- 1Department of Gastroenterology, Peking University Third Hospital, Beijing, China
- 2International Institute of Population Health, Peking University Health Science Center, Beijing, China
Introduction: Small intestinal bacterial overgrowth (SIBO) leads to non-specific abdominal discomfort and nutrient malabsorption. Currently, rifaximin is widely applied in SIBO based on its antibacterial and non-absorbable nature. Berberine is a natural component of many popular medicine plants that ameliorates intestinal inflammation in humans through its modification of the gut microbiota. Potential effect of berberine to the gut may provide therapeutic target for SIBO. We aimed to evaluate the effect of berberine compared with rifaximin on SIBO patients.
Methods: This is an investigator-initiated, single-center, open-label, double-arm randomized controlled trial, termed BRIEF-SIBO (Berberine and rifaximin effects for small intestinal bacterial overgrowth). In total, 180 patients will be recruited and allocated to an intervention group (berberine) and a control group (rifaximin). Each participant will receive one 400 mg drug twice a day (800 mg daily) for 2 weeks. The total follow-up period is 6 weeks from the start of medication. The primary outcome is a negative breath test. The secondary outcomes include abdominal symptom relief and alteration in gut microbiota. Efficacy assessment will be performed every 2 weeks, as well as safety assessment during the treatment. The primary hypothesis is that berberine is not inferior to rifaximin for SIBO.
Discussion: The BRIEF-SIBO study is the first clinical trial assessing the eradication effects of 2 weeks of berberine treatment in SIBO patients. The effect of berberine will be fully verified by using rifaximin as the positive control. The findings of this study may have implications for the management of SIBO, especially increasing the awareness of both physicians and patients who are suffering from long-term abdominal discomfort and avoiding excessive examination.
1 Introduction
Small intestinal bacterial overgrowth (SIBO) is defined as the presence of an abnormally excessive amount of bacterial colonization in the small bowel (Quigley et al., 2020; Bushyhead and Quigley, 2022). Primary or secondary motility abnormalities destroy the ability of the small intestine to prevent colon bacterial translocation (Bohm et al., 2013). Meanwhile, ileocecal valve dysfunction leads to colonic bacterial regurgitation (Miller et al., 2012). It is believed that symptoms linked to SIBO consist of bloating, diarrhea and abdominal pain/discomfort. Steatorrhea, vitamin B12 deficiency and malnutrition can be seen in more severe cases.
The gold standard for the diagnosis of SIBO is quantitative culture of small intestine aspirates. The American Gastroenterology Association (AGA) recently recommended a new threshold at >103 colony-forming units per milliliter (CFU/mL) on fresh aspirate culture instead of >105 CFU/mL based on a large-scale study (Leite et al., 2019), derived from subjects with altered intestinal anatomy because the bacterial level in normal subjects rarely exceeds 102 CFU/mL (Pimentel et al., 2020). An alternative method is the measurement of exhaled hydrogen and methane gas, which is considered a non-invasive, safe, useful, and cost-efficient test (Hammer et al., 2022). The North American Consensus recommended that a rise in hydrogen of ≥20 part per million (ppm) or methane levels ≥10 ppm by 90 min during glucose or lactulose breath test was considered positive (Rezaie et al., 2017).
SIBO is now commonly diagnosed and closely associated with many gastrointestinal diseases, such as irritable bowel syndrome (IBS) (Ghoshal et al., 2020), inflammatory bowel disease (IBD) (Shah et al., 2019), pancreatitis (El Kurdi et al., 2019), non-alcoholic liver disease (Wijarnpreecha et al., 2020), colorectal cancer and abdominal surgery. Based on the similarity of the clinical manifestation profiles between IBS and SIBO, the prevalence of SIBO in IBS patients has been widely reported to range from 4% to 84%, with an overall pooled prevalence rate of 38% (Chen et al., 2018). A meta-analysis of case‒control studies found that SIBO prevalence in patients with IBS was 35.5% (95% CI 33.6–37.4) and 29.7% (95% CI 27.6–31.8) in controls based on breath tests, while culture-based studies yielded a SIBO prevalence of 33.5% (95% CI 30.1–36.9) in patients with IBS and 8.2% (95% CI 6.8–9.6) in controls with a cutoff value of 103 CFU/mL (Shah et al., 2020). The diagnostic modality actually influences the prevalence of SIBO in consideration of the sensitivity and specificity of breath tests.
