
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 30 January 2023
Sec. Gastrointestinal and Hepatic Pharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1092476
This article is part of the Research Topic Exploration of Genetic Variation, Drug Response, and Interactions Between Gastrointestinal Disorders and Other Diseases View all 5 articles
 Sherry Seah1†
Sherry Seah1† Yen Kheng Tan2†
Yen Kheng Tan2† Kevin Teh3
Kevin Teh3 Wann Jia Loh4
Wann Jia Loh4 Pei Ting Tan5
Pei Ting Tan5 Leng Chuan Goh6
Leng Chuan Goh6 Roy Debajyoti Malakar7
Roy Debajyoti Malakar7 Tar Choon Aw8
Tar Choon Aw8 Chin Shern Lau8
Chin Shern Lau8 Trishpal Dhalliwal9
Trishpal Dhalliwal9 Swee Leng Kui10
Swee Leng Kui10 Jia Wen Kam5
Jia Wen Kam5 Joan Khoo4
Joan Khoo4 Tunn Lin Tay4
Tunn Lin Tay4 Eberta Tan4
Eberta Tan4 Vanessa Au4
Vanessa Au4 Shui Boon Soh4
Shui Boon Soh4 Meifen Zhang4
Meifen Zhang4 Thomas F. King4
Thomas F. King4 Linsey Gani4‡
Linsey Gani4‡ Troy H. Puar4*‡
Troy H. Puar4*‡Introduction: Long-term proton pump inhibitor (PPI) use has been associated with hypomagnesemia. It is unknown how frequently PPI use is implicated in patients with severe hypomagnesemia, and its clinical course or risk factors.
Methods: All patients with severe hypomagnesemia from 2013 to 2016 in a tertiary center were assessed for likelihood of PPI-related hypomagnesemia using Naranjo algorithm, and we described the clinical course. The clinical characteristics of each case of PPI-related severe hypomagnesemia was compared with three controls on long-term PPI without hypomagnesemia, to assess for risk factors of developing severe hypomagnesemia.
Results: Amongst 53,149 patients with serum magnesium measurements, 360 patients had severe hypomagnesemia (<0.4 mmol/L). 189 of 360 (52.5%) patients had at least possible PPI-related hypomagnesemia (128 possible, 59 probable, two definite). 49 of 189 (24.7%) patients had no other etiology for hypomagnesemia. PPI was stopped in 43 (22.8%) patients. Seventy (37.0%) patients had no indication for long-term PPI use. Hypomagnesemia resolved in most patients after supplementation, but recurrence was higher in patients who continued PPI, 69.7% versus 35.7%, p = 0.009. On multivariate analysis, risk factors for hypomagnesemia were female gender (OR 1.73; 95% CI: 1.17–2.57), diabetes mellitus (OR, 4.62; 95% CI: 3.05–7.00), low BMI (OR, 0.90; 95% CI: 0.86–0.94), high-dose PPI (OR, 1.96; 95% CI: 1.29–2.98), renal impairment (OR, 3.85; 95% CI: 2.58–5.75), and diuretic use (OR, 1.68; 95% CI: 1.09–2.61).
Conclusion: In patients with severe hypomagnesemia, clinicians should consider the possibility of PPI-related hypomagnesemia and re-examine the indication for continued PPI use, or consider a lower dose.
Proton-pump inhibitors (PPI) are one of the most widely prescribed medications. While PPI are generally well-tolerated, they have been linked to increased risk of fractures, dementia, infections, and hypomagnesemia (Sheen and Triadafilopoulos, 2011). Since Epstein first described a case of severe hypomagnesemia with PPI use in 2006 (Epstein et al., 2006), other cases or case series have been reported (Hess et al., 2012; Danziger et al., 2013; Janett et al., 2015; Pasina et al., 2016). While some case-control and cross-sectional studies have demonstrated an association of PPI use with hypomagnesemia, other studies have not (Faulhaber et al., 2013; Koulouridis et al., 2013; Markovits et al., 2014; Van Ende et al., 2014; Zipursky et al., 2014; Kim et al., 2015; Pisani et al., 2016; Biyik et al., 2017). These conflicting findings may lead to uncertainty amongst clinicians of this potential adverse effect of PPI. Severe hypomagnesemia can lead to life-threatening arrythmias or seizures, and it is important to prevent recurrent episodes. Previous studies did not conduct a detailed review of individual patients to assess for causality, and it is currently unknown how frequent severe hypomagnesemia could be attributed to PPI use, or if appropriate management is taken to avoid recurrence of hypomagnesemia.
PPI-related hypomagnesemia appears to occur only after prolonged use of PPI, usually at least 3 months (Pisani et al., 2016). Current understanding is that PPI may impair gastrointestinal absorption of magnesium, via alteration of intestinal mucosal pH and interference with transient potential melastatin-6 (TRPM6)-mediated active absorption of magnesium (Bai et al., 2012; Lameris et al., 2015). In a prospective study by Begley et al. (2016), patients using a prolonged course of PPI were monitored prospectively for 8 months, with none of the patients developing clinically significant hypomagnesemia. This suggests that hypomagnesemia may only affect a small proportion of patients with genetic or clinical predisposition (Pisani et al., 2016). Previously identified risk factors include chronic renal impairment or concomitant use of diuretics (Danziger et al., 2013).
Amongst patients with severe hypomagnesemia, we aimed to assess the likelihood of PPI-related hypomagnesemia using the Naranjo algorithm (Naranjo et al., 1981), and described the clinical course of patients with at least possible PPI-related severe hypomagnesemia. We subsequently aimed to identify risk factors for developing severe hypomagnesemia amongst long-term PPI users, by comparing these cases with matched controls of patients on long-term PPI without hypomagnesemia.
We conducted a retrospective study of all patients treated at a single tertiary center, Changi General Hospital, Singapore, from 2013 to 2016. Local ethics approval was obtained, and waiver of consent was granted for all patients. The study was approved by the Singapore Health Services ethics committee and institutional review board (IRB 2017/2112), and is registered with Clinicaltrials.gov (NCT04426994).
