- 1Cardiology Department of the Second Medical Center, National Clinical Research Center for Geriatric Diseases, Chinese PLA General Hospital, Beijing, China
- 2The Forth Healthcare Department of the Second Medical Center, Chinese PLA General Hospital, Beijing, China
Objectives: Long-term use of evidence-based antiplatelet therapy is recommended for management of stable coronary artery disease (SCAD). However, non-adherence to antiplatelet drugs is common in older patients. This study aimed to evaluate the incidence and impact of antiplatelet therapy cessation on clinical outcomes of older patients with SCAD.
Methods: A total of 351 consecutive eligible very older patients (≥80 years) with SCAD from the PLA General Hospital were included. Baseline demographics, clinical characteristics, and clinical outcomes were collected during follow-up. Patients were divided into cessation group and standard group based on whether discontinuing of antiplatelet drugs. The primary outcome was major adverse cardiovascular events (MACE) and secondary outcomes were minor bleeding and all-cause mortality.
Results: A total of 351 participants, with a mean age of 91.76 ± 5.01 years old (range 80–106 years) were included in statistical analysis. The antiplatelet drug cessation rate was 60.1%. There were 211 patients in cessation group and 140 patients in standard group. During a median follow-up of 98.6 months, the primary outcome of MACE occurred in 155 patients (73.5%) in the cessation group and 84 patients (60.0%) in the standard group (HR = 1.476, 95% CI:1.124-1.938, p = 0.005). Cessation of antiplatelet drugs increased the rates of angina (HR = 1.724, 95% CI:1.211-2.453, p = 0.002) and non-fatal MI (HR = 1.569, 95% CI:1.093-2.251, p = 0.014). The secondary outcomes of minor bleeding and all-cause mortality were similar between the two groups.
Conclusion: Among very older patients with SCAD, antiplatelet therapy cessation significantly increased the risk of MACE, and continuous antiplatelet drug therapy didn’t increase the risk of minor bleeding.
Introduction
Coronary artery disease (CAD) is a leading cause of death worldwide. The Chinese Cardiovascular Health and Disease Report 2020 estimated that there were 11.39 million patients diagnosed CAD, which caused 43.81 and 46.66 deaths per 100 individuals in rural and urban areas, respectively (National Center for Cardiovascular Diseases, 2021). Although the mortality and prevalence of CAD have decreased significantly in recent decades, management of stable coronary artery disease (SCAD) remains an important issue.
Oral antiplatelet agents (OAA) including aspirin and a P2Y12 inhibitor (such as clopidogrel) can prevent myocardial infarction (MI), stroke, and death in cardiovascular disease (Antithrom botic Trialists and Collaboration, 2002). Guidelines recommend long-term use of evidence-based medications for management of SCAD (Fihn et al., 2012; Task Force Memberes Montalescot et al., 2013; Section of Interventional Cardiology of Chinese Society of Cardiology et al., 2018). However, adherence is crucial for achieving optimal benefit from antiplatelet therapy and improving clinical outcomes (Bosworth et al., 2011; Reuter et al., 2015). Medication adherence is suboptimal after MI, with a report from 2008 indicating that 1 in 4 patients did not fill their initial cardiac medication prescriptions in the first month after MI, which is associated with higher mortality and cardiovascular events (Jackevicius et al., 2008). However, non-adherence to medications is common among older patients with cardiovascular diseases. Among the reasons for non-adherence in these older patients, independent functional ability non-adherence is common and may represent a significant barrier to optimal adherence.
A previous study showed that patients with a recent interruption in OAA-intake displayed worse clinical outcomes, and OAA withdrawal was found to be an independent predictor of both mortality and bleedings at 30 days (Collet et al., 2004). However, the incidence and impact of recent OAA cessation on clinical outcomes among very older patients with SCAD are still lacking. Therefore, we prospectively investigated whether continuous use or cessation of OAA influences long-term clinical outcomes in very older patients with SCAD.
Materials and methods
Study population
A total of 377 patients (age ≥ 80 years) with SCAD were recruited from the Chinese PLA General Hospital from January 2009 to December 2010 and were followed up until December 2020. The inclusion criteria were as follows: 1) age ≥80 years and 2) compliance with SCAD diagnostic criteria. We excluded 26 patients based on the following criteria: 1) major bleeding, cerebrovascular disease, or other unstable conditions over the past one month (n = 16) and 2) incomplete data on outcomes during the follow-up period (n = 10). Ultimately, a total of 351 patients were found to be eligible for statistical analysis.
