocardial hypertrophy by inhibiting the expression of GATA-4. In these two studies, the regulation of the expression of GATA-4 by AS-IV was inconsistent, which might be related to the different intervention methods. HDAC are enzymes that regulate histone acetylation. Zhang et al. (Zhang et al., 2021) found that AS-IV (12.5 and 50 µM) could inhibit HDAC activity, thereby protecting HL-1 mouse cardiomyocytes from oxidative damage of ox-LDL. However, Yang et al. (Matthews et al., 2006) found that AS-IV (40 mg/kg/day) promoted angiogenesis in the myocardial tissue of AMI rats by upregulating the HDAC5/VEGF signaling pathway. In these two studies, the regulation of HDAC expression by AS-IV was inconsistent, which may be related to the target cell type. The Notch signaling pathway is also associated with angiogenesis. Liang et al. (Liang et al., 2020) found that AS-IV (40 mg/kg/day) promoted angiogenesis in SD rats with middle cerebral artery occlusion by activating the Notch signaling pathway. Yao et al. (Yao et al., 2021) found that AS-IV (20 mol/L) mitigated hypoxia-induced PASMC proliferation by inhibiting the Notch signaling pathway. In these two studies, AS-IV showed inconsistent regulation of the Notch signaling pathway, which may be related to the cell type or drug dose. In future studies, systems biology methods should be used to further study the specific molecular targets of AS-IV so that the mechanism of action of AS-IV can be fundamentally clarified.
6 Conclusion
In conclusion, AS-IV has multiple targets and pathways that play important roles in CVD. AS-IV protects the myocardium through anti-oxidative stress, anti-inflammatory effects, regulation of calcium homeostasis, improvement of myocardial energy metabolism, anti-apoptosis, anti-cardiomyocyte hypertrophy, anti-myocardial fibrosis, regulation of myocardial autophagy, and improvement of myocardial microcirculation. AS-IV protects VECs through anti-inflammatory and antioxidant stress pathways. In addition, AS-IV stabilized atherosclerotic plaques and inhibited VSMC proliferation and migration. AS-IV is a complementary or alternative medicine that cannot replace the primary therapeutic agent. In addition, most of the efficacy of AS-IV has been observed under laboratory conditions, and clinical studies have been relatively inadequate. Thus, AS-IV is an important drug worth exploring.
Author contributions
CY and JL: conceptualization, methodology. CY and QP: writing-original draft. KJ, ZT, HZ, SL, and CL: visualization. JL: supervision. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by grants from General Program of National Natural Science Foundation of China (Grant no. 81973836), Science and Technology Innovation Project of China Academy of Chinese Medical Sciences (Grant no. CI2021A00902).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abderrazak, A., Syrovets, T., Couchie, D., El Hadri, K., Friguet, B., Simmet, T., et al. (2015). NLRP3 inflammasome: From a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biol. 4, 296–307. doi:10.1016/j.redox.2015.01.008
Alonso, F., Domingos-Pereira, S., Le Gal, L., Derré, L., Meda, P., Jichlinski, P., et al. (2016). Targeting endothelial connexin40 inhibits tumor growth by reducing angiogenesis and improving vessel perfusion. Oncotarget 7 (12), 14015–14028. doi:10.18632/oncotarget.7370
Bauer, T. M., and Murphy, E. (2020). Role of mitochondrial calcium and the permeability transition pore in regulating cell death. Circ. Res. 126 (2), 280–293. doi:10.1161/CIRCRESAHA.119.316306
Bian, K., Doursout, M. F., and Murad, F. (2008). Vascular system: Role of nitric oxide in cardiovascular diseases. J. Clin. Hypertens. (Greenwich) 10 (4), 304–310. doi:10.1111/j.1751-7176.2008.06632.x
Biernacka, A., Dobaczewski, M., and Frangogiannis, N. G. (2011). TGF-β signaling in fibrosis. Growth factors. 29 (5), 196–202. doi:10.3109/08977194.2011.595714
Bone, R., Balk, R., Cerra, F., Dellinger, R. P., Fein, A. M., Knaus, W. A., et al. (1992). Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM consensus conference committee. American college of chest physicians/society of critical care medicine. Chest 101, 1644–1655. doi:10.1378/chest.101.6.1644
Chen, P., Xie, Y., Shen, E., Yu, Y., Zhang, C. B., et al. (2011). Astragaloside IV attenuates myocardial fibrosis by inhibiting TGF-β1 signaling in coxsackievirus B3-induced cardiomyopathy. Eur. J. Pharmacol. 658 (2-3), 168–174. doi:10.1016/j.ejphar.2011.02.040
Chen, Q., Paillard, M., Gomez, L., Ross, T., Hu, Y., Xu, A., et al. (2011). Activation of mitochondrial μ-calpain increases AIF cleavage in cardiac mitochondria during ischemia-reperfusion. Biochem. Biophys. Res. Commun. 415 (4), 533–538. doi:10.1016/j.bbrc.2011.10.037
Chen, Z., Cai, Y., Zhang, W., Liu, X., and Liu, S. (2014). Astragaloside IV inhibits platelet-derived growth factor-BB-stimulated proliferation and migration of vascular smooth muscle cells via the inhibition of p38 MAPK signaling. Exp. Ther. Med. 8 (4), 1253–1258. doi:10.3892/etm.2014.1905
Cheng, S., Zhang, X., Feng, Q., Chen, J., Shen, L., Yu, P., et al. (2019). Astragaloside IV exerts angiogenesis and cardioprotection after myocardial infarction via regulating PTEN/PI3K/Akt signaling pathway. Life Sci. 227, 82–93. doi:10.1016/j.lfs.2019.04.040
Chouchani, E. T., Pell, V. R., Gaude, E., Aksentijević, D., Sundier, S. Y., Robb, E. L., et al. (2014). Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515 (7527), 431–435. doi:10.1038/nature13909
