
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 28 February 2023
Sec. Gastrointestinal and Hepatic Pharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1131974
This article is part of the Research Topic Advances in Novel Drugs and Targets for Hepatic and Gastrointestinal Diseases View all 33 articles
Background: The therapeutic value of neostigmine as a prokinetic drug in acute pancreatitis (AP), especially in non-mild AP, including moderately severe and severe AP remains controversial. This meta-analysis aimed to investigate the efficacy of neostigmine treatment in patients with non-mild AP.
Methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, China National Knowledge Infrastructure (CNKI), and Wanfang databases up to 24 December 2022 for RCTs comparing neostigmine plus conventional treatment versus the conventional treatment alone in patients with non-mild AP. Trial sequential analyses (TSA) were used to assess the risk of random errors and the results.
Results: Six RCTs with 318 participants were included. Compared with conventional treatment, patients who received neostigmine plus conventional treatment had a shorter time duration for their first defecation (MD: −1.74; 95% CI: −2.10 to −1.38; p < 0.00001; n = 205; RCTs = 4; low quality of evidence) and better relief time of abdominal symptoms (MD: −1.59, 95% CI: −2.07 to −1.11; p < 0.00001; n = 155; RCTs = 3; low quality of evidence) as primary outcomes, and a faster percentage decrease of IAP at 24 h (p = 0.0005; moderate quality of evidence) and a shorter length of ICU stay (p < 0.00001; moderate quality of evidence) as partial secondary outcomes. TSA suggested the sample size was limited, but the cumulative Z curves of the primary outcomes crossed the conventional boundary and the trial sequential monitoring boundary.
Conclusion: For patients with non-mild AP, neostigmine promotes the recovery of gastrointestinal motility and may have positive effects on the improvement of a clinical prognosis. Further large-sample studies are needed for a definite conclusion.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/; Identifier: CRD 42022381417.
Acute pancreatitis (AP) is acute inflammation of the pancreas with a variable involvement of the nearby tissues or other organs, causing substantial mortality and morbidity (Bradley, 1993; Boxhoorn et al., 2020). It is one of the most common causes for gastroenterology-related hospitalization, with an increase in the annual incidence of AP in the past decades (Peery et al., 2012; Roberts et al., 2013). Based on the presence of organ failure and local and systemic complications, AP was classified into mild AP (MAP) and non-mild AP, including moderately severe AP (MSAP) and severe AP (SAP), according to the revised Atlanta Classification. MAP presents with a self-limitation process, but non-mild AP accounts for substantial morbidity and mortality and needs aggressive treatment (Banks et al., 2013).
Gut dysfunction, with ileus as the most frequently encountered complication, is common in AP, especially SAP (Uhl et al., 1996; Wang et al., 2003). It often aggravates intra-abdominal hypertension (IAH) and may even give rise to abdominal compartment syndrome (ACS) (Kirkpatrick et al., 2013; van Brunschot et al., 2014). In addition, gut dysfunction also limits the starting and delivery of enteral nutrition, which has been confirmed to reduce infections, surgical interventions, organ failures, and mortality (Cao et al., 2008; Al-Omran et al., 2010). Therefore, the concept of ‘gut rousing’ was proposed to maintain the gut function, while there was no effective evidence-based drugs except for supportive treatment in clinical practice until now (Petrov and Windsor, 2013; Moggia et al., 2017).
Neostigmine is an anti-cholinesterase drug that enhances intestinal peristalsis, promoting the passage of flatus and defecation. It is widely used as a prokinetic drug in the treatment of AP, especially with gut dysfunction and IAH. Several previous studies showed that it had positive effects on AP with the latest randomized controlled trials (RCTs) published in March 2022, suggesting that neostigmine could reduce IAH and promote defecation (Schneider et al., 2014; He et al., 2022; Mancilla Asencio and Berger Fleiszig, 2022). However, its clinical value for AP has remained debatable so far, and the current international clinical guidelines did not attribute enough importance to this drug. To date, no systemic review has addressed this topic either (Tenner et al., 2013; Greenberg et al., 2016; Crockett et al., 2018a; Leppaniemi et al., 2019). Therefore, we planned to conduct a systematic review and meta-analysis to explore the efficacy of neostigmine for the treatment of non-mild AP, aiming to provide current evidence for clinical practice.
This study was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocol (Supplementary Table S1, PRISMA checklist) and the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (The Cochrane Collaboration, 2011; Page et al., 2021). The study protocol was prospectively registered on PROSPERO (CRD 42022381417).
Our study only included RCTs, while other types of studies such as case series, case reports, and observational cohort studies were excluded. Studies without sufficient data, primary data, or full text were also excluded, and neither were duplicate publications. Languages were not limited.
Our study included adults (aged over 18) with non-mild AP including MSAP and SAP, and participants with contraindications to neostigmine, such as an allergy to neostigmine, comorbidity with epilepsy, angina pectoris or asthma, and pregnancy or lactation, were excluded (Neely et al., 2022). MSAP was defined as AP that had local complications with or without transient organ failures (<48 h). SAP was characterized by persistent organ failure (>48 h) with or without local complications. The diagnosis of AP and definitions of MSAP and SAP were made according to the established guidelines (Tenner et al., 2013; Greenberg et al., 2016; Crockett et al., 2018a; Leppaniemi et al., 2019; Chinese Pancreatic Surgery Association CSoSCMA, 2021).