Data collection and definitions
Patient baseline demographics, clinical characteristics, and follow-up outcomes were collected during the follow-up period.
The diagnostic criteria for SCAD were based on the 2013 ESC guidelines on the management of SCAD (Task Force Memberes Montalescot et al., 2013; Section of Interventional Cardiology of Chinese Society of Cardiology et al., 2018) and 2018 Chinese guidelines on the diagnosis and treatment of SCAD (Section of Interventional Cardiology of Chinese Society of Cardiology et al., 2018). Major bleeding corresponded to overt bleeding resulting in death; a bleed in a retroperitoneal, intracranial, or intraocular location; a hemoglobin drop of ≥3 g/dL; or the need for transfusion of ≥2 U blood. Minor bleeding was any clinically important bleeding that did not qualify as major (Collet et al., 2004).
The criteria for antiplatelet therapy cessation were as follows (Levine et al., 2016): aspirin or clopidogrel therapy which had been interrupted for >7 days without a specific reason or without advice from the prescribing practitioner, and no other antithrombotic drugs were adopted during the interruption.
Follow-up and outcomes
All participants were followed-up until December 2020. Outcome events were recorded through outpatient visits, rehospitalizations, and telephone calls. The primary outcome was major adverse cardiovascular events (MACE), the secondary outcomes were minor bleeding and all-cause mortality. MACE include cardiovascular death, non-fatal MI, restenosis, and angina, as reported in a previous study (Baber et al., 2018).
Statistical analysis
The baseline characteristics were compared between the standard and cessation groups. Continuous variables were displayed as mean ± SD or median (25th-75th percentile) and compared using the t-test or Wilcoxon rank test, as appropriate. Categorical variables are presented as numbers (percentages) and were compared using the chi-square test. Kaplan-Meier survival curves were used to determine the cumulative survival rates at the end of the follow-up period among patients in the two groups.
To explore the association between outcomes and antiplatelet therapy cessation, univariate and multivariate Cox regression analyses were performed to calculate hazard ratios (HRs) with 95% confidence intervals (CIs). The following adjusted covariates were included in the multivariate Cox regression analysis: age, sex, BMI, smoking, drinking, antidiabetic, hypertension, and cancer.
All analyses were conducted using the SPSS Statistics software version 22.0. Statistical significance was set at p < 0.05.
Results
Baseline characteristics
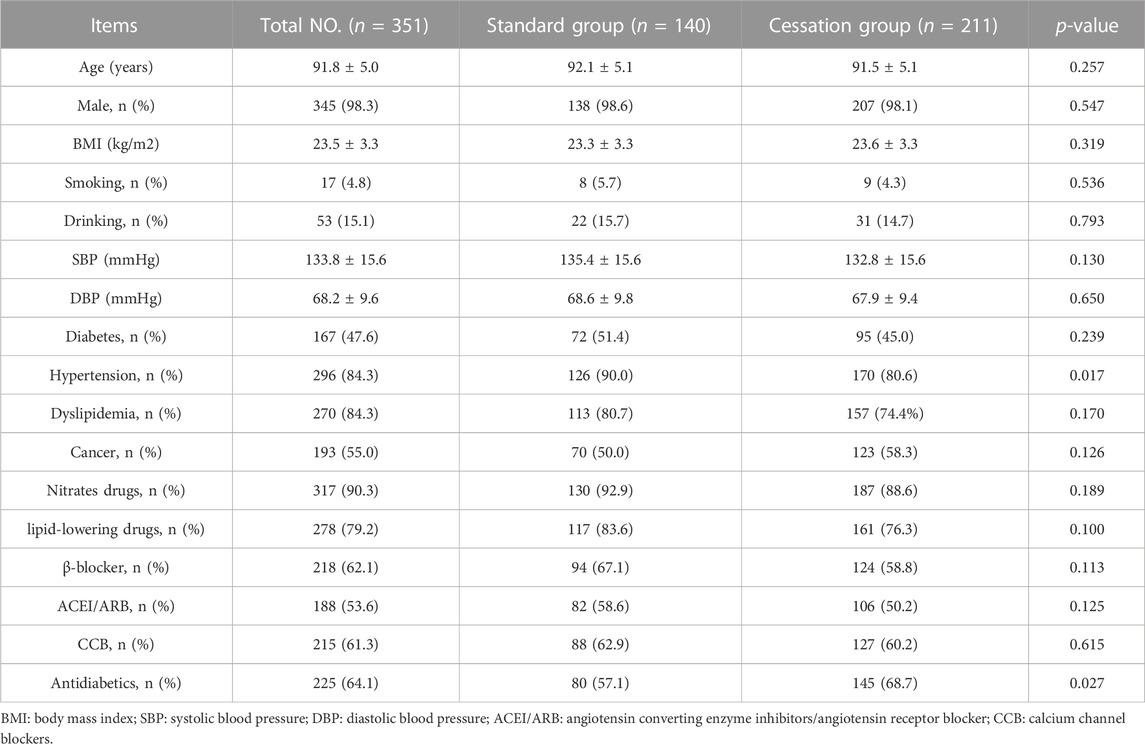
A total of 351 patients were included in this study; their characteristics are shown in Table 1. Mean age was 91.76 ± 5.01 years (range 80–106 years), and 98.3% of patients were male. A total of 211 (60.1%) patients experienced antiplatelet drug cessation and were placed in the cessation group. Compared with the patients in the cessation group, those in the standard group were more likely to have hypertension and receive antidiabetic therapy. The other baseline characteristics were well balanced between the two groups.
