- Department of Korean Rehabilitation Medicine, College of Korean Medicine, Daejeon University, Daejeon, Republic of Korea
Purpose: This study aimed to comprehensively review the effect of combining herbal medicine (HM) with Western Medicine (WM) compared to WM alone on bone mineral density (BMD) improvement for osteoporosis in patients with rheumatoid arthritis (RA).
Methods: Randomized controlled trials (RCTs) were searched using 10 databases, including PubMed, Embase, Cochrane Library, China National Knowledge Infrastructure, and Nation Information by NII. We selected studies that used BMD as an evaluation index and administered HM treatment for osteoporosis in patients with RA. Subsequently, a meta-analysis was conducted using BMD as a continuous variable using RevMan version 5.4.
Results: Eighteen RCTs that met the eligibility criteria of this study were selected. The total number of study participants was 1,491 (481 men and 1,010 women). The mean age of participants was 52.4 ± 7.4 years, and the mean morbidity period of RA was 6.8 ± 1.3 years. In all studies, disease-modifying anti-rheumatic drugs (DMARDs; 16 RCTs) or bisphosphonates (two RCTs) were used as WM co-intervention with HMs (17 types of HM, 18 RCTs). Overall, the combination of HM and WM improved the BMD score, producing better results than WM alone. In particular, when HM was used in combination with DMARDs, which were used in most studies, BMD improved by 0.04 g/cm2 (95% confidence interval [CI]: 0.03–0.05, p < 0.001, I2 = 19%) in the lumbar spine and 0.03 g/cm2 (95% CI: 0.02–0.03, p < 0.001, I2 = 0%) in the femoral neck compared to the DMARDs alone group after treatment. In addition to BMD, bone markers and inflammatory indicators evaluated by each RCT showed significant improvement after HM plus WM treatment. In the analysis of frequently prescribed HMs, the BMD after treatment was higher by 0.04 g/cm2 (95% CI: 0.03–0.04, p < 0.001, I2 = 45%) in the Xianlinggubao-capsule plus methotrexate (MTX) group and by 0.02 g/cm2 (95% CI: 0.00–0.03, p = 0.04, I2 = 0) in the Hanbikang-tang plus MTX group compared to the MTX alone group.
Conclusion: This systematic review cautiously provides evidence for the combined therapeutic effect of HM and WM for osteoporosis in patients with RA. However, well-designed, large-scale clinical trials are necessary before recommending this combination therapy for osteoporosis in patients with RA.
Systematic Review Registration: [https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=331854], identifier [CRD42022331854].
1 Introduction
Osteoporosis is one of the most well-known extra-articular complications in patients with rheumatoid arthritis (RA). It increases the risk of fragility fractures, impairing the quality of life and life expectancy of patients with RA (Haugeberg et al., 2000a; Haugeberg et al., 2000b; Lodder et al., 2004; van Staa et al., 2006).
The global prevalence of osteoporosis in patients with RA was estimated to be 27.6% (95% confidence interval [CI]: 23.9–31.3) in 2022 (Moshayedi et al., 2022), approximately 1.5 times higher than that of the general population (19.7%; 95% CI: 18.0%–21.4%) (Xiao et al., 2022). Up to 50% higher prevalence of osteoporosis has been reported in postmenopausal women with RA compared to those without RA (Haugeberg et al., 2000a; Hauser et al., 2014). In particular, it has been observed that the risk of osteoporotic fracture in the femoral neck and spine is approximately twice as high in patients with RA than in the general population (Haugeberg et al., 2000a; Sinigaglia et al., 2000; Hoes et al., 2015; Choi et al., 2018). Moreover, patients with RA have longer fracture healing times and higher rates of complications, including non-unions, owing to systemic inflammatory conditions (Claes et al., 2012). Therefore, osteoporosis in patients with RA has become an important medical challenge to manage and prevent because it is a major disease that reduces the quality of life and increases the burden of medical expenses (Wu et al., 2021).
As the main risk factor for low bone mineral density (BMD) in patients with RA is a persistent inflammatory response, it is important to lower the inflammatory response to reduce disease activity and inhibit bone loss (Lodder et al., 2004; van Staa et al., 2006). Therefore, major societies for rheumatology recommend RA disease treatment as the primary treatment strategy for preventing bone loss in patients with RA (Singh et al., 2016; Smolen et al., 2017; Lau et al., 2019; Moshayedi et al., 2022). Treatments for bone loss in patients with RA, include disease-modifying anti-rheumatic drugs (DMARDs) such as methotrexate (MTX), leflunomide, and hydroxychloroquine as well as osteoporosis drugs, including bisphosphonates (BPs), and are used in clinics depending on the patient’s condition (Raterman et al., 2020). However, some RA standard treatments may cause osteoporosis as an adverse reaction (Machado-Alba et al., 2014; Lin et al., 2020) and have been reported to be ineffective in inhibiting bone loss (Mazzantini et al., 2000). Therefore, it is necessary to develop a safer and more effective treatment for inhibiting bone loss in patients with RA.
Herbal medicines (HMs) have been traditionally used for thousands of years and have been proven safe and accessible (Wang et al., 2021). Hence, they have been considered a complementary alternative treatment commonly used in patients with RA over the past decades (Huang et al., 2015; Daily et al., 2017; Li et al., 2019). In several recent studies, herbal extracts and their major compounds were found to act as anti-RA ingredients (Wang et al., 2021), and the combination of DMARDs and HMs significantly improved the disease activity of RA compared to DMARDs alone in patients with RA (Gong et al., 2021). In addition, some herbal extracts showed improvement in BMD and bone turnover markers via various mechanisms (He et al., 2017).
Based on the results of these previous studies, HMs may potentially be new therapeutic agents effective in preventing and treating osteoporosis in patients with RA. However, to our knowledge, no studies to date have analyzed the overall effect of HM on osteoporosis in patients with RA by reviewing previous studies. Therefore, a systematic review and a meta-analysis were conducted to address the research question of whether HMs are effective and safe in improving BMD in RA. Thus, this study suggested the possibility of HM as a new treatment for secondary osteoporosis.
2 Methods
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement. The review protocol was registered in the International Prospective Register of Systematic Reviews (Open Science Framework) with the Registration ID CRD42022331854.
2.1 Search strategy
Data from randomized controlled trials (RCTs) for patients with RA having osteoporosis published up to 24 April 2022, were searched and obtained without language restrictions. A total of 10 databases, including PubMed, Embase, Cochrane library, China National Knowledge Infrastructure, Wanfang, Nation Information by NII, KoreaMed, Kmbase, Korean studies Information Service System, and ScienceOn, were used to conduct a systematic review. The search keywords were (rheumat*[Title/Abstract] OR reumat*[Title/Abstract]), (osteoporo*[Title/Abstract] OR “bone loss” [Title/Abstract] OR “low bone densit*” [Title/Abstract] OR osteopeni*[Title/Abstract], and “Korean medicine*” [Title/Abstract] OR herb*[Title/Abstract] OR TCM [Title/Abstract] OR decoction*[Title/Abstract] OR “kampo medicine*” (Title/Abstract). These keywords were combined and modified as required, according to whether the database was in English, Chinese, and Korean. Detailed search strategies are reported in Supplementary Tables S1–S3.
2.2 Eligibility criteria
The inclusion criteria were as follows: 1) The study participants had RA with osteoporosis according to the criteria for osteoporosis defined by the World Health Organization in 1994 (WHO Study Group, 1994); 2) The intervention included HMs or herbal extracts taken orally with no restriction on the formulation; 3) The control group was administered WM (anti-osteoporosis or anti-rheumatic drugs) or placebo. There were no restrictions on the method of administration or whether supplements, such as calcium and vitamin D, were used; 4) Outcome measurement had to include the BMD value (lumbar spine or femoral neck) measured using dual-energy X-ray absorptiometry. There was no restriction on other frequently used measurements in the included studies; 5) The study design had to be an RCT on humans. The following studies were excluded: 1) Studies without full texts or those where the BMD measurement site was not specified; 2) Studies where BMD could not be estimated using dual-energy X-ray absorptiometry; 3) Studies with a Jadad score <2 points, which was judged to indicate low quality. Additionally, unpublished studies and ongoing trials were not considered in this study.
