- Center of Integrative Medicine, Beijing Ditan Hospital, Capital Medical University, Beijing, China
Objective: To delineate the curative effect and safety of anti-fibrosis Chinese patent medicines (CPMs) combined with ursodeoxycholic acid (UDCA) for primary biliary cholangitis (PBC).
Methods: A literature search was conducted using PubMed, Web of Science, Embase, Cochrane Library, Wanfang database, VIP database, China Biology Medicine Database, and Chinese National Knowledge Infrastructure from their inception until August 2022. Randomized controlled trials (RCTs) of the treatment of PBC with anti-fibrotic CPMs were collected. The eligibility of the publications was assessed using the Cochrane risk-of-bias tool. The evaluation indicators were the clinical efficacy rate, liver fibrosis, liver function, immune function, and symptom score. Meta-analysis and subgroup analysis were conducted to evaluate the effectiveness of anti-fibrosis CPMs. Risk ratio (RR) was used to assess dichotomous variables, and continuous variables with a 95% confidence interval were calculated using mean difference.
Results: Twenty-two RCTs including 1,725 patients were selected. The findings demonstrated that anti-fibrotic CPMs combined with UDCA improved the efficacy rate, liver function, liver fibrosis, immunological indicators, and clinical symptoms compared with UDCA alone (all p < 0.05).
Conclusion: This study demonstrates that the combination of anti-fibrotic CPMs and UDCA can improve both clinical symptoms and outcomes. Nevertheless, more high-quality RCTs are needed to assess the effectiveness of anti-fibrosis CPMs for PBC.
1 Introduction
Primary biliary cholangitis (PBC), also called primary biliary cirrhosis, is a chronic autoimmune cholestatic liver disease whose pathogenesis has not been fully elucidated (European Association for the Study of the Liver, 2017). PBC frequently occurs in middle-aged women, and its clinical serological characteristics include positive anti-mitochondrial antibodies and elevated levels of alkaline phosphatase (ALP) or gamma-glutamyl transpeptidase (GGT) (You et al., 2022). The main pathological features of the liver include progressive, non-suppurative, and destructive intrahepatic cholangitis, leading to fibrosis and eventually cirrhosis (Zhang et al., 2021; You et al., 2022; European Association for the Study of the Liver, 2017). PBC is mainly caused by genetic and environmental factors, with unknown pathogenesis and hidden clinical manifestations (You et al., 2022). Some patients with PBC have cirrhosis at the time of diagnosis (Zhang et al., 2021), making anti-fibrotic therapy particularly important.
Ursodeoxycholic acid (UDCA) is an effective treatment for PBC (You et al., 2022), and its mechanism of action includes cholelithiasis, cytoprotection, anti-inflammatory effects, and immune regulation (Zhang et al., 2021). UDCA has greatly improved longevity in patients with PBC. However, 40% of patients with PBC still respond poorly to it (Cheung et al., 2016), and non-responders have a lower survival rate than the general population. Owing to rapid disease progression and poor long-term prognosis, patients who respond poorly to UDCA may require alternative treatment methods urgently; however, currently, no unified therapies exist.
Traditional Chinese medicine (TCM) has advantages in treating liver fibrosis, owing to its combination of ingredients and multiple pathways and targets. Previous studies have shown that Fuzheng Huayu capsules (FZHY) (Liu et al., 2019), Fufang Biejia Ruangan tablets (FFBJRG) (Ji et al., 2022), and Anluo Huaxian pills (ALHX) (Lu et al., 2017) are commonly used clinically as Chinese patent medicines (CPMs) for the anti-fibrosis treatment of liver these have been approved by the State Food and Drug Administration of China, with national medicine permission numbers of Z20020074 (FZHY), Z19991011 (FFBJRG), and Z20010098 (ALHX). The fibrosis stage is important in the progression of PBC. If anti-fibrosis treatment is administered in time at this stage, the occurrence of liver cancer or even liver failure will be reversed (Prince et al., 2002). Additionally, a recent study demonstrated that the treatment of PBC with TCM exerts anti-fibrotic effects and helps improve patients’ pruritus, fatigue, and the response rate to UDCA (Chen et al., 2018). Meanwhile, several studies have revealed that FZHY, FFBJRG, and ALHX have unique advantages in improving biochemical indices, anti-fibrosis, and quality of life in patients with PBC (Chen et al., 2019; Jiang et al., 2019; Wang et al., 2020). Furthermore, a previous real-world cohort study (Chen et al., 2018) conducted by our research group found that TCM combined with UDCA increased the 1-year biochemical response rate of patients with PBC by 15.1% compared with that of UDCA alone (43.0% vs. 27.9%, p < 0.05).
Therefore, it is of great significance to explore the clinical curative effects of CPMs combined with UDCA on PBC. Therefore, a meta-analysis of randomized controlled trials (RCTs) was conducted to measure the efficacy of CPMs plus UDCA for treating PBC.
2 Materials and methods
2.1 Standards for inclusion and exclusion of literature
The inclusion criteria were: 1) the studies reported RCTs; 2) the participants were diagnosed with PBC based on the consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL); 3) the treatment involved anti-fibrotic CPMs plus UDCA; and 4) at least one of the following outcome indices was used: clinical efficacy rate, ALP, GGT, alanine aminotransferase (ALT), aspartate aminotransferase (AST), hyaluronic acid (HA), laminin (LN), collagen type IV (IV-C), type III procollagen (PC-III), immunoglobulin M (IgM), immunoglobulin G (IgG), and clinical symptoms. The primary outcome was the clinical efficacy rate, whereas the secondary outcomes were liver function, hepatic fibrosis, immunological indicators, and clinical symptoms.
The exclusion criteria were: 1) the experimental group used none of the three aforementioned anti-fibrotic CPMs or other TCMs; 2) duplicate studies; 3) studies with incomplete research data; and 4) animal experiments, conferencing articles, reviews, non-RCTs, and other unrelated studies.
2.2 Search strategy
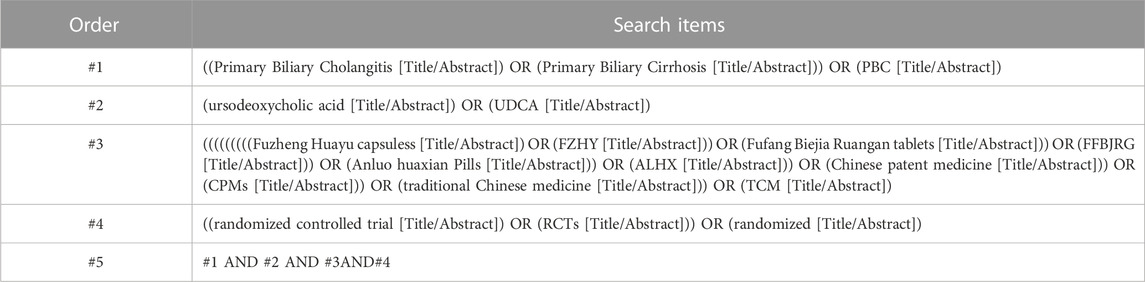
The meta-analysis followed the newest Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Supplementary Table S1) (Page et al., 2021). A literature search was conducted using PubMed, Web of Science, Embase, Cochrane Library, Wanfang database, VIP database, China Biology Medicine Database, and Chinese National Knowledge Infrastructure from their inception until August 2022, with no language restriction. The search terms included: “Primary Biliary Cirrhosis,” “Primary Biliary Cholangitis,” “PBC,” “Chinese patent medicine,” “CPMs,” “traditional Chinese medicine,” “TCM,” “Ursodeoxycholic Acid,” “UDCA,” “Fuzheng Huayu capsules,” “FZHY,” “Fufang Biejia Ruangan tablets,” “FFBJRG,” “Anluo huaxian Pills,” “ALHX,” “ursodeoxycholic acid,” “UDCA,” “randomized controlled trials,” and “RCT.” The search strategy is presented in Table 1.
