- 1Department of Internal Korean Medicine, Kyung Hee University Hospital at Gangdong, Seoul, Republic of Korea
- 2Department of Clinical Korean Medicine, Graduate School of Kyung Hee University, Seoul, Republic of Korea
- 3Department of Gastroenterology, College of Korean Medicine, Kyung Hee University, Seoul, Republic of Korea
Background: The demand for complementary and alternative medicine for the management of functional dyspepsia (FD) is increasing due to the insufficient efficacy of conventional treatment options. In Asia, the Chinese herbal medicine formula Banxia-xiexin tang (BXT) has been used to treat FD.
Methods: We searched 11 digital medical databases on 1 September 2021. Randomized controlled trials (RCTs) that investigated the efficacy of BXT or combination therapy (BXT plus Western medicines) for FD were selected. The outcome parameters were total clinical efficacy rate (TCE), motilin level, symptom checklist-90-revised (SCL-90-R), and visual analog scale (VAS) for dyspepsia and adverse events. Cochrane risk of bias tool 2.0 (RoB 2) was used for the quality assessment of included studies.
Results: The meta-analysis comprised 57 RCTs with 5,525 participants. BXT was more efficacious, with a higher TCE than Western medicine. Combination therapy (BXT plus Western medicine) also resulted in a higher TCE than Western medicine. Combination therapy improved motilin levels and psychological symptoms to a greater extent than Western medicine, evidenced by a higher SCL-90-R score. However, no significant difference in VAS scores was observed between the BXT and placebo groups. BXT and combination therapy were associated with fewer adverse events than Western medicine or placebo.
Conclusion: Our findings suggest that BXT and its combination therapy may be an effective and safe alternative treatment for FD. More RCTs with better methodologies are required to strengthen this evidence.
Systematic Review Registration: [https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019123285], identifier [CRD42019123285].
1 Introduction
Functional dyspepsia (FD) is a common clinical disorder characterized by dyspeptic symptoms, such as early satiation, postprandial fullness, epigastric pain, or burning, that persist despite routine medical evaluations (Tack et al., 2006). The global prevalence of FD ranges from 11% to 29.12% (Mahadeva and Goh, 2006). A recent study reported that FD was the most common gastroduodenal disease, with pooled prevalence rates of 7.2% on the Internet and 4.8%, on a household survey (Sperber et al., 2020). The pathophysiology of FD is multifactorial and has not been completely explained (Stanghellini et al., 2016). Gastric motility, sensory disorders, mucosal permeability, low-grade immune activation, dysregulation of the gut-brain axis, and environmental exposure are all potential causative factors of FD (Vanheel and Farré, 2013). Helicobacter pylori (Helicobacter pylori) eradication, prokinetic (PK) agents, acid suppressants, and central neuromodulators are conventional treatments for FD (Moayyedi et al., 2017). However, an incomplete understanding of the pathophysiology of FD makes the treatment difficult (Ford et al., 2020). The need for complementary and alternative treatments, including herbal medicines, is growing because conventional treatments are less efficacious (Suzuki et al., 2009).
Banxia-xiexin tang (BXT; 半夏瀉心湯; Banha-sasim tang in traditional Korean medicine; Hange-shashin-to in Kampo medicine) is an herbal medicine formula that comprises 7 botanical drugs and originally recorded in the old Chinese literature “Shan han za bing lin (傷寒雜病論)”. It has been used in various versions by adding or subtracting botanical drugs. In Korea, BXT is produced in granules according to Korean Good Manufacturing Practice under the regulation of the Ministry of Food and Drug Safety. BXT (1/3 pack dose of the formula [貼] is as follows: Pinellia ternata (Thunb.) Makino [Araceae; Pinellia ternata rhizoma] 1.67g, Panax ginseng C.A.Mey. [Araliaceae; Panax ginseng root] 1.00g, Zingiber officinale Roscoe [Zingiberaceae; Zingiber officinale rhizoma] 0.83g, Coptis chinensis Franch. [Ranunculaceae; Coptis chinensis rhizoma] 0.33g, Scutellaria baicalensis Georgi [Lamiaceae; Scutellaria baicalensis root] 1.00g, Ziziphus jujuba Mill. [Rhamnaceae; Zizyphus jujuba fruit] 1.00 g) is extracted (0.91 g) in boiling water and mixed with lactose (0.52 g) and starch (1.57 g) then given 3 g of granules (Park et al., 2010). In traditional Chinese medicine, this formula has been administered to treat “epigastric stuffiness,” a symptom of FD (Park et al., 2010). In addition to FD, BXT has been administered to treat a wide range of gastrointestinal (GI) diseases, including gastroesophageal reflux disease (GERD), acute gastroenteritis, chronic gastritis, peptic ulcers, and ulcerative colitis (Ji et al., 2017). A systematic review reported that modified BXT is an efficacious treatment option for GERD (Dai et al., 2017), and another meta-analysis reported that BXT is more efficacious than Western medicine for treating diabetic gastroparesis (Tian et al., 2013). BXT and combination therapy (BXT plus Western medicine) had a better effect on ulcerative colitis, according to another meta-analysis (Zhu et al., 2016). One systematic review reported that combination therapy (BXT plus Western medicine) was more efficacious than Western medicine alone, in treating peptic ulcers (Chen et al., 2014).
Systematic reviews that investigated the effects of BXT on FD have been published earlier. However, some studies only compared BXT and Western medicine and did not investigate the effects of combination therapy (BXT plus Western medicine) (Gan et al., 2014; Zhang, 2015; Li and Li, 2016; Hu et al., 2020). One review was based on a Chinese database (Li and Li, 2016). In another meta-analysis, it was difficult to focus on the effects of BXT because of the high heterogeneity of the experimental group (Zhang, 2015). Consequently, limited evidence supports BXT as an efficacious treatment option for FD. Therefore, this review aimed to systematically investigate the efficacy and safety of BXT and combination therapy (BXT plus Western medicine) in the treatment of FD.
2 Methods
2.1 Protocol and registration
The study protocol was registered in the International Prospective Register of Systematic Reviews. The registration number is CRD42019123285 (Ko et al., 2019). This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines (Liberati et al., 2009).
2.2 Inclusion and exclusion criteria
2.2.1 Types of studies
This systematic review included randomized controlled trials (RCTs) and quasi-RCTs.
2.2.2 Types of participants
This systemic review included patients diagnosed with FD according to the ROME criteria. No limitations were observed on the participant’s age, sex, or ethnicity. The ROME criteria were used as the diagnostic criteria for screening functional GI disorders (FGID). The ROME IV criteria were finalized in 2016, after being announced in 1992 and after several revisions. The inclusions of studies published before adopting the ROME I criteria in 1992 were decided by the consensus of two reviewers (KK and SC), who assessed whether the diagnostic criteria were compatible with the ROME I criteria. Patients with dyspepsia caused by drugs or secondary pathologies (e.g., GERD and irritable bowel syndrome) were excluded.
2.2.3 Types of interventions
This systematic review included studies on BXT, modified BXT, and combination therapy (BXT and Western medicine). Modified BXT is BXT with additional medicinal botanical drugs, for example, Chaizhi BXT (CZBXT; 柴枳半夏瀉心湯; BXT added to Bupleurum falcatum L. [Apiaceae; Bupleurum falcatum root] and Citrus trifoliata L. [Rutaceae; Citrus trifoliata immature fruit]. We ruled out modified BXT if the botanical drugs added to BXT resulted in another herbal medicine formula. Combinations of BXT and other treatments in complementary and alternative medicine, such as acupuncture and moxibustion, were excluded. The following medication groups were compared in this study: a) the BXT and modified BXT groups with Western medicine (such as PK agents and proton pump inhibitors [PPIs]); b) the BXT group with placebo (same taste, shape, color, and odor as BXT) group; and c) combination therapy (BXT plus Western medicine) group with Western medicine group.
2.2.4 Types of outcome measures
The total clinical efficacy rate (TCE) was the primary outcome. TCE is the percentage of patients who responded to treatment (Tang, 2015; Zou, 2015). The patients’ improvements to the interventions were graded into three or four levels after treatment, and TCE was calculated as the total number of improved patients. TCE with three levels includes “cured” or “excellently improved,” “improved,” and “not improved,” while TCE with four levels includes “cured,” “excellently improved,” “improved,” and “not improved.” Although the number of evaluation levels differs, it is the same standard in that the ratio of numbers excluding ‘not improved’ from the total is calculated.
The secondary outcomes included motilin levels, symptom checklist-90-revised (SCL-90-R) score, visual analog scale (VAS) pain scores, and adverse events. Motilin, a GI hormone, induces GI motor activity (Naito et al., 2002), and increased motilin secretion might improve GI mobility. The SCL-90-R is a self-rating scale used to evaluate psychological symptoms, and a higher SCL-90-R score indicates greater psychiatric distress (Faramarzi et al., 2014). The VAS was used to quantify the degree of indigestion symptoms felt by the patient, and the number of side effects was compared.
2.3 Search strategy
A literature search was conducted using Medline (via PubMed), Cochrane Central Register of Controlled Trials, EMBASE, and Allied and Complementary Medicine Databases on 1 September 2021. Medical databases in Korea, including the National Digital Science Library, Korean Medical Database, Korean Studies Information Service System, KoreaMed, and Oriental Medicine Advanced Searching Integrated System, were also searched. Additionally, other Asian databases, including China National Knowledge Infrastructure Database in Chinese and Citation Information by Nii in Japanese, were searched.
The search terms were composed of disease- and intervention-related terms. Disease-related terms included “indigestion,” “dyspepsia,” “discomfort,” “disturbance,” “pain,” “dysfunction,” “intestine,” “stomach,” and “gut.” Terms such as “Banha sasim,” “Banxia xiexin,” “Hange shashin,” “herbal medicine,” and “botanical” were used as intervention-related terms. Language and publication dates were not restricted.