Currently, rifaximin is widely applied considering its broad-spectrum and non-absorbed nature to achieve low gastrointestinal absorption while retaining good antibacterial activity, which is thought to be effective and safe for the treatment of SIBO (Gatta and Scarpignato, 2017). Studies have shown significant symptom remission of rifaximin therapy in IBS patients with SIBO (Moraru et al., 2014; Liu et al., 2016; Leite et al., 2018; Rezaie et al., 2019; Tuteja et al., 2019). Unfortunately, no responsiveness and recurrence after eradication of SIBO limit antibiotic usage. Rezaie et al. (2019) reported that 48/93 (51.6%) were non-responders after 2 weeks of low-dose rifaximin treatment. For those responders, the average time of recurrence was 94.86 ± 38.6 days, and 38 (84.4%) of the 45 patients experienced symptom recurrence by the end of the 18-week observation phase. Moreover, we must take into account that rotating antibiotic regimens lead to drug resistance and risks of Clostridium difficile infection. Medication for SIBO remains confusing and limited.
Berberine is a natural pentacyclic isoquinoline alkaloid extracted from many popular medicine plants such as the genus Berberis, Coptis and Hydrastis (Wang et al., 2017). There is already evidence that the structural and numerical changes in the gut microbiota under pathological conditions can be reversed by berberine (Jia et al., 2019), which mediates modulatory effects on microglial activation and visceral hypersensitivity (Zhang et al., 2021), and ameliorates intestinal inflammation in humans through antibacterial action (Gong et al., 2017; Habtemariam, 2020). Berberine enhanced the composition of beneficial bacteria such as Bacteroides, Bifidobacterium, Lactobacillus, and Akkermansia (Fang et al., 2021). Furthermore, it could alleviate colon inflammation by regulating interferon-gamma and interleukin-17A-producing lamina propria CD4 (+) T cells (Takahara et al., 2019). It has been proven to significantly decrease diarrhea and abdominal pain scores in diarrhea-predominant IBS patients (Chen et al., 2015). Berberine effects to the gut may provide therapeutic targets for SIBO. Chedid et al. (2014) confirmed that herbal therapy containing berberine is equivalent to rifaximin for the resolution of SIBO. However, there is little evidence investigating the potential effect of berberine as a single agent in SIBO patients. In the present study, we aimed to evaluate the effects of berberine and rifaximin on patients with SIBO (BRIEF-SIBO study). The effectiveness outcomes included a negative lactulose hydrogen methane breath test (LHMBT) and clinical symptom remission. Different gut microbiome spectra in SIBO patients treated with berberine and rifaximin may provide a potential explanation.
2 Methods and analysis
2.1 Trial design
This is an investigator-initiated, single-center, open-label, two-arm randomized controlled trial from Peking University Third Hospital, Beijing, China, termed BRIEF-SIBO. The study aims to evaluate the efficacy and safety of berberine for SIBO patients compared with rifaximin as the positive control. This study was registered on the Chinese Clinical Trial Registry platform under number ChiCTR2200057554. The study protocol, informed consent and other documents were reviewed and approved by the Peking University Third Hospital Medical Science Research Ethics Committee under number 2022-021-02. This protocol complies with the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) (Chan et al., 2013).
2.2 Patient recruitment and eligibility
Patients who have at least one of the major symptoms, including abdominal pain, distension, diarrhea or constipation, with positive LHMBT will be enrolled in this study. The inclusion criteria were as follows: 1) Aged ≥18 and ≤65 years old; 2) Complained of abdominal pain, distension, constipation or diarrhea for over 6 months, and at least one of them up to a moderate or severe degree according to the gastrointestinal symptom rating scale (GSRS); 3) Positive hydrogen breath test with elevated or normal methane gas; 4) Voluntarily joined the study and completed the case report form, LHMBT, colonoscopy and blood biochemical examination; and 5) Took drugs according to the required rules with good compliance.
Patients who were found to have gastrointestinal organic diseases detected by endoscopy or digestive tract surgery history were excluded. Patients with severe heart, liver, lung, kidney, blood, endocrine, and nervous system diseases, or severe respiratory tract, digestive tract, urinary tract infections or mental disorders will temporarily stop entering treatment until professional clinicians access their conditions. Patients who were taking antibiotics and acid suppression drugs for more than 3 days during the past month or probiotics, laxatives, antidiarrheal or prokinetic agents within 2 weeks will not be eligible for participation. Pregnant or lactating women will also be excluded. Patients who firmly report rifaximin or rifamycin allergy will not participate, as it is possible to be randomized to the rifaximin group. All patients will be identified through gastroenterologists for their patients with non-specific abdominal symptoms. Two principal researchers of the project team are responsible for explaining the background, purpose, process, risks and benefits of this study and obtaining informed consent.
