- 1Department of Radiation Oncology, Shaoxing People’s Hospital, Shaoxing, Zhejiang Province, China
- 2Department of Radiology, Shaoxing People’s Hospital, Shaoxing, Zhejiang Province, China
- 3Emergency Department, Shaoxing People’s Hospital, Shaoxing, Zhejiang Province, China
For patients with locally unresectable recurrent nasopharyngeal carcinoma who relapsed after 2 years of radiotherapy, re-radiotherapy is also the preferred treatment. However, for patients relapsed within 2 years, the use of re-radiotherapy would be greatly limited by its adverse effects. Consequently, finding a new strategy to prolong the time of re-radiotherapy for locally recurrent nasopharyngeal carcinoma is very necessary to reduce the related side effects and improve the curative effect. Anlotinib is an orally available small molecule multi-target tyrosine kinase inhibitor that primarily inhibits VEGFR2/3, FGFR1–4, PDGFR α/β, c-Kit, and Ret. However, whether recurrent nasopharyngeal carcinoma patients can be treated with anlotinib combined with ticeorgio (also called S-1) remains unknown. Herein, we report a nasopharyngeal carcinoma patient with local recurrence after radical radiotherapy who benefited from combination treatment of anlotinib with ticeorgio.
Introduction
Nasopharyngeal carcinoma (NPC) is a malignant tumor arising from the nasopharyngeal epithelium. With an estimated 133,354 new cases and 80,008 associated deaths worldwide, NPC is a relatively rare disease, representing approximately 0.7% of cancer diagnoses and 0.8% of cancer-related deaths (Sung et al., 2021). The distribution of NPC has strong regional characteristics, with approximately half of NPC cases arising in China (Chen et al., 2019). Induction chemotherapy followed by concurrent chemoradiotherapy is the standard treatment for locoregionally advanced NPC. For patients who cannot tolerate or are unwilling to receive chemotherapy, radiotherapy combined with nimotuzumab can be an option in China (Radiation Oncology Physicians Branch of Chinese Medical Doctor Association ROBoCMA, 2022).
With the increased application of intensity modulated radiotherapy (IMRT) and comprehensive treatment plans, the 5-year local and regional relapse-free survival rates in patients with newly diagnosed nonmetastatic NPC are between 83.0% and 91.8% and between 91.0% and 96.4%, respectively (He, 2021); however, it has been reported that between 10% and 20% of patients still experience local recurrence (Li et al., 2018; Lee et al., 2019). According to the National Comprehensive Cancer Network guidelines for unresectable head and neck cancer with locoregional recurrence previously treated with radiotherapy, radiotherapy is the recommended treatment for unresectable locally recurrent NPC. Nevertheless, incidences of reirradiation-related grade 3–4 late toxic effects after salvage IMRT remain between 34% and 70% (Han et al., 2012; Hua et al., 2012). These toxic effects can be particularly pronounced within 2 years after primary radiotherapy. Thus, choosing an appropriate treatment strategy for patients with unresectable NPC who have relapsed within 2 years after radiotherapy is a challenge for oncologists.
Anlotinib is an orally available small molecule multi-target tyrosine kinase inhibitor that primarily inhibits VEGFR2/3, FGFR1–4, PDGFR α/β, c-Kit, and Ret and has been widely used in the clinic (Shen et al., 2018). A recent study reported that anlotinib combined with ticeorgio showed favorable efficacy for advanced NPC patients who had failed first-line treatment (Yang and Chen, 2022). However, whether patients with advanced recurrent NPC can be treated with anlotinib combined with ticeorgio remains unknown. Herein, we report the case of a patient with locally recurrent NPC after radical radiotherapy who benefited from anlotinib in combination with ticeorgio.
Case presentation
A previously healthy 60-year-old man visited the ENT Department owing to right ear tinnitus and stuffy discomfort for 20 days. The first doctor performed a contrast-enhanced magnetic resonance scan of the nasopharynx, which showed a mass on the posterior wall of the nasopharynx, then we reexamined a 3-T contrast-enhanced MRI (Figures 1A, B). The patient was subsequently diagnosed with NPC by pathological biopsy (Figures 1C, D). The pathological classification was non-keratinizing undifferentiated carcinoma, and the TNM staging was stage III (T3N2M0) according to the eighth edition of the American Joint Committee on Cancer guidelines.
