
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Pharmacol. , 05 April 2023
Sec. Obstetric and Pediatric Pharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1094435
This article is part of the Research Topic Safety of Drugs and CAM Products in Pregnancy and Breastfeeding: Evidence From Clinical Toxicology View all 9 articles
Acetaminophen (APAP) is a widely used as analgesic and antipyretic drug. APAP is also added as an active ingredient in various medications to relieve pain and reduce fever. APAP has been widely used in pregnant women in the past decades because it is considered a relatively safe drug with recommended dose in different countries. However, an increasing number of epidemiological and experimental studies have shown that APAP exposure during pregnancy may increase the risk of inducing reproductive and neurobehavior dysfunctions, hepatotoxicity in offspring. This review aims to assess the potential effects of prenatal APAP exposure on offspring growth and development.
APAP is a common antipyretic that has been used widely in family population, including pregnant woman (Nilsen et al., 2023). As a household remedy, pregnant women through self-directed or unintentional use of APAP is unavoidable (Bandoli et al., 2020). It is estimated that parts of pregnant women may expose to APAP in gestation period (Patel et al., 2022). It is reported that pregnant women usually take over-the-counter drugs (including APAP) without medical guidance and it may adversely affect the fetus development (Thiele et al., 2013). Experimental data in vivo show neonatal APAP exposure (single low dose with 30 mg/kg) may induce adverse actions on the developing brain (Philippot et al., 2022). Prenatal APAP exposure may induce autism spectrum disorders in childhood, suggesting that gestational APAP use should be clinically guided (Ji et al., 2020). Pharmacokinetic analysis of oral APAP dose (single intake 1,000 mg) shows that the contents are highly correlated in maternal venous blood (12.3 μg/mL) and fetal blood (11.2 μg/mL) (Nitsche et al., 2017). It is reported that APAP content in umbilical cord blood is approximate 3.6 mg/L as the median molar dose proportion metabolized to acetaminophen-sulphate and N-acetyl-p-benzoquinone imine is 0.8% and 0.06% (Mian et al., 2020). Compared to non-pregnant women, an increase in volume of distribution and an increase in clearance of APAP in pregnant women is 3.5%–60.7% and 36.8%–84.4% respectively. Notably, the toxic metabolite N-acetyl-p-benzoquinone imine is greatest in the first trimester, followed by the second and third trimester in pregnant women (Brookhuis et al., 2021). During pregnancy and breastfeeding periods, woman may use APAP to relieve acute or chronic and it may cause negative consequence to offspring (Scialli et al., 2010). Maternal APAP use during pregnancy is implicated in certain adverse outcomes in offspring, including attention and sleep problems (Sznajder et al., 2022). The cohort studies report that prenatal APAP exposure results in adverse neurodevelopment, including attention-deficit/hyperactivity disorder associated with frontoparietal network brain connectivity (Ystrom et al., 2017). Relief from maternal pain is an important factor for smooth delivery, and it is particularly important to use drugs rationally according to the type and duration of symptoms (Roberge et al., 2016). However, for various reasons, they are reported to take at least one or two medications, both prescription and over-the-counter, during pregnancy (Lupattelli et al., 2014). APAP is commonly used as antipyretic and analgesic, including pregnant women (Bauer et al., 2021). Despite the controversy occurs, APAP has potential benefits in treating clinical symptoms in pregnant women and it is considered to be low-risk (Toda, 2017). Collectively, current evidences partially reason that prenatal exposure of APAP may affect the development and function in offspring.
