
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 23 February 2023
Sec. Ethnopharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1120457
This article is part of the Research TopicNutraceuticals Modulation for Oxidative Stress in Disease and HealthView all 11 articles
 Putri Ayu Jayusman1
Putri Ayu Jayusman1 Nurrul Shaqinah Nasruddin1
Nurrul Shaqinah Nasruddin1 Badiah Baharin2
Badiah Baharin2 Nurul ‘Izzah Ibrahim3
Nurul ‘Izzah Ibrahim3 Haryati Ahmad Hairi4
Haryati Ahmad Hairi4 Ahmad Nazrun Shuid5*
Ahmad Nazrun Shuid5*Osteoporosis and periodontitis are two major chronic diseases of postmenopausal women. The association between these two diseases are evident through systemic bone loss and alveolar bone loss. Both postmenopausal osteoporosis and periodontitis impose a considerable personal and socioeconomic burden. Biphosphonate and hormone replacement therapy are effective in preventing bone loss in postmenopausal osteoporosis and periodontitis, but they are coupled with severe adverse effects. Phytoestrogens are plant-based estrogen-like compounds, which have been used for the treatment of menopause-related symptoms. In the last decades, numerous preclinical and clinical studies have been carried out to evaluate the therapeutic effects of phytoestrogens including bone health. The aim of this article is to give an overview of the bidirectional interrelationship between postmenopausal osteoporosis and periodontitis, summarize the skeletal effects of phytoestrogens and report the most studied phytoestrogens with promising alveolar bone protective effect in postmenopausal osteoporosis model, with and without experimental periodontitis. To date, there are limited studies on the effects of phytoestrogens on alveolar bone in postmenopausal osteoporosis. Phytoestrogens may have exerted their bone protective effect by inhibiting bone resorption and enhancing bone formation. With the reported findings on the protective effects of phytoestrogens on bone, well-designed trials are needed to better investigate their therapeutic effects. The compilation of outcomes presented in this review may provide an overview of the recent research findings in this field and direct further in vivo and clinical studies in the future.
• Osteoporosis is an age-related bone disease characterized by low bone mass and deterioration of bone tissue micro-architecture resulting in increased bone fragility and susceptibility to fracture (Sözen et al., 2017). It is an emerging geriatric condition in developing nations. It affects men and women of all races, but the prevalence of osteoporosis is higher among women compared to men (Clynes et al., 2020). The global increase in life expectancy, being 74 years for women has lead them to suffer from many debilitating diseases such as osteoporosis (Manyonda et al., 2020).
• The alveolar bone is the thick ridge of bone located on the jaw bones. It contains the tooth sockets which hold the teeth. Anatomically, these human bones are called the maxilla and mandible. Alveolar bone loss is one of the hallmarks of periodontitis. It causes weakening of the supporting structures of the teeth and predisposes to tooth mobility and loss. Postmenopausal osteoporosis is closely related to the development of periodontitis (Contaldo et al., 2020). Periodontitis, the sixth most prevalent disease worldwide, is multifactorial inflammatory disease mediated by host response and dysbiotic plaque biofilms, resulting in periodontal tissue destruction, alveolar bone loss and eventually tooth loss (Tonetti et al., 2017; Papapanou et al., 2018).
• Both osteoporosis and periodontitis are prevalent inflammation-associated bone disorders that have common features of bone resorption, being silent and asymptomatic (Mashalkar et al., 2018; Ayed et al., 2019). These diseases remain a major public health problem particularly in the aging population (Yu & Wang, 2022). It is projected that osteoporosis and periodontitis cases will increase as the population advances in age and is predicted to cause great health challenges (Wang and McClauley, 2016). As periodontitis leads to alveolar bone loss, tooth loss, edentulism and masticatory dysfunction, it could indirectly affect nutrition, impair quality of life and self-esteem of the affected individuals (Chang et al., 2020). Postmenopausal osteoporosis and periodontitis may impose huge socioeconomic impacts and healthcare costs (Brandão et al., 2012; Mohd-Dom et al., 2014; Mohd Dom et al., 2016). Both diseases share a number of risk factors such as age, smoking, alcohol consumption and diabetes, and common features of bone resorption that might require mutual concomitant management (Wang & McCauley, 2016).
• Anti-resorptive drugs such as biphosphonates are the commonly used pharmacological agents for the treatment of osteoporosis. Zoledronate is a long-acting bisphosphonate and most potent anti-resorptive drug that has been reported in the literature to have a positive effect on bone density in patients with osteoporosis. Apart from that, zoledronate could also improve periodontal disease and prevent tooth loss (Taguchi et al., 2019). Postmenopausal women on hormone replacement therapy (HRT) were found to have better natural teeth retention than those not receiving HRT (Han et al., 2016). However, the use of these treatment modalities is associated with unwanted side effects. Anti-resorptive agent biphosphonate may lead to renal toxicity, acute-phase reactions, gastro-intestinal toxicity, and osteonecrosis of the jaw (Ralston, 2015). The use of HRT is also associated with side effects and risks, including stroke, thromboembolism, vascular diseases and breast cancer. It was reported that to avoid the HRT adverse effects, women nowadays are shifting to herbal medicine, particularly for the prevention and treatment of menopause related symptoms (Gerbarg & Brown, 2016; Djapardy and Panay. 2022). The search of natural substances with promising results for the treatment of postmenopausal osteoporosis and periodontitis therefore is highly desirable.
• In this regard, phytomedicine or plant-based medicine with therapeutic and healing properties have gain scientific and clinical interest. Phytoestrogens are naturally occurring non-steroidal polyphenolic compounds that have structural and biological similarity to 17-β-estradiol, the main female sex hormone. Even though the affinity is lesser than that of endogenous estrogens, phytoestrogens can bind to estrogen receptors (ER) and exert anti-estrogenic or pro-estrogenic effects (Rowe and Barber, 2021). Most of phytoestrogens are also antioxidant and anti-inflammatory agents and these properties contribute to their distinguished therapeutic health effects Kładna et al., 2016. A growing body of evidence supported their therapeutic potential in preventing and treating several dysfunctions and diseases related to aging including menopausal symptoms and osteoporosis (Sirotkin & Harrath, 2014). In fact, phytoestrogens are used as a dietary supplement and as an alternative to HRT as they are believed to be safe and effective. Such properties turn these substances into promising targets for development as adjunctive preventive and therapeutic strategies for postmenopausal osteoporosis and periodontitis. In this review, we summarized the effects of the most studied phytoestrogens on bone health and screened phytoestrogens with the most promising alveolar bone protective effect in postmenopausal osteoporosis model with and without experimental periodontitis. This review may provide important insights for further in vivo and clinical studies of postmenopausal osteoporosis and periodontitis.
The association between postmenopausal osteoporosis and periodontitis has been reported extensively in epidemiologic and experimental studies (Luo et al., 2014; Juluri et al., 2015; Ayed et al., 2019). Though the mechanism of periodontitis in postmenopausal women has not been fully elucidated, an explicit understanding on the mechanistic link between the two diseases and their interplay is important for the prevention and management of these disorders, particularly in the elderly. The pathogenesis of postmenopausal osteoporosis involved the activation of systemic inflammation and dysregulation of immune response (Al-Daghri et al., 2017; Fang et al., 2022). Healthy bone continuously remodels through osteoblast-mediated bone formation and osteoclast-mediated bone resorption until the fourth to sixth decade of life when resorption exceeded the formation, causing a continuous loss of bone mass and a progressive decline in bone mineral density (BMD) (Kirk et al., 2020; Munoz et al., 2020). The cessation of ovarian function at menopause is one of the main causes of osteoporosis (Wu et al., 2021). Withdrawal of the protective effect of estrogen and immune cells alteration contribute to ongoing bone destruction in postmenopausal osteoporosis (Fischer and Haffner-Luntzer, 2022). The crosstalk between the immune system and the bone has been reported in the literature since the past decades (Takayanagi et al., 2000). Various immune cells interact with bone cells, the osteoblasts and osteoclasts, through cell-cell direct contact or via paracrine mechanisms (Fischer and Haffner-Luntzer, 2022). Immune cells including subtypes of T lymphocytes, B lymphocytes, macrophages, neutrophils and mast cells influence bone cells via factors including inflammatory cytokines, the interleukin (IL)-6 and tumor necrosis factor -α (TNF-α), osteoprotegerin/receptor activator of nuclear factor kappa-β ligand (OPG/RANKL) and other mediators, by increasing osteoblast apoptosis and stimulating osteoclastogenesis, thereby triggering bone loss during postmenopausal osteoporosis (Du et al., 2018; Zhang et al., 2020; Fischer and Haffner-Luntzer, 2022).
Bacterial dental plaque or biofilm is the primary etiological factor that dissociate periodontitis from osteoporosis. Though the pathogenesis and progression of periodontitis is primarily dependent on host interaction with the dysbiotic biofilm, the subsequent exacerbation of inflammatory response and its influence on bone homeostasis play crucial roles in both osteoporosis and periodontitis (Yu & Wang, 2022). Inflammatory response, the recruitment of polymorphonuclear neutrophils in particular, is the first line of defense against the invading periodontal pathogens in subgingival dental biofilm that is intended to eliminate the initial cause of tissue injury. As inflammation involve the activation of immune cells in the innate and adaptive immunity, the interplay between microbes and immune components initiate and propagate periodontal inflammation (Hajishengallis, 2014). The activation of lymphocyte and amplification of local inflammatory signaling cascade could stimulate RANKL signal, promoting osteoclastogenesis and inhibiting osteoblast lineage cells, thereby causing an uncoupling of bone remodeling process (Kawai et al., 2006; Pacios et al., 2015). These events are thought to be responsible for bone resorptive lesion and periodontal bone loss in periodontitis.
