- 1Department of Laboratory Medicine, Shengjing Hospital of China Medical University, Shenyang, China
- 2Liaoning Clinical Research Center for Laboratory Medicine, Shenyang, China
- 3Department of Pulmonary and Critical Care Medicine, Shengjing Hospital of China Medical University, Shenyang, China
Introduction: Lung cancer is the leading cause of cancer-related deaths worldwide, and non-small cell lung carcinoma (NSCLC) accounts for approximately 80% of all cases. Immune checkpoint inhibitors (ICIs) are widely used to treat NSCLC owing to their remarkable efficacy. In this study, we analyzed the scientific collaboration network, defined the hotspots of research on the use of ICIs for NSCLC treatment, analyzed its evolution over the past few years, and forecasted the field’s future development using bibliometric analysis and a graphical study.
Methods: Research articles and reviews regarding ICIs for NSCLC were retrieved and obtained from the Web of Science Core Collection on 26 September 2022. CtieSpace and VOSviewer were thereafter used to conduct the bibliometric and knowledge-map analysis.
Results: We included 8,149 articles for this literature analysis. Our analysis showed that the USA had the highest number of publications and citations. We also noted that research trends in this field have changed drastically over the past 20 years, from the early development of ICIs, such as CTLA-4 inhibitors, to the development of recent ones, such as PD-1 and PD-L1 blockers. Further, the focus of research in this field has also gradually shifted from mechanisms to treatment effects and adverse events, suggesting that the field is maturing. Clinical applications are also being explored, including studies on how to enhance efficacy, reduce adverse effects, and expand to other specific cancer types.
Conclusion: To the best of our knowledge, this is the first study to construct a comprehensive knowledge map on ICIs for NSCLC. It can help researchers rapidly grasp the status and focus of current research in this area, offer direction, and serve as a reference for conducting similar studies.
1 Introduction
Lung cancer is the leading cause of cancer-related deaths globally (Bray et al., 2018), and small cell carcinoma (SCLC) accounts for approximately 20% of all cases, whereas non-small cell lung carcinoma (NSCLC), which includes the subtypes, adenocarcinoma (AD), squamous cell carcinoma, and large cell carcinoma, accounts for approximately 80% of all cases. The prognosis is poor for over 70% of patients with NSCLC, who are initially diagnosed when the disease is already at an advanced stage (Bodor et al., 2020). The 5-year survival rate of patients with advanced NSCLC is less than 3% (Ozkaya et al., 2012). In the past, chemotherapy regimens combined with radiotherapy were the recommended standard of care for patients with NSCLC who presented with advanced-stage disease (De Ruysscher et al., 2020). With a median progression-free survival (PFS) of only 4–6 months and overall survival (OS) of approximately 12–18 months, the treatment provided relatively modest responses (Assi et al., 2018; Suresh et al., 2018). Recent advancements in immunotherapy, which boosts antitumor activity using cytotoxic T-lymphocyte antigen 4 (CTLA-4), programmed cell death-1 (PD-1), and PD ligand 1 (PD-L1) inhibitors, have altered the therapeutic approach for a variety of cancers (Naidoo et al., 2020). Specifically, tumor immunotherapy, a cutting-edge and effective therapeutic approach for advanced NSCLC, has several potential applications (Lurienne et al., 2020; De Giglio et al., 2021). Immune checkpoint inhibitors (ICIs) are a class of immunotherapeutic agents capable of harnessing intrinsic immune response against tumor antigens by inhibiting T-cell activation by antigen-presenting cells. The first checkpoint to be identified was CTLA-4, and an ICI, ipilimumab, was developed to improve OS in patients with previously treated metastatic melanomas (Hodi et al., 2010; Larkin et al., 2015; Robert et al., 2015; Weber et al., 2017). Over the past few years, immunotherapeutic drugs that target immune checkpoint pathways have made significant strides in clinical trials and have quickly become the standard of care for advanced-stage NSCLC (Guibert and Mazières, 2015; Socinski et al., 2021; Reck and Hellmann, 2022).
Bibliometrics, which was first used in 1969, is a strategy for comprehensively reviewing a subject of study using mathematical and statistical techniques to examine literature statistically (Pritchard, 1969). This strategy is predominantly used in healthcare research to assess the influence or impact of research papers. Thus, the results of bibliometric studies, which analyze the influence or impact of a particular research paper on subsequent studies, are particularly useful for subjects that are progressively gaining interest. Traditional reviews usually reflect the latest developments in one aspect of a subject rather than the overall picture of the discipline. However, bibliometrics is a measurable informatics approach that addresses these limitations by analyzing the structure of knowledge related to a particular discipline to obtain quantifiable data. This multi-perspective, time-phased, and dynamic technique of visual analysis of literature can show the evolution of knowledge disciplines and automatically identify the research frontiers of the discipline through citation nodes and co-citation clustering, providing an important and viable systematic method for determining the importance of published literature by showing author networks and scholarly communication, connections between scholars, and developments in a field of knowledge.
Although bibliometric analysis has been used in several other domains, there is currently no bibliometric study on the use of ICIs for NSCLC treatment (Chen et al., 2020; Xin et al., 2021; Shen et al., 2022). To perform knowledge mapping and discover the hotspots or frontiers on ICIs for NSCLC treatment, we used CiteSpace, VOSviewer, and R tools in this study to create scientific knowledge maps and analyze publications from 2000 to 2022. The clinical trial characteristics were reviewed, and their development processes were summarized and visually represented in this study. We believe that this study can serve as a resource for future research.
2 Materials and methods
2.1 Data collection and search strategy
The most well-known and important database of scientific literature, the Web of Science Core Collection (WoSCC), was selected as the data source for this study. WoSCC, the most widely used database in prior bibliometric studies (Dai et al., 2022; Miao et al., 2022; Qin et al., 2022), is an ideal database containing information corresponding to over 10,000 high-quality journals. It was chosen for this bibliometric analysis because it offers a wealth of bibliometric indicators, such as publication and reference information. For this study, data were retrieved and downloaded from WoSCC on 26 September 2022. We set the search formula as follows: Immune checkpoint inhibitors search term: #1:TS=(ipilimumab) OR TI=(ipilimumab) OR AB=(ipilimumab); #2:TS=(pembrolizumab) OR TI=(pembrolizumab) OR AB=(pembrolizumab); #3:TS=(nivolumab) OR TI=(nivolumab) OR AB=(nivolumab); #4:TS=(immunotherapy) OR TI=(immunotherapy) OR AB=(immunotherapy) OR TS=(immune checkpoint blockade) OR TI=(immune checkpoint blockade) OR AB=(immune checkpoint blockade) OR TS=(immune checkpoint inhibitor) OR TI=(immune checkpoint inhibitor) OR AB=(immune checkpoint inhibitor); #5:TS=(PD-1) OR TI=(PD-1) OR AB=(PD-1) OR TS=(PD-L1) OR TI=(PD-L1) OR AB=(PD-L1) OR TS=(CTLA-4) OR TI=(CTLA-4) OR AB=(CTLA-4); #6; TS=(yervoy) OR TI=(yervoy) OR AB=(yervoy) OR TS=(keytruda) OR TI=(keytruda) OR AB=(keytruda) OR TS=(opdivo) TI=(opdivo) OR AB=(opdivo); #7:#1 OR #2 OR #3 OR #4 OR #5 OR #6; non-small cell lung cancer search term: #8: TS=(non-small cell lung cancer) OR TI=(non-small cell lung cancer) OR AB=(non-small cell lung cancer); #9:TS=(non-small cell lung carcinoma) OR TI=(non-small cell lung carcinoma) OR AB=(non-small cell lung carcinoma); #10:TS=(NSCLC) OR TI=(NSCLC) OR AB=(NSCLC); #11:#8 OR #9 OR #10. Final Data Sources: #7 and #11.
2.2 Inclusion and exclusion criteria
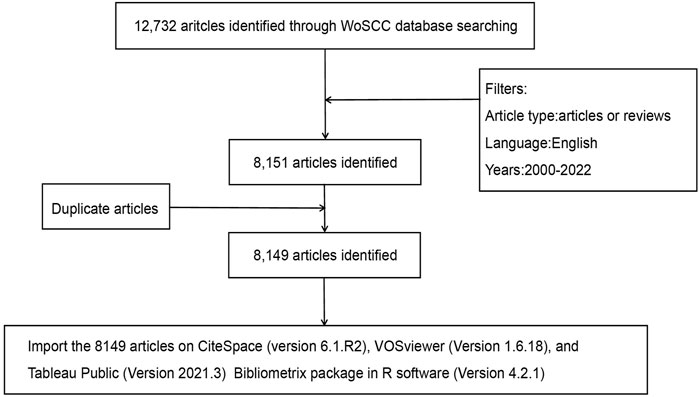
The study period was from 1 January 2000, to 26 September 2022, with English as the only permitted language. The only available documents were research and review articles. Figure 1 displays a flowchart of the study’s procedures.
