- 1Department of General and Vascular Surgery, Xiangya Hospital, Central South University, Changsha, China
- 2National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China
- 3Department of Surgery, Stanford University School of Medicine, Stanford, CA, United States
Background: It is well-accepted that antihypertensive therapy is the cornerstone of treatment for abdominal aortic aneurysm (AAA) patients with hypertension. Direct-acting vasodilators were used in the treatment of hypertension by directly relaxing vascular smooth muscle but may have destructive effects on the aortic wall by activating the renin–angiotensin system axis. Their roles in AAA disease remain to be elucidated. In this study, we used hydralazine and minoxidil, two classical direct-acting vasodilators, to investigate their influence and potential mechanisms on AAA disease.
Methods and results: In this study, we investigated the plasma renin level and plasma renin activity in AAA patients. Simultaneously, age and gender ratio-matched patients diagnosed with peripheral artery disease and varicose veins were selected as the control group using a ratio of 1:1:1. Our regression analysis suggested both the plasma renin level and plasma renin activity are positively associated with AAA development. In view of the well-established relationship between direct-acting vasodilators and increased plasma renin concentration, we established a porcine pancreatic elastase-infused AAA mouse model, followed by oral administration of hydralazine (250 mg/L) and minoxidil (120 mg/L) to investigate effects of direct-acting vasodilators on AAA disease. Our results suggested both hydralazine and minoxidil promoted the progression of AAA with increased aortic degeneration. Mechanistically, the vasodilators aggravated aortic inflammation by increased leukocyte infiltration and inflammatory cytokine secretion.
Conclusion and relevance: The plasma renin level and plasma renin activity are positively associated with AAA development. Direct vasodilators aggravated experimental AAA progression, which raised cautionary concerns about their applications in AAA disease.
Introduction
Abdominal aortic aneurysm (AAA) is the dilation of the abdominal aorta 1.5 times its original size, generally more than 3 cm, with structural disruptions of the aortic vasculature (Golledge, 2019). The incidence of AAAs increases significantly in men aged >55 years and women aged >70 years, posing a high risk to the elderly due to the high morbidity and mortality associated with aortic rupture (Bossone and Eagle, 2021). Although previous studies reported successful medical stabilization of growing AAAs in different animal models, the current management of AAA mainly relies on the prophylactic operative repair of larger aneurysms (Liao et al., 2001; Golledge et al., 2006; Sakalihasan et al., 2018). Currently, medical management during aneurysm surveillance warrants further studies.
Arterial hypertension is an important population-attributable risk factor for AAA, which is linked to an increased risk of cardiovascular events and adverse prognosis, following AAA (Kobeissi et al., 2019). Previous studies reported that for every 20-mmHg increase in systolic blood pressure and 10-mmHg increase in diastolic blood pressure, the relative risk for AAA increases by 14% and 28%, respectively (Kobeissi et al., 2019; Wanhainen et al., 2019). Although antihypertensive therapy has not been shown to inhibit aneurysm expansion, it could benefit patients by reducing the risks of cardiovascular events (Bicknell et al., 2016). Thus, guidelines encouraged AAA patients to seek appropriate medical management for hypertension (Chaikof et al., 2018; NICE guideline [NG156]). However, specific recommendations for the choice of antihypertensive medication remain poorly described, which is mainly referred to the local guidelines, as demonstrated in the ESVS and NICE guidelines (Wanhainen et al., 2019).
Direct-acting vasodilators are a heterogeneous group of drugs, which can directly dilate peripheral arterioles and lower blood pressure, and are generally used in systemic hypertension, especially in patients with refractory hypertension, stage III hypertension, or renal dysfunction (Cassis et al., 2009; Lu et al., 2012). Despite the decline in their use due to adverse effects, direct vasodilators are still valuable in clinical practice for these niche indications. Hydralazine and minoxidil are the most commonly prescribed direct vasodilators for hypertension (Laurent, 2017). However, it is reported that arterial vasodilation induced by direct vasodilators can activate the peripheral sympathetic nervous system via carotid and aortic baroreceptor reflexes, casing increased circulating renin levels and subsequent activation of the renin–angiotensin system, which eventually leads to tachycardia and fluid retention (Gottlieb et al., 1972; Campese et al., 1979; Sica and Gehr, 2001). As the only enzyme known to cleave angiotensinogen and the rate-limiting enzyme of the renin-angiotensin system, plasma renin can increase the synthesis of angiotensin II and aldosterone, which plays a critical role in cardiovascular disease (Ferrario and Strawn, 2006; Miyake et al., 2017). Considering the essential role of the renin–angiotensin system in the progression of AAA disease, it is worth investigating whether neurohumoral regulation by direct-acting vasodilators aggravates AAA disease.
In the study, we investigated the relationship between the plasma renin level (PRL) and plasma renin activity (PRA) with AAA in patients using a case–control design, which suggested PRL and PRA are positively associated with AAA development. Given that direct vasodilators may increase the PRL and PRA, we explored the effect of direct vasodilators on AAA disease using hydralazine and minoxidil. Our results suggested direct vasodilators aggravated experimental AAA progression, raising cautionary concerns about their applications on arterial disease and AAA disease.
Methods
Study subjects
Cases
Patients diagnosed with AAA were selected for pre-onset PRL and PRA determination according to the disease diagnosis code of the 10th edition of the International Classification of Diseases (ICD) as the case group. AAA is defined as 50% dilation of the abdominal aorta, generally more than 3 cm of normal (Golledge, 2019). At the same time, two investigators examined and determined the aortic angiography and computed tomography findings and classification of all cases to exclude false positives. Patients with connective tissue disease, Marfan syndrome, vasculitis, and traumatic aortic dissection were excluded. In addition, patients who had a definite diagnosis of secondary hypertension at the time of aldosterone testing (renal vascular hypertension, renal hypertension, and aldosterone-producing adenoma with adenoma larger than 1 cm, Cushing’s syndrome, pheochromocytoma, and coarctation of the aorta) were excluded.
Control
Given the possibility of increased plasma renin levels in arterial disease (Marchesi et al., 2008), we included peripheral arterial disease (PAD) and varicose vein (VV) as controls using a case–control ratio of 1:1:1 matching age and sex with the same exclusion criteria. In addition, in order to explore the effects of AAA on the PRL and PRA, the PAD and VV groups were combined into the non-AAA group and compared with the AAA group.
Data collection
We obtained patient data from the hospital electronic medical database. The following general, clinical, and laboratory data were collected: general data (age, gender, and cigarette consumption), anthropometric data [body mass index, systolic blood pressure, diastolic blood pressure, pulse rate, and abdominal aorta diameter], biochemical measurements [total cholesterol, triglycerides, LDL cholesterol, HDL cholesterol, alanine aminotransferase, aspartate aminotransferase, serum creatinine, serum uric acid, serum potassium, serum sodium, plasma renin level (PRL), and plasma renin activity (PRA)], comorbidities [coronary heart disease diabetes, hypertension, chronic obstructive pulmonary diseases, and chronic kidney disease], and medication history (hypoglycemic medicine, lipid-lowering drugs, and antihypertensive drugs).
PRL and PRA measurements
After approval of Xiangya Hospital Institutes’ Review Board and Ethics Committee of the Xiangya Hospital, Central South University, blood collection was carried out at dawn of the second day of patients’ admission to ensure the measurements of recumbent position PRL and PRA are comparable. Then, the blood samples were transported to and evaluated at the clinical laboratory of Xiangya Hospital immediately.
Animals and animal care
Ten-week-old male C57BL/6J mice were purchased from the Xiangya School of Medicine, Central South University. Mice were given unique numbers after quarantine and then randomly divided into a vehicle group, hydralazine group, and minoxidil group, according to different administrations by stratified random sampling. The mice were housed under specific pathogen-free conditions in a 12-h light/dark cycle with ad libitum access to food and water. Calculation of the sample size was determined by power analysis, as previously reported (Festing and Altman, 2002). According to previous research on experimental AAA disease, n = 8–15 specimens per group are required. The sample size was calculated by referring to the previously published literature of our research group, and a total of 40 mice were used in this study (Liu et al., 2020). Each mouse constituted an experimental unit. The mice were divided into the vehicle group (n = 15), hydralazine group (n = 9), and minoxidil group (n = 13), and we declared no excluding animals. Hydralazine (250 mg/L) and minoxidil (120 mg/L) were administered via drinking water and were replaced every other day. The dosage, time points, and route of administration were chosen based on the prior literature (Tsoporis et al., 1998; Knutsen et al., 2018; Kobeissi et al., 2019).