2.3 Visit and procedure
2.3.1 Screening
The study flow diagram is shown in Figure 1 (Schulz et al., 2010). We will first perform LHMBT for those suspected to have SIBO and exclude negative patients. The next examination is colonoscopy to ensure that they do not have organic colon diseases. Baseline measures include a case report form (shown in Supplementary Material), regular blood tests, stool collection for calprotein and 16S rRNA sequencing. Blood samples will be collected and shipped to the core laboratory. Stool samples will be stored at −80°C.
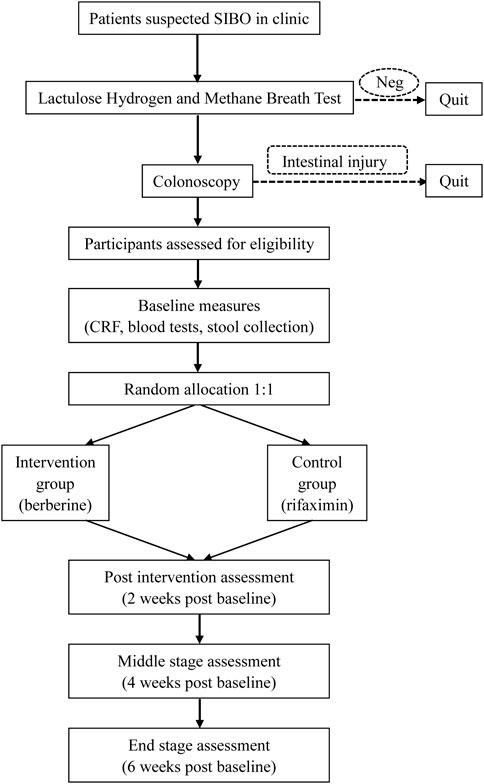
FIGURE 1. Flow diagram based on the Consolidated Standards of Reporting Trials guidelines (CONSORT): Neg, negative; CRF, case report form.
Before LHMBT, all subjects are supposed to refrain from antibiotic use and discontinue probiotics, laxatives, antidiarrheal and prokinetic agents for 2 weeks. To minimize basal hydrogen excretion, dietary restriction and avoidance of smoking for at least 24 h prior to the test and during the test are recommended. Furthermore, patients are required to avoid coarse grains, milk, juice, and alcohol in the evening before the test. Fasting for 8–12 h before the procedure is needed. Before the examination, subjects use 20 mL antiseptic mouthwash (0.05% chlorhexidine) to eliminate fermentation by oral bacteria. End-expiratory breath samples will be collected just before the ingestion of 10 g (15 mL) of lactulose in a 250 mL water solution. Gas samples are collected every 15 min until 90 min and tested immediately by the methane-hydrogen breathing analyzer DA6000 (Sunvou Medical Electronics Co., Ltd., Wuxi, China). A rise in hydrogen of ≥20 part per million (ppm) by 90 min with elevated or normal methane was considered positive. Patients with only methane levels ≥10 ppm are not eligible (Rezaie et al., 2017).
2.3.2 Intervention
In total, 180 patients will be recruited and divided into two groups: the berberine group (Berberine Hydrochloride Tablets, H51022193, Chengdu Jinhua Pharmaceutical Co., Ltd., China) and the rifaximin group (Xifaxan, H20181212, Alfasigma S.p.A, Italy). Each participant will receive only one 400 mg drug twice a day (800 mg daily) for 2 weeks. The recommended dose of berberine is mainly determined by the advice of pharmacological experts, while that of rifaximin is based on past clinical trial conclusions. The first participant was enrolled on 15 March, 2022, and the study is expected to be terminated by July 2023 with the anticipated inclusion of 180 participants.
2.3.3 Follow-up
The total follow-up period is 6 weeks from the start of medication. Efficacy assessment will be performed every 2 weeks using GSRS (Dimenäs et al., 1993), Bristol Stool Form (BSF) and breath tests, as well as safety assessment during the first 2 weeks of treatment. Each specific visit and measurement are summarized in Table 1. Multiple methods, such as telephone and online follow-up, are carried out to improve patient compliance if room consultation is impossible. Participants will return unused tablets and report reasons for non-compliance at the first visit (Kini and Ho, 2018). If a patient decides to withdraw, the follow-up will be stopped.