2.3 Data analysis and visualization
CiteSpace (version 6.1. R2), VOSviewer (version 1.6.18), Tableau Public (version 2021.3), and R (version 4.2.1) software were used for data analysis and visualization. CiteSpace, created in 2004 by Chaomei Chen at Drexel University, is typically used to analyze, spot, and display trends and patterns in scientific publications (Chen, 2004). Thus, researchers can use it to predict the research and development trend in their area of interest and facilitate an intuitive understanding of research hotspots and the evolution process. In this study, it was primarily used to visualize articles that had the largest number of citations. Further, VOSviewer, which is more focused on the visualization of scientific information, is a Java-based bibliometric mapping program that has a powerful capability to handle large maps and display large bibliometric maps in an easily interpretable manner (van Eck and Waltman, 2010). In this study, co-authorship analysis in terms of country, author, and institution; co-citation analysis of the journal and author; and keyword co-occurrence analysis were performed using VOSviewer. The spatial distribution of the global publications was realized using the Tableau Public software. Further, the Bibliometrix package in R software offered the possibility to visualize the keywords with the largest number of citations. Together, these analytical tools offered unbiased and varied perspectives on the advancement of the use of ICIs for NSCLC treatment.
3 Results
3.1 Obtaining relevant literature
A total of 12,732 articles were first identified in the WoSCC database. According to the exclusion criteria, 3,803 meeting abstracts, 401 editorial materials, 151 letters, 67 revisions, 53 online publications, and 6 other categories of literature were excluded (a total of 4,481 articles). Further, a total of 100 non-English articles (49 in German, 41 in French, 4 in Spanish, 3 in Polish, 1 in Hungarian, 1 in Japanese, and 1 in Portuguese) and 2 duplicate articles were also excluded (Figure 1).
3.2 Analysis of annual publications and citation trends
From 2000 to 2022, 8,149 research and review articles were published on the use of ICIs for NSCLC treatment. We noted that between 2000 and 2014, only 410 were published articles, and these showed no evident research trends, i.e., the number of publications remained relatively constant. As more scholars focused on ICIs for NSCLC treatment, the annual growth rate of papers varied. After 2015, the annual production of relevant papers exhibited a sharp increase, peaking in 2021. There were approximately 60 times more publications in 2021 (1,862) than in 2011 (30). We also constructed models based on exponential smoothing to estimate the number of papers that will be published and cited in 2022 and 2023, forecasted at approximately 2,122 and 2,474 publications, respectively (Figure 2) (JO, 1971).
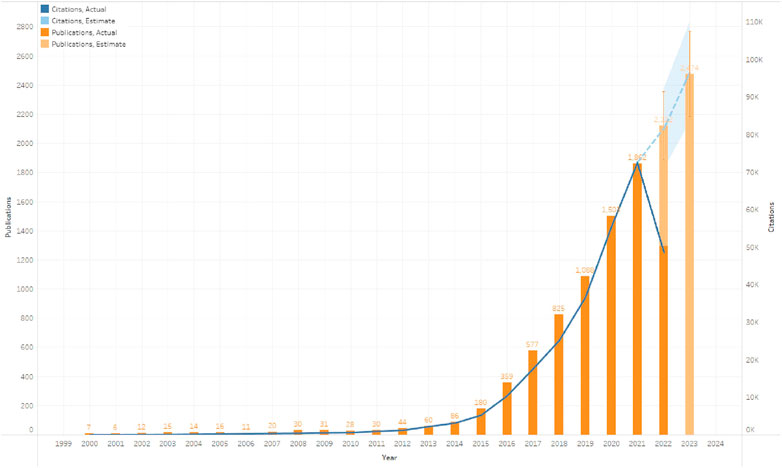
FIGURE 2. Global trend of publications and total citations on the use of immune checkpoint inhibitors (ICIs) for non-small cell lung cancer (NSCLC) treatment from 2000 to 2022.
3.3 Analysis of countries/regions
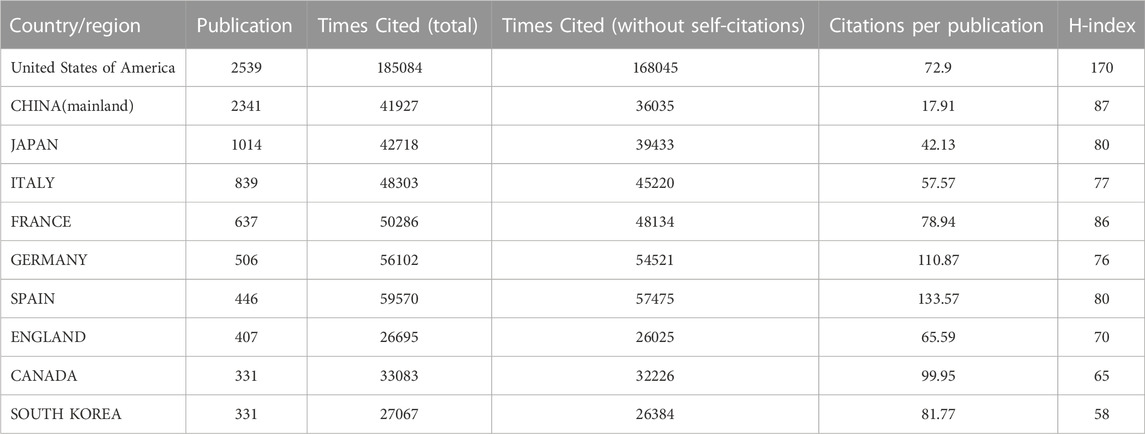
Thirty-five countries with more than 30 publications in the field of ICIs for NSCLC treatment were identified (Figure 3A). The top ten productive nations/regions were ranked, as shown in Table 1. Notably, the top five nations were the USA (2,539/31.2%), China (mainland) (2,341/28.7%), Japan (1,041/12.8%), Italy (839/10.3%), and France (637/7.8%), with the USA taking the top spot. Although the number of publications in China was similar to that in the USA, the total number of citations (36,035) was much lower than that for the USA (168,045). VOSviewer was used to build a co-authorship network map to study the cooperation among countries/regions. The map of global collaboration shows how closely nations were working together. The two nations with the most international partnerships were the USA and China (Figure 3B). Further, the VOSviewer-based overlay visualization map of organizational cooperation was used to analyze the publication time. Notably, China started publishing more recently than most other productive nations (Figure 3C).
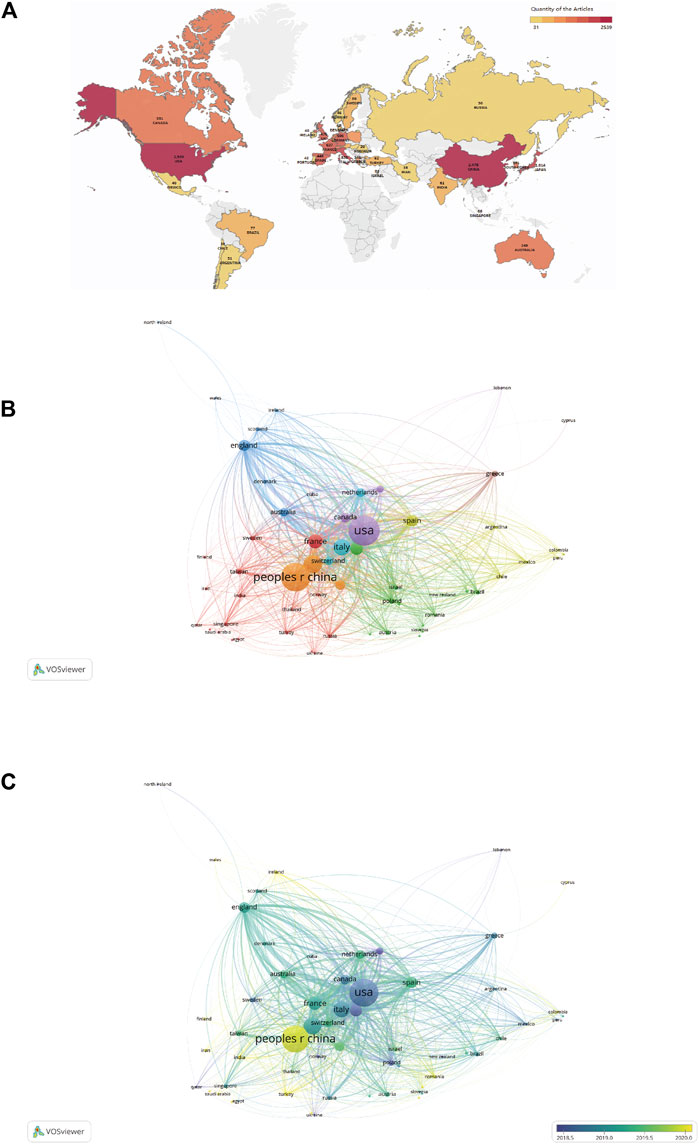
FIGURE 3. (A) Geographic distribution map based on publications from different countries/regions (n ≥ 30). (B) Countries/regions citation network visualization map. The number of publications is represented by the size of each circle/node. Further, the strength of the connections between the circles/nodes is represented by the thickness of the connecting lines, and each color represents a cluster, i.e., a group of objects with related characteristics within a network. Each circle or node represents a separate nation or region. (C) Countries/regions citation overlay visualization map. The institutions that earlier commenced research in this field of research are depicted using purple nodes, whereas the yellow nodes represent institutions that commenced research in this field later.