As described above, it is known that osteoporosis and periodontitis are closely related with inflammation and aging. Apart from inflammation, oxidative stress, is another major causative factor implicated in the pathogenesis and progression of these diseases. During aging, the accumulation of intracellular reactive oxygen species (ROS) and depletion of antioxidant enzymes lead to the elevation of oxidative stress in skeletal system (Manolagas, 2010). Excessive production of ROS, which is also responsible for elevation of immune cytokines could trigger the increase in osteoclastogenesis and osteoblast apoptosis as well as decrease in osteoblastogenesis. A population-based study suggested an interplay between oxidative stress and bone resorption that possibly underlies the development of postmenopausal osteoporosis (Cervellati et al., 2014). Menopause-related estrogen withdrawal increase the risk of postmenopausal osteoporosis basically by making the bone more vulnerable to oxidative injury. The theory that oxidative stress affects BMD was also supported by numerous clinical studies. Oxidant and antioxidant status imbalance in postmenopausal osteoporosis could affect the osteoclastic and osteoblastic activity. With regards to its involvement in periodontitis, oxidative stress has been linked to periodontal tissue destruction. ROS initially act as an antimicrobial defense system by killing the invaded pathogenic microorganism triggered by the infiltration of polymorphonuclear neutrophil (Wang, 2015). The generation of ROS is also considered as a “double-edge” sword as it helps to kill invading pathogen and it can be cytotoxic to host cells when the inflammatory response is exaggerated. Overproduction of ROS within the affected tissues leads to oxidant-antioxidant imbalance which then results in oxidative stress and pathological changes and consequently cause destruction of host tissues (Sczepanik et al., 2020). Hence, ROS is responsible for destruction of periodontal tissues and tooth loss in periodontitis. Due to these facts, periodontitis is also referred to as an inflammatory disease of oxidative stress.
The evolution of classification and diagnostic criteria for osteoporosis and periodontitis have been described and reported in the literature. Osteoporosis is determined by the measurement of BMD, expressed in terms of the number of standard deviations (SD) from the mean BMD of healthy individuals that matched to age and sex (Z-score), and the number of SD from the mean BMD of healthy young sex-matched individuals (T-score). According to WHO criteria, osteoporosis is present when BMD lies 2.5 SD or more below the BMD of young healthy women. Meanwhile, osteopenia or low bone mass is defined as BMD levels between one SD and 2.5 SD below the normal BMD (Lorentzon & Cummings, 2015). The most widely recognized tools used to measure BMD is dual energy x-ray absorptiometry (DXA). This technique is a standard non-invasive diagnosis approach that is reliably used worldwide to identify patients with low BMD due to its high precision and resolution but low radiation and cost. Additionally, quantitative ultrasound (QUS) methods have been used in the diagnosis and follow-up treatment in osteoporosis. Advances in computed tomography (CT) and magnetic resonance imaging (MRI) are promising in clinical and research settings in terms of capturing bone microarchitecture and also characterizing processes at the molecular level (Oei et al., 2016).
The diagnosis of periodontitis is primarily based on clinical evaluation whereby radiographs are used to confirm the clinical manifestation. The patient is considered to have periodontitis when there are at least four teeth with 4 mm probing depth in one or more sites, clinical attachment loss (CAL) up to 3 mm at the same site and presence of bleeding on probing (Ayed et al., 2019). The American Academy of Periodontology and the European Federation of Periodontology has modified the definition and classification framework for periodontitis based on staging and grading system (Tonetti et al., 2017). The staging refers to the severity and extent of periodontitis at present while grading refers to the rate of its progression. In clinical setting, the periodontal health can be evaluated by the measurement of CAL using probing pocket depths (PPD) and gingival recession but the reliability of this method is limited in terms of probing force, angulation, placement and tip diameter (Trombelli et al., 2018). Radiographic bone loss (RBL) should be used in cases if the CAL is unavailable (Tonetti et al., 2017). Loss of alveolar crestal height (ACH), oral hygiene simplified (OHI-S) and sites with bleeding on probing (BOP) percentage are among other important clinical parameters in the evaluation of periodontal status (Qi et al., 2021). Apart from that, identification of cavities and periodontal lesions, maxillary sinusitis and other lesions in the oral and maxillofacial field as well as osteoporosis can be done with the use of computer-aided diagnosis (CAD) (Wang and McCauley. 2016).
Tooth loss, periodontal disease, ill-fitting or loose dentures and severe bone loss around the teeth, are among the early indicators of osteoporosis detected in the oral cavity (Ayed et al., 2019). In the clinical setting, patients at risk can be identified by a dentist from medical history, clinical examination and radiographic findings while patient’s osteoporosis status can be obtained by further determination of BMD together with the evaluation of dental radiography. Knowledge on the relationship between postmenopausal osteoporosis and periodontitis, as well as sufficient clinical and radiographic information would enable dentists to play their role in early diagnosis and screening of osteoporosis in postmenopausal women. Regardless of gender, patients with osteoporosis may have two-fold increase in the risk of periodontitis. Though osteoporosis is not an etiologic factor in periodontal disease, osteoporotic women presented a higher risk and greater severity of periodontitis than men (Pepelassi et al., 2012; Manjunath et al., 2019). This association suggested that patients with osteoporosis should be evaluated for periodontal health and vice versa. Efforts towards the prevention of periodontal disease in patients at risk of osteoporosis particularly in postmenopausal women would be enviable. Although the mechanisms underlying this association has not been not fully elucidated, information collected from the literature strongly explained the association.
Estrogen is a steroid hormone that is not only responsible for female sexual characteristics development but also has other non-reproductive physiological roles. It is one of the key regulators of bone metabolism with significant influence on skeletal growth and homeostasis in both women and men. Estrogens act directly on osteoblasts, osteocytes, immune cells and other cells via the ER found on these bone cells (Bord et al., 2001; Braidman et al., 2001; Crusodé-Souza et al., 2009). In general, ER signaling pathway activation stimulated differentiation of osteoblast and suppressed osteoclastic activity (Manolagas, 2000). Estrogen also prevented apoptosis of osteocytes and its anti-apoptotic effect is related to their autophagy regulation in osteocytes (Florencio-Silva et al., 2018). Estrogen deficiency increased osteoclastogenesis, prolonged osteoclast lifespan and increased the rate of bone turnover, causing accelerated resorption than formation (Manolagas, 2000; Manolagas et al., 2013). A recent study showed that estrogen deficiency decreased autophagy and increased apoptosis in alveolar process osteocytes, whereby estrogen replacement enhanced osteocyte viability simply by inhibiting apoptosis and maintaining autophagy in these cells (Florencio-Silva et al., 2018).
Apart from its direct effects on bone cells, research has revealed estrogen regulated bone hemostasis through its influence on the immune system and on oxidative stress. In essence, the proinflammatory cytokines, IL-1, IL-6, TNF-α, granulocyte macrophage colony-stimulating factor, macrophage colony-stimulating factor (M-CSF), and prostaglandin-E2 (PGE2) played a significant role in bone metabolism by increasing bone resorption (Riggs, 2000). These proinflammatory cytokines particularly IL-1, IL-6 and TNF-α were considered as osteoclastogenic bone resorption-inducing cytokines (Scheidt-Nave et al., 2001). Activation of inflammatory cascades due to estrogen deficiency led to increased production of M-CSF and RANKL. The binding of M-CSF to its receptor stimulated the proliferation of osteoclast and the survival of its precursors as well as mature osteoclasts. The binding of RANKL to RANK receptors stimulated differentiation and activity of osteoclast and prevented their apoptosis (Feng et al., 2019). In osteoporotic patients, the increased in systemic levels of IL-6 could predict BMD change and fracture rate (Barbour et al., 2014). A significant elevation in IL-6 as well as TNF-α were also observed in ovariectomized (OVX) animals compared to the SHAM group (Eminov et al., 2018; Delgobo et al., 2019). Apart from the increase in IL-6 expression, OVX rats also showed increased RANKL and to a lesser extent, OPG expression. OVX rat is a suitable model for studying postmenopausal bone loss as it mimics the decline in endogenous estrogen production by the ovaries during menopause (Johnston & Ward, 2015). This model resembles deterioration of bone tissue in the hip and spine in postmenopausal osteoporosis and hence it can also be used to study mineral and structural changes in alveolar bone.
Alveolar bone loss due to periodontitis is a frequent complication in postmenopausal women suffering from osteoporosis due to estrogen deficiency (Tanaka et al., 2020). As described earlier, changes in hormone level particularly estrogen have impact on systemic bone homeostasis and inflammatory response. Following menopause, estrogen levels in the circulation fall drastically as the production by the ovaries cease. Estrogen reduced osteoclast activity and prevented apoptosis of osteocytes, and for that reason rapid decline in estrogen may lead to systemic bone loss due to disruption of bone homeostasis (Grover et al., 2014). Other than bones, estrogen receptors can also be found in the oral mucosa, gingiva and salivary glands. ER-β is the predominant ER present in the gingival epithelium (Valimaa et al., 2004). Therefore, hormone fluctuations may also contribute to changes in the oral cavity and acceleration of inflammatory response (Jafri et al., 2015). Upregulation of immune cells and osteoclasts due to estrogen deficiency eventually result in a greater production of bone-resorbing cytokines. The increase in inflammatory cytokines and other factors in the circulation may not only have impact on systemic bone remodeling but also may locally compromise the tissue response to periodontal disease (Golub et al., 2006).
Estrogen deficiency in postmenopausal women or in experimental OVX rodents has been markedly linked to alterations in trabecular and cortical bone including the alveolar bone of mandible (Johnston & Ward, 2015; Jonasson et al., 2018). Studies also showed that estrogen deficiency aggravates the severity of experimental periodontitis (Anbinder et al., 2016). Clinically, postmenopausal women with osteoporosis and concurrent periodontitis have been found to exhibit an exaggerated response to dental plaque, higher periodontal attachment loss and a significant reduction of alveolar bone compared to healthy women (Singh et al., 2014). These changes are evident through the assessment of periodontal health such as increased gingival bleeding and periodontal pocket depth, decreased BMD of the alveolar crestal and subcrestal bone, loss of dentoalveolar bone height, or loss of clincial attachment and tooth (Marjanovic et al., 2013; Jang et al., 2015; Juluri et al., 2015; Savić Pavičin et al., 2017).
