- 1Department of Pathology, Xiangya Changde Hospital, Changde, Hunan, China
- 2Department of Pharmacy, Xiangya Hospital, Central South University, Changsha, Hunan, China
- 3National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, Hunan, China
- 4Department of Pathology, Xiangya Hospital, Central South University, Changsha, Hunan, China
- 5Pritzker School of Molecular Engineering, Ben May Department for Cancer Research, University of Chicago, Chicago, IL, United States
Exosomes are nanoscale extracellular vesicles secreted by a variety of cells, affecting the physiological and pathological homeostasis. They carry various cargoes including proteins, lipids, DNA, and RNA and have emerged as critical mediators of intercellular communication. During cell–cell communication, they can internalize either by autologous or heterologous recipient cells, which activate different signaling pathways, facilitating malignant progression of cancer. Among different types of cargoes in exosomes, the endogenous non-coding RNAs, such as circular RNAs (or circRNAs), have gained tremendous attention for their high stability and concentration, playing promising functional roles in cancer chemotherapeutic response by regulating the targeted gene expression. In this review, we primarily described the emerging evidence demonstrating the important roles of circular RNAs derived from exosomes in the regulation of cancer-associated signaling pathways that were involved in cancer research and therapeutic interventions. Additionally, the relevant profiles of exosomal circRNAs and their biological implications have been discussed, which is under investigation for their potential effect on the control of cancer therapeutic resistance.
1 Introduction
Globally, cancers serve as one of the primary reasons for deaths, including in China (Bray et al., 2021), resulting in a serious economic and social burden (Stanway et al., 2021). Up to now, a variety of therapeutic strategies, such as chemotherapy (Okusaka and Furuse, 2020), immunotherapy (Sharma et al., 2017; Keenan and Tolaney, 2020), and targeted therapy (Yang et al., 2021a), have ameliorated the clinical treatment effects for cancer patients (Tsimberidou, 2015). However, due to their heterogeneity, cancer cells often exhibit primary or acquired treatment resistance, thereby resulting in treatment failure (Xu et al., 2020a; Liu et al., 2021a).
Exosomes are a subset of cell-derived nanovesicles with a diameter approximately ranging from 30 to 200 nm and participate in intracellular communication (Thakur et al., 2020a; Gaurav et al., 2021a; Thakur et al., 2021). They are released from almost all types of cells and remain more stable for a long time while participating in intracellular communication (Nie et al., 2021). During cell–cell communication, they can be internalized either by their own parent cells (referred as autologous uptake) or by different recipient cells (referred as heterologous uptake), which trigger different phenotypes in the recipient cells. Also, they can impersonate the constituents of their originating parent cells. This infers that the different cargoes from exosomes could be utilized as reliable prognostic and diagnostic biomarkers for multiple cancers (Thakur et al., 2017; Thakur et al., 2020b; Gaurav et al., 2021b). As one of the several different types of cargoes, circular RNAs (or circRNAs) have been found to interfere with the therapeutic response. A plethora of research studies have been conducted, which contributed toward the advancement of our knowledge about exosomal circRNAs (Hon et al., 2019; Wang et al., 2022a; Long et al., 2022).
Recently, circRNAs have emerged as promising regulatory factors for the malignant progression and treatment resistance (Liu et al., 2020; Yang et al., 2021b). In 1976, Sanger et al. used electron microscopy and preliminarily discovered the single-stranded closed-loop RNA molecules, circRNAs, in the virus (Sanger et al., 1976). Subsequent studies have pointed out that exosomes mainly comprise the following four categories, namely, circular intronic circRNAs, exon–intron circRNAs, exon circRNAs, and fusion circRNAs (Zhang et al., 2013; Guarnerio et al., 2016). Because of the deficiency of 5′ and 3′ terminals in structures, circRNAs are more stable than linear RNAs (Li et al., 2015). Studies have showed the important roles of aberrant circRNA-associated signaling in cancer pathogenesis and therapeutic response (Lai et al., 2021; Chen et al., 2022). Because of their unique closed-loop form and structural stability, circRNAs have been serving as novel prognostic and therapeutic targets for human cancers (Jiao et al., 2021; Lan et al., 2021).
Therapeutic resistance is one of the main challenges to cancer treatment (Wade and Kyprianou, 2018; Long et al., 2019). Because of the primary or acquired resistance to therapeutic agents, the overall therapeutic efficiency on cancer patients was limited, ultimately resulting in severe clinical and social problems. Meanwhile, the underlying molecular mechanisms for treatment resistance are varied and complex (Yan et al., 2019a; Yan et al., 2019b). Among these, the profiles of circRNAs have also been reported in exosomes, which can determine cancer heterogeneity and responses to therapeutic resistances. Apparently, in this review, we have discussed in detail about the different aspects of exosomal circRNAs in the context of drug resistance development, causing malignant progression of cancer.
2 Implication of exosomal circRNAs for cancer development and prognosis
2.1 Application of exosomal circRNAs as cancer biomarkers
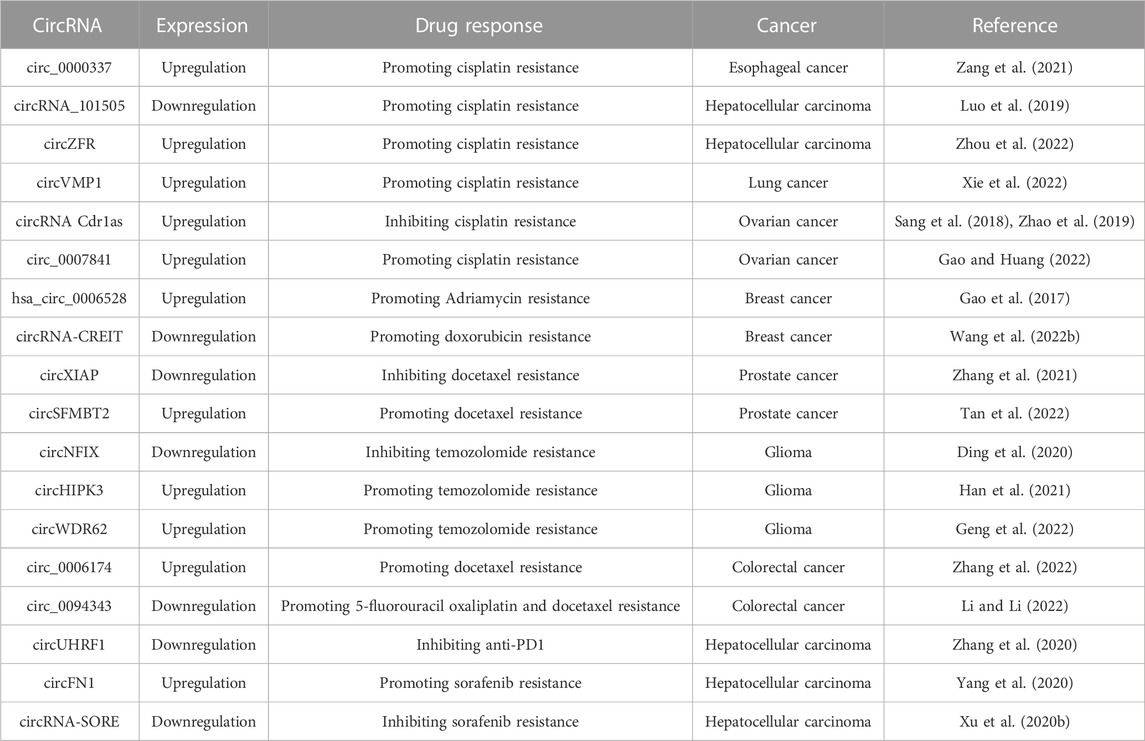
It is an important medical concern to explore the precise bio-targets for cancer treatment. Currently, some studies have showed the promising biological functions of non-coding RNAs in cancer pathogenesis and therapeutic response, such as microRNAs and long non-coding RNAs (Dumache, 2017; Zhao et al., 2018; Fadhil et al., 2020). As a new member of non-coding RNAs, circRNAs are released from almost all types of cells and could maintain the balance of body fluids (Figure 1). Customarily, exosomal circRNAs are balanced in the body fluids (Wang et al., 2019a; Verduci et al., 2021), participating in the pathogenesis of cancers (Zhang et al., 2018; Wu et al., 2022). Recently, Zhang et al. have evaluated the profiles of circRNAs in the colorectal cancer tissue. The results from this report demonstrated that the overexpression of circZNF609 could reduce cell proliferation and promote cell apoptosis by upregulating p53 expression (Zhang et al., 2019). Thus, exosomal circRNAs can be used as promising biomarkers for cancer development (Wang et al., 2020a; Wang et al., 2021a).
