- 1The First Clinical Medical College, Shaanxi University of Chinese Medicine, Xianyang, China
- 2Department of Gastroenterology, The Affiliated Hospital of Shaanxi University of Chinese Medicine, Xianyang, China
Background: Antibiotics alter the microbial balance commonly resulting in antibiotic-associated diarrhea (AAD). Probiotics may prevent and treat AAD by providing the gut barrier and restoring the gut microflora. This study will overview the Systematic Reviews (SRs) of probiotics in preventing and treating AAD in children. It will also assess the reporting, methodological, and evidence quality of the included SRs to provide evidence for their clinical practice.
Methods: After searching PubMed, Embase, Cochrane Library, CNKI, CBM, VIP, and WanFang Data databases, and finally included SRs of probiotics in the prevention and treatment of AAD in children, which were published before 1 October 2022. The reporting, methodological, and evidence quality of the included SRs were assessed by PRISMA 2020 statement, AMSTAR 2 tool, and GRADE system.
Results: A total of 20 SRs were included, and the results of PRISMA 2020 showed that 4 out of 20 SRs with relatively complete reporting, and the others within some reporting deficiencies, with scores ranging from 17 points to 26.5 points; the results of AMSTAR 2 showed that 3 SRs belonged to moderate quality level, 10 SRs belonged to low-quality level and 7 SRs being extremely low-quality level; the results of the GRADE system showed that a total of 47 outcomes were reported for the included SRs, three were high-level evidence quality, 16 were medium-level evidence quality, 24 were low-level evidence quality, and four were extremely low-level evidence quality; the results of the Meta-analysis showed that high doses (5–40 billion CFUs per day) of probiotics had a significant effect in the prevention of AAD, but it is too early to conclude the effectiveness and safety of other probiotic drugs for AAD in children, except for Lacticaseibacillus rhamnosus and Saccharomyces boulardii.
Conclusion: Current evidence shows that probiotics effectively prevent and treat AAD in children, and the effect of probiotics on pediatric AAD may be a potential dose-response effect. However, the conclusion should be treated with caution due to deficiencies in the methodological, reporting, and evidence quality of the included SRs. Therefore, the methodological, reporting, and evidence quality of relevant SRs still need further improvement.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42022362328
1 Introduction
Antibiotic-associated diarrhea (AAD) is defined as diarrhea that occurs in the long-term use of antimicrobial drugs leading to dysbiosis of the intestinal flora (Bartlett, 2002). With the increasing degree of intestinal dysbiosis, the clinical manifestations of AAD can progress from mild diarrhea to acute and severe disease such as pseudomembranous colitis or toxic megacolon (seen in Clostridium difficile infection) (Bartlett, 2002; Zheng et al., 2021). The incidence and severity of clinical manifestations of AAD are related to the type of antibiotic, duration of use, patient health status, and the type of pathogen to which the patient is exposed (McFarland, 2008; Hayes and Vargas, 2016). Some studies showed that the incidence of childhood AAD in the United States ranged from 6% in outpatients to 80% in hospitalized children (McFarland et al., 2016). The incidence of childhood AAD in China has only been studied in hospitalized children, with incidence rates ranging from 16.80% to 70.59% (Zheng et al., 2021).
Currently, antibiotic-induced dysbiosis of the intestinal flora is the primary mechanism of AAD pathogenesis, and the basic therapeutic approach is re-establishing intestinal flora homeostasis (Zheng et al., 2021). Clinical commonly used bioactive agents, such as probiotics (living microorganisms, when administered with sufficient amounts of probiotics, may bring health benefits to the host) (Hill et al., 2014), prebiotics (a substrate that is selectively utilized by the microorganisms of the host, conferring a health benefit) (Gibson et al., 2017), synbiotics (a mixture comprising live microorganisms and substrates selectively utilized by host microorganisms that confers a health benefit on the host) (Swanson et al., 2020), and postbiotics (preparation of inanimate microorganisms and their components that confers a health benefit on the host) (Salminen et al., 2021). The European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) recommendations for probiotics to prevent antibiotic-associated diarrhea high doses (≥5 billion CFU/day) of Lacticaseibacillus rhamnosus (L. rhamnosus) GG or Saccharomyces boulardii (S. boulardii) started simultaneously with antibiotic treatment (certainty of evidence: moderate; grade of recommendation: strong) (Szajewska et al., 2023) There are many systematic reviews (SRs) that have explored the efficacy and adverse effects of probiotics in pediatric AAD (Johnston et al., 2006; Szajewska et al., 2006; Johnston et al., 2011; Szajewska and Kołodziej, 2015a; Szajewska and Kołodziej, 2015b; Goldenberg et al., 2015; Szajewska et al., 2016; Xu et al., 2017; Guo et al., 2019; Storr and Stengel, 2021), however, their methodological, reporting and evidence quality of evidence are unclear. An overview of systematic reviews is a comprehensive approach that collects relevant systematic reviews of the treatment, etiology, diagnosis, and prognosis of the same disease or health problem (Lunny et al., 2017; Lunny et al., 2018). The principal objective of this overview was to clarify the benefits of probiotics for the prevention or treatment of AAD in children, which promotes evidence-based decision-making. Therefore, this study will overview SRs related to probiotics in preventing and treating AAD in children. It will also assess the methodological, reporting, and evidence quality of the included SRs to provide evidence for their clinical practice.
2 Methods
2.1 Project registration
This study was registered in the PROSPERO platform at the beginning of the project, ID: CRD42022362328.
2.2 Data sources
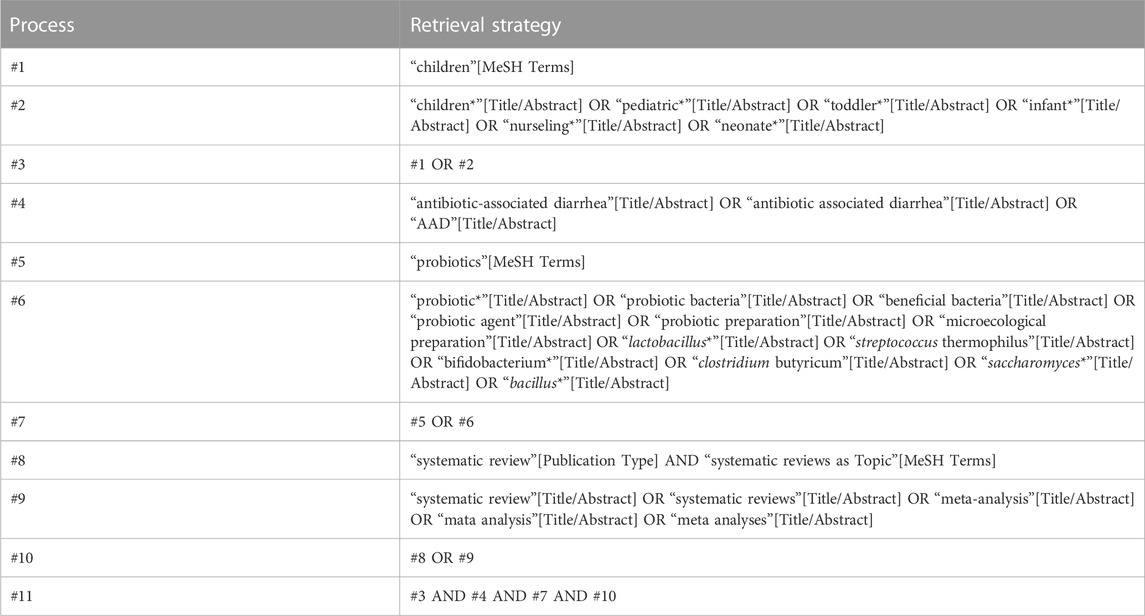
The databases of PubMed, Embase, Cochrane Library, Chinese Biomedical Literature Database (CBM), Chinese Journal Full Text Database (CNKI), Vipers Database (VIP), and WanFang Data Knowledge Service Platform (WanFang Data) were searched from their inception to 1 October 2022. The languages were limited to Chinese and English. The search terms included: probiotics, microecological agents, children, antibiotic associated diarrhea, systematic reviews, Mata analysis, Child, Antibiotic-associated diarrhea, Diarrhea, Systematic Review, and Meta-analysis. The specific search strategy for the PubMed database, for example, is shown in Table 1.