We identified all patients with documented severe hypomagnesemia from 2013 to 2016. 175,943 measurements of serum magnesium were taken in 53,149 patients during this period. 6,902 patients had at least one episode of hypomagnesemia (<0.65 mmol/L). The first episode of severe hypomagnesemia (<0.4 mmol/L) was taken for patients with more than one episode. All patients were admitted during the episode of severe hypomagnesemia, and reconciliation of medication use was conducted by a pharmacist upon admission. For evaluation of PPI-related hypomagnesemia, we included patients on long-term PPI use (at least 3 months) prior to development of severe hypomagnesemia. We excluded patients with spurious hypomagnesemia results (Figure 1).
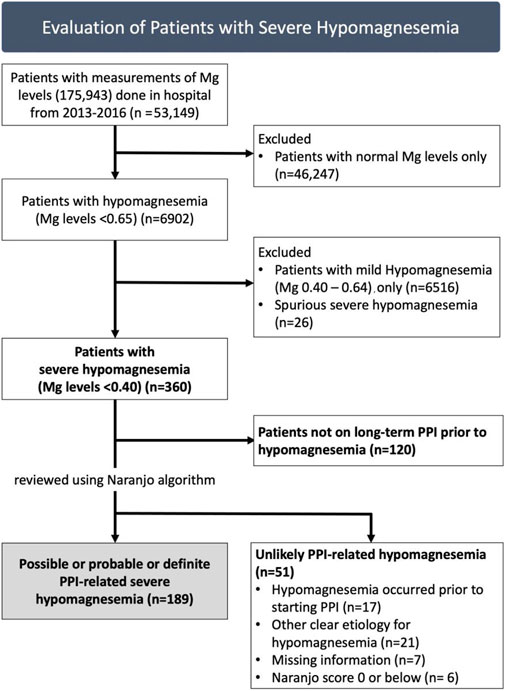
FIGURE 1. Patients with severe hypomagnesemia assessed for likelihood of proton-pump inhibitor (PPI)-related hypomagnesemia, with 189 identified to have at least possible PPI-related severe hypomagnesemia.
All patients were evaluated using the Naranjo algorithm (Naranjo et al., 1981) which indicates likelihood of an adverse drug reaction due to PPI: ≤0 doubtful, one to four possible, five to eight probable, and ≥9 definite (details in Supplementary Material). All patients were scored by two investigators (S.S and T.P), and discrepancies were settled by mutual consensus, or by a third investigator (Y.K.T). Electronic medical records were comprehensive, including data of previous medication prescriptions, laboratory tests from year 2000 onwards, from both tertiary and primary healthcare systems. Patients were assessed for any recurrence or resolution of hypomagnesemia until 30th September 2017. Medical information obtained included demographics, Charlson’s Comorbidity Index (CCI) (Charlson et al., 1987), concomitant medications, and laboratory data (including serum calcium, serum magnesium, urine magnesium, serum potassium, parathyroid hormone, where available). Glomerular filtration rate was estimated (eGFR) using CKD-EPI equation (Levey et al., 2009). In patients with severe hypomagnesemia, any associated complications were noted, including development of hypokalemia, hypocalcemia, arrthythmias and seizures.
Information was collected regarding magnesium levels prior to PPI initiation, and action (if any) taken after hypomagnesemia occurred (withdrawal, or continuation of PPI). We used the NICE guidelines to define standard-dosage PPI (e.g. omeprazole daily dose of 20 mg or equivalent) (NICE, 2014), and dosages above that as high-dosage PPI. We assessed if patients had an indication for long-term PPI use (Freedberg et al., 2017). This was defined as single antiplatelet therapy (SAPT) with additional risk factors including (Sheen and Triadafilopoulos, 2011) dual antiplatelet therapy (DAPT), Epstein et al. (2006) concomitant anticoagulant, NSAIDs or steroid use, Hoorn et al. (2010) history of gastrointestinal bleeding or ulcer, or (Pasina et al., 2016) age above 65 years; or gastroesophageal reflux disease (GERD), complicated by either Barrett’s esophagus or esophagitis (Freedberg et al., 2017).
To identify risk factors for developing severe hypomagnesemia, we compared the patients identified with at least possible PPI-related severe hypomagnesemia (cases), with patients on long-term PPI without hypomagnesemia (controls). Controls were patients prescribed long-term PPI at the same center over the same period, without documented hypomagnesemia. A total of 649,374 prescriptions of PPI were given to 158,523 patients from 2013 to 2016. We excluded prescriptions given for a duration of fewer than 3 months, pro re nata (when necessary), and patients with hypomagnesemia. We randomly selected three controls per case, matched for the date of PPI prescription. Duration of PPI therapy was date of first-ever PPI prescription till most recent prescription, or date of hypomagnesemia (if occurred).
Statistical analyses were performed using SPSS version 25, IBM Corp., Armonk, New York, United States.
Continuous variables were expressed as mean (SD), or median (IQR) and compared using independent t-test, or Mann-Whitney test as appropriate. Categorical variables were compared using Chi-square test. Spearman correlation was used to compare the association between serum magnesium, with calcium and potassium, during the episode of severe hypomagnesemia. We conducted backwards stepwise selection process. For risk factors of severe hypomagnesemia, variables associated with severe hypomagnesemia in the univariate analysis (p < 0.1) or previously described in the literature were included in the multivariate logistic regression model. Missing values were imputed for the purpose of the multivariate logistic regression model, with median values used. Individual components of CCI were used in the multivariate model. Statistical significance was set at p less than 0.05.
Of 175,943 magnesium measurements done in 53,149 patients from 2013 to 2016, there were 360 patients with severe hypomagnesemia (Figure 1). We excluded 120 patients who were not taking PPI prior to hypomagnesemia. Hence, we reviewed 240 patients with severe hypomagnesemia for PPI-related hypomagnesemia. Fifty-one patients were deemed unlikely to have PPI-related hypomagnesemia: 17 patients with hypomagnesemia which occurred prior to starting PPI, 21 patients with other clear etiology for hypomagnesemia (e.g. gastrointestinal malignancy, chronic alcoholism), 7 patients with missing information and six patients with Naranjo score of 0 or below (doubtful for PPI-related hypomagnesemia).
