- 1Department of Clinical Research and Data Management, Center of Respiratory Medicine, China-Japan Friendship Hospital, National Center for Respiratory Medicine, Institute of Respiratory Medicine, Chinese Academy of Medical Sciences, National Clinical Research Center for Respiratory Diseases, Beijing, China
- 2Department of Traditional Chinese Medicine for Pulmonary Diseases, Center of Respiratory Medicine, China-Japan Friendship Hospital, National Center for Respiratory Medicine, Institute of Respiratory Medicine, Chinese Academy of Medical Sciences, National Clinical Research Center for Respiratory Diseases, Beijing, China
- 3Department of Pulmonary and Critical Care Medicine, Center of Respiratory Medicine, China-Japan Friendship Hospital, National Center for Respiratory Medicine, Institute of Respiratory Medicine, Chinese Academy of Medical Sciences, National Clinical Research Center for Respiratory Diseases, Beijing, China
- 4Beijing Key Laboratory of Respiratory and Pulmonary Circulation Disorders, Department of Pulmonary and Critical Care Medicine, Beijing Chao-Yang Hospital, Capital Medical University, Beijing Institute of Respiratory Medicine, Beijing, China
- 5Department of Respiratory and Critical Care Medicine, Peking University Third Hospital, Beijing, China
Objective: Our aim was to systematically investigate the efficacy of Tanreqing (TRQ) injection on in-hospital outcomes among inpatients with frequent or infrequent AECOPD.
Methods: In this ongoing, nationwide multicenter registry designed to investigate clinical characteristics, management, and prognoses of Chinese patients admitted for AECOPD in real-world settings, we collected characteristics, comorbidities, in-hospital prognoses, and information on the COPD assessment test (CAT) questionnaire, PEACE questionnaire, and modified British Medical Research Council (mMRC) questionnaire from each enrolled patient. Frequent AECOPD was determined as being admitted to the hospital ≥1 time or visiting the emergency room (ER) ≥ 2 times due to AECOPD within a year. A propensity match method and univariable and multivariable regression models were performed to analyze the efficacy of TRQ on clinical outcomes for inpatients with frequent AECOPD.
Results: A total of 4135 inpatients were involved in the analysis, including 868 administered with TRQ and 3267 not administered with TRQ. After propensity score match, among those administered with TRQ, 493 had frequent AECOPD and 358 had infrequent AECOPD. A significant reduction of CAT score at discharge (TRQ median 12, IQR 8.0–16.0; non-TRQ median 13, IQR 9.0–18.0, p = 0.0297), a lower rate of ICU admission (TRQ 0.8% vs. non-TRQ 2.6%, p = 0.0191), and a shorter length of stay (LOS) (TRQ median 11, IQR 9.0–14.0; non-TRQ median 11, IQR 8.0–14.0, p = 0.004) were observed in the TRQ group, compared with the non-TRQ group among frequent AECOPD patients. In the subgroup analysis, for those with a PEACE score >7 on admission, TRQ contributed to a significantly lower CAT score at discharge (p = 0.0084) and a numerically lower ICU admission rate with a marginal statistical significance. Among those with phlegm-heat symptom complex on admission ≥2, a lower CAT score at discharge and a lower ICU admission were also observed in the TRQ group.
Conclusion: TRQ injection had better efficacy in patients with frequent AECOPD in reducing ICU admission and alleviating respiratory symptoms, especially for those with higher severity on admission or more phlegm-heat symptoms.
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by chronic irreversible airflow limitation, and its high prevalence and mortality continue to lead to a heavy disease burden globally (GBD Chronic Respiratory Disease Collaborators, 2020; Global strategy for the diagnosis and management and prevention of chronic obstructive pulmonary disease, 2022). In China, the prevalence of COPD was 8.6%, and it was estimated that there were nearly 100 million patients among those over 20 years old in 2015.
Acute exacerbation of COPD (AECOPD) is an acute event where a worsening of respiratory symptoms beyond normal daily variations occurs, resulting in the need for a change in therapy (Wang et al., 2018). AECOPD is thought to be caused by complex interactions between the human body, pathogens, and the external environment, which leads to an increase in the inflammatory burden, and it is associated with increased airway and systemic inflammation and physiological changes (Wedzicha and Terence, 2007). Frequent exacerbation increases hospitalization and promotes disease progression, thus negatively impacting the management of COPD. A large cohort study revealed that AECOPD frequency in a single year predicts long-term AECOPD rate. Increasing frequency and severity of AECOPD is associated with the risk of death (Rothnie et al., 2018). The GOLD report uses a threshold of two or more acute exacerbations in the previous year, or at least one hospital admission related to acute exacerbation to identify individuals at a high risk of future events (Ouaalaya et al., 2020). Patients who have frequent exacerbations have higher mortality, worse quality of life, more future exacerbation events, and faster FEV1 decline than those with infrequent exacerbations (0–1/time per year) (Seemungal et al., 2000; Wedzicha et al., 2013; Mullerova et al., 2014). These patients also have an increased airway inflammation, which contributes to a higher risk of hospital admission and disease progression (Seemungal et al., 2000).
