- 1Institute of Chronic Disease Risks Assessment, Henan University, Kaifeng, China
- 2Institute of Nursing and Health, School of Nursing and Health, Henan University, Kaifeng, Henan, China
- 3School of Clinical Medicine, Henan University, Kaifeng, Henan, China
- 4School of Basic Medical Sciences, Henan University, Kaifeng, Henan, China
Inflammasomes play an important role in innate immunity. As a signal platform, they deal with the excessive pathogenic products and cellular products related to stress and injury. So far, the best studied and most characteristic inflammasome is the NLR-family pyrin domain-containing protein 3(NLRP3) inflammasome, which is composed of NLRP3, apoptosis associated speck like protein (ASC) and pro-caspase-1. The formation of NLRP3 inflammasome complexes results in the activation of caspase-1, the maturation of interleukin (IL)-1β and IL-18, and pyroptosis. Many studies have demonstrated that NLRP3 inflammasome not only participates in tumorigenesis, but also plays a protective role in some cancers. Hepatocellular carcinoma (HCC) is a major cause of cancer-related mortality. Currently, due to the lack of effective treatment methods for HCC, the therapeutic effect of HCC has not been ideal. Therefore, it is particularly urgent to explore the pathogenesis of HCC and find its effective treatment methods. The increasing evidences indicate that NLRP3 inflammasome plays a vital role in HCC, however, the related mechanisms are not fully understood. Hence, we focused on the recent progress about the role of NLRP3 inflammasome in HCC, and analyzed the relevant mechanisms in detail to provide reference for the future in-depth researches.
1 Introduction
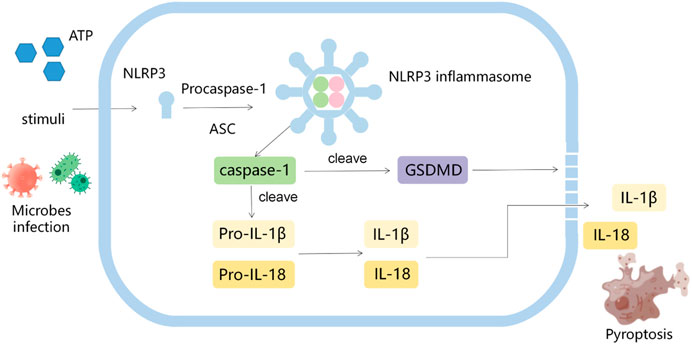
Inflammasomes, firstly proposed by Tschopp et al., in 2002, are multi-protein complexes that can activate procaspase-1 in response to “damaged-self” signals or infection (Hoffman et al., 2001; Martinon et al., 2002; Martinon et al., 2002; Broz and Dixit, 2016; Collison, 2019). The activated caspase-1 in turn transforms pro-IL-1β and pro-IL-18 into their mature forms to induce inflammation (Broz and Dixit, 2016). A variety of inflammasomes have been found: NLR-family pyrin domain-containing protein 1(NLRP1), NLRP2, NLRP3, NLRP6, NLRP7, NLRP12, NLRC4, IPAF and AIM2. Among them, NLRP3 inflammasome is the most thoroughly studied one, consisting of NLRP3, apoptosis associated speck like protein (ASC) and pro-caspase-1 (Volt et al., 2016; Sharif et al., 2019). Pathogen-associated molecular patterns (PAMPs) (including microorganisms, misfolded proteins, crystals and nanoparticles) and damage-associated molecular patterns (DAMPs) (such as extracellular ATP), induce the formation of NLRP3 oligomers, and the recruitment of ASC and pro-caspase-1, resulting in the activation of caspase-1 and the cleavage of gasdermin D (GSDMD). The activated caspase-1 cleaved pro-IL-1β and pro-IL-18 into their active forms. The N-terminal domain of cleaved GSDMD drills pores in the cell membrane and promotes the release of IL-1β and IL-18, thus inducing pyroptosis (Figure 1) (Ding et al., 2021; Coll et al., 2022). The evidences indicate that NLRP3 inflammasome is closely related to the tumorigenesis, including proliferation, invasion, angiogenesis and metastasis (Moossavi et al., 2018; Ershaid et al., 2019; Sharma and Kanneganti, 2021), however, the relevant mechanisms are not completely clear.
Liver cancer is the leading cause of cancer-related deaths and a major health problem in the world. There are more than 850,000 cases of liver cancer every year (Forner et al., 2018; Sim and Knox, 2018). Hepatocellular carcinoma (HCC) is the most common tumor and accounts for the vast majority of primary liver cancers (Ganne-Carrié and Nahon, 2019; Lurje et al., 2019). The incidence rate of HCC related to non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) is increasing. It is estimated that by 2030, the global mortality rate of HCC will reach 1 million people per year (Fitzmaurice et al., 2017; Anstee et al., 2019). The occurrence of HCC is related to many factors, including alcoholism, cirrhosis (chronic liver damage caused by fibrosis), hepatitis B virus (HBV) infection, hepatitis C virus (HCV) infection and metabolic syndrome (Piñero et al., 2020). The pathogenesis of HCC is complex, including cell cycle disorder, immune regulation disorder, microRNA (miRNA) disorder, DNA methylation change, chromosome instability, epithelial-to-mesenchymal cell transformation (EMT) and the increase of HCC stem cells (Chidambaranathan-Reghupaty et al., 2021). HCC stem cells have many characteristics similar to those of normal liver stem cells. In addition to self-renewal and tumorigenesis, it is also associated with patient resistance to treatment and relapse (Ogunwobi et al., 2019). At present, the treatment schemes for HCC include surgical resection, liver transplantation and minimally invasive local treatment including percutaneous ablation, transcatheter arterial chemoembolization (TACE) and transcatheter arterial radiation embolism (TARE) (Tellapuri et al., 2018). Patients diagnosed in the late stage of the disease are not eligible for therapeutic surgery, and treatment options for patients with advanced HCC are limited in terms of availability and effectiveness. In order to improve the status of early diagnosis and treatment, the better understanding of molecular biology of HCC is very needed (Kim and Viatour, 2020). More and more evidence indicates that NLRP3 inflammasome plays an important role in HCC, however, the relevant mechanisms are needed to be further studied. In this review, we summarized the literatures on the role of NLRP3 in HCC in recent years, and deeply analyzed the relevant mechanisms, hoping to reveal the role of NLRP3 inflammasome in HCC and provide theoretical reference for further researches in the future.
