
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 13 February 2023
Sec. Inflammation Pharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1126580
This article is part of the Research Topic Streaming Inflammation: From Damage to Healing and Resilience - Volume II View all 11 articles
Background: Voriconazole (VCZ) metabolism is influenced by many factors. Identifying independent influencing factors helps optimize VCZ dosing regimens and maintain its trough concentration (C0) in the therapeutic window.
Methods: We conducted a prospective study investigating independent factors influencing VCZ C0 and the VCZ C0 to VCZ N-oxide concentration ratio (C0/CN) in younger adults and elderly patients. A stepwise multivariate linear regression model, including the IL-6 inflammatory marker, was used. The receiver operating characteristic (ROC) curve analysis was used to evaluate the predictive effect of the indicator.
Results: A total of 463 VCZ C0 were analyzed from 304 patients. In younger adult patients, the independent factors that influenced VCZ C0 were the levels of total bile acid (TBA) and glutamic-pyruvic transaminase (ALT) and the use of proton-pump inhibitors. The independent factors influencing VCZ C0/CN were IL-6, age, direct bilirubin, and TBA. The TBA level was positively associated with VCZ C0 (ρ = 0.176, p = 0.019). VCZ C0 increased significantly when the TBA levels were higher than 10 μmol/L (p = 0.027). ROC curve analysis indicated that when the TBA level ≥4.05 μmol/L, the incidence of a VCZ C0 greater than 5 μg/ml (95% CI = 0.54–0.74) (p = 0.007) increased. In elderly patients, the influencing factors of VCZ C0 were DBIL, albumin, and estimated glomerular filtration rate (eGFR). The independent factors that affected VCZ C0/CN were eGFR, ALT, γ-glutamyl transferase, TBA, and platelet count. TBA levels showed a positive association with VCZ C0 (ρ = 0.204, p = 0.006) and C0/CN (ρ = 0.342, p < 0.001). VCZ C0/CN increased significantly when TBA levels were greater than 10 μmol/L (p = 0.025). ROC curve analysis indicated that when the TBA level ≥14.55 μmol/L, the incidence of a VCZ C0 greater than 5 μg/ml (95% CI = 0.52–0.71) (p = 0.048) increased.
Conclusion: TBA level may serve as a novel marker for VCZ metabolism. eGFR and platelet count should also be considered when using VCZ, especially in elderly patients.
Invasive fungal infections (IFIs) remain a clinical problem with high morbidity and mortality despite recent advances in diagnosis and treatment (Jenks et al., 2020). Common pathogens of IFIs are Candida, Cryptococcus, Aspergillus, and Mucormycetes. Except for patients with underlying hematologic malignancies, solid organ transplant recipients, and critically ill patients, high rates of IFIs and mortality are also observed among patients 65 years or older (Vallabhaneni et al., 2017; Matthaiou et al., 2018; Hesstvedt et al., 2019; Tsay et al., 2020). Voriconazole (VCZ) is an essential drug for treating IFIs, especially those caused by Aspergillus and Candida. It is a first-line therapy for patients with invasive Aspergillosis (Ullmann et al., 2018). However, VCZ has a narrow therapeutic range. A trough level of 1–5.5 mg/L is recommended for most European patients on VCZ prophylaxis or treatment (Ullmann et al., 2018), while a range of 0.5–5 mg/L is considered adequate for Chinese patients (Chen et al., 2018). Maintaining VCZ trough concentration (C0) in the therapeutic range is crucial in enhancing its treatment effect.
VCZ exhibits non-linear pharmacokinetics with large interindividual and intraindividual variabilities (Purkins et al., 2002; Theuretzbacher et al., 2006). Many factors influence VCZ C0, such as age, sex, VCZ dose and administration route, albumin, total bilirubin (TBIL), glutamic-pyruvic transaminase (ALT), glutamic-oxalacetic transaminase (AST), γ-glutamyl transferase (γ-GT), CYP2C19 gene polymorphisms, and inflammatory state. (Vanstraelen et al., 2014; Niioka et al., 2017; Veringa et al., 2017). However, the specificity of each index has certain limitations. Both clinical symptoms and test results must be considered to diagnose and treat infectious diseases. VCZ dosing regimens also require modification according to patients’ conditions.
Our previous study found that VCZ C0 in elderly patients was significantly higher than in younger adult patients. The proportion of patients with C0 greater than 5 mg/L was higher in older adults (Cheng et al., 2020). VCZ C0 in elderly patients was not significantly affected by CYP2C19 polymorphisms (Shang et al., 2020). Inflammation could affect liver function, C0, and the concentration ratio of VCZ C0 to VCZ N-oxide (C0/CN) in younger and older patients (Liang et al., 2022). Therefore, disease state and patient status could confer significant dynamic markers that contribute to the fluctuation of VCZ concentrations (Chantharit et al., 2020).