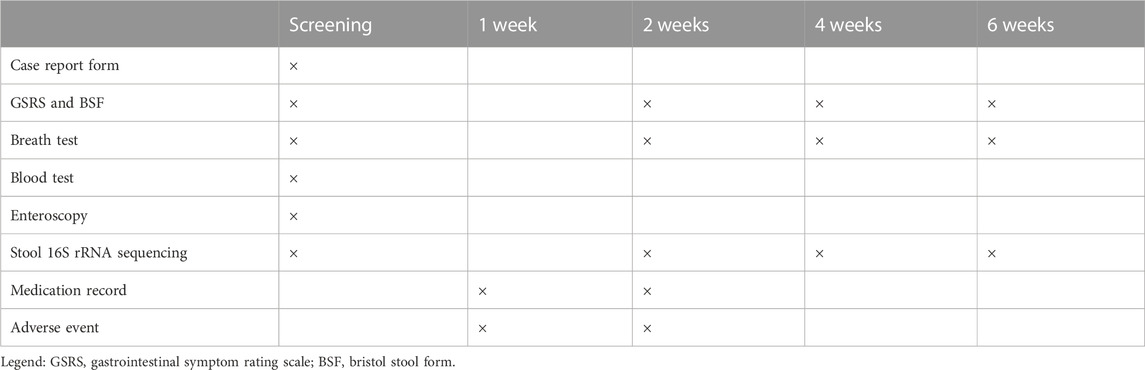
TABLE 1. Standard protocol items: Adapted recommendations for interventional trials (SPIRIT) schedule: Enrollment, interventions, and follow-up.
2.4 Results
2.4.1 Effectiveness outcomes
The primary outcome is a negative LHMBT. It is defined as a rise of hydrogen less than 20 ppm and methane less than 10 ppm within 90 min compared with screening tests.
The secondary outcomes include abdominal symptom relief and alterations in gut microbiota. The key secondary outcome we designed was similar to the relief of IBS global symptoms (Pimentel et al., 2011). Abdominal symptom remission is required to meet at least one of the following criteria: 1) The pain or distension scores of GSRS decrease at least 30% from baseline; 2) Hard stool (1/2 in BSF) and loose stool (6/7 in BSF) reduce more than 50% from baseline.
2.4.2 Assessment of harms
The study investigator will exclude those with any physical problems that may limit participation with berberine or rifaximin exposures. Participants will be asked to report any adverse events (AEs) experienced during the study period. Participants with adverse events (e.g., dizziness, headache, loss of taste, dull sensation, diplopia, vertigo, palpitations, hot flashes, dyspnea, hematochezia, rash, back pain, muscle spasm, muscle weakness, hematuria, fever, flu-like symptoms after intervention) will be advised to consult their physician to provide healthcare as appropriate. The investigator will record the details of the frequency and severity of adverse events.
2.5 Randomization and allocation
Participants will be randomized after baseline assessment to either berberine or rifaximin intervention with a 1:1 allocation. A laboratory staff member, not the investigator, used Statistical Package for Social Science Version 26.0 software (SPSS V.26.0) to produce 180 random numbers after setting the starting point according to the simple randomization. The patient codes were divided into two groups (A for berberine and B for rifaximin) according to the size of the number to form a random allocation table. The corresponding information of each code was assembled into the envelope seal. Thus, the principal investigator will remain blinded to the randomization process. Participants will be aware of the group to which they are allocated.
2.6 Sample size
We calculated the sample size based on the primary outcome of eradication rate. Gatta and Scarpignato (2017) published a meta-analysis in which the overall eradication rate of rifaximin for SIBO patients according to per-protocol analysis was 72.9%. Therefore, the null hypothesis is postulated to be a rate of 72.9% (P0). We proposed that the effective rate of berberine is 69.8% (P1) (Wang et al., 2002; Chen et al., 2015). Using the non-inferiority design with unilateral α = 0.025 and power 1-β = 0.8, 82 participants will be enrolled in each group. However, assuming a 10% attrition rate, we will aim to recruit 90 participants per group.
2.7 Criteria for discontinuing or modifying allocated interventions
If a severe or unanticipated adverse event that may influence the risk-benefit ratio of the study occurs, the principal investigator must report to the ethics committee and permanently discontinue the treatment. Peking University Third Hospital, the sponsor of this study, will pay for medical expenses and provide economic compensation for the injuries related to this study. Study treatment must be stopped permanently in the event of unintended pregnancies. In addition, participants can withdraw from the trial at any stage for any reason. For patients with limited symptom response, we will provide alternative interventions including switching medication, probiotics and dietary guidance.