We planned to include studies comparing neostigmine plus conventional therapy as the neostigmine treatment (NT) group and conventional therapy alone as the conventional treatment (CT) group. Conventional therapy included fluid management with early fluid resuscitation and volume control for IAH, analgesics, nutrition support, symptomatic treatment, gastrointestinal decompression, and traditional Chinese medicine (TCM) components such as rhubarb, Glauber’s salt, and Da-Cheng-Qi decoction (Qiong et al., 2005; Boxhoorn et al., 2020). Studies with different kinds of conventional treatment between NT and CT were excluded. The route of neostigmine included intramuscular injections and Zusanli (stomach meridian, ST36), which is an acupoint 2 cm below the knee joint on the anterior aspect of the lower limb based on the TCM theory of acupuncture and had potential effects on the recovery of gastrointestinal disorders (Ng et al., 2013).
Primary outcomes: a. Time to the first defecation; b. time to the relief of abdominal symptoms.
Secondary outcomes: a. Percentage decrease of intra-abdominal pressure (IAP) at 24 h; b. new-onset ACS that is defined as a sustained IAP>20 mmHg with organ failure after treatment (Kirkpatrick et al., 2013); c. in-hospital mortality; e. multiple organ failure; d. interventional drainage and operation events; e. length of ICU stay; f. length of hospital stay; and g. serious adverse events caused by neostigmine, which include nervous system dysfunctions such as ataxia, convulsions, coma, slurred speech, anxiety or fear, malignant arrhythmia, or bronchospasm (Smedley et al., 2020).
We conducted a literature search up to 24 December 2022 in the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, China National Knowledge Infrastructure (CNKI), and Wanfang databases with the designed search strategies (Supplementary Table S2). The reference lists of relevant articles were also checked for additional references.
Two reviewers (JL and XB) independently completed study screening and selection. First, the reviewers screened the titles and abstracts of potential studies from the literature search and retrieved the qualified articles. Afterward, the reviewers screened the full text and identified eligible studies for the inclusion based on inclusion and exclusion criteria. Disagreements were resolved through discussions. In addition, we identified and excluded duplicates and multiple reports of the same study. Another set of reviewers (YW and ZY) independently conducted data extraction and recorded all relevant details from the included studies using a standardized data extraction form including methods, participants, interventions, outcomes, and additional related information. Similarly, disagreements were resolved through discussions.
Two reviewers (XB and ZY) independently assessed the risk of bias for each study and recorded it in ‘Risk of bias’ tables with conflicts resolved through discussions. The risk of bias of RCTs was assessed with items in the Cochrane Collaboration’s tool (The Cochrane Collaboration, 2011).
We applied the Grading of Recommendations Assessment, Development, and Evaluations (GRADE) framework to assess the certainty of evidence on the main outcomes, including primary outcomes and several important secondary outcomes, such as the time to the first defecation, time to the relief of abdominal symptoms, percentage decrease of IAP at 24 h, and the length of ICU stay (Atkins et al., 2004).
Trial sequential analyses (TSA) were used to control the risk of random errors and assess the conclusions. Based on previous clinical experiences and RCTs in this field, we used the mean difference (MD) and a variance of power of 80% to calculate the required sample size and assess the clinical significance of the primary outcome in our review (He et al., 2022). Decisions were made based on the position of cumulative Z curves with the conventional boundary, trial sequential monitoring boundary, and futility boundary (Wetterslev et al., 2017).
We utilized the I2 statistic to measure the heterogeneity among the RCTs. We considered an I2 value greater than or equal to 60% as the evidence of moderate to substantial levels of heterogeneity. In the event of I2 > 80% (substantial heterogeneity), we did not plan to perform the meta-analysis but instead presented the results using forest plots without pooled estimates (Higgins, 2011). We planned to explore the potential reasons by a subgroup analysis based on the severity of AP and the detailed therapeutic regimen of neostigmine if heterogeneity was found, and there were sufficient relevant data (Higgins, 2011).
Funnel plots for measuring the publication bias were performed if there were 10 or more included studies in this meta-analysis, with Egger’s test being used to determine the statistical significance of the publication bias. A p-value of less than 0.05 was considered to indicate the statistically significant publication bias (Egger et al., 1997). If fewer than 10 studies were included, the publication bias was assessed based on the characteristics of the included studies instead.
Data were analyzed using RevMan (version 5.4.1). A random-effects model was used to combine the eligible trials, in which the DerSimonian and Laird method was used to estimate the between-study variance. The results were presented as forest plots and risk ratios (RRs) with 95% confidence interval (CI) for dichotomous data and mean MD with 95% CI for continuous data. If the data were reported as the median, the minimum and maximum values, and/or the first and third quartiles, we transformed the data to the mean value and standard deviation (SD) to pool the results in a consistent format (Wan et al., 2014; Luo et al., 2018).
As shown in Figure 1, six RCTs fulfilling all eligible criteria from 194 records were included in this meta-analysis (Feng, 2011; Chen and Zhang, 2013; Wang and Xiao, 2013; Chen, 2016; Wang, 2016; He et al., 2022). Table 1 shows the details of the included studies with a total of 318 participants. A total of 161 participants were in NT, and the remaining 157 were in CT.
