- 1Department of Thoracic Surgery, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Obstetrics and Gynecology, West China Second University Hospital of Sichuan University, Chengdu, Sichuan, China
- 3Lung Cancer Center, West China Hospital, Sichuan University, Chengdu, China
Background and purpose: Previous studies have found that metformin can inhibit tumor growth and improve outcomes for cancer patients. However, the association between the addition of metformin to the treatment regimen and survival in non-small cell lung cancer (NSCLC) patients receiving antineoplastic agents such as chemotherapy drugs, epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs), and immune checkpoint inhibitors (ICIs) remains unclear. This study aimed to evaluate the effect of metformin in NSCLC patients who received the aforementioned antineoplastic therapies.
Methods: Several electronic databases were searched for relevant studies published by 10 September 2022. The primary and secondary outcomes were overall survival (OS) and progression-free survival (PFS); eligible studies were those comparing patients with and without the addition of metformin. Hazard ratios (HRs) and 95% confidence intervals (CIs) were combined, with all statistical analyses performed using STATA 15.0.
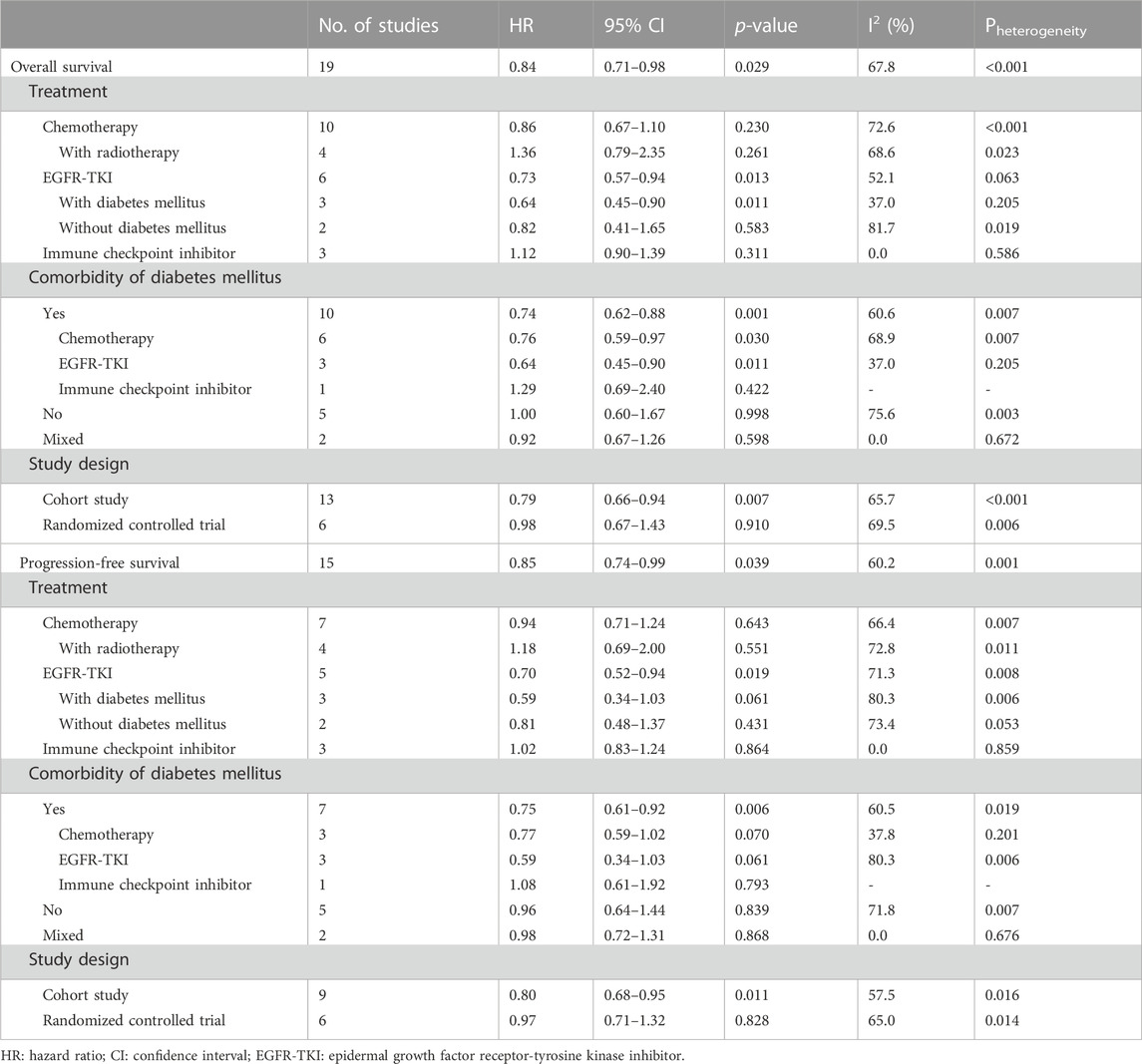
Results: A total of 19 studies involving 6,419 participants were included, of which six were randomized controlled trials. The overall pooled results indicate that the addition of metformin improved OS (HR = 0.84, 95% CI: 0.71–0.98, p = 0.029) and PFS (HR = 0.85, 95% CI: 0.74–0.99, p = 0.039). However, subgroup analysis based on treatment type and comorbidity of diabetes mellitus demonstrated that improvements in OS and PFS were observed only in diabetic and EGFR-TKI-treated patients (OS: HR = 0.64, 95% CI: 0.45–0.90, p = 0.011; PFS: HR = 0.59, 95% CI: 0.34–1.03, p = 0.061).
Conclusion: Overall, this meta-analysis found that metformin use could improve outcomes for diabetic patients receiving EGFR-TKIs. However, no significant association between the addition of metformin and the survival of non-diabetic NSCLC patients receiving chemotherapy or ICI therapy was identified based on the current evidence.
Introduction
The survival rate of patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) remains poor, with the opportunity for radical resection having been lost by the time of diagnosis for a significant proportion of NSCLC patients (Kang et al., 2022; Xia et al., 2022; Xiang et al., 2022). Chemotherapy, epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs), and immune checkpoint inhibitors (ICIs) are currently the main treatments for advanced NSCLC patients. However, the overall therapeutic effects of these treatments are not satisfactory, and their clinical use is usually limited for various reasons, such as primary and acquired resistance. Therefore, other medical measures are needed to improve the therapeutic effects of these antineoplastic agents.
Metformin (1,1-dimethylbiguanide) is the most commonly used drug for treating type 2 diabetes mellitus. In recent decades, substantial evidence has suggested that there is a clear beneficial effect of metformin in cases of malignancies, and that metformin plays a significant role in reducing cancer risk and improving outcomes for cancer patients (Mu et al., 2022; Yao et al., 2022; Zhang Q. et al., 2022; Han et al., 2023). A number of studies have found that metformin could reduce cancer morbidity and mortality rates in diabetic patients (Brancher et al., 2021; Kang et al., 2021). The underlying mechanisms are complicated and involve numerous pathways, such as the liver kinase B1 (LKB1)-dependent AMPK pathway and the GRB/IRS-1/PI3K/AKT/mTOR pathway, and the regulation of certain targets, such as the silent information regulator T1 (SIRT1) and YAP (Han et al., 2023).