3.4 Analysis of productive organizations
In total, 8,149 documents were released by 8,353 institutions. Further, 265 organizations that qualified for inclusion (publications >20) were included in the visualization analysis. Table 2 shows the institutions with the highest number of publications and citations. The top three productive organizations were the University of Texas MD Anderson Cancer Center (572/7.0%), the Memorial Sloan-Kettering Cancer Center (307/3.8%), and SUN YAT-SEN University(293/3.5%). Four of the top 10 institutions were US-based, while the remaining six were in China. Regarding organizations with the most citations, the top three were the Memorial Sloan-Kettering Cancer Center (37,968 citations), Dana-Farber Cancer Institute (28,147 citations), and Yale University (25,653 citations). Further, eight of the top 10 institutions were US-based. The cumulative annual publications of the top 10 institutions are shown in Figure 4A. We also examined the organizations’ co-authorship (Figure 4B). VOSviewer was used to build a co-authorship network map of countries to study cooperation among organizations. All 265 top publishing institutions could be divided into six clusters, each roughly denoting one country. The National Cancer Institute, Memorial Sloan Kettering Cancer Center, and MD Anderson Cancer Center are a few American institutions represented by the green clusters. With limited connections to other clusters, the deep blue cluster represented Chinese universities and institutions, including SUN YAT-SEN University and Tongji University. Universities and other institutions in Japan are represented using the yellow cluster.

TABLE 2. Top 10 productive organizations and the top 10 organizations with the most citations in the field of ICIs for NSCLC.
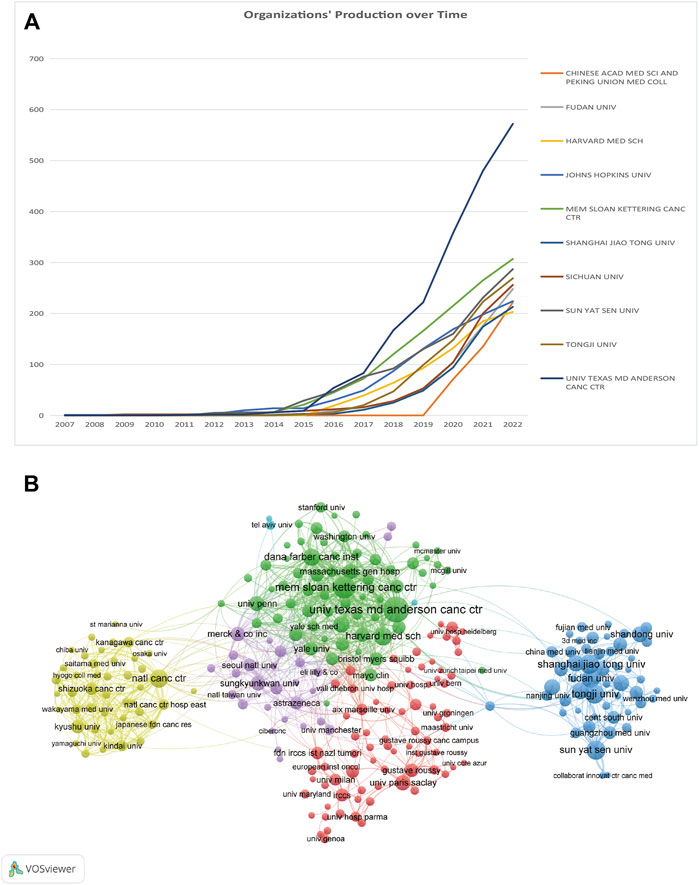
FIGURE 4. (A) Chronological changes in the number of publications from the top 10 organizations. (B) Network visualization maps of organizations.
3.5 Analysis of authors and Co-cited authors
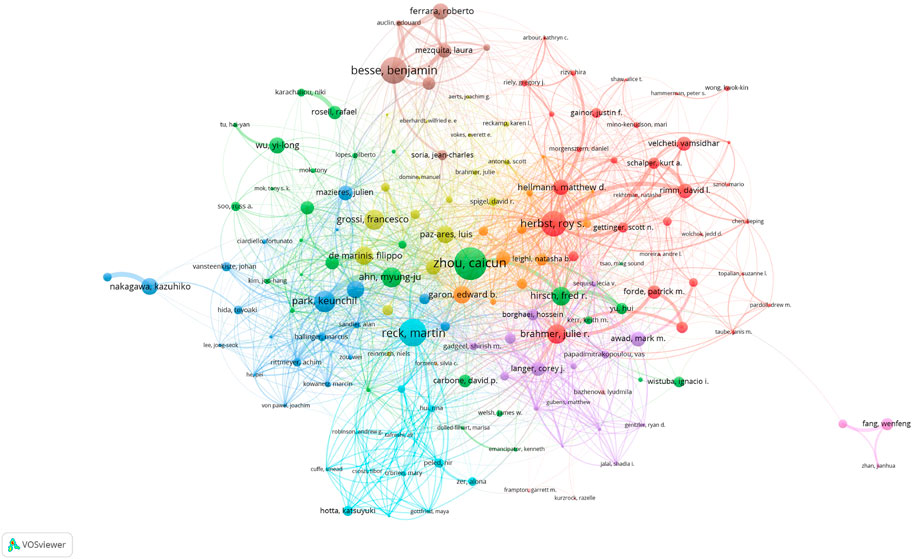
More than 30,000 researchers have been involved in the study of ICIs for NSCLC treatment. Among these, the top three authors with the most publications were Caicun Zhou (68), Martin Reck (57), and Benjamin Besse (55) (Table 3). Among the top 10 most-cited authors (Table 3), Julie R Brahmer, Martin Reck, and Gregory M Lubiniecki (total citations: 13,819, 13,669, and 10,803) ranked first, second, and third, respectively. Additionally, as shown in Figure 5, each author (n > 5, total citations >100) is represented by a single node, while authors with large publishing volumes, such as Caicun Zhou, Martin Reck, and Benjamin Besse, created several autonomous core author groups. These groups exhibited close collaboration and few ties with other author groups.
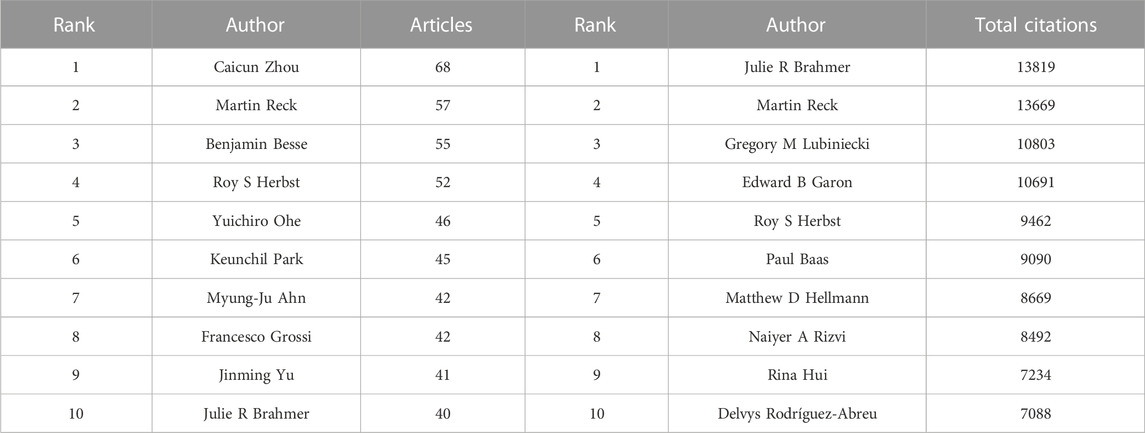
TABLE 3. The 10 most productive authors and the top 10 authors with most citations in the field of ICIs for NSCLC.