In total, 189 of 360 (52.5%) patients with severe hypomagnesmia were identified to have at least possible PPI-related hypomagnesemia using Naranjo algorithm: 128 possible, 59 probable, two definite. The mean age was 71.8 [11.0] years, with 103 females (54.5%) (Table 1). Majority of patients were using omeprazole (97.9%), with a median duration of PPI use of 57 (28–86) months. At baseline, between patients with lower (<5) or higher (≥ 5) Naranjo scores, there were no differences in demographics, co-morbidities, duration or dose of PPI.
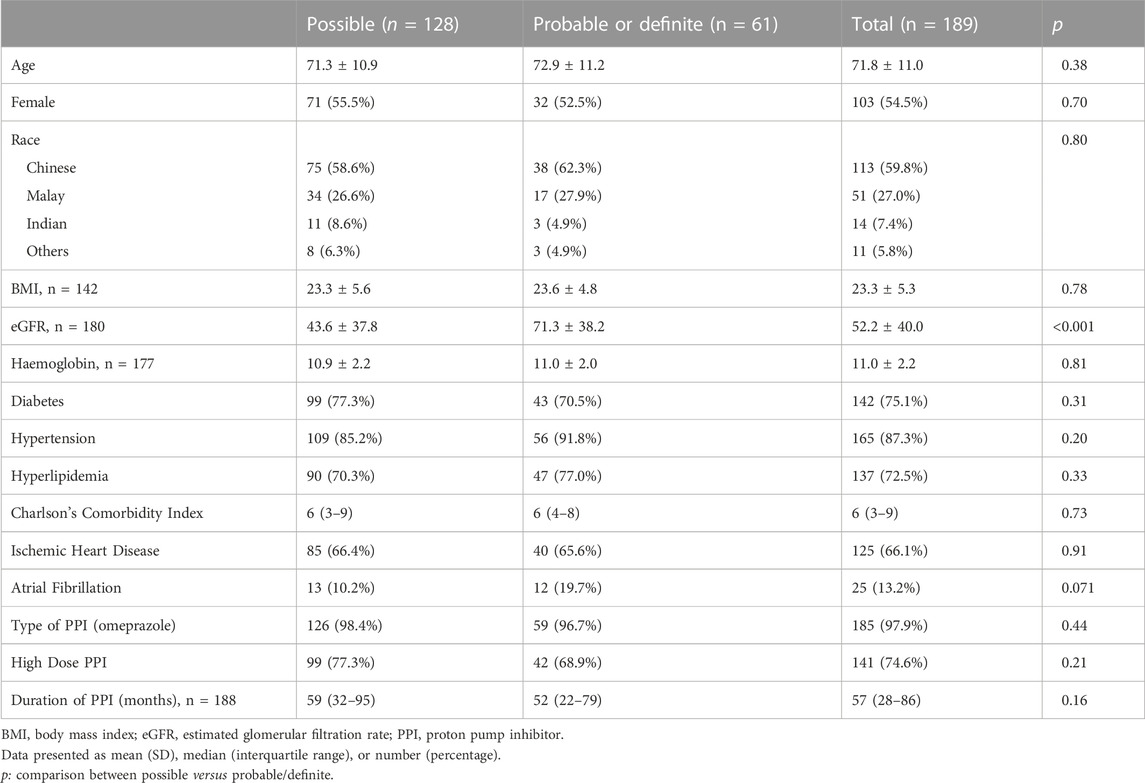
TABLE 1. Baseline Characteristics of 189 patients using proton pump inhibitor (PPI) prior to severe hypomagnesemia, evaluated with Naranjo algorithm for likelihood of PPI-related hypomagnesemia, 1–4 (possible), 5–8 (probable), ≥9 (definite).
Patients with at least probable PPI-related hypomagnesemia (Naranjo score ≥ 5) were more likely to have no alternative etiology for hypomagnesemia, compared to those with possible PPI-related hypomagnesemia, 75.4% versus 3.1%, p < 0.001 (Table 2). Overall, 39.7% of patients were using diuretic therapy, 66.7% had renal impairment, and 20.6% had a history of gastrointestinal loss prior to development of hypomagnesemia. PPI therapy was stopped in only 43 of 189 patients (22.8%) of patients after the episode of severe hypomagnesemia, and this was more often in patients with probable PPI-related hypomagnesemia, 47.5%, compared with possible PPI-related hypomagnesemia, 10.9%, p < 0.001. Forty-two patients were switched to a histamine-2 receptor antagonist, while one patient had cessation of PPI therapy. Only 63% of cases had a strong indication for long-term PPI use, which was most often SAPT usage in a patient above 65 years.