It was hypothesized that osteoporosis could accelerate alveolar bone resorption and bone loss in periodontitis because the loss of alveolar BMD allowed deeper bacterial penetration into the enlarged periodontal space. In response to local inflammation, alveolar resorption could be further amplified and accelerated as focal infection of the periodontium may also release inflammatory cytokines into the system (Barbato et al., 2015). Additionally, overexpression of proinflammatory cytokines with osteoclastic activity occured in both osteoporosis and periodontitis (Barbato et al., 2015; Inchingolo et al., 2020). The impact of estrogen deficiency and the association between postmenopausal osteoporosis and periodontitis are shown in the schematic diagram (Figure 1).
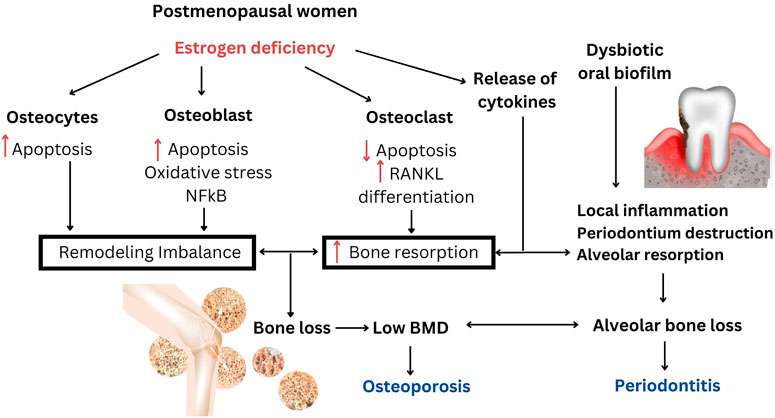
FIGURE 1. The impact of estrogen deficiency and the association between postmenopausal osteoporosis and periodontitis. Estrogen deficiency is responsible for the increase in systemic bone resorption and remodeling imbalance that would also accelerate the increase in alveolar bone resorption. Loss of BMD in postmenopausal osteoporosis may be responsible for increasing alveolar bone loss in periodontitis. Local inflammation in periodontitis may activate systemic osteoclastogenic cytokines and further aggravates bone loss.
Phytoestrogens are generally divided into four major groups: isoflavones, stilbenes, coumestans and lignans. Isoflavones are the most widely used and studied phytoestrogens. They are found primarily in soybeans and other legumes, which constitute the major dietary source of phytoestrogens in Asian communities (Rowe & Baber, 2021). Genistein and daidzein are the two well-characterized isoflavones that have also been shown to have estrogenic potential. Resveratrol is the most common and the main dietary source of phytoestrogenic stilbenes. Its estrogenic activity is dependent on the two isomers, cis and trans. Trans has been reported to have higher estrogenic activity (Desmawati & Sulastri, 2019). Coumestans are biosynthetically related to isoflavones but only a small number of coumestans have shown estrogenic activity (Poluzzi et al., 2014). Lignans, the major dietary source in Western diets, are mostly derived from fruit, vegetables, legumes and whole grains. Lignan dimers that are not estrogenic themselves can be converted by gut microflora to mammalian lignans, the enterodiol and enterolactone which are estrogenic (Cornwell et al., 2004).
Phytoestrogens have been extensively studied for their potential role to prevent and treat diseases related to aging such as menopausal symptoms and skin aging, cardiovascular, neurodegenerative, immune and metabolic diseases and cancer. Recent systematic review and meta-analysis reported that consumption of low doses of phytoestrogen (25 mg/d ≤ dose ≤100 mg/d) for a long-term duration were effective in relieving depression symptoms in postmenopausal women (Li et al., 2021). Some reported adverse effect associated to phytoestrogens in menopausal women however are not yet clear and required more supportive evidence from high-quality randomized control trial (RCT) studies. Animal studies showed that high-dose administration of phytoestrogens (equol and puerarian mirica) modulated female reproductive system by enhancing the levels of serum luteinizing hormone and reducing urinary follicular stimulating hormone in ovariectomized rats and cynomolgus monkeys respectively (Rachoń et al., 2007; Trisomboon et al., 2007). Exogenous estrogen-like molecules could promote reproductive function and it also could possibly destroy reproductive processes (Sirotkin & Harrath, 2014). Nevertheless, no adverse effects of phytoestrogens on human reproduction has been reported yet. In a RCT, supplemention of 100 mg isoflavones-rich, concentrated soy extract daily to postmenopausal women for 6 months has improved their skin health by increasing epithelial thickness, number of elastic and collagen fibres (Accorsi-Neto et al., 2009).
Soy genistein has been proposed to have a promising therapeutic effect for metabolism improvement and treatment of metabolic disorder (Behloul & Wu, 2013). In an RCT, 250 mg per day of genistein administered to non-alcoholic fatty liver patients for 2 months demonstrated reduction in insulin levels, indicating the ability of phytoestrogen to modulate human endocrine system (Amanat et al., 2018). Supplementation of soy protein with 66 mg isoflavones for 6 months has been found to significantly improve cardiovascular disease risk markers in women during the early menopause (Sathyapalan et al., 2018). A meta-analysis of epidemiological studies has found that the intake of soy isoflavones by pre- and post-menopausal women in Asian countries could lower the risk of breast cancer (Chen et al., 2014). Several other studies have shown the potential effect of phytoestrogen consumption in reducing the risks of lung cancer (Shimazu et al., 2011), stomach cancer (Ko et al., 2013), prostate cancer (He et al. 2015; Hwang et al., 2009), endometrial and ovarian cancer (Bandera et al., 2009; Qu et al., 2014).
The therapeutic potential described above indicated that phytoestrogens possess beneficial effect on the health of various organs and systems at different doses. Though the main mechanism of action of phytoestrogens is due to ER binding, their antioxidant, anti-inflammatory and other properties could also contribute to their pro-health effects. For example, the interaction of phytoestrogens with ER as well as their antioxidant properties might contribute to its neuroprotective effects (Gorzkiewicz et al., 2021). Several studies showed that different forms of soybean including soy isoflavones have antibacterial activity against oral microbes (Lee & Kim, 2006; Wang et al., 2010; Laodheerasiri and Horana Pathirage, 2017; Choo et al., 2020; How et al., 2020). Antimicrobial activity of phytoestrogens may be useful for the treatment and prevention of periodontal disease as periodontitis is known to have bacterial cause. Dental plaque and polymicrobial infections play a pivotal role in the initiation of periodontitis. For this reason, elimination or controlling the bacteria could be a beneficial approach in managing periodontal diseases.
In view of its role as the key regulator of bone metabolism, it has been hypothesized that phytoestrogens exerted bone health effects through their estrogenic potential, usually by binding to estrogen receptors (Chiang and Pan. 2013). As described earlier, soy isoflavones is one of the most widely studied phytoestrogens and they have received considerable attention in the management of postmenopausal bone loss. In the last decades, numerous clinical studies have been conducted in a wide range of populations including Western and Asian counterparts using different types of isoflavones preparations. Observational studies showed that women who consume higher amounts of soy foods have lower risk of postmenopausal fracture (Setchell & Lydeking-Olsen, 2003; Messina et al., 2004; McCarty, 2006) and as a consequent many clinical trials have attempted to evaluate the protective role of soy isoflavones on bone health (Lagari & Levis, 2013). Reported findings on the skeletal effects of isoflavones from clinical trials however are inconsistent due to the variation of study design and length of intervention, study population, dose and types of isoflavones preparation. The inconclusive results from human clinical trials are generally contributed by their heterogeneity and poor quality (Rowe & Baber, 2021).
A systematic review of RCT in 2016 compiled 23 eligible studies that ranged from 7 weeks to 3 years of interventions, which mainly assessed the effectiveness of phytoestrogens intervention through the measurement of whole body or regional BMD or bone mineral content, T-scores and bone metabolism biomarkers during the menopause transition (Abdi et al., 2016). Though there were controversial reports about changes in BMD, different types of soy isoflavones extracts (including genistein and daidzein), dietary products containing different amounts of phytoestrogens and red clover extract may have beneficial effects on bone health in postmenopausal women. A recent meta-analysis and systemic review of RCT concluded that isoflavones could be beneficial in preserving BMD and reducing bone resorption in premenopausal and postmenopausal women (Lambert et al., 2017). This could be linked to the effect of phytoestrogens principally as antiresorptive agents rather than their potential in bone formation. The use of isoflavones aglycones with well-controlled, standardized and defined isoflavones interventions revealed greater efficacy in treating BMD loss in estrogen-deficient women compared to glycosides and less well-defined isoflavones formulation. One year intake of novel red clover extract rich in isoflavones aglycones and probiotics has been found to potently attenuated BMD loss and improved bone turnover in postmenopausal osteopenic women (Lambert et al., 2017).
Although human clinical trials are inconsistent and some reported negative findings, numerous in vitro and animal studies on phytoestrogens revealed encouraging bone sparing effects. Phytoestrogen could promote osteogenesis by specifically targeting osteoblast and osteoclast. The administration of phytoestrogens such as isoflavones and flavonoids promoted bone formation, which stimulate the expression of osteogenic markers such as Runx2, ALP, osteocalcin, type 1 collagen (COL-1), osteopontin, and morphogenetic protein-2 (BMP-2) for osteoblast differentiation and bone matrix mineralization (Ramesh et al., 2021; Ortiz et al., 2022; Sekaran et al., 2022). Additionally, by virtue of its similar structure to 17β-estradiol in conformational binding to estrogen receptors, phytoestrogen has capability to reciprocally affect osteogenic versus adipogenic differentiation of mesenchymal stem cells (MSCs) in a dose dependent manner. For instance, phytoestrogen stimulated osteoblast differentiation from MSCs via the activation of important signalling pathways such as Smad, Wnt/β-catenin and Sirt1 pathways (Schilling et al., 2014; Gorabi et al., 2019; Khezri et al., 2021; Wang et al., 2021). Concomitantly, there is evidence that phytoestrogen suppressed the adipogenic differentiation signalling pathway including PPAR and C/EBP pathways in a dose dependent manner (Schilling et al., 2014; Ahmed 2014).