2.8 Data management
The original data will be correctly, completely and clearly recorded in the written case report form and observation questionnaire. After being reviewed and signed by the principal investigator, the records should be sent in a timely manner to the clinical research data manager. The Microsoft Excel database will be used by two-person and two-machine input for checking. Data review will be performed regularly by the study monitoring committee to ensure completeness and accuracy. The monitor will finish a data inspection report for each participant, including the study completion, inclusion and exclusion criteria, pharmaceutical compliance, concomitant medication, adverse events, etc. During this period, the investigator should be required to answer if any problem is found, and the monitor will be informed in time. The exchange of questions and answers between them should be sent in the form of a question sheet for future reference.
The losses and dropouts will be carefully recorded to ensure study reliability. The next participant who meets the eligibility criteria will replace the last participant who withdraws from the research until reaching the calculated sample size. All participant information will be stored in a safe place anonymously by the responsible researcher as required by Chinese relevant laws to guarantee the security of privacy. The study results will be disseminated in a peer-reviewed journal and at conferences without any privacy information of participants.
2.9 Statistical analysis
Patients who finish at least one postmedication evaluation after randomization and subsequent treatment constitute the study full analysis set (FAS) as well as the primary group of efficacy assessment, according to the intention to treatment (ITT) principle. Patients who meet the criteria and complete the observation before medication without other affected treatments during the trial will be included in the study per protocol set (PPS) as the secondary group of efficacy assessment. The study safety set consists of all cases who have safety evaluation data after taking at least one kind of drug. Rubin’s method of multiple imputation will be performed for missing values to restore natural variability and uncertainty. The complete and imputed datasets will be combined and analyzed (Little et al., 2012). Complete case analysis is used for fecal microbiota on the nature of the outcome.
Analysis will be conducted using SPSS V.26.0. The quantitative and qualitative variables were reported as mean ± standard deviation (SD), median ± interquartile range (IQR), and number (frequency). Univariate analysis of variance (ANOVA) will examine differences between groups for variables with continuous data. χ2 tests will examine differences between groups for categorical variables. Unadjusted ANOVA and adjusted analysis of covariance models will compare differences in scores from baseline and postintervention data within and between groups using FAS and PPS. Variable correlations will be analyzed through Spearman’s correlation analysis. Statistical significance was defined as a p-value less than 0.05.
3 Discussion
The pharmacology of berberine has been extensively explored and revealed multifunctional activities, including anti-inflammatory (Wang et al., 2017), anticancer (Chen et al., 2019; Sun et al., 2019; Gong et al., 2020), antidiabetic (Gong et al., 2017), antihyperlipidemic and cardioprotective effects, based on its regulation of gut microbiota. To the best of our knowledge, the BRIEF-SIBO study is the first clinical trial assessing the eradication effects of a two-week intervention with berberine in SIBO patients. The effect of berberine will be fully verified by using rifaximin as the positive control. The findings of this study may have implications for the management of SIBO, especially increasing the awareness of both clinicians and patients who suffer from long-term abdominal discomfort and avoiding excessive examination. We also expect to promote clinical diagnosis and efficient management. Intervention patterns will refine and inform the development of future berberine medication designed for patients with SIBO.
The goal of therapy in SIBO is not only to eradicate the small intestinal microbiota but also to improve symptoms (Quigley et al., 2020). Thus, we set abdominal symptom remission as an important point of effectiveness. The secondary outcomes also include other symptom improvements from GSRS and alterations in the gut microbiome. Different microbiome spectra in SIBO patients after treatment with berberine and rifaximin may provide a potential explanation. Predictors may be considered in clinical practice to target SIBO patients most likely to benefit from the varied intervention.
4 Trial status
This study was prospectively registered on the Chinese Clinical Trial Registry platform under number ChiCTR2200057554 (15 March, 2022). The study is ongoing and has not completed participant recruitment at the time of submission. Recruitment is expected to be completed by July 2023.
Ethics statement
The studies involving human participants were reviewed and approved by the Peking University Third Hospital Medical Science Research Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
Author contributions
HG is involved in all aspects of the protocol design and the lead author of the manuscript. SL, JZ, CC, YD, and KW provided their clinical expertise and patient recruitment through the clinic. LD is the senior author on the protocol, and got the funding, as well as guided the work of this research. All authors reviewed the manuscript for study design, provided critical insight into manuscript content and approved the final version for submission.
