
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 26 April 2023
Sec. Pharmacology of Anti-Cancer Drugs
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1158421
This article is part of the Research TopicDisputes and Challenges of Immune Checkpoint Inhibitors in Gastrointestinal CancersView all 13 articles
Purpose: Older cancer patients are more likely to develop and die from chemotherapy-related toxicity. However, evidence on drug safety and optimal effective doses is relatively limited in this group. The aim of this study was to develop a tool to identify elderly patients vulnerable to chemotherapy toxicity.
Patients and methods: Elderly cancer patients ≥60 years old who visited the oncology department of Peking Union Medical College Hospital between 2008 and 2012 were included. Each round of chemotherapy was regarded as a separate case. Clinical factors included age, gender, physical status, chemotherapy regimen and laboratory tests results were recorded. Severe (grade ≥3) chemotherapy-related toxicity of each case was captured according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0. Univariate analysis was performed by chi-square statistics to determine which factors were significantly associated with severe chemotherapy toxicity. Logistic regression was used to build the predictive model. The prediction model was validated by calculating the area under the curve of receiver operating characteristic (ROC).
Results: A total of 253 patients and 1,770 cases were included. The average age of the patients was 68.9 years. The incidence of grade 3–5 adverse events was 24.17%. Cancer type (non-GI cancers), BMI<20 kg/m2, KPS<90%, severe comorbidity, polychemotherapy, standard dose chemotherapy, low white blood cells count, anemia, low platelet cells count, low creatine level and hypoalbuminemia were associated with severe chemotherapy-related toxicity. We used these factors to construct a chemotherapy toxicity prediction model and the area under the ROC curve was 0.723 (95% CI, 0.687–0.759). Risk of toxicity increased with higher risk score (11.98% low, 31.51% medium, 70.83% high risk; p < 0.001).
Conclusion: We constructed a predictive model of chemotherapy toxicity in elderly cancer patients based on a Chinese population. The model can be used to guide clinicians to identify vulnerable population and adjust treatment regimens accordingly.
Cancers are age-related diseases (Chang et al., 2019). According to the data on GLOBOCAN, approximately 50% of new diagnosed cancer patients in 2020 are elderly people over 65 years old (Sung et al., 2021). However, there are still many knowledge gaps in the treatment of elderly cancer patients (Hurria et al., 2014).
While older patients may respond similarly to anticancer treatments as younger patients, treatment-related toxicity remains a concern (Macchini et al., 2019). Studies have found that older patients are more likely to experience chemotherapy-related adverse events (Trumper et al., 2006; Muss et al., 2007; Asmis et al., 2008). Poor tolerability in the elderly population may be due to many factors, including age-related deterioration of multiple organ functions, comorbidities, polypharmacy, and other problems that can lead to altered pharmacokinetics and pharmacodynamics of chemotherapy drugs (Brunello et al., 2009; Feliu et al., 2018).
Dose reduction as a strategy to improve patient tolerability while preserving the antitumor effect has been identified as a promising approach (Hall et al., 2021). However, the elderly population is a highly heterogeneous group, with chronological age often not reflecting functional status and chemotherapy tolerance (Hernandez Torres and Hsu, 2017). Moreover, current guidelines for cancer treatment primarily rely on evidence obtained from clinical trials, which often exclude older patient population (Joharatnam-Hogan et al., 2020). Therefore, more evidence is needed to identify elderly populations at risk and guide adjustments of antitumor drug doses.
Several predictive tools have been developed to assess the risk of chemotherapy toxicity, including the Cancer and Aging Research Group (CARG) score and the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) Score (Hurria et al., 2011; Extermann et al., 2012). However, it should be noted that these models were primarily based on data from the Caucasian population and have limited applicability to Asian populations.
In this article, we collected data of elderly cancers patients from a tertiary hospital in inland China and analyzed the incidence of severe chemotherapy-related adverse events. We aimed at predicting the risk of chemotherapy toxicity using a logistic regression model and this predictive model should give more suggestion when discussing the risks and benefits of chemotherapy with older adults.