In the case of lung cancer, a meta-analysis by Xiao et al. (2020) demonstrated that metformin treatment is significantly associated with reduced NSCLC incidence (HR = 0.78, 95% CI: 0.70–0.86). Many studies have verified the beneficial role of metformin in NSCLC patients after surgical resection (Medairos et al., 2016; Yendamuri et al., 2019). However, as mentioned previously, a certain proportion of patients with advanced or inoperable NSCLC receive non-surgical treatments, including chemotherapy, EGFR-TKIs, and ICI therapy, which have become common in recent years. It remains unclear whether the concurrent use of metformin could enhance the efficacy of the aforementioned medications and improve the survival of NSCLC patients.
Luo et al. (2021) conducted a meta-analysis to explore the value of metformin as an adjunct treatment alongside antineoplastic agents in lung cancer; they showed that the addition of metformin might improve survival outcomes for lung cancer patients. However, their results were limited in terms of identifying an association between metformin use and the survival of NSCLC patients receiving the aforementioned antineoplastic agents.
Therefore, the aim of this meta-analysis was to identify the value of the concurrent use of metformin during treatment of NSCLC patients with chemotherapy, EGFR-TKIs, and ICIs. The results might contribute to the clinical application of metformin in NSCLC patients receiving the aforementioned antineoplastic agents.
Materials and methods
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (2020) (Zhang et al., 2020).
Literature search
The PubMed, Embase, and Web of Science electronic databases were searched for articles published in the period from their inception to 10 September 2022. The following keywords were used during literature retrieval: metformin, lung, pulmonary, tumor, cancer, carcinoma, neoplasm, survival, prognosis, and prognostic. To avoid omissions, a relatively broad search strategy was developed and implemented, using the expression: metformin AND (lung OR pulmonary) AND (tumor OR cancer OR carcinoma OR neoplasm) AND (survival OR prognosis OR prognostic). In addition, MeSH terms and free words were applied, and references cited in the included publications were also reviewed.
Inclusion and exclusion criteria
The inclusion criteria were as follows: 1) patients were pathologically diagnosed with primary NSCLC and received chemotherapy, EGFR-TKIs, or ICI therapy; 2) overall survival (OS) and/or progression-free survival (PFS) were compared between patients who did and did not also receive metformin during treatment with the aforementioned therapies; 3) hazard ratios (HRs) with 95% confidence intervals (CIs) were directly reported by the article.
The exclusion criteria were as follows: 1) letters, editorials, meeting abstracts, case reports, and reviews; 2) articles reporting insufficient, overlapping, or duplicated data.
Data extraction
The following information was collected from each included study: the name of the first author; publication year; country; sample size; study design, including randomized controlled trials (RCTs) and cohort studies; tumor–node–metastasis (TNM) stage; treatment strategy; comorbidity of diabetes mellitus; endpoint; and HR and 95% CI.
Methodological quality assessment
The quality of the RCTs and cohort studies was assessed using the Jadad scale and the Newcastle–Ottawa scale (NOS), respectively (Bhandari et al., 2001; Wang J. L. et al., 2021). Studies with a Jadad score of 4 or a NOS score of 6 or higher were defined as high-quality studies.
The literature search, selection, data collection, and quality assessment were all performed by two authors independently, and all disagreements were resolved by team discussion.
Statistical analysis
All statistical analyses carried out in this meta-analysis were conducted using STATA 15.0 software. HRs with 95% CIs were combined to compare OS and PFS between patients who did and did not receive metformin. Heterogeneity among the included studies was evaluated via I2 statistics and Q tests. When significant heterogeneity was observed, in the form of I2 > 50% or p < 0.1, a random-effects model was applied; otherwise, a fixed-effects model was used (Barili et al., 2018). Subgroup analysis, stratified by treatment type, comorbidity of diabetes mellitus, and study design, was additionally conducted. In addition, a sensitivity analysis was conducted to identify the sources of heterogeneity and evaluate the stability of the pooled results. Furthermore, Begg’s funnel plots were constructed and Egger’s tests were conducted to detect publication bias (Begg and Mazumdar, 1994; Egger et al., 1997). Significant publication bias was defined as p < 0.05.
Results
Literature search and retrieval
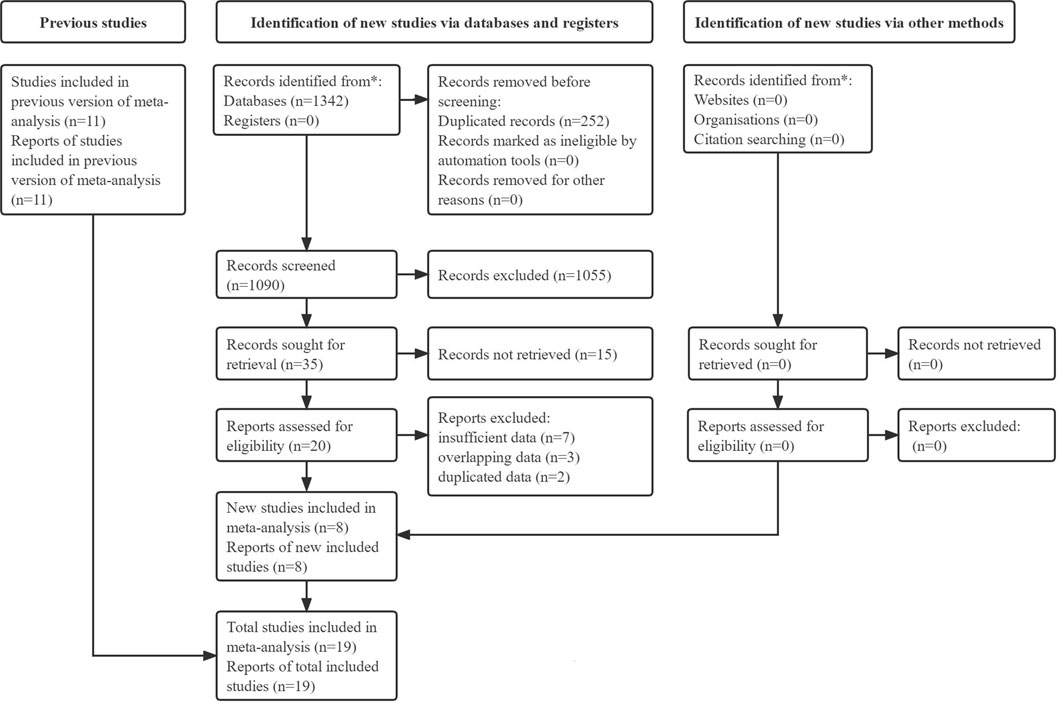
Initially, 1,342 records were identified from electronic databases, and 252 duplicated records were removed. Next, 1,055 irrelevant publications and 15 unavailable publications were excluded after review of the titles and abstracts, respectively. Eight of the remaining reports were included after review of the full texts, and 11 available studies were included from previous relevant meta-analyses. Thus, a total of 19 studies were included in this meta-analysis (Tan et al., 2011; Ahmed et al., 2015; Chen et al., 2015; Lin et al., 2015; Sayed et al., 2015; Wink et al., 2016; Wen-Xiu et al., 2018; Afzal et al., 2019; Arrieta et al., 2019; Hung et al., 2019; Li et al., 2019; Su et al., 2020; Cortellini et al., 2021; Han et al., 2021; Jacobi et al., 2021; Lee et al., 2021; Skinner et al., 2021; Tsakiridis et al., 2021; Wang Y. et al., 2021). The specific literature retrieval process is presented in Figure 1.