In addition, the process of bone resorption and formation are closely coupled through the RANKL/OPG system. The integrity of the skeleton is maintained through bone formation followed by a balanced cycle of bone resorption (Narducci et al., 2011; Sharma et al., 2022). Intriguingly, phytoestrogen was able to regulate the RANKL/OPG system, thereby playing an important role in the pathogenesis of osteoporosis (Ni et al., 2023; Hooshiar et al., 2022; Zakłos-Szyda et al., 2020). Furthermore, phytoestrogen exerted antiresorptive activities by suppressing the expression of osteoclast differentiation markers such as matrix metalloproteinase 9 (MMP9), cathepsin-K and tartrate-resistant acid phosphatase (TRAP) via downregulating the activation of NF-κB and MAPK signaling pathways (Xu et al., 2022; Tanaka et al., 2020). These findings demonstrated that phytoestrogen treatments could inhibit osteoclast activation and therefore have therapeutic potential for osteoporosis.
The primary endpoints that generally measured following hormone ablation in in vivo studies include, trabecular bone and/or cortical bone mass, BMD and mechanical strength. Changes in bone turnover markers and uterine weight were also measured. Phytoestrogens significantly increased both trabecular and/or cortical bone volume in OVX-induced bone loss with no uterotrophic effect in animal models (Inchingolo et al., 2020; Long et al., 2022). Phytoestrogen was also shown to significantly decreased urinary excretion of deoxypyridinoline (DPD), which is one of the bone resorption markers (Li et al., 2021; Song et al., 2016). Furthermore, phytoestrogen exerted antioxidant effects as shown by the increased expression of antioxidant enzymes such as superoxide dismutase (SOD) and gluthathione (GSH), that serve to scavenge the excess free radicals. This antioxidative effect of phytoestrogens also inhibit osteoclast differentiation (Oršolić et al., 2022). Overall, findings from in vivo studies are in line with in vitro studies, which demonstrated the bone-conserving effects of phytoestrogens in reducing bone loss due to estrogen deficiency.
Being a systemic disease, the manifestation of bone loss in osteoporosis is not only evident in vertebrae and appendicular skeleton but also in alveolar bone (Muslita et al., 2012). For this reason, osteoporosis is expected to accelerate alveolar bone resorption caused by periodontitis (Guiglia et al., 2013). Additionally, osteoporosis results in an increase in certain inflammatory cytokines which are also affected in the progression of periodontitis. Oxidative stress is also an indicator of periodontitis development, which is evident by the accumulation of ROS (Kanzaki et al., 2017). Studies on the potential skeletal effects of phytoestrogens in postmenopausal osteoporosis at the preclinical (in vitro and in animal models) and clinical level have been substantially reported in the literature. To the best of our knowledge, studies on the effect of phytoestrogens on postmenopausal osteoporosis with periodontal disease in human are still lacking. Nonetheless, several animal studies have been carried out recently to evaluate the protective effects of phytoestrogens against alveolar bone loss in postmenopausal osteoporosis with or without experimental periodontitis.
Table 1 summarizes the effect of phytoestrogens against alveolar bone loss. In a recently published study, oral administration of soy isoflavones was found to alleviate experimental periodontitis in estrogen-deficient rats as revealed by the increased expression of tight junction proteins in the gingiva, reduced proinflammatory cytokines, IL-17 and ROS levels (Liu et al., 2022). Attenuation of alveolar bone loss was observed through micro-CT and histologic observation. Experimental periodontitis in OVX rats was established by silk ligature and inoculation with Porphyromonas gingivalis, a Gram-negative anaerobe, which is one of the well-characterized periodontal pathogens involved in periodontitis. P. gingivalis possibly modulate the immune response through the inactivation of certain cytokines (Baek et al., 2015). An in vitro P. gingivalis infection model was also used to determine whether grainyhead-like 2 (GRHL2), the epithelial transcription factor and ER-binding partner (He et al., 2020) is required by soy isoflavones to enhance the oral epithelial barrier. It was found that the enhancement of oral gingival epithelial barrier function by soy isoflavones treatment was partially dependent on GRHL2 (Liu et al., 2022).
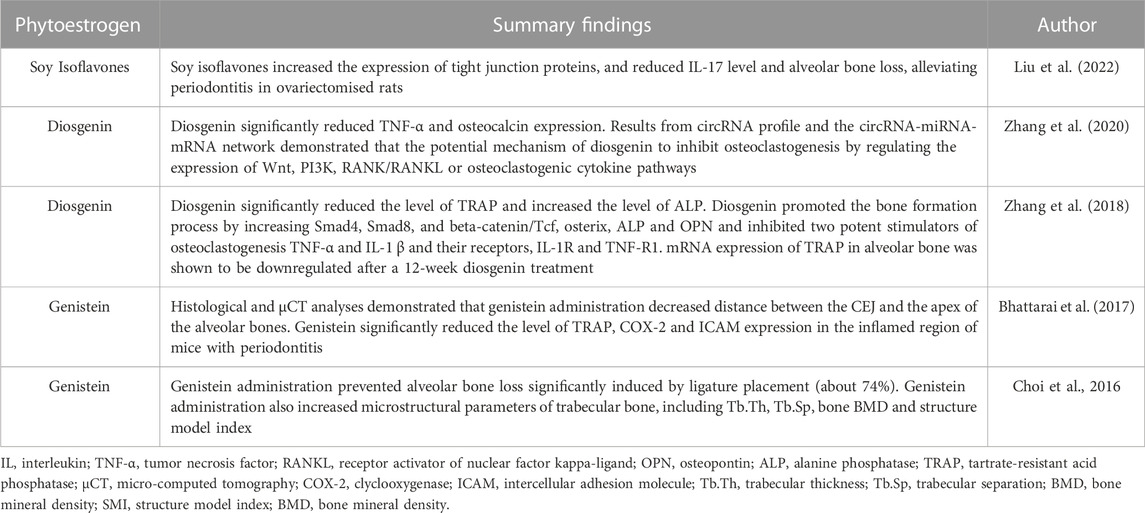
TABLE 1. Summary findings on the therapeutic potential of phytoestrogens against alveolar bone loss.
Earlier studies showed that genistein, a major subclass of isoflavones found in soybean was protective against periodontitis-induced alveolar bone loss (Choi et al., 2016; Bhattarai et al., 2017). Genistein was found to significantly attenuated Prevotella intermedia LPS-induced production of inducible nitric oxide synthase (iNOS) and IL-6 coupled with the decreased in their mRNA expression in RAW264.7 cells. In experimental animal, alveolar bone height and bone volume fraction were decreased and microstructural parameters of trabecular bone were improved with administration of genistein (Choi et al., 2016). These findings were in line with a study by Bhattarai et al. (2017) that also showed a reduction in LPS-induced alveolar bone loss with genistein administration. Additionally, genistein significantly prevented osteoclast differentiation by suppressing the expression of osteoclast-specific molecules in NFκB ligand- or LPS-stimulated macrophages. Apart from its inhibitory effect on osteoclast activation, the protection against LPS-mediated stresses by genistein was indicated by the reduction of mitochondrial impairment and ROS accumulation, which lead to the reduction of periodontal damage. The reported antioxidant and anti-osteoclastic potential of phytoestrogen genistein might be protective against alveolar bone loss in postmenopausal osteoporosis condition.
A study done by Zhang et al. (2018) has found that 12-week oral treatment with diosgenin, a natural steroidal saponin and a phytoestrogen, suppressed alveolar bone loss in OVX rats by promoting bone formation. Though the effects of estradiol valerate on alveolar bone volume was greater than diosgenin, both treatments showed reduced alveolar bone loss compared to OVX rats as indicated by 3-D bone microstructure analysis and histological observation. The protective role of diosgenin on bone loss was also described earlier in the peripheral skeletal of OVX rats (Zhang et al., 2014; Folwarczna et al., 2016). The bone protective effect of diosgenin might be associated with the modulation of RANKL/OPG ratio (Zhang et al., 2014). As with other phytoestrogens, diosgenin may be one of the sparse compounds that have the potential to increase bone formation and inhibit bone resorption. In this study, lncRNA and mRNA profiles were evaluated using a microarray to confirm the anti-osteoporotic effects of diosgenin on alveolar bone. Diosgenin may have exerted this effect by increasing the Wnt and BMPs pathways, the two recognized signaling pathways that regulate the osteogenic differentiation of mesenchymal stem cells or preosteoblasts (Lin and Hankenson, 2011; Marcellini et al., 2012). This finding was further supported by another study by Zhang et al. (2020). The study revealed that anti-bone loss action of diosgenin on alveolar bone was attributed by the regulation of important molecules expression in the Wnt, P13K, RANK/RANKL or osteoclastogenic cytokine pathways. The possible molecular mechanisms underlying the protection against alveolar bone loss by phytoestrogens are summarized in Figure 2.
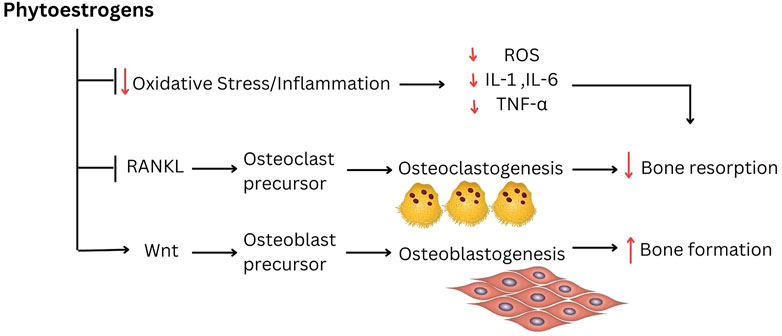
FIGURE 2. The possible molecular mechanism underlying the protection against alveolar bone loss by phytoestrogens.