Mounting data suggest that APAP may exert neuroendocrine disrupting effects, in which APAP exposure may affect endocrinological functions from fetal life to adulthood. Increasing reports manifest that APAP is a hormone disrupter that interferes with sex and thyroid hormones required for normal brain development (Aminoshariae and Khan, 2015). Exposure of APAP during a key period of brain development can lead to a long-term effect on cognitive functions (Viberg et al., 2014). As APAP can pass through the blood-brain barrier, it can act both centrally and peripherally through different molecular regulatory mechanisms. For example, one of the mechanisms of APAP action is thought to relieve pain is through competitive suppression of the peroxidase moiety of prostaglandin H2 synthase and regulation of cannabinoid receptor signaling pathway (Bührer et al., 2021). Additionally, APAP has also been found to inhibit serotonergic mechanisms for relieving pain in clinical studies (Pickering et al., 2008). However, the oxidative brain impairment and mitochondrial dysfunction are found in APAP acute exposure in vivo under 600 mg/kg for 5 h (da Silva et al., 2012). Collectively, understanding the exact mechanisms regarding APAP-caused neuroendocrinological dysfunction may be indefinable. However, the potential disrupting effects of APAP seem to be closely related to dosing dependence. High dose of APAP exposure may induce oxidative stress and affect Nrf2 function resulting in reduction of oxidative stability and increment of toxic response to cause direct toxicity to neuron and astroglia. Instead, low dose of APAP exposure may mediate neuroprotective actions via reducing ischemic and amyloid impairment. Both low and high APAP doses can play analgesic and antipyretic effects via modulation of cannabinoid system (Figure 1).
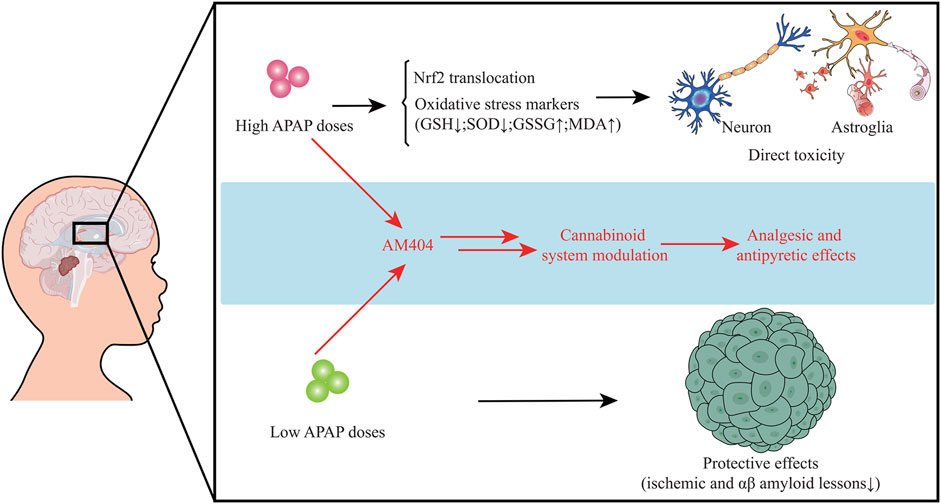
FIGURE 1. High dose of APAP may induce oxidative stress and affect Nrf2 function resulting in reduction of oxidative stability and increment of toxic response to cause direct toxicity to neuron and astroglia. However, low dose of APAP may mediate neuroprotective actions. Low and high APAP doses can exert analgesic and antipyretic actions through regulation of cannabinoid system.
Exposure of APAP in utero is involved in an increased risk of developing male genital tract abnormalities. An in vitro study from isolating adult human testis suggests that 10−4–10−5 mol/L APAP exposure for 24–48 h may alter testosterone and insulin-like factor 3 in Leydig cells (Albert et al., 2013). Another ex vivo study from isolating human ovarian fragments displays that 10−3–10−8 mol/L APAP exposure for 7 days may decrease the total cell number ovaries and the KI67-positive cell density, induce cell death. Human fetal ovarian steroidogenesis is affected (Lecante et al., 2022). A Danish National Birth Cohort study investigating 47,400 live-born singleton sons reports that maternal exposure of APAP over 4 weeks during pregnancy, especially within the first and second trimesters, may potentially elevate the presence of cryptorchidism in offspring (Henriksen and Olsen et al., 2010). A prospective birth cohort study involving 2,500 pregnant women shows that exposure to analgesics including APAP during pregnancy is related to a reduced anogenital distance (AGD) in offspring boys that may affect normal reproductive development (Lind et al., 2017). A prospective study from 2,229 recruited women in UK exhibits that intrauterine exposure of APAP during 8–14 weeks (masculinisation programming window) of gestation is correlated to shortened AGD from birth to 24 months (Fisher et al., 2016). A longitudinal Puberty Cohort study in Danish demonstrates that APAP use during pregnancy and postpartum may induce advance appearance of female pubertal development around 1.5–3 months earlier (Ernst et al., 2019). In addition to experimental data, there are still few human studies on the long-term reproductive functions affected by prenatal APAP exposure, especially for intergenerational and transgenerational outcomes. In brief, current evidences partially reason that prenatal exposure of APAP may potentially disrupt human reproductive functions both male and female offspring (Figure 2).