Porcine pancreatic elastase (PPE)-induced AAA model in mice
AAAs were created via transient intra-infrarenal aortic infusion of PPE, based on the study conducted by Liu et al. (2022). Mice were randomly selected from each cage in turn, according to the stratified sampling results for the following steps. Briefly, the mice were anesthetized with inhaled isoflurane, and surgical procedures were performed under sterile conditions. The mouse was subjected to transient infusion of type I PPE (4.0 U/mL; E1250; Sigma-Aldrich) dissolved in saline at a pressure of 150 mmHg. The aortic diameters of pre-infusion and post-infusion were measured to ensure consistency and minimize potential confounders for each group (Liu et al., 2022). Following the closure of laparotomy, all mice were housed under specific pathogen-free conditions as the preoperative environment. At the end, mice were anesthetized using ketamine/xylazine (100 and 20 mg/kg i.p., respectively) and euthanized by exsanguination before aortic tissue collection on day 14. AAA was defined as more than 50% enlargement of the maximum abdominal aortic diameter. All processes were implemented under double-blind conditions.
Imaging AAA formation and progression
AAA was monitored via serial transabdominal high-frequency ultrasound (Vevo® 2100 Imaging System, VisualSonics, Toronto, ON, Canada) for determining the internal diameter or using a digital camera for the external diameter. Measurements of the aortic extra-diameter were performed before and after perfusion on day 0, and before euthanasia on day 14. Measurements of the aortic intro-diameter were performed on days 0 and 14 by investigators blinded to the group assignment.
Histological analyses
Mice were euthanized 14 days after PPE infusion. Aortae were harvested, fixed in 4% paraformaldehyde, embedded in paraffin, and horizontally cut into sections. For histological analyses, hematoxylin–eosin (H&E) staining, Masson, elastic Van Gieson (EVG) staining, and a two-step standard immunoperoxidase procedure for immunohistochemistry were conducted to identify CD3+ T cells (ab16669; Abcam), CD68+ macrophages (ab125212; Abcam), CD31+ mural vessels (ab28364; Abcam), and MMP9 (ab38898; Abcam). The same concentration of rabbit control IgG (AC005, ABclonal) serves as the negative control.
Real-time polymerase chain reaction (PCR)
Mice were euthanized 14 days after PPE infusion. The total RNA of aortae was extracted using the TRIzol reagent (TaKaRa, Japan), according to the manufacturer’s instruction. A measure of 500 nanograms of total RNA was used for reverse transcription using a PrimeScript RT Reagent Kit (TaKaRa). Quantitative RT-PCR on a real-time PCR system (Applied Biosystems) was performed using SYBR Premix Ex Taq qRT-PCR assays (TaKaRa). The Ct value of mRNA was normalized to GAPDH, and the fold change was calculated using the ΔΔCt method. The primer sequences for genes are listed as follows: ACTB, forward primer: GTGCTATGTTGCTCTAGACTTCG and reverse primer: ATGCCACAGGATTCCATACC; NOS2, forward primer: GTTCTCAGCCCAACAATACAAGA and reverse primer: GTGGACGGGTCGATGTCAC; CCL2, forward primer: TAAAAACCTGGATCGGAACCAAA and reverse primer: GCATTAGCTTCACATTTACGGGT; IFN-γ, forward primer: CAGCAACAGCAAGGCGAAAAAGG and reverse primer: TTTCCGCTTCCTGAGGCTGGAT; IL-1β, forward primer: GAAATGCCACCTTTTGACAGTG and reverse primer: TGGATGCTCTCATCAGGACAG.
Statistical analysis
Continuous variables are expressed as mean values ± standard deviation, unless otherwise stated. For the duration of hypertension, triglyceride, alanine aminotransferase, aspartate aminotransferase, serum creatinine, serum uric acid, PRL, and PRA, the medians in the 25th and 75th percentile ranges are provided because they do not satisfy the normal distribution. One- or Two-way ANOVA was used to compare the normal distribution of continuous variables including age, body mass index, systolic blood pressure, diastolic blood pressure, pulse rate, AAA diameter (for both humans and mice), low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, serum K+, and serum Na+ among the three groups, and the independent sample t-test was used between two groups. If the data did not meet the normal distribution, the quantile-quantile graph was used to check its distribution. If the data were located near the diagonal, there was an approximate normal distribution between the groups (including total cholesterol and low-density lipoprotein cholesterol). Non-parametric tests (including Kruskal Wallis and Mann Whitney tests) were provided to compare the difference among ranked data and continuous variables that do not satisfy normal distribution or homogeneity of variance (including the duration of hypertension, triglyceride, ALT, AST, serum creatinine, serum uric acid, PRL, PRA, EVG, and SMA score). We used the χ2 analysis (χ2 test) to compare categorical variables, frequencies, and proportions (including sex, cigarette consumption, coronary heart disease, diabetes, hypertension chronic obstructive, pulmonary diseases, chronic kidney disease, and medication history). Multivariate logistic regression analysis was used to evaluate the relationship between the PRL, PRA and AAA among the three groups, and binary logistic regression analysis was used for two groups. The multivariate linear regression analysis was used to test the collinearity between variables. Odds ratio and 95% CI were reported. A two-tailed p-value of 0.05 is considered significant. SPSS 26.0 version was used for data management and analysis.
Result
Baseline characteristics of patients enrolled
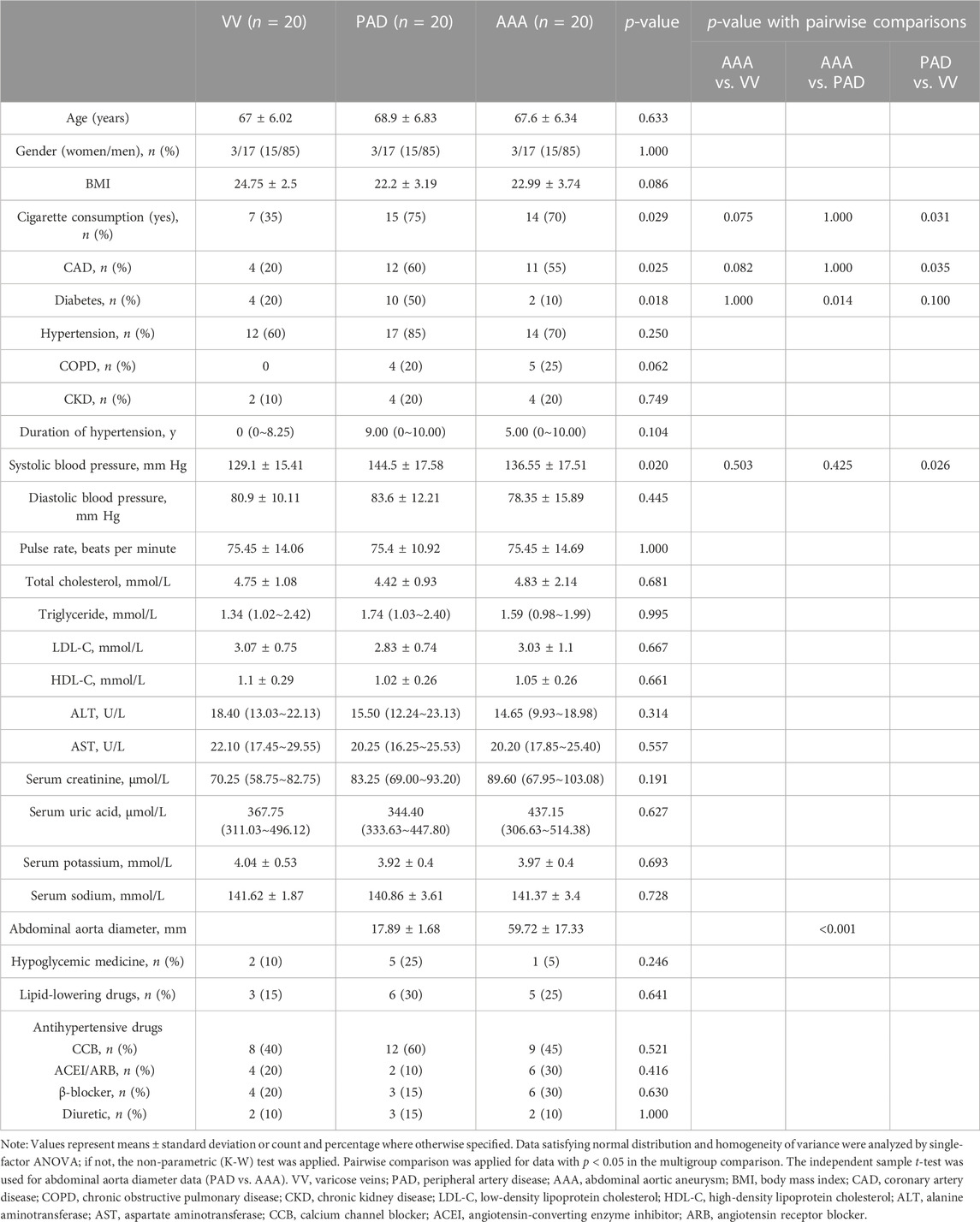
Finally, 20 patients diagnosed with AAA from 01/01/2022 to 01/11/2022 were enrolled in the case group. Meanwhile, 20 patients diagnosed with PAD and VV matched with age and sex were included, respectively. The baseline characteristics of the 60 patients are given in Table 1. As is shown, cigarette consumption, coronary heart disease, the diabetes composition ratio, and systolic blood pressure revealed significant differences among the three groups (Table 1). Specifically, the diabetes composition ratio (10% versus 50%, AAA versus PAD, and p = 0.014) showed statistically significant differences between AAA and other two groups when compared in a pairwise manner (Table 1). Statistical differences in cigarette consumption (75% versus 35%, PAD versus VV, and p = 0.031), the coronary heart disease composition ratio (60% versus 20%, PAD versus VV, and p = 0.035), and systolic blood pressure (144.5 ± 17.58 mmHg versus 129.1 ± 15.41 mmHg, PAD versus VV, and p = 0.026) were observed when comparing the PAD group with the VV group (Table 1).