2 NLRP3 inflammasome-mediated pyroptosis plays a protective role in HCC
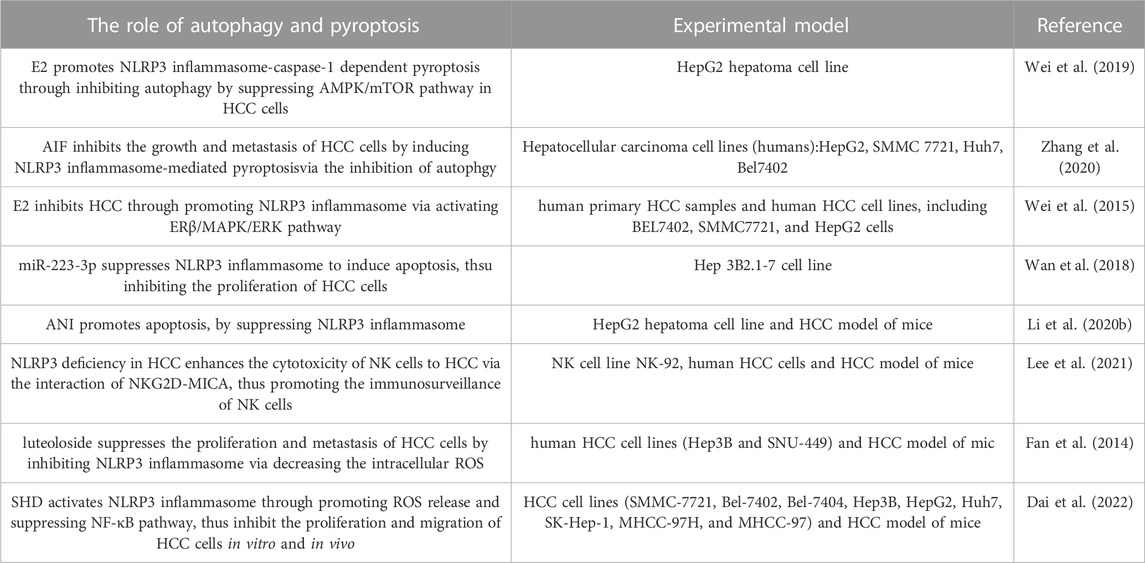
It has been reported that 17β-estradiol (E2) is involved in HCC via promoting the NLRP3 inflammasome activation (Wei et al., 2015), however, the related mechanisms are not fully understood. Qing Wei and others found that E2 promoted NLRP3 inflammasome activation through increasing the expression levels of caspase-1 and IL-1β in HCC cells. Meanwhile, E2 also decreased the viability of HCC cells and increased HCC cells mortality rate. The caspase-1-specific inhibitor YVAD-cmk significantly abolished the E2-induced inhibition of growth of HCC cells effect, suggesting that E2 induced the death of HCC cells by promoting NLRP3 inflammasome-caspase-1 dependent pyroptosis. The in-depth experiments revealed that E2 significantly inhibited autophagy in HCC cells, which was abolished by YVAD-cmk, indicating that NLRP3 inflammasome mediated E2-induced inhibition of autophagy. E2 inhibited AMPK/mTOR pathway by downregulating AMPK phosphorylation level and upregulating mTOR phosphorylation level, and AMPK overexpression and rapamycin (an mTOR inhibitor) reversed E2-induced autophagy inhibition, suggesting that E2 suppressed autophagy by inhibiting AMPK/mTOR pathway. In addition, 3-MA, an autophagy inhibitor significantly upregulated E2-induced pyroptosis, which was reversed by YVAD-cmk, suggesting that autophagy negatively regulated inflammasome-caspase-1 dependent pyroptosis induced by E2. Collectively, E2 induced NLRP3 inflammasome-caspase-1 dependent pyroptosis by suppressing autophagy through inhibiting AMPK/mTOR pathway in HCC cells. E2-induced NLRP3 inflammasome promoted HCC via upregulating caspase-1-dependent pyroptosis of HCC cells (Wei et al., 2019). The interaction between NLRP3 inflammasome and autophagy participates in many pathological processes (Tao et al., 2020; Ding et al., 2021; Lv et al., 2021). In the above study, autophagy inhibition promotes E2-induced NLRP3 inflammasome of HCC cells, thus providing a new idea for HCC treatment by regulating autophagy. Conversely, E2-induced autophagy can also be negatively regulated by NLRP3 inflammasome in HCC cells. Therefore, the relationship between NLRP3 inflammasome and autophagy in HCC deserves further study in the future, which will help to explore the pathogenesis of HCC.
Another study has confirmed the above conclusion that promoting NLRP3 inflammasome-mediated pyropsis significantly inhibits HCC. Alpinumisoflavone (AIF) is a dimethyl pyran derivative that is isoprenoized on the ring A of genistein. It is the main component of Derris eriocarpa F.C. (Ateba et al., 2019). AIF has been reported to have a variety of pharmacological activities, including estrogenic (Mvondo et al., 2012), atherosclerosis protection (Ateba et al., 2019), estrogen and antibacterial (Chukwujekwu et al., 2011). In recent years, AIF has been found to have potential anti-tumor effects (Zhang et al., 2021). However, the relevant mechanisms are not yet fully understood. Yan Zhang and others studied the role of AIF and pyroptosis in HCC, and the results showed that AIF could inhibit the growth and clonogenic capacity of HCC cells, but not normal hepatocytes. In HCC cells treated with AIF, the number of HCC cells with invasion and migration ability was significantly reduced, the protein expression levels of matrix metalloproteinase-2 (MMP-2) and matrix metalloproteinase-9 (MMP-9) were significantly reduced, while the expression level of metalloproteinases-3 (TIMP3) was significantly increased, indicating that AIF inhibited the migration and invasion of HCC cells. The in-depth research showed that AIF increased the release of lactate dehydrogenase (LDH), and upregulated the expression levels of NLRP3, caspase- 1, IL-18 and IL-1β, suggesting that AIF induced the pyroptosis of HCC cells through activating NLRP3-caspase-1-IL-1β pathways. The treatment of MCC950 (NLRP3 inflammasome inhibitor) abolished the effect of AIF on pyroptosis-related genes. Moreover, NLRP3 knockdown inhibited AIF-induced increase of cleaved caspase-1, mature IL-1β, IL-18, and GSDMD-N, and weakened the inhibitory effects of AIF on the viability and motility of HCC cells. These results indicated that AIF-induced pyroptosis of HCC cell was dependent on the activation of NLRP3, and AIF inhibition of the viability and motility of HCC cells was mediated by pyroptosis. Furthermore, AIF treatment enhanced autophagy by upregulating the expression levels of the autophagy-related proteins including LCII, and beclin 1 and downregulating p62 expression in HCC cells. Further, CQ (autophagy inhibitor) or Atg siRNA promoted AIF-induced NLRP3 inflammasome-mediated pyroptosis of HCC cells, which seemed to contradict with the above that AIF promoted autophagy. Does AIF-upregulated autophagy inhibit AIF-induced NLRP3 inflammasome? The reason needed to be clarified. Collectively, AIF inhibited the growth and metastasis of HCC cells by inducing NLRP3 inflammasome-mediated pyroptosis, (Zhang et al., 2020). In the above study, the relationship between autophagy and NLRP3 inflammasome in HCC cells deserves further study in the future. In addition, it has been reported that AIF can promote the apoptosis of cancer cells (Han et al., 2016; Hong et al., 2022). Therefore, whether AIF can induce apoptosis of HCC cells through NLRP3 inflammasome remains to be clarified.
Estrogen mediates the various systemic effects in women and men, and regulates the physiological and pathological processes of reproductive, skeletal, nervous, cardiovascular, endocrine and immune systems (Tang et al., 2019). The effect of estrogen is mainly mediated by estrogen receptor (ER)ɑ and ERβ (Song et al., 2019). It has been reported that 17β-estradiol (E2, a form of estrogen) or ERβ is invovled in HCC (Teng et al., 2014; Ren et al., 2016; Xu et al., 2018). However, the role and mechanism of E2 and ERβ in HCC have not been clarified. Qing Wei and others found that compared with normal liver tissue, the ERβ expression in HCC tissue was notably decreased. Furthermore, the protein level of ERβ was negatively correlated with the pathological grades and clinical stages of the HCC patients, and positively correlated with the levels of NLRP3 inflammasome components. In addition, E2 significantly increased the expression of NLRP3 inflammasome, and activated MAPK/ERK pathway in HCC cells. The ERβ-specific inhibitor or the inhibitor of MAPK pathway could abolish E2-induced increase of the expression of NLRP3 inflammasome, indicating that E2 upregulated the expression of NLRP3 inflammasome through activating ERβ/MAPK/ERK pathway. The experiments using HCC cells transfected with the plasmids encoding NLRP3 inflammasome demonstrated that the levels of NLRP3 inflammasome, caspase-1 and IL-1β were significantly increased, the proliferation of these HCC cells was inhibited, and the LDH release was upregulated, indicating that overexpression of NLRP3 suppressed the growth of HCC cells and promoted the death of HCC cells. Moreover, the inhibition of ERβ/MAPK/ERK pathway abolished E2 inhibition of the cell viability, colony formation capability and migration of HCC cells, suggesting that E2 suppressed HCC by activating NLRP3 inflammasome via ERβ/MAPK/ERK pathway (Wei et al., 2015). The above conclusions well explain why the incidence of HCC has gender differences. The promotion of pyroptosis and apoptosis has been reported to inhibit HCC (Wan et al., 2018; Shen et al., 2021; Yan et al., 2022). Therefore, from the above, it can be deduced that E2 suppressed HCC through promoting NLRP3 inflammasome-mediated pyroptosis (Wei et al., 2015).