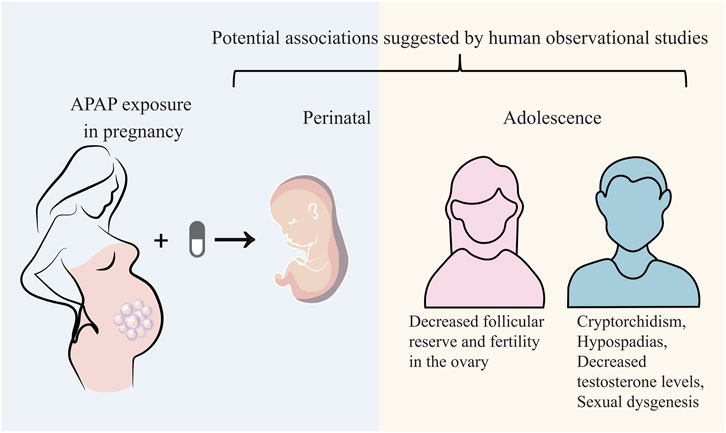
FIGURE 2. Prenatal APAP exposure may potentially disrupt human reproductive functions both male and female offspring.
Increasing evidences data indicate APAP use during pregnancy may induce the potential risk of developing neurobehavior problems and hyperkinetic symptoms (Liew et al., 2014). Another An Auckland Birthweight Collaborative study from 871 infants shows that APAP exposure during pregnancy may increase the risk of developing attention-deficit/hyperactivity disorder (ADHD)-like symptoms in children at 7 and 11 years of age (Thompson et al., 2014). Prenatal and postnatal exposure of APAP up to 18 months result in autism spectrum conditions (ASC) and ADHD in offspring (Alemany et al., 2021). A prospective birth cohort from 7,796 mothers presents that APAP use in 18–32 weeks of pregnant women results in increased risk of multiple behavioral problems, including emotional symptom (Stergiakouli et al., 2016). The meta-analysis systematic review, meta-analysis, and meta-regression analysis of cohort studies from 132,738 mother-child pairs suggests that prenatal APAP use is involve in the elevated for inducing ADHD, autism spectrum disorder (ASD) and hyperactivity syndromes (Masarwa et al., 2018). Eight cohort studies included 244,940 participants exhibits that APAP exposure to pregnancy may raise the risk of ADHD in offspring, in which a longer duration of prenatal APAP exposure is likely associated with a higher risk outcome (Gou et al., 2019). The available data is of observational nature only. However, current demographical data is of observational nature only, and pathological mechanism regarding APAP-induced human neurobehavior dysfunction is limitedly revealed in details.
Usually, most of patients take around 12 g or more APAP before inducing serious hepatotoxicity, and the peak serum transaminase activity can occur between 48 and 96 h. It may cause liver failure in APAP-used patients after days (Fisher et al., 2016). It is preclinically showed that prenatal APAP exposure may induce hepatic toxicity in offspring owing to oxidative stress and inflammatory injury (Rofaeil et al., 2023). Other preclinical evidences indicate that an increased susceptibility towards APAP-induced liver injury in pregnant mice, and hematopoietic stem cells in fetal liver is functionally affected (Karimi et al., 2015). The liver is the largest detoxifying organ in human body. When APAP enters the body, it will undergo “first pass” metabolism in the liver tissue approximately 25% of APAP before being excreted in the urine as glucuronide and sulphate conjugates (Prescott, 1980). Physiologically, most of APAP is metabolized by stage II binding enzymes characterized as UDP-glucuronate transferase (UGT) and sulfonyltransferase (SULT) for