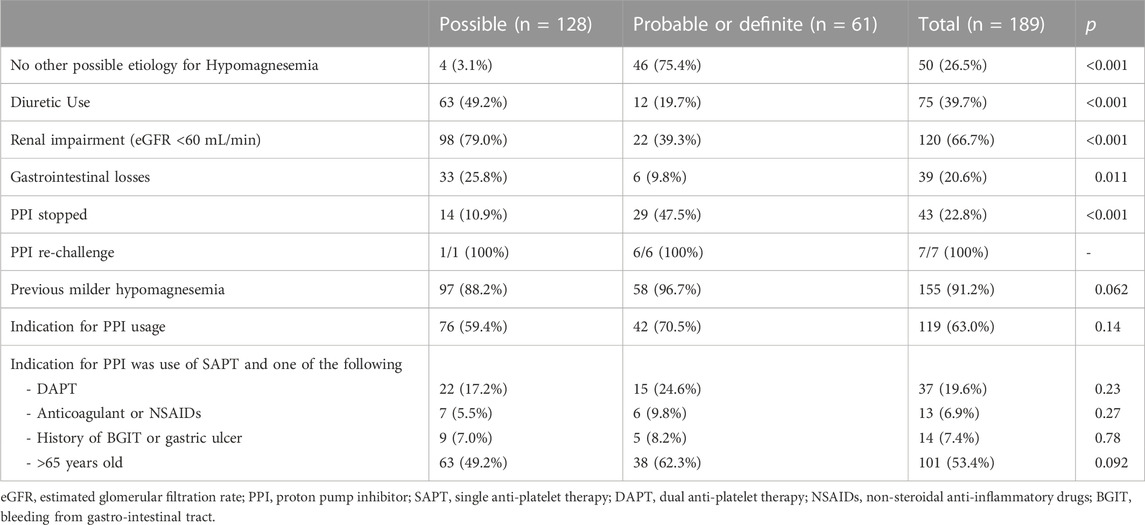
TABLE 2. Etiology and Outcomes of 189 patients with proton pump inhibitor (PPI)-related severe hypomagnesemia as evaluated with Naranjo algorithm, 1–4 (possible), 5–8 (probable), ≥9 (definite).
All patients were given oral or intravenous magnesium replacement during the admission, and most patients had resolution of hypomagnesemia within a few days. Patients who stopped using PPI were less likely to develop a subsequent episode of hypomagnesemia, compared to those who were continued on PPI therapy, five of 14 (35.7%), versus 115 of 165 (69.7%), p = 0.009. Out of the five patients who stopped PPI but had recurrent hypomagnesemia, three patients had alternative etiologies (renal impairment, diuretic use) to account for this.
Overall, 56.4% of patients developed hypocalcaemia, while 40.2% developed hypokalaemia (Table 3). In addition, this was complicated by an episode of arrhythmia in 4.8% of patients, and seizure in 5.3%. There was a weak positive correlation between magnesium and calcium, rs = 0.360, p < 0.001, while there was no correlation between magnesium and potassium, rs = −0.008, p = 0.109 (Supplementary Figure S1A and S1B). In 13 patients, urine Mg was measured, which were normal/low in all but two patients, one of whom was on a diuretic and had renal impairment. In 32 patients with hypocalcaemia, iPTH was normal in 10 patients, and elevated in 22 patients, of whom 86.3% had renal impairment.
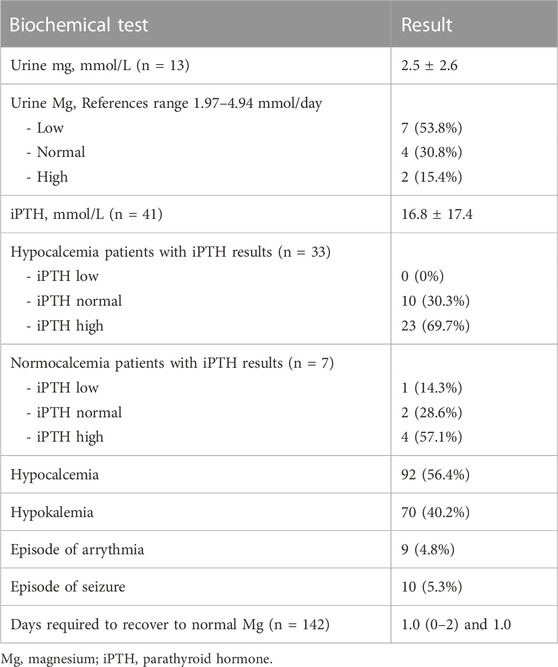
TABLE 3. Characteristics during severe Hypomagnesemia amongst 189 patients with proton pump inhibitor (PPI)-related hypomagnesemia.
Each of the 189 cases with severe hypomagnesemia were matched with three controls, who were on PPI therapy without documented hypomagnesemia (Figure 2). On univariate analysis, patients with severe hypomagnesemia were more likely to be older (OR, 1.04; 95% CI, 1.02–1.05), female (OR, 1.85; 95% CI, 1.35–2.61), lower BMI (OR, 0.95; 95% CI 0.92–0.99), on high dose PPI (OR, 2.10; 95% CI, 1.46–3.04), renal impairment (OR, 4.21; 95% CI, 2.88–6.16), and had a higher CCI score (OR, 1.77; 95% CI, 1.61–1.96) (Table 4). On multivariate analysis, variables included were age, gender, ethnicity, BMI, PPI dosage, duration of PPI, renal impairment, use of diuretics, CCI score and presence of diabetes. The significant risk factors on multivariate analysis for severe hypomagnesemia were female gender (OR 1.73; 95% CI, 1.17–2.57), presence of diabetes mellitus (OR, 4.62; 95% CI, 3.05–7.00), low BMI (OR, 0.90; 95% CI, 0.86–0.94), high dose PPI therapy (OR, 1.96; 95% CI, 1.29–2.98), renal impairment (OR, 3.85; 95% CI, 2.58–5.75), and use of diuretics (OR, 1.68; 95% CI, 1.09–2.61) (Table 4).