According to the US Food and Drug Administration Adverse Event Reporting System (2004–2021 data), the VCZ-induced liver injury ratio is 32.45% (Zhou et al., 2022). Intrinsic and idiosyncratic drug-induced hepatotoxicity causes alterations in bile acid homeostasis (Mosedale and Watkins, 2017). Thus, the total bile acid (TBA) level can influence VCZ metabolism. Platelets are key effector cells for inflammatory responses and have particular advantages (Jenne et al., 2013). Platelet count was one of the determinants of VCZ C0 in kidney transplant recipients (Zhao Y. C. et al., 2021). VCZ clearance was also significantly associated with platelet count in patients with liver dysfunction (Tang et al., 2019; Tang et al., 2021). The worsening of renal function was significantly associated with a cumulative dose of intravenous VCZ (≥400 mg/kg) (Yasu et al., 2018). Elderly patients often have impaired liver function, renal function, and chronic inflammation induced by chronic disease conditions. Therefore, we hypothesized that platelet count and renal function might affect VCZ metabolism in elderly patients.
This study aimed to identify the factors affecting VCZ C0 and C0/CN in younger adults and elderly patients using the stepwise multivariate linear regression model. In addition to the influencing factors reported in the literature, the TBA, IL-6, platelet count, hemoglobin, and renal function indexes were also included in the study.
A single-center prospective study was conducted from January 2018 to June 2022. The study analyzed patients who received both VCZ prophylaxis and treatment. The inclusion criteria were patients who: (a) received VCZ therapeutic drug monitoring (TDM); (b) aged ≥18 years; (c) with steady-state VCZ C0 ≥ 0.4 μg/ml; (d) with available IL-6 concentration data measured on the same day of VCZ C0 measurement (IL-6 level was routinely detected in our hospital); (e) with available routine blood, liver function, and renal function results measured on the same day of VCZ C0 measurement; and (f) agreed to the use of their blood samples for VCZ CN determination and signed informed consent forms.
This study was approved by the Ethics Committee of the First Affiliated Hospital of the Army Medical University. Patients were divided into two cohorts according to age: the elderly cohort (≥60 years) and the younger adult cohort (<60 years).
The following data were collected from the medical chart: (a) demographic and clinical characteristics, including age, sex, weight, underlying diseases, fungal infection, VCZ dose and administration route, and combined use of proton-pump inhibitors (PPIs); (b) inflammation marker IL-6 levels; (c) routine blood examination indices, including hemoglobin levels and platelet count; (d) liver function indices, including alkaline phosphatase (ALP), ALT, AST, γ-GT, TBA, albumin, TBIL, direct bilirubin (DBIL), and indirect bilirubin (IBIL) levels; and (e) renal function indices, including urea nitrogen, creatinine levels, and estimated glomerular filtration rate (eGFR). VCZ dosing was adjusted according to the TDM result at the VCZ C0 measurement.
VCZ C0 was measured routinely in the clinic. The steady state of VCZ C0 was defined as the concentration obtained after 3 days of intravenous VCZ therapy (a loading dose of 6 mg/kg) or oral VCZ therapy (a loading dose of 400 mg) or the concentration obtained after 5 days of VCZ therapy without a loading dose. VCZ N-oxide is the primary metabolite in plasma, accounting for 72% of circulating VCZ metabolites (Geist et al., 2013). The plasma VCZ C0/CN ratio may provide information about VCZ clearance. Therefore, the VCZ CN was detected. VCZ CN was measured together with VCZ C0 using liquid chromatography-tandem mass spectrometry (LC-MS/MS) as previously described (Shang et al., 2020). The limit of detection (LOD) of VCZ and VCZ N-oxide was 8 ng/ml and 10 ng/ml, respectively. The lower limit of quantification (LLOQ) of VCZ and VCZ N-oxide were both 400 ng/ml.
IBM SPSS 19.0 (IBM Corp., Armonk, NY, USA) was used to perform the analysis. Categorical data were compared with the chi-square test. Data that do not conform to a normal distribution are represented by the median and interquartile range (IQR). Data from the two cohorts were compared using independent sample t-tests and Mann-Whitney U tests. A stepwise multivariate linear regression model was used to identify the factors influencing the VCZ C0 and C0/CN ratios.
A total of 20 factors were used in the analysis, including sex, age, route of administration of VCZ, VCZ dose, combined use of PPIs, platelet count, and levels of hemoglobin, ALP, ALT, AST, γ-GT, TBA, albumin, TBIL, DBIL, IBIL, urea nitrogen, creatinine, eGFR, and IL-6. Additionally, the Spearman correlation test was performed to assess the association of the TBA level with VCZ C0 and VCZ C0/CN. The receiver operating characteristic (ROC) curve analysis was used to evaluate the predictive effect of indicators. Covariates with a p-value < 0.1 in the univariate analysis were entered into the multivariate analysis. A p-value < 0.05 was considered statistically significant.
A total of 161 younger adult patients were included, with 229 VCZ C0 and 102 VCZ C0/CN. The primary baseline diseases were pneumonia, kidney disease, and leukemia. Almost a third of the patients had negative fungus detection results. Most patients received VCZ intravenously, with a dose of 3.6 ± 0.9 mg/kg, twice daily. Almost half of the patients received PPIs when taking VCZ (Table 1). The percentages of ALP, ALT, AST, TBA, albumin, TBIL, and DBIL within the normal range were 58.3%, 53.4%, 50.0%, 75.7%, 69.3%, 71.9%, and 61.6%, respectively (Table 2).
The independent influencing factors of VCZ C0 were levels of TBA and ALT and the use of PPIs. The independent influencing factors of VCZ C0/CN were IL-6, age, DBIL, and TBA levels (Table 3). TBA values showed a positive association with VCZ C0 (ρ = 0.176, p = 0.019) but not with VCZ C0/CN (ρ = 0.114, p = 0.305) (Figure 1). As shown in Figure 2, VCZ C0 increased significantly when TBA levels were higher than 10 μmol/L (p = 0.027). The analysis of the ROC curve indicated that TBA levels of ≥4.05 μmol/L, as well as the platelet count less than 31, increased the incidence of VCZ C0 greater than 5 μg/ml (95% CI = 0.54–0.74) (p = 0.007) (Figure 3). The ROC curve was not used for C0/CN due to the lack of a clinically significant threshold.
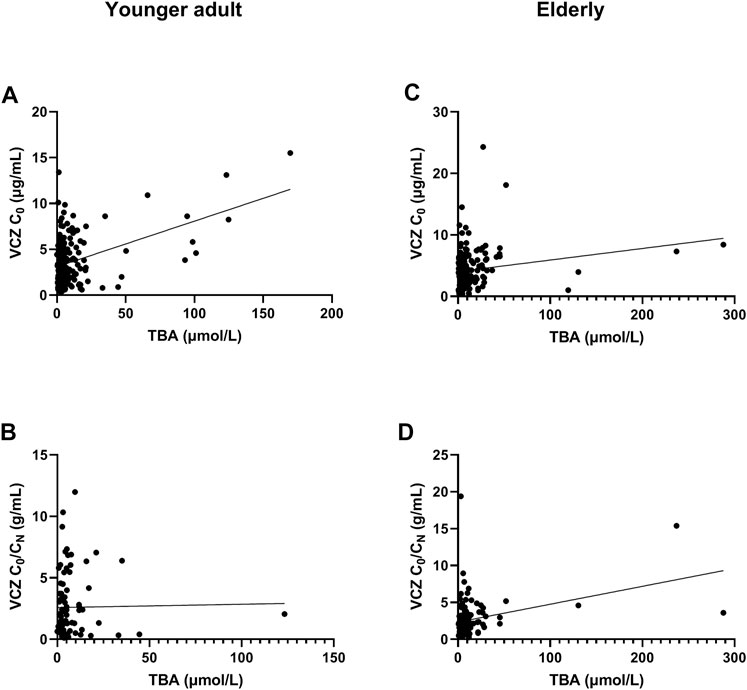
FIGURE 1. Association of total bile acid (TBA) level with voriconazole (VCZ) trough concentration (C0) and VCZ-to-VCZ N-oxide concentration ratio (C0/CN). (A). The TBA level was positively associated with VCZ C0 in younger adult patients; (B). There was no association between TBA level and VCZ C0/CN in younger adult patients; (C). There was a positive association between TBA level and VCZ C0 in elderly patients; (D). The TBA level was positively associated with VCZ C0/CN in elderly patients.
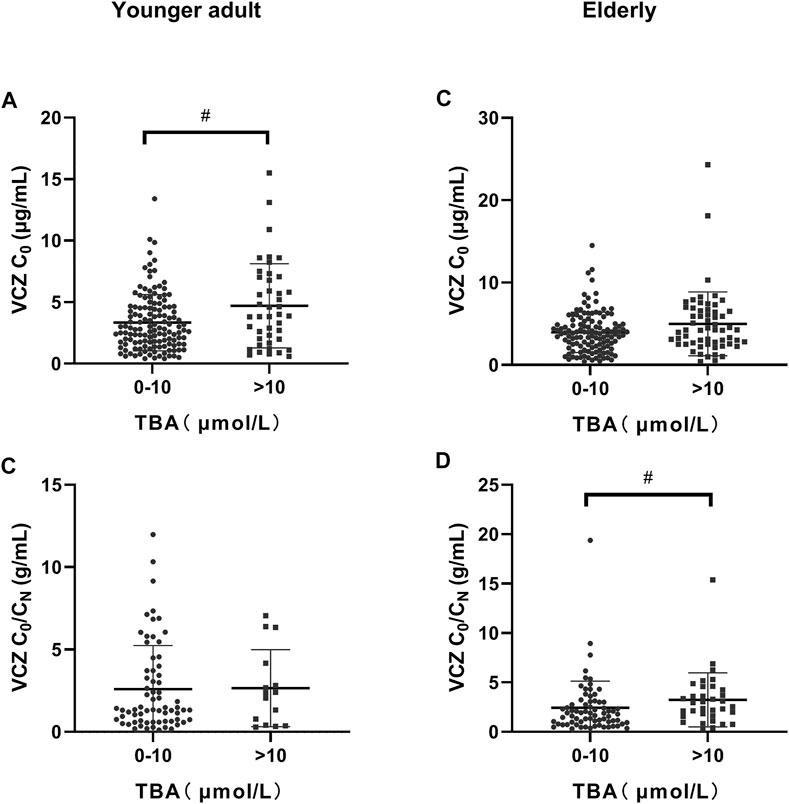
FIGURE 2. Distribution of voriconazole (VCZ) trough concentration (C0) and the VCZ-to-VCZ N-oxide concentration ratio (C0/CN) according to total bile acid (TBA) level. (A). VCZ C0 in younger adult patients was significantly increased when TBA levels were higher than 10 μmol/L; (B). VCZ C0/CN in younger adult patients was similar when TBA levels were between 0 and 10 μmol/L and higher than 10 μmol/L; (C). VCZ C0 in elderly patients was similar when TBA levels were between 0 and 10 μmol/L and greater than 10 μmol/L; (D). VCZ C0/CN in elderly patients increased significantly when TBA levels were higher than 10 μmol/L. #p < 0.05.