2.3 Inclusion criteria
2.3.1 Type of study
Systematic review or Meta-analysis.
2.3.2 Study population
(1) Patient type: patients with AAD; (2) Referring to the definition of children in Pediatrics: children aged ≤18 years old (Pomerance, 1997). There was no restriction on their gender or duration of illness.
2.3.3 Interventions
The treatment group was probiotics or probiotics combined with conventional Western medical treatment (CWM), and the control group was CWM, placebo, or blank control. The type, usage, dose, and duration of probiotics were not limited.
2.3.4 Outcome indexes
Any efficacy and safety indexes.
2.4 Exclusion criteria
(1) Duplicate published literature; (2) Literature with inaccessible full text or incomplete data; (4) Studies containing a systematic review and Meta-analysis of other types of diarrhea; (3) Probiotic-related review studies.
2.5 Literature screening and data extraction
Two researchers (YL and XL) independently screened the literature and extracted data. They cross-checked them in parallel and negotiated, discussed, or consulted a third researcher (XD) in case of disagreement. Data extraction included: authors, disease names, sample size and interventions, and Meta-analysis results.
2.6 Quality assessment
Two researchers independently evaluated the reporting, methodological, and evidence quality of the included SRs using PRISMA 2020 (Page et al., 2021a; Page et al., 2021b), AMSTAR 2 (Shea et al., 2017) and the GRADE system (Atkins et al., 2004; Balshem et al., 2011), cross-checking in parallel and consulting a third party in case of disagreement. PRISMA 2020 consists of 27 items, and each item is scored as 1) fully satisfied (i.e., complete reporting) is scored as 1; 2) partially satisfied (i.e., partial reporting) is scored as 0.5; and 3) not satisfied (i.e., not reported) is scored as 0. AMSTAR 2 consists of 16 items, of which 7 are key items; each item is evaluated as “yes” (fully reported), “partially yes” (partially reported), and “partially yes” (partially reported). Combining the results of the key and non-key item assessments, each included SR was rated as high, moderate, low or very low in quality. Escalation factors for GRADE are large effect size, dose-effect relationship, and negative bias, and the downgrading factors are risk of bias, inconsistency, indirectness, imprecision, and publication bias. The level of evidence for the indicators was evaluated as high, moderate, low, or very low. Two researchers (YL and XL) independently assessed the evidence quality.
2.7 Statistical analysis
The extracted information was collated using Excel 2020—descriptive statistical analysis of frequency and percentage of the included studies. The risk ratio (RR), odds ratio (OR), 95% confidence interval (CI), weighted mean difference (WMD), standard mean difference (SMD), Relative Risk Reduction (RRR), and number needed to treat (NNT) were included to summarize the results. The heterogeneity of each included SR was extracted, which was detected by I2 statistics.
3 Results
3.1 Literature search
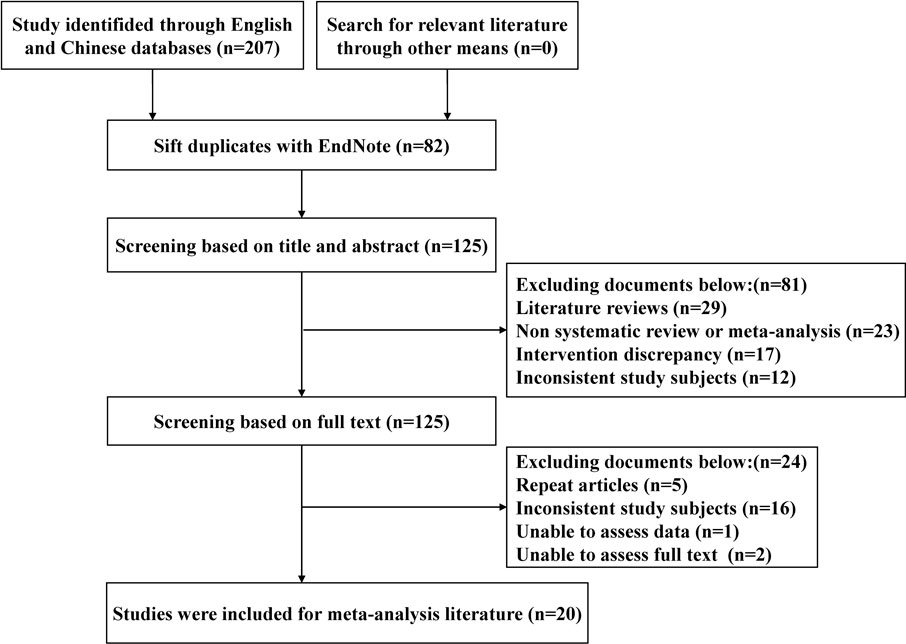
A total of 207 studies were obtained for the initial review, and 20 SRs were finally included after a hierarchical screening process (Johnston et al., 2006; Shi et al., 2006; Szajewska et al., 2006; Chen et al., 2010; Lu, 2010; Johnston et al., 2011; Fang et al., 2013; Chai et al., 2015; Goldenberg et al., 2015; You and Gao, 2015; Yang et al., 2016a; Yang et al., 2016b; Szajewska et al., 2016; Zhou et al., 2016; Chai et al., 2017; He et al., 2017; Xu et al., 2017; Guo et al., 2019; Liu et al., 2020; Liu et al., 2022), and the literature screening process and results are shown in Figure 1.