The management of AECOPD includes short-acting inhaled bronchodilators, systemic corticosteroids, antibiotics, oxygen therapy, and mechanical ventilation if required (Ko et al., 2016). Some clinical trials have evaluated the efficacy and safety of Chinese medicine injections for AECOPD patients and proved their effectiveness in inhibiting inflammation, regulating immune function, and alleviating symptoms (Li et al., 2010; Hu et al., 2021). Tanreqing (TRQ), an injectable prescription from traditional Chinese medicine with functions of clearing the heart, detoxifying, and resolving phlegm has been approved to treat acute respiratory infection (National Medical Products Administration, China, Number Z20030054). Several randomized clinical trials (RCTs) with limited sample sizes demonstrated the efficacy of TRQ for the treatment of AECOPD and severe pneumonia (Wang et al., 2020; Hu et al., 2021; Chen et al., 2022). However, the effectiveness of TRQ on frequent AECOPD in real-world applications has never been discussed. In our study, we conducted an analysis among AECOPD inpatients who were prescribed TRQ injection using the propensity score match (PSM) method, compared with those who did not use TRQ, to systematically investigate its efficacy on in-hospital outcomes of frequent or infrequent AECOPD.
Materials and methods
Study design and participants
Our study analyzed data from the acute exacerbation of chronic obstructive pulmonary disease inpatient registry (ACURE) study. The ACURE study is an ongoing, nationwide multicenter registry designed to investigate clinical characteristics, management, and prognoses of Chinese patients admitted for AECOPD in real-world settings (ClinicalTrials. gov identifier: NCT02657525). It started on 1 September 2017 and planned to recruit 7600 in-hospital AECOPD patients with a 3-year follow-up. The protocol and phase 1 results of the registry have been previously described (Pei et al., 2020; Liang et al., 2021). The study was approved by the ethics committee of China-Japan Friendship Hospital (No. 2015-88) and informed consent was obtained from all involved participants. The study was conducted in accordance with the Declaration of Helsinki.
Measurements and outcomes
For each patient, a baseline survey was conducted within 1–3 days after hospitalization to collect information on medical history, physical examination, and inpatient diagnosis. During the hospital stay, the COPD assessment test (CAT) questionnaire, PEACE questionnaire (consisting of eight questions assessing daily variance of COPD symptoms, i.e., dyspnea, purulent sputum, sputum volume, upper respiratory tract infection, fever, wheezing, cough, and breath rate), modified British Medical Research Council (mMRC) questionnaire, medical examinations, laboratory tests, and treatments were recorded. Comorbidities including respiratory diseases, cardiovascular diseases, metabolic diseases (diabetes and osteoporosis), and digestive diseases, as well as malignancies other than lung cancer, peripheral arterial disease, venous thromboembolism, cerebrovascular disease, anxiety/depression, musculoskeletal dysfunction, chronic kidney disease, etc., were recorded.
During hospitalization, treatment (including the application of TRQ) and auxiliary examination results including laboratory and lung function tests were recorded if available. All auxiliary examinations were conducted at local sites, and the results were uploaded to the database by investigators. If laboratory data were unavailable during hospitalization, the most recent results within 3 days before admission were used for imputation. If multiple tests were conducted after admission, the earliest one was used. The severity of airflow limitation was classified into four grades based on the 2017 Global Initiative for Chronic Obstructive Lung Disease (GOLD) report: GOLD 1 (forced expiratory volume in one second [FEV1]% predicted ≥80), GOLD 2 (50 ≤ FEV1% predicted <80), GOLD 3 (30 ≤ FEV1% predicted <50), and GOLD 4 (FEV1% predicted <30) (Global strategy for the diagnosis and management and prevention of chronic obstructive pulmonary disease, 2017). Total direct costs were calculated in US dollars using the average exchange rate in 2019 (one US dollar was equivalent to 6.90 yuan).
Patients were divided into “frequent AECOPD” if they were admitted to the hospital ≥1 time or visited the emergency room (ER) ≥ 2 times due to AECOPD within a year and “infrequent AECOPD” if they were never admitted to the hospital or visited the emergency room (ER) < 2 times due to AECOPD, according to the GOLD report (Global strategy for the diagnosis and management and prevention of chronic obstructive pulmonary disease, 2017). According to the theory of traditional Chinese medicine, patients were defined as having “phlegm and heat” syndrome if they had ≥ two of the following symptoms: fever, pharyngalgia, purulent sputum, and sputum over 50 mL/day. Those without a single of the above symptoms were defined as having “no phlegm and heat” syndrome.
The primary outcome was a PEACE score at discharge (Zheng et al., 2008). The secondary outcomes were ICU admission, the change of CAT score at discharge, mMRC dyspnea grade at discharge, length of hospitalization, and total cost of hospitalization.
Statistical analysis
Baseline patient characteristics were expressed in terms of descriptive statistics. Categorical variables were summarized as frequency (percentage). Continuous variables were presented as mean (standard deviation, SD) or median (interquartile range, IQR). p values were calculated by students’ t-test, χ2 test, or Fisher exact test where appropriate.
A propensity score was estimated by logistic regression to determine the probability of TRQ treatment of each patient conditionally on observed covariates. Based on the propensity score, we performed 2:1 match-to-match patients who were not administered with TRQ to those who were on a range of ±0.0001 to ± 0.1. The match started with the range of ± 0.0001, and those who were matched were extracted from the database and excluded from the following ranges. If more than two patients who were not administered TRQ were detected, only two of them were selected randomly. Variables involved in the propensity score estimation included age, drug therapy, PEACE score at admission, mMRC score at admission, hospitalization frequency, diagnosis as COPD for the first time, cor pulmonale, non-drug therapy, cough frequency, expectoration, and fever. The propensity score matches were performed in the analyses between frequent AECOPD and infrequent AECOPD, PEACE score >7 and ≤7 on admission, phlegm-heat symptom complex ≥2 and <2 on admission, or phlegm-heat symptom complex ≥1 and <1 on admission. Univariable and multivariable analyses of the efficacy of TRQ on clinical outcomes for inpatients with frequent AECOPD were performed by the logistic regression model or general linear model to investigate the efficacy of TRQ. In the multivariable model, covariates including age, drug therapy, PEACE score at admission, mMRC score at admission, hospitalization frequency, diagnosis as COPD for the first time, cor pulmonale, non-drug therapy, cough frequency, expectoration, and fever were adjusted.
