
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 06 March 2023
Sec. Ethnopharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1072991
This article is part of the Research Topic New opportunities in drug design for the management and treatment of type 2 diabetes View all 5 articles
 Min Song1,2
Min Song1,2 Baogeng Huai1
Baogeng Huai1 Zhenpeng Shi1
Zhenpeng Shi1 Wenyi Li1
Wenyi Li1 Yutan Xi3
Yutan Xi3 Zhenguo Liu1
Zhenguo Liu1 Jihang Zhang1
Jihang Zhang1 Junyu Zhou1
Junyu Zhou1 Yun Qiao4
Yun Qiao4 Deshan Liu4*
Deshan Liu4*Objective: The objective of this systematic review and meta-analysis is to assess the effectiveness and security of Chinese herbal medicine (CHM) in the therapy of painful diabetic neuropathy (PDN).
Methods: We searched databases for randomized controlled trials (RCTs) of CHM in the treatment of PDN. Outcome indicators included nerve conduction velocity, clinical efficiency, pain score, TCM syndrome score, and adverse events. Stata 16.0 was used to carry out the Meta-analysis.
Results: A total of 21 RCTs with 1,737 participants were included. This meta-analysis found that using CHM as adjuvant treatment or as monotherapy for PDN can improve SCV of median nerve [mean difference (MD) = 3.56, 95% Confidence interval (CI) (2.19, 4.92) ], MCV of median nerve [ MD = 3.82, 95% CI (2.51, 5.12) ], SCV of common peroneal nerve [ MD = 4.16, 95% CI (1.62, 6.70) ], MCV of common peroneal nerve [ MD = 4.37, 95% CI (1.82, 6.93) ], SCV of gastrocnemius nerve [ MD = 4.95, 95% CI (3.52, 6.37) ], SCV of tibial nerve [ MD = 3.17, 95% CI (−2.64, 8.99) ], MCV of tibial nerve [MD = 6.30, 95%CI (5.00, 7.60)] and clinical effective rate [ odds ratio (OR) = 4.00, 95% CI (2.89, 5.52) ] and reduce pain score [standardized mean difference (SMD) = -2.23, 95% CI (-3.04, -1.41) ], TCM syndrome score [ MD = -4.70, 95% CI (-6.61, -2.80) ]. In addition, compared to the control group, adverse events of Chinese medicine intervention occurred less.
Conclusion: CHM as adjuvant therapy or single treatment has a good curative effect and is safe for patients with PDN, which is worthy of clinical promotion and use, however; higher quality clinical studies are still needed to prove.
Systematic Review Registration: https://www.crd.york.ac.uk/, identifier CRD42022327967
Painful diabetic neuropathy (PDN) is a common complication of type 2 diabetes, and about 16%–26% of diabetic patients will progress to PDN (Jensen et al., 2006; Tesfaye et al., 2011). The disease mostly starts from the distal limb, and its symptoms are mostly symmetrically distributed, with burning, electric shock, or acupuncture-like pain (Kulkantrakorn and Lorsuwansiri, 2013). The patient’s quality of life is drastically decreased by the pain, which gets worse over time, particularly at night, and may even lead to serious sleep disorders, anxiety, or depression (Veves et al., 2008; Sun et al., 2020). Despite considerable advancements in our knowledge of the pathophysiology of this condition, PDN is not currently managed with a specific medication (Duran et al., 2022). Presently, the clinical treatment of PDN refers to the combination of antidepressants, anticonvulsants, or opioids that are based on controlling blood glucose. These treatments result in only one-third of patients relieving half of their pain, while these treatments are often accompanied by serious side effects (Schreiber et al., 2015; Nayak et al., 2021). Due to the complexity and risk of disease, the development of an alternative or complementary therapies is urgent.
Chinese herbal medicine which is an essential part of traditional Chinese medicine, has been utilized successfully in China as a supplement and alternative form of therapy for thousands of years. Due to its “comprehensive, multi-channel, multi-target” therapy qualities, CHM has garnered increasing attention in the management of disease and comorbidities (Qinghua et al., 2019; Yue et al., 2019). In fact CHM has certain pharmacological effects mainly because of the active compounds contained in it. It has been found that the natural active compounds in CHM have a higher biological activity and structural diversity than artificial monomers, making it easier for them to enter the body and exert their medicinal effects, and possessing higher biological activity (Glevitzky et al., 2019; Fernandez-Ochoa et al., 2022).