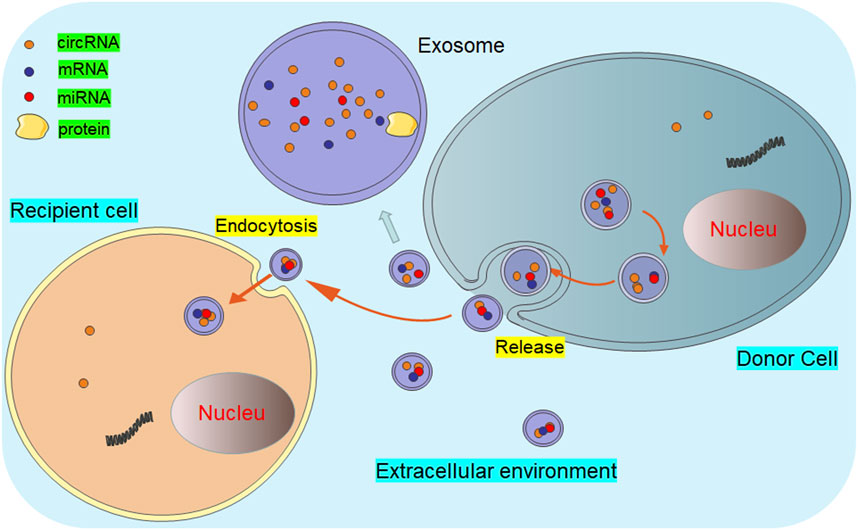
FIGURE 1. Diagram of exosomal circRNAs released from donor cells to recipient cells. The circRNA-containing exosomes are secreted into the extracellular environment by the donor cells. Thereafter, these exosomal circRNAs are transferred to the recipient cells through endocytosis or membrane fusion, executing multiple biological functions.
2.2 Early diagnosis of cancers via the detection of exosomal circRNAs
Due to the absence of obvious symptoms and lack of specific diagnosis markers at an early stage, cancer patients are commonly diagnosed with the advanced stage of the disease and miss the optimal therapeutic opportunities. Owing to several advantages, including their convenient availability in biofluids like blood, cerebrospinal fluid, urine, and saliva, and their ability to mimic the constituents of their parent cells, exosomes have been reported to be excellent nano-theranostic tools (Thakur et al., 2022a; Thakur et al., 2022b). A recent study by Wang et al. (2020b) has observed the significantly upregulated hsa_circRNA_002178 in plasma-derived exosomes from lung adenocarcinoma patients, and this serum exosomal circRNA could act as the potential biomarker for patients’ diagnoses. Tian et al. (2019) have confirmed that exosomal circRASSF2 can be effectively released into the serum from laryngeal squamous cell carcinoma patients. Exosomal circRASSF2 is significantly overexpressed in the serum and, hence, could be considered a novel diagnostic target for laryngeal squamous cell carcinoma patients. Furthermore, overexpression of exosomal hsa_circ_0004771 in the serum could effectively distinguish the cancer patients from the healthy populations, which indicated the possible diagnostic roles of serum exosomal hsa_circ_0004771 for the patients with colorectal cancer (Lasda and Parker, 2016; Pan et al., 2019).
2.3 Monitoring of cancer prognosis via the detection of exosomal circRNAs
Exosomes have been extensively studied as a potential tool for prognosis of different diseases, including cancer progression (Wang et al., 2019b; Zhou et al., 2021). This provides the ability to monitor the disease progression via a non-invasive technique. Among various cargoes, several circRNAs have also been detected in exosomes for disease prognosis purposes (Sun et al., 2021). The upregulated exosomal circPRMT5 in the urine and serum was found to be closely related to tumor progression of bladder urethral epithelial carcinoma patients. Also, exosomal circPRMT5 has been expected to be a non-invasive biomarker for the evaluation of prognostic values and therapeutic efficacy of patients with bladder urethral epithelial carcinoma (Chen et al., 2018). Moreover, survival analysis verified that pancreatic cancer patients with a high level of exo-circPDE8A displays unfavorable prognosis (Li et al., 2018). In addition, FLI1 exonic circRNAs, a newly identified oncogenic driver, could also serve as an unfavorable prognostic biomarker for small-cell lung cancer patients (Li et al., 2019). Taken together, these aforementioned studies suggested the functional roles of exosomal circRNAs as promising markers for cancer patients’ prognoses.
3 Exosomal circRNAs in cancer therapeutic resistance
3.1 Significance of exosomal circRNAs in chemotherapy resistance
As one of the most effective strategies, chemotherapy is widely used for the clinical management of cancer patients (Carceles-Cordon et al., 2020). Recently, exosomes could transfer multiple circRNAs from donor cells to recipient cells, participating in the regulation of chemotherapeutic response (Figure 2). Emerging studies have implied that exosomal circRNAs are expected to become the bio-targets for the improvement of therapeutic efficacy.