All tests were two-sided and were considered statistically significant at a p-value of <0.05. All analyses were performed using SAS 9.4 software (Cary, NC, United States).
Results
Baseline characteristics
As is shown in the flow chart of this study, 5334 AECOPD inpatients were enrolled from 153 sites between 1 September 2017 and 25 February 2020. After excluding 1199 inpatients, 4135 inpatients were involved in the analysis, including 868 administered with TRQ and 3267 not administered with TRQ. After propensity score match, among those administered with TRQ, 493 had frequent AECOPD and 358 had infrequent AECOPD (Figure 1).
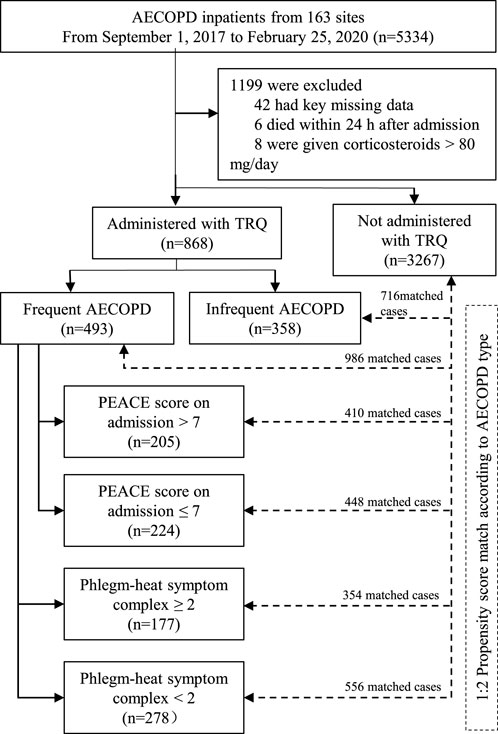
FIGURE 1. Flowchart of this study. Abbreviations: COPD, chronic obstructive pulmonary disease; AECOPD, acute exacerbation of COPD; TRQ, Tanreqing injection.
Among frequent AECOPD patients (n = 2179), those who have been prescribed TRQ (n = 505) had a significantly higher rate of cor pulmonale, cough, a larger amount of sputum, fever, and lower C-reactive protein (CRP) level on admission. The PEACE scores were higher among the TRQ group, compared with the non-TRQ group (TRQ median 8.0, interquartile range [IQR] 6.0–9.0 vs. non-TRQ median 7.0, IQR 6.0–9.0, p = 0.0038), indicating that those injected with TRQ had more severe symptoms on admission. Similar results were revealed among the infrequent AECOPD patients (Table 1). After the propensity match, the demographic and baseline characteristics were balanced between TRQ and non-TRQ groups in patients with frequent AECOPD or infrequent AECOPD (Supplementary Table S1).
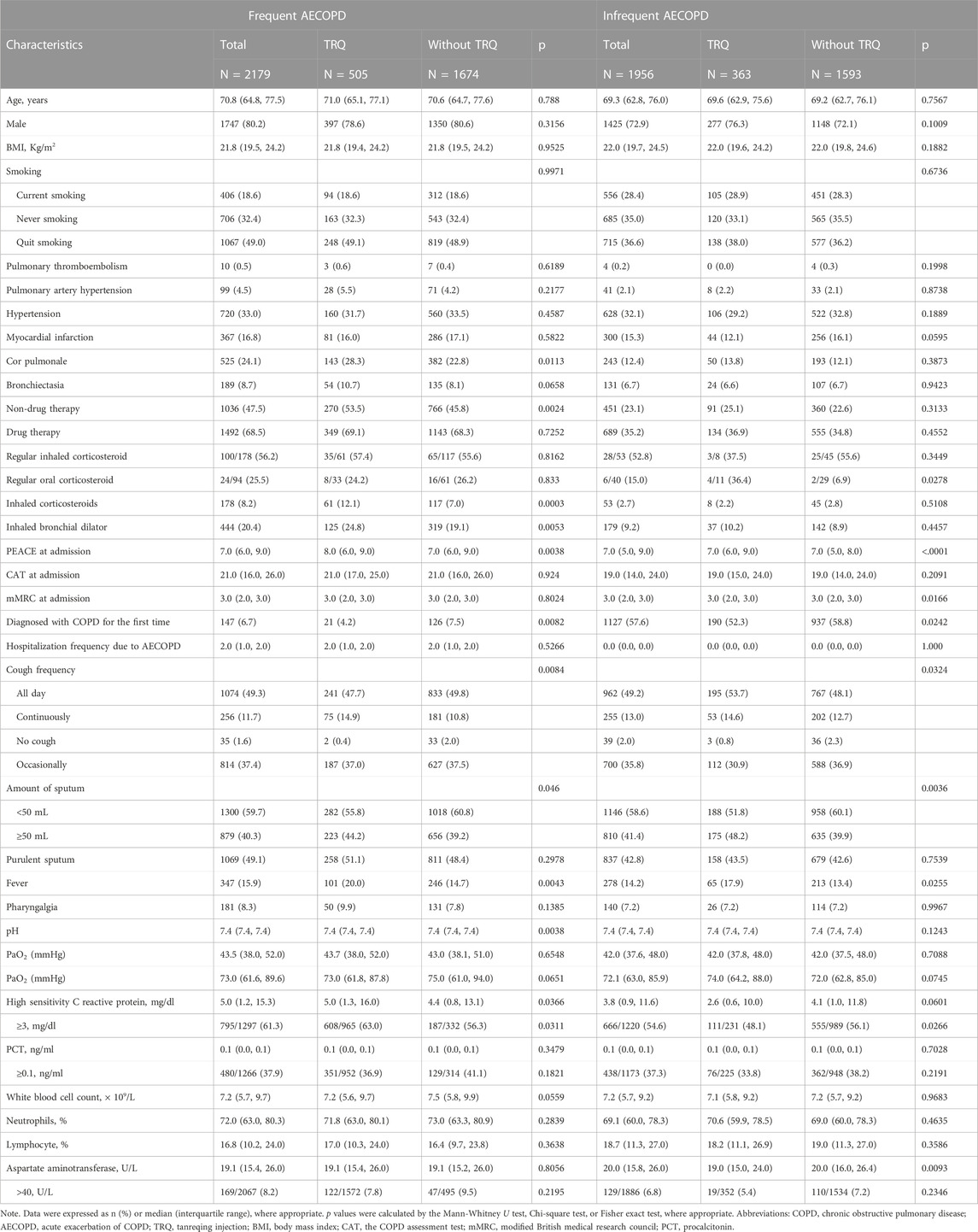
TABLE 1. Characteristics of inpatients with frequent AECOPD or Infrequent AECOPD before propensity score match.