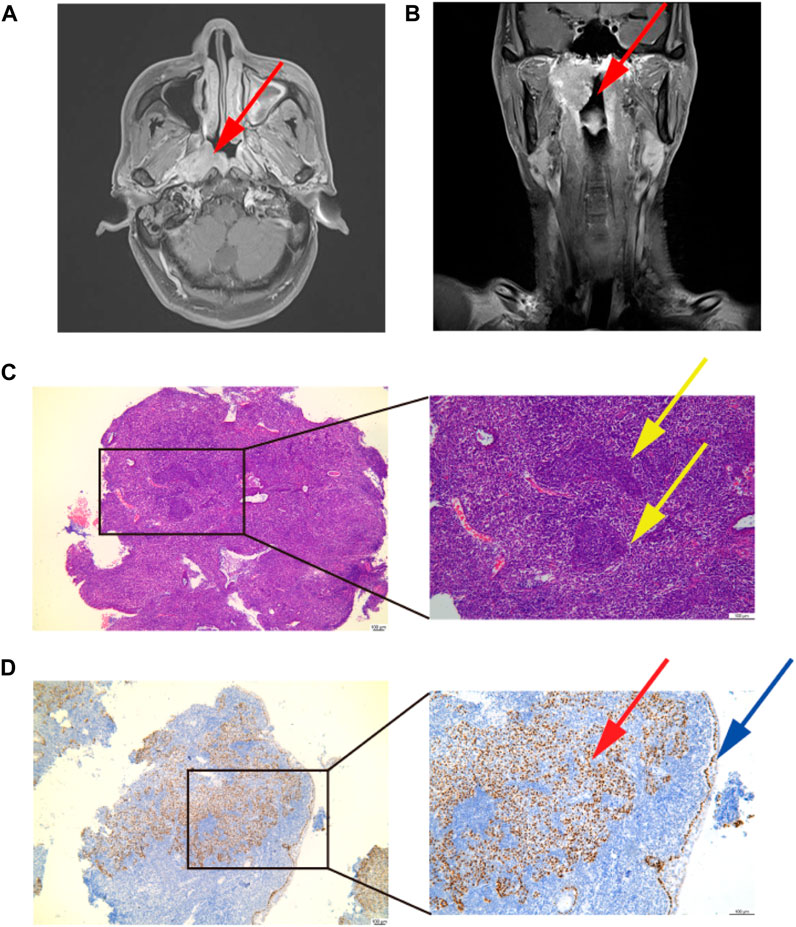
FIGURE 1. Diagnosis of nasopharynx cancer. (A,B) Diagnostic imaging result. A mass on the posterior wall of the nasopharynx: the red arrow points to. (C) Image of pathological diagnosis. Magnification; ×40 and×100. A paraffin section of the mass was stained with HE, showing obvious tumor nests, oval nuclei: the yellow arrow points to. (D) P63 immunohistochemical staining. Magnification;×40 and ×100. A paraffin section of the mass was stained with P63, a large number of P63-positive cells can be seen under the basement membrane, squamous cell carcinoma is positive for P63: the red arrow points to. P63-positive cells indicated by blue arrows are the basement membrane.
The patient was then referred to the Radiotherapy Department and received three cycles of induction chemotherapy with the TP regimen (paclitaxel [270 mg] + cisplatin [135 mg]), which resulted in partial response (PR). Then, treatment was performed with radical radiotherapy at a dose delivered to the gross tumor volume of the nasopharynx (PGTVnx) and gross tumor volume of the lymph nodes (PGTVnd) of at least 6,996 cGy in 33 fractions, with 212 cGy daily five times per week; targeted therapy with nimotuzumab once weekly for 6 weeks and one cycle of cisplatin chemotherapy were performed concurrently. After radiotherapy, three cycles of consolidation TP chemotherapy (paclitaxel [270 mg] + cisplatin [135 mg]) were performed. All treatments resulted in PR (Figure 2A). During the timely periodic follow-up checks, residual tumor foci decreased further in size, and the final assessment of efficacy was cCR (Figure 2B 2019.07.03). In the following 5 months, there is no sign of recurrence discovered. However, the result of the sixth month’s follow-up examination revealed parapharyngeal lymph node exhibited a trend toward increasing size (Figure 2B 2020.02.10). Five months later the patient developed a slight restriction of mouth opening. And a subsequent MR (Figure 2B 2020.07.17) scan showed further enlargement of the parapharyngeal lymph nodes, leading to invasion of the parapharyngeal space and the lateral pterygoid muscle. According to the Expert consensus on the treatment of recurrent nasopharyngeal carcinoma, recurrent NPC is defined as 6 months after radical treatment of the first diagnosis of nasopharyngeal carcinoma, during which the tumour tissue has reached cCR or pCR, followed by a recurrence of tumour growth (Lin et al., 2018). Recurrence was defined on the basis of radiographic and clinical findings.