Clinical outcomes
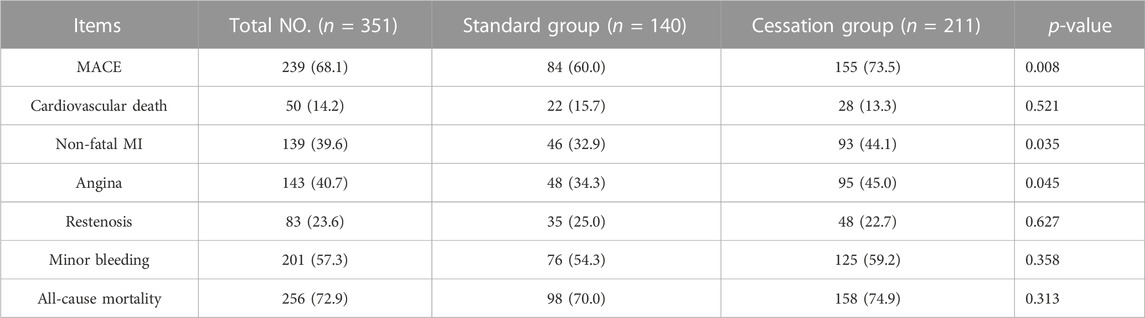
The median follow-up period in our study was 98.6 months (range 2–126 months). The median follow-up period for standard and cessation group were 87.2 months (range 5–125 months) and 88.6 months (range 2–126 months), respectively. There was no significant difference between them during the follow-up period (p = 0.401). With regard to the primary outcome of MACE, patients in the cessation group experienced higher MACE rates than those in the standard group (73.5% vs. 60.0%, p = 0.008), and also displayed higher rates of non-fatal MI (44.1% vs. 32.9%, p = 0.035) and angina (45.0% vs. 34.3%, p = 0.045). However, the patients in the two groups displayed similar rates on the secondary outcomes of all-cause mortality and minor bleeding. Table 2 shows all outcomes between the two groups.
Kaplan-Meier Survival Curves
The Kaplan-Meier curves of the two groups are shown in Figure 1. The cumulative survival proportions of MACE at the end of follow-up were statistically different between the standard and cessation groups. Compared with patients in the standard group, those in the cessation group had higher survival rates for non-fatal MI and angina. There were no statistically significant differences in the survival rates of all-cause mortality between the two groups.