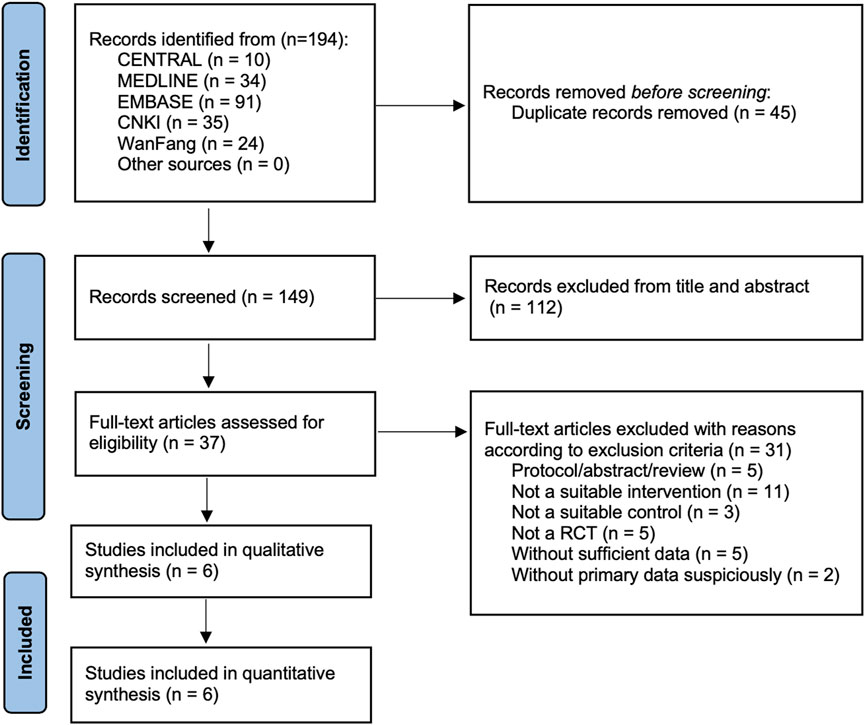
FIGURE 1. PRISMA flowchart represents the flow of information through the different phases of the systematic review and meta-analysis.
The risk of bias of eligible studies is shown in Figure 2. All RCTs, except the latest one (He 2022), had some concerns of bias. The quality of evidence for the main outcomes mentioned previously using the GRADE methodology is shown in Table 2.
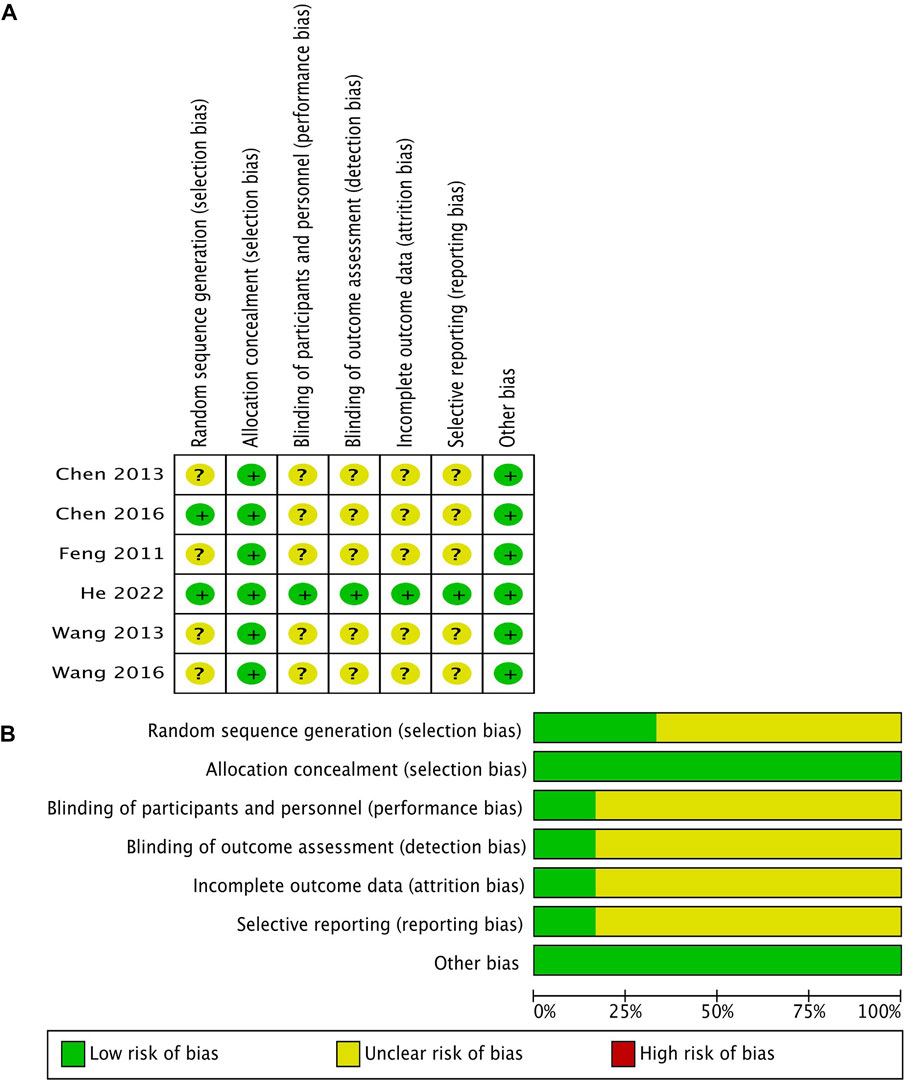
FIGURE 2. Methodological quality of the included studies according to the Cochrane Collaboration’s tool for assessing the risk of bias. (A) Risk of bias summary; (B) risk of bias graph.
We assessed the publication bias on the basis of the characteristics of the included studies rather than the funnel plots because fewer than the 10 included studies were analyzed. We assessed the risk of bias to be unclear because the protocols and registered information on five RCTs in our review were not found despite the fact that we obtained the registered information on the last RCT (He 2022) from the clinical trial registration website (No. NCT02543658).
Four RCTs were analyzed for the time to the first defecation. A meta-analysis showed that NT had a significantly shorter time to the first defecation than CT (MD: −1.74; 95% CI: −2.10 to −1.38; p < 0.00001; I2 55%; n = 205; RCTs = 4; low quality of evidence) (Figure 3A). The TSA result showed the required information size was 529. Although the cumulative Z curve did not reach the required information size, it crossed the conventional boundary and the trial sequential monitoring boundary, suggesting a reliable positive result (Figure 4A).