3 NLRP3 inflammasome-mediated apoptosis plays a protective role in HCC
MicroRNAs (miRNAs) are a group of highly conserved non-protein-coding small RNAs, which are post-transcriptional regulators of gene expression. Many studies have shown that miRNA can regulate many biological processes, including cancer, inflammation and metabolism (Wurdinger and Costa, 2007; Quintanilha et al., 2017). MiR-223-3p has been reported to interact with the 3′-untranslated region fragment of NLRP3 and suppress its expression (Deng et al., 2020; Wang et al., 2021). In addition, many evidences indicate that miR-223-3p is involved in HCC (Pratedrat et al., 2020; Si et al., 2021). However, the role and mechanism of miR-223-3p in regulating NLRP3 in HCC have not been understood. To clarify this, LINGFENG WAN and others committed a series of experiments and MTT assay showed that the proliferation of HCC cells transfected with miR-223-3p decreased significantly. At the same time, after transfection with miR-223-3p, the apoptosis rate increased significantly. These results indicated that miR-223-3p had antitumor effect on HCC cells. The dual-luciferase reporter assay showed that the co-transfection of miR-223-3p decreased luciferase activity of the plasmid containing the fragment of NLRP3 3′-UTR. However, the luciferase activity of the plasmid containing the mutant fragment of NLRP3 3′-UTR was not influenced by co-transfection with the miR-223 analog, indicating that miR-223-3p directly interacted with 3′- UTR of NLRP3 mRNA.Further experiments showed that after transfection with miR-223-3p, the expression of NLRP3, caspase-1, IL-1β and IL-18 in HCC cells was inhibited, indicating that miR-223-3p suppressed NLRP3 inflammasome. Moreover, the inhibition of NLRP3 inflammasome, IL-1β and IL-18 promoted the apoptosis of HCC cells. Collectively, miR-223-3p inhibited NLRP3 inflammasome to inducing apoptosis, thus inhibiting the proliferation of HCC cells (Wan et al., 2018). At present, it has been reported that NLRP3 inflammasome positively regulates apoptosis of cancer cells (Jabir et al., 2021), which is inconsistent with the conclusion that inhibition of NLRP3 inflammasome induces apoptosis of HCC cells in above studies. Therefore, the relationship between NLRP3 inflammasome and apoptosis, especially in cancer, needs further study.
Anisodamine (ANI) is a belladonna alkaloid. Like other drugs in the family, it is not only a nicotine choline receptor antagonist, but also a non-subtype-selective muscarine (Zhao et al., 1993). It has been reported that ANI also has a significant anti-inflammatory effect, which can reduce the damage of kidney and heart by inhibiting the activation of inflammasomes and decreasing the expression levels of inflammatory factors. However, the role of ANI in cancers has not yet been clarified (Yuan et al., 2017; Yao et al., 2018). ANI can alleviate the tissue damage in diseases by regulating NLRP3 inflammasome (Yuan et al., 2017; Li et al., 2020a). However, the role of ANI in regulating NLRP3 in HCC is unclear. Ping Li et al. studied the molecular mechanism of the anti-cancer role of ANI in HCC through inhibiting NLRP3 inflammasome by constructing a xenotransplantation mouse model of HCC. The results showed that ANI could inhibit the formation of tumor, increase the survival rate of HCC xenograft mice, and reduce the levels of TG, TC, HDL and LDL in serum. In addition, The results of HE staining showed that ANI treatment improved the histopathological damage of HCC xenotransplantation mice in a dose-dependent manner. These findings indicated that ANI could improve HCC. Moreover, NLRP3 inflammasome was inhibited by ANI evidenced by the decreased levels of ASC, caspase-1, IL-18 and IL-1β. Further analysis showed that NLRP3 overexpression resulted in the increase of tumor volume and decreased survival rate of HCC xenograft mice, indicating that NLRP3 overexpression counteracted the therapeutic effects of ANI on HCC. In-depth mechanism studies showed that ANI reduced the expressions of Ki67 (the proliferating cell related antigen) and vascular endothelial-derived growth factor (VEGF), and induced HCC cells apoptosis, which were reversed by NLRP3 overexpression, indicating that ANI inhibited the growth and motility of HCC cells and induced apoptosis by inhibiting NLRP3 inflammasome. In addition, ANI increased the levels of INF-γ and IL-27, and reduced the levels of TNF-α and IL-4, while overexpression of NLRP3 counteracted this effect. Furthermore, overexpression of NLRP3 reversed ANI-induced reduction in the number of Th1 and Th2 cells. These results indicated that ANI could achieve anti-inflammatory effect to play anti-cancer roles by inhibiting the expression of NLRP3 inflammasome. Furthermore, the knocking down NLRP3 with shRNA enhanced the ANI inhibition of the development of HCC in xenotransplantation mice. Summarily, ANI promoted apoptosis of HCC cells through inhibiting NLRP3 inflammasome (Li et al., 2020b). At present, the relationship between Th1/Th2 balance and cancer has been studied extensively (Shahid and Bharadwaj, 2019; Hetland et al., 2020). In the above study, ANI reduced the number of Th1 and Th2 cells by inhibiting NLRP3 inflammasome (Li et al., 2020b). Therefore, whether ANI can regulate the balance of Th1/Th2 via NLRP3 inflammasome in HCC needs to be further explored. Besides, that ANI inhibits the metastasis of HCC through reducing VEGF expression by inhibiting NLRP3 inflammasome needs further validation.
4 NLRP3 inflammasome inhibition enhanced HCC sensitivity to the cytotoxicity of natural killer cells
Natural killer (NK) cells are important natural immune cells which can kill infected viruses and cancer cells (Lim et al., 2015; Fang et al., 2018; Sung and Jang, 2018), and are involved in the first immune defense against viral infection and cancer (Liu et al., 2018). NK cells play an important role in HCC (Guillerey et al., 2016). Although there have been many studies on the role of NK cells in HCC, the role and mechanism of NK cells and NLRP3 inflammasome in HCC are still unclear. Hwan Hee Lee et al. deleted NLRP3 gene in HCC using lentivirus CRISPR-cas9 system, then studied how NLRP3 KO influenced the cytotoxicity of NK cells in HCC. The results showed that NK cytotoxicity to HCC cells lacking NLRP3 was significantly increased. NK cytotoxicity is affected by its NK-activating receptors (NKG2D). The expression of NKG2D on NK cells was notably upregulated in a co-culture of NK cells and NLRP3 KO HCC cells (Lee et al., 2021). It has been reported that NK-activating receptors interact with various ligands on the surface of cancer cells, thereby inducing the toxicity of NK cells to cancer cells (Thompson et al., 2017). MICA/B is a ligand expressed in many cancer cells, which can bind to the NKG2D receptor on NK cells to increase cytotoxicity. Its expression was found to be upregulated on the surface of NLRP3 KO HCC cells through the downregulation of the expression of matrix metalloproteinase in liver tissues of mice implanted with NLRP3 KO HCC cells. Moreover, in a xenograft mice model, NLRP3 KO HCC cells inhibited HCC development and metastasis, and increased HCC sensitivity to the cytotoxicity of NK cells. Collectively, NLRP3 deficiency in HCC increased the cytotoxicity of NK cells to HCC via the interaction of NKG2D-MICA, thus enhancing the immunosurveillance of NK cells (Lee et al., 2021). The immunosurveillance of NK cells is important for HCC aggressiveness (Wang et al., 2016; Cadoux et al., 2021). In the above study, the deletion of NLRP3 inflammasome can enhance the immunosurveillance of NK cells on HCC, which clarifies a new mechanism for the role of NLRP3 inflammasome in HCC. However, the further research is needed on this issue. For example, how NLRP3 inflammasome deficiency upregulates the NK-activating receptors on NK cells and corresponding ligands on HCC cells.
