- Institut des Sciences Cognitives Marc Jeannerod, UMR5229 CNRS Université de Lyon, Bron, France
Selective serotonin reuptake inhibitors (SSRIs) increase serotonin activity in the brain. While they are mostly known for their antidepressant properties, they have been shown to improve visual functions in amblyopia and impact cognitive functions ranging from attention to motivation and sensitivity to reward. Yet, a clear understanding of the specific action of serotonin to each of bottom-up sensory and top-down cognitive control components and their interaction is still missing. To address this question, we characterize, in two adult male macaques, the behavioral effects of fluoxetine, a specific SSRI, on visual perception under varying bottom-up (luminosity, distractors) and top-down (uncertainty, reward biases) constraints while they are performing three different visual tasks. We first manipulate target luminosity in a visual detection task, and we show that fluoxetine degrades luminance perceptual thresholds. We then use a target detection task in the presence of spatial distractors, and we show that under fluoxetine, monkeys display both more liberal responses as well as a degraded perceptual spatial resolution. In a last target selection task, involving free choice in the presence of reward biases, we show that monkeys display an increased sensitivity to reward outcome under fluoxetine. In addition, we report that monkeys produce, under fluoxetine, more trials and less aborts, increased pupil size, shorter blink durations, as well as task-dependent changes in reaction times. Overall, while low level vision appears to be degraded by fluoxetine, performances in the visual tasks are maintained under fluoxetine due to enhanced top-down control based on task outcome and reward maximization.
Introduction
While selective serotonin reuptake inhibitors (SSRIs) are mostly known for their antidepressant properties (Bauer et al., 2008), serotonin concentration in the brain impacts multiple sensory and cognitive functions ranging from retinal (for review, see Masson, 2019; Pootanakit and Brunken, 2000) and visual functions (Lansner et al., 2019), to higher order cognitive functions such as sustained attention (Carter et al., 2005; Scholes et al., 2007; Wingen et al., 2008; Enge et al., 2011; Li et al., 2018), impulsivity (Brown et al., 2012; Worbe et al., 2014; Meyniel et al., 2016), working memory and learning (Meneses and Liy-Salmeron, 2012) and emotional and affective processing (Harmer et al., 2006; Bar-Haim et al., 2007; Harmer, 2008; Pérez-Edgar et al., 2010). Serotonin has also been proposed to play a crucial role in remodeling visual cortical circuits (Maya-Vetencourt et al., 2008; 2011; Umemori et al., 2018) as well as in cognitive flexibility (Clarke et al., 2004). In this context, the specific contribution of serotonin to low-level visual luminosity perception on the one hand and to the top-down control mechanisms of visual perception on the other hand, such as attentional selection and distractor suppression (Di Bello et al., 2022) or reward-based decision making (Homberg, 2012; Seymour et al., 2012) is still unknown.
Serotonin brain concentrations can be modulated by increasing the circulating levels of tryptophan, its precursor, by the action of selective serotonin agonists, or yet by the action of SSRIs. These bind selectively to serotonin transporters and inhibit their ability to reuptake serotonin into presynaptic terminals, resulting in an increase in the levels of extracellular serotonin (Wong et al., 1995; Clark et al., 1996). Thus, increased release in serotonin enhances the neurotransmitter likelihood to bind to a post-synaptic receptor. In addition, the decrease of serotonin reuptake also inhibits the negative feedback regulation, resulting in increased serotonin release in the synaptic cleft (Cerrito and Raiteri, 1979). A particular SSRI, fluoxetine (Prozac), expresses a strong binding to receptors 5-HT2c (Pälvimäki et al., 1996; Ni and Miledi, 1997) and 5-HT2a (Koch, 2002), the latter having the highest concentration in the visual system (Beliveau et al., 2017; Hansen et al., 2022), as well as in the pre-frontal cortex (Puig and Gulledge, 2011). In particular, serotonin modulates the neuronal activity in the visual system, in a dose- and species dependent-manner, such that increase or depletion of 5-HT regulates the switch between single-spike activity and rhythmic burst firing specifically in brain regions involved in visual processing, such as the retina, the visual cortex, and the thalamus (McCormick and Wang, 1991; Brunken et al., 1993; Monckton and McCormick, 2002; Moreau et al., 2013). In addition, fluoxetine decreases extracellular GABA levels (Santana et al., 2004; Vetencourt et al., 2008; Baroncelli et al., 2011; Beshara et al., 2016) thus leading to enhanced cortical excitability through a reduction of global inhibition. Relevant to the study of the effects of serotonin on top-down and bottom-up visual processes, GABAa receptor concentrations are higher in the visual cortex than in the rest of the brain and higher in the ventral part of the striate and extrastriate cortex than in its dorsal part (Kaulen et al., 2022). All this taken together indicates complex interactions between serotonin circulating levels and behavioral and cognitive markers of visual perception.
In the present work, we precisely characterize the effects of fluoxetine on visual perception under varying bottom-up (luminosity, distractors) and top-down (uncertainty, reward biases) constraints, while two monkeys perform three different visual tasks. The first task is a visual detection task in the presence of target stimuli of varying luminosity and mostly involves bottom-up visual processes. This task thus allows to characterize the effects of fluoxetine on luminosity perceptual thresholds. The second task is a visual detection task in the presence of spatial distractors and involves a combination of bottom-up visual processes and top-down target selection and reactive distractor suppression mechanisms (Di Bello et al., 2022). This task thus allows to characterize the effects of fluoxetine on perception under spatial uncertainty. The third task is a free choice task in the presence of reward biases (Chelazzi et al., 2014). This task thus allows to characterize the effects of fluoxetine on reward-based decision making. Overall, we report longer time on this reward-biased free choice task, increased pupil size and shorter blink durations under fluoxetine, increased luminance perceptual thresholds, such that higher levels of luminosity are needed to reach a 50% correct detection, more liberal decision thresholds thus producing more responses to both targets and distractors, a degraded perceptual spatial resolution under spatial uncertainty, and an enhanced sensitivity to both the positive incentive of high rewards as well as to the negative outcome of low rewards. We finally show that fluoxetine can either speed up or slow down manual reaction times, depending on the nature of the task. Overall, we show that the effects of fluoxetine on perception result from interference with both bottom-up perceptual mechanisms, namely, degraded luminosity thresholds or degraded spatial resolution and top-down perceptual mechanisms, namely, relaxed decision thresholds and increased sensitivity to reward outcomes. In other words, while low level vision appears to be degraded by fluoxetine, performance in the visual tasks may be maintained under fluoxetine due to enhanced top-down control based on task outcome and reward maximization.
Material and methods
Animals and ethical approval
Two healthy adult male rhesus macaques (macaca mulatta) took part in the study (M1: 11 kgs, 12 years; M2: 8.5 kgs, 13 years). The project was authorized by the French Ministry for Higher Education and Research (# 2016120910476056 and #1588–2015090114042892) in accordance with the French transposition texts of Directive 2010/63/UE. This authorization was based on an ethical evaluation by the French Committee on the Ethics of Experiments in Animals (C2EA) CELYNE registered at the national level as C2EA number 42.
Surgery
The animals were implanted with a peek MRI-compatible headset covered by dental acrylic. The anesthesia for the surgery was induced by Zoletil (Tiletamine-Zolazepam, Virbac, 5 mg/kg) and maintained by isoflurane (Belamont, 1%–2%). Post-surgery analgesia was ensured thanks to Temgesic (buprenorphine, 0.3 mg/mL, 0.01 mg/kg). During recovery, proper analgesic and antibiotic coverage was provided. The surgical procedures conformed to European and National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Fluoxetine preparation
Fluoxetine hydrochloryde is a SSRI which binds to the human 5-HT transporter with a Ki of 0.9 nmol/L and is between 150- and 900-fold selective over 5-HT1A, 5-HT2A, 5-HT2c, H1, α1, α2-adrenergic, and muscarinic receptors (Ambati et al., 2021). The fluoxetine (N-Methyl-3-[(4-trifluoromethyl) phenoxy]-3-phenylpropylamine hydrochloride) used in the present study has a molecular weight of 345,78 g/mol. Powder galenic form (BioTechne©, ToCris BioScience) was diluted in a saline vehicle (NaCl) as follows. In order to inject the smallest possible volume to the monkeys, we dissolved fluoxetine in a saline solution at a concentration of 8 mg/mL, vortexed 10 s and heated the suspension at 60°C in bain-marie to increase solubility while not degrading the active compound. This preparation was frozen at −20°C immediately so as to avoid the molecule degradation and heated back to body temperature when necessary.
Fluoxetine administration
In order to reduce the stress potentially induced by the injection, monkeys were progressively trained to spontaneously receive subcutaneous saline injections with clicker training. In contrast with intramuscular injections, subcutaneous injections allow a slow distribution of injected product, thus a longer half-life in the body. Injection site and side of injection was changed daily. Injection sites were carefully monitored and sanitized. Once animals reached stable performance and were habituated to subcutaneous injections, behavioral data collection started under either placebo (saline) or fluoxetine injections (2.5 mg/kg/day). This fluoxetine concentration was chosen based on its specific serum concentration decay time in macaques (half-life <16 h, Sawyer and Howell, 2011) and the reported threshold for behavioral effects (Fontenot et al., 2009; Chen et al., 2012). Two different injection schedules were used. Acute schedule involving, over a full working week, 1 day of saline injection, followed by 1 day of fluoxetine, followed by 3 days of saline injections (Free choice task). Chronical injections involved daily fluoxetine injections during one full month (Luminance perceptual task and target detection task under spatial uncertainty). The effect of this latter schedule was compared to that of 1 month of saline injections. Monkeys were always injected in the morning, at the same time, and behavioral data was collected 4–6 h later based on the pharmacokinetics of fluoxetine (Sawyer and Howell, 2011). Table 1 describes the number of placebo and fluoxetine sessions collected for each task, under each injection schedule, as well as general trial statistics.
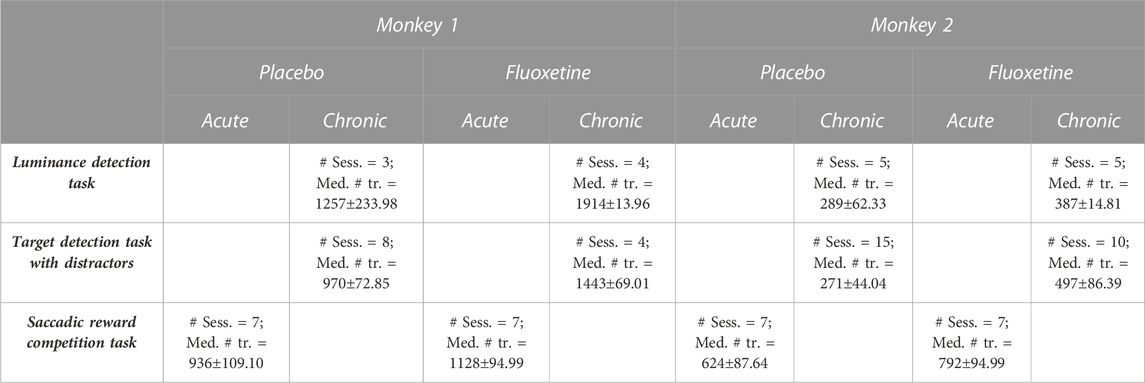
TABLE 1. Description of number of sessions and trial statistics. Number of sessions and trial statistics (median +se) are described per type of tasks (luminance detection task, target detection task with distractors and saccadic reward competition task), condition (placebo and fluoxetine) and monkeys. Injections for the saccadic reward competition task, were performed acutely (1 day per week). For the other tasks, injections were performed according to a chronic injection schedule.
Experimental setup
Monkeys sat in a primate chair in sphinx position head-fixated thanks to a surgically implanted head post. They were positioned in front of a screen. The eye to screen distance was of 60 cm and screen resolution was 1200 × 1900pixels with a 60 Hz refresh rate. Gaze location was sampled at 120 Hz using an infrared video-eye tracking system (ISCAN). Eye movement data acquisition software interfaced with an inhouse program for stimulus delivery and experimental control (Presentation©). Monkey hand responses were produced by releasing a bar, the effect of which was to restore the continuity of an infra-red optic beam.