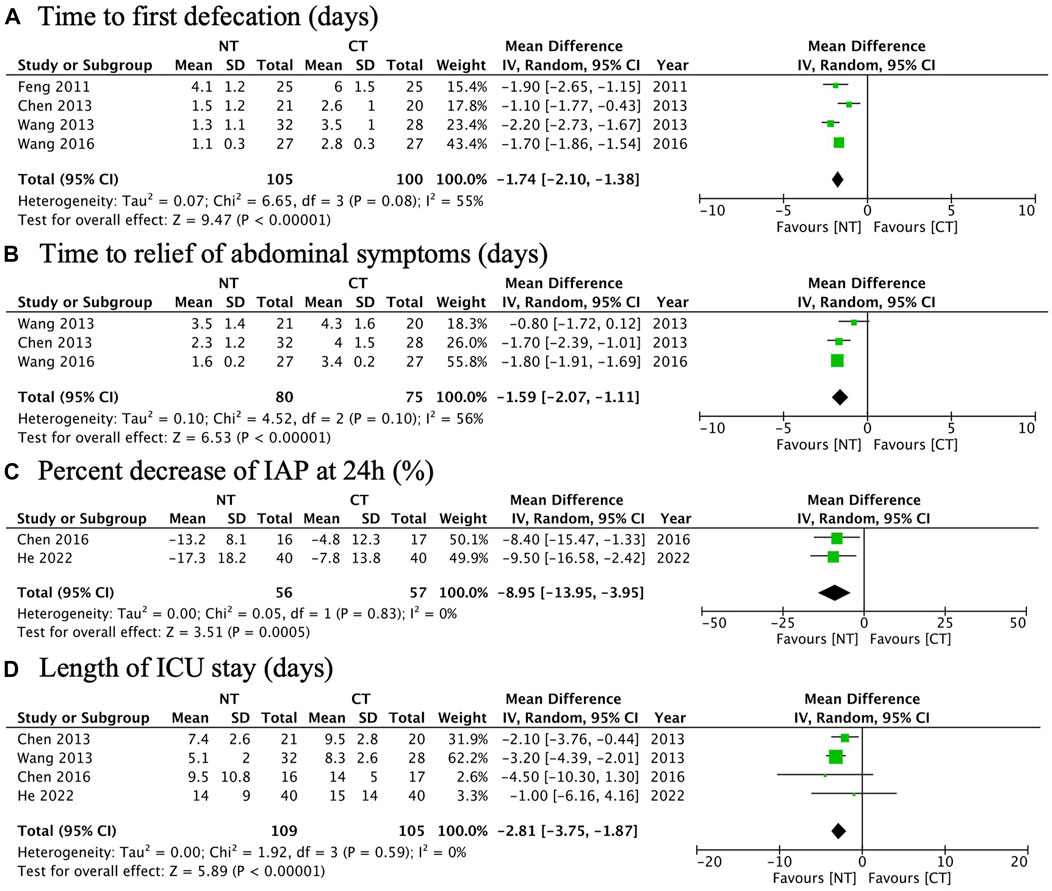
FIGURE 3. Forest plots illustrating the main outcomes (the primary outcome and some secondary outcomes): (A) time to the first defecation; (B) time to the relief of abdominal symptoms; (C) percentage decrease of IAP at 24 h; (D) length of ICU stay.
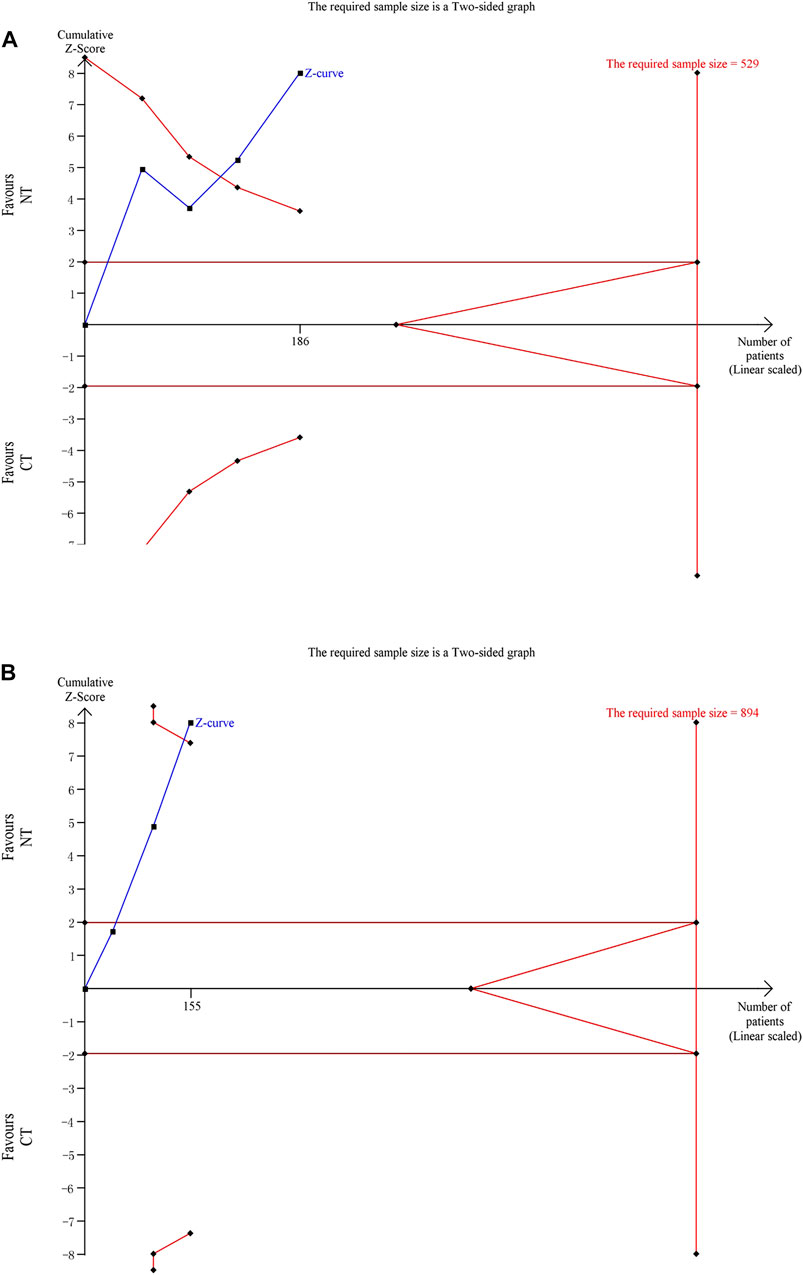
FIGURE 4. Trial sequential analyses for primary outcomes: (A) time to the first defecation and (B) time to the relief of abdominal symptoms.
