- 1Department of Pharmacy, Mindong Hospital Affiliated to Fujian Medical University, Ningde, Fujian, China
- 2Department of Pharmacy, Mengchao Hepatobiliary Hospital of Fujian Medical University, Fuzhou, Fujian, China
Background: The EMPOWER-LUNG 3 clinical trial has shown that cemiplimab plus chemotherapy (CCT) significantly extended overall survival (OS) and progression-free survival (PFS) for patients with advanced non-small cell cancer (NSCLC) compared to placebo plus chemotherapy (PCT). However, the cost-effectiveness of this new treatment option remains unknown. Thus, we evaluated the cost-effectiveness of CCT versus (vs.) PCT as the first-line treatment for patients with advanced NSCLC from the perspective of the Chinese healthcare system.
Methods: We constructed a Markov model to evaluate the cost-effectiveness of CCT as the first-line treatment for patients with advanced NSCLC. The transition probabilities were extracted from the survival data of the EMPOWER-LUNG 3 trial. The drugs’ costs were referred from national tender prices, while other model input parameters were derived from the EMPOWER-LUNG 3 trial and published literature. The outcome parameters mainly included quality-adjusted life years (QALYs) and incremental cost-effectiveness ratios (ICERs). One-way sensitivity analysis and probabilistic sensitivity analysis were performed to evaluate the robustness of the model outcomes.
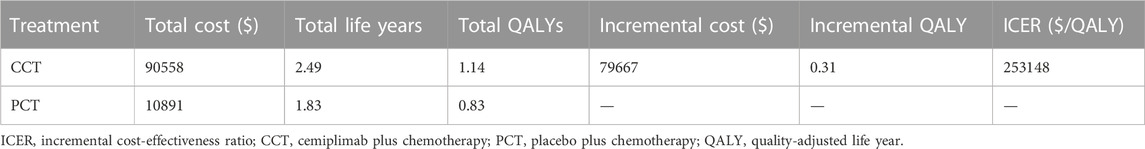
Results: Compared to PCT, in the CCT regimen, an additional $79,667 was spent in terms of the total cost and with an additional 0.31 QALYs, resulting in an ICER value of $253,148/QALY. Sensitivity analysis indicated that the hazard ratio (HR) of OS, the cost of cemiplimab (100 mg), and the HR of PFS, all significantly impacted the model’s results. The probability of CCT (vs. PCT) being cost-effective was 0% at a willingness-to-pay threshold of $38,201/QALYs in China. The scenario analysis showed that when the price of cemiplimab was reduced to less than $184.09/100 mg, the CCT regimen could be considered cost-effective as the first-line treatment for patients with advanced NSCLC compared to the PCT.
Conclusion: In China, the CCT was not cost-effective as the first-line treatment for patients with advanced NSCLC.
1 Introduction
Lung cancer is a common public health concern with the second highest incidence rate among all cancers worldwide and is the leading cause of cancer-related deaths (Sung et al., 2021). Non-small cell lung cancer (NSCLC) accounts for approximately 80%–85% of the common subtypes of lung cancer (Chen et al., 2022b). Unfortunately, the majority of cases (nearly 85%) are already at an advanced stage at the time of first diagnosis (Miller et al., 2022). The overall 5-year survival rate has been less than 5% in the past decade (Arbour and Riely, 2019). Conventional platinum-based chemotherapy is the standard first-line treatment for patients with advanced NSCLC without epidermal growth factor receptor (EGFR) mutations, anaplastic lymphoma kinase (ALK) translocations, or ROS proto-oncogene 1 (ROS1) fusions (Lee, 2019; Ettinger et al., 2022; Leone et al., 2023). However, the clinical benefit of standard chemotherapy remains unsatisfactory, with a 5-year survival rate of only 15% (Rocco et al., 2019; Ibodeng et al., 2023; Lahiri et al., 2023). Therefore, searching for new treatment options is necessary.
In recent years, immune checkpoint inhibitors (ICIs) targeting programmed cell death protein 1 (PD-1) and programmed cell death ligand 1 (PD-L1) have attracted global attention, and their development and application have fundamentally changed the landscape of treatment for newly diagnosed patients with advanced NSCLC, especially those without targeted genetic mutations (Peters et al., 2019). By 2018, approximately 33% of patients diagnosed with advanced NSCLC had received this emerging therapy (American College of Surgeons Commission on Cancer, 2019).
Cemiplimab is an anti-PD-1 monoclonal antibody (Lee et al., 2020). Gogishvili et al. (2022) recently conducted a multicenter phase III clinical trial (EMPOWER-Lung 3; identifier: NCT03409614) and compared the efficacy and safety of cemiplimab plus chemotherapy (CCT) versus (vs.) placebo plus chemotherapy (PCT) as the first-line therapy for untreated patients with locally advanced (stage IIIB or IIIC) or stage IV NSCLC. The outcomes showed that CCT, compared to PCT, had a significant clinical benefit as the first-line treatment for patients with advanced NSCLC, regardless of the levels of PD-L1 expression [median overall survival (OS): 21.9 vs. 13.0 months, hazard ratio (HR) = 0.71; median progression-free survival (PFS): 8.2 vs. 5.0 months, HR = 0.56] (Gogishvili et al., 2022).
Although the CCT regimen, compared to the PCT regimen, showed clear superiority in clinical efficacy as the treatment of advanced NSCLC (Gogishvili et al., 2022), its cost also increased dramatically, which prompted us to consider the following question: is the cost of CCT proportional to its clinical value? Therefore, we evaluated the cost-effectiveness of the CCT regimen as the first-line treatment for patients with advanced NSCLC from the perspective of the Chinese healthcare system in this study. The study aimed to provide healthcare decision makers with economic references for the CCT treatment option and improve the efficient use of limited healthcare resources, especially in cemiplimab pricing and reimbursement. Currently, no other study has evaluated the economics of CCT as the first-line treatment strategy for patients with advanced NSCLC.
2 Materials and methods
2.1 Model structure
The study’s design is based on the consolidated health economic evaluation reporting standards (CHEERS 2022) (Husereau et al., 2022) (Supplementary Table S1). A Markov model was constructed using TreeAge Pro 2022 (TreeAge Software, Williamstown, MA, United States) for evaluating the cost and health outcomes of both CCT and PCT strategies for patients with advanced NSCLC. The model involved three different health states [PFS, progressive disease (PD), and death], according to the progression of advanced NSCLC and relevant references (Liu G. et al., 2021; Luo et al., 2022), as shown in Figure 1. The patient can only be in one of the health states at any given time point. Since both cemiplimab and chemotherapy were administered once every 3 weeks in the EMPOWER-Lung 3 trial, the cycle length of the model was set at 21 days. In each cycle, patients either remained in their previous health state or developed a new one and were not allowed to return to their previous health state. The model’s time horizon was approximately 6 years (determined by assuming the death of 99% of the patients). We assumed that the patients in the model all started with the PFS state and randomly received CCT or PCT.
