
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pharmacol. , 06 February 2023
Sec. Neuropharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1126235
This article is part of the Research Topic Novel Therapeutic Target and Drug Discovery for Neurological Diseases, Volume II View all 33 articles
Anlotinib is an oral multi-targeted tyrosine kinase inhibitor as a third-line and subsequent treatment for patients with small cell lung cancer (SCLC) in China. The neurotoxicity is less reported. Posterior reversible encephalopathy syndrome (PRES) is characterized by headaches, seizures, encephalopathy, and visual disturbances, as well as focal reversible vasogenic edema seen on neuroimages. Here, we presented a case of PRES in a small cell lung cancer (SCLC) patient associated with anlotinib. A 37-year-old female patient, who had a history of diabetes, with extensive-stage SCLC received anlotinib after third-line chemotherapy. Ten cycles of anlotinib later, the patient experienced visual disturbance and was diagnosed with PRES based on the typical demyelination of white matter obtained in the brain magnetic resonance. During anlotinib therapy, the patient did not develop anti-VEGF therapy-induced hypertension. Subsequently, the patient stopped anlotinib, but she did not recover from symptoms. We also summarized the characteristics of fifty-four cases of PRES caused by antiangiogenic drugs in the literature. Based on our experience and the literature review, the incidence of PRES induced by antiangiogenic drugs is low, and the symptom can resolve upon stopping the medications. However, some cases still have a poor prognosis and the underlying mechanism requires further investigation. In addition, early detection and treatment of PRES are essential for physicians.
Small cell lung cancer (SCLC) accounts for approximately 15% of all newly diagnosed lung cancer cases and about 60%–70% of patients present with extensive-stage disease (ES-SCLC) at the initial visit (Siegel et al., 2017; Huang et al., 2021). Progress in the treatment of ES-SCLC over the past 30 years has been modest. Chemotherapy with platinum (cisplatin or carboplatin) plus etoposide is the standard first-line therapy for patients with ES-SCLC (Zugazagoitia and Paz-Ares, 2022). To date, topotecan is the only Food and Drug Administration (FDA) approved second-line drug for the treatment of recurrent metastatic SCLC (Ganti et al., 2021). Currently, there was no standard third-line therapy recommended in guidelines (Dingemans et al., 2021; Ganti et al., 2021).
Anlotinib hydrochloride is an oral multi-targeted tyrosine kinase inhibitor of vascular endothelial growth factor receptor (VEGFR)-1/2/3, fibroblast growth factor receptor (FGFR)-1-4, platelet-derived growth factor receptor-α/β (PDGFR-α/β) and c-kit-proto-oncogene-protein (c-Kit). Cheng et al. launched a Phase 2 trial to evaluate the efficacy and safety of anlotinib as a third-line and subsequent treatment for patients with SCLC in China (ALTER 1202) and found that anlotinib showed improved PFS and OS than placebo with favorable safety profile (Cheng et al., 2021). In this trial, the most common adverse events (AEs) of anlotinib were hypertension, fatigue, thyroid-stimulating hormone elevation, anorexia, hypertriglyceridemia, hand-foot syndrome, and hypercholesterolemia (Cheng et al., 2021). And the most common grade 3 or higher AEs were hypertension (11 [13.6%]), gamma-glutamyl transpeptidase elevation (4 [4.9%]), and hand and foot skin reaction (4 [4.9%]) (Cheng et al., 2021). Similar results were seen in the ALTER0303, ALTER 0703, ALTER 01031 and a double-blind randomized phase 2 trials (Cheng et al., 2021; Chi et al., 2021; Huang et al., 2021; Huang et al., 2021; Li et al., 2021). However, the neurotoxicity was less reported. The most common AEs related to nervous system disorder was reported to be headache (33 [11.2%]) in the ALTER0303 trial (Han et al., 2018). In addition, there were a few rare AEs reported such as insomnia, sensory neurotoxicity, and seizure (Huang et al., 2021; Liu et al., 2021; Lu et al., 2022; Nie et al., 2022; Shao et al., 2022; Zou et al., 2022). Although a few cases of posterior reversible encephalopathy syndrome (PRES) caused by some anti-angiogenic drugs were reported in the literature (Koopman et al., 2008; Chelis et al., 2012; Myint et al., 2014; Sharma et al., 2016; Saraceno et al., 2017; Miaris et al., 2019; Van Pelt et al., 2020), no PRES has been previously reported in SCLC patients treated with anlotinib.
Here, we present a case with ES-SCLC who achieved a significant progression-free survival benefit from anlotinib as fourth-line therapy, report the PRES during anlotinib treatment, and review the characteristics of PRES induced by antiangiogenic therapy.
A 37-year-old Chinese woman was admitted to the hospital with repeated cough in February 2019. The patient had a 3-year history of diabetes and no smoking history. Chest computed tomography (CT) scan revealed a mass in the lower lobe of the left lung. Fiberoptic bronchoscopy revealed a tumor in the left lower lobe. Pathologic examination of the biopsies showed SCLC. Positron emission tomography (PET)/CT scan showed a huge soft tissue mass in the lower lobe of the left lung and left hilar nodule metastasis. Accordingly, she was diagnosed with left lung SCLC with limited-stage (LS-SCLC) based on the Veterans Administration Lung Study Group (VALSG) 2-stage classification scheme (Kalemkerian and Gadgeel, 2013). Subsequently, she received first-line treatment with etoposide/cisplatin (EP) for four cycles plus definitive thoracic radiotherapy (RT). However, 1 month after the completion of initial therapy, abdominal CT revealed right adrenal metastasis. Then she received four cycles of irinotecan as the second-line treatment from November 2019 to January 2020 and followed by radiation therapy for right adrenal metastasis. On 29 March 2020, her brain magnetic resonance imaging (MRI) showed the left basal ganglia metastasis (Figure 1A). Meanwhile, the abdominal CT scan discovered left adrenal metastasis. And she was diagnosed with hyponatremia (121 mmol/L) and the serum neuron-specific enolase (NSE) (45.1 ng/mL) level was higher than the normal range. Then she received whole-brain radiation therapy (WBRT) of a total dose of 37.5 Gy (in 15 fractions), which had achieved radiographic complete regression (Figure 1B) and left adrenal gland radiation of a total dose of 46.0 Gy (in 23 fractions) from 9 April to 11 May 2020. At the same time, she received two cycles of chemotherapy with cyclophosphamide/adriamycin/cisplatin (CAP).