Behavioral tasks
Animals had free access to food and were maintained under a water regulation schedule individually optimized to keep a stable motivation and performance. They were trained on three different behavioral tasks. In all of these tasks, monkeys had to fixate a central fixation point on a screen for a variable duration (1-to-2 s) while stimuli (size: 0.5°; duration: 100 ms) were presented at an eccentricity of 8°.
Luminance detection task
This task aims at assessing changes in luminance perception thresholds under fluoxetine as compared to saline placebo injections. Monkeys had to fixate a central cross. One thousand to 2000 ms from fixation onset, a 200 ms target appeared randomly at one of the four possible following positions: (8.5, 8.5), (−8.5, 8.5), (−8.5, −8.5) or (8.5, −8.5). This task was designed to be dominated by bottom-up perceptual processes as we did not use any spatial cue to indicate to the monkeys the position of the upcoming target (Reynolds et al., 2000; Carrasco et al., 2004; Reynolds and Chelazzi, 2004; Ibos et al., 2009). The luminance of the target varied from the background luminance, from easy to hard, on a scale of seven equidistant luminance values (Figure 1). Each target position was sampled for each luminance 10 times per session. Monkeys were rewarded for producing a hand response to target presentation, within a response time window of [150 ms–1000 ms]. Misses or false alarms are not rewarded. Note that by construction the task does not produce correct rejections. This task was tested twice, in two sets of recording sessions spaced by 10 months and a wash out period of at least two consecutive months in between (first data collection: M1: 7 sessions, M2: 10 sessions; second data collection: M1: 3 sessions, M2: 4 sessions (Table 1). In each block of trials, stimuli presentations were pseudo-randomized such that for each block of trials and each target position, each target luminosity was presented at least 10 times. If trials were aborted by the monkeys prior to target presentation, the trial was presented again. A given target luminance could not appear more than 3 times in a row. Monkeys performed a minimum of 3 such blocks of trials per sessions. Individual psychometric luminance perception curves are constructed for each of the four target positions independently for the placebo and the fluoxetine conditions. In the results, we discuss the data collected during the first data collection sessions. The data from the second data collection sessions are presented in Supplementary Material (Supplementary Figure S1). They are not significantly different from those reported for the first sessions, indicating a stable and reproducible effect in time.
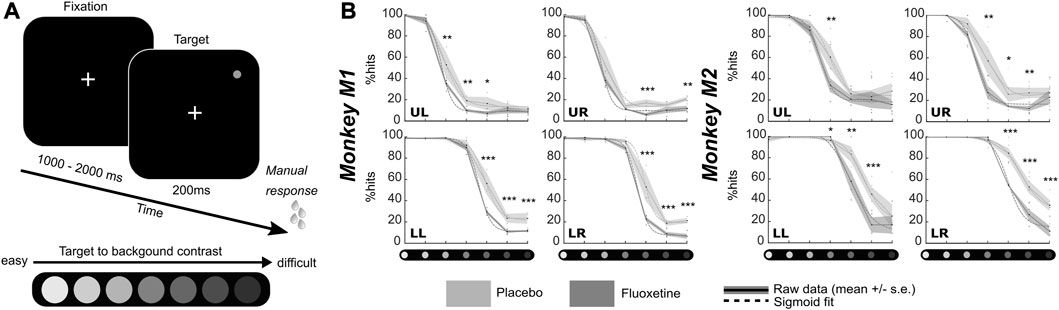
FIGURE 1. Effect of fluoxetine on perceptual thresholds in a luminance detection task. (A) Monkeys had to detect a target presented in one of four quadrants. Target luminosity ranged from high to low luminosity in 7 steps. Monkeys were rewarded for a speeded detection of target presentation. Targets were presented at four different locations, in the upper left (UL), upper right (UR), lower right (LR) and lower left (LL) quadrants, at 8° of eccentricity from the center of the screen, for 200 ms. (B) For both monkeys, % of hits were computed independently for each target luminosity. Dots represent individual sessions, continuous lines represent average % hits across all sessions (+/-median absolute error) and dashed lines represent sigmoid fit of the data. Placebo data are represented in light gray and fluoxetine data are represented in dark gray. Behavioral data are represented independently for each target position. Statistical significance is represented as follows: ***, p < 0.001; **, p < 0.01; *, p < 0.05; n. s., p > 0.05. See also companion Supplementary Table S2 for detailed statistics.
Target detection task in the presence of distractors
The previous task allows to identify possible changes in individual perception thresholds. However, changes in such metrics can be due to bottom-up changes in perceptual sensitivity (or dprime) or to changes in individual subject response criterion. In order to refine our understanding of the effect of fluoxetine on perception and decision-making, we used a peripheral target detection task in the presence of spatial distractors (Figure 2). Monkeys had to fixate a central cross. One thousand to 2000 ms from fixation onset, a 200 ms target appeared randomly at one of the four possible following positions: (8.5, 8.5), (−8.5, 8.5), (−8.5, −8.5) or (8.5, −8.5). Target luminance was defined as target luminance associated with a 70% correct detection threshold in the placebo sessions of the luminance task. Prior to actual target presentation, a 200 ms spatial distractor could randomly appear within of virtual circle of 2° around the expected target location (as learned from the previous task). Distractors were identical to the target and only differed in their position. Distractors were present in 3:4 of the trials and appeared [200–500 ms] prior the target presentation. Monkeys were rewarded for producing a hand response to target presentation, within a response time window of [150 ms–1000 ms]. This response consisted in the release of a lever, thus restoring an infra-red beam. It was thus spatially non-oriented to the target. Data on this task were collected from 8 (M1) and 15 (M2) placebo sessions and 4 (M1) and 10 (M2) fluoxetine sessions (Table 1). Outliers (response duration <150 ms) were removed from the datasets to differentiate responses to distractors from anticipated responses due to the distractor presentation.
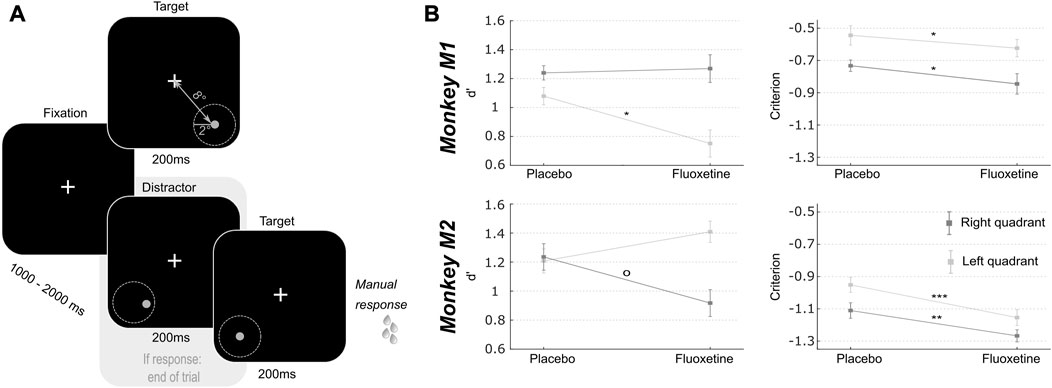
FIGURE 2. Effect of fluoxetine on spatial sensitivity d-prime and response criterion in a target detection task in the presence of spatial distractors. (A) Monkeys had to detect a target presented in one of two quadrants (lower left or lower right). Target luminosity was kept high and presented at a fixed location, at 8° of eccentricity from the center of the screen, for 200 ms. Monkeys were rewarded for a speeded detection of target presentation. On 75% of the trials, targets were preceded by a distractor, undistinguishable from the target except for its spatial location. These distractors were located within a circle of 2° of eccentricity around the target. Responses to these distractors interrupted the trial and monkeys were not rewarded. (B) For both monkeys, d’ and criterion were computed independently for each target (left, light gray; right, dark gray). Median+/-median absolute error are presented for placebo and fluoxetine conditions. Statistical significance is represented as follows: ***, p < 0.001; **, p < 0.01; *, p < 0.05; °, p < 0.07. See also companion Supplementary Table S3 for detailed statistics.
Saccadic reward competition task
In order to investigate the possible contribution of fluoxetine to the learning of reward biases, we used a saccadic competitive task, towards stimuli the spatial position of which was associated, with a specific reward probability schedule (Figure 3). The specific spatial reward contingencies changed from one session to the next. Monkeys had to fixate a central cross. One thousand to 2000 ms from fixation onset, two identical stimuli were presented. Stimuli were drawn from a virtual array of eight stimuli organized along a circle of 8° of eccentricity. From one session to the other, each location in this virtual array was associated with a different reward probability (stable across trials of the same session), which the monkeys discovered at the beginning of the session, thus building a reward based spatial priority map (Chelazzi et al., 2014; Della Libera et al., 2017), then exploited during the rest of the session. Possible high reward probabilities were 80%, 50% and 20%, according to a fixed spatial relationship, such that the extreme reward probabilities (80% and 20%) were neighbored intermediate reward probability targets (50%) (Figure 3). Monkeys had to make a saccade to one of the two presented stimuli and were rewarded according to the reward probability associated with the chosen target location. The spatial reward contingency map was rotated from 1 day to the next, leading to seven different spatial reward contingency maps, played several times over independent sessions (as the initial spatial contingency map on which initial training was performed was not used). For this experiment, we used a 3-week chronic saline injection schedule followed by and 8-week chronic fluoxetine injection schedule.
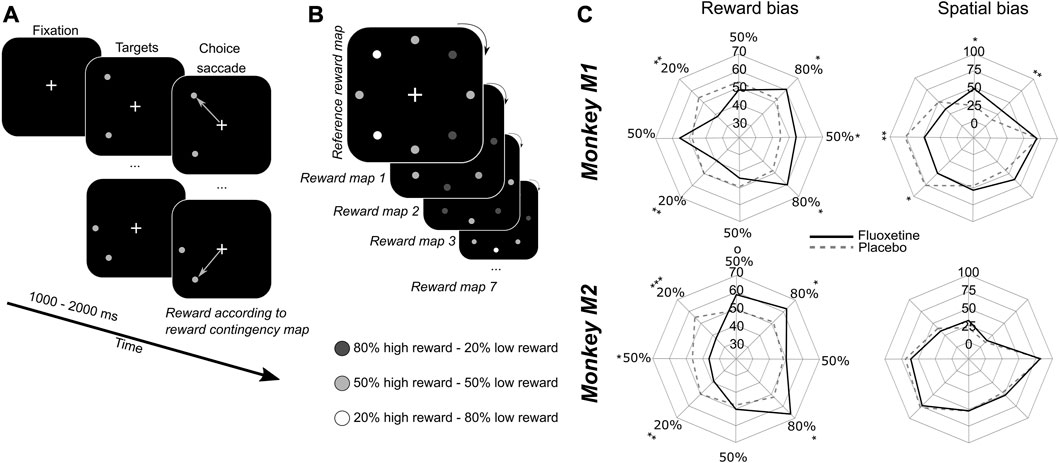
FIGURE 3. Effect of fluoxetine on saccadic choices towards targets of different reward contingencies. (A) Monkeys had to fixate a central cross on a screen 60 cm away from their eyes. After an interval of 1–2 s, two stimuli appeared simultaneously at two different locations out of eight. All of the 8 possible target locations were organized along a virtual circle of 8° of eccentricity from the fixation cross, equidistant one from the other. Monkeys were rewarded to make a saccadic eye movement to any of the two targets. (B) Each target was associated with two possible reward quantities, but with a different probability. High expected reward targets were associated with 80% of high reward probability and 20% of low reward probability. Low expected reward targets were associated with 20% of high reward probability and 80% of low reward probability. Intermediate expected reward targets were associated with 50% of high reward probability and 50% of low reward probability. Reward contingencies between neighbors were kept constant as follows: 80% high reward (HR)—50% HR—80% HR—50% HR—20% HR—50% HR—20% HR—50% HR. However, the actual location of high and low rewarding targets changed pseudo-randomly from 1 day to the next. Thus, monkeys had to learn the new reward contingencies every day. We did not evaluate how much monkeys built a representation of the reference contingency map. (C) Polar plots represent the probability that monkeys choose any given target either as a function of the reward contingency map (i.e., irrespective of actual spatial position, left) or as a function of the spatial map (i.e., irrespective of actual reward contingency maps, right). Median are presented for placebo (dashed lines) and fluoxetine (continuous lines) conditions. Statistical significance is represented as follows: ***, p < 0.001; **, p < 0.01; *, p < 0.05. See also companion Supplementary Tables S4, S5 for detailed statistics.