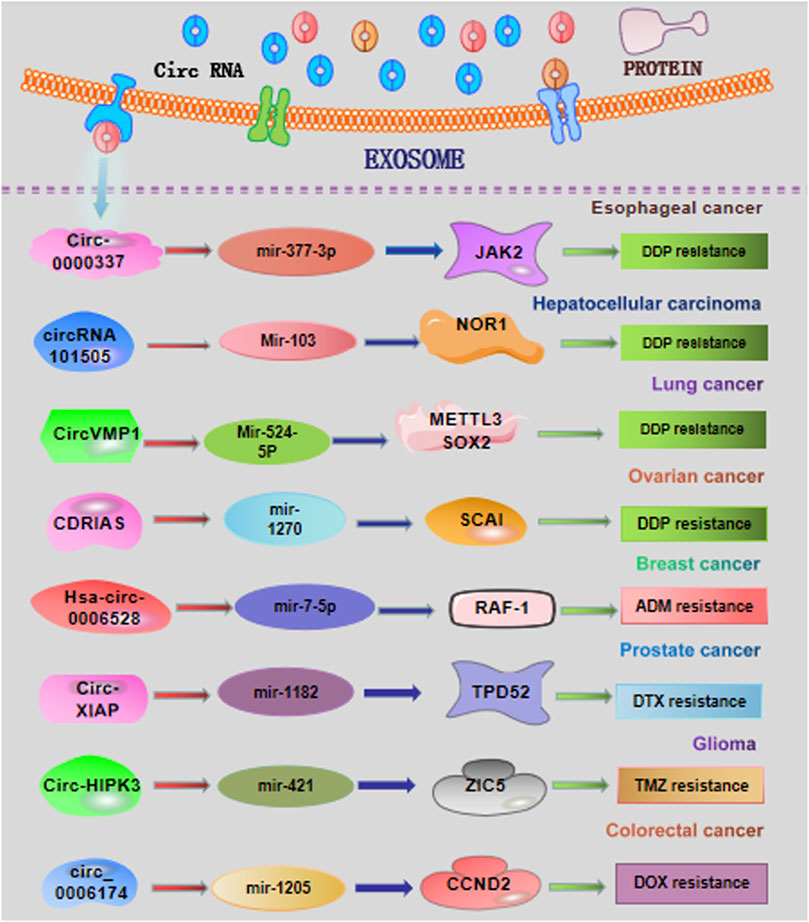
FIGURE 2. Overview of exosomal circRNAs in cancer chemotherapy resistance. DDP, cisplatin; ADM, Adriamycin; DTX, docetaxel; TMZ, temozolomide.
3.1.1 Esophageal cancer
Zang et al. (2021) have showed that circ-0000337 and JAK2 were highly expressed in cisplatin (DDP)-resistant esophageal cancer cells and tissues. The highly expressed circ-0000337 derived from exosomes obviously promotes cell proliferation and DDP resistance of esophageal cancer cells in vitro and in vivo. The exosomal circ-0000337 from DDP-resistant esophageal cancer cells could be transported into DDP-sensitive cells, consequentially resulting in therapeutic resistance. Mechanistically, serving as a sponge of miR-377-3p, exosomal circ-0000337 could promote the activation of JAK2 signaling, accelerating the DDP resistance (Zang et al., 2021).
3.1.2 Hepatocellular carcinoma
CircRNA-101505 has recently been demonstrated to be downregulated in hepatocellular carcinoma (HCC) cells and tissues (Luo et al., 2019). Overexpression of circZFR displays the important inhibitory effects on cancer development and DDP chemotherapy. CircRNA_101505 could upregulate the expression of oxidized-nitro domain-containing protein 1 via sponging miR-103, facilitating the DDP sensitization of HCC patients (Luo et al., 2019). Zhou et al. found that cancer-associated fibroblasts (CAFs) could transfer circZFR into HCC cells, restraining the STAT3/NF-κB pathway and promoting HCC development and chemoresistance (Zhou et al., 2022).
3.1.3 Lung cancer
Xie et al. have recently demonstrated the upregulated levels of exosomal circVMP1 in non-small cell lung cancer (NSCLC) cells. The findings in this study also revealed that circVMP1 expression was markedly elevated in DDP-resistant NSCLC cell lines A549/DDP and H1299/DDP (Xie et al., 2022). Acting as a ceRNA, circVMP1 could sponge microRNA-524-5p (miR-524-5p) to upregulate the expression of methyltransferase 3, the N6-adenosine-methyltransferase complex catalytic subunit (METTL3), and SOX2. CircVMP1 silencing significantly restrained the malignant behaviors and DDP resistance of A549/DDP and H1299/DDP cells.
3.1.4 Ovarian cancer
Several studies have found the essential roles of exosomal circRNAs in influencing the drug sensitivity. The DDP sensitivity of ovarian cancer cells could be enhanced by upregulation of the exosomal circRNA Cdr1as (Sang et al., 2018; Zhao et al., 2019). The results from these studies reveal that exosomal circRNA Cdr1as could be transferred to the cancer cells and sponge miR-1270 to alleviate DDP resistance in ovarian cancer. Hence, it might be a hopeful strategy to sensitize the treatment response for cancer patients by regulating the release of exosomal circRNAs. Gao and Huang revealed that circ_0007841 induced cell apoptosis in DDP-resistant ovarian cancer cells by acting as a miR-532-5p sponge, consequently weakening the DDP resistance and malignant behaviors in DDP-resistant ovarian cancer cells (Gao and Huang, 2022).
3.1.5 Breast cancer
Through exploring the profiles of circRNAs in Adriamycin (ADM)-resistant breast cancer cell MCF-7/ADM, Gao et al. identified the significantly upregulated hsa_circ_0006528 in MCF-7/ADM cells. Several algorithms, such as TargetScan and miRanda, indicated miR-7-5p as the potential target of hsa_circ_0006528. Thereafter, cellular function analysis displayed that hsa_circ_0006528 overexpression promoted the chemoresistance to ADM in breast cancer cells though sponging miR-7-5p (Gao et al., 2017). These results pointed out the important biological roles of circRNAs in chemo-resistance of breast cancer patients. Another recent study revealed that circRNA-CREIT was aberrantly downregulated in doxorubicin (DOX)-resistant triple-negative breast cancer (TNBC) cells and associated with a poor prognosis. Mechanistically, circRNA-CREIT can activate the RACK1/MTK1-mediated apoptosis signaling pathway. In addition, circRNA-CREIT could be packaged into exosomes and disseminate DOX sensitivity among TNBC cells (Wang et al., 2022b).
3.1.6 Prostate cancer
A recent study has shown that knockdown of the exosomal circRNA X-linked inhibitor of apoptosis (circXIAP) could restrain cell migration and proliferation, and induce cell apoptosis, leading to docetaxel (DTX) sensitization in prostate cancer cells (Zhang et al., 2021). Downregulation of miR-1182 could effectively overturn the sensitization effects of circ-XIAP knockdown on DTX chemotherapy. Consequently, circ-XIAP derived from exosomes could enhance the therapeutic effects of DTX, offering predictive chemotherapy sensitizing targets for prostate cancer patients. Tan et al. revealed that circSFMBT2 was upregulated in DTX-resistant prostate cells and could increase the DTX resistance of prostate cells by regulating the miR-136-5p/TRIB1 axis (Tan et al., 2022).
3.1.7 Glioma
Ding et al. observed that depletion of exosomal circNFIX increased temozolomide (TMZ) sensitization in glioma cells (Ding et al., 2020). This study proved that circNFIX transferred by exosomes could weaken the killing effect of TMZ on glioma cells by acting as a sponge of miR-132. Analogously, Han et al. demonstrated that aberrantly expressed exosomal circHIPK3 could accelerate the cancer progression and TMZ resistance by adjusting the miR-421/ZIC5 signaling axis (Han et al., 2021). Geng et al. demonstrated the exosome-mediated delivery of circWDR62 in TMZ-resistant glioma cells U251-R. High expression of exosomal circWDR62 can enhance TMZ resistance and malignant progression via regulating the miR-370-3p/MGMT axis (Geng et al., 2022).