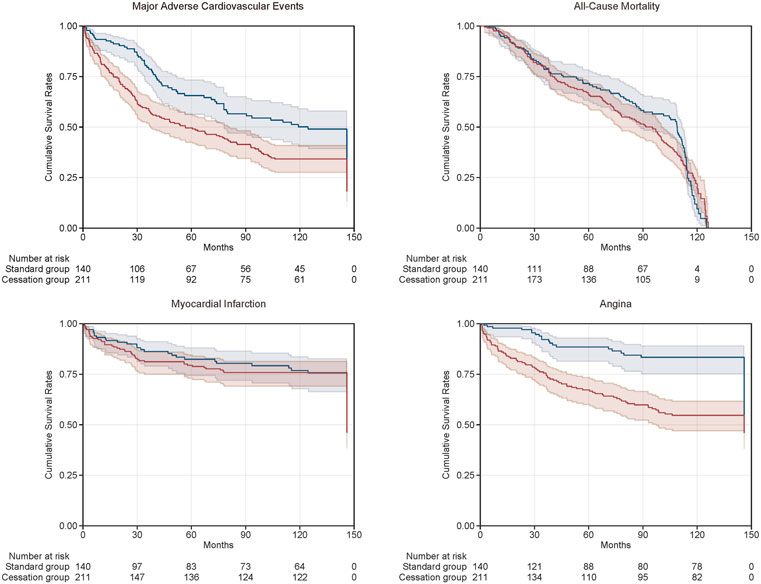
FIGURE 1. Kaplan-Meier curves of cumulative survival rates of clinical outcomes between standard group (the blue curve) and cessation group (the red curve).
Associations between antiplatelet therapy cessation and outcomes
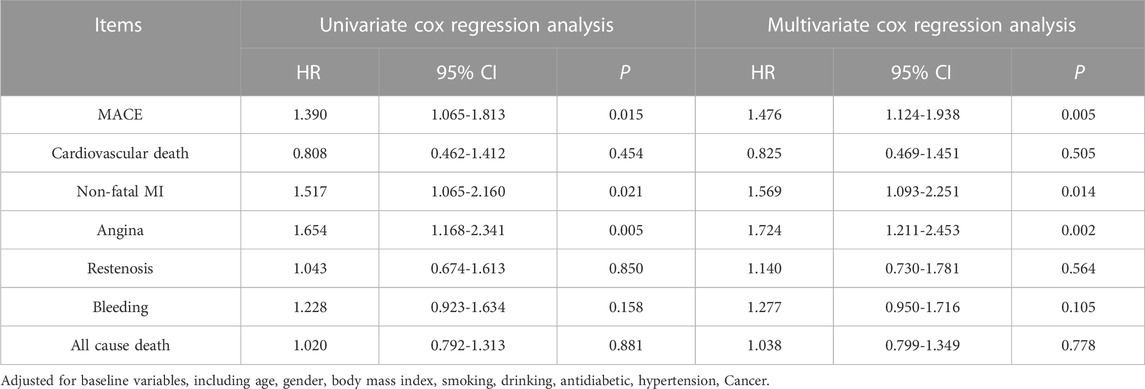
Multivariate Cox regression analyses were performed to evaluate the association between cessation of antiplatelet therapy and outcomes. We performed the univariate Cox analyses first, and then the multivariate Cox analyses, which were fully adjusted for clinical variables (Table 3); the multivariate Cox analyses showed similar results to univariate models. Patients in the cessation group were more likely to have MACE than those in the standard group (HR = 1.390, 95% CI:1.065-1.813, p = 0.015). Similarly, patients in the cessation group were more likely to have non-fatal MI (HR = 1.517, 95% CI:1.065-2.160, p = 0.021) and angina (HR = 1.654, 95% CI:1.168-2.341, p = 0.005). The interaction between antiplatelet therapy cessation and the secondary outcomes of minor bleeding and all-cause mortality was not statistically significant. These results suggest that cessation of antiplatelet therapy increased the risk of MACE, especially non-fatal MI and angina.
Discussion
Our study provides insights into the incidence and association between antiplatelet therapy cessation and clinical outcomes in older patients with SCAD. The present study found that 60.1% of patients had experienced antiplatelet therapy cessation. This is the first time that a variable (antiplatelet therapy cessation) has been systematically collected in very older patients with SCAD. Subsequently, we found that antiplatelet therapy cessation appeared to be associated with a higher risk of MACE, especially increased risk of non-fatal MI and angina. However, continuous antiplatelet drug therapy did not increase the risk of minor bleeding in these patients.
Aspirin (or another oral antiplatelet drug) has a protective effect following long-term use in patients at increased risk of occlusive vascular events, including those with ACS or SCAD (Antithrom botic Trialists and Collaboration, 2002). Guidelines have suggested that all patients with SCAD should receive antiplatelet drugs every day for their whole lives unless there are contraindications (Juul-Moller et al., 1992; Committee, 1996; Section of Interventional Cardiology of Chinese Society of Cardiology et al., 2018). Adherence to taking P2Y12 inhibitors is an important determinant of efficacy, with lower rates of compliance being associated with MACE (Lordkipanidze et al., 2020). While aspirin/clopidogrel cessation still occurs for many reasons, with prevalence ranging from 5.4% to 47.4% in previous studies (Collet et al., 2004; Derogar et al., 2013; Baber et al., 2018; Koshy et al., 2022). In our study, all patients were initially administered aspirin or clopidogrel therapy, and 60.1% of patients displayed aspirin/clopidogrel cessation, which was higher than that reported in previous studies. The main reason was that most of the very older patients had comorbidities and dysfunctional activities of daily living.