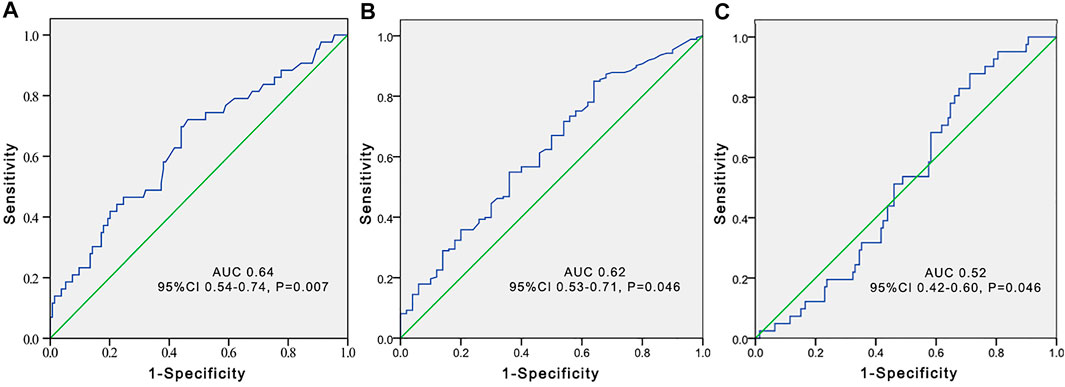
FIGURE 3. Receiver operating characteristic (ROC) curve to predict voriconazole trough concentrations greater than 5 μg/ml according to the total bile acid level (A), platelet count (B), and estimated glomerular filtration rate (C) in younger adult patients.
A total of 143 elderly patients were included, with 234 VCZ C0 and 131 VCZ C0/CN. The primary baseline diseases were pneumonia, hypertension, and kidney disease. Thirty-four patients (23.8%) had negative fungi detection results. The proportion of men in the elderly cohort was higher than that in the younger adult cohort (p = 0.001). The route of VCZ administration in patients in the two cohorts was similar (p > 0.05). In contrast, the dose of VCZ in the elderly cohort was significantly lower than that in the younger adult cohort (p < 0.001) (Table 1).
VCZ C0 in the elderly cohort was significantly higher than that in the younger adult cohort (p < 0.05), while the VCZ C0/CN ratio in the two cohorts was similar (p > 0.05). The percentages of ALP, ALT, AST, TBA, albumin, TBIL, and DBIL within the normal range were 57.9%, 67.5%, 58.4%, 67.8%, 73.4%, 71.6%, and 59.5%, respectively. The levels of IL-6, platelet count, hemoglobin, TBA, and urea nitrogen in the elderly cohort were significantly higher than those of the younger adult cohort (p < 0.05) (Table 2).
The independent influencing factors of VCZ C0 were the levels of DBIL, albumin, and eGFR. The independent influencing factors of VCZ C0/CN were eGFR, ALT, γ-GT, TBA, and platelet count (Table 4). The TBA level showed a positive association with VCZ C0 (ρ = 0.204, p = 0.006) and C0/CN (ρ = 0.342, p < 0.001), respectively (Figure 1). VCZ C0/CN significantly increased when TBA levels were higher than 10 μmol/L (p = 0.025) (Figure 2). ROC curve analysis indicated that when the TBA level ≥14.55 μmol/L, the incidence of a VCZ C0 greater than 5 μg/ml (95% CI = 0.52–0.71) (p = 0.048) increased (Figure 4).
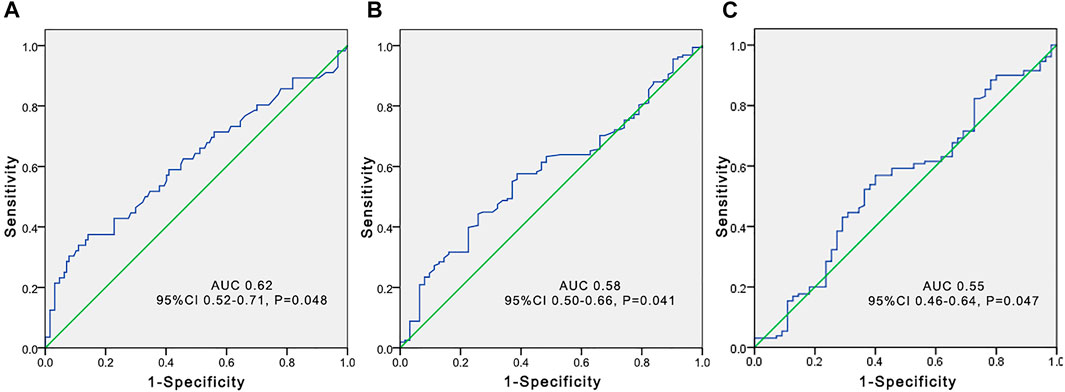
FIGURE 4. Receiver operating characteristic (ROC) curve to predict voriconazole trough concentrations greater than 5 μg/ml according to the total bile acid level (A), platelet count (B), and estimated glomerular filtration rate (C) in elderly patients.
