- 1Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2TCM Regulating Metabolic Diseases Key Laboratory of Sichuan Province, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 3Chengdu University of Traditional Chinese Medicine, Chengdu, China
Background: Chinese herbal medicine (CHM) has the advantage of being safe and effective and has been widely used in clinical practice for the treatment of type 2 diabetes mellitus (T2DM) with hyperuricemia (HUA), but its overall efficacy and safety remain unclear. This study aimed to evaluate the efficacy and safety of CHM for the treatment of T2DM with HUA based on randomized controlled trials (RCTs) to provide clinical evidence.
Methods: The protocol evaluated in this study is registered with PROSPERO (CRD42022351519). As of November 2022, eight databases were searched, and RCTs of CHM for the treatment of T2DM with HUA were included. Outcome indicators observed included fasting blood glucose (FBG), 2-h postprandial glucose (2hPG), glycated hemoglobin (HbA1c), uric acid (UA), triglycerides (TG), total cholesterol (TC), overall effectiveness, and adverse events. Utilizing Review Manager 5.4, Stata V14.0, and GRADEpro, the included studies were evaluated, and the quality of the evidence was determined.
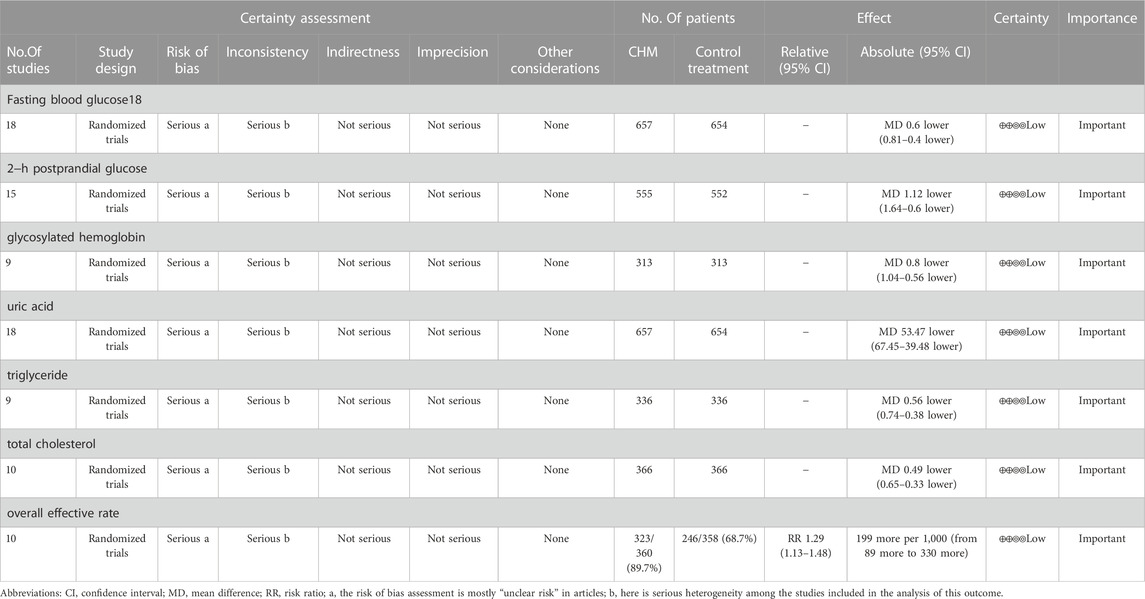
Results: 18 RCTs covering 1,311 patients were included in this study. The results of the study demonstrated that the combination of CHM and western medicine (WM) was more effective in treating patients with T2DM with HUA than WM alone, with significant improvements in FBG (weighted mean differences (WMD) = −0.60.95% confidence interval (CI) [−0.81, −0.40], p < 0.00001), 2hPG (WMD = −1.12.95% CI [−1.64, −0.60], p < 0.0001), HbA1c (WMD = −0.80.95% CI [−1.04, −0.56], p < 0.00001), UA (WMD = −53.47.95% CI [−67.45, −39.48], p < 0.00001), TG (WMD = −0.56.95% CI [−0.74, −0.38], p < 0.00001), TC (WMD = −0.49.95% CI [−0.65, −0.33], p < 0.00001), and overall effective rate (risk ratio (RR) = 1.29.95%CI [1.13, 1.48], p = 0.0002). The quality of evidence for all outcomes was low.
Conclusion: Compared with WM alone, the combination of CHM and WM was more effective in treating patients with T2DM with HUA, with significant improvements in glucose metabolism, uric acid, and lipids. However, further evaluation by high−quality RCT results is needed due to the low quality and high heterogeneity of the evidence.
Systematic Review Registration: [https://systematicreview.gov/], identifier [CRD42022351519].
1 Introduction
Type 2 diabetes mellitus (T2DM), a chronic systemic disease characterized by metabolic disorders (Xu et al., 2018), is one of the most challenging global public health issues of the 21st century (Lou et al., 2020). Hyperuricemia (HUA) is a metabolic disorder characterized by abnormally high levels of uric acid in the blood. It is associated with arthritis and the formation of gout stones, with gout further developing HUA (Gois and Souza, 2017; Kojima et al., 2019). The prevalence of HUA in people diagnosed with T2DM ranges from 16% to 38% (Donkeng et al., 2021). There are positive correlation and bidirectional causality between T2DM and HUA (Krishnan et al., 2013; Ahmadieh and Azar, 2017). On the one hand, diabetic insulin resistance competitively inhibits uric acid secretion (Gao et al., 2019). At the same time, hyperinsulinemia to insulin resistance secondary increases uric acid reabsorption and promotes the development of HUA (Li and Ye, 2017). On the other hand, excessive uric acid levels can trigger inflammatory response and oxidative stress that result in insulin resistance (Su et al., 2021); while uric acid leads to pancreatic β−cell dysfunction by decreasing nitric oxide bioavailability (Ghasemi, 2021), two alterations that are central to the physiopathology of T2DM. Another study revealed that the co−existence of T2DM and HUA increased the risk of cardiovascular disease and kidney disease (Viazzi et al., 2017; Lu et al., 2020; Liu et al., 2021), two complications that are significant causes of death and increase the public health burden on society (Xu et al., 2013).
The main risk factors for T2DM and HUA are insulin resistance and obesity, which often co−exist and interact with each other (Li et al., 2013). Due to the increased prevalence and co−existence of T2DM and HUA leading to serious complications and few clinical trials investigating the use of drugs in both diseases (Mortada, 2017), active and effective measures should be sought to improve T2DM combined with HUA. The current treatment of T2DM with HUA with western medicine (WM) is controversial and is associated with unavoidable adverse effects. Available clinical trials have shown that febuxostat and benzbromarone both have uric acid-lowering and hypoglycemic effects (Shang et al., 2017), but the common adverse effects of febuxostat are abnormal liver function, rash, nausea, arthralgia, and high cardiovascular risk (Love et al., 2010; White et al., 2018), while benzbromarone has serious hepatotoxicity and was withdrawn from the European market in 2003 (Azevedo et al., 2014). New glucose−lowering drugs can achieve glucose−lowering and uric acid−lowering effects. However, the current research is small, the applicable population is not limited, and urinary adverse effects may occur (Zhang J. Y. et al., 2022). Some patients may not respond to existing uric acid−lowering drugs (Major et al., 2018), and existing uric acid−lowering drugs have been found to increase cardiovascular events and cause severe liver damage (Zhang J. Y. et al., 2022). The use of uric acid−lowering therapy can increase the risk of hypersensitivity reactions and severe skin reactions if there are comorbidities, predominantly renal and cardiovascular disease (Bardin and Richette, 2017). Uric acid can be obtained through the external intake of purine−rich foods such as seafood and meat, so healthy dietary management is also essential for preventing and treating HUA (Adachi et al., 2017). T2DM and HUA have the potential for a bi−directional causal relationship. They are physiopathologically mutually reinforcing, but there are many constraints on treatment options, so the search for alternative therapies is urgent.
Traditional Chinese medicine (TCM) has been widely used as a complementary and alternative therapy throughout Chinese history. TCM is used in accordance with TCM theory, which obtains clinical information through the four diagnoses and then provides individualized treatment plans for patients. As TCM continues to develop, its clinical value is becoming more recognized worldwide. Chinese herbal medicine (CHM), as the primary therapy of TCM, has the characteristics of being easily accessible, safe, and effective. It has been proved that CHM can exert the efficacy of lowering glucose and lipid, and uric acid through multi−target and multi−channel (Zhou et al., 2013; Li Z. N. et al., 2020). However, there is no systematic evaluation or meta−analysis to date on the efficacy and safety of CHM for the treatment of T2DM with HUA. Therefore, this study aims to evaluate the efficacy and safety of CHM in the treatment of T2DM with HUA and to provide high−quality clinical evidence and scientifically optimized treatment strategies.
2 Methods
This systematic evaluation and meta−analysis were performed according to the Preferred Reporting Items for Systematic Evaluation and Meta−Analysis (PRISMA−P) guidelines (Moher et al., 2015) (Supplementary File S1). Moreover, the protocol of this evaluation is registered with PROSPERO (CRD42022351519).
2.1 Search strategy
A comprehensive computer search of eight databases was conducted: Pubmed, Cochrane Library, Embase, Web of Science, China National Knowledge Infrastructure (CNKI), Wanfang database, China Biomedical Database (CBM), and China Science and Technology Journal Database (VIP). Ongoing or completed but unpublished randomized controlled trials (RCTs) were also searched on the China Clinical Trials Registry (CHiCTR) (http://www.chictr.org.cn/index.aspx) and ClinicalTrials.gov. The search period is from the establishment of the database until November 2022. All RCTs of CHM for the treatment of T2DM with HUA were included, with no restrictions on language. The search was conducted using a combination of subject terms and free words, including “type 2 diabetes”, “diabetes, type 2″, “hyperuricemia,” “Chinese medicine,” “traditional medicine,” “Chinese herbal medicine,” etc. Comprehensive search strategies for the databases are shown in the (Supplementary Table S1).
2.2 Inclusion and exclusion criteria
RCTs assessing the effect of CHM on T2DM with HUA were included in this study. The inclusion criteria were as follows: 1) Study format: RCTs; 2) Study subjects: patients with clinically confirmed T2DM with HUA or with gout (regardless of age, gender, race or nationality); 3) Interventions: CHM (regardless of dosage form and duration) combined with WM in the intervention group and WM in the control group; 4) Outcome evaluation indicators: the primary indicators of the study were fasting blood glucose (FBG), 2-h postprandial glucose (2hPG), glycated hemoglobin (HbA1c), and uric acid (UA). The secondary outcome indicators were triglycerides (TG), total cholesterol (TC), overall effective rate and adverse effects. The exclusion criteria were as follows: 1) non−RCTs, such as famous doctors’ experiences, animal experiments, retrospective studies, reviews, and other types of studies; 2) repeated publications; 3) lack of article results or data; and 4) Other TCM interventions besides CHM, such as acupuncture, moxibustion, and tui na, as well as herbal enemas.
2.3 Literature screening and data extraction
Two investigators (SP and YH) screened the literature by pre−established inclusion and exclusion criteria and imported the literature to be screened into EndNote X9 software to manage the literature. Two reviewers (HY and JT) independently extracted relevant data to the data statistics table, which included: the first author, year of publication, sample size, patient characteristics (age, gender, duration of disease, etc.), interventions (drug name, composition, etc.), duration of treatment, outcome indicators, and adverse effects. Two reviewers (HY and JT) check each other’s extracted data, and a third reviewer (HL) will assess and resolve any conflicts.
2.4 Risk of bias assessment
Two investigators (HL and SP) independently used the Cochrane Risk of Bias Assessment Tool (Higgins et al., 2022) to assess the quality of the included RCTs and determined “low risk of bias,” “high risk of bias,” and “unclear” for six types of bias, including selection bias, implementation bias, measurement bias, follow−up bias, reporting bias, and other biases.
2.5 Data analysis
Review Manager 5.4 software was used for data analysis. This study was assessed using the weighted mean differences (WMD) and 95% confidence interval (CI) for continuous type variables. The X2 test and I2 test responded to the heterogeneity of the data. If heterogeneity (I2 > 50%, p < 0.05) exists, a random−effects model is used; conversely, a fixed−effects model is used. When heterogeneity exists, we will explore the sources of heterogeneity through subgroup analysis. In addition, the funnel plot detected publication bias, and Egger’s test of Stata V14.0 was performed.
2.6 Sensitivity analysis
Sensitivity analysis was performed using Stata V14.0 to exclude the effect of a single RCT on the remaining study results. The robustness and reliability of the results were good if there was no significant difference before and after the exclusion of individual trials.
3 Results
3.1 Literature selection
Based on the pre−defined search strategy, we initially screened 790 documents, 241 of which were duplicates. After excluding duplicates, 488 non−conforming articles were excluded according to their titles and abstracts. The full text of the 61 remaining articles was read, and 31 were excluded for the following reasons: lack of outcome details (n = 21), not high−quality articles (n = 7), use of other TCM therapies (n = 5), and inconsistent interventions (n = 10). 18 RCTs (Yu, 2010; Peng et al., 2014; Tang, 2014; Wu, 2015; Yin, 2015; Lou, 2017; Tang et al., 2017; Zhou, 2018; Hou, 2019; Hu and Qu, 2019; Liu, 2020; Ma et al., 2020; Shao, 2020; Gu et al., 2021; Liu, 2021; Xie et al., 2021; Zhang et al., 2021; Zou and Jiang, 2021) were eventually included for systematic evaluation. The flow diagram of the selection process is shown in Figure 1.
3.2 Study characteristics
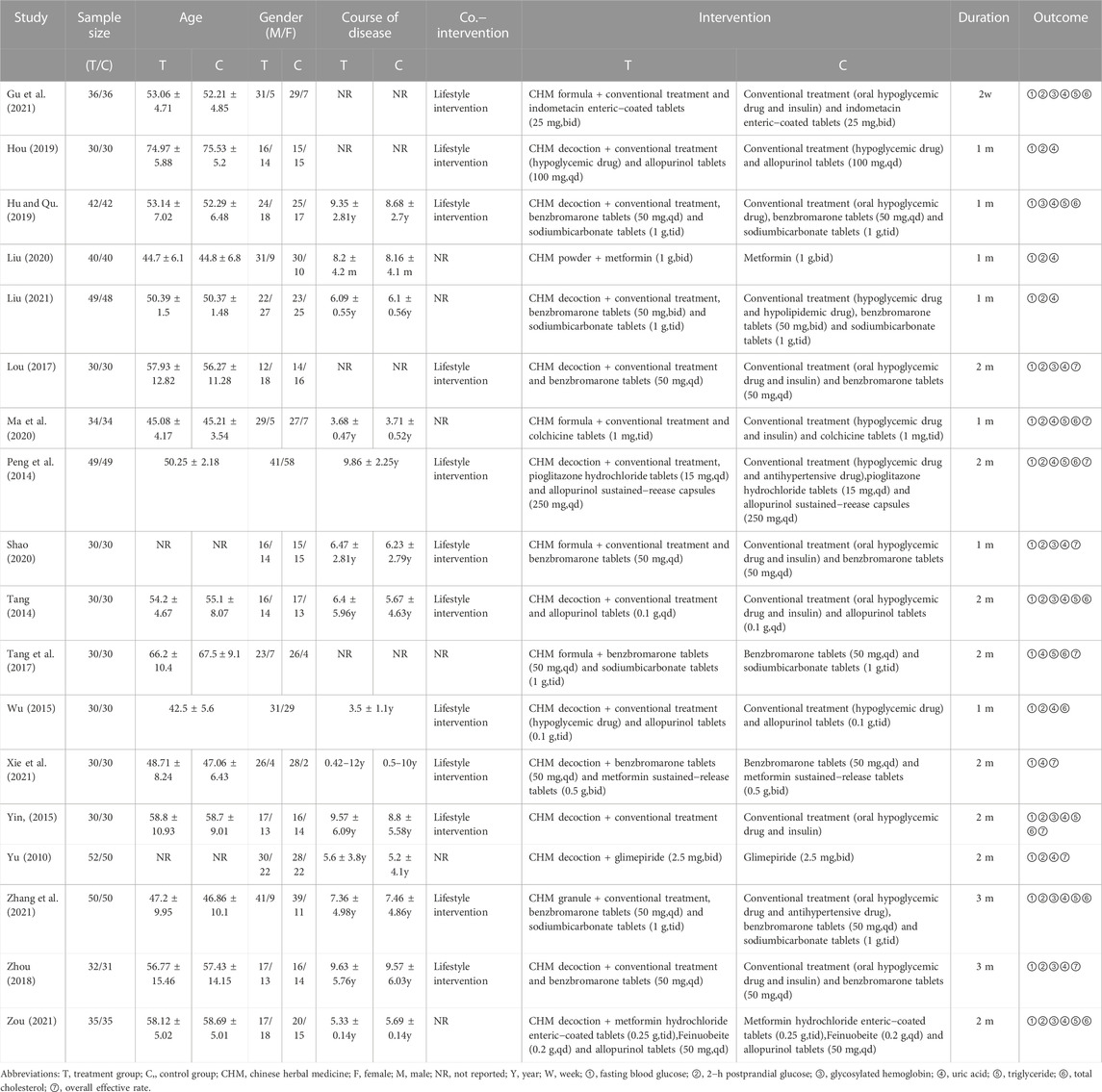
A total of 18 RCTs of CHM for T2DM with HUA conducted in China from 2014 to 2021 were included in this paper, involving a total of 1,311 patients, including 657 in the treatment group and 654 in the control group. Among the 18 included studies, the shortest intervention duration was 2 weeks (Gu et al., 2021), and the longest was 3 months (Zhou, 2018; Zhang et al., 2021). Looking at the interventions in the control group included in the study, only three studies (Yu, 2010; Yin, 2015; Liu, 2020) treated with glucose−lowering drugs alone, while 15 studies (Peng et al., 2014; Tang, 2014; Wu, 2015; Lou, 2017; Tang et al., 2017; Zhou, 2018; Ma et al., 2020; Shao, 2020; Liu, 2021; Xie et al., 2021; Zhang et al., 2021; Zou and Jiang, 2021) used a combination of glucose−lowering drugs and uric acid−lowering drugs. Table 1 summarizes the characteristics of the 18 RCTs, and Supplementary Table S2 lists specific information on CHM.