2.3 Data acquisition and quality evaluation
The literature was independently screened by two researchers (BI and SHI) in terms of the inclusion and exclusion standards. Data acquisition consisted of 1) general information: title, first author, and publication year; 2) sex, age, sample size, intervention measures, and treatment course; and 3) observed outcome indicators.
Literature eligibility was estimated using the Cochrane collaboration tool, including incomplete outcome data, selective reporting, allocation concealment, random-sequence generation, blinding of outcome assessment, blinding of participants and personnel, and other biases. Based on these standards, the literature eligibility was categorized as three levels of risk of bias: high, unclear, and low.
2.4 Statistical methods
The statistical analysis was performed using RevMan 5.4 software. According to the type of outcome, continuous data are depicted as mean difference (MD) or standardized mean difference (SMD), while categorical data are presented as risk ratios (RRs); all are expressed with a 95% confidence interval (CI). Furthermore, the χ2 and I2 tests were used for heterogeneity analysis. The fixed-effects model was used for analysis if p ≥ 0.1 and I2 ≤ 50% in the subgroup or overall. Conversely, the random-effects model was employed if p < 0.1 and I2 > 50%. Additionally, we searched for sources of heterogeneity in the results and employed a sensitivity analysis to affirm whether the results were stable. Moreover, subgroup analyses were performed based on the use of three different CPMs. A p-value less than 0.05 was considered statistically significant. Publication bias was analyzed using funnel plots.
3 Results
3.1 Study selection
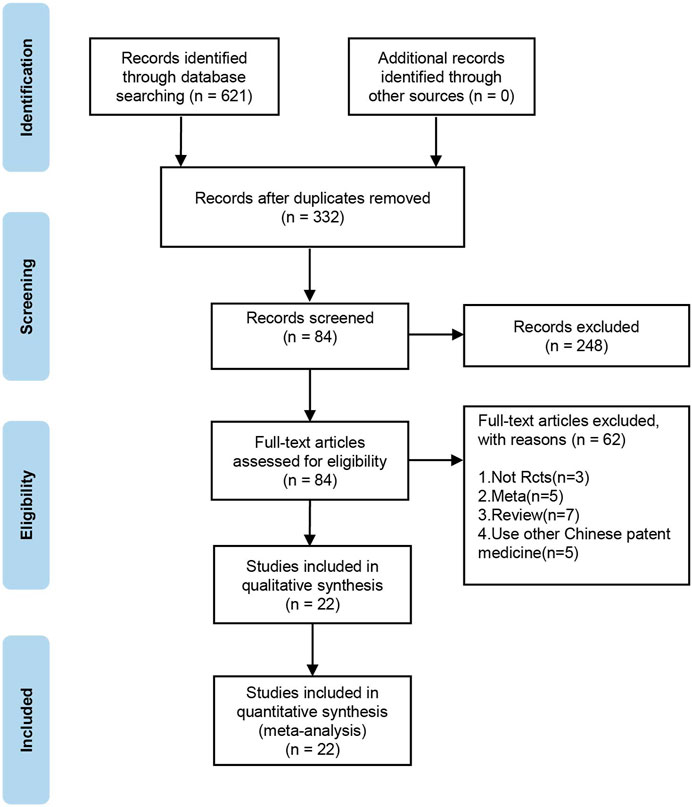
In total, 621 relevant studies were selected in the initial search. After duplicates were removed, 332 studies remained. After screening the titles, abstracts, and introductions of each article, 84 articles were deleted. Finally, after reading the full-text articles for further screening and evaluation, 22 publications (Wu et al., 2012; Xie et al., 2012; Ying, 2012; Li and Wu, 2013; Zhang et al., 2014; Gao et al., 2015; Huang et al., 2015; Qiu and Rao, 2015; Wang, 2015; Wang et al., 2015; Pang, 2017; Yang, 2017; Chen et al., 2019; Jiang et al., 2019; Zhang, 2019; Liu, 2020b; Wang, 2020; Zhang et al., 2020; Ai, 2021; Zhang, 2021) were included. The selection procedure for publications is shown in Figure 1.
3.2 Study characteristics
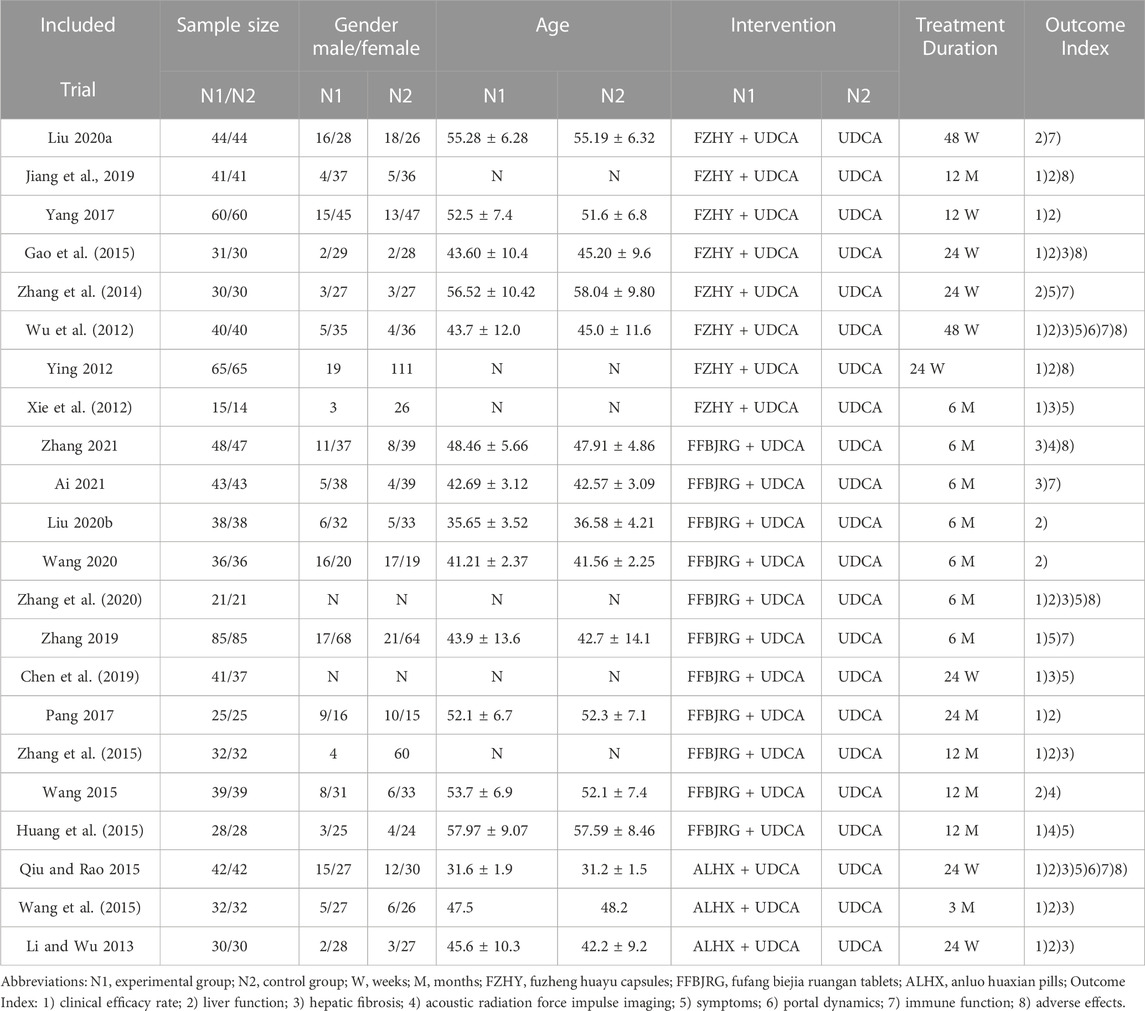
All 22 included studies were RCTs conducted in China and involved 1,725 patients with PBC. All studies were classified into an experimental group and a control group to compare the efficacies of CMPs combined with UDCA and UDCA monotherapy. The basic traits of each study are summarized in Table 2.
3.3 Quality assessment
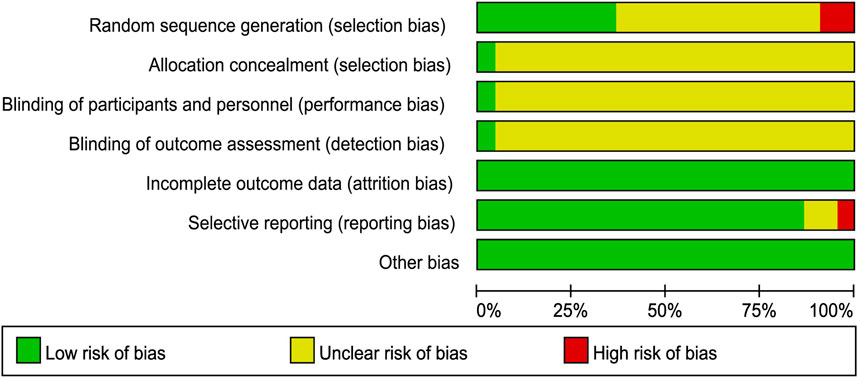
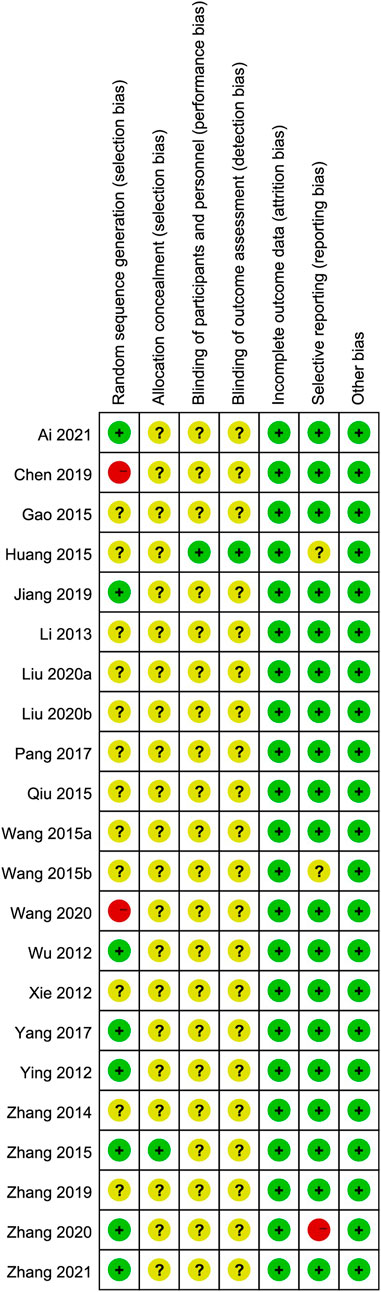
We used the Cochrane risk-of-bias tool to estimate article quality. Of these, eight publications (Wu et al., 2012; Ying, 2012; Zhang et al., 2015; Yang, 2017; Jiang et al., 2019; Zhang et al., 2020; Ai, 2021; Zhang, 2021) used a random number table, 12 (Xie et al., 2012; Li and Wu, 2013; Zhang et al., 2014; Huang et al., 2015; Qiu and Rao, 2015; Wang, 2015; Wang et al., 2015; Cheung et al., 2016; Pang, 2017; Zhang, 2019; Liu, 2020a; Liu, 2020b) did not describe the randomization method, and two (Wang 2020; Chen et al., 2019 did not mention the word “random.” Only one publication (Zhang et al., 2015) used a double-blind design and reported the number of missing cases in the experimental and control groups. In terms of hidden allocation, one publication (Huang et al., 2015) adopted a non-transparent envelope. The details of the risk of bias assessment are shown in Figures 2, 3.
3.4 CPMs drug composition
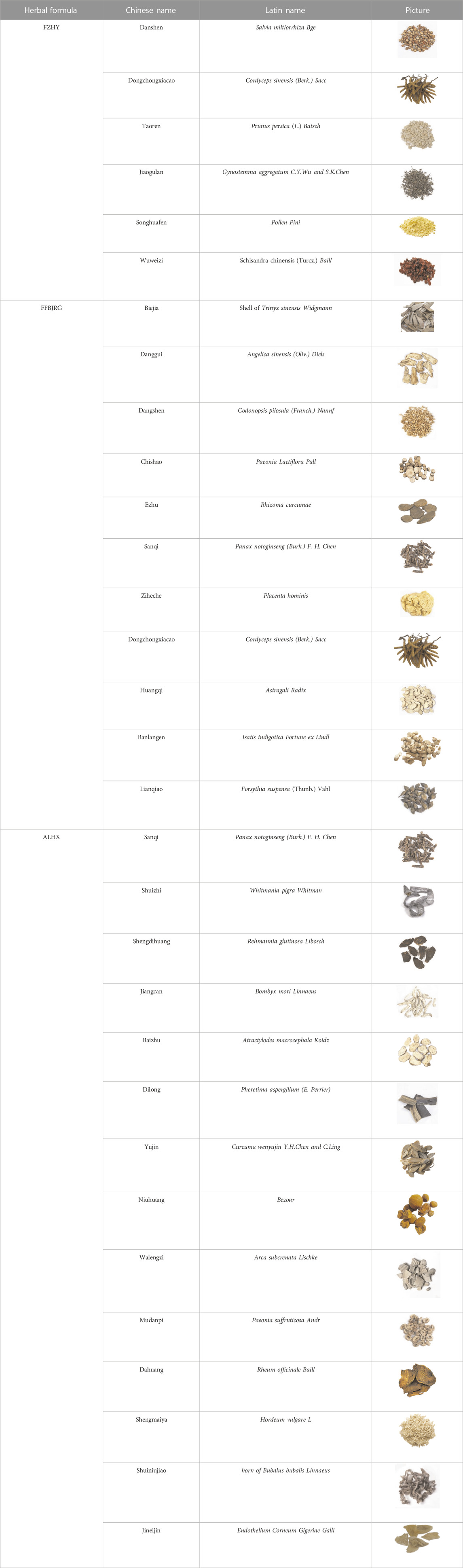
These studies used three anti-fibrosis CPMs, including FZHY, FFBJRG, and ALHX, and listed the TCMs used. Dongchongxiacao (Cordyceps sinensis (Berk.) Sacc) is an ingredient in FZHY and FFBJRG, while Sanqi (Panax notoginseng (Burkill) F. H. Chen) is an ingredient in ALHX and FFBJRG. The compositions of the anti-hepatic fibrosis herbal formulations are listed in Table 3.
3.5 Outcome index
3.5.1 Clinical efficacy rate
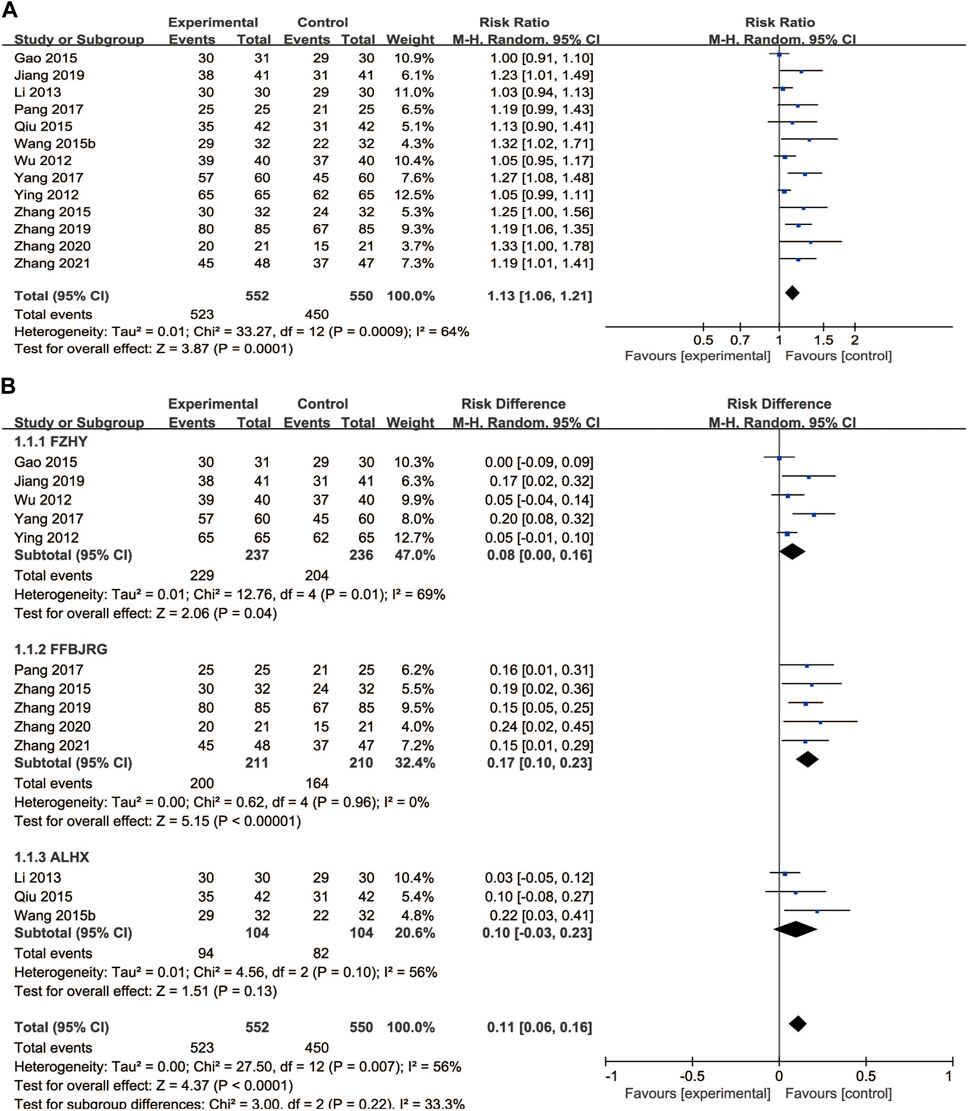
A total of 13 clinical trials described the clinical efficacy rate as the main outcome, which was classified as markedly effective, effective, and ineffective grades. A random-effects model was implemented for analysis regarding heterogeneity testing (p = 0.0009, I2 = 64%), and anti-fibrosis CPMs plus UDCA improved the clinical efficacy rate compared with UDCA alone (RR = 0.11, 95% CI: 0.06, 0.16; p < 0.00001) (Figure 4A). Subgroup analysis showed that the clinical efficacy rate of FFBJRG was better than that of the other two CPMs and had no heterogeneity (p = 0.96, I2 = 0%) (Figure 4B).
3.6 Liver function
3.6.1 Alkaline phosphatase
We found 10 trials that reported the effects of anti-fibrotic CPMs plus UDCA on ALP levels. A random-effects model was implemented based on the p-value and I2 value. The subgroup analyses showed that anti-fibrotic CPMs plus UDCA were superior to UDCA alone in terms of serum ALP levels (RR = −28.83, 95% CI: −36.57, −21.10; p < 0.00001) (Figure 5A).
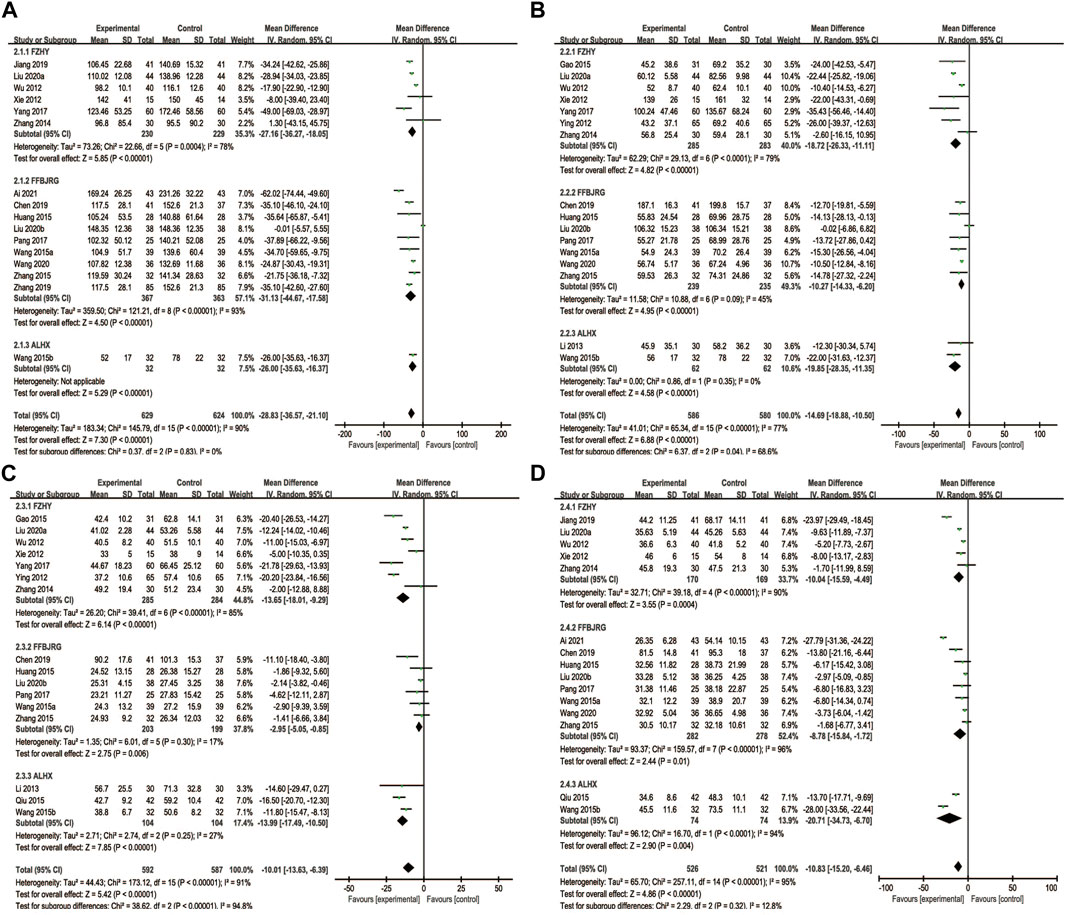
FIGURE 5. Forest plot of meta-analysis of liver function (A) alkaline phosphatase; (B) gamma-glutamyl transpeptidase (C) alanine aminotransferase; and (D) aspartate aminotransferase.
3.6.2 Gamma-glutamyl transpeptidase
A total of 16 articles reported GGT levels as the outcome. In the subgroup analyses, random-effects models were used due to heterogeneity (p < 0.00001, I2 = 77%). Consequently, the anti-fibrotic CPMs plus UDCA were more effective at decreasing GGT levels than UDCA alone (MD = −14.69, 95% CI: −18.88, −10.50; p < 0.00001) (Figure 5B).
3.6.3 Alanine aminotransferase
Sixteen studies described ALT levels as the main outcome. A random-effects model was implemented for the analysis based on the heterogeneity test (p < 0.00001, I2 = 91%). Overall, ALT levels significantly decreased after the combination of anti-fibrotic CPMs and UDCA (MD = −10.01, 95% CI: −13.63, −6.39; p < 0.00001) (Figure 5C).
3.6.4 Aspartate aminotransferase
Sixteen studies reported serum AST levels as the main outcome. The AST data were processed using a random-effects model because AST was heterogeneous (p < 0.00001, I 2 = 95%). The use of anti-fibrotic CMPs plus UDCA showed a greater decrease in serum AST levels than that with UDCA alone (MD = −10.83, 95% CI: −15.20, −6.46; p < 0.00001) (Figure 5D).
3.7 Liver fibrosis
Nine trials reported the serum LN and IV-C levels, and we performed a subgroup analysis on these. The findings revealed that the LN (MD = −19.67, 95% CI: −22.73, −16.61) and IV-C (MD = −18.01, 95% CI: −20.96, −15.06) notably decreased after treatment with the combination of anti-fibrotic CMPs plus UDCA (p < 0.00001) (Figures 6A, B).
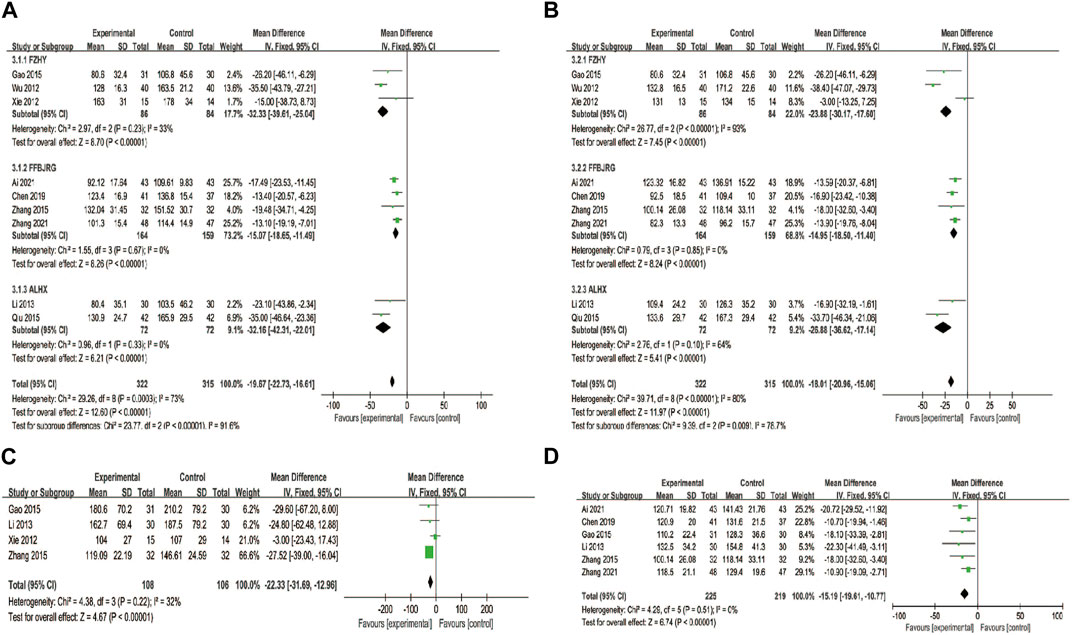
FIGURE 6. Forest plot of meta-analysis of liver fibrosis (A) laminin; (B) type IV collagen (C) hyaluronic acid; and (D) type III procollagen.
HA levels were reported in four clinical studies, and PC-III was reported as an outcome indicator in six. After testing for heterogeneity, HA was analyzed using a fixed-effects model (p = 0.22, I2 = 32%) and PC-III (p = 0.51, I2 = 0) levels. The experimental group showed a greater decrease in serum HA (MD = −22.33, 95% CI: −31.69, −12.96) and PC-Ⅲ (MD = −15.19, 95% CI: −19.61, −10.77) levels than the control group (p < 0.00001) (Figures 6C, D).
3.8 Immune function
3.8.1 Immunoglobulin M and immunoglobulin G
The IgG data were processed using a fixed-effects model based on the heterogeneity test (p = 0.96, I2 = 0%), and a random-effects model was used for the IgM data with high heterogeneity (p = 0.03, I2 = 63%). The MD (95% CI) of IgG and IgM were −2.86 (−3.63, −2.08) and −1.28 (−1.62, −0.94), respectively. Compared with UDCA alone, the additional use of anti-fibrosis CPMs was more effective in reducing IgG and IgM levels (Figures 7A, B).
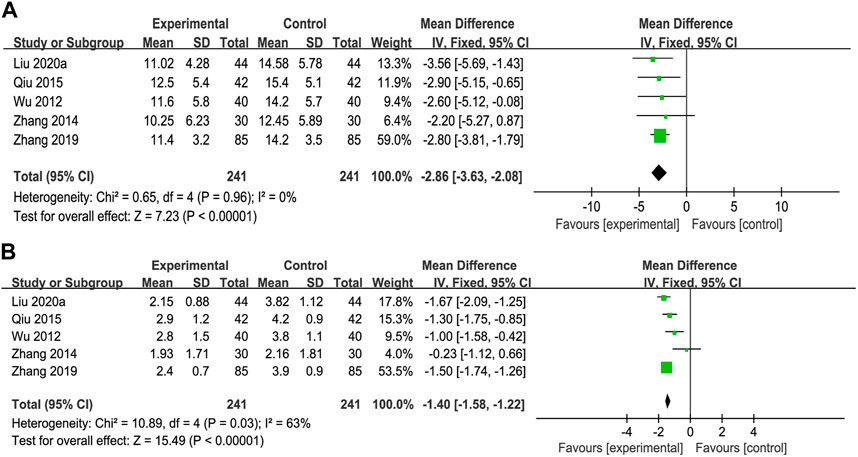
FIGURE 7. Forest plot of meta-analysis of immune function (A) immunoglobulin G (IgG); and (B) immunoglobulin M (IgM).
3.9 Symptom score
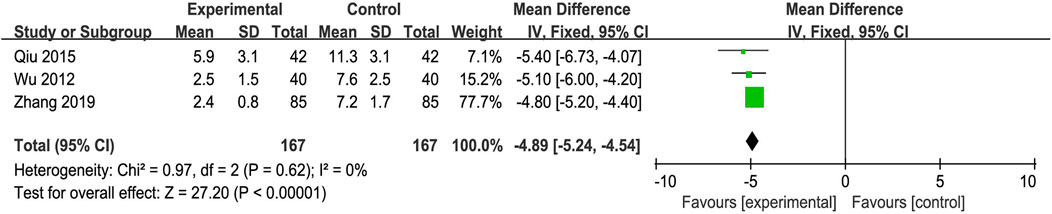
Three trials reported Symptom Score Change as the main outcome. The fixed-effects model was adopted because of the absence of heterogeneity (p = 0.62, I2 = 0%). The improvement in the Symptom Score Change was better in the experimental group than in the control group (MD = −4.89, 95% CI: −5.24, −4.54; p < 0.00001) (Figure 8).
3.10 Adverse events
Adverse events were described in seven studies. Three articles reported a total of nine patients who developed mild diarrhea and nausea after taking CPM; however, these symptoms were not severe and did not require treatment. Four studies reported no adverse reactions.
3.11 Publication bias
An inverted funnel plot analysis of the clinical efficacy rate revealed that there may be publication bias in the asymmetric distribution of these publications (Figure 9).
4 Discussion
PBC is the most common autoimmune cirrhotic hepatic disease, occurring in all ethnic groups worldwide (Lv et al., 2020). The prognosis of patients with PBC mostly depends on the degree of liver fibrosis and its complications (Lammers et al., 2015). Patients with PBC tend to seek additional pharmacological treatments because UDCA is not uniformly effective (Lammers et al., 2015). In recent years, TCM has been widely studied and discussed as a complementary therapy. Many studies have shown that TCM plus UDCA has diverse advantages in relieving the clinical symptoms and improving the prognosis of PBC.
This meta-analysis validated the advantages of FZHY, FFBJRG, and ALHX combined with UDCA in the treatment of PBC. Compared with UDCA treatment alone, anti-fibrotic CPMs plus UDCA improved efficacy rates. Furthermore, liver function tests are widely used in clinical practice as indicators of the degree of liver damage. ALP, ALT, AST, and GGT levels decreased after combined treatment with anti-fibrotic CPMs and UDCA. LN, IV-C, PC-III, and HA are indicators for the detection of liver fibrosis, and the addition of anti-fibrosis CPMs to UDCA resulted in decreased levels of these compared with UDCA treatment alone. Moreover, immunological indicators (IgM and IgG) and clinical symptoms also notably improved with the combined treatment of anti-fibrotic CPMs and UDCA. In conclusion, anti-fibrotic CPMs combined with UDCA in the treatment of PBC effectively relieved various clinical indicators. These results provide hope for the treatment and prevention of liver fibrosis and cirrhosis in the future.
The pathobiology of PBC is characterized by inflammation, bile duct damage, and fibrosis (You et al., 2022), of which fibrosis appears in stage Ⅱ. It is believed that TCM is hepatoprotective and anti-inflammatory and suppresses the activation of hepatic stellate cells, which is advantageous in the treatment of liver fibrosis. Additionally, TCM may have multi-level, multi-pathway, and multi-target pharmacological actions on the comprehensive pathogenesis of PBC. For example, P. notoginseng is an ingredient in FFBJRG and ALHX, and P. notoginseng saponins are its main active constituent, which play an immunomodulatory role by reducing the levels of pro-inflammatory cytokines (Jiang et al., 2013). Furthermore, Ophiacordyceps sinensis, as a duplicate herb in FZHY and FFBJRG, can attenuate liver inflammation and fibrosis by regulating the expression of the TGF-β/MAPK pathway (Fu et al., 2021). Moreover, a mechanistic study has revealed that FZHY can decrease the expression levels of α-SMA, CTGF, TIMP-1, TGF-β1, and Smads, thereby reducing hepatic apoptosis, acute liver injury, and liver fibrosis (Cheng et al., 2013; Xie et al., 2013). An animal experiment reported that FFBJRG ameliorates hepatic disease by reducing the serum collagen levels of LN, HA, and IV-C and downregulating TGF-β-Smad pathway fibroblast signal transduction (Yang et al., 2013). The possible mechanism of ALHX for the inhibition of hepatic fibrosis is related to enhancing MMP2 activity in liver tissue and promoting extracellular matrix degradation by hepatoprotective enzymes (Tan et al., 2010). In brief, certain evidence support the anti-inflammatory and anti-fibrosis effects of FZHY, FFBJRG, and ALHX as the appropriate CPMs for treating PBC.
However, this meta-analysis had some limitations. First, the 22 studies included had a small sample size, all were Chinese, and only a small number of studies reported several outcome indicators. Second, most publications only mentioned random assignment, and only one-third of the studies described a specific randomization method, such as a random number table. Therefore, the findings need to be further evaluated using high-quality trials. Third, different studies had different experimental periods, ranging between 12 and 48 weeks, which may be a source of heterogeneity. Fourth, although anti-fibrosis CPMs were always used in the experimental group, there were three different types—FZHY, FFBJRG, and ALHX, which could also be a source of heterogeneity. Finally, because half of the studies did not mention adverse events, the safety of CPMs as an anti-fibrosis therapy for PBC should be further evaluated, and caution is needed when drawing conclusions.
5 Conclusion
Our research shows that the combination of anti-fibrosis CPMs and UDCA is more effective than UDCA alone in treating PBC in improving clinical efficacy rate, liver fibrosis, liver function, immune function, and symptom score. This systematic review and meta-analysis provides reliable clinical evidence for PBC treatment. Anti-fibrotic CPMs are a promising therapeutic approach to supplement the conventional treatment of PBC. However, further evidence from high-quality and multi-center studies with larger samples is warranted to confirm the curative effect of anti-fibrosis CPMs during follow-up periods.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Author contributions
XW and YB designed the study. KS and JC contributed to the data collection and analysis. The results were interpreted by YB. KS drafted the original manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Beijing Municipal Science Technology Commission (no. Z191100006619033). Beijing Ditan Hospital scientific research fund project (DTYM202102).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1159222/full#supplementary-material
Abbreviations
ALHX, anluo huaxian pills; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; APASL, asian pacific association for the study of the liver; CI, confidence interval; CPM, Chinese patent medicine; FDA, United States Food and Drug Administration; FFBJRG, Fufang Biejia Ruangan tablets; FZHY, Fuzheng Huayu capsules; GGT, gamma-glutamyl transpeptidase; HA, hyaluronidase; IgG, immunoglobulin G; IgM, immunoglobulin M; IV-C, type IV collagen; LN, laminin; MD, mean difference; PBC, primary biliary cholangitis; PC-III, type III procollagen; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCTs, randomized controlled trials; RR, risk ratio; SMD, standardized mean difference; TCM, traditional Chinese medicine; UDCA, ursodeoxycholic acid.
References
Ai, Y. G. (2021). The effect of ursodeoxycholic acid combined with compound Biejia ruangan tablet on liver function and immune function of PBC/AI yuanguang. Chin. Foreign Med. Res. 19, 49–51.
Chen, J. L., Yang, X., Zhang, Q., Liu, Y., and Wang, X. B. (2018). Effect of ursodeoxycholic acid with traditional Chinese medicine on biochemical response in patients with primary biliary cholangitis: A real-world cohort study. Chin. J. Hepatol. 26, 900–915.
Chen, M. M., Duan, X. Y., and Cao, H. X. (2019). Therapeutic effect of Biejia Ruangan Tablet on patients with primary biliary cirrhosis. J. Pract. Hepatology 22, 880–882.
Cheng, Q., Li, N., Chen, M., Zheng, J., Qian, Z., Wang, X., et al. (2013). Fuzheng Huayu inhibits carbon tetrachloride-induced liver fibrosis in mice through activating hepatic NK cells. J. Ethnopharmacol. 145, 175–181. doi:10.1016/j.jep.2012.10.047
Cheung, A. C., Lapointe-Shaw, L., Kowgier, M., Meza-Cardona, J., Hirschfield, G. M., Janssen, H. L., et al. (2016). Combined ursodeoxycholic acid (UDCA) and fenofibrate in primary biliary cholangitis patients with incomplete UDCA response may improve outcomes. Aliment. Pharmacol. Ther. 43, 283–293. doi:10.1111/apt.13465
European Association For The Study Of The Liver (2017). EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J. Hepatol. 67, 145–172. doi:10.1016/j.jhep.2017.03.022
Fu, X. L., Xiao, J., and Niu, L. (2021). Cordyceps sinensis alleviating CCl4-induced liver inflammation and fibrosis in rats by regulating MAPK pathway. China Pharm. 24, 1657–1664.
Gao, F., Xun, J., and Jiao, J. L. (2015). Clinical observation of Fuzhenghuayu capsules combined with ursodeoxycholic acid capsules in the treatment of primary biliary cirrhosis. J. Shanxi Med. Coll. Continuing Educ. 25, 17–19.
Huang, L. Y., Zhou, Z. H., Sun, X. H., and Gao, Y. Q. (2015). Clinical efficacy of Biejiaruangan compound tablets in treatment of primary biliary cirrhosis. J. Clin. Hepatol. 31, 181–184.
Ji, D., Chen, Y., Bi, J., Shang, Q., Liu, H., Wang, J. B., et al. (2022). Entecavir plus Biejia-Ruangan compound reduces the risk of hepatocellular carcinoma in Chinese patients with chronic Hepatitis B. J. Hepatol. 77, 1515–1524. doi:10.1016/j.jhep.2022.07.018
Jiang, H., Wang, Y. Z., Liu, X. C., and Xue, X. (2013). Effect of Panax notoginseng saponins on cytokines in liver fibrosis rats. J. Chin. Med. Mater. 36, 1123–1127.
Jiang, X. Y., Wu, W. J., and Ou, Y. (2019). Therapeutic effect of Fuzheng huayu capsule combined with ursodeoxycholic acid on primary biliary cholangitis. Pub Med. Forum Mag. 23, 3667–3668.
Lammers, W. J., Hirschfield, G. M., Corpechot, C., Nevens, F., Lindor, K. D., Janssen, H. L. A., et al. (2015). Development and validation of a scoring system to predict outcomes of patients with primary biliary cirrhosis receiving ursodeoxycholic acid therapy. Gastroenterology 149, 1804–1812.e4. doi:10.1053/j.gastro.2015.07.061
Li, Z. Q., and Wu, D. 2013. Clinical observation of Anluo Huaxian pill combined with ursodeoxycholic acid in the treatment of primary biliary cirrhosis. Chin. J. Integr. Traditional West. Med. Liver Dis., 23, 277–278.
Liu, F. (2020a). Clinical effect of Compound Biejia Ruangan Tablet on patients with primary biliary cirrhosis. Chin. Health Care 38, 5–6.
Liu, F. (2020b). Influence of fuzheng huayu capsule on liver function and immune function in patients with primary biliary cirrhosis. Guangming J. Chin. Med. 35, 304–306.