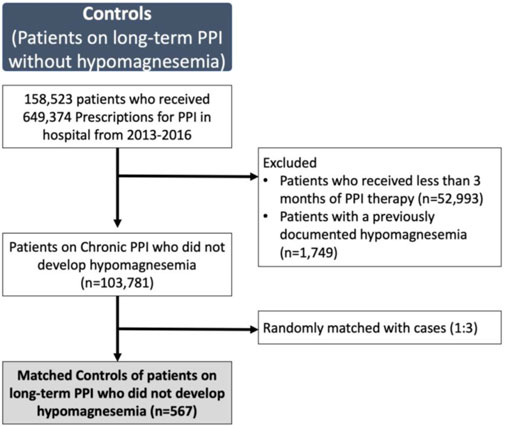
FIGURE 2. Patients on long-term proton-pump inhibitor (PPI) without documented hypomagnesemia selected as controls (n = 567)
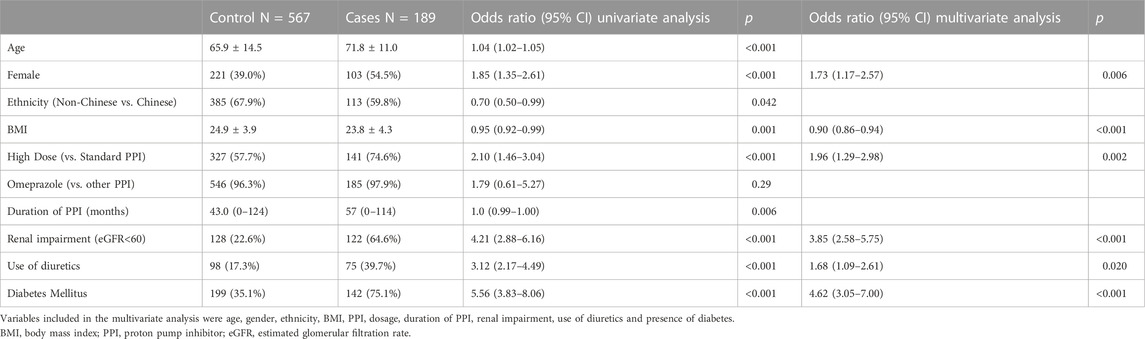
TABLE 4. Univariate and Multivariate analysis of factors associated with severe hypomagnesemia in 187 Cases with proton pump inhibitor (PPI)-related severe hypomagnesemia compared with 567 controls on PPI use without document hypomagnesemia.
In our study, we found that long-term PPI use was implicated in half of the patients with severe hypomagnesemia. Despite this, PPI therapy was stopped in less than a quarter of the patients. In addition to hypomagnesemia, PPI are also associated with other adverse effects, such as osteoporosis and renal impairment (Al-Aly et al., 2020), leading to increased calls for deprescription of PPI (Clarke et al., 2022). However, a third of our patients continued their PPI therapy despite no compelling indication for long-term PPI. When compared to controls on long-term PPI without hypomagnesemia, we also identified female gender, renal impairment, use of diuretics and dose of PPI as risk factors for PPI-related hypomagnesemia.
In our study, 66.7% (240 of 360) of patients with severe hypomagnesemia were taking PPI prior to admission, with 52.5% (189 of 360) being attributed to PPI use (128 possible, 59 probable, two definite). While two previous studies similarly found a high proportion of patients with hypomagnesemia using PPI, 54.5% (Koulouridis et al., 2013) and 41.4% (Zipursky et al., 2014), they did not assess for causality in these patients. Many of our patients had other risk factors for developing hypomagnesemia, such as use of diuretics and renal impairment. However, in 25.7% of our cases, there was no other known trigger for hypomagnesemia, such as renal or gastrointestinal losses, which strengthens the likelihood of causality. To assess for likelihood of adverse drug reactions, various algorithms are available. The Naranjo algorithm compares favourably with other algorithms like the World Health Organization-Uppsala Monitoring Center (WHO-UMC) system, or Liverpool algorithm (Gallagher et al., 2011). Unlike the WHO-UMC system, which provides a global score, the Naranjo algorithm allows a more objective measure of drug-related adverse events by assigning fixed scores, thereby reducing inter-rater variability. Because we only included patients with severe hypomagnesemia, this may explain the high proportion of PPI-related hypomagnesemia. We were conservative in our analysis, such as excluding patients if hypomagnesemia occurred prior to the first use of PPI, and it is possible that the prevalence of PPI-related hypomagnesemia could be higher. We only identified two patients with ‘definite’ PPI-related hypomagnesemia. This is because algorithms, such as Naranjo algorithm and WHO-UMC system, put a high weightage for recurrence of an adverse event after drug re-challenge. However, re-challenge with PPI was uncommon if drug causality was clear, especially with the concern of causing arrhythmias or seizures with severe hypomagnesemia.
On multi-variate analysis, we identified several factors associated with increased risk of hypomagnesemia, including use of diuretics, renal impairment, diabetes mellitus, higher dose of PPI, female gender, and lower BMI. Diuretics and renal impairment both cause hypomagnesemia via renal losses. Conversely, PPI use has also been associated with acute kidney injury, acute interstitial nephritis or chronic kidney disease (Al-Aly et al., 2020). Diabetes mellitus may cause hypomagnesemia, via poor dietary intake, increased renal losses, impaired insulin secretion and insulin resistance (Gommers et al., 2016). Meta-analyses have shown that use of PPI is associated with increased risk of hypomagnesemia, with higher dose of PPI a risk factor (Park et al., 2014; Cheungpasitporn et al., 2015; Srinutta et al., 2019). We similarly found an increased risk with higher doses of PPI, supporting the causality link between PPI use and hypomagnesemia. In view of this, clinicians may consider using the lowest required dose in patients with a strong indication for continued PPI use (Freedberg et al., 2017). We did not find duration of PPI to be associated with greater risk, but this may be due to our choice to include only patients with at least 3 month use of PPI (Kieboom et al., 2015). In one previous study, omeprazole and pantoprazole were found to have higher risk for PPI-related hypomagnesemia compared to other PPI (Luk et al., 2013). Since the cost of omeprazole is subsidized locally, a large majority of our patients were using omeprazole. We did not find any difference in risk between omeprazole and other non-omeprazole PPI. Interestingly, we found that females were more likely to develop hypomagnesemia, with 54.5% of our cases being females. Previous studies similarly found a higher proportion of females amongst cases with PPI-related hypomagnesemia, of 53.1% (Janett et al., 2015) and 52.8% (Luk et al., 2013). However, the latter study analysed adverse events reported to the FDA and found female gender to be associated with a lower risk of hypomagnesemia. A possible explanation is that they compared cases reporting of PPI-related hypomagnesemia, with controls reporting of any PPI-related adverse events, and females may be more likely to develop other PPI-related adverse events such as fractures (Thaler et al., 2016). We also found an association of PPI use with fractures (Nehra et al., 2018) but not dementia (Supplementary Table S1), and consistent with recent studies (Khan et al., 2020).