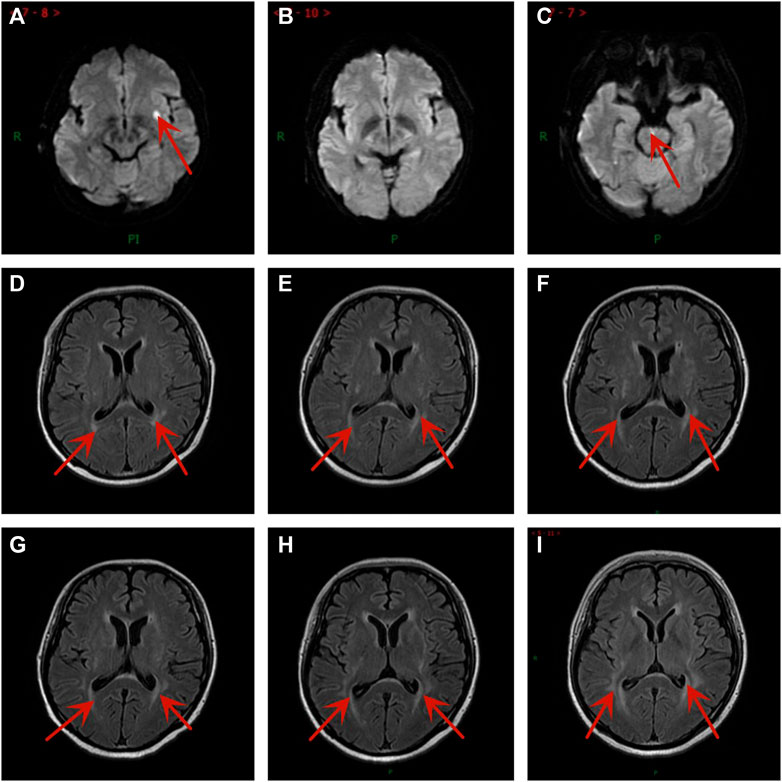
FIGURE 1. Brain magnetic resonance imaging images at different time points. (A) Brain metastases were newly diagnosed after second-line treatment. (B) One month after brain radiation therapy. (C) New brain metastasis after taking anlotinib 10 cycles. The PRES developed gradually after taking anlotinib for (D) 1 cycle, (E) 3 cycles, (F) 4 cycles, (G) 6 cycles, (H) 8 cycles, and (I) 10 cycles.
However, the patient complained about increasing cough with dyspnea on exertion and fatigue. The contrast-enhanced CT (10 June 2020) showed a 46 mm × 39 mm mass in the left lower lobe, which was larger than before (Figures 2A,B). No evidence of metastasis was found by the brain MRI. In addition, the serum NSE (35.4 ng/mL) level was also elevated. According to Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1), efficacy assessment was progression of disease (PD). Subsequently, we administered anlotinib (10 mg/day once daily orally, 2 weeks on and 1 week off) as the fourth-line treatment on 18 June 2020 according to the result of ALTER 1202 (Cheng et al., 2021).
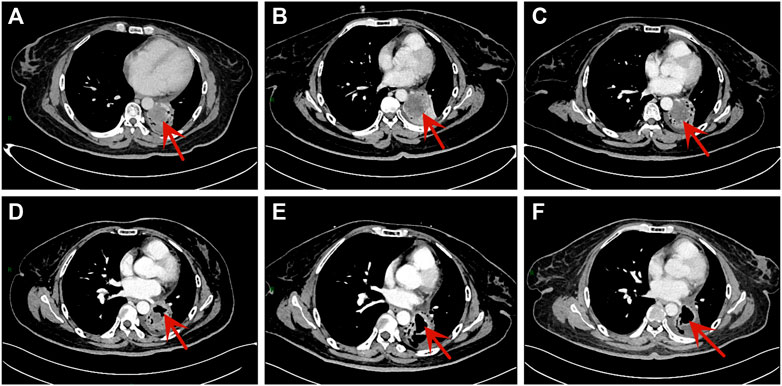
FIGURE 2. Chest CT scans of the patient. (A) The scan was performed after second-line treatment. (B) The scan was performed after third-line treatment. (C) The scan was performed after taking anlotinib for one cycle. (D)The scan showed tumor cavity formation after taking anlotinib for six cycles. (E) The scan showed tumor cavity was larger after taking anlotinib for eight cycles. (F) The tumor cavity didn’t change significantly after taking anlotinib for 10 cycles.
After one cycle of anlotinib, we performed the first evaluation by CT and brain MRI, which showed a stable disease (SD) (Figure 2C). Simultaneously, not only the level of NSE (21.2 ng/mL) declined rapidly, but also the sodium level was restored to normal. Then the efficacy was evaluated once every two cycles. After six cycles, she had a reduced cough with her Eastern Cooperative Oncology Group (ECOG) performance status ≤1 point. Additionally, the serum sodium and NSE level remained normal. Chest CT showed tumor cavity formation and tumor assessment was SD (Figures 2D, E, F).
During the treatment with anlotinib (10 mg), the most severe adverse effect was oral ulcer, which was evaluated as graded 3 according to National Cancer Institute Common Toxicity Criteria for Adverse Events (NCI-CTCAE, version 5.0). As a result, we reduced the dose of anlotinib from 10mg to 8 mg on 18 November 2020. Her brain MRI showed mild demyelination of white matter after one cycle with anlotinib and the extension of the demyelinating lesions increased slowly (Figures 1D–I), but she had no neurological symptoms. Encouraged by the result, the treatment was not interrupted.