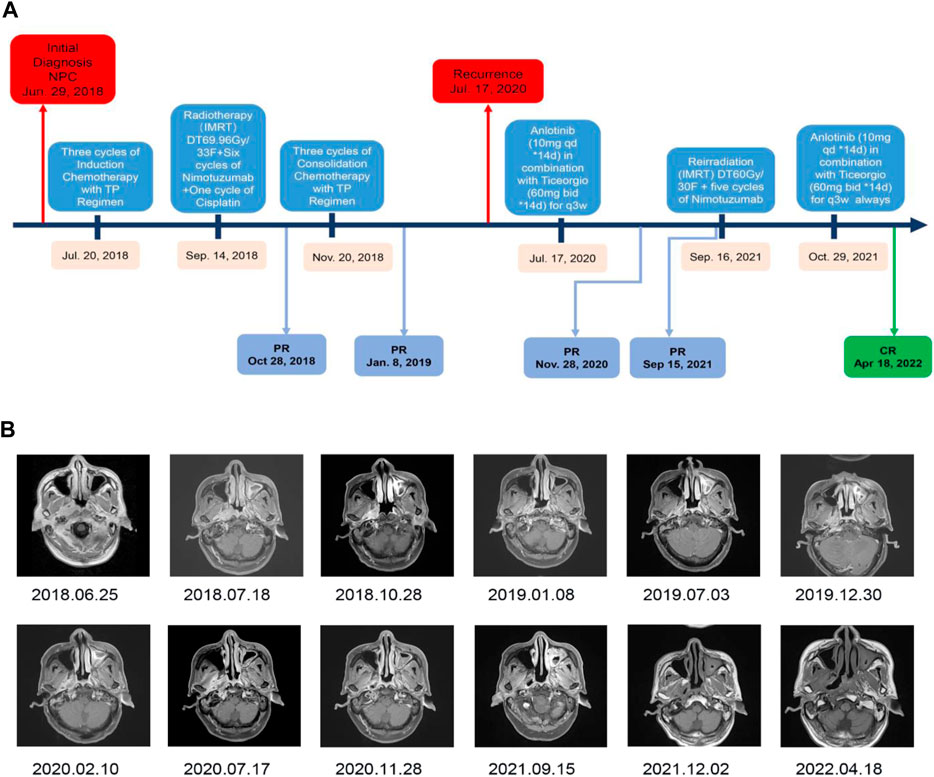
FIGURE 2. Timeline of treatment and radiographic responses. (A) Timeline of treatment. (B) Imaging results showing regression of the lesion and demonstrating the effectiveness of the anlotinib combined with ticeorgio regimen.
In conclusion, 18 months after completing treatment, the patient was diagnosed with recurrent NPC in the parapharyngeal space by contrast-enhanced magnetic resonance imaging of the nasopharynx. Thereafter, the patient received anlotinib (10 mg qd for 14 days) combined with ticeorgio (60 mg bid for 14 days, based on 60 mg per dose for body surface area >1.5 m2, twice a day) every 3 weeks, drugs administration were performed daily by an oral rout, which resulted in PR. Common adverse drug reactions of anlotinib and ticeorgio include hypertension, proteinuria, nausea, vomiting, liver and kidney function injury, bone marrow suppression, fatigue, and so on. Despite that, the actual toxic effects were just mild fatigue during the co-medications period for this patient. Considering the effectiveness of the combination therapy regimen and its mild side effects, we tried to use this treatment as much as possible to gain time and space for a further re-radiotherapy. After 14 months of treatment with anlotinib and ticeorgio, the patient received re-radiotherapy at a dose delivered to the recurrent gross tumor volume of the nasopharynx (PTV) of at least 6,000 cGy in 30 fractions, with 200 cGy daily five times per week concurrently with nimotuzumab once weekly for 6 weeks. Maintenance treatment with anlotinib (10 mg qd for 14 days) combined with ticeorgio (60 mg bid for 14 days) every 3 weeks was continued when re-radiotherapy had finished. The lesion was evaluated as complete response on 2 December 2021. As of 28 April 2022, the lesion remains stable and no residual cancer has been found on imaging.