In past decades, the number of RCTs assessing the safety and effectiveness of CHM as a single or adjuvant therapy for PDN has significantly expanded. However, there is still no systematic evaluation and meta-analysis of this issue to date. The purpose of this study is to systematically evaluate the efficacy and safety of CHM in the treatment of PDN, so as to provide high-quality evidence-based basis and treatment strategies for CHN in the treatment of PDN.
We completed this study using the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines (Page et al., 2021) (Supplementary Material). In addition, the review has been registered in PROSPERO (CRD42022327967).
PubMed, Embase, The Cochrane Library, Web of Science, CNKI, Wanfang, VIP, and CBM were the eight databases we scanned through. All databases are available from their inception to 21 April 2022. The main search terms are: “Chinese herbal medicine”, “painful diabetic neuropathy”, “Chinese medicine”, and “diabetic neuropathic pain”.
Two researchers (ZP-S and WY-L) independently imported all the retrieved literature into the software EndnoteX9.0 for management and screening. For controversial literature, two researchers negotiate with a third researcher (YT-X).
The inclusion criteria were:1) participants: PDN patients (regardless of race, gender, or age); 2) study format: RCTs; 3) Interventions: the intervention group received CHM treatment (whether CHM as a single therapy or adjuvant therapy) and the control group used western medicine (WM) or placebo; and 4) outcomes: the main outcome indicators include SCV, MCV, pain score, and clinical efficiency The Secondary outcome indicators include TCM syndrome score and adverse events. The exclusion criteria were:1) treatment using acupuncture, massage, or other Chinese medicine; 2) Intervention time is not appropriate; and 3) patients without clear diagnostic criteria or accompanied by other diseases.
Herein, the definition of clinical efficiency in each trial is not the same, and the clinical efficiency of the included trials is based on the following criteria. a: Effectiveness: peripheral nerve function or clinical symptoms improved. b: Ineffectiveness: peripheral nerve function or clinical symptoms were not significantly improved or not improved (Xiaoyu, 2002).
Data was separately extracted and cross-checked by the two researchers (ZG-L and JH-Z). The extracted data primarily include: 1) the fundamental information of the selected research; 2) key elements of bias risk assessment; 3) outcome data: if the data type were measurement data, the mean and standard deviation were extracted, if the data were count data, the number of events and the total number were extracted.
Using the Risk of Bias instrument developed by the Cochrane Collaboration (Higgins et al., 2011), two researchers (MS and JY-Z) independently assessed the caliber of RCTs and cross-checked their results. The following seven components made up the evaluation content: the creation of random sequences, the concealment of allocations, the blinding of individuals and researchers, the integrity of outcome data, report bias, and other biases.
The meta-analysis of the research data was carried out using Stata16.0 software. The effect analysis statistic for categorical data was OR, while the effect analysis statistic for continuous data was either MD or SMD. For each effect, the 95% CI was calculated. The χ2 test (test level = 0.10) was utilized to examine the heterogeneity of the outcomes of these studies, and the I2 test was used to quantify the heterogeneity. If p≥0.10 and I2<50%, the fixed effect model was used for analysis. If p<0.10 and I2 ≥ 50%, it indicates that a huge heterogeneity appeared among the studies, and then subgroup analyses have been conducted so as to make out the origin of heterogeneity. The analysis was carried out using a random effect model when methodological heterogeneity and clinical heterogeneity are absent. α = 0.05 was used as the meta-analysis test level. Through sensitivity analysis, the stability and reliability of the analysis’s results are examined. Publication bias was evaluated using the funnel plot, Begg’s test, and Egger’s test.
A sum of 492 papers was found during the preliminary screening, however, 184 papers were eliminated due to repetition, 247 papers were eliminated by reading their titles and abstracts, and 40 papers were eliminated by reading the complete text. Finally, the quantitative analysis covered 21 articles (Hongwei et al., 2008; Honggang, 2011; Laibiao et al., 2012; Tsai et al., 2013; Fanrong and Mingkong, 2014; Laibiao et al., 2015; Ruixia et al., 2015; Haibo, 2016; Xiaorui, 2016; Di et al., 2017; Cizhen, 2018; Honggang et al., 2018; Liting et al., 2018; Qiaren and Xiaohong, 2018; Tao et al., 2018; Ye et al., 2018; Dianrong et al., 2020; Haiyan and Yange, 2020; Yanli et al., 2020; Qingqing, 2021; Shuquan et al., 2021). Figure 1 depicts the literature screening procedure.