We also found that only a minority of patients prescribed with PPI develop severe hypomagnesemia. In our single referral center serving a population of ∼700,000 individuals, we had 158,523 patients prescribed with PPI over 4 years. During that same period, 189 patients had possible PPI-related severe hypomagnesemia, providing a crude estimate of 0.12%. This is slightly below a previous estimate of ∼1% (Luk et al., 2013), and likely because we included only severe hypomagnesemia (<0.4 mmol/L). This low incidence may explain why studies comparing magnesium levels in PPI users versus non-PPI users have either found no difference (Chowdhry et al., 2018), or marginally lower magnesium levels of 0.022 mmol/L (Kieboom et al., 2015). It is possible that patients who develop hypomagnesemia have a genetic predisposition. Hess and others found two common single nucleotide polymorphism, rs3750425 and rs2274924, in patients with PPI-associated hypomagnesemia (Hess et al., 2017). Despite PPI being contributory to severe hypomagnesemia in almost half of our patients, it was alarming that PPI were only stopped in a minority of patients. It is possible that clinicians may have attributed hypomagnesemia to other possible etiologies for hypomagnesemia that were coexistent, such as renal impairment or diuretics. In medical practice, PPI are very commonly prescribed; Severe hypomagnesemia is known to cause life-threatening arrythmia and seizures and it will be wise to prevent such episodes (Chrysant, 2019). In ∼30% of our patients, there was no indication for long-term PPI, such as DAPT or previous gastrointestinal bleeding (Freedberg et al., 2017). In addition, many patients with indications for long-term PPI were also taking higher than standard dose of PPI. However, in 42 of 43 patients where PPI was stopped, patients were prescribed histamine two receptor antagonist instead, suggesting that they had ongoing symptoms requiring treatment. In light of this, we suggest that if hypomagnesemia occurs during the course of PPI therapy, it will be prudent for clinicians to review its indication from time to time, or considering switching to histamine two receptor antagonist (Al-Aly et al., 2020).
Urinary magnesium levels in our patients were generally low. This supports the current hypothesis that PPI impair magnesium absorption, in particular active uptake of magnesium (Hess et al., 2012; Janett et al., 2015). Since magnesium absorption can also occur via a passive paracellular pathway, this explains why in many of our patients, magnesium supplementation alone corrected hypomagnesemia rapidly, despite continuation of PPI. However, only 1% of total body magnesium is present in blood, with the remaining 99% in bones, muscles and soft tissue (Elin, 2010). Hence, even after early normalisation of magnesium levels, these patients may have a significant total body magnesium deficit. To illustrate this, several of our patients (Supplementary Figure S2) had recurrences of hypomagnesemia, and complete normalisation occurred months later. Hypomagnesemia also improved when patients switched to a histamine two receptor antagonist, consistent with other reports that hypomagnesemia is due to a class effect of PPI (Hess et al., 2012; Janett et al., 2015).
The present study had several limitations. First, it was a retrospective study, and management of hypomagnesemia was not standardised. A prospective study with a protocol for stopping or reducing PPI dose may guide the optimal management of patients with PPI-related hypomagnesemia. Second, the temporal relationship (cause-effect) of PPI with development of hypomagnesemia could not be clearly demonstrated in all patients. However, the Naranjo algorithm includes an item which scores patients with documented normomagnesemia prior to PPI use, and development of hypomagnesemia only after PPI use. 53% of patients fulfilled this criterion which supports the temporal relationship of PPI-related hypomagnesemia. Third, we elected to focus only on severe hypomagnesemia which was more clinically relevant. Hence, we were not able to evaluate the impact of milder degrees of hypomagnesemia. Finally, all cases with severe hypomagnesemia were admitted for treatment, whereas controls were selected from all patients prescribed PPI in the same time period, potentially leading to differences in the two groups. However, the risk factors we identified (renal impairment and use of diuretics) are consistent with other studies and we did not find differences in unrelated conditions such as gastritis or peptic ulcer.
We have found that use of long-term PPI played a contributory role in almost half of all patients with severe hypomagnesemia. Although magnesium levels transiently returned to normal after supplementation, recurrence were frequent in patients continued on PPI. Clinicians need to be aware of this uncommon but serious side-effect of PPI, and constantly review the indication to continue long-term PPI to prevent potential hypomagnesemia episodes which can lead to life-threatening arrythmias or seizures.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by SingHealth Institutional Review Board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
SS, YT, TP had full access to all the data in the study and take responsibility of the integrity of the data and accuracy of data analysis. SS and YT contributed equally and share first authorship. TP and LG contributed equally and share final authorship. Study concept and design: SS, LG, KT, WJ, LC, TK, SK, RD. Data collection: KT, WJ, LC, TK, SK, TC, ML. Data analysis: PT, JW. Drafting of the manuscript: SS, TK, LG, KT, WJ, TC, ML, RD, JK, TL, ET, VA, SB, MZ, TK, TP. All authors made critical revisions of the report and approved the final manuscript.