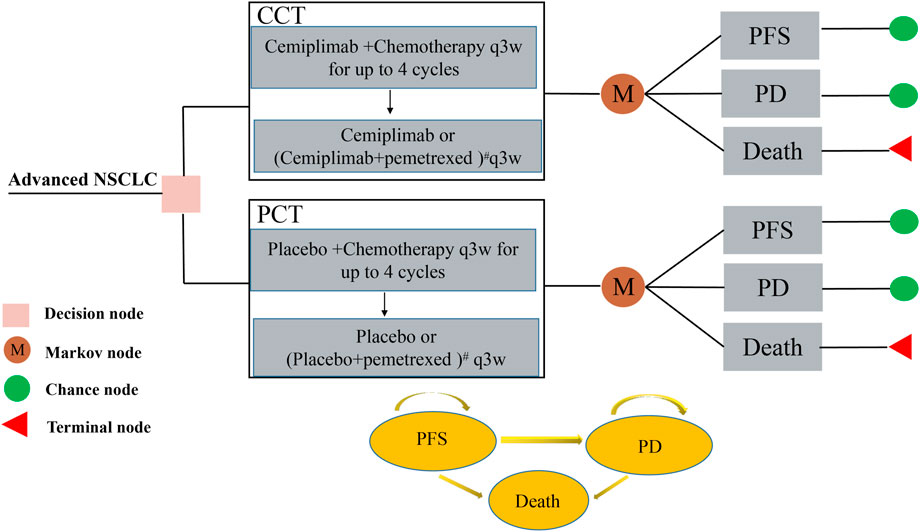
FIGURE 1. The Markov model used to compare CCT with PCT for treating patients with advanced NSCLC. All patients started with PFS state and randomly received CCT or PCT. # For patients with non-squamous histology who were assigned a pemetrexed-containing regimen, the maintenance regimen was cemiplimab or placebo plus pemetrexed. CCT, cemiplimab plus chemotherapy; NSCLC, non-small cell lung cancer; PCT, placebo plus chemotherapy; PD, progressive disease; PFS, progression-free survival.
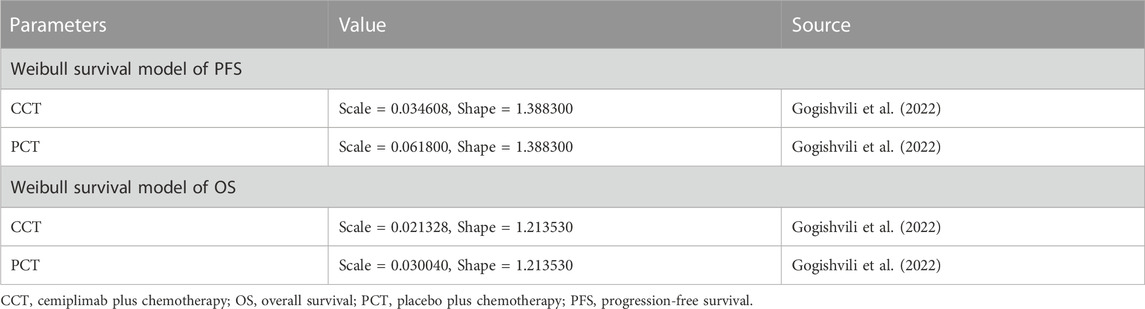
First, Data points from the Kaplan-Meier survival curves of the PCT group in the EMPOWER-Lung 3 trial were extracted using GetData Graph Digitizer (version 2.26). Pseudo-individual patient data were reconstructed using R software (version 4.2.0) following the algorithm described by Hoyle and Henley (2011). These data were fitted with the following standard parameter models (exponential, Weibull, log-logistic, and log-normal distributions) to obtain survival information over the observation period. Visual inspection and the values of Akaike information criterion (AIC) and Bayesian information criteria (BIC), as indicators of goodness of fit, were compared among the different distribution functions. The lower the values of AIC and BIC, the better the selected model’s fitness (Ishak et al., 2013; Bullement et al., 2019). The Weibull distribution function was found to provide the best fit for the survival data of the PCT group (Supplementary Figure S1). We also estimated the scale parameter (λ) and shape parameter (γ) of the PCT group (Table 1). For the CCT group, λ and γ were calculated according to the following equations: γ CCT = γ PCT and λ CCT = HR × λ PCT (Table 1), where HR is the HR of PFS and OS obtained from the EMPOWER-Lung 3 trial. Finally, the transition probabilities for each cycle in the Markov model were calculated.
2.2 Clinical data
The enrolled population, interventions, and survival data for our study were obtained from the EMPOWER-LUNG 3 trial, which enrolled 466 adult patients (≥18 years of age) from 74 medical participating centers of multiple countries with squamous or non-squamous stage IIIB/C (if deemed unsuitable for definitive chemoradiotherapy) or stage IV NSCLC. Patients with tumors positive for EGFR mutations, ALK translocations, or ROS1 fusions were excluded, as well as those who had previously undergone anti-PD-1/PD-L1 therapy (Supplementary Table S2). The patients were randomly assigned in a ratio of 2:1 to the CCT (n = 312) or PCT group (n = 154) for treatment. Baseline characteristics were generally well-balanced between the two arms. The CCT and PCT arms received cemiplimab and placebo, respectively, 350 mg once every 3 weeks for up to 108 weeks, in combination with a maximum of four cycles of chemotherapy. The chemotherapy regimens were selected based on the patient’s NSCLC histology (Table 2; Supplementary Table S3). After four cycles, pemetrexed (500 mg/m2, once every 3 weeks) was maintained for patients with non-squamous histology who were previously assigned to a pemetrexed-containing regimen. Patients were treated until disease progression, unacceptable toxicity, or completion of 108 weeks, whichever occurred first.
Because the EMPOWER-LUNG 3 trial lacked subsequent regimens after patients entered the PD state, we referred to some studies (Wu et al., 2020; Chen et al., 2022a; You et al., 2022b), assuming that patients in both the CCT and PCT groups received the best supportive care (BSC) after their disease progressed until death.
2.3 Cost and utility estimates
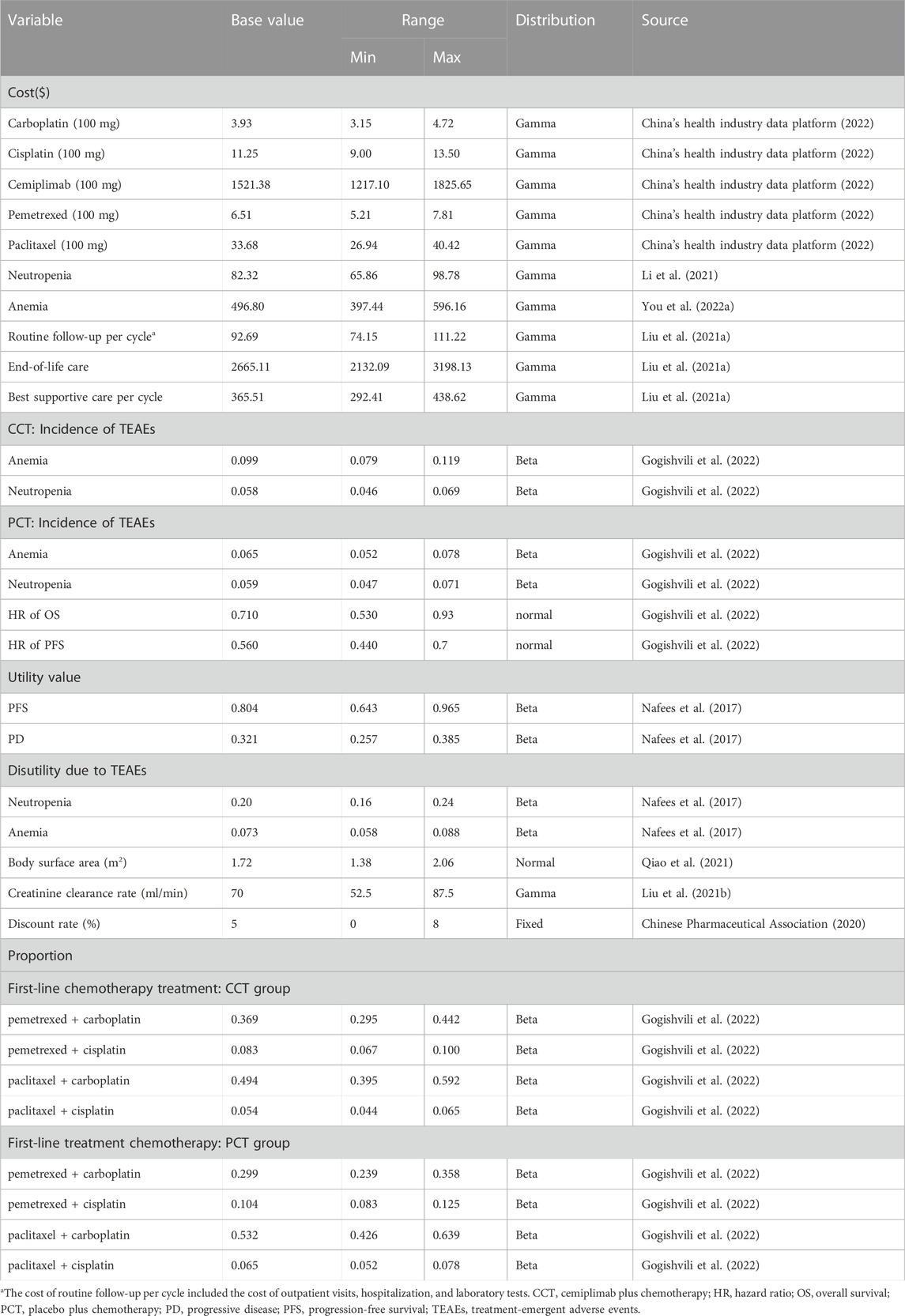
Only direct medical costs were considered, including those of medications, routine follow-up, BSC, end-of-life care, and management of treatment-emergent adverse events (TEAEs) (grade ≥3 and incidence >5%). The prices of drugs were obtained from the national public data platform (China’s health industry data platform, 2022). However, information on the price of cemiplimab in China could not be obtained as it is still unavailable in the Chinese market. Thus, we referred to the Chinese price of a classical PD-1 inhibitor (pembrolizumab, US$2662.41/100 mg) (China’s health industry data platform, 2022) available in China and assumed the same cost of the two drugs for one cycle. Additional data were obtained from published literature (Table 2), wherein costs from previous years were adjusted to those in 2022 in US dollars using the China Statistics Bureau Medical Price Index (National Bureau of Statistics, 2022). All costs were converted to US dollars using an average exchange rate of 1 USD = 6.73 CNY. We obtained utility values of 0.804 and 0.321 for PFS and PD, respectively, from published articles (Nafees et al., 2017; Liu et al., 2020; Zhu et al., 2021). We also considered the disutility of TEAEs (grade ≥3 and incidence >5%) (Zhu et al., 2021). All cost and utility values were discounted at a rate of 5% per year as recommended by the Chinese Guidelines for Pharmacoeconomic Evaluations (Chinese Pharmaceutical Association, 2020). The details of the model input parameter are shown in Table 2.
The major output parameters of the model were total costs, life years (LYs), quality-adjusted life years (QALYs), and incremental cost-effectiveness ratios (ICERs). The QALY value for each treatment option is the sum of the survival time for each state multiplied by the corresponding utility value. The ICER value is the ratio of the difference in cost and the difference in effectiveness between the two groups, which is compared with the given willingness to pay (WTP) threshold. If the ICER was below the WTP threshold, the CCT treatment was considered more cost-effective vs. PCT; The WTP threshold for QALYs, as recommended by the World Health Organization, is three times the national gross domestic product per capita in the current year (herein, 2022) (National Bureau of Statistics, 2022), i.e., $38,201/QALY.
2.4 Sensitivity analysis
To investigate the effect of the model parameter’s uncertainty on the results, one-way sensitivity analysis and probabilistic sensitivity analysis (PSA) were performed In one-way sensitivity analysis, the ranges of the relevant parameters were their 95% confidence intervals from the EMPOWER-Lung 3 trials or set to be ± 20% of the baseline values if the former were not available (Zhang et al., 2012). For PSA, appropriate distributions were assigned for different types of parameters (Table 2) and 1,000 iterative Monte Carlo simulations were performed. Moreover, we repeatedly calculated the acceptable probabilities of the cost-effectiveness for the CCT regimen by continuously reducing the price of cemiplimab. Then, an appropriate price of cemiplimab was obtained when the acceptance probability was 50% at a WTP threshold of $38,201. When the acceptable probability was above 50%, CCT was considered cost-effective as the first-line treatment for patients with advanced NSCLC.