Funding
The study is supported by JKCJ202101 from the International Institute of Population Health of Peking University Health Science Center, China. This funding source had no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data, or decision to submit results.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1121435/full#supplementary-material
References
Bohm, M., Siwiec, R. M., and Wo, J. M. (2013). Diagnosis and management of small intestinal bacterial overgrowth. Nutr. Clin. Pract. 28 (3), 289–299. doi:10.1177/0884533613485882
Bushyhead, D., and Quigley, E. M. M. (2022). Small intestinal bacterial overgrowth pathophysiology and its implications for definition and management. Gastroenterology 163 (3), 593–607. doi:10.1053/j.gastro.2022.04.002
Chan, A.-W., Tetzlaff, J. M., Altman, D. G., Laupacis, A., Gøtzsche, P. C., Krleža-Jerić, K., et al. (2013). SPIRIT 2013 statement: Defining standard protocol items for clinical trials. Ann. Intern Med. 158 (3), 200–207. doi:10.7326/0003-4819-158-3-201302050-00583
Chedid, V., Dhalla, S., Clarke, J. O., Roland, B. C., Dunbar, K. B., Koh, J., et al. (2014). Herbal therapy is equivalent to rifaximin for the treatment of small intestinal bacterial overgrowth. Glob. Adv. Health Med. 3 (3), 16–24. doi:10.7453/gahmj.2014.019
Chen, B., Kim, J. J.-W., Zhang, Y., Du, L., and Dai, N. (2018). Prevalence and predictors of small intestinal bacterial overgrowth in irritable bowel syndrome: A systematic review and meta-analysis. J. Gastroenterol. 53 (7), 807–818. doi:10.1007/s00535-018-1476-9
Chen, C., Tao, C., Liu, Z., Lu, M., Pan, Q., Zheng, L., et al. (2015). A randomized clinical trial of berberine Hydrochloride in patients with diarrhea-predominant irritable bowel syndrome. Phytother. Res. 29 (11), 1822–1827. doi:10.1002/ptr.5475
Chen, Q. Q., Shi, J. M., Ding, Z., Xia, Q., Zheng, T. S., Ren, Y. B., et al. (2019). Berberine induces apoptosis in non-small-cell lung cancer cells by upregulating miR-19a targeting tissue factor. Cancer Manag. Res. 11, 9005–9015. doi:10.2147/CMAR.S207677
Dimenäs, E., Glise, H., Hallerbäck, B., Hernqvist, H., Svedlund, J., and Wiklund, I. (1993). Quality of life in patients with upper gastrointestinal symptoms. An improved evaluation of treatment regimens? Scand. J. Gastroenterol. 28 (8), 681–687. doi:10.3109/00365529309098272
El Kurdi, B., Babar, S., El Iskandarani, M., Bataineh, A., Lerch, M. M., Young, M., et al. (2019). Factors that affect prevalence of small intestinal bacterial overgrowth in chronic pancreatitis: A systematic review, meta-analysis, and meta-regression. Clin. Transl. Gastroenterol. 10, e00072. doi:10.14309/ctg.0000000000000072
Fang, Y., Zhang, J., Zhu, S., He, M., Ma, S., Jia, Q., et al. (2021). Berberine ameliorates ovariectomy-induced anxiety-like behaviors by enrichment in equol generating gut microbiota. Pharmacol. Res. 165, 105439. doi:10.1016/j.phrs.2021.105439
Gatta, L., and Scarpignato, C. (2017). Systematic review with meta-analysis: Rifaximin is effective and safe for the treatment of small intestine bacterial overgrowth. Aliment. Pharmacol. Ther. 45 (5), 604–616. doi:10.1111/apt.13928
Ghoshal, U. A.-O., Nehra, A., Mathur, A., and Rai, S. (2020). A meta-analysis on small intestinal bacterial overgrowth in patients with different subtypes of irritable bowel syndrome. J. Gastroenterol. Hepatol. 35 (6), 922–931. doi:10.1111/jgh.14938