There are 1737 patients altogether in the sample size of the 21 studies, which include 871 patients in the intervention group and 866 patients in the control group. The intervention group and the control group had equivalent pre-treatment data (such as age, sex ratio, outcome indicators, etc.). All 21 studies were from China (Table 1, Table 2).
Among the 21 studies included, 7 studies (Tsai et al., 2013; Fanrong and Mingkong, 2014; Ruixia et al., 2015; Di et al., 2017; Cizhen, 2018; Tao et al., 2018; Shuquan et al., 2021) were grouped by a random number table, 1 study (Yanli et al., 2020)used a draw for grouping, 1 study (Haibo, 2016) adopted the odd-even number method for grouping, and the remaining studies did not explain the specific methods. The allocation concealment method was not discussed in any of the 21 studies. One study (Tsai et al., 2013) used double-blind, and the remaining research failed to state whether or not blinding was utilized. All studies’ outcome data were complete, and there were no other biases or selective reporting results (Figure 2).
14 articles (Honggang, 2011; Laibiao et al., 2012; Tsai et al., 2013; Fanrong and Mingkong, 2014; Ruixia et al., 2015; Xiaorui, 2016; Di et al., 2017; Qiaren and Xiaohong, 2018; Tao et al., 2018; Ye et al., 2018; Dianrong et al., 2020; Haiyan and Yange, 2020; Yanli et al., 2020; Qingqing, 2021) talked about pain scores. The studies’ significant heterogeneity was shown using the heterogeneity test (p < 0.10, I2 = 97.1%). Thus, to combine the effect sizes, we employ a random effects model. The outcomes demonstrated that, in comparison to the control group, the intervention group was able to significantly reduce pain and increase pain scores and the change was statistically meaningful [SMD = − 2.23, 95% CI (−3.04, − 1.41), p < 0.05; Figure 3]. The kind of pain scale and the length of the intervention were significantly different among subgroups according to subgroup analysis (p < 0.05 and p < 0.05, respectively). But the sample size and intervention strategies did not significantly differ (p = 0.566 and p = 0.48, respectively) (Table 3).
The SCV of median nerve was reported in 10 articles (Hongwei et al., 2008; Laibiao et al., 2012; Fanrong and Mingkong, 2014; Laibiao et al., 2015; Di et al., 2017; Honggang et al., 2018; Qiaren and Xiaohong, 2018; Dianrong et al., 2020; Haiyan and Yange, 2020; Shuquan et al., 2021). Following the heterogeneity test, there was significant homogeneity between the papers (p < 0.10, I2 = 82%). The results of a random effect model that combined effect sizes revealed that the intervention group considerably improved SCV of the median nerve compared to the control group, and the results of this experiment were statistically significant [MD = 3.56, 95% CI (2.19, 4.92), p < 0.05; Figure 4]. According to subgroup analysis, there were significant differences across subgroups with various intervention times (p < 0.05), sample sizes (p = 0.003), and intervention methods (p < 0.05) (Table 3).
The MCV of median nerve appeared in eight articles (Hongwei et al., 2008; Laibiao et al., 2012; Fanrong and Mingkong, 2014; Laibiao et al., 2015; Honggang et al., 2018; Qiaren and Xiaohong, 2018; Dianrong et al., 2020; Haiyan and Yange, 2020). Based on the heterogeneity test, there was significant heterogeneity amongst the data (p < 0.10, I2 = 79.7%). The improvement of the MCV of the median nerve was discovered to be better in the intervention group than in the control group, and this experiment’s findings were statistically significant. [MD = 3.82, 95% CI (2.51, 5.12), p < 0.05; Figure 5] by the random effect model combining with the effect size. There were obvious differences between sample sizes (p < 0.05), as revealed by subgroup analysis, but there was no statistically significant difference between the intervention groups (p = 0.121) (Table 3).
Nine articles (Hongwei et al., 2008; Laibiao et al., 2012; Fanrong and Mingkong, 2014; Laibiao et al., 2015; Haibo, 2016; Di et al., 2017; Qiaