- 1International Joint Research Centre on Purinergic Signalling, School of Acupuncture and Tuina/ School of Health and Rehabilitation, Chengdu University of Traditional Medicine, Chengdu, China
- 2Department of Neurology, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China
- 3School of Clinical Medicine, Chengdu University of Traditional Medicine, Chengdu, China
- 4Rudolf Boehm Institute for Pharmacology and Toxicology, University of Leipzig, Leipzig, Germany
- 5Acupuncture and Chronobiology Key Laboratory of Sichuan Province, Chengdu, China
Single-nucleotide polymorphisms are connected with the risk of epilepsy on occurrence, progress, and the individual response to drugs. Progress in genomic technology is exposing the complex genetic architecture of epilepsy. Compelling evidence has demonstrated that purines and adenosine are key mediators in the epileptic process. Our previous study found the interconnection of P2Y12 receptor single-nucleotide polymorphisms and epilepsy. However, little is known about the interaction between the purine nucleoside A2A receptor and rate-limiting enzyme ecto-5′-nucleotidase/CD73 and epilepsy from the genetic polymorphism aspect. The aim of the study is to evaluate the impact of A2AR and CD73 polymorphisms on epilepsy cases. The study group encompassed 181 patients with epilepsy and 55 healthy volunteers. A significant correlation was confirmed between CD73 rs4431401 and epilepsy (p < 0.001), with TT genotype frequency being higher and C allele being lower among epilepsy patients in comparison with healthy individuals, indicating that the presence of the TT genotype is related to an increased risk of epilepsy (OR = 2.742, p = 0.006) while carriers of the C allele demonstrated a decreased risk of epilepsy (OR = 0.304, p < 0.001). According to analysis based on gender, the allele and genotype of rs4431401 in CD73 were associated with both male and female cases (p < 0.0001, p = 0.026, respectively). Of note, we found that A2AR genetic variants rs2267076 T>C (p = 0.031), rs2298383 C>T (p = 0.045), rs4822492 T>G (p = 0.034), and rs4822489 T>G (p = 0.029) were only associated with epilepsy in female subjects instead of male. It is evident that the TT genotype and T allele of rs4431401 in CD73 were genetic risk factors for epilepsy, whereas rs2267076, rs2298383, rs4822492, and rs4822489 polymorphisms of the A2AR were mainly associated with female subjects.
Introduction
Single-nucleotide polymorphisms (SNPs) are valuable for diagnosis and treatment guidance in epilepsy (Pal et al., 2010). As one of the prominent forms of gene variations in the human genome (Kim and Misra, 2007), SNPs are utilized to detect encoded proteins for prevention and treatment in epilepsy genetic studies. With progress in genomic technology, almost a thousand genes have been verified to relate to epilepsy etiology (Wang et al., 2017). Observational publications have reported that the GABA receptor and GABA transporter-1 SNPs are associated with the risk of epilepsy (Sesarini et al., 2015; Schijns et al., 2020). Several autophagy-related protein 5 gene polymorphisms show significant associations with the susceptibility to late-onset epilepsy and temporal lobe epilepsy (Zhang et al., 2021). Additionally, there is an increasing focus on the role of purinergic signaling receptors in various central nervous system diseases, including epilepsy (Scheffer et al., 2017; Nikolic et al., 2020; Beamer et al., 2021). We reported in our former study that the polymorphisms of the P2Y12 receptor are related to epilepsy susceptibility, and one of the polymorphisms may be specifically associated with seizure frequency (Wang et al., 2022). CD73 plays a key role in ATP metabolism, which generates the adenosine that activates the A2AR. The overfunction of A2AR is sufficient to trigger brain dysfunction and induce neuronal excitotoxicity (Cunha et al, 2016). In addition, genetic deletion of CD73 was found to attenuate neuron degeneration in mice (Augusto et al., 2021). However, the relationship between epilepsy and SNPs from the purinergic signaling facet, particularly adenosine A2A receptors (A2AR) and 5′-nucleotidase (CD73), currently has only a limited number of investigations.
It has already been widely studied that A2AR and CD73 participate in the etiology of epilepsy, whether in experimental or observational studies (Xu et al., 2022; El Yacoubi et al., 2009; Augusto et al., 2021). A2AR exists in both synapses and neurons (Rebola et al., 2005; Borea et al., 2018), is associated with adenylyl cyclase activation, and is thought to have an excitatory effect on neurons upon activation (Corvol et al., 2001). In the hippocampus of both animal models and those of human brains, A2AR upregulation in synapses has been demonstrated to be one of the pathogenic characteristics of epilepsy (Canas et al., 2018; Crespo et al., 2018; Barros-Barbosa et al., 2016). Patients with mesial temporal lobe epilepsy have an elevated proportion of A2AR during epileptogenesis, and this enhanced astrocytic A2AR has more public involvement in disorders linked to neuroexcitotoxicity (Barros-Barbosa et al., 2016). Neuronal excitation in epilepsy may increase synaptic A2A activation, which aggravates synaptotoxicity and causes standard circuitry to deteriorate, resulting in epilepsy progression (Barros-Barbosa et al., 2016). In other words, A2AR overfunction plays an essential role in the cumulative aggravation of epilepsy rather than in the onset of seizure activity (Moreira-de-Sá et al., 2021). These studies support that A2AR may be involved in the pathophysiology of epilepsy by controlling the function of glial cells. Accordingly, the corresponding gene (ADORA2A) is studied as a promising candidate for epilepsy. ADORA2A is located on chromosome 22q 11.23 and has two coding exons spanning about 9 kb (MacCollin et al., 1994; Peterfreund et al., 1996). rs2298383 was proven to be associated with childhood epilepsy and a predisposition to childhood epilepsy (Fan et al., 2020). However, whether the association exists in a wider age range remains unclear. CD73 is an enzyme that catalyzes the last step in the extracellular metabolism of ATP to form adenosine (Alcedo et al., 2021) and is positioned ideally to promote A2AR activation after the conversion of released adenine nucleotides into adenosine (Cunha et al., 1996). A rodent study demonstrates that CD73 lost its activity along with the decreasing density of A2AR 48 h after hyperthermia-evoked convulsions. The amount and distribution of CD73 in the hippocampus of mesial temporal lobe epilepsy patients were higher and broader than that in control individuals, and hippocampal astrogliosis was observed in patients (Barros-Barbosa et al., 2016). Together, CD73 may be associated with epilepsy by promoting the A2AR activation after the conversion of released adenine nucleotides into adenosine. Furthermore, genetic variations in enzymes influencing extracellular adenosine homeostasis, including CD73, have been significantly associated with epilepsy. SNPs of CD73 have been significantly connected with epileptogenesis in the Caucasian race since variants may alter the function of CD73 to regulate the extracellular adenosine and seizure activity (Diamond et al., 2015), whereas the functions of ATP-related CD73 SNPs have not been completely illuminated in epileptic disease in the Asian race yet.
Given the substantiation that the adenosine A2AR and CD73 take part in the etiology of epilepsy in both clinical and rodent experiments, we designed this study to investigate changes between epilepsy cases and control individuals and from genetic variations aspects which are worth exploring as therapeutic targets for treatment development.
Participants and methods
Subjects
Between August 2020 and August 2021, 181 epilepsy cases (92 male and 89 female) were diagnosed according to the 2014 International League against Epilepsy criteria (Thijs et al., 2019), and there were 50 healthy participants (22 male and 28 female). The medians (ranges of the first quartile to the third quartile) of age for the cases and volunteers were 28 (23–47) and 26 (25–28), respectively. The subjects were recruited at the Sichuan Academy of Medical Science and Sichuan Provincial People’s Hospital in China. Clinical data of patients were collected, including gender, age, disease diagnosis, seizure onset frequency, medical history, drug treatment, and imaging examination. Individuals with missing abovementioned clinical data were excluded from the study. Those with a history of pseudo-epileptic seizures, as well as with impaired hepatic and/or renal function, were excluded. Healthy controls were neurologically normal, with no personal or family history of epilepsy.
Following approval of the Sichuan Academy of Medical Science and Sichuan Provincial People’s Hospital Ethics Committees, written informed consent was obtained from the individuals before participation in the study. Blood samples were taken with the consent of the individuals, and 2 ml blood from each participant was collected in EDTA tubes and kept at −20°C for extraction of DNA and genotyping. Samples were stored at −70°C until analysis.
DNA extraction and genotyping
Genomic DNA was isolated from peripheral blood using a QIAGEN kit (QIAGEN, Hilden, Germany). Extracted DNA was quantified using a NanoDrop analyzer (ND-2000) spectrophotometer (NanoDrop Technologies Inc., Wilmington, DE, United States). Qualified DNA samples were stored at −80°C until further use.
We selected fourteen single-nucleotide polymorphisms from two genes involved in the adenosine cycle for analysis. Genotyping of CD73 rs4431401 T>C, rs2065114 A>G, rs2229523 A>G, rs4579322 T>A, rs9444348 G>A, rs9450282 A>G, rs6922 T>G, rs4373337 A>C, rs2267076 T>C, and A2AR rs3761422 T> rs2298383 C>T, rs4822492 C>G, rs2236624 T>C, and rs4822489 T>G polymorphisms was performed by the MassARRAY platform (Agena Bioscience, San Diego, CA, United States) at CapitalBio (Beijing, China). The primers for PCR amplification and extension were designed using the MassARRAY Assay Design v4.0 software. The PCR cycle program, as well as shrimp alkaline phosphatase digestion and extension, was performed according to the manufacturer’s protocol. Extension products were desalted and detected using matrix-assisted laser desorption ionization time-of-fight. Finally, the data were processed with Typer v4.0 software (Agena Bioscience, San Diego, CA, United States).
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS), version 26.0 (IBM, Chicago, IL, United States). Categorical variables were expressed as numbers and percentages and compared by using the Pearson chi-squared test and Fisher’s exact test. Numerical variables were expressed as medians with interquartile ranges and compared by the non-parametric independent-sample Wilcoxon signed-rank test. The χ2 test was used to assess the deviation from the Hardy–Weinberg equilibrium. The χ2 statistics or Fisher’s exact test was used to compare the statistical differences in genotype distributions and allele frequencies between the cases and controls. The odds ratio (OR) was calculated with 95% confidence intervals (CIs). Statistical significance was defined as two-tailed p < 0.05.
Results
Genotype frequencies of all investigated ADORA2A and CD73 SNPs conformed to the Hardy–Weinberg equilibrium in epilepsy and the healthy control samples.
Clinical characteristics of the study participants
Demographic and clinical characteristics of the 231 enrolled participants are shown in Table 1.
The study included 181 patients (92 male and 89 female) and 50 volunteers (22 male and 28 female) with median ages of 28 years and 26 years, respectively. There were no statistically significant differences between epileptic patients and healthy controls in terms of gender or age.