Discussion
This study investigated whether anlotinib combined with ticeorgio could be an effective regimen for patients with locally recurrent NPC who had received comprehensive treatments, including induction chemotherapy plus concurrent chemoradiotherapy, concurrent targeted therapy, and consolidation chemotherapy after radical radiotherapy. Anlotinib is a novel anti-angiogenesis drug that was designed to primarily inhibit VEGFR2/3, FGFR1–4, PDGFR α/β, c-Kit, and Ret, and it shows broad-spectrum antitumor potential in advanced refractory solid tumors (Sun et al., 2016) and seemed to be a promising therapeutic option for recurrent or metastatic NPC with acceptable toxicity profiles (Cai et al., 2020). Recently, anlotinib has been increasingly used in clinical practice for advanced tumors, and not only as an individual agent (Sun Y. et al., 2021; Cheng et al., 2021; Chi et al., 2021; Cui et al., 2021; Zhu et al., 2021) but also combined with immunotherapy (Han et al., 2021; Wang et al., 2021; Zhang et al., 2021; Su et al., 2022; Xu et al., 2022), radiofrequency ablation (Zhou et al., 2021), radiotherapy (He et al., 2021), and chemotherapy (Sun W. et al., 2021; She et al., 2021; Xiang et al., 2021). These studies have all shown promising efficacy and safety data for anlotinib. Ticeorgio is the Chinese version of S-1, which is a novel oral fluoropyrimidine agent containing tegafur, gimeracil and oteracil potassium (Oki et al., 2016; Zheng et al., 2019). Clinical trials showed that ticeorgio plus gemcitabine or nedaplatin have provided a satisfactory and safe clinical activity for patients with recurrent and metastatic NPC after platinum-containing chemotherapy failed (Peng et al., 2016; Peng et al., 2017). These studies suggested that anlotinib and ticeorgio are likely to be effective in recurrent metastatic nasopharyngeal carcinoma, meanwhile the adverse effects are tolerable. Notably, one clinical trial showed that anlotinib combined with ticeorgio had favorable efficacy for advanced NPC patients who had failed first-line treatment (Yang and Chen, 2022). However, there are no reported cases of locally recurrent NPC treated with anlotinib, not to mention the combination of anlotinib and ticeorgio.
In this case, the patient experienced recurrence within 2 years after radical radiotherapy. According to the National Comprehensive Cancer Network guidelines for head and neck cancer with locoregional recurrence previously treated with radiotherapy, surgical resection is the preferred approach for resectable recurrent tumors. However, given that the recurrent tumor was located within the parapharyngeal space, surgical treatment would have been quite difficult, with the potential for serious complications. Thus, the patient was not a candidate for surgery. For locally unresectable recurrent NPC, re-radiotherapy is the preferred choice. However, if the patient had received radiotherapy again in such a short period of time, there would have been a high probability of serious and even lethal toxic side effects. It is important to extend the interval between re-radiotherapy for the patient. Therefor platinum-based systemic chemotherapy is a good choice to effectively control tumor progression. Due to unpleasant side effects from previous chemotherapy, the patient was resistant to platinum-based chemotherapy. Given the patient’s wishes, the above reasons and in accordance with previous reports, we selected the anlotinib plus ticeorgio regimen to extend the interval between the two radiotherapy courses. Therefore, in this case, the patient received anlotinib (10 mg once daily) and ticeorgio (60 mg twice daily) for 2 weeks on with 1 week off. The patient responded well to this therapy, with the lesion dramatically decreasing in size and forming tumor cavitation after 4 months of therapy. Then the patient continued to be treated with anlotinib and ticeorgio for 10 months, which resulted in PR and further enlargement of the tumor cavity. Tumor cavitation is an independent predictor of better PFS in NPC (Chen et al., 2020). During anlotinib therapy, tumor cavitation occurred in our patient and grew over treatment. However, effective candidate biomarkers for anlotinib remain unclear, and further investigation is warranted.