further being converted into non-toxic compounds. Another part of APAP can react with cytochrome P450 enzymes (CYP) and is eventually metabolized to the highly reactive intermediate metabolite N-acetyl-p-benzoquinoneimide (NAPQI), a strong hepatotoxic molecule (Lancaster et al., 2015). It is reported that exposure to NAPQI metabolized by APAP may lead to hepatotoxicity and acute liver failure, in which this outcome may affect the fetus and newborn developments (Brune et al., 2015). Typically, NAPQI is rapidly detoxified by glutathione (GSH) action. However, APAP overdose use can cause functional insufficiency of specific metabolic enzymes owing to threshold saturation, resulting in NAPQI exhausting GSH (James et al., 2003). APAP in human body is metabolized and detoxified in liver tissue, as reveled in a graphical briefing (Figure 3). Mitochondrial oxidative stress is one of the leading causes in APAP-induced liver damage. Additionally, certain cellular events including autophagy, endoplasmic reticulum stress, inflammatory infiltration and microcirculatory dysfunction, have been found involvement with the pathogenesis of APAP-caused liver injury (Yan et al., 2018). An animal study shows that prenatal exposure to APAP may reduce the expressions of insulin receptor substrate 1 (IRS1), phosphorylated glycogen synthase kinase-3beta (GSK-3β) and protein kinase B (AKT), and downregulate hepatic glucose transporter 2 (GLUT2) in offspring livers. And the underlying mechanism regarding hepatic dysmetabolism caused by prenatal APAP exposure may be involved in disturbance of insulin-dependent AKT pathway (Wu et al., 2016). APAP administered intraperitoneally to mice (250 mg/kg) shows that mitochondrial GSH depletion appears to be more severe than cytoplasmic depletion (Tirmenstein and Nelson, 1989). Excessive APAP induces mitochondrial dysfunction through production of NAPQI binding to mitochondrial proteins, resulting in release of reactive oxygen species (ROS) to damage mitochondrial respiration and to affect ATP synthesis (Burcham and Harman, 1991; Ramsay et al., 1989; Jaeschke, 1990). The massive production of ROS in the liver is involved in APAP-induced hepatotoxicity via mediating endoplasmic reticulum (ER) stress (Uzi et al., 2013). In addition, high expression of peroxynitrite participation in the cytotoxicity of APAP may impair antioxidant function and cell homeostasis, gradually causing apoptotic or necrotic cell death in liver tissue (Denicola and Radi, 2005). Overall, current reports comprehensively uncover the molecular mechanism of APAP-induced hepatotoxicity, especially via mitochondrial avenue (Figure 4).
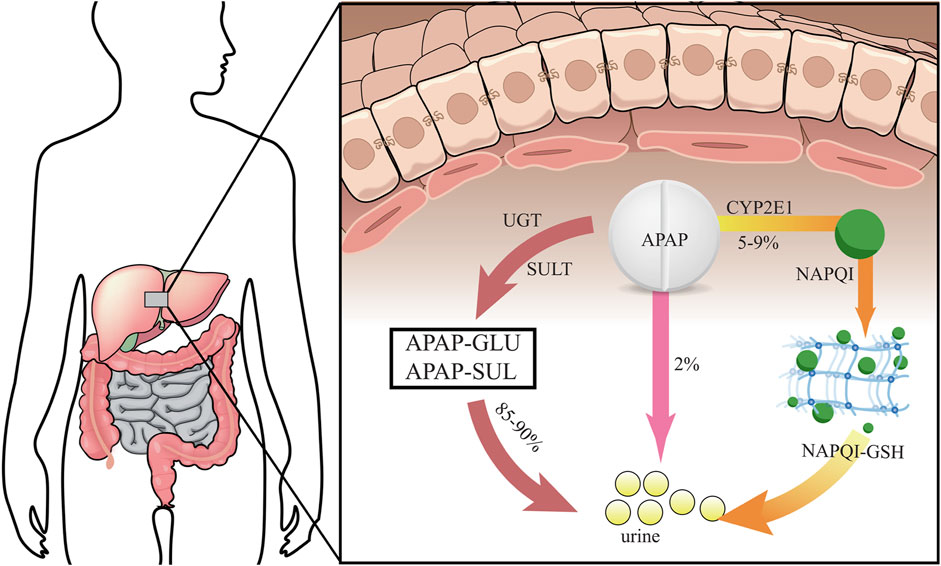
FIGURE 3. Human APAP is metabolized and detoxified in liver tissue via different processes before urine excretion.