Liu, H. L., Lv, J., Zhao, Z. M., Xiong, A. M., Tan, Y., Glenn, J. S., et al. (2019). Fuzhenghuayu Decoction ameliorates hepatic fibrosis by attenuating experimental sinusoidal capillarization and liver angiogenesis. Sci. Rep. 9, 18719. doi:10.1038/s41598-019-54663-4
Lu, W., Gao, Y. H., Wang, Z. Z., Zhang, H., Yang, Y. Q., Miao, Y. Q., et al. (2017). Effect of Anluo huaxian Wan on transforming growth factor β 1 and its signal pathway in rats with liver fibrosis Chin. J. Hepatol. 25, 257–262. doi:10.3760/cma.j.issn.1007-3418.2017.04.005
Lv, T., Chen, S., Li, M., Zhang, D., Kong, Y., and Jia, J. (2020). Regional variation and temporal trend of primary biliary cholangitis epidemiology: A systematic review and meta-analysis. J. Gastroenterology Hepatology 36, 1423–1434. doi:10.1111/jgh.15329
Page, M. J., Moher, D., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. Bmj 29, n160. doi:10.1136/bmj.n160
Pang, Y. M. (2017). Clinical research of Biejia Ruangan tablets combined with ursodeoxycholic acid in treatment of the primary biliary cirrhosis. Aero Med. 28, 880–882.
Prince, M., Chetwynd, A., Newman, W., Metcalf, J. V., and James, O. F. (2002). Survival and symptom progression in a geographically based cohort of patients with primary biliary cirrhosis: Follow-up for up to 28 years. Gastroenterology 123, 1044–1051. doi:10.1053/gast.2002.36027
Qiu, H. Q., and Rao, L. P. (2015). Anluo huaxian pill in treatment of primary biliary cirrhosis patients and influence on Index of immunology. Chin. Archives Traditional Chin. Med. 33, 2922–2925.
Tan, X. H., Li, C. Q., Zou, S. R., Xie, M., Zhang, A. M., Li, W. L., et al. (2010). Inhibitory effect of anluohuaxianwan on experimental hepatic fibrosis in rats. Zhonghua Gan Zang Bing Za Zhi 18, 9–12. doi:10.3760/cma.j.issn.1007-3418.2010.01.003
Wang, D. T. (2015). Observation of curative effect Biejia Ruangan tablets combined with ursodeoxycholic acid on the primary biliary cirrhosis. Contem Med. Forum 13, 138–139.
Wang, J. (2020). Clinical effect of compound Biejia Ruangan Tablet on patients with primary biliary cirrhosis. Front. Med. 223.
Wang, W., Tian, Y., Li, Y. F., and Lu, Y. J. (2015). Observation of curative effects of Anluohuaxian pills combined with ursodeoxycholic acid capsules on patients with primary biliary cirrhosis. Chin. J. Liver Dis. Version) 7, 100–102.
Wang, X. B., Liu, Y., and Wang, X. B. (2020). Biochemical response and integrated traditional Chinese and Western medicine therapy for primary biliary cholangitis. J. Clin. Hepatol. 36, 26–30.
Wu, Y., Yao, D. K., and Zhu, L. (2012). Clinical observation on the safety and efficacy of ursodeoxycholic acid and Fuzheng huayu capsule in the treatment of primary biliary cirrhosis. Chin. J. Integr. Trad. West Med. 32, 1477–1482.
Xie, H., Tao, Y., Lv, J., Liu, P., and Liu, C. 2013. Proteomic analysis of the effect of fuzheng huayu recipe on fibrotic liver in rats. Evid. Based Complement. Altern. Med., 2013, 972863, doi:10.1155/2013/972863
Xie, X. R., Hu, B., and Du, W. X. (2012). Clinical efficacy of ursodeoxycholic acid tablets combined with Biejia Ruangan tablets on primary biliary cirrhosis. Shanxi Med. J. 25, 257–262.
Yang, F. R., Fang, B. W., and Lou, J. S. (2013). Effects of Fufang Biejia ruangan pills on hepatic fibrosis in vivo and in vitro. World J. Gastroenterol. 19, 5326–5333. doi:10.3748/wjg.v19.i32.5326
Yang, Z. G. (2017). Curative effect of Fuzheng Huayu capsule combined with ursodeoxycholic acid capsule on primary biliary cirrhosis. Psychol. Dr. 23, 175–176.
Ying, H. J. (2012). Observation and nursing of the short-term effect of ursodeoxycholic acid and Fuzheng huayu capsule on primary biliary cirrhosis. Strait Pharm. J. 24, 217–218.
You, H., Ma, X., Efe, C., Wang, G., Jeong, S. H., Abe, K., et al. (2022). APASL clinical practice guidance: The diagnosis and management of patients with primary biliary cholangitis. Hepatol. Int. 16, 1–23. doi:10.1007/s12072-021-10276-6
Zhang, F. C., Wang, L., Shuai, Z. W., Wu, Z. L., Zhang, W., Zhang, Z. L., et al. (2021). Recommendations for diagnosis and treatment of primary biliary cholangitis in China. Zhonghua Nei Ke Za Zhi 60, 709–715. doi:10.3760/cma.j.cn112138-20210520-00360
Zhang, H., Wang, J. B., Liu, X. B., and Yin, X. Y. (2020). Effect of compound Biejia Ruangan tablet combined with ursodeoxycholic acid on primary biliary cirrhosis. Med. Front. 10.
Zhang, J. Y., Zhang, Y. P., and Zhang, L. (2014). Clinical research of Fuzheng huayu capsule and ursodeoxcholic acid in the treatment of primary biliary cirrhosis. Chin. J. Hepatol. 6, 63–66.
Zhang, S.-S., Li, Y., and Xu, F. (2015). Clinical effects of ursodeoxycholic acid combined with compound Biejia Ruangan tablets in treatment of the early- or mid-stage primary biliary cirrhosis. World Chin. J. Dig. 23, 4251. doi:10.11569/wcjd.v23.i26.4251
Zhang, W. (2021). Clinical effect of compound Biejia ruangan tablets combined with ursodeoxycholic acid on primary biliary cirrhosis. Pract. Clin. Med. 22.
Keywords: primary biliary cholangitis, Chinese patent medicine, anti-fibrosis, ursodeoxycholic acid, meta-analysis
Citation: Bi Y, Shi K, Chen J and Wang X (2023) Curative effect of anti-fibrosis Chinese patent medicines combined with ursodeoxycholic acid for primary biliary cholangitis: A systematic review and meta-analysis. Front. Pharmacol. 14:1159222. doi: 10.3389/fphar.2023.1159222
Received: 05 February 2023; Accepted: 13 March 2023;
Published: 21 March 2023.
Edited by:
Zhengsheng Zou, Fifth Medical Center of the PLA General Hospital, ChinaReviewed by:
Aimin Zhou, Cleveland State University, United StatesMao Qianguo, Xiamen Hospital of Traditional Chinese Medicine, China
Copyright © 2023 Bi, Shi, Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianbo Wang, d2FuZ3hiQGNjbXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Yufei Bi
Yufei Bi Ke Shi
Ke Shi Jialiang Chen
Jialiang Chen Xianbo Wang
Xianbo Wang