Genotypic and allelic distribution of the CD73 and A2AR SNPs
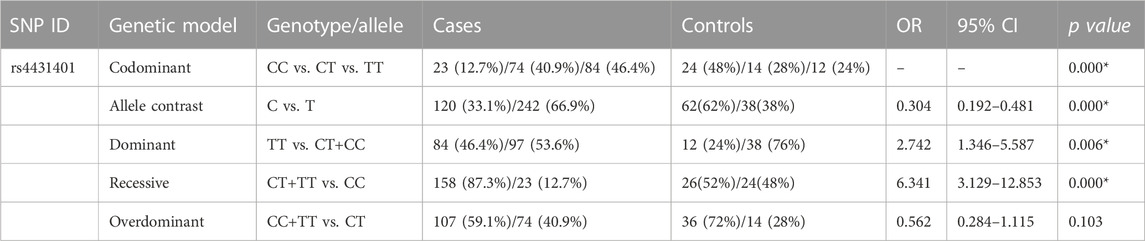
The frequency distributions of the CD73 and A2AR polymorphisms were compared between epilepsy patients and healthy controls. Of the fourteen investigated SNPs, a significant difference was observed in the CD73 SNP rs4431401. The genotype frequencies of CD73 rs4431401 T>C polymorphism CC, CT, and TT genotypes were found in 12.7%, 40.9%, and 46.4% of cases and 48%, 28%, and 24% of the control group, respectively. The allelic frequency was 33.1% for the C allele and 66.9% for the T allele in patients, while it was 62% for the C allele and 38% for the T allele in the volunteer group. The T allele and TT genotype were conspicuously higher among patients than in healthy controls (OR = 0.305, 95% CI = 0.193–0.483, p = 0.0001 for C vs. T; OR = 2.714, 95% CI = 1.333–5.529, p = 0.006 for TT vs. CT/CC), indicating that individuals with the T allele and TT genotype of rs4431401 T>C might have higher risks for epilepsy (Table 2). However, the risk of epilepsy did not differ in other CD73 and A2AR polymorphisms between the cases and the control group (Supplementary Tables S1, S2).
Genotypic and allelic distribution of CD73 and A2AR SNPs in different genders
The CD73 and A2AR genotype and allele frequencies between the patients and controls of different genders are summarized in Table 3, Table 4, and Supplementary Tables S3 and S4. We found that the frequencies of the alleles and genotypes in CD73 rs4431401 between both male and female groups varied significantly (p < 0.001). With the T allele and TT genotype frequency being lower among the healthy in comparison with the epilepsy subjects, the presence of the T allele and TT genotype was connected with an increased risk of epilepsy (OR = 0.149, CI = 0.069–0.322, p < 0.001; OR = 5.092, CI = 1.408–18.407, p = 0.013, respectively) (Table 3). Females carrying the C allele/CC variant in CD73 rs4431401 had a lower risk of epilepsy in contrast with females who carried no copies (OR = 0.483, 95% CI = 0.263–0.890, p = 0.026 for C vs. T; OR = 3.039, 95% CI = 1.119–8.258, p = 0.045 for CT/TT vs. CC) (Table 3). No additional significant genotypic and allelic distribution between the female and male patients was observed in our study (Supplementary Table S3).
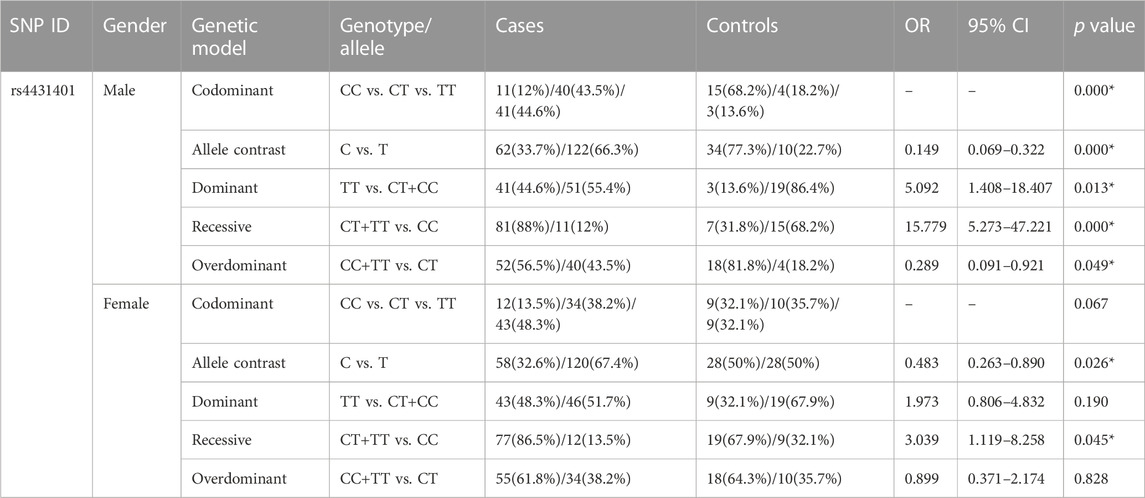
TABLE 3. Genotypic and allelic distribution of the CD73 gene between all patients and controls in different genders.