Gong, C., Hu, X., Xu, Y., Yang, J., Zong, L., Wang, C., et al. (2020). Berberine inhibits proliferation and migration of colorectal cancer cells by downregulation of GRP78. Anti-Cancer Drugs 31 (2), 141–149. doi:10.1097/CAD.0000000000000835
Gong, J., Hu, M., Huang, Z., Fang, K., Wang, D., Chen, Q., et al. (2017). Berberine attenuates intestinal mucosal barrier dysfunction in type 2 diabetic rats. Front. Pharmacol. 8, 42. doi:10.3389/fphar.2017.00042
Habtemariam, S. (2020). Berberine pharmacology and the gut microbiota: A hidden therapeutic link. Pharmacol. Res. 155, 104722. doi:10.1016/j.phrs.2020.104722
Hammer, H. F., Fox, M. R., Keller, J., Salvatore, S., Basilisco, G., Hammer, J., et al. (2022). European guideline on indications, performance, and clinical impact of hydrogen and methane breath tests in adult and pediatric patients: European association for Gastroenterology, endoscopy and nutrition, European society of neurogastroenterology and motility, and European society for paediatric Gastroenterology hepatology and nutrition consensus. United Eur. Gastroenterol. J. 10 (1), 15–40. doi:10.1002/ueg2.12133
Jia, Q., Zhang, L., Zhang, J., Pei, F., Zhu, S., Sun, Q., et al. (2019). Fecal microbiota of diarrhea-predominant irritable bowel syndrome patients causes hepatic inflammation of germ-free rats and berberine reverses it partially. Biomed. Res. Int. 2019, 4530203. doi:10.1155/2019/4530203
Kini, V., and Ho, P. M. (2018). Interventions to improve medication adherence: A review. JAMA, J. Am. Med. Assoc. 320 (23), 2461–2473. doi:10.1001/jama.2018.19271
Leite, G., Morales, W., Weitsman, S., Celly, S., Parodi, G., Barlow, G., et al. (2018). Sa1216 - the convergence of bacterial overgrowth, systemic inflammatory biomarkers, and anti-vinculin antibodies in determining the response to rifaximin treatment in diarrhea-predominant ibs (D-Ibs) subjects. Gastroenterology 154 (6), S-280. doi:10.1016/S0016-5085(18)31297-6
Leite, G., Villanueva-Millan, M. J., Celly, S., Sedighi, R., Morales, W., Rezaie, A., et al. (2019). First large scale study defining the characteristic microbiome signatures of small intestinal bacterial overgrowth (SIBO): Detailed analysis from the reimagine study. Gastroenterology 156 (6), S1–S2. doi:10.1016/s0016-5085(19)36776-9
Little, R. J., D'Agostino, R., Cohen, M. L., Dickersin, K., Emerson, S. S., Farrar, J. T., et al. (2012). The prevention and treatment of missing data in clinical trials. N. Engl. J. Med. 367 (14), 1355–1360. doi:10.1056/NEJMsr1203730
Liu, Z. J., Wei, H., Duan, L. P., Zhu, S. W., Zhang, L., and Wang, K. (2016). Clinical features of irritable bowel syndrome with small intestinal bacterial overgrowth and a preliminary study of effectiveness of Rifaximin. Zhonghua Yi Xue Za Zhi 96 (24), 1896–1902. doi:10.3760/cma.j.issn.0376-2491.2016.24.005
Miller, L. S., Vegesna, A. K., Sampath, A. M., Prabhu, S., Kotapati, S. K., and Makipour, K. (2012). Ileocecal valve dysfunction in small intestinal bacterial overgrowth: A pilot study. World J. Gastroenterol. 18 (46), 6801–6808. doi:10.3748/wjg.v18.i46.6801
Moraru, I. G., Moraru, A. G., Andrei, M., Iordache, T., Drug, V., Diculescu, M., et al. (2014). Small intestinal bacterial overgrowth is associated to symptoms in irritable bowel syndrome. Evidence from a multicentre study in Romania. Rom. J. Intern Med. 52 (3), 143–150.