After taking anlotinib for 7.5 months, the patient suffered from loss of appetite, weight loss, excessive fatigue, and visual disturbance. The enhanced CT scan showed multiple metastatic lesions in the bilateral ovaries on 2 February 2021. Her contrast-enhanced MRI showed the demyelination of white matter in T2-weighted fluid attenuated inversion recovery (FLAIR) images and the pons had enhancement lesions, which was considered metastasis (Figure 1C). We thought the visual disturbance was related to PRES, not brain metastases. The tumor assessment was PD. So anlotinib was discontinued. During the anlotinib therapy, the patient did not develop anti-VEGF therapy-induced hypertension. Two months later, the blurred vision was not restored, and there were no significant changes in her brain MRI. In conclusion, she gained a progression-free survival (PFS) of 7.5 months. Later, the patient had received a clinical trial and the best supportive treatment. Sadly, she passed away on 14 January 2022.
PRES, also known as Reversible posterior leukoencephalopathy syndrome (RPLS), is a brain-capillary leak syndrome related to hypertension, fluid retention, and the cytotoxic effects of immunosuppressive agents on the vascular endothelium, characterized by seizures, headache, altered mental status, and visual disturbances (Mergen et al., 2021). At present, the diagnosis of PRES mainly relies on MRI findings (Lee et al., 2012), characterized by white matter vasogenic edema affecting the brain’s posterior occipital and parietal lobes (Fugate and Rabinstein, 2015). Classical MRI findings include high signal intensity on T2-weighted and FLAIR images, predominantly in the posterior regions, particularly parieto-occipital lobes (Lee et al., 2012). However, there were PRES cases also occurring in the frontal lobes, basal ganglia, thalamus, and brainstem (Ahn et al., 2004). If promptly recognized and treated, the prognosis is usually favorable and the changes, seen in MRI, resolve over days to weeks (Fugate and Rabinstein, 2015).
Many drugs can cause PRES (Abughanimeh et al., 2018; Vilas-Boas and Corte-Real, 2019; Kaur et al., 2020). With new antitumor agents widely used in the clinic in recent years, accumulating case reports have reported the occurrence of PRES in patients receiving targeted therapies, particularly antiangiogenic agents, such as bevacizumab (Hamid et al., 2018; Katada et al., 2018), sunitinib (Saraceno et al., 2017; Rifino et al., 2020), sorafenib (Dogan et al., 2010; Laruelle et al., 2018), pazopanib (Deguchi et al., 2018; Tatsumichi et al., 2021) and regorafenib (Aanes et al., 2018).
From 2006 to 2022, 54 cases of PRES receiving antiangiogenic therapies from the “PubMed” database were reported. Articles were limited to human cases and be published in English. The characteristics of 54 included cases were shown in Table 1. Of the 54 patients, 41 were females and 13 males, and the female-to-male ratio was about 3:1. The median age of patients was 56 years old at the time of diagnosis of PRES. Fifteen patients had a hypertension history and two patients had a diabetes history. Of these patients, 7 cases didn’t develop hypertension and 41 cases developed grade 3 to 4 hypertension according to NCI-CTCAE (version 5.0) during the antiangiogenic therapy. The median time interval from the start of antiangiogenic agents to PRES diagnosis was 33.5 days. As for the patients who had a hypertension history before receiving antiangiogenic therapies, the median time interval from the start of antiangiogenic agents to PRES diagnosis was 21 days, which was earlier than patients without a hypertension history. After discontinuing antiangiogenic therapy and receiving symptomatic treatments, almost all (98.2%) patients achieved complete response, but one patient died.
Table 2 summarized the common MRI findings in 54 PRES patients. Thirty-three patients’ imaging findings were symmetrical hemispheric vasogenic edema in the parietal occipital regions, fourteen in basal ganglia, frontal lobe, brainstem, cerebellar hemisphere and unilateral lesion, and seven in the watershed areas of the frontal, parietal, and occipital lobes.
Anlotinib demonstrated its efficacy and safety in the ALTER01031 (Li et al., 2021), ALTER0303 (Zhou et al., 2019), and ALTER1202 trials (Cheng et al., 2021), and no PRES was reported. We presented a female patient who contributed to PRES caused by anlotinib. This patient had only hyperglycemia without any other clinical or metabolic derangements and didn’t develop hypertension during the treatment. Of note, after discontinuation of anlotinib, she did not recover from the blurred vision and her brain MRI showed no decrease in cerebral white matter lesions.
The mechanism of PRES induced by antiangiogenic drugs is still unclear. A leading theory of the pathophysiological changes underlying PRES was that rapidly developing hypertension exceeded the upper limit of cerebral blood flow auto-regulation and caused hyper-perfusion (Fugate and Rabinstein, 2015; Parasher and Jhamb, 2020). Based on the literature review, 41 of 54 patients with PRES developed grade 3 to 4 hypertension. Therefore, anti-VEGF therapy-induced hypertension might be a major mechanism of PRES. However, this mechanism could not explain the development of PRES in the 15%–20% of patients who have no hypertension. Additional mechanisms to cause PRES in patients receiving antiangiogenic therapies might exist. Antiangiogenic drugs could lead to vasoconstriction and hypo-perfusion, leading to cerebral ischemia and subsequent vasogenic edema (Morbidelli et al., 2016; Shah, 2017; Gewirtz et al., 2021) (Supplementary Figure 1). Previous studies demonstrated that increased serum pro-inflammatory cytokines including circulating levels of interleukin (IL)-6 and tumor necrosis factor-alpha (TNF-alpha) regulated the expression of vascular endothelial growth factor and they might increase vascular permeability leading to vasogenic edema and cause endothelial damage or dysfunction in PRES (Esposito et al., 2002; Fugate and Rabinstein, 2015). Development of hyperglycemia results in increased serum pro-inflammatory cytokines, such as interleukin (IL)-6 and IL-1b, and C-reactive protein, which stimulates endothelial cells to secrete vasoactive factors and increases vascular permeability (Pickup, 2004; Vaarala and Yki-Järvinen, 2012; Li et al., 2014). Nishanth et al. previously reported one case with hyperglycemia-induced PRES (Dev et al., 2019). From what has been discussed above, hyperglycemia and anlotinib might jointly lead to PRES.