3.1.8 Colorectal cancer
A recent study demonstrated that the circ_0006174 derived from exosomes was significantly upregulated in DOX-resistant colorectal cancer tissues and cells (Zhang et al., 2022). This research found that the effect of exosomal circ_0006174 on DOX resistance was dependent on the miR-1205-mediated CCND2 upregulation. Li et al. found that exosome-derived circ_0094343 was remarkably downregulated in chemotherapy-resistant colorectal cancer tissues and metastatic colorectal cancer tissues (Li and Li, 2022). Mechanistic validation demonstrated that circ_0094343 could inhibit HCT116 cell proliferation and improve the therapeutic resistance to 5-fluorouracil (5-FU), oxaliplatin, and DOX via the miR-766-5p/TRIM67 axis.
3.2 Implication of exosomal circRNAs in immunotherapeutic resistance
Nowadays, immunotherapy is becoming an attractive therapeutic method that helps in effectively resisting the cancer cells by ameliorating the host immune response. Targeting the host immune system could initiate a systemically permanent anti-tumor immune response. Despite the unprecedented tumor regressions and long-term survival benefits observed with anti-programmed death 1 (anti-PD1) therapy in patients with advanced cancers, there is still a subset of patients who do not obtain a curative benefit from immunotherapy (Topalian et al., 2020). Recently, exosomal circRNAs have been proved to affect the therapeutic response to anti-PD1 and provided hopeful biomarkers for improving immunotherapy. Accordingly, circ-UHRF1 knockdown in HCC cells could sensitize the anti-PD1 treatment, consequently ameliorating the patients’ survival rate (Zhang et al., 2020). In addition, exosomal circ-UHRF1 released from HCC cells effectively inhibits the function of natural killer cells by degrading miR-449c-5p and inducing TIM-3 expression, finally reinforcing the immunosuppressive tumor microenvironment (Huang et al., 2020) (Figure 3).
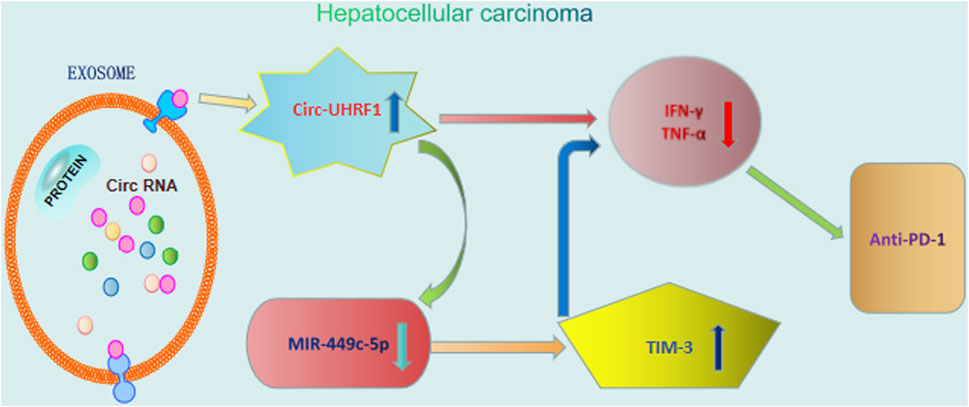
FIGURE 3. Roles of exosomal circRNAs in immunotherapy of hepatocellular carcinoma. Exosomal circ-UHRF1 released from HCC cells effectively inhibits the function of natural killer cells by degrading miR-449c-5p and inducing TIM-3 expression, finally reinforcing anti-PD1 immunotherapy.
3.3 Involvement of exosomal circRNAs in targeted therapy resistance
It has been suggested that cancer cells might become resistant to targeted therapy-mediated cellular cytotoxicity, interfering with the clinical therapeutic effects (Table 1). For example, Yang et al. found the upregulated circFN1 in both sorafenib-resistant HCC cells and tissues (Yang et al., 2020). Overexpression of circFN1 may function as a sponge for miR-1205 to facilitate sorafenib resistance and tumorigenesis of HCC cells.
Similarly, circRNA-SORE transported by exosomes plays a promoting role in sorafenib resistance in HCC (Xu et al., 2020b). Depletion of circRNA-SORE could enhance the cell-killing ability of sorafenib through improving the stability of the oncogenic protein YBX1.
3.4 Importance of exosomal circRNAs in radiotherapy resistance
He et al. (2022) demonstrated that exosomal circPRRX1 affected cell proliferation, motility, invasion, and radiation sensitivity in vitro and in vivo. Mechanistically, exosomal circPRRX1 affected the cancer-associated biological functions through regulating miR-596 and its downstream target NKAP (He et al., 2022). This study demonstrated the important roles of the exosomal circRNA–miRNA ceRNA crosstalk in adjusting tumorigenesis and radiation sensitivity. Another group’s study showed that low-dose radiation-induced exosomal circ-METRN played an oncogenic role in glioblastoma progression and radioresistance through the miR-4709-3p/GRB14/PDGFRα pathway (Wang et al., 2021b), providing novel mechanistic insights into the potential roles of exosomal circRNAs as therapeutic targets in glioblastoma.
4 Discussion and perspective
Because of the stable structures and attractive biological functions (Huang et al., 2021; Papaspyropoulos et al., 2021), circRNAs are intrinsically resistant to degradation mediated by RNA exonuclease or RNase R and easily transferred into the body fluids by exosomes (Burd et al., 2010). More important, exosome-carried circRNAs have been proved to be the candidate non-invasive biomarkers for the diagnosis and prognosis of cancer patients (Wang et al., 2021c; Li et al., 2021). Meanwhile, exosomal circRNAs, as available therapeutic targets, have also been obtained for clinical practice (Harper et al., 2021).
The preexisting drug-resistant cells and the continuous self-differentiation of cancer cells lead to the accumulated therapy-resistant characteristics of cancer patients (Haider et al., 2020). Cancer-related exosomes, released by cancer cells and other cells in the tumor micro-environment, play regulatory roles in the progression, migration, and deterioration of multiple cancers (Sandua et al., 2021). CircRNAs can be a novel type of therapeutic targets, by functioning as tumor suppressors or oncogenes (He et al., 2021; Pan et al., 2023). Accumulated studies have revealed that cancer cells could release circRNAs to the surrounding recipient cells via exosome-mediated transport strategies, thus affecting the carcinogenesis and therapeutic response (Liu et al., 2021b; Fontemaggi et al., 2021; Zhang et al., 2023). For example, the synthetic tumor-suppressing circRNAs can be packaged into exosomes and transported into certain target cells to suppress cancer metastasis and invasion (Dai et al., 2020). The circRNA-based research field has identified the vital functional roles of exosomal circRNAs in cancer biology (Harper et al., 2021). They could act as the pivotal biomarkers for early cancer detection, diagnosis, prognosis, and evaluation of therapeutic efficacy.
5 Conclusion
Cancers are the highest malignant diseases, resulting in the leading cause of death worldwide. Therapeutic resistance limits the effectiveness of therapies, presenting a major challenge for the clinical treatment of cancer patients. Exosomal circRNAs are recently demonstrated to regulate the response to therapeutic strategies. As biological delivery carriers, exosomes are of prodigious interest in cancer research and treatment. Nevertheless, the detailed mechanisms underlying the regulation and biological functions of exosomal circRNAs remain to be further explained. Therefore, clarifying the underlying molecular mechanisms regulated by the exosome-containing circRNAs may help strengthen the therapeutic sensitivity by modulating the tumor microenvironment.