Dai, H., Jia, G., Lu, M., Liang, C., Wang, Y., and Wang, H. (2017). Astragaloside IV inhibits isoprenaline-induced cardiac fibrosis by targeting the reactive oxygen species/mitogen-activated protein kinase signaling axis. Mol. Med. Rep. 15 (4), 1765–1770. doi:10.3892/mmr.2017.6220
De Boer, L., Oakes, V., Beamish, H., Giles, N., Stevens, F., Somodevilla-Torres, M., et al. (2008). Cyclin A/cdk2 coordinates centrosomal and nuclear mitotic events. Oncogene 27 (31), 4261–4268. doi:10.1038/onc.2008.74
de la Ballina, L. R., Munson, M. J., and Simonsen, A. (2020). Lipids and lipid-binding proteins in selective autophagy. J. Mol. Biol. 432 (1), 135–159. doi:10.1016/j.jmb.2019.05.051
Di Benedetto, G., Scalzotto, E., Mongillo, M., and Pozzan, T. (2013). Mitochondrial Ca2⁺ uptake induces cyclic AMP generation in the matrix and modulates organelle ATP levels. Cell Metab. 17 (6), 965–975. doi:10.1016/j.cmet.2013.05.003
di Blasio, L., Droetto, S., Norman, J., Bussolino, F., and Primo, L. (2010). Protein kinase D1 regulates VEGF-A-induced alphavbeta3 integrin trafficking and endothelial cell migration. Traffic 11 (8), 1107–1118. doi:10.1111/j.1600-0854.2010.01077.x
Dong, Z., Zhao, P., Xu, M., Zhang, C., Guo, W., Chen, H., et al. (2017). Astragaloside IV alleviates heart failure via activating PPARα to switch glycolysis to fatty acid β-oxidation. Sci. Rep. 7 (1), 2691. doi:10.1038/s41598-017-02360-5
Donovan, J., Abraham, D., and Norman, J. (2013). Platelet-derived growth factor signaling in mesenchymal cells. Front. Biosci. (Landmark Ed. 18 (1), 106–119. doi:10.2741/4090
Du, X. Q., Shi, L. P., Chen, Z. W., Hu, J. Y., Zuo, B., Xiong, Y., et al. (2022). Astragaloside IV ameliorates isoprenaline-induced cardiac fibrosis in mice via modulating gut microbiota and fecal metabolites. Front. Cell Infect. Microbiol. 12, 836150. doi:10.3389/fcimb.2022.836150
Evans, J. L., Goldfine, I. D., Maddux, B. A., and Grodsky, G. M. (2002). Oxidative stress and stress-activated signaling pathways: A unifying hypothesis of type 2 diabetes. Endocr. Rev. 23 (5), 599–622. doi:10.1210/er.2001-0039
Frame, S., and Cohen, P. (2001). GSK3 takes centre stage more than 20 years after its discovery. Biochem. J. 359 (1), 1–16. doi:10.1042/0264-6021:3590001
Friedman, S. L., Sheppard, D., Duffield, J. S., and Violette, S. (2013). Therapy for fibrotic diseases: Nearing the starting line. Sci. Transl. Med. 5 (167), 167sr1. doi:10.1126/scitranslmed.3004700
Fu, S., Zhang, J., Menniti-Ippolito, F., Gao, X., Galeotti, F., Massari, M., et al. (2011). Huangqi injection (a traditional Chinese patent medicine) for chronic heart failure: A systematic review. PLoS One 6 (5), e19604. doi:10.1371/journal.pone.0019604
Garcia, C., Feve, B., Ferré, P., Halimi, S., Baizri, H., Bordier, L., et al. (2010). Diabetes and inflammation: Fundamental aspects and clinical implications. Diabetes Metab. 36 (5), 327–338. doi:10.1016/j.diabet.2010.07.001
Gu, Y., Wang, G., Pan, G., Fawcett, J. P., A, J., and Sun, J. (2004). Transport and bioavailability studies of astragaloside IV, an active ingredient in Radix Astragali. Basic Clin. Pharmacol. Toxicol. 95 (6), 295–298. doi:10.1111/j.1742-7843.2004.t01-1-pto950508.x
Guan, Y., Zhou, L., Zhang, Y., Tian, H., Li, A., and Han, X. (2019). Effects of PP2A/Nrf2 on experimental diabetes mellitus-related cardiomyopathy by regulation of autophagy and apoptosis through ROS dependent pathway. Cell Signal 62, 109339. doi:10.1016/j.cellsig.2019.06.004
Gui, J., Chen, R., Xu, W., and Xiong, S. (2015). Remission of CVB3-induced myocarditis with Astragaloside IV treatment requires A20 (TNFAIP3) up-regulation. J. Cell Mol. Med. 19 (4), 850–864. doi:10.1111/jcmm.12459
Han, X. H., Liu, P., Zhang, Y. Y., Zhang, N., Chen, F. R., and Cai, J. F. (2011). Astragaloside IV regulates expression of ATP-sensitive potassium channel subunits after ischemia-reperfusion in rat ventricular cardiomyocytes. J. Tradit. Chin. Med. 31 (4), 321–326. doi:10.1016/s0254-6272(12)60012-0
He, K., Yan, L., Lin, S. Q., Liu, Y. Y., Hu, B. H., Chang, X., et al. (2022). Implication of IGF1R signaling in the protective effect of Astragaloside IV on ischemia and reperfusion-induced cardiac microvascular endothelial hyperpermeability. Phytomedicine 100, 154045. doi:10.1016/j.phymed.2022.154045
He, Y., Xi, J., Zheng, H., Zhang, Y., Jin, Y., and Xu, Z. (2012). Astragaloside IV inhibits oxidative stress-induced mitochondrial permeability transition pore opening by inactivating GSK-3β via nitric oxide in H9c2 cardiac cells. Oxid. Med. Cell Longev. 2012, 935738. doi:10.1155/2012/935738
Higashikuni, Y., Tanaka, K., Kato, M., Nureki, O., Hirata, Y., Nagai, R., et al. (2013). Toll-like receptor-2 mediates adaptive cardiac hypertrophy in response to pressure overload through interleukin-1β upregulation via nuclear factor κB activation. J. Am. Heart Assoc. 2 (6), e000267. doi:10.1161/JAHA.113.000267
Huang, K. Y., Yu, Y. W., Liu, S., Zhou, Y. Y., Wang, J. S., Peng, Y. P., et al. (2021). A single, acute astragaloside IV therapy protects cardiomyocyte through attenuating superoxide anion-mediated accumulation of autophagosomes in myocardial ischemia-reperfusion injury. Front. Pharmacol. 12, 642925. doi:10.3389/fphar.2021.642925
Huang, R., Cui, Y. C., Wei, X. H., Pan, C. S., Li, Q., He, S. Y., et al. (2019). A novel traditional Chinese medicine ameliorates fatigue-induced cardiac hypertrophy and dysfunction via regulation of energy metabolism. Am. J. Physiol. Heart Circ. Physiol. 316 (6), H1378–H1388. doi:10.1152/ajpheart.00731.2018
Huang, X., Zhang, M. Z., Liu, B., Ma, S. Y., Yin, X., and Guo, L. H. (2021). Astragaloside IV attenuates polymicrobial sepsis-induced cardiac dysfunction in rats via IKK/NF-κB pathway. Chin. J. Integr. Med. 27 (11), 825–831. doi:10.1007/s11655-021-2869-9