Data analysis
All analyses are implemented in Matlab® using ad-hoc scripts.
Extracted behavioral and physiological measures
For each task and each session, we quantified session length (overall number of trials) and overall behavioral performance (percentage of correct trials relative to the sum of correct and miss trials). For the luminance detection task, we computed the behavioral performance independently for each target contrast level to right and left targets. We then fit a sigmoid model to the data. Using a sigmoid function Sigm_fit (https://www.mathworks.com/matlabcentral/fileexchange/42641-sigm_fit), we determined the p50 (target luminance associated with a 50% chance performance), the slope (sensitivity to contrast changes) and the baseline (response level to noise) for each mean number of trials per session (see Table 1), in both placebo and fluoxetine condition. On the two target detection tasks, manual reaction times (RT) were extracted, defined as the time between target presentation and hand lever release. Pupil size variation (McGuirk and Silverstone, 1990; Phillips et al., 2000; Dumont et al., 2005) and blinks were identified (Wilson et al., 1983; Semlitsch et al., 1993) and we quantified, for each session, the distribution of blink duration and pupil size variations. We quantified durations of all blinks while monkeys were engaged in tasks in both placebo and fluoxetine conditions and determined the median value. As for measuring pupil dilation, we estimated pupil size during repeated measurements of periods of 3 s of rest before each task initiation while monkeys were sat in the dark, and during periods of 3 s during each task execution, sampled after target presentation. Prior to these analyses, data were pre-processed to remove blinks and artefacts. Percentage pupil size variation in placebo and fluoxetine conditions were computed by estimating the average pupil size on these two epochs and normalizing it by average pupil size over the entire task duration.
RT analyses
All RTs above 1000 ms were excluded from the analysis. RT distributions were then analyzed using the Later model (Noorani and Carpenter, 2016). This model distributes data according to their frequency of distribution. The LATER model allows to segregate RTs in two categories: controlled and anticipated/express responses. We thus segregated, for each task, RT as a function of target position, and we used the LATER model analysis in order to identify the cutoff between anticipatory and controlled responses (Table 2).
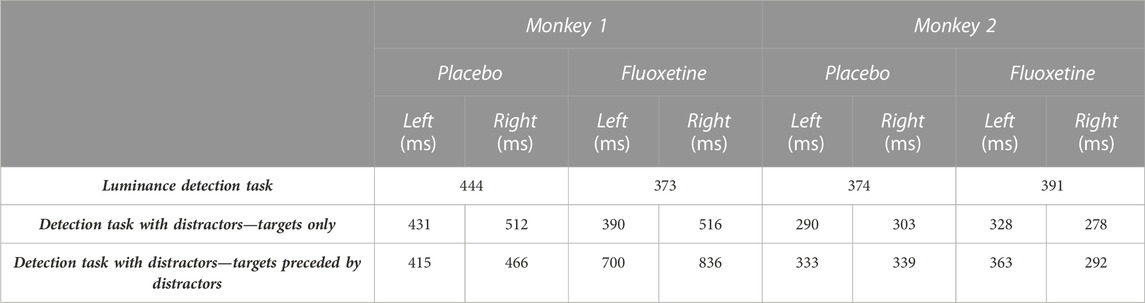
TABLE 2. RT threshold between anticipatory and controlled responses as defined by the LATER model. This threshold was defined independently for each monkey, each task and each target position, except for the luminance detection task, for which all four positions were considered together.
Signal detection theory
In the target detection task in the presence of distractors, we used signal-detection-theory and computed the monkey’s sensitivity to the location of the target relative to the distractors randomly presented around the target location (d’, reflecting bottom-up sensory features) as well as their response criterion (reflecting top-down control in the decision-making process). These metrics were independently computed per session (see Table 1).
Spatial (SSI) and reward (RSI) selectivity index
In the saccadic reward competition task, we calculated, for each session (Table 1), the choice performance of a given singleton for each possible pair of stimuli. We then calculated, for each session, and each spatial position the SSI as follows. For each position i, we computed the median choice percentage SSIi that a singleton at position i was chosen, irrespective of the second singleton in the pair and irrespective of their associated rewards. In other words, it is the median, over all pairs containing singleton i, of the percentage of times i was actually chosen. SSIi thus reflects the average preference of the monkey for singleton i.
Likewise, we computed the RSI as follows. For each reward contingency i, we computed the median choice percentage RSIi that a singleton with that specific reward contingency i was chosen, irrespective of the reward associated with the second singleton in the pair, and irrespective of their spatial position. RSIi thus reflects the average preference of the monkey for a given reward contingency i.
Statistical analyses
All statistical analyses are non-parametric Wilcoxon or Kruskall-Wallis tests, except when two-way ANOVAs are required. p-value < 0.05 were considered as statistically significant. All statistical analyses are implemented in Matlab® using ad-hoc scripts.
Results
Under fluoxetine, monkeys work longer and produce less aborts
Based on serum concentration decay time in macaques (half-life <16 h, Sawyer and Howell, 2011) and to the reported threshold for behavioral effects (Fontenot et al., 2009; Chen et al., 2012), monkeys were injected with 2.5 mg/kg of Fluoxetine or an equivalent volume of saline. On each session, subjects were allowed to work for as long as they were motivated to. Monkeys were considered as less motivated and were brought back to their home cage when their compliance to the central fixation constrain in the task decreased beyond a certain threshold (85% overall fixation in a block of 280 trials for both the luminance detection task and the target detection task with distractors and 75% for the saccadic reward competition task).
For all three tasks, a significant increase in the number of trials as well as a significant decrease in abort trials (i.e., trials discontinued prior to the onset of task response signal) is observed when monkeys are on fluoxetine compared to placebo sessions (Figure 4). Specifically, during the luminance detection task, both monkeys M1 and M2 performed more trials per session in fluoxetine sessions as compared to placebo sessions and less aborted trials (Supplementary Table S1). This was also true for the detection task with distractors (Supplementary Table S1). However, we did not observe this effect for the saccadic reward competition task (Supplementary Table S1). Overall, fluoxetine thus enhances both the motivation of the monkeys to work (more trials) as well as their compliance on the task (less aborts). Please note that, due to the nature of the tasks, performance defined as the percentage of correct trials cannot be computed.
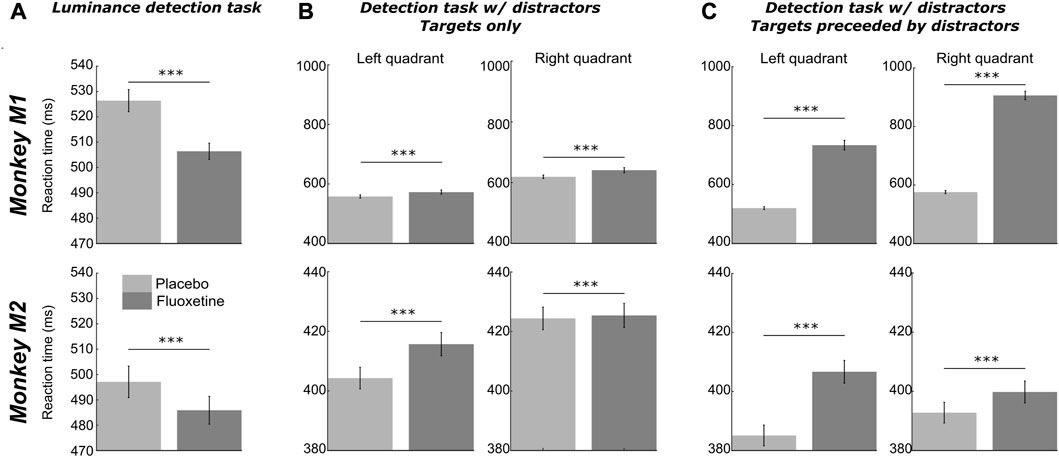
FIGURE 4. Effect of fluoxetine on controlled reaction times. Median+/-median absolute error of reaction times, for monkeys M1 (top) and M2 (bottom), in the placebo and fluoxetine conditions in the luminance task (A), all positions collapsed, high luminance targets only), in the detection tasks with distractors, on target only trials (B), and in trials with distractors (C). Statistical significance is represented as follows: ***, p < 0.001; **, p < 0.01; *, p < 0.05. See also companion Supplementary Table S7 for detailed statistics.
Under fluoxetine, perceptual thresholds are increased
In order to assess the effect of fluoxetine on perceptual thresholds, we had monkeys detect targets of varying luminosities ranging for very high luminance to very low, in seven steps (Figure 1). Targets could appear in one of four locations on the screen (upper left, upper right, lower left, lower right). It is to be noted that this experiment was conducted twice, at a 10 months’ interval and all observations reported below are reproduced (Supplementary Figure S1).
Both monkeys had a hit rate of 100% in both the placebo and fluoxetine conditions for the high luminosity targets, indicating that they were well trained and highly motivated in this task. Behavioral performance (% Hits, i.e., correct responses) were extracted as a function of target location and target luminosity for each monkey and each session and fitted with a sigmoid fit (Figure 1B, Supplementary Figure S1).
Two different effects of fluoxetine can be described. First, fluoxetine increases perceptual thresholds such that lower luminosity targets are less perceived under fluoxetine relative to placebo condition. This is quantified by a shift in the
Second, under fluoxetine, monkeys had lower hit rates than on placebo condition, producing significantly less hits for lower luminosity targets (Figure 1B). A two-way ANOVA on target position x condition indicates a significant effect of condition and quadrant with no interaction for both monkeys (M1: target position, F (1,83) = 78.365, p < 0.001; condition, F (3,249) = 77.638, p < 0.001; interaction, F (3,249) = 1.639, p = 0.181; M2: target position, F (1,76) = 40.803, p < 0.001; condition, F (3,228) = 41.768, p < 0.001; interaction, F (3,228) = 1.702, p = 0.167). Post-hoc Wilcoxon tests (Benjamini–Hochberg corrected) indicate that this holds significant in M1 for three quadrants out of four and in M2, for one quadrant out of four (Figure 1B). This indicates that their perception for low luminosity target is strongly degraded under fluoxetine. Alternatively, this possibly indicates that monkeys become more conservative under fluoxetine, i.e., they select more carefully their responses to minimize errors. These hypotheses are evaluated in the next section, using a second behavioral task.
Under spatial uncertainty, fluoxetine relaxes perceptual decision thresholds and degrades perceived spatial resolution.
In order to further investigate the effects of fluoxetine on perceptual sensitivity and response decision thresholds described in the previous experiment, we had monkeys perform luminosity target detection task in the presence of spatial distractors (Figure 2A). In this task, targets and distractors were only distinguishable by their spatial position, and were set at a perceptual threshold of 70%, as characterized in the luminance detection task (Figure 1). Targets and distractors could either be presented in the lower left or in the lower right quadrants. As for the previous task, this experiment was conducted twice, at a 10 months’ interval and all observations reported below are reproduced. In this task, the target, although supra-threshold, is perceived on circa 70% of the trials. This is thus a target detection task. Comparing the target only trials with and without fluoxetine allows to characterize the effects of fluoxetine on target detection. In addition, this task involves a spatial discrimination component, as what differentiates the target from the distractors is their spatial location. Comparing the target only trials to the target with distractor trials with and without fluoxetine allows to characterize its effects on this spatial discrimination component. Last, this task involves a response inhibition component. Indeed, ¾ of the trials included distractors that the monkeys had to refrain respond to. We have previously shown that the proportion of non-target trials in a task adjusts the level of response inhibition (Wardak et al., 2012). Unfortunately, we are missing a reference condition to reproduce the previously described effects of fluoxetine on impulsivity (Brown et al., 2012; Worbe et al., 2014; Meyniel et al., 2016).