2.4 Selection and data extraction
Two authors (KK and SC) independently screened the studies to evaluate their eligibility for inclusion. The selected paper’s titles, abstracts, and full texts were screened. Endnote X9 (Clarivate Analytics, Philadelphia, PA, United States) was used to manage the search results. Furthermore, these authors independently extracted data from the studies and filled out a standard data extraction form. The form included information on the studies, such as the first authors, titles, publication years, journals, research design, interventions, sample sizes, treatment period, and outcomes. Disagreements between the two reviewers (KK and SC) were resolved through discussion. If the two reviewers could not reach an agreement, an arbiter (SK) intervened and resolved the discrepancies.
2.5 Quality assessment
Two reviewers (KK and SC) independently evaluated the risk of bias (RoB) using the Cochrane RoB tool 2.0 with the following items: a) bias arising from the randomization process, b) bias due to deviations from intended interventions, c) bias due to missing outcome data, d) bias in the measurement of the outcome, e) bias in the selection of the reported result, and f) overall bias. The assessment results were divided into three categories: low, high, and some concerns. All disagreements between the two evaluators (KK and SC) were discussed. When needed, the arbiter (SK) intervened and resolved the disagreement.
2.6 Data analysis and synthesis
Review Manager (V5.3 Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2014) was used for data analysis. Dichotomous data were assessed for relative risk (RR) with a 95% confidence interval (CI), and continuous data were evaluated using the mean difference (MD) with 95% CI. A random-effects model was used for meta-analysis. TCE, the only ordinal scale in this study, was treated as a dichotomous scale by dividing it into “not improved” and “improved.” To judge the heterogeneity of the selected studies, we used the chi-squared (χ2) test and I-squared statistics (I2). A p-value < 0.1 and I2 ≥ 50 indicated substantial heterogeneity. Subgroup analyses were performed. The subgroups were formed based on the type of Western medicine administered. A funnel plot was used to present small-study effects or publication bias.
2.7 Level of evidence
The level of evidence was examined using the Grading of Recommendations, Assessment, Development, and Evaluation approach. The level of evidence was classified as high, moderate, low, or very low. The evaluation was performed for domains such as the RoB, imprecision, inconsistency, and indirectness.
3 Results
3.1 Selection of study
The initial search identified 504 studies, of which 83 duplicates were excluded. After screening the titles and abstracts, 340 studies were excluded. Fifteen studies were excluded because 14 did not meet the inclusion criteria, and one presented inaccurate data. Finally, 66 studies were included, and 57 were selected for the meta-analysis (Figure 1).
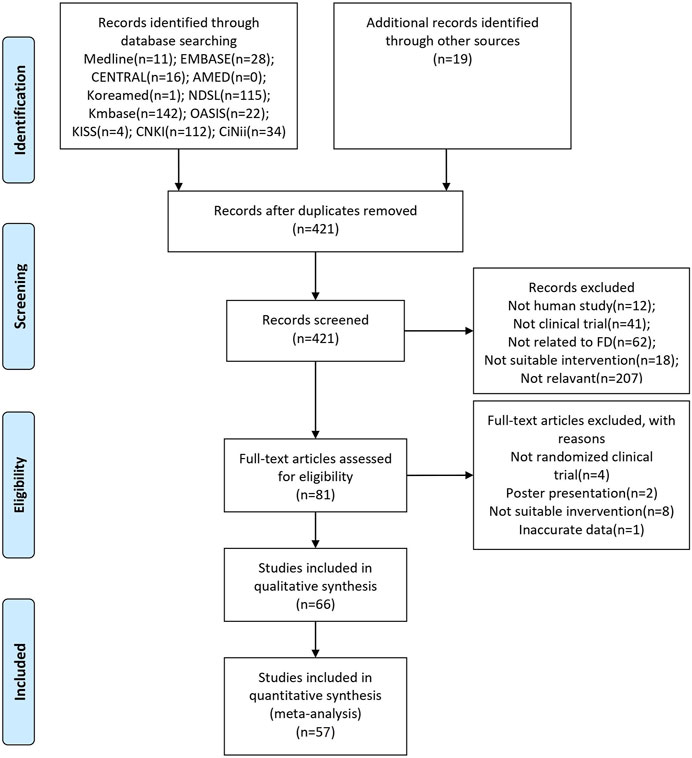
FIGURE 1. PRISMA flow chart of the selection process. EMBASE: Excerpta Medica Database; CENTRAL: Cochrane Central Register of Controlled Trials; AMED: Allied and Complementary Medicine Database; NDSL: National Digital Science Library; Kmbase: Korean Medical Database; OASIS: Oriental Medicine Advanced Searching Integrated System; KISS: Korean Studies Information Service System; CNKI: China National Knowledge Infrastructure Database; CiNii: Citation Information by Nii; FD: Functional dyspepsia.
3.2 Characteristics of included studies
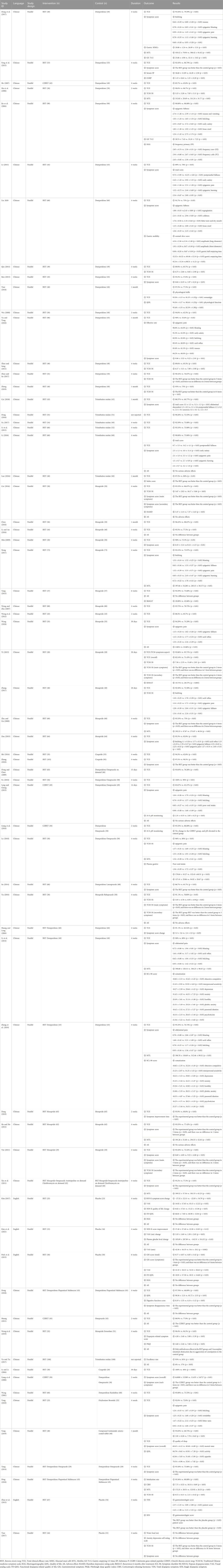
We included 66 studies published between 2001 and 2021. All articles were RCTs and parallel-design studies. Four studies were written in English and 62 in Chinese. Three randomized trials were conducted in Korea and 63 in China. BXT was compared with Western medicine in 51 RCTs. In 10 articles, combination therapy (BXT plus Western medicine), and Western medicine were compared. BXT was also compared with a placebo in five RCTs. The total number of patients included in the meta-analysis was 5,615. In each study, 31–200 patients were included. Table 1 shows a summary of the analyzed RCTs.
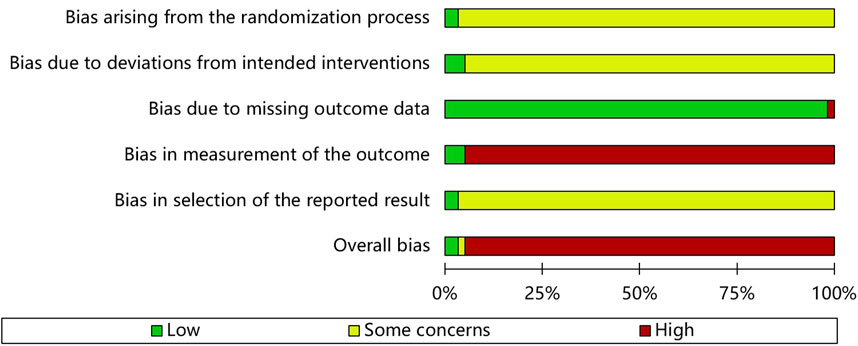
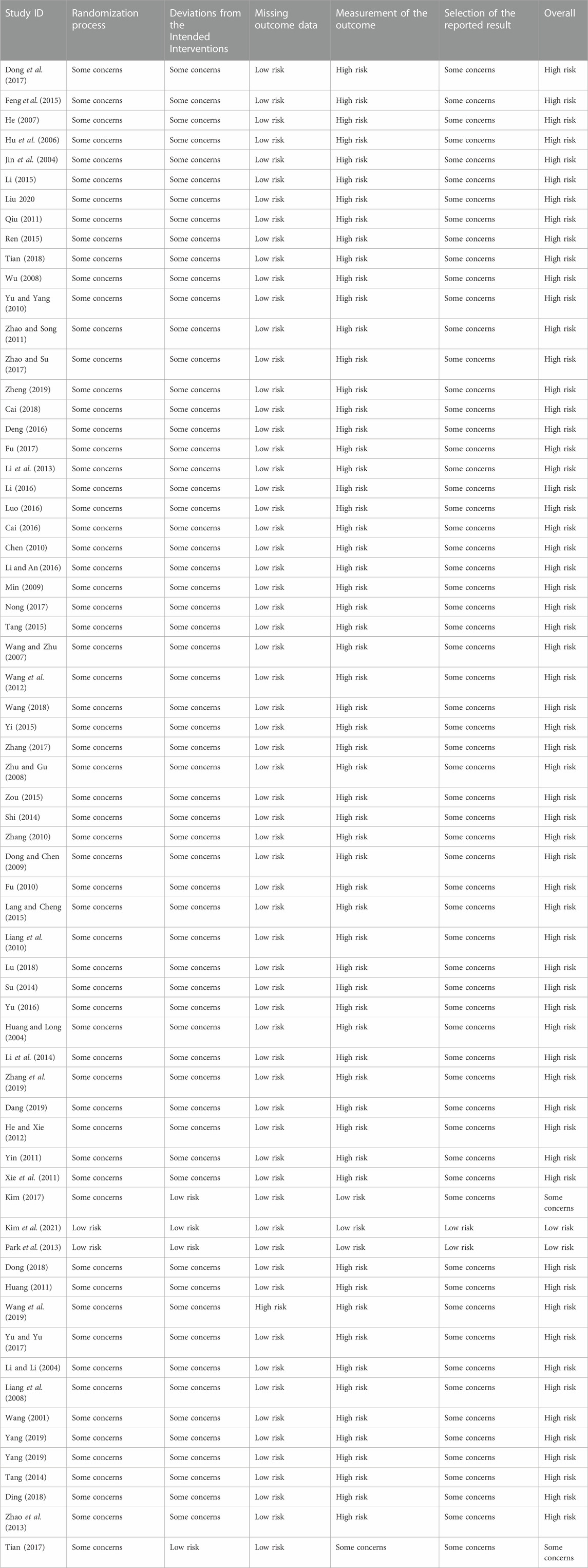
3.3 Assessment of risk of bias
Figure 2 shows the quality assessment results of the 57 studies selected for this meta-analysis using the RoB 2 tool. Table 2 shows the ratings for the individual domains at the study level for the 66 included studies.