3.2 Study characteristics
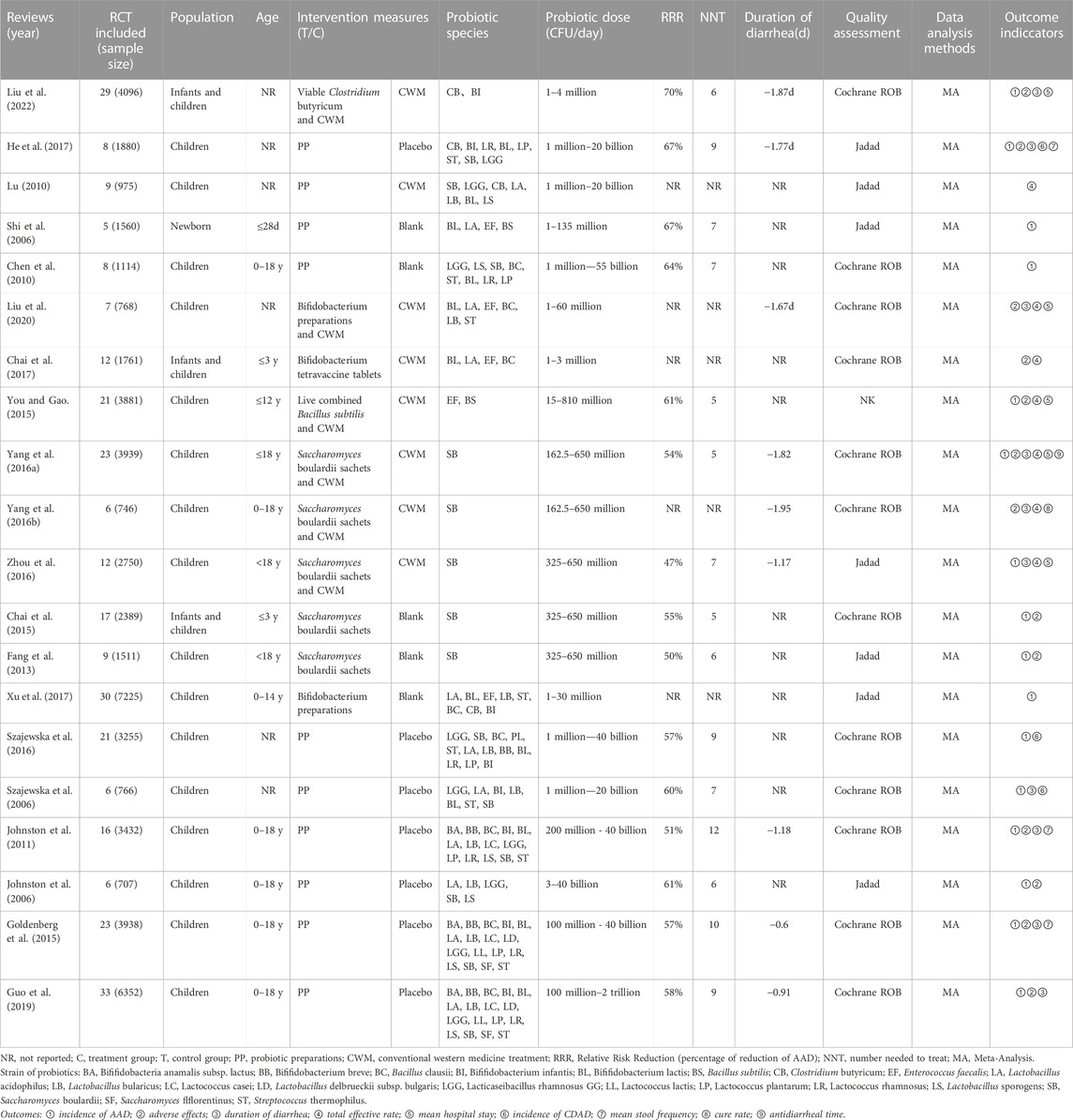
The basic information of the included studies is shown in Table 2. Among the 20 SRs included, 13 SRs (Shi et al., 2006; Chen et al., 2010; Lu, 2010; Fang et al., 2013; Chai et al., 2015; You and Gao, 2015; Yang et al., 2016a; Yang et al., 2016b; Zhou et al., 2016; Chai et al., 2017; He et al., 2017; Liu et al., 2020; Liu et al., 2022) were in Chinese and seven SRs (Johnston et al., 2006; Szajewska et al., 2006; Johnston et al., 2011; Goldenberg et al., 2015; Szajewska et al., 2016; Xu et al., 2017; Guo et al., 2019) were in English, published from 2006 to 2022. All SRs were included in randomized controlled trial studies (RCTs), and all used Meta-analysis to process the data. The main probiotics include Bacillus spp., Bifidobacterium spp., Lacticaseibacillus spp., Lactococcus spp., Saccharomyces spp., and Streptococcus spp. The daily dosage of probiotics varied greatly from 1 million to 2 trillion CFUs/day. Twelve SRs (Szajewska et al., 2006; Chen et al., 2010; Johnston et al., 2011; Chai et al., 2015; Goldenberg et al., 2015; Yang et al., 2016a; Yang et al., 2016b; Szajewska et al., 2016; Chai et al., 2017; Guo et al., 2019; Liu et al., 2020; Liu et al., 2022) used the Cochrane systematic review tool, seven SRs (Johnston et al., 2006; Shi et al., 2006; Lu, 2010; Fang et al., 2013; Zhou et al., 2016; He et al., 2017; Xu et al., 2017) used the Jadad scale, and one SR (You and Gao, 2015) did not report a risk of the bias assessment tool.
3.3 Reporting quality
The results of the PRISMA 2020 are shown in Table 3: the scores of the included 20 SRs ranged from 16.5 to 26.5, four SRs (Johnston et al., 2006; Johnston et al., 2011; Goldenberg et al., 2015; Guo et al., 2019) (20%) had relatively complete reports, and 16 SRs (Shi et al., 2006; Szajewska et al., 2006; Chen et al., 2010; Lu, 2010; Fang et al., 2013; Chai et al., 2015; You and Gao, 2015; Yang et al., 2016a; Yang et al., 2016b; Szajewska et al., 2016; Zhou et al., 2016; Chai et al., 2017; He et al., 2017; Xu et al., 2017; Liu et al., 2020; Liu et al., 2022) (80%) had some reporting deficiencies. Among the reporting deficiencies, the main ones were found in item 24: Program and registration, followed by item 15: Other analysis in the methods section and item 22: Other analysis in the results section, as well as other information related to the item on funding.
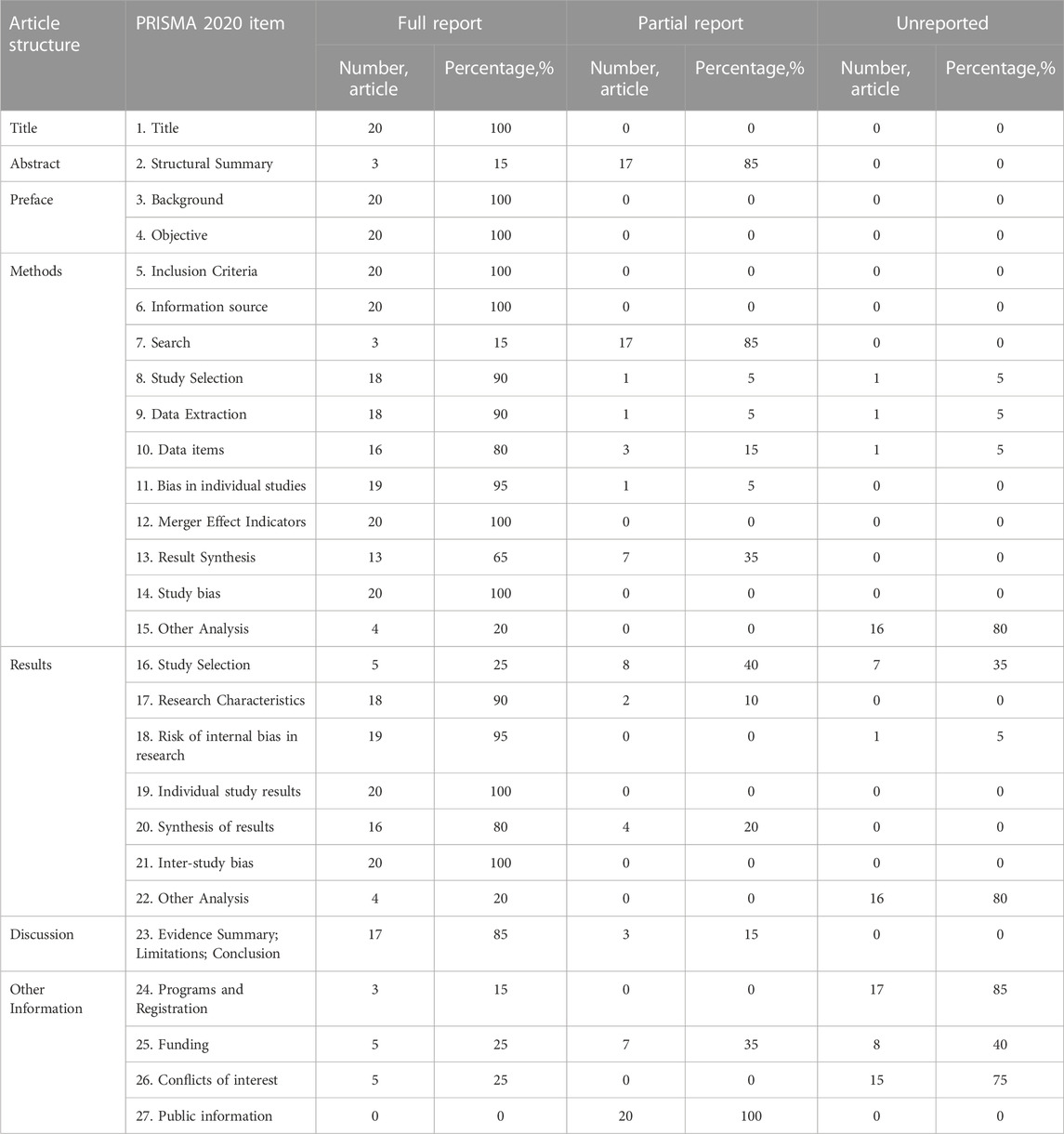
TABLE 3. Quality of reporting of included systematic reviews assessed using the PRISMA 2020 statement.