Based on the monkeys’ response in this task (Hits: correct target detections; Misses: no response to target presentation; False alarms: erroneous responses to distractors and Correct rejections: correct no response to distractors), we calculated the criterion (reflecting the willingness to respond that the signal is present in an ambiguous situation, independently of the subject’s sensitivity to the signal) and d-prime (reflects the actual sensitivity of the subject to the signal) over all sessions in both the fluoxetine and placebo conditions. A high criterion corresponds to a conservative behavior (i.e., less responses but mostly correct) while a low criterion corresponds to a liberal behavior (i.e., more responses but more false alarms). A high d’ indicates the signal is easily detected in the face of noise while a low d’ reflects a difficulty to detect the signal. Because reaction times differed between left and right targets, these trials were considered independently. This spatial uncertainty task was very difficult; thus, overall criteria were negative. Yet, for both monkeys and both hemifields, we observe a significant decrease in criterion after fluoxetine administration compared to the placebo condition (detailed statistics are described in Supplementary Table S3). Under fluoxetine, both subjects lowered their response decision thresholds, so that they allowed themselves more mistakes. This effect was present irrespective of whether distractors were closest to the target location, closest to the center of the visual field or further away in the periphery (Supplementary Figures S2A, B, Two-way ANOVA condition x distractor location, M1: condition, F (1,91) = 4.266, p = 0.041; location, F (2,182) = 12.772, p < 0.001; interaction, F (2,182) = 0.139, p = 0.870; M2: condition, F (1,62) = 19.484, p < 0.001; location, F (2,124) = 11.389, p < 0.001; interaction, F (2,124) = 0.829, p = 0.439, this analysis cumulates both test and retest tasks to increase samples per distractor location categories). In this task, changes in d-prime (assessing sensitivity to spatial location) were inconsistent across subjects and across hemifields (detailed statistics are described in Supplementary Table S3). Because fluoxetine is expected to change excitatory/inhibitory balance in favor of inhibition through its effect on GABAergic circuitry and thus change the coding spatial resolution in the visual cortex (Robinson et al., 2003), we reasoned that changes in spatial d-primes might depend on the actual distance of the distractors to the target. For both monkeys, fluoxetine resulted in significantly decreased d-primes for close distractors but not for intermediate and far distractors (Supplementary Figure S2B, intermediate distractors, M1: Wilcoxon non-parametric test, p = 0.126; M2, p = 0.470; close distractors, M1: p = 0.041; M2, p = 0.043; far distractors, M1: p = 0.155; M2: p = 0.481). Post-hoc analyses however indicate that this effect was driven, in both monkeys, by a right quadrant effect. This suggests a degraded spatial resolution in the visual cortex, compatible with a related excitatory/inhibitory balance under fluoxetine.
Fluoxetine results in increased sensitivity to reward during free choice
Decision-making in non-human primates is most often guided by reward expectation. Recent fMRI observations suggest that spatial biases induced by reward incentives are subtended by a cortical network that is functionally distinct from spatial biases induced by spatial attention (Zubair et al., 2021). Additionally, in ecological conditions, foraging often takes place in a changing environment, where the actual location of rewards change dynamically with time and the actions taken in this environment. Continuously updating expected reward locations is thus crucial. We extensively trained the two macaques included in the present study on a saccadic reward competition in a stable environment (Figure 3A). On every trial, the monkeys had to make a saccade to one of two possible targets. Each successful saccade was rewarded, but the delivered reward depended on which target was selected on this specific trial. Some targets were associated with an 80% probability of high reward and a 20% probability of low reward (High expected reward). Some targets were associated with the opposite reward contingencies: 20% probability of high reward and 80% probability of low reward (Low expected rewards). Some others yet were associated with 50% probability of high or low reward (Intermediate expected reward). Reward contingencies were fixed from one trial to the next, and were spatially organized such that each High or Low expected reward target was neighbored by Intermediate expected reward targets. Prior to our measurements, monkeys were training on the contingency map task, in a fixed reference configuration. They were then trained under a daily rotational change in this overlearned contingency map for 4 weeks. In order to evaluate the effect of fluoxetine on reward-based decision-making in a changing environment, we then performed acute fluoxetine (or placebo) injections while the monkeys performed the saccade reward competition task described above, to the exception that reward contingency maps varied from 1 day to the next in a pseudo-random manner (Figure 3B). This allowed to have monkeys engage in the active inference of the reward contingency maps on each day. In addition, this manipulation allowed to dissociate possible effects of fluoxetine on each of reward biases and spatial biases. On every week, a placebo session was recorded. The next day, monkeys received an acute injection of 2.5 mg/kg of fluoxetine. They then worked on the remaining weekdays on the same task, but these days were considered as washout days. Because subjects, whether human or non-human, have individual reward sensitives as well as individual spatial response biases, we independently characterize the effects of fluoxetine on reward and spatial biases as presented next.
Effect of fluoxetine on reward biases
In order to assess the effect of fluoxetine on reward-induced biases, we computed for each individual reward contingency, a reward selectivity index (RSI) as follows. For each rewarded contingency, we estimate the median proportion of instances in which this contingency was chosen, irrespective of the reward contingency associated with the other singleton in the pair, as well as irrespective of spatial positions. Thus, RSI reflects preference for a given reward contingency irrespective of other sources of variation in the trial. Hence, a high RSI indicates that monkeys prefer this contingency relative to the others. An increase in reward selectivity index under fluoxetine indicates that the preference for this specific spatial position is enhanced. In both monkeys, we observe a significant increase in the RSI on the highly rewarded items (80% of high reward probability neighboring them (Figure 3C, left, detailed statistics are provided in Supplementary Table S4). This indicates an increase in the monkeys’ preference for these rewards under fluoxetine. We also observe, for both monkeys, a significant decrease in the RSI on both the low reward items and for M2 on the intermediate reward items neighboring them (Figure 3C, Supplementary Table S5). This indicates a decrease in the monkeys’ preference for these rewards under fluoxetine. Thus, overall, this demonstrates that fluoxetine significantly alters reward–based decision making such that subjects are more sensitive to the positive incentive of high reward probabilities as well as to the negative outcome of low reward probabilities.
Effect of fluoxetine on spatial biases
In order to assess the effect of fluoxetine on intrinsic spatial biases, we computed for each individual target position, a spatial selectivity index (SSI) as follows. For each spatial location, we estimated the median proportion of instances in which this position was chosen, irrespective of the spatial position of the other singleton in the pair, as well as irrespective of reward contingencies. Hence, a high SSI indicates that monkeys prefer this contingency relative to the others. An increase in spatial selectivity index under fluoxetine indicates that the preference for this specific reward contingency is enhanced. Under fluoxetine, Monkey M1 shows a decreased SSI specifically for the left targets relative to the placebo and an increased SSI in upper positions (Figure 3C, right). Because spatial positions on the left hemifield were associated with a high SSI in the placebo condition relative to the right targets, this indicates that the monkeys often preferred targets on this side on the placebo condition and that this spatial bias decreased under fluoxetine (detailed statistics are presented in Supplementary Table S5). Likewise, Monkey M1 had a low SSI toward upper positions in placebo position relative to lower positions targets and this spatial bias decreased under fluoxetine (Supplementary Table S5). Thus, in this monkey, fluoxetine resulted in a reduction in overall spatial biases. Monkey M2 show no significant difference in SSIs between the placebo and the fluoxetine condition (Figure 3C, right).
Pupil size is enlarged and blink duration decreased under fluoxetine
Changes in perceptual thresholds as described in the first luminance detection task can be accounted for by local changes in excitatory/inhibitory balance in the visual cortex. However, this can also be accounted for by changes in oculomotor functions such as pupil size changes and blink duration (LeDoux et al., 1998). We thus quantified these two parameters independently in the fluoxetine and placebo conditions.
Overall, pupil size significantly increases under fluoxetine relative to placebo, both at rest and in the task (Figure 5A, two-way ANOVA, condition x epoch: M1: main condition effect, F (1,2999) = 1910, p < 0.001; main epoch effect: F (1,2999) = 64926, p < 0.001; interaction: F (1,83) = 6027, p < 0.001, ntasks-placebo = 15, ntasks-fluoxetine = 15; M2: main condition effect, F (1,2999) = 58539, p < 0.001; main epoch effect: F (1,2999) = 57, p < 0.001; interaction: F (1,2999) = 55, p < 0.001, ntasks-placebo = 22, ntasks-fluoxetine = 22). This observation is in agreement with what has already been described in the literature (McGuirk and Silverstone, 1990; Cazettes et al., 2021). However, enlarged pupil size is associated with enhanced visual acuity (Leibowitz, 1952). Thus, this observation on pupil size is at odds with our observation of degraded perceptual thresholds in the luminance detection task.
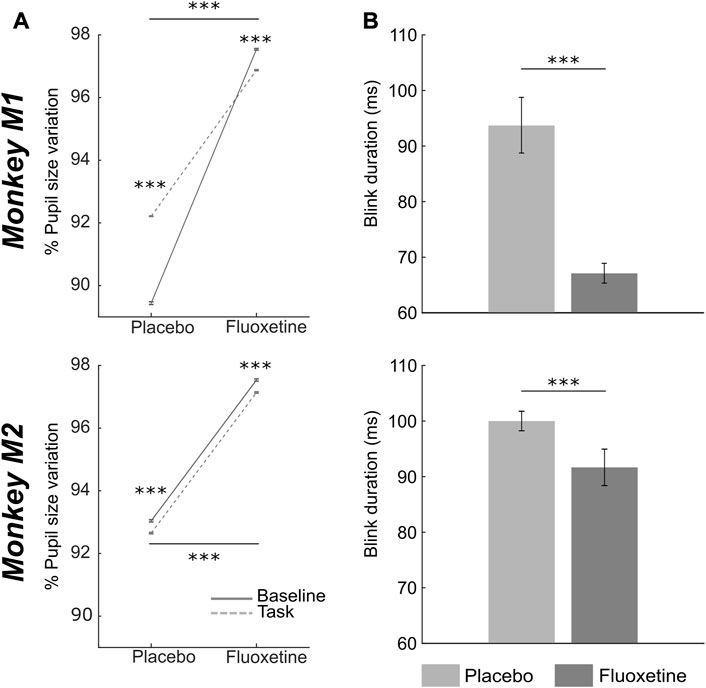
FIGURE 5. Effect of fluoxetine on pupil size and eye blink duration. (A) Median+/-median absolute error of %pupil size changes relative to the entire task, for monkeys M1 (top) and M2 (bottom), during rest (baseline, continuous line) and after target presentation (task, dashed lines), in the placebo and fluoxetine conditions. (B) Median+/-median absolute error of blink duration, for monkeys M1 (top) and M2 (bottom), in the placebo and fluoxetine conditions. Statistical significance is represented as follows: ***, p < 0.001; **, p < 0.01; *, p < 0.05. See also companion Supplementary Table S6 for detailed statistics.
We also measured eye blink statistics as a proxy of attentional engagement. Blink rate in the task was not affected by fluoxetine (statistics are detailed in Supplementary Table S6). However, blink duration was significantly shorter under fluoxetine relative to placebo (Figure 5B, Wilcoxon test, M1: p < 0.001, ntasks-placebo= 15, ntasks-fluoxetine = 15; M2: p < 0.001, ntasks-placebo = 22, ntasks-fluoxetine = 22). Because epochs of blinking have been shown to interfere with cognition (Irwin, 2014), shorter eye blink under fluoxetine might be associated with stronger involvement in the task.
Paradoxical effects of fluoxetine on manual reaction times
Reaction times (RT) correspond to a complex behavioral variable that is subject to modulations by multiple cognitive functions ranging from spatial attention (Wardak et al., 2012a; 2011), to temporal expectation and anticipation (Wardak et al., 2012b; Cravo et al., 2013), decision making (Hanks et al., 2006; Noorani and Carpenter, 2016; Fujimoto et al., 2021), perception (Song et al., 2008), reinforcement learning (Viejo et al., 2018), arousal (Eason et al., 1969; Davranche et al., 2006; Fujimoto et al., 2021), reward (Firestone and Douglas, 1975; Procyk et al., 2000; Simen et al., 2009; Epstein et al., 2011), to name a few. In the following, we characterize the effect of fluoxetine on RT distributions (Figure 6). For the sake of clarity, in the following, we focus on manual reaction times in the two first tasks, as saccadic reaction time from the saccadic reward choice task are confounded by possible spatial and reward biases. We used the LATER model in order to classify reaction times in anticipatory reaction times and controlled reaction times (Supplementary Figure S3).