Three RCTs were analyzed for the time to the relief of abdominal symptoms. A meta-analysis showed that NT had a significantly shorter time to the relief of abdominal symptoms than CT (MD: −1.59; 95% CI: −2.07 to −1.11; p < 0.00001; I2 56%; n = 155; RCTs = 3; low quality of evidence) (Figure 3B). The TSA result showed the required information size was 894. The cumulative Z curve crossed the conventional boundary and the trial sequential monitoring boundary despite the fact that it did not reach the required information size, suggesting a reliable positive result (Figure 4B).
Two RCTs were analyzed for the percentage decrease of IAP at 24 h. A meta-analysis showed that NT had a significantly faster rate of the percentage decrease of IAP at 24 h than CT (MD: −8.95%; 95% CI: −13.95 to −3.95%; p = 0.0005; I2 0%; n = 113; RCTs = 2; moderate quality of evidence) (Figure 3C).
Four RCTs were analyzed for the length of ICU stay. A meta-analysis showed that NT had a significantly shorter length of ICU stay than CT (MD: −2.81; 95% CI: −3.75 to −1.87; p < 0.00001; I2 0%; n = 214; RCTs = 4; moderate quality of evidence) (Figure 3D).
There was one RCT that reported the safety outcome of neostigmine as serious adverse events (He 2022). The latest RCT reported six adverse events including circulatory failure, respiratory failure, renal failure, and bradycardia. After careful analyses, the investigators considered neostigmine treatment unlikely to be related to all these adverse events, which were attributed to the progression of AP and the withdrawal of a cardio-selective beta receptor blocker.
Meta-analysis showed there was no significant difference between NT and CT in the secondary outcomes, shown as follows:
New-onset ACS (RR: 0.61; 95% CI: 0.15 to 2.49; p = 0.49; I2 0%; n = 113; RCTs = 2) (Figure 5A).
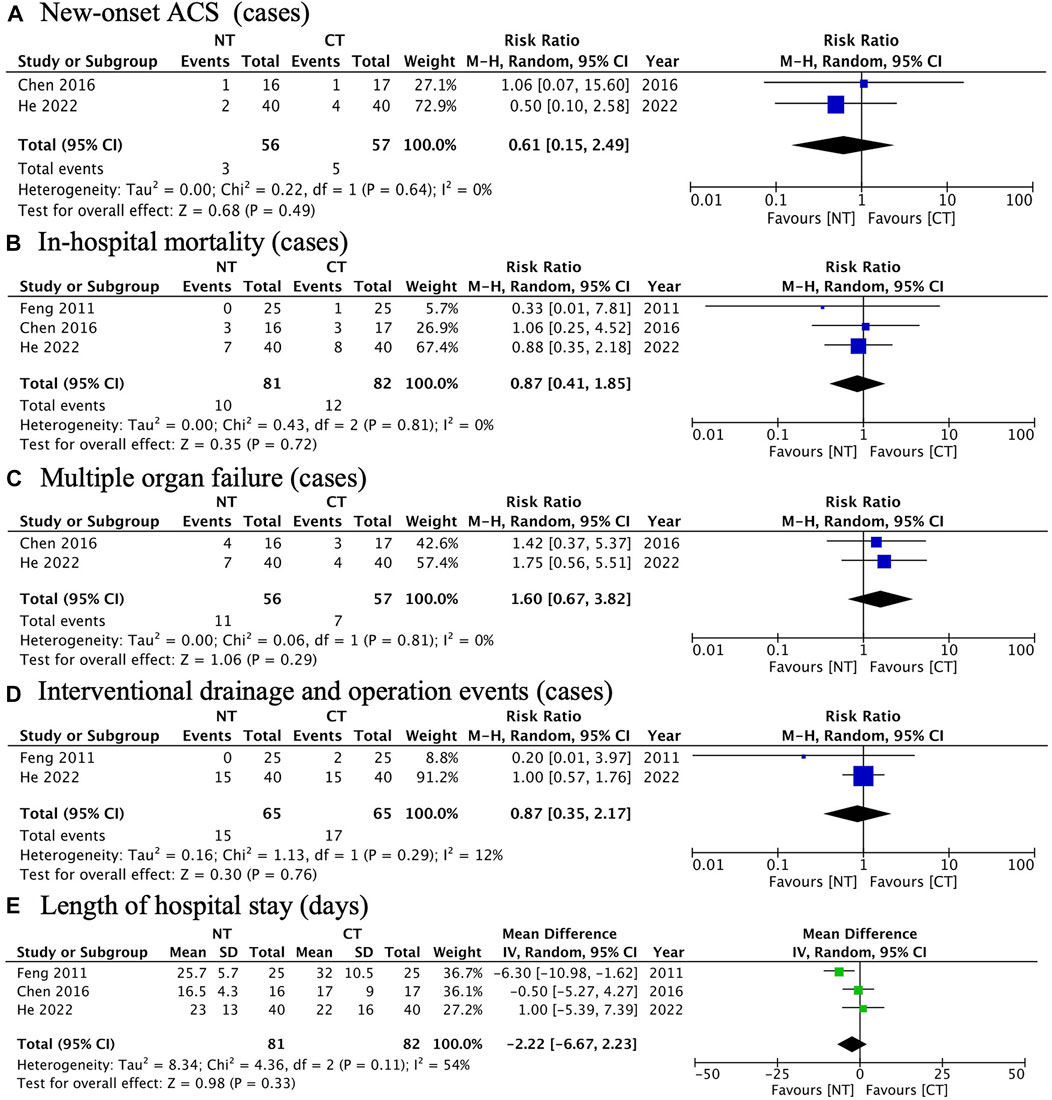
FIGURE 5. Forest plots illustrating other secondary outcomes: (A) new-onset ACS; (B) in-hospital mortality; (C) multiple organ failure; (D) interventional drainage and operation events; (E) length of hospital stay.