2.5 Subgroup analysis
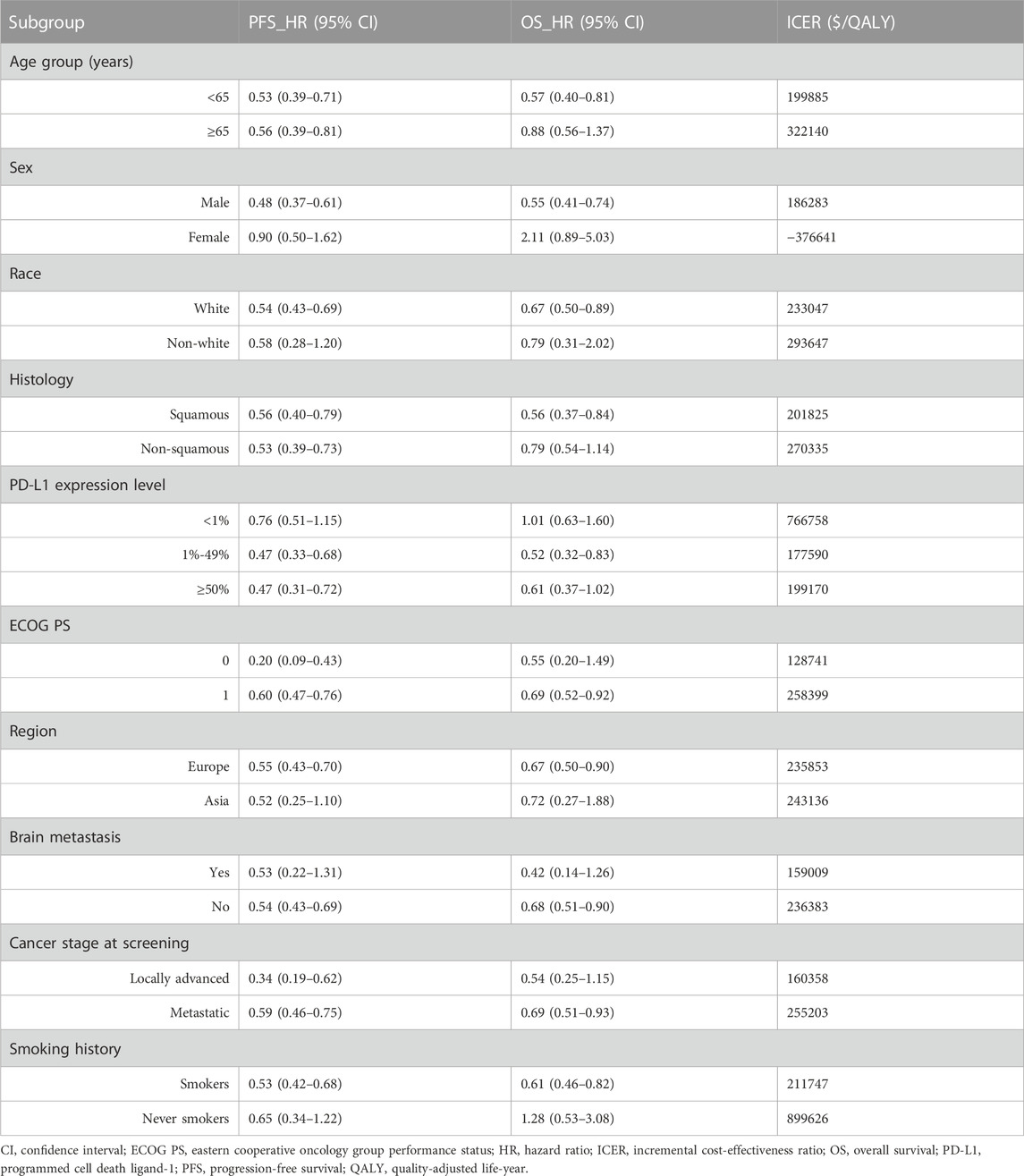
Subgroup analysis was performed for group characteristics including age, sex, histological characteristics, and PD-L1 expression (Table 3), among others, to determine whether the corresponding performance was better in terms of cost-effectiveness in specific subgroups. Given the lack of Kaplan-Meier survival curves of the subgroup population in the EMPOWER-Lung 3 trial, based on the method described by Hoyle et al. (2010), we assumed that survival data of all subgroups in the PCT group followed the Weibull distribution, while the survival functions of all subgroups in the CCT group was estimated based on the subgroup-specific HRs (Table 3) from the EMPOWER-Lung 3 trial. It needs to be emphasized that all parameters were assumed to be consistent with those of the entire patient population, except for subgroup-specific HRs. Finally, ICERs and cost-effectiveness probabilities were obtained for each subgroup.
3 Results
3.1 Base-case analysis
For the entire patient population, $79,667 was spent more in the CCT group vs. PCT group with an additional 0.31 QALYs, resulting in an ICER value of $253,148/QALY (Table 4), which was much higher than the WTP threshold ($38,201/QALYs) in China. Thus, the CCT was not cost-effective compared to PCT as the first-line treatment for patients with advanced NSCLC in China.
3.2 Sensitivity analyses
3.2.1 One-way sensitivity analysis
The results of the one-way sensitivity analysis are presented as a tornado diagram (Figure 2). The HR of OS, the cost of cemiplimab (100 mg), the HR of PFS, the shape parameter value of the OS in the PCT group, and the utility values of PFS and PD all had a significant impact on the results of the model. However, the ICER value was always above the WTP threshold regardless of the variation in each parameter within the preset upper and lower limits, thereby confirming that our model outcomes were reliable.