Pimentel, M., Lembo, A., Chey, W. D., Zakko, S., Ringel, Y., Yu, J., et al. (2011). Rifaximin therapy for patients with irritable bowel syndrome without constipation. N. Engl. J. Med. 364 (1), 22–32. doi:10.1056/NEJMoa1004409
Pimentel, M., Saad, R. J., Long, M. D., and Rao, S. S. C. (2020). ACG clinical guideline: Small intestinal bacterial overgrowth. Am. J. Gastroenterol. 115 (2), 165–178. doi:10.14309/ajg.0000000000000501
Quigley, E. M. M., Murray, J. A., and Pimentel, M. (2020). AGA clinical practice update on small intestinal bacterial overgrowth: Expert review. Gastroenterology 159 (4), 1526–1532. doi:10.1053/j.gastro.2020.06.090
Rezaie, A., Buresi, M., Lembo, A., Lin, H., McCallum, R., Rao, S., et al. (2017). Hydrogen and methane-based breath testing in gastrointestinal disorders: The north American consensus. Am. J. Gastroenterol. 112 (5), 775–784. doi:10.1038/ajg.2017.46
Rezaie, A., Heimanson, Z., McCallum, R., and Pimentel, M. (2019). Lactulose breath testing as a predictor of response to rifaximin in patients with irritable bowel syndrome with diarrhea. Am. J. Gastroenterol. 114 (12), 1886–1893. doi:10.14309/ajg.0000000000000444
Schulz, K. F., Altman Dg Fau - Moher, D., and Moher, D. (2010). CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. Br. Med. J. Clin. Res. ed.) 340, c332. doi: doi:doi:10.1136/bmj.c332
Shah, A., Morrison, M., Burger, D., Martin, N., Rich, J., Jones, M., et al. (2019). Systematic review with meta-analysis: The prevalence of small intestinal bacterial overgrowth in inflammatory bowel disease. Aliment. Pharmacol. Ther. 49 (6), 624–635. doi:10.1111/apt.15133
Shah, A., Talley, N. J., Jones, M., Kendall, B. J., Koloski, N., Walker, M. M., et al. (2020). Small intestinal bacterial overgrowth in irritable bowel syndrome: A systematic review and meta-analysis of case-control studies. Am. J. Gastroenterol. 115 (2), 190–201. doi:10.14309/ajg.0000000000000504
Sun, Y., Wang, W., and Tong, Y. (2019). Berberine inhibits proliferative ability of breast cancer cells by reducing metadherin. Med. Sci. Monit. 25, 9058–9066. doi:10.12659/MSM.914486
Takahara, M., Takaki, A., Hiraoka, S., Adachi, T., Shimomura, Y., Matsushita, H., et al. (2019). Berberine improved experimental chronic colitis by regulating interferon-gamma- and IL-17A-producing lamina propria CD4(+) T cells through AMPK activation. Sci. Rep. 9 (1), 11934. doi:10.1038/s41598-019-48331-w
Tuteja, A. K., Talley, N. J., Stoddard, G. J., and Verne, G. N. (2019). Double-blind placebo-controlled study of rifaximin and lactulose hydrogen breath test in gulf war veterans with irritable bowel syndrome. Dig. Dis. Sci. 64 (3), 838–845. doi:10.1007/s10620-018-5344-5
Wang, K., Feng, X., Chai, L., Cao, S., and Qiu, F. (2017). The metabolism of berberine and its contribution to the pharmacological effects. Drug Metab. Rev. 49 (2), 139–157. doi:10.1080/03602532.2017.1306544
Wang, Y. B., Tang, X. S., and Yang, Z. X. (2002). Effects of berberine on patients with IBS. J. Cap. Univ. Med. Sci. 02, 151–152.
Wijarnpreecha, K., Lou, S., Watthanasuntorn, K., Kroner, P. T., Cheungpasitporn, W., Lukens, F. J., et al. (2020). Small intestinal bacterial overgrowth and nonalcoholic fatty liver disease: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 32 (5), p601–p608. doi:10.1097/MEG.0000000000001541
Keywords: small intestinal bacteria overgrowth (SIBO), berberine (BBR), rifaximin, microbiota, breath test
Citation: Guo H, Lu S, Zhang J, Chen C, Du Y, Wang K and Duan L (2023) Berberine and rifaximin effects on small intestinal bacterial overgrowth: Study protocol for an investigator-initiated, double-arm, open-label, randomized clinical trial (BRIEF-SIBO study). Front. Pharmacol. 14:1121435. doi: 10.3389/fphar.2023.1121435
Received: 11 December 2022; Accepted: 31 January 2023;
Published: 15 February 2023.
Edited by:
Yong Gao, Guangzhou University of Chinese Medicine, ChinaReviewed by:
Yu Zhao, Shanghai University of Traditional Chinese Medicine, ChinaJoshua Goldenberg, National University of Natural Medicine, United States
Copyright © 2023 Guo, Lu, Zhang, Chen, Du, Wang and Duan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liping Duan, ZHVhbmxwQGJqbXUuZWR1LmNu
 Huaizhu Guo
Huaizhu Guo Siqi Lu1
Siqi Lu1 Jindong Zhang
Jindong Zhang Chen Chen
Chen Chen Liping Duan
Liping Duan