We thank Amanda Tan and the department of Clinical Trials and Research Unit, Changi General Hospital for their assistance in the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1092476/full#supplementary-material
Al-Aly, Z., Maddukuri, G., and Xie, Y. (2020). Proton pump inhibitors and the kidney: Implications of current evidence for clinical practice and when and how to deprescribe. Am. J. Kidney Dis. 75 (4), 497–507. doi:10.1053/j.ajkd.2019.07.012
Bai, J. P. F., Hausman, E., Lionberger, R., and Zhang, X. (2012). Modeling and simulation of the effect of proton pump inhibitors on magnesium homeostasis. 1. Oral absorption of magnesium. Mol. Pharm. 9 (12), 3495–3505. doi:10.1021/mp300323q
Begley, J., Smith, T., Barnett, K., Strike, P., Azim, A., Spake, C., et al. (2016). Proton pump inhibitor associated hypomagnasaemia - a cause for concern? Br. J. Clin. Pharmacol. 81 (4), 753–758. doi:10.1111/bcp.12846
Biyik, M., Solak, Y., Ucar, R., Cifci, S., Tekis, D., Polat, İ., et al. (2017). Hypomagnesemia among outpatient long-term proton pump inhibitor users. Am. J. Ther. 24 (1), e52–e55. doi:10.1097/MJT.0000000000000154
Charlson, M. E., Pompei, P., Ales, K. L., and MacKenzie, C. R. (1987). A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 40 (5), 373–383. doi:10.1016/0021-9681(87)90171-8
Cheungpasitporn, W., Thongprayoon, C., Kittanamongkolchai, W., Srivali, N., Edmonds, P. J., Ungprasert, P., et al. (2015). Proton pump inhibitors linked to hypomagnesemia: A systematic review and meta-analysis of observational studies. Ren. Fail 37 (7), 1237–1241. doi:10.3109/0886022X.2015.1057800
Chowdhry, M., Shah, K., Kemper, S., Zekan, D., Carter, W., and McJunkin, B. (2018). Proton pump inhibitors not associated with hypomagnesemia, regardless of dose or concomitant diuretic use. J. Gastroenterol. Hepatol. 33 (10), 1717–1721. doi:10.1111/jgh.14141
Chrysant, S. G. (2019). Proton pump inhibitor-induced hypomagnesemia complicated with serious cardiac arrhythmias. Expert Rev. Cardiovasc Ther. 17 (5), 345–351. doi:10.1080/14779072.2019.1615446
Clarke, K., Adler, N., Agrawal, D., Bhakta, D., Sata, S. S., Singh, S., et al. (2022). Indications for the use of proton pump inhibitors for stress ulcer prophylaxis and peptic ulcer bleeding in hospitalized patients. Am. J. Med. 135 (3), 313–317. doi:10.1016/j.amjmed.2021.09.010
Danziger, J., William, J. H., Scott, D. J., Lee, J., wei, L. L., Mark, R. G., et al. (2013). Proton-pump inhibitor use is associated with low serum magnesium concentrations. Kidney Int. 83 (4), 692–699. doi:10.1038/ki.2012.452
Elin, R. J. (2010). Assessment of magnesium status for diagnosis and therapy. Magnes. Res. 23 (4), S194–S198. doi:10.1684/mrh.2010.0213
Epstein, M., McGrath, S., and Law, F. (2006). Proton-pump inhibitors and hypomagnesemic hypoparathyroidism. N. Engl. J. Med. 355 (17), 1834–1836. doi:10.1056/NEJMc066308
Faulhaber, G. A. M., Ascoli, B. M., Lubini, A., Mossmann, M., Rossi, G., Geib, G., et al. (2013). Serum magnesium and proton-pump inhibitors use: A cross-sectional study. Rev. Assoc. Med. Bras. 59 (3), 276–279. doi:10.1016/j.ramb.2012.12.007
Freedberg, D. E., Kim, L. S., and Yang, Y. X. (2017). The risks and benefits of long-term use of proton pump inhibitors: Expert review and best practice advice from the American gastroenterological association. Gastroenterology 152 (4), 706–715. doi:10.1053/j.gastro.2017.01.031
Gallagher, R. M., Kirkham, J. J., Mason, J. R., Bird, K. A., Williamson, P. R., Nunn, A. J., et al. (2011). Development and inter-rater reliability of the Liverpool adverse drug reaction causality assessment tool. PLoS ONE 6 (12), e28096. doi:10.1371/journal.pone.0028096
Gommers, L. M. M., Hoenderop, J. G. J., Bindels, R. J. M., and de Baaij, J. H. F. (2016). Hypomagnesemia in type 2 diabetes: A vicious circle? Diabetes 65 (1), 3–13. doi:10.2337/db15-1028
Hess, M. W., de Baaij, J. H. F., Broekman, M. M. T. J., Bisseling, T. M., Bjt, H., Tan, A., et al. (2017). Common single nucleotide polymorphisms in transient receptor potential melastatin type 6 increase the risk for proton pump inhibitor-induced hypomagnesemia: A case-control study. Pharmacogenet Genomics 27 (3), 83–88. doi:10.1097/FPC.0000000000000259
Hess, M. W., Hoenderop, J. G. J., Bindels, R. J. M., and Drenth, J. P. H. (2012). Systematic review: Hypomagnesaemia induced by proton pump inhibition. Aliment. Pharmacol. Ther. 36 (5), 405–413. doi:10.1111/j.1365-2036.2012.05201.x
Hoorn, E. J., van der Hoek, J., de Man, R. A., Kuipers, E. J., Bolwerk, C., and Zietse, R. (2010). A case series of proton pump inhibitor-induced hypomagnesemia. Am. J. Kidney Dis. 56 (1), 112–116. doi:10.1053/j.ajkd.2009.11.019
Janett, S., Camozzi, P., Peeters, G. G. A. M., Lava, S. A. G., Simonetti, G. D., Goeggel Simonetti, B., et al. (2015). Hypomagnesemia induced by long-term treatment with proton-pump inhibitors. Gastroenterol. Res. Pract. 2015, 951768. doi:10.1155/2015/951768
Khan, M. A., Yuan, Y., Iqbal, U., Kamal, S., Khan, M., Khan, Z., et al. (2020). No association linking short-term proton pump inhibitor use to dementia: Systematic review and meta-analysis of observational studies. Am. J. Gastroenterol. 115 (5), 671–678. doi:10.14309/ajg.0000000000000500