FIGURE 6. Effect of fluoxetine on on-task motivation (# of trials) and on-task compliance (% aborts), on the Luminance detection task (A), the detection task with distractors (B) and the saccadic reward competition task (C). For all plots, median+/-median absolute error. of the median are represented. Placebo data are represented in light gray and fluoxetine data are represented in dark gray. Statistical significance is represented as follows: ***, p < 0.001; **, p < 0.01; *, p < 0.05; n. s., p > 0.05. See also companion Supplementary Table S1 for detailed statistics.
For the luminance detection task, because reaction times vary as a function of target luminance, we focused on the trials with two highest target luminance. On these trials, both monkeys had a 100% hit rate. We pooled the trials corresponding to the two easiest targets on all four positions. We report, in both monkeys, a significant decrease in controlled reaction time under fluoxetine relative to placebo (Figure 6, detailed statistics are presented in Supplementary Table S7). Fluoxetine did not have the same impact on the rate of anticipatory responses in each monkey. M1 had fewer anticipations in the fluoxetine condition (2.89%) relative to the placebo condition (7.83%, p < 0.001) and faster responses in the fluoxetine condition (median RT+/-median absolute error, 263.8 ms+/-2.99) relative to the placebo condition (329.3 ms+/-4.30; p < 0.001). In M2, anticipation rate was not significantly different in the fluoxetine condition (7.08%) relative to the placebo condition (6.06%, p = 0.344) and these anticipatory responses were slower in the fluoxetine condition (median RT+/-median absolute error, 286.7 ms+/-6.16) relative to the placebo condition (234.4 ms+/-4.67; p = 0.029).
For the target detection task in the presence of distractors, we report the opposite observations. Indeed, RT increased under fluoxetine in target only trials (Supplementary Table S7). On these trials, we also report less percentage of anticipatory responses under fluoxetine relative to placebo (Figure 6, M1, left quadrant, placebo = 3.62%, fluoxetine = 1.25%, p < 0.001; right quadrant, placebo = 8.86%, fluoxetine = 1.25%, p = 0.008; M2, left quadrant, placebo = 1.79%, fluoxetine = 0.55%, p < 0.001; right quadrant, placebo = 0.92%, fluoxetine = 0.59%, p < 0.001).
On trials with a distractor preceding target presentation, RT also increased under fluoxetine in target only trials (Supplementary Table S7). In addition, we observe a marked increase of the overall percentage of anticipatory responses (M1, left quadrant, placebo = 25.74%, fluoxetine = 91.75%, p < 0.001; right quadrant, placebo = 47.49%, fluoxetine = 97.74%, p < 0.001. M2, left quadrant, placebo = 9.71%, fluoxetine = 10.06%, p < 0.001; right quadrant, placebo = 3.37%, fluoxetine = 25.48%, p < 0.001), and significantly more when distractor is preceding the target compared to target only trials under fluoxetine than in the placebo condition (Two-way ANOVA condition distractor presence, M1: left quadrant, condition, F(1,8) = 3586.177, p < 0.001; location, F(1,8) = 1123.003, p < 0.001; interaction, F(1,8) = 857.459, p < 0.001; right quadrant, condition, F(1,8) = 3586.177, p < 0.001; location, F(1,8) = 1123.003, p < 0.001; interaction, F(1,8) = 857.459, p < 0.001; M2: left quadrant, condition, F(1,15) = 3586.177, p < 0.001; location, F(1,15) = 1123.003, p < 0.001; interaction, F(1,15) = 857.459, p < 0.001; right quadrant, condition, F(1,15) = 3586.177, p < 0.001; location, F(1,15) = 1123.003, p < 0.001; interaction, F(1,15) = 857.459, p < 0.001). Overall, on this task, we thus report a paradoxical effect of fluoxetine, associated with more anticipatory RTs on distractor trials, indicating a stronger release of proactive inhibitory mechanisms (Wardak et al., 2012a; 2012b; Criaud et al., 2012), while at the same time we report longer controlled RT on these same trials, indicating stronger cognitive control.
Discussion
In the present work, we precisely characterize the effects of fluoxetine on behavioral and physiological metrics while monkeys are performing three different visual tasks. We report a set of specific effects of fluoxetine as well as several non-specific effects of fluoxetine, including longer time on the task and shorter blink durations. Luminance perceptual thresholds are increased, such that higher levels of luminosity are needed to reach a 50% correct detection. Under sensory uncertainty, decision thresholds are released and perceptual spatial resolution is degraded. We additionally show that fluoxetine increases sensitivity to reward outcome. Last, we show that fluoxetine can either speed up or slow down manual reaction times, depending on the nature of the task. Thus, overall, while low level vision appears to be degraded by fluoxetine, performance in the visual tasks are maintained under fluoxetine due to enhanced top-down control based on task outcome and reward maximization. In the following, we discuss these observations in the light of the current knowledge on fluoxetine.
Fluoxetine interferes with luminance perception
We here show that fluoxetine results in increased visual perceptual thresholds, higher levels of luminosity being required to achieve similar detection thresholds as in placebo. This can be accounted for by the reported role of serotonin (5-hydroxytryptamine or 5-HT) in the physiology of the retinal of vertebrates (for review, see Masson, 2019; Pootanakit and Brunken, 2000). 5-HT is synthesized as a precursor for melanopsin in both photoreceptors and amacrine cells (Vaney, 1986; Millar et al., 1988; Pourcho, 1996) and its uptake occurs in bipolar and retinal ganglion cells (RGC). Fluoxetine enhances serotonin accumulation in bipolar (Schuette and Chappell, 1998) and retinal ganglion cells (Wassle et al., 1987), thus suppressing their spontaneous firing (Hughes et al., 2016). This mechanism possibly accounts for our experimental observation of a
Fluoxetine interferes with attention
Shorter blinks (Hsieh and Tai, 2013) as well as enlarged pupil (Aston-Jones and Cohen, 2005; Wang et al., 2018) as we report here following fluoxetine injections have been associated with higher arousal. Improved arousal could thus be at the origin of the enhanced commitment to the task that we observed. Beyond this non-specific arousal effect, enhanced performance in task could also be due to enhanced attention, taking place independently from motivational factors. Indeed, it has been shown that 5-HTP (the immediate serotonin precursor) uptake increases attention in low baseline attention individuals (Weinberg-Wolf et al., 2018). Likewise, fluoxetine is shown to selectively modulate, prefrontal synaptic growth during macaque brain development (Golub et al., 2017), to activate cortical structures involved in sustained attention, such as the thalamus and caudate nucleus in healthy subjects (Wingen et al., 2008) as well as the dorsolateral prefrontal cortex in attention deficit hyperactivity disorder and autism spectrum disorder patients (Hollander et al., 2005; Quintana et al., 2007; Riggs et al., 2007; Chantiluke et al., 2015; Strawn et al., 2015). At the metabolic level, fluoxetine increases the cerebrospinal fluid GABA levels (Gören et al., 2007), indirectly affecting the GABA levels in the brain (Santana et al., 2004; Beshara et al., 2016). This results in a change in the excitatory/inhibitory balance in the brain to the benefit of a stronger inhibition (Yin et al., 2021). More particularly, frontoparietal inhibitory mechanisms have been shown to be closely linked with individual differences in attentional processing such that GABA concentrations in the prefrontal cortex are negatively related to attentional blink magnitude while GABA concentrations in the posterior parietal cortex are positively correlated with attentional blink magnitude (Kihara et al., 2016). This suggests a specific impact of fluoxetine on the fronto-parietal attentional network (Ibos et al., 2013). A direct role of fluoxetine on the correlated activation of the fronto-parietal attentional network is observed in the same macaques as those included in the present study, during the performance of a perceptual task during an fMRI protocol (Gacoin, 2023). This thus confirms the impact of fluoxetine on the cortical substrates of the attentional function.
Attentional control on perception involves both changes in perceptual sensitivity and changes in the decision response threshold. The neuronal activity in the prefrontal cortex has been associated to both (Luo and Maunsell, 2018), while the neuronal activity of extrastriate cortex has mostly been associated with changes in sensitivity (Martinez-Trujillo and Gulli, 2018). Here, in a spatial decision task involving a spatial uncertainty, we report both a change in response criterion, monkeys becoming more liberal, as well as a loss of spatial resolution in visual processing. This further confirms the impact of fluoxetine on the cortical substrates of the attentional function. However, attentional processes are strongly impacted by motivational factors including reward processing (Arnsten and Rubia, 2012; for review, see Hélie et al., 2017). The possible role of fluoxetine on this cognitive component is discussed next.
Fluoxetine enhances motivation on task by modulating the sensitivity to reward
Fluoxetine is proposed to mediate cognitive functions through the reward valuation pathways. Indeed, inhibition of central serotonin reuptake decreases probabilistic learning (Chamberlain et al., 2006) and SSRI enhances reward processing in healthy adults (McCabe et al., 2010; Macoveanu, 2014; Scholl et al., 2017), although these effects are highly dose-dependent (Bari et al., 2010). The serotoninergic cells of the dorsal raphe nucleus project to both the bed nucleus of the stria terminalis, an anxiety-related structure, the ventral tegmental area, a reward-related structure, and are shown to respond to emotional salience (Paquelet et al., 2022). The activity of this neuronal population is additionally shown to correlate with learning rate, both in a context of expected and unexpected uncertainty (Grossman et al., 2022).
While fluoxetine has been shown to decrease hunger and thirst (McGuirk and Silverstone, 1990), it has also been associated with a reduction of effort cost, or to an increased valuation of reward (Meyniel et al., 2016). Accordingly, we report that monkeys make more trials and produce less abort trials under fluoxetine relative to the placebo condition, irrespective of the task. They also expressed a higher willingness to initiate working sessions, at all stages of experimental preparation and execution (higher willingness to come out of the cage, and go in monkey chair, faster eye calibration, no signs of restlessness at the end of the working session that would indicate that the monkey wants to go back to its home cage). In addition, in the free choice task, we manipulated reward contingency and we measured the monkeys’ sensitivity to reward. Fluoxetine significantly altered reward based-decision making such that subjects were more sensitive to the positive incentive of high reward probability as well as to the negative outcome of low reward probabilities, thus increasing the effect of aversion loss among individuals, and facilitating sensitivity to the reward outcome (Macoveanu et al., 2013). In other words, monkeys’ decision-making was more impacted by expected reward under fluoxetine. Thus, not only did monkeys put more effort to get a reward under fluoxetine (Meyniel et al., 2016), but they also better used reward information in order to guide their behavior. This parameter also influenced their reaction times (RT), for it have recently been found to be prolonged in the context of decision-making under SSRIs (Khalighinejad et al., 2022).
Fluoxetine and reaction times
Reaction times (RT) are modulated by multiple cognitive functions ranging from spatial attention (Wardak et al., 2012a; 2011), to temporal expectation and anticipation (Wardak et al., 2012b; Cravo et al., 2013), decision making (Hanks et al., 2006; Noorani and Carpenter, 2016; Fujimoto et al., 2021), perception (Song et al., 2008), reinforcement learning (Viejo et al., 2018), arousal (Eason et al., 1969; Davranche et al., 2006; Fujimoto et al., 2021) and reward processing (Firestone and Douglas, 1975; Procyk et al., 2000; Simen et al., 2009; Epstein et al., 2011). While the effects of fluoxetine on attentional and motivational processes are expected to speed up reaction time distributions, our observations lead to a more complex picture. Indeed, we reproduce the observation of prolonged RTs under fluoxetine in the detection task under spatial uncertainty as well as in the free choice task based on reward incentives. However, we report the opposite trend (i.e., speeded up RTs under fluoxetine) in a luminance detection task. We propose to interpret this paradoxical effect in the context of stochastic resonance. A recent study (Groen et al., 2018) shows that adding noise to the visual cortex using transcranial random noise stimulation enhanced decision-making when stimuli were just below perceptual threshold, but not when they were well below or above threshold. Stretching this observation, we would like to propose that the observed paradoxical effects of fluoxetine on RT depend on the specific noise functions associated with each task, noise being defined as both neuronal noise possibly effected by fluoxetine due to changes in the excitatory/inhibitory balance in the brain (Yin et al., 2021), as well as task related noise or uncertainty, be it spatial uncertainty or reward-related uncertainty.