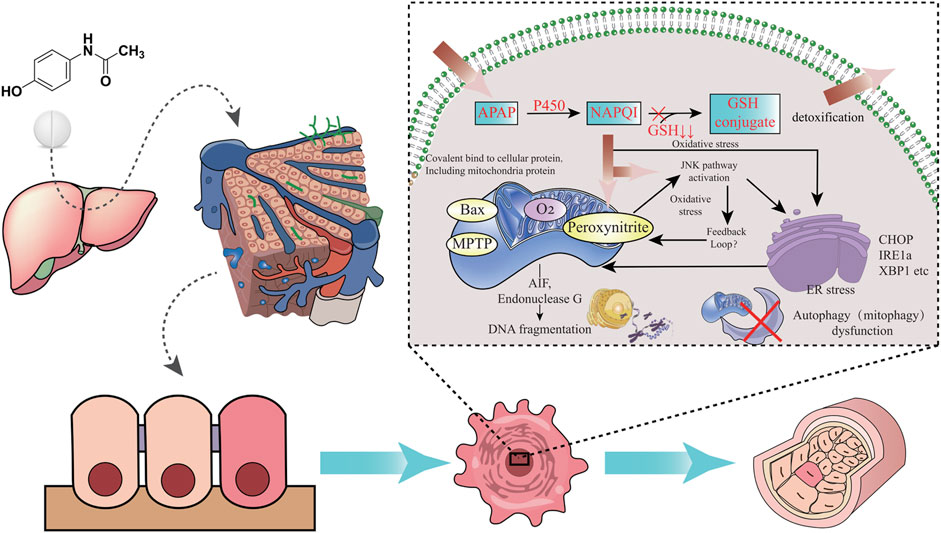
FIGURE 4. Molecular mechanism of APAP-induced hepatotoxicity is revealed through integrated pathways, including oxidative stress and ER stress.
APAP exposure during pregnancy to treat pain or other symptoms may induce certain harmful effects on both the mother and the fetus. Other evidences indicate that prenatal APAP exposure may disrupt endocrine functions, including brain and liver tissues. Both intracellular and extracellular events are involved in pathophysiological processes in APAP-induced cytotoxicity, including drug metabolism, mitochondrial oxidative stress, DNA damage and microcirculatory dysfunction. As limited in current reference reports, more human data and toxicological investigation is needed to further elucidate the adverse actions of prenatal APAP exposure to offspring. More notably, we should pay close attention to the household use of APAP for safety, especially pregnant women before self-directed use.
KW and XY contributed to the conception, design of the manuscript. WL contributed to the acquisition, analysis, and interpretation of data in this manuscript. KW drafted this manuscript. XY revised this manuscript. All authors agree to be accountable for all aspects of work ensuring integrity and accuracy.
This study is granted by Natural Science Foundation of Guangxi Province (No. 2018GXNSFAA281153), and National Natural Science Foundation of China (No. 82060740).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Albert, O., Desdoits-Lethimonier, C., Lesné, L., Legrand, A., Guillé, F., Bensalah, K., et al. (2013). Paracetamol, aspirin and indomethacin display endocrine disrupting properties in the adult human testis in vitro. Hum. Reprod. 28, 1890–1898. doi:10.1093/humrep/det112
Alemany, S., Avella-García, C., Liew, Z., García-Esteban, R., Inoue, K., Cadman, T., et al. (2021). Prenatal and postnatal exposure to acetaminophen in relation to autism spectrum and attention-deficit and hyperactivity symptoms in childhood: Meta-analysis in six European population-based cohorts. Eur. J. Epidemiol. 36, 993–1004. doi:10.1007/s10654-021-00754-4
Aminoshariae, A., and Khan, A. (2015). Acetaminophen: Old drug, new issues. J. Endod. 41, 588–593. doi:10.1016/j.joen.2015.01.024
Bandoli, G., Palmsten, K., and Chambers, C. (2020). Acetaminophen use in pregnancy: Examining prevalence, timing, and indication of use in a prospective birth cohort. Paediatr. Perinat. Epidemiol. 34, 237–246. doi:10.1111/ppe.12595
Bauer, A. Z., Swan, S. H., Kriebel, D., Liew, Z., Taylor, H. S., Bornehag, C. G., et al. (2021). Paracetamol use during pregnancy - a call for precautionary action. Nat. Rev. Endocrinol. 17, 757–766. doi:10.1038/s41574-021-00553-7
Brookhuis, S. A. M., Allegaert, K., Hanff, L. M., Lub-de Hooge, M. N., Dallmann, A., and Mian, P. (2021). Modelling tools to characterize acetaminophen pharmacokinetics in the pregnant population. Pharmaceutics 13, 1302. doi:10.3390/pharmaceutics13081302
Brune, K., Renner, B., and Tiegs, G. (2015). Acetaminophen/paracetamol: A history of errors, failures and false decisions. Eur. J. Pain 19, 953–965. doi:10.1002/ejp.621
Bührer, C., Endesfelder, S., Scheuer, T., and Schmitz, T. (2021). Paracetamol (acetaminophen) and the developing brain. Int. J. Mol. Sci. 22, 11156. doi:10.3390/ijms222011156
Burcham, P. C., and Harman, A. W. (1991). Acetaminophen toxicity results in site-specific mitochondrial damage in isolated mouse hepatocytes. J. Biol. Chem. 266, 5049–5054. doi:10.1016/s0021-9258(19)67754-9
da Silva, M. H., da Rosa, E. J., de Carvalho, N. R., Dobrachinski, F., da Rocha, J. B., Mauriz, J. L., et al. (2012). Acute brain damage induced by acetaminophen in mice: Effect of diphenyl diselenide on oxidative stress and mitochondrial dysfunction. Neurotox. Res. 21, 334–344. doi:10.1007/s12640-011-9288-1
Denicola, A., and Radi, R. (2005). Peroxynitrite and drug-dependent toxicity. Toxicology 208, 273–288. doi:10.1016/j.tox.2004.11.023
Ernst, A., Brix, N., Lauridsen, L., Olsen, J., Parner, E. T., Liew, Z., et al. (2019). Acetaminophen (paracetamol) exposure during pregnancy and pubertal development in boys and girls from a nationwide puberty cohort. Am. J. Epidemiol. 188, 34–46. doi:10.1093/aje/kwy193
Fisher, B. G., Thankamony, A., Hughes, I. A., Ong, K. K., Dunger, D. B., and Acerini, C. L. (2016). Prenatal paracetamol exposure is associated with shorter anogenital distance in male infants. Hum. Reprod. 31, 2642–2650. doi:10.1093/humrep/dew196
Gou, X., Wang, Y., Tang, Y., Qu, Y., Tang, J., Shi, J., et al. (2019). Association of maternal prenatal acetaminophen use with the risk of attention deficit/hyperactivity disorder in offspring: A meta-analysis. Aust. N. Z. J. Psychiatry 53, 195–206. doi:10.1177/0004867418823276
Henriksen, T. B., and Olsen, J. (2010). Maternal use of acetaminophen, ibuprofen, and acetylsalicylic acid during pregnancy and risk of cryptorchidism. Epidemiology 21 (6), 779–785. doi:10.1097/EDE.0b013e3181f20bed
James, L. P., Mayeux, P. R., and Hinson, J. A. (2003). Acetaminophen-induced hepatotoxicity. Drug Metab. Dispos. 31, 1499–1506. doi:10.1124/dmd.31.12.1499
Jaeschke, H. (1990). Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: The protective effect of allopurinol. J. Pharmacol. Exp. Ther. 255, 935–941.
Ji, Y., Azuine, R. E., Zhang, Y., Hou, W., Hong, X., Wang, G., et al. (2020). Association of cord plasma biomarkers of in utero acetaminophen exposure with risk of attention-deficit/hyperactivity disorder and autism spectrum disorder in childhood. JAMA Psychiatry 77, 180–189. doi:10.1001/jamapsychiatry.2019.3259
Karimi, K., Keßler, T., Thiele, K., Ramisch, K., Erhardt, A., Huebener, P., et al. (2015). Prenatal acetaminophen induces liver toxicity in dams, reduces fetal liver stem cells, and increases airway inflammation in adult offspring. J. Hepatol. 62, 1085–1091. doi:10.1016/j.jhep.2014.12.020
Lancaster, E. M., Hiatt, J. R., and Zarrinpar, A. (2015). Acetaminophen hepatotoxicity: An updated review. Arch. Toxicol. 89, 193–199. doi:10.1007/s00204-014-1432-2
Lecante, L. L., Leverrier-Penna, S., Gicquel, T., Giton, F., Costet, N., Desdoits-Lethimonier, C., et al. (2022). Acetaminophen (APAP, paracetamol) interferes with the first trimester human fetal ovary development in an ex vivo model. J. Clin. Endocrinol. Metab. 107, 1647–1661. doi:10.1210/clinem/dgac080
Liew, Z., Ritz, B., Rebordosa, C., Lee, P. C., and Olsen, J. (2014). Acetaminophen use during pregnancy, behavioral problems, and hyperkinetic disorders. JAMA Pediatr. 168, 313–320. doi:10.1001/jamapediatrics.2013.4914
Lind, D. V., Main, K. M., Kyhl, H. B., Kristensen, D. M., Toppari, J., Andersen, H. R., et al. (2017). Maternal use of mild analgesics during pregnancy associated with reduced anogenital distance in sons: A cohort study of 1027 mother-child pairs. Hum. Reprod. 32, 223–231. doi:10.1093/humrep/dew285
Lupattelli, A., Spigset, O., Twigg, M. J., Zagorodnikova, K., Mardby, A. C., Moretti, M. E., et al. (2014). Medication use in pregnancy: A cross-sectional, multinational web-based study. BMJ Open 4, e004365. doi:10.1136/bmjopen-2013-004365
Masarwa, R., Levine, H., Gorelik, E., Reif, S., Perlman, A., and Matok, I. (2018). Prenatal exposure to acetaminophen and risk for attention deficit hyperactivity disorder and autistic spectrum disorder: A systematic review, meta-analysis, and meta-regression analysis of cohort studies. Am. J. Epidemiol. 187, 1817–1827. doi:10.1093/aje/kwy086
Mian, P., Allegaert, K., Conings, S., Annaert, P., Tibboel, D., Pfister, M., et al. (2020). Integration of placental transfer in a fetal-maternal physiologically based pharmacokinetic model to characterize acetaminophen exposure and metabolic clearance in the fetus. Clin. Pharmacokinet. 59, 911–925. doi:10.1007/s40262-020-00861-7
Nilsen, K., Staff, A. C., and Krogsrud, S. K. (2023). Paracetamol use in pregnancy: Not as safe as we may think? Acta Obstet. Gynecol. Scand. doi:10.1111/aogs.14557
Nitsche, J. F., Patil, A. S., Langman, L. J., Penn, H. J., Derleth, D., Watson, W. J., et al. (2017). Transplacental passage of acetaminophen in term pregnancy. Am. J. Perinatol. 34, 541–543. doi:10.1055/s-0036-1593845
Patel, E., Jones, J. P., Bono-Lunn, D., Kuchibhatla, M., Palkar, A., Cendejas Hernandez, J., et al. (2022). The safety of pediatric use of paracetamol (acetaminophen): A narrative review of direct and indirect evidence. Minerva Pediatr. (Torino) 74, 774–788. doi:10.23736/S2724-5276.22.06932-4
Philippot, G., Hosseini, K., Yakub, A., Mhajar, Y., Hamid, M., Buratovic, S., et al. (2022). Paracetamol (acetaminophen) and its effect on the developing mouse brain. Front. Toxicol. 4, 867748. doi:10.3389/ftox.2022.867748
Pickering, G., Esteve, V., Loriot, M. A., Eschalier, A., and Dubray, C. (2008). Acetaminophen reinforces descending inhibitory pain pathways. Clin. Pharmacol. Ther. 84, 47–51. doi:10.1038/sj.clpt.6100403
Prescott, L. F. (1980). Kinetics and metabolism of paracetamol and phenacetin. Br. J. Clin. Pharmacol. 10, 291–298. doi:10.1111/j.1365-2125.1980.tb01812.x
Ramsay, R. R., Rashed, M. S., and Nelson, S. D. (1989). In vitro effects of acetaminophen metabolites and analogs on the respiration of mouse liver mitochondria. Arch. Biochem. Biophys. 273, 449–457. doi:10.1016/0003-9861(89)90504-3
Roberge, S., Odibo, A. O., and Bujold, E. (2016). Aspirin for the prevention of preeclampsia and intrauterine growth restriction. Clin. Lab. Med. 36, 319–329. doi:10.1016/j.cll.2016.01.013
Rofaeil, R. R., Welson, N. N., Fawzy, M. A., Ahmed, A. F., Atta, M., Bahaa El-Deen, M. A., et al. (2023). The IL-6/HO-1/STAT3 signaling pathway is implicated in the amelioration of acetaminophen-induced hepatic toxicity: A neonatal rat model. Hum. Exp. Toxicol. 42, 9603271231151376. doi:10.1177/09603271231151376
Scialli, A. R., Ang, R., Breitmeyer, J., and Royal, M. A. (2010). A review of the literature on the effects of acetaminophen on pregnancy outcome. Reprod. Toxicol. 30, 495–507. doi:10.1016/j.reprotox.2010.07.007
Stergiakouli, E., Thapar, A., and Davey, S. G. (2016). Association of acetaminophen use during pregnancy with behavioral problems in childhood: Evidence against confounding. JAMA Pediatr. 170, 964–970. doi:10.1001/jamapediatrics.2016.1775
Sznajder, K. K., Teti, D. M., and Kjerulff, K. H. (2022). Maternal use of acetaminophen during pregnancy and neurobehavioral problems in offspring at 3 years: A prospective cohort study. PLoS One 17, e0272593. doi:10.1371/journal.pone.0272593
Thiele, K., Kessler, T., Arck, P., Erhardt, A., and Tiegs, G. (2013). Acetaminophen and pregnancy: Short- and long-term consequences for mother and child. J. Reprod. Immunol. 97, 128–139. doi:10.1016/j.jri.2012.10.014
Thompson, J. M., Waldie, K. E., Wall, C. R., Murphy, R., and Mitchell, E. A.ABC study group (2014). Associations between acetaminophen use during pregnancy and ADHD symptoms measured at ages 7 and 11 years. PLoS One 9, e108210. doi:10.1371/journal.pone.0108210
Tirmenstein, M. A., and Nelson, S. D. (1989). Subcellular binding and effects on calcium homeostasis produced by acetaminophen and a nonhepatotoxic regioisomer, 3’-hydroxyacetanilide, in mouse liver. J. Biol. Chem. 264, 9814–9819. doi:10.1016/s0021-9258(18)81731-8
Toda, K. (2017). Is acetaminophen safe in pregnancy? Scand J. Pain 17, 445–446. doi:10.1016/j.sjpain.2017.09.007
Uzi, D., Barda, L., Scaiewicz, V., Mills, M., Mueller, T., Gonzalez-Rodriguez, A., et al. (2013). CHOP is a critical regulator of acetaminophen-induced hepatotoxicity. J. Hepatol. 59, 495–503. doi:10.1016/j.jhep.2013.04.024
Viberg, H., Eriksson, P., Gordh, T., and Fredriksson, A. (2014). Paracetamol (acetaminophen) administration during neonatal brain development affects cognitive function and alters its analgesic and anxiolytic response in adult male mice. Toxicol. Sci. 138, 139–147. doi:10.1093/toxsci/kft329
Wu, K., Guo, C., Lu, X., Wu, X., Pan, H., and Su, M. (2016). Impact of perinatal exposure to acetaminophen on hepatocellular metabolic function in offspring. Am. J. Transl. Res. 8, 5646–5652.
Yan, M., Huo, Y., Yin, S., and Hu, H. (2018). Mechanisms of acetaminophen-induced liver injury and its implications for therapeutic interventions. Redox Biol. 17, 274–283. doi:10.1016/j.redox.2018.04.019
Keywords: prenatal exposure, acetaminophen, offspring, disruption effect, hepatotoxicity
Citation: Wu K, Lu W and Yan X (2023) Potential adverse actions of prenatal exposure of acetaminophen to offspring. Front. Pharmacol. 14:1094435. doi: 10.3389/fphar.2023.1094435
Received: 10 November 2022; Accepted: 28 March 2023;
Published: 05 April 2023.
Edited by:
Giada Crescioli, University of Florence, ItalyReviewed by:
Rolando Garcia-Milian, Yale University, United StatesCopyright © 2023 Wu, Lu and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Yan, eWFueGluODIwNjI3QDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.