Association between postmenopausal osteoporosis and periodontitis has long been postulated though its causal relationship is yet to be determined. In vitro and animal studies have demonstrated phytoestrogens favorable effects on skeletal health. Phytoestrogens may exert their bone protective effect by inhibiting bone resorption and promoting bone formation. Phytoestrogens mainly isoflavones, may offer protection against alveolar bone loss in postmenopausal osteoporosis condition. Well-designed clinical trials are needed to determine the therapeutic potential of phytoestrogen on skeletal health particularly in postmenopausal women. Phytoestrogens can be potentially developed as adjunctive preventive and therapeutic cost-effective strategies in the treatment and prevention of bone loss in postmenopausal osteoporosis with periodontitis.
PJ and AS contributed toward conceptualization, planning and writing the paper. PJ and HA took the lead in drafting and writing the manuscript. AS and BB revise the manuscript for important intellectual content. NN and NI contributed toward conceptualization and editing of the manuscript. All authors read and approved the final manuscript.
We thank Universiti Kebangsaan Malaysia, Universiti Teknologi MARA and Manipal University College Malaysia for the support in completing this project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdi, F., Alimoradi, Z., Haqi, P., and Mahdizad, F. (2016). Effects of phytoestrogens on bone mineral density during the menopause transition: A systematic review of randomized, controlled trials. Climacteric 19 (6), 535–545. doi:10.1080/13697137.2016.1238451
Accorsi-Neto, A., Haidar, M., Simões, R., Simões, M., Soares, J., and Baracat, E. (2009). Effects of isoflavones on the skin of postmenopausal women:a pilot study. Clinics 64 (6), 505–510. doi:10.1590/s1807-59322009000600004
Ahmed, B. (2014). Combinations of genistein, EGCG and/or resveratrol synergistically inhibit pre-adipocyte differentiation by suppressing PPAR-γ/C/EBP-α pathway (Doctoral dissertation, Tennessee State University)
Al-Daghri, N. M., Aziz, I., Yakout, S., Aljohani, N. J., Al-Saleh, Y., Amer, O. E., et al. (2017). Inflammation as a contributing factor among postmenopausal Saudi women with osteoporosis. Medicine 96 (4), e5780. doi:10.1097/MD.0000000000005780
Amanat, S., Eftekhari, M. S., Fararouei, M., BagheriLankarani, K. B., and Massoumi, S. J. (2018). Genistein supplementation improves insulin resistance and inflammatory state in non-alcoholic fatty liver patients: A randomized, controlled trial. Clin. Nutr. 37 (4), 1210–1215. doi:10.1016/j.clnu.2017.05.028
Anbinder, A. L., Moraes, R. M., Lima, G. M., Oliveira, F. E., Campos, D. R., Rossoni, R. D., et al. (2016). Periodontal disease exacerbates systemic ovariectomy-induced bone loss in mice. Bone 83, 241–247. doi:10.1016/j.bone.2015.11.014
Ayed, M. S., Alsharif, A. F., Divakar, D. D., Jhugroo, C., Alosaimi, B., and Mustafa, M. (2019). Evaluating the possible association between systemic osteoporosis and periodontal disease progression in postmenopausal women. Disease-a-month. 65 (6), 193–215. doi:10.1016/j.disamonth.2018.11.001
Baek, K. J., Ji, S., Kim, Y. C., and Choi, Y. (2015). Association of the invasion ability of Porphyromonas gingivalis with the severity of periodontitis. Virulence 6 (3), 274–281. doi:10.1080/21505594.2014.1000764
Bandera, E. V., Williams, M. G., Sima, C., Bayuga, S., Pulick, K., Wilcox, H., et al. (2009). Phytoestrogen consumption and endometrial cancer risk: A population-based case–control study in New Jersey. Cancer Causes Control 20, 1117–1127. doi:10.1007/s10552-009-9336-9
Barbato, L., Francioni, E., Bianchi, M., Mascitelli, E., Marco, L. B., and Tonelli, D. P. (2015). Periodontitis and bone metabolism. Clin. Cases Min. Bone Metab. 12 (2), 174–177. doi:10.11138/ccmbm/2015.12.2.174
Barbour, K. E., Lui, L. Y., Ensrud, K. E., Hillier, T. A., LeBlanc, E. S., Ing, S. W., et al. (2014). Inflammatory markers and risk of hip fracture in older white women: The study of osteoporotic fractures. J. Bone Min. Res. 29 (9), 2057–2064. doi:10.1002/jbmr.2245
Behloul, N., and Wu, G. (2013). Genistein: A promising therapeutic agent for obesity and diabetes treatment. Eur. J. Pharmacol. 698 (1-3), 31–38. doi:10.1016/j.ejphar.2012.11.013
Bhattarai, G., Poudel, S. B., Kook, S. H., and Lee, J. C. (2017). Anti-inflammatory, anti-osteoclastic, and antioxidant activities of genistein protect against alveolar bone loss and periodontal tissue degradation in a mouse model of periodontitis. J. Biomed. Mat. Res. Part A 105 (9), 2510–2521. doi:10.1002/jbm.a.36109
Bord, S., Horner, A., Beavan, S., and Compston, J. (2001). Estrogen receptors α and β are differentially expressed in developing human bone. J. Clin. Endocrinol. Metab. 86 (5), 2309–2314. doi:10.1210/jcem.86.5.7513
Braidman, I. P., Hainey, L., Batra, G., Selby, P. L., Saunders, P. T., and Hoyland, J. A. (2001). Localization of estrogen receptor β protein expression in adult human bone. J. Bone Min. Res. 16 (2), 214–220. doi:10.1359/jbmr.2001.16.2.214
Brandão, C. M. R., Machado, G. P. D. M., and Acurcio, F. D. A. (2012). Análise farmacoeconômica das estratégias de tratamento da osteoporose em mulheres na pós-menopausa: uma revisão sistemática. Rev. Bras. Reumatol. 52, 924–937. doi:10.1590/s0482-50042012000600010
Cervellati, C., Bonaccorsi, G., Cremonini, E., Romani, A., Fila, E., Castaldini, M. C., et al. (2014). Oxidative stress and bone resorption interplay as a possible trigger for postmenopausal osteoporosis. Biomed. Res. Int. 2014, 569563. doi:10.1155/2014/569563
Chang, H. J., Lee, S. J., Yong, T. H., Shin, N. Y., Jang, B. G., Kim, J. E., et al. (2020). Deep learning hybrid method to automatically diagnose periodontal bone loss and stage periodontitis. Sci. Rep. 10 (1), 7531–7538. doi:10.1038/s41598-020-64509-z
Chen, M., Rao, Y., Zheng, Y., Wei, S., Li, Y., Guo, T., et al. (2014). Association between soy isoflavone intake and breast cancer risk for pre-and post-menopausal women: A meta-analysis of epidemiological studies. PloS One 9 (2), e89288. doi:10.1371/journal.pone.0089288
Chiang, S. S., and Pan, T. M. (2013). Beneficial effects of phytoestrogens and their metabolites produced by intestinal microflora on bone health. Appl. Microbiol. Biotechnol. 97 (4), 1489–1500. doi:10.1007/s00253-012-4675-y
Choi, E. Y., Bae, S. H., Ha, M. H., Choe, S. H., Hyeon, J. Y., Choi, J. I., et al. (2016). Genistein suppresses Prevotella intermedia lipopolysaccharide-induced inflammatory response in macrophages and attenuates alveolar bone loss in ligature-induced periodontitis. Arch. Oral Biol. 62, 70–79. doi:10.1016/j.archoralbio.2015.11.019
Choo, S. M., Yap, K. Y., Yap, W. H., and Ewe, J. A. (2020). Soy fermentation by orally isolated putative probiotic Streptococcus salivarius for healthy oral. J. Int. Oral Health 12 (1), 33. doi:10.4103/jioh.jioh_196_19
Clynes, M. A., Harvey, N. C., Curtis, E. M., Fuggle, N. R., Dennison, E. M., and Cooper, C. (2020). The epidemiology of osteoporosis. Br. Med. Bull. 133, 105–117. doi:10.1093/bmb/ldaa005
Contaldo, M., Itro, A., Lajolo, C., Gioco, G., Inchingolo, F., and Serpico, R. (2020). Overview on osteoporosis, periodontitis and oral dysbiosis: The emerging role of oral microbiota. Appl. Sci. 10 (17), 6000. doi:10.3390/app10176000
Cornwell, T., Cohick, W., and Raskin, I. (2004). Dietary phytoestrogens and health. Phytochem 65 (8), 995–1016. doi:10.1016/j.phytochem.2004.03.005
Cruzoé-Souza, M., Sasso-Cerri, E., and Cerri, P. S. (2009). Immunohistochemical detection of estrogen receptor β in alveolar bone cells of estradiol-treated female rats: Possible direct action of estrogen on osteoclast life span. J. Anat. 215 (6), 673–681. doi:10.1111/j.1469-7580.2009.01158.x
Delgobo, M., Agnes, J. P., Goncalves, R. M., Dos Santos, V. W., Parisotto, E. B., Zamoner, A., et al. (2019). N-acetylcysteine and alpha-lipoic acid improve antioxidant defenses and decrease oxidative stress, inflammation and serum lipid levels in ovariectomized rats via estrogen-independent mechanisms. J. Nutr. Biochem. 67, 190–200. doi:10.1016/j.jnutbio.2019.02.012
Desmawati, D., and Sulastri, D. (2019). Phytoestrogens and their health effect. Open Access Maced. J. Med. Sci. 7 (3), 495–499. doi:10.3889/oamjms.2019.044
Djapardy, V., and Panay, N. (2022). Alternative and non-hormonal treatments to symptoms of menopause. Best. Pract. Res. Clin. Obstet. Gynaecol. 81, 45–60. doi:10.1016/j.bpobgyn.2021.09.012