Fluoxetine interferes with pupil and blink physiology
Pupil diameter and blink frequencies have been associated with both changes in attention and arousal (Aston-Jones and Cohen, 2005; Wang et al., 2018). SSRIs have been shown to result in increased pupil dilation (Schmitt et al., 2002; Hughes et al., 2016) and slower pupillary contraction (Rodriguez et al., 2020). It is unclear whether these effects are also associated with low level changes in visual accommodation (Rodriguez et al., 2020). Enlarged pupil diameter has been associated with higher thresholds at detecting the frequency at which a flickering light is perceived as a steady light source (Schmitt et al., 2002) as well as with enhanced letter identification report at very short presentation timings (Lansner et al., 2019). Thus, while dilated pupil size could account for our experimental observation of fluoxetine induced changes in luminance perception, actual pupil size changes could result (at least in part) from the attentional and motivational fluoxetine effects described above.
To our knowledge, there are no reports that fluoxetine impacts blink duration. Capitão et al. (2015) show that fluoxetine modulates emotional processing, suppressing, for example, the motion-potentiated startle effect. Here, we show a significant decrease in blink duration, in the absence of change in blink frequency. In the context of our task, these blinks are considered as spontaneous rather than reflex blinks in response to external events. Spontaneous blinks have been shown to correlate with the activation of a network involving somatosensory primary and secondary areas, as well as parietal, cingulate, insular, as well as striate and extrastriate visual areas (Guipponi et al., 2015). Shorter blinks possibly correlate with weaker activations in this network. This remains to be explored as well as the possible link between blink duration, attention, motivation and perception.
Limitation of systemic drug injections
Systemic drug injection protocols closely mimic the clinical protocols that mostly rely on the oral intake of drugs that reach the brain systemically, in a non-specific and non-targeted manner. Such systemic drug injection protocols thus allow to characterize the behavioral effects that are expected to arise in patients under similar medication. These protocols do not however allow a precise characterization of the molecular, cellular and network effects of these drugs. Fluoxetine has a strong affinity for 5HT2A receptors, the concentration of which is high in the visual striate and extrastriate cortex (Beliveau et al., 2017; Hansen et al., 2022), as well as in the pre-frontal cortex (Puig and Gulledge, 2011). While our protocol does not allow to specifically characterize the serotoninergic projections responsible for the observed behavioral effects, functional magnetic resonance imaging in these same animals reveals large range network effects of fluoxetine both on resting-state activity and on task-related activations (Gacoin, 2023).
Another possible limitation of systemic injections is the fact that repeated fluoxetine administration can lead to adaptation effects in less than 6 months on a chronic administration schedule (Sawyer and Howell, 2011). The SSRI effect can be impaired by the plateau effect, which can be overcome with wash-out periods. The duration of this wash-out period depends on fluoxetine dose used as well as on the subject’s basal serotonin levels (Fontenot et al., 2009; Weinberg-Wolf et al., 2018). In order to circumvent these long term effects of repeated fluoxetine administration, in our protocol, chronic fluoxetine administration periods never exceeded 2 consecutive months and were always separated by wash-out and rest periods. As a result, we expect possible habituation effects to be marginal in our data.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by This authorization was based on an ethical evaluation by the French Committee on the Ethics of Experiments in Animals (C2EA) CELYNE registered at the national level as C2EA number 42.
Author contributions
Conceptualization, SB and MG; Stimuli preparation, MG; Data Acquisition, MG; Methodology, MG and SB; Investigation, MG and SB; Writing—Original Draft, MG and SB; Writing—Review & Editing, MG and SB; Funding Acquisition, SB; Supervision, SB.
Funding
With financial support from UNADEV (National Union for Blind and Visually Impaired People) in partnership with ITMO NNP (Multi-Organism Thematic Institute Neurosciences, cognitive sciences, neurology and psychiatry)/AVIESAN (national alliance for life science and health) as part of research on vision disorders to SB as well as LABEX CORTEX funding (ANR-11-LABX-0042) from the Université de Lyon, within the program Investissements d’Avenir (ANR-11-IDEX-0007) operated by the French National Research Agency (ANR).
Acknowledgments
We thank Fidji Francioly and Laurence Boes for animal care. We thank Serge Pinède and Julian Amengual for technical assistance on the project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1103999/full#supplementary-material
References
Ambati, M., Apicella, I., Wang, S., Narendran, S., Leung, H., Pereira, F., et al. (2021). Identification of fluoxetine as a direct NLRP3 inhibitor to treat atrophic macular degeneration. Proc. Natl. Acad. Sci. 118, e2102975118. doi:10.1073/pnas.2102975118
Arnsten, A. F. T., and Rubia, K. (2012). Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: Disruptions in neurodevelopmental psychiatric disorders. J. Am. Acad. Child Adolesc. Psychiatry 51, 356–367. doi:10.1016/j.jaac.2012.01.008
Aston-Jones, G., and Cohen, J. D. (2005). An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annu. Rev. Neurosci. 28, 403–450. doi:10.1146/annurev.neuro.28.061604.135709
Bar-Haim, Y., Lamy, D., Pergamin, L., Bakermans-Kranenburg, M. J., and van Ijzendoorn, M. H. (2007). Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychol. Bull. 133, 1–24. doi:10.1037/0033-2909.133.1.1
Bari, A., Theobald, D. E., Caprioli, D., Mar, A. C., Aidoo-Micah, A., Dalley, J. W., et al. (2010). Serotonin modulates sensitivity to reward and negative feedback in a probabilistic reversal learning task in rats. Neuropsychopharmacol 35, 1290–1301. doi:10.1038/npp.2009.233
Baroncelli, L., Maffei, L., and Sale, A. (2011). New perspectives in amblyopia therapy on adults: A critical role for the excitatory/inhibitory balance. Front. Cell. Neurosci. 5, 25. doi:10.3389/fncel.2011.00025
Bauer, M., Monz, B. U., Montejo, A. L., Quail, D., Dantchev, N., Demyttenaere, K., et al. (2008). Prescribing patterns of antidepressants in europe: Results from the factors influencing depression endpoints research (FINDER) study. Eur. Psychiatry 23, 66–73. doi:10.1016/j.eurpsy.2007.11.001
Beliveau, V., Ganz, M., Feng, L., Ozenne, B., Højgaard, L., Fisher, P. M., et al. (2017). A high-resolution in vivo atlas of the human brain’s serotonin system. J. Neurosci. 37, 120–128. doi:10.1523/JNEUROSCI.2830-16.2016
Beshara, S., Beston, B. R., Pinto, J. G. A., and Murphy, K. M. (2016). Effects of fluoxetine and visual experience on glutamatergic and GABAergic synaptic proteins in adult rat visual cortex. eNeuro 2, ENEURO.0126–15.2015. doi:10.1523/ENEURO.0126-15.2015
Brown, H. D., Amodeo, D. A., Sweeney, J. A., and Ragozzino, M. E. (2012). The selective serotonin reuptake inhibitor, escitalopram, enhances inhibition of prepotent responding and spatial reversal learning. J. Psychopharmacol. 26, 1443–1455. doi:10.1177/0269881111430749
Brunken, W. J., Jin, X. T., and Pis-Lopez, A. M. (1993). Chapter 4 the properties of the serotoninergic system in the retina. Prog. Retin. Res. 12, 75–99. doi:10.1016/0278-4327(93)90005-E
Capitão, L. P., Murphy, S. E., Browning, M., Cowen, P. J., and Harmer, C. J. (2015). Acute fluoxetine modulates emotional processing in young adult volunteers. Psychol. Med. 45, 2295–2308. doi:10.1017/S0033291715000240
Carrasco, M., Ling, S., and Read, S. (2004). Attention alters appearance. Nat. Neurosci. 7, 308–313. doi:10.1038/nn1194
Carter, O. L., Burr, D. C., Pettigrew, J. D., and Vollenweider, F. X. (2005). Using psilocybin to investigate the relationship between attention, working memory and the serotonin 5-HT1A and 5-HT2A receptors. J. Vis. 5, 683. doi:10.1167/5.8.683
Cazettes, F., Reato, D., Morais, J. P., Renart, A., and Mainen, Z. F. (2021). Phasic activation of dorsal raphe serotonergic neurons increases pupil size. Curr. Biol. 31, 192–197.e4. doi:10.1016/j.cub.2020.09.090
Cerrito, F., and Raiteri, M. (1979). Serotonin release is modulated by presynaptic autoreceptors. Eur. J. Pharmacol. 57, 427–430. doi:10.1016/0014-2999(79)90506-5
Chamberlain, S. R., Müller, U., Blackwell, A. D., Clark, L., Robbins, T. W., and Sahakian, B. J. (2006). Neurochemical modulation of response inhibition and probabilistic learning in humans. Science 311, 861–863. doi:10.1126/science.1121218
Chantiluke, K., Barrett, N., Giampietro, V., Brammer, M., Simmons, A., and Rubia, K. (2015). Disorder-dissociated effects of fluoxetine on brain function of working memory in attention deficit hyperactivity disorder and autism spectrum disorder. Psychol. Med. 45, 1195–1205. doi:10.1017/S0033291714002232
Chelazzi, L., E to inova, J., Calletti, R., Lo Gerfo, E., Sani, I., Della Libera, C., et al. (2014). Altering spatial priority maps via reward-based learning. J. Neurosci. 34, 8594–8604. doi:10.1523/JNEUROSCI.0277-14.2014
Chen, Y.-A., Huang, W.-S., Lin, Y.-S., Cheng, C.-Y., Liu, R.-S., Wang, S.-J., et al. (2012). Characterization of 4-[18F]-ADAM as an imaging agent for SERT in non-human primate brain using PET: A dynamic study. Nucl. Med. Biol. 39, 279–285. doi:10.1016/j.nucmedbio.2011.08.002
Clark, R. N., Ashby, C. R., Dewey, S. L., Ramachandran, P. V., and Strecker, R. E. (1996). Effect of acute and chronic fluoxetine on extracellular dopamine levels in the caudate-putamen and nucleus accumbens of rat. Synapse 23, 125–131. doi:10.1002/(SICI)1098-2396(199607)23:3<125::AID-SYN1>3.0.CO;2-A
Clarke, H. F., Dalley, J. W., Crofts, H. S., Robbins, T. W., and Roberts, A. C. (2004). Cognitive inflexibility after prefrontal serotonin depletion. Science 304, 878–880. doi:10.1126/science.1094987
Cravo, A. M., Rohenkohl, G., Wyart, V., and Nobre, A. C. (2013). Temporal expectation enhances contrast sensitivity by phase entrainment of low-frequency oscillations in visual cortex. J. Neurosci. 33, 4002–4010. doi:10.1523/JNEUROSCI.4675-12.2013
Criaud, M., Wardak, C., Ben Hamed, S., Ballanger, B., and Boulinguez, P. (2012). Proactive inhibitory control of response as the default state of executive control. Front. Psychol. 3, 59. doi:10.3389/fpsyg.2012.00059
Davranche, K., Audiffren, M., and Denjean, A. (2006). A distributional analysis of the effect of physical exercise on a choice reaction time task. J. Sports Sci. 24, 323–329. doi:10.1080/02640410500132165
Della Libera, C., Calletti, R., Eštočinová, J., Chelazzi, L., and Santandrea, E. (2017). Reward-based plasticity of spatial priority maps: Exploiting inter-subject variability to probe the underlying neurobiology. Cogn. Neurosci. 8, 85–101. doi:10.1080/17588928.2016.1213226
Di Bello, F., Ben Hadj Hassen, S., Astrand, E., and Ben Hamed, S. (2022). Prefrontal control of proactive and reactive mechanisms of visual suppression. Cereb. Cortex 32, 2745–2761. doi:10.1093/cercor/bhab378
Dumont, G. J. H., De Visser, S. J., Cohen, A. F., and Van Gerven, J. M. A.Biomarker Working Group of the German Association for Applied Human Pharmacology (2005). Biomarkers for the effects of selective serotonin reuptake inhibitors (SSRIs) in healthy subjects. Br. J. Clin. Pharmacol. 59, 495–510. doi:10.1111/j.1365-2125.2005.02342.x
Eason, R. G., Harter, M. R., and White, C. T. (1969). Effects of attention and arousal on visually evoked cortical potentials and reaction time in man. Physiology Behav. 4, 283–289. doi:10.1016/0031-9384(69)90176-0
Enge, S., Fleischhauer, M., Lesch, K.-P., and Strobel, A. (2011). On the role of serotonin and effort in voluntary attention: Evidence of genetic variation in N1 modulation. Behav. Brain Res. 216, 122–128. doi:10.1016/j.bbr.2010.07.021
Epstein, J. N., Langberg, J. M., Rosen, P. J., Graham, A., Narad, M. E., Antonini, T. N., et al. (2011). Evidence for higher reaction time variability for children with ADHD on a range of cognitive tasks including reward and event rate manipulations. Neuropsychology 25, 427–441. doi:10.1037/a0022155
Firestone, P., and Douglas, V. (1975). The effects of reward and punishment on reaction times and autonomic activity in hyperactive and normal children. J. Abnorm Child. Psychol. 3, 201–216. doi:10.1007/BF00916751
Fontenot, M. B., Musso, M. W., McFatter, R. M., and Anderson, G. M. (2009). Dose-finding study of fluoxetine and venlafaxine for the treatment of self- injurious and stereotypic behavior in rhesus macaques (Macaca mulatta). J. Am. Assoc. Laboratory Animal Sci. 48, 176–184.