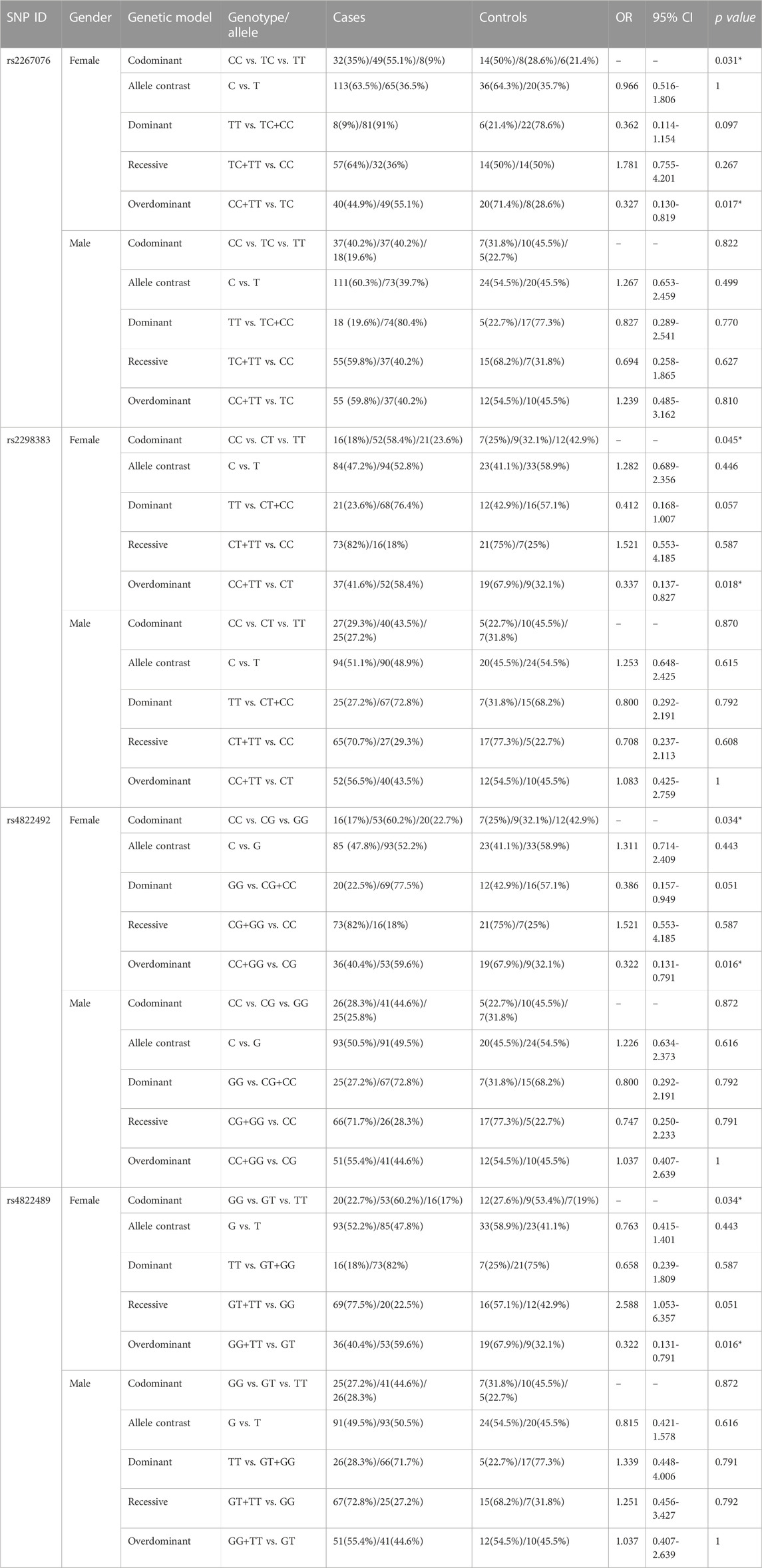
TABLE 4. Genotypic and allelic distribution of the A2AR gene between all patients and controls in different genders.
In the A2AR gene, there were no associations of the identified SNPs, rs3761422 and rs2236624, with analysis based on gender (Supplementary Table S4). Interestingly, we observed that SNPs for the A2AR gene differed between female cases and controls, including rs2267076 T>C (p = 0.031), rs2298383 C>T (p = 0.045), rs4822492 T>G (p = 0.034), and rs4822489 T>G (p = 0.034). The differences were mainly attributed to a greater proportion of heterozygotes and fewer homozygotes. They were observed in A2AR gene polymorphisms, where female cases with CT in rs2267076 (OR = 0.327, CI = 0.130–0.819, p = 0.017), TC in rs2298383 (OR = 0.337, CI = 0.137–0.827, p = 0.018), CG in rs4822492 (OR = 0.322, CI = 0.131–0.791, p = 0.016), and GT in rs4822489 (OR = 0.322, CI = 0.131–0.791, p = 0.016) had a higher proportion of heterozygotes than homozygotes (Table 4). Therefore, females who are heterozygous genotype carriers of rs2267076, rs229838, rs4822489, and rs4822492 polymorphisms have a higher risk of epilepsy.
Subgroup analysis of A2AR and CD73
We conducted sub-analyses to determine whether risk varied by subgroups differing in drug treatment, neuroimaging, or epileptic seizure frequencies. We did not find any significant connection between neuroimaging and seizure frequency subgroups but did find an association between genotypes and drug therapy among epilepsy cases (Supplementary Tables S5, S6). This relationship was evident only for single-nucleotide polymorphisms on the A2AR gene (Table 5). The frequency of the TT genotype (38.6% for polytherapy; 46.7% for monotherapy) and T allele (60.5% for polytherapy; 46.7% for monotherapy) for rs2298383 in the A2AR gene was higher among cases of polypharmacy than in single drug treatment groups, suggesting that patients that had the TT genotype and T allele (TT vs. CT/CC: OR = 0.390, CI = 0.194–0.781, p = 0.010; C vs. T: OR = 1.749, CI = 1.113–2.478, p = 0.017) had a higher potential of requiring two or more antiepileptic drugs. Similar to former results, in rs4822492, the GG genotype and G allele were associated with polytherapy (GG vs. CG/CC: OR = 0.370, CI = 0.184–0.744, p = 0.006; C vs. G: OR = 1.844, CI = 1.172–2.902, p = 0.009). In addition, we found that patients carrying the GG variant in A2AR rs4822489 were associated with polypharmacy (OR = 2.706, CI = 1.343–5.449, p = 0.006).