This study retrospectively analyzed elderly cancer patients who attended Peking Union Medical College Hospital between 2008 and 2012. Patient data were extracted, encrypted, and de-identified by 2 professional researchers in 2013. The Ethics Committee at Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College approved the study and waived the need for patient consent because of the retrospective design of this study.
1,453 cancer patients were reviewed, and 253 patients were enrolled. The inclusion criteria were patients (1) who were older than or equal to 60 years; (2) with a clear pathological diagnosis of malignancy or lymphoma; and (3) receiving at least one chemotherapy treatment. Patients whose diagnosis was unclear or who did not receive chemotherapy were excluded.
Each chemotherapy cycle received by each patient is considered as a separate case in our study. In total, 1,770 cases are included.
The primary endpoint was the occurrence of severe hematologic and non-hematologic chemotherapy-related toxicity (grade 3 [hospitalization indicated], grade 4 [life threatening], and grade 5 [treatment-related death]), graded using National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) v5.0 criteria (National Cancer Institute, 2017). This endpoint was chosen because most guidelines recommend dose adjustment when severe toxicity (grade≥ 3) occurs.
Patient demographic data (gender and age), tumor-related conditions (type and stage), body mass index (BMI), and comorbidities were collected. The Charlson comorbidity index (CCI) score was used to assess the severity of comorbidity (Charlson et al., 1987). In calculating the CCI score, metastatic solid tumor was excluded as comorbid conditions, given that our data itself is a cohort of tumor patients and most of them had distant metastases.
Before each chemotherapy cycle, the following data were captured: (1) Chemotherapy regimen, number of chemotherapy drugs, and chemotherapy doses. (2) Eastern Cooperative Oncology Group (ECOG) Performance Status Scale and Karnofsky score (KPS). (3) Laboratory variables included but are not limited to white blood cells, hemoglobin, platelets, transaminases, creatinine, and albumin.
We performed descriptive statistics of chemotherapy-related adverse events in all patients and calculated the incidence of hematological and non-hematological toxicities.
First, we used chi-square (χ2) tests to identify variables associated with grade 3–5 chemotherapy-related adverse events. Variables included age, gender, tumor type (non-gastrointestinal cancers or gastrointestinal cancers), stage (≤3 or 4), number of chemotherapy drugs (single or multiple drugs), chemotherapy doses (reduced or standard doses), BMI (<20 kg/m2 or ≥20 kg/m2), ECOG (>1 or ≤1), KPS (<90% or ≥90%), comorbidity score (CCI≥4 or <4) and multiple laboratory variables.
Variables with p-values less than 0.1 and certain clinical variables strongly associated with the outcome would be selected as model factors. We established the predictive model by a multivariate logistic regression model. The Youden Index (Youden, 1950) was used to identify the cut point with the highest sensitivity and specificity when classifying the presence or absence of toxicity. The discrimination of models was evaluated by calculating the area under the receiver operating characteristic (ROC) curve.
A risk score for each risk factor was calculated by dividing the coefficient of the variable by the lowest coefficient in the model, rounded to the nearest 0.5 times. (Concato et al., 1993; Walter et al., 2001). After that, the sum of scores for each chemotherapy case was calculated. The sample was divided into three risk strata (low, medium, and high risk) based on approximate quartiles of risk score with the middle two quartiles combined. The difference in toxicity incidence among the strata was evaluated by χ2 test.
The model was internally validated. We obtain the area under the ROC curve (AUC) of the model. If the AUC is larger than 0.7, it means the model is valid. All statistical analyses were performed by using SPSS.
The basic information of the patients was listed in Table1. Male patients accounted for 66.4% and female patients accounted for 33.6%. The average age of patients was 68.9 years old. More than 75% of patients were older than 65 years old, but the proportion of the oldest old patients (≥80 years old) was relatively small, accounting for only about 5%. Staging IV account for 43.48%. The most common cancer type was gastrointestinal cancer (172 cases, about 67.98% of 253 patients). Among GI cancer, gastric cancer accounted for about 20% (34 cases) and colorectal cancer accounted for about 45% (78 cases). Among non-GI tumors, lung cancer accounted for the largest proportion (56 cases, 70%). As for patients’ treatments, 18.18% of patients experienced 1–3 rounds of chemotherapy, 28.46% experienced 4-6 rounds, and 53.36% experienced more than 7 rounds of chemotherapy. 76.28% of patients had none or less severe comorbidity (CCI <4), whereas 23.72% of patients had severe comorbidity (CCI≥ 4). The most common comorbidities were cardiovascular disease, diabetes mellitus and chronic respiratory disease, consistent with previous surveys of chronic disease burden in the elderly population (Supplementary Figure S1) (Prince et al., 2015).