Author contributions
ZX, AT, and WZ: conception and design. YY, YL, QL, WZ, and FK: writing and revising the manuscript. All authors have contributed to and approved this work.
Funding
This study is supported by grants from the National Natural Science Foundation of China (82272659), the Science and Technology Innovation Program of Hunan Province (2022RC1210), and the horizontal project (2022, 1 43010100; 2021-021, 143010100).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bray, F., Laversanne, M., Weiderpass, E., and Soerjomataram, I. (2021). The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 127 (16), 3029–3030. doi:10.1002/cncr.33587
Burd, C. E., Jeck, W. R., Liu, Y., Sanoff, H. K., Wang, Z., and Sharpless, N. E. (2010). Expression of linear and novel circular forms of an INK4/ARF-associated non-coding RNA correlates with atherosclerosis risk. PLoS Genet. 6 (12), e1001233. doi:10.1371/journal.pgen.1001233
Carceles-Cordon, M., Kelly, W. K., Gomella, L., Knudsen, K. E., Rodriguez-Bravo, V., and Domingo-Domenech, J. (2020). Cellular rewiring in lethal prostate cancer: The architect of drug resistance. Nat. Rev. Urol. 17 (5), 292–307. doi:10.1038/s41585-020-0298-8
Chen, J., Shi, P., Zhang, J., Li, Y., Ma, J., Zhang, Y., et al. (2022). CircRNA_0044556 diminishes the sensitivity of triple-negative breast cancer cells to adriamycin by sponging miR-145 and regulating NRAS. Mol. Med. Rep. 25 (2), 51. doi:10.3892/mmr.2021.12567
Chen, X., Chen, R. X., Wei, W. S., Li, Y. H., Feng, Z. H., Tan, L., et al. (2018). PRMT5 circular RNA promotes metastasis of urothelial carcinoma of the bladder through sponging miR-30c to induce epithelial-mesenchymal transition. Clin. Cancer Res. 24 (24), 6319–6330. doi:10.1158/1078-0432.Ccr-18-1270
Dai, J., Su, Y., Zhong, S., Cong, L., Liu, B., Yang, J., et al. (2020). Exosomes: Key players in cancer and potential therapeutic strategy. Signal Transduct. Target. Ther. 5 (1), 145. doi:10.1038/s41392-020-00261-0
Ding, C., Yi, X., Wu, X., Bu, X., Wang, D., Wu, Z., et al. (2020). Exosome-mediated transfer of circRNA CircNFIX enhances temozolomide resistance in glioma. Cancer Lett. 479, 1–12. doi:10.1016/j.canlet.2020.03.002
Dumache, R. (2017). Early diagnosis of oral squamous cell carcinoma by salivary microRNAs. Clin. Lab. 63 (11), 1771–1776. doi:10.7754/Clin.Lab.2017.170607
Fadhil, R. S., Wei, M. Q., Nikolarakos, D., Good, D., and Nair, R. G. (2020). Salivary microRNA miR-let-7a-5p and miR-3928 could be used as potential diagnostic bio-markers for head and neck squamous cell carcinoma. PLoS One 15 (3), e0221779. doi:10.1371/journal.pone.0221779
Fontemaggi, G., Turco, C., Esposito, G., and Di Agostino, S. (2021). New molecular mechanisms and clinical impact of circRNAs in human cancer. Cancers 13 (13), 3154. PubMed PMID: 34202482; PubMed Central PMCID: PMC8268751. doi:10.3390/cancers13133154
Gao, D., Zhang, X., Liu, B., Meng, D., Fang, K., Guo, Z., et al. (2017). Screening circular RNA related to chemotherapeutic resistance in breast cancer. Epigenomics 9 (9), 1175–1188. doi:10.2217/epi-2017-0055
Gao, Y., and Huang, Y. (2022). Circ_0007841 knockdown confers cisplatin sensitivity to ovarian cancer cells by down-regulation of NFIB expression in a miR-532-5p-dependent manner. J. Chemother., 1–14. doi:10.1080/1120009X.2022.2056995
Gaurav, I., Thakur, A., Iyaswamy, A., Wang, X., Chen, X., and Yang, Z. (2021). Factors affecting extracellular vesicles based drug delivery systems. Molecules 26 (6), 1544. doi:10.3390/molecules26061544
Gaurav, I., Wang, X., Thakur, A., Iyaswamy, A., Thakur, S., Chen, X., et al. (2021). Peptide-conjugated nano delivery systems for therapy and diagnosis of cancer. Pharmaceutics 13 (9), 1433. doi:10.3390/pharmaceutics13091433
Geng, X., Zhang, Y., Lin, X., Zeng, Z., Hu, J., Hao, L., et al. (2022). Exosomal circWDR62 promotes temozolomide resistance and malignant progression through regulation of the miR-370-3p/MGMT axis in glioma. Cell death Dis. 13 (7), 596. doi:10.1038/s41419-022-05056-5
Guarnerio, J., Bezzi, M., Jeong, J. C., Paffenholz, S. V., Berry, K., Naldini, M. M., et al. (2016). Oncogenic role of fusion-circRNAs derived from cancer-associated chromosomal translocations. Cell 165 (2), 289–302. doi:10.1016/j.cell.2016.03.020
Haider, T., Pandey, V., Banjare, N., Gupta, P. N., and Soni, V. (2020). Drug resistance in cancer: Mechanisms and tackling strategies. Pharmacol. Rep. P. R. 72 (5), 1125–1151. doi:10.1007/s43440-020-00138-7
Han, C., Wang, S., Wang, H., and Zhang, J. (2021). Exosomal circ-HIPK3 facilitates tumor progression and temozolomide resistance by regulating miR-421/ZIC5 Axis in glioma. Cancer Biother Radiopharm. 36 (7), 537–548. doi:10.1089/cbr.2019.3492
Harper, K. L., Mottram, T. J., and Whitehouse, A. (2021). Insights into the evolving roles of circular RNAs in cancer. Cancers 13 (16), 4180. doi:10.3390/cancers13164180
He, R. Z., Jiang, J., and Luo, D. X. (2021). M6A modification of circNSUN2 promotes colorectal liver metastasis. Genes & Dis. 8 (1), 6–7. doi:10.1016/j.gendis.2019.12.002
He, Y., Zheng, L., Yuan, M., Fan, J., Rong, L., Zhan, T., et al. (2022). Exosomal circPRRX1 functions as a ceRNA for miR-596 to promote the proliferation, migration, invasion, and reduce radiation sensitivity of gastric cancer cells via the upregulation of NF-κB activating protein. Anti-cancer drugs 33 (10), 1114–1125. doi:10.1097/CAD.0000000000001358
Hon, K. W., Ab-Mutalib, N. S., Abdullah, N. M. A., Jamal, R., and Abu, N. (2019). Extracellular Vesicle-derived circular RNAs confers chemoresistance in Colorectal cancer. Sci. Rep. 9 (1), 16497. doi:10.1038/s41598-019-53063-y