After 14 months of combined treatment with anlotinib and ticeorgio, the patient received re-radiotherapy with concurrent targeted sensitization therapy with nimotuzumab. After re-radiotherapy, the patient continued treatment with anlotinib (10 mg qd for 14 days) combined with ticeorgio (60 mg bid for 14 days) every 3 weeks. Subsequently, the lesion was evaluated as complete response (CR) on 2 December 2021. As of 28 April 2022, the lesion has remained stable, with no signs of residual cancer on imaging. Ongoing follow-up will continue, and we will look for opportunities to provide further updates on the patient’s condition.
Conclusion
Overall, this case highlights the potential efficacy of anlotinib combined with ticeorgio for recurrent refractory NPC. In this patient, anlotinib plus ticeorgio had a miraculous effect on recurrent refractory NPC. However, testing of this regimen has had some limitations, such as small case samples. In future studies, we aim to further explore this option in larger cohorts.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
JM and WY contributed equally to this work. WM and YZ performed the radiological analysis of MRI images. JM wrote the first draft of the manuscript. DW wrote sections of the manuscript. JL and TL are responsible for data collection and patient follow-up. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from the Medical and Health Science and Technology Programs of Shaoxing (#2020A13017), Health Science and Technology Plan Project of Shaoxing (#2022KY005) and Medical Science and Technology Project of Zhejiang Province (#2023RC287).
Acknowledgments
We thank the patient in this report and his family. Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Cai, Q., Su, N., Fang, Y., Ma, S., Xia, Y., Zhang, X., et al. (2020). 929P Anlotinib in patients with recurrent or metastatic nasopharyngeal carcinoma: An interim analysis of a phase II clinical trial. Ann. Oncol. 31, S668. doi:10.1016/j.annonc.2020.08.1044
Chen, D., Xu, J., Zhao, Y., Chu, T., Zhong, H., Han, B., et al. (2020). Prognostic value of tumor cavitation in extensive-stage small-cell lung cancer patients treated with anlotinib. J. Cancer Res. Clin. Oncol. 146 (2), 401–406. doi:10.1007/s00432-019-03064-1
Chen, Y. P., Chan, A. T. C., Qt, L., Blanchard, P., Sun, Y., and Ma, J. (2019). Nasopharyngeal carcinoma. Lancet 394 (10192), 64–80. doi:10.1016/s0140-6736(19)30956-0
Cheng, Y., Wang, Q., Li, K., Shi, J., Liu, Y., Wu, L., et al. (2021). Anlotinib vs placebo as third- or further-line treatment for patients with small cell lung cancer: A randomised, double-blind, placebo-controlled phase 2 study. Br. J. Cancer 125 (3), 366–371. doi:10.1038/s41416-021-01356-3
Chi, Y., Shu, Y., Ba, Y., Bai, Y., Qin, B., Wang, X., et al. (2021). Anlotinib monotherapy for refractory metastatic colorectal cancer: A double-blinded, placebo-controlled, randomized phase III trial (ALTER0703). Oncologist 26 (10), e1693–e1703. doi:10.1002/onco.13857
Cui, Q., Hu, Y., Ma, D., and Liu, H. (2021). A retrospective observational study of anlotinib in patients with platinum-resistant or platinum-refractory epithelial ovarian cancer. Drug Des. Devel Ther. 15, 339–347. doi:10.2147/dddt.S286529
Han, C., Ye, S., Hu, C., Shen, L., Qin, Q., Bai, Y., et al. (2021). Clinical activity and safety of penpulimab (Anti-PD-1) with anlotinib as first-line therapy for unresectable hepatocellular carcinoma: An open-label, multicenter, phase ib/II trial (AK105-203). Front. Oncol. 11, 684867. doi:10.3389/fonc.2021.684867