Most patients had relatively good physical status during each chemotherapy cycle: Among the 1,429 cases, which KPS scores were available, 77.19% of patients (1,103 cases) had a KPS score≥ 90%. Among the 1,470 cases, which ECOG scores were available, 90.88% (1,336 cases) had an ECOG score ≤1. More than half of the cases (58.05%, 865 cases from total 1,490 valid BMI records) had normal weight, 31.82% (474 cases) were overweight or obese (BMI >24 and 28 kg/m2, based on the new Chinese criteria (Pan et al., 2021)) and 10.13% (151 cases) were underweight (BMI <18 kg/m2). Besides, in 1757 total valid data of the number of chemotherapy agents, approximately 80% of cases were treated with a multidrug chemotherapy regimen and about 20% were treated with single-agent chemotherapy. In detail, 56.55% of the regimen contained fluoropyrimidine (such as capecitabine, fluorouracil and tegafur), 60.28% contained platinum, and about 20% contained taxanes (Supplementary Table S1). In addition, in 1,489 total valid data of chemotherapy dose, 50.44% of the patients received physician-determined reduced-dose chemotherapy, while the others used the guideline-recommended standard dose. Of patients who underwent dose adjustment, more than half of the patients (64.85%) received chemotherapy with a reduction of 20%–35%, 29.69% with a reduction of 35%–50%, and only 5.46% with a reduction of more than 50% (Supplementary Table S2).
Consistent with previous studies (Trumper et al., 2006; Muss et al., 2007; Asmis et al., 2008), the incidence of chemotherapy-related toxicity in elderly tumor patients was high, with 79.72% of patients experiencing any grade of adverse events, of which about 24.16% were grade 3–4 adverse events (Table 2). 12 patients died but were considered not to be directly related to chemotherapy toxicity.
Among all non-hematologic adverse events in total 1770 cases, nausea (398 [22.49%]), lack of appetite (376 [21.24%]), hypoalbuminemia (120 [21.47%]) and weakness (257 [14.52%]) were most common, but mostly to a lesser extent. Although a low proportion of the overall cases, infection was the most common severe non-hematologic adverse events: In 1770 cases, only 21 cases had infection but 6 of them (1.19%) were grade 3–5 toxicity, which was the highest proportion of grade 3–5 toxicity among all non-hematologic adverse events, accounting for 28.57%.
The incidence of hematological toxicity was higher than non-hematological toxicity. Among valid data: The most common hematologic adverse events were white blood cell decreased (578 [70.66%]), followed by anemia (576 [64%]) and thrombocytopenia (306 [33.66%]). Among severe hematologic adverse events, neutropenia was the most common (150 [35.63%]).
We assessed the association between severe chemotherapy-related toxicity (Grade≥ 3) and multiple clinical variables (Table 3). There are 12 variables significantly associated with severe chemotherapy-related toxicity: cancer type (non-GI, p < 0.001), number of chemotherapy agents (polychemotherapy, p = 0.042), chemotherapy dose (standard dose, p = 0.078), BMI (<20 kg/m2, p < 0.001), KPS (<90%, p < 0.001), ECOG (>1, p = 0.036), comorbidity (CCI≥4, p = 0.002), low white blood cell (<4 × 109/L, p < 0.001), low neutrophils (<2×109/L, p < 0.001), anemia (hemoglobin<110 g/L, p < 0.001), low platelets (<100×109/L, p < 0.001), hypoalbuminemia (albumin<35 g/L, p < 0.001), and low creatine level (<59 μmol/L, p < 0.001). Outliers in these factors can significantly increase the probability of severe chemotherapy-related toxicity. Focusing on BMI, for example, of the 1,176 non-severe toxicity cases, the proportion of BMI<20 kg/m2 is 20.07% (236 cases), which is increasing significantly to 28.66% (90 cases) in 314 cases with severe toxicity. At the same time, the chi-square (χ2) test obtained p < 0.001, which showed that lower BMI was significantly positively correlated with the occurrence of severe chemotherapy-related toxicity.