Treatment and clinical outcomes after PSM
After the propensity score match, a significant reduction of CAT score at discharge (TRQ median 12, IQR 8.0–16.0; non-TRQ median 13, IQR 9.0–18.0, p = 0.0297), a lower rate of ICU admission (TRQ 0.8% Vs. non-TRQ 2.6%, p = 0.0191), and a shorter length of stay (LOS) (TRQ median 11 days, IQR 9.0–14.0; non-TRQ median 11 days, IQR 8.0–14.0, p = 0.004) were observed in the TRQ group, compared with the non-TRQ group among frequent AECOPD patients. Treatment and clinical outcomes including PEACE at discharge, PEACE difference, mMRC at discharge, mMRC difference, death or worsened cases, cost, antibiotics, and systemic corticosteroid were compared between TRQ and non-TRQ groups among frequent AECOPD patients. No significant difference in outcomes was shown in infrequent AECOPD patients (Table 2). After adjusting for covariates, the TRQ group was independently associated with a lower CAT score at discharge (β −0.90, 95% confidence interval [95% CI] −1.53 to −0.27, p = 0.0050) and lower ICU admission (odds ratio [OR] 0.30, 95% CI 0.10–0.87, p = 0.0050) (Table 3).
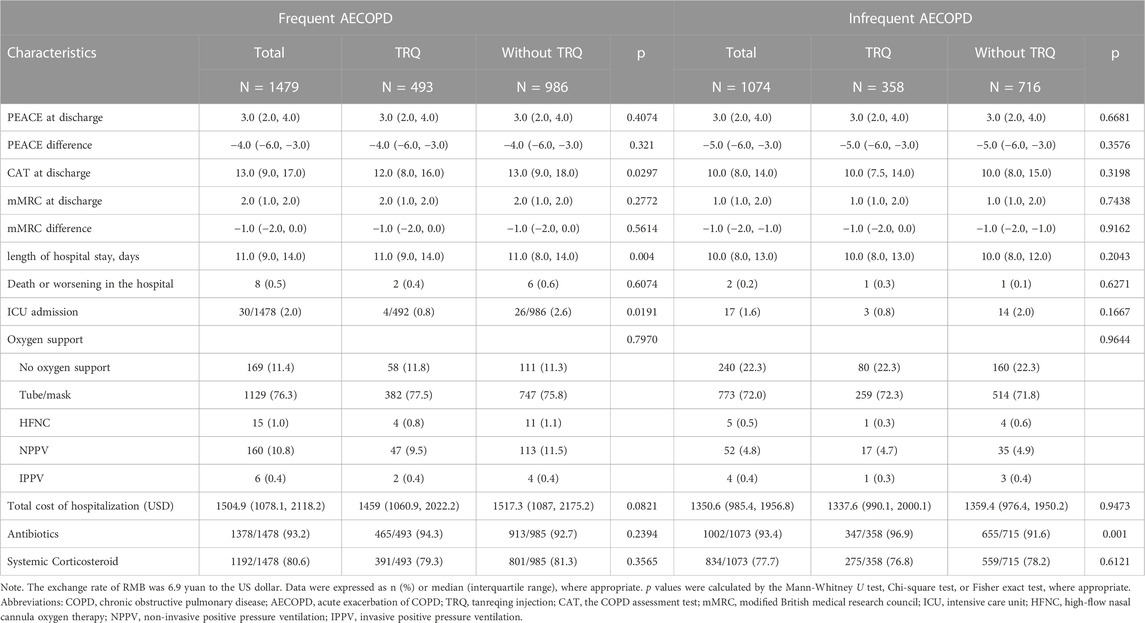
TABLE 2. Treatment and clinical outcomes of inpatients with frequent AECOPD or Infrequent AECOPD after propensity score match.