3.6 Analysis of influential journals and Co-cited journals
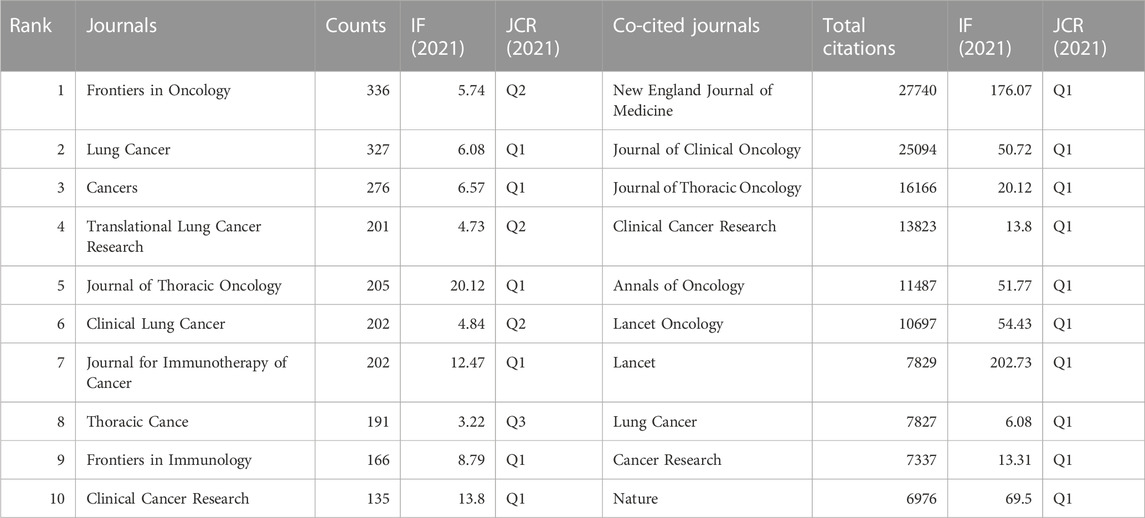
A total of 890 journals were included in this study, with Frontiers in Oncology (N = 336, impact factor (IF) = 5.74, Q2), Lung Cancer (N = 327, IF = 6.08, Q1), and Cancers (N = 276, IF = 6.57, Q1) identified as the top three journals with the highest number of publications (Table 4). Journal of Thoracic Oncology had the highest IF among the 10 most productive journals (N = 205, IF = 20.12, Q1). Further, all the top 10 co-cited publications, shown in Table 4, received more than 6,500 citations, with New England Journal of Medicine, Journal of Clinical Oncology, Journal of Thoracic Oncology, Clinical Cancer Research, and Annals of Oncology identified as the top five most co-cited journals (total citations: 27,740, 25,094, 16,166, 13,823, and 11,487, respectively). The network visualization maps of the citing and co-cited journals were generated using VOSviewer. As shown in Figures 6A, B, many journals co-occurred in both maps and had live citation links.
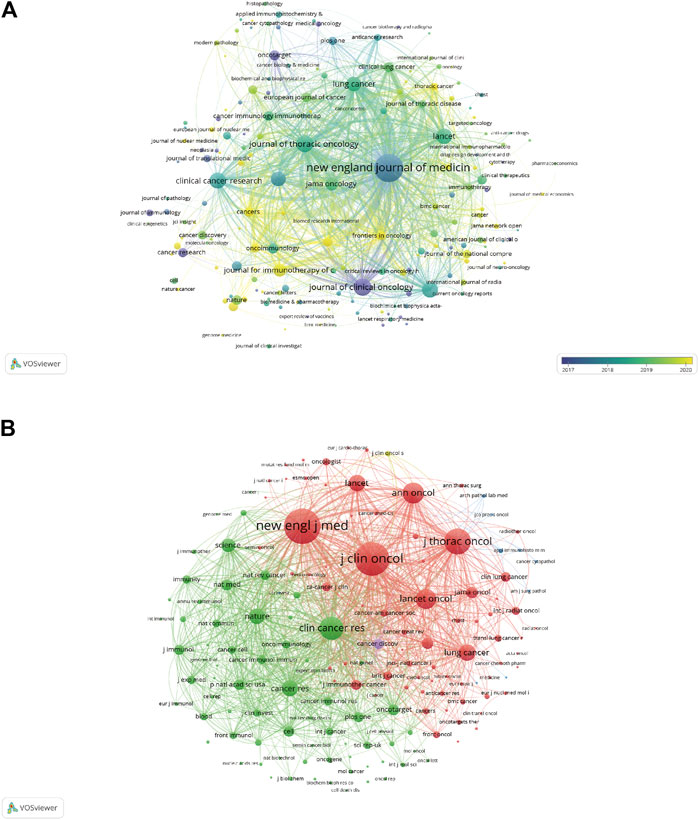
FIGURE 6. (A) Overlay visualization maps of citing journals. (B) Network visualization maps of co-cited journals.
3.7 Analysis of references and Co-cited references
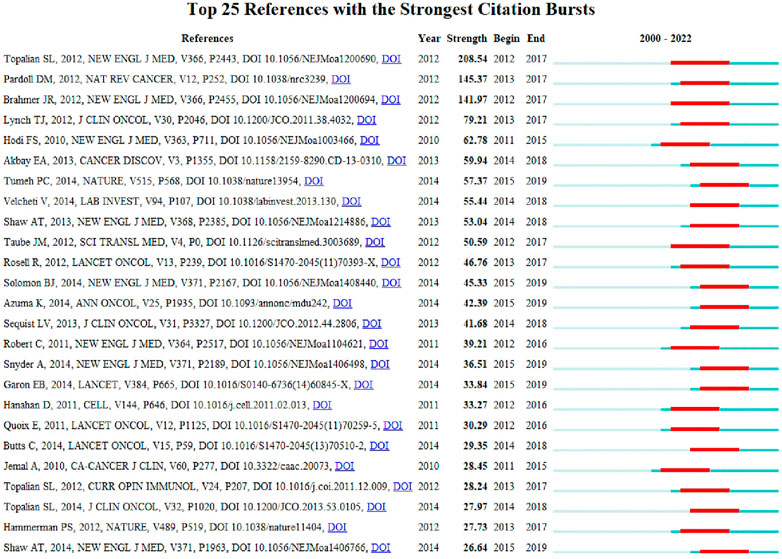
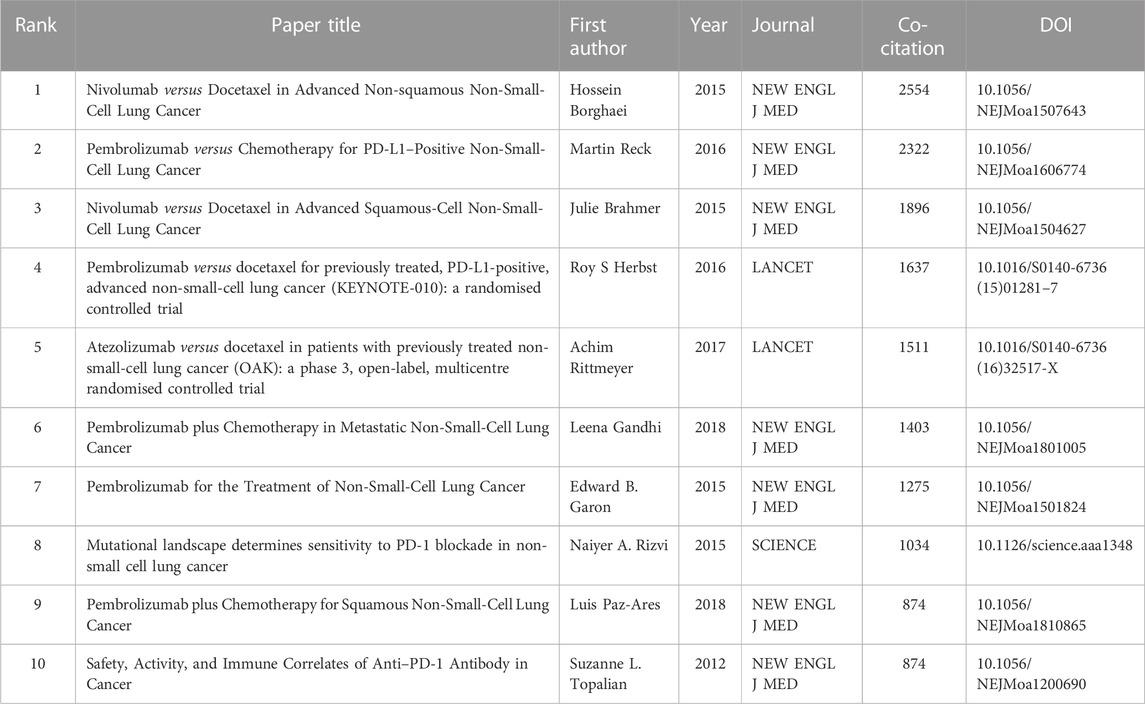
In this study, reference analysis was performed to understand advances in research on the use of ICIs in NSCLC treatment. Table 5 outlines the top 10 cited and/or co-cited references. The titles of the top three most frequently cited articles were “Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer,” authored by Suzanne L. Topalian (Topalian et al., 2012) (total citations: 8,542; Publication Year: 2012); “Nivolumab versus Docetaxel in Advanced Non-squamous Non-small Cell Lung Cancer,” authored by Hossein Borghaei (Borghaei et al., 2015) (total citations: 6,110; Publication Year: 2015); and “Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer,” authored by Martin Reck (Reck et al., 2016) (total citations: 5,560; Publication Year: 2016). Seven of the top ten most-cited articles were published in the New England Journal of Medicine. As a result, articles with the highest number of citations were examined, and CiteSpace was used to display the network of articles’ co-citations. In addition, articles on ICIs for NSCLC treatment with the strongest citation bursts were identified using CiteSpace. The top 25 images are shown in Figure 7. Further, the titles of the top three articles with the most frequent citation bursts were “Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer” (Topalian et al., 2012) (Strength: 