Du, D., Zhou, Z., Zhu, L., Hu, X., Lu, J., Shi, C., et al. (2018). TNF-α suppresses osteogenic differentiation of MSCs by accelerating P2Y2 receptor in estrogen-deficiency induced osteoporosis. Bone 117, 161–170. doi:10.1016/j.bone.2018.09.012
Eminov, E., Hortu, I., Akman, L., Erbas, O., Yavasoglu, A., and Cirpan, T. (2018). Exenatide preserves trabecular bone microarchitecture in experimental ovariectomized rat model. Arch. Gynecol. Obstet. 297 (6), 1587–1593. doi:10.1007/s00404-018-4776-7
Fang, H., Deng, Z., Liu, J., Chen, S., Deng, Z., and Li, W. (2022). The mechanism of bone remodeling after bone aging. Clin. Interv. Aging. 405, 405–415. doi:10.2147/CIA.S349604
Feng, W., Guo, J., and Li, M. (2019). RANKL-independent modulation of osteoclastogenesis. J. Oral Biosci. 61 (1), 16–21. doi:10.1016/j.job.2019.01.001
Fischer, V., and Haffner-Luntzer, M. (2022). Interaction between bone and immune cells: Implications for postmenopausal osteoporosis. Seminars Cell and Dev. Biol. 123, 14–21. doi:10.1016/j.semcdb.2021.05.014
Florencio-Silva, R., Sasso, G. R., Sasso-Cerri, E., Simões, M. J., and Cerri, P. S. (2018). Effects of estrogen status in osteocyte autophagy and its relation to osteocyte viability in alveolar process of ovariectomized rats. Biomed. Pharmacother. 98, 406–415. doi:10.1016/j.biopha.2017.12.08910.1016/j.biopha.2017.12.089
Folwarczna, J., Zych, M., Nowińska, B., Pytlik, M., Bialik, M., Jagusiak, A., et al. (2016). Effect of diosgenin, a steroidal sapogenin, on the rat skeletal system. Acta Biochim. Pol. 63 (2), 287–295. doi:10.18388/abp.2015_1095
Gerbarg, P. L., and Brown, R. P. (2016). Pause menopause with Rhodiola rosea, a natural selective estrogen receptor modulator. Phytomedicine 23 (7), 763–769. doi:10.1016/j.phymed.2015.11.013
Golub, L. M., Payne, J. B., Reinhardt, R. A., and Nieman, G. (2006). Can systemic diseases co-induce (not just exacerbate) periodontitis? A hypothetical “two-hit” model. J. Dent. Res. 85 (2), 102–105. doi:10.1177/154405910608500201
Gorabi, A. M., Kiaie, N., Hajighasemi, S., Jamialahmadi, T., Majeed, M., and Sahebkar, A. (2019). The effect of curcumin on the differentiation of mesenchymal stem cells into mesodermal lineage. Molecules 24 (22), 4029. doi:10.3390/molecules24224029
Gorzkiewicz, J., Bartosz, G., and Sadowska-Bartosz, I. (2021). The potential effects of phytoestrogens: The role in neuroprotection. Molecules 26 (10), 2954. doi:10.3390/molecules26102954
Grover, C. M., More, V. P., Singh, N., and Grover, S. (2014). Crosstalk between hormones and oral health in the mid-life of women: A comprehensive review. J. Int. Soc. Prev. Community Dent. 4, S5–S10. doi:10.4103/2231-0762.144559
Guiglia, R., Di-Fede, O., Lo-Russo, L., Sprini, D., Rini, G. B., and Campisi, G. (2013). Osteoporosis, jawbones and periodontal disease. Med. Oral Patol. Oral Cir. Bucal 18 (1), e93–e99. doi:10.4317/medoral.18298
Hajishengallis, G. (2014). Immunomicrobial pathogenesis of periodontitis: Keystones, pathobionts, and host response. Trends Immunol. 35 (1), 3–11. doi:10.1016/j.it.2013.09.001
Han, K., Ko, Y., Park, Y. G., and Park, J. B. (2016). Associations between the number of natural teeth in postmenopausal women and hormone replacement therapy. Maturitas 94, 125–130. doi:10.1016/j.maturitas.2016.10.005
He, J., Feng, C., Zhu, H., Wu, S., Jin, P., and Xu, T. (2020). Grainyhead-like 2 as a double-edged sword in development and cancer. Am. J. Trans. Res. 12 (2), 310–331.
He, J., Wang, S., Zhou, M., Yu, W., Zhang, Y., and He, X. (2015). Phytoestrogens and risk of prostate cancer: A meta-analysis of observational studies. World J. Surg. Oncol. 13 (1), 1–11. doi:10.1186/s12957-015-0648-9
How, Y. H., Ewe, J. A., Song, K. P., Kuan, C. H., Kuan, C. S., and Yeo, S. K. (2020). Soy fermentation by indigenous oral probiotic Streptococcus spp. and its antimicrobial activity against oral pathogens. Int. Food Res. J. 27 (2), 357–365.
Hooshiar, S. H., Tobeiha, M., and Jafarnejad, S. (2022). Soy Isoflavones and Bone Health: Focus on the RANKL/RANK/OPG Pathway. BioMed Res. Int., 1–10. doi:10.1155/2022/8862278
Hwang, Y. W., Kim, S. Y., Jee, S. H., Kim, Y. N., and Nam, C. M. (2009). Soy food consumption and risk of prostate cancer: A meta-analysis of observational studies. Nutr. Cancer. 61 (5), 598–606. doi:10.1080/01635580902825639
Inchingolo, F., Martelli, F. S., Gargiulo Isacco, C., Borsani, E., Cantore, S., Corcioli, F., et al. (2020). Chronic periodontitis and immunity, towards the implementation of a personalized medicine: A translational research on gene single nucleotide polymorphisms (SNPs) linked to chronic oral dysbiosis in 96 caucasian patients. Biomedicines 8 (5), 115. doi:10.3390/biomedicines8050115
Jafri, Z., Bhardwaj, A., Sawai, M., and Sultan, N. (2015). Influence of female sex hormones on periodontium: A case series. J. Nat. Sci. Biol. Med. 6, S146–S149. doi:10.4103/0976-9668.166124
Jang, K. M., Cho, K. H., Lee, S. H., Han, S. B., Han, K. D., and Kim, Y. H. (2015). Tooth loss and bone mineral density in postmenopausal south Korean women: The 2008–2010 Korea national health and nutrition examination survey. Maturitas 82 (4), 360–364. doi:10.1016/j.maturitas.2015.07.016
Johnston, B. D., and Ward, W. E. (2015). The ovariectomized rat as a model for studying alveolar bone loss in postmenopausal women. Biomed. Res. Int. 2015, 635023. doi:10.1155/2015/635023
Jonasson, G., Skoglund, I., and Rythén, M. (2018). The rise and fall of the alveolar process: Dependency of teeth and metabolic aspects. Arch. Oral Biol. 96, 195–200. doi:10.1016/j.archoralbio.2018.09.016
Juluri, R., Prashanth, E., Gopalakrishnan, D., Kathariya, R., Devanoorkar, A., Viswanathan, V., et al. (2015). Association of postmenopausal osteoporosis and periodontal disease: A double-blind case-control study. J. Int. Oral Health. 7 (9), 119–123.
Kanzaki, H., Wada, S., Narimiya, T., Yamaguchi, Y., Katsumata, Y., Itohiya, K., et al. (2017). Pathways that regulate ROS scavenging enzymes, and their role in defense against tissue destruction in periodontitis. Front. Physiol. 8, 351. doi:10.3389/fphys.2017.00351
Kawai, T., Matsuyama, T., Hosokawa, Y., Makihira, S., Seki, M., Karimbux, N. Y., et al. (2006). B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am. J. Pathol. 169 (3), 987–998. doi:10.2353/ajpath.2006.060180
Khezri, K., Maleki Dizaj, S., Rahbar Saadat, Y., Sharifi, S., Shahi, S., Ahmadian, E., et al. (2021). Osteogenic differentiation of mesenchymal stem cells via curcumin-containing nanoscaffolds. Stem Cells Int. 2021, 1520052. doi:10.1155/2021/1520052
Kirk, B., Feehan, J., Lombardi, G., and Duque, G. (2020). Muscle, bone, and fat crosstalk: The biological role of myokines, osteokines, and adipokines. Curr. Osteoporos. Rep. 18 (4), 388–400. doi:10.1007/s11914-020-00599-y
Kładna, A., Berczy ´nski, P., Kruk, I., Piechowska, T., and Aboul-Enein, H. Y. (2016). Studies on the antioxidant properties of some phytoestrogens. Luminescence 31, 1201–1206. doi:10.1002/bio.3091
Ko, K. P., Park, S. K., Yang, J. J., Ma, S. H., Gwack, J., Shin, A., et al. (2013). Intake of soy products and other foods and gastric cancer risk: A prospective study. J. Epidemiol. 23 (5), 337–343. doi:10.2188/jea.JE20120232
Lagari, V. S., and Levis, S. (2013). Phytoestrogens in the prevention of postmenopausal bone loss. J. Clin. Densitom. 16 (4), 445–449. doi:10.1016/j.jocd.2013.08.011
Lambert, M. N. T., Hu, L. M., and Jeppesen, P. B. (2017). A systematic review and meta-analysis of the effects of isoflavone formulations against estrogen-deficient bone resorption in peri-and postmenopausal women. Am. J. Clin. Nutr. 106 (3), 801–811. doi:10.3945/ajcn.116.151464
Lambert, M. N. T., Thybo, C. B., Lykkeboe, S., Rasmussen, L. M., Frette, X., Christensen, L. P., et al. (2017). Combined bioavailable isoflavones and probiotics improve bone status and estrogen metabolism in postmenopausal osteopenic women: A randomized controlled trial. Am. J. Clin. Nutr. 106 (3), 909–920. doi:10.3945/ajcn.117.153353
Laodheerasiri, S., and Horana Pathirage, N. (2017). Antimicrobial activity of raw soybean, soybean flour and roasted soybean extracted by ethanol-hexane method. Br. Food J. 119 (10), 2277–2286. doi:10.1108/BFJ-10-2016-0499
Lee, S. L., and Kim, J. G. (2006). Anti-microbial activity of soybean extract against oral microbes. J. Environ. Health Sci. 32 (2), 192–197.