3.3.1 Bias arising from the randomization process
Concerns were raised in 55 of the RCTs. These randomization studies did not report the sequence of allocation concealment. Two studies (Park et al., 2013; Kim et al., 2021) used a center-controlled method to randomize participants and were assessed to have a low RoB.
3.3.2 Bias due to deviations from intended interventions
Three trials (Park et al., 2013; Kim, 2017; Kim et al., 2021) had low RoB because the participants and personnel were blinded to the placebo. Fifty-five studies had high RoB because participants and those delivering the interventions were aware of their assigned interventions. Deviations from the intended intervention occurred in these studies because of the trial context and group balance, which probably affected the outcome.
3.3.3 Bias due to missing outcome data
(Wang et al., 2019) conducted a per-protocol analysis and had a high RoB. However, the other studies had a low RoB. Fifty-four trials did not have missing patients (Park et al., 2013). conducted an intention-to-treat analysis, while (Kim, 2017) had one missing patient before administering the test drug.
3.3.4 Bias in measurement of the outcome
While three RCTs (Park et al., 2013; Kim, 2017; Kim et al., 2021) blinded outcome assessment, 54 did not report blinding of statistical analyses, and the outcome assessment was probably influenced by knowledge of the intervention received.
3.3.5 Bias in selection of the reported result
Two RCTs (Park et al., 2013; Kim et al., 2021) with published study protocols had a low RoB. The remaining 55 studies were biased due to insufficient information.
3.3.6 Overall bias
Of the 57 studies included in this meta-analysis, two had a low risk, one had a moderate risk, and 54 had a high overall RoB.
3.4 Primary outcome: total clinical efficacy rate
This systematic review examined the efficacy of BXT and combination therapy (BXT plus Western medicine) in treating FD using TCE.
3.4.1 BXT versus western medicine
Western medicine groups were subdivided into PK agent groups, such as domperidone, trimebutine maleate, mosapride, and cisapride, as well as combinations of the PK and PPI groups. BXT was more efficacious than domperidone (RR: 1.19; 95% CI:1.11–1.21; p < 0.001), trimebutine maleate (RR: 1.24; 95% CI: 1.15–1.33; p < 0.001), mosapride (RR: 1.18; 95% CI: 1.13 to 1.24; p < 0.001), and combinations of PK and PPI (RR: 1.20; 95% CI: 1.12–1.29; p < 0.001). Heterogeneity was not significant in the trimebutine maleate (p = 0.99; I2 = 0%), mosapride (p = 0.89; I2 = 0%), or the combination of the PK and PPI groups (p = 0.31; I2 = 16%).
In the integrated analysis of the five groups, 43 RCTs with 4,183 patients were included. They showed that BXT was more efficacious than Western medicine against FD (RR: 1.19; 95% CI: 1.15–1.23; p < 0.001) and had moderate heterogeneity (p = 0.02; I2 = 33%) (Figure 3).
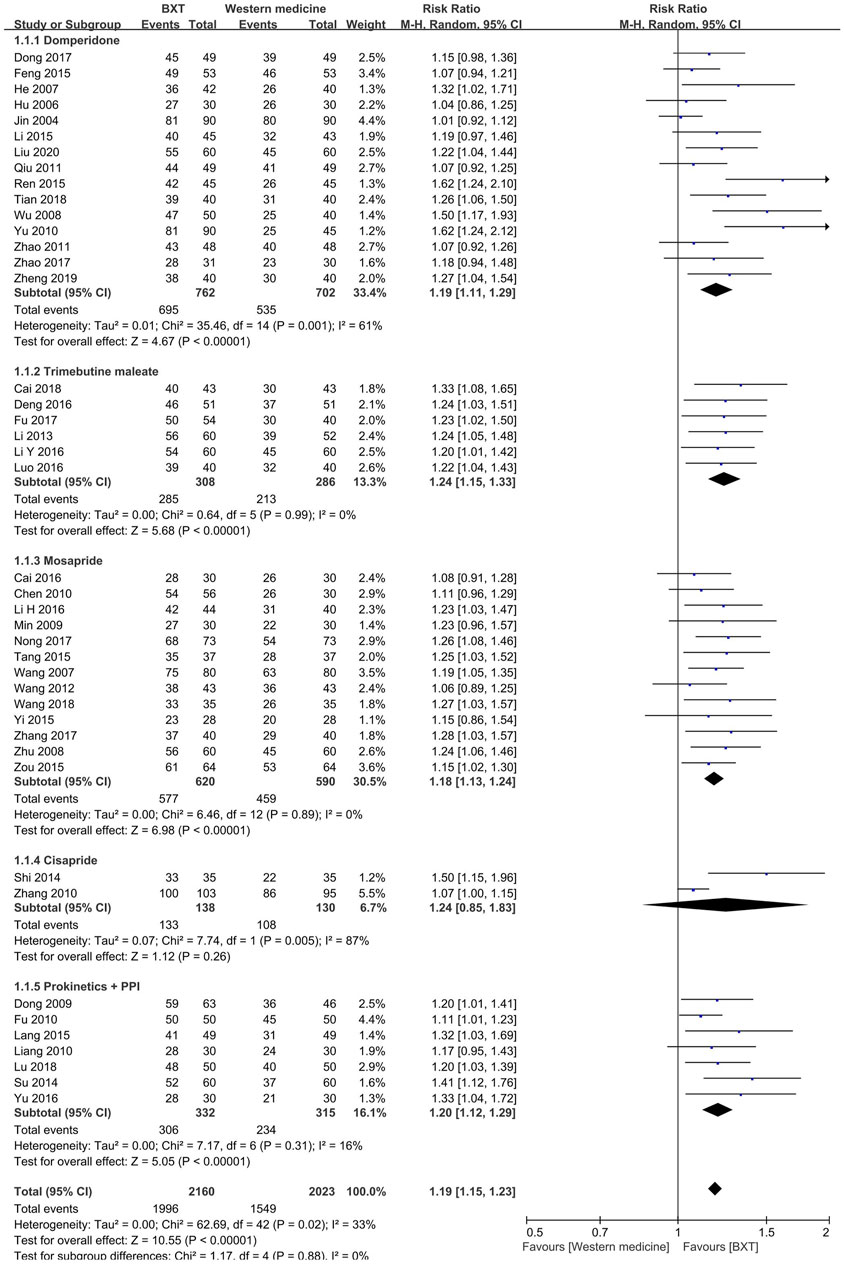
FIGURE 3. Forest plot of TCE between BXT and Western medicine. BXT: Banxia-xiexin tang; CI: confidence interval; TCE: total clinical efficacy rate.
3.4.2. Combination of BXT and western medicine versus western medicine
The Western medicine group was divided into two subgroups: domperidone and mosapride. The combination of BXT and Western medicine was more efficacious than domperidone (RR: 1.16; 95% CI: 1.06–1.27; p = 0.002) and mosapride (RR: 1.21; 95% CI: 1.10–1.33; p < 0.001). Heterogeneity was insignificant in either subgroup (p = 0.66, I2 = 0%; p = 0.79, I2 = 0%).
Six studies with 601 patients were analyzed by integrating the subgroups. Combination therapy (BXT plus Western medicine) was more efficacious (RR: 1.18; 95% CI: 1.11–1.26; p < 0.001) and had lower heterogeneity (p = 0.88; I2 = 0%) than Western medicine (Figure 4).
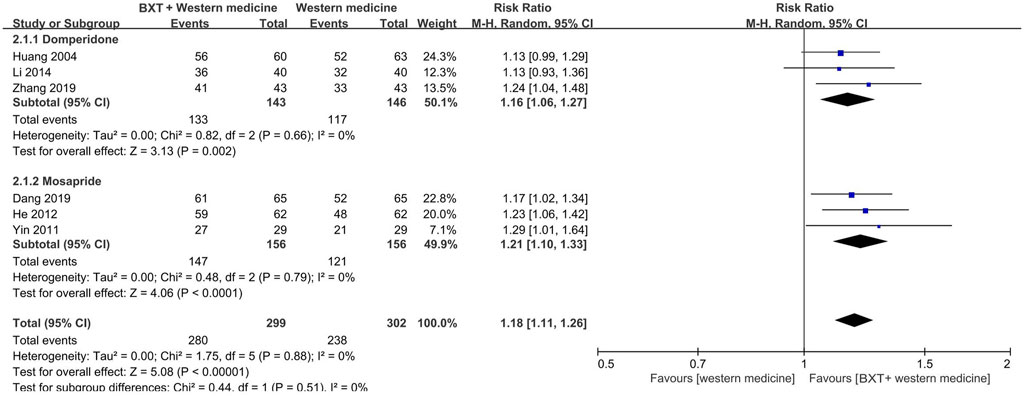
FIGURE 4. Forest plot of TCE between combination therapy and Western medicine alone. BXT: Banxia-xiexin tang; CI: confidence interval; TCE: total clinical efficacy rate.