3.3 Risk of bias
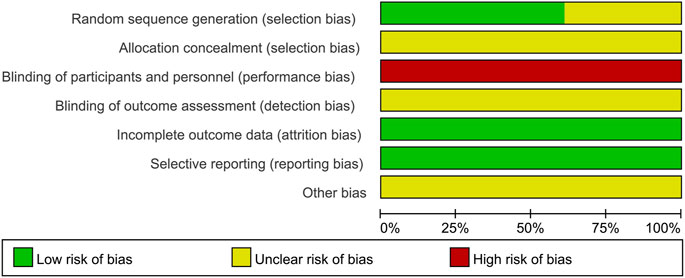
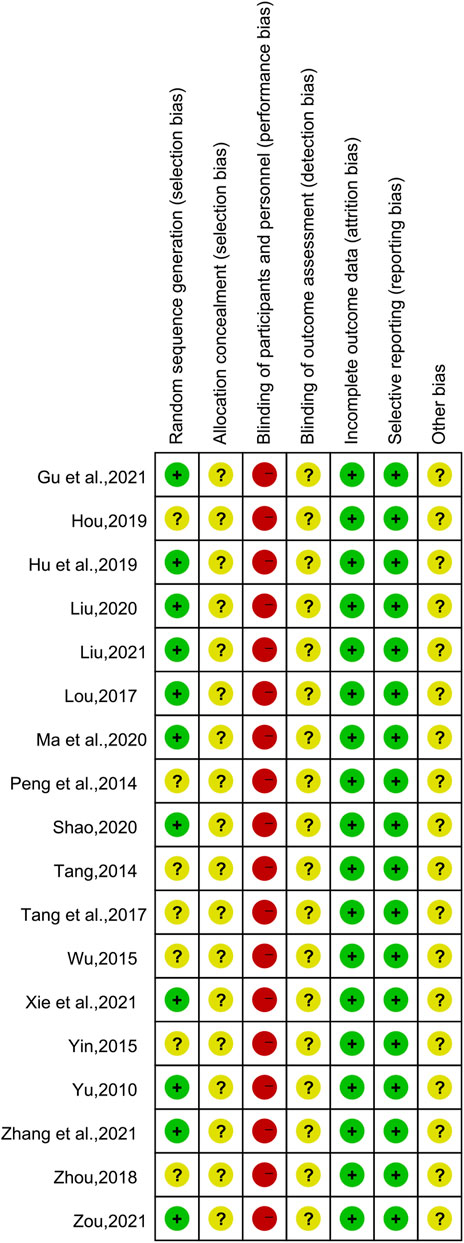
Of the 18 included studies, 11 studies (Yu, 2010; Lou, 2017; Hu and Qu, 2019; Liu, 2020; Ma et al., 2020; Shao, 2020; Gu et al., 2021; Liu, 2021; Xie et al., 2021; Zhang et al., 2021; Zou and Jiang, 2021) used the random number table method to generate random sequences and were at low risk of bias, while the remaining seven studies (Peng et al., 2014; Tang, 2014; Wu, 2015; Yin, 2015; Tang et al., 2017; Zhou, 2018; Hou, 2019) had unclear randomization and were at “unclear risk.” None of the studies demonstrated participant or study staff blindness and were therefore assessed as high risk of bias. All studies were assessed as low risk of bias in terms of incomplete data and reporting bias. All studies had unclear allocation concealment and did not have insufficient information to report other biases and were therefore assessed as “unclear risk.” The results of the risk bias assessment for the included studies are shown in Figure 2 and Figure 3.