3.4 Methodological quality
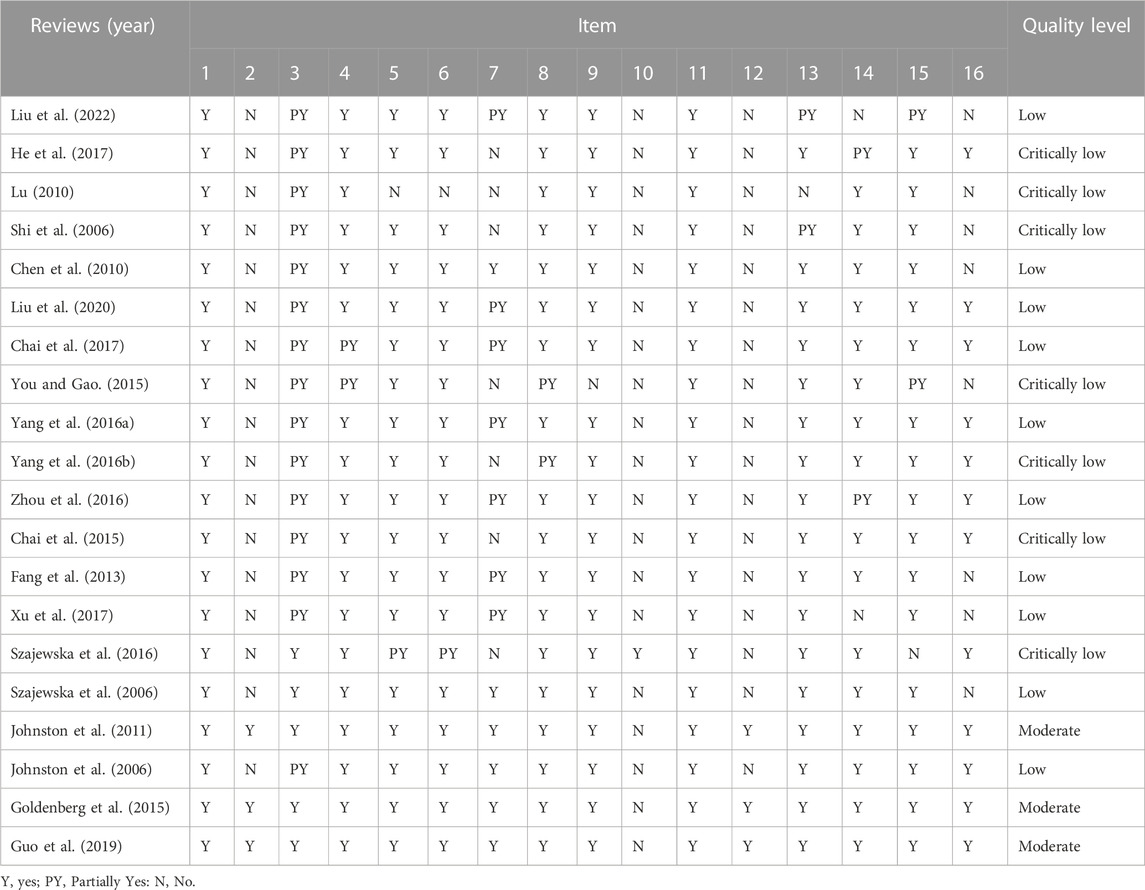
The results of the AMSTAR 2 are shown in Table 4: in the 20 SRs included, three SRs (Johnston et al., 2011; Goldenberg et al., 2015; Guo et al., 2019) (15%) were of medium quality, 10 SRs (Johnston et al., 2006; Szajewska et al., 2006; Chen et al., 2010; Fang et al., 2013; Yang et al., 2016a; Zhou et al., 2016; Chai et al., 2017; Xu et al., 2017; Liu et al., 2020; Liu et al., 2022) (50%) were of low quality, and seven SRs (Shi et al., 2006; Lu, 2010; Chai et al., 2015; You and Gao, 2015; Yang et al., 2016b; Szajewska et al., 2016; He et al., 2017) (35%) were of very low quality. The main reason for the lower quality level was that item 10, item 2, item 12, item 3, item 7, and item 16 were not reported.
3.5 GRADE quality of evidence
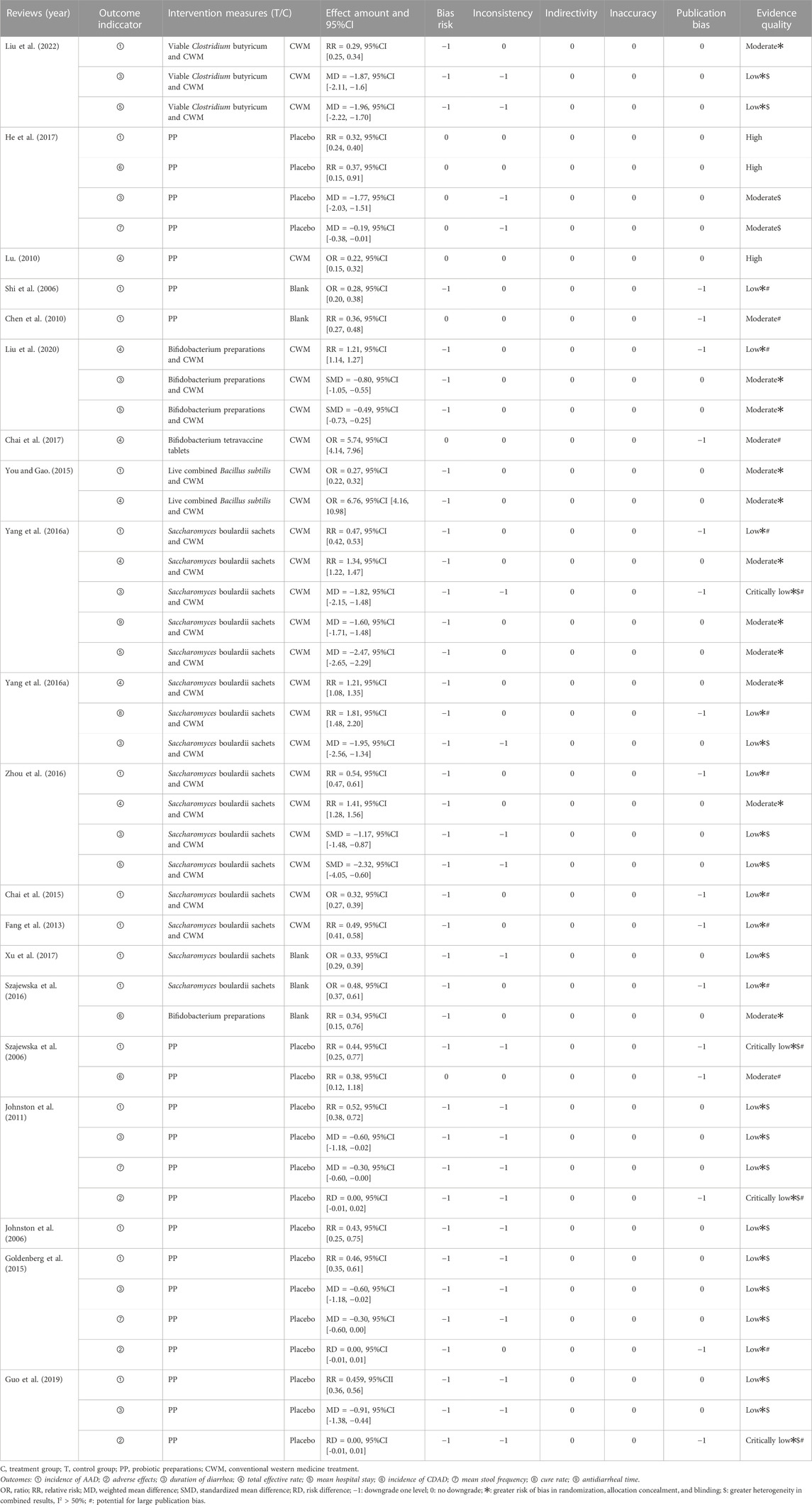
Based on the preventive and therapeutic effects of probiotics on ADD in clinical studies, as well as the adverse effects produced, the results of the quantitative analysis of the outcome indicators and the quality of evidence results of the 20 SRs from these three aspects are summarized and reported below, as detailed in Table 5.