3.5 Motilin
The meta-analysis included only plasma motilin levels (pg/ml) measured using radio-immunoassays before meals. Analysis of three RCTs with 286 participants showed that combination therapy (BXT plus Western medication) was more efficacious than Western medicine alone in boosting motilin secretion (MD: 96.89; 95% CI: 82.49–111.30; p < 0.001). Western medicine groups included domperidone (Li et al., 2014), mosapride (He and Xie, 2012), and a combination of mosapride, omeprazole, amitriptyline (on demand), and clarithromycin (on demand) (Xie et al., 2011). Heterogeneity was high (p < 0.001; I2 = 0%) (Figure 5).

FIGURE 5. Forest plot of variation in motilin levels between combination therapy and Western medicine alone. BXT: Banxia-xiexin tang; SD: standard deviation; IV: inverse-variance; CI: confidence interval.
3.6 Symptom checklist-90-revised
Two studies (Li et al., 2014; Zhang et al., 2019) compared combination therapy (BXT plus domperidone) with domperidone alone and used the SCL-90-R to evaluate psychological symptoms. These studies included 166 patients. The SCL-90-R has nine subscales. Combination therapy (BXT plus domperidone) was more efficacious than domperidone alone in all aspects of the SCL-90-R.
The somatization score was significantly lower in the combination therapy group (BXT plus domperidone) than in the domperidone group (MD: –13.51; 95% CI: [–14.52]—[–12.49]; p < 0.001). Obsessive-compulsive symptoms improved remarkably in the combination therapy group (BXT plus domperidone) than in the domperidone group (MD: –10.63; 95% CI: [–12.05]- [–9.20]; p < 0.001), and interpersonal sensitivity was found to be more significantly improved in the combination therapy group than in the domperidone group (MD: –11.08; 95% CI: [–12.16] -[–10.01]; p < 0.001). Depression scores were significantly lower in the combination therapy group (BXT plus domperidone) than in the domperidone group (MD: –13.00; 95% CI: [–14.39]—[–11.60]; p < 0.001). Combination therapy (BXT plus domperidone) was found to be remarkably more efficacious against anxiety than domperidone (MD: –14.14; 95% CI: [–21.01]—[ −7.27]; p < 0.001) and reduced hostile symptoms more significantly than domperidone (MD: –7.46; 95% CI: [–8.47]—[–6.45]; p < 0.001). Combination therapy (BXT plus domperidone) was more efficacious in reducing phobic anxiety than domperidone (MD: –8.97; 95% CI [–10.00] - [–7.94]; p < 0.001). The paranoid ideation score was significantly lower in the combination therapy group (BXT plus domperidone) than in the domperidone group (MD: –12.19; 95% CI: [–13.25]—[–11.14]; p < 0.001). Combination therapy (BXT plus domperidone) was more efficacious in reducing the psychoticism score than domperidone (MD: –13.09; 95% CI [–14.42] - [–11.77]; p < 0.001) (Figure 6).

FIGURE 6. Forest plot of SCL-90-R between combination therapy and Western medicine alone. BXT: Banxia-xiexin tang; SD: standard deviation; IV: inverse-variance; CI: confidence interval.
3.7 Visual analog scale
Three RCTs (Park et al., 2013; Kim, 2017; Kim et al., 2021) with 179 participants compared the BXT and placebo groups using the VAS. No significant difference was observed between the BXT and placebo groups (MD: –4.31; 95% CI: [–17.40]—[–8.77]; p = 0.52). Heterogeneity was high (p = 0.01; I2 = 78%) (Figure 7).
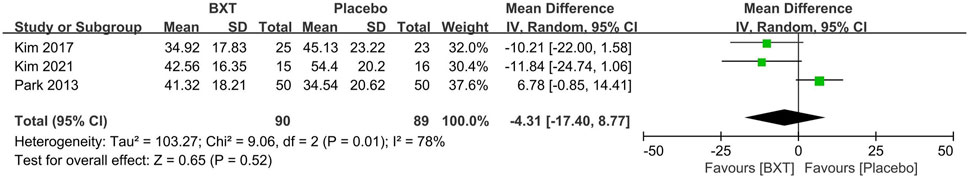
FIGURE 7. Forest plot of VAS between BXT and placebo. BXT: Banxia-xiexin tang; SD: standard deviation; IV: inverse-variance; CI: confidence interval.
3.8 Subgroup analysis: Chaizhi-BXT versus western medicine
In this meta-analysis, the modified BXT group was integrated with the BXT group. A subgroup analysis was performed to minimize heterogeneity in the experimental groups. Three RCTs (He, 2007; Liang et al., 2010; Lang and Chen, 2015) with 240 participants showed that Chaizhi-BXT had a significantly higher TCE than Western medicine (RR: 1.25; 95% CI: 1.09–1.43; p = 0.001) (Figure 8).
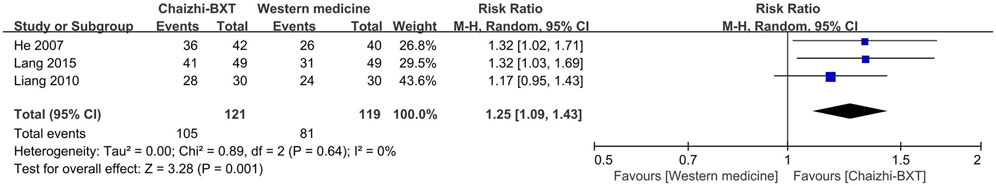
FIGURE 8. Forest plot of TCE between Chaizhi-BXT and Western medicine. BXT: Banxia-xiexin tang; CI: confidence interval; TCE: total clinical efficacy rate.
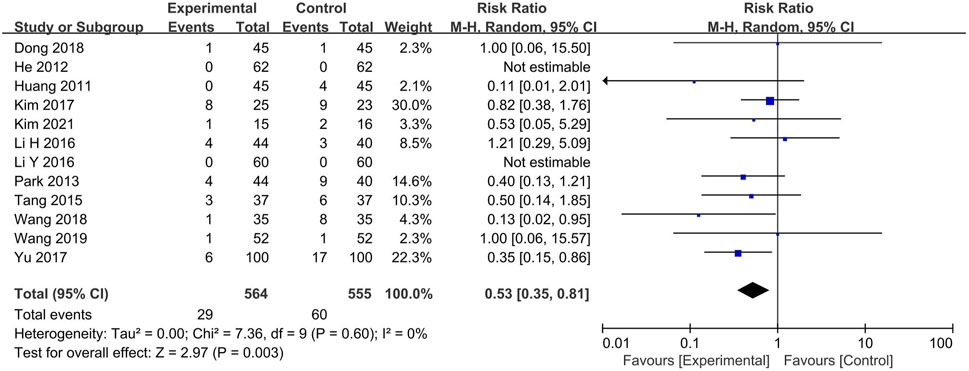
3.9 Adverse events
Of the 66 studies, 54 did not report any adverse events. The experimental group included BXT and Western medicine, and the control group included Western medicine and placebo. Two articles (He and Xie, 2012; Li, 2016) reported no adverse events, and one (Tang, 2015) reported the number of occurrences alone. The remaining nine RCTs reported mild adverse events. The number of adverse events was significantly lower in the experimental group than in the control group (RR: 0.53; 95% CI: 0.35–0.81; p = 0.003) (Figure 9).
Relatively mild adverse events such as nausea, vomiting, diarrhea, abdominal pain, acid reflux, epigastric fullness, dizziness, headache, urticaria, and insomnia were reported in the experimental groups. Adverse events in the control group were similar to those in the experimental groups and were mostly mild. However (Wang et al., 2019), reported intestinal obstruction (n = 1) due to progressive aggravation of constipation in the Western medicine (mosapride and estazolam) group.
3.10 Publication bias
Figure 10 shows a funnel plot of TCE comparing BXT and Western medicine. It is possible that smaller studies with lower estimates of benefits have not been published. However, the asymmetry is difficult to equate with publication bias because most of the included RCTs were published in Chinese, and the overall methodological quality of the studies was low.
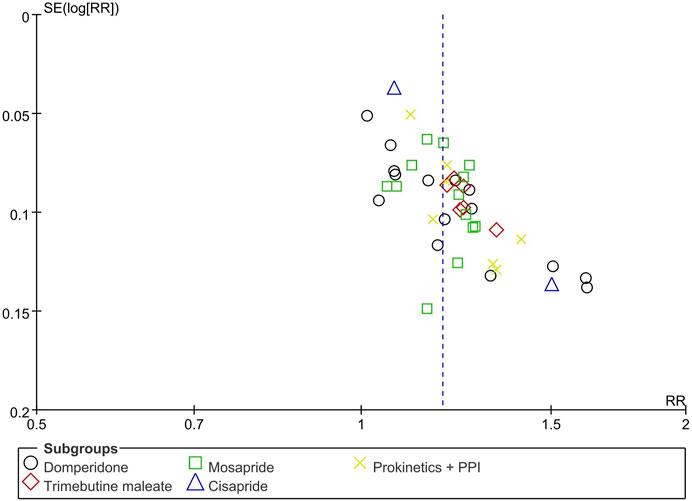
FIGURE 10. Funnel plot of TCE between BXT and Western medicine. BXT: Banxia-xiexin tang; RR: relative risk; TCE: total clinical efficacy rate.
3.11 Level of evidence
Table 3 shows the level of evidence for each outcome. Regarding TCE, BXT was more efficacious than Western medicine against FD. Because of the high RoB, the level of evidence was moderate. In the subgroup analysis, the level of evidence for BXT was lower than that of domperidone, considering the unexplained heterogeneity. The level of evidence for BXT was lower than that of cisapride due to unexplained heterogeneity and the small sample size. Combination therapy (BXT plus Western medicine) was more efficacious than Western medicine against FD. A high RoB led to a moderate level of evidence. In the subgroup analysis, the level of evidence was low because of the small sample size. Combination therapy (BXT plus Western medicine) was more efficacious than Western medicine alone in boosting motilin secretion. Because of the high RoB and the small sample size, the level of evidence was low. Combination therapy (BXT plus domperidone) had significantly better efficacy than domperidone in all aspects of the SCL-90-R. The level of evidence was low because of the high RoB and small sample size. The VAS scores showed no significant differences between the BXT and placebo groups. The RoB was low in the VAS; however, the small sample size led to a moderate level of evidence. The number of adverse events was significantly lower in the experimental group than in the control group. The level of evidence was moderate because of the high RoB.