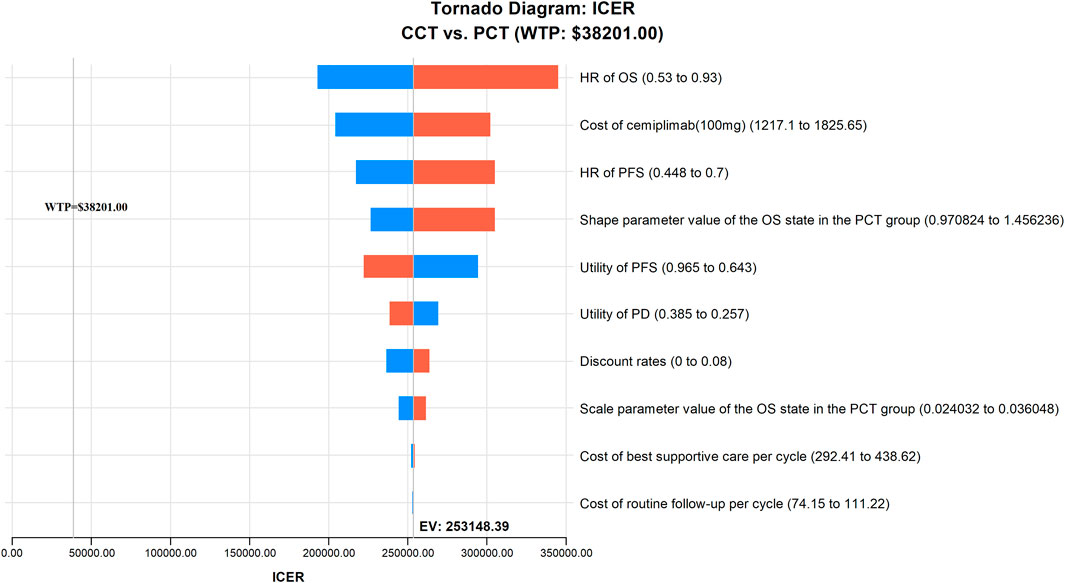
FIGURE 2. The results of one-way sensitivity analysis indicating the most influential parameters for model’s results in China. CCT, cemiplimab plus chemotherapy; HR, hazard ratio; ICER, incremental cost-effectiveness ratio; OS, overall survival; PCT, placebo plus chemotherapy; PFS, progression-free survival; WTP, willingness-to-pay.
3.2.2 PSA
The results of PSA are presented as a scatter plot (Figure 3) and cost-effectiveness acceptability curves (Figure 4). As shown, the probability that CCT was cost-effective relative to PCT was 0% in China, at a WTP threshold of $38,201. We also found that when the original price of cemiplimab was reduced to 12.10% (i.e., $184.09/100 mg), the probability of CCT being cost-effective rose to 50%, implying that the CCT regimen could be considered cost-effective as the first-line treatment for patients with advanced NSCLC compared to PCT if the price of cemiplimab dropped below $184.09/100 mg.
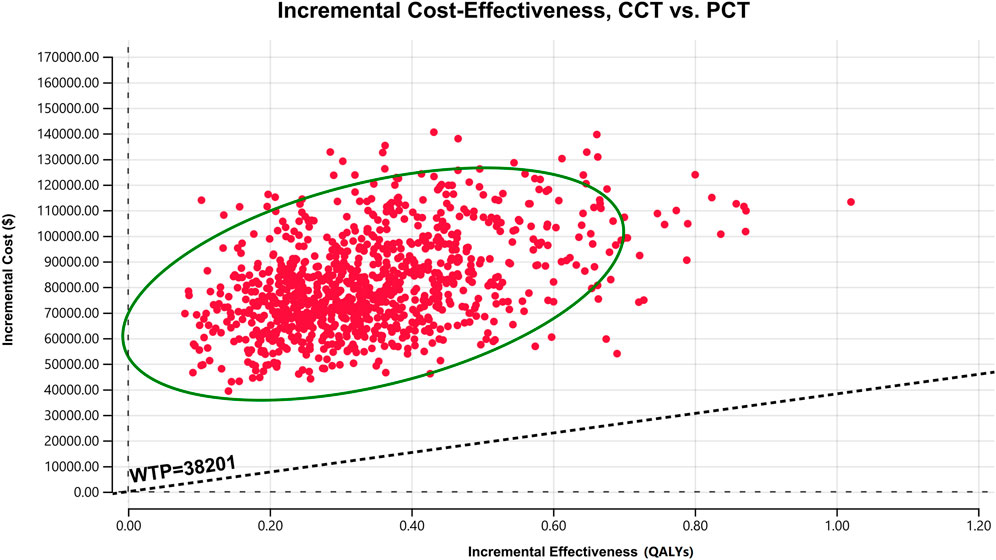
FIGURE 3. A scatter plot of ICER for probabilistic sensitivity analysis. The points in the graph are obtained by Monte Carlo simulation of 1,000 iterations. Points above the WTP threshold line show the cost-effectiveness of PCT over CCT; conversely, CCT is cost-effective. CCT, cemiplimab plus chemotherapy; ICER, incremental cost-effectiveness ratio; PCT, placebo plus chemotherapy; QALYs, quality-adjusted life years; WTP, willingness-to-pay.
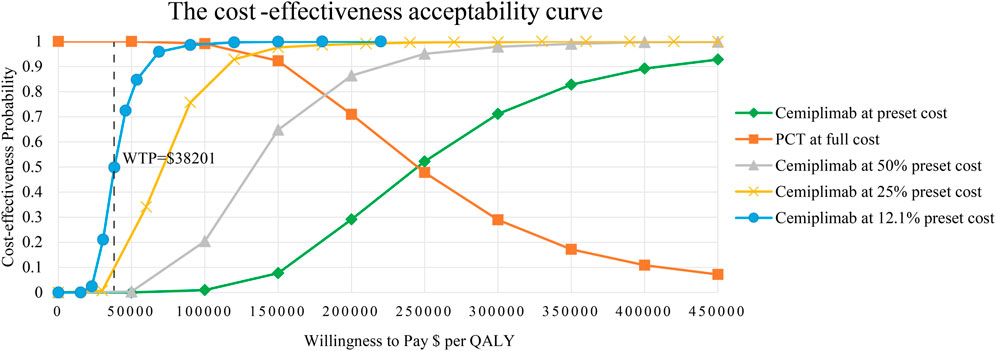
FIGURE 4. The cost-effectiveness acceptability curves for CCT versus PCT. CCT, cemiplimab plus chemotherapy; PCT, placebo plus chemotherapy; QALY, quality-adjusted life year; WTP, willingness-to-pay.
3.3 Subgroup analysis
The results of our subgroup analysis are shown in Table 3. For all subgroups of the population with different characteristics, the ICERs of CCT were all above the WTP threshold of $38,201 with 0% probabilities of being cost-effective compared to PCT.
4 Discussion
Indeed, in the era of targeted therapy and immunotherapy, platinum-based compounds and other standard chemotherapeutic agents still represent key treatments for the survival of NSCLC patients (Hellmann et al., 2016; Lee, 2019; Falzone et al., 2023). However, chemotherapy alone has a limited survival benefit and poor prognosis for NSCLC patients (Rocco et al., 2019). According to the recommendations of Guidelines of Chinese Society of Clinical Oncology (CSCO) for NSCLC (2022 edition) (Guidelines Working Committee of Chinese Society of Clinical Oncology, 2022), immunotherapy represented by immune checkpoint inhibitors (e.g., PD-1/PD-L1 inhibitors) has been shown to significantly improve the survival of patients with advanced NSCLC in recent years.