VCZ-induced adverse reactions are generally considered the main reason for drug discontinuation and treatment failure associated with C0 (Jin et al., 2016; Hamada et al., 2020). Our previous study also showed a considerable number of VCZ C0 greater than 5 μg/ml, with a ratio of 23.4% in the younger adult cohort and 31.3% in the elderly cohort (Cheng et al., 2020). Therefore, it is crucial to investigate factors affecting VCZ metabolism. A significant correlation was found between VCZ C0 and age (Allegra et al., 2020; Bolcato et al., 2021). Niioka et al. found that older Japanese patients had higher VCZ C0/CN ratios (Niioka et al., 2017). Age was also a predictor of VCZ trough levels >5 μg/ml (Chen et al., 2022). Therefore, we investigated the factors affecting VCZ C0 and C0/CN in younger and elderly patients.
Our previous study found that IL-6 levels were associated with the VCZ C0/CN ratio in both younger and elderly patients (r = 0.355, p = 0.003; r = 0.386, p = 0.001). Therefore, this study included IL-6 as an inflammatory marker. IL-6 can directly target liver cells and down-regulate CYP2C19 and CYP3A4 gene expression during inflammation (Li et al., 2014; Klein et al., 2015), affecting VCZ metabolism. Our results showed that IL-6 level was an independent influencing factor of VCZ C0/CN in younger adults, which further confirmed the results of our previous study (Cheng et al., 2020; Liang et al., 2022).
Data on the effect of TBA on VCZ metabolism are limited. In the current study, TBA level was the independent influencing factor of VCZ C0 and C0/CN in younger adult patients and the independent influencing factor of C0/CN in older patients. TBA can effectively reflect the liver cell injury and the secretion and synthesis function of liver cells. TBA levels rise before the increase of bilirubin, which may partially explain our findings. Furthermore, the ROC curve identified the good predictive effects of TBA for VCZ C0 greater than 5 μg/ml. Our results indicate that TBA could be a good predictor of VCZ C0 in younger adult patients.
Platelets emerge as key players in inflammation and are key elements in the early phases of the inflammatory response (Nicolai and Massberg, 2020; Portier and Campbell, 2021). Accumulating evidence demonstrates that platelets contribute to the initiation and propagation of both local and systemic inflammatory processes (Manne et al., 2017). Since platelet count is routinely measured at our hospital, it was chosen as a key element in the inflammatory response. C-reactive protein (CRP) is an inflammatory marker commonly investigated in association with VCZ C0 and VCZ C0/CN in IFI patients (Dote et al., 2016; Encalada Ventura et al., 2016; Veringa et al., 2017; Vreugdenhil et al., 2018). We did not include CRP in this study due to the limited CRP data in the elderly. We also omitted procalcitonin because its association with VCZ C0/CN was insignificant in our previous study (Liang et al., 2022). Our results showed that platelet count was an independent influencing factor of VCZ C0/CN in elderly individuals. Therefore, platelet count could be considered in patients on VCZ therapies.
Liver function is generally considered to influence VCZ metabolism. VCZ is bound to albumin. Decreased albumin levels increase the unbound fraction of VCZ (Vanstraelen et al., 2014). Serum albumin and γ-GT levels were significantly correlated with the VCZ clearance rate (Chantharit et al., 2020). This study found that albumin level was an independent influencing factor of VCZ C0, and the γ-GT level was an independent influencing factor of VCZ C0/CN in elderly patients. Plasma TBIL concentration significantly influenced VCZ protein-protein binding (Vanstraelen et al., 2014). The TBIL level was associated with VCZ clearance in IFI patients with liver dysfunction (Tang et al., 2021). TBIL level was also considered an independent factor influencing VCZ C0 (Cheng et al., 2020; Zeng et al., 2020; Zhao Y. et al., 2021). However, our results showed that levels of DBIL but not TBIL influenced VCZ C0 and C0/CN. The liver is rich in a smooth endoplasmic reticulum (ER) equipped with enzymes that metabolize several drugs, including VCZ. DBIL is bioconverted to IBIL in the ER. DBIL levels may reflect the state of the ER and then exhibit an association with the metabolism of VCZ.
We found that eGFR was an independent influencing factor of VCZ C0 and VCZ C0/CN in elderly individuals. Our results showed that the eGFR in the elderly cohort was lower than that in the younger adult cohort, indicating an impaired renal function in the elderly cohort. Although VCZ dose adjustment is not recommended for patients with renal impairment, we should still pay attention to its use in the elderly based on our results. Furthermore, the degree of inflammation in the elderly cohort was more severe than in the younger adult cohort, with impaired liver and kidney function. Therefore, the use of VCZ in elderly patients should be monitored.