Considering the critical role of renin in vascular disease (Wu et al., 2018), the PAD and VV groups were roughly combined into the non-AAA group to exclude the bias of the plasma renin level and activity in arterial disease. Characteristics of AAA and non-AAA groups are given in Supplementary Table S1. The AAA group showed no significant differences compared to the non-AAA group.
PRL and PRA are increased in AAA disease
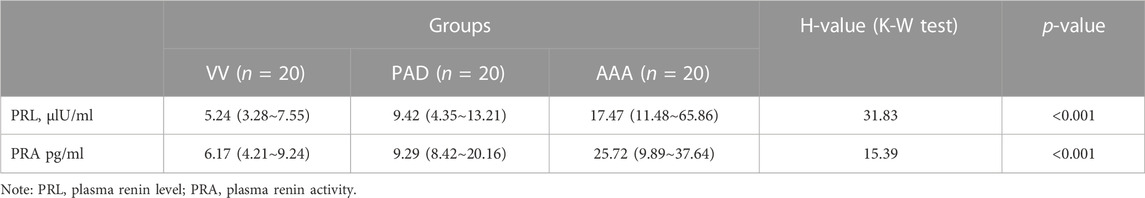
As shown in Table 2 and Figure 1, the AAA group showed a significantly higher PRL [17.47 (11.48∼65.86) versus 9.42 (4.35∼13.21) versus 5.24 (3.28∼7.55), AAA versus PAD versus VV ulU/ml, and p < 0.001] and higher PRA [25.72 (9.89∼37.64) versus 9.29 (8.42∼20.16) versus 6.17 (4.21∼9.24), AAA versus PAD versus VV pg/ml, and p < 0.001] than the PAD and VV groups. Similarly, compared to the non-AAA group, the AAA group also showed a higher PRL [17.47 (11.48∼65.86) versus 7.02 (3.67∼9.65) ulU/ml, and p < 0.001] and higher PRA [25.72 (9.89∼37.64) versus 8.28 (6.05∼13.45) pg/ml, and p = 0.001] (Supplementary Table S2; Supplementary Figure S1).
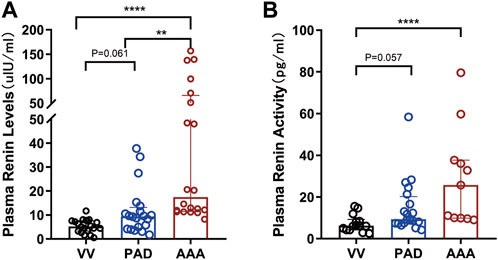
FIGURE 1. Comparison of PRL and PRA among AAA, PAD, and VV groups. Plasma renin levels and renin activity were measured in the included patients to evaluate the differences in plasma renin levels and activity among the AAA (n = 20), PAD (n = 20), and VV (n = 20) groups. (A) Plasma renin level (PRL) measurement (left). Data were expressed as the median with an interquartile range. (B) Plasma renin activity (PRA) measurement (right). Data were expressed as the median with an interquartile range. The K-W test and pairwise comparisons were applied. **p < 0.01 and ****p < 0.0001.
PRL and PRA are positively associated with AAA development
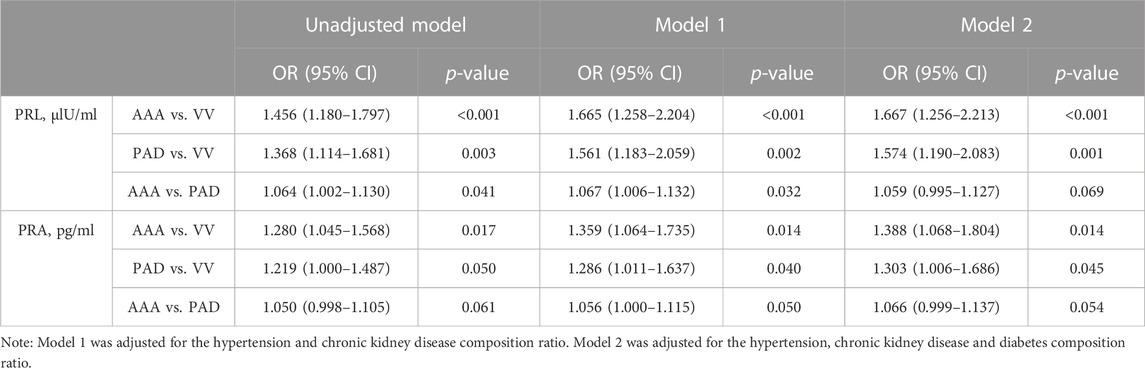
Multiple logistic regression analysis was conducted to identify whether the progression of abdominal aortic aneurysms could be attributed to increased plasma renin levels and activity. It is important to mention that a detailed collinearity analysis (SPSS collinearity tests) was performed between the PRL and PRA, with the piece of evidence that the Spearman correlation coefficient of the PRL and PRA is 0.883 (Supplementary Table S3). Therefore, we analyzed the two indicators separately. A univariate analysis was conducted to evaluate the relationship between the increased PRL, and PRA and AAA development. Our results showed higher PRL was significantly associated with AAA development with an unadjusted OR of 1.456 [(95% CI, 1.180–1.797), and p < 0.001] compared with VV and 1.064 [(95% CI, 1.002–1.130), and p = 0.041] compared with PAD (Table 3). Similarly, higher PRA was also associated with AAA development with an unadjusted OR of 1.280 [(95% CI, 1.045–1.568), and p = 0.017] compared with VV (Table 3). Similar trends were observed after adjustment of hypertension and the chronic kidney disease composition ratio in model 1, although the difference was not significant in model 2 (Table 3). Surprisingly, we also found that increased PRL and PRA were correlated with a higher risk of PAD (unadjusted OR, 1.368 and 1.219, respectively) than the VV group (Table 3).
In addition, a binary logistic regression was conducted between AAA and non-AAA groups, which demonstrated that both high-level PRL and PRA were positively associated with AAA development with an unadjusted OR of 1.099 [(95% CI, 1.026–1.176), and p = 0.007] for the PRL and 1.070 [(95% CI, 1.013–1.129), and p = 0.014] for PRA, and adjusted OR of 1.100 [(1.028–1.176); p = 0.005] for PRL and 1.075 [(1.015–1.138); p = 0.014] for PRA in model 1 and 1.102 [(1.024–1.186); p = 0.009] for PRL and 1.088 [(1.019–1.162); p = 0.012] for PRA in model 2, respectively (Supplementary Table S4).