Basic characteristics of the included studies
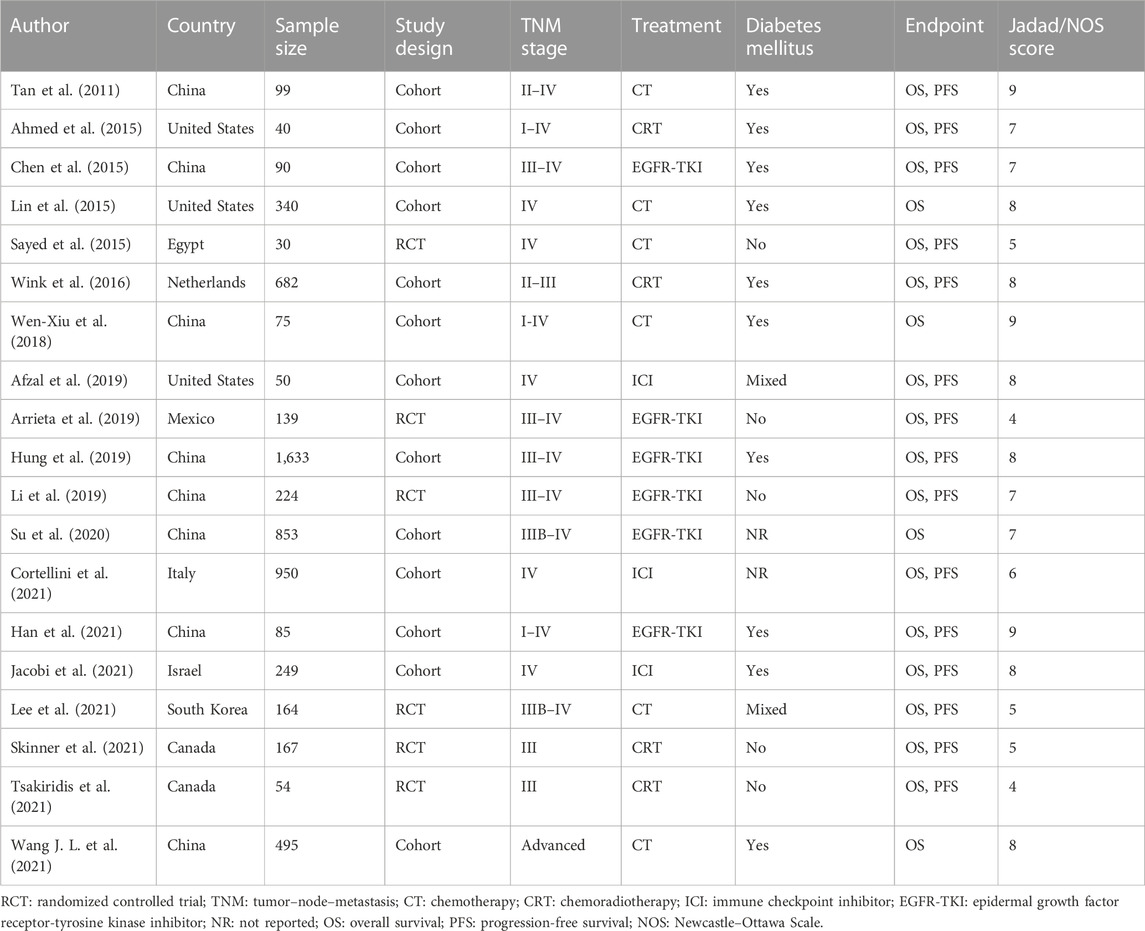
The included studies were published between 2011 and 2021, and their sample sizes ranged from 40 to 1,633, for a total of 6,419 patients. Six of the studies were RCTs (Sayed et al., 2015; Arrieta et al., 2019; Li et al., 2019; Lee et al., 2021; Skinner et al., 2021; Tsakiridis et al., 2021); the remainder were cohort studies (Tan et al., 2011; Ahmed et al., 2015; Chen et al., 2015; Lin et al., 2015; Wink et al., 2016; Wen-Xiu et al., 2018; Afzal et al., 2019; Hung et al., 2019; Su et al., 2020; Cortellini et al., 2021; Han et al., 2021; Jacobi et al., 2021; Wang Y. et al., 2021). All included studies were high-quality studies with a Jadad score ≥4 or NOS score ≥6. Detailed information is presented in Table 1.
The association between addition of metformin and OS of NSCLC patients receiving antineoplastic agents
All included studies explored the association between the addition of metformin and the OS of NSCLC patients receiving antineoplastic agents (Afzal et al., 2019; Ahmed et al., 2015; Arrieta et al., 2019; Chen et al., 2015; Cortellini et al., 2021; Han et al., 2021; Hung et al., 2019; Jacobi et al., 2021; Lee et al., 2021; Li et al., 2019; J. J; Lin et al., 2015; Sayed et al., 2015; Skinner et al., 2021; Su et al., 2020; Tan et al., 2011; Tsakiridis et al., 2021; Wang J. L. et al., 2021; Wen-Xiu et al., 2018; Wink et al., 2016). The overall pooled results indicated that patients who were treated with metformin had better rates of OS (HR = 0.84, 95% CI: 0.71–0.98, p = 0.029; I2 = 67.8%, p < 0.001) (Figure 2A). However, subgroup analysis based on treatment type and comorbidity of diabetes mellitus showed that the effect of improved OS was observed only for patients receiving EGFR-TKIs (HR = 0.73, 95% CI: 0.57–0.94, p = 0.013) (Figure 2B) and those with diabetes mellitus (HR = 0.74, 95% CI: 0.62–0.88, p = 0.001) (Figure 2C). In contrast, the association between metformin use and the OS of NSCLC patients receiving chemoradiotherapy was negative (HR = 1.36, 95% CI: 0.79–2.35, p = 0.261). In addition, subgroup analysis based on study design showed that the association between metformin use and improved OS in NSCLC patients was observed only in cohort studies (HR = 0.79, 95% CI: 0.66–0.94, p = 0.007) (Figure 2D).