We selected 11 variables to construct a chemotherapy-related toxicity predictive model (Table 4). The variables included cancer type (non-GI cancer), BMI< 20 kg/m2, KPS< 90%, severe comorbidity, polychemotherapy, standard dose chemotherapy and 5 laboratory variables (low white blood cells count, anemia, low platelet cells count, low creatine level and hypoalbuminemia.) Each variable was assigned a different risk score (ranged 1–3), with a total score of 21.
Risk score ranges from 0 to 21 points and was divided into three groups (low-risk group, 0 to 6 points; medium-risk group, 6.5 to 12 points; high-risk group, 12.5 to 21 points). Most patients (57.54%) were classified as low-risk group, 40.26% of patients were classified as medium-risk group, and 2.2% were classified as high-risk group. The risk of toxicity increased with increasing risk score (11.98% in the low-risk group, 31.51% in the medium-risk group, and 70.83% in the high-risk group; p < 0.001; Table 5). We examined the internal validation of this model: The area under the ROC curve for the predictive model is 0.723 ([95% CI, 0.687 to 0.759]. Figure 1), suggesting good predictive power of severe chemotherapy toxicity.
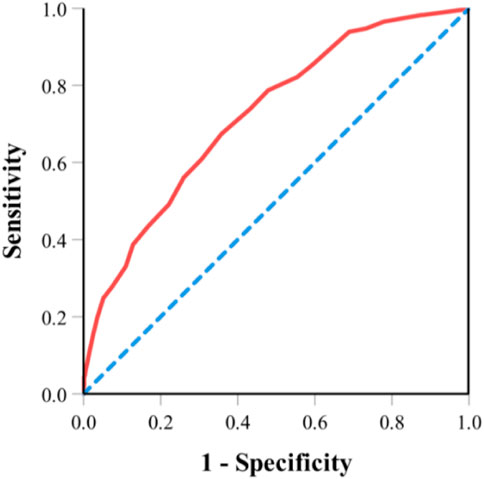
FIGURE 1. ROC curve for the predictive model. Note: Area under the ROC curve for the predictive model is 0.723 ([95% CI, 0.687 to 0.759]. ROC, receiver operating characteristic.
The tolerance of chemotherapy in elderly cancer patients is a concern. Predicting the risk of chemotherapy toxicity in advance can help clinicians identify vulnerable populations.
Several models have been developed to predict chemotherapy toxicity. In 2011, Hurria et al. constructed the CARG score, which is a predictive tool and a risk stratification schema that aims to identify older adults at low, intermediate, or high risk of chemotherapy toxicity (Hurria et al., 2011). The variables included in the model are age, tumor type, treatment intensity, laboratory test values, and a 5-question brief geriatric assessment. The CARG tool is simple to use and has been validated in many studies (Kotzerke et al., 2019; Zhang et al., 2019; Ostwal et al., 2021). In the same year, Extermann et al. published the CRASH Score, which is more comprehensive but relatively complex to use. Among Asian populations (Extermann et al., 2012), the Korean Cancer Study Group (KCSG) score have been developed by Kim and his colleagues but has not yet been widely used (Kim et al., 2018).
In this retrospective single-center study, we sought to develop an objective predictive model based on a Chinese population cohort. In our model, we included tumor types (non-GI cancers), BMI, KPS, severe comorbidity, chemotherapy regimens (multidrug and standard dose chemotherapy) and 5 laboratory variables to predict the risk of chemotherapy-related toxicity.
The effect of BMI, KPS or ECOG score, comorbidity and chemotherapy regimens on chemotherapy tolerance have been widely discussed ((Hurria et al., 2011; Lee et al., 2011; Extermann et al., 2012; Dotan et al., 2020).