Fujimoto, A., Murray, E. A., and Rudebeck, P. H. (2021). Interaction between decision-making and interoceptive representations of bodily arousal in frontal cortex (preprint). Neuroscience. doi:10.1101/2021.02.04.429750
Gacoin, M. (2023). Behavioral and pharmacological enhancement of neural plasticity in the adult visual cortex. Lyon: Université Claude Bernard.
Golub, M. S., Hackett, E. P., Hogrefe, C. E., Leranth, C., Elsworth, J. D., and Roth, R. H. (2017). Cognitive performance of juvenile monkeys after chronic fluoxetine treatment. Dev. Cogn. Neurosci. 26, 52–61. doi:10.1016/j.dcn.2017.04.008
Gören, M. Z., Küçükibrahimoglu, E., Berkman, K., and Terzioglu, B. (2007). Fluoxetine partly exerts its actions through GABA: A neurochemical evidence. Neurochem. Res. 32, 1559–1565. doi:10.1007/s11064-007-9357-2
Groen, O. van der, Tang, M. F., Wenderoth, N., and Mattingley, J. B. (2018). Stochastic resonance enhances the rate of evidence accumulation during combined brain stimulation and perceptual decision-making. PLOS Comput. Biol. 14, e1006301. doi:10.1371/journal.pcbi.1006301
Grossman, C. D., Bari, B. A., and Cohen, J. Y. (2022). Serotonin neurons modulate learning rate through uncertainty. Curr. Biol. 32, 586–599.e7. doi:10.1016/j.cub.2021.12.006
Guipponi, O., Odouard, S., Pinède, S., Wardak, C., and Ben Hamed, S. (2015). fMRI cortical correlates of spontaneous eye blinks in the nonhuman primate. Cereb. Cortex 25, 2333–2345. doi:10.1093/cercor/bhu038
Hanks, T. D., Ditterich, J., and Shadlen, M. N. (2006). Microstimulation of macaque area LIP affects decision-making in a motion discrimination task. Nat. Neurosci. 9, 682–689. doi:10.1038/nn1683
Hansen, J. Y., Shafiei, G., Markello, R. D., Smart, K., Cox, S. M. L., Nørgaard, M., et al. (2022). Mapping neurotransmitter systems to the structural and functional organization of the human neocortex. Nat. Neurosci. 25, 1569–1581. doi:10.1038/s41593-022-01186-3
Harmer, C. J., Mackay, C. E., Reid, C. B., Cowen, P. J., and Goodwin, G. M. (2006). Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol. Psychiatry 59, 816–820. doi:10.1016/j.biopsych.2005.10.015
Harmer, C. J. (2008). Serotonin and emotional processing: Does it help explain antidepressant drug action? Neuropharmacol. Curr. Res. Serot. Gene Funct. Ther. 55, 1023–1028. doi:10.1016/j.neuropharm.2008.06.036
Hélie, S., Shamloo, F., Novak, K., and Foti, D. (2017). The roles of valuation and reward processing in cognitive function and psychiatric disorders. Ann. N. Y. Acad. Sci. 1395, 33–48. doi:10.1111/nyas.13327
Hollander, E., Phillips, A., Chaplin, W., Zagursky, K., Novotny, S., Wasserman, S., et al. (2005). A placebo controlled crossover trial of liquid fluoxetine on repetitive behaviors in childhood and adolescent autism. Neuropsychopharmacol 30, 582–589. doi:10.1038/sj.npp.1300627
Homberg, J. R. (2012). Serotonin and decision making processes. Neurosci. Biobehav. Rev. 36, 218–236. doi:10.1016/j.neubiorev.2011.06.001
Hsieh, C.-S., and Tai, C.-C. (2013). An improved and portable eye-blink duration detection system to warn of driver fatigue. Instrum. Sci. Technol. 41, 429–444. doi:10.1080/10739149.2013.796560
Hughes, S., Foster, R. G., Peirson, S. N., and Hankins, M. W. (2016). Inhibitory effects of fluoxetine on photosensitive retinal ganglion cells. Investigative Ophthalmol. Vis. Sci. 57, 4660.
Ibos, G., Duhamel, J.-R., and Hamed, S. B. (2013). A functional hierarchy within the parietofrontal network in stimulus selection and attention control. J. Neurosci. 33, 8359–8369. doi:10.1523/JNEUROSCI.4058-12.2013
Ibos, G., Duhamel, J.-R., and Hamed, S. B. (2009). The spatial and temporal deployment of voluntary attention across the visual field. PLOS ONE 4, e6716. doi:10.1371/journal.pone.0006716
Kaulen, N., Rajkumar, R., Régio Brambilla, C., Mauler, J., Ramkiran, S., Orth, L., et al. (2022). mGluR5 and GABAA receptor-specific parametric PET atlas construction—PET/MR data processing pipeline, validation, and application. Hum. Brain Mapp. 43, 2148–2163. doi:10.1002/hbm.25778
Khalighinejad, N., Manohar, S., Husain, M., and Rushworth, M. F. S. (2022). Complementary roles of serotonergic and cholinergic systems in decisions about when to act. Curr. Biol. 32, 1150–1162.e7. doi:10.1016/j.cub.2022.01.042
Kihara, K., Kondo, H. M., and Kawahara, J. I. (2016). Differential contributions of GABA concentration in frontal and parietal regions to individual differences in attentional blink. J. Neurosci. 36, 8895–8901. doi:10.1523/JNEUROSCI.0764-16.2016
Koch, S., Perry, K. W., Nelson, D. L., Conway, R. G., Threlkeld, P. G., and Bymaster, F. P. (2002). R-Fluoxetine increases extracellular DA, NE, as well as 5-HT in rat prefrontal cortex and hypothalamus an in vivo microdialysis and receptor binding study. Neuropsychopharmacology 27, 949–959. doi:10.1016/S0893-133X(02)00377-9
Lansner, J., Jensen, C. G., Petersen, A., Fisher, P. M., Frokjaer, V. G., Vangkilde, S., et al. (2019). Three weeks of SSRI administration enhances the visual perceptual threshold - a randomized placebo-controlled study. Psychopharmacology 236, 1759–1769. doi:10.1007/s00213-018-5158-3
LeDoux, M. S., Lorden, J. F., Smith, J. M., and Mays, L. E. (1998). Serotonergic modulation of eye blinks in cat and monkey. Neurosci. Lett. 253, 61–64. doi:10.1016/S0304-3940(98)00616-8
Leibowitz, H. (1952). The effect of pupil size on visual acuity for photometrically equated test fields at various levels of luminance. J. Opt. Soc. Am. JOSA 42, 416–422. doi:10.1364/JOSA.42.000416
Li, X., Chen, W., Pan, K., Li, H., Pang, P., Guo, Y., et al. (2018). Serotonin receptor 2c-expressing cells in the ventral CA1 control attention via innervation of the Edinger–Westphal nucleus. Nat. Neurosci. 21, 1239–1250. doi:10.1038/s41593-018-0207-0
Luo, T. Z., and Maunsell, J. H. R. (2018). Attentional changes in either criterion or sensitivity are associated with robust modulations in lateral prefrontal cortex. Neuron 97, 1382–1393.e7. doi:10.1016/j.neuron.2018.02.007
Macoveanu, J., Rowe, J. B., Hornboll, B., Elliott, R., Paulson, O. B., Knudsen, G. M., et al. (2013). Serotonin 2A receptors contribute to the regulation of risk-averse decisions. NeuroImage 83, 35–44. doi:10.1016/j.neuroimage.2013.06.063
Macoveanu, J. (2014). Serotonergic modulation of reward and punishment: Evidence from pharmacological fMRI studies. Brain Res. 1556, 19–27. doi:10.1016/j.brainres.2014.02.003
Martinez-Trujillo, J., and Gulli, R. A. (2018). Dissecting modulatory effects of visual attention in primate lateral prefrontal cortex using signal detection theory. Neuron 97, 1208–1210. doi:10.1016/j.neuron.2018.03.012
Masson, J. (2019). Serotonin in retina. Biochimie, 70 years Serot. 161, 51–55. doi:10.1016/j.biochi.2018.11.006
McCabe, C., Mishor, Z., Cowen, P. J., and Harmer, C. J. (2010). Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biol. Psychiatry 67, 439–445. doi:10.1016/j.biopsych.2009.11.001
McCormick, D. A., and Wang, Z. (1991). Serotonin and noradrenaline excite GABAergic neurones of the Guinea-pig and cat nucleus reticularis thalami. J. Physiology 442, 235–255. doi:10.1113/jphysiol.1991.sp018791
McGuirk, J., and Silverstone, T. (1990). The effect of the 5-HT re-uptake inhibitor fluoxetine on food intake and body weight in healthy male subjects. Int. J. Obes. 14, 361–372.