Han, F., Zhao, C., Huang, S. M., Lu, L. X., Huang, Y., Deng, X. W., et al. (2012). Long-term outcomes and prognostic factors of re-irradiation for locally recurrent nasopharyngeal carcinoma using intensity-modulated radiotherapy. Clin. Oncol. R. Coll. Radiol. 24 (8), 569–576. doi:10.1016/j.clon.2011.11.010
He, M. Y. J. (2021). Current status of treatment for recurrent nasopharyngeal carcinoma[J]. Chin. J. Radiat. Oncol. 30 (11), 1202–1208. doi:10.3760/cma.j.cn113030-20200220-00065
He, Z., Liu, J., Ma, Y., Jiang, H., Cui, Z., Wang, G., et al. (2021). Anlotinib combined with cranial radiotherapy for non-small cell lung cancer patients with brain metastasis: A retrospectively, control study. Cancer Manag. Res. 13, 6101–6111. doi:10.2147/cmar.S319650
Hua, Y. J., Han, F., Lu, L. X., Mai, H. Q., Guo, X., Hong, M. H., et al. (2012). Long-term treatment outcome of recurrent nasopharyngeal carcinoma treated with salvage intensity modulated radiotherapy. Eur. J. Cancer 48 (18), 3422–3428. doi:10.1016/j.ejca.2012.06.016
Lee, A. W. M., Ng, W. T., Chan, J. Y. W., Corry, J., Mäkitie, A., Mendenhall, W. M., et al. (2019). Management of locally recurrent nasopharyngeal carcinoma. Cancer Treat. Rev. 79, 101890. doi:10.1016/j.ctrv.2019.101890
Li, Y. Q., Tian, Y. M., Tan, S. H., Liu, M. Z., Kusumawidjaja, G., Ong, E. H. W., et al. (2018). Prognostic model for stratification of radioresistant nasopharynx carcinoma to curative salvage radiotherapy. J. Clin. Oncol. 36 (9), 891–899. doi:10.1200/jco.2017.75.5165
Lin, S. J., Chen, X. Z., and Li, J. G., (2018). Expert consensus on the treatment of recurrent nasopharyngeal carcinoma[J]. Chin. J. Radiat. Oncol. 27 (1), 7. doi:10.3760/cma.j.issn.1004-4221.2018.01.004
Oki, E., Murata, A., Yoshida, K., Maeda, K., Ikejiri, K., Munemoto, Y., et al. (2016). A randomized phase III trial comparing S-1 versus UFT as adjuvant chemotherapy for stage II/III rectal cancer (JFMC35-C1: ACTS-RC). Ann. Oncol. 27 (7), 1266–1272. doi:10.1093/annonc/mdw162
Peng, P., Ou, X., Liao, H., Liu, Y., Wang, S., Cheng, Z., et al. (2016). Phase II study of gemcitabine plus S-1 chemotherapy in recurrent and metastatic nasopharyngeal carcinoma patients after failure of platinum-based chemotherapy. Ther. Adv. Med. Oncol. 8 (3), 153–159. doi:10.1177/1758834016637592
Peng, P. J., Lv, B. J., Wang, Z. H., Liao, H., Liu, Y. M., Lin, Z., et al. (2017). Multi-institutional prospective study of nedaplatin plus S-1 chemotherapy in recurrent and metastatic nasopharyngeal carcinoma patients after failure of platinum-containing regimens. Ther. Adv. Med. Oncol. 9 (2), 68–74. doi:10.1177/1758834016675099
Radiation Oncology Physicians Branch of Chinese Medical Doctor Association ROBoCMA (2022). Guidelines for radiation therapy of nasopharyngeal carcinoma in China (2022 edition) [J]. Chin. J. Cancer Prev. Treat. 029 (009), 611–622. doi:10.16073/j.cnki.cicpt.2022.9.1
She, L., Su, L., Shen, L., and Liu, C. (2021). Retrospective study of the safety and efficacy of anlotinib combined with dose-dense temozolomide in patients with recurrent glioblastoma. Front. Oncol. 11, 687564. doi:10.3389/fonc.2021.687564
Shen, G., Zheng, F., Ren, D., Du, F., Dong, Q., Wang, Z., et al. (2018). Anlotinib: A novel multi-targeting tyrosine kinase inhibitor in clinical development. J. Hematol. Oncol. 11 (1), 120. doi:10.1186/s13045-018-0664-7
Su, Y., Luo, B., Lu, Y., Wang, D., Yan, J., Zheng, J., et al. (2022). Anlotinib induces a T cell-inflamed tumor microenvironment by facilitating vessel normalization and enhances the efficacy of PD-1 checkpoint blockade in neuroblastoma. Clin. Cancer Res. 28 (4), 793–809. doi:10.1158/1078-0432.Ccr-21-2241