In the reviewed cases, we found that after drug withdrawal almost all (98.2%) patients achieved complete response, which was higher than the incidence (56%–72%) of PRES induced by other causative factors (Bartynski and Boardman, 2007; Legriel et al., 2012). The underlying reversible mechanism was related to reversible dysregulation of the cerebral vasculature (Singhal, 2021), which was completely different from the neurotoxic transformation caused by neurofibrillary tangles in Alzheimer’s disease (Zeng et al., 2022). Higher glycemia on day 1 was a strong predictor of poor outcomes by day 90 after admission for severe PRES in a retrospective cohort study (Legriel et al., 2012). Our case gave the similar outcome, the irreversible changes such as blurred vision. In addition, the patient received WBRT before taking anlotinib. Late radiation-related complications usually occurred more than 6 months after radiotherapy, tended to be irreversible and often progressive (Wujanto et al., 2021). The pathogenesis of late complications was often seen in the white matter and was linked to persistent demyelination, reduced neurogenesis with altered neural stem cell differentiation, inflammatory response through oxidative damage, and disruption of microvasculature resulting in ischaemia and toxic neuro-excitation (Wilke et al., 2018). Thus, hyperglycemia and WBRT was likely to exacerbate the irreversible neurological injury of this case. As we know, once PRES induced by anti-VEGF therapy was confirmed, the therapy should be discontinued. However, how to improve the therapeutic effects for unrecovered patients still requires further investigation.
Based on our experience and the literature review, the incidence of PRES induced by antiangiogenic drugs including anlotinib is low, and the symptom can resolve upon stopping the medications. However, some cases still have a poor prognosis and the underlying mechanism requires further investigation. In addition, early detection and treatment of PRES are very important for physicians.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This work was supported by the Natural Science Foundation of Shandong Province (ZR2020MH210).
Thanks to the support of the Natural Science Foundation of Shandong Province (ZR2020MH210).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1126235/full#supplementary-material
Aanes, S. G., Nieder, C., Prytz, J., and Odeh, F. (2018). A man in his 50s with neurological symptoms during cancer treatment. Tidsskr. Nor. Laegeforen 138 (17). doi:10.4045/tidsskr.18.0096
Abbas, O., Shamseddin, A., Temraz, S., and Haydar, A. (2013). Posterior reversible encephalopathy syndrome after bevacizumab therapy in a normotensive patient. BMJ Case Rep. 2013, bcr2012007995. doi:10.1136/bcr-2012-007995
Abughanimeh, O., Abu Ghanimeh, M., Qasrawi, A., Al Momani, L. A., and Madhusudhana, S. (2018). Trastuzumab-associated posterior reversible encephalopathy syndrome. Cureus 10 (5), e2686. doi:10.7759/cureus.2686
Ahn, K. J., You, W. J., Jeong, S. L., Lee, J. W., Kim, B. S., Lee, J. H., et al. (2004). Atypical manifestations of reversible posterior leukoencephalopathy syndrome: Findings on diffusion imaging and ADC mapping. Neuroradiology 46 (12), 978–983. doi:10.1007/s00234-004-1276-1
Allen, J. A., Adlakha, A., and Bergethon, P. R. (2006). Reversible posterior leukoencephalopathy syndrome after bevacizumab/FOLFIRI regimen for metastatic colon cancer. Arch. Neurol. 63 (10), 1475–1478. doi:10.1001/archneur.63.10.1475
Arslan, B. M., Bajrami, A., Demir, E., Cabalar, M., and Yayla, V. (2017). Pazopanib induced unilateral posterior reversible encephalopathy syndrome. Ideggyogy Sz. 70 (3-4), 140–144. doi:10.18071/isz.70.0001
Asaithambi, G., Peters, B. R., Hurliman, E., Moran, B. P., Khan, A. S., and Taylor, R. A. (2013). Posterior reversible encephalopathy syndrome induced by pazopanib for renal cell carcinoma. J. Clin. Pharm. Ther. 38 (2), 175–176. doi:10.1111/jcpt.12031
Bartynski, W. S., and Boardman, J. F. (2007). Distinct imaging patterns and lesion distribution in posterior reversible encephalopathy syndrome. AJNR Am. J. Neuroradiol. 28 (7), 1320–1327. doi:10.3174/ajnr.A0549
Burki, F., Badie, K., Bartoli, P., Bernard, P., Montastruc, J. L., and Bagheri, H. (2008). Reversible posterior leukoencephalopathy syndrome associated with bevacizumab/doxorubicin regimen. Br. J. Clin. Pharmacol. 65 (5), 793–794. doi:10.1111/j.1365-2125.2008.03119.x
Chang, Y., Mbeo, G., and Littman, S. J. (2012). Reversible posterior leukoencephalopathy syndrome associated with concurrent bevacizumab, gemcitabine, and oxaliplatin for cholangiocarcinoma. J. Gastrointest. Cancer 43 (3), 505–507. doi:10.1007/s12029-011-9279-8
Chelis, L., Souftas, V., Amarantidis, K., Xenidis, N., Chamalidou, E., Dimopoulos, P., et al. (2012). Reversible posterior leukoencephalopathy syndrome induced by pazopanib. BMC Cancer 12, 489. doi:10.1186/1471-2407-12-489
Chen, A., and Agarwal, N. (2009). Reversible posterior leucoencephalopathy syndrome associated with sunitinib. Intern Med. J. 39 (5), 341–342. doi:10.1111/j.1445-5994.2009.01908.x