3.5.1 Indicators of preventive effects
3.5.1.1 AAD incidence
Sixteen SRs (Johnston et al., 2006; Shi et al., 2006; Szajewska et al., 2006; Chen et al., 2010; Johnston et al., 2011; Fang et al., 2013; Chai et al., 2015; Goldenberg et al., 2015; You and Gao, 2015; Yang et al., 2016a; Szajewska et al., 2016; Zhou et al., 2016; He et al., 2017; Xu et al., 2017; Guo et al., 2019; Liu et al., 2022) reported the incidence of ADD. The GRADE system showed that one was of high quality, three were of moderate quality, eleven were of low quality, and one was of very low quality. The results showed that probiotics and probiotics combined with conventional western medical treatment were superior to conventional western medical treatment, placebo, and blank control in reducing the incidence of AAD, with statistically significant differences (p < 0.05).
3.5.1.2 CDAD incidence
Three SRs (Szajewska et al., 2006; Szajewska et al., 2016; He et al., 2017) reported the incidence of Clostridium difficile-associated diarrhea (CDAD) belonging to severe AAD. The GRADE system showed that one was of high quality and two were of moderate quality, suggesting that probiotics were superior to placebo in the incidence of CDAD, with a statistically significant difference (p < 0.05).
3.5.2 Indicators of treatment effects
3.5.2.1 Duration of diarrhea
Duration of diarrhea was reported in 10 SRs (Szajewska et al., 2006; Johnston et al., 2011; Goldenberg et al., 2015; Yang et al., 2016a; Yang et al., 2016b; Zhou et al., 2016; He et al., 2017; Guo et al., 2019; Liu et al., 2020; Liu et al., 2022) and data were not combined for Meta-analysis in one SR (Szajewska et al., 2006). The GRADE system showed that two were of medium quality, six were of low quality, and one very low quality. Four of the SRs (Johnston et al., 2011; Goldenberg et al., 2015; He et al., 2017; Guo et al., 2019) with probiotics alone and five SRs (Yang et al., 2016a; Yang et al., 2016b; Zhou et al., 2016; Liu et al., 2020; Liu et al., 2022) by probiotics combined with conventional Western medical treatment showed superiority overview placebo and conventional Western medical treatment in reducing the duration of diarrhea, with statistically significant differences (p < 0.05).
3.5.2.2 Total effective rate
Seven SRs (Lu, 2010; You and Gao, 2015; Yang et al., 2016a; Yang et al., 2016b; Zhou et al., 2016; Chai et al., 2017; Liu et al., 2020) mentioned the total effective rate. The GRADE system showed that one was high quality, five were medium quality, and one was low quality. The results suggest that the total effective rate of both probiotics and probiotics combined with conventional western medical treatment was better than conventional western medical treatment for AAD in children, and the difference was statistically significant (p < 0.05).
3.5.2.3 Mean hospital stay
Five SRs (You and Gao, 2015; Yang et al., 2016a; Zhou et al., 2016; Liu et al., 2020; Liu et al., 2022) reported mean hospital stay, but one study (You and Gao, 2015) had a pooled data results of mean length of stay (MD: −53.19, 95% CI: −79.63 to −26.75), which was considered synthetically as a data error. Therefore, the quality of evidence was not evaluated for the outcome indicators in this overview. The GRADE system showed that two were medium quality and two were low quality. The results suggest that probiotic combined with conventional western medical treatment was superior to conventional western medical treatment in reducing the mean hospital stay in all cases, with a statistically significant difference (p < 0.05).
3.5.2.4 Mean frequency of diarrhea
Three SRs (Johnston et al., 2011; Goldenberg et al., 2015; He et al., 2017) assessed the mean diarrhea frequency. The GRADE system showed that one was of medium quality and two were of low quality. The results of only one of these SRs (He et al., 2017) suggested a statistically significant difference in the mean frequency of diarrhea in children with AAD treated with probiotics compared with placebo (p < 0.05).
3.5.2.5 Cure rate
Only one SRs (Yang et al., 2016b) analyzed the cure rate index. GRADE system the results as high quality, and the study showed that probiotics combined with conventional western medical treatment improved the cure rate of AAD in children compared with conventional western medical treatment alone, and the difference was statistically significant (p < 0.05).
3.5.2.6 Antidiarrheal time
Only one SRs (Yang et al., 2016a) analyzed the time to stop diarrhea index, and the GRADE system the results as high quality. The study showed that using probiotics on top of conventional western medical treatment could be better than conventional western medical treatment in reducing the time to stop diarrhea, and the difference was statistically significant (p < 0.05).
3.5.3 Adverse effects
Adverse Drug Reaction (ADR) was not defined in advance in all studies. 13 SRs (Johnston et al., 2006; Johnston et al., 2011; Fang et al., 2013; Chai et al., 2015; Goldenberg et al., 2015; You and Gao, 2015; Yang et al., 2016a; Yang et al., 2016b; Chai et al., 2017; He et al., 2017; Guo et al., 2019; Liu et al., 2020; Liu et al., 2022) reported ADRs, which mainly manifested as damage to the gastrointestinal digestive system and skin mucosa, including dry mouth, nausea, vomiting, belching, sputum, taste disturbance, loss of appetite, headache, chest pain, gastrointestinal distention, reflux, abdominal pain, constipation, rash, allergic reaction to antibiotics and mycosis stomatitis, but most studies did not report the group in which the ADR occurred (treatment or control group). There are six SRs (Johnston et al., 2006; Johnston et al., 2011; Goldenberg et al., 2015; Yang et al., 2016a; Guo et al., 2019; Liu et al., 2022) in the literature describing the specifics of ADR, of which only three SRs (Johnston et al., 2011; Goldenberg et al., 2015; Guo et al., 2019) combined data for Meta-analysis of ADR, and the GRADE system showed one of low quality and two of very low quality, which showed no statistically significant differences between the two groups (p > 0.05). In addition, two SRs (Szajewska et al., 2006; Szajewska et al., 2016) did not mention the specific occurrence of ADR, and five SRs (You and Gao, 2015; Yang et al., 2016b; Chai et al., 2017; He et al., 2017; Liu et al., 2020) mentioned that no ADR was seen. The above indicates that the incidence of ADRs in probiotics is low, suggesting that probiotics are safe to prevent and treat AAD.