As PAD and AAA shared similar etiology and pathological mechanisms, the elevated risk of AAA was reported in PAD patients (Hicks et al., 2021; Cai et al., 2022). Thus, a correlation analysis was performed to determine the relationship between aortic diameters and PRL and PRA in AAA and PAD groups. Our results demonstrated a significant positive correlation between the aortic diameter and both PRL (γ = 0.529; p < 0.001) and PRA (γ = 0.412; p = 0.021) (Supplementary Table S5). However, the correlation was not significant in the AAA group (γ = −0.025; p = 0.917) and PRA (γ = −0.335; p = 0.285).
Influence of direct-acting vasodilators on PRL and PRA
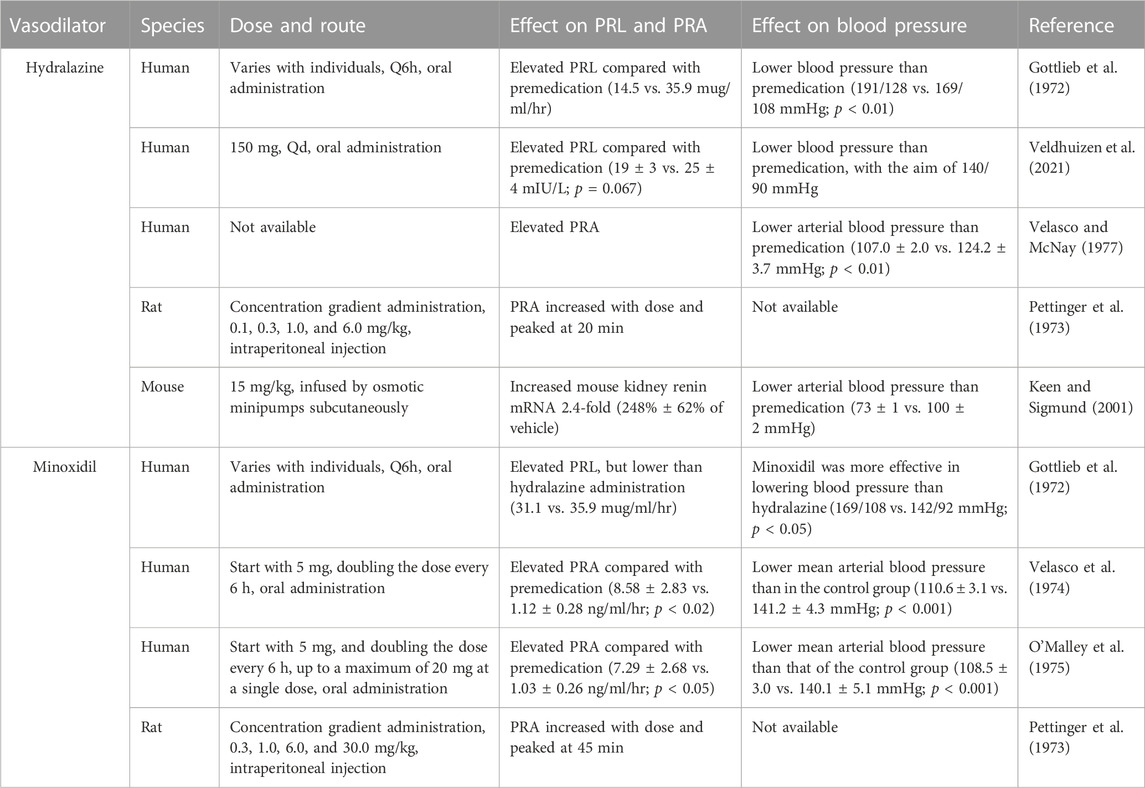
Hydralazine and minoxidil, the most commonly prescribed direct-acting vasodilators in hypertension (Laurent, 2017), were selected as representatives of direct vasodilators in our study. Given the well-established relationship between the administration of direct-acting vasodilators and PRL and PRA, a review of the published literature showed that the administration of minoxidil and hydralazine increased the PRL and PRA in both clinical and animal experiments, as shown in Table 4.
Direct-acting vasodilators aggravated experimental AAA
Considering the critical role of renin in AAA disease, we then established a PPE-induced experimental AAA mouse model to verify whether direct-acting vasodilators aggravated AAA development and progression. Hydrazine, minoxidil, and vehicle treatment were administrated 3 days before PPE infusion until euthanasia, while blood pressure and aortic diameters were monitored (Figure 2A). Our results suggested hydralazine administration significantly decreased systolic blood pressure and diastolic blood pressure compared to the vehicle group on days 7 and 14, respectively (Figure 2B). Although minoxidil-treated mice displayed a significant decrease in blood pressure (106.3 ± 19.04 versus 130.41 ± 7.18 mmHg and p < 0.001 for systolic blood pressure 82.45 ± 22.17 versus 103.06 ± 10.45 mmHg and p = 0.007 for diastolic blood pressure) on day 7, this significant difference disappeared on day 14 (Figures 2B, C), supporting the notion that the neurohumoral changes induced by minoxidil can offset its benefits to arteriolar vasodilatation (Baer et al., 1980; Sica and Gehr, 2001).
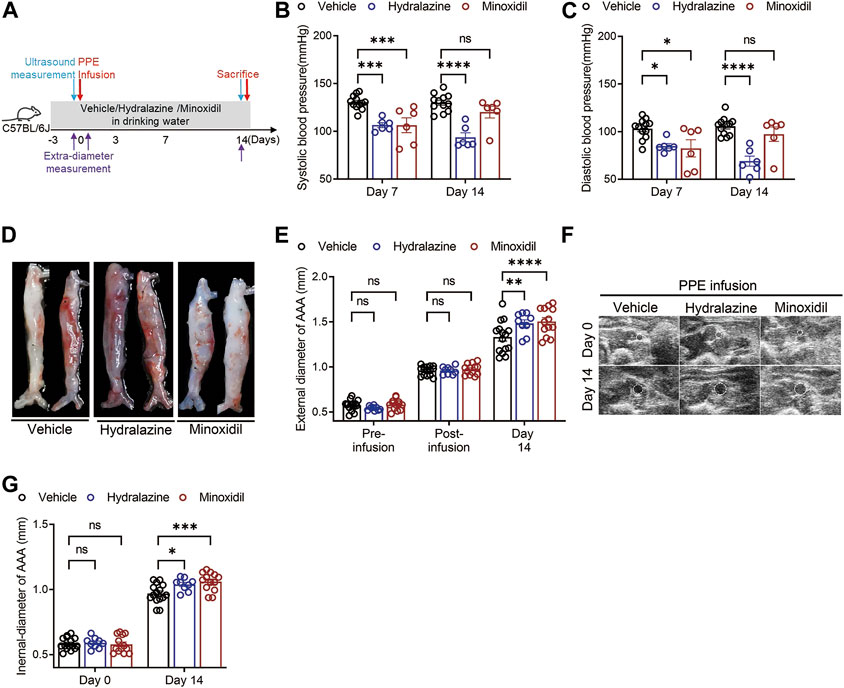
FIGURE 2. Hydrazine and minoxidil aggravated experimental AAA disease. (A) Scheme of the PPE-induced experimental AAA mouse model and monitoring of AAA diameters. (B,C) Effect of hydrazine and minoxidil on systolic blood pressure and diastolic blood pressure in experimental AAA. (D,E) Representative AAA images and quantitative analysis of aortic extra-diameters in the vehicle group (n = 15), hydrazine group (n = 9), and minoxidil group (n = 13). (F,G) Representative ultrasound AAA images and quantitative analysis of aortic intra-diameters and growth rate in the three groups. Data are presented as the mean ± SEM. Significance was analyzed using two-way ANOVA with the Bonferroni correction. *p < 0.05; **p < 0.01; and ***p < 0.001 vs. vehicle group.
Monitoring external diameters using a digital camera, our results suggested AAA diameters in the three groups were comparable after PPE infusion but increased more rapidly in hydrazine and minoxidil groups than those in the vehicle group (1.48 ± 0.12 mm versus 1.33 ± 0.17 mm, hydralazine versus vehicle, p = 0.037; 1.50 ± 0.15 mm versus 1.33 ± 0.17 mm, minoxidil versus vehicle, p = 0.018) (Figures 2D, E). These images of all aneurysms are given in Supplementary Figure S2A. Likewise, hydrazine and minoxidil aggravated AAA progression, which was observed by monitoring internal diameters by ultrasonography (1.04 ± 0.05 mm versus 0.97 ± 0.07 mm, hydralazine versus vehicle, and p = 0.017; 1.06 ± 0.07 mm versus 0.97 ± 0.07 mm, minoxidil versus vehicle, and p < 0.001).