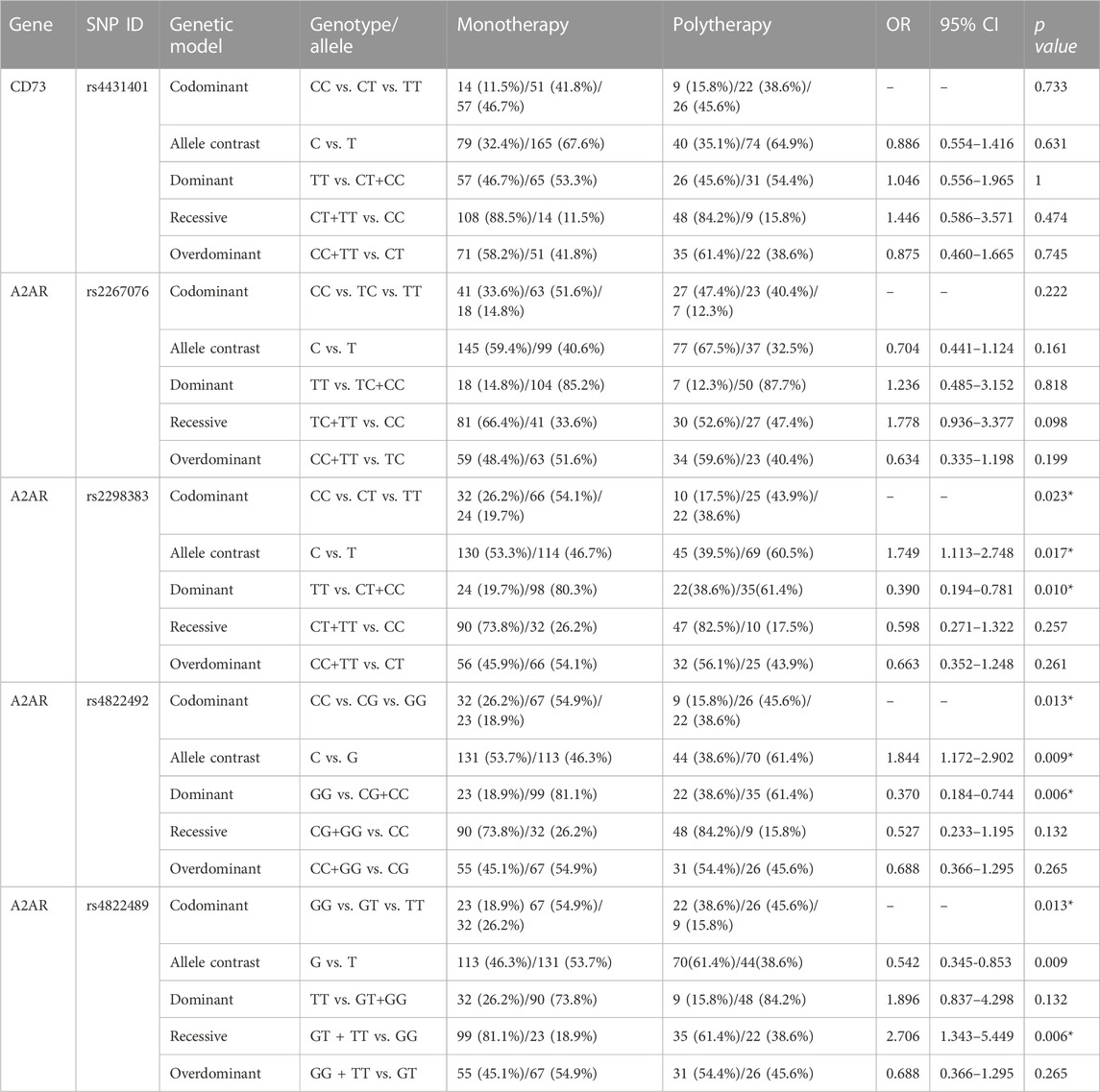
TABLE 5. Genotypic and allelic distribution of CD73 and A2AR genes between patients with monotherapy and polytherapy.
Discussion
We tested the hypothesis that adenosine-related SNPs are connected with the risk of epilepsy.
Our data showed that the T allele and TT genotype of SNP rs4431401 of CD73 was associated with a more pronounced predisposition to an increased risk of epilepsy, while A2AR gene rs2267076, rs2298383, rs4822492, and rs4822489 polymorphisms were more strongly linked with female epileptic patients. In contrast, there was no evidence for interactions of the identified SNPs: rs2065114, rs2229523, rs4579322, rs9444348, rs9450282, rs6922, and rs4373337 of CD73, rs3761422, and rs2236624 of A2AR. Noteworthily, rs2298383, rs4822492, and rs4822489 on the A2AR gene were associated with medication administered among epilepsy cases. These findings provide insight into the genetic susceptibility of epileptic disease and assistance for clinical drug therapy.
We found that carriers of rs4431401 in CD73, with a higher proportion of T allele and TT genotype, may have a higher predisposition for epilepsy. Observational studies focused on nephrotic syndrome (NS) (Yang et al., 2018; Zaorska et al., 2021) and uremia patients (Rothe et al., 2017) and found that rs4431401 (T>C) was significantly correlated with both differed NS risk and altered hormone sensitivity to NS. We also observed that both female and male subjects have a higher frequency of TT genotype compared to the controls. Since the risk factors of epilepsy are complex, such as family history, excessive sleep deprivation, and use of alcohol (Gavvala and Schuele, 2016), we speculate that one of the possible mechanisms is that gender difference influences the cognitive strategies on brain activation, such as women preferring the left hemisphere while men favoring the right hemisphere (Koepp, 2011). In addition, endogenous sex hormones may play a role. In an earlier cohort study, rs9444348 of CD73 was reported to have been significantly associated with a shorter time to first seizure and an increased seizure rate within 3 years of post-traumatic brain injury in Caucasian patients (Diamond et al., 2015). However, no difference was found in our present study. Race, the pathogenesis of epilepsy, and the statistical method were regarded to be the feasible reasons which are causing differences in results.