In-hospital mortality (RR: 0.87; 95% CI: 0.41 to 1.85; p = 0.72; I2 0%; n = 163; RCTs = 2) (Figure 5B).
Multiple organ failure (RR: 1.60; 95% CI: 0.67 to 3.82; p = 0.29; I2 0%; n = 113; RCTs = 2) (Figure 5C).
Interventional drainage and operation events (RR: 1.60; 95% CI: 0.67 to 3.82; p = 0.29; I2 0%; n = 130; RCTs = 2) (Figure 5D).
Length of hospital stay (MD: −2.22; 95% CI: −6.67 to 2.23; p = 0.33; I2 54%; n = 163; RCTs = 3) (Figure 5E).
Our systemic review and meta-analysis showed that compared with the conventional treatment, the application of neostigmine promoted the recovery of bowel movements in patients with non-mild AP and fastened the percentage decrease of IAP at 24 h and shortened the length of ICU stay, suggesting that neostigmine may be beneficial to promote the recovery of the gastrointestinal function and improve clinical prognoses. The TSA showed that the results for primary outcomes were reliable, regardless of the relatively small sample size. The quality of evidence for the main outcomes was low to moderate due to the paucity of the included studies and risk of bias.
Neostigmine is a reversible acetylcholinesterase inhibitor, which increases acetylcholine and stimulates both nicotinic and muscarinic receptors. Its distribution and elimination half-lives are 3.4 and 77 min, respectively. Therefore, neostigmine is usually used to reverse the effects of non-depolarizing muscle relaxants at the end of an operation, showing a dose-dependent effect and ceiling effect (Miller, 1976; Miller and Roderick, 1977). In the field of gastroenterology, it has been proved that neostigmine can induce colonic decompression in pseudo-obstruction and is recommended in the treatment of patients with colonic obstruction and IAH, who show poor response to other measures. It further supports the beneficial effect of neostigmine in non-mild AP on the promotion of gastrointestinal motility and reduction of IAP revealed by our review (Ponec et al., 1999; Kirkpatrick et al., 2013). As for this, there was no significant difference in secondary outcomes as the new-onset ACS, in-hospital mortality, multiple organ failure, interventional drainage, and operation events and the length of hospital stay, we speculated that the reason may be due to the paucity of the included participants, and further large-sample size studies are needed.
However, as for AP, the international clinical guidelines paid less attention to neostigmine despite the fact that neostigmine with or without TCM was widely used in clinical practice in the department of gastroenterology and ICUs in lots of hospitals in China for non-mild AP, especially with enteroparalysis or IAH (Tenner et al., 2013; Greenberg et al., 2016; Crockett et al., 2018a; Crockett et al., 2018b; Leppaniemi et al., 2019). We speculated that the reason was that all previous related studies were published in the Chinese language in the local journals until He et al. published a high-quality RCT in an international journal in 2022 (He et al., 2022; Mancilla Asencio and Berger Fleiszig, 2022). To the best of our knowledge, this systematic review was the first review that included all RCTs with the latest review published in March, 2022, about neostigmine treatment for non-mild AP, providing strong current evidence in this field.
Nevertheless, our study had several limitations. First, the main limitation was the small sample size of the included studies and participants and the risk of bias due to the inappropriate methodology of several included RCTs published in the Chinese language, which limited its clinical application (Wu et al., 2006). However, TSA revealed that the result of primary outcomes seemed to be reliable, regardless of the unsatisfied sample size. Certainly, large-sample, prospective, multi-center RCTs were required to confirm the final results in the future. Second, the lack of sufficient clinical data and the paucity of the included studies made it difficult to perform further statistical analysis, such as the safety of neostigmine treatment, efficacy of neostigmine versus neostigmine and TCM, exploration of the appropriate dosage, frequency, route of administration, location of the injection, and treatment course for neostigmine treatment.
Our meta-analysis suggested that for patients with non-mild AP, neostigmine promotes the recovery of the gastrointestinal motility and may have positive effects on the clinical prognosis. Future studies such as large-sample prospective multi-center studies are needed for conclusive results and further detailed analyses.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
All author contributions to the manuscript are as follows: conception or design of the study: XH and DW; data collection: KH and YW; data analysis and interpretation: KH, YW, JL, XB, and ZY; writing the manuscript: KH and YW; critical revision of the manuscript: XH and DW; and approval of the final draft: KH, YW, JL, XB, ZY, XH, and DW.
This research received financial support from the National Natural Science Foundation of China (No. 32170788), the National High Level Hospital Clinical Research Funding (2022-PUMCH-B-023), and the National Key Clinical Specialty Construction Project (ZK108000).