Direct-acting vasodilators promoted aortic degeneration
Next, histological analyses demonstrated that both hydrazine and minoxidil promoted inflammatory cell infiltration and deposited collagen fibers compared to the vehicle group in H&E and Masson staining (Figure 3A). Furthermore, hydrazine and minoxidil aggravated vascular structure disorder, as evidenced by increased elastic destruction scores and vascular smooth muscle cell degeneration scores qualified by EVG and α-SMA staining (Figures 3A, B). These results histologically confirmed aortic degeneration was aggravated by hydrazine/minoxidil administration.

FIGURE 3. Hydrazine and minoxidil promoted aortic degeneration in experimental AAA. (A) Representative images of HE, Masson, EVG, and anti-SMC α-actin staining in the vehicle group (n = 5), hydrazine group (n = 6), and minoxidil group (n = 5). (B) Quantitative analysis of EVG and anti-SMC α-actin staining in the three groups. Data are presented as the mean ± SEM. Significance was analyzed using the Mann–Whitney test. *p < 0.05; **p < 0.01 vs. vehicle group.
Direct-acting vasodilators aggravated aortic inflammation
As mural inflammation promoted aortic degeneration in AAA disease, immunohistological analysis was conducted to evaluate inflammatory cell infiltration in the study. Our results revealed that both hydrazine and minoxidil aggravated mural angiogenesis and accumulation of CD3+ T cells, CD68+ macrophages, and MMP9 (Figure 4A; Supplementary Figure S2B). Furthermore, the relative mRNA level of inflammatory factors of aortic aneurysm was examined by quantitative PCR, which suggested minoxidil significantly upregulated the expression levels of IFN-γ, NOS2, and CCL2 (Figure 4B). Similar trends were observed in the hydralazine group, but the difference was not significant, which may be due to the small sample size.
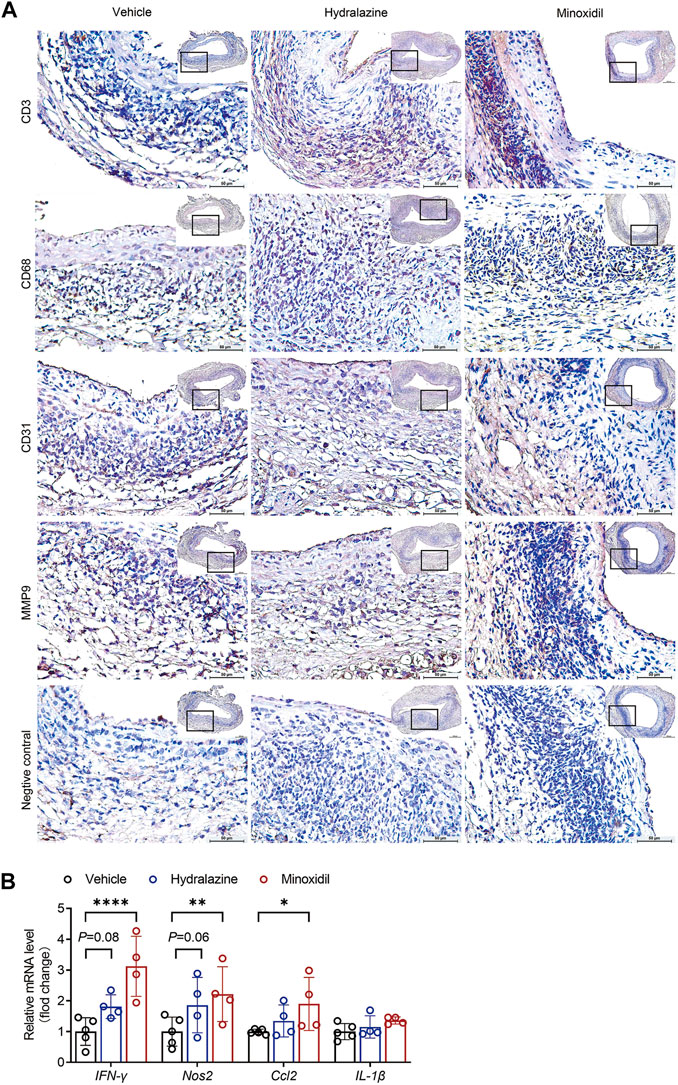
FIGURE 4. Hydrazine and minoxidil aggravated aortic inflammation in experimental AAA. (A) Representative immunohistological staining of CD3+ T cells, CD68+ macrophages, CD31+ mural angiogenesis, and MMP9. Rabbit control IgG serves as the negative control. (B) IFN-γ, NOS2, CCL2, and Il-1β mRNA expression were normalized to GAPDH endogenous control mRNA expression by quantitative RT-PCR. Significance was analyzed using two-way ANOVA. *p < 0.05; **p < 0.01 vs. vehicle group.
Discussion
Antihypertensive medication is a cornerstone therapy among AAA patients with hypertension, which minimizes risks of cardiovascular events and AAA rupture. However, specific recommendations for the choice of antihypertensive medication in AAA patients remain poorly described. In the current study, we demonstrated that the PRL and PRA increased in patients with AAA compared to patients with PAD and VV, and were positively associated with AAA development after further analysis of clinical characteristics. Despite their potent blood pressure-lowering effect, direct-acting vasodilators, such as hydralazine and minoxidil, were reported to increase PRL and PRA and upregulated the renin-angiotensin system which implied an increased risk of AAA development and progression. Hence, animal experiments were performed and confirmed that administration of hydralazine and minoxidil aggravated PPE-induced experimental AAA progression with increased aortic degeneration and inflammatory infiltration. Our results highlighted the critical roles of renin in AAA disease, which raised cautionary concerns about the applications of direct vasodilators on AAA and other arterial diseases.
The involvement of the renin-angiotensin system in the pathophysiology of AAA disease has been well-established (Lu et al., 2012). Particularly secreted by juxtaglomerular cells of the kidney, renin is the rate-limiting enzyme of the renin-angiotensin system, and regulates blood pressure and cardiovascular functions. As renin catalyzes the first step in the renin-angiotensin system cascade, numerous studies have been conducted to investigate the link between PRL/PRA and the subsequent cardiovascular risk. Although the majority of these studies demonstrated a positive relationship between PRL and PRA, and cardiovascular morbidity and mortality, no definitive conclusions were obtained due to differences in the methodology among these trials (Volpe and Unger, 2013). In view of the critical role of the renin-angiotensin system in AAA disease, several previous studies demonstrated the potential role of plasma renin in AAA (Daugherty and Cassis, 2004; Steckelings and Bader, 2018). Early research addressed that excessive salt intake increased AAA incidence and rupture risk in hypertensive angiotensin and renin transgenic mice, which was protected by angiotensin converting enzyme inhibitor or angiotensin receptor blocker (Nishijo et al., 1998; Liao et al., 2001). The prorenin receptor, a specific receptor for renin and prorenin, aggravated Ang II-induced AAA on the Aope−/− background, which was reversed by blocking the Ang II receptor using telmisartan (Ma et al., 2020). Furthermore, aliskiren, a direct renin inhibitor, was shown to limit atherosclerosis and AAA in an Ang II-infused mouse model by reducing prorenin receptor expression and Mitogen-activated protein kinases activity (Seto et al., 2014; Miyake et al., 2017). However, a recent case–control study reported plasma aldosterone concentrations increased in patients diagnosed with aortic dissection and aneurysms, accompanied by suppressed PRA in women rather than men (Zhu et al., 2022). In the study, we demonstrated that the PRL and PRA were increased in patients with AAA compared to those with PAD and VV, and considered risk factors for AAA, which indicated their potential role in arterial disease and AAA development. However, for patients with existing AAA, no significant correlation was found between PRL and PRA and AAA diameters. Considering the effect of Ang II on renin release through a negative feedback regulatory mechanism cannot be ignored, it suggested the roles of the renin-angiotensin system on AAA remain to be further clarified (Schweda et al., 2007).