2.3 Review process and data extraction
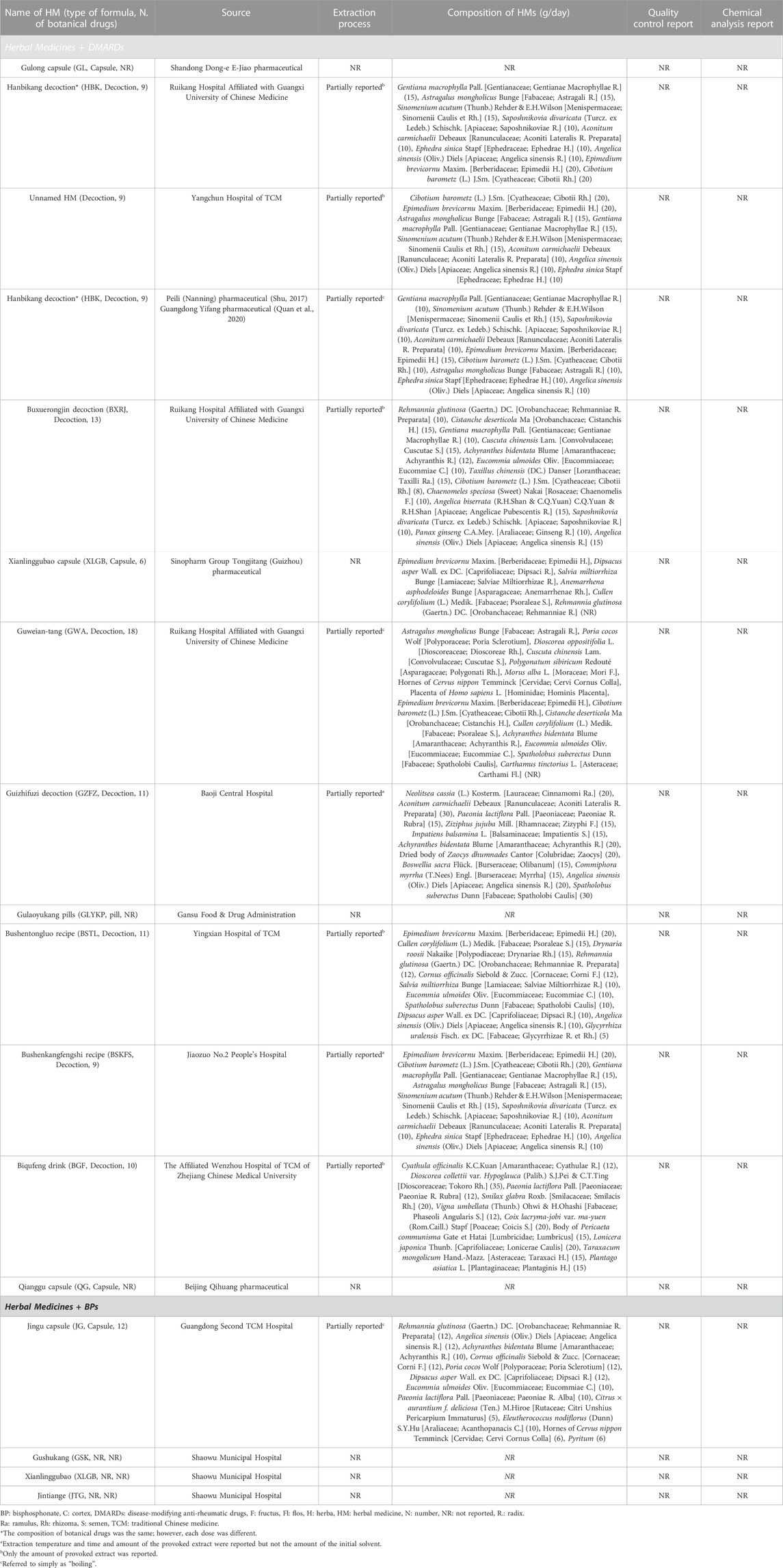
Two independent reviewers (DK and JG) checked the title and abstract of the searched articles and primarily screened them according to the inclusion and exclusion criteria. After obtaining the full text of RCTs, the full text of the first selected studies was rechecked and the studies to be analyzed were finally determined. In cases of disagreement between the two reviewers, the corresponding studies were reviewed together until agreement was reached. In cases where agreement was not reached, the opinion of a third reviewer (E-JL) was adopted. The reviewers extracted the characteristics of RCTs (characteristics of participants, such as sex and age; sample size; and duration of RA), interventions (type of HM, composition of prescription, dose, extraction process, quality control, chemical analysis, and treatment duration), and control (BMD score, bone turnover markers, inflammatory indicators, and adverse events), and performed a quality assessment of studies. When extracting data of the botanical drugs used in each HM treatment, the notation of botanic drugs was as shown in “scientific plant name” [family; synonyms]. In cases of animal-derived material, the scientific name and the used part were presented together. In addition, an analysis was performed with respect to the frequency of HMs used in RCTs.
2.4 Statistical analysis
All mean data described in this study regarding the characteristics of participants and interventions are the mean of means, which were presented as the mean and standard deviation (SD) computed by Microsoft Excel software.
2.5 Meta-analysis
In this study, a meta-analysis was conducted using BMD values as the primary outcome to draw meaningful conclusions regarding the clinical effect of HMs on BMD. These analyses were conducted in terms of comparison between the HM with WM group and the WM alone group before and after administration of HMs and WMs (before and after BMD). First, a meta-analysis was performed on the BMD data of the lumbar spine and femoral neck. In the analysis of the HM plus WM group and WM alone group, the BMDs of the lumbar spine and femoral neck showed high heterogeneity. Therefore, the included RCTs were classified based on the use of DMARDs and BPs and according to the type of drugs administered in the treatment and control groups. Subsequently, a meta-analysis was conducted to compare the final value of BMD after the intervention. We conducted a meta-analysis to confirm the effect of the prescriptions frequently used in the selected studies on the difference in BMD.
However, when subgroup analysis was performed on BMD of the lumbar spine and femoral neck of studies classified according to the type of WM used, the heterogeneity of the merged results was very high. Therefore, we conducted a meta-analysis for each type of WM and each BMD measurement site. Nevertheless, due to the high heterogeneity (Supplementary Figures S1, S2), additional sensitivity analysis was conducted, and as a result, some studies (Wang, 2018a; Wang and Liu, 2018b; Liu, 2018) were found to be the cause of heterogeneity. For the reliability of the results, we conducted a meta-analysis excluding three studies that caused heterogeneity.
In addition, for a comprehensive evaluation of the improvement of inflammatory indicators, a meta-analysis was conducted using inflammatory indicators (erythrocyte sedimentation rate [ESR] and C-reactive protein [CRP] as secondary outcomes). The validity rate, an outcome used in most selected studies, was excluded from the meta-analysis because there were differences in the standards and calculation methods used in each study.
The analysis program used was Cochrane’s Review Manager (RevMan) 5.4 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). The BMD scores, ESR, and CRP were considered continuous variables, and a meta-analysis was conducted with a 95% CI using the SD and mean of the final value. A fixed-effect (I2<50%) or a random-effect model (I2 ≥ 50%) was applied according to the I2 value obtained by Higgin’s homogeneity test.
2.6 Quality assessment
Two reviewers (DK and JG) assessed the quality of studies by a Jadad score evaluating randomization, blinding, withdrawals, and dropouts of study participants (Jadad et al., 1996). In the final selected studies, Cochrane’s Risk of bias 2 (RoB 2) (Cochrane Handbook for Systematic Reviews of Interventions 6.3) was used to assess the risk of bias (Higgins and Green, 2011).
To assess the quality of evidence of each meta-analysis result, we used the Grading of Recommendations, Assessment, Development, and Evaluations approach (Guyatt et al., 2008).
3 Results
3.1 Characteristics of the included RCTs
Of the 851 RCTs, 18 were selected according to the eligibility criteria (Figure 1). All selected studies had been published after 2015. In the 18 RCTs, the total number of participants was 1,491 (481 men and 1,010 women), with 731 in the intervention group and 760 in the control group. The average number of participants was 82.8 ± 22.6 (an average of 26.1 ± 11.7 for men and 56.1 ± 18.7 for women). The average mean age of participants presented in each study was 52.4 ± 7.4 years, and the mean RA duration was 6.8 ± 1.3 years (Table 1).

FIGURE 1. Flow chart of the study selection process. BMD: bone mineral density, RA: rheumatoid arthritis, RCT: randomized controlled trial.
All intervention groups received combined treatment with HM and WM, whereas the control groups received WM alone. The prescribed WM was classified into 16 DMARDs (seven RCTs with DMARDs and nine RCTs with DMARDs plus supplements), and two bisphosphonates (BPs) (one RCT with alendronate sodium, ibandronate sodium and one RCT with pamidronate disodium plus supplements). The average duration of treatment was 4.2 ± 2.5 months (Table 1).
The mean initial BMD value of 15 studies, excluding three studies without initial BMD records, was 0.766 ± 0.104 g/cm2 in the lumbar spine; 0.717 ± 0.086 g/cm2 in the femoral neck in the experimental group and 0.752 ± 0.708 g/cm2 in the lumbar spine; 0.714 ± 0.087 g/cm2 in the femoral neck in the control group. No significant difference was observed between the groups in each study (p > 0.05) (Table 1).
All RCTs used BMD as an outcome measurement; therefore, we set BMD as the primary outcome. In addition to BMD, bone markers and inflammatory indicators were used as evaluation indicators in the selected RCTs. Bone markers included one bone resorption marker (C-telopeptide of type 1 collagen [CTX-I] in three studies), three bone formation markers (alkaline phosphatase [ALP] in three studies, bone alkaline phosphatase [BALP] in eight studies, and bone gamma-carboxyglutamic acid protein [BGP] in four studies), five mineral composition and metabolic markers (calcium in nine studies, phosphorus in four studies, 25-hydroxyvitamin D [25-OHD] in two studies, urinary calcium/creatinine [Ca/Cr] in three studies, and parathyroid hormone [PTH] in three studies), and three cytokines (receptor activator of nuclear factor κB ligand [RANKL] in one study, osteoprotegerin [OPG] in two studies, and RANKL/OPG in one study). As inflammatory indicators, anti-cyclic citrullinated peptide [anti-CCP], rheumatoid factor [RF], ESR, and CRP were used in three, four, eight, and five studies, respectively (Figure 8).
3.2 Analysis of HMs used in the RCTs
A total of 17 types of HMs were used in the 18 RCTs, all of which were multi-HMs. The formulations of the 17 HM were capsules in four, decoctions in nine, pill in one, and unknown in three RCTs. Except for one RCT that selected three HMs, other RCTs applied one treatment (one HM). Six studies used the same prescription name and composition: Hanbikang-tang (HBK) in three studies (Pang et al., 2015; Shu, 2017; Quan et al., 2020) and Xianlinggubao-capsule (XLGB) in three studies. Of the three studies that used HBK, two (Shu, 2017; Quan et al., 2020) used the same drug dose but one (Pang et al., 2015) used a different dose. The XLGB used in three studies (Luo et al., 2018; Zhou, 2019; Zhu and He, 2019) had the same China Food and Drug Administration registration number; therefore, they were considered the same prescription. However, the dose of each botanical drug was unknown (Table 2).