4 Discussion
4.1 Major findings
The principle objective of this overview was to clarify the benefits of probiotics for preventing or treating AAD in children, which promotes evidence-based decision-making. The main used microorganisms in probiotic preparations in 20 SRs are bacteria of the Lactobacillaceae family, particularly L. rhamnosus and L. acidophilus, as well as L. plantarum, L. casei, L. lactis and L. bulgaricus. Probiotics frequently contain bacteria of the genera Bifidobacterium (B. longum, B. infantis, B. breve), Clostridium, Lactococcus, Enterococcus, Bacillus, and strains of S. thermophiles. In addition, strains of Saccharomyces species, such as S. boulardii also present in these preparations (Table 2). We established some interesting findings through an in-depth review of the 20 studies.
Firstly, 16 studies reported the incidence of AAD, and five of them (Johnston et al., 2006; Johnston et al., 2011; Goldenberg et al., 2015; Xu et al., 2017; Guo et al., 2019) analyzed the incidence of AAD by intention-to-treat (ITT) analysis (overall patients as randomized were analyzed), results showed definite benefits of probiotics compared to active, placebo or no treatment controls. The pooled results of a per-protocol (PP) analysis (patients for whom data were available were analyzed as randomized) of one study (Xu et al., 2017) were similar to the ITT analysis (bifidobacterial preparations for the prevention or treatment of AAD in children). However, the ITT analysis was unreliable if the rate of lost to follow-up (LTFU) was high. Therefore, we chose the PP analysis for the pooled data results of the other four studies (Johnston et al., 2006; Johnston et al., 2011; Goldenberg et al., 2015; Guo et al., 2019). In addition, given that the definition of probiotics requires that “sufficient amounts” be given to achieve health benefits, it is unclear what the daily dose of probiotics should be. No dose-ranging studies have been reported to determine the minimum effective dose of probiotics in the prevention of AAD, and some studies (Ouwehand, 2017) suggest that doses near the lower range may not provide benefit, while doses in the higher range may be associated with an increased risk of adverse events. The daily doses of probiotics included in the 20 SRs were highly variable (1 million to 2 trillion CFU/d), with reductions in the incidence of AAD ranging from 47% to 70% after treatment with different probiotic dose interventions (corresponding probiotic doses of 325–650 million CPU/d for S. boulardii and 1-4 million CPU/d for C. butyricum and B. infantis) and a reduction in the duration of diarrhea of 0.6d–1.95 d (corresponding probiotic doses of L. GG 100 million-40 billion CPU/d and S. boulardii 162.5–650 million CPU/d) (Table 2). It suggests that the effect of probiotics on pediatric AAD may be a potential dose-response effect and that the use of probiotics during antibiotic use reduces the incidence of AAD. Notably, the SRs published in English are more in-depth than most published in Chinese regarding diarrhea incidence, especially in exploring the heterogeneity of the combined results. Several studies (Johnston et al., 2006; Johnston et al., 2011; Goldenberg et al., 2015; Guo et al., 2019) have critically evaluated each subgroup (e.g., probiotic type, probiotic dose, antibiotic class, and definition of diarrhea) by using multiple criteria. Subgroup analyses regarding probiotic dose compared low doses (<5 billion CFU/day) with high doses (≥5 billion CFU/day). For example, one study (Guo et al., 2019) reported a benefit of high-dose probiotics in AAD prevention, with a 63% reduction in the incidence of AAD with high-dose probiotics compared to controls (RR: 0.37, 95% CI: 0.30 to 0.46, p = 0.06, I2 = 36%) and the NNT (i.e., number needed to treat) of 6 for prevention of one case of diarrhea (NNT: 6, 95% CI: 5–9).
4.2 Outcome indicators for systematic reviews
The included 20 SRs had some limitations in their analysis of outcome indicators. First, clinical efficacy may be affected because the effects of probiotics are strain-specific and dose-specific, and it is challenging to standardize specific interventions, doses, and regimens in clinical studies. For the preventive effect of probiotics, eight SRs (Johnston et al., 2006; Szajewska et al., 2006; Chen et al., 2010; Johnston et al., 2011; Goldenberg et al., 2015; Szajewska et al., 2016; Xu et al., 2017; Guo et al., 2019) have performed subgroup analyses of AAD incidence according to probiotic species, and the results suggest that it is too early to conclude the efficacy and safety of other probiotic drugs for AAD in children, except L. rhamnosus and S. boulardii. Four SRs (Johnston et al., 2006; Johnston et al., 2011; Goldenberg et al., 2015; Guo et al., 2019) performed subgroup analyses of AAD incidence according to probiotic dose subgroup analysis, with moderate quality evidence suggesting a significant role for high-dose (5–40 billion colony forming units per day) probiotics in the prevention of AAD. In addition, since multiple SRs were studying the same disease and data were collated and evaluated for the analysis of the same outcome indicators, there may be some overlap in the original studies included in different SRs. For example, two SRs (Johnston et al., 2011; Goldenberg et al., 2015) had the same Meta-analysis results for two outcome indicators (Table 5). On the other hand, the naming of the outcome indicators included in the SRs is highly variable, irregular, and even contradictory. Using of outcome indicators with different definition criteria may potentially affect the credibility of the conclusions. Therefore, there is a need to further promote the development of Core Outcome Set (COS) studies in the future, intending to address the problems of arbitrariness, inconsistency, and lack of recognition of clinical research outcome indicators (Williamson et al., 2012; Zhang et al., 2021).
4.3 Reporting quality of systematic reviews
According to the results of PRISMA 2020, certain reporting deficiencies exist: ① 85% of SRs failed to fully meet the requirements of structured abstracts, especially the study protocols and registration numbers of the original studies were not reported, which affected the reliability and rigour of the results; ② 85% of SRs only reported the search strategies of some databases, which affected the reproducibility of the results; ③ in terms of result synthesis, 35% of the SRs were not fully reported on data synthesis, mainly reflecting the lack of detailed and transparent methodological analysis of result heterogeneity and stability, which may potentially affect the reliability of the results; ④ 80% of SRs did not report content related to the strength of evidence for outcome indicators; ⑤ 35% of SRs did not provide a flow chart of the literature screening process, and 75% did not provide a list of excluded literature, which would affect the transparency and reproducibility of SRs production; ⑥ In terms of financial support, 40% of SRs did not report the source of funding for the study, while 75% of SRs did not report the role of the funder in completing the study, which could potentially have a conflict of interest and thus affect the study results; ⑦ All SRs did not fully disclose details about the data processing, which affected the recalibration and use of these data. In general, there is much room for future improvement in the standardization and rigour of report writing.