3.4 Effects of CHM on glucose metabolism
3.4.1 FBG
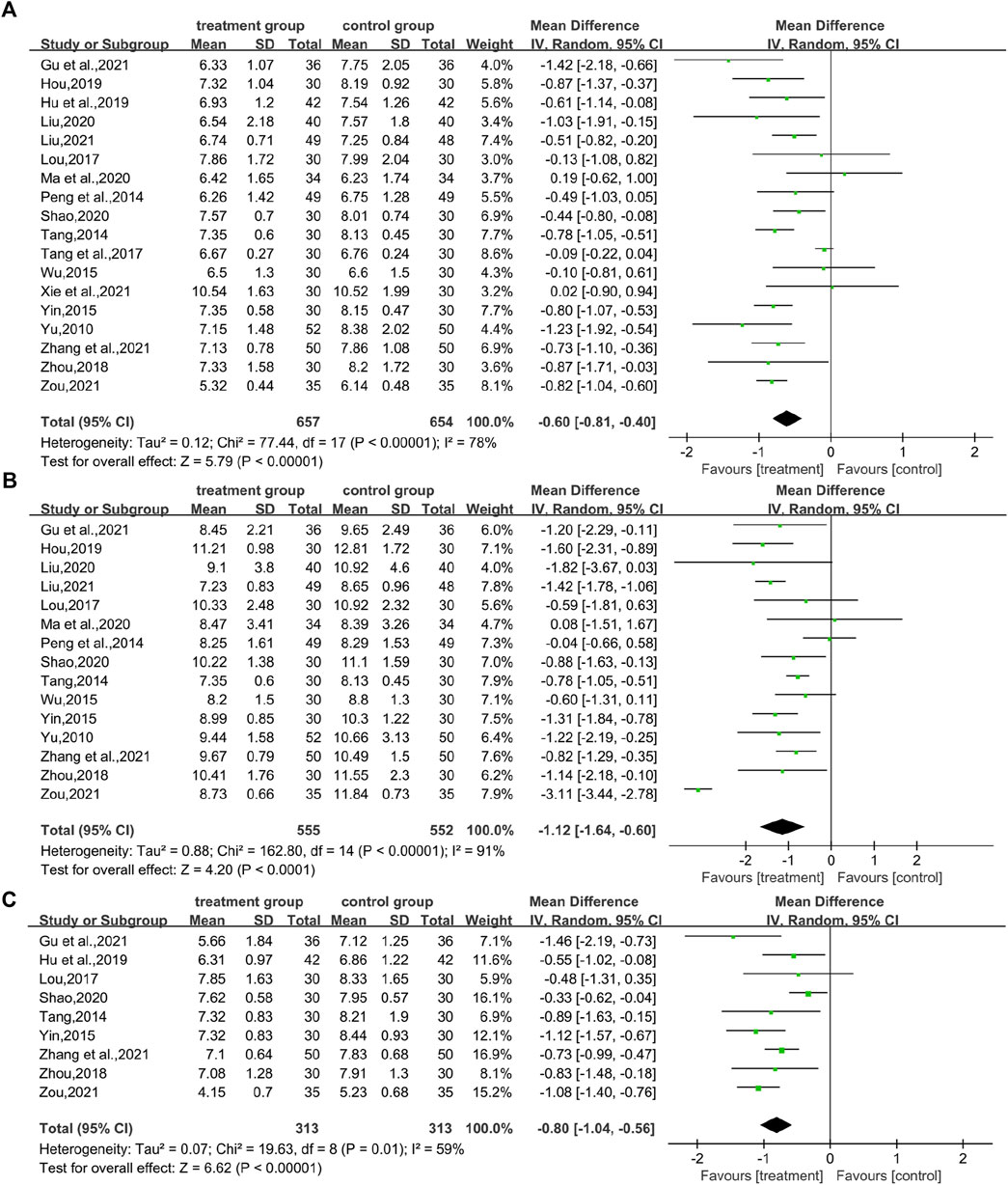
In total, 18 studies (Yu, 2010; Peng et al., 2014; Tang, 2014; Wu, 2015; Lou, 2017; Tang et al., 2017; Zhou, 2018; Hou, 2019; Hu and Qu, 2019; Liu, 2020; Ma et al., 2020; Shao, 2020; Gu et al., 2021; Liu, 2021; Xie et al., 2021; Zhang et al., 2021; Zou and Jiang, 2021) reported FBG levels in a total of 1,311 patients. The analysis showed that the combination of CHM and WM was effective in improving FBG levels in T2DM with HUA with statistical significance (WMD = −0.60.95% CI [−0.81, −0.40], p < 0.00001, I2 = 78, high heterogeneity, random effect model; Figure 4). In subgroup analysis results showed no differences between different intervention durations (p = 0.12), and different control treatments (p = 0.05), while differences were found between different types of drugs (p = 0.03). (Table 2; Supplementary Figure S1).
3.4.2 2hPG
In total, 15 studies (Yu, 2010; Peng et al., 2014; Tang, 2014; Wu, 2015; Lou, 2017; Zhou, 2018; Hou, 2019; Liu, 2020; Ma et al., 2020; Shao, 2020; Gu et al., 2021; Liu, 2021; Zhang et al., 2021; Zou and Jiang, 2021) evaluated 2hPG levels, involving a total of 1,107 patients. The overall analysis showed that the combination of CHM and WM improved the 2hPG level in T2DM with HUA better than WM alone with statistical significance (WMD = −1.12.95% CI [−1.64, −0.60], p < 0.0001, I2 = 91, high heterogeneity, random effect model; Figure 4). In the results of the subgroup analysis, there were no differences between the different intervention durations, different types of drugs, and different control treatments (p = 0.83, 0.82, and 0.5, respectively). (Table 2; Supplementary Figure S1).
3.4.3 HbA1c
A total of nine studies (involving a total of 626 patients) (Tang, 2014; Yin, 2015; Lou, 2017; Zhou, 2018; Hu and Qu, 2019; Shao, 2020; Gu et al., 2021; Zhang et al., 2021; Zou and Jiang, 2021) reported on HbA1c levels in patients with T2DM with HUA in CHM. The results showed that the combination of CHM and WM had a statistically significant reduction in HbA1c levels in T2DM with HUA (WMD = −0.80.95% CI [−1.04, −0.56], p < 0.00001, I2 = 59, high heterogeneity, random effect model; Figure 4). Subgroup analysis showed differences by intervention durations (p = 0.0008) and by types of drugs (p = 0.0005), while there were no differences by control treatments (p = 0.16). (Table 2; Supplementary Figure S1).
3.5 Effect of CHM on uric acid
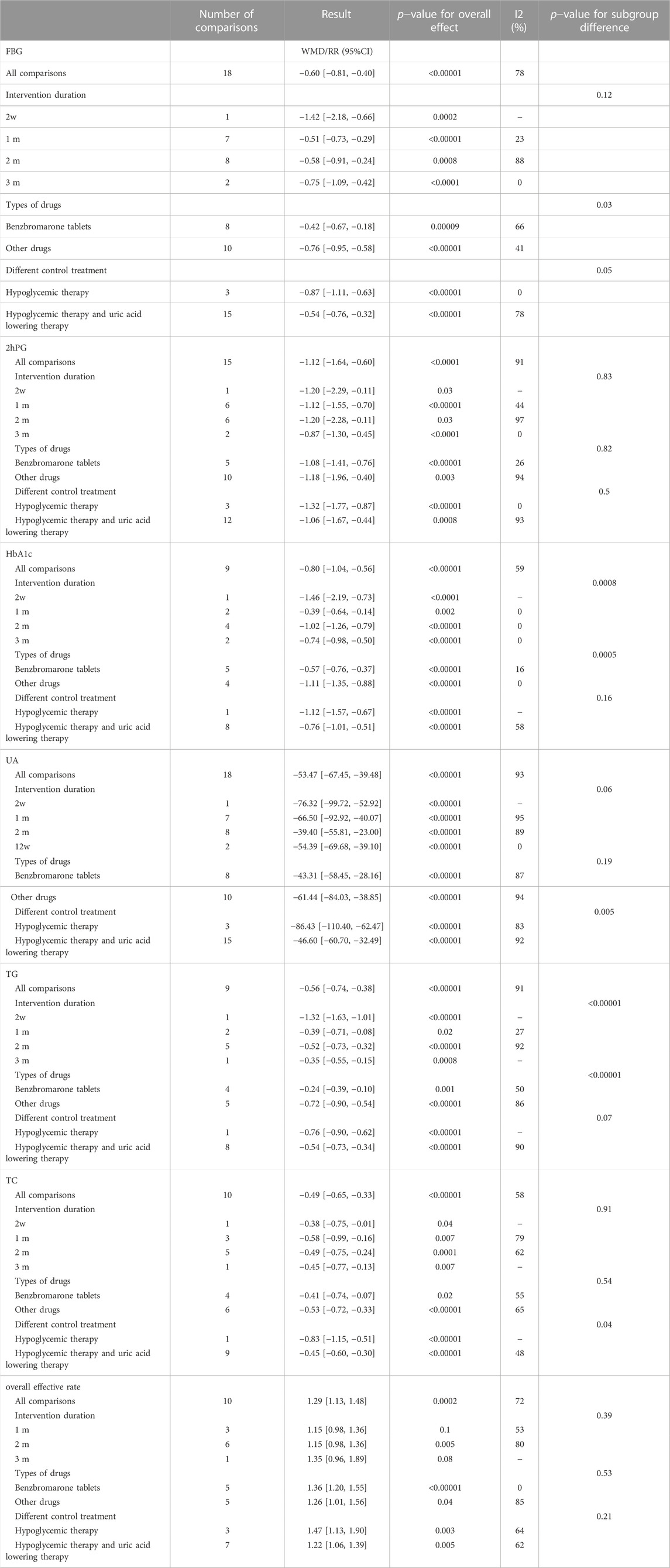
A total of 18 studies (Yu, 2010; Peng et al., 2014; Tang, 2014; Wu, 2015; Lou, 2017; Tang et al., 2017; Zhou, 2018; Hou, 2019; Hu and Qu, 2019; Liu, 2020; Ma et al., 2020; Shao, 2020; Gu et al., 2021; Liu, 2021; Xie et al., 2021; Zhang et al., 2021; Zou and Jiang, 2021) used uric acid levels as an outcome indicator, involving a total of 1,311 patients. In terms of improving uric acid levels, the combination of CHM and WM was statistically more effective (WMD = −53.47.95% CI [−67.45, −39.48], p < 0.00001, I2 = 93, high heterogeneity, random effects model; Figure 5). The results of the subgroup analysis showed no differences between the different intervention durations (p = 0.06), between the different types of drugs (p = 0.19), and differences between the different control treatments (p = 0.005). (Table 2; Supplementary Figure S1).
3.6 Effects of CHM on blood lipids
3.6.1 TG
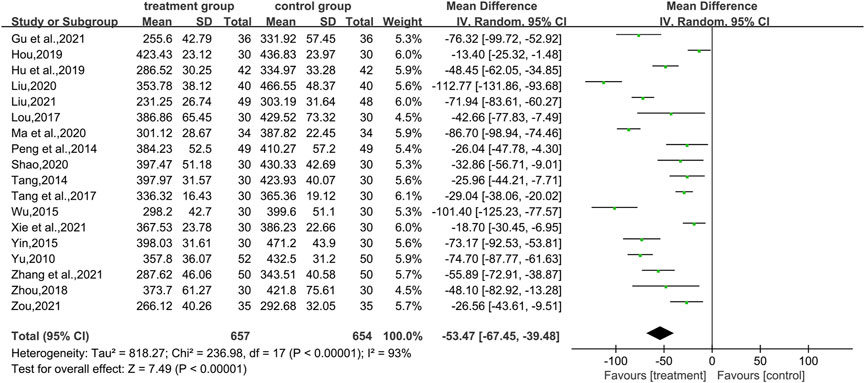
In total, nine studies (Peng et al., 2014; Tang, 2014; Yin, 2015; Tang et al., 2017; Hu and Qu, 2019; Ma et al., 2020; Gu et al., 2021; Zhang et al., 2021; Zou and Jiang, 2021) reported TG levels, covering 672 patients. There was a difference between the two groups (WMD = −0.56.95% CI [−0.74, −0.38], p < 0.00001, I2 = 91, high heterogeneity, random effects model; Figure 6). It indicates that the combination of CHM and WM effectively improved TG levels in patients with T2DM with HUA, which was statistically significant. Regarding the subgroup analysis, differences were found by intervention durations (p < 0.00001) and by types of drugs (p < 0.00001), while there were no differences by control treatments (p = 0.07). (Table 2; Supplementary Figure S1).
3.6.2 TC
The TC index was mentioned in 10 studies (Peng et al., 2014; Tang, 2014; Wu, 2015; Yin, 2015; Tang et al., 2017; Hu and Qu, 2019; Ma et al., 2020; Gu et al., 2021; Zhang et al., 2021; Zou and Jiang, 2021) involving a total of 732 patients. The analysis differed between the two groups (WMD = −0.49.95% CI [−0.65, −0.33], p < 0.00001, I2 = 58, high heterogeneity, random effects model; Figure 6). In terms of subgroup analysis, there were no differences by intervention durations (p = 0.91) and by types of drugs (p = 0.54), while differences existed by control treatments (p = 0.04). (Table 2; Supplementary Figure S1).
3.7 Overall effective rate and adverse effects
3.7.1 Overall effective rate
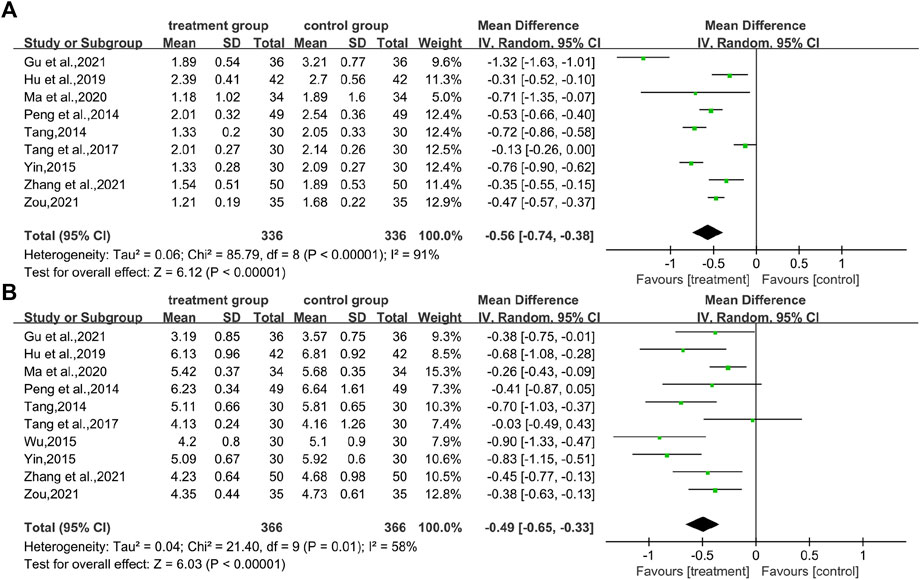
In total, 10 studies (Yu, 2010; Peng et al., 2014; Yin, 2015; Lou, 2017; Tang et al., 2017; Zhou, 2018; Liu, 2020; Ma et al., 2020; Shao, 2020; Xie et al., 2021) reported total effective rates, involving a total of 718 patients. The analysis showed that the combination of CHM and WM effectively improved the overall effective rate of T2DM with HUA in a statistically significant manner compared with WM alone (RR = 1.29.95% CI [1.13, 1.48], p = 0.0002, I2 = 72%, high heterogeneity, random effects model; Figure 7). In the results of the subgroup analysis, there were no differences between the different intervention durations, different types of drugs, and different control treatments (p = 0.39, 0.53, and 0.21, respectively). (Table 2; Supplementary Figure S1).
3.7.2 Adverse effects
Adverse effects were mentioned in nine studies. Six of these studies (Yu, 2010; Yin, 2015; Zhou, 2018; Hou, 2019; Ma et al., 2020; Shao, 2020) showed no adverse events in patients while taking the drug, and three other studies (Hu and Qu, 2019; Liu, 2021; Zhang et al., 2021) reported adverse events. The most common adverse reactions that occur when combining CHM and WM are gastrointestinal discomfort, such as bloating and diarrhea; skin abnormalities such as itching and rash may also occur.
3.8 Publication bias
Results from ten or more included studies were assessed for publication bias. Publication bias needs to be assessed by funnel plot and Egger’s test. There was a slight asymmetry in the funnel plots (Figures 8B,C,E) and Egger’s test indicated possible publication bias for 2hPG (t = −3.95, p = 0.002), UA (t = −3.21, p = 0.005) and overall effective rate (t = 6.51 p < 0.0001) (Supplementary Figure S2). While the rest of the funnel plots (Figures 8A,D) showed symmetrical funnel plots, Egger’s test indicated no publication bias for FBG (t = −1.36, p = 0.191) and TC (t = −2.09, p = 0.07) (Supplementary Figure S2).

FIGURE 8. Funnel plots for assessing publication bias. (A) FBG (B) 2hPG (C) UA (D) TC; and (E) overall effective rate.
3.9 Sensitivity analysis
To ensure the robustness and reliability of the analysis results, sensitivity analysis was performed using Stata V14.0. No significant changes in the results of the remaining trials were observed after the exclusion of each study, indicating that the results were robust and reliable (Supplementary Figure S3).
4 Discussion
4.1 Research results
A total of 790 publications were retrieved for this study, and 18 RCTs involving 1,311 patients were finally included after a detailed review of the literature. This systematic evaluation and meta−analysis assessed the efficacy and safety of CHM in the treatment of T2DM with HUA. The results of the study showed that the combination of CHM and WM was more effective in treating patients with T2DM with HUA than WM alone, with significant improvements in glucose metabolism (FBG, 2hPG, HbA1c), UA, and lipids (TG, TC). The high heterogeneity present in the data analysis stemmed from differences in interventions. The sources of heterogeneity were examined by subgroup analysis, using different intervention durations, different types of drugs, and different control treatments to explain the effect of heterogeneity. In the literature assessing safety, most of the adverse reactions were not reported, and a few had non−serious adverse reactions. Funnel plot analysis and Egger’s test revealed possible publication bias for some indicators. The heterogeneity of this study is high and the methodological quality is low, but sensitivity analysis indicates that the results are robust.
4.2 Quality of evidence
We used GRADEpro to describe the quality of evidence for the results. The results, assessed according to study limitations, inconsistencies, indirectness, imprecision, and publication bias, indicated that the quality of evidence was low for all outcomes (Table 3). The low quality of evidence is mainly due to the low methodological quality and high heterogeneity. Therefore, further assessment of clinical effectiveness and safety through large−scale, high−quality, comprehensive, and standardized RCT results is needed.
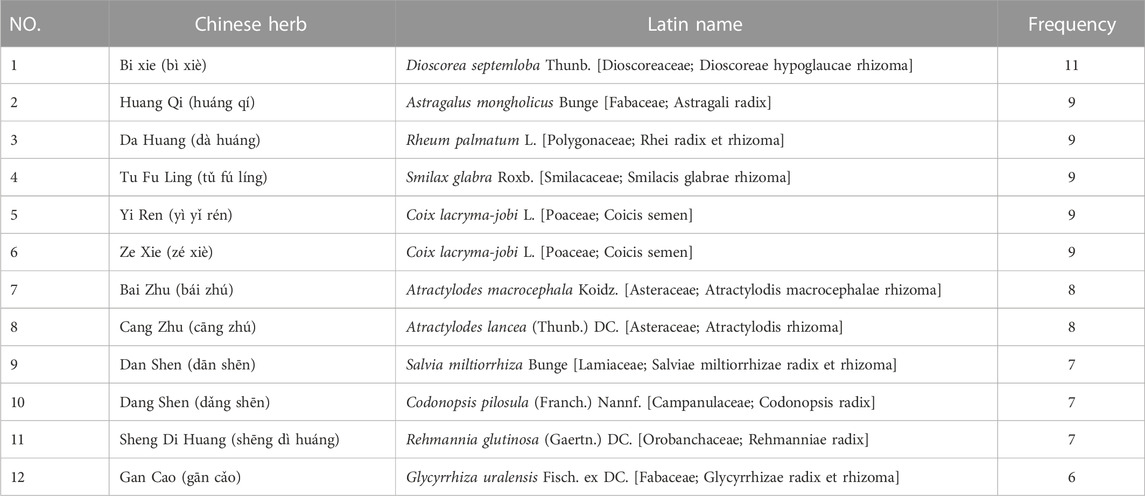
4.3 Frequency of Chinese herb medicines
70 single CHMs were recorded in the 18 RCTs included in this study, and those with more than five occurrences are listed in the table in descending order of frequency (Table 4). The most frequently occurring CHMs was Dioscorea septemloba Thunb (bì xiè), which appeared 11 times. The results of the study of the pharmacological effects of Dioscorea septemloba Thunb. Showed that the total saponin of Dioscorea is its main component and the primary substance that exerts its pharmacological activity (Chen et al., 2017), with hyporheic acid, renal protection, and anti−inflammatory and immunomodulatory effects (Xiao and Li, 2018). Total saponin of Dioscorea reduces uric acid reabsorption by down−regulating the expression of renal tubular uric acid transporter one and up−regulates the expression of organic anion transporter, thereby increasing uric acid excretion (Chen et al., 2013; Zhu, 2013; Chen et al., 2015); total saponin of Dioscorea attenuates sodium urate−induced inflammatory response and improves insulin resistance (Li G. Y. et al., 2020; Guoying et al., 2021). Other CHMs such as Astragalus mongholicus Bunge also have related pharmacological effects. Astragalus mongholicus Bunge has a complex chemical composition with a high content of flavonoids, polysaccharides, and saponins, which are the main active components of Astragalus (Zhang S. J. et al., 2022). Astragalus polysaccharide can exert hypoglycemic effects by upregulating the expression of glucose transporter protein four mRNA; in addition, astragaloside inhibits hepatic gluconeogenesis by regulating the PI3K/Akt/FoxO1 signaling pathway to regulate blood glucose, while protecting against the damage caused to pancreatic β−cells after activation of uric acid by this pathway, and also effectively improves insulin resistance by inhibiting protein tyrosine phosphatase 1B in insulin−resistant human hepatocytes HepG2 cells (Dai et al., 2022; Jiang et al., 2022). In terms of improving uric acid levels, astragalus polysaccharides achieve inhibition of uric acid production by inhibiting xanthine oxidase activity (Li et al., 2021).
4.4 Research strengths and limitations
This study is the first to focus on and evaluate CHM’s effectiveness in treating T2DM with HUA. The methods and requirements of systematic evaluation were strictly followed during the study, and GRADEpro was used to describe the quality of evidence for the results. In contrast, the results were interpreted with care. The results showed that the combination of CHM and WM treatment improved glucolipid metabolism, uric acid levels, and overall efficiency in patients with T2DM with HUA with fewer adverse effects than WM alone. Therefore, it can provide a new idea and direction for clinical treatment.
However, this study also has some limitations. First, significant heterogeneity was demonstrated in the analysis. Although subgroup analysis was performed to explain the source of the high heterogeneity, the heterogeneity may also be related to patient gender, herbal dosage form, and other reasons. Second, the quality of evidence was poor for all outcomes, and the presence of low methodological quality and high heterogeneity due to uncertainty in publication bias mostly may have affected the results. In addition, although two investigators independently performed the assessment of risk bias, there was subjectivity in the evaluation of risk bias. More, obesity is a common influencing factor for T2DM and HUA, while contributing to each other’s development through inflammatory responses. However, the present study outcome indicators did not involve body mass index and some inflammatory indicators. Finally, external intake of purine−rich foods is an essential source of HUA, making dietary management particularly important for improving T2DM with HUA. Moreover, this study could not determine the effect of health management on patients with T2DM with HUA, which provides a direction for further studies to follow.
5 Conclusion
In conclusion, this study shows that CHM combined with WM is effective in treating patients with T2DM with HUA and improving glucolipid metabolism and high uric acid levels, with high overall clinical efficiency. However, due to the low quality of the evidence, it should be used with caution in clinical settings. Therefore, high−quality, large−sample RCTs will be needed in the future to provide more accurate, credible, and convincing evidence for the efficacy and safety of CHM in the treatment of T2DM with HUA.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
HL and XZ conceived and designed the study. HL, SP, YH, HY, and JT conducted this meta−analysis. SP and YH drafted the manuscript. HY and JT revised this article. XZ supervised all aspects of this study. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Natural Science Foundation of Sichuan Provincial Science and Technology Department (2022NSFSC1277), and Science and Technology Development Fund of Affiliated Hospital of Chengdu University of Traditional Chinese Medicine (Y2022091).
Acknowledgments
We would like to thank all the authors who contributed to the systematic review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1102513/full#supplementary-material
Abbreviations
2hPG, 2-h postprandial glucose; CHM, chinese herbal medicine; CI, confidence interval; CNKI, china national knowledge infrastructure; FBG, fasting blood glucose; GRADE, grading of recommendations assessment, development, and evaluation; HbA1c, glycated hemoglobin; HUA, hyperuricemia; PRISMA-P, preferred reporting items for systematic review and meta-analysis protocols; RCTs, randomized controlled trials; T2DM, type 2 diabetes mellitus; TC, total cholesterol; TG: triglyceride; RR, risk ratio; TCM, traditional chinese medicine; UA, uric acid; WM, western medicine; WMD, weighted mean differences.