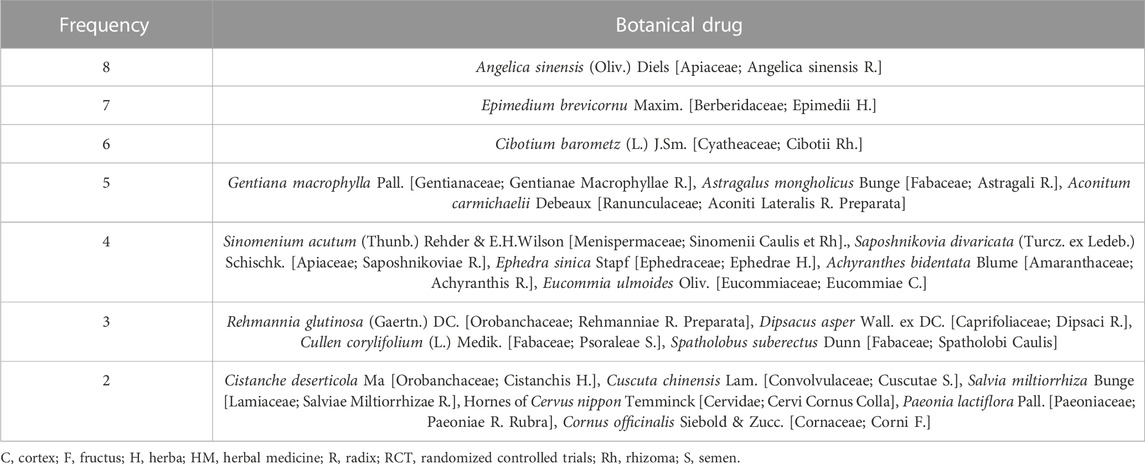
In total, 54 botanical drugs were used in the 18 RCTs, including four animal-derived, 49 plant-derived, and one mineral-derived compositions. Angelica sinensis (Oliv.) Diels [Apiaceae; Angelica Sinensis Radix] was used the most in eight HMs, followed by Epimedium brevicornu Maxim. [Berberidaceae; Epimedii Herba] (in seven HMs); Cibotium barometz (L.) J.Sm. [Cyatheaceae; Cibotii Rhizoma] (in six HMs); Gentiana macrophylla Pall. [Gentianaceae; Gentianae Macrophyllae Radix], Astragalus mongholicus Bunge [Fabaceae; Astragali Radix], and Aconitum carmichaelii Debeaux [Ranunculaceae; Aconiti Lateralis Radix Preparata] (each in five HMs); Sinomenium acutum (Thunb.) Rehder & E.H.Wilson [Menispermaceae; Sinomenii Caulis et Rhizoma], Saposhnikovia divaricata (Turcz. ex Ledeb.) Schischk. [Apiaceae; Saposhnikoviae Radix], Ephedra sinica Stapf [Ephedraceae; Ephedrae Herba], Achyranthis Radix, and Eucommia ulmoides Oliv. [Eucommiaceae; Eucommiae Cortex] (each in four HMs); Rehmannia glutinosa (Gaertn.) DC. [Orobanchaceae; Rehmanniae Radix Preparata], Dipsacus asper Wall. ex DC. [Caprifoliaceae; Dipsaci Radix], Cullen corylifolium (L.) Medik. [Fabaceae; Psoraleae Semen], and Spatholobus suberectus Dunn [Fabaceae; Spatholobi Caulis] (each in three HMs); and Cistanche deserticola Ma [Orobanchaceae; Cistanchis Herba], Cuscuta chinensis Lam. [Convolvulaceae; Cuscutae Semen], Salvia miltiorrhiza Bunge [Lamiaceae; Salviae Miltiorrhizae Radix], Hornes of Cervus nippon Temminck [Cervidae; Cervi Cornus Colla], Paeonia lactiflora Pall. [Paeoniaceae; Paeoniae Radix Rubra], and Cornus officinalis Siebold & Zucc. [Cornaceae; Corni Fructus] (each in two HMs) (Tables 2, 3).
3.3 Meta-analysis of changes in BMD
3.3.1 HM plus DMARDs vs. DMARDs alone
When a meta-analysis was conducted after excluding three studies (Wang, 2018a; Wang and Liu, 2018b; Liu, 2018) that caused heterogeneity, in 13 RCTs that used DMARDs, the HM plus DMARDs group showed a more significant synergistic effect than the DMARDs alone group on the BMD of each site. Compared to that in the DMARDs alone group, BMD improved by 0.04 g/cm2 (95% CI: 0.03–0.05; p < 0.00001; I2 = 19%) in the lumbar spine and 0.03 g/cm2 (95% CI: 0.02–0.03; p < 0.00001; I2 = 0%) in the femoral neck (Figures 2, 3). When treated in parallel with DMARDs and HM, BMD increased by 0.08 g/cm2 (95% CI: 0.05–0.12; p < 0.00001; I2 = 80%) in the lumbar spine and 0.06 g/cm2 (95% CI: 0.03–0.08; p < 0.00001; I2 = 78%) in the femoral neck compared to the corresponding values before treatment (Supplementary Figure S3).
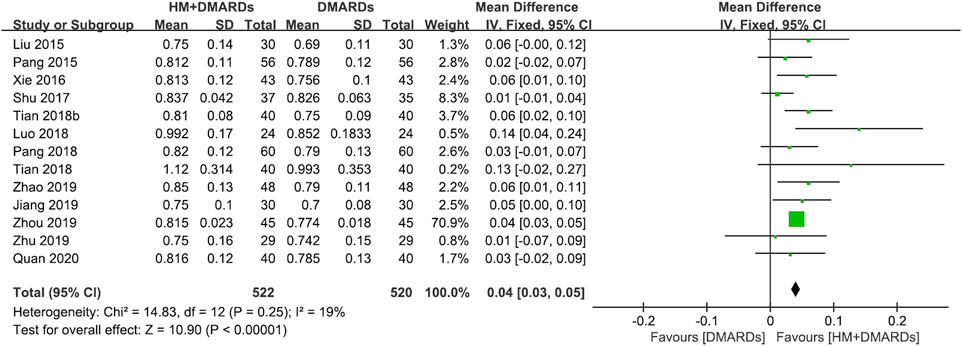
FIGURE 2. Comparison of BMD at the lumbar spine between HM plus DMARDs vs. DMARDs. CI: confidence interval, DMARDs: disease-modifying anti-rheumatic drugs, HM: herbal medicine, SD: standard deviation.
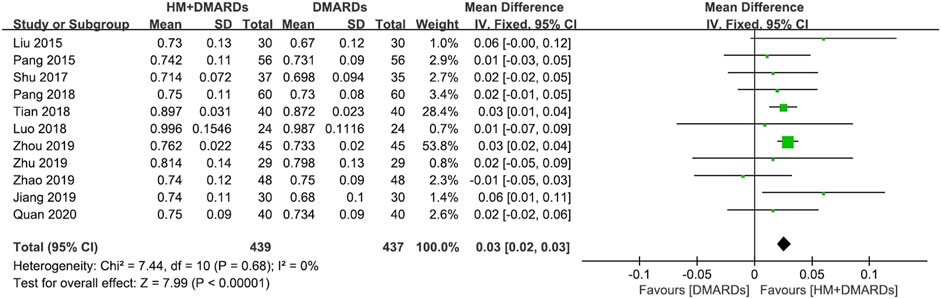
FIGURE 3. Comparison of BMD at the femoral neck between HM plus DMARDs vs. DMARDs. CI: confidence interval, DMARDs: disease-modifying anti-rheumatic drugs, HM: herbal medicine, SD: standard deviation.
3.3.2 HM plus BPs vs. BPs alone
In the two RCTs that used BPs, the BMD of both the lumbar spine and femoral neck increased significantly in the HM plus BPs group compared to that in BPs alone group. In each analysis according to the measurement site, a significant difference in BMD improvement was observed compared to the control group in both sites, with 0.13 g/cm2 (95% CI: 0.12–0.15; p < 0.00001; I2 = 0%) in the lumbar spine and 0.17 g/cm2 (95% CI: 0.05–0.30; p = 0.006; I2 = 97%) in the femoral neck (Figures 4, 5). When HM was used in parallel with BPs in the treatment, BMD increased by 0.19 g/cm2 (95% CI: 0.17–0.20; p < 0.00001; I2 = 0%) in the lumbar spine and 0.24 g/cm2 (95% CI: 0.20–0.29; p < 0.00001; I2 = 89%) in the femoral neck compared to before treatment (Supplementary Figure S3).
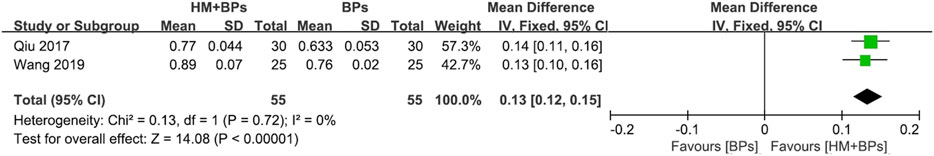
FIGURE 4. Comparison of BMD at the lumbar spine between HM plus BPs vs. BPs. BPs: bisphosphonates, CI: confidence interval, HM: herbal medicine, SD: standard deviation.
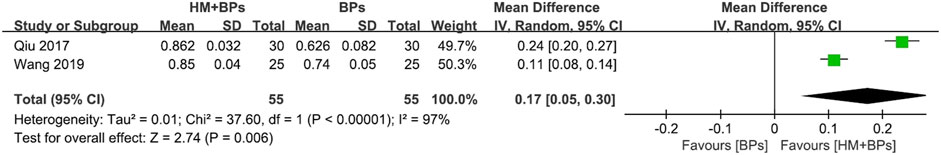
FIGURE 5. Comparison of BMD at the femoral neck between HM plus BPs vs. BPs. BPs: bisphosphonates, CI: confidence interval, HM: herbal medicine, SD: standard deviation.
Comparing mean differences in BMD according to the type of co-intervention, the mean difference was significantly greater in both lumbar spine and femoral neck in the HM plus BPs group than in the HM plus DMARDs group (Figure 6A).