In November 2022, the FDA approved a new indication for cemiplimab based on the results of the EMPOWER-LUNG 3 trial, a phase III clinical trial for cemiplimab, as the first-line treatment of patients with advanced NSCLC without EGFR, ALK, or ROS1 mutations, regardless of the levels of PD-L1 expression. The EMPOWER-LUNG 3 trial showed that the CCT arm demonstrated better clinical benefits compared to the PCT arm, as evidenced by the prolonged OS and PFS of patients with advanced NSCLC However, as in some other countries (Godman et al., 2021), the affordability of innovative anticancer medicines (e.g., PD-1/PD-L1 inhibitors) is a hitherto unknown and grave challenge for the majority of Chinese patients with cancer. One study predicts that the financial burden of cancer in China may continue to rise in the coming decades (Chen et al., 2016). For example, without insurance, patients who were prescribed trastuzumab had to pay more than $50,000 in that year, almost 24 times the Chinese annual per capita disposable income during the same period (Chen et al., 2009). The high cost of these innovative anticancer drugs not only places a monetary burden on cancer patients but also exacerbates the drain on healthcare resources, especially for developing countries like China with limited healthcare resources. Therefore, it is essential for us to evaluate the economics of the CCT treatment strategy for these patients compared to PCT from a Chinese perspective.
This is the first study to evaluate the cost-effectiveness of CCT as a first-line treatment option for advanced NSCLC from the Chinese healthcare perspective. Our study included patients with different levels of PD-1 expression and no genetic mutations. The results of this study showed that the ICER of $253,148/QALY in China was above the WTP threshold ($38,201/QALYs), indicating that the CCT treatment strategy was unlikely to provide a proportionate and reasonable value for the money spent. Sensitivity analysis verified that the results were reliable in general. The results of the subgroup analysis showed that the CCT was not a cost-effective choice for the first-line treatment of advanced NSCLC in different subgroups of patients.
According to the recommendations of Guidelines of Chinese Society of Clinical Oncology for NSCLC (2022 edition) (Guidelines Working Committee of Chinese Society of Clinical Oncology, 2022), PD-1/PD-L1 inhibitors in combination with chemotherapy is the major first-line treatment regimen for patients with advanced NSCLC without driver mutations, including pembrolizumab, atezolizumab, camrelizumab, and sugemalimab. Several existing studies (Liu G. et al., 2021) (Wan et al., 2020; Xiang et al., 2021; Zheng et al., 2022) have evaluated the cost-effectiveness of these PD-1/PD-L1 inhibitors in combination with chemotherapy as the first-line treatment for advanced NSCLC from the perspective of Chinese payers. The results of all these studies suggested that the combination of the PD-1/PD-L1 inhibitor and chemotherapy is unlikely to be a cost-effective option for patients with advanced NSCLC compared to chemotherapy alone, consistent with our results. This is due to their modest incremental effect and the high cost of PD-1/PD-L1 inhibitors. However, the previous findings should not be a reason to restrict the use of PD-1/PD-L1 inhibitors, as we risk missing beneficial therapeutic options but rather be treated as economic references for healthcare policymakers in setting a reasonable market price for cemiplimab and in national drug price negotiations. Therefore, we conducted a scenario analysis by continuously reducing the price of cemiplimab to make CCT cost-effective and affordable for patients with advanced NSCLC. The results suggested that the CCT regimen became economical compared to PCT only when the price of cemiplimab was below $184.09/100 mg (probability of cost-effectiveness, >50%).
Some limitations of our study warrant further consideration. First, we extrapolated the survival curves by fitting parameter functions, which inevitably resulted in deviations between the model’s results and the actual situation, an unavoidable drawback of most cost-effective analyses. We will revise our analysis as long-term survival data become available. Second, only a minority of the study population in the EMPOWER-LUNG 3 trial was Chinese (5.58%), which may not accurately reflect the efficacy of Chinese patients. However, we performed a one-way sensitivity analysis of the two key parameters (λ, γ) of the survival curve from the EMPOWER-LUNG 3 trial. The results of the sensitivity analysis suggested that this does not affect the results of our economic evaluation. Third, since cemiplimab is not yet available in China, we used the price of another PD-1 inhibitor, pembrolizumab, which is available in China. We will update the results of our study when the price of cemiplimab is available. Fourth, we assumed that all the patients received BSC after entering the PD state, as EMPOWER-LUNG 3 results for subsequent treatment were not available. Although this assumption may differ from clinical practice, one-way sensitivity analysis suggested that the cost of subsequent treatment was not sensitive to the outcome. When the subsequent treatment plan is released, we will refine the corresponding results. Fifth, due to the lack of corresponding head-to-head trials, we could not directly evaluate the cost-effectiveness of the CCT regimen vs. other PD-1/PD-L1 inhibitors in combination with chemotherapy, recommended by the Guidelines of CSCO for NSCLC (Guidelines Working Committee of Chinese Society of Clinical Oncology, 2022) as potential first-line regimens for patients with advanced NSCLC, like pembrolizumab plus chemotherapy. More clinical trials are required in the future to support this type of cost-effectiveness analysis. Finally, the results of the subgroup analysis should be cautiously interpreted because it is an exploratory study with several unknown parameters. Despite these limitations, we believe that our findings have important economic implications for Chinese decision makers as a reference for drug pricing and health insurance access negotiations.
5 Conclusion
In conclusion, for the first time, we conducted an economic evaluation of CCT for patients with advanced NSCLC from the Chinese healthcare perspective. Compared to PCT, CCT was not a cost-effective choice for patients with advanced NSCLC without EGFR mutations or ALK rearrangements in China. A price reduction for cemiplimab may be a potential measure to make CCT cost-effective and affordable after entering China, thus providing more economic options for these patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
No human studies are presented in this manuscript. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Study design and supervision: TL, RC; data analysis and interpretation: ZC, WL, and XC; data collection: YuH and YiH; manuscript writing: TL; All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Natural Science Foundation of Fujian Province of China (Grant number: 2023J011910; 2021J011288); Social Development Project of Fuzhou Science and Technology Department (Grant number: 2021-S-108); Startup Fund for scientific research, Fujian Medical University (Grant number: 2019QH1301; 2021QH1203); Mindong Hospital Science Fund (Grant number: 2022YN005). This study was not supported by any pharmaceutical company.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1171302/full#supplementary-material
References
American College of Surgeons Commission on Cancer (2019). National cancer database. Available at: https://www.facs.org/quality-programs/cancer-programs/national-cancer-database/ (Accessed February 21, 2023).