Huang, X. P., Tan, H., Chen, B. Y., and Deng, C. Q. (2012). Astragalus extract alleviates nerve injury after cerebral ischemia by improving energy metabolism and inhibiting apoptosis. Biol. Pharm. Bull. 35 (4), 449–454. doi:10.1248/bpb.35.449
Ip, Y. T., and Davis, R. J. (1998). Signal transduction by the c-Jun N-terminal kinase (JNK)-from inflammation to development. Curr. Opin. Cell Biol. 10 (2), 205–219. doi:10.1016/s0955-0674(98)80143-9
Iwasaki, H., Kawamoto, A., Tjwa, M., Horii, M., Hayashi, S., Oyamada, A., et al. (2011). PlGF repairs myocardial ischemia through mechanisms of angiogenesis, cardioprotection and recruitment of myo-angiogenic competent marrow progenitors. PLoS One 6 (9), e24872. doi:10.1371/journal.pone.0024872
Jiang, B. H., Zheng, J. Z., Aoki, M., and Vogt, P. K. (2000). Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc. Natl. Acad. Sci. U. S. A. 97 (4), 1749–1753. doi:10.1073/pnas.040560897
Jiang, M., Ni, J., Cao, Y., Xing, X., Wu, Q., and Fan, G. (2019). Astragaloside IV attenuates myocardial ischemia-reperfusion injury from oxidative stress by regulating succinate, lysophospholipid metabolism, and ROS scavenging system. Oxid. Med. Cell Longev. 2019, 9137654. doi:10.1155/2019/9137654
Jiangbo, Z., Xuying, W., Yuping, Z., Xili, M., Yiwen, Z., and Tianbao, Z. (2009). Effect of astragaloside IV on the embryo-fetal development of Sprague-Dawley rats and New Zealand White rabbits. J. Appl. Toxicol. 29 (5), 381–385. doi:10.1002/jat.1422
Jin, H., Jiao, Y., Guo, L., Ma, Y., Zhao, R., Li, X., et al. (2021). Astragaloside IV blocks monocrotaline-induced pulmonary arterial hypertension by improving inflammation and pulmonary artery remodeling. Int. J. Mol. Med. 47 (2), 595–606. doi:10.3892/ijmm.2020.4813
Kania, G., Blyszczuk, P., and Eriksson, U. (2009). Mechanisms of cardiac fibrosis in inflammatory heart disease. Trends Cardiovasc Med. 19 (8), 247–252. doi:10.1016/j.tcm.2010.02.005
Karbach, S. H., Schönfelder, T., Brandão, I., Wilms, E., Hörmann, N., Jäckel, S., et al. (2016). Gut microbiota promote angiotensin II-induced arterial hypertension and vascular dysfunction. J. Am. Heart Assoc. 5 (9), e003698. doi:10.1161/JAHA.116.003698
Ko, J. K., Lam, F. Y., and Cheung, A. P. (2005). Amelioration of experimental colitis by Astragalus membranaceus through anti-oxidation and inhibition of adhesion molecule synthesis. World J. Gastroenterol. 11 (37), 5787–5794. doi:10.3748/wjg.v11.i37.5787
Koh, J. H., Hancock, C. R., Terada, S., Higashida, K., Holloszy, J. O., and Han, D. H. (2017). PPARβ is essential for maintaining normal levels of PGC-1α and mitochondria and for the increase in muscle mitochondria induced by exercise. Cell Metab. 25 (5), 1176–1185. doi:10.1016/j.cmet.2017.04.029
Koss, K. L., and Kranias, E. G. (1996). Phospholamban: A prominent regulator of myocardial contractility. Circ. Res. 79 (6), 1059–1063. doi:10.1161/01.res.79.6.1059
Kubli, D. A., and Gustafsson, A. B. (2015). Unbreak my heart: Targeting mitochondrial autophagy in diabetic cardiomyopathy. Antioxid. Redox Signal 22 (17), 1527–1544. doi:10.1089/ars.2015.6322
Landmesser, U., Dikalov, S., Price, S. R., McCann, L., Fukai, T., Holland, S. M., et al. (2003). Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J. Clin. Invest. 111 (8), 1201–1209. doi:10.1172/JCI14172
Lavine, K. J., Kovacs, A., and Ornitz, D. M. (2008). Hedgehog signaling is critical for maintenance of the adult coronary vasculature in mice. J. Clin. Invest. 118 (7), 2404–2414. doi:10.1172/JCI34561
Leng, B., Tang, F., Lu, M., Zhang, Z., Wang, H., and Zhang, Y. (2018). Astragaloside IV improves vascular endothelial dysfunction by inhibiting the TLR4/NF-κB signaling pathway. Life Sci. 209, 111–121. doi:10.1016/j.lfs.2018.07.053
Leng, B., Zhang, Y., Liu, X., Zhang, Z., Wang, H., et al. (2019). Astragaloside IV suppresses high glucose-induced NLRP3 inflammasome activation by inhibiting TLR4/NF-κB and CaSR. Mediat. Inflamm. 2019, 1082497. doi:10.1155/2019/1082497
Leybaert, L., Lampe, P. D., Dhein, S., Kwak, B. R., Ferdinandy, P., Beyer, E. C., et al. (2017). Connexins in cardiovascular and neurovascular health and disease: Pharmacological implications. Pharmacol. Rev. 69 (4), 396–478. doi:10.1124/pr.115.012062
Li, C., Li, Q., Liu, Y. Y., Wang, M. X., Pan, C. S., Yan, L., et al. (2014). Protective effects of Notoginsenoside R1 on intestinal ischemia-reperfusion injury in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 306 (2), G111–G122. doi:10.1152/ajpgi.00123.2013
Li, Y., Liu, Y., Wang, C., Xia, W. R., Zheng, J. Y., Yang, J., et al. (2018). Succinate induces synovial angiogenesis in rheumatoid arthritis through metabolic remodeling and HIF-1α/VEGF axis. Free Radic. Biol. Med. 126, 1–14. doi:10.1016/j.freeradbiomed.2018.07.009
Li, Z., Zhang, S., Cao, L., Li, W., Ye, Y. C., Shi, Z. X., et al. (2018). Tanshinone IIA and Astragaloside IV promote the angiogenesis of mesenchymal stem cell-derived endothelial cell-like cells via upregulation of Cx37, Cx40 and Cx43. Exp. Ther. Med. 15 (2), 1847–1854. doi:10.3892/etm.2017.5636
Liang, C., Ni, G. X., Shi, X. L., Jia, L., and Wang, Y. L. (2020). Astragaloside IV regulates the HIF/VEGF/Notch signaling pathway through miRNA-210 to promote angiogenesis after ischemic stroke. Restor. Neurol. Neurosci. 38 (3), 271–282. doi:10.3233/RNN-201001
Lin, J., Fang, L., Li, H., Li, Z., Lyu, L., Wang, H., et al. (2019). Astragaloside IV alleviates doxorubicin induced cardiomyopathy by inhibiting NADPH oxidase derived oxidative stress. Eur. J. Pharmacol. 859, 172490. doi:10.1016/j.ejphar.2019.172490