Females who carried a greater proportion of heterozygotes in rs2298383, rs2267076, rs4822492, and rs4822489 polymorphisms on the A2AR gene were identified to have an increased risk of epilepsy. On the contrary, no significant difference was observed in male patients. Consistent with this, a rodent study showed that female rats were more susceptible to acquiring seizures than male rats (Dai et al., 2014). Since sex hormones are associated with neuronal development, neuronal excitability, and epileptic susceptibility (Patrone et al., 1999; Zupanc, 2006; Liu et al., 2012), the possible reason is the effect of endogenous sex hormones, such as androgen, estrogen, and progesterone, as well as their metabolites. In addition, a recent study based on southern Chinese children with epileptic diseases shows that the carriers of the rs2298383 TT genotype tended to have a lower chance of epilepsy (Fan et al., 2020). In addition, the haplotype C frequency at rs2298383 and rs4822492 polymorphisms was reported to be significantly higher than in controls in acute encephalopathy with biphasic seizures and late reduced diffusion in children from Japan (Shinohara et al., 2013). The fact that these results are incompatible with ours could be attributed to the differences in age and the regions from where the participants were enrolled. As for s2267076 and rs4822489, we found no directly comparable studies when we searched PubMed for studies published in English that investigated the association between them and the risk of epilepsy. We have, therefore, provided comparisons with broader literature. The current study indicates that a higher risk of rheumatoid arthritis was found in patients who consume more caffeine with a CT genotype of rs2267076 (Soukup et al., 2020). rs4822489 is associated with chronic heart failure and type 1 diabetes (Charles et al., 2011; Zhai et al., 2015). To our knowledge, the genetic polymorphisms rs2267076 and rs4822489 were first identified to be associated with epilepsy in the present study.
We also observed an increased risk of polypharmacy in individuals with the TT genotype and T allele of rs2298383. An earlier case-control study reported that rs2298383 polymorphisms associated with tear volume increase after caffeine intake (Arita et al., 2012). Caffeine has various pharmacologic effects on the human body, including stimulation of the central nervous system (van Dam et al., 2020). A2AR, as one of the main target receptors of caffeine, has already been proven to play an important role in caffeine metabolism (Cappelletti et al., 2015). In addition, genetic factors are revealed to be associated with the direct effects of caffeine (Yang et al., 2010). In this way, an in-depth study needs to investigate whether rs2298383 is linked with polypharmacy of antiepileptic drugs and caffeine. In addition, though our results suggest that A2AR and CD73 gene polymorphisms do not correlate with the epileptic seizure frequency or abnormal/normal neuroimaging, they still require further investigation.
Some potential limitations should be considered. First, we only analyzed the population in southwestern China because representation from other regions of the country is lacking. Future studies should involve patients from the greater China region. Second, the SNPs of CD73 have been rarely reported in epilepsy. Hence, the discussion concerning the SNPs of CD73 is limited. Further validation and studies are necessary to confirm the relationship between CD73 and epilepsy. Finally, this study was confined to the association of SNPs with epilepsy and lacked specific epileptic sub-types due to the limited sample size. Therefore, more in-depth research is needed to improve our understanding of the association between CD73 and A2A receptors and the pathophysiology of epilepsy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Sichuan Academy of Medical Science and Sichuan Provincial People’s Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
N-RS, QW, and JL performed the study, analyzed the data, and wrote the manuscript; J-ZZ, B-LD, X-MH, JY, XW, XC, Y-QZ, T-TL, and J-LZ, performed the study; and XY, PI, and YT designed the study and wrote the manuscript.
Funding
This work was supported by grants from the Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (ZYYCXTDD-202003) and the Science and Technology Program of Sichuan Province, China (2022YFH0006 and 23RCYJ0059).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1152667/full#supplementary-material
References
Alcedo, K. P., Bowser, J. L., and Snider, N. T. (2021). The elegant complexity of mammalian ecto-5'-nucleotidase (CD73). Trends Cell. Biol. 31 (10), 829–842. doi:10.1016/j.tcb.2021.05.008
Arita, R., Yanagi, Y., Honda, N., Maeda, S., Maeda, K., Kuchiba, A., et al. (2012). Caffeine increases tear volume depending on polymorphisms within the adenosine A2a receptor gene and cytochrome P450 1A2. Ophthalmology 119 (5), 972–978. doi:10.1016/j.ophtha.2011.11.033
Augusto, E., Gonçalves, F. Q., Real, J. E., Silva, H. B., Pochmann, D., Silva, T. S., et al. (2021). Increased ATP release and CD73-mediated adenosine A2A receptor activation mediate convulsion-associated neuronal damage and hippocampal dysfunction. Neurobiol. Dis. 157, 105441. doi:10.1016/j.nbd.2021.105441