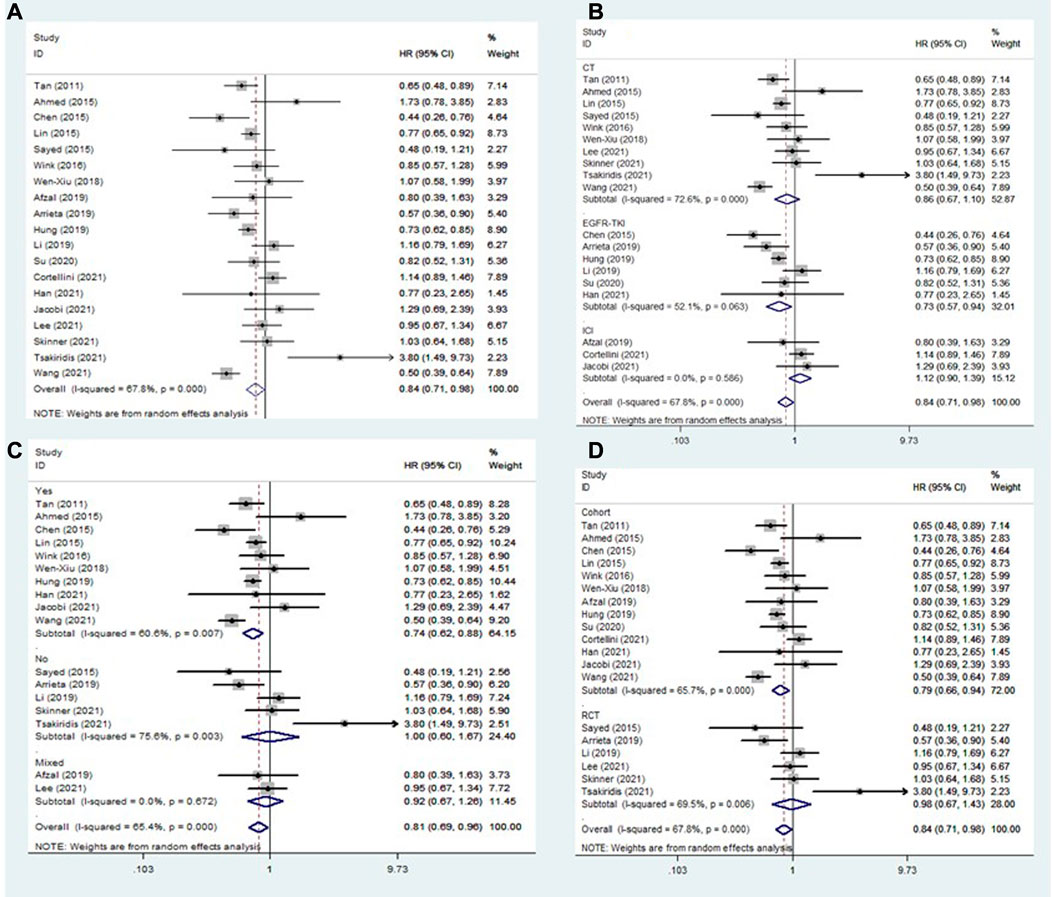
FIGURE 2. The association between metformin use and overall survival in (A) all NSCLC patients; (B) subgroups by treatment type; (C) subgroups by comorbidity of diabetes mellitus; and (D) subgroups by study design. NSCLC: non-small cell lung cancer.
To further clarify the nature of the association between metformin use and OS in EGFR-TKI-treated and diabetic NSCLC patients, subgroup analyses were conducted focusing on EGFR-TKI-treated patients (according to comorbidity of diabetes mellitus) and diabetic patients (according to treatment type). The pooled results demonstrated that the addition of metformin could significantly improve the OS of diabetic NSCLC patients receiving EGFR-TKIs (HR = 0.64, 95% CI: 0.45–0.90, p = 0.011) (Table 2).
The association between addition of metformin and PFS of NSCLC patients receiving antineoplastic agents
Fifteen studies explored the association between the addition of metformin and PFS in NSCLC patients receiving antineoplastic agents (Tan et al., 2011; Ahmed et al., 2015; Chen et al., 2015; Sayed et al., 2015; Wink et al., 2016; Afzal et al., 2019; Arrieta et al., 2019; Hung et al., 2019; Li et al., 2019; Cortellini et al., 2021; Han et al., 2021; Jacobi et al., 2021; Lee et al., 2021; Skinner et al., 2021; Tsakiridis et al., 2021). The overall results showed that metformin use was clearly related to better rates of PFS (HR = 0.85, 95% CI: 0.74–0.99, p = 0.039; I2 = 60.2%, p = 0.001) (Figure 3A). However, subgroup analysis stratified by treatment type and comorbidity of diabetes mellitus also indicated that the effect of improved PFS was observed only in patients receiving EGFR-TKIs (HR = 0.70, 95% CI: 0.52–0.94, p = 0.019) (Figure 3B) and those with diabetes mellitus (HR = 0.75, 95% CI: 0.61–0.92, p = 0.006) (Figure 3C). In contrast, the association between metformin use and PFS of NSCLC patients receiving chemoradiotherapy was negative (HR = 1.18, 95% CI: 0.69–2.00, p = 0.551). In addition, subgroup analysis based on study design showed that the association between metformin use and improved PFS in patients with NSCLC was observed only in cohort studies (HR = 0.80, 95% CI: 0.68–0.95, p = 0.011) (Figure 3D).
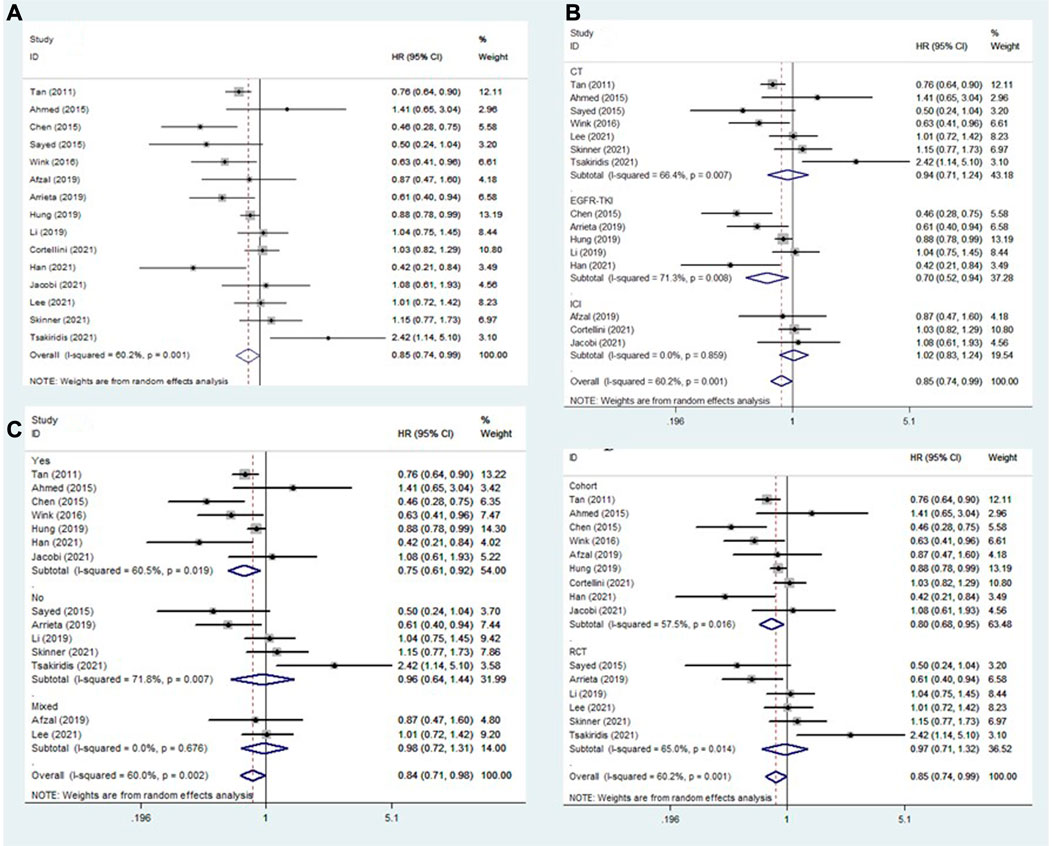
FIGURE 3. The association between metformin use and progression-free survival in (A) all NSCLC patients; (B) subgroups by treatment type; (C) subgroups by comorbidity of diabetes mellitus; and (D) subgroups by study design. NSCLC: non-small cell lung cancer.
Similarly, subgroup analyses were performed focusing on patients receiving EGFR-TKI treatment (according to comorbidity of diabetes mellitus) and diabetic patients (according to treatment type). The pooled results demonstrated that the addition of metformin was related to better PFS in diabetic NSCLC patients receiving EGFR-TKIs (HR = 0.59, 95% CI: 0.34–1.03, p = 0.061), although the difference was not significant (Table 2).
Sensitivity analysis
Sensitivity analyses were conducted for OS and PFS, focusing on all NSCLC patients (Figures 4A, 5A), EGFR-TKI-treated patients (Figures 4B, 5B), and diabetic patients (Figures 4C, 5C). Overall, a small number of the included studies had a clear impact on the results. More RCTs with large samples are needed to verify our findings.

FIGURE 4. Sensitivity analysis for the association between metformin use and overall survival in (A) all NSCLC patients; (B) patients receiving EGFR-TKI therapy; and (C) patients with diabetes mellitus. NSCLC: non-small cell lung cancer; EGFR-TKI: epidermal growth factor receptor-tyrosine kinase inhibitor.

FIGURE 5. Sensitivity analysis for the association between metformin use and progression-free survival in (A) all NSCLC patients; (B) patients receiving EGFR-TKI therapy; and (C) patients with diabetes mellitus. NSCLC: non-small cell lung cancer; EGFR-TKI: epidermal growth factor receptor-tyrosine kinase inhibitor.
Publication bias
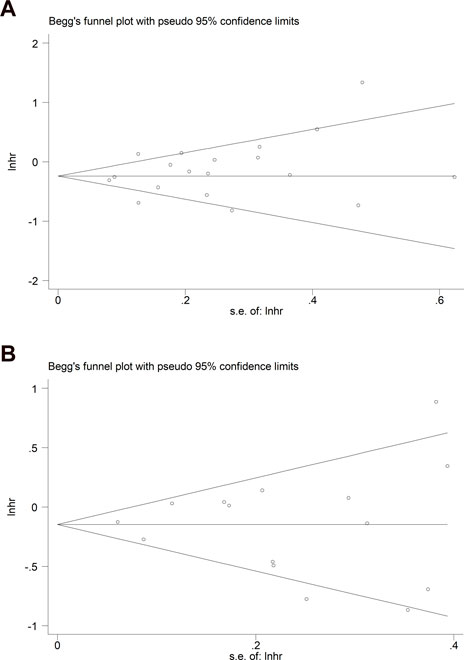
Begg’s funnel plots for OS and PFS were both symmetrical (Figures 6A, B), and the p-values in Egger’s tests for OS and PFS were 0.218 and 0.900, respectively, indicating that no significant publication bias existed in this meta-analysis.
Discussion
The current meta-analysis demonstrated that the addition of metformin was beneficial for diabetic NSCLC patients who received EGFR-TKI therapy and that metformin use could significantly improve the survival rate of this group of patients. However, no significant association between the addition of metformin and the survival of non-diabetic patients receiving chemotherapy or ICI was observed. Furthermore, due to the limitations of the included studies, more RCTs with larger samples are needed to verify the beneficial value of metformin use in diabetic and EGFR-TKI-treated NSCLC patients.
In a previous similar meta-analysis, Luo et al. (2021) included three RCTs and 11 observational cohort studies involving 3,856 lung cancer patients, and showed that antineoplastic agents combined with metformin significantly improve OS (HR = 0.73, p < 0.001) and PFS (HR = 0.72, p = 0.001). Similar results were observed when they combined cohort studies, but no significant association between metformin use and survival of lung cancer patients was detected based on limited data from RCTs. The authors conducted additional subgroup analyses based on type of therapy (chemotherapy vs EGFR-TKI), histology (NSCLC vs small cell lung cancer), and stage (III–IV vs I–IV), which produced consistent results, indicating that the addition of metformin could induce clear improvement in the efficacy of antineoplastic agents in lung cancer patients (Luo et al., 2021). However, this conclusion is obviously crude, and several factors—such as therapeutic methods and histology—may affect the objectivity and authenticity of the results.
Similarly, several other meta-analyses have explored the anticancer effect of metformin in cases of lung cancer in recent years, but there are considerable limitations to these analyses (Zhang Q. et al., 2022). Zhang F. et al. (2022) explored the anticancer role of metformin in NSCLC patients receiving EGFR-TKIs, but only three studies published before August 2020 were included. Brancher et al. (2021) included ten cohort studies and four RCTs and came to the tentative conclusion that metformin use might be associated with improved OS of lung cancer patients; this result was similar to that of several other meta-analyses (Wan et al., 2016; Cao et al., 2017; Zhong et al., 2017; Zhang et al., 2018; Zeng et al., 2019; Xiao et al., 2020; Brancher et al., 2021). Thus, we conducted the current meta-analysis to clarify the value of metformin use in NSCLC patients receiving the aforementioned antineoplastic agents. We demonstrated that benefits of metformin were only experienced by diabetic and EGFR-TKI-treated NSCLC patients.
Several studies have investigated the underlying mechanisms by which metformin affects the therapeutic effects of EGFR-TKIs in NSCLC patients. It has been reported that metformin and EGFR-TKIs have a synergistic therapeutic effect in NSCLC patients with type 2 diabetes (Nguyen et al., 2011). In one study focusing on the treatment of LKB1 wild-type NSCLC cells, it was found that the addition of gefitinib to metformin could inhibit EGFR phosphorylation and its downstream signaling; in addition, increased c-Raf/B-Raf isomerization was found to cause MAPK activation, which induced significant apoptosis in vitro and in vivo (Morgillo et al., 2013). Furthermore, metformin plays a role in overcoming resistance to EGFR-TKIs by inhibiting the PI3K/AKT/mTOR signaling pathway (Tan, 2020). Li et al. (2014) also reported on the effects of metformin in inhibiting the IL-6/STAT3 signaling pathway, reversing epithelial–mesenchymal transition (EMT), and overcoming EGFR-TKI drug (gefitinib and erlotinib) resistance in NSCLC cells. However, most relevant studies have been conducted in vitro and in vivo, and the mechanisms are still unclear.
Although we found that metformin was not significantly associated with the survival of NSCLC patients receiving chemotherapy drugs, a number of studies have indicated a role for metformin in increasing the sensitivity of chemotherapy drugs such as doxorubicin, cisplatin, and paclitaxel in NSCLC (Iliopoulos et al., 2011; Tan et al., 2011; Teixeira et al., 2013; Tseng et al., 2013). For example, cisplatin resistance is associated with signal transducer and activator of transcription 3 (STAT3) phosphorylation, production of reactive oxygen species (ROS), and IL-6 secretion, but metformin could inhibit cisplatin-induced STAT3 phosphorylation (through the LKB1-AMPK and mTOR pathway-dependent mechanisms), ROS generation, and autocrine IL-6 secretion (Khan et al., 2013; Lin et al., 2013). Metformin thus plays a role in enhancing cisplatin cytotoxicity and improving the cisplatin resistance of cancer cells (Wang et al., 2014; Wang et al., 2015).