208.54; Publication Year: 2012), “The Blockade of Immune Checkpoints in Cancer Immunotherapy” (Pardoll, 2012) (Strength: 145.37; Publication Year: 2012), and “Safety and Activity of Anti-PD-L1 Antibody in Patients with Advanced Cancer” (Brahmer et al., 2012) (Strength: 141.97; Publication Year: 2012). We also noted that the titles of the top three articles with the most frequent citation bursts were “Nivolumab versus Docetaxel in Advanced Non-squamous Non-Small-Cell Lung Cancer” (Borghaei et al., 2015) (Co-citations: 2,554; Publication Year: 2015), “Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer” (Reck et al., 2016) (Co-citations: 2,322; Publication Year: 2016), and “Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer” (Brahmer et al., 2015) (Co-citations: 1,896; Publication Year: 2015) (Table 6).
3.8 Emerging Trends and Research Focus Based on Keywords Analysis
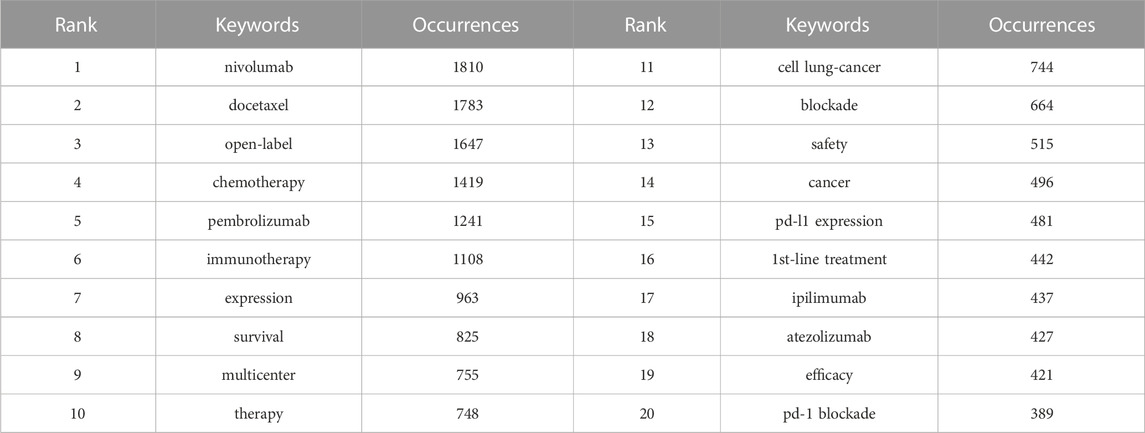
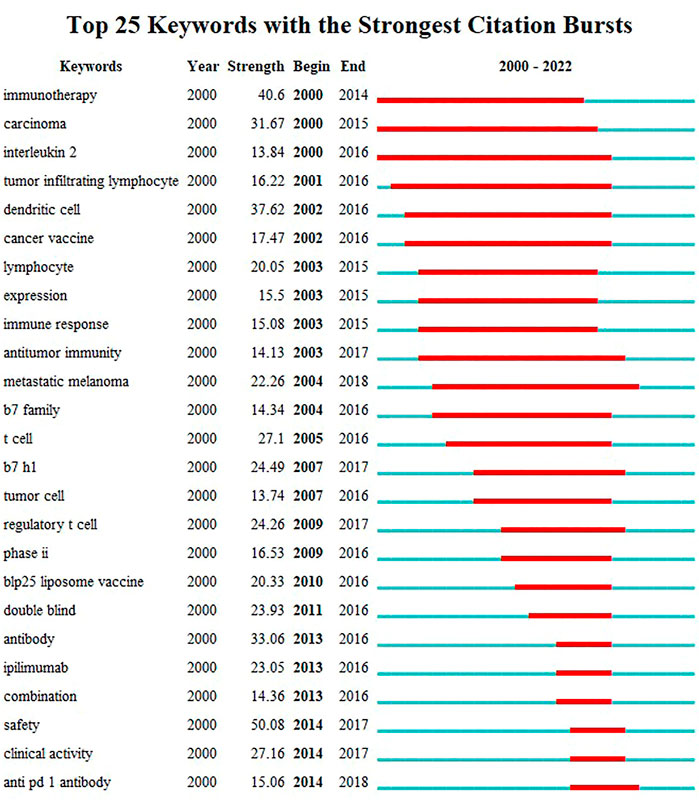
The visualization map of keywords was generated using VOSviewer software after combining the synonyms and eliminating irrelevant terms. Thus, a total of 13,814 keywords were included, and 220 terms with at least 50 occurrences were identified and grouped based on publication date (2018–2020) (Figure 8A) and into four clusters (Figure 8B). According to the sequence in which these technologies advance, hot research regions changed with time, transitioning from “antibody” to “atezolizumab,” “pneumonitis,” and so on, as seen by the overlay map of keywords classified based on publication date. Further, the keywords were separated into four clusters for the network map. One of the clusters shown in red included “lung cancer,” “PD-L1,” “cancer,” and “immunotherapy.” The other cluster, shown in green, generally contained terms associated with clinical oncology issues, such as “radiotherapy,” “chemotherapy,” “double-blind,” and “open-label.” The blue cluster included the names of antibodies approved by the US Food and Drug Administration (FDA), such as “nivolumab,” “pembrolizumab,” “atezolizumab,” and “ipilimumab,” and the yellow cluster included “multicenter” and “phase 3". The top 45 terms in the keyword trend map are shown in Figure 8C, from which it is evident that the keywords have undergone a significant shift recently. In recent years (2021–2022), researchers have been interested in “plus chemotherapy,” “1st line nivolumab,” and other aspects. Additionally, Table 7 outlines the top 20 co-occurrence author keywords frequently appearing in this study, including nivolumab and docetaxel. The burst for keywords revealed that “immunotherapy” is still the research front in the field of ICIs for NSCLC (Figure 9).