Cheng, Y., Wang, Q., Li, K., Shi, J., Liu, Y., Wu, L., et al. (2021). Anlotinib vs placebo as third- or further-line treatment for patients with small cell lung cancer: A randomised, double-blind, placebo-controlled phase 2 study. Br. J. Cancer 125 (3), 366–371. doi:10.1038/s41416-021-01356-3
Chi, Y., Shu, Y., Ba, Y., Bai, Y., Qin, B., Wang, X., et al. (2021). Anlotinib monotherapy for refractory metastatic colorectal cancer: A double-blinded, placebo-controlled, randomized phase III trial (ALTER0703). Oncologist 26 (10), e1693–e1703. doi:10.1002/onco.13857
Cumurciuc, R., Martinez-Almoyna, L., Henry, C., Husson, H., and de Broucker, T. (2008). Posterior reversible encephalopathy syndrome during sunitinib therapy. Rev. Neurol. Paris. 164 (6-7), 605–607. doi:10.1016/j.neurol.2008.03.007
Deguchi, S., Mitsuya, K., Nakasu, Y., Hayashi, N., Katagiri, H., Murata, H., et al. (2018). Posterior reversible encephalopathy syndrome (PRES) induced by pazopanib, a multi-targeting tyrosine kinase inhibitor, in a patient with soft-tissue sarcoma: Case report and review of the literature. Invest. New Drugs 36 (2), 346–349. doi:10.1007/s10637-017-0521-5
Dersch, R., Stich, O., Goller, K., Meckel, S., Dechent, F., Doostkam, S., et al. (2013). Atypical posterior reversible encephalopathy syndrome associated with chemotherapy with Bevacizumab, Gemcitabine and Cisplatin. J. Neurol. 260 (5), 1406–1407. doi:10.1007/s00415-013-6866-6
Dev, N., Kumar, R., Kumar, P., and Kumawat, A. (2019). Hyperglycemia-induced posterior reversible encephalopathy syndrome: A rare cause of reversible blindness. J. Fam. Med. Prim. Care 8 (10), 3431–3433. doi:10.4103/jfmpc.jfmpc_695_19
Dingemans, A. C., Früh, M., Ardizzoni, A., Besse, B., Faivre-Finn, C., Hendriks, L. E., et al. (2021). Small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up(☆). Ann. Oncol. 32 (7), 839–853. doi:10.1016/j.annonc.2021.03.207
Dogan, E., Aksoy, S., Arslan, C., Dede, D. S., and Altundag, K. (2010). Probable sorafenib-induced reversible encephalopathy in a patient with hepatocellular carcinoma. Med. Oncol. 27 (4), 1436–1437. doi:10.1007/s12032-009-9378-6
Dos Reis Simões da Silva, F. M., Burgos Pêgo, P. M., Henriques Vendrell, M. C., de Azevedo Batalha Ferreira Dos Santos Farias, M. J., Ribeiro Timóteo Â, C., Martins da Costa, M. C., et al. (2011). Posterior reversible encephalopathy syndrome and anti-angiogenic agents: A case report. Neuroophthalmology 35 (1), 32–37. doi:10.3109/01658107.2010.539763
Duchnowska, R., Miciuk, B., Bodnar, L., Waśniewski, L., and Szczylik, C. (2013). Severe neurological symptoms in a patient with advanced renal cell carcinoma treated with sunitinib. J. Oncol. Pharm. Pract. 19 (2), 186–189. doi:10.1177/1078155212457967
Elmalik, H. H., ElAzzazy, S., Salem, K. S., and Bujassoum, S. (2015). A grave outcome of posterior reversible encephalopathy syndrome in a patient receiving avastin (bevacizumab) for metastatic high-grade serous ovarian cancer. Case Rep. Oncol. 8 (2), 290–294. doi:10.1159/000435805
Eryılmaz, M. K., Mutlu, H., Salim, D. K., Musri, F. Y., and Coşkun, H. (2016). Fatal posterior revesible leukoencephalopathy syndrome associated coma induced by bevacizumab in metastatic colorectal cancer and review of literature. J. Oncol. Pharm. Pract. 22 (6), 806–810. doi:10.1177/1078155215611048
Esposito, K., Nappo, F., Marfella, R., Giugliano, G., Giugliano, F., Ciotola, M., et al. (2002). Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: Role of oxidative stress. Circulation 106 (16), 2067–2072. doi:10.1161/01.cir.0000034509.14906.ae
Foerster, R., Welzel, T., Debus, J., Gruellich, C., Jaeger, D., and Potthoff, K. (2013). Posterior reversible leukoencephalopathy syndrome associated with pazopanib. Case Rep. Oncol. 6 (1), 204–208. doi:10.1159/000350742
Frantzen, L., Rondeau-Lutz, M., Mosquera, F., Martinez, C., Labani, A., and Weber, J. C. (2016). Reversible posterior encephalopathy syndrome and cardiomyopathy after bevacizumab therapy. Rev. Med. Interne 37 (1), 50–52. doi:10.1016/j.revmed.2015.04.011
Fugate, J. E., and Rabinstein, A. A. (2015). Posterior reversible encephalopathy syndrome: Clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 14 (9), 914–925. doi:10.1016/s1474-4422(15)00111-8
Furubayashi, N., Negishi, T., Iwai, H., Nagase, K., and Nakamura, M. (2017). Sorafenib-induced reversible posterior leukoencephalopathy in patients with renal cell carcinoma: A report of two cases. Mol. Clin. Oncol. 7 (2), 281–284. doi:10.3892/mco.2017.1291
Ganti, A. K. P., Loo, B. W., Bassetti, M., Blakely, C., Chiang, A., D'Amico, T. A., et al. (2021). Small cell lung cancer, version 2.2022, NCCN clinical practice guidelines in Oncology. J. Natl. Compr. Canc Netw. 19 (12), 1441–1464. doi:10.6004/jnccn.2021.0058
Gewirtz, A. N., Gao, V., Parauda, S. C., and Robbins, M. S. (2021). Posterior reversible encephalopathy syndrome. Curr. Pain Headache Rep. 25 (3), 19. doi:10.1007/s11916-020-00932-1
Goto, N., and Mimura, J. (2014). Gastrointestinal: Bevacizumab-induced reversible posterior leukoencephalopathy syndrome in patient with rectal cancer. J. Gastroenterol. Hepatol. 29 (5), 895. doi:10.1111/jgh.12569
Hamid, M., Ghani, A., Micaily, I., Sarwar, U., Lashari, B., and Malik, F. (2018). Posterior reversible encephalopathy syndrome (PRES) after bevacizumab therapy for metastatic colorectal cancer. J. Community Hosp. Intern Med. Perspect. 8 (3), 130–133. doi:10.1080/20009666.2018.1478563
Han, B., Li, K., Wang, Q., Zhang, L., Shi, J., Wang, Z., et al. (2018). Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: The ALTER 0303 phase 3 randomized clinical trial. JAMA Oncol. 4 (11), 1569–1575. doi:10.1001/jamaoncol.2018.3039
Huang, J., Xiao, J., Fang, W., Lu, P., Fan, Q., Shu, Y., et al. (2021a). Anlotinib for previously treated advanced or metastatic esophageal squamous cell carcinoma: A double-blind randomized phase 2 trial. Cancer Med. 10 (5), 1681–1689. doi:10.1002/cam4.3771
Huang, L. L., Hu, X. S., Wang, Y., Li, J. L., Wang, H. Y., Liu, P., et al. (2021b). Survival and pretreatment prognostic factors for extensive-stage small cell lung cancer: A comprehensive analysis of 358 patients. Thorac. Cancer 12 (13), 1943–1951. doi:10.1111/1759-7714.13977
Huang, N. S., Wei, W. J., Xiang, J., Chen, J. Y., Guan, Q., Lu, Z. W., et al. (2021c). The efficacy and safety of anlotinib in neoadjuvant treatment of locally advanced thyroid cancer: A single-arm phase II clinical trial. Thyroid 31 (12), 1808–1813. doi:10.1089/thy.2021.0307
Kalemkerian, G. P., and Gadgeel, S. M. (2013). Modern staging of small cell lung cancer. J. Natl. Compr. Canc Netw. 11 (1), 99–104. doi:10.6004/jnccn.2013.0012
Katada, E., Mitsui, A., Sasaki, S., Uematsu, N., and Anan, C. (2018). Posterior reversible encephalopathy syndrome after a variety of combined chemotherapies containing bevacizumab for metastatic colon cancer. Intern Med. 57 (16), 2403–2407. doi:10.2169/internalmedicine.0284-17
Kaur, G., Ashraf, I., Peck, M. M., Maram, R., Mohamed, A., Ochoa Crespo, D., et al. (2020). Chemotherapy and immunosuppressant therapy-induced posterior reversible encephalopathy syndrome. Cureus 12 (10), e11163. doi:10.7759/cureus.11163
Khan, K. H., Fenton, A., Murtagh, E., McAleer, J. J., and Clayton, A. (2012). Reversible posterior leukoencephalopathy syndrome following sunitinib therapy: A case report and review of the literature. Tumori 98 (5), 139e–142e. doi:10.1700/1190.13216
Kim, C. A., Price-Hiller, J., Chu, Q. S., Tankel, K., Hennig, R., Sawyer, M. B., et al. (2014). Atypical reversible posterior leukoencephalopathy syndrome (RPLS) induced by cediranib in a patient with metastatic rectal cancer. Invest. New Drugs 32 (5), 1036–1045. doi:10.1007/s10637-014-0113-6
Koopman, M., Muller, E. W., and Punt, C. J. (2008). Reversible posterior leukoencephalopathy syndrome caused by bevacizumab: Report of a case. Dis. Colon Rectum 51 (9), 1425–1426. doi:10.1007/s10350-008-9282-8
Laruelle, M., Filleul, B., Duprez, T., and Machiels, J. P. (2018). Posterior reversible encephalopathy syndrome associated with sorafenib and successful retreatment. Urol. Int. 100 (3), 357–360. doi:10.1159/000443970
Lau, P. C., and Paunipagar, B. (2011). Posterior reversible encephalopathy syndrome with bevacizumab. Hong Kong Med. J. 17 (1), 80–81.doi:10.1080/20009666.2018.1478563
Lazarus, M., Amundson, S., and Belani, R. (2012). An association between bevacizumab and recurrent posterior reversible encephalopathy syndrome in a patient presenting with deep vein thrombosis: A case report and review of the literature. Case Rep. Oncol. Med. 2012, 819546. doi:10.1155/2012/819546
Lee, E. Q., Arrillaga-Romany, I. C., and Wen, P. Y. (2012). Neurologic complications of cancer drug therapies. Contin. (Minneap Minn) 18 (2), 355–365. doi:10.1212/01.Con.0000413663.42798.64
Legriel, S., Schraub, O., Azoulay, E., Hantson, P., Magalhaes, E., Coquet, I., et al. (2012). Determinants of recovery from severe posterior reversible encephalopathy syndrome. PLoS One 7 (9), e44534. doi:10.1371/journal.pone.0044534
Levy, A., Benmoussa, L., Ammari, S., Albiges, L., and Escudier, B. (2014). Reversible posterior leukoencephalopathy syndrome induced by axitinib. Clin. Genitourin. Cancer 12 (1), e33–e34. doi:10.1016/j.clgc.2013.08.008
Li, D., Chi, Y., Chen, X., Ge, M., Zhang, Y., Guo, Z., et al. (2021). Anlotinib in locally advanced or metastatic medullary thyroid carcinoma: A randomized, double-blind phase IIB trial. Clin. Cancer Res. 27 (13), 3567–3575. doi:10.1158/1078-0432.Ccr-20-2950
Li, J., Huang, M., and Shen, X. (2014). The association of oxidative stress and pro-inflammatory cytokines in diabetic patients with hyperglycemic crisis. J. Diabetes Complicat. 28 (5), 662–666. doi:10.1016/j.jdiacomp.2014.06.008
Li, X., Chai, J., Wang, Z., Lu, L., Zhao, Q., Zhou, J., et al. (2018). Reversible posterior leukoencephalopathy syndrome induced by apatinib: A case report and literature review. Onco Targets Ther. 11, 4407–4411. doi:10.2147/ott.S166605