Li, J., Li, H., Yan, P., Guo, L., Li, J., Han, J., et al. (2021). Efficacy and safety of phytoestrogens in the treatment of perimenopausal and postmenopausal depressive disorders: A systematic review and meta-analysis. Int. J. Clin. Pract. 75 (10), e14360. doi:10.1111/ijcp.14360
Lin, G. L., and Hankenson, K. D. (2011). Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J. Cell. Biochem. 112 (12), 3491–3501. doi:10.1002/jcb.23287
Liu, C., Zhang, S., Bai, H., Zhang, Y., Jiang, Y., Yang, Z., et al. (2022). Soy isoflavones alleviate periodontal destruction in ovariectomized rats. J. Periodontal. Res. 57 (3), 519–532. doi:10.1111/jre.12981
Long, L., Wang, X., Lei, Y., Guo, S., Wang, C., and Dai, W. (2022). Icariin: A Potential Alternative Against Osteoporosis. Nat. Prod. Commun. 17 (11), 1–15. doi:10.1177/1934578X221134881
Lorentzon, M., and Cummings, S. R. (2015). Osteoporosis: The evolution of a diagnosis. J. Intern. Med. 277 (6), 650–661. doi:10.1111/joim.12369
Luo, K., Ma, S., Guo, J., Huang, Y., Yan, F., and Xiao, Y. (2014). Association between postmenopausal osteoporosis and experimental periodontitis. Biomed. Res. Int. 2014, 316134. doi:10.1155/2014/316134
Manjunath, S. H., Rakhewar, P., Nahar, P., Tambe, V., Gabhane, M., and Kharde, A. (2019). Evaluation of the prevalence and severity of periodontal diseases between osteoporotic and nonosteoporotic subjects: A cross-sectional comparative study. J. Contemp. Dent. Pract. 20 (10), 1223–1228. doi:10.5005/jp-journals-10024-2717
Manolagas, S. C. (2000). Birth and death of bone cells: Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 21 (2), 115–137. doi:10.1210/edrv.21.2.0395
Manolagas, S. C. (2010). From estrogen-centric to aging and oxidative stress: A revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. 31 (3), 266–300. doi:10.1210/er.2009-0024
Manolagas, S. C., O'brien, C. A., and Almeida, M. (2013). The role of estrogen and androgen receptors in bone health and disease. Nat. Rev. Endocrinol. 9 (12), 699–712. doi:10.1038/nrendo.2013.179
Manyonda, I., S Talaulikar, V., Pirhadi, R., and Onwude, J. (2020). Progestogens are the problem in hormone replacement therapy: Time to reappraise their use. Post. Reprod. Health 26 (1), 26–31. doi:10.1177/2053369119876490
Marcellini, S., Henriquez, J. P., and Bertin, A. (2012). Control of osteogenesis by the canonical Wnt and BMP pathways in vivo: Cooperation and antagonism between the canonical Wnt and BMP pathways as cells differentiate from osteochondroprogenitors to osteoblasts and osteocytes. Bioessays 34 (11), 953–962. doi:10.1002/bies.201200061
Marjanovic, E. J., Southern, H. N., Coates, P., Adams, J. E., Walsh, T., Horner, K., et al. (2013). Do patients with osteoporosis have an increased prevalence of periodontal disease? A cross-sectional study. Osteoporos. Int. 24 (7), 1973–1979. doi:10.1007/s00198-012-2246-9
Mashalkar, V. N., Suragimath, G., Zope, S. A., and Varma, S. A. (2018). A cross-sectional study to assess and correlate osteoporosis and periodontitis among postmenopausal women: A dual energy X-ray absorptiometry study. J. Mid-life Health. 9 (1), 2–7. doi:10.4103/jmh.JMH_39_17
McCarty, M. F. (2006). Isoflavones made simple–genistein’s agonist activity for the beta-type estrogen receptor mediates their health benefits. Med. Hypotheses. 66 (6), 1093–1114. doi:10.1016/j.mehy.2004.11.046
Messina, M., Ho, S., and Alekel, D. L. (2004). Skeletal benefits of soy isoflavones: A review of the clinical trial and epidemiologic data. Curr. Opin. Clin. Nutr. Metab. Care. 7 (6), 649–658. doi:10.1097/00075197-200411000-00010
Mohd Dom, T. N., Ayob, R., Abd Muttalib, K., and Aljunid, S. M. (2016). National economic burden associated with management of periodontitis in Malaysia. Int. J. Dent. 2016, 1891074. doi:10.1155/2016/189107410.1155/2016/1891074
Mohd-Dom, T., Ayob, R., Mohd-Nur, A., Abdul-Manaf, M. R., Ishak, N., Abdul-Muttalib, K., et al. (2014). Cost analysis of periodontitis management in public sector specialist dental clinics. BMC Oral Health 14 (1), 56–10. doi:10.1186/1472-6831-14-5610.1186/1472-6831-14-56
Munoz, M., Robinson, K., and Shibli-Rahhal, A. (2020). Bone health and osteoporosis prevention and treatment. Clin. Obstet. Gynecol. 63 (4), 770–787. doi:10.1097/GRF.0000000000000572
Muslita, I., Lindawati, S. K., and Henni, K. (2012). Resorption level of edentulous alveolar bone in normal, osteopenia and osteoporosis postmenopausal women. Int. J. Clin. Prev. Dent. 8 (3), 141–146.
Narducci, P., Bareggi, R., and Nicolin, V. (2011). Receptor Activator for Nuclear Factor kappa B Ligand (RANKL) as an osteoimmune key regulator in bone physiology and pathology. Acta histochem. 113 (2), 73–81. doi:10.1016/j.acthis.2009.10.003
Ni, X., Wu, B., Li, S., Zhu, W., Xu, Z., Zhang, G., et al. (2023). Equol exerts a protective effect on postmenopausal osteoporosis by upregulating OPG/RANKL pathway. Phytomedicine 1, 108–154509. doi:10.1016/j.phymed.2022.154509
Oei, L., Koromani, F., Rivadeneira, F., Zillikens, M. C., and Oei, E. H. (2016). Quantitative imaging methods in osteoporosis. Quant. Imaging Med. Surg. 6 (6), 680–698. doi:10.21037/qims.2016.12.13
Oršolić, N., Nemrava, J., Jelec, Z., Kukolj, M., Odeh, D., and Jakopovic, B. (2022). Antioxidative and Anti-Inflammatory Activities of Chrysin and Naringenin in a Drug-Induced Bone Loss Model in Rats. Int. J. Mol. Sci. 23 (5), 2872–2895. doi:10.3390/ijms23052872
Ortiz, A. D., Fideles, S. O., Reis, C. H., Bellini, M. Z., Pereira, E. D., Pilon, J. P., et al. (2022). Therapeutic effects of citrus flavonoids neohesperidin, hesperidin and its aglycone, hesperetin on bone health. Biomolecules 12 (5), 626. doi:10.3390/biom12050626
Pacios, S., Xiao, W., Mattos, M., Lim, J., Tarapore, R. S., Alsadun, S., et al. (2015). Osteoblast lineage cells play an essential role in periodontal bone loss through activation of nuclear factor-kappa B. Sci. Rep. 5 (1), 1–12. doi:10.1038/srep16694
Papapanou, P. N., Sanz, M., Buduneli, N., Dietrich, T., Feres, M., Fine, D. H., et al. (2018). Periodontitis: Consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J. Periodontol. 89, S173–S182. doi:10.1002/JPER.17-0721
Pepelassi, E., Nicopoulou-Karayianni, K., Archontopoulou, A. D., Mitsea, A., Kavadella, A., Tsiklakis, K., et al. (2012). The relationship between osteoporosis and periodontitis in women aged 45–70 years. Oral Dis. 18 (4), 353–359. doi:10.1111/j.1601-0825.2011.01881.x
Poluzzi, E., Piccinni, C., Raschi, E., Rampa, A., Recanatini, M., and De Ponti, F. (2014). Phytoestrogens in postmenopause: The state of the art from a chemical, pharmacological and regulatory perspective. Curr. Med. Chem. 21 (4), 417–436. doi:10.2174/09298673113206660297
Qi, J., Liu, E., Guo, Y. F., Hu, J. M., Liu, Y. T., Chen, G., et al. (2021). Association between periodontal disease and osteoporosis in postmenopausal women: A protocol for systematic review and meta-analysis. BMJ Open 11 (9), e049277. doi:10.1136/bmjopen-2021-049277
Qu, X. L., Fang, Y., Zhang, M., and Zhang, Y. Z. (2014). Phytoestrogen intake and risk of ovarian cancer: A meta-analysis of 10 observational studies. Asian pac. J. Cancer Prev. 15 (21), 9085–9091. doi:10.7314/apjcp.2014.15.21.9085
Rachoń, D., Vortherms, T., Seidlová-Wuttke, D., and Wuttke, W. (2007). Effects of dietary equol on the pituitary of the ovariectomized rats. Horm. Metab. Res. 39, 256–261. doi:10.1055/s-2007-973074
Ramesh, P., Jagadeesan, R., Sekaran, S., Dhanasekaran, A., and Vimalraj, S. (2021). Flavonoids: Classification, function, and molecular mechanisms involved in bone remodelling. Front. Endocrinol. 12, 779638. doi:10.3389/fendo.2021.779638
Riggs, B. L. (2000). The mechanisms of estrogen regulation of bone resorption. J. Clin. Investig. 106 (10), 1203–1204. doi:10.1172/JCI11468
Rowe, I. J., and Baber, R. J. (2021). The effects of phytoestrogens on postmenopausal health. Climacteric 24 (1), 57–63. doi:10.1080/13697137.2020.1863356