References
Adachi, S. I., Yoshizawa, F., and Yagasaki, K. (2017). Hyperuricemia in type 2 diabetic model KK-A(y)/Ta mice: A potent animal model with positive correlation between insulin resistance and plasma high uric acid levels. BMC Res. Notes 10 (1), 577. doi:10.1186/s13104-017-2897-x
Ahmadieh, H., and Azar, S. (2017). Effects of sodium glucose cotransporter-2 inhibitors on serum uric acid in type 2 diabetes mellitus. Diabetes Technol. Ther. 19 (9), 507–512. doi:10.1089/dia.2017.0070
Azevedo, V. F., Buiar, P. G., Giovanella, L. H., Severo, C. R., and Carvalho, M. (2014). Allopurinol, benzbromarone, or a combination in treating patients with gout: Analysis of a series of outpatients. Int. J. Rheumatol. 2014, 263720. doi:10.1155/2014/263720
Bardin, T., and Richette, P. (2017). Impact of comorbidities on gout and hyperuricaemia: An update on prevalence and treatment options. BMC Med. 15 (1), 123. doi:10.1186/s12916-017-0890-9
Chen, C., Zeng, C. H., Zhang, S. Q., and Wei, L. (2017). Research progress of bixie. China J. Chin. Materia Medica 42 (18), 3488–3496. doi:10.19540/j.cnki.cjcmm.20170731.005
Chen, G. L., Zhu, L. R., Na, S., and Li, L. (2013). Effect of total saponin of Dioscorea on chronic hyperuricemia and expression of URAT1 in rats. China J. Chin. Materia Medica 38 (14), 2348–2353. doi:10.4268/cjcmm20131427
Chen, Y., Chen, X. L., Liu, M. T., Luo, H. X., Xiang, T., Zhang, S. J., et al. (2015). Exploration of the mechanism of uric acid reduction by total saponin of Dioscorea based on oatp1a1 expression in hyperuricemic rats. Lishizhen Med. Materia Medica Res. 26 (10), 2330–2332.
Dai, Y. T., Zhang, X. Y., Wang, Y. X., Fan, Y. S., Yang, D., Liu, Y. Q., et al. (2022). Research progress on Astragali Radix and prediction of its quality markers (Q-markers). China J. Chin. Materia Medica 47 (07), 1754–1764. doi:10.19540/j.cnki.cjcmm.20211213.201
Donkeng, M., Kuaté, D., Koudjou, P. N., Noubiap, J. J., and Kuiate, J. R. (2021). Association between hyperuricemia and glycated hemoglobin in type 2 diabetes at the District Hospital of Dschang. Pan Afr. Med. J. 40, 177. doi:10.11604/pamj.2021.40.177.30207
Gao, Z., Zuo, M., Han, F., Yuan, X., Sun, M., Li, X., et al. (2019). Renal impairment markers in type 2 diabetes patients with different types of hyperuricemia. J. Diabetes Investig. 10 (1), 118–123. doi:10.1111/jdi.12850
Ghasemi, A. (2021). Uric acid-induced pancreatic β-cell dysfunction. BMC Endocr. Disord. 21 (1), 24. doi:10.1186/s12902-021-00698-6
Gois, P. H. F., and Souza, E. R. M. (2017). Pharmacotherapy for hyperuricemia in hypertensive patients. Cochrane Database Syst. Rev. 4 (4), Cd008652. doi:10.1002/14651858.CD008652.pub3
Gu, J., Lin, L. Y., and Hu, Y. (2021). Clinical observation on 72 cases of type 2 diabetes mellitus complicated with gouty arthritis treated by integrated traditional Chinese and Western medicine. Chin. J. General Pract. 19 (04), 656–659. doi:10.16766/j.cnki.issn.1674-4152.001886
Guoying, L., Li, L., Siyue, Y., Lei, L., and Guangliang, C. (2021). Total saponin of Dioscorea collettii attenuates MSU crystal-induced inflammation by inhibiting the activation of the TLR4/NF-κB signaling pathway. Evid. Based Complement. Altern. Med. 2021, 8728473. doi:10.1155/2021/8728473
Higgins, T. J., Thomas, J., Cumpston, M., and Chandler, J. (2022). “Cochrane handbook for systematic reviews of interventions,”. Version 6.3.
Hou, Z. (2019). Clinical study of kaiyu yunpi decoction in the treatment of senile type 2 diabetes mellitus with hyperuricemia. Master’s thesis. Shandong, Jinan: Shandong University of Traditional Chinese Medicine.
Hu, Y., and Qu, H. X. (2019). The treatment of type 2 diabetes mellitus with hyperuricemia (damp-heat stagnation syndrome) with Self-made Jianpi Yishen Huazhuo decoction to resolve turbidity. acidemia (damp heat and stagnation), glucose and lipid metabolism, blood uric acid and insulin resistance. J. Sichuan Traditional Chin. Med. 37 (02), 127–129.
Jiang, Z., Wang, G., Meng, L., Tang, Y., Yang, M., and Ni, C. (2022). Protective effects of astragaloside IV on uric acid-induced pancreatic β-cell injury through PI3K/AKT pathway activation. Evid. Based Complement. Altern. Med. 2022, 2429162. doi:10.1155/2022/2429162
Kojima, S., Matsui, K., Hiramitsu, S., Hisatome, I., Waki, M., Uchiyama, K., et al. (2019). Febuxostat for cerebral and CaRdiorenovascular events PrEvEntion StuDy. Eur. Heart J. 40 (22), 1778–1786. doi:10.1093/eurheartj/ehz119
Krishnan, E., Akhras, K. S., Sharma, H., Marynchenko, M., Wu, E. Q., Tawk, R., et al. (2013). Relative and attributable diabetes risk associated with hyperuricemia in US veterans with gout. Qjm 106 (8), 721–729. doi:10.1093/qjmed/hct093
Li, C., Hsieh, M. C., and Chang, S. J. (2013). Metabolic syndrome, diabetes, and hyperuricemia. Curr. Opin. Rheumatol. 25 (2), 210–216. doi:10.1097/BOR.0b013e32835d951e
Li, F. Q., and Ye, Z. B. (2017). Research progress of hyperuricemia associated with diabetes mellitus and diabetic complications. Chin. J. Pract. Intern. Med. 37 (06), 569–572. doi:10.19538/j.nk2017060122
Li, G. Y., Zhang, W. Z., Jiang, L., and Chen, G. L. (2020a). Effect of total saponin of dioscoreae collettii rhizoma on toll-like receptor/nuclear factor-κb (TLR/NF-κB) signaling pathway induced by monosodium urate in THP-1 cells. Chin. J. Exp. Traditional Med. Formulae 26 (05), 34–41. doi:10.13422/j.cnki.syfjx.20200401
Li, J., Jiang, L. L., Li, M. X., Deng, F., Xu, S. Y., Xue, X., et al. (2021). Inhibitory effects of Astragalus polysaccharide on activity of xanthine oxidase. J. Food Sci. Biotechnol. 40 (10), 16–20.
Li, Z. N., Zhou, Y. Y., Xing, Y. T., Xing, Y. Y., Ma, S. H., and Dou, D. M. (2020b). Effect of biling qutong decoction on expression of visfatin protein in myocardial tissue of diabetic gout rats. J. Liaoning Univ. Traditional Chin. Med. 22 (04), 17–20. doi:10.13194/j.issn.1673-842x.2020.04.005
Liu, J. (2021). Effect of Jianpi Yishen Huazhuo decoction on patients with type 2 diabetes mellitus with hyperuricemia. Heilongjiang Med. Pharm. 44 (05), 127–128.
Liu, J. H., Wu, M. Z., Li, S. M., Chen, Y., Ren, Q. W., Lin, Q. S., et al. (2021). Association of serum uric acid with biventricular myocardial dysfunction in patients with type 2 diabetes mellitus. Nutr. Metab. Cardiovasc Dis. 31 (10), 2912–2920. doi:10.1016/j.numecd.2021.06.012
Liu, W. C. (2020). Clinical observation on the treatment of asymptomatic hyperuricemia with type 2 diabetes mellitus by combining Qiwei Baizhu powder with metformin. Diabetes New World 23 (01), 52–53. doi:10.16658/j.cnki.1672-4062.2020.01.052
Lou, Y., Qin, P., Wang, C., Ma, J., Peng, X., Xu, S., et al. (2020). Sex-specific association of serum uric acid level and change in hyperuricemia status with risk of type 2 diabetes mellitus: A large cohort study in China. J. Diabetes Res. 2020, 9637365. doi:10.1155/2020/9637365
Lou, Y. (2017). The clinical observation of treating Type 2 Diabetes with Hyperuricemia by Baihu Erdi decoction. Master’s thesis. China: Nanjing University of Chinese Medicine.