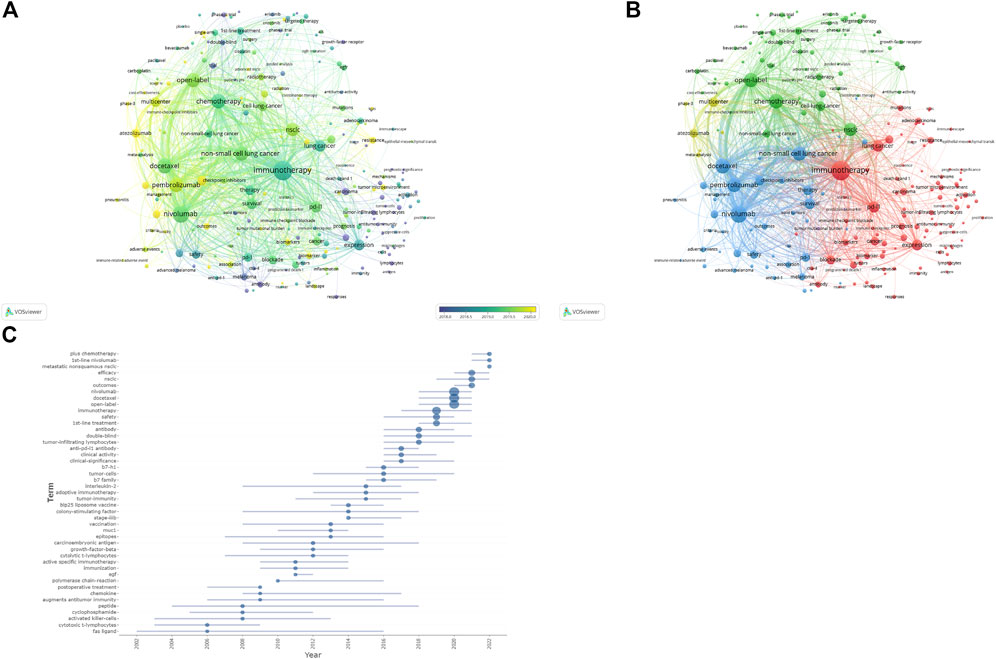
FIGURE 8. (A) Overlay visualization maps of keywords. (B) Network visualization maps of keywords. (C) Trend map of top 45 keywords.
4 Discussion
To the best of our knowledge, this is the first bibliometric analysis comparing the outcomes of ICIs in NSCLC treatment. We performed a bibliometric analysis involving 8,149 articles from the WoSCC database corresponding to the 2000–2022 period. We further summarized the research themes, trends, and sources of ICIs for NSCLC treatment and the global impact of research in this field.
One of the most prevalent cancers in both men and women is NSCLC, and treating it is still challenging on a global scale. Based on our results, the number of papers related to ICIs for NSCLC has been steadily increasing. This indicates that this subject has recently received considerable attention. Additionally, a notable trend shift occurred in 2015. This may be related to the FDA approval of the PD-1 checkpoint drugs (nivolumab and pembrolizumab) for the immunotherapy of NSCLC and melanoma at the end of 2014 (Borghaei et al., 2015; Herbst et al., 2016). This marked the beginning of a new era of ICI use for NSCLC. Our chronological distribution results indicated that the research field on the use of ICIs for NSCLC has steadily been a research hot zone and is currently undergoing a significant development stage. During this period, the theory advanced quickly, and the volume of papers also grew rapidly. Moreover, the growth curve was abrupt, and its gradient did not lessen in 2022. Therefore, it can be expected that in the upcoming years, this field would continue to advance.
The USA, China, Japan, Italy, and France published the most articles (Table 1), and the highest level of international cooperation corresponded to the USA (Figure 3). This may be due to the rapid economic development in these countries and increased investment in related fields, resulting in more publications. Additionally, cooperation with developed countries can help increase scientific productivity given that these developed countries have sophisticated medical standards and a wealth of resources for scientific research; therefore, to encourage the advancement of global ICI research, we recommend that developed countries strengthen their partnerships and connections with more nations and regions, particularly with those that are still relatively underdeveloped. More than a quarter of all publications came from China, the only developing nation listed among the top ten most productive nations or regions. Even though China is the second-largest publishing nation, none of the top ten journals were Chinese, suggesting that China should improve the quality of its periodic productions in this area. Only less than half of all the countries published more than 30 publications. Therefore, to ensure improvement in this area, we need to strengthen international cooperation while improving the quality of publications.
Institutional cooperation is an aggregative trend from the perspective of cooperative relationships. Most cooperative Chinese institutions are located in China. American institutions have collaborated more with other countries. More multi-institutional collaborations, such as academic exchanges and integration, are required to develop the field. This will help improve the overall level of institutions, and the leading institutions in the field will exchange advanced technologies and ideas with relatively less apt institutions.
Among the co-cited journals, the New England Journal of Medicine may be the most important journal on the use of ICIs for NSCLC treatment, according to the total number of citations and its IF. This is because clinical trials of ICIs for NSCLC treatment have been published in these journals, attracting a great deal of attention. Simultaneously, our findings provide a reference for researchers in this field to choose journals for submission and reading articles.
Regarding authorship, Martin Reck ranked second in the number of publications and the total number of citations. He also published the article with the third highest number of citations titled “Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer,” indicating his important role in this field. Martin Reck is a chief physician at the Lung Clinic Grosshansdorf, Germany, and principal investigator in KEYNOTE-024 (NCT02142738) (Reck et al., 2016; Brahmer et al., 2017; Reck et al., 2021), a phase 3 study for PD-L1-positive NSCLC, which compared pembrolizumab with platinum-based doublet chemotherapy. In patients with EGFR/ALK-negative advanced NSCLC with ≥50% PD-L1 tumor cell positivity, pablizumab significantly improved objective remission rates (44.8% vs. 27.8%), prolonged PFS (10.3 months vs. 6.0 months; HR = 0.50, 95% CI: 0.37–0.68) and OS (26.3 months vs. 13.4 months; HR = 0.62, 95% CI: 0.48–0.81), and exhibited a lower rate of grade 3 or higher treatment-related adverse events (26.6% vs. 53.3%) compared to their counterparts who received chemotherapy.
The study by Topalian et al. demonstrated that individuals with specific advanced malignancies, including NSCLC, experience long-lasting tumor reduction and disease stabilization when their PD-L1 was blocked by an antibody (Topalian et al., 2012). These results support the importance of targeting the PD-1–PD-L1 pathway as a therapeutic target for some patients with cancer. The second highly-cited article, authored by Hossein (Borghaei et al., 2015) and published in the New England Journal of Medicine (cited 6,110 times), demonstrated that nivolumab outperformed docetaxel in terms of OS, with patients with advanced non-squamous NSCLC for whom platinum-based chemotherapy had failed benefiting from the increased efficacy of this drug owing to PD-L1 expression. Compared with docetaxel, nivolumab has a better safety profile. The third highly-cited article, authored by Martin Reck (Reck et al., 2016) and also published in the New England Journal of Medicine, noted that in patients with advanced NSCLC exhibiting PD-L1 expression on at least 50% of tumor cells, pembrolizumab was associated with considerably longer PFS and OS and with fewer adverse effects than for the platinum-based treatment.