Lin, J., Wang, Q., Zhou, S., Xu, S., and Yao, K. (2022). Tetramethylpyrazine: A review on its mechanisms and functions. Biomed. Pharmacother. 150, 113005. doi:10.1016/j.biopha.2022.113005
Lin, X. P., Cui, H. J., Yang, A. L., Luo, J. K., and Tang, T. (2018). Astragaloside IV improves vasodilatation function by regulating the PI3K/Akt/eNOS signaling pathway in rat aorta endothelial cells. J. Vasc. Res. 55 (3), 169–176. doi:10.1159/000489958
Liu, C. J., Cheng, Y. C., Lee, K. W., Hsu, H. H., Chu, C. H., Tsai, F. J., et al. (2008). Lipopolysaccharide induces cellular hypertrophy through calcineurin/NFAT-3 signaling pathway in H9c2 myocardiac cells. Mol. Cell Biochem. 313 (1-2), 167–178. doi:10.1007/s11010-008-9754-0
Liu, T., Yang, F., Liu, J., Zhang, M., Sun, J., Xiao, Y., et al. (2019). Astragaloside IV reduces cardiomyocyte apoptosis in a murine model of coxsackievirus B3-induced viral myocarditis. Exp. Anim. 68 (4), 549–558. doi:10.1538/expanim.19-0037
Liu, X., Zhang, Z., Ruan, J., Pan, Y., Magupalli, V. G., Wu, H., et al. (2016). Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535 (7610), 153–158. doi:10.1038/nature18629
Liu, Z. H., Liu, H. B., and Wang, J. (2018). Astragaloside IV protects against the pathological cardiac hypertrophy in mice. Biomed. Pharmacother. 97, 1468–1478. doi:10.1016/j.biopha.2017.09.092
Lu, F. H., Fu, S. B., Leng, X., Zhang, X., Dong, S., Zhao, Y. J., et al. (2013). Role of the calcium-sensing receptor in cardiomyocyte apoptosis via the sarcoplasmic reticulum and mitochondrial death pathway in cardiac hypertrophy and heart failure. Cell Physiol. Biochem. 31 (4-5), 728–743. doi:10.1159/000350091
Lu, M., Leng, B., He, X., Zhang, Z., Wang, H., and Tang, F. (2018). Calcium sensing receptor-related pathway contributes to cardiac injury and the mechanism of astragaloside IV on cardioprotection. Front. Pharmacol. 9, 1163. doi:10.3389/fphar.2018.01163
Lu, M., Wang, H., Wang, J., Zhang, J., Yang, J., Liang, L., et al. (2014). Astragaloside IV protects against cardiac hypertrophy via inhibiting the Ca2+/CaN signaling pathway. Planta Med. 80 (1), 63–69. doi:10.1055/s-0033-1360129
Lu, S., Chen, K. J., Yang, Q. Y., and Sun, H. r. (2011). Progress in the research of radix astragali in treating chronic heart failure: Effective ingredients, dose-effect relationship and adverse reaction. Chin. J. Integr. Med. 17 (6), 473–477. doi:10.1007/s11655-011-0756-5
Lu, Y., Li, S., Wu, H., Bian, Z., Xu, J., Gu, C., et al. (2015). Beneficial effects of astragaloside IV against angiotensin II-induced mitochondrial dysfunction in rat vascular smooth muscle cells. Int. J. Mol. Med. 36 (5), 1223–1232. doi:10.3892/ijmm.2015.2345
Luo, L. F., Guan, P., Qin, L. Y., Wang, J. X., Wang, N., and Ji, E. S. (2021). Astragaloside IV inhibits adriamycin-induced cardiac ferroptosis by enhancing Nrf2 signaling. Mol. Cell Biochem. 476 (7), 2603–2611. doi:10.1007/s11010-021-04112-6
Luo, L. F., Qin, L. Y., Wang, J. X., Guan, P., and Ji, E. S. (2021). Astragaloside IV attenuates the myocardial injury caused by adriamycin by inhibiting autophagy. Front. Pharmacol. 12, 669782. doi:10.3389/fphar.2021.669782
Luo, P., Zhu, Y., Chen, M., Yan, H., Yang, B., Yang, X., et al. (2018). HMGB1 contributes to adriamycin-induced cardiotoxicity via up-regulating autophagy. Toxicol. Lett. 292, 115–122. doi:10.1016/j.toxlet.2018.04.034
Lv, L., Wu, S. Y., Wang, G. F., Zhang, J. J., Pang, J. X., Liu, Z. Q., et al. (2010). Effect of astragaloside IV on hepatic glucose-regulating enzymes in diabetic mice induced by a high-fat diet and streptozotocin. Phytother. Res. 24 (2), 219–224. doi:10.1002/ptr.2915
Ma, T., Tian, X., Zhang, B., Li, M., Wang, Y., Yang, C., et al. (2022). Low-dose metformin targets the lysosomal AMPK pathway through PEN2. Nature 603 (7899), 159–165. doi:10.1038/s41586-022-04431-8
Ma, X., Liu, H., Foyil, S. R., Godar, R. J., Weinheimer, C. J., Hill, J. A., et al. (2012). Impaired autophagosome clearance contributes to cardiomyocyte death in ischemia/reperfusion injury. Circulation 125 (25), 3170–3181. doi:10.1161/CIRCULATIONAHA.111.041814
Mackay, K., and Mochly-Rosen, D. (1999). An inhibitor of p38 mitogen-activated protein kinase protects neonatal cardiac myocytes from ischemia. J. Biol. Chem. 274 (10), 6272–6279. doi:10.1074/jbc.274.10.6272
Matsui, Y., Takagi, H., Qu, X., Abdellatif, M., Sakoda, H., Asano, T., et al. (2007). Distinct roles of autophagy in the heart during ischemia and reperfusion: Roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ. Res. 100 (6), 914–922. doi:10.1161/01.RES.0000261924.76669.36
Matthews, S. A., Liu, P., Spitaler, M., Olson, E. N., McKinsey, T. A., Cantrell, D. A., et al. (2006). Essential role for protein kinase D family kinases in the regulation of class II histone deacetylases in B lymphocytes. Mol. Cell Biol. 26 (4), 1569–1577. doi:10.1128/MCB.26.4.1569-1577.2006
McLaughlin, D., Zhao, Y., O'Neill, K. M., Edgar, K. S., Dunne, P. D., Kearney, A. M., et al. (2017). Signalling mechanisms underlying doxorubicin and Nox2 NADPH oxidase-induced cardiomyopathy: Involvement of mitofusin-2. Br. J. Pharmacol. 174 (21), 3677–3695. doi:10.1111/bph.13773
Mei, M., Tang, F., Lu, M., He, X., Wang, H., Hou, X., et al. (2015). Astragaloside IV attenuates apoptosis of hypertrophic cardiomyocyte through inhibiting oxidative stress and calpain-1 activation. Environ. Toxicol. Pharmacol. 40 (3), 764–773. doi:10.1016/j.etap.2015.09.007
Miele, L., Giorgio, V., Alberelli, M. A., De Candia, E., Gasbarrini, A., and Grieco, A. (2015). Impact of gut microbiota on obesity, diabetes, and cardiovascular disease risk. Curr. Cardiol. Rep. 17 (12), 120. doi:10.1007/s11886-015-0671-z