Among the laboratory variables, anemia is frequently diagnosed in elderly people and is associated with reduced overall survival. (Knight et al., 2004; Stauder et al., 2018). Anemia leads to increased serum free concentrations of many chemotherapeutic agents that need to bind to red blood cells, thereby increasing toxicity. (Schrijvers et al., 1999; Feliu et al., 2018). Similarly, hypoalbuminemia, an indicator of malnutrition, increases the serum concentration of some drugs and leads to increased chemotherapy toxicity. (Schrijvers et al., 1999; Feliu et al., 2018).
The association between malnutrition and chemotherapy tolerance has been demonstrated in many studies. (Arrieta et al., 2010; Barret et al., 2011; Bozzetti, 2017). Low serum creatinine is a marker of reduced muscle mass associated with malnutrition, aging, and chronic disease, but is often overlooked in clinical practice (Cartin-Ceba et al., 2007; de Jong et al., 2022). In our cohort, there is a large proportion of patients (308/1,500) had a lower-than-normal serum creatinine level which is associated with high risk of chemotherapy-related toxicity. We also analyzed the effect of elevated serum creatinine level on the risk of toxicity but did not obtain statistically significant results, probably because of the small number of this group of people.
Interestingly, non-GI cancer type is associated with severe toxicity in our cohort, which is the opposite of the CARG study results. In the CARG study, patients with gastrointestinal (GI) or genitourinary (GU) tumors had a higher risk of chemotherapy toxicity (Hurria et al., 2011). We thought this may be related to the different toxicity profiles between different human races. Fluoropyrimidine drugs such as 5-fluorouracil, S-1 and capecitabine are recommended by many guidelines and frequently used in the treatment of GI cancers. (Park and Chun, 2013). Studies have found that fluoropyrimidine drugs cause a higher incidence of severe gastrointestinal toxicity in Caucasians than in Asians, partly because of polymorphic differences in the CYP2A6 gene. (Ajani et al., 2005; Haller et al., 2008; Chuah et al., 2011; Ma et al., 2012).
Notably, in our data, the risk of chemotherapy toxicity was not increased with age. This may be because half of our patients had already received dose-reduced regimen. In clinical practice, oncologists often reduce the doses for elderly and frail patients based on their clinical assessment to prevent severe toxicity. (Gajra et al., 2015). However, it is currently unknown whether the empirical adjustment of drug doses will result in optimal clinical outcomes. This uncertainty prompted the development of this predictive model to assess the risk of chemotherapy toxicity in older patients.
There are some limitations to this study. First, this study was a retrospective single-center analysis and more external validation is needed. A prospective clinical study is underway at our center, and further data is expected to confirm the utility of this predictive model.
Second, we did not include comprehensive geriatric assessments such as functional capabilities, cognitive status, emotional status, and social support. Although the ECOG score, comorbidity score, and laboratory variables can partially reflect the health status of elderly patients, they cannot replace the comprehensive geriatric assessment (CGA). However, the comprehensive geriatric assessment is still rarely used in China. In a recent study, the use of CGA tools was found to be only 56.9% in tertiary hospitals in China. (Wu et al., 2022). We are actively collaborating with geriatricians to introduce geriatric assessments into our center and plan to incorporate additional CGA components in future iterations of this model.
This study has some future directions. Many novel anti-tumor therapies such as targeted therapy, immunotherapy, and CAR-T therapy have emerged. However, there are less evidence on the use of these drugs in elderly patients. Toxicity prediction models should also be constructed and validated for these treatments. In addition, even though we successfully stratified patients according to their risk of chemotherapy toxicity, it is still unclear what percent of dose reduction should be applied to each group of patients.
In conclusion, we constructed a simple and objective model with 11 variables to predict chemotherapy-related toxicity in elderly cancer patients. This model aims to help clinicians identify vulnerable populations as well as formulate the best treatment and nursing strategies for elderly cancer patients.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
MG conceived and designed the study. YPZ, YH, and FL collected the data. YLZ performed the statistical analysis. YH and YLZ wrote the draft of the manuscript. All authors reviewed the results and approved the final version of the manuscript.
This research was supported by National High Level Hospital Clinical Research Funding No. 2022-PUMCH-B-049.