4.4 Methodological quality of systematic reviews
According to the results of AMSTAR 2, the deficiencies of key item 2 (reported the predefined protocol) and item 7 (List of excluded studies and reason) were found to be more obvious: ① 85% of the SRs did not provide a pre-study design plan, which would affect the rigour of the study results; ② 70% of the SRs did not provide a list of excluded literature in the screening process, which might have literature inclusion bias. In addition, the results of nine non-critical item assessments showed that: ① 75% of SRs did not describe the basis of study design selection (item 3), which may prevent a complete efficacy assessment of a certain intervention due to the type of study design included; ② 95% of SRs did not give information on the source of funds for inclusion in the original study (item 10), and 40% of SRs did not report potential conflicts of interest (item 16), which may affect the credibility of evidence-based conclusions; ③ 85% of SRs did not evaluate the impact of individual study risk of bias on the results of Meta-analysis (item 12), and inadequate assessment of the risk of bias may lead to biased results. Therefore, the methodological quality of relevant SRs still needs to be improved.
4.5 Quality of evidence for systematic reviews
The results of the GRADE system showed that high-quality evidence accounted for 6.25%, moderate-quality evidence for 33.33%, low-quality evidence for 52.08%, and very low-quality evidence for 8.33%. Major downgrading factors exist for risk of bias, inconsistency and publication bias. There is the unreasonable or incorrect implementation of random grouping, allocation concealment and blind implementation in the methodology design; the inconsistency is mainly reflected in large heterogeneity and low interval overlap; publication bias is reflected in small studies, funnel plot asymmetry or Egger’s test. In summary, the included studies were positive for the efficacy of probiotics in treating AAD in children, but the quality of evidence was generally low.
4.6 Study limitations
(1) A comprehensive literature search was conducted for this study, but due to language limitations, only Chinese and English SRs were included, which may be subject to potential publication bias. (2) The methodological, report, and evidence quality of the included SRs have certain shortcomings. There may be subjectivity in the study process, which reduces the reliability of the study results.
5 Conclusion
Overviews, as a comprehensive and relatively novel research method, assess the evidence from systematic reviews at a higher level, contain a richer and more comprehensive amount of information and can provide more focused evidence support for clinical researchers (McKenzie and Brennan, 2017; López-López et al., 2022). A total of 20 SRs were included in this study, which comprehensively compared the efficacy of probiotics in preventing and treating AAD in children. The results showed that probiotics alone or probiotics combined with conventional western medical treatment could not only effectively prevent the incidence of AAD and CDAD, but also improve the overall efficiency and clinical cure rate, shorten the duration of diarrhea, mean frequency of diarrhea, the average hospitalization time and antidiarrheal time, and the incidence of adverse effects was low, the safety of probiotics was good. However, the results of existing evidence show that the methodological, reporting and evidence quality of SRs of probiotics for AAD in children are generally low. There is still a need to improve the quality of evidence-based evidence to better explain the clinical application value of probiotics for AAD in children in the future. The results of this study need to be applied with reasonable interpretation.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Author contributions
Conceptualization: QY and ZH. Data curation: YL and XL. Formal analysis: YL and XL. Investigation: CX and JZ. Methodology: HL and XD. Resources: XD. Writing—original draft: QY. Writing—review and editing: ZH. All authors contributed to the article and approved the submitted version.
Funding
The Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (No: ZYYCXTD-C-202010).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Atkins, D., Best, D., Briss, P. A., Eccles, M., Falck-Ytter, Y., Flottorp, S., et al. (2004). Grading quality of evidence and strength of recommendations. BMJ 328 (7454), 1490. doi:10.1136/bmj.328.7454.1490
Balshem, H., Helfand, M., Schünemann, H. J., Oxman, A. D., Kunz, R., Brozek, J., et al. (2011). GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 64, 401–406. doi:10.1016/j.jclinepi.2010.07.015
Bartlett, J. G. (2002). Clinical practice. Antibiotic-associated diarrhea. N. Engl. J. Med. 346, 334–339. doi:10.1056/NEJMcp011603
Chai, J., Chang, H., and Li, L. (2015). Prevention of antibiotic associated diarrhea by Saccharomyces boulardii in infants in China: A meta analysis. Chin. J. Microecol. 27, 284–288. doi:10.13381/j.cnki.cjm.201503010
Chai, J., Chang, H., and Li, L. (2017). Treatment of antibiotic associated diarrhea by Bifidobacterium tetravaccine in infants in China: A meta-analysis. West China Med. J. 32, 395–399. doi:10.7507/1002-0179.201502139
Chen, X., Wang, L., Shan, Q., and Chen, L. (2010). Meta-analyses of the effect of probiotics in prevention of antibiotic-associated diarrhea in children. Chin. J. Pract. Pediatr. 25, 303–308.
Fang, B., Wu, S., Chen, F., Shao, H., and Zhu, X. (2013). Prevention of antibiotic associated diarrhea by Saccharomyces boulardii in children: A meta analysis. J. Pediatr. Pharm. 19, 5–7. doi:10.13407/j.cnki.jpp.1672-108x.2013.10.007
Gibson, G. R., Hutkins, R., Sanders, M. E., Prescott, S. L., Reimer, R. A., Salminen, S. J., et al. (2017). Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 14 (8), 491–502. doi:10.1038/nrgastro.2017.75
Goldenberg, J. Z., Lytvyn, L., Steurich, J., Parkin, P., Mahant, S., and Johnston, B. C. (2015). Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst. Rev. (12), CD004827. doi:10.1002/14651858.CD004827.pub4
Guo, Q., Goldenberg, J. Z., Humphrey, C., El Dib, R., and Johnston, B. C. (2019). Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst. Rev. 4, CD004827. doi:10.1002/14651858.CD004827.pub5
Hayes, S. R., and Vargas, A. J. (2016). Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Explore (NY) 12, 463–466. doi:10.1016/j.explore.2016.08.015
He, X., Zhang, Y., Xu, J., Wang, X., and Xu, J. (2017). Effect of probiotics on prevention of antibiotic-associated diarrhea in children: A meta-analysis of randomized controlled trials. Chin. J. Nosocomiol 27, 185–189. doi:10.11816/cn.ni.2017-153518
Hill, C., Guarner, F., Reid, G., Gibson, G. R., Merenstein, D. J., Pot, B., et al. (2014). Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11 (8), 506–514. doi:10.1038/nrgastro.2014.66
Johnston, B. C., Supina, A. L., and Vohra, S. (2006). Probiotics for pediatric antibiotic-associated diarrhea: A meta-analysis of randomized placebo-controlled trials. CMAJ 175, 377–383. doi:10.1503/cmaj.051603
Johnston, B. C., Goldenberg, J. Z., Vandvik, P. O., Sun, X., and Guyatt, G. H. (2011). Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst. Rev. (11), CD004827. doi:10.1002/14651858.CD004827.pub3
Liu, J., Du, X., and Shi, Y. (2020). Bifidobacterium as adjuvant in treatment of antibiotic related diarrhea in children: A system review and meta-analysis. Chin. J. Microecol. 32, 151–155. doi:10.13381/j.cnki.cjm.202002006