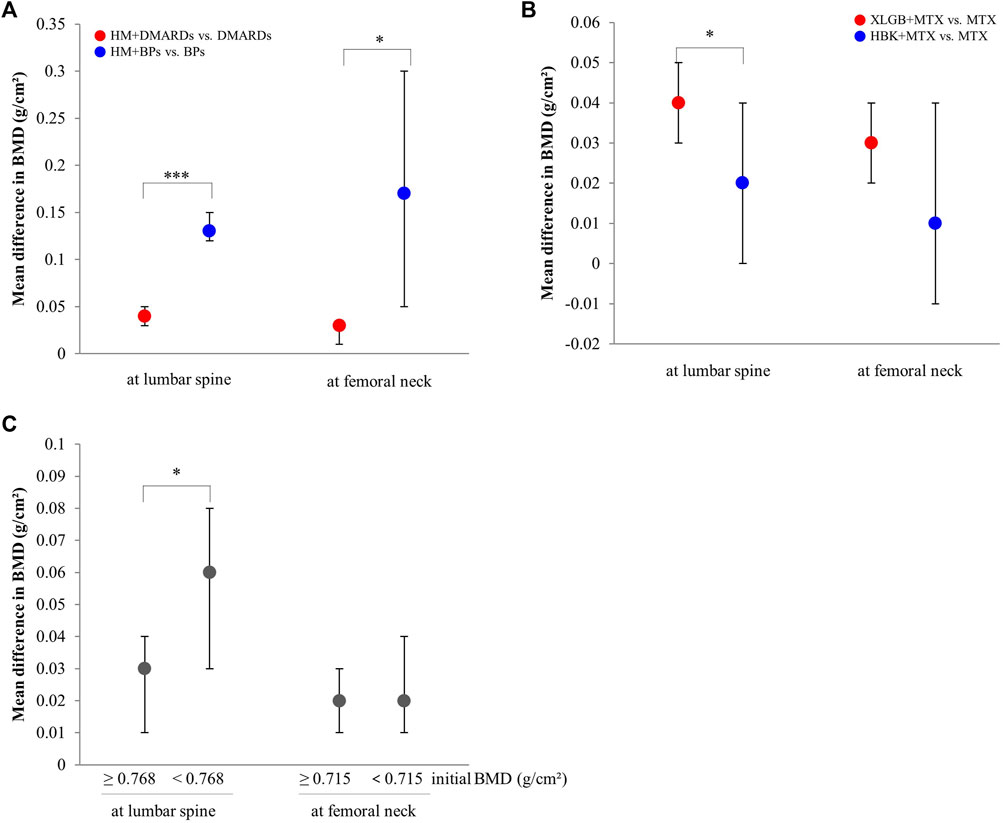
FIGURE 6. Comparison of the mean difference in BMD by subgroups. (A) By the type of combined western medicine. (B) By the prescriptions frequently used. (C) By the initial BMD scores. BMD: bone mineral density, BPs: bisphosphonates, DMARDs: disease-modifying anti-rheumatic drugs, HBK: Hanbikang-decoction, HM: herbal medicine, MTX: methotrexate, XLGB: Xianlinggubao-capsule. *: The p-value of subgroup differences was <0.05. ***: The p-value of subgroup differences was <0.0005.
3.3.3 Changes in BMD according to frequently prescribed HMs
The frequently used prescriptions in the selected studies were XLGB (Luo et al., 2018; Zhou, 2019; Zhu and He, 2019) and HBK (Pang et al., 2015; Shu, 2017; Quan et al., 2020), which were used in three studies each. All six studies that used XLGB or HBK used MTX as the main Western treatment.
A meta-analysis of BMD after treatment showed that the group treated with XLGB and MTX, or other DMARDs containing MTX, improved BMD by 0.04 g/cm2 (95% CI: 0.03–0.05; p < 0.00001; I2 = 55%) in the lumbar spine and by 0.03 g/cm2 (95% CI: 0.02–0.04; p < 0.00001; I2 = 0%) in the femoral neck. This was higher compared to the DMARDs alone treatment group containing MTX (Figure 7A). At this time, as the heterogeneity was >55%, we conducted a sensitivity analysis, and identified a specific study to be the cause of heterogeneity (Luo et al., 2018). However, the mean difference (MD) and 95% CI was the same before and after excluding the study, therefore, it was retained. In the group treated with HBK and MTX, or other DMARDs, including MTX, BMD was found to be higher after treatment by 0.02 g/cm2 (95% CI: −0.00–0.04) in the lumbar spine and by 0.01 g/cm2 (95% CI: −0.01–0.04) in the femoral neck, higher than that observed in the DMARDs only treatment group, including MTX (Figure 7B). However, as the p-values were p = 0.11 in the lumbar spine and p = 0.21 in the femoral neck, there was no significant difference from the BMD after treatment of the MTX alone treatment group.
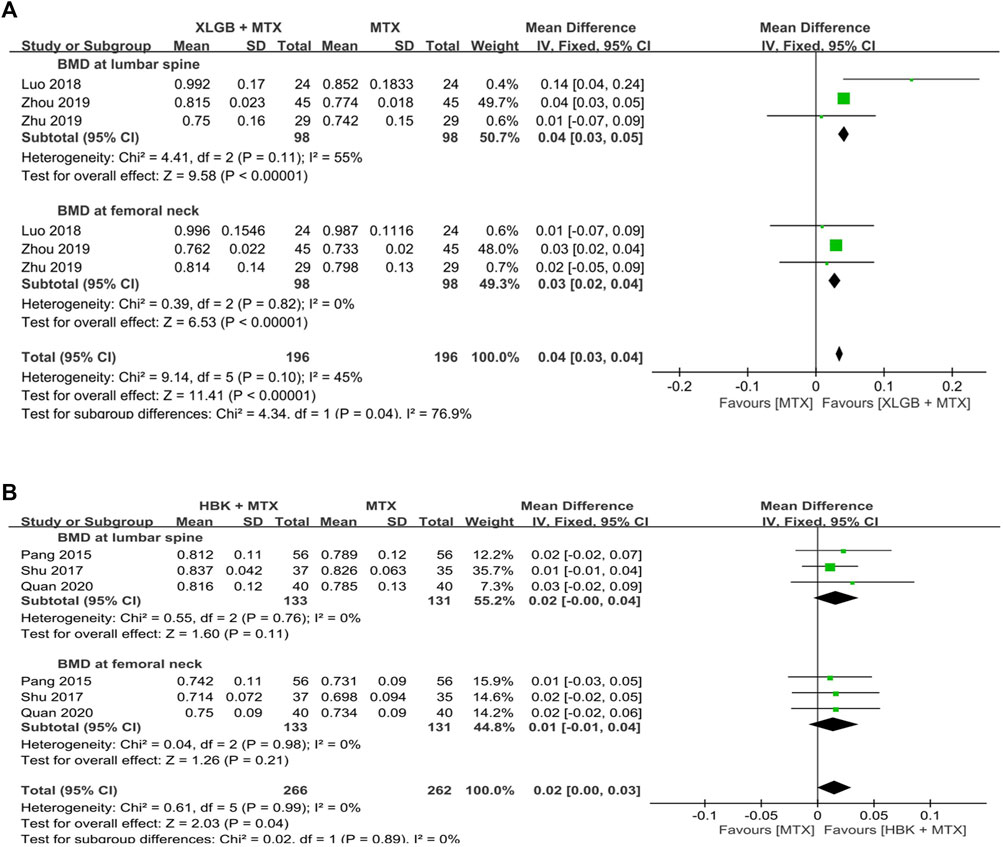
FIGURE 7. Comparison of BMD between the frequently used HM plus MTX group and the MTX group. (A) The XLGB + MTX group vs. the MTX group. (B) The HBK + MTX group vs. the MTX group. BMD: bone mineral density, CI: confidence interval, HBK: Hanbikang-decoction, HM: herbal medicine, MTX: methotrexate, SD: standard deviation, XLGB: Xianlinggubao-capsule.
When comparing the two prescriptions, the combined treatment group with XLGB and MTX had a greater BMD difference compared to the control group in the lumbar spine than that observed in the combined treatment group that used HBK and MTX (Figure 6B).
3.3.4 Changes in BMD scores by initial BMD
A meta-analysis was conducted to compare the improvement of BMD between the HM plus WM and WM alone groups. The median value of the initial BMD was measured in the lumbar spine and later in the femoral neck. The group with relatively high and low initial BMD was classified based on the median value. In this analysis, two RCTs that used BPs as intervention (Qiu et al., 2017; Wang, 2019) were excluded because the change in BMD before and after treatment was significantly greater than that observed in studies that used WM as intervention, and both studies involved the group with low initial BMD values, which could excessively affect heterogeneity and results.
In the lumbar spine (median, 0.768 g/cm2), the effect of BMD improvement was greater in the HM plus DMARDs subgroup with low initial BMD than in the DMARDs alone group. Conversely, in the femoral neck (median, 0.715 g/cm2), there was no significant difference in BMD improvement between the group with relatively high initial BMD value and that with low initial BMD value (Figure 6C).
3.4 Changes in bone markers and inflammatory indicators, including BMD
All three studies that used CTX-I as an evaluation index showed a significant reduction effect compared to that in the control group after treatment.
With respect to the bone formation markers, all four studies used BGP as a measurement and reported a significant increase compared to the control group after treatment. Two of the three studies that evaluated ALP showed a significant improvement effect compared to the control group after treatment. However, in one study (Tian et al., 2018a), it was unknown whether there was a significant difference from the control group. All eight studies that evaluated BALP showed significant differences compared to the control group after treatment.
Among the mineral composition and metabolic markers, all studies that used 25-OHD, a urinary Ca/Cr, and PTH as outcome measurements showed significant improvement compared with the control group before and after treatment. Ca increased significantly in seven studies except for one (Tian et al., 2018b) compared to the control group after treatment. However, no significant or unknown effect was observed in two of four studies that used P as evaluation indicators. RANKL, OPG, and RANKL/OPG were used as outcome indices in two of 18 studies and showed significant improvement effects.
The results showed that anti-CCP decreased significantly in all three studies, while RF also decreased significantly in all four studies that evaluated these indicators. In addition, all eight studies that evaluated ESR showed a distinct reduction effect compared to the control group, except for one study, in which the effect of the comparison with the control group was unknown. CRP also decreased significantly in all but one of five studies that evaluated it (Figure 8).

FIGURE 8. Summary of BMD values and bone marker outcomes in RCTs. 25-OHD: 25-hydroxyvitamin D, ALP: alkaline phosphatase, Anti-CCP: anti-cyclic citullinated peptide, BALP: bone alkaline phosphatase, Ca: calcium, Cr: creatinine, CRP: C-reactive protein, CTX: C-telopeptide of type 1 collagen, ESR: erythrocyte sedimentation rate, OPG: osteoprotegerin, P: phosphorus, PTH: parathyroid hormone, RANKL: receptor activator of nuclear factors κB ligand, RCT, randomized clinical trial, RF, rheumatoid factor.
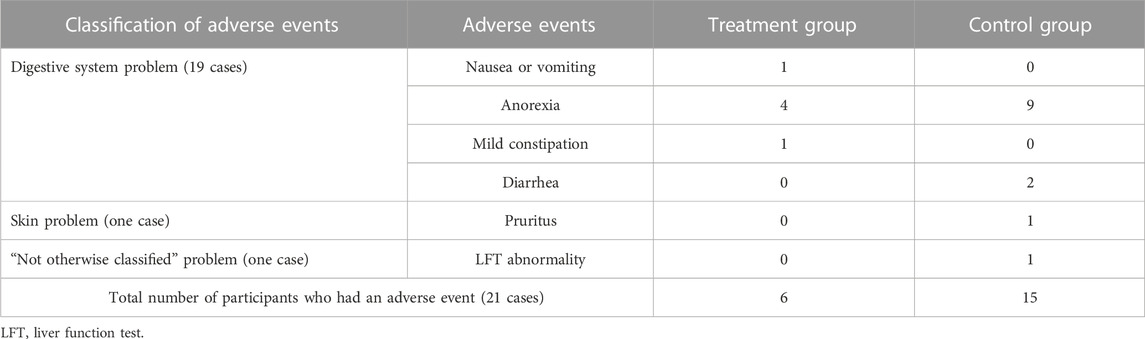
3.5 Adverse events
Only three of the 18 RCTs mentioned adverse events (Qiu et al., 2017; Shu, 2017; Liu, 2018). Of these, two (Shu, 2017; Liu, 2018) reported side effects, whereas one (Qiu et al., 2017) reported no side effects. The types of adverse events reported were 19 cases of digestive system problems, such as nausea, anorexia, vomiting, mild constipation, and diarrhea; one case of skin problems, such as pruritus; and one case of liver function test abnormality. Nineteen digestive-related symptoms occurred in six cases in the experimental group and in 13 cases in the control group. The most frequently reported adverse event was anorexia. In addition, both skin-related symptoms and liver function test abnormality occurred in the control group (Table 4).
All adverse events shown in both studies were not severe enough to affect the study. Therefore, the study was resumed after confirming that the patients’ condition was improved after symptomatic treatment.
3.6 Risk of bias
As there were more than 10 selected studies, we plotted a funnel plot with the X-axis set to the effect size and the Y-axis set to the standard error. Although there were a few outliers, the left and right sides of the funnel plot were generally symmetrical (Supplementary Figure S4). However, compared to other studies, there were studies with relatively small sample sizes; thus, it is estimated that there is some visual asymmetry.
The risk of bias of each study was assessed and is presented in Supplementary Figures S5, S6. There were two studies reporting the specific randomization process and allocation concealment for participants (Shu, 2017; Liu, 2018), whereas other studies did not mention the randomization method or allocation concealment. None of the selected studies set placebo or sham intervention. There was no risk of missing outcome data because some studies produced dropouts (Shu, 2017; Liu, 2018), whereas the other studies showed no dropouts. In most studies, it was not clear whether participants were blinded for their intervention, which is a concern because it could affect the outcome values of symptom-related indicators. However, four studies (Tian et al., 2018a; Luo et al., 2018; Pang et al., 2018; Wang, 2019) were evaluated as low risk because they used only outcome values that intervention blinding could not affect the results. As all studies did not have a protocol or were not accessible, selection of the reported result was evaluated as some concerns.
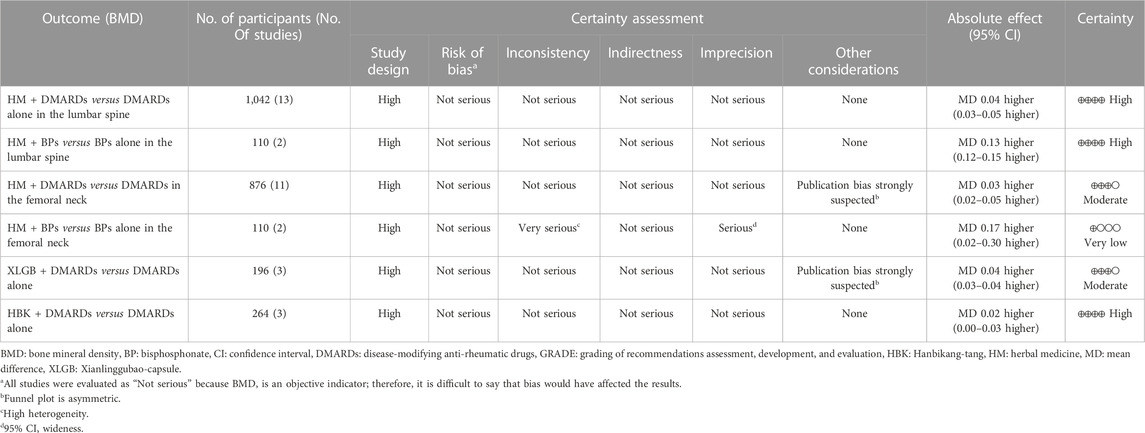
3.7 Certainty of evidence
All the included studies were RCTs, therefore the study design was evaluated as “high.” Most of the meta-analysis using BMD as an evaluation index was judged as high certainty, whereas some analysis was suspected to have publication bias owing to the asymmetry of the funnel plot. Therefore, the certainty of evidence was “moderate.” The comparison of femoral neck BMD after treatment in the group treated with HM and BPs and only BPs was judged to have very low certainty because of its serious inconsistency and imprecision due to very high heterogeneity (I2 = 97%) and very wide 95% CI (0.05–0.30) (Table 5).
4 Discussion
Osteoporosis in patients with RA remains a medical challenge that needs to be overcome despite advances in research on the mechanism of RA and drug treatment (Herrera et al., 2015). Several previous studies have reported the efficacy of HMs, including multi-target and multi-compound products, that can promote bone formation, inhibit bone resorption, and reduce ongoing inflammation (Daily et al., 2017; He et al., 2017; Gong et al., 2021; Hong and Lee, 2021; Wang et al., 2021; Han et al., 2022). To evaluate these potential effects of HM as a new alternative treatment for bone loss in patients with RA, a systematic review and a meta-analysis was comprehensively conducted on 15 RCTs. Although 18 RCTs met the eligibility criteria, the heterogeneity was very high when the first meta-analysis was conducted (Supplementary Figures S1, S2), and three studies (Wang, 2018a; Wang and Liu, 2018b; Liu, 2018) were identified to be the cause of increased heterogeneity through sensitivity analysis. Among them, in Liu. (2018) the initial BMD was too high compared to that of other studies, and it is controversial whether the participants were selected well. In the study by Wang. (2018a), the calculated SD was lower than the corresponding value in other studies, which is assumed to have affected heterogeneity. Therefore, a meta-analysis was conducted with 15 RCTs, excluding the three studies that affected heterogeneity. Our data showed that patients with RA having osteoporosis had a higher BMD when administered additional HM compared to using WM alone (DMARDs or BPs). Particularly, when HM was added to DMARDs for approximately 4.2 ± 2.5 months, approximately 9.8% improvement in BMD (improvement by 0.08 g/cm2 in the lumbar spine and 0.06 g/cm2 in the femoral neck) was observed compared to before treatment, whereas there was an improvement of 0.04 g/cm2 in the lumbar spine and 0.03 g/cm2 in the femoral neck than when using DMARDs alone (Figures 2, 3). When HM was added to BPs, there was an approximately 34.3% improvement in BMD (approximately 0.19 g/cm2 in the lumbar spine and 0.24 g/cm2 in the femoral neck) compared to before treatment and an improvement of 0.13 g/cm2 in the lumbar spine and 0.15 g/cm2 in the femoral neck than when using BPs alone (Figures 4, 5). When HM and WM were combined, the HM plus DMARDs group increased BMD by 0.08 g/cm2 in the lumbar spine and by 0.06 g/cm2 in the femoral neck than before treatment (Supplementary Figure S3). In general, adult women and men have a peak BMD at the age of 30–39 and 20–29 years, respectively, which gradually decreases with age. On average, men and women in their 50s–60 s reduce BMD by 0.061 g/cm2 in the lumbar spine and 0.066 g/cm2 in the femoral neck (Zhang et al., 2014). Therefore, the increase in BMD by 0.06 g/cm2 by the combination of HM and DMARD for approximately 4.2 months clinically means that bone health improved from 60 to 50 s younger.
In particular, in the HM plus DMARDs group, a lower initial BMD value of the lumbar spine resulted in a greater BMD difference in the DMARDs alone group after treatment (Figure 6C). However, the meta-analysis comparing BMD in the femoral neck after treatment in the HM plus BPs versus BPs alone group not only had very high heterogeneity of I2 = 97% but also had a “very low” certainty of evidence (Figure 5; Table 5). Therefore, it is controversial to judge the synergetic effect of HM plus BPs on the femoral neck in this study.
This requires caution in interpreting the results, given the low quality of the included RCTs (average Jadad score of 2.5) and the high RoB. In particular, all studies were evaluated as having “some concerns” in RoB 2 (Supplementary Figures S5, S6), because most studies did not mention the specific randomization process, allocation concealment, and blinding using placebo. This can be considered as indicating the low quality of the selected studies; however, the BMD, set as the primary outcome in this study, is an objective indicator in which bias cannot occur by knowing whether the participants took HM. Therefore, it was assumed that it would not have affected the results of the study.
The short duration of treatment (approximately 4.2 months) in the RCTs included in this review and previous studies showing an average increase in BMD of 3.1% when BP was used for 12 months in a population with an average age of 63–78 years (Lyu et al., 2019) may suggest that adding HM to the primary treatment of patients with RA has sufficient potential efficacy for bone loss.
Of the 1,491 participants included in this study, 481 were men and 1,010 women, approximately twice as many women as men. The average age of the study participants was 52.4 ± 7.4 years, and the mean morbidity due to RA was 6.8 ± 1.3 years. Participants’ characteristics were consistent with those of previous studies showing that women had a higher incidence of osteoporosis among patients with RA, especially postmenopausal women (Hauser et al., 2014; Moshayedi et al., 2022). The mean initial BMD of the participants was 0.753 ± 0.109 g/cm2 in the lumbar spine and 0.715 ± 0.087 g/cm2 in the femoral neck. Compared to an observational study conducted in South China, in which the mean BMD of 405 participants of the same age group with an RA disease duration of 5.5 years was 0.833 ± 0.149 g/cm2 in the lumbar spine and 0.667 ± 0.130 g/cm2 in the femoral neck (Hu et al., 2021), the initial BMD value of the participants included in our review was found to be lower by 10.6% in the lumbar spine and higher by 6.7% in the femoral neck.
The effect of BMD improvement in the combination treatment group of HM plus WM was also confirmed in the bone markers and inflammation-related indicators identified in each study. Bone resorption markers (CTX-I), bone formation markers (ALP, BALP, and BGP), minor composition and metabolic markers (Ca, P, 25-OHD, urinary Ca/Cr, and PTH), cytokines (RANKL, OPG, and RANKP/OPG), and inflammatory indicators (anti-CCP, RF, ESR, and CRP) were all significantly improved compared to using WM alone in 14 RCTs. In particular, in a meta-analysis of ESR and CRP changes, ESR and CRP decreased significantly by −8.25 mm/h and −3.48 mg/L on average, respectively, compared to the corresponding values in the WM group (Supplementary Figures S7A, B). This was interpreted as the decrease in the inflammatory response being related to the increase in BMD. Evaluating indicators, such as 25-OHD, urinary Ca/Cr, RANKL, OPG, and RANKL/OPG, was considered to have limitations in interpreting positive results because the number of studies using them as evaluation indicators was small, even if each study reported improvement.
Of these, Guizhifuzi-tang was used by Tian et al. (2018a) and presented had the most significant CRP reduction effect. Moreover, Spatholobus suberectus Dunn [Fabaceae; Spatholobi Caulis] was the primary botanical drug used in the highest dose. Spatholobus suberectus Dunn is known to reduce the influx of inflammatory cells into the vein wall where thrombosis is formed and the serum level of inflammatory cells, inflammatory cytokines, and CRP increase; therefore, it is believed to be related to the results of this study (Tang et al., 2020). Gentiana macrophylla Pall. and E. brevicornu Maxim. Were the most commonly used botanical drugs in the prescriptions of RCTs that had a reducing effect on ESR. Iridoid, a herbal ingredient of G. macrophylla Pall., and Quercetin and Icariin, ingredients of E. brevicornu Maxim., are known to have anti-inflammatory effects by inhibiting the expression of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) in macrophages (Liu et al., 2021; Zhang et al., 2022). Therefore, it is presumed that this mechanism influenced the results of this study.
In most studies, other RA-related symptom indicators, such as pain, swelling, stiffness, and function of the joint, had significant effects compared with those observed in the treatment group before intervention and in the control group after treatment. However, most studies did not specify the measurement method of the symptom index, and even if it was specified, the measurement method used for each study was different; hence, attention should be paid to the interpretation of the results.
The included RCTs used a total of 17 kinds of HMs, and the most frequently used prescriptions were HBK (Pang et al., 2015; Shu, 2017; Quan et al., 2020) and XLGB (Luo et al., 2018; Zhou, 2019; Zhu and He, 2019), used each in three studies. HBK is presumed to alleviate RA activity by reducing the levels of inflammatory cytokines, especially tumor necrosis factor (TNF)-α and interleukin (IL)-1β (Pang et al., 2012). Conversely, XLGB is a commonly used prescription for osteoporosis, as it has already been proven to have anti-osteoporotic effects in previous studies (Hu and Cheng, 2000; Zhu et al., 2012; Wang et al., 2015). In particular, it is believed to exert its anti-osteoporotic effect through processes, such as inhibiting reactive oxygen species, promoting the organonitrogen compound response, and cell migration (Bao et al., 2020). Therefore, our results of using these two prescriptions were consistent with those of previous studies. However, the prescriptions used in the other 12 studies differed in name, composition, and capacity. This can act increase the heterogeneity among studies. However, the heterogeneity was somewhat higher in the meta-analysis comparing XLGB and DMARDs group with the DMARDs alone group. Therefore, we conducted a sensitivity analysis and the study by Luo et al. (2018) was found to be the cause of heterogeneity. However, as the MD and 95% CI before and after excluding the study were same, it was retained. However, all studies did not mention other factors that could affect bone density (e.g., smoking, drinking, exercise, diet, sleep status, etc.). Although it was reported that there was no significant difference in general characteristics between the experimental group and the control group, it was not known whether this meant other factors than age, sex ratio, and duration of RA, so attention should be paid to the interpretation of the results.
The most frequently used botanical drugs in the RCTs were the following six: A. sinensis (Oliv.) Diels [Apiaceae; Angelica Sinensis Radix], E. brevicornu Maxim. [Berberidaceae; Epimedii Herba], C. barometz (L.). J.Sm. [Cyatheaceae; Cibotii Rhizoma], G. macrophylla Pall. [Gentianaceae; Gentianae Macrophyllae Radix], A. mongholicus Bunge [Fabaceae; Astragali Radix], and A. carmichaelii Debeaux [Ranunculaceae; Aconiti Lateralis Radix Preparata] (Table 3). These botanical drugs have been reported to have anti-osteoporotic effects. Angelica sinensis (Oliv.) Diels has been reported to have anti-osteoporotic effects by reducing CTX-I and osteocalcin (Lim and Kim, 2014), whereas C. barometz (L.) J.Sm. Was reported to have anti-osteoporotic effects by reducing RANKL (Kim et al., 2007). In particular, G. macrophylla Pall. and E. brevicornu Maxim. Have been reported to have excellent anti-osteoporosis and anti-inflammatory effects. Gentiana macrophylla Pall. Inhibits the production of inflammatory cytokines, nitric oxide, and COX-2 (Wang et al., 2013), and reduces the differentiation of RANKL-induced osteoclasts and the expression of Nucleic Factor Kappa B (Yang et al., 2020). Moreover, Icarin, the main ingredient of E. brevicornu Maxim., has been reported to reduce COX and inflammatory mediators, such as COX-2 and iNOS, as well as IL-1, IL-6, and TNF-α (Zhang et al., 2022). Therefore, these botanical drugs may be considered for clinical treatment of secondary osteoporosis in patients with RA in the future.
Of the 21 adverse events reported in three of the 18 studies, 90% involved the gastrointestinal system. Of all side effects, 72.4% were found in the control group that only used WM. Therefore, it is worth researching whether adding HMs to WM can attenuate the side effects of DMARDs, including nausea, vomiting, blood problems, muscle pain, and liver problems (Farzaei et al., 2016).
This study had some critical limitations, such as high heterogeneity in some analysis, high bias (i.e., rare blinding and publication), and insufficient reports on the manufacturing process of the HMs used. Especially, concerning HM, all included RCTs did not report the quality control and chemical analysis of HM. Specific manufacturing processes were not specified, and some studies did not mention the dose of components or the component itself. This may mean that the HMs used were not standardized, thus, resulting in heterogeneity of the results. Because of these limitations, caution should be exercised when interpreting the results of this review conclusively. Therefore, based on our findings, further RCTs with low heterogeneity and bias should be conducted in the future aiming at examining the effect of HMs on osteoporosis in patients with RA. Moreover, a description of the extraction process of the HMs and a document on the legal basis for the collection and processing of the HMs used are also necessary. In addition to this problem, all studies should be conducted under complete control of the confounding factors that affect BMD.
Despite these limitations, this study is meaningful in that it is the first systematic review to provide evidence that BMD, inflammation levels, and bone markers are further improved when HM is combined with WM (DMARDs or BP) for treating patients with RA having osteoporosis. In the future, high-quality clinical studies designed to supplement the limitations of this study and existing studies are required.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
DK and JG searched the literature, extracted, and analyzed the data; DK, JG, MO, and E-JL participated in the discussion; DK and E-JL wrote the manuscript. E-JL supervised the whole process of this study with the initial design. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Ministry of Education, Science, and Technology (NRF-2021R1A2C2013483).
Acknowledgments
We would like to express our gratitude to Professor Son Chang-gyu, who always spares no encouragement and guidance in research activities.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1164898/full#supplementary-material
References
Bao, H., Guo, H., Feng, Z., and Li, X. (2020). Deciphering the underlying mechanism of Xianlinggubao capsule against osteoporosis by network pharmacology. BMC Complement. Med. Ther. 20, 208. doi:10.1186/s12906-020-03007-1
Choi, S. T., Kwon, S. R., Jung, J. Y., Kim, H. A., Kim, S. S., Kim, S. H., et al. (2018). Prevalence and fracture risk of osteoporosis in patients with rheumatoid arthritis: a multicenter comparative study of the FRAX and WHO criteria. J. Clin. Med. 7, 507. doi:10.3390/jcm7120507
Claes, L., Recknagel, S., and Ignatius, A. (2012). Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol. 8, 133–143. doi:10.1038/nrrheum.2012.1
Daily, J. W., Zhang, T., Cao, S., and Park, S. (2017). Efficacy and safety of guizhi-shaoyao-zhimu decoction for treating rheumatoid arthritis: a systematic review and meta-analysis of randomized clinical trials. J. Altern. Complement. Med. 23, 756–770. doi:10.1089/acm.2017.0098
Farzaei, M. H., Farzaei, F., Abdollahi, M., Abdollahi, M., Abbasabadi, Z., Abdolghaffari, A. H., et al. (2016). A mechanistic review on medicinal plants used for rheumatoid arthritis in traditional Persian medicine. J. Pharm. Pharmacol. 68, 1233–1248. doi:10.1111/jphp.12606
Gong, X., Liu, W. X., Tang, X. P., Wang, J., Liu, J., Huang, Q. C., et al. (2021). Traditional Chinese medicine qingre huoxue treatment vs. the combination of methotrexate and hydroxychloroquine for active rheumatoid arthritis: a multicenter, double-blind, randomized controlled trial. Front. Pharmacol. 12, 679588. doi:10.3389/fphar.2021.679588
Guyatt, G. H., Oxman, A. D., Vist, G. E., Kunz, R., Falck-Ytter, Y., Alonso-Coello, P., et al. (2008). Grade: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336, 924–926. doi:10.1136/bmj.39489.470347.AD
Han, R., Ren, H. C., Zhou, S., Gu, S., Gu, Y. Y., Sze, D. M. Y., et al. (2022). Conventional disease-modifying anti-rheumatic drugs combined with Chinese herbal medicines for rheumatoid arthritis: a systematic review and meta-analysis. J. Tradit. Complement. Med. 12, 437–446. doi:10.1016/j.jtcme.2022.01.005
Haugeberg, G., Uhlig, T., Falch, J. A., Halse, J. I., and Kvien, T. K. (2000a). Bone mineral density and frequency of osteoporosis in female patients with rheumatoid arthritis: results from 394 patients in the oslo county rheumatoid arthritis register. Arthritis Rheum. 43, 522–530. doi:10.1002/1529-0131(200003)43:3<522::AID-ANR7>3.0
Haugeberg, G., Uhlig, T., Falch, J. A., Halse, J. I., and Kvien, T. K. (2000b). Reduced bone mineral density in male rheumatoid arthritis patients: frequencies and associations with demographic and disease variables in ninety-four patients in the oslo county rheumatoid arthritis register. Arthritis Rheum. 43, 2776–2784. doi:10.1002/1529-0131(200012)43:12<2776::AID-ANR18>3.0
Hauser, B., Riches, P. L., Wilson, J. F., Horne, A. E., and Ralston, S. H. (2014). Prevalence and clinical prediction of osteoporosis in a contemporary cohort of patients with rheumatoid arthritis. Rheumatol. Oxf. 53, 1759–1766. doi:10.1093/rheumatology/keu162
He, J. B., Chen, M. H., and Lin, D. K. (2017). New insights into the tonifying kidney-yin herbs and formulas for the treatment of osteoporosis. Arch. Osteoporos. 12, 14. doi:10.1007/s11657-016-0301-4
Herrera, A., Mateo, J., Gil-Albarova, J., Lobo- Escolar, A., Artigas, J. M., Lopez-Prats, F., et al. (2015). Prevalence of osteoporotic vertebral fracture in Spanish women over age 45. Maturitas 80, 288–295. doi:10.1016/j.maturitas.2014.12.004
Higgins, J. P. T., and Green, S. (2011). Cochrane Handbook for systematic reviews of interventions version 5.1.0. Avialble at; http://www.cochrane.org/training/cochrane.-
Hoes, J. N., Bultink, I. E., and Lems, W. F. (2015). Management of osteoporosis in rheumatoid arthritis patients. Expert Opin. Pharmacother. 16, 559–571. doi:10.1517/14656566.2015.997709
Hong, S. M., and Lee, E. J. (2021). Effects of herbal medicines on bone mineral density score in osteoporosis or osteopenia: study protocol for a systematic review and meta-analysis. J. Korean Med. Rehabil. 31, 49–55. doi:10.18325/jkmr.2021.31.2.49
Hu, C., and Cheng, M. (2000). Clinical observation on 30 cases of Xianlinggubao capsules in treatment of osteoporosis. Chin. Tradit. Pat. Med. 22, 246–247.
Hu, Z., Zhang, L., Lin, Z., Zhao, C., Xu, S., Lin, H., et al. (2021). Prevalence and risk factors for bone loss in rheumatoid arthritis patients from South China:modeled by three methods. BMC Musculoskelet. Disord. 22, 534. doi:10.1186/s12891-021-04403-5
Huang, M. C., Pai, F. T., Lin, C. C., Chang, C. M., Chang, H. H., Lee, Y. C., et al. (2015). Characteristics of traditional Chinese medicine use in patients with rheumatoid arthritis in taiwan: a nationwide population-based study. J. Ethnopharmacol. 176, 9–16. doi:10.1016/j.jep.2015.10.024
Jadad, A. R., Moore, R. A., Carroll, D., Jenkinson, C., Reynolds, D. J., Gavaghan, D. J., et al. (1996). Assessing the quality of reports of randomized clinical trials: is blinding necessary. Control Clin. Trials. 17, 1–12. doi:10.1016/0197-2456(95)00134-4
Jiang, Y., Lin, Y. Q., Wang, C. C., et al. (2019). Clinical study of Xie Qifeng Yin combined with methotrexate in patients with rheumatoid arthritis and osteoporosis. Chin. Remedies Clin. 19, 3366–3368. doi:10.11655/zgywylc2019.19.049
Kim, Y. O., Kim, G. S., Lee, S. E., Seong, N. S., Cha, S. W., Jekal, S. J., et al. (2007). Effects of cibotium barometz on RANKL from collagen-induced rheumatoid arthritis Mice. J. Acupunct. Res. 24, 207–213.
Lau, C. S., Chia, F., Dans, L., Harrison, A., Hsieh, T. Y., Jain, R., et al. (2019). 2018 update of the APLAR recommendations for treatment of rheumatoid arthritis. Int. J. Rheum. Dis. 22, 357–375. doi:10.1111/1756-185X.13513
Li, H. H., Livneh, H., Yeh, C. C., Guo, H. R., Lai, N. S., Lu, M. C., et al. (2019). Association between use of Chinese herbal medicine and depression risk in patients with rheumatoid arthritis: a nationwide retrospective cohort study. Int. J. Rheum. Dis. 22, 986–994. doi:10.1111/1756-185X.13571
Lim, D. W., and Kim, Y. T. (2014). Anti-osteoporotic effects of Angelica sinensis (Oliv) Diels extract on ovariectomized rats and its oral toxicity in rats. Nutrients 6, 4362–4372. doi:10.3390/nu6104362
Lin, Y. J., Anzaghe, M., and Schülke, S. (2020). Update on the pathomechanism, diagnosis, and treatment options for rheumatoid arthritis. Cells 9, 880. doi:10.3390/cells9040880
Liu, H. (2015). Gu Long Capsule combined with methotrexate and thiazepine in the treatment of rheumatoid arthritis complicated osteoporosis. Hebei Med. J. 37, 2949–2951. doi:10.3969/j.issn.1002-7386.2015.19.022
Liu, H., Zhao, H., Hu, W., Hu, H., Hou, S., and Chen, G. (2021). A new strategy for the preparation of total iridoids from Radix Gentianae Macrophyllae and anti-inflammatory profile digesting by UPLC-Q-TOF-MS characterization coupled with PLS analysis. Ind. Crops Prod. 168, 113586. doi:10.1016/j.indcrop.2021.113586
Liu, S. S. (2018). The treatment method of Tonifying the liver and kidney deficiency of the liver and kidney of rheumatoid arthritis. Clinical study of secondary osteoporosis (China: Guangxi University of Traditional Chinese Medicine). [Master's Thesis].
Lodder, M. C., de Jong, Z., Kostense, P. J., Molenaar, E., Staal, K., Voskuyl, A., et al. (2004). Bone mineral density in patients with rheumatoid arthritis: relation between disease severity and low bone mineral density. Ann. Rheum. Dis. 63, 1576–1580. doi:10.1136/ard.2003.016253
Luo, X. G., Zeng, P. P., and Yan, B. (2018). Observation on treating osteoporosis after rheumatoid arthritis with the Xianling Gubao capsule. Clinical Study of Trad. Chin. Med. 10, 78–80. doi:10.3969/j.issn.1674-7860.2018.04.039
Lyu, H., Jundi, B., Xu, C., Tedeschi, S. K., Yoshida, K., Zhao, S., et al. (2019). Comparison of denosumab and bisphosphonates in patients with osteoporosis: a meta-analysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 104, 1753–1765. doi:10.1210/jc.2018-02236
Machado-Alba, J. E., Ruiz, A. F., and Machado-Duque, M. E. (2014). Adverse drug reactions associated with the use of disease-modifying anti-rheumatic drugs in patients with rheumatoid arthritis. Rev. Panam. Salud. Publica. 36, 396–401. doi:10.1016/j.jval.2015.03.891
Mazzantini, M., Di Munno, O., Incerti-Vecchi, L., and Pasero, G. (2000). Vertebral bone mineral density changes in female rheumatoid arthritis patients treated with low-dose methotrexate. Clin. Exp. Rheumatol. 18, 327–331.