Minnich, A., Tian, N., Byan, L., and Bilder, G. (2001). A potent PPARalpha agonist stimulates mitochondrial fatty acid beta-oxidation in liver and skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 280 (2), E270–E279. doi:10.1152/ajpendo.2001.280.2.E270
Mizukami, Y., Okamura, T., Miura, T., Kimura, M., Mogami, K., Todoroki-Ikeda, N., et al. (2001). Phosphorylation of proteins and apoptosis induced by c-Jun N-terminal kinase1 activation in rat cardiomyocytes by H(2)O(2) stimulation. Biochim. Biophys. Acta 1540 (3), 213–220. doi:10.1016/s0167-4889(01)00137-9
Moens, A. L., Claeys, M. J., Timmermans, J. P., and Vrints, C. J. (2005). Myocardial ischemia/reperfusion-injury, a clinical view on a complex pathophysiological process. Int. J. Cardiol. 100 (2), 179–190. doi:10.1016/j.ijcard.2004.04.013
Nie, P., Meng, F., Zhang, J., Wei, X., and Shen, C. (2019). Astragaloside IV exerts a myocardial protective effect against cardiac hypertrophy in rats, partially via activating the Nrf2/HO-1 signaling pathway. Oxid. Med. Cell Longev. 2019, 4625912. doi:10.1155/2019/4625912
Nie, Q., Zhu, L., Zhang, L., Leng, B., and Wang, H. (2019). Astragaloside IV protects against hyperglycemia-induced vascular endothelial dysfunction by inhibiting oxidative stress and Calpain-1 activation. Life Sci. 232, 116662. doi:10.1016/j.lfs.2019.116662
Oh, J., Riek, A. E., Darwech, I., Funai, K., Shao, J., Chin, K., et al. (2015). Deletion of macrophage Vitamin D receptor promotes insulin resistance and monocyte cholesterol transport to accelerate atherosclerosis in mice. Cell Rep. 10 (11), 1872–1886. doi:10.1016/j.celrep.2015.02.043
Oka, T., Maillet, M., Watt, A. J., Schwartz, R. J., Aronow, B. J., Duncan, S. A., et al. (2006). Cardiac-specific deletion of Gata4 reveals its requirement for hypertrophy, compensation, and myocyte viability. Circ. Res. 98 (6), 837–845. doi:10.1161/01.RES.0000215985.18538.c4
Oslowski, C. M., Hara, T., O'Sullivan-Murphy, B., Kanekura, K., Lu, S., et al. (2012). Thioredoxin-interacting protein mediates ER stress-induced β cell death through initiation of the inflammasome. Cell Metab. 16 (2), 265–273. doi:10.1016/j.cmet.2012.07.005
Osugi, T., Oshima, Y., Fujio, Y., Funamoto, M., Yamashita, A., Negoro, S., et al. (2002). Cardiac-specific activation of signal transducer and activator of transcription 3 promotes vascular formation in the heart. J. Biol. Chem. 277 (8), 6676–6681. doi:10.1074/jbc.M108246200
Ozaki, E., Campbell, M., and Doyle, S. L. (2015). Targeting the NLRP3 inflammasome in chronic inflammatory diseases: Current perspectives. J. Inflamm. Res. 8, 15–27. doi:10.2147/JIR.S51250
Padro, T., Manfrini, O., Bugiardini, R., Canty, J., Cenko, E., De Luca, G., et al. (2020). ESC Working Group on Coronary Pathophysiology and Microcirculation position paper on 'coronary microvascular dysfunction in cardiovascular disease. Cardiovasc Res. 116 (4), 741–755. doi:10.1093/cvr/cvaa003
Pola, R., Ling, L. E., Silver, M., Corbley, M. J., Kearney, M., Blake Pepinsky, R., et al. (2001). The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat. Med. 7 (6), 706–711. doi:10.1038/89083
Qiao, Y., Fan, C. L., and Tang, M. K. (2017). Astragaloside IV protects rat retinal capillary endothelial cells against high glucose-induced oxidative injury. Drug Des. Devel Ther. 11, 3567–3577. doi:10.2147/DDDT.S152489
Qin, H., Liu, P., and Lin, S. (2015). Effects of astragaloside IV on the SDF-1/CXCR4 expression in atherosclerosis of apoE(-/-) mice induced by hyperlipaemia. Evid. Based Complement. Altern. Med. 2015, 385154. doi:10.1155/2015/385154
Qiu, L. H., Xie, X. J., and Zhang, B. Q. (2010). Astragaloside IV improves homocysteine-induced acute phase endothelial dysfunction via antioxidation. Biol. Pharm. Bull. 33 (4), 641–646. doi:10.1248/bpb.33.641
Ren, S., Zhang, H., Mu, Y., Sun, M., and Liu, P. (2013). Pharmacological effects of astragaloside IV: A literature review. J. Tradit. Chin. Med. 33 (3), 413–416. doi:10.1016/s0254-6272(13)60189-2
Rosenfeld, M. E., Polinsky, P., Virmani, R., Kauser, K., Rubanyi, G., and Schwartz, S. M. (2000). Advanced atherosclerotic lesions in the innominate artery of the ApoE knockout mouse. Arterioscler. Thromb. Vasc. Biol. 20 (12), 2587–2592. doi:10.1161/01.atv.20.12.2587
Sankaralingam, S., and Lopaschuk, G. D. (2015). Cardiac energy metabolic alterations in pressure overload-induced left and right heart failure (2013 Grover Conference Series). Pulm. Circ. 5 (1), 15–28. doi:10.1086/679608
Saraste, A., Pulkki, K., Kallajoki, M., Henriksen, K., Parvinen, M., and Voipio-Pulkki, L. M. (1997). Apoptosis in human acute myocardial infarction. Circulation 95 (2), 320–323. doi:10.1161/01.cir.95.2.320
Scioli, M. G., Storti, G., D'Amico, F., Rodríguez Guzmán, R., Centofanti, F., Doldo, E., et al. (2020). Oxidative stress and new pathogenetic mechanisms in endothelial dysfunction: Potential diagnostic biomarkers and therapeutic targets. J. Clin. Med. 9 (6), 1995. doi:10.3390/jcm9061995
Semenza, G. L. (2012). Hypoxia-inducible factors in physiology and medicine. Cell 148 (3), 399–408. doi:10.1016/j.cell.2012.01.021
Shan, Z. X., Lin, Q. X., Fu, Y. H., Deng, C. Y., Zhou, Z. L., Zhu, J. N., et al. (2009). Upregulated expression of miR-1/miR-206 in a rat model of myocardial infarction. Biochem. Biophys. Res. Commun. 381 (4), 597–601. doi:10.1016/j.bbrc.2009.02.097
Shao, X., Liu, Z., Liu, S., Lin, N., and Deng, Y. (2021). Astragaloside IV alleviates atherosclerosis through targeting circ_0000231/miR-135a-5p/CLIC4 axis in AS cell model in vitro. Mol. Cell Biochem. 476 (4), 1783–1795. doi:10.1007/s11010-020-04035-8