We would like to express our gratitude to all the medical staff of the Department of Oncology of Peking Union Medical College Hospital.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1158421/full#supplementary-material
Ajani, J. A., Faust, J., Ikeda, K., Yao, J. C., Anbe, H., Carr, K. L., et al. (2005). Phase I pharmacokinetic study of S-1 plus cisplatin in patients with advanced gastric carcinoma. J. Clin. Oncol. 23, 6957–6965. doi:10.1200/JCO.2005.01.917
Arrieta, O., Michel Ortega, R. M., Villanueva-Rodríguez, G., Serna-Thomé, M. G., Flores-Estrada, D., Diaz-Romero, C., et al. (2010). Association of nutritional status and serum albumin levels with development of toxicity in patients with advanced non-small cell lung cancer treated with paclitaxel-cisplatin chemotherapy: A prospective study. BMC Cancer 10, 50. doi:10.1186/1471-2407-10-50
Asmis, T. R., Ding, K., Seymour, L., Shepherd, F. A., Leighl, N. B., Winton, T. L., et al. (2008). Age and comorbidity as independent prognostic factors in the treatment of non small-cell lung cancer: A review of national cancer Institute of Canada clinical trials group trials. J. Clin. Oncol. 26, 54–59. doi:10.1200/JCO.2007.12.8322
Barret, M., Malka, D., Aparicio, T., Dalban, C., Locher, C., Sabate, J. M., et al. (2011). Nutritional status affects treatment tolerability and survival in metastatic colorectal cancer patients: Results of an AGEO prospective multicenter study. Oncology 81, 395–402. doi:10.1159/000335478
Bozzetti, F. (2017). Forcing the vicious circle: Sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Ann. Oncol. 28, 2107–2118. doi:10.1093/annonc/mdx271
Brunello, A., Loaldi, E., and Balducci, L. (2009). Dose adjustment and supportive care before and during treatment. Cancer Treat. Rev. 35, 493–498. doi:10.1016/j.ctrv.2009.04.009
Cartin-Ceba, R., Afessa, B., and Gajic, O. (2007). Low baseline serum creatinine concentration predicts mortality in critically ill patients independent of body mass index. Crit. Care Med. 35, 2420–2423. doi:10.1097/01.ccm.0000281856.78526.f4
Chang, A. Y., Skirbekk, V. F., Tyrovolas, S., Kassebaum, N. J., and Dieleman, J. L. (2019). Measuring population ageing: An analysis of the global burden of disease study 2017. Lancet Public Health 4, e159–e167. doi:10.1016/S2468-2667(19)30019-2
Charlson, M. E., Pompei, P., Ales, K. L., and MacKenzie, C. R. (1987). A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 40, 373–383. doi:10.1016/0021-9681(87)90171-8
Chuah, B., Goh, B. C., Lee, S. C., Soong, R., Lau, F., Mulay, M., et al. (2011). Comparison of the pharmacokinetics and pharmacodynamics of S-1 between caucasian and east asian patients. Cancer Sci. 102, 478–483. doi:10.1111/j.1349-7006.2010.01793.x
Concato, J., Feinstein, A. R., and Holford, T. R. (1993). The risk of determining risk with multivariable models. Ann. Intern Med. 118, 201–210. doi:10.7326/0003-4819-118-3-199302010-00009
de Jong, C., Chargi, N., Herder, G. J. M., van Haarlem, S. W. A., van der Meer, F., van Lindert, A. S. R., et al. (2022). The association between skeletal muscle measures and chemotherapy-induced toxicity in non-small cell lung cancer patients. J. Cachexia Sarcopenia Muscle 13, 1554–1564. doi:10.1002/jcsm.12967
Dotan, E., Tew, W. P., Mohile, S. G., Ma, H., Kim, H., Sun, C. L., et al. (2020). Associations between nutritional factors and chemotherapy toxicity in older adults with solid tumors. Cancer 126, 1708–1716. doi:10.1002/cncr.32718
Extermann, M., Boler, I., Reich, R. R., Lyman, G. H., Brown, R. H., DeFelice, J., et al. (2012). Predicting the risk of chemotherapy toxicity in older patients: The chemotherapy risk assessment Scale for high-age patients (CRASH) score. Cancer 118, 3377–3386. doi:10.1002/cncr.26646
Feliu, J., Heredia-Soto, V., Gironés, R., Jiménez-Munarriz, B., Saldaña, J., Guillén-Ponce, C., et al. (2018). Can we avoid the toxicity of chemotherapy in elderly cancer patients? Crit. Rev. Oncol. Hematol. 131, 16–23. doi:10.1016/j.critrevonc.2018.08.008