4 Discussion
4.1 Summary of the main findings
This systematic review investigated the efficacy and safety of BXT and combination therapy (BXT plus Western medicine) against FD. The TCE results showed that BXT and combination therapy (BXT plus Western medicine) had a stronger therapeutic effect on FD than Western medicine alone. The motilin assays results showed that the combination therapy (BXT plus Western medicine) had more clinical benefits than Western medicine alone. Combination therapy (BXT plus domperidone) was more efficacious than domperidone alone in SCL-90-R. In the subgroup analysis, the Chaizhi-BXT group was more efficacious than the Western medicine group based on TCE. No significant difference was observed between the BXT and placebo groups regarding the VAS scores. None of the included RCTs reported severe adverse events in the BXT and combination therapy (BXT plus Western medicine) groups. The incidence of adverse reactions was lower in the BXT and combination therapy (BXT plus Western medicine) groups than in the Western medicine and placebo groups.
4.2 Comparison with previous reviews
Several previous studies have reported the efficacy and safety of BXT for treating FD. One meta-analysis, including 10 RCTs, reported that BXT was more efficacious than Western medicines (domperidone, mosapride, and a combination of domperidone and omeprazole) against FD. No adverse events were reported in the BXT group. However, side effects, such as GI symptoms and headaches, occurred in the control group (Gan et al., 2014). Another systematic review involving 30 studies from Chinese databases compared BXT with Western medicines, including PK agents (domperidone, mosapride, trimebutine, and cisapride), PPIs (omeprazole and lansoprazole), and H2 receptor antagonists (famotidine). The review reported that BXT was better than Western medicine in terms of TCE, recovery rate, and symptom improvement. However, there were no significant differences between the groups when plasma motilin levels and gastric dynamics (Li and Li, 2016) were considered. A systematic review of 20 trials indicated that Chinese herbal medicines, including modified BXT, were more efficacious than conventional Western medicines against FD and did not cause side effects (Zhang, 2015). A systematic review of 28 RCTs reported that BXT was more efficacious than Western medicine in increasing the TCE, reducing several symptoms and recurrence rates. However, BXT was less efficacious than Western medicine in reducing symptoms, such as early satiation and plasma motilin levels (Hu et al., 2020).
4.3 Components of BXT
BXT is a complex of seven botanical drugs: Pinellia ternata (Thunb.) Makino [Araceae; Pinellia ternata rhizoma], Panax ginseng C.A.Mey. [Araliaceae; Panax ginseng root], Zingiber officinale Roscoe [Zingiberaceae; Zingiber officinale rhizoma], Coptis chinensis Franch. [Ranunculaceae; Coptis chinensis rhizoma], Scutellaria baicalensis Georgi [Lamiaceae; Scutellaria baicalensis root], Glycyrrhiza uralensis Fisch. ex DC. [Fabaceae; Glycyrrhiza uralensis root], Ziziphus jujuba Mill. [Rhamnaceae; Zizyphus jujuba fruit] (Park et al., 2010). The pharmacological effects of Pinellia ternata (Thunb.) Makino [Araceae; Pinellia ternata rhizoma], such as anti-vomiting, anti-coughing, antidepressant, anti-inflammatory, and sedative-hypnotic activities, have been demonstrated in modern pharmacological studies (Mao and He, 2020). Scutellaria baicalensis Georgi [Lamiaceae; Scutellaria baicalensis root] contains several flavones, including baicalin, baicalein, wogonin, and wogonoside, and is known to have anti-inflammatory, anti-tumor, and anticonvulsant effects (Li and Zuo, 2011). Zingiber officinale Roscoe [Zingiberaceae; Zingiber officinale rhizoma], the root of ginger, has anti-emetic, anti-diarrheal, anti-oxidative, anti-inflammatory, anti-tumor, and anti-lipidemic effects (Chrubasik et al., 2005). The therapeutic effects of BXT against FD may be attributed to the efficacy of each BXT component. Combinations of various active ingredients may have advantages over other treatment options against FD, considering its heterogeneous pathophysiology and symptoms (Rösch et al., 2006). Additionally, interactions between multiple bioactive components in the herbal medicine formula can create synergistic effects, and further studies are required to reveal these interactions (Zhou et al., 2016).
4.4 Implication for the clinical practice
The pathophysiology of FD is unknown; however, the deterioration of gastric motility is considered one of the leading causes of FD symptoms (Vanheel and Farré, 2013). GI hormones, such as gastrin, somatostatin, and motilin, regulate secretion, digestion, absorption, appetite, and GI motility (Ahmed and Ahmed, 2019). Motilin stimulates antral contractions and improves gastric emptying (Van den Houte et al., 2020). This systematic review showed that BXT increases motilin secretion. In a previous pharmacological study, BXT enhanced somatostatin- and motilin-immunoreactive substances levels in human plasma (Naito et al., 2002). These results suggest that BXT may affect FD by normalizing gastric motility. However, further studies using sensitive measurements of gastric emptying, such as scintigraphy, isotope respiration tests, ultrasonography, and magnetic resonance imaging, are required to investigate the association between BXT and gastric motility.
The brain-gut axis plays an essential role in the pathophysiology of FD (Wauters et al., 2020). FD is associated with central modulation (brain-to-gut) and visceral sensory signaling (gut-to-brain). Bidirectional pathways are likely regulated by psychological influences and stress responses of the hypothalamic-pituitary-adrenal (HPA) axis (Van Oudenhove and Aziz, 2013; Wauters et al., 2020). Several epidemiological studies have reported a higher prevalence of psychiatric disorders in patients with FD than in healthy individuals (Van Oudenhove and Aziz, 2013). In some patients, mood or anxiety disorders precede the occurrence of FGID (Jones et al., 2017), and FD symptoms can induce anxiety and depression. A previous prospective study reported that anxiety was an independent predictor of FGID (Koloski et al., 2012).
The SCL-90-R is a self-rating clinical symptom scale of 90 questions associated with nine psychiatric subsections. In a previous study, the SCL-90-R was used to evaluate psychiatric distress in patients with FD, and the FD group had significantly higher scores than the healthy control group in all subsections (Faramarzi et al., 2014). In this systematic review, combination therapy (BXT plus domperidone) was better than domperidone alone in improving the SCL-90-R scores in patients with FD. The therapeutic effects of BXT on dyspeptic symptoms may relieve stress in patients and decrease their SCL-90-R scores. However, BXT exerts regulatory effects on the HPA axis by regulating the plasma cortisol and adrenocorticotropic hormone levels under stressful conditions (Naito et al., 2003).
Furthermore, a recent network pharmacological study reported that BXT could influence the process of depression by modulating the 5-hydroxytryptamine synaptic signaling pathway, arachidonic acid metabolism, and hypoxia-inducible factor-1 signaling pathway (Yu et al., 2020). One RCT suggested that combination therapy with BXT and antidepressants (paroxetine) had a more rapid effect than antidepressants (paroxetine) alone in female patients with somatoform disorders (Sun and Li, 2014). In another trial, combination therapy (BXT, flupentixol, and melitracen) was found to be more efficacious than Western medicines (flupentixol and melitracen) in reducing anxiety and depression, as well as improving the quality of life of perimenopausal depressed patients (Chen, 2019). BXT improved the psychological state of the patients and provided symptomatic relief. However, human data supporting the brain-gut axis are limited (Wauters et al., 2020). Further studies are required to investigate the interaction between the gut and brain in humans and the effects of BXT on bidirectional pathways.
4.5 Strengths of this study
This study has several strengths. First, it included the latest results on the effects of BXT against FD. Although a previous meta-analysis published in 2016 used Chinese databases alone (Li and Li, 2016), we searched global databases and did not apply language restrictions. Second, the superior effect of combination therapy (BXT plus Western medicine) against FD was indicated in this systematic review. Previous studies have compared the effect of BXT versus Western medicine or placebo; however, this analysis examined the synergistic effect of BXT and Western medicine on FD. Conventional medications are less efficacious in managing the symptoms of FD, and the demand for complementary medicine, such as herbal medicine, is increasing (Suzuki et al., 2009). In some patients, herbal and conventional Western medicines are being co-administered. Therefore, it is important to investigate the efficacy and safety of combination therapy (herbal medicine plus conventional Western medicine) for treating FD. Third, a subgroup analysis was performed for both the BXT and control groups. Chaizhi-BXT was found to be more efficacious than Western medicine. The Western medicine group was further subcategorized into domperidone, trimebutine maleate, mosapride, cisapride, and a combination of PK and PPI groups. Although cisapride was withdrawn from the global market because of its severe side effects on the cardiovascular system, it was included in the subgroup analysis to focus on the therapeutic effects of BXT against FD. Fourth, this review suggests that BXT could treat FD in addition to conventional Western medicine.
The American College of Gastroenterology and Canadian Association of Gastroenterology guidelines on dyspepsia recommend H. pylori eradication, if positive, as the first line of treatment. As secondary strategies, PPI, tricyclic antidepressants, and PK agents have been recommended for treating FD (Moayyedi et al., 2017). The Korean clinical guidelines strongly recommend PK agents and PPIs for treating FD. As a first-line treatment, PK agents for patients with postprandial distress syndrome (PDS) and PPI for patients with epigastric pain syndrome (EPS) have been suggested (Oh et al., 2020). PK agents constitute a significant part of the Western medicine used in this study. BXT and combination therapy (BXT plus Western medicine) were more efficacious than Western medicine alone. Furthermore, the psychological effects of BXT may benefit patients with FD.