In the present study, aspirin/clopidogrel cessation patients experienced a higher prevalence of MACE compared to patients who did not cease taking aspirin or clopidogrel (73.5% vs. 60.0%), and also displayed higher rates of non-fatal MI (44.1% vs. 32.9%) and angina (45.0% vs. 34.3%). Ferrari et al. (2005) published 51 cases of coronary events after aspirin cessation. Similarly, they found a higher incidence of ST-segment elevation ACS in aspirin cessation patients. They reported that aspirin cessation in patients with coronary disease may represent a risk for occurrence of new coronary events.
Non-compliance with aspirin/clopidogrel treatment has ominous prognostic implications in subjects at moderate to high risk for CAD. A meta-analysis showed that aspirin non-adherence/withdrawal was associated with a three-fold higher risk of MACE (Biondi-Zoccai et al., 2006). Baber et al. (2018) reported that disruption of antiplatelet therapy was associated with higher risks for MACE among patients with and without chronic kidney disease. A prospective multicenter observational registry study with 5018 PCI-treated patients also found that antiplatelet therapy cessation was associated with a higher risk of MI and MACE (Faggioni et al., 2019). In our study, although the participants were very older patients with SCAD, we found a similar result showing antiplatelet drug cessation was associated with a 1.47-fold higher risk of MACE. The impact of medication on cessation and outcomes was consistent with previous literature.
The prevalence of clinical outcomes in our study was higher than previous studies (Collet et al., 2004; Derogar et al., 2013; Reuter et al., 2015) as the patients in our study differed from those in the other studies. First, the mean age was 91.76 ± 5.01 years, and the median follow-up period of our study was 98.6 months (range 2–126 months); patient age was significantly higher and follow-up time was significantly longer than other studies. Second, most very older patients have more than two comorbidities. These variabilities may have led to the higher rate of clinical outcomes in our study. A similar result was found in our previous study of older patients (Zou et al., 2022).
Baber et al. (2018) published a study on the pattern and impact of various modes of antiplatelet therapy cessation for patients undergoing PCI, and found no significant interaction between the risk of clinical outcomes and each cessation mode. Antiplatelet therapy cessation modes included physician-recommended discontinuation, temporary interruption (≤14 days), and disruption due to bleeding or noncompliance. Hennigan et al. (2022) recently studied the relationship between antiplatelet therapy cessation and clinical outcomes. They found that cessation of any type led to a substantial increase in platelet reactivity, with differential effects on different pathways of platelet activation when aspirin or P2Y12 inhibitors were stopped. Recent years, some studies also evaluate the adherence of other antiplatelet drugs-ticagrelor and prasugrel. Previous study showed 22% of patients with ACS discontinued ticagrelor because of side effects, age >75 years, and bleeding during the follow-up. Koskinas et al. report a study about 1830 patients (17% women, mean age 59 years), they found that prasugrel cessation had occurred in 13.8% of the patients at 1 year, early prasugrel cessation also associated with statistically significant excess in ischemic risk within 1 year after PCI (Koskinas et al., 2018). Further study is required to determine which patients could receive a net benefit from long-term continuation of dual antiplatelet therapy.
Study limitations
This study has several limitations. Our study was based on patients from a hospital in a single center, and therefore may not be representative of the entire older population. In addition, although the association of antiplatelet therapy cessation with a higher rate of MACE was particularly strong, the special nature of older patients and the presence of unmeasured confounders may have contributed to the strength of the association and survival bias. Lastly, in our retrospective study, we did not classify cessation types due to the insufficient sample size and data limitations. In our future study, we will continue the prospective study to improve the results.
Conclusion
Among very older patients with SCAD, antiplatelet therapy cessation significantly increased the risk of MACE, and continuous antiplatelet drug therapy did not increase the risk of minor bleeding.