Arbour, K. C., and Riely, G. J. (2019). Systemic therapy for locally advanced and metastatic non-small cell lung cancer: A review. Jama 322 (8), 764–774. doi:10.1001/jama.2019.11058
Bullement, A., Meng, Y., Cooper, M., Lee, D., Harding, T. L., O'Regan, C., et al. (2019). A review and validation of overall survival extrapolation in health technology assessments of cancer immunotherapy by the national Institute for health and care excellence: How did the initial best estimate compare to trial data subsequently made available? J. Med. Econ. 22 (3), 205–214. doi:10.1080/13696998.2018.1547303
Chen, P., Li, Y., Jing, X., Chen, J., Chen, S., and Yang, Q. (2022a). Cost-effectiveness analysis of sugemalimab in combination with chemotherapy as first-line treatment in Chinese patients with metastatic NSCLC. Lung Cancer 174, 157–164. doi:10.1016/j.lungcan.2022.11.008
Chen, P., Liu, Y., Wen, Y., and Zhou, C. (2022b). Non-small cell lung cancer in China. Cancer Commun. (Lond) 42 (10), 937–970. doi:10.1002/cac2.12359
Chen, W., Jiang, Z., Shao, Z., Sun, Q., and Shen, K. (2009). An economic evaluation of adjuvant trastuzumab therapy in HER2-positive early breast cancer. Value Health 12 (Suppl. 3), S82–S84. doi:10.1111/j.1524-4733.2009.00634.x
Chen, W., Zheng, R., Baade, P. D., Zhang, S., Zeng, H., Bray, F., et al. (2016). Cancer statistics in China, 2015. CA Cancer J. Clin. 66 (2), 115–132. doi:10.3322/caac.21338
China’s health industry data platform (2022). Bid winning information of drugs. Available at: https://www.yaozh.com/ (Accessed December 26, 2022).
Chinese Pharmaceutical Association (2020). Chinese Guidelines for pharmacoeconomic evaluations. Available at: https://www.cpa.org.cn/cpadmn/attached/file/20201203/1606977380634185.pdf.
Ettinger, D. S., Wood, D. E., Aisner, D. L., Akerley, W., Bauman, J. R., Bharat, A., et al. (2022). Non-small cell lung cancer, version 3.2022, NCCN clinical practice Guidelines in oncology. J. Natl. Compr. Canc Netw. 20 (5), 497–530. doi:10.6004/jnccn.2022.0025
Falzone, L., Bordonaro, R., and Libra, M. (2023). SnapShot: Cancer chemotherapy. Cell 186 (8), 1816–1816.E1. doi:10.1016/j.cell.2023.02.038
Godman, B., Hill, A., Simoens, S., Selke, G., Selke Krulichová, I., Zampirolli Dias, C., et al. (2021). Potential approaches for the pricing of cancer medicines across Europe to enhance the sustainability of healthcare systems and the implications. Expert Rev. Pharmacoecon Outcomes Res. 21 (4), 527–540. doi:10.1080/14737167.2021.1884546
Gogishvili, M., Melkadze, T., Makharadze, T., Giorgadze, D., Dvorkin, M., Penkov, K., et al. (2022). Cemiplimab plus chemotherapy versus chemotherapy alone in non-small cell lung cancer: A randomized, controlled, double-blind phase 3 trial. Nat. Med. 28 (11), 2374–2380. doi:10.1038/s41591-022-01977-y
Guidelines Working Committee of Chinese Society of Clinical Oncology (2022). Guidelines of Chinese society of clinical oncology (CSCO) for non-small cell lung cancer. Beijing: People's Medical Publishing House.
Hellmann, M. D., Li, B. T., Chaft, J. E., and Kris, M. G. (2016). Chemotherapy remains an essential element of personalized care for persons with lung cancers. Ann. Oncol. 27 (10), 1829–1835. doi:10.1093/annonc/mdw271
Hoyle, M., Green, C., Thompson-Coon, J., Liu, Z., Welch, K., Moxham, T., et al. (2010). Cost-effectiveness of temsirolimus for first line treatment of advanced renal cell carcinoma. Value Health 13 (1), 61–68. doi:10.1111/j.1524-4733.2009.00617.x
Hoyle, M. W., and Henley, W. (2011). Improved curve fits to summary survival data: Application to economic evaluation of health technologies. BMC Med. Res. Methodol. 11, 139. doi:10.1186/1471-2288-11-139
Husereau, D., Drummond, M., Augustovski, F., de Bekker-Grob, E., Briggs, A. H., Carswell, C., et al. (2022). Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: Updated reporting guidance for health economic evaluations. Value Health 25 (1), 3–9. doi:10.1016/j.jval.2021.11.1351
Ibodeng, G.-o., Uche, I. N., Mokua, R., Galo, M., Odigwe, B., Galeas, J. N., et al. (2023). A snapshot of lung cancer: Where are we now?—a narrative review. Ann. Transl. Med. 11 (6), 261. doi:10.21037/atm-22-4479
Ishak, K. J., Kreif, N., Benedict, A., and Muszbek, N. (2013). Overview of parametric survival analysis for health-economic applications. Pharmacoeconomics 31 (8), 663–675. doi:10.1007/s40273-013-0064-3
Lahiri, A., Maji, A., Potdar, P. D., Singh, N., Parikh, P., Bisht, B., et al. (2023). Lung cancer immunotherapy: Progress, pitfalls, and promises. Mol. Cancer 22 (1), 40. doi:10.1186/s12943-023-01740-y
Lee, A., Duggan, S., and Deeks, E. D. (2020). Cemiplimab: A review in advanced cutaneous squamous cell carcinoma. Drugs 80 (8), 813–819. doi:10.1007/s40265-020-01302-2
Lee, S. H. (2019). Chemotherapy for lung cancer in the era of personalized medicine. Tuberc. Respir. Dis. Seoul. 82 (3), 179–189. doi:10.4046/trd.2018.0068
Leone, G. M., Candido, S., Lavoro, A., Vivarelli, S., Gattuso, G., Calina, D., et al. (2023). Clinical relevance of targeted therapy and immune-checkpoint inhibition in lung cancer. Pharmaceutics 15 (4), 1252. doi:10.3390/pharmaceutics15041252
Li, M., Lin, S., Wilson, L., Huang, P., Wang, H., Lai, S., et al. (2021). Cost-effectiveness analysis of hepatic arterial infusion of FOLFOX combined sorafenib for advanced hepatocellular carcinoma with portal vein invasion. Front. Oncol. 11, 562135. doi:10.3389/fonc.2021.562135