The authors would like to thank Tao Wang for his invaluable advice in the preparation of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1131974/full#supplementary-material
Al-Omran, M., Albalawi, Z. H., Tashkandi, M. F., and Al-Ansary, L. A. (2010). Enteral versus parenteral nutrition for acute pancreatitis. Cochrane Database Syst. Rev. 1, CD002837. doi:10.1002/14651858.CD002837.pub2
Atkins, D., Eccles, M., Flottorp, S., Guyatt, G. H., Henry, D., Hill, S., et al. (2004). Systems for grading the quality of evidence and the strength of recommendations I: Critical appraisal of existing approaches the GRADE working group. BMC Health Serv. Res. 4 (1), 38. doi:10.1186/1472-6963-4-38
Banks, P. A., Bollen, T. L., Dervenis, C., Gooszen, H. G., Johnson, C. D., Sarr, M. G., et al. (2013). Classification of acute pancreatitis-2012: Revision of the Atlanta classification and definitions by international consensus. Gut 62 (1), 102–111. doi:10.1136/gutjnl-2012-302779
Boxhoorn, L., Voermans, R. P., Bouwense, S. A., Bruno, M. J., Verdonk, R. C., Boermeester, M. A., et al. (2020). Acute pancreatitis. Lancet 396 (10252), 726–734. doi:10.1016/S0140-6736(20)31310-6
Bradley, E. L. (1993). A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch. Surg. 128 (5), 586–590. doi:10.1001/archsurg.1993.01420170122019
Cao, Y., Xu, Y., Lu, T., Gao, F., and Mo, Z. (2008). Meta-analysis of enteral nutrition versus total parenteral nutrition in patients with severe acute pancreatitis. Ann. Nutr. Metab. 53 (3-4), 268–275. doi:10.1159/000189382
Chen, P. (2016). Clinical study of neostigmine in the treatment of acute pancreatitis with intra-abdominal hypertension. Nanchang, China: Nanchang University.
Chen, X., and Zhang, Y. (2013). Observation on therapeutic effect of injection of neostigmine at zusanli combined with qingyi decoction on severe pancreatitis complicated with gastrointestinal paralysis. J. Mod. Integr. Chin. West. Med. dai zhong xi yi jie he] 22 (02), 128–130. doi:10.3969/j.issn.1008-8849.2013.02.006
Chinese Pancreatic Surgery Association CSoSCMA (2021). Guidelines for diagnosis and treatment of acute pancreatitis in China (2021). Zhonghua Wai Ke Za Zhi 59 (7), 578–587. doi:10.3760/cma.j.cn112139-20210416-00172
Crockett, S., Falck-Ytter, Y., Wani, S., and Gardner, T. B. (2018). Acute pancreatitis guideline. Gastroenterology 154 (4), 1102. doi:10.1053/j.gastro.2018.02.029
Crockett, S. D., Wani, S., Gardner, T. B., Falck-Ytter, Y., and Barkun, A. N., and American Gastroenterological Association Institute Clinical Guidelines C (2018). American gastroenterological association Institute guideline on initial management of acute pancreatitis. Gastroenterology 154 (4), 1096–1101. doi:10.1053/j.gastro.2018.01.032
Egger, M., Davey Smith, G., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ 315 (7109), 629–634. doi:10.1136/bmj.315.7109.629
Feng, R. (2011). Effect of injection of neostigmine at Zusanli point on gastrointestinal dysfunction in patients with severe acute pancreatitis. Chengdu: Chengdu University of TCM[cheng du zhong yi yao].
Greenberg, J. A., Hsu, J., Bawazeer, M., Marshall, J., Friedrich, J. O., Nathens, A., et al. (2016). Clinical practice guideline: Management of acute pancreatitis. Can. J. Surg. 59 (2), 128–140. doi:10.1503/cjs.015015
He, W., Chen, P., Lei, Y., Xia, L., Liu, P., Zhu, Y., et al. (2022). Randomized controlled trial: Neostigmine for intra-abdominal hypertension in acute pancreatitis. Crit. Care 26 (1), 52. doi:10.1186/s13054-022-03922-4
Higgins, J. P. G. S. (2011). Cochrane Handbook for systematic reviews of interventions Version 5.1.0 The Cochrane Collaboration. (updated March 2011).