To analyze research trends, we also performed keyword and burst term analyses. Thus, we observed a significant change in focus over time. Specifically, we noted that research trends in this field changed drastically over the last 20 years, from the early development of ICIs, such as CTLA-4 inhibitors, to recent ones, such as PD-1 and PD-L1 blockers. Research focus has also gradually shifted from causes to side effects and adverse occurrences of treatments. Through translational research, clinical studies into the interactions of immune checkpoints with costimulatory molecules, cancer drive genes, and cancer hallmarks, as well as basic studies on the mechanism of action of ICIs have advanced from the early research stage with a focus on the mechanisms of immunotherapy, including the alteration of immune cells and immune molecules under ICI treatment. This suggests that this field of research is maturing. Further, clinical applications are being explored, including how to enhance efficacy, reduce adverse effects, and translate the findings to other specific cancer types. Currently, “plus chemotherapy” and “1st line nivolumab” are new research hotspots with respect to the use of ICI for NSCLC treatment. Nivolumab restores the antitumor function of T cells. Further, nivolumab plus ipilimumab, as a combination immunotherapy, increases long-term survival in patients with advanced malignancies, including melanoma, renal cell carcinoma, malignant pleural mesothelioma, and NSCLC (NCT01844505, NCT02231749, NCT02899299) (Larkin et al., 2019; Motzer et al., 2020; Baas et al., 2021). First-line therapy with nivolumab and ipilimumab for advanced NSCLC drastically decreased progression in Part 1 of the randomized phase 3 CheckMate 227 trial compared to the platinum-doublet chemotherapy and significantly prolonged OS in patients with tumor PD-L1 expression ≥1% and disease-free survival in patients with a high tumor mutational load (NCT02477826, NCT02477826) (Hellmann et al., 2018; Hellmann et al., 2019). In recent years, it has been reported that in addition to PD1/PD-L1 and CTLA-4, a second group of co-inhibitory molecules, including lymphocyte-activation gene-3 (Lag-3 or CD223), T-cell immunoglobulin and mucin domain-3 (TIM-3), and T-cell immunoglobulin and ITIM domain (TIGIT), are also involved in controlling immunological responses, particularly at regions of tissue inflammation. Even though the expression patterns of LAG-3, TIM-3, and TIGIT largely overlap, their distinct signaling tails provide a foundation for both their distinct regulatory activities and the synergistic impact of medicines that target them in diseases (Goldberg and Drake, 2010; Sakuishi et al., 2010; Anderson Ana et al., 2016; Attanasio and Wherry, 2016). LAG-3 is highly similar to the CD4 protein in terms of structure and can bind MHC class II molecules (Andrews et al., 2017). Although there are active clinical trials of LAG-3-targeting therapeutics for NSCLC (NCT03625323, NCT01968109, NCT02750514, NCT02460224, and NCT03538028), it is still unknown how its expression is related to clinicopathologic factors and how it affects NSCLC prognosis. Additionally, TIM-3 is a type I transmembrane protein that includes a mucin stalk domain and a variable N-terminal Ig domain (Huang et al., 2015), and TIGIT is a receptor that modulates immunity and serves as an immunological checkpoint in both innate and adaptive immunity. In mice as well as in humans, TIGIT and PD-1 are co-expressed on tumor antigen-specific CD8 T cells and CD8 tumor-infiltrating lymphocytes (TILs) in cancer. Other ICIs also being investigated in clinical trials. For example, preliminary results have shown that sabatolimab plus spartalizumab therapy is well tolerated and shows signs of antitumor activity in NSCLC (NCT02608268) (Curigliano et al., 2021). Further, vibostolimab plus pembrolizumab shows anticancer effects in patients with advanced solid tumors, including advanced NSCLC, and is well tolerated (NCT02964013) (Niu et al., 2022). Molecular understanding of adaptive immunity over the past 10 years has made it possible to develop ICIs that are effective against a variety of malignancies; however, these often have unique side effects, known as immune-related adverse events (irAEs) (Naidoo et al., 2016; Sullivan and Weber, 2022). Considering the current research progress, we forecasted that in this developing field of study, expanding the indications for ICIs, exploring medication combinations, improving the efficacy of ICIs, reducing adverse effects, and screening biomarkers to predict efficacy and unfavorable effects will all likely become hot issues.
This study had some limitations. First, we used only one data source (WoSCC), indicating that the literature included may not be exhaustive; hence, some pertinent papers in other data sources may have been left out. Second, we only utilized papers written in the English language, which introduces some linguistic bias. Thus, the publications may not accurately represent all studies on ICIs for NSCLC. However, we believe our research will help readers gain a thorough understanding of this subject area.
Author contributions
RZ designed and supervised the study, and YZ performed the computational analysis. All the authors discussed the results and wrote the manuscript. All authors agree to be accountable for all aspects of the work, ensuring its integrity and accuracy.
Funding
This work was supported in part by the Key Research and Development Plan Guidance Program of Liaoning Province (grant number 2018225006), the Liaoning Province Key Research and Development Program (grant number 2020JH2/10300125), and the 345 Talent Project of Shengjing Hospital of China Medical University (grant number M0346).
Acknowledgments
We would like to thank editage (www.editage.cn) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1140771/full#supplementary-material
Abbreviations
AD, adenocarcinoma; CTLA-4, cytotoxic T-lymphocyte antigen 4; FDA, US Food and Drug Administration; ICI, immune checkpoint inhibitor; NSCLC, non-small cell lung cancer; OS, overall survival; PD-1, programmed cell death-1; PD-L1, PD ligand 1; PFS, progression-free survival; SCLC, small cell carcinoma; WoSCC, Web of Science Core Collection.
References
Anderson Ana, C., Joller, N., and Kuchroo Vijay, K. (2016). Lag-3, tim-3, and TIGIT: Co-Inhibitory receptors with specialized functions in immune regulation. Immunity 44 (5), 989–1004. doi:10.1016/j.immuni.2016.05.001
Andrews, L. P., Marciscano, A. E., Drake, C. G., and Vignali, D. A. A. (2017). LAG3 (CD223) as a cancer immunotherapy target. Immunol. Rev. 276 (1), 80–96. doi:10.1111/imr.12519
Assi, H. I., Kamphorst, A. O., Moukalled, N. M., and Ramalingam, S. S. (2018). Immune checkpoint inhibitors in advanced non-small cell lung cancer. Cancer 124 (2), 248–261. doi:10.1002/cncr.31105
Attanasio, J., and Wherry, E. J. (2016). Costimulatory and coinhibitory receptor pathways in infectious disease. Immunity 44 (5), 1052–1068. doi:10.1016/j.immuni.2016.04.022
Baas, P., Scherpereel, A., Nowak, A. K., Fujimoto, N., Peters, S., Tsao, A. S., et al. (2021). First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): A multicentre, randomised, open-label, phase 3 trial. Lancet 397 (10272), 375–386. doi:10.1016/S0140-6736(20)32714-8
Bodor, J. N., Boumber, Y., and Borghaei, H. (2020). Biomarkers for immune checkpoint inhibition in non-small cell lung cancer (NSCLC). Cancer 126 (2), 260–270. doi:10.1002/cncr.32468
Borghaei, H., Paz-Ares, L., Horn, L., Spigel, D. R., Steins, M., Ready, N. E., et al. (2015). Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 373 (17), 1627–1639. doi:10.1056/NEJMoa1507643
Brahmer, J., Reckamp, K. L., Baas, P., Crino, L., Eberhardt, W. E., Poddubskaya, E., et al. (2015). Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 373 (2), 123–135. doi:10.1056/NEJMoa1504627
Brahmer, J. R., Tykodi, S. S., Chow, L. Q., Hwu, W. J., Topalian, S. L., Hwu, P., et al. (2012). Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366 (26), 2455–2465. doi:10.1056/NEJMoa1200694
Brahmer, R-A. D., Robinson, A. G., Hui, R., Csőszi, T., Fülöp, A., Gottfried, M., et al. (2017). Health-related quality-of-life results for pembrolizumab versus chemotherapy in advanced, PD-L1-positive NSCLC (KEYNOTE-024): A multicentre, international, randomised, open-label phase 3 trial. Lancet Oncol. 18 (12), 1600–1609. doi:10.1016/S1470-2045(17)30690-3
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68 (6), 394–424. doi:10.3322/caac.21492
Chen, C. (2004). Searching for intellectual turning points: Progressive knowledge domain visualization. Proc. Natl. Acad. Sci. U. S. A. 1 (1), 5303–5310. doi:10.1073/pnas.0307513100
Chen, L., Wei, Q., Li, J., Liao, D., and Feng, D. (2020). A scientometric visualization analysis for global toxicology and pharmacology research of natural products from 1962 to 2018. Phytomedicine 68, 153190. doi:10.1016/j.phymed.2020.153190
Curigliano, G., Gelderblom, H., Mach, N., Doi, T., Tai, D., Forde, P. M., et al. (2021). Phase I/ib clinical trial of sabatolimab, an anti-TIM-3 antibody, alone and in combination with spartalizumab, an anti-PD-1 antibody, in advanced solid tumors. Clin. Cancer Res. 27 (13), 3620–3629. doi:10.1158/1078-0432.CCR-20-4746
Dai, Y. K., Zhao, Z. M., and Liu, C. (2022). Treatment of liver fibrosis: A 20-year bibliometric and knowledge-map analysis. Front. Pharmacol. 13, 942841. doi:10.3389/fphar.2022.942841
De Giglio, A., Di Federico, A., Nuvola, G., Deiana, C., and Gelsomino, F. (2021). The landscape of immunotherapy in advanced NSCLC: Driving beyond PD-1/PD-L1 inhibitors (CTLA-4, LAG3, ido, OX40, TIGIT, vaccines). Curr. Oncol. Rep. 23 (11), 126. doi:10.1007/s11912-021-01124-9
De Ruysscher, D., Faivre-Finn, C., Nackaerts, K., Jordan, K., Arends, J., Douillard, J. Y., et al. (2020). Recommendation for supportive care in patients receiving concurrent chemotherapy and radiotherapy for lung cancer. Ann. Oncol. 31 (1), 41–49. doi:10.1016/j.annonc.2019.10.003
Goldberg, M. V., and Drake, C. G. (2010). LAG-3 in cancer immunotherapy. Curr. Top. Microbiol. Immunol. 344, 269–278. doi:10.1007/82_2010_114
Guibert, N. M. J., and Mazières, J. (2015). Nivolumab for treating non-small cell lung cancer. Expert Opin. Biol. Ther. 15 (12), 1789–1797. doi:10.1517/14712598.2015.1114097
Hellmann, M. D., Ciuleanu, T. E., Pluzanski, A., Lee, J. S., Otterson, G. A., Audigier-Valette, C., et al. (2018). Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N. Engl. J. Med. 378 (22), 2093–2104. doi:10.1056/NEJMoa1801946
Hellmann, M. D., Paz-Ares, L., Bernabe Caro, R., Zurawski, B., Kim, S. W., Carcereny Costa, E., et al. (2019). Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N. Engl. J. Med. 381 (21), 2020–2031. doi:10.1056/NEJMoa1910231
Herbst, R. S. B. P., Kim, D. W., Felip, E., Pérez-Gracia, J. L., Han, J. Y., Molina, J., et al. (2016). Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 387 (10027), 1540–1550. doi:10.1016/S0140-6736(15)01281-7
Hodi, F. S., O'Day, S. J., McDermott, D. F., Weber, R. W., Sosman, J. A., Haanen, J. B., et al. (2010). Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363 (8), 711–723. doi:10.1056/NEJMoa1003466
Huang, Y. H., Zhu, C., Kondo, Y., Anderson, A. C., Gandhi, A., Russell, A., et al. (2015). CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature 517 (7534), 386–390. doi:10.1038/nature13848
Jo, M. (1971). Exponential smoothing: Appropriate and inappropriate applications. Health Serv. Res. 6 (3), 256–259.