Shi, H., Zhou, P., Gao, G., Liu, P. P., Wang, S. S., Song, R., et al. (2021). Astragaloside IV prevents acute myocardial infarction by inhibiting the TLR4/MyD88/NF-κB signaling pathway. J. Food Biochem. 45 (7), e13757. doi:10.1111/jfbc.13757
Shostak, K., and Chariot, A. (2015). EGFR and NF-κB: Partners in cancer. Trends Mol. Med. 21 (6), 385–393. doi:10.1016/j.molmed.2015.04.001
Si, J., Wang, N., Wang, H., Xie, J., Yang, J., Yi, H., et al. (2014). HIF-1α signaling activation by post-ischemia treatment with astragaloside IV attenuates myocardial ischemia-reperfusion injury. PLoS One 9 (9), e107832. doi:10.1371/journal.pone.0107832
Simon, A. M., and McWhorter, A. R. (2003). Decreased intercellular dye-transfer and downregulation of non-ablated connexins in aortic endothelium deficient in connexin37 or connexin40. J. Cell Sci. 116 (11), 2223–2236. doi:10.1242/jcs.00429
Song, Z., Wei, D., Chen, Y., Chen, L., Bian, Y., Shen, Y., et al. (2019). Association of astragaloside IV-inhibited autophagy and mineralization in vascular smooth muscle cells with lncRNA H19 and DUSP5-mediated ERK signaling. Toxicol. Appl. Pharmacol. 364, 45–54. doi:10.1016/j.taap.2018.12.002
Su, Y., Yin, X., Huang, X., Guo, Q., Ma, M., and Guo, L. (2022). Astragaloside IV ameliorates sepsis-induced myocardial dysfunction by regulating NOX4/JNK/BAX pathway. Life Sci. 310, 121123. doi:10.1016/j.lfs.2022.121123
Sui, Y. B., Wang, Y., Liu, L., Liu, F., and Zhang, Y. Q. (2019). Astragaloside IV alleviates heart failure by promoting angiogenesis through the JAK-STAT3 pathway. Pharm. Biol. 57 (1), 48–54. doi:10.1080/13880209.2019.1569697
Sun, B., Rui, R., Pan, H., Zhang, L., and Wang, X. (2018). Effect of combined use of astragaloside IV (asIV) and atorvastatin (AV) on expression of PPAR-γ and inflammation-associated cytokines in atherosclerosis rats. Med. Sci. Monit. 24, 6229–6236. doi:10.12659/MSM.908480
Sun, C., Zeng, G., Wang, T., Ren, H., An, H., Lian, C., et al. (2021). Astragaloside IV ameliorates myocardial infarction induced apoptosis and restores cardiac function. Front. Cell Dev. Biol. 9, 671255. doi:10.3389/fcell.2021.671255
Sun, Y., Lu, M., Sun, T., and Wang, H. (2021). Astragaloside IV attenuates inflammatory response mediated by NLRP-3/calpain-1 is involved in the development of pulmonary hypertension. J. Cell Mol. Med. 25 (1), 586–590. doi:10.1111/jcmm.15671
Suryakumar, G., Kasiganesan, H., Balasubramanian, S., and Kuppuswamy, D. (2010). Lack of beta3 integrin signaling contributes to calpain-mediated myocardial cell loss in pressure-overloaded myocardium. J. Cardiovasc Pharmacol. 55 (6), 567–573. doi:10.1097/FJC.0b013e3181d9f5d4
Takahashi, M. (2014). NLRP3 inflammasome as a novel player in myocardial infarction. Int. Heart J. 55 (2), 101–105. doi:10.1536/ihj.13-388
Tan, Y. Q., Chen, H. W., and Li, J. (2020). Astragaloside IV: An effective drug for the treatment of cardiovascular diseases. Drug Des. Devel Ther. 14, 3731–3746. doi:10.2147/DDDT.S272355
Tang, B., Zhang, J. G., Tan, H. Y., and Wei, X. Q. (2018). Astragaloside IV inhibits ventricular remodeling and improves fatty acid utilization in rats with chronic heart failure. Biosci. Rep. 38 (3), BSR20171036. doi:10.1042/BSR20171036
Tesauro, M., Thompson, W. C., and Moss, J. (2006). Effect of staurosporine-induced apoptosis on endothelial nitric oxide synthase in transfected COS-7 cells and primary endothelial cells. Cell Death Differ. 13 (4), 597–606. doi:10.1038/sj.cdd.4401770
Tu, L., Pan, C. S., Wei, X. H., Yan, L., Liu, Y. Y., Fan, J. Y., et al. (2013). Astragaloside IV protects heart from ischemia and reperfusion injury via energy regulation mechanisms. Microcirculation 20 (8), 736–747. doi:10.1111/micc.12074
Varadharaj, S., Porter, K., Pleister, A., Wannemacher, J., Sow, A., Jarjoura, D., et al. (2015). Endothelial nitric oxide synthase uncoupling: A novel pathway in OSA induced vascular endothelial dysfunction. Respir. Physiol. Neurobiol. 207, 40–47. doi:10.1016/j.resp.2014.12.012
Wan, Y., Xu, L., Wang, Y., Tuerdi, N., Ye, M., and Qi, R. (2018). Preventive effects of astragaloside IV and its active sapogenin cycloastragenol on cardiac fibrosis of mice by inhibiting the NLRP3 inflammasome. Eur. J. Pharmacol. 833, 545–554. doi:10.1016/j.ejphar.2018.06.016
Wang, B., Zhang, C., Chu, D., Ma, X., Yu, T., Liu, X., et al. (2021). Astragaloside IV improves angiogenesis under hypoxic conditions by enhancing hypoxia-inducible factor-1α SUMOylation. Mol. Med. Rep. 23 (4), 244. doi:10.3892/mmr.2021.11883
Wang, C., Li, Y., Yang, X., Bi, S., Zhang, Y., Han, D., et al. (2017). Tetramethylpyrazine and astragaloside IV synergistically ameliorate left ventricular remodeling and preserve cardiac function in a rat myocardial infarction model. J. Cardiovasc Pharmacol. 69 (1), 34–40. doi:10.1097/FJC.0000000000000437
Wang, N., Zhang, X., Ma, Z., Niu, J., Ma, S., Wenjie, W., et al. (2020). Combination of tanshinone IIA and astragaloside IV attenuate atherosclerotic plaque vulnerability in ApoE(-/-) mice by activating PI3K/AKT signaling and suppressing TRL4/NF-κB signaling. Biomed. Pharmacother. 123, 109729. doi:10.1016/j.biopha.2019.109729
Wang, Q., Chen, W., Yang, X., Song, Y., Sun, X., Tao, G., et al. (2022). Inhibition of miRNA-1-mediated inflammation and autophagy by astragaloside IV improves lipopolysaccharide-induced cardiac dysfunction in rats. J. Inflamm. Res. 15, 2617–2629. doi:10.2147/JIR.S362368
Wang, Q., Yang, X., Song, Y., Sun, X., Li, W., Zhang, L., et al. (2021). Astragaloside IV-targeting miRNA-1 attenuates lipopolysaccharide-induced cardiac dysfunction in rats through inhibition of apoptosis and autophagy. Life Sci. 275, 119414. doi:10.1016/j.lfs.2021.119414