Huang, X. Y., Zhang, P. F., Wei, C. Y., Peng, R., Lu, J. C., Gao, C., et al. (2020). Circular RNA circMET drives immunosuppression and anti-PD1 therapy resistance in hepatocellular carcinoma via the miR-30-5p/snail/DPP4 axis. Mol. Cancer 19 (1), 92. doi:10.1186/s12943-020-01213-6
Huang, Y., Zhang, C., Xiong, J., and Ren, H. (2021). Emerging important roles of circRNAs in human cancer and other diseases. Genes & Dis. 8 (4), 412–423. doi:10.1016/j.gendis.2020.07.012
Jiao, S., Wu, S., Huang, S., Liu, M., and Gao, B. (2021). Advances in the identification of circular RNAs and research into circRNAs in human diseases. Front. Genet. 12, 665233. doi:10.3389/fgene.2021.665233
Keenan, T. E., and Tolaney, S. M. (2020). Role of immunotherapy in triple-negative breast cancer. J. Natl. Compr. Canc Netw. 18 (4), 479–489. doi:10.6004/jnccn.2020.7554
Lai, Q., Wang, M., Hu, C., Tang, Y., Li, Y., and Hao, S. (2021). Circular RNA regulates the onset and progression of cancer through the mitogen-activated protein kinase signaling pathway. Oncol. Lett. 22 (6), 817. doi:10.3892/ol.2021.13078
Lan, H., Yuan, J., Zeng, D., Liu, C., Guo, X., Yong, J., et al. (2021). The emerging role of non-coding RNAs in drug resistance of ovarian cancer. Front. Genet. 12, 693259. doi:10.3389/fgene.2021.693259
Lasda, E., and Parker, R. (2016). Circular RNAs Co-precipitate with extracellular vesicles: A possible mechanism for circRNA clearance. PLoS One 11 (2), e0148407. doi:10.1371/journal.pone.0148407
Li, C., and Li, X. (2022). Exosome-derived Circ_0094343 promotes chemosensitivity of colorectal cancer cells by regulating glycolysis via the miR-766-5p/TRIM67 Axis. Contrast media & Mol. imaging, 2878557. doi:10.1155/2022/2878557
Li, L., Li, W., Chen, N., Zhao, H., Xu, G., Zhao, Y., et al. (2019). FLI1 exonic circular RNAs as a novel oncogenic driver to promote tumor metastasis in small cell lung cancer. Clin. Cancer Res. 25 (4), 1302–1317. doi:10.1158/1078-0432.Ccr-18-1447
Li, X., Wang, J., Qian, H., Wu, Y., Zhang, Z., Hu, Z., et al. (2021). Serum exosomal circular RNA expression profile and regulative role in proliferative diabetic retinopathy. Front. Genet. 12, 719312. doi:10.3389/fgene.2021.719312
Li, Y., Zheng, Q., Bao, C., Li, S., Guo, W., Zhao, J., et al. (2015). Circular RNA is enriched and stable in exosomes: A promising biomarker for cancer diagnosis. Cell Res. 25 (8), 981–984. doi:10.1038/cr.2015.82
Li, Z., Yanfang, W., Li, J., Jiang, P., Peng, T., Chen, K., et al. (2018). Tumor-released exosomal circular RNA PDE8A promotes invasive growth via the miR-338/MACC1/MET pathway in pancreatic cancer. Cancer Lett. 432, 237–250. doi:10.1016/j.canlet.2018.04.035
Liu, J., Zhang, X., Yan, M., and Li, H. (2020). Emerging role of circular RNAs in cancer. Front. Oncol. 10, 663. doi:10.3389/fonc.2020.00663
Liu, J., Zhu, H., Fu, L., and Xu, T. (2021). Investigating the underlying mechanisms of circular RNAs and their application in clinical research of cervical cancer. Front. Genet. 12, 653051. doi:10.3389/fgene.2021.653051
Liu, X., Xu, J., Zhou, J., and Shen, Q. (2021). Oridonin and its derivatives for cancer treatment and overcoming therapeutic resistance. Genes & Dis. 8 (4), 448–462. doi:10.1016/j.gendis.2020.06.010
Long, G., Ma, S., Shi, R., Sun, Y., Hu, Z., and Chen, K. (2022). Circular RNAs and drug resistance in genitourinary cancers: A literature review. Cancers (Basel) 14 (4), 866. doi:10.3390/cancers14040866
Long, K. B., Collier, A. I., and Beatty, G. L. (2019). Macrophages: Key orchestrators of a tumor microenvironment defined by therapeutic resistance. Mol. Immunol. 110, 3–12. doi:10.1016/j.molimm.2017.12.003
Luo, Y., Fu, Y., Huang, R., Gao, M., Liu, F., Gui, R., et al. (2019). CircRNA_101505 sensitizes hepatocellular carcinoma cells to cisplatin by sponging miR-103 and promotes oxidored-nitro domain-containing protein 1 expression. Cell death Discov. 5, 121. doi:10.1038/s41420-019-0202-6
Nie, H., Liao, Z., Wang, Y., Zhou, J., He, X., and Ou, C. (2021). Exosomal long non-coding RNAs: Emerging players in cancer metastasis and potential diagnostic biomarkers for personalized oncology. Genes & Dis. 8 (6), 769–780. doi:10.1016/j.gendis.2020.12.004
Okusaka, T., and Furuse, J. (2020). Recent advances in chemotherapy for pancreatic cancer: Evidence from Japan and recommendations in guidelines. J. Gastroenterol. 55 (4), 369–382. doi:10.1007/s00535-020-01666-y
Pan, B., Qin, J., Liu, X., He, B., Wang, X., Pan, Y., et al. (2019). Identification of serum exosomal hsa-circ-0004771 as a novel diagnostic biomarker of colorectal cancer. Front. Genet. 10, 1096. doi:10.3389/fgene.2019.01096
Pan, Y., Liu, Y., Wei, W., Yang, X., Wang, Z., and Xin, W. (2023). Extracellular vesicles as delivery shippers for noncoding RNA-based modulation of angiogenesis: Insights from ischemic stroke and cancer. Small, e2205739. doi:10.1002/smll.202205739
Papaspyropoulos, A., Hazapis, O., Lagopati, N., Polyzou, A., Papanastasiou, A. D., Liontos, M., et al. (2021). The role of circular RNAs in DNA damage response and repair. Cancers 13 (21), 5352. doi:10.3390/cancers13215352
Sandua, A., Alegre, E., and Gonzalez, A. (2021). Exosomes in lung cancer: Actors and heralds of tumor development. Cancers 13 (17), 4330. doi:10.3390/cancers13174330
Sang, M., Meng, L., Sang, Y., Liu, S., Ding, P., Ju, Y., et al. (2018). Corrigendum to "Circular RNA ciRS-7 accelerates ESCC progression through acting as a miR-876-5p sponge to enhance MAGE-A family expression" [Cancer Lett. 426 (2018) 37-46]. Cancer Lett. 426526, 37364–37465. doi:10.1016/j.canlet.2021.12.001
Sanger, H. L., Klotz, G., Riesner, D., Gross, H. J., and Kleinschmidt, A. K. (1976). Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc. Natl. Acad. Sci. U. S. A. 73 (11), 3852–3856. doi:10.1073/pnas.73.11.3852