CYP2C19, CYP3A4, and CYP2C9 enzymes metabolize PPIs. The combined use of PPIs with VCZ can affect VCZ concentration (Yan et al., 2018). PPIs also significantly affected VCZ C0 in younger adult patients in our study.
This study has several limitations. First, we did not include samples with VCZ C0 lower than 0.4 mg/L because the LLOQ of VCZ and VCZ N-oxide were both 400 ng/ml. Second, although the polymorphisms of CYP2C19*2 and *3 are critical for examining the pharmacokinetics of VCZ (Moriyama et al., 2017), the CYP2C19 genotypes were not assessed since testing is not routinely performed. Finally, this study had a relatively small sample size. A large multicenter, prospective study is needed to confirm our results.
In conclusion, we report for the first time that TBA, eGFR, and platelet count were associated with VCZ C0 and C0/CN. Furthermore, the TBA level had a good predictive effect on VCZ C0 in younger adult patients and may serve as a novel marker of VCZ metabolism. eGFR and platelet count should also be considered when using VCZ, especially in elderly patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee of the First Affiliated Hospital of Army Medical University. The patients/participants provided their written informed consent to participate in this study.
LC and FS designed the study, performed the data analysis, and drafted the manuscript. ZL searched the data and performed the data analysis. MY performed the detection. FL, LL, JZ, and LX searched the data. All authors approved the final version of the manuscript.
This study was supported by the Medical Research Project of Science and Health of Chongqing (2021MSXM218).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Allegra, S., De Francia, S., De Nicolo, A., Cusato, J., Avataneo, V., Manca, A., et al. (2020). Effect of gender and age on voriconazole trough concentrations in Italian adult patients. Eur. J. Drug Metab. Pharmacokinet. 45 (3), 405–412. doi:10.1007/s13318-019-00603-6
Bolcato, L., Khouri, C., Veringa, A., Alffenaar, J. W. C., Yamada, T., Naito, T., et al. (2021). Combined impact of inflammation and pharmacogenomic variants on voriconazole trough concentrations: A meta-analysis of individual data. J. Clin. Med. 10 (10), 2089. doi:10.3390/jcm10102089
Chantharit, P., Tantasawat, M., Kasai, H., and Tanigawara, Y. (2020). Population pharmacokinetics of voriconazole in patients with invasive aspergillosis: Serum albumin level as a novel marker for clearance and dosage optimization. Ther. Drug Monit. 42 (6), 872–879. doi:10.1097/FTD.0000000000000799
Chen, C., Xu, T., Zhou, K., and Zhu, S. (2022). Factors affecting voriconazole concentration to dose ratio changes according to route of administration. Eur. J. Hosp. Pharm. ejhpharm-2021-003173. doi:10.1136/ejhpharm-2021-003173
Chen, K., Zhang, X., Ke, X., Du, G., Yang, K., and Zhai, S. (2018). Individualized medication of voriconazole: A practice guideline of the division of therapeutic drug monitoring, Chinese pharmacological society. Ther. Drug Monit. 40 (6), 663–674. doi:10.1097/FTD.0000000000000561
Cheng, L., Xiang, R., Liu, F., Li, Y., Chen, H., Yao, P., et al. (2020). Therapeutic drug monitoring and safety of voriconazole in elderly patients. Int. Immunopharmacol. 78, 106078. doi:10.1016/j.intimp.2019.106078
Dote, S., Sawai, M., Nozaki, A., Naruhashi, K., Kobayashi, Y., and Nakanishi, H. (2016). A retrospective analysis of patient-specific factors on voriconazole clearance. J. Pharm. Health Care Sci. 2, 10. doi:10.1186/s40780-016-0044-9
Encalada Ventura, M. A., van Wanrooy, M. J., Span, L. F., Rodgers, M. G., van den Heuvel, E. R., Uges, D. R., et al. (2016). Longitudinal analysis of the effect of inflammation on voriconazole trough concentrations. Antimicrob. Agents Chemother. 60 (5), 2727–2731. doi:10.1128/AAC.02830-15
Geist, M. J., Egerer, G., Burhenne, J., Riedel, K. D., Weiss, J., and Mikus, G. (2013). Steady-state pharmacokinetics and metabolism of voriconazole in patients. J. Antimicrob. Chemother. 68 (11), 2592–2599. doi:10.1093/jac/dkt229
Hamada, Y., Ueda, T., Miyazaki, Y., Nakajima, K., Fukunaga, K., Miyazaki, T., et al. (2020). Effects of antifungal stewardship using therapeutic drug monitoring in voriconazole therapy on the prevention and control of hepatotoxicity and visual symptoms: A multicentre study conducted in Japan. Mycoses 63 (8), 779–786. doi:10.1111/myc.13129