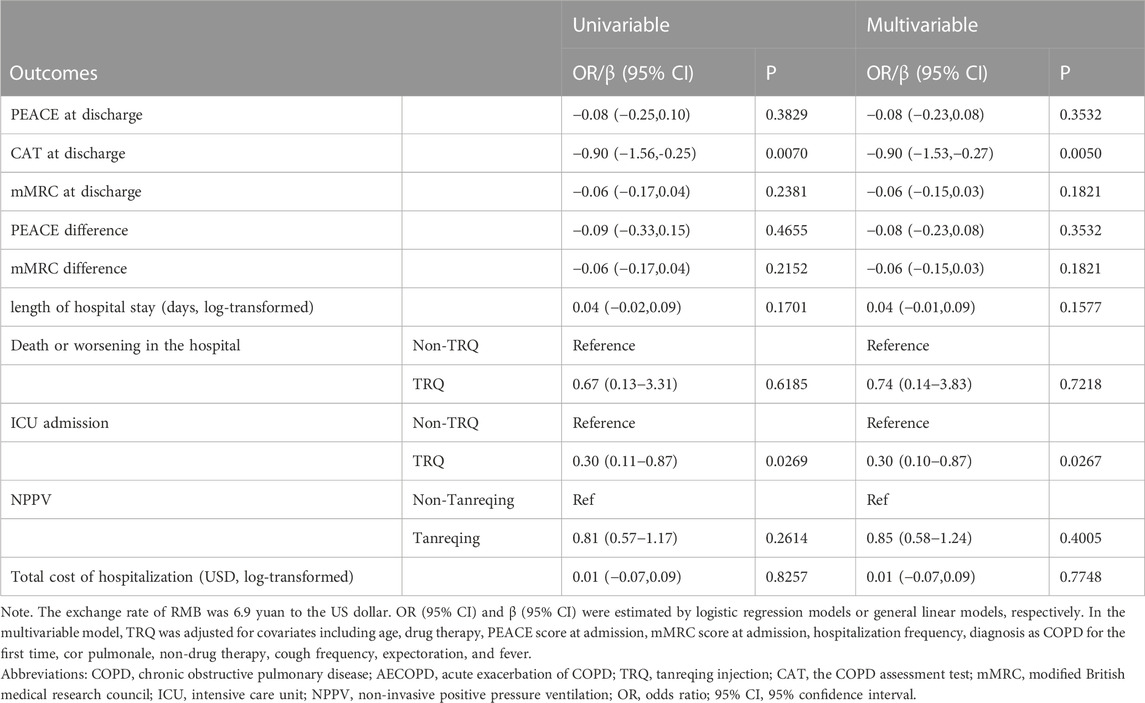
TABLE 3. Univariable and multivariable analyses of the efficacy of TRQ on clinical outcomes for inpatients with frequent AECOPD.
Subgroup analysis after PSM
To further analyze the potential of susceptible patients treated with TRQ, a series of subgroup analyses were conducted among frequent AECOPD inpatients with PEACE scores >7 or ≤7 on admission and different phlegm-heat symptom complex. As shown in Table 4 and supplementary table 2, among those with PEACE score >7 on admission, TRQ contributed to a significantly lower CAT score at discharge (TRQ median 12.0, IQR 8.5–16.0; non-TRQ median 13.0, median 10.0–19.0, p = 0.0084) and numerically lower ICU admission rate with marginal statistical significance (TRQ 1.0%; non-TRQ 3.7%, p = 0.0557). Among those with phlegm-heat symptom complex on admission ≥2, lower CAT score at discharge and lower ICU admission were also observed in the TRQ group (p = 0.0145 and 0.0434, respectively); among those with phlegm-heat symptom complex on admission <2, TRQ contributed to the lower total cost of hospitalization (p = 0.0126) (Table 4). The treatment of TRQ may prolong the length of hospital stay in patients with phlegm-heat symptom complex on admission ≥1 (p = 0.0271) but still be effective in lowering CAT score at discharge among those without any phlegm-heat symptom complex (p = 0.0438) (Supplementary Table S2).