Despite the lack of evidence that hypertension is linked to AAA progression and remains a potential risk factor for the development and prognosis of AAA (Rapsomaniki et al., 2014; Kobeissi et al., 2019; Li et al., 2019). Thus, it is encouraged to apply antihypertensive therapy in AAA patients with hypertension. Despite not being the first choice of antihypertensive agents, direct vasodilators, such as minoxidil and hydralazine, are still widely used in clinical therapeutics (Dormois et al., 1975; Mehta et al., 1975; Lu et al., 2012). Hydralazine, as a classical direct vasodilator, remains an option for the treatment of hypertension and systolic heart failure (Kandler et al., 2011). It is used as step 3 in the Hypertension Detection and Follow-up Program (HDFP) cooperative group trial and the Antihypertensive and Lipid-lowering Treatment to Prevent Heart Attack Trial (ALLHAT) to reduce the incidence of hypertension and related complications (Five-year findings of the hypertension, 1979; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial, 2002). It has been proposed to inhibit IP3-induced sarcoplasmic reticulum calcium release and to inhibit myosin phosphorylation in arterial smooth muscle cells, although its mechanism is not fully elucidated (McComb et al., 2016). Minoxidil, another direct vasodilator, was introduced in the early 1970s for treating refractory hypertension in cases where multidrug regimens had failed. As an ATP-modulated potassium (KATP) channel-opening agent in the vascular smooth muscle cells, minoxidil allows potassium efflux and reduces calcium influx, which ultimately relaxes vascular smooth muscle cells, dilates the aortic lumen, and lowers blood pressure (McComb et al., 2016). In our current study, we found that administration of hydralazine and minoxidil increased AAA progression with increased infiltration of local inflammatory cells and arterial degeneration. Not coincidentally, this is not the first study to investigate the relationship between vasodilating-effect drugs and AAAs. It has been reported that hydralazine showed a trend toward aggravated AAA (1.59 ± 0.21 vs. 1.38 ± 0.03; hydralazine vs. vehicle) and atherosclerotic lesion areas (3.00 ± 0.40 vs. 1.74 ± 0.50 mm2, hydralazine vs. vehicle, and p = 0.06) in Ang II-infused mice, although the differences were not significant (Cassis et al., 2009). In addition, sildenafil (Viagra), another vasodilator used to treat impotence and pulmonary hypertension, aggravated elastin degeneration and experimental AAA progression by dysregulating cyclic guanosine monophosphate and contractile signaling in vascular smooth muscle cells (Zhang et al., 2022). Other direct-acting vasodilators, such as calcium channel blockers, were also proved to increase perioperative mortality in both acute and elective aortic aneurysm surgery (Wilmink et al., 2002; Kertai et al., 2008). Consistently, as one of the dihydropyridine calcium channel blocker, amlodipine has been demonstrated to significantly promote elastin degradation and enhance matrix metalloproteinase-9 activity in experimental porcine aneurysm (Boyle et al., 1998). However, studies reported that the calcium channel blocker protected from Ang II induced AAA by reducing inflammatory infiltration and preserving eNOS coupling (Kurobe et al., 2013; Miao et al., 2015). These completely opposite results may attribute to the differences in animal models, to be precise, that Ang II negative regulates renin levels (Schweda et al., 2007). Therefore, the risk of direct-acting vasodilators to AAA diseases should be considered.
It is worth mentioning that as a direct vasodilator, minoxidil has a direct antihypertensive effect when initially administered. However, long-term administration can weaken this hypotensive effect, which is attributed to the desensitization of K+ channels in vascular smooth muscle cells in the aorta. This phenomenon was also observed in another ATP-dependent K+ channel opener, levcromakalim, which attenuated vascular relaxation in NO-donors after 2 weeks of administration in rats (Trongvanichnam et al., 1996). Now, minoxidil is widely used to treat androgenetic alopecia mainly by topical application and oral administration at a low dose (Stoehr et al., 2019; Randolph and Tosti, 2021). Adverse cardiovascular events induced by topical minoxidil suggest that it can be systemically absorbed, implying its cardiovascular risks in AAA patients (Lu et al., 2012). Although there is no clinical research exploring the relationship between minoxidil and AAA, minoxidil should be used with caution in androgenetic alopecia patients with AAA or other arterial diseases.
The risk factors for AAA are mainly old age, male gender, hypertension, and hyperlipidemia, which are consistent with the risk factors for PAD (Morley et al., 2018; Golledge, 2019). A prospective analysis of 14,148-participants also indicated that symptomatic PAD had a higher hazard ratio of incident AAA [2.96 (95% CI 1.73–5.07)], as did asymptomatic PAD [1.52 (95% CI 1.00–2.30)] (Hicks et al., 2021). Moreover, our current study showed that PRL and PRA were correlated with a higher risk of PAD [odds ratio (OR), 1.368 and 1.219, respectively] than VV (Table 3), which was consistent with previous studies. To eliminate the selective bias of the current study, we included patients with PAD and VV together in the control group and compared them separately with patients with AAAs and matched them by age, sex, and history of hypertension to enhance comparability among the clinical data. In addition, patients with PAD also showed increased PRL and PRA compared to VV disease, which raised cautionary concerns about increased renin phenotypes and applications of direct vasodilators in PAD and other arterial diseases.
Nonetheless, this study has several limitations. First, a small sample size was a major drawback of the present study. A more comprehensive analysis is needed to determine the relationship between renin and AAA disease. Second, direct-acting vasodilators are a heterogeneous group of drugs. Although hydralazine and minoxidil are the most commonly prescribed direct vasodilators, they may not be representative of all vasodilators in the study. More retrospective studies are needed to assess their safety in vascular disease. Therefore, future studies with larger samples and longitudinal measurements of people taking vasodilators are warranted to overcome the limitations to our study and establish cause-and-effect relationships.
Conclusion
PRL and PRA are positively associated with AAA development. Hydralazine and minoxidil promote the progression of AAA in a mouse model, which might be associated with increased PRL and PRA. Direct-acting vasodilators should be used with caution in patients with AAA, as well as in those at high risk for AAA disease and other artery diseases.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials; further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Xiangya Hospital Institutes’ Review Board and Ethics Committee of the Xiangya Hospital, Central South University. The patients/participants provided written informed consent to participate in this study. The animal study was reviewed and approved by the Animal Care and Use Committee of Ethics of the Xiangya Hospital, Central South University (NO. 201803481).
Author contributions
Conception and design: YL, SL, and WW. Analysis and interpretation: YL, SL, and JZ. Data collection: YL, SL, JZ, and KW. Writing the article: YL and SL. Critical revision of the article: YL, SL, and BX. Final approval of the article: YL, SL, and WW. Statistical analysis: YL and SL. Fund acquisition: WW. YL, and SL contributed equally to this article and share co-first authorship. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (Nos 81873525 and 82070491).
Acknowledgments
The authors thank Yongping Bai at the Department of Cardiovascular Medicine, Xiangya Hospital, Central South University, for guidance during the experiments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1174278/full#supplementary-material
SUPPLEMENTARY TABLE S1 | Characteristics of study participants stratified by AAA occurrence.
SUPPLEMENTARY TABLE S2 | Comparison of PRL and PRA between AAA and non-AAA groups.
SUPPLEMENTARY TABLE S3 | Multicollinearity analysis.
SUPPLEMENTARY TABLE S4 | Logistic regression analysis for the association between PRL/PRA and AAA.
SUPPLEMENTARY TABLE S5 | Relationship between PRL/PRA and AAA diameter.
SUPPLEMENTARY FIGURE S1 | Comparison of PRL and PRA between AAA and non-AAA groups. Plasma renin levels and renin activity were measured in the included patients to evaluate the differences in plasma renin levels and activity among the AAA (n = 20) and non-AAA (n = 40) groups. (A) Plasma renin level (PRL) measurement (left). Data were expressed as the median with an interquartile range. (B) Plasma renin activity (PRA) measurement (right). Data were expressed as the median with an interquartile range. The M-W test was applied. **p < 0.01 and ****p < 0.0001.
SUPPLEMENTARY FIGURE S2 | All the images of PPE-induced aneurysms and statistical analysis of immunohistological staining in the three groups. (A) All the images of PPE-induced aneurysms in the vehicle group (n = 15), hydrazine group (n = 9), and minoxidil group (n = 11) are shown. (B) Quantitative analysis of CD3+ T cells, CD68+ macrophages, and CD31+ mural angiogenesis staining in the three groups. Data are presented as the mean ± SEM. Significance was analyzed using one-way ANOVA. *p < 0.05 vs. the vehicle group.
Abbreviations
AAA, abdominal aortic aneurysm; PAD, peripheral artery disease; PPE, porcine pancreatic elastase; PRA, plasma renin activity; PRL, plasma renin level; VV, varicose vein.
References
1979). Five-year findings of the hypertension detection and follow-up prograMajor outcomes in highm. I. Reduction in mortality of persons with high blood pressure, including mild hypertension. Hypertension Detection and Follow-up Program Cooperative Group. Jama 242 (23), 2562–2571.
ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (2002). Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Jama 288 (23), 2981–2997. doi:10.1001/jama.288.23.2981
Baer, L., Radichevich, I., and Williams, G. S. (1980). Treatment of drug-resistant hypertension with minoxidil or angiotensin-converting enzyme inhibitor: Blood pressure, renin, aldosterone, and electrolyte responses. J. Cardiovasc. Pharmacol. 2 (Suppl. 2), S206–S216. doi:10.1097/00005344-198000022-00015
Bicknell, C. D., Kiru, G., Falaschetti, E., Powell, J. T., and Poulter, N. R., (2016). An evaluation of the effect of an angiotensin-converting enzyme inhibitor on the growth rate of small abdominal aortic aneurysms: A randomized placebo-controlled trial (AARDVARK). Eur. Heart J. 37 (42), 3213–3221. doi:10.1093/eurheartj/ehw257
Bossone, E., and Eagle, K. A. (2021). Epidemiology and management of aortic disease: Aortic aneurysms and acute aortic syndromes. Nat. Rev. Cardiol. 18 (5), 331–348.
Boyle, J. R., Loftus, I. M., Goodall, S., Crowther, M., Bell, P. R., and Thompson, M. M. (1998). Amlodipine potentiates metalloproteinase activity and accelerates elastin degradation in a model of aneurysmal disease. Eur. J. Vasc. Endovasc. Surg. 16 (5), 408–414. doi:10.1016/s1078-5884(98)80008-7
Cai, H., Pan, B., Xu, J., Liu, S., Wang, L., Wu, K., et al. (2022). D-dimer is a diagnostic biomarker of abdominal aortic aneurysm in patients with peripheral artery disease. Front. Cardiovasc Med. 9, 890228. doi:10.3389/fcvm.2022.890228
Campese, V. M., Stein, D., and DeQuattro, V. (1979). Treatment of severe hypertension with minoxidil: Advantages and limitations. J. Clin. Pharmacol. 19 (4), 231–241. doi:10.1002/j.1552-4604.1979.tb01657.x
Cassis, L. A., Gupte, M., Thayer, S., Zhang, X., Charnigo, R., Howatt, D. A., et al. (2009). ANG II infusion promotes abdominal aortic aneurysms independent of increased blood pressure in hypercholesterolemic mice. Am. J. Physiol. Heart Circ. Physiol. 296 (5), H1660–H1665. doi:10.1152/ajpheart.00028.2009
Chaikof, E. L., Dalman, R. L., Eskandari, M. K., Jackson, B. M., Lee, W. A., Mansour, M. A., et al. (2018). The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J. Vasc. Surg. 67 (1), 2–77. doi:10.1016/j.jvs.2017.10.044
Daugherty, A., and Cassis, L. (2004). Angiotensin II and abdominal aortic aneurysms. Curr. Hypertens. Rep. 6 (6), 442–446. doi:10.1007/s11906-004-0038-0
Dormois, J. C., Young, J. L., and Nies, A. S. (1975). Minoxidil in severe hypertension: Value when conventional drugs have failed. Am. Heart J. 90 (3), 360–368. doi:10.1016/0002-8703(75)90326-9
Ferrario, C. M., and Strawn, W. B. (2006). Role of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease. Am. J. Cardiol. 98 (1), 121–128. doi:10.1016/j.amjcard.2006.01.059
Festing, M. F. W., and Altman, D. G. (2002). Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J. 43 (4), 244–258. doi:10.1093/ilar.43.4.244
Golledge, J., Muller, J., Daugherty, A., and Norman, P. (2006). Abdominal aortic aneurysm: Pathogenesis and implications for management. Arterioscler. Thromb. Vasc. Biol. 26 (12), 2605–2613. doi:10.1161/01.ATV.0000245819.32762.cb
Golledge, J. (2019). Abdominal aortic aneurysm: Update on pathogenesis and medical treatments. Nat. Rev. Cardiol. 16 (4), 225–242.
Gottlieb, T. B., Katz, F. H., and Chidsey, C. A. (1972). Combined therapy with vasodilator drugs and beta-adrenergic blockade in hypertension. A comparative study of minoxidil and hydralazine. Circulation 45 (3), 571–582. doi:10.1161/01.cir.45.3.571
Hicks, C. W., Al-Qunaibet, A., Ding, N., Kwak, L., Folsom, A. R., Tanaka, H., et al. (2021). Symptomatic and asymptomatic peripheral artery disease and the risk of abdominal aortic aneurysm: The Atherosclerosis Risk in Communities (ARIC) study. Atherosclerosis 333, 32–38. doi:10.1016/j.atherosclerosis.2021.08.016
Kandler, M. R., Mah, G. T., Tejani, A. M., Stabler, S. N., and Salzwedel, D. M. (2011). Hydralazine for essential hypertension. Cochrane Database Syst. Rev. 11, Cd004934. doi:10.1002/14651858.CD004934.pub4
Keen, H. L., and Sigmund, C. D. (2001). Paradoxical regulation of short promoter human renin transgene by angiotensin ii. Hypertension 37 (2 Pt 2), 403–407. doi:10.1161/01.hyp.37.2.403
Kertai, M. D., Westerhout, C. M., Varga, K. S., Acsady, G., and Gal, J. (2008). Dihydropiridine calcium-channel blockers and perioperative mortality in aortic aneurysm surgery. Br. J. Anaesth. 101 (4), 458–465. doi:10.1093/bja/aen173
Knutsen, R. H., Beeman, S. C., Broekelmann, T. J., Liu, D., Tsang, K. M., Kovacs, A., et al. (2018). Minoxidil improves vascular compliance, restores cerebral blood flow, and alters extracellular matrix gene expression in a model of chronic vascular stiffness. Am. J. Physiol. Heart Circ. Physiol. 315 (1), H18–H32. doi:10.1152/ajpheart.00683.2017
Kobeissi, E., Hibino, M., Pan, H., and Aune, D. (2019). Blood pressure, hypertension and the risk of abdominal aortic aneurysms: A systematic review and meta-analysis of cohort studies. Eur. J. Epidemiol. 34 (6), 547–555. doi:10.1007/s10654-019-00510-9
Kurobe, H., Matsuoka, Y., Hirata, Y., Sugasawa, N., Maxfield, M. W., Sata, M., et al. (2013). Azelnidipine suppresses the progression of aortic aneurysm in wild mice model through anti-inflammatory effects. J. Thorac. Cardiovasc Surg. 146 (6), 1501–1508. doi:10.1016/j.jtcvs.2013.02.073
Laurent, S. (2017). Antihypertensive drugs. Pharmacol. Res. 124, 116–125. doi:10.1016/j.phrs.2017.07.026
Li, Q., Youn, J. Y., Siu, K. L., Murugesan, P., Zhang, Y., and Cai, H. (2019). Knockout of dihydrofolate reductase in mice induces hypertension and abdominal aortic aneurysm via mitochondrial dysfunction. Redox Biol. 24, 101185. doi:10.1016/j.redox.2019.101185
Liao, S., Miralles, M., Kelley, B. J., Curci, J. A., Borhani, M., and Thompson, R. W. (2001). Suppression of experimental abdominal aortic aneurysms in the rat by treatment with angiotensin-converting enzyme inhibitors. J. Vasc. Surg. 33 (5), 1057–1064.(
Liu, S., Huang, T., Liu, R., Cai, H., Pan, B., Liao, M., et al. (2020). Spermidine suppresses development of experimental abdominal aortic aneurysms. J. Am. Heart Assoc. 9 (8), e014757. doi:10.1161/JAHA.119.014757
Liu, S., Liu, Y., Zhao, J., Yang, P., Wang, W., and Liao, M. (2022). Effects of spermidine on gut microbiota modulation in experimental abdominal aortic aneurysm mice. Nutrients 14 (16), 3349. doi:10.3390/nu14163349
Lu, H., Rateri, D. L., Bruemmer, D., Cassis, L. A., and Daugherty, A. (2012). Involvement of the renin-angiotensin system in abdominal and thoracic aortic aneurysms. Clin. Sci. (Lond). 123 (9), 531–543. doi:10.1042/CS20120097
Ma, H., Dong, X-F., Cao, X-R., Hei, N-H., Li, J-L., Wang, Y-L., et al. (2020). Pro-renin receptor overexpression promotes angiotensin II-induced abdominal aortic aneurysm formation in apolipoprotein E-knockout mice. Hum. Gene Ther. 31 (11-12), 639–650. doi:10.1089/hum.2019.124
Marchesi, C., Paradis, P., and Schiffrin, E. L. (2008). Role of the renin-angiotensin system in vascular inflammation. Trends Pharmacol. Sci. 29 (7), 367–374. doi:10.1016/j.tips.2008.05.003
McComb, M. N., Chao, J. Y., and Ng, T. M. (2016). Direct vasodilators and sympatholytic agents. J. Cardiovasc Pharmacol. Ther. 21 (1), 3–19. doi:10.1177/1074248415587969
Mehta, P. K., Mamdani, B., Shansky, R. M., Mahurkar, S. D., and Dunea, G. (1975). Severe hypertension. Treatment with minoxidil. Jama 233 (3), 249–252. doi:10.1001/jama.233.3.249
Miao, X. N., Siu, K. L., and Cai, H. (2015). Nifedipine attenuation of abdominal aortic aneurysm in hypertensive and non-hypertensive mice: Mechanisms and implications. J. Mol. Cell Cardiol. 87, 152–159. doi:10.1016/j.yjmcc.2015.07.031
Miyake, T., Miyake, T., Shimizu, H., and Morishita, R. (2017). Inhibition of aneurysm progression by direct renin inhibition in a rabbit model. Hypertension 70 (6), 1201–1209. doi:10.1161/HYPERTENSIONAHA.117.09815
Morley, R. L., Sharma, A., Horsch, A. D., and Hinchliffe, R. J. (2018). Peripheral artery disease. Bmj 360, j5842. doi:10.1136/bmj.j5842
NICE guideline [NG156]. Available from: https://www.nice.org.uk/guidance/ng156 (Accessed March 1, 2023).