Moshayedi, S., Tasorian, B., and Almasi-Hashiani, A. (2022). The prevalence of osteoporosis in rheumatoid arthritis patient: a systematic review and meta-analysis. Sci. Rep. 12, 15844. doi:10.1038/s41598-022-20016-x
Pang, X. F., Liao, J. Y., Li, Y. L., et al. (2018). Clinical observation on 60 cases osteoporosis secondary to rheumatoid arthritis treated by Guwei'an Tang combined with Western medicine. Arthritis Rheum. 7, 26–27. doi:10.3969/j.issn.2095-4174.2018.07.005
Pang, X. F., Liu, H., Wu, Y. H., et al. (2015). Clinical study on the treatment of secondary osteoporosis of rheumatoid arthritis with kidney tonifying and anti-Rheumatoid Arthritis. World J. Integr. Trad. West. Med. 10, 47–49. doi:10.13935/j.cnki.sjzx.150101
Pang, X. F., Wu, Y. H., Meng, Y. H., et al. (2012). Regulatory effect of Hanbikang granules on rheumatoid arthritis inflammatory cytokines. Proc. 8th Natl. Congr. Immunol., 587.
Qiu, J. M., Lu, C. H., and Dong, M. (2017). Clinical observation on the treatment of rheumatoid arthritis complicated osteoporosis with muscle and bone capsules. Chin. J. Trauma Disabil. Med. 25, 11–13. doi:10.13214/j.cnki.cjotadm.2017.21.007
Quan, X. L., Liu, F., Yue, F., et al. (2020). Effect of methotrexate combined with Hanbikang decoction on rheumatoid arthritis with osteoporosis and its effect on bone density and biochemical markers of bone metabolism. Mod. J. Integr. Traditional Chin. West. Med. 29, 4068–4079. doi:10.3969/j.issn.1008-8849.2020.36.019
Raterman, H. G., Bultink, I. E., and Lems, W. F. (2020). Osteoporosis in patients with rheumatoid arthritis: an update in epidemiology, pathogenesis, and fracture prevention. Expert Opin. Pharmacother. 21, 1725–1737. doi:10.1080/14656566.2020.1787381
Shu, J. L. (2017). Effect of Han Bi Kang decoction on patients with secondary osteoporosis of rheumatoid arthritis in peripheral blood RANKL/OPG (China: Guangxi University of Traditional Chinese Medicine). [Master's Thesis].
Singh, J. A., Saag, K. G., Bridges, S. L., Akl, E. A., Bannuru, R. R., Sullivan, M. C., et al. (2016). 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 68, 1–26. doi:10.1002/art.39480
Sinigaglia, L., Nervetti, A., Mela, Q., Bianchi, G., Del Puente, A., Di Munno, O., et al. (2000). A multicenter cross sectional study on bone mineral density in rheumatoid arthritis. Italian Study Group on Bone Mass in Rheumatoid Arthritis. J. Rheumatol. 27, 2582–2589.
Smolen, J. S., Landewé, R., Bijlsma, J., Burmester, G., Chatzidionysioy, K., Dougados, M., et al. (2017). EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann. Rheum. Dis. 76, 960–977. doi:10.1136/annrheumdis-2016-210715
Tang, P., Liu, H., Lin, B., Yang, W., Chen, W., Lu, Z., et al. (2020). Spatholobi Caulis dispensing granule reduces deep vein thrombus burden through antiinflammation via SIRT1 and Nrf2. Phytomedicine 77, 153285. doi:10.1016/j.phymed.2020.153285
Tian, J. X., Liu, H. T., Wang, G., et al. (2018b). Clinical observation of the efficacy of nourishing kidney and activating meridian recipe combined with methotrexate on the treatment of rheumatoid arthritis with osteoporosis. Chin. J. Osteoporos. 24, 1502–1505. doi:10.3969/j.issn.1006-7108.2018.11.022
Tian, Y. P., Chen, F. Y., Chen, T., et al. (2018a). Observation on the curative effect of Guizhi Fuzi Decoction combined with western medicine on secondary osteoporosis of rheumatoid arthritis. Mod. J. Integr. Traditional Chin. West. Med. 27, 430–432. doi:10.3969/j.issn.1008-8849.2018.04.028
van Staa, T. P., Geusens, P., Bijlsma, J. W., Leufkens, H. G. M., and Cooper, C. (2006). Clinical assessment of the long-term risk of fracture in patients with rheumatoid arthritis. Arthritis Rheum. 54, 3104–3112. doi:10.1002/art.22117
Wang, L., and Liu, Z. Y. (2018b). Effect of Chinese herbal medicine on secondary osteoporosis of rheumatoid arthritis. Clin. Res. 26, 177–178.
Wang, Q. R. (2019). Clinical observation on combined Chinese and Western medicine in treatment of secondary osteoporosis of rheumatoid arthritis. Chin. J. Clin. Ration. Drug Use. 12, 66–67. doi:10.15887/j.cnki.13-1389/r.2019.29.041
Wang, S., Xu, Y., Jiang, W., and Zhang, Y. (2013). Isolation and identification of constituents with activity of inhibiting nitric oxide production in RAW 264.7 macrophages from Gentiana triflora. Planta Med. 79, 680–686. doi:10.1055/s-0032-1328460
Wang, W. P. (2018a). Clinical observation on tonifying the kidney and dredging collaterals prescription combined with Western medicine in treating osteoporosis secondary to rheumatoid arthritis. Guangming Tradit. Chin. Med. 33, 3395–3397. doi:10.3969/j.issn.1003-8914.2018.22.048
Wang, X., He, Y., Guo, B., Tsang, M. C., Tu, F., Dai, Y., et al. (2015). In vivo screening for anti-osteoporotic fraction from extract of herbal formula Xianlinggubao in ovariectomized mice. PLoS One 10, e0118184. doi:10.1371/journal.pone.0118184
Wang, Y., Chen, S., Du, K., Liang, C., Wang, S., Boadi, E. O., et al. (2021). Traditional herbal medicine: therapeutic potential in rheumatoid arthritis. J. Ethnopharmacol. 279, 114368. doi:10.1016/j.jep.2021.114368
WHO Study Group (1994). Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ. Tech. Rep. Ser. 843, 1–129.
Wu, H., Cheng, K., Guo, Q., Yang, W., Tong, L., Wang, Y., et al. (2021). Mapping knowledge structure and themes trends of osteoporosis in rheumatoid arthritis: a bibliometric analysis. Front. Med. (Lausanne). 8, 787228. doi:10.3389/fmed.2021.787228
Xiao, P. L., Cui, A. Y., Hsu, C. J., Peng, R., Jiang, N., Xu, X. H., et al. (2022). Global, regional prevalence, and risk factors of osteoporosis according to the World health organization diagnostic criteria: a systematic review and meta-analysis. Osteoporos. Int. 33, 2137–2153. doi:10.1007/s00198-022-06454-3
Xie, J. N., and Wang, A. (2017). Clinical observation of 43 cases of osteoporosis secondary to rheumatoid arthritis treated by Chinese herbal prescription. Yunnan J. Traditional Chin. Med. 37, 58–60.
Yang, K., Kim, J. H., Kim, M. S., Ryu, G. H., Moon, J. H., Lee, H. I., et al. (2020). Gentianae Macrophyllae Radix water extract inhibits RANKL-induced osteoclastogenesis and osteoclast specific genes. Korean J. Acupunct. 37, 63–75. doi:10.14406/acu.2020.002
Zhang, L. B., Yan, Y., He, J., Wang, P. P., Chen, X., Lan, T. Y., et al. (2022). Epimedii herba: an ancient Chinese herbal medicine in the prevention and treatment of rheumatoid arthritis. Front. Chem. 10, 1023779. doi:10.3389/fchem.2022.1023779
Zhang, Z. Q., Ho, S. C., Chen, Z. Q., Zhang, C. X., and Chen, Y. M. (2014). Reference values of bone mineral density and prevalence of osteoporosis in Chinese adults. Osteoporos. Int. 25, 497–507. doi:10.1007/s00198-013-2418-2
Zhao, Y. D., and Xu, W. D. (2019). Clinical observation on Qianggu capsule combined with sulfasalazine on bone metabolism in patients with rheumatoid arthritis complicated with osteoporosis. World Chin. Med. 14, 438–441. doi:10.3969/j.issn.1673-7202.2019.02.038
Zhou, J. L. (2019). Effect of Xilinggubao capsule and methotrexate on rheumatoid arthritis secondary osteoporosis. J. Math. Med. 32, 596–597. doi:10.3969/j.issn.1004-4337.2019.04.060
Zhu, H. B., and He, Z. B. (2019). Effect of methotrexate combined with Xilinggubao capsule on secondary osteoporosis of rheumatoid arthritis. Inn. Mong. J. Traditional Chin. Med. 38, 32–33. doi:10.16040/j.cnki.cn15-1101.2019.07.022
Keywords: herbal medicine, meta-analysis, osteoporosis, rheumatoid arthritis, systematic review
Citation: Kwon DY, Gu JH, Oh M and Lee E-J (2023) Combination effects of herbal and western medicines on osteoporosis in rheumatoid arthritis: systematic review and meta-analysis. Front. Pharmacol. 14:1164898. doi: 10.3389/fphar.2023.1164898
Received: 13 February 2023; Accepted: 24 July 2023;
Published: 10 August 2023.
Edited by:
Xiaomeng You, Harvard Medical School, United StatesReviewed by:
Jing Luo, China-Japan Friendship Hospital, ChinaGuang Chen, Beijing University of Chinese Medicine, China
Copyright © 2023 Kwon, Gu, Oh and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eun-Jung Lee, anVuZ2thaG5AZGp1Lmty
†These authors have contributed equally to this work and share first authorship
 Do Young Kwon
Do Young Kwon Ji Hyang Gu
Ji Hyang Gu Minseok Oh
Minseok Oh Eun-Jung Lee
Eun-Jung Lee