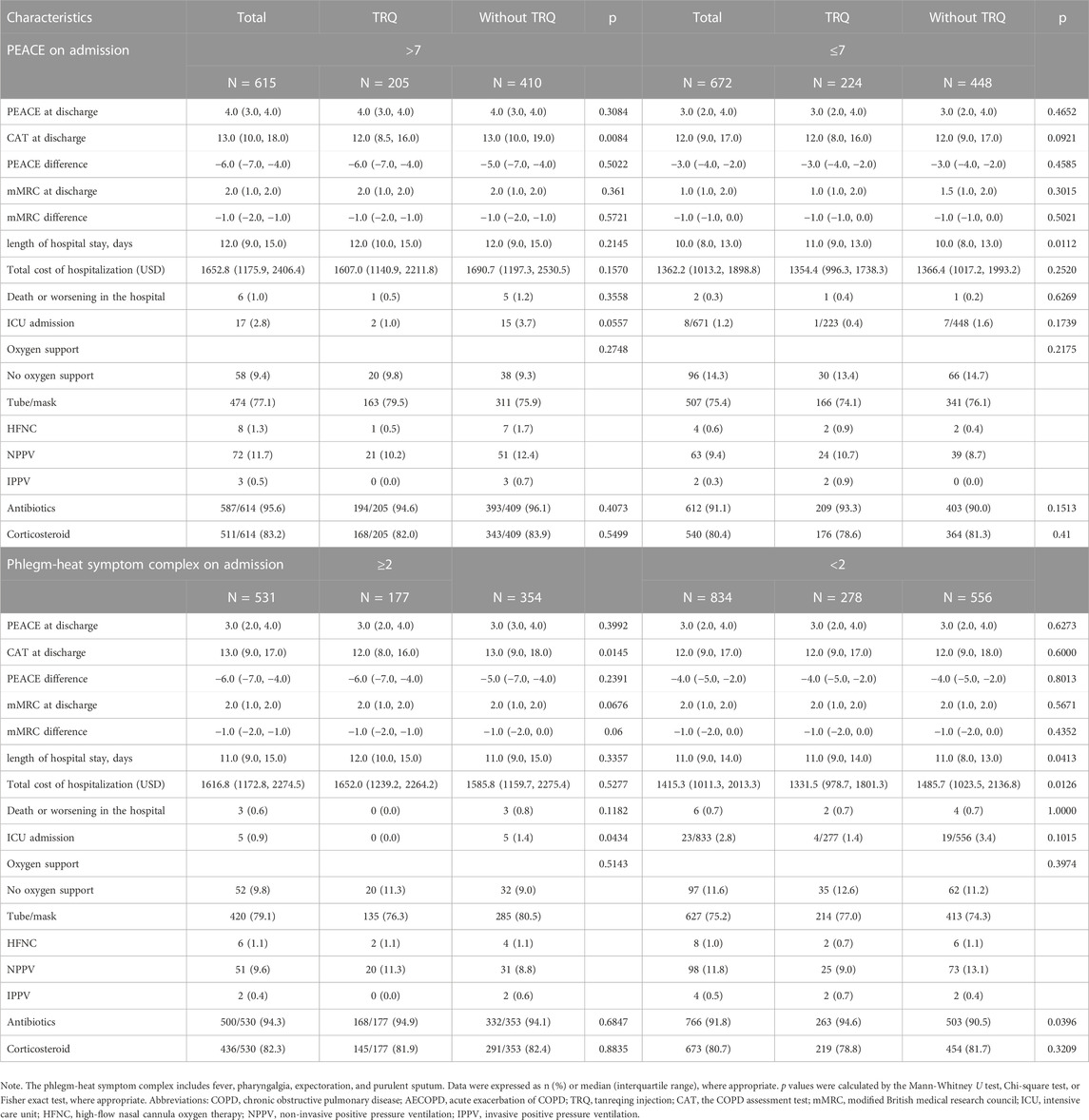
TABLE 4. Subgroup analyses on treatment and clinical outcomes of inpatients with frequent AECOPD after propensity match.
Discussion
This was a real-world, national wide, multi-center registry study investigating the efficacy of TQR injection in the treatment of AECOPD Patients. In this study, we found TRQ was effective in lowering CAT score at discharge and ICU admission rate, especially for those with phlegm-heat symptom complex on admission ≥2 and PEACE score >7 on admission among frequent AECOPD patients. For those with infrequent AECOPD, the efficacy is to be further explored. The results of our study provided robust support to the real-world evidence on the clinical use of TRQ and clues for the future investigation of mechanisms of TRQ.
In traditional Chinese medicine, COPD is placed in the same category as cough, dyspnea, and lung distention. Patients with AECOPD often have a series of symptoms of exacerbated cough, increased amounts of sputum, purulent sputum, and fever belonging to the Chinese medicine syndrome of phlegm-heat congestion of the lungs. For such a syndrome, the common treatment principle is clearing the heat and dissipating the phlegm (Li et al., 2010). TRQ consists of Scutellariae radix (SR, Scutellaria baicalensis Georgi), bear bile powder (BBP, Selenaretos thibetanus Cuvier), Cornu Caprae Hicus (CCH, Naemorhedus goral Hardwicke), Lonicerae japonicae flos (LJF, Lonicera japonica Thunb.), and Forsythiae fructus (FF, Forsythia suspensa (Thunb.) Vahl), and it is in accordance with the formulation of Tanreqing injection for treating syndromes including fever, cough, and expectoration (Han et al., 2022).
In our study, TRQ injection was effective in frequent AECOPD inpatients instead of infrequent patients. Frequent and infrequent AECOPD has been recently considered as different phenotypes: patients with frequent exacerbations may have increased airway inflammation in a stable state. More frequent exacerbations were associated with greater impairment in health status, a history of gastroesophageal reflux, and an elevated white-cell count (Hurst et al., 2010).
Modern pharmacological studies have found that Scutellaria baicalensis contained in TRQ injection has antioxidant, free radical scavenging, anti-infection, and antiviral effects; bear gall has anti-infective, sedative, and antispasmodic effects; goat horn has a strong antipyretic effect; honeysuckle contains chlorogenic acid and isochlorogenic acid, which has broad-spectrum antibacterial effect (Jiao et al., 2019). Moreover, laboratory studies showed that effective constituents of TRQ injection promoted the anti-inflammation progress in AECOPD patients. TRQ may improve lung function by inhibiting airway mucus hypersecretion and alleviating airway obstruction and inflammatory injury. Its action pathway may include inhibiting MAPK/NF-κB, which regulates IL-10 and TNF-α release, as well as regulating MUC5AC mRNA expression, which attenuates airway inflammation, airway damage, and mucus hypersecretion (Han et al., 2022). Several clinical studies have suggested that TRQ can regulate cytokines in AECOPD patients, reduce the activation and recruitment of neutrophils and other inflammatory cells in the respiratory tract, slow down the inflammatory process, and promote patient recovery (Yang et al., 2014; Yong et al., 2015). In our study, inpatients with frequent AECOPD were with significantly higher inflammation levels, compared to those with infrequent AECOPD. The drug reaction of TRQ may be influenced by inflammation status, but more studies are needed to verify this finding.