Kieboom, B. C. T., Kiefte-de Jong, J. C., Eijgelsheim, M., Franco, O. H., Kuipers, E. J., Hofman, A., et al. (2015). Proton pump inhibitors and hypomagnesemia in the general population: A population-based cohort study. Am. J. Kidney Dis. 66 (5), 775–782. doi:10.1053/j.ajkd.2015.05.012
Kim, S., Lee, H., Park, C. H., Shim, C. N., Lee, H. J., Park, J. C., et al. (2015). Clinical predictors associated with proton pump inhibitor-induced hypomagnesemia. Am. J. Ther. 22 (1), 14–21. doi:10.1097/MJT.0b013e31829c4c71
Koulouridis, I., Alfayez, M., Tighiouart, H., Madias, N. E., Kent, D. M., Paulus, J. K., et al. (2013). Out-of-hospital use of proton pump inhibitors and hypomagnesemia at hospital admission: A nested case-control study. Am. J. Kidney Dis. 62 (4), 730–737. doi:10.1053/j.ajkd.2013.02.373
Lameris, A. L., Nevalainen, P. I., Reijnen, D., Simons, E., Eygensteyn, J., Monnens, L., et al. (2015). Segmental transport of Ca2+ and Mg2+ along the gastrointestinal tract. Am. J. Physiol. Gastrointest. Liver Physiol. 308 (3), G206–G216. doi:10.1152/ajpgi.00093.2014
Levey, A. S., Stevens, L. A., Schmid, C. H., Zhang, Y. L., Castro, A. F., Feldman, H. I., et al. (2009). A new equation to estimate glomerular filtration rate. Ann. Intern Med. 150 (9), 604–612. doi:10.7326/0003-4819-150-9-200905050-00006
Luk, C. P., Parsons, R., Lee, Y. P., and Hughes, J. D. (2013). Proton pump inhibitor-associated hypomagnesemia: What do FDA data tell us? Ann. Pharmacother. 47 (6), 773–780. doi:10.1345/aph.1R556
Markovits, N., Loebstein, R., Halkin, H., Bialik, M., Landes-Westerman, J., Lomnicky, J., et al. (2014). The association of proton pump inhibitors and hypomagnesemia in the community setting. J. Clin. Pharmacol. 54 (8), 889–895. doi:10.1002/jcph.316
Naranjo, C. A., Busto, U., Sellers, E. M., Sandor, P., Ruiz, I., Roberts, E. A., et al. (1981). A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 30 (2), 239–245. doi:10.1038/clpt.1981.154
Nehra, A. K., Alexander, J. A., Loftus, C. G., and Nehra, V. (2018). Proton pump inhibitors: Review of emerging concerns. Mayo Clin. Proc. 93 (2), 240–246. doi:10.1016/j.mayocp.2017.10.022
NICE (2014). Appendix A: Dosage information on proton pump inhibitors | gastro-oesophageal reflux disease and dyspepsia in adults: Investigation and management | guidance | NICE. Available from: https://www.nice.org.uk/guidance/cg184/chapter/Appendix-A-Dosage-information-on-proton-pump-inhibitors.
Park, C. H., Kim, E. H., Roh, Y. H., Kim, H. Y., and Lee, S. K. (2014). The association between the use of proton pump inhibitors and the risk of hypomagnesemia: A systematic review and meta-analysis. PLoS One 9 (11), e112558. doi:10.1371/journal.pone.0112558
Pasina, L., Zanotta, D., Puricelli, S., and Bonoldi, G. (2016). Acute neurological symptoms secondary to hypomagnesemia induced by proton pump inhibitors: A case series. Eur. J. Clin. Pharmacol. 72 (5), 641–643. doi:10.1007/s00228-016-2024-2
Pisani, L. F., Filippi, E., Vavassori, S., Munizio, N., Vecchi, M., and Pastorelli, L. (2016). Effect of proton pump inhibitors on magnesium balance: Is there a link to cardiovascular risk? Magnes. Res. 29 (1), 1–10. doi:10.1684/mrh.2016.0397
Sheen, E., and Triadafilopoulos, G. (2011). Adverse effects of long-term proton pump inhibitor therapy. Dig. Dis. Sci. 56 (4), 931–950. doi:10.1007/s10620-010-1560-3
Srinutta, T., Chewcharat, A., Takkavatakarn, K., Praditpornsilpa, K., Eiam-Ong, S., Jaber, B. L., et al. (2019). Proton pump inhibitors and hypomagnesemia: A meta-analysis of observational studies. Med. Baltim. 98 (44), e17788. doi:10.1097/MD.0000000000017788
Thaler, H. W., Sterke, C. S., and van der Cammen, T. J. M. (2016). Association of proton pump inhibitor use with recurrent falls and risk of fractures in older women: A study of medication use in older fallers. J. Nutr. Health Aging 20 (1), 77–81. doi:10.1007/s12603-016-0679-0
Van Ende, C., Van Laecke, S., Marechal, C., Verbeke, F., Kanaan, N., Goffin, E., et al. (2014). Proton-pump inhibitors do not influence serum magnesium levels in renal transplant recipients. J. Nephrol. 27 (6), 707–711. doi:10.1007/s40620-014-0105-9
Keywords: omeprazole, magnesium, chronic kidney disease, drug adverse effects, medication safety, toxicity
Citation: Seah S, Tan YK, Teh K, Loh WJ, Tan PT, Goh LC, Malakar RD, Aw TC, Lau CS, Dhalliwal T, Kui SL, Kam JW, Khoo J, Tay TL, Tan E, Au V, Soh SB, Zhang M, King TF, Gani L and Puar TH (2023) Proton-pump inhibitor use amongst patients with severe hypomagnesemia. Front. Pharmacol. 14:1092476. doi: 10.3389/fphar.2023.1092476
Received: 08 November 2022; Accepted: 16 January 2023;
Published: 30 January 2023.
Edited by:
Weihong Sha, Guangdong Academy of Medical Sciences, ChinaReviewed by:
Tatyana Kugler, Donetsk National Medical University, UkraineCopyright © 2023 Seah, Tan, Teh, Loh, Tan, Goh, Malakar, Aw, Lau, Dhalliwal, Kui, Kam, Khoo, Tay, Tan, Au, Soh, Zhang, King, Gani and Puar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Troy H. Puar, dHJveS5wdWFyLmgua0BzaW5naGVhbHRoLmNvbS5zZw==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.