4.6 Limitations and implications for further research
This systematic review has some limitations. First, the general methodological quality of the included RCTs was low because of moderate or high RoB in the randomization process, deviations from intended interventions, and measurement of the outcome domains. Most studies did not have a pre-published study protocol or sufficient information to assess the risk of reporting bias. Double-blinding was performed in five of the 66 studies, which resulted in poor methodological quality. Second, all the included studies were published in Asia, and most of them were from China. Third, clinical heterogeneity may exist among the intervention groups. Modified BXTs, which varied by species and the number of added botanical drugs, were included in the meta-analysis. High heterogeneity was observed in VAS scores among the outcomes. It is difficult to resolve the heterogeneity because of the small sample size; therefore, it is necessary to include more participants in future studies. In addition, the effects of different doses of BXT could not be compared due to the heterogeneity of constituent herbs and their doses. Fourth, in the included RCTs, there is lack of description of extraction procedure and quality control of the botanical drugs. Finally, pattern identification was not considered in the meta-analysis. In traditional Chinese medicine, FD can be differentiated into several patterns based on clinical signs and symptoms. BXT has often been regarded as an herbal formula treating “Mixed cold and heat” FD patterns (Ji et al., 2017). However, it might have different effects on other FD patterns. Consequently, well-designed RCTs with clear randomization and double-blinding are required.
5 Conclusion
BXT and combination therapy (BXT plus Western medicines) may have therapeutic effects against FD. BXT can be considered a treatment option for FD with fewer adverse events. However, the methodological quality of the included studies was low; hence, the validity of the evidence obtained is controversial. More robust, large-scale, high-quality RCTs are required for more credible evidence.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
Conceptualization: KK and S-JK. Data curation: KK, S-JK, SC, JK, and J-WP. Formal analysis: KK and SC. Investigation: S-JK and SC. Methodology: S-JK and SC. Resources: S-JK and J-WP. Writing—original draft: KK. Writing—review and editing: S-JK. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2022R1C1C1004937) to S-JK, and by the Bio and Medical Technology Development Program of the NRF funded by the MSIT (No. 2022M3A9E4082682) to J-WP.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1130257/full#supplementary-material
References
Ahmed, M., and Ahmed, S. (2019). Functional, diagnostic and therapeutic aspects of gastrointestinal hormones. Gastroenterol. Res. 12, 233–244. doi:10.14740/gr1219
Cai, M. (2018). Investigation of the clinical effect of Banxia Xiexin decoction on functional dyspepsia. World Latest Med. Inf. 18, 167. doi:10.19613/j.cnki.1671-3141.2018.40.136
Cai, R. (2016). The clinical observation of functional dyspepsia of mixed cold and heat syndrome with the treatment of Banxiaxiexin decoction. Nanjing: Nanjing Nanjing Univ. Chinese Med.
Chen, S., Peng, X., Jiang, Y., and Huang, H. (2014). Effectiveness of Banxia xiexin decoction combined with Western medicine in treating peptic ulcer: A systematic review. J. Liaoning Univ. TCM 16, 50–53. doi:10.13194/j.issn.1673-842x.2014.11.017
Chen, W. (2019). Clinical analysis of Banxia Xiexin decoction in treating Pperimenopausal depression. Heal. Everyone 23, 451.
Chen, Y. (2010). Clinical observation on treatment of 56 cases of functional dyspepsia with Banxia Xiexin decoction. Jiangsu J. Tradit. Chin. Med. 42, 39.
Chrubasik, S., Pittler, M. H., and Roufogalis, B. D. (2005). Zingiberis rhizoma: A comprehensive review on the ginger effect and efficacy profiles. Phytomedicine 12, 684–701. doi:10.1016/j.phymed.2004.07.0099
Dai, Y., Zhang, Y., Li, D., Ye, J., Chen, W., and Hu, L. (2017). Efficacy and safety of modified Banxia xiexin decoction (Pinellia decoction for draining the heart) for gastroesophageal reflux disease in adults: A systematic review and meta-analysis. Evidence-Based Complement. Altern. Med. 2017, 1–17. doi:10.1155/2017/9591319
Dang, W. (2019). Analysis of the effect of Banxia Xiexin decoction in the treatment of dyspepsia in children. Cap. Food Med. 1, 167.
Deng, X. (2016). Observation on the efficacy of Banxia Xiexin decoction in treating functional dyspepsia. Clin. Res. Pract. 1, 64. doi:10.19347/j.cnki.2096-1413.2016.03.050
Ding, X. (2018). Observation on the clinical efficacy and safety of Banxia Xiexin decoction in the treatment of functional dyspepsia. World Latest Med. Inf. 18, 127. doi:10.19613/j.cnki.1671-3141.2018.19.104
Dong, H., Xu, X., and Yu, Z. (2017). Clinical effect of Pinelliae Decoction for purging stomach-fire on gastric mucosal mast cells and plasma motilin in patients with functional dyspepsia. Hainan Med. J. 28, 566–568. doi:10.3969/j.issn.1003-6350.2017.04.015
Dong, J., and Chen, K. (2009). Clinical observation on treatment of 63 cases of functional dyspepsia with Banxia Xiexin decoction. China Foreign Med. Treat. 19, 107. doi:10.16662/j.cnki.1674-0742.2009.19.018
Dong, X. (2018). Clinical efficacy and safety of Banxia Xiexin decoction in the treatment of functional dyspepsia. Guid. China Med. 16, 190–191. doi:10.15912/j.cnki.gocm.2018.29.068
Faramarzi, M., Kheirkhah, F., Shokri-Shirvani, J., Mosavi, S., and Zarini, S. (2014). Psychological factors in patients with peptic ulcer and functional dyspepsia. Casp. J. Intern. Med. 5, 71–76.
Feng, H., Zang, L., and Zhang, D. (2015). Influence of Banxia Xiexin decoction on substance P (SP) in the blood serum and calcitonin gene-related peptide (CGRP) in the gastric antrum mucosa of patients with functional dyspepsia. J. Nanjing Univ. TCM 31, 310–313. doi:10.14148/j.issn.1672-0482.2015.0310
Ford, A. C., Mahadeva, S., Carbone, M. F., Lacy, B. E., and Talley, N. J. (2020). Functional dyspepsia. Lancet 396, 1689–1702. doi:10.1016/S0140-6736(20)30469-4
Fu, G. (2010). Treatment of 50 cases of functional dyspepsia with Banxia Xiexin decoction. Guid. J. Tradit. Chin. Med. Pharm. 16, 45–46. doi:10.13862/j.cnki.cn43-1446/r.2010.08.030
Fu, S. (2017). Clinical analysis of Banxia Xiexin decoction for functional dyspepsia. World Latest Med. Inf. 17, 200–201. doi:10.3969/j.issn.1671-3141.2017.52.103
Gan, Y., Liu, H., Yang, L., and Yang, K. (2014). Effect of Banxiaxiexin tang on treatment of functional dyspepsia: A meta-analysis of randomized controlled trials. J. Tradit. Chin. Med. 34, 140–144. doi:10.1016/s0254-6272(14)60067-4
He, M. (2007). Treatment of functional dyspepsia with Banxia Xiexin decoction. China Trop. Med. 7, 487–488.
He, R., and Xie, X. (2012). Observation on the efficacy of Mosapride combined with Banxia Xiexin decoction in the treatment of functional dyspepsia and its effect on plasma motilin. J. New Chin. Med. 44, 39. doi:10.13457/j.cnki.jncm.2012.04.077
Hu, X., Liu, J., Xiao, K., Dong, M., Cui, R., Gan, A., et al. (2020). Banxia xiexin decoction in the treatment of functional dyspepsia: A meta-analysis of randomized controlled trials. Chin. J. Integr. Tradit. West. Med. Dig. 28, 923–931. doi:10.3969/j.issn.1671-038X.2020.12.06
Hu, X., Zhu, D., Zhou, H., Xiong, X., and Wang, D. (2006). Clinical study of Banxia Xiexin decoction in treating functional dyspepsia due to combination of cold and heat. J. TCM Univ. Hunan 26, 40–43.
Huang, X., and Long, J. (2004). Clinical observation of Banxia Xiexin decoction combined with Western medicine for functional dyspepsia. Acta Med. Sin. 17, 559–560.
Huang, Y. (2011). The effect of Chaizhi Banxia Xiexin decoction on clinical effect of treating patients with functional indigestion. Clin. J. Chin. Med. 3, 34–35.
Ji, Y., Wang, T., Liu, C., Wang, B., and Meng, J. (2017). Current status of clinical and experimental research on the treatment of digestive system diseases with Banxia Xiexin decoction. J. Tianjin Univ. Tradit. Chin. Med. 36, 156–160. doi:10.11656/j.issn.1673-9043.2017.02.20
Jin, P., Qu, B., and Chang, Y. (2004). Clinical observation on treatment of functional dyspepsia due to combination of cold and heat by Banxia Xiexin granule. Inf. Tradit. Chin. Med. 21, 50–52.
Jones, M. P., Tack, J., Van Oudenhove, L., Walker, M. M., Holtmann, G., Koloski, N. A., et al. (2017). Mood and anxiety disorders precede development of functional gastrointestinal disorders in patients but not in the population. Clin. Gastroenterol. Hepatol. 15, 1014–1020. doi:10.1016/j.cgh.2016.12.032
Kim, J. (2017). Effects and safety of Banhasasim-tang Depending on pattern identification and Subtypes of functional dyspepsia: A randomized, double-blind, placebo-controlled. Daejoen: Dajoen Univ.