Nishijo, N., Sugiyama, F., Kimoto, K., Taniguchi, K., Murakami, K., Suzuki, S., et al. (1998). Salt-sensitive aortic aneurysm and rupture in hypertensive transgenic mice that overproduce angiotensin II. Lab. Invest. 78 (9), 1059–1066.
O'Malley, K., Velasco, M., Wells, J., and McNay, J. L. (1975). Control plasma renin activity and changes in sympathetic tone as determinants of minoxidil-induced increase in plasma renin activity. J. Clin. Invest. 55 (2), 230–235. doi:10.1172/JCI107926
Pettinger, W. A., Campbell, W. B., and Keeton, K. (1973). Adrenergic component of renin release induced by vasodilating antihypertensive drugs in the rat. Circ. Res. 33 (1), 82–86. doi:10.1161/01.res.33.1.82
Randolph, M., and Tosti, A. (2021). Oral minoxidil treatment for hair loss: A review of efficacy and safety. J. Am. Acad. Dermatol 84 (3), 737–746. doi:10.1016/j.jaad.2020.06.1009
Rapsomaniki, E., Timmis, A., George, J., Pujades-Rodriguez, M., Shah, A. D., Denaxas, S., et al. (2014). Blood pressure and incidence of twelve cardiovascular diseases: Lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. Lancet 383 (9932), 1899–1911. doi:10.1016/S0140-6736(14)60685-1
Sakalihasan, N., Michel, J. B., Katsargyris, A., Kuivaniemi, H., Defraigne, J. O., Nchimi, A., et al. (2018). Abdominal aortic aneurysms. Nat. Rev. Dis. Prim. 4 (1), 34. doi:10.1038/s41572-018-0030-7
Schweda, F., Friis, U., Wagner, C., Skott, O., and Kurtz, A. (2007). Renin release. Renin release. Physiol. (Bethesda) 22, 310–319. doi:10.1152/physiol.00024.2007
Seto, S. W., Krishna, S. M., Moran, C. S., Liu, D., and Golledge, J. (2014). Aliskiren limits abdominal aortic aneurysm, ventricular hypertrophy and atherosclerosis in an apolipoprotein-E-deficient mouse model. Clin. Sci. (Lond). 127 (2), 123–134. doi:10.1042/CS20130382
Sica, D. A., and Gehr, T. W. (2001). Direct vasodilators and their role in hypertension management: Minoxidil. J. Clin. Hypertens. (Greenwich) 3 (2), 110–114. doi:10.1111/j.1524-6175.2001.00455.x
Steckelings, U. M., and Bader, M. (2018). Renin-angiotensin system in aortic aneurysm. Hypertension 72 (3), 579–581. doi:10.1161/HYPERTENSIONAHA.118.11238
Stoehr, J. R., Choi, J. N., Colavincenzo, M., and Vanderweil, S. (2019). Off-label use of topical minoxidil in alopecia: A review. Am. J. Clin. Dermatol 20 (2), 237–250. doi:10.1007/s40257-018-0409-y
Trongvanichnam, K., Mitsui-Saito, M., Ozaki, H., and Karaki, H. (1996). Effects of chronic oral administration of levcromakalim on in vitro contractile responses of arterial smooth muscle. Eur. J. Pharmacol. 303 (1-2), 39–45. doi:10.1016/0014-2999(96)00031-3
Tsoporis, J., Keeley, F. W., Lee, R. M., and Leenen, F. H. (1998). Arterial vasodilation and vascular connective tissue changes in spontaneously hypertensive rats. J. Cardiovasc. Pharmacol. 31 (6), 960–962. doi:10.1097/00005344-199806000-00022
Velasco, M., and McNay, J. L. (1977). Physiologic mechanisms of bupicomide- and hydralazine-induced increase in plasma renin activity in hypertensive patients. Mayo Clin. Proc. 52 (7), 430–432.
Velasco, M., O'Malley, K., Robie, N. W., Wells, J., Israili, Z. H., and McNay, J. L. (1974). Differential effects of propranolol on heart rate and plasma renin activity in patients treated with minoxidil. Clin. Pharmacol. Ther. 16 (6), 1031–1038. doi:10.1002/cpt19741661031
Veldhuizen, G. P., Alnazer, R. M., de Leeuw, P. W., and Kroon, A. A. (2021). The effects of verapamil, hydralazine, and doxazosin on renin, aldosterone, and the ratio thereof. Cardiovasc Drugs Ther. 37, 283–289. doi:10.1007/s10557-021-07262-3
Volpe, M., and Unger, T. (2013). Plasma renin and cardiovascular risk: What is the evidence for an association? Cardiology 125 (1), 50–59. doi:10.1159/000348365
Wanhainen, A., Verzini, F., Van Herzeele, I., Allaire, E., Bown, M., Cohnert, T., et al. (2019). Editor's choice - European society for vascular surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur. J. Vasc. Endovasc. Surg. 57 (1), 8–93. doi:10.1016/j.ejvs.2018.09.020
Wilmink, A. B. M., Vardulaki, K. A., Hubbard, C. S. F., Day, N. E., Ashton, H. A., Scott, A. P., et al. (2002). Are antihypertensive drugs associated with abdominal aortic aneurysms? J. Vasc. Surg. 36 (4), 751–757. doi:10.1016/s0741-5214(02)00129-5
Wu, C. H., Mohammadmoradi, S., Chen, J. Z., Sawada, H., Daugherty, A., and Lu, H. S. (2018). Renin-angiotensin system and cardiovascular functions. Arterioscler. Thromb. Vasc. Biol. 38 (7), e108–e116. doi:10.1161/ATVBAHA.118.311282
Zhang, C., Mohan, A., Shi, H., and Yan, C. (2022). Sildenafil (viagra) aggravates the development of experimental abdominal aortic aneurysm. J. Am. Heart Assoc. 11 (2), e023053. doi:10.1161/JAHA.121.023053
Keywords: abdominal aortic aneurysm, hypertension, antihypertensive drugs, plasma renin level, plasma renin activity, vasodilator
Citation: Liu Y, Liu S, Zhao J, Wu K, Xu B and Wang W (2023) Increased plasma renin by vasodilators promotes the progression of abdominal aortic aneurysm. Front. Pharmacol. 14:1174278. doi: 10.3389/fphar.2023.1174278
Received: 26 February 2023; Accepted: 22 May 2023;
Published: 13 June 2023.
Edited by:
Suren Soghomonyan, The Ohio State University, United StatesReviewed by:
Ben Li, University of Toronto, CanadaQian Fleming, The Ohio State University, United States
Copyright © 2023 Liu, Liu, Zhao, Wu, Xu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Wang, d2Vpd2FuZ2NzdUBjc3UuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Yu Liu
Yu Liu Shuai Liu
Shuai Liu Jiani Zhao1
Jiani Zhao1 Baohui Xu
Baohui Xu Wei Wang
Wei Wang