5 The role of reactive oxygen species (ROS)/NLRP3 inflammasome in HCC
Luteoloside (luteolin-7-O-glucoside; cymaroside), an active ingredient isolated from Reseda odorata L., has the effects of anti-tumor, anti-inflammatory, antibacterial and free radical scavenging (Baskar et al., 2011; Sun et al., 2011; Xiong et al., 2013). Although the role of luteolide in cancer has been widely studied (hao et al., 2018; Zhou et al., 2017), the mechanism of action is still unclear, especially in HCC. The results of Shao hua Fan and others showed that luteoloside inhibited the proliferation, migration and invasion of HCC cells in vitro. Luteoloside has no significant effect on the expressions of caspase-3, IL-CII and Beclin 1 in HCC cells, indicating that luteoloside has no effect on inducing apoptosis and autophagy of HCC cells. Further mechanism studies showed that luteoloside could reduce the accumulation of intracellular ROS, enhance the inhibition of ROS induced by NAC (a specific ROS inhibitor), and reverse the upregulation of ROS induced by H2O2 (a ROS inducer). In addition, luteoloside downregulated the expressions of NLRP3 inflammasome and IL-1β in HCC cells. In vivo, experiments also verified that luteoloside inhibited the proliferation and metastasis of HCC (Fan et al., 2014). Previous studies have revealed that NLRP3 inflammasome can positively regulate the proliferation and metastasis of tumor cells (Ershaid et al., 2019; Hofbauer et al., 2021), and the activation of NLRP3 inflammasome depends on the production of ROS (Li et al., 2021). Therefore, it could be deduced that luteoloside inhibits the proliferation and metastasis of HCC cells by inhibiting NLRP3 inflammasome through reducing intracellular ROS (Fan et al., 2014). In the above study, luteoloside has no signifcant effect on apoptosis and autophagy of HCC cells, indicating that aopoptosis and autophagy-related cell death are not involved in luteoloside inhibition of HCC. Therefore, the mechanism of NLRP3 inflammasome inhibiting the proliferation and metastasis of HCC cells needs to be clarified. The evidences indicate that autophagy, NLRP3 inflammasome and apoptosis are interrelated and play an important role in cancer (Jabir et al., 2021). While in the above study, luteoloside only inhibits NLRP3 inflammasome, but has no effect on apoptosis and autophagy of HCC cells. This indicates that NLRP3 inflammasome appears to be unrelated to apoptosis and autophagy in HCC cells, which contradicts the previous research conclusions. The reason may be related to different types of tumor cells and needs to be clarified.
Shuanghua decoction (SHD) consists of Oldenlandia diffusa Willd., Prunella vulgaris L., Panax ginseng C. A., Meyer, and Lonicera japonica Thunb., and is a traditional folk prescription in China. It has been reported that SHD plays an anti-tumor role through suppressing the growth of tumor cells and promoting the apoptosis of tumor cells, and strengthens the immunity (Zhu et al., 2018; Wang et al., 2019a; Ge et al., 2019; Luo et al., 2020; Zhao et al., 2020). The anticancer mechanism of SHD is far from clear. Bingling Dai and others studied the role and mechanism of SHD and NLRP3 inflammasome in HCC, and the results showed that two ingredients of SHD, Oldenlandia and OP (Oldenlandia 15 g: Prunella spike 1.5 g = 10:1), significantly inhibited the growth of HCC cells and xenograft tumors evidenced by the decreased size and weight of tumour, and downregulated Ki67. Oldenlandia could inhibit the colony formation of HCC cells, make more cells stop at S phase of the cell cycle, and increase the release of LDH. Furthermore, Oldenlandia significantly induced apoptosis of HCC cells by increasing the levels of Bax and cleaved PARP, and decreasing Bcl-2 level. In addition, Oldenlandia and OP significantly inhibited the migration of HCC cells. Further studies showed that Oldenlandia upregulated the expressions of NLRP3 inflammasome, pro-IL-1β and cleaved-caspase-1 in a concentration-dependent manner. Oldenlandia also significantly enhanced the activation of NLRP3 inflammasome induced by LPS + ATP. Mechanism studies revealed that Oldenlandia increased ROS release in a concentration and time-dependent manner, while NAC (a ROS scavenger) reduced Oldenlandia-induced ROS release, and weakened the activation of Oldenlandia-induced NLRP3 inflammasome activation, indicating that Oldenlandia activated NLRP3 inflammasome in HCC cells by promoting ROS release. In addition, Oldenlandia reduced IKKβ expression and inhibited LPS (NF-κB activator)-induced phosphorylation of IkBα and NF-κB. PDTC and MG132 (two NF-κB inhibitors) reduced NF-κB phosphorylation, while the combination of Oldenlandia, PDTC and MG132 enhanced the inhibition of NF-κB phosphorylation. These indicated that Oldenlandia inhibited NF-κB pathway. OP could obtain the similar results as Oldenlandia, and also inhibit the expressions of N-cadherin, snail, MMP9, MMP2 and vimentin. Summarily, it can be deduced that SHD activates NLRP3 inflammasome through promting ROS release and inhibiting NF-κB signal pathway to induce apoptosis and cell cycle arrest of HCC cells in vitro and in vivo, which need to be further confirmed by using the inhibitor of NF-κB signal pathway (Dai et al., 2022). NLRP3 inflammasome-mediated pyroptosis has been reported to play an important role in HCC (Zhang et al., 2020). Whether NLRP3 inflammasome-mediated pyroptosis is involved in SHD inhibition of HCC needs to be studied in the future. ROS/NLRP3 inflammasome will provide an important target for the treatment of HCC.
6 Conclusion
In recent years, the increasing evidences show that NLRP3 inflammasome plays an important role in HCC. In this review, we summarize the role of NLRP3 inflammasome in HCC as follows: 1)E2 promotes NLRP3 inflammasome-caspase-1 dependent pyroptosis through inhibiting autophagy by suppressing AMPK/mTOR pathway in HCC cells; 2)AIF inhibits the growth and metastasis of HCC cells by inducing NLRP3 inflammasome-mediated pyroptosis via the inhibition of autophgy; 3)E2 inhibits HCC through promoting NLRP3 inflammasome via activating ERβ/MAPK/ERK pathway; 4)miR-223-3p suppresses NLRP3 inflammasome to induce apoptosis, thus inhibiting the proliferation of HCC cells; 5)ANI promotes apoptosis of HCC cells by suppressing NLRP3 inflammasome; 6)NLRP3 deficiency in HCC enhances the cytotoxicity of NK cells to HCC via the interaction of NKG2D-MICA, thus promoting the immunosurveillance of NK cells; 7) Luteoloside suppresses the proliferation and metastasis of HCC cells by inhibiting NLRP3 inflammasome via decreasing the intracellular ROS; 8)SHD activates NLRP3 inflammasome through promoting ROS release and suppressing NF-κB pathway, thus inducing apoptosis and cell cycle arrest of HCC cells (Table 1). Our previous research team showed that exogenous H2S can regulate NLRP3 inflammasome through AMPK/mTOR pathway (Wang et al., 2019b). In this review, in addition to AMPK/mTOR pathway, ERβ/MAPK/ERK pathway and NF-κB pathway are also involved in the role of NLRP3 inflammasome in HCC. Whether there are other pathways involved in the role of NLRP3 inflammasome in HCC remains to be clarified. The purinergic ligand gated ion channel 7 receptor (P2X7 receptor) is an adenosine triphosphate (ATP) gated ion channel that is widely distributed in various tissues and cells, including HCC cells (Li et al., 2023). The activation of P2X7 receptor can promote the assembly of NLRP3 inflammasome, thereby activating NLRP3 inflammasome (Di Virgilio et al., 2017). The evidence indicates that melatonin inhibits the activation of NLRP3 inflammasome in a mouse model of non-alcoholic steatohepatitis induced by high fat diet by inhibiting P2X7R receptors (Saha et al., 2022). Hence, whether the inhibition of NLRP3 inflammasome via P2X7R receptors can improve HCC is worth studying in the future.