Barros-Barbosa, A. R., Ferreirinha, F., Oliveira, Â., Mendes, M., Lobo, M. G., Santos, A., et al. (2016). Adenosine A2Areceptor, and ecto-5'-nucleotidase/CD73 are upregulated in hippocampal astrocytes of human patients with mesial temporal lobe epilepsy (MTLE). Purinergic Signal 12 (4), 719–734. doi:10.1007/s11302-016-9535-2
Beamer, E., Kuchukulla, M., Boison, D., and Engel, T. (2021). ATP and adenosine-Two players in the control of seizures and epilepsy development. Prog. Neurobiol. 204, 102105. doi:10.1016/j.pneurobio.2021.102105
Borea, P. A., Gessi, S., Merighi, S., Vincenzi, F., and Varani, K. (2018). Pharmacology of adenosine receptors: The state of the art. Physiol. Rev. 98 (3), 1591–1625. doi:10.1152/physrev.00049.2017
Canas, P. M., Porciúncula, L. O., Simões, A. P., Augusto, E., Silva, H. B., Machado, N. J., et al. (2018). Neuronal adenosine A2A receptors are critical mediators of neurodegeneration triggered by convulsions. eNeuro 5 (6), ENEURO.0385–18.2018. doi:10.1523/ENEURO.0385-18.2018
Cappelletti, S., Piacentino, D., Sani, G., and Aromatario, M. (2015). Caffeine: Cognitive and physical performance enhancer or psychoactive drug? Curr. Neuropharmacol. 13 (1), 71–88. doi:10.2174/1570159X13666141210215655
Charles, B. A., Conley, Y. P., Chen, G., Miller, R. G., Dorman, J. S., Gorin, M. B., et al. (2011). Variants of the adenosine A(2A) receptor gene are protective against proliferative diabetic retinopathy in patients with type 1 diabetes. Ophthalmic Res. 46 (1), 1–8. doi:10.1159/000317057
Corvol, J. C., Studler, J. M., Schonn, J. S., Girault, J. A., and Hervé, D. (2001). Galpha(olf) is necessary for coupling D1 and A2a receptors to adenylyl cyclase in the striatum. J. Neurochem. 76 (5), 1585–1588. doi:10.1046/j.1471-4159.2001.00201.x
Crespo, M., León-Navarro, D. A., and Martín, M. (2018). Early-life hyperthermic seizures upregulate adenosine A2A receptors in the cortex and promote depressive-like behavior in adult rats. Epilepsy Behav. 86, 173–178. doi:10.1016/j.yebeh.2018.06.048
Cunha, R. A., Correia-de-Sá, P., Sebastião, A. M., and Ribeiro, J. A. (1996). Preferential activation of excitatory adenosine receptors at rat hippocampal and neuromuscular synapses by adenosine formed from released adenine nucleotides. Br. J. Pharmacol. 119, 253–260. doi:10.1111/j.1476-5381.1996.tb15979.x
Cunha, R. A. (2016). How does adenosine control neuronal dysfunction and neurodegeneration? J. Neurochem. 139 (6), 1019–1055. doi:10.1111/jnc.13724
Dai, Y. J., Xu, Z. H., Feng, B., Xu, C. L., Zhao, H. W., and Wu, D. C. (2014). Gender difference in acquired seizure susceptibility in adult rats after early complex febrile seizures. Neurosci. Bull. 30(6), 913–922. doi:10.1007/s12264-014-1482-8
Diamond, M. L., Ritter, A. C., Jackson, E. K., Conley, Y. P., Kochanek, P. M., Boison, D., et al. (2015). Genetic variation in the adenosine regulatory cycle is associated with posttraumatic epilepsy development. Epilepsia 56 (8), 1198–1206. doi:10.1111/epi.13044
El Yacoubi, M., Ledent, C., Parmentier, M., Costentin, J., and Vaugeois, J. M. (2009). Adenosine A2A receptor deficient mice are partially resistant to limbic seizures. Naunyn Schmiedeb. Arch. Pharmacol. 380 (3), 223–232. doi:10.1007/s00210-009-0426-8
Fan, X., Chen, Y., Li, W., Xia, H., Liu, B., Guo, H., et al. (2020). Genetic polymorphism ofADORA2A is associated with the risk of epilepsy and predisposition to neurologic comorbidity in Chinese southern children. Front. Neurosci. 14, 590605. doi:10.3389/fnins.2020.590605
Gavvala, J. R., and Schuele, S. U. (2016). New-onset seizure in adults and adolescents: A review. JAMA 316 (24), 2657–2668. doi:10.1001/jama.2016.18625
Kim, S., and Misra, A. (2007). SNP genotyping: Technologies and biomedical applications. Annu. Rev. Biomed. Eng. 9, 289–320. doi:10.1146/annurev.bioeng.9.060906.152037
Koepp, M. J. (2011). Gender and drug effects on neuroimaging in epilepsy. Epilepsia 52 (4), 35–37. doi:10.1111/j.1528-1167.2011.03150.x
Liu, S. B., Zhang, N., Guo, Y. Y., Zhao, R., Shi, T. y., Feng, S. f., et al. (2012). G-protein-coupled receptor 30 mediates rapid neuroprotective effects of estrogen via depression of NR2B-containing NMDA receptors. J. Neurosci. 32 (14), 4887–4900. doi:10.1523/JNEUROSCI.5828-11.2012
MacCollin, M., Peterfreund, R., MacDonald, M., Fink, J. S., and Gusella, J. (1994). Mapping of a human A2a adenosine receptor (ADORA2) to chromosome 22. Genomics 20 (2), 332–333. doi:10.1006/geno.1994.1181
Moreira-de-Sá, A., Lourenço, V. S., Canas, P. M., and Cunha, R. A. (2021). Adenosine A2A receptors as biomarkers of brain diseases. Front. Neurosci. 15, 702581. doi:10.3389/fnins.2021.702581
Nikolic, L., Nobili, P., Shen, W., and Audinat, E. (2020). Role of astrocyte purinergic signaling in epilepsy. Glia 68 (9), 1677–1691. doi:10.1002/glia.23747
Pal, D. K., Pong, A. W., and Chung, W. K. (2010). Genetic evaluation and counseling for epilepsy. Nat. Rev. Neurol. 6 (8), 445–453. doi:10.1038/nrneurol.2010.92
Patrone, C., Andersson, S., Korhonen, L., and Lindholm, D. (1999). Estrogen receptor-dependent regulation of sensory neuron survival in developing dorsal root ganglion. Proc. Natl. Acad. Sci. U. S. A. 96 (19), 10905–10910. doi:10.1073/pnas.96.19.10905
Peterfreund, R. A., MacCollin, M., Gusella, J., and Fink, J. S. (1996). Characterization and expression of the human A2a adenosine receptor gene. J. Neurochem. 66 (1), 362–368. doi:10.1046/j.1471-4159.1996.66010362.x