Sharma, P., Hu-Lieskovan, S., Wargo, J. A., and Ribas, A. (2017). Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 168 (4), 707–723. doi:10.1016/j.cell.2017.01.017
Stanway, S., Lodge, M., Sullivan, R., Diprose, K., Young, A. M., Crisp, N., et al. (2021). The UK's contribution to cancer control in low-income and middle-income countries. Lancet Oncol. 22 (9), e410–e418. doi:10.1016/s1470-2045(21)00380-6
Sun, R., Liu, W., Zhao, Y., Chen, H., Wang, Z., Zhang, Y., et al. (2021). Exosomal circRNA as a novel potential therapeutic target for multiple myeloma-related myocardial damage. Cancer Cell Int. 21 (1), 311. doi:10.1186/s12935-021-02011-w
Tan, X., Song, X., Fan, B., Li, M., Zhang, A., and Pei, L. (2022). Exosomal circRNA Scm-like with four malignant brain tumor domains 2 (circ-SFMBT2) enhances the docetaxel resistance of prostate cancer via the microRNA-136-5p/tribbles homolog 1 pathway. Anti-cancer drugs 33 (9), 871–882. doi:10.1097/CAD.0000000000001365
Thakur, A., Johnson, A., Jacobs, E., Zhang, K., Chen, J., Wei, Z., et al. (2022). Energy sources for exosome communication in a cancer microenvironment. Cancers (Basel) 14 (7), 1698. doi:10.3390/cancers14071698
Thakur, A., Parra, D. C., Motallebnejad, P., Brocchi, M., and Chen, H. J. (2022). Exosomes: Small vesicles with big roles in cancer, vaccine development, and therapeutics. Bioact. Mater. 10, 281–294. doi:10.1016/j.bioactmat.2021.08.029
Thakur, A., Qiu, G., Ng, S. P., Guan, J., Yue, J., Lee, Y., et al. (2017). Direct detection of two different tumor-derived extracellular vesicles by SAM-AuNIs LSPR biosensor. Biosens. Bioelectron. 94, 400–407. doi:10.1016/j.bios.2017.03.036
Thakur, A., Qiu, G., Xu, C., Han, X., Yang, T., Ng, S. P., et al. (2020). Label-free sensing of exosomal MCT1 and CD147 for tracking metabolic reprogramming and malignant progression in glioma. Sci. Adv. 6 (26), eaaz6119. doi:10.1126/sciadv.aaz6119
Thakur, A., Sidu, R. K., Zou, H., Alam, M. K., Yang, M., and Lee, Y. (2020). Inhibition of glioma cells' proliferation by doxorubicin-loaded exosomes via microfluidics. Int. J. nanomedicine 15, 8331–8343. doi:10.2147/IJN.S263956
Thakur, A., Xu, C., Li, W. K., Qiu, G., He, B., Ng, S. P., et al. (2021). In vivo liquid biopsy for glioblastoma malignancy by the AFM and LSPR based sensing of exosomal CD44 and CD133 in a mouse model. Biosens. Bioelectron. 191, 113476. doi:10.1016/j.bios.2021.113476
Tian, L., Cao, J., Jiao, H., Zhang, J., Ren, X., Liu, X., et al. (2019). CircRASSF2 promotes laryngeal squamous cell carcinoma progression by regulating the miR-302b-3p/IGF-1R axis. Clin. Sci. (Lond) 133 (9), 1053–1066. doi:10.1042/cs20190110
Topalian, S. L., Taube, J. M., and Pardoll, D. M. (2020). Neoadjuvant checkpoint blockade for cancer immunotherapy. Science 367 (6477), eaax0182. doi:10.1126/science.aax0182
Tsimberidou, A. M. (2015). Targeted therapy in cancer. Cancer Chemother. Pharmacol. 76 (6), 1113–1132. doi:10.1007/s00280-015-2861-1
Verduci, L., Tarcitano, E., Strano, S., Yarden, Y., and Blandino, G. (2021). CircRNAs: Role in human diseases and potential use as biomarkers. Cell Death Dis. 12 (5), 468. doi:10.1038/s41419-021-03743-3
Wade, C. A., and Kyprianou, N. (2018). Profiling prostate cancer therapeutic resistance. Int. J. Mol. Sci. 19 (3), 904. doi:10.3390/ijms19030904
Wang, H., Lu, Z., and Zhao, X. (2019). Tumorigenesis, diagnosis, and therapeutic potential of exosomes in liver cancer. J. Hematol. Oncol. 12 (1), 133. doi:10.1186/s13045-019-0806-6
Wang, J., Zhao, X., Wang, Y., Ren, F., Sun, D., Yan, Y., et al. (2020). circRNA-002178 act as a ceRNA to promote PDL1/PD1 expression in lung adenocarcinoma. Cell Death Dis. 11 (1), 32. doi:10.1038/s41419-020-2230-9
Wang, M., Yu, F., Zhang, Y., Zhang, L., Chang, W., and Wang, K. (2022). The emerging roles of circular RNAs in the chemoresistance of gastrointestinal cancer. Front. Cell Dev. Biol. 10, 821609. doi:10.3389/fcell.2022.821609
Wang, S., Dong, Y., Gong, A., Kong, H., Gao, J., Hao, X., et al. (2021). Exosomal circRNAs as novel cancer biomarkers: Challenges and opportunities. Int. J. Biol. Sci. 17 (2), 562–573. doi:10.7150/ijbs.48782
Wang, X., Cao, Q., Shi, Y., Wu, X., Mi, Y., Liu, K., et al. (2021). Identification of low-dose radiation-induced exosomal circ-METRN and miR-4709-3p/GRB14/PDGFRα pathway as a key regulatory mechanism in Glioblastoma progression and radioresistance: Functional validation and clinical theranostic significance. Int. J. Biol. Sci. 17 (4), 1061–1078. doi:10.7150/ijbs.57168
Wang, X., Chen, T., Li, C., Li, W., Zhou, X., Li, Y., et al. (2022). CircRNA-CREIT inhibits stress granule assembly and overcomes doxorubicin resistance in TNBC by destabilizing PKR. J. Hematol. Oncol. 15 (1), 122. doi:10.1186/s13045-022-01345-w
Wang, Y., Li, Z., Xu, S., and Guo, J. (2020). Novel potential tumor biomarkers: Circular RNAs and exosomal circular RNAs in gastrointestinal malignancies. J. Clin. Lab. Anal. 34 (7), e23359. doi:10.1002/jcla.23359
Wang, Y., Liu, J., Ma, J., Sun, T., Zhou, Q., Wang, W., et al. (2019). Exosomal circRNAs: Biogenesis, effect and application in human diseases. Mol. Cancer 18 (1), 116. doi:10.1186/s12943-019-1041-z
Wang, Y., Pei, L., Yue, Z., Jia, M., Wang, H., and Cao, L. L. (2021). The potential of serum exosomal hsa_circ_0028861 as the novel diagnostic biomarker of HBV-derived hepatocellular cancer. Front. Genet. 12, 703205. doi:10.3389/fgene.2021.703205
Wu, Y., Niu, D., Deng, S., Lei, X., Xie, Z., and Yang, X. (2022). Tumor-derived or non-tumor-derived exosomal noncodingRNAs and signaling pathways in tumor microenvironment. Int. Immunopharmacol. 106, 108626. doi:10.1016/j.intimp.2022.108626