Larkin, J., Chiarion-Sileni, V., Gonzalez, R., Grob, J. J., Cowey, C. L., Lao, C. D., et al. (2015). Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 373 (1), 23–34. doi:10.1056/NEJMoa1504030
Larkin, J., Chiarion-Sileni, V., Gonzalez, R., Grob, J. J., Rutkowski, P., Lao, C. D., et al. (2019). Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 381 (16), 1535–1546. doi:10.1056/NEJMoa1910836
Lurienne, L., Cervesi, J., Duhalde, L., de Gunzburg, J., Andremont, A., Zalcman, G., et al. (2020). NSCLC immunotherapy efficacy and antibiotic use: A systematic review and meta-analysis. J. Thorac. Oncol. 15 (7), 1147–1159. doi:10.1016/j.jtho.2020.03.002
Miao, L., Zhang, J., Zhang, Z., Wang, S., Tang, F., Teng, M., et al. (2022). A bibliometric and knowledge-map analysis of CAR-T cells from 2009 to 2021. Front. Immunol. 13, 840956. doi:10.3389/fimmu.2022.840956
Motzer, R. J., Escudier, B., McDermott, D. F., Aren Frontera, O., Melichar, B., Powles, T., et al. (2020). Survival outcomes and independent response assessment with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma: 42-month follow-up of a randomized phase 3 clinical trial. J. Immunother. Cancer 8 (2), e000891. doi:10.1136/jitc-2020-000891
Naidoo, J., Nishino, M., Patel, S. P., Shankar, B., Rekhtman, N., Illei, P., et al. (2020). Immune-related pneumonitis after chemoradiotherapy and subsequent immune checkpoint blockade in unresectable stage III non-small-cell lung cancer. Clin. Lung Cancer 21 (5), e435–e444. doi:10.1016/j.cllc.2020.02.025
Naidoo, J., Schindler, K., Querfeld, C., Busam, K., Cunningham, J., Page, D. B., et al. (2016). Autoimmune bullous skin disorders with immune checkpoint inhibitors targeting PD-1 and PD-L1. Cancer Immunol. Res. 4 (5), 383–389. doi:10.1158/2326-6066.CIR-15-0123
Niu, J., Maurice-Dror, C., Lee, D. H., Kim, D. W., Nagrial, A., Voskoboynik, M., et al. (2022). First-in-human phase 1 study of the anti-TIGIT antibody vibostolimab as monotherapy or with pembrolizumab for advanced solid tumors, including non-small-cell lung cancer(☆). Ann. Oncol. 33 (2), 169–180. doi:10.1016/j.annonc.2021.11.002
Ozkaya, S., Findik, S., Dirican, A., and Atici, A. G. (2012). Long-term survival rates of patients with stage IIIB and IV non-small cell lung cancer treated with cisplatin plus vinorelbine or gemcitabine. Exp. Ther. Med. 4 (6), 1035–1038. doi:10.3892/etm.2012.714
Pardoll, D. M. (2012). The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12 (4), 252–264. doi:10.1038/nrc3239
Qin, Y. F., Ren, S. H., Shao, B., Qin, H., Wang, H. D., Li, G. M., et al. (2022). The intellectual base and research fronts of IL-37: A bibliometric review of the literature from WoSCC. Front. Immunol. 13, 931783. doi:10.3389/fimmu.2022.931783
Reck, M. R-A. D., Robinson, A. G., Hui, R., Csőszi, T., Fülöp, A., Gottfried, M., et al. (2021). Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50. J. Clin. Oncol. 39 (21), 2339–2349. doi:10.1200/JCO.21.00174
Reck, M. R. J., and Hellmann, M. D. (2022). First-line immunotherapy for non-small-cell lung cancer. J. Clin. Oncol. 40 (6), 586–597. doi:10.1200/jco.21.01497
Reck, M., Rodriguez-Abreu, D., Robinson, A. G., Hui, R., Csoszi, T., Fulop, A., et al. (2016). Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 375 (19), 1823–1833. doi:10.1056/NEJMoa1606774
Robert, C., Schachter, J., Long, G. V., Arance, A., Grob, J. J., Mortier, L., et al. (2015). Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 372 (26), 2521–2532. doi:10.1056/NEJMoa1503093
Sakuishi, K., Apetoh, L., Sullivan, J. M., Blazar, B. R., Kuchroo, V. K., and Anderson, A. C. (2010). Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 207 (10), 2187–2194. doi:10.1084/jem.20100643
Shen, J., Shen, H., Ke, L., Chen, J., Dang, X., Liu, B., et al. (2022). Knowledge mapping of immunotherapy for hepatocellular carcinoma: A bibliometric study. Front. Immunol. 13, 815575. doi:10.3389/fimmu.2022.815575
Socinski, M. A., Nishio, M., Jotte, R. M., Cappuzzo, F., Orlandi, F., Stroyakovskiy, D., et al. (2021). IMpower150 final overall survival analyses for atezolizumab plus bevacizumab and chemotherapy in first-line metastatic nonsquamous NSCLC. J. Thorac. Oncol. 16 (11), 1909–1924. doi:10.1016/j.jtho.2021.07.009
Sullivan, R. J., and Weber, J. S. (2022). Immune-related toxicities of checkpoint inhibitors: Mechanisms and mitigation strategies. Nat. Rev. Drug Discov. 21 (7), 495–508. doi:10.1038/s41573-021-00259-5
Suresh, K., Naidoo, J., Lin, C. T., and Danoff, S. (2018). Immune checkpoint immunotherapy for non-small cell lung cancer: Benefits and pulmonary toxicities. Chest 154 (6), 1416–1423. doi:10.1016/j.chest.2018.08.1048
Topalian, S. L., Hodi, F. S., Brahmer, J. R., Gettinger, S. N., Smith, D. C., McDermott, D. F., et al. (2012). Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366 (26), 2443–2454. doi:10.1056/NEJMoa1200690
van Eck, N. J., and Waltman, L. (2010). Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 84 (2), 523–538. doi:10.1007/s11192-009-0146-3
Weber, J., Mandala, M., Del Vecchio, M., Gogas, H. J., Arance, A. M., Cowey, C. L., et al. (2017). Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N. Engl. J. Med. 377 (19), 1824–1835. doi:10.1056/NEJMoa1709030
Keywords: non-small cell lung carcinoma, immune checkpoint inhibitors, bibliometric analysis, CiteSpace, VOSviewer
Citation: Zhang Y, Lu L and Zheng R (2023) Emerging trends and focus on immune checkpoint inhibitors for non-small cell lung cancer treatment: visualization and bibliometric analysis. Front. Pharmacol. 14:1140771. doi: 10.3389/fphar.2023.1140771
Received: 09 January 2023; Accepted: 24 April 2023;
Published: 04 May 2023.
Edited by:
Sungpil Yoon, Sungkyunkwan University, Republic of KoreaReviewed by:
Hashem Obaid Alsaab, Taif University, Saudi ArabiaZhijian Lin, Beijing University of Chinese Medicine, China
Copyright © 2023 Zhang, Lu and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui Zheng, emhlbmdyQHNqLWhvc3BpdGFsLm9yZw==
 Yue Zhang
Yue Zhang Lishan Lu1,2
Lishan Lu1,2 Rui Zheng
Rui Zheng