Liu, J., Deng, Y. T., and Jiang, Y. (2021). Switch maintenance therapy with anlotinib after chemotherapy in unresectable or metastatic soft tissue sarcoma: A single-center retrospective study. Invest. New Drugs 39 (2), 330–336. doi:10.1007/s10637-020-01015-z
Lou, E., Turner, S., Sumrall, A., Reardon, D. A., Desjardins, A., Peters, K. B., et al. (2011). Bevacizumab-induced reversible posterior leukoencephalopathy syndrome and successful retreatment in a patient with glioblastoma. J. Clin. Oncol. 29 (28), e739–e742. doi:10.1200/jco.2011.36.1865
Lu, S., Hong, Y., Chen, H., Wu, L., Sun, F., Wang, J., et al. (2022). The efficacy and safety of anlotinib in pediatric patients with refractory or recurrent Solid tumors. Front. Pharmacol. 13, 711704. doi:10.3389/fphar.2022.711704
Lv, Y., Zhang, Y., Zhang, J., Liang, N., Liu, F., and Liu, R. (2019). Reversible posterior leukoencephalopathy syndrome following apatinib for gastric cancer in an adult: A case report and a review of the literature. Med. Baltim. 98 (46), e17787. doi:10.1097/MD.0000000000017787
Martin, G., Bellido, L., and Cruz, J. J. (2007). Reversible posterior leukoencephalopathy syndrome induced by sunitinib. J. Clin. Oncol. 25 (23), 3559. doi:10.1200/JCO.2007.12.8710
Massey, J. (2017). Posterior reversible encephalopathy syndrome (PRES) with sub-arachnoid haemorrhage after bevacizumab and 5-FU. J. Clin. Neurosci. 40, 57–59. doi:10.1016/j.jocn.2017.01.005
Mergen, S., Long, B., and Matlock, A. (2021). Posterior reversible encephalopathy syndrome: A narrative review for emergency clinicians. J. Emerg. Med. 61 (6), 666–673. doi:10.1016/j.jemermed.2021.09.005
Miaris, N., Maltezou, M., Papaxoinis, G., Visvikis, A., and Samantas, E. (2017). Posterior reversible encephalopathy syndrome with concurrent nephrotic syndrome in a patient treated with pazopanib for metastatic renal cell carcinoma: Case report and review of the literature. Clin. Genitourin. Cancer 15 (1), e99–e103. doi:10.1016/j.clgc.2016.08.005
Miaris, N., Sgouros, J., Gerolympou, M., Spyropoulos, B., Drakopoulos, D., Gkoura, S., et al. (2019). Posterior reversible encephalopathy syndrome during treatment with aflibercept, 5-fluorouracil, leucovorin, and irinotecan for metastatic colorectal cancer. J. Gastrointest. Cancer 50 (1), 123–126. doi:10.1007/s12029-017-9986-x
Miller, A. H., Monteiro de Oliveira Novaes, J. A., Brock, P. A., and Sandoval, M. (2016). Posterior reversible encephalopathy syndrome after bevacizumab treatment presenting to the ED as chest pain and headache. Am. J. Emerg. Med. 34 (9), .e1911–1916. doi:10.1016/j.ajem.2016.02.033
Morbidelli, L., Donnini, S., and Ziche, M. (2016). Targeting endothelial cell metabolism for cardio-protection from the toxicity of antitumor agents. Cardiooncology 2 (1), 3. doi:10.1186/s40959-016-0010-6
Myint, Z. W., Sen, J. M., Watts, N. L., Druzgal, T. J., Nathan, B. R., Ward, M. D., et al. (2014). Reversible posterior leukoencephalopathy syndrome during regorafenib treatment: A case report and literature review of reversible posterior leukoencephalopathy syndrome associated with multikinase inhibitors. Clin. Colorectal Cancer 13 (2), 127–130. doi:10.1016/j.clcc.2013.12.003
Nakamura, K., Saiki, H., Muramatsu, H., Morinaga, S., Kobayashi, I., Kajikawa, K., et al. (2017). Axitinib-induced reversible posterior leukoencephalopathy syndrome in a patient with metastatic renal cell carcinoma. Int. Cancer Conf. J. 6 (4), 197–199. doi:10.1007/s13691-017-0306-x
Nie, C., He, Y., Lv, H., Gao, M., Gao, X., Chen, B., et al. (2022). Clinical study of anlotinib as third-line or above therapy in patients with advanced or metastatic gastric cancer: A multicenter retrospective study. Front. Oncol. 12, 885350. doi:10.3389/fonc.2022.885350
Ozcan, C., Wong, S. J., and Hari, P. (2006). Reversible posterior leukoencephalopathy syndrome and bevacizumab. N. Engl. J. Med. 354 (9), 980–982. doi:10.1155/2012/819546
Padhy, B. M., Shanmugam, S. P., Gupta, Y. K., and Goyal, A. (2011). Reversible posterior leucoencephalopathy syndrome in an elderly male on sunitinib therapy. Br. J. Clin. Pharmacol. 71 (5), 777–779. doi:10.1111/j.1365-2125.2010.03893.x
Parasher, A., and Jhamb, R. (2020). Posterior reversible encephalopathy syndrome (PRES): Presentation, diagnosis and treatment. Postgrad. Med. J. 96 (1140), 623–628. doi:10.1136/postgradmedj-2020-137706
Peter, S., Hausmann, N., Schuster, A., and Boehm, H. F. (2008). Reversible posterior leukoencephalopathy syndrome and intravenous bevacizumab. Clin. Exp. Ophthalmol. 36 (1), 94–96. doi:10.1111/j.1442-9071.2007.01658.x
Pickup, J. C. (2004). Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 27 (3), 813–823. doi:10.2337/diacare.27.3.813
Rifino, N., Mantero, V., Filizzolo, M. G., Basilico, P., Scaccabarozzi, C., Arnoffi, J., et al. (2020). Sunitinib associated posterior reversible encephalopathy syndrome in a patient treated for GIST. Acta Neurol. Belg 120 (4), 995–997. doi:10.1007/s13760-020-01367-6
Saraceno, L., Ricigliano, V. A. G., Cavalli, M., and Meola, G. (2017). Posterior reversible encephalopathy syndrome after long-term treatment with low-dose sunitinib: A case report. Neurol. Sci. 38 (6), 1119–1121. doi:10.1007/s10072-017-2851-7