No significant relationship between metformin use and the survival of ICI-treated NSCLC patients was observed in our meta-analysis. A few previous studies have suggested that metformin enhances the efficacy of ICI therapy in NSCLC patients (Yendamuri et al., 2019; Shen et al., 2020; Kim et al., 2021). The expression levels of LKB1 and PD-L1 are closely correlated, and AMPK inhibition reduces PD-L1 levels in NSCLC cells through LKB1 (Shen et al., 2020). Metformin has the ability to enhance the expression of LKB1 and the activation of AMPK, which improves the therapeutic effect of ICIs in NSCLC (Shen et al., 2020). However, most relevant studies investigating the therapeutic role of metformin in NSCLC patients receiving chemotherapy and ICIs have been based on cellular and animal trials. Therefore, more prospective RCTs are needed to clarify the therapeutic role of metformin in NSCLC patients receiving the aforementioned therapies.
There are several limitations to our meta-analysis. First, most of the studies included were retrospective cohort studies with small sample sizes, which might cause some bias. Second, although stratification analysis was performed based specifically on treatment type (EGFR-TKI therapy) and comorbidity of diabetes mellitus and strongly indicated a significant relationship between metformin use and better outcomes for diabetic and EGFR-TKI-treated NSCLC patients, only 1808 patients from three cohort studies were enrolled (Chen et al., 2015; Hung et al., 2019; Han et al., 2021) in this group. Third, due to a lack of original data, we were unable to conduct additional subgroup analyses based on other important parameters, such as metformin dose, TNM stage, and age. Fourth, we were unable to calculate the base and recommended doses of metformin to determine its effect in enhancing the efficacy of EGFR-TKIs in this meta-analysis. Five clear instances of heterogeneity among the included studies were observed in the analysis; however, unfortunately, the sources of heterogeneity were not clarified in the subgroup analyses, and due to the limited amount of evidence, we were unable to conduct more subgroup analyses based on other parameters.
Conclusion
The addition of metformin to the treatment regimen was beneficial for diabetic NSCLC patients who received EGFR-TKI therapy, and metformin use could significantly improve the survival rate of this group of patients. However, no significant association between the addition of metformin and the survival of non-diabetic NSCLC patients receiving chemotherapy or ICI therapy was identified based on the current evidence. Meanwhile, more RCTs with larger samples are needed to verify the aforementioned findings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies that involved human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Author contributions
Conceptualization: LL and GC. Literature search: YW and TW. Independent review and risk of bias assessment: YW and TW. Data curation: YH. Data analysis and synthesis: YW, TW, and YH. Initial draft of manuscript: YW and TW. Final manuscript: all authors.
Funding
This work was supported by the Regional Innovation Cooperation Project of the Sichuan Science and Technology Program (2021YFQ0029).
Acknowledgments
Thanks are due to Prof. Wei Huang for assistance with the data analysis and valuable discussion.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Afzal, M. Z., Dragnev, K., Sarwar, T., and Shirai, K. (2019). Clinical outcomes in non-small-cell lung cancer patients receiving concurrent metformin and immune checkpoint inhibitors. Lung Cancer Manag. 8 (2), LMT11. doi:10.2217/lmt-2018-0016
Ahmed, I., Ferro, A., Cohler, A., Langenfeld, J., Surakanti, S. G., Aisner, J., et al. (2015). Impact of metformin use on survival in locally-advanced, inoperable non-small cell lung cancer treated with definitive chemoradiation. J. Of Thorac. Dis. 7 (3), 346–355. doi:10.3978/j.issn.2072-1439.2014.12.32
Arrieta, O., Barron, F., Padilla, M. A. S., Aviles-Salas, A., Ramirez-Tirado, L. A., and Arguelles Jimenez, M. J. (2019). Effect of metformin plus tyrosine kinase inhibitors compared with tyrosine kinase inhibitors alone in patients with epidermal growth factor receptor-mutated lung adenocarcinoma: A phase 2 randomized clinical trial. Jama Oncol. 5 (11), e192553. doi:10.1001/jamaoncol.2019.2553
Barili, F., Parolari, A., Kappetein, P. A., and Freemantle, N. (2018). Statistical primer: Heterogeneity, random- or fixed-effects model analyses? Interact. Cardiovasc Thorac. Surg. 27 (3), 317–321. doi:10.1093/icvts/ivy163
Begg, C. B., and Mazumdar, M. (1994). Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101.
Bhandari, M., Richards, R. R., Sprague, S., and Schemitsch, E. H. (2001). Quality in the reporting of randomized trials in surgery: Is the Jadad scale reliable? Control Clin. Trials 22 (6), 687–688. doi:10.1016/s0197-2456(01)00147-7
Brancher, S., Ribeiro, A. E., Toporcov, T. N., and Weiderpass, E. (2021). The role of metformin on lung cancer survival: The first systematic review and meta-analysis of observational studies and randomized clinical trials. J. Cancer Res. Clin. Oncol. 147 (10), 2819–2836. doi:10.1007/s00432-021-03728-x
Cao, X., Wen, Z. S., Wang, X. D., Li, Y., Liu, K. Y., and Wang, X. (2017). The clinical effect of metformin on the survival of lung cancer patients with diabetes: A comprehensive systematic review and meta-analysis of retrospective studies. J. Cancer 8 (13), 2532–2541. doi:10.7150/jca.19750
Chen, H., Yao, W., Chu, Q., Han, R., Sun, J., Wang, D., et al. (2015). Synergistic effects of metformin in combination with EGFR-TKI in the treatment of patients with advanced non-small cell lung cancer and type 2 diabetes. Cancer Lett. 369 (1), 97–102. doi:10.1016/j.canlet.2015.08.024
Cortellini, A., Di Maio, M., Nigro, O., Leonetti, A., Cortinovis, D. L., Aerts, J. G., et al. (2021). Differential influence of antibiotic therapy and other medications on oncological outcomes of patients with non-small cell lung cancer treated with first-line pembrolizumab versus cytotoxic chemotherapy. J. Immunother. Cancer 9, e002421. doi:10.1136/jitc-2021-002421
Egger, M., Davey Smith, G., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. Bmj 315 (7109), 629–634. doi:10.1136/bmj.315.7109.629
Han, P., Zhou, J., Xiang, J., Liu, Q., and Sun, K. (2023). Research progress on the therapeutic effect and mechanism of metformin for lung cancer (Review). Oncol. Rep. 49, 3. doi:10.3892/or.2022.8440