Hesstvedt, L., Gaustad, P., Muller, F., Torp Andersen, C., Brunborg, C., Mylvaganam, H., et al. (2019). The impact of age on risk assessment, therapeutic practice and outcome in candidemia. Infect. Dis. (Lond) 51 (6), 425–434. doi:10.1080/23744235.2019.1595709
Jenks, J. D., Cornely, O. A., Chen, S. C., Thompson, G. R., and Hoenigl, M. (2020). Breakthrough invasive fungal infections: Who is at risk? Mycoses 63 (10), 1021–1032. doi:10.1111/myc.13148
Jenne, C. N., Urrutia, R., and Kubes, P. (2013). Platelets: Bridging hemostasis, inflammation, and immunity. Int. J. Lab. Hematol. 35 (3), 254–261. doi:10.1111/ijlh.12084
Jin, H., Wang, T., Falcione, B. A., Olsen, K. M., Chen, K., Tang, H., et al. (2016). trough concentration of voriconazole and its relationship with efficacy and safety: A systematic review and meta-analysis. J. Antimicrob. Chemother. 71 (7), 1772–1785. doi:10.1093/jac/dkw045
Klein, M., Thomas, M., Hofmann, U., Seehofer, D., Damm, G., and Zanger, U. M. (2015). A systematic comparison of the impact of inflammatory signaling on absorption, distribution, metabolism, and excretion gene expression and activity in primary human hepatocytes and HepaRG cells. Drug Metab. Dispos. 43 (2), 273–283. doi:10.1124/dmd.114.060962
Li, A. P., Yang, Q., Vermet, H., Raoust, N., Klieber, S., and Fabre, G. (2014). Evaluation of human hepatocytes under prolonged culture in a novel medium for the maintenance of hepatic differentiation: Results with the model pro-inflammatory cytokine interleukin 6. Drug Metab. Lett. 8 (1), 12–18. doi:10.2174/187231280801140929155351
Liang, Z., Yu, M., Liu, Z., Liu, F., Jia, C., Xiong, L., et al. (2022). Inflammation affects liver function and the metabolism of voriconazole to voriconazole-N-oxide in adult and elderly patients. Front. Pharmacol. 13, 835871. doi:10.3389/fphar.2022.835871
Manne, B. K., Xiang, S. C., and Rondina, M. T. (2017). Platelet secretion in inflammatory and infectious diseases. Platelets 28 (2), 155–164. doi:10.1080/09537104.2016.1240766
Matthaiou, D. K., Dimopoulos, G., Taccone, F. S., Bulpa, P., Van den Abeele, A. M., Misset, B., et al. (2018). Elderly versus nonelderly patients with invasive aspergillosis in the ICU: A comparison and risk factor analysis for mortality from the aspICU cohort. Med. Mycol. 56 (6), 668–678. doi:10.1093/mmy/myx117
Moriyama, B., Obeng, A. O., Barbarino, J., Penzak, S. R., Henning, S. A., Scott, S. A., et al. (2017). Clinical pharmacogenetics implementation consortium (CPIC) guidelines for CYP2C19 and voriconazole therapy. Clin. Pharmacol. Ther. 102 (1), 45–51. doi:10.1002/cpt.583
Mosedale, M., and Watkins, P. B. (2017). Drug-induced liver injury: Advances in mechanistic understanding that will inform risk management. Clin. Pharmacol. Ther. 101 (4), 469–480. doi:10.1002/cpt.564
Nicolai, L., and Massberg, S. (2020). Platelets as key players in inflammation and infection. Curr. Opin. Hematol. 27 (1), 34–40. doi:10.1097/MOH.0000000000000551
Niioka, T., Fujishima, N., Abumiya, M., Yamashita, T., Ubukawa, K., Nara, M., et al. (2017). Relationship between the CYP2C19 phenotype using the voriconazole-to-voriconazole N-oxide plasma concentration ratio and demographic and clinical characteristics of Japanese patients with different CYP2C19 genotypes. Ther. Drug Monit. 39 (5), 514–521. doi:10.1097/FTD.0000000000000441
Portier, I., and Campbell, R. A. (2021). Role of platelets in detection and regulation of infection. Arterioscler. Thromb. Vasc. Biol. 41 (1), 70–78. doi:10.1161/ATVBAHA.120.314645
Purkins, L., Wood, N., Ghahramani, P., Greenhalgh, K., Allen, M. J., and Kleinermans, D. (2002). Pharmacokinetics and safety of voriconazole following intravenous-to oral-dose escalation regimens. Antimicrob. Agents Chemother. 46 (8), 2546–2553. doi:10.1128/AAC.46.8.2546-2553.2002
Shang, S., Cheng, L., Li, X., Xiang, R., Yu, M., Xiong, L., et al. (2020). Effect of CYP2C19 polymorphism on the plasma voriconazole concentration and voriconazole-to-voriconazole-N-oxide concentration ratio in elderly patients. Mycoses 63 (11), 1181–1190. doi:10.1111/myc.13105