Sathyapalan, T., Aye, M., Rigby, A. S., Thatcher, N. J., Dargham, S. R., Kilpatrick, E. S., et al. (2018). Soy isoflavones improve cardiovascular disease risk markers in women during the early menopause. Nutr. Metab. Cardiovas. Dis. 28 (7), 691–697. doi:10.1016/j.numecd.2018.03.007
Savić Pavičin, I., Dumančić, J., Jukić, T., and Badel, T. (2017). The relationship between periodontal disease, tooth loss and decreased skeletal bone mineral density in ageing women. Gerodontology 34 (4), 441–445. doi:10.1111/ger.12290
Scheidt-Nave, C., Bismar, H., Leidig-Bruckner, G., Woitge, H., Seibel, M. J., Ziegler, R., et al. (2001). Serum interleukin 6 is a major predictor of bone loss in women specific to the first decade past menopause. J. Clin. Endocrinol. Metab. 86 (5), 2032–2042. doi:10.1210/jcem.86.5.7445
Schilling, T., Ebert, R., Raaijmakers, N., Schütze, N., and Jakob, F. (2014). Effects of phytoestrogens and other plant-derived compounds on mesenchymal stem cells, bone maintenance and regeneration. J. Steroid Biochem. Mol. Biol. 139, 252–261. doi:10.1016/j.jsbmb.2012.12.006
Sczepanik, F. S. C., Grossi, M. L., Casati, M., Goldberg, M., Glogauer, M., Fine, N., et al. (2020). Periodontitis is an inflammatory disease of oxidative stress: We should treat it that way. Periodontol 84 (1), 45–68. doi:10.1111/prd.12342
Sekaran, S., Roy, A., and Thangavelu, L. (2022). Re-appraising the role of flavonols, flavones and flavonones on osteoblasts and osteoclasts-A review on its molecular mode of action. Chem-Biol. Interact. 355, 109831. doi:10.1016/j.cbi.2022.109831
Setchell, K. D., and Lydeking-Olsen, E. (2003). Dietary phytoestrogens and their effect on bone: Evidence from in vitro and in vivo, human observational, and dietary intervention studies. Am. J. Clin. Nutr. 78 (3), 593S–609S. doi:10.1093/ajcn/78.3.593S
Sharma, G., Sultana, A., Abdullah, K. M., Pothuraju, R., Nasser, M. W., Batra, S. K., et al. (2022). Epigenetic regulation of bone remodeling and bone metastasis. Semin. Cell and Dev. Biol. doi:10.1016/j.semcdb.2022.11.002
Shimazu, T., Inoue, M., Sasazuki, S., Iwasaki, M., Sawada, N., Yamaji, T., et al. (2011). Plasma isoflavones and the risk of lung cancer in women: A nested case–control study in Japan. Cancer Epidemiol. Biomarkers Prev. 20 (3), 419–427. doi:10.1158/1055-9965.EPI-10-1025
Singh, P., Gupta, N. D., Bey, A., and Khan, S. (2014). Salivary TNF-alpha: A potential marker of periodontal destruction. J. Indian Soc. Periodontol. 18 (3), 306–310. doi:10.4103/0972-124X.134566
Sirotkin, A. V., and Harrath, A. H. (2014). Phytoestrogens and their effects. Eur. J. Pharmacol. 741, 230–236. doi:10.1016/j.ejphar.2014.07.057
Song, S. H., Zhai, Y. K., Li, C. Q., Yu, Q., Lu, Y., and Zhang, Y. (2016). Effects of total flavonoids from Drynariae Rhizoma prevent bone loss in vivo and in vitro. Bone Rep. 5, 262–273. doi:10.1016/j.bonr.2016.09.001
Sözen, T., Özışık, L., and Başaran, N. Ç. (2017). An overview and management of osteoporosis. Eur. J. Rheumatol. 4 (1), 46–56. doi:10.5152/eurjrheum.2016.048
Taguchi, A., Shiraki, M., Tanaka, S., Ohshige, H., and Nakamura, T. (2019). Improved periodontal disease and prevention of tooth loss in osteoporosis patients receiving once-yearly zoledronic acid: A randomized clinical trial. Menopause 26 (11), 1277–1283. doi:10.1097/GME.0000000000001393
Takayanagi, H., Ogasawara, K., Hida, S., Chiba, T., Murata, S., Sato, K., et al. (2000). T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature 408 (6812), 600–605. doi:10.1038/35046102
Tanaka, R., Tanaka, T., Yeung, A. W. K., Taguchi, A., Katsumata, A., and Bornstein, M. M. (2020). Mandibular radiomorphometric indices and tooth loss as predictors for the risk of osteoporosis using panoramic radiographs. Oral Health Prev. Dent. 18 (1), 773–782. doi:10.3290/j.ohpd.a45081
Tonetti, M. S., Jepsen, S., Jin, L., and Otomo-Corgel, J. (2017). Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J. Clin. Periodontol. 44 (5), 456–462. doi:10.1111/jcpe.12732
Trisomboon, H., Malaivijitnond, S., Cherdshewasart, W., Watanabe, G., and Taya, K. (2007). Assessment of urinary gonadotropin and steroid hormone profiles of female cynomolgus monkeys after treatment with Pueraria mirifica. J. Reprod. Dev. 53, 395–403. doi:10.1262/jrd.18079
Trombelli, L., Farina, R., Silva, C. O., and Tatakis, D. N. (2018). Plaque-induced gingivitis: Case definition and diagnostic considerations. J. Clin. Periodontol. 45, S44–S67. doi:10.1111/jcpe.12939
Valimaa, H., Savolainen, S., Soukka, T., Silvoniemi, P., Makela, S., Kujari, H., et al. (2004). Estrogen receptor-beta is the predominant estrogen receptor subtype in human oral epithelium and salivary glands. J. Endocrinol. 180 (1), 55–62. doi:10.1677/joe.0.1800055
Wang, C. W. J., and McCauley, L. K. (2016). Osteoporosis and periodontitis. Curr. Osteoporos. Rep. 14 (6), 284–291. doi:10.1007/s11914-016-0330-3
Wang, G. P. (2015). Defining functional signatures of dysbiosis in periodontitis progression. Genome Med. 7 (1), 40–43. doi:10.1186/s13073-015-0165-z
Wang, Q., Wang, H., and Xie, M. (2010). Antibacterial mechanism of soybean isoflavone on Staphylococcus aureus. Arch. Microbiol. 192 (11), 893–898. doi:10.1007/s00203-010-0617-1
Wang, X., Zheng, A., Xin, X. Z., Peng, L. J., Wang, J., Cao, L. Y., et al. (2021). Osteogenic induction of low-dose ipriflavone on bone marrow mesenchymal stem cells extracted from osteoporosis rats. Chin. J. Dent. Res. 24, 153–158. doi:10.3290/j.cjdr.b1965039
Wu, D., Cline-Smith, A., Shashkova, E., Perla, A., Katyal, A., and Aurora, R. (2021). T-cell mediated inflammation in postmenopausal osteoporosis. Front. Immunol. 12, 687551. doi:10.3389/fimmu.2021.687551
Xu, Q., Cao, Z., Xu, J., Dai, M., Zhang, B., and Lai, Q. (2022). Effects and mechanisms of natural plant active compounds for the treatment of osteoclast-mediated bone destructive diseases. J. Drug Target. 30(4), 394-412. doi:10.1080/1061186X.2021.2013488
Yu, B., and Wang, C. Y. (2022). Osteoporosis and periodontal diseases–An update on their association and mechanistic links. Periodontology 89 (1), 99–113. doi:10.1111/prd.12422
Zakłos-Szyda, M., Budryn, G., Grzelczyk, J., Perez-Sanchez, H., and Zyzelewicz, D. (2020). Evaluation of Isoflavones as bone resorption inhibitors upon interactions with receptor activator of nuclear factor-κb ligand (RANKL). Molecules. 25(1), 206-225. doi:10.3390/molecules25010206
Zhang, W., Dang, K., Huai, Y., and Qian, A. (2020). Osteoimmunology: The regulatory roles of T lymphocytes in osteoporosis. Front. Endocrinol. 11, 465. doi:10.3389/fendo.2020.00465
Zhang, Z., Chen, Y., Xiang, L., Wang, Z., Xiao, G. G., and Ju, D. (2018). Diosgenin protects against alveolar bone loss in ovariectomized rats via regulating long non-coding RNAs. Exp. Ther. Med. 16 (5), 3939–3950. doi:10.3892/etm.2018.6681
Zhang, Z., Song, C., Fu, X., Liu, M., Li, Y., Pan, J., et al. (2014). High-dose diosgenin reduces bone loss in ovariectomized rats via attenuation of the RANKL/OPG ratio. Int. J. Mol. Sci. 15 (9), 17130–17147. doi:10.3390/ijms150917130
Keywords: periodontitis, postmenopausal osteoporosis, alveolar bone loss, phytoestrogens, estrogen deficiency
Citation: Jayusman PA, Nasruddin NS, Baharin B, Ibrahim N‘I, Ahmad Hairi H and Shuid AN (2023) Overview on postmenopausal osteoporosis and periodontitis: The therapeutic potential of phytoestrogens against alveolar bone loss. Front. Pharmacol. 14:1120457. doi: 10.3389/fphar.2023.1120457
Received: 10 December 2022; Accepted: 10 February 2023;
Published: 23 February 2023.
Edited by:
Wei Peng, Chengdu University of Traditional Chinese Medicine, ChinaCopyright © 2023 Jayusman, Nasruddin, Baharin, Ibrahim, Ahmad Hairi and Shuid. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmad Nazrun Shuid, YW5henJ1bkB1aXRtLmVkdS5teQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.