Rebola, N., Canas, P. M., Oliveira, C. R., and Cunha, R. A. (2005). Different synaptic and subsynaptic localization of adenosine A2A receptors in the hippocampus and striatum of the rat. Neuroscience 132 (4), 893–903. doi:10.1016/j.neuroscience.2005.01.014
Rothe, H., Brandenburg, V., Haun, M., Kollerits, B., Kronenberg, F., Ketteler, M., et al. (2017). Ecto-5' -Nucleotidase CD73 (NT5E), vitamin D receptor and FGF23 gene polymorphisms may play a role in the development of calcific uremic arteriolopathy in dialysis patients - data from the German Calciphylaxis Registry. PLoS One 12 (2), e0172407. doi:10.1371/journal.pone.0172407
Scheffer, I. E., Berkovic, S., Capovilla, G., Connolly, M. B., French, J., Guilhoto, L., et al. (2017). ILAE classification of the epilepsies: Position paper of the ILAE commission for classification and terminology. Epilepsia 58 (4), 512–521. doi:10.1111/epi.13709
Schijns, O. E., Bisschop, J., Rijkers, K., Dings, J., Vanherle, S., Lindsey, P., et al. (2020). GAT-1 (rs2697153) and GAT-3 (rs2272400) polymorphisms are associated with febrile seizures and temporal lobe epilepsy. Epileptic Disord. 22 (2), 176–182. doi:10.1684/epd.2020.1154
Sesarini, C. V., Costa, L., Grañana, N., Coto, M. G., Pallia, R. C., and Argibay, P. F. (2015). Association between GABA(A) receptor subunit polymorphisms and autism spectrum disorder (ASD). Psychiatry Res. 229 (1-2), 580–582. doi:10.1016/j.psychres.2015.07.077
Shinohara, M., Saitoh, M., Nishizawa, D., Ikeda, K., Hirose, S., Takanashi, J. i., et al. (2013). ADORA2A polymorphism predisposes children to encephalopathy with febrile status epilepticus. Neurology 80 (17), 1571–1576. doi:10.1212/WNL.0b013e31828f18d8
Soukup, T., Hloch, K., Doseděl, M., Tebbens, J. D., Nekvindova, J., Šembera, Š., et al. (2020). The influence of coffee intake and genetics on adenosine pathway in rheumatoid arthritis. Pharmacogenomics 21 (11), 735–749. doi:10.2217/pgs-2020-0042
Thijs, R. D., Surges, R., O'Brien, T. J., and Sander, J. W. (2019). Epilepsy in adults. Lancet. 393 (10172), 689–701. doi:10.1016/S0140-6736(18)32596-0
van Dam, R. M., Hu, F. B., and Willett, W. C. (2020). Coffee, caffeine, and health. N. Engl. J. Med. 383 (4), 369–378. doi:10.1056/nejmra1816604
Wang, J., Lin, Z. J., Liu, L., Xu, H. Q., Shi, Y. W., Yi, Y. H., et al. (2017). Epilepsy-associated genes. Seizure 44, 11–20. doi:10.1016/j.seizure.2016.11.030
Wang, Q., Shi, N. R., Lv, P., Liu, J., Zhang, J. Z., Deng, B. L., et al. (2022). P2Y12 receptor gene polymorphisms are associated with epilepsy. Purinergic Signal 19, 155–162. doi:10.1007/s11302-022-09848-4
Xu, X., Beleza, R. O., Gonçalves, F. Q., Valbuena, S., Alçada-Morais, S., Gonçalves, N., et al. (2022). Adenosine A2A receptors control synaptic remodeling in the adult brain. Sci. Rep. 12 (1), 14690. doi:10.1038/s41598-022-18884-4
Yang, A., Palmer, A. A., and de Wit, H. (2010). Genetics of caffeine consumption and responses to caffeine. Psychopharmacol. Berl. 211 (3), 245–257. doi:10.1007/s00213-010-1900-1
Yang, R., Hong, H., Wang, M., and Ma, Z. (2018). Correlation between single-nucleotide polymorphisms within miR-30a and related target genes and risk or prognosis of nephrotic syndrome. DNA Cell. Biol. 37 (3), 233–243. doi:10.1089/dna.2017.4024
Zaorska, K., Zawierucha, P., Świerczewska, M., Ostalska-Nowicka, D., Zachwieja, J., and Nowicki, M. (2021). Prediction of steroid resistance and steroid dependence in nephrotic syndrome children. J. Transl. Med. 19 (1), 130. doi:10.1186/s12967-021-02790-w
Zhai, Y. J., Liu, P., He, H. R., Zheng, X. W., Wang, Y., Yang, Q. T., et al. (2015). The association of ADORA2A and ADORA2B polymorphisms with the risk and severity of chronic heart failure: A case-control study of a northern Chinese population. Int. J. Mol. Sci. 16 (2), 2732–2746. doi:10.3390/ijms16022732
Zhang, Y. X., Qiao, S., Cai, M. T., Lai, Q. L., Shen, C. H., and Ding, M. P. (2021). Association between autophagy-related protein 5 gene polymorphisms and epilepsy in Chinese patients. Neurosci. Lett. 753, 135870. doi:10.1016/j.neulet.2021.135870
Keywords: A2A receptor, CD73, single-nucleotide polymorphism, epilepsy, purinergic receptor
Citation: Shi N-R, Wang Q, Liu J, Zhang J-Z, Deng B-L, Hu X-M, Yang J, Wang X, Chen X, Zuo Y-Q, Liu T-T, Zheng J-L, Yang X, Illes P and Tang Y (2023) Association of the ADORA2A receptor and CD73 polymorphisms with epilepsy. Front. Pharmacol. 14:1152667. doi: 10.3389/fphar.2023.1152667
Received: 28 January 2023; Accepted: 14 March 2023;
Published: 29 March 2023.
Edited by:
Man Li, Huazhong University of Science and Technology, ChinaReviewed by:
Shangdong Liang, Nanchang University, ChinaSheng-Feng Lu, Nanjing University of Chinese Medicine, China
Copyright © 2023 Shi, Wang, Liu, Zhang, Deng, Hu, Yang, Wang, Chen, Zuo, Liu, Zheng, Yang, Illes and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Yang, eWFuZ3hpbkBjZHV0Y20uZWR1LmNu; Peter Illes, UGV0ZXIuSWxsZXNAbWVkaXppbi51bmktbGVpcHppZy5kZQ==; Yong Tang, dGFuZ3lvbmdAY2R1dGNtLmVkdS5jbg==
†These authors have contributed equally to this work
 Nan-Rui Shi1†
Nan-Rui Shi1† Jie Liu
Jie Liu Bin-Lu Deng
Bin-Lu Deng Xiang Chen
Xiang Chen Xin Yang
Xin Yang Peter Illes
Peter Illes Yong Tang
Yong Tang