Meneses, A., and Liy-Salmeron, G. (2012). Serotonin and emotion, learning and memory. Rev. Neurosci. 23, 543–553. doi:10.1515/revneuro-2012-0060
Meyniel, F., Goodwin, G. M., Deakin, J. W., Klinge, C., MacFadyen, C., Milligan, H., et al. (2016). A specific role for serotonin in overcoming effort cost. eLife 5, e17282. doi:10.7554/eLife.17282
Millar, T. J., Winder, C., Ishimoto, I., and Morgan, I. G. (1988). Putative serotonergic bipolar and amacrine cells in the chicken retina. Brain Res. 439, 77–87. doi:10.1016/0006-8993(88)91463-1
Monckton, J. E., and McCormick, D. A. (2002). Neuromodulatory role of serotonin in the ferret thalamus. J. Neurophysiology 87, 2124–2136. doi:10.1152/jn.00650.2001
Moreau, A. W., Amar, M., Callebert, J., and Fossier, P. (2013). Serotonergic modulation of LTP at excitatory and inhibitory synapses in the developing rat visual cortex. Neuroscience 238, 148–158. doi:10.1016/j.neuroscience.2013.02.013
Ni, Y. G., and Miledi, R. (1997). Blockage of 5HT 2C serotonin receptors by fluoxetine (Prozac). Proc. Natl. Acad. Sci. U.S.A. 94, 2036–2040. doi:10.1073/pnas.94.5.2036
Noorani, I., and Carpenter, R. H. S. (2016). The LATER model of reaction time and decision. Neurosci. Biobehav. Rev. 64, 229–251. doi:10.1016/j.neubiorev.2016.02.018
Pälvimäki, E.-P., Majasuo, H., Laakso, A., Kuoppamäki, M., Syvälahti, E., Roth, B. L., et al. (1996). Interactions of selective serotonin reuptake inhibitors with the serotonin 5-HT2C receptor. Psychopharmacology 126, 234–240. doi:10.1007/BF02246453
Paquelet, G. E., Carrion, K., Lacefield, C. O., Zhou, P., Hen, R., and Miller, B. R. (2022). Single-cell activity and network properties of dorsal raphe nucleus serotonin neurons during emotionally salient behaviors. Neuron 110, 2664–2679.e8. doi:10.1016/j.neuron.2022.05.015
Pérez-Edgar, K., Bar-Haim, Y., McDermott, J. M., Gorodetsky, E., Hodgkinson, C. A., Goldman, D., et al. (2010). Variations in the serotonin-transporter gene are associated with attention bias patterns to positive and negative emotion faces. Biol. Psychol. 83, 269–271. doi:10.1016/j.biopsycho.2009.08.009
Phillips, M. A., Bitsios, P., Szabadi, E., and Bradshaw, C. M. (2000). Comparison of the antidepressants reboxetine, fluvoxamine and amitriptyline upon spontaneous pupillary fluctuations in healthy human volunteers. Psychopharmacology 149, 72–76. doi:10.1007/s002139900334
Pootanakit, K., and Brunken, W. J. (2000). 5-HT1A and 5-HT7 receptor expression in the mammalian retina. Brain Res. 875, 152–156. doi:10.1016/S0006-8993(00)02553-1
Pourcho, R. G. (1996). Neurotransmitters in the retina. Curr. Eye Res. 15, 797–803. doi:10.3109/02713689609003465
Procyk, E., Ford Dominey, P., Amiez, C., and Joseph, J.-P. (2000). The effects of sequence structure and reward schedule on serial reaction time learning in the monkey. Cognitive Brain Res. 9, 239–248. doi:10.1016/S0926-6410(00)00002-1
Puig, M. V., and Gulledge, A. T. (2011). Serotonin and prefrontal cortex function: Neurons, networks, and circuits. Mol. Neurobiol. 44, 449–464. doi:10.1007/s12035-011-8214-0
Quintana, H., Butterbaugh, G. J., Purnell, W., and Layman, A. K. (2007). Fluoxetine monotherapy in attention-deficit/hyperactivity disorder and comorbid non-bipolar mood disorders in children and adolescents. Child. Psychiatry Hum. Dev. 37, 241–253. doi:10.1007/s10578-006-0032-7
Reynolds, J. H., and Chelazzi, L. (2004). Attentional modulation of visual processing. Annu. Rev. Neurosci. 27, 611–647. doi:10.1146/annurev.neuro.26.041002.131039
Reynolds, J. H., Pasternak, T., and Desimone, R. (2000). Attention increases sensitivity of V4 neurons. Neuron 26, 703–714. doi:10.1016/S0896-6273(00)81206-4
Riggs, P. D., Mikulich-Gilbertson, S. K., Davies, R. D., Lohman, M., Klein, C., and Stover, S. K. (2007). A randomized controlled trial of fluoxetine and cognitive behavioral therapy in adolescents with major depression, behavior problems, and substance use disorders. Archives Pediatr. Adolesc. Med. 161, 1026–1034. doi:10.1001/archpedi.161.11.1026
Robinson, R. T., Drafts, B. C., and Fisher, J. L. (2003). Fluoxetine increases GABA(A) receptor activity through a novel modulatory site. J. Pharmacol. Exp. Ther. 304, 978–984. doi:10.1124/jpet.102.044834
Rodriguez, L., Joly, S., Zine-Eddine, F., Mdzomba, J. B., and Pernet, V. (2020). Tau modulates visual plasticity in adult and old mice. Neurobiol. Aging 95, 214–224. doi:10.1016/j.neurobiolaging.2020.07.024
Santana, N., Bortolozzi, A., Serrats, J., Mengod, G., and Artigas, F. (2004). Expression of Serotonin1A and Serotonin2A receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb. Cortex 14, 1100–1109. doi:10.1093/cercor/bhh070
Sawyer, E. K., and Howell, L. L. (2011). Pharmacokinetics of fluoxetine in rhesus macaques following multiple routes of administration. PHA 88, 44–49. doi:10.1159/000329417
Schmitt, J. A., Riedel, W. J., Vuurman, E. F., Kruizinga, M., and Ramaekers, J. G. (2002). Modulation of the Critical Flicker Fusion effects of serotonin reuptake inhibitors by concomitant pupillary changes. Psychopharmacology 160, 381–386. doi:10.1007/s00213-001-0993-y
Scholes, K. E., Harrison, B. J., O’Neill, B. V., Leung, S., Croft, R. J., Pipingas, A., et al. (2007). Acute serotonin and dopamine depletion improves attentional control: Findings from the stroop task. Neuropsychopharmacol 32, 1600–1610. doi:10.1038/sj.npp.1301262
Scholl, J., Kolling, N., Nelissen, N., Browning, M., Rushworth, M. F. S., and Harmer, C. J. (2017). Beyond negative valence: 2-week administration of a serotonergic antidepressant enhances both reward and effort learning signals. PLOS Biol. 15, e2000756. doi:10.1371/journal.pbio.2000756
Schuette, E., and Chappell, R. L. (1998). Excitatory amino acids and serotonin uptake blockers reveal two physiologically distinct serotonin systems in the retina of the skate, Raja erinacea. Int. J. Neurosci. 95, 115–132. doi:10.3109/00207459809000655
Semlitsch, H. V., Anderer, P., Saletu, B., Binder, G. A., and Decker, K. A. (1993). Acute effects of the novel antidepressant venlafaxine on cognitive event-related potentials (P300), eye blink rate and mood in young healthy subjects. Int. Clin. Psychopharmacol. 8, 155–166. doi:10.1097/00004850-199300830-00004
Seymour, B., Daw, N. D., Roiser, J. P., Dayan, P., and Dolan, R. (2012). Serotonin selectively modulates reward value in human decision-making. J. Neurosci. 32, 5833–5842. doi:10.1523/JNEUROSCI.0053-12.2012
Simen, P., Contreras, D., Buck, C., Hu, P., Holmes, P., and Cohen, J. D. (2009). Reward rate optimization in two-alternative decision making: Empirical tests of theoretical predictions. J. Exp. Psychol. Hum. Percept. Perform. 35, 1865–1897. doi:10.1037/a0016926
Song, S., Howard, J. H., and Howard, D. V. (2008). Perceptual sequence learning in a serial reaction time task. Exp. Brain Res. 189, 145–158. doi:10.1007/s00221-008-1411-z
Strawn, J. R., Welge, J. A., Wehry, A. M., Keeshin, B., and Rynn, M. A. (2015). Efficacy and tolerability of antidepressants in pediatric anxiety disorders: A systematic review and meta-analysis. Depress. Anxiety 32, 149–157. doi:10.1002/da.22329
Umemori, J., Winkel, F., Didio, G., Llach Pou, M., and Castrén, E. (2018). iPlasticity: Induced juvenile-like plasticity in the adult brain as a mechanism of antidepressants. Psychiatry Clin. Neurosci. 72, 633–653. doi:10.1111/pcn.12683
Vaney, D. I. (1986). Morphological identification of serotonin-accumulating neurons in the living retina. Science 233, 444–446. doi:10.1126/science.3726538
Vetencourt, J. F. M., Sale, A., Viegi, A., Baroncelli, L., De Pasquale, R., O’Leary, F. O., et al. (2008). The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science 320, 385–388. doi:10.1126/science.1150516
Vetencourt, J. F. M., Tiraboschi, E., Spolidoro, M., Castrén, E., and Maffei, L. (2011). Serotonin triggers a transient epigenetic mechanism that reinstates adult visual cortex plasticity in rats: Epigenetics of serotonin-induced adult cortical plasticity. Eur. J. Neurosci. 33, 49–57. doi:10.1111/j.1460-9568.2010.07488.x
Viejo, G., Girard, B., Procyk, E., and Khamassi, M. (2018). Adaptive coordination of working-memory and reinforcement learning in non-human primates performing a trial-and-error problem solving task. Behav. Brain Res. SI MCC 355, 76–89. doi:10.1016/j.bbr.2017.09.030
Wang, C.-A., Baird, T., Huang, J., Coutinho, J. D., Brien, D. C., and Munoz, D. P. (2018). Arousal effects on pupil size, heart rate, and skin conductance in an emotional face task. Front. Neurology 9, 1029. doi:10.3389/fneur.2018.01029
Wardak, C., Ben Hamed, S., Olivier, E., and Duhamel, J.-R. (2012a). Differential effects of parietal and frontal inactivations on reaction times distributions in a visual search task. Front. Integr. Neurosci. 6, 39. doi:10.3389/fnint.2012.00039
Wardak, C., Olivier, E., and Duhamel, J.-R. (2011). The relationship between spatial attention and saccades in the frontoparietal network of the monkey. Eur. J. Neurosci. 33, 1973–1981. doi:10.1111/j.1460-9568.2011.07710.x
Wardak, C., Ramanoël, S., Guipponi, O., Boulinguez, P., and Ben Hamed, S. B. (2012b). Proactive inhibitory control varies with task context. Eur. J. Neurosci. 36, 3568–3579. doi:10.1111/j.1460-9568.2012.08264.x
Wassle, H., Voigt, T., and Patel, B. (1987). Morphological and immunocytochemical identification of indoleamine-accumulating neurons in the cat retina. J. Neurosci. 7, 1574–1585. doi:10.1523/JNEUROSCI.07-05-01574.1987
Weinberg-Wolf, H., Fagan, N. A., Anderson, G. M., Tringides, M., Dal Monte, O., and Chang, S. W. C. (2018). The effects of 5-hydroxytryptophan on attention and central serotonin neurochemistry in the rhesus macaque. Neuropsychopharmacol 43, 1589–1598. doi:10.1038/s41386-017-0003-7
Wilson, W. H., Higano, H., Papadatos, Y., Kelwala, S., and Ban, T. A. (1983). A double-blind placebo controlled study to compare the autonomic effects of fluvoxamine with those of amitriptyline and doxepin in healthy volunteers. Br. J. Clin. Pharmacol. 15, 385S–392S. doi:10.1111/j.1365-2125.1983.tb02129.x
Wingen, M., Kuypers, K. P. C., van de Ven, V., Formisano, E., and Ramaekers, J. G. (2008). Sustained attention and serotonin: A pharmaco-fMRI study. Hum. Psychopharmacol. Clin. Exp. 23, 221–230. doi:10.1002/hup.923
Wong, D. T., Bymaster, F. P., and Engleman, E. A. (1995). Prozac (fluoxetine, lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: Twenty years since its first publication. Life Sci. 57, 411–441. doi:10.1016/0024-3205(95)00209-O
Worbe, Y., Savulich, G., Voon, V., Fernandez-Egea, E., and Robbins, T. W. (2014). Serotonin depletion induces ‘waiting impulsivity’ on the human four-choice serial reaction time task: Cross-species translational significance. Neuropsychopharmacol 39, 1519–1526. doi:10.1038/npp.2013.351
Yin, Y.-Y., Wang, Y.-H., Liu, W.-G., Yao, J.-Q., Yuan, J., Li, Z.-H., et al. (2021). The role of the excitation:inhibition functional balance in the mPFC in the onset of antidepressants. Neuropharmacology 191, 108573. doi:10.1016/j.neuropharm.2021.108573
Keywords: fluoxetine, serotonin, macaque, visual perception, luminosity thresholds, response criterion, reward sensitivity
Citation: Gacoin M and Ben Hamed S (2023) Fluoxetine degrades luminance perceptual thresholds while enhancing motivation and reward sensitivity. Front. Pharmacol. 14:1103999. doi: 10.3389/fphar.2023.1103999
Received: 21 November 2022; Accepted: 30 March 2023;
Published: 20 April 2023.
Edited by:
Erik B. Oleson, University of Colorado Denver, United StatesReviewed by:
Todor Vassilev Gerdjikov, University of Leicester, United KingdomGiovanni Hernandez, McGill University, Canada
Copyright © 2023 Gacoin and Ben Hamed. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maëva Gacoin, bWFldmEuZ2Fjb2luQGlzYy5jbnJzLmZy; Suliann Ben Hamed, YmVuaGFtZWRAaXNjLmNucnMuZnI=
 Maëva Gacoin
Maëva Gacoin Suliann Ben Hamed
Suliann Ben Hamed