Sawaya, R., Radwan, W., and Hammoud, S. (2014). Benign reversible encephalopathy syndrome after bevacizumab therapy for metastatic ovarian cancer. Med. Oncol. 31 (2), 831. doi:10.1007/s12032-013-0831-1
Sclafani, F., Giuseppe, G., Mezynksi, J., Collins, C., and Crown, J. (2012). Reversible posterior leukoencephalopathy syndrome and bevacizumab in breast cancer. J. Clin. Oncol. 30 (26), e257–e259. doi:10.1200/jco.2011.38.8942
Seet, R. C., and Rabinstein, A. A. (2012). Clinical features and outcomes of posterior reversible encephalopathy syndrome following bevacizumab treatment. Qjm 105 (1), 69–75. doi:10.1093/qjmed/hcr139
Shah, R. R. (2017). Anti-angiogenic tyrosine kinase inhibitors and reversible posterior leukoencephalopathy syndrome: Could hypomagnesaemia Be the trigger? Drug Saf. 40 (5), 373–386. doi:10.1007/s40264-017-0508-3
Shao, Y., Luo, Z., Yu, Y., He, Y., Liu, C., Chen, Q., et al. (2022). A real-world study of anlotinib as third-line or above therapy in patients with her-2 negative metastatic breast cancer. Front. Oncol. 12, 939343. doi:10.3389/fonc.2022.939343
Sharma, P., Abbas, M. K., and Huynh, M. (2016). Posterior reversible encephalopathy syndrome (PRES) presenting as status epilepticus: A case report and literature review. Conn Med. 80 (8), 475–478. doi:10.1016/j.amsu.2021.01.095
Siegel, R. L., Miller, K. D., and Jemal, A. (2017). Cancer statistics, 2017. CA Cancer J. Clin. 67 (1), 7–30. doi:10.3322/caac.21387
Singhal, A. B. (2021). Posterior reversible encephalopathy syndrome and reversible cerebral vasoconstriction syndrome as syndromes of cerebrovascular dysregulation. Contin. (Minneap Minn) 27 (5), 1301–1320. doi:10.1212/con.0000000000001037
Tatsumichi, T., Tanaka, H., Okazaki, T., Takahashi, K., Suzuki, K., Kawakita, K., et al. (2021). Uterine sarcoma with posterior reversible encephalopathy syndrome associated with pazopanib. J. Clin. Pharm. Ther. 46 (1), 223–226. doi:10.1111/jcpt.13261
Vaarala, O., and Yki-Järvinen, H. (2012). Diabetes Should we treat infection or inflammation to prevent T2DM? Nat. Rev. Endocrinol. 8 (6), 323–325. doi:10.1038/nrendo.2012.31
Van Pelt, Q., Stragier, E., Roelandt, P., and Van Cutsem, E. (2020). Posterior reversible encephalopathy syndrome secondary to oxaliplatin-based chemotherapy and regorafenib in metastastic colorectal cancer: Case reports and literature review. Acta Gastroenterol. Belg 83 (1), 47–50.doi:10.3816/CCC.2007.n.009
Vilas-Boas, S., and Corte-Real, A. (2019). Posterior reversible encephalopathy syndrome and azathioprine. Eur. J. Case Rep. Intern Med. 6 (1), 001032. doi:10.12890/2019_001032
Wang, W., Zhao, L. R., Lin, X. Q., and Feng, F. (2014). Reversible posterior leukoencephalopathy syndrome induced by bevacizumab plus chemotherapy in colorectal cancer. World J. Gastroenterol. 20 (21), 6691–6697. doi:10.3748/wjg.v20.i21.6691
Wilke, C., Grosshans, D., Duman, J., Brown, P., and Li, J. (2018). Radiation-induced cognitive toxicity: Pathophysiology and interventions to reduce toxicity in adults. Neuro Oncol. 20 (5), 597–607. doi:10.1093/neuonc/nox195
Wujanto, C., Vellayappan, B., Chang, E. L., Chao, S. T., Sahgal, A., and Lo, S. S. (2021). Radiotherapy to the brain: What are the consequences of this age-old treatment? Ann. Palliat. Med. 10 (1), 936–952. doi:10.21037/apm-20-856
Zeng, L., Jiang, H., Ashraf, G. M., Liu, J., Wang, L., Zhao, K., et al. (2022). Implications of miR-148a-3p/p35/PTEN signaling in tau hyperphosphorylation and autoregulatory feedforward of Akt/CREB in Alzheimer's disease. Mol. Ther. Nucleic Acids 27, 256–275. doi:10.1016/j.omtn.2021.11.019
Zhou, M., Chen, X., Zhang, H., Xia, L., Tong, X., Zou, L., et al. (2019). China national medical products administration approval summary: Anlotinib for the treatment of advanced non-small cell lung cancer after two lines of chemotherapy. Cancer Commun. (Lond) 39 (1), 36. doi:10.1186/s40880-019-0383-7
Zou, H., Xia, L., Jin, G., Wu, H., Qian, W., Jia, D., et al. (2022). Retrospective review of efficacy and safety of anlotinib in advanced leiomyosarcoma: A real-world study. Cancer Manag. Res. 14, 1703–1711. doi:10.2147/cmar.S357334
Keywords: posterior reversible encephalopathy syndrome, anlotinib, antiangiogenic therapy, small cell lung cancer, case report
Citation: Zou X, Zhou P, Lv W, Liu C and Liu J (2023) Posterior reversible encephalopathy syndrome after anlotinib treatment for small cell lung cancer: A case report and literature review. Front. Pharmacol. 14:1126235. doi: 10.3389/fphar.2023.1126235
Received: 17 December 2022; Accepted: 23 January 2023;
Published: 06 February 2023.
Edited by:
Rui Liu, Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences, ChinaReviewed by:
Li Zeng, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaCopyright © 2023 Zou, Zhou, Lv, Liu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Liu, c2RqbmxqamllQDEyNi5jb20=; Chuanyong Liuand, Y3lsMDkzNkBzaW5hLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.