Patients with frequent AECOPD were known for risk factors such as a long time of COPD diagnosis, a larger amount of daily sputum production, higher mMRC score, lower predicted FEV1, and hospitalization during the previous year (Le Rouzic et al., 2018). Our study was consistent with previous findings and also found that inpatients with frequent AECOPD had a higher rate of death, ICU admission, and systemic corticosteroid use, compared with those with infrequent AECOPD. This indicated that among frequent AECOPD patients, antibiotic resistance may be common and inflammation status of the host has been changed for long-term, high-dose corticosteroids as well. As a classic traditional Chinese medicine, TRQ functions in the regulation of homeostasis and rebalance, thus contributing to the recovery of frequent AECOPD, especially for those with more than two phlegm-heat symptoms on admission.
Our study has some limitations. Firstly, we lacked data on biomarkers in our study, making it difficult to analyze the efficacy of TRQ combined with inflammation status. Secondly, the information on the use of antibiotics was missing. Patients with frequent or infrequent AECOPD may be administered with different grades of antibiotics and have different drug resistance statuses, which possibly impacts the course of treatment, length of hospital stay, and clinical outcomes as well. Thirdly, the follow-up data on rehospitalization were temporarily unavailable, therefore we were unable to evaluate the long-term efficacy of TRQ. However, our study revealed that the short-term efficacy was significant and provided important clues for future studies.
Conclusion
TRQ injection had better efficacy in patients with frequent AECOPD in reducing ICU admission and alleviating respiratory symptoms, especially for those with higher severity on admission or more phlegm-heat symptoms. TRQ may have the potential to improve the long-term prognosis of AECOPD, but more studies are needed to verify this.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the ethics committee of China-Japan Friendship Hospital (No. 2015-88). The patients/participants provided their written informed consent to participate in this study.
Author contributions
GF, DW, and SW had full access to all the data in this study, conceived the analysis, and took responsibility for the integrity and accuracy of the analysis. Concept and design: GF, DW, SW, FD, KH, YC, HZ, CW, and TY. Data collection and management: GF, DW, KH, FD, YC, HZ, CW, and TY. Statistical analysis: GF and DW. Drafting of the manuscript: GF and DW. All authors: Clinical interpretation of results, critical revision of the manuscript for intellectual content, agreement on the journal to which the manuscript should be submitted, approval of the final manuscript to be submitted, and agreement to be accountable for the contents of the manuscript.
Funding
National High Level Hospital Clinical Research Funding (2022-NHLHCRF-LX-01-01-01); CAMS Innovation Fund for Medical Sciences (2021-I2M-1-049); Respiratory Disease Clinical Research Public Welfare Program of China Song Qingling Foundation (2018MZFC-032). The funders of the study had no role in study design, data collection and management, data analysis, data interpretation, writing of the manuscript, and the decision to submit the manuscript for publication.
Acknowledgments
The authors thank all participants, staff, and investigators involved in the ACURE from all participating sites for their efforts in providing and collecting the data used in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1118143/full#supplementary-material
References
Chen, X., Kang, F., Lai, J., Deng, X., Guo, X., and Liu, S. (2022). Comparative effectiveness of phlegm-heat clearing Chinese medicine injections for AECOPD: A systematic review and network meta-analysis. J. Ethnopharmacol. 292, 115043. doi:10.1016/j.jep.2022.115043
GBD Chronic Respiratory Disease Collaborators (2020). Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet Respir. Med. 8 (6), 585–596.
Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease (2017). Global initiative for chronic obstructive lung disease (GOLD) committees. Available at: https://goldcopd.org/wp-content/uploads/2017/02/wms-GOLD-2017-FINAL.pdf (AccessedDecember1, 2018).
Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease (2022). Global initiative for chronic obstructive lung disease (GOLD) committees. Available at: https://goldcopd.org/wp-content/uploads/2021/12/GOLD-REPORT-2022-v1.1-22Nov2021_WMV.pdf (AccessedNovember3, 2022).
Han, X. X., Tian, Y. G., Liu, X. F., Zhao, D., Du, X. H., Dong, H. R., et al. (2022). Network pharmacology combined with pharmacodynamics revealed the anti-inflammatory mechanism of Tanreqing capsule against acute-exacerbation chronic obstructive pulmonary disease. Sci. Rep. 12 (1), 13967. doi:10.1038/s41598-022-18326-1
Hu, H., Ji, Z., Qiang, X., Liu, S., Sheng, X., Chen, Z., et al. (2021). Chinese medical injections for acute exacerbation of chronic obstructive pulmonary disease: A network meta-analysis. Int. J. Chron. Obstruct Pulmon Dis. 16, 3363–3386. doi:10.2147/COPD.S335579
Hurst, J. R. V. J., Anzueto, A., Locantore, N., Müllerova, H., Tal-Singer, R., Miller, B., et al. (2010). Susceptibility to exacerbation in chronic obstructive pulmonary disease. N. Engl. J. Med. 363 (12), 1128–1138. doi:10.1056/NEJMoa0909883
Jiao, W. U., Wang, C., Hai-Chuan, Y. U., Pharmacy, S. O., and XmjcjoETMF, U. (2019). Chemical constituents and pharmacological effect of Lonicerae japonicae flos.