The role of NLRP3 inflammasome in HCC remains controversial. It has been reported that NLRP3 inflammasome plays a dual role in cancer (Moossavi et al., 2018; Hamarsheh and Zeiser, 2020; Sharma and Kanneganti, 2021). In the literature cited in this review, sometimes NLRP3 inflammasome plays a role in inhibiting HCC, and sometimes it plays the opposite role. The reason may be related to the different HCC cell line and the different course of HCC. As the core part of inflammation, the activation of NLRP3 inflammasome promotes the release of IL-1β and IL-18, thereby accelerating the growth and metastasis of tumors. On the contrary, when the tissue is damaged to a certain extent, the activation of inflammasome inhibits tumor cells by promoting pyroptosis and apoptosis. The conditions under which NLRP3 inflammasomes are beneficial to HCC and inhibit HCC need to be clarified by future researches. In addition, it can be seen from this review that NLRP3 inflammasome inhibits HCC through inducing pyroptosis, apoptosis, cell cycle arrest and the enhancement of the immunosurveillance of NK cells. Moreover, autophagy and ROS play a role in HCC by associating with NLRP3 inflammasome (Figure 2). However, the mechanism of autophagy and ROS remains unclear and needs further study. At present, there are few studies on the role of NLRP3 inflammasome in cancer by regulating NK. Therefore, how NLRP3 inflammasome regulates NK cells in HCC is a topic worthy of study.
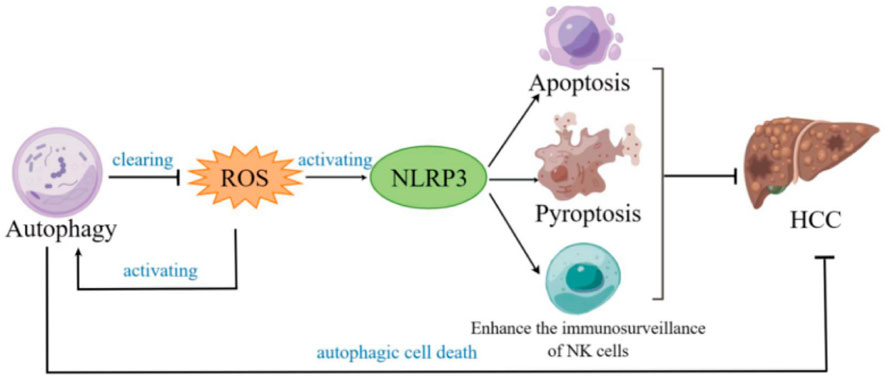
FIGURE 2. Diagram of the role of autophagy, NLRP3 and reactive oxygen species (ROS) in hepatocellular carcinoma.
It is believed that with the deepening of research, ROS/NLRP3 inflammasome will provide an important target for the treatment of HCC.
Author contributions
HW and CC devised and wrote the manuscript; HZ wrote and funded with the manuscript; YiZ, YaZ, YY and HL wrote the manuscript. All authors read and approved the final manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Anstee, Q. M., Reeves, H. L., Kotsiliti, E., Govaere, O., and Heikenwalder, M. (2019). From NASH to HCC: Current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol. 16, 411–428. doi:10.1038/s41575-019-0145-7
Ateba, S. B., Mvondo, M. A., Djiogue, S., Zingué, S., Krenn, L., and Njamen, D. (2019). A pharmacological overview of alpinumisoflavone, a natural prenylated isoflavonoid. Front. Pharmacol. 10, 952. doi:10.3389/fphar.2019.00952
Baskar, A. A., Ignacimuthu, S., Michael, G. P., and Al Numair, K. S. (2011). Cancer chemopreventive potential of luteolin-7-O-glucoside isolated from Ophiorrhiza mungos Linn. Nutr. cancer 63, 130–138. doi:10.1080/01635581.2010.516869
Broz, P., and Dixit, V. M. (2016). Inflammasomes: Mechanism of assembly, regulation and signalling Nat. Rev. Immunol., 16, pp. 407–420. doi:10.1038/nri.2016.58
Cadoux, M., Caruso, S., Pham, S., Gougelet, A., Pophillat, C., Riou, R., et al. (2021). Expression of NKG2D ligands is downregulated by β-catenin signalling and associates with HCC aggressiveness. J. Hepatol. 74 (6), 1386–1397. doi:10.1016/j.jhep.2021.01.017
Chen, B., Song, Y., Zhan, Y., Zhou, S., Ke, J., Ao, W., et al. (2022). Fangchinoline inhibits non-small cell lung cancer metastasis by reversing epithelial-mesenchymal transition and suppressing the cytosolic ROS-related Akt-mTOR signaling pathway. Cancer Lett. 543, 215783. doi:10.1016/j.canlet.2022.215783
Chidambaranathan-Reghupaty, S., Fisher, P. B., and Sarkar, D. (2021). Hepatocellular carcinoma (HCC): Epidemiology, etiology and molecular classification. Adv. Cancer Res. 149, 1–61. doi:10.1016/bs.acr.2020.10.001
Chukwujekwu, J. C., Van Heerden, F. R., and Van Staden, J. (2011). Antibacterial activity of flavonoids from the stem bark of Erythrina caffra thunb. Phytotherapy Res. 25, 46–48. doi:10.1002/ptr.3159
Coll, R. C., Schroder, K., and Pelegrín, P. (2022). NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol. Sci. 43 (8), 653–668. doi:10.1016/j.tips.2022.04.003
Collison, J. (2019). Oncogenes and inflammasomes in lupus nephritis Nat. Rev. Rheumatol., 15, p. 190, doi:10.1038/s41584-019-0194-x
Dai, B., Fan, M., Huang, X., Gong, Z., Cao, H., Hu, Y., et al. (2022). Shuanghua decoction exerts anticancer activity by activating NLRP3 inflammasome via ROS and inhibiting NF-κB signaling in hepatocellular carcinoma cells. Phytomedicine 103, 154249. doi:10.1016/j.phymed.2022.154249
Deng, B., Hu, Y., Sheng, X., Zeng, H., and Huo, Y. (2020). miR-223-3p reduces high glucose and high fat-induced endothelial cell injury in diabetic mice by regulating NLRP3 expression. Exp. Ther. Med. 20 (2), 1514–1520. doi:10.3892/etm.2020.8864
Di Virgilio, F., Dal Ben, D., Sarti, A. C., Giuliani, A. L., and Falzoni, S. (2017). The P2X7 receptor in infection and inflammation. Immunity 47 (1), 15–31. doi:10.1016/j.immuni.2017.06.020
Ding, Y., Fu, X., Wang, Q., Liu, H., Wang, H., and Wu, D. (2021). The complex Interplay between autophagy and NLRP3 inflammasome in renal diseases. Int. J. Mol. Sci. 22 (23), 12766. doi:10.3390/ijms222312766
Ershaid, N., Erez, N., Doron, H., Raz, Y., Shani, O., Cohen, N., et al. (2019). NLRP3 inflammasome in fibroblasts links tissue damage with inflammation in breast cancer progression and metastasis. Nat. Commun. 10 (1), 4375. doi:10.1038/s41467-019-12370-8
Fan, S. H., Wang, Y. Y., Lu, J., Zheng, Y. L., Wu, D. M., Li, M. Q., et al. (2014). Luteoloside suppresses proliferation and metastasis of hepatocellular carcinoma cells by inhibition of NLRP3 inflammasome. PLoS One 9 (2), e89961. doi:10.1371/journal.pone.0089961
Fang, F., Xiao, W., and Tian, Z. (2018). Challenges of NK cell-based immunotherapy in the new era. Front. Med. 12, 440–450. doi:10.1007/s11684-018-0653-9