Love, B. L., Barrons, R., Veverka, A., and Snider, K. M. (2010). Urate-lowering therapy for gout: Focus on febuxostat. Pharmacotherapy 30 (6), 594–608. doi:10.1592/phco.30.6.594
Lu, Y. H., Chang, Y. P., Li, T., Han, F., Li, C. J., Li, X. Y., et al. (2020). Empagliflozin attenuates hyperuricemia by upregulation of ABCG2 via AMPK/AKT/CREB signaling pathway in type 2 diabetic mice. Int. J. Biol. Sci. 16 (3), 529–542. doi:10.7150/ijbs.33007
Ma, J., Ji, Y. L., Liu, M. J., Zhang, Y., Rong, H., and Zhao, N. (2020). Clinical Research of Qingli Xiaotong Decoction on Type 2 Diabetes Mellitus Complicated with Gout Arthritis (Dampness and Heat Accumulation Syndrome). J. Emerg. Traditional Chin. Med. 29 (02), 233–235+240. doi:10.3969/j.issn.1004-745X.2020.02.012
Major, T. J., Dalbeth, N., Stahl, E. A., and Merriman, T. R. (2018). An update on the genetics of hyperuricaemia and gout. Nat. Rev. Rheumatol. 14 (6), 341–353. doi:10.1038/s41584-018-0004-x
Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 4 (1), 1. doi:10.1186/2046-4053-4-1
Mortada, I. (2017). Hyperuricemia, type 2 diabetes mellitus, and hypertension: An emerging association. Curr. Hypertens. Rep. 19 (9), 69. doi:10.1007/s11906-017-0770-x
Peng, Y. H., Ye, H. H., and Zhao, N. (2014). Clinical analysis on Kaiyu Qingre treatment for type 2 diabetes mellitus with hyperuricemia. Clin. J. Chin. Med. 6 (09), 53–55. doi:10.3969/j.issn.1674-7860.2014.09.025
Shang, X. Y., Deng, L., Song, Y. L., and Han, L. L. (2017). Benefit and safety of benzbromarone and febuxostat in the treatment of type 2 diabetes with hyperuricemia. Pract. Pharm. Clin. Remedies 20 (04), 402–405. doi:10.14053/j.cnki.ppcr.201704010
Shao, M. S. (2020). Clinical Study of Jiedu Tongluo Baoshen Jiangzhuo Decoction inthe Treatment of type 2 diabetes mellitus complicated with hyperuricemia. Master’s thesis. China: Changchun University Of Chinese Medicine.
Su, H., Liu, T., Li, Y., Fan, Y., Wang, B., Liu, M., et al. (2021). Serum uric acid and its change with the risk of type 2 diabetes: A prospective study in China. Prim. Care Diabetes 15 (6), 1002–1006. doi:10.1016/j.pcd.2021.06.010
Tang, C. M. (2014). Spleen and kidney, bloos stasis, turbidity method to treat diabetes combined with high uric acid hematic disease the clinical research. Master’s thesis. China: Shandong University of Traditional Chinese Medicine.
Tang, F., Zhang, X. Y., and Xu, W. D. (2017). Clinical observation of gout and type 2 diabetes patients by treatment of yinshandan decoction and western medicine. J. Jiangxi Univ. Chin. Med. 29 (06), 48–50.
Viazzi, F., Piscitelli, P., Giorda, C., Ceriello, A., Genovese, S., Russo, G., et al. (2017). Metabolic syndrome, serum uric acid and renal risk in patients with T2D. PLoS One 12 (4), e0176058. doi:10.1371/journal.pone.0176058
White, W. B., Saag, K. G., Becker, M. A., Borer, J. S., Gorelick, P. B., Whelton, A., et al. (2018). Cardiovascular safety of febuxostat or allopurinol in patients with gout. N. Engl. J. Med. 378 (13), 1200–1210. doi:10.1056/NEJMoa1710895
Wu, S. L. (2015). Huang ling ze xie soup treatment of type 2 diabetes mellitus and high uric acid hematic disease. Women's Health Res. 23 (24), 182–182,191.
Xiao, Y., and Li, G. Z. (2018). Progress in the study of the pharmacological effects of Bixie. Shanxi J. Traditional Chin. Med. 34 (07), 54–56.
Xie, X. C., Gong, M., Chen, W. W., Yang, A. H., and Liu, M. (2021). Clinical observation of Lingbi Simiao Decoction combined with Western medicine in the treatment of type 2 diabetes with hyperuricemia in spleen-kidney deficiency and turbid toxin internal resistance syndrome. China J. Traditional Chin. Med. Pharm. 36 (09), 5686–5688.
Xu, L., Li, Y., Dai, Y., and Peng, J. (2018). Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharmacol. Res. 130, 451–465. doi:10.1016/j.phrs.2018.01.015
Xu, Y., Zhu, J., Gao, L., Liu, Y., Shen, J., Shen, C., et al. (2013). Hyperuricemia as an independent predictor of vascular complications and mortality in type 2 diabetes patients: A meta-analysis. PLoS One 8 (10), e78206. doi:10.1371/journal.pone.0078206
Yin, J. (2015). The clinical and experimental research of huazhuo decoction improves insulin resistance of type 2 diabetes mellitus patients with high uric acid hematic disease. China: Master’s thesis, Shandong University of Traditional Chinese Medicine.
Yu, C. L. (2010). Combination of Chinese and Western medicine in the treatment of hyperuricemia with Type 2 diabetes mellitus 52 cases summary. Hunan J. Traditional Chin. Med. 26 (05), 30–31. doi:10.16808/j.cnki.issn1003-7705.2010.05.015
Zhang, J. Y., Zang, J. F., Zhang, C., and Yang, H. J. (2022a). Treatment of type 2 diabetes mellitus with hyperuricemia from the perspective of traditional Chinese and western medicine. Guangming J. Chin. Med. 37 (13), 2449–2451.
Zhang, Q. J., Wu, R., Wang, Z. F., Qin, L., Guo, F. N., An, R., et al. (2021). Clinical study of modified Dachaihu Decoction on type 2 diabetes mellitus complicated with hyperuricemia. J. Clin. Exp. Med. 20 (05), 478–482.
Zhang, S. J., Zhang, Y. G., Niu, J. T., Si, X. L., Li, D. H., Wu, H. W., et al. (2022b). Research progress of huangqi(astragali radix) and prediction of its quality markers. Chin. Archives Traditional Chin. Med. 40 (02), 151–155. doi:10.13193/j.issn.1673-7717.2022.02.035
Zhou, J. Y., Yang, J. C., Hao, Z. Y., Shen, H. P., and Weng, S. Y. (2013). Effect of xiezhuo Yin on SD rats cysteine protease inhibitor protein C of type 2 diabetes mellitus with hyperuricemia. Chin. Archives Traditional Chin. Med. 31 (09), 1909–1911. doi:10.13193/j.issn.1673-7717.2013.09.117
Zhou, K. L. (2018). “Clinical observation on Shenlingerzhu decoction treating Type 2 diabetes mellitus with Hyperuricemia,”. Master’s thesis, Nanjing University of Chinese Medicine.
Zhu, L. R. (2013). “Effect of total saponins of Dioscorea on expression of uric acid transporters in hyperuricemia rats,”. Master’s thesis, Anhui University of Traditional Chinese Medicine.
Keywords: type 2 diabetes mellitus, hyperuricemia, Chinese herbal medicine, meta–analysis, systematic review
Citation: Liu H, Peng S, Yuan H, He Y, Tang J and Zhang X (2023) Chinese herbal medicine combined with western medicine for the treatment of type 2 diabetes mellitus with hyperuricemia: A systematic review and meta-analysis. Front. Pharmacol. 14:1102513. doi: 10.3389/fphar.2023.1102513
Received: 19 November 2022; Accepted: 11 January 2023;
Published: 24 January 2023.
Edited by:
Juei-Tang Cheng, Chang Jung Christian University, TaiwanReviewed by:
Sujan Banik, Noakhali Science and Technology University, BangladeshKrit Pongpirul, Chulalongkorn University, Thailand
Copyright © 2023 Liu, Peng, Yuan, He, Tang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiyu Zhang, emhhbmd4aXl1dGNtQHlhaG9vLmNvbQ==
 Hongyan Liu
Hongyan Liu Sihan Peng
Sihan Peng Haipo Yuan2
Haipo Yuan2 Xiyu Zhang
Xiyu Zhang