Kim, S. K., Joung, J. Y., Ahn, Y. C., Jung, I. C., and Son, C. G. (2021). Beneficial potential of Banha-sasim-tang for stress-sensitive functional dyspepsia via modulation of ghrelin: A randomized controlled trial. Front. Pharmacol. 12, 636752–636759. doi:10.3389/fphar.2021.636752
Kim, Y. H., Kim, J. Y., Jung, S. Y., Kwon, O. J., Lee, J. H., and Son, C. G. (2017). Efficacy of Banha-sasim-tang on functional dyspepsia classified as excess pattern: Study protocol for a randomized controlled trial. Trials 18, 525–527. doi:10.1186/s13063-017-2282-z
Ko, S.-J., Cho, S., Kim, K.-J., Kim, J., Ha, N., and Park, J.-W. (2019). Herbal medicine Banha-sasim-tang for the treatment of functional dyspepsia protocol for a systematic review of randomized controlled trials. Med. Baltim. 98, e15760. doi:10.1097/MD.0000000000015760
Koloski, N. A., Jones, M., Kalantar, J., Weltman, M., Zaguirre, J., and Talley, N. J. (2012). The brain-gut pathway in functional gastrointestinal disorders is bidirectional: A 12-year prospective population-based study. Gut 61, 1284–1290. doi:10.1136/gutjnl-2011-300474
Lang, H., and Chen, F. (2015). Clinical observation of using Banxia Xiexin decoction in the treatment of functional dyspepsia. J. Sichuan Tradit. Chin. Med. 33, 90–92.
Li, C., and Zuo, Z. (2011). Pharmacological effects and pharmacokinetics properties of Radix Scutellariae and its bioactive flavones. Drug Dispos. 32, 427–445. doi:10.1002/bdd.771
Li, D., Tang, R., and Zhao, S. (2014). Clinical observation on effect of Banxia Xiexin decoction combined with Domperidone in treating patients with functional dyspepsia. Chin. J. Exp. Tradit. Med. Formulae 20, 203–206. doi:10.13422/j.cnki.syfjx.2014220203
Li, H., and An, Y. (2016). Clinical observation on the treatment of 44 cases of functional dyspepsia with Banxia Xiexin decoction. Hunan J. Tradit. Chin. Med. 32, 54–56. doi:10.16808/j.cnki.issn1003-7705.2016.07.026
Li, J., Li, T., Xu, Z., Chen, P., and Mao, R. (2004). 50 cases of functional dyspepsia treated by Banxia Xiexin decoction. Shaanxi J. Tradit. Chin. Med. 25, 21–22.
Li, S., and Li, D. (2016). Systematic review of Banxia Xiexin decoction for the treatment of functional dyspepsia. Beijing J. Tradit. Chin. Med. 35, 456–461. doi:10.16025/j.1674-1307.2016.05.012
Li, X., Lin, L., Wei, W., and Dai, L. Y. (2013). Stage specific effect of leptin on the expressions of estrogen receptor and extracellular matrix in a model of chondrocyte differentiation. China J. Tradit. Chin. Med. Pharm. 28, 876–884. doi:10.1016/j.cyto.2012.12.017
Li, Y. (2016). Clinical study on modification of Banxia Xiexin decoction for functional dyspepsia. Chronic Pathematology J. 17, 67–68. doi:10.16440/j.cnki.1674-8166.2016.01.020
Li, Y. (2015). Therapeutic observation of Banxia Xiexin decoction for functional dyspepsia. Mod. J. Integr. Tradit. Chin. West. Med. 24, 1507–1509. doi:10.3969/j.issn.1008-8849.2015.14.009
Liang, X., He, H., Li, B., Peng, Z., Huang, G., Deng, Y., et al. (2010). Effect of Chaiqin Banxia Xiexin decoction on clinical efficacy and gastric acid in patients with ulcer-like functional dyspepsia. Lishizhen Med. Mater. Medica Res. 21, 442–443.
Liang, X., He, H., Li, G., Peng, Z., Li, B., Deng, C., et al. (2008). Clinical observation on 30 cases of functional dyspepsia treated with Chaizhi Banxia Xiexin decoction. Jiangsu J. Tradit. Chin. Med. 40, 42–43.
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gøtzsche, P. C., Ioannidis, J. P. A., et al. (2009). The PRISMA Statement for reporting systematic reviews and meta-analyses of studies that evaluate Health Care interventions: Explanation and Elaboration. PLoS Med. 6, e1000100. doi:10.1371/journal.pmed.1000100
Liu, L. (2020). Clinical observation of Banxia Xiexin decoction in treating cold-heat complex type FD. Guangming J. Chin. Med. 35, 482–484.
Lu, X. (2018). Effect of Banxia Xiexin decoction on functional dyspepsia. Mod. Diagn. Treat. 29, 2211–2213.
Luo, J. (2016). Application experience of Banxia Xiexin decoction in patients with functional dyspepsia. all Heal 10, 33–34.
Mahadeva, S., and Goh, K. L. (2006). Epidemiology of functional dyspepsia: A global perspective. World J. Gastroenterol. 12, 2661–2666. doi:10.3748/wjg.v12.i17.2661
Mao, R., and He, Z. (2020). Pinellia ternata (Thunb) Breit: A review of its germplasm resources, genetic diversity and active components. J. Ethnopharmacol. 263, 113252. doi:10.1016/j.jep.2020.113252
Min, G. (2009). Observation on the efficacy of Banxia Xiexin decoction in the treatment of functional dyspepsia due to mixed cold and heat syndrome. Guangxi J. Tradit. Chin. Med. 32, 30–31.
Moayyedi, P. M., Lacy, B. E., Andrews, C. N., Enns, R. A., Howden, C. W., and Vakil, N. (2017). ACG and CAG clinical guideline: Management of dyspepsia. Am. J. Gastroenterol. 112, 988–1013. doi:10.1038/ajg.2017.154
Naito, T., Itoh, H., and Takeyama, M. (2003). Some gastrointestinal function regulatory Kampo medicines have modulatory effects on human plasma adrenocorticotropic hormone and cortisol levels with continual stress exposure. Biol. Pharm. Bull. 26, 101–104. doi:10.1248/bpb.26.101
Naito, T., Itoh, H., Yasunaga, F., and Takeyama, M. (2002). Hange-shashin-to raises levels of somatostatin, motilin, and gastrin in the plasma of healthy subjects. Biol. Pharm. Bull. 25, 327–331. doi:10.1248/bpb.25.327
Nong, Y. (2017). Observation of clinical effect of Banxia Xiexin decoction in the treatment of functional dyspepsia and its influence on the plasma motilin. China Foreign Med. Treat. 25, 14–16. doi:10.16662/j.cnki.1674-0742.2017.25.014
Oh, J. H., Kwon, J. G., Jung, H. K., Tae, C. H., Song, K. H., Kang, S. J., et al. (2020). Clinical practice guidelines for functional dyspepsia in Korea. J. Neurogastroenterol. Motil. 26, 29–50. doi:10.5056/jnm19209
Park, J.-W., Ko, S.-J., Han, G., Yeo, I., Ryu, B., and Kim, J. (2013). The effects of Banha-sasim-tang on dyspeptic symptoms and gastric motility in cases of functional dyspepsia: A randomized, double-blind, placebo-controlled, and two-center trial. Evidence-Based Complement. Altern. Med. 2013, 265035. doi:10.1155/2013/265035
Park, J. W., Ryu, B., Yeo, I., Jerng, U. M., Han, G., Oh, S., et al. (2010). Banha-sasim-tang as an herbal formula for the treatment of functional dyspepsia: A randomized, double-blind, placebo-controlled, two-center trial. Trials 11, 83–87. doi:10.1186/1745-6215-11-83
Qiu, C. (2011). Therapeutic effect of Banxia Xiexin decoction in treating functional dyspepsia with cold and heat syndromes. China Mod. Dr. 49, 54–55.
Ren, J. (2015). Clinical effect of Banxia Xiexin decoction in the treatment of elderly patients with functional dyspepsia. J. Community Med. 13, 10–12.
Rösch, W., Liebregts, T., Gundermann, K. J., Vinson, B., and Holtmann, G. (2006). Phytotherapy for functional dyspepsia: A review of the clinical evidence for the herbal preparation STW 5. Phytomedicine 13, 114–121. doi:10.1016/j.phymed.2006.03.022
Shi, Y. (2014). Clinical observation on functional dyspepsia treated with Pinelliae decoction for purging stomach-fire. J. Liaoning Univ. TCM 16, 198–199. doi:10.13194/j.issn.1673-842x.2014.10.071
Sperber, A. D., Bangdiwala, S. I., Drossman, D. A., Ghoshal, U. C., Simren, M., Tack, J., et al. (2020). Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome foundation global study. Gastroenterology 160, 99–114.e3. doi:10.1053/j.gastro.2020.04.014
Stanghellini, V., Chan, F. K. L., Hasler, W. L., Malagelada, J. R., Suzuki, H., Tack, J., et al. (2016). Gastroduodenal disorders. Gastroenterology 150, 1380–1392. doi:10.1053/j.gastro.2016.02.011
Su, Z. (2014). Observation on the efficacy of Banxia Xiexin decoction in the treatment of functional dyspepsia. Nei Mong. J. Tradit. Chin. Med. 1, 10.
Sun, H., and Li, J. (2014). A comparative study of Banxia-xiexin decoction combined with paroxetine in treatment of female patients with somatoform disorder. J. Psychiatry 27, 128–130. doi:10.3969/j.issn.1009-7201.2014.02.015
Suzuki, H., Inadomi, J. M., and Hibi, T. (2009). Japanese herbal medicine in functional gastrointestinal disorders. Neurogastroenterol. Motil. 21, 688–696. doi:10.1111/j.1365-2982.2009.01290.x
Tack, J., Talley, N. J., Camilleri, M., Holtmann, G., Hu, P., Malagelada, J. R., et al. (2006). Functional gastroduodenal disorders. Gastroenterology 130, 1466–1479. doi:10.1053/j.gastro.2005.11.059
Tang, H. (2015). Curative effect and recurrence prevention of Banxiaxiexin decoction in the treatment of the patients with functional dyspepsia. Chin. Community Dr. 31, 103–104. doi:10.3969/j.issn.1007-614x.2015.7.64
Tang, L. (2014). Therapeutic effect of Banxia Xiexin decoction on functional dyspepsia. J. Mudanjang Med. Univ. 35, 80–81. doi:10.13799/j.cnki.mdjyxyxb.2014.03.042
Tian, J., Li, M., Liao, J., Li, J., and Tong, X. (2013). Chinese herbal medicine Banxiaxiexin decoction treating diabetic gastroparesis: A systematic review of randomized controlled trials. Evidence-Based Complement. Altern. Med. 2013, 1–11. doi:10.1155/2013/749495
Tian, L. (2018). Clinical effect of modified Banxia Xiexin decoction in treatment of patients with functional dyspepsia. Doctor 10, 45–46. doi:10.19604/j.cnki.dys.2018.10.022
Tian, T. (2017). Study on the effects of Banxia Xiexin decoction on visceral sensitivity and psychology of patients with functional dyspepsia. Beijing Beijing Univ. Chinese Med.