Liu, G., Kang, S., Wang, X., and Shang, F. (2021a). Cost-effectiveness analysis of atezolizumab versus chemotherapy as first-line treatment for metastatic non-small-cell lung cancer with different PD-L1 expression status. Front. Oncol. 11, 669195. doi:10.3389/fonc.2021.669195
Liu, Q., Luo, X., Peng, L., Yi, L., Wan, X., Zeng, X., et al. (2020). Nivolumab versus docetaxel for previously treated advanced non-small cell lung cancer in China: A cost-effectiveness analysis. Clin. Drug Investig. 40 (2), 129–137. doi:10.1007/s40261-019-00869-3
Liu, Q., Zhou, Z., Luo, X., Yi, L., Peng, L., Wan, X., et al. (2021b). First-line ICI monotherapies for advanced non-small-cell lung cancer patients with PD-L1 of at least 50%: A cost-effectiveness analysis. Front. Pharmacol. 12, 788569. doi:10.3389/fphar.2021.788569
Luo, X., Zhou, Z., Zeng, X., and Liu, Q. (2022). The cost-effectiveness of tislelizumab plus chemotherapy for locally advanced or metastatic nonsquamous non-small cell lung cancer. Front. Pharmacol. 13, 935581. doi:10.3389/fphar.2022.935581
Miller, K. D., Nogueira, L., Devasia, T., Mariotto, A. B., Yabroff, K. R., Jemal, A., et al. (2022). Cancer treatment and survivorship statistics, 2022. CA Cancer J. Clin. 72 (5), 409–436. doi:10.3322/caac.21731
Nafees, B., Lloyd, A. J., Dewilde, S., Rajan, N., and Lorenzo, M. (2017). Health state utilities in non-small cell lung cancer: An international study. Asia Pac J. Clin. Oncol. 13 (5), e195–e203. doi:10.1111/ajco.12477
National Bureau of Statistics (2022). China statistical yearbook 2022. Available at: http://www.stats.gov.cn (Accessed February 21, 2023)
Peters, S., Reck, M., Smit, E. F., Mok, T., and Hellmann, M. D. (2019). How to make the best use of immunotherapy as first-line treatment of advanced/metastatic non-small-cell lung cancer. Ann. Oncol. 30 (6), 884–896. doi:10.1093/annonc/mdz109
Qiao, L., Zhou, Z., Zeng, X., and Tan, C. (2021). Cost-effectiveness of domestic PD-1 inhibitor camrelizumab combined with chemotherapy in the first-line treatment of advanced nonsquamous non-small-cell lung cancer in China. Front. Pharmacol. 12, 728440. doi:10.3389/fphar.2021.728440
Rocco, D., Della Gravara, L., Battiloro, C., and Gridelli, C. (2019). The role of combination chemo-immunotherapy in advanced non-small cell lung cancer. Expert Rev. Anticancer Ther. 19 (7), 561–568. doi:10.1080/14737140.2019.1631800
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Wan, N., Zhang, T. T., Hua, S. H., Lu, Z. L., Ji, B., Li, L. X., et al. (2020). Cost-effectiveness analysis of pembrolizumab plus chemotherapy with PD-L1 test for the first-line treatment of NSCLC. Cancer Med. 9 (5), 1683–1693. doi:10.1002/cam4.2793
Wu, Q., Liao, W., Zhang, M., Huang, J., Zhang, P., and Li, Q. (2020). Cost-effectiveness of tucatinib in human epidermal growth factor receptor 2-positive metastatic breast cancer from the US and Chinese perspectives. Front. Oncol. 10, 1336. doi:10.3389/fonc.2020.01336
Xiang, G., Gu, L., Chen, X., Wang, F., Chen, B., Zhao, J., et al. (2021). Economic evaluation of first-line camrelizumab for advanced non-small-cell lung cancer in China. Front. Public Health 9, 743558. doi:10.3389/fpubh.2021.743558
You, M., Chen, R., Wu, Q., Zhu, W., He, Y., and Huang, Y. (2022a). Cost-effectiveness analysis of adebrelimab combined with chemotherapy for extensive-stage small cell lung cancer. Front. Pharmacol. 13, 1019826. doi:10.3389/fphar.2022.1019826
You, M., Huang, Y., Cai, Z., Wu, Q., Zhu, W., He, Y., et al. (2022b). Cost-effectiveness analysis of sintilimab plus chemotherapy for advanced or metastatic esophageal squamous cell carcinoma. Front. Oncol. 12, 986762. doi:10.3389/fonc.2022.986762
Zhang, Y., Baik, S. H., Fendrick, A. M., and Baicker, K. (2012). Comparing local and regional variation in health care spending. N. Engl. J. Med. 367 (18), 1724–1731. doi:10.1056/NEJMsa1203980
Zheng, Z., Zhu, H., Fang, L., and Cai, H. (2022). Cost-effectiveness analysis of sugemalimab vs. chemotherapy as first-line treatment of metastatic nonsquamous non-small cell lung cancer. Front. Pharmacol. 13, 996914. doi:10.3389/fphar.2022.996914
Zhu, C., Xing, X.-x., Wu, B., Liang, G., Han, G., Lin, C.-x., et al. (2021). Cost-effectiveness analysis of camrelizumab plus chemotherapy vs. Chemotherapy alone as the first-line treatment in patients with IIIB–IV non-squamous non-small cell lung cancer (NSCLC) without EGFR and ALK alteration from a perspective of health-care system in China. Front. Pharmacol. 12, 735536. doi:10.3389/fphar.2021.735536
Keywords: cost-effectiveness, cemiplimab, chemotherapy, advanced non-small cell lung cancer, first-line treatment
Citation: Lu T, Huang Y, Cai Z, Lin W, Chen X, Chen R and Hu Y (2023) The cost-effectiveness of cemiplimab plus chemotherapy as the first-line treatment for advanced non-small cell lung cancer. Front. Pharmacol. 14:1171302. doi: 10.3389/fphar.2023.1171302
Received: 22 February 2023; Accepted: 10 July 2023;
Published: 26 July 2023.
Edited by:
Brian Godman, University of Strathclyde, United KingdomReviewed by:
Guangming Tian, Beijing Cancer Hospital, ChinaLuca Falzone, G. Pascale National Cancer Institute Foundation (IRCCS), Italy
Copyright © 2023 Lu, Huang, Cai, Lin, Chen, Chen and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruijia Chen, cnVpamlhY2hlbjUwM0AxNjMuY29t; Yingying Hu, Y2VsaWFoeXlAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Tingting Lu
Tingting Lu Yufan Huang
Yufan Huang Zhongjie Cai1
Zhongjie Cai1 Ruijia Chen
Ruijia Chen Yingying Hu
Yingying Hu