Wang, S., Chen, J., Fu, Y., and Chen, X. (2015). Promotion of astragaloside IV for EA-hy926 cell proliferation and angiogenic activity via ERK1/2 pathway. J. Nanosci. Nanotechnol. 15 (6), 4239–4244. doi:10.1166/jnn.2015.9785
Wang, S. G., Xu, Y., Chen, J. D., and Yang, C. H. (2013). Astragaloside IV stimulates angiogenesis and increases nitric oxide accumulation via JAK2/STAT3 and ERK1/2 pathway. Molecules 18 (10), 12809–12819. doi:10.3390/molecules181012809
Wang, X., Liang, X., Liang, H., and Wang, B. (2018). SENP1/HIF-1α feedback loop modulates hypoxia-induced cell proliferation, invasion, and EMT in human osteosarcoma cells. J. Cell Biochem. 119 (2), 1819–1826. doi:10.1002/jcb.26342
Wei, D., Xu, H., Gai, X., and Jiang, Y. (2019). Astragaloside IV alleviates myocardial ischemia-reperfusion injury in rats through regulating PI3K/AKT/GSK-3β signaling pathways. Acta Cir. Bras. 34 (7), e201900708. doi:10.1590/s0102-865020190070000008
Wei, Y., Wu, Y., Feng, K., Zhao, Y., Tao, R., Xu, H., et al. (2020). Astragaloside IV inhibits cardiac fibrosis via miR-135a-TRPM7-TGF-β/Smads pathway. J. Ethnopharmacol. 249, 112404. doi:10.1016/j.jep.2019.112404
Weis, S. M. (2008). Vascular permeability in cardiovascular disease and cancer. Curr. Opin. Hematol. 15 (3), 243–249. doi:10.1097/MOH.0b013e3282f97d86
Xu, C., Tang, F., Lu, M., Yang, J., Han, R., Mei, M., et al. (2016). Astragaloside IV improves the isoproterenol-induced vascular dysfunction via attenuating eNOS uncoupling-mediated oxidative stress and inhibiting ROS-NF-κB pathways. Int. Immunopharmacol. 33, 119–127. doi:10.1016/j.intimp.2016.02.009
Xu, C., Tang, F., Lu, M., Yang, J., Han, R., Mei, M., et al. (2016). Pretreatment with Astragaloside IV protects human umbilical vein endothelial cells from hydrogen peroxide induced oxidative stress and cell dysfunction via inhibiting eNOS uncoupling and NADPH oxidase - ROS - NF-κB pathway. Can. J. Physiol. Pharmacol. 94 (11), 1132–1140. doi:10.1139/cjpp-2015-0572
Xu, X. L., Chen, X. J., Ji, H., Li, P., Bian, Y. Y., Yang, D., et al. (2008). Astragaloside IV improved intracellular calcium handling in hypoxia-reoxygenated cardiomyocytes via the sarcoplasmic reticulum Ca-ATPase. Pharmacology 81 (4), 325–332. doi:10.1159/000121335
Xu, X. L., Ji, H., Gu, S. Y., Shao, Q., Huang, Q. J., and Cheng, Y. P. (2007). Modification of alterations in cardiac function and sarcoplasmic reticulum by astragaloside IV in myocardial injury in vivo. Eur. J. Pharmacol. 568 (1-3), 203–212. doi:10.1016/j.ejphar.2007.04.007
Xuying, W., Jiangbo, Z., Yuping, Z., Xili, M., Yiwen, Z., Tianbao, Z., et al. (2010). Effect of astragaloside IV on the general and peripartum reproductive toxicity in Sprague-Dawley rats. Int. J. Toxicol. 29 (5), 505–516. doi:10.1177/1091581810376840
Yang, J., Wang, H. X., Zhang, Y. J., Yang, Y. H., Lu, M. L., Zhang, J., et al. (2013). Astragaloside IV attenuates inflammatory cytokines by inhibiting TLR4/NF-кB signaling pathway in isoproterenol-induced myocardial hypertrophy. J. Ethnopharmacol. 150 (3), 1062–1070. doi:10.1016/j.jep.2013.10.017
Yang, J. J., Zhang, X. H., Ma, X. H., Duan, W. J., Xu, N. G., Chen, Y. J., et al. (2020). Astragaloside IV enhances GATA-4 mediated myocardial protection effect in hypoxia/reoxygenation injured H9c2 cells. Nutr. Metab. Cardiovasc Dis. 30 (5), 829–842. doi:10.1016/j.numecd.2020.01.009
Yang, L., Liu, N., Zhao, W., Li, X., Han, L., Zhang, Z., et al. (2019). Angiogenic function of astragaloside IV in rats with myocardial infarction occurs via the PKD1-HDAC5-VEGF pathway. Exp. Ther. Med. 17 (4), 2511–2518. doi:10.3892/etm.2019.7273
Yao, J., Fang, X., Zhang, C., Yang, Y., Wang, D., Chen, Q., et al. (2021). Astragaloside IV attenuates hypoxia-induced pulmonary vascular remodeling via the Notch signaling pathway. Mol. Med. Rep. 23 (1), 89. doi:10.3892/mmr.2020.11726
Yin, B., Hou, X. W., and Lu, M. L. (2019). Astragaloside IV attenuates myocardial ischemia/reperfusion injury in rats via inhibition of calcium-sensing receptor-mediated apoptotic signaling pathways. Acta Pharmacol. Sin. 40 (5), 599–607. doi:10.1038/s41401-018-0082-y
You, L., Fang, Z., Shen, G., Wang, Q., He, Y., Ye, S., et al. (2019). Astragaloside IV prevents high glucose-induced cell apoptosis and inflammatory reactions through inhibition of the JNK pathway in human umbilical vein endothelial cells. Mol. Med. Rep. 19 (3), 1603–1612. doi:10.3892/mmr.2019.9812
Yu, L. M., and Xu, Y. (2015). Epigenetic regulation in cardiac fibrosis. World J. Cardiol. 7 (11), 784–791. doi:10.4330/wjc.v7.i11.784
Yu, S. Y., Ouyang, H. T., Yang, J. Y., Huang, X. L., Duan, J. P., et al. (2007). Subchronic toxicity studies of Radix Astragali extract in rats and dogs. J. Ethnopharmacol. 110 (2), 352–355. doi:10.1016/j.jep.2006.09.024
Zanotti-Cavazzoni, S. L., and Hollenberg, S. M. (2009). Cardiac dysfunction in severe sepsis and septic shock. Curr. Opin. Crit. Care 15 (5), 392–397. doi:10.1097/MCC.0b013e3283307a4e
Zhang, D. Q., Li, J. S., Zhang, Y. M., Gao, F., and Dai, R. Z. (2018). Astragaloside IV inhibits Angiotensin II-stimulated proliferation of rat vascular smooth muscle cells via the regulation of CDK2 activity. Life Sci. 200, 105–109. doi:10.1016/j.lfs.2018.03.036
Zhang, L., Liu, Q., Lu, L., Zhao, X., Gao, X., and Wang, Y. (2011). Astragaloside IV stimulates angiogenesis and increases hypoxia-inducible factor-1α accumulation via phosphatidylinositol 3-kinase/Akt pathway. J. Pharmacol. Exp. Ther. 338 (2), 485–491. doi:10.1124/jpet.111.180992