Ko, F. W., Chan, K. P., Hui, D. S., Goddard, J. R., Shaw, J. G., Reid, D. W., et al. (2016). Acute exacerbation of COPD. Respirology 21 (7), 1152–1165. doi:10.1111/resp.12780
Le Rouzic, O., Roche, N., Cortot, A. B., Tillie-Leblond, I., Masure, F., Perez, T., et al. (2018). Defining the "frequent exacerbator" phenotype in COPD: A hypothesis-free approach. Chest 153 (5), 1106–1115. doi:10.1016/j.chest.2017.10.009
Li, W., Mao, B., Wang, G., Wang, L., Chang, J., Zhang, Y., et al. (2010). Effect of Tanreqing Injection on treatment of acute exacerbation of chronic obstructive pulmonary disease with Chinese medicine syndrome of retention of phlegm and heat in Fei. Chin. J. Integr. Med. 16 (2), 131–137. doi:10.1007/s11655-010-0131-y
Liang, C., Mao, X., Niu, H., Huang, K., Dong, F., Chen, Y., et al. (2021). Characteristics, management and in-hospital clinical outcomes among inpatients with acute exacerbation of chronic obstructive pulmonary disease in China: Results from the phase I data of ACURE study. Int. J. Chron. Obstruct Pulmon Dis. 16, 451–465. doi:10.2147/COPD.S281957
Mullerova, H., Shukla, A., Hawkins, A., and Quint, J. (2014). Risk factors for acute exacerbations of COPD in a primary care population: A retrospective observational cohort study. BMJ Open 4 (12), e006171. doi:10.1136/bmjopen-2014-006171
Ouaalaya, E. H., Falque, L., Dupis, J. M., Sabatini, M., Bernady, A., Nguyen, L., et al. (2020). Susceptibility to frequent exacerbation in COPD patients: Impact of the exacerbations history, vaccinations and comorbidities? Respir. Med. 169, 106018. doi:10.1016/j.rmed.2020.106018
Pei, Z., Sun, Y., Wang, S., Chen, Y., Yang, T., Huang, K., et al. (2020). Estimating mortality among inpatients with acute exacerbation of chronic obstructive pulmonary disease using registry data. NPJ Prim. Care Respir. Med. 30 (1), 28. doi:10.1038/s41533-020-0186-y
Rothnie, K. J., Mullerova, H., Smeeth, L., and Quint, J. K. (2018). Natural history of chronic obstructive pulmonary disease exacerbations in a general practice-based population with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 198 (4), 464–471. doi:10.1164/rccm.201710-2029OC
Seemungal, T. A. D. G., Bhowmik, A., Jeffries, D. J., and Wedzicha, J. A. (2000). Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 161 (5), 1608–1613. doi:10.1164/ajrccm.161.5.9908022
Wang, C., Xu, J., Yang, L., Xu, Y., Zhang, X., Bai, C., et al. (2018). Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China pulmonary health [CPH] study): A national cross-sectional study. Lancet 391 (10131), 1706–1717. doi:10.1016/S0140-6736(18)30841-9
Wang, L., Fan, Y., Xu, J., Deng, H., Geng, C., and Jia, B. (2020). The efficacy and safety of tanreqing injection combined with Western medicine for severe pneumonia: A protocol for systematic review and meta-analysis. Med. Baltim. 99 (35), e22010. doi:10.1097/MD.0000000000022010
Wedzicha, J. A. B. S., Allinson, J. P., and Donaldson, G. C. (2013). Mechanisms and impact of the frequent exacerbator phenotype in chronic obstructive pulmonary disease. BMC Med. 14 (11), 181. doi:10.1186/1741-7015-11-181
Wedzicha, J. A., and Terence, A. S. (2007). COPD exacerbations: Defining their cause and prevention. Lancet 370 (9589), 786–796. doi:10.1016/S0140-6736(07)61382-8
Yang, J., Chen, X. X., Huang, F., Sun, S. Y., and Stomatology, D. (2014). Effect of LPS on expression of TLR4 and the downstream cytokines in PDLCs.
Yong, D. U., Jie, Z. J., and HejcjoN, W. (2015). Study of influence of Tanreqing injection on serum high-sensitivity C-reactive protein and procalcitonin of patients with acute exacerbation of chronic obstructive pulmonary disease.
Keywords: Tanreqing injection, frequent AECOPD, comparative study, real-world, efficacy
Citation: Fan G, Wang D, Wu S, Li D, Ren X, Dong F, Huang K, Chen Y, Zhang H, Wang C and Yang T (2023) Better response to Tanreqing injection in frequent acute exacerbation of chronic obstructive pulmonary disease (AECOPD) patients—Real-world evidence from a nationwide registry (ACURE) study. Front. Pharmacol. 14:1118143. doi: 10.3389/fphar.2023.1118143
Received: 07 December 2022; Accepted: 20 February 2023;
Published: 16 March 2023.
Edited by:
Haiyang Tang, University of Arizona, United StatesReviewed by:
Yongchun Shen, Sichuan University, ChinaRaffaele Campisi, Azienda Ospedaliera Universitaria Policlinico G. Rodolico-San Marco, Italy
Copyright © 2023 Fan, Wang, Wu, Li, Ren, Dong, Huang, Chen, Zhang, Wang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ting Yang, ZHJ5YW5ndGluZ0BxcS5jb20=; Chen Wang, Y3loLWJpcm1AMjYzLm5ldA==
†These authors have contributed equally to this work and share first authorship
 Guohui Fan
Guohui Fan Dingyi Wang
Dingyi Wang Sinan Wu1†
Sinan Wu1† Xiaoxia Ren
Xiaoxia Ren Fen Dong
Fen Dong Kewu Huang
Kewu Huang Yahong Chen
Yahong Chen Chen Wang
Chen Wang Ting Yang
Ting Yang