Gajra, A., Klepin, H. D., Feng, T., Tew, W. P., Mohile, S. G., Owusu, C., et al. (2015). Predictors of chemotherapy dose reduction at first cycle in patients age 65 years and older with solid tumors. J. Geriatr. Oncol. 6, 133–140. doi:10.1016/j.jgo.2014.12.002
Hall, P. S., Swinson, D., Cairns, D. A., Waters, J. S., Petty, R., Allmark, C., et al. (2021). Efficacy of reduced-intensity chemotherapy with oxaliplatin and capecitabine on quality of life and cancer control among older and frail patients with advanced gastroesophageal cancer: The GO2 phase 3 randomized clinical trial. JAMA Oncol. 7, 869–877. doi:10.1001/jamaoncol.2021.0848
Haller, D. G., Cassidy, J., Clarke, S. J., Cunningham, D., Van Cutsem, E., Hoff, P. M., et al. (2008). Potential regional differences for the tolerability profiles of fluoropyrimidines. J. Clin. Oncol. 26, 2118–2123. doi:10.1200/JCO.2007.15.2090
Hernandez Torres, C., and Hsu, T. (2017). Comprehensive geriatric assessment in the older adult with cancer: A review. Eur. Urol. Focus 3, 330–339. doi:10.1016/j.euf.2017.10.010
Hurria, A., Dale, W., Mooney, M., Rowland, J. H., Ballman, K. V., Cohen, H. J., et al. (2014). Designing therapeutic clinical trials for older and frail adults with cancer: U13 conference recommendations. J. Clin. Oncol. 32, 2587–2594. doi:10.1200/JCO.2013.55.0418
Hurria, A., Togawa, K., Mohile, S. G., Owusu, C., Klepin, H. D., Gross, C. P., et al. (2011). Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J. Clin. Oncol. 29, 3457–3465. doi:10.1200/JCO.2011.34.7625
Joharatnam-Hogan, N., Shiu, K. K., and Khan, K. (2020). Challenges in the treatment of gastric cancer in the older patient. Cancer Treat. Rev. 85, 101980. doi:10.1016/j.ctrv.2020.101980
Kim, J. W., Lee, Y. G., Hwang, I. G., Song, H. S., Koh, S. J., Ko, Y. H., et al. (2018). Predicting cumulative incidence of adverse events in older patients with cancer undergoing first-line palliative chemotherapy: Korean cancer study group (KCSG) multicentre prospective study. Br. J. Cancer 118, 1169–1175. doi:10.1038/s41416-018-0037-6
Knight, K., Wade, S., and Balducci, L. (2004). Prevalence and outcomes of anemia in cancer: A systematic review of the literature. Am. J. Med. 116 (7), 11S–26S. doi:10.1016/j.amjmed.2003.12.008
Kotzerke, D., Moritz, F., Mantovani, L., Hambsch, P., Hering, K., Kuhnt, T., et al. (2019). The performance of three oncogeriatric screening tools - G8, optimised G8 and CARG - in predicting chemotherapy-related toxicity in older patients with cancer. A prospective clinical study. J. Geriatr. Oncol. 10, 937–943. doi:10.1016/j.jgo.2019.04.004
Lee, L., Cheung, W. Y., Atkinson, E., and Krzyzanowska, M. K. (2011). Impact of comorbidity on chemotherapy use and outcomes in solid tumors: A systematic review. J. Clin. Oncol. 29, 106–117. doi:10.1200/JCO.2010.31.3049
Ma, Y., Tang, L., Wang, H. X., Xu, Y. C., Ma, Y., and Zhang, F. C. (2012). Capecitabine for the treatment for advanced gastric cancer: Efficacy, safety and ethnicity. J. Clin. Pharm. Ther. 37, 266–275. doi:10.1111/j.1365-2710.2011.01289.x
Macchini, M., Chiaravalli, M., Zanon, S., Peretti, U., Mazza, E., Gianni, L., et al. (2019). Chemotherapy in elderly patients with pancreatic cancer: Efficacy, feasibility and future perspectives. Cancer Treat. Rev. 72, 1–6. doi:10.1016/j.ctrv.2018.10.013
Muss, H. B., Berry, D. A., Cirrincione, C., Budman, D. R., Henderson, I. C., Citron, M. L., et al. (2007). Toxicity of older and younger patients treated with adjuvant chemotherapy for node-positive breast cancer: The cancer and leukemia group B experience. J. Clin. Oncol. 25, 3699–3704. doi:10.1200/JCO.2007.10.9710
National Cancer Institute (2017). Common Terminology criteria for adverse events (CTCAE). National Cancer Institute. U.S. Department of Health and Human Services.