Xie, H., Yao, J., Wang, Y., and Ni, B. (2022). Exosome-transmitted circVMP1 facilitates the progression and cisplatin resistance of non-small cell lung cancer by targeting miR-524-5p-METTL3/SOX2 axis. Drug Deliv. 29 (1), 1257–1271. doi:10.1080/10717544.2022.2057617
Xu, J., Ji, L., Liang, Y., Wan, Z., Zheng, W., Song, X., et al. (2020). CircRNA-SORE mediates sorafenib resistance in hepatocellular carcinoma by stabilizing YBX1. Signal Transduct. Target. Ther. 5 (1), 298. doi:10.1038/s41392-020-00375-5
Xu, Z., Peng, B., Cai, Y., Wu, G., Huang, J., Gao, M., et al. (2020). N6-methyladenosine RNA modification in cancer therapeutic resistance: Current status and perspectives. Biochem. Pharmacol. 182, 114258. doi:10.1016/j.bcp.2020.114258
Yan, Y., Chen, X., Wang, X., Zhao, Z., Hu, W., Zeng, S., et al. (2019). The effects and the mechanisms of autophagy on the cancer-associated fibroblasts in cancer. J. Exp. Clin. Cancer Res. 38 (1), 171. doi:10.1186/s13046-019-1172-5
Yan, Y., Xu, Z., Chen, X., Wang, X., Zeng, S., Zhao, Z., et al. (2019). Novel function of lncRNA ADAMTS9-AS2 in promoting temozolomide resistance in glioblastoma via upregulating the FUS/MDM2 ubiquitination Axis. Front. Cell Dev. Biol. 7, 217. doi:10.3389/fcell.2019.00217
Yang, B., Teng, F., Chang, L., Wang, J., Liu, D. L., Cui, Y. S., et al. (2021). Tumor-derived exosomal circRNA_102481 contributes to EGFR-TKIs resistance via the miR-30a-5p/ROR1 axis in non-small cell lung cancer. Aging 13 (9), 13264–13286. doi:10.18632/aging.203011
Yang, C., Dong, Z., Hong, H., Dai, B., Song, F., Geng, L., et al. (2020). circFN1 mediates sorafenib resistance of hepatocellular carcinoma cells by sponging miR-1205 and regulating E2F1 expression. Mol. Ther. Nucleic Acids 22, 421–433. doi:10.1016/j.omtn.2020.08.039
Yang, J., Zhang, X., Cao, J., Xu, P., Chen, Z., Wang, S., et al. (2021). Circular RNA UBE2Q2 promotes malignant progression of gastric cancer by regulating signal transducer and activator of transcription 3-mediated autophagy and glycolysis. Cell Death Dis. 12 (10), 910. doi:10.1038/s41419-021-04216-3
Zang, R., Qiu, X., Song, Y., and Wang, Y. (2021). Exosomes mediated transfer of Circ_0000337 contributes to cisplatin (CDDP) resistance of esophageal cancer by regulating JAK2 via miR-377-3p. Front. Cell Dev. Biol. 9, 673237. doi:10.3389/fcell.2021.673237
Zhang, G., Hou, J., Mei, C., Wang, X., Wang, Y., and Wang, K. (2023). Effect of circular RNAs and N6-methyladenosine (m6A) modification on cancer biology. Biomed. Pharmacother. = Biomedecine Pharmacother. 159, 114260. doi:10.1016/j.biopha.2023.114260
Zhang, H., Li, M., Zhang, J., Shen, Y., and Gui, Q. (2021). Exosomal circ-XIAP promotes docetaxel resistance in prostate cancer by regulating miR-1182/TPD52 Axis. Drug Des. Devel Ther. 15, 1835–1849. doi:10.2147/dddt.S300376
Zhang, P. F., Gao, C., Huang, X. Y., Lu, J. C., Guo, X. J., Shi, G. M., et al. (2020). Cancer cell-derived exosomal circUHRF1 induces natural killer cell exhaustion and may cause resistance to anti-PD1 therapy in hepatocellular carcinoma. Mol. Cancer 19 (1), 110. doi:10.1186/s12943-020-01222-5
Zhang, X., Zhao, Y., Kong, P., Han, M., and Li, B. (2019). Expression of circZNF609 is down-regulated in colorectal cancer tissue and promotes apoptosis in colorectal cancer cells by upregulating p53. Med. Sci. Monit. 25, 5977–5985. doi:10.12659/msm.915926
Zhang, Y., Tan, X., and Lu, Y. (2022). Exosomal transfer of circ_0006174 contributes to the chemoresistance of doxorubicin in colorectal cancer by depending on the miR-1205/CCND2 axis. J. physiology Biochem. 78 (1), 39–50. doi:10.1007/s13105-021-00831-y
Zhang, Y., Zhang, X. O., Chen, T., Xiang, J. F., Yin, Q. F., Xing, Y. H., et al. (2013). Circular intronic long noncoding RNAs. Mol. Cell 51 (6), 792–806. doi:10.1016/j.molcel.2013.08.017
Zhang, Z., Xie, Q., He, D., Ling, Y., Li, Y., Li, J., et al. (2018). Circular RNA: New star, new hope in cancer. BMC Cancer 18 (1), 834. doi:10.1186/s12885-018-4689-7
Zhao, S. Y., Wang, J., Ouyang, S. B., Huang, Z. K., and Liao, L. (2018). Salivary circular RNAs Hsa_Circ_0001874 and Hsa_Circ_0001971 as novel biomarkers for the diagnosis of oral squamous cell carcinoma. Cell Physiol. Biochem. 47 (6), 2511–2521. doi:10.1159/000491624
Zhao, Z., Ji, M., Wang, Q., He, N., and Li, Y. (2019). Circular RNA Cdr1as upregulates SCAI to suppress cisplatin resistance in ovarian cancer via miR-1270 suppression. Mol. Ther. Nucleic Acids 18, 24–33. doi:10.1016/j.omtn.2019.07.012
Zhou, Y., Tang, W., Zhuo, H., Zhu, D., Rong, D., Sun, J., et al. (2022). Cancer-associated fibroblast exosomes promote chemoresistance to cisplatin in hepatocellular carcinoma through circZFR targeting signal transducers and activators of transcription (STAT3)/nuclear factor -kappa B (NF-κB) pathway. Bioengineered 13 (3), 4786–4797. doi:10.1080/21655979.2022.2032972
Keywords: exosomes, extracellular vesicles, cancer, circular RNAs, drug resistance
Citation: Kang F, Yan Y, Liu Y, Liang Q, Xu Z, Zhu W and Thakur A (2023) Unraveling the significance of exosomal circRNAs in cancer therapeutic resistance. Front. Pharmacol. 14:1093175. doi: 10.3389/fphar.2023.1093175
Received: 08 November 2022; Accepted: 30 January 2023;
Published: 15 February 2023.
Edited by:
Christian Celia, University of Studies G. d’Annunzio Chieti and Pescara, ItalyReviewed by:
Jinhui Liu, Nanjing Medical University, ChinaYiqun Jiang, Hunan Normal University, China
Copyright © 2023 Kang, Yan, Liu, Liang, Xu, Zhu and Thakur. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Zhu, emh1d2VpODYxMjA0QGNzdS5lZHUuY24=
†These authors have contributed equally to this work
 Fanhua Kang
Fanhua Kang Yuanliang Yan
Yuanliang Yan Yuanhong Liu
Yuanhong Liu Qiuju Liang
Qiuju Liang Zhijie Xu4
Zhijie Xu4 Abhimanyu Thakur
Abhimanyu Thakur