Liu, K., Zhao, J., He, L., Qiao, G., Liang, Y., Li, J., et al. (2022). Safety and efficacy of viable Clostridium butyricum in the prevention and treatment of antibiotic-associated diarrhea in infants and young children: A meta analysis. Chin. J. Microecol. 34, 168–174. doi:10.13381/j.cnki.cjm.202202007
López-López, J. A., Rubio-Aparicio, M., and Sánchez-Meca, J. (2022). Overviews of reviews: Concept and development. Psicothema 34, 175–181. doi:10.7334/psicothema2021.586
Lu, L. (2010). Probiotics in the prevention of antibiotics-associated diarrhea in children: A meta-analysis of randomized controlled trials. J. Clin. Pediatr. 28 (11), 1083–1085. doi:10.3969/j.issn.1000-3606.2010.11.023
Lunny, C., Brennan, S. E., McDonald, S., and McKenzie, J. E. (2017). Toward a comprehensive evidence map of overview of systematic review methods: Paper 1-purpose, eligibility, search and data extraction. Syst. Rev. 6, 231. doi:10.1186/s13643-017-0617-1
Lunny, C., Brennan, S. E., McDonald, S., and McKenzie, J. E. (2018). Toward a comprehensive evidence map of overview of systematic review methods: Paper 2-risk of bias assessment; synthesis, presentation and summary of the findings; and assessment of the certainty of the evidence. Syst. Rev. 7, 159. doi:10.1186/s13643-018-0784-8
McFarland, L. V., Ozen, M., Dinleyici, E. C., and Goh, S. (2016). Comparison of pediatric and adult antibiotic-associated diarrhea and Clostridium difficile infections. World J. Gastroenterol. 22, 3078–3104. doi:10.3748/wjg.v22.i11.3078
McFarland, L. V. (2008). Antibiotic-associated diarrhea: Epidemiology, trends and treatment. Future Microbiol. 3, 563–578. doi:10.2217/17460913.3.5.563
McKenzie, J. E., and Brennan, S. E. (2017). Overviews of systematic reviews: Great promise, greater challenge. Syst. Rev. 6, 185. doi:10.1186/s13643-017-0582-8
Ouwehand, A. C. (2017). A review of dose-responses of probiotics in human studies. Benef. Microbes 8 (2), 143–151. doi:10.3920/BM2016.0140
Page, M. J., Moher, D., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021a). PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 372, n160. doi:10.1136/bmj.n160
Page, M. J., Moher, D., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021b). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 134, 178–189. doi:10.1016/j.jclinepi.2021.03.001
Pomerance, H. H. (1997). Nelson textbook of pediatrics. Arch. Pediatr. Adolesc. Med. 151, 324. doi:10.1001/archpedi.1997.02170400110025
Salminen, S., Collado, M. C., Endo, A., Hill, C., Lebeer, S., Quigley, E. M. M., et al. (2021). The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 18 (9), 649–667. doi:10.1038/s41575-021-00440-6
Shea, B. J., Reeves, B. C., Wells, G., Thuku, M., Hamel, C., Moran, J., et al. (2017). Amstar 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 358, j4008. doi:10.1136/bmj.j4008
Shi, Y., Wang, M., Ma, Y., and Guo, J. (2006). Meta-analyses of the effect of probiotics in prevention of antibiotic-associated diarrhea in newborns. Chin. J. Microecol. 18, 252–254. doi:10.3969/j.issn.1005-376X.2006.03.043
Storr, M., and Stengel, A. (2021). Systematic review: Clinical evidence of probiotics in the prevention of antibiotic-associated diarrhoea. MMW Fortschr Med. 163, 19–26. doi:10.1007/s15006-021-9762-5
Swanson, K. S., Gibson, G. R., Hutkins, R., Reimer, R. A., Reid, G., Verbeke, K., et al. (2020). The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 17 (11), 687–701. doi:10.1038/s41575-020-0344-2
Szajewska, H., and Kołodziej, M. (2015a). Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment. Pharmacol. Ther. 42, 793–801. doi:10.1111/apt.13344
Szajewska, H., and Kołodziej, M. (2015b). Systematic review with meta-analysis: Lactobacillus rhamnosus GG in the prevention of antibiotic-associated diarrhoea in children and adults. Aliment. Pharmacol. Ther. 42, 1149–1157. doi:10.1111/apt.13404
Szajewska, H., Ruszczyński, M., and Radzikowski, A. (2006). Probiotics in the prevention of antibiotic-associated diarrhea in children: A meta-analysis of randomized controlled trials. J. Pediatr. 149, 367–372. doi:10.1016/j.jpeds.2006.04.053
Szajewska, H., Canani, R. B., Guarino, A., Hojsak, I., Indrio, F., Kolacek, S., et al. (2016). Probiotics for the prevention of antibiotic-associated diarrhea in children. J. Pediatr. Gastroenterol. Nutr. 62, 495–506. doi:10.1097/MPG.0000000000001081
Szajewska, H., Canani, R. B., Domellöf, M., Guarino, A., Hojsak, I., Indrio, F., et al. (2023). Probiotics for the management of pediatric gastrointestinal disorders: Position paper of the ESPGHAN special interest group on gut microbiota and modifications. J. Pediatr. Gastroenterol. Nutr. 76 (2), 232–247. doi:10.1097/MPG.0000000000003633
Williamson, P. R., Altman, D. G., Blazeby, J. M., Clarke, M., Devane, D., Gargon, E., et al. (2012). Developing core outcome sets for clinical trials: Issues to consider. Trials 13, 132. doi:10.1186/1745-6215-13-132
Xu, H., Jiang, R., and Sheng, H. (2017). Meta-analysis of the effects of Bifidobacterium preparations for the prevention and treatment of pediatric antibiotic-associated diarrhea in China. Complement. Ther. Med. 33, 105–113. doi:10.1016/j.ctim.2017.07.001
Yang, C., Zhang, L., Zhang, S., Yang, Y., and Zhu, C. (2016a). Efficacy and safety of Saccharomyces boulardii in the prevention of antibiotic associated diarrhea in children: Meta analysis. Her. Med. 35, 1211–1219. doi:10.3807/j.issn.1004-0781.2016.11.014
Yang, C., Zhang, L., Yan, P., Li, L., and Zhu, C. (2016b). Saccharomyces boulardii powder in the treatment of antibiotic associated diarrhea in children: A meta analysis. China Pharm. 27, 334–336. doi:10.6039/j.issn.1001-0408.2016.03.17
You, Y., and Gao, Y. (2015). Systematic review of the efficacy of live combined balillus subtilis and Enterococcus faecium in the prevention of and treatment antibiotic associated diarrhea. China Pharm. 26, 3806–3808. doi:10.6039/j.issn.1001-0408.2015.27.23
Zhang, M., Li, K., Cai, H., Niu, B., Zhang, J., Liu, Z., et al. (2021). Effect of surface property on the release and oral absorption of solid sirolimus-containing self-microemulsifying drug delivery system. J. Tradit. Chin. Med. 62, 108–113. doi:10.1208/s12249-021-01978-z
Zheng, Y., Wu, Q., Fang, F., Chen, J., Shang, Y., Fu, Z., et al. (2021). Expert consensus on diagnosis, treatment and prevention of antibiotic-associated diarrhea in children. Chin. J. Appl. Clin. Pediatr. 36, 424–430. doi:10.3760/cma.j.cn101070-20210201-00137
Keywords: children, antibiotic-associated diarrhea, probiotics, systematic review, overview of systematic reviews, PRISMA 2020, AMSTAR 2, grade
Citation: Yang Q, Hu Z, Lei Y, Li X, Xu C, Zhang J, Liu H and Du X (2023) Overview of systematic reviews of probiotics in the prevention and treatment of antibiotic-associated diarrhea in children. Front. Pharmacol. 14:1153070. doi: 10.3389/fphar.2023.1153070
Received: 28 January 2023; Accepted: 10 July 2023;
Published: 24 July 2023.
Edited by:
Catherine M. T. Sherwin, Wright State University, United StatesReviewed by:
Silvia Salvatore, University of Insubria, ItalyJulio Plaza-Diaz, Children’s Hospital of Eastern Ontario (CHEO), Canada
Copyright © 2023 Yang, Hu, Lei, Li, Xu, Zhang, Liu and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoquan Du, ZHV4aWFvcXVhbjE5OTdAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Qingrui Yang
Qingrui Yang Zeyu Hu1†
Zeyu Hu1†