Ostwal, V., Ramaswamy, A., Bhargava, P., Hatkhambkar, T., Swami, R., Rastogi, S., et al. (2021). Cancer aging research group (CARG) score in older adults undergoing curative intent chemotherapy: A prospective cohort study. BMJ Open 11, e047376. doi:10.1136/bmjopen-2020-047376
Park, S. C., and Chun, H. J. (2013). Chemotherapy for advanced gastric cancer: Review and update of current practices. Gut Liver 7, 385–393. doi:10.5009/gnl.2013.7.4.385
Pan, X- F., Wang, L., and Pan, A. (2005). Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 9. doi:10.1016/S2213-8587(21)00045-0
Prince, M. J., Wu, F., Guo, MY., Gutierrez Robledo, L. M., O’Donnell, M., Sullivan, R., et al. (2015). The burden of disease in older people and implications for health policy and practice. London, England: Lancet 385. doi:10.1016/S0140-6736(14)61347-7
Schrijvers, D., Highley, M., De Bruyn, E., Van Oosterom, A. T., and Vermorken, J. B. (1999). Role of red blood cells in pharmacokinetics of chemotherapeutic agents. Anticancer Drugs 10, 147–153. doi:10.1097/00001813-199902000-00002
Stauder, R., Valent, P., and Theurl, I. (2018). Anemia at older age: Etiologies, clinical implications, and management. Blood 131, 505–514. doi:10.1182/blood-2017-07-746446
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. doi:10.3322/caac.21660
Trumper, M., Ross, P. J., Cunningham, D., Norman, A. R., Hawkins, R., Seymour, M., et al. (2006). Efficacy and tolerability of chemotherapy in elderly patients with advanced oesophago-gastric cancer: A pooled analysis of three clinical trials. Eur. J. Cancer 42, 827–834. doi:10.1016/j.ejca.2005.08.044
Walter, L. C., Brand, R. J., Counsell, S. R., Palmer, R. M., Landefeld, C. S., Fortinsky, R. H., et al. (2001). Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA 285, 2987–2994. doi:10.1001/jama.285.23.2987
Wu, W., Shen, J., Zhang, J., Li, H., Li, J., and Shi, H. (2022). Pulmonary vascular volume is associated with DLCO and fibrotic score in idiopathic pulmonary fibrosis: An observational study. Chin. J. Geriatrics 41, 76–79. doi:10.1186/s12880-022-00803-8
Youden, W. J. (1950). Index for rating diagnostic tests. Cancer 3, 32–35. doi:10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3
Keywords: chemotherapy, gastrointestinal cancers, drug-related adverse effects, elderly, cancer
Citation: Hua Y, Zou Y, Guan M, Yuan H-Y, Zhou Y and Liu F (2023) Predictive model of chemotherapy-related toxicity in elderly Chinese cancer patients. Front. Pharmacol. 14:1158421. doi: 10.3389/fphar.2023.1158421
Received: 03 February 2023; Accepted: 10 April 2023;
Published: 26 April 2023.
Edited by:
Hongwei Yao, Beijing Friendship Hospital, ChinaReviewed by:
Chee Kin Then, University of Oxford, United KingdomCopyright © 2023 Hua, Zou, Guan, Yuan, Zhou and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mei Guan, Z3Vhbm1laTcxQDEyNi5jb20=; Hsiang-Yu Yuan, c2Vhbi55dWFuQGNpdHl1LmVkdS5oaw==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.