Zhang, Q., Zhu, L. L., Chen, G. G., and Du, Y. (2007). Pharmacokinetics of astragaloside iv in beagle dogs. Eur. J. Drug Metab. Pharmacokinet. 32 (2), 75–79. doi:10.1007/BF03190995
Zhang, S., Tang, F., Yang, Y., Lu, M., Luan, A., Zhang, J., et al. (2015). Astragaloside IV protects against isoproterenol-induced cardiac hypertrophy by regulating NF-κB/PGC-1α signaling mediated energy biosynthesis. PLoS One 10 (3), e0118759. doi:10.1371/journal.pone.0118759
Zhang, W., Wang, X., Li, J., Xu, M., Ren, X., Liu, H., et al. (2021). Astragaloside IV reduces OxLDL-induced BNP overexpression by regulating HDAC. J. Healthc. Eng. 2021, 3433615. doi:10.1155/2021/3433615
Zhang, W. D., Zhang, C., Liu, R. H., Li, H. L., Zhang, J. T., Mao, C., et al. (2006). Preclinical pharmacokinetics and tissue distribution of a natural cardioprotective agent astragaloside IV in rats and dogs. Life Sci. 79 (8), 808–815. doi:10.1016/j.lfs.2006.02.032
Zhang, W. J., and Frei, B. (2015). Astragaloside IV inhibits NF- κ B activation and inflammatory gene expression in LPS-treated mice. Mediat. Inflamm. 2015, 274314. doi:10.1155/2015/274314
Zhang, X., Chen, J., Xu, P., and Tian, X. (2018). Protective effects of astragaloside IV against hypoxic pulmonary hypertension. Medchemcomm 9 (10), 1715–1721. doi:10.1039/c8md00341f
Zhang, X., Li, M., and Wang, H. (2019). Astragaloside IV alleviates the myocardial damage induced by lipopolysaccharide via the toll-like receptor 4 (TLR4)/Nuclear factor kappa B (NF-κB)/Proliferator-Activated receptor α (PPARα) signaling pathway. Med. Sci. Monit. 25, 7158–7168. doi:10.12659/MSM.916030
Zhang, X., Qu, H., Yang, T., Liu, Q., and Zhou, H. (2022). Astragaloside IV attenuate MI-induced myocardial fibrosis and cardiac remodeling by inhibiting ROS/caspase-1/GSDMD signaling pathway. Cell Cycle 21 (21), 2309–2322. doi:10.1080/15384101.2022.2093598
Zhang, Y., Du, M., Wang, J., and Liu, P. (2022). Astragaloside IV relieves atherosclerosis and hepatic steatosis via MAPK/NF-κB signaling pathway in LDLR-/- mice. Front. Pharmacol. 13, 828161. doi:10.3389/fphar.2022.828161
Zhang, Y., Hu, G., Li, S., Li, Z. H., Lam, C. O., Hong, S. J., et al. (2012). Pro-angiogenic activity of astragaloside IV in HUVECs in vitro and zebrafish in vivo. Mol. Med. Rep. 5 (3), 805–811. doi:10.3892/mmr.2011.716
Zhang, Y., Rong, H., Zhang, F. X., Wu, K., Mu, L., Meng, J., et al. (2018). A membrane potential- and calpain-dependent reversal of caspase-1 inhibition regulates canonical NLRP3 inflammasome. Cell Rep. 24 (9), 2356–2369. doi:10.1016/j.celrep.2018.07.098
Zhang, Y., Zhu, H., Huang, C., Cui, X., Gao, Y., Huang, Y., et al. (2006). Astragaloside IV exerts antiviral effects against coxsackievirus B3 by upregulating interferon-gamma. J. Cardiovasc Pharmacol. 47 (2), 190–195. doi:10.1097/01.fjc.0000199683.43448.64
Zhang, Z., Wang, J., Zhu, Y., Zhang, H., and Wang, H. (2019). Astragaloside IV alleviates myocardial damage induced by type 2 diabetes via improving energy metabolism. Mol. Med. Rep. 20 (5), 4612–4622. doi:10.3892/mmr.2019.10716
Zhao, P., Wang, Y., Zeng, S., Lu, J., Jiang, T. M., and Li, Y. M. (2015). Protective effect of astragaloside IV on lipopolysaccharide-induced cardiac dysfunction via downregulation of inflammatory signaling in mice. Immunopharmacol. Immunotoxicol. 37 (5), 428–433. doi:10.3109/08923973.2015.1080266
Zhao, Y., Li, Q., Zhao, W., Li, J., Sun, Y., Liu, K., et al. (2015). Astragaloside IV and cycloastragenol are equally effective in inhibition of endoplasmic reticulum stress-associated TXNIP/NLRP3 inflammasome activation in the endothelium. J. Ethnopharmacol. 169, 210–218. doi:10.1016/j.jep.2015.04.030
Zhou, R. N., Song, Y. L., Ruan, J. Q., Wang, Y. T., and Yan, R. (2012). Pharmacokinetic evidence on the contribution of intestinal bacterial conversion to beneficial effects of astragaloside IV, a marker compound of astragali radix, in traditional oral use of the herb. Drug Metab. Pharmacokinet. 27 (6), 586–597. doi:10.2133/dmpk.dmpk-11-rg-160
Zhu, Y., Qian, X., Li, J., Lin, X., Luo, J., Huang, J., et al. (2019). Astragaloside-IV protects H9C2(2-1) cardiomyocytes from high glucose-induced injury via miR-34a-mediated autophagy pathway. Artif. Cells Nanomed Biotechnol. 47 (1), 4172–4181. doi:10.1080/21691401.2019.1687492
Zhu, Z., Li, J., and Zhang, X. (2019). Astragaloside IV protects against oxidized low-density lipoprotein (ox-LDL)-Induced endothelial cell injury by reducing oxidative stress and inflammation. Med. Sci. Monit. 25, 2132–2140. doi:10.12659/MSM.912894
Zucchi, R., Ronca, F., and Ronca-Testoni, S. (2001). Modulation of sarcoplasmic reticulum function: A new strategy in cardioprotection? Pharmacol. Ther. 89 (1), 47–65. doi:10.1016/s0163-7258(00)00103-0
Glossary
Keywords: astragaloside IV, anti-oxidative stress, apoptosis, fibrosis, inflammation, energy metabolism, cardiovascular diseases
Citation: Yang C, Pan Q, Ji K, Tian Z, Zhou H, Li S, Luo C and Li J (2023) Review on the protective mechanism of astragaloside IV against cardiovascular diseases. Front. Pharmacol. 14:1187910. doi: 10.3389/fphar.2023.1187910
Received: 16 March 2023; Accepted: 03 May 2023;
Published: 11 May 2023.
Edited by:
Zhengyu Cao, China Pharmaceutical University, ChinaReviewed by:
Fang Zhao, China Pharmaceutical University, ChinaJunhui Xing, The First Affiliated Hospital of Zhengzhou University, China
Copyright © 2023 Yang, Pan, Ji, Tian, Zhou, Li, Luo and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Li, Z2FteXlsakAxNjMuY29t
†These authors have contributed equally to this work
 Chunkun Yang
Chunkun Yang Qingquan Pan2†
Qingquan Pan2†