Data availability statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the ethics committee of the Chinese PLA general hospital, and all the participants submitted a written informed consent prior to enrolment. All the procedures were performed in accordance with the relevant guidelines and regulations to this effect.
Author contributions
Conception and design: LF and H-BL. Performance, analysis and interpretation of data: XZ, LW, S-SS, Y-XH, H-WL, HW, and JC. Drafting of the manuscript: XZ. Revising manuscript for important intellectual content: LW and JC. Final approval of the manuscript submitted: XZ and LW. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Military Healthcare Fund of China (18BJZ32), the Miao Pu Fund of Chinese PLA General Hospital (17KMM23), and the Second Medical Center Foundation of Chinese PLA General Hospital (ZXFH 2003).
Acknowledgments
The authors would like to thank doctors and technologists who supported this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Antithrombotic Trialists, Collaboration (2002). Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 324, 71–86. doi:10.1136/bmj.324.7329.71
Baber, U., Li, S. X., Pinnelas, R., Pocock, S. J., Krucoff, M. W., Ariti, C., et al. (2018). Incidence, patterns, and impact of dual antiplatelet therapy cessation among patients with and without chronic kidney disease undergoing percutaneous coronary intervention: Results from the PARIS registry (patterns of non-adherence to anti-platelet regimens in stented patients). Circ. Cardiovasc Interv. 11, e006144. doi:10.1161/CIRCINTERVENTIONS.117.006144
Biondi-Zoccai, G. G., Lotrionte, M., Agostoni, P., Abbate, A., Fusaro, M., et al. (2006). A systematic review and meta-analysis on the hazards of discontinuing or not adhering to aspirin among 50,279 patients at risk for coronary artery disease. Eur. Heart J. 27, 2667–2674. doi:10.1093/eurheartj/ehl334
Bosworth, H. B., Granger, B. B., Mendys, P., Brindis, R., Burkholder, R., et al. (2011). Medication adherence: A call for action. Am. Heart J. 162, 412–424. doi:10.1016/j.ahj.2011.06.007
Collet, J. P., Montalescot, G., Blanchet, B., Tanguy, M. L., Golmard, J. L., et al. (2004). Impact of prior use or recent withdrawal of oral antiplatelet agents on acute coronary syndromes. Circulation 110, 2361–2367. doi:10.1161/01.CIR.0000145171.89690.B4
Committee, C. S. (1996). A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet 348, 1329–1339. doi:10.1016/s0140-6736(96)09457-3
Derogar, M., Sandblom, G., Lundell, L., Orsini, N., Bottai, M., et al. (2013). Discontinuation of low-dose aspirin therapy after peptic ulcer bleeding increases risk of death and acute cardiovascular events. Clin. Gastroenterol. Hepatol. 11, 38–42. doi:10.1016/j.cgh.2012.08.034
Faggioni, M., Baber, U., Sartori, S., Chandrasekhar, J., Cohen, D. J., et al. (2019). Influence of baseline anemia on dual antiplatelet therapy cessation and risk of adverse events after percutaneous coronary intervention. Circ. Cardiovasc Interv. 12, e007133. doi:10.1161/CIRCINTERVENTIONS.118.007133
Ferrari, E., Benhamou, M., Cerboni, P., and Marcel, B. (2005). Coronary syndromes following aspirin withdrawal: A special risk for late stent thrombosis. J. Am. Coll. Cardiol. 45, 456–459. doi:10.1016/j.jacc.2004.11.041
Fihn, S. D., Gardin, J. M., Abrams, J., Berra, K., Blankenship, J. C., Dallas, A. P., et al. (2012). 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: A report of the American College of Cardiology foundation/American heart association task force on practice guidelines, and the American College of physicians, American association for thoracic surgery, preventive cardiovascular nurses association, society for cardiovascular angiography and interventions, and society of thoracic surgeons. Circulation 126, e354–e471. doi:10.1161/CIR.0b013e318277d6a0