Han, R., Jia, Y., Li, X., Zhao, C., Zhao, S., Liu, S., et al. (2021). Concurrent use of metformin enhances the efficacy of EGFR-TKIs in patients with advanced EGFR-mutant non-small cell lung cancer-an option for overcoming EGFR-TKI resistance. Transl. Lung Cancer Res. 10 (3), 1277–1291. doi:10.21037/tlcr-20-1153
Hung, M. S., Chuang, M. C., Chen, Y. C., Lee, C. P., Yang, T. M., Chen, P. C., et al. (2019). Metformin prolongs survival in type 2 diabetes lung cancer patients with EGFR-TKIs. Integr. Cancer Ther. 18, 1534735419869491. doi:10.1177/1534735419869491
Iliopoulos, D., Hirsch, H. A., and Struhl, K. (2011). Metformin decreases the dose of chemotherapy for prolonging tumor remission in mouse xenografts involving multiple cancer cell types. Cancer Res. 71 (9), 3196–3201. doi:10.1158/0008-5472.CAN-10-3471
Jacobi, O., Landman, Y., Reinhorn, D., Icht, O., Sternschuss, M., Rotem, O., et al. (2021). The relationship of diabetes mellitus to efficacy of immune checkpoint inhibitors in patients with advanced non-small cell lung cancer. Oncol. Switz. 99 (9), 555–561. doi:10.1159/000516671
Kang, J., Jeong, S. M., Shin, D. W., Cho, M., Cho, J. H., and Kim, J. (2021). The associations of aspirin, statins, and metformin with lung cancer risk and related mortality: A time-dependent analysis of population-based nationally representative data. J. Thorac. Oncol. 16 (1), 76–88. doi:10.1016/j.jtho.2020.08.021
Kang, M. J., Won, Y. J., Lee, J. J., Jung, K. W., Kim, H. J., Kong, H. J., et al. (2022). Cancer statistics in korea: Incidence, mortality, survival, and prevalence in 2019. Cancer Res. Treat. 54 (2), 330–344. doi:10.4143/crt.2022.128
Khan, K. H., Yap, T. A., Yan, L., and Cunningham, D. (2013). Targeting the PI3K-AKT-mTOR signaling network in cancer. Chin. J. Cancer 32 (5), 253–265. doi:10.5732/cjc.013.10057
Kim, Y., Vagia, E., Viveiros, P., Kang, C. Y., Lee, J. Y., Gim, G., et al. (2021). Overcoming acquired resistance to PD-1 inhibitor with the addition of metformin in small cell lung cancer (SCLC). Cancer Immunol. Immunother. 70 (4), 961–965. doi:10.1007/s00262-020-02703-8
Lee, Y., Joo, J., Lee, Y. J., Lee, E. K., Park, S., Kim, T. S., et al. (2021). Randomized phase II study of platinum-based chemotherapy plus controlled diet with or without metformin in patients with advanced non-small cell lung cancer. Lung Cancer 151, 8–15. doi:10.1016/j.lungcan.2020.11.011
Li, L., Han, R., Xiao, H., Lin, C., Wang, Y., Liu, H., et al. (2014). Metformin sensitizes EGFR-TKI-resistant human lung cancer cells in vitro and in vivo through inhibition of IL-6 signaling and EMT reversal. Clin. Cancer Res. 20 (10), 2714–2726. doi:10.1158/1078-0432.CCR-13-2613
Li, L., Jiang, L., Wang, Y., Zhao, Y., Zhang, X. J., Wu, G., et al. (2019). Combination of metformin and gefitinib as first-line therapy for nondiabetic advanced NSCLC patients with EGFR mutations: A randomized, double-blind phase II trial. Clin. Cancer Res. 25 (23), 6967–6975. doi:10.1158/1078-0432.CCR-19-0437
Lin, C. C., Yeh, H. H., Huang, W. L., Yan, J. J., Lai, W. W., Su, W. P., et al. (2013). Metformin enhances cisplatin cytotoxicity by suppressing signal transducer and activator of transcription-3 activity independently of the liver kinase B1-AMP-activated protein kinase pathway. Am. J. Respir. Cell Mol. Biol. 49 (2), 241–250. doi:10.1165/rcmb.2012-0244OC
Lin, J. J., Gallagher, E. J., Sigel, K., Mhango, G., Galsky, M. D., Smith, C. B., et al. (2015). Survival of patients with stage IV lung cancer with diabetes treated with metformin. Am. J. Of Respir. And Crit. Care Med. 191 (4), 448–454. doi:10.1164/rccm.201407-1395OC
Luo, X., Chen, X., Wang, L., Yang, B., and Cai, S. (2021). Metformin adjunct with antineoplastic agents for the treatment of lung cancer: A meta-analysis of randomized controlled trials and observational cohort studies. Front. Pharmacol. 12, 639016. doi:10.3389/fphar.2021.639016
Medairos, R. A., Clark, J., Holoubek, S., Kubasiak, J. C., Pithadia, R., Hamid, F., et al. (2016). Metformin exposure is associated with improved progression-free survival in diabetic patients after resection for early-stage non-small cell lung cancer. J. Thorac. Cardiovasc Surg. 152 (1), 55–61. doi:10.1016/j.jtcvs.2016.03.094
Morgillo, F., Sasso, F. C., Della Corte, C. M., Vitagliano, D., D'Aiuto, E., Troiani, T., et al. (2013). Synergistic effects of metformin treatment in combination with gefitinib, a selective EGFR tyrosine kinase inhibitor, in LKB1 wild-type NSCLC cell lines. Clin. Cancer Res. 19 (13), 3508–3519. doi:10.1158/1078-0432.CCR-12-2777
Mu, W., Jiang, Y., Liang, G., Feng, Y., and Qu, F. (2022). Metformin: A promising antidiabetic medication for cancer treatment. Curr. Drug Targets 24, 41–54. doi:10.2174/1389450124666221104094918
Nguyen, G. H., Murph, M. M., and Chang, J. Y. (2011). Cancer stem cell radioresistance and enrichment: Where frontline radiation therapy may fail in lung and esophageal cancers. Cancers (Basel) 3 (1), 1232–1252. doi:10.3390/cancers3011232
Sayed, R., Saad, A. S., El Wakeel, L., Elkholy, E., and Badary, O. (2015). Metformin addition to chemotherapy in stage IV non-small cell lung cancer: An open label randomized controlled study. Asian Pac. J. cancer Prev. 16 (15), 6621–6626. doi:10.7314/apjcp.2015.16.15.6621
Shen, X., Zhao, Y., Liu, G., Zhou, H. L., Fan, J., Zhang, L., et al. (2020). Upregulation of programmed death ligand 1 by liver kinase B1 and its implication in programmed death 1 blockade therapy in non-small cell lung cancer. Life Sci. 256, 117923. doi:10.1016/j.lfs.2020.117923
Skinner, H., Hu, C., Tsakiridis, T., Santana-Davila, R., Lu, B., Erasmus, J. J., et al. (2021). Addition of metformin to concurrent chemoradiation in patients with locally advanced non-small cell lung cancer: The NRG-lu001 phase 2 randomized clinical trial. Jama Oncol. 7 (9), 1324–1332. doi:10.1001/jamaoncol.2021.2318
Su, V. Y., Yang, K. Y., Huang, T. Y., Hsu, C. C., Chen, Y. M., Yen, J. C., et al. (2020). The efficacy of first-line tyrosine kinase inhibitors combined with co-medications in Asian patients with EGFR mutation non-small cell lung cancer. Sci. Rep. 10 (1), 14965. doi:10.1038/s41598-020-71583-w
Tan, A. C. (2020). Targeting the PI3K/Akt/mTOR pathway in non-small cell lung cancer (NSCLC). Thorac. Cancer 11 (3), 511–518. doi:10.1111/1759-7714.13328