Fitzmaurice, C., Akinyemiju, T., Abera, S., Ahmed, M., Alam, N., Alemayohu, M. A., et al. (2017). The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level results from the global burden of disease study 2015. JAMA Oncol. 3, 1683–1691. doi:10.1001/jamaoncol.2017.3055
Forner, A., Reig, M., and Bruix, J. (2018). Hepatocellular carcinoma. Lancet 391 (10127), 1301–1314. doi:10.1016/S0140-6736(18)30010-2
Ganne-Carrié, N., and Nahon, P. (2019). Hepatocellular carcinoma in the setting of alcohol-related liver disease. J. Hepatol. 70 (2), 284–293. doi:10.1016/j.jhep.2018.10.008
Gao, C., Yan, Y., Chen, G., Wang, T., Luo, C., Zhang, M., et al. (2020). Autophagy activation Represses pyroptosis through the IL-13 and JAK1/STAT1 pathways in a mouse model of Moderate Traumatic Brain injury. ACS Chem. Neurosci. 11 (24), 4231–4239. doi:10.1021/acschemneuro.0c00517
Ge, L., Xiao, L., Wan, H., Li, J., Lv, K., Peng, S., et al. (2019). Chemical constituents from Lonicera japonica flower buds and their anti-hepatoma and anti HBV activities. Bioorg. Chem. 92, 103198. doi:10.1016/j.bioorg.2019.103198
Guillerey, C., Huntington, N. D., and Smyth, M. J. (2016). Targeting natural killer cells in cancer immunotherapy. Nat. Immunol. 17, 1025–1036. doi:10.1038/ni.3518
Hamarsheh, S., and Zeiser, R. (2020). NLRP3 inflammasome activation in cancer: A Double-Edged Sword. Front. Immunol. 11, 1444. doi:10.3389/fimmu.2020.01444
Han, Y., Yang, X., Zhao, N., Peng, J., Gao, H., and Qiu, X. (2016). Alpinumisoflavone induces apoptosis in esophageal squamous cell carcinoma by modulating miR-370/PIM1 signaling. Am. J. Cancer Res. 6 (12), 2755–2771.
hao, J., Wang, C., Li, L., Liang, H., Dai, J., Ling, X., et al. (2018). Luteoloside inhibits proliferation and promotes Intrinsic and Extrinsic pathway-mediated apoptosis involving MAPK and mTOR signaling pathways in human Cervical cancer cells. Int. J. Mol. Sci. 19 (6), 1664. doi:10.3390/ijms19061664
Hetland, G., Tangen, J. M., Mahmood, F., Mirlashari, M. R., Nissen-Meyer, L. S. H., Nentwich, I., et al. (2020). Antitumor, anti-inflammatory and Antiallergic effects of Agaricus blazei Mushroom Extract and the related Medicinal Basidiomycetes Mushrooms, Hericium erinaceus and Grifolafrondosa: A review of Preclinical and clinical studies. Nutrients 12 (5), 1339. doi:10.3390/nu12051339
Hofbauer, D., Mougiakakos, D., Broggini, L., Zaiss, M., Büttner-Herold, M., Bach, C., et al. (2021). β2-microglobulin triggers NLRP3 inflammasome activation in tumor-associated macrophages to promote multiple myeloma progression. Immunity 54 (8), 1772–1787.e9. doi:10.1016/j.immuni.2021.07.002
Hoffman, H. M., Mueller, J. L., Broide, D. H., Wanderer, A. A., and Kolodner, R. D. (2001) Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Genet., 29, pp. 301–305. doi:10.1038/ng756
Hong, T., Ham, J., Song, G., and Lim, W. (2022). Alpinumisoflavone Disrupts endoplasmic reticulum and Mitochondria leading to apoptosis in human Ovarian cancer. Pharmaceutics 14 (3), 564. doi:10.3390/pharmaceutics14030564
Jabir, M. S., Saleh, Y. M., Sulaiman, G. M., Yaseen, N. Y., Sahib, U. I., Dewir, Y. H., et al. (2021). Green Synthesis of Silver nanoparticles using Annona muricata Extract as an inducer of apoptosis in cancer cells and inhibitor for NLRP3 inflammasome via enhanced autophagy. Nanomater. (Basel) 11 (2), 384. doi:10.3390/nano11020384
Jiang, C., Jiang, L., Li, Q., Liu, X., Zhang, T., Dong, L., et al. (2018). Acrolein induces NLRP3 inflammasome-mediated pyroptosis and suppresses migration via ROS-dependent autophagy in vascular endothelial cells. Toxicology 410, 26–40. doi:10.1016/j.tox.2018.09.002
Jin, M., Wang, J., Ji, X., Cao, H., Zhu, J., Chen, Y., et al. (2019). MCUR1 facilitates epithelial-mesenchymal transition and metastasis via the mitochondrial calcium dependent ROS/Nrf2/Notch pathway in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 38 (1), 136. doi:10.1186/s13046-019-1135-x
Kim, E., and Viatour, P. (2020). Hepatocellular carcinoma: Old friends and new tricks. Exp. Mol. Med. 52 (12), 1898–1907. doi:10.1038/s12276-020-00527-1
Lee, H. H., Kim, D., Jung, J., Kang, H., and Cho, H. (2021). NLRP3 deficiency in hepatocellular carcinoma enhances Surveillance of NK-92 through a Modulation of MICA/B. Int. J. Mol. Sci. 22 (17), 9285. doi:10.3390/ijms22179285
Lee, W., Song, G., and Bae, H. (2022). Laminarin Attenuates ROS-mediated cell migration and Invasiveness through mitochondrial dysfunction in pancreatic cancer cells. Antioxidants (Basel) 11 (9), 1714. doi:10.3390/antiox11091714
Li, M. Y., Zhu, X. L., Zhao, B. X., Shi, L., Wang, W., Hu, W., et al. (2019). Adrenomedullin alleviates the pyroptosis of Leydig cells by promoting autophagy via the ROS-AMPK-mTOR axis. Cell Death Dis. 10 (7), 489. doi:10.1038/s41419-019-1728-5
Li, P., Liu, Y., and He, Q. (2020). Anisodamine suppressed the growth of hepatocellular carcinoma cells, induced apoptosis and regulated the levels of inflammatory factors by inhibiting NLRP3 inflammasome activation. Drug Des. Devel Ther. 14, 1609–1620. doi:10.2147/DDDT.S243383
Li, X., Bai, X., Tang, Y., Qiao, C., Zhao, R., and Peng, X. (2023). Research progress on the P2X7 receptor in liver injury and hepatocellular carcinoma. Chem. Biol. Drug Des. 101 (3), 794–808. doi:10.1111/cbdd.14182
Li, Z., Chi, H., Zhu, W., Yang, G., Song, J., Mo, L., et al. (2021). Cadmium induces renal inflammation by activating the NLRP3 inflammasome through ROS/MAPK/NF-κB pathway in vitro and in vivo. Arch. Toxicol. 95 (11), 3497–3513. doi:10.1007/s00204-021-03157-2
Li, Z., Xu, C., Tao, Y., Liang, Y., Liang, Q., Li, J., et al. (2020). Anisodamine alleviates lipopolysaccharide-induced pancreatic acinar cell injury through NLRP3 inflammasome and NF-κB signaling pathway. J. Recept Signal Transduct. Res. 40 (1), 58–66. doi:10.1080/10799893.2020.1713808