Kirkpatrick, A. W., Roberts, D. J., De Waele, J., Jaeschke, R., Malbrain, M. L., De Keulenaer, B., et al. (2013). Intra-abdominal hypertension and the abdominal compartment syndrome: Updated consensus definitions and clinical practice guidelines from the world society of the abdominal compartment syndrome. Intensive Care Med. 39 (7), 1190–1206. doi:10.1007/s00134-013-2906-z
Leppaniemi, A., Tolonen, M., Tarasconi, A., Segovia-Lohse, H., Gamberini, E., Kirkpatrick, A. W., et al. (2019). 2019 WSES guidelines for the management of severe acute pancreatitis. World J. Emerg. Surg. 14, 27. doi:10.1186/s13017-019-0247-0
Luo, D., Wan, X., Liu, J., and Tong, T. (2018). Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 27 (6), 1785–1805. doi:10.1177/0962280216669183
Mancilla Asencio, C., and Berger Fleiszig, Z. (2022). Intra-abdominal hypertension: A systemic complication of severe acute pancreatitis. Med. Kaunas. 58 (6), 785. doi:10.3390/medicina58060785
Miller, R. D. (1976). Antagonism of neuromuscular blockade. Anesthesiology 44 (4), 318–329. doi:10.1097/00000542-197604000-00010
Miller, R. D., and Roderick, L. L. (1977). Pancuronium-induced neuromuscular blockade, and its antagonism by neostigmine, at 29, 37, and 41 c. Anesthesiology 46 (5), 333–335. doi:10.1097/00000542-197705000-00006
Moggia, E., Koti, R., Belgaumkar, A. P., Fazio, F., Pereira, S. P., Davidson, B. R., et al. (2017). Pharmacological interventions for acute pancreatitis. Cochrane Database Syst. Rev. 4, CD011384. doi:10.1002/14651858.CD011384.pub2
Ng, S. S. M., Leung, W. W., Mak, T. W. C., Hon, S. S. F., Li, J. C. M., Wong, C. Y. N., et al. (2013). Electroacupuncture reduces duration of postoperative ileus after laparoscopic surgery for colorectal cancer. Gastroenterology 144 (2), 307–313.e1. doi:10.1053/j.gastro.2012.10.050
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Peery, A. F., Dellon, E. S., Lund, J., Crockett, S. D., McGowan, C. E., Bulsiewicz, W. J., et al. (2012). Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 143 (5), 1179–1187.e3. doi:10.1053/j.gastro.2012.08.002
Petrov, M. S., and Windsor, J. A. (2013). Nutritional management of acute pancreatitis: The concept of 'gut rousing. Curr. Opin. Clin. Nutr. Metab. Care 16 (5), 557–563. doi:10.1097/MCO.0b013e3283638ed1
Ponec, R. J., Saunders, M. D., and Kimmey, M. B. (1999). Neostigmine for the treatment of acute colonic pseudo-obstruction. N. Engl. J. Med. 341 (3), 137–141. doi:10.1056/NEJM199907153410301
Qiong, W., Yiping, W., Jinlin, Y., Tao, G., Zhen, G., and Pengcheng, Z. (2005). Chinese medicinal herbs for acute pancreatitis. Cochrane Database Syst. Rev. 1, CD003631. doi:10.1002/14651858.CD003631.pub2
Roberts, S. E., Akbari, A., Thorne, K., Atkinson, M., and Evans, P. A. (2013). The incidence of acute pancreatitis: Impact of social deprivation, alcohol consumption, seasonal and demographic factors. Aliment. Pharmacol. Ther. 38 (5), 539–548. doi:10.1111/apt.12408
Schneider, L., Jabrailova, B., Soliman, H., Hofer, S., Strobel, O., Hackert, T., et al. (2014). Pharmacological cholinergic stimulation as a therapeutic tool in experimental necrotizing pancreatitis. Pancreas 43 (1), 41–46. doi:10.1097/MPA.0b013e3182a85c21
Smedley, L. W., Foster, D. B., Barthol, C. A., Hall, R., and Gutierrez, G. C. (2020). Safety and efficacy of intermittent bolus and continuous infusion neostigmine for acute colonic pseudo-obstruction. J. Intensive Care Med. 35 (10), 1039–1043. doi:10.1177/0885066618809010
Tenner, S., Baillie, J., DeWitt, J., Vege, S. S., and American College of, G. (2013). American college of gastroenterology guideline: Management of acute pancreatitis. Am. J. Gastroenterol. 108 (9), 1400–1415. doi:10.1038/ajg.2013.218
The Cochrane Collaboration (2011). Cochrane Handbook for systematic reviews of interventions, Version 5.1.0. http://handbook.cochrane.org/. [updated March 2011].
Uhl, W., Isenmann, R., Curti, G., Vogel, R., Beger, H. G., and Buchler, M. W. (1996). Influence of etiology on the course and outcome of acute pancreatitis. Pancreas 13 (4), 335–343. doi:10.1097/00006676-199611000-00002
van Brunschot, S., Schut, A. J., Bouwense, S. A., Besselink, M. G., Bakker, O. J., van Goor, H., et al. (2014). Abdominal compartment syndrome in acute pancreatitis: A systematic review. Pancreas 43 (5), 665–674. doi:10.1097/MPA.0000000000000108
Wan, X., Wang, W., Liu, J., and Tong, T. (2014). Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 14, 135. doi:10.1186/1471-2288-14-135
Wang, X., Gong, Z., Wu, K., Wang, B., and Yuang, Y. (2003). Gastrointestinal dysmotility in patients with acute pancreatitis. J. Gastroenterol. Hepatol. 18 (1), 57–62. doi:10.1046/j.1440-1746.2003.02898.x
Wang, X., and Xiao, J. (2013). Effect of injection of neostigmine at zusanli combined with rhubarb on severe pancreatitis complicated with gastrointestinal paralysis. J. Youjiang Med. Coll. Natl. jiang min zu xue yuan] 35 (06), 793–795. doi:10.3969/j.issn.1001-5817.2013.06.022
Wang, Y. (2016). Effect of injection of neostigmine at zusanli on abdominal distension in severe acute pancreatitis. J. Chin. Pract. Med. guo shi yong yi yao] 2016 (7), 190–191. doi:10.14163/j.cnki.11-5547/r.2016.07.141
Wetterslev, J., Jakobsen, J. C., and Gluud, C. (2017). Trial Sequential Analysis in systematic reviews with meta-analysis. BMC Med. Res. Methodol. 17 (1), 39. doi:10.1186/s12874-017-0315-7
Keywords: acute pancreatitis, neostigmine, prokinetic drug, gastrointestinal motility, prognosis
Citation: He K, Wang Y, Li J, Bai X, Yang Z, Han X and Wu D (2023) Neostigmine for non-mild acute pancreatitis: A systematic review and meta-analysis of randomized controlled trials. Front. Pharmacol. 14:1131974. doi: 10.3389/fphar.2023.1131974
Received: 26 December 2022; Accepted: 13 February 2023;
Published: 28 February 2023.
Edited by:
Yong Gao, Guangzhou University of Chinese Medicine, ChinaReviewed by:
Wenhao Cai, University of Liverpool, United KingdomCopyright © 2023 He, Wang, Li, Bai, Yang, Han and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianlin Han, aGFueGlhbmxpbkBwdW1jaC5jbg==; Dong Wu, d3Vkb25nQHB1bWNoLmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.