Tan, B. X., Yao, W. X., Ge, J., Peng, X. C., Du, X. B., Zhang, R., et al. (2011). Prognostic influence of metformin as first-line chemotherapy for advanced nonsmall cell lung cancer in patients with type 2 diabetes. Cancer 117 (22), 5103–5111. doi:10.1002/cncr.26151
Teixeira, S. F., Guimarães Idos, S., Madeira, K. P., Daltoé, R. D., Silva, I. V., and Rangel, L. B. (2013). Metformin synergistically enhances antiproliferative effects of cisplatin and etoposide in NCI-H460 human lung cancer cells. J. Bras. Pneumol. 39 (6), 644–649. doi:10.1590/S1806-37132013000600002
Tsakiridis, T., Pond, G. R., Wright, J., Ellis, P. M., Ahmed, N., Abdulkarim, B., et al. (2021). Metformin in combination with chemoradiotherapy in locally advanced non-small cell lung cancer: The OCOG-ALMERA randomized clinical trial. Jama Oncol. 7 (9), 1333–1341. doi:10.1001/jamaoncol.2021.2328
Tseng, S. C., Huang, Y. C., Chen, H. J., Chiu, H. C., Huang, Y. J., Wo, T. Y., et al. (2013). Metformin-mediated downregulation of p38 mitogen-activated protein kinase-dependent excision repair cross-complementing 1 decreases DNA repair capacity and sensitizes human lung cancer cells to paclitaxel. Biochem. Pharmacol. 85 (4), 583–594. doi:10.1016/j.bcp.2012.12.001
Wan, G., Yu, X., Chen, P., Wang, X., Pan, D., Wang, X., et al. (2016). Metformin therapy associated with survival benefit in lung cancer patients with diabetes. Oncotarget 7 (23), 35437–35445. doi:10.18632/oncotarget.8881
Wang, J.-L., Tsai, Y.-T., Lin, C.-H., Cidem, A., Staniczek, T., and Chang, G. R.-L. (2021). Benefits of metformin combined with pemetrexed-based platinum doublets as a first-line therapy for advanced lung adenocarcinoma patients with diabetes. Biomolecules 11, 11081252. doi:10.3390/biom11081252
Wang, J., Xia, S., and Zhu, Z. (2015). Synergistic effect of phenformin in non-small cell lung cancer (NSCLC) ionizing radiation treatment. Cell Biochem. Biophys. 71 (2), 513–518. doi:10.1007/s12013-014-0283-z
Wang, Y., Li, J., Chang, S., Dong, Y., and Che, G. (2021). Risk and influencing factors for subsequent primary lung cancer after treatment of breast cancer: A systematic review and two meta-analyses based on four million cases. J. Thorac. Oncol. 16 (11), 1893–1908. doi:10.1016/j.jtho.2021.07.001
Wang, Y., Lin, B., Wu, J., Zhang, H., and Wu, B. (2014). Metformin inhibits the proliferation of A549/CDDP cells by activating p38 mitogen-activated protein kinase. Oncol. Lett. 8 (3), 1269–1274. doi:10.3892/ol.2014.2270
Wen-Xiu, X., Xiao-Wei, Z., Hai-Ying, D., Ying-Hui, T., Si-Si, K., Xiao-Fang, Z., et al. (2018). Impact of metformin use on survival outcomes in non-small cell lung cancer treated with platinum. Med. Baltim. 97 (51), e13652. doi:10.1097/MD.0000000000013652
Wink, K. C. J., Belderbos, J. S. A., Dieleman, E. M. T., Rossi, M., Rasch, C. R. N., Damhuis, R. A. M., et al. (2016). Improved progression free survival for patients with diabetes and locally advanced non-small cell lung cancer (NSCLC) using metformin during concurrent chemoradiotherapy. Radiotherapy And Oncol. 118 (3), 453–459. doi:10.1016/j.radonc.2016.01.012
Xia, C., Dong, X., Li, H., Cao, M., Sun, D., He, S., et al. (2022). Cancer statistics in China and United States, 2022: Profiles, trends, and determinants. Chin. Med. J. Engl. 135 (5), 584–590. doi:10.1097/CM9.0000000000002108
Xiang, D., Hu, S., Mai, T., Zhang, X., Zhang, L., Wang, S., et al. (2022). Worldwide cancer statistics of adults over 75 years old in 2019: A systematic analysis of the global burden of disease study 2019. BMC Public Health 22 (1), 1979. doi:10.1186/s12889-022-14412-1
Xiao, K., Liu, F., Liu, J., Xu, J., Wu, Q., and Li, X. (2020). The effect of metformin on lung cancer risk and survival in patients with type 2 diabetes mellitus: A meta-analysis. J. Clin. Pharm. Ther. 45 (4), 783–792. doi:10.1111/jcpt.13167
Yao, K., Zheng, H., and Li, T. (2022). Association between metformin use and the risk, prognosis of gynecologic cancer. Front. Oncol. 12, 942380. doi:10.3389/fonc.2022.942380
Yendamuri, S., Barbi, J., Pabla, S., Petrucci, C., Punnanitinont, A., Nesline, M., et al. (2019). Body mass index influences the salutary effects of metformin on survival after lobectomy for stage I NSCLC. J. Thorac. Oncol. 14 (12), 2181–2187. doi:10.1016/j.jtho.2019.07.020
Zeng, S., Gan, H. X., Xu, J. X., and Liu, J. Y. (2019). Metformin improves survival in lung cancer patients with type 2 diabetes mellitus: A meta-analysis. Med. Clin. Barc. 152 (8), 291–297. doi:10.1016/j.medcli.2018.06.026
Zhang, F., Han, S., and Song, W. (2022). Anticancer effects of metformin in experimental animal models of different types of cancer: A systematic review and meta-analysis. Lab. Anim. Res. 38 (1), 22. doi:10.1186/s42826-022-00131-6
Zhang, J., Wu, J., He, Q., Liang, W., and He, J. (2018). The prognostic value of metformin for advanced non-small cell lung cancer: A systematic review and meta-analysis. Transl. Lung Cancer Res. 7 (3), 389–396. doi:10.21037/tlcr.2018.03.14
Zhang, Q., Zheng, J., Wang, W., Cornett, E. M., Kaye, A. D., Urits, I., et al. (2022). The anticancer effect of metformin combined with epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer patients with or without type 2 diabetes mellitus: A systematic review and meta-analysis. Oncol. Ther. 10 (2), 363–375. doi:10.1007/s40487-022-00209-0
Zhang, X., Tan, R., Lam, W. C., Yao, L., Wang, X., Cheng, C. W., et al. (2020). PRISMA (preferred reporting Items for systematic reviews and meta-analyses) extension for Chinese herbal medicines 2020 (PRISMA-CHM 2020). Am. J. Chin. Med. 48 (6), 1279–1313. doi:10.1142/S0192415X20500639
Keywords: metformin, non-small cell lung cancer, epidermal growth factor receptor tyrosine kinase inhibitor, chemotherapy, immune checkpoint inhibitor, prognosis
Citation: Wang Y, Hu Y, Wang T, Che G and Li L (2023) Addition of metformin for non-small cell lung cancer patients receiving antineoplastic agents. Front. Pharmacol. 14:1123834. doi: 10.3389/fphar.2023.1123834
Received: 14 December 2022; Accepted: 21 February 2023;
Published: 10 March 2023.
Edited by:
Mo Aljofan, Nazarbayev University School of Medicine, KazakhstanReviewed by:
Yang Yang, University of Chinese Academy of Sciences, ChinaSamson Mathews Samuel, Weill Cornell Medicine- Qatar, Qatar
Copyright © 2023 Wang, Hu, Wang, Che and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guowei Che, Y2hlZ3Vvd2VpeHdAMTI2LmNvbQ==; Lu Li, d2luZGZsb3dlcjE5OTFAdmlwLjE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Yan Wang
Yan Wang Yuanyuan Hu2†
Yuanyuan Hu2† Guowei Che
Guowei Che