Tang, D., Song, B. L., Yan, M., Zou, J. J., Zhang, M., Zhou, H. Y., et al. (2019). Identifying factors affecting the pharmacokinetics of voriconazole in patients with liver dysfunction: A population pharmacokinetic approach. Basic Clin. Pharmacol. Toxicol. 125 (1), 34–43. doi:10.1111/bcpt.13208
Tang, D., Yan, M., Song, B. L., Zhao, Y. C., Xiao, Y. W., Wang, F., et al. (2021). Population pharmacokinetics, safety and dosing optimization of voriconazole in patients with liver dysfunction: A prospective observational study. Br. J. Clin. Pharmacol. 87 (4), 1890–1902. doi:10.1111/bcp.14578
Theuretzbacher, U., Ihle, F., and Derendorf, H. (2006). Pharmacokinetic/pharmacodynamic profile of voriconazole. Clin. Pharmacokinet. 45 (7), 649–663. doi:10.2165/00003088-200645070-00002
Tsay, S. V., Mu, Y., Williams, S., Epson, E., Nadle, J., Bamberg, W. M., et al. (2020). Burden of candidemia in the United States, 2017. Clin. Infect. Dis. 71 (9), e449–e453. doi:10.1093/cid/ciaa193
Ullmann, A. J., Aguado, J. M., Arikan-Akdagli, S., Denning, D. W., Groll, A. H., Lagrou, K., et al. (2018). Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 24 (1), e1–e38. doi:10.1016/j.cmi.2018.01.002
Vallabhaneni, S., Benedict, K., Derado, G., and Mody, R. K. (2017). Trends in hospitalizations related to invasive aspergillosis and mucormycosis in the United States, 2000-2013. Open Forum Infect. Dis. 4 (1), ofw268. doi:10.1093/ofid/ofw268
Vanstraelen, K., Wauters, J., Vercammen, I., de Loor, H., Maertens, J., Lagrou, K., et al. (2014). Impact of hypoalbuminemia on voriconazole pharmacokinetics in critically ill adult patients. Antimicrob. Agents Chemother. 58 (11), 6782–6789. doi:10.1128/AAC.03641-14
Veringa, A., Ter Avest, M., Span, L. F., van den Heuvel, E. R., Touw, D. J., Zijlstra, J. G., et al. (2017). Voriconazole metabolism is influenced by severe inflammation: A prospective study. J. Antimicrob. Chemother. 72 (1), 261–267. doi:10.1093/jac/dkw349
Vreugdenhil, B., van der Velden, W., Feuth, T., Kox, M., Pickkers, P., van de Veerdonk, F. L., et al. (2018). Moderate correlation between systemic IL-6 responses and CRP with trough concentrations of voriconazole. Br. J. Clin. Pharmacol. 84 (9), 1980–1988. doi:10.1111/bcp.13627
Yan, M., Wu, Z. F., Tang, D., Wang, F., Xiao, Y. W., Xu, P., et al. (2018). The impact of proton pump inhibitors on the pharmacokinetics of voriconazole in vitro and in vivo. Biomed. Pharmacother. 108, 60–64. doi:10.1016/j.biopha.2018.08.121
Yasu, T., Konuma, T., Kuroda, S., Takahashi, S., and Tojo, A. (2018). Effect of cumulative intravenous voriconazole dose on renal function in hematological patients. Antimicrob. Agents Chemother. 62 (9), e00507–e00518. doi:10.1128/AAC.00507-18
Zeng, G., Wang, L., Shi, L., Li, H., Zhu, M., Luo, J., et al. (2020). Variability of voriconazole concentrations in patients with hematopoietic stem cell transplantation and hematological malignancies: Influence of loading dose, procalcitonin, and pregnane X receptor polymorphisms. Eur. J. Clin. Pharmacol. 76 (4), 515–523. doi:10.1007/s00228-020-02831-1
Zhao, Y. C., Lin, X. B., Zhang, B. K., Xiao, Y. W., Xu, P., Wang, F., et al. (2021b). Predictors of adverse events and determinants of the voriconazole trough concentration in kidney transplantation recipients. Clin. Transl. Sci. 14 (2), 702–711. doi:10.1111/cts.12932
Zhao, Y., Xiao, C., Hou, J., Wu, J., Xiao, Y., Zhang, B., et al. (2021a). A large sample retrospective study on the distinction of voriconazole concentration in asian patients from different clinical departments. Pharm. (Basel) 14 (12), 1239. doi:10.3390/ph14121239
Keywords: voriconazole, voriconazole-N-oxide, total bile acid, platelet count, estimated glomerular filtration rate, IL-6
Citation: Cheng L, Liang Z, Liu F, Lin L, Zhang J, Xie L, Yu M and Sun F (2023) Factors influencing plasma concentration of voriconazole and voriconazole- N-oxide in younger adult and elderly patients. Front. Pharmacol. 14:1126580. doi: 10.3389/fphar.2023.1126580
Received: 18 December 2022; Accepted: 03 February 2023;
Published: 13 February 2023.
Edited by:
Pallavi R. Devchand, University of Calgary, CanadaReviewed by:
Fu Liu, Affiliated Hospital of North Sichuan Medical College, ChinaCopyright © 2023 Cheng, Liang, Liu, Lin, Zhang, Xie, Yu and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingjie Yu, eW1qeG55eUAxNjMuY29t; Fengjun Sun, ZmVuZ2pfc3VuQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.