Sun, W., Zou, X., Zhang, W., Hu, S., and Ge, K. (2021). Clinical efficacy of anlotinib plus S-1 as a second-line therapy for recurrent or metastatic esophageal squamous cell carcinoma. Nan Fang. Yi Ke Da Xue Xue Bao 41 (2), 250–255. doi:10.12122/j.issn.1673-4254.2021.02.13
Sun, Y., Niu, W., Du, F., Du, C., and Wang, J., (2016). Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J. Hematol. Oncol. 9 (1), 105. doi:10.1186/s13045-016-0332-8
Sun, Y., Zhou, A., Zhang, W., Jiang, Z., Chen, B., Zhao, J., et al. (2021). Anlotinib in the treatment of advanced hepatocellular carcinoma: An open-label phase II study (ALTER-0802 study). Hepatol. Int. 15 (3), 621–629. doi:10.1007/s12072-021-10171-0
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Wang, P., Fang, X., Yin, T., Tian, H., Yu, J., and Teng, F. (2021). Efficacy and safety of anti-PD-1 plus anlotinib in patients with advanced non-small-cell lung cancer after previous systemic treatment failure-A retrospective study. Front. Oncol. 11, 628124. doi:10.3389/fonc.2021.628124
Xiang, M., Yang, X., Ren, S., Du, H., Geng, L., Yuan, L., et al. (2021). Anlotinib combined with S-1 in third- or later-line stage IV non-small cell lung cancer treatment: A phase II clinical trial. Oncologist 26 (12), e2130–e2135. doi:10.1002/onco.13950
Xu, Q., Wang, J., Sun, Y., Lin, Y., Liu, J., Zhuo, Y., et al. (2022). Efficacy and safety of sintilimab plus anlotinib for PD-L1-positive recurrent or metastatic cervical cancer: A multicenter, single-arm, prospective phase II trial. J. Clin. Oncol. 40 (16), 1795–1805. doi:10.1200/jco.21.02091
Yang, C. W. Z. Z., and Chen, Y. Q., (2022). Clinical study of anlotinib combined with ticeorgio in the second-line treatment of advanced nasopharyngeal carcinoma[J]. Chin. Health Stand. Adm. 13 (10), 148–150. doi:10.3969/j.issn.1674-9316.2022.10.039
Zhang, X., Zeng, L., Li, Y., Xu, Q., Yang, H., Lizaso, A., et al. (2021). Anlotinib combined with PD-1 blockade for the treatment of lung cancer: A real-world retrospective study in China. Cancer Immunol. Immunother. 70 (9), 2517–2528. doi:10.1007/s00262-021-02869-9
Zheng, W., Ying, J., Zhou, Y., Lu, Z., Min, K., Wang, W., et al. (2019). The efficacy and safety of first-line chemotherapies for advanced biliary tract cancer: A Network meta-analysis. J. Cancer 10 (1), 257–266. doi:10.7150/jca.27487
Zhou, W., Gao, Y., Tong, Y., Wu, Q., Zhou, Y., and Li, Y. (2021). Anlotinib enhances the antitumor activity of radiofrequency ablation on lung squamous cell carcinoma. Pharmacol. Res. 164, 105392. doi:10.1016/j.phrs.2020.105392
Keywords: anlotinib, combined therapy, recurrent nasopharyngeal carcinoma, ticeorgio, side effects of re-radiotherapy, S-1
Citation: Mao J, Ye W, Wu D, Liu J, Li T, Ma W and Zhou Y (2023) Effect of anlotinib combined with ticeorgio for recurrent nasopharyngeal carcinoma: a case report. Front. Pharmacol. 14:1166809. doi: 10.3389/fphar.2023.1166809
Received: 15 February 2023; Accepted: 03 July 2023;
Published: 14 July 2023.
Edited by:
Rajeev K. Tyagi, Institute of Microbial Technology (CSIR), IndiaReviewed by:
Rahul Shukla, National Institute of Pharmaceutical Education and Research, IndiaYingming Sun, Fujian Medical University, China
Copyright © 2023 Mao, Ye, Wu, Liu, Li, Ma and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wanli Ye, yewanli319@163.com
 Jiwei Mao
Jiwei Mao Wanli Ye1*
Wanli Ye1* Yang Zhou
Yang Zhou