Hennigan, B. W., Good, R., Adamson, C., Parker, W. A. E., Martin, L., Anderson, L., et al. (2022). Recovery of platelet reactivity following cessation of either aspirin or ticagrelor in patients treated with dual antiplatelet therapy following percutaneous coronary intervention: A GLOBAL LEADERS substudy. Platelets 33, 141–146. doi:10.1080/09537104.2020.1863937
Jackevicius, C. A., Li, P., and Tu, J. V. (2008). Prevalence, predictors, and outcomes of primary nonadherence after acute myocardial infarction. Circulation 117, 1028–1036. doi:10.1161/CIRCULATIONAHA.107.706820
Juul-Moller, S., Edvardsson, N., Jahnmatz, B., Rosén, A., Sørensen, S., Omblus, R., et al. (1992). Double-blind trial of aspirin in primary prevention of myocardial infarction in patients with stable chronic angina pectoris. The Swedish Angina Pectoris Aspirin Trial (SAPAT) Group. Lancet 340, 1421–1425. doi:10.1016/0140-6736(92)92619-q
Koshy, A. N., Cao, D., Levin, M. A., Sartori, S., Giustino, G., Kyaw, H., et al. (2022). Predictors of antiplatelet cessation in a real-world patient population undergoing non-cardiac surgery after PCI. Int. J. Cardiol. 364, 27–30. doi:10.1016/j.ijcard.2022.06.023
Koskinas, K. C., Zanchin, T., Klingenberg, R., Gencer, B., Temperli, F., Baumbach, A., et al. (2018). Incidence, predictors, and clinical impact of early prasugrel cessation in patients with ST-elevation myocardial infarction. J. Am. Heart Assoc. 7, e008085. doi:10.1161/JAHA.117.008085
Levine, G. N., Bates, E. R., Bittl, J. A., Brindis, R. G., Fihn, S. D., Fleisher, L. A., et al. (2016). 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J. Am. Coll. Cardiol. 68, 1082–1115. doi:10.1016/j.jacc.2016.03.513
Lordkipanidzé, M., Marquis-Gravel, G., Tanguay, J. F., Mehta, S. R., and So, D. Y. F. (2020). Implications of the antiplatelet therapy gap left with discontinuation of Prasugrel in Canada. CJC Open 3, 814–821. doi:10.1016/j.cjco.2020.11.021
Task Force Memberes Montalescot, G., Sechtem, U., Achenbach, S., Andreotti, F., Arden, C., et al. (2013). 2013 ESC guidelines on the management of stable coronary artery disease: The task force on the management of stable coronary artery disease of the European society of Cardiology. Eur. Heart J. 34, 2949–3003. doi:10.1093/eurheartj/eht296
National Center for Cardiovascular Diseases (2021). Annual report on cardiovascular health and diseases in China 2020. J. Cardiovasc. &Pulmonary Dis. 40. doi:10.3969/j.issn.1007-5062.2021.09.001
Reuter, H., Markhof, A., Scholz, S., Wegmann, C., Seck, C., Adler, C., et al. (2015). Long-term medication adherence in patients with ST-elevation myocardial infarction and primary percutaneous coronary intervention. Eur. J. Prev. Cardiol. 22, 890–898. doi:10.1177/2047487314540385
Section of Interventional Cardiology of Chinese Society of CardiologySection of Atherosclerosis and Coronary Artery Disease of Chinese Society of CardiologySpecialty Committee on Prevention and Treatment of Thrombosis of Chinese College of Cardiovascular Physicians (2018). Guideline on the diagnosis and treatment of stable coronary artery disease. Zhonghua Xin Xue Guan Bing Za Zhi 46, 680–694. doi:10.3760/cma.j.issn.0253-3758.2018.09.004
Keywords: stable coronary artery disease, antiplatelet therapy, MACE, bleeding, mortality
Citation: Zou X, Wang L, Sun S-S, Hu Y-X, Liu H-W, Wang H, Cao J, Liu H-B and Fan L (2023) Incidence and impact of antiplatelet therapy cessation among very older patients with stable coronary artery disease. Front. Pharmacol. 14:1183839. doi: 10.3389/fphar.2023.1183839
Received: 10 March 2023; Accepted: 23 May 2023;
Published: 05 June 2023.
Edited by:
Brian Godman, University of Strathclyde, United KingdomReviewed by:
Maja Ortner Hadžiabdić, University of Zagreb, CroatiaMarta Belmonte, OLV Aalst, Belgium
Copyright © 2023 Zou, Wang, Sun, Hu, Liu, Wang, Cao, Liu and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Fan, Zmw2Njk4QDE2My5jb20=; Hong-Bin Liu, bGl1aGJpbjMwMUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Xiao Zou
Xiao Zou Liang Wang1†
Liang Wang1† Sha-Sha Sun
Sha-Sha Sun Yi-Xin Hu
Yi-Xin Hu Jian Cao
Jian Cao