Van den Houte, K., Scarpellini, E., Verbeure, W., Mori, H., Schol, J., Masuy, I., et al. (2020). The role of GI peptides in functional dyspepsia and gastroparesis: A systematic review. Front. Psychiatry 11, 1–12. doi:10.3389/fpsyt.2020.00172
Van Oudenhove, L., and Aziz, Q. (2013). The role of psychosocial factors and psychiatric disorders in functional dyspepsia. Nat. Rev. Gastroenterol. Hepatol. 10, 158–167. doi:10.1038/nrgastro.2013.10
Vanheel, H., and Farré, R. (2013). Changes in gastrointestinal tract function and structure in functional dyspepsia. Nat. Rev. Gastroenterol. Hepatol. 10, 142–149. doi:10.1038/nrgastro.2012.255
Wang, H. (2001). 60 cases of functional dyspepsia treated with Banxia Xiexin decoction. Zhejiang J. Tradit. Chin. Med. 4, 239.
Wang, Q., Chen, H., Wang, T., and Xiao, G. (2019). Modeling and control of mine main fan switchover system. World Latest Med. Inf. 19, 189–199. doi:10.1016/j.isatra.2018.10.024
Wang, Q., Li, Z., Zhang, Y., Wang, L., Zhao, Z., He, S., et al. (2012). Oncogenic reg IV is a novel prognostic marker for glioma patient survival. Clin. J. Chin. Med. 4, 69–71. doi:10.1186/1746-1596-7-69
Wang, W. (2018). Observation on the efficacy of Banxia Xiexin decoction in the treatment of functional dyspepsia. J. Pract. Tradit. Chin. Med. 34, 660–661.
Wang, X., and Zhu, R. (2007). Effect of modified Banxia Xiexin decoction on functional dyspepsia. Liaoning J. Tradit. Chin. Med. 34, 1274–1275. doi:10.13192/j.ljtcm.2007.09.94.wangxp.041
Wauters, L., Talley, N. J., Walker, M. M., Tack, J., and Vanuytsel, T. (2020). Novel concepts in the pathophysiology and treatment of functional dyspepsia. Gut 69, 591–600. doi:10.1136/gutjnl-2019-318536
Wu, J. (2008). Treatment of 50 cases of functional dyspepsia with Banxia Xiexin decoction. Henan Tradit. Chin. Med. 28, 19.
Xie, X., Zhang, L., and Peng, Y. (2011). Banxia Xiexin decoction in the treatment of 42 cases of functional dyspepsia. J. Anhui Tradit. Chin. Med. Coll. 30, 30–31. doi:10.16194/j.cnki.31-1059/g4.2011.07.016
Yang, F., Liu, M., Qin, N., Li, S., Yu, M., Wang, C., et al. (2019a). Lipidomics coupled with pathway analysis characterizes serum metabolic changes in response to potassium oxonate induced hyperuricemic rats. Clin. J. Chin. Med. 11, 112–113. doi:10.1186/s12944-019-1054-z
Yang, L., Wang, Q., Liu, Y., Zhang, L., Lv, W., and Liu, B. (2019b). Expression profiles of interferon-stimulated gene 15 and prostaglandin synthases in the ovine lymph nodes during early pregnancy. Yunnan J. Tradit. Chin. Med. Mater. Medica 40, 100–108. doi:10.1002/mrd.23085
Yi, W. (2015). The clinical observation of functional dyspepsia of mixed cold and heat syndrome with the treatment of Banxiaxiexin decoction. Wuhan: Wuhan Hubei Univ. Chinese Med.
Yin, Q. (2011). Clinical research on the therapy of Banxiaxiexin soup joint Mosapride to FD with cold and heat. Nanjing: Nanjing Nanjing Univ. Chinese Med.
Yu, S., and Yu, X. (2017). Investigating clinical effect and safety of modified Pinelliae decoction for purging stomach-fire in treatment of functional dyspepsia. Smart Healthc. 21, 14–16. doi:10.19335/j.cnki.2096-1219.2017.21.007
Yu, X. (2016). Clinical research of Banxiaxiexin decoction in treating functional dyspepsia-epigastric pain syndrome of cold-heat complicated type. Nanjing: Nanjing Nanjing Univ. Chinese Med.
Yu, Y., and Yang, X. (2010). Clinical observation on BanXia XieXin decoction in treating 90 cases of functional indigestion. Gansu J. TCM 23, 34–35.
Yu, Y., Zhang, G., Han, T., and Huang, H. (2020). Analysis of the pharmacological mechanism of Banxia Xiexin decoction in treating depression and ulcerative colitis based on a biological network module. BMC Complement. Med. Ther. 20, 1–13. doi:10.1186/s12906-020-02988-3
Zhang, H., Chen, H., and Luo, J. (2019). Clinical study on modified Banxia Xiexin tang combined with Domperidone for functional dyspepsia. J. New Chin. Med. 51, 59–61. doi:10.13457/j.cnki.jncm.2019.06.017
Zhang, L., Hou, S. x., Zhang, J. q., Hu, W. j., and Wang, C. y. (2010). Preparation, characterization, and in vivo evaluation of mitoxantrone-loaded, folate-conjugated albumin nanoparticles. J. Integr. Tradit. Chin. West. Med. 19, 1193–1198. doi:10.1007/s12272-010-0809-x
Zhang, S., Wang, Q., Tan, W., Wang, Z., and Li, J. (2015). The use of a modified, oscillating positive expiratory pressure device reduced fever and length of hospital stay in patients after thoracic and upper abdominal surgery: A randomised trial. J. Shaanxi Coll. Tradit. Chin. Med. 38, 16–20. doi:10.1016/j.jphys.2014.11.013
Zhang, Y. (2017). Banxia Xiexin decoction combined with mosapride in the treatment of functional dyspepsia randomized controlled study. J. Pract. Tradit. Chin. Intern. Med. 31, 73–75. doi:10.13729/j.issn.1671-7813.2017.10.27
Zhao, L., and Su, X. (2017). Clinical observation of Banxia Xiexin decoction in treating functional dyspepsia. Guangming J. Chin. Med. 32, 1432–1434. doi:10.3969/j.issn.1003-8914.2017.10.025
Zhao, L., Zhang, S., Wang, Z., Wang, C., Huang, S., Shen, H., et al. (2013). Efficacy of modified ban Xia Xie Xin decoction on functional dyspepsia of cold and heat in complexity syndrome: A randomized controlled trial. Evidence-Based Complement. Altern. Med. 2013, 812143. doi:10.1155/2013/812143
Zhao, Y., and Song, Q. (2011). Banxia Xiexin decoction for 48 cases of functional dyspepsia with cold and heat syndrome. Shaanxi J. Tradit. Chin. Med. 32, 1144–1146. doi:10.16194/j.cnki.31-1059/g4.2011.07.016
Zheng, Y. (2019). Clinical observation of Banxia Xiexin decoction in the treatment of functional dyspepsia. Contin. Med. Educ. 33, 150–152. doi:10.3969/j.issn.1004-6763.2019.03.080
Zhou, X., Seto, S. W., Chang, D., Kiat, H., Razmovski-Naumovski, V., Chan, K., et al. (2016). Synergistic effects of Chinese herbal medicine: A comprehensive review of methodology and current research. Front. Pharmacol. 7, 201–216. doi:10.3389/fphar.2016.00201
Zhu, J., Du, J., Guan, J., Dong, Y., Du, Y., Wang, C., et al. (2016). Meta-analysis on random-control experiment of treatment of ulcerative colitis with Banxia Xiexin decoction and its decomposed recipe. J. Liaoning Univ. TCM 18, 104–108. doi:10.13194/j.issn.1673-842x.2016.12.030
Zhu, L., and Gu, X. (2008). Influence of Banxia Xiexin decoction on functional dyspepsia and motilin concentration. Shanghai J. Tradit. Chin. Med. 42, 36–37.
Keywords: functional dyspepsia, Banxia-xiexin tang, herbal medicine, meta-analysis, systematic review
Citation: Kim K, Ko S-J, Cho SH, Kim J and Park J-W (2023) Herbal medicine, Banxia-xiexin tang, for functional dyspepsia: a systematic review and meta-analysis. Front. Pharmacol. 14:1130257. doi: 10.3389/fphar.2023.1130257
Received: 23 December 2022; Accepted: 04 May 2023;
Published: 19 May 2023.
Edited by:
Yong Gao, Guangzhou University of Chinese Medicine, ChinaReviewed by:
Chuangpeng Shen, Guangzhou University of Chinese Medicine, ChinaShota Takemi, Saitama University, Japan
Copyright © 2023 Kim, Ko, Cho, Kim and Park. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jae-Woo Park, cGp3MjkwN0BraHUuYWMua3I=
†These authors have contributed equally to this work and share first authorship
 Keumji Kim1,2†
Keumji Kim1,2† Seok-Jae Ko
Seok-Jae Ko Soo Ho Cho
Soo Ho Cho Jinsung Kim
Jinsung Kim Jae-Woo Park
Jae-Woo Park