Lim, O., Jung, M. Y., Hwang, Y. K., and Shin, E. C. (2015). Present and future of allogeneic natural killer cell therapy. Front. Immunol. 6, 286. doi:10.3389/fimmu.2015.00286
Liu, P., Chen, L., and Zhang, H. (2018). Natural killer cells in liver disease and hepatocellular carcinoma and the NK cell-based immunotherapy. J. Immunol. Res. 2018, 1206737. doi:10.1155/2018/1206737
Luo, C. H., Ma, L. L., Liu, H. M., Liao, W., Xu, R. C., Ci, Z. M., et al. (2020). Research progress on main symptoms of novel coronavirus pneumonia improved by traditional Chinese medicine. Front. Pharmacol. 11, 556885. doi:10.3389/fphar.2020.556885
Lurje, I., Czigany, Z., Bednarsch, J., Roderburg, C., Isfort, P., Neumann, U. P., et al. (2019). Treatment Strategies for hepatocellular carcinoma ⁻ a Multidisciplinary Approach. Int. J. Mol. Sci. 20 (6), 1465. doi:10.3390/ijms20061465
Lv, S., Liu, H., and Wang, H. (2021). The Interplay between autophagy and NLRP3 inflammasome in ischemia/reperfusion injury. Int. J. Mol. Sci. 22 (16), 8773. doi:10.3390/ijms22168773
Martinon, F., Burns, K., Tschopp, J., et al. (2002). The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Cell, 10, pp. 417–426. doi:10.1016/s1097-2765(02)00599-3
Martinon, F., Burns, K., and Tschopp, J. (2002). The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 10, 417–426. doi:10.1016/s1097-2765(02)00599-3
Moossavi, M., Parsamanesh, N., Bahrami, A., Atkin, S. L., and Sahebkar, A. (2018). Role of the NLRP3 inflammasome in cancer. Mol. Cancer 17 (1), 158. doi:10.1186/s12943-018-0900-3
Mvondo, M. A., Njamen, D., Tanee Fomum, S., and Wandji, J. (2012). Effects of alpinumisoflavone and abyssinone V-4′-methyl ether derived from Erythrina lysistemon (Fabaceae) on the genital tract of ovariectomized female Wistar rat. Phytotherapy Res. 26, 1029–1036. doi:10.1002/ptr.3685
Ogunwobi, O. O., Harricharran, T., Huaman, J., Galuza, A., Odumuwagun, O., Tan, Y., et al. (2019). Mechanisms of hepatocellular carcinoma progression. World J. Gastroenterol. 25 (19), 2279–2293. doi:10.3748/wjg.v25.i19.2279
Piñero, F., Dirchwolf, M., and Pessôa, M. G. (2020). Biomarkers in hepatocellular carcinoma: Diagnosis, Prognosis and treatment response Assessment. Cells 9 (6), 1370. doi:10.3390/cells9061370
Pratedrat, P., Chuaypen, N., Nimsamer, P., Payungporn, S., Pinjaroen, N., Sirichindakul, B., et al. (2020). Diagnostic and prognostic roles of circulating miRNA-223-3p in Hepatitis B virus-related hepatocellular carcinoma. PLoS One 15 (4), e0232211. doi:10.1371/journal.pone.0232211
Quintanilha, B. J., Reis, B. Z., Nutrimiromics, D. G. B. S., Cozzolino, S. M. F., and Rogero, M. M. (2017). Nutrimiromics: Role of microRNAs and Nutrition in modulating inflammation and chronic diseases. Nutrients 9 (11), E1168. doi:10.3390/nu9111168
Ren, J., Chen, G. G., Liu, Y., Su, X., Hu, B., Leung, B. C., et al. (2016). Cytochrome P450 1A2 Metabolizes 17β-estradiol to suppress hepatocellular carcinoma. PLoS One 11 (4), e0153863. doi:10.1371/journal.pone.0153863
Saha, M., Manna, K., and Das Saha, K. (2022). Melatonin suppresses NLRP3 inflammasome activation via TLR4/NF-κB and P2X7R signaling in high-fat diet-induced Murine NASH model. J. Inflamm. Res. 15, 3235–3258. doi:10.2147/jir.s343236
Shahid, A., and Bharadwaj, M. (2019). The connection between the Th17 cell related cytokines and cancer stem cells in cancer: Novel therapeutic targets. Immunol. Lett. 213, 9–20. doi:10.1016/j.imlet.2019.07.001
Sharif, H., Wang, L., Wang, W. L., Magupalli, V. G., Andreeva, L., Qiao, Q., et al. (2019). Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature 570 (7761), 338–343. doi:10.1038/s41586-019-1295-z
Sharma, B. R., and Kanneganti, T. D. (2021). NLRP3 inflammasome in cancer and metabolic diseases. Nat. Immunol. 22 (5), 550–559. doi:10.1038/s41590-021-00886-5
Shen, Z., Zhou, H., Li, A., Wu, T., Ji, X., Guo, L., et al. (2021). Metformin inhibits hepatocellular carcinoma development by inducing apoptosis and pyroptosis through regulating FOXO3. Aging (Albany NY) 13 (18), 22120–22133. doi:10.18632/aging.203464
Si, H., Wang, H., Xiao, H., Fang, Y., and Wu, Z. (2021). Anti-tumor effect of Celastrol on hepatocellular carcinoma by the circ_SLIT3/miR-223-3p/CXCR4 Axis. Cancer Manag. Res. 13, 1099–1111. doi:10.2147/CMAR.S278023
Sim, H. W., and Knox, J. (2018). Hepatocellular carcinoma in the era of immunotherapy. Curr. Probl. Cancer 42 (1), 40–48. doi:10.1016/j.currproblcancer.2017.10.007
Song, P., Li, Y., Dong, Y., Liang, Y., Qu, H., Qi, D., et al. (2019). Estrogen receptor β inhibits breast cancer cells migration and invasion through CLDN6-mediated autophagy. J. Exp. Clin. Cancer Res. 38 (1), 354. doi:10.1186/s13046-019-1359-9
Sun, X., Sun, G. B., Wang, M., Xiao, J., and Sun, X. B. (2011). Protective effects of cynaroside against H₂O₂-induced apoptosis in H9c2 cardiomyoblasts. J. Cell. Biochem. 112, 2019–2029. doi:10.1002/jcb.23121
Sung, P. S., and Jang, J. W. (2018). Natural killer cell dysfunction in hepatocellular carcinoma: Pathogenesis and clinical implications. Int. J. Mol. Sci. 19, 3648. doi:10.3390/ijms19113648
Tang, Z. R., Zhang, R., Lian, Z. X., Deng, S. L., and Yu, K. (2019). Estrogen-receptor expression and Function in female reproductive disease. Cells 8 (10), 1123. doi:10.3390/cells8101123
Tao, Y., Wang, N., Qiu, T., and Sun, X. (2020). The role of autophagy and NLRP3 inflammasome in liver fibrosis. Biomed. Res. Int. 2020, 7269150. doi:10.1155/2020/7269150
Tellapuri, S., Sutphin, P. D., Beg, M. S., Singal, A. G., and Kalva, S. P. (2018). Staging systems of hepatocellular carcinoma: A review. Indian J. Gastroenterol. 37 (6), 481–491. doi:10.1007/s12664-018-0915-0
Teng, Y., Litchfield, L. M., Ivanova, M. M., Prough, R. A., Clark, B. J., and Klinge, C. M. (2014). Dehydroepiandrosterone-induces miR-21 transcription in HepG2 cells through estrogen receptor β and androgen receptor. Mol. Cell Endocrinol. 392 (1-2), 23–36. doi:10.1016/j.mce.2014.05.007
Thompson, T. W., Kim, A. B., Li, P. J., Wang, J., Jackson, B. T., Huang, K. T. H., et al. (2017). Endothelial cells express NKG2D ligands and desensitize antitumor NK responses. Elife 6, e30881. doi:10.7554/eLife.30881
Volt, H., García, J. A., Doerrier, C., Díaz-Casado, M. E., Guerra-Librero, A., López, L. C., et al. (2016). Same molecule but different expression: Aging and sepsis trigger NLRP3 inflammasome activation, a target of melatonin. J. Pineal Res. 60 (2), 193–205. doi:10.1111/jpi.12303
Wan, L., Yuan, X., Liu, M., and Xue, B. (2018). miRNA-223-3p regulates NLRP3 to promote apoptosis and inhibit proliferation of hep3B cells. Exp. Ther. Med. 15 (3), 2429–2435. doi:10.3892/etm.2017.5667
Wang, H., Zhong, P., and Sun, L. (2019). Exogenous hydrogen sulfide mitigates NLRP3 inflammasome-mediated inflammation through promoting autophagy via the AMPK-mTOR pathway. Biol. Open 8 (7), bio043653. doi:10.1242/bio.043653
Wang, H., Zhong, W., Zhao, J., Zhang, H., Zhang, Q., Liang, Y., et al. (2019). Oleanolic acid inhibits epithelial mesenchymal transition of hepatocellular carcinoma by promoting iNOS dimerization. Mol. Cancer Ther. 18, 62–74. doi:10.1158/1535-7163.MCT-18-0448
Wang, T., Sun, F., Xie, W., Tang, M., He, H., Jia, X., et al. (2016). A bispecific protein rG7S-MICA recruits natural killer cells and enhances NKG2D-mediated immunosurveillance against hepatocellular carcinoma. Cancer Lett. 372 (2), 166–178. doi:10.1016/j.canlet.2016.01.001
Wang, X., Chi, J., Dong, B., Xu, L., Zhou, Y., Huang, Y., et al. (2021). MiR-223-3p and miR-22-3p inhibit monosodium urate-induced gouty inflammation by targeting NLRP3. Int. J. Rheum. Dis. 24 (4), 599–607. doi:10.1111/1756-185X.14089
Wei, Q., Guo, P., Mu, K., Zhang, Y., Zhao, W., Huai, W., et al. (2015). Estrogen suppresses hepatocellular carcinoma cells through ERβ-mediated upregulation of the NLRP3 inflammasome. Lab. Invest 95 (7), 804–816. doi:10.1038/labinvest.2015.63
Wei, Q., Zhu, R., Zhu, J., Zhao, R., and Li, M. (2019). E2-Induced activation of the NLRP3 inflammasome triggers pyroptosis and inhibits autophagy in HCC cells. Oncol. Res. 27, 827–834. doi:10.3727/096504018X15462920753012
Wurdinger, T., and Costa, F. F. (2007). Molecular therapy in the microRNA era. Pharmacogenomics J. 7 (5), 297–304. doi:10.1038/sj.tpj.6500429
Xiong, J., Li, S., Wang, W., Hong, Y., Tang, K., and Luo, Q. (2013). Screening and identification of the antibacterial bioactive compounds from Lonicera japonica Thunb. Leaves. Food Chem. 138, 327–333. doi:10.1016/j.foodchem.2012.10.127
Xu, Z., Liu, J., Jianxin, C., Yongliang, Z., and Pan, X. (2018). 17β-Estradiol inhibits testosterone-induced cell proliferation in HepG2 by modulating the relative ratios of 3 estrogen receptor isoforms to the androgen receptor. J. Cell Biochem. 119 (10), 8659–8671. doi:10.1002/jcb.27111
Yan, Z., Da, Q., Li, Z., Lin, Q., Yi, J., Su, Y., et al. (2022). Inhibition of NEK7 suppressed hepatocellular carcinoma progression by mediating cancer cell pyroptosis. Front. Oncol. 12, 812655. doi:10.3389/fonc.2022.812655
Yao, B. J., He, X. Q., Lin, Y. H., and Dai, W. J. (2018). Cardioprotective effects of anisodamine against myocardial ischemia/reperfusion injury through the inhibition of oxidative stress, inflammation and apoptosis. Mol. MedRep 17 (1), 1253–1260. doi:10.3892/mmr.2017.8009
Yuan, X., Zheng, Y., Chen, C., and Wang, C. (2017). Anisodamine inhibits endoplasmic reticulum stress-associated TXNIP/NLRP3 inflammasome activation in rhabdomyolysis-induced acute kidney injury. Apoptosis 22 (12), 1524–1531. doi:10.1007/s10495-017-1414-y
Zhang, B., Zhu, W. Y., Tian, H., and Zhang, H. R. (2021). Alpinumisoflavone triggers GSDME-dependent pyroptosis in esophageal squamous cell carcinomas. Anat. Rec. Hob. 304 (2), 323–332. doi:10.1002/ar.24414
Zhang, Y., Yang, H., Sun, M., He, T., Liu, Y., Yang, X., et al. (2020). Alpinumisoflavone suppresses hepatocellular carcinoma cell growth and metastasis via NLRP3 inflammasome-mediated pyroptosis. Pharmacol. Rep. 72 (5), 1370–1382. doi:10.1007/s43440-020-00064-8
Zhao, C. L., Ron, S., Liu, C. G., He, X. P., and Xie, Z. P. (1993). Blocking effect of anisodamine on acetylcholine receptor channels. Zhongguo Yao Li Xue Bao 14, 190–192.
Zhao, C., Li, S., Zhang, J., Huang, Y., Zhang, L., Zhao, F., et al. (2020). Current state and future perspective of cardiovascular medicines derived from natural products. Pharmacol. Ther. 216, 107698. doi:10.1016/j.pharmthera.2020.107698
Zhou, M., Shen, S., Zhao, X., and Gong, X. (2017). Luteoloside induces G0/G1 arrest and pro-death autophagy through the ROS-mediated AKT/mTOR/p70S6K signalling pathway in human non-small cell lung cancer cell lines. Biochem. Biophys. Res. Commun. 494 (1-2), 263–269. doi:10.1016/j.bbrc.2017.10.042
Keywords: hepatocellular carcinoma, NLRP3 inflammasome, pyroptosis, apoptosis, reactive oxygen species
Citation: Zhao H, Zhang Y, Zhang Y, Chen C, Liu H, Yang Y and Wang H (2023) The role of NLRP3 inflammasome in hepatocellular carcinoma. Front. Pharmacol. 14:1150325. doi: 10.3389/fphar.2023.1150325
Received: 24 January 2023; Accepted: 10 April 2023;
Published: 20 April 2023.
Edited by:
Jianqiang Xu, Dalian University of Technology, ChinaReviewed by:
Moumita Saha, Indian Institute of Chemical Biology (CSIR), IndiaHongqin Yang, Fujian Normal University, China
Copyright © 2023 Zhao, Zhang, Zhang, Chen, Liu, Yang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Honggang Wang, d2hnMTk3MTY3QHZpcC5oZW51LmVkdS5jbg==; Chaoran Chen, a2ZjY3JAaGVudS5lZHUuY24=
 Huijie Zhao
Huijie Zhao Yiming Zhang
Yiming Zhang Yanting Zhang3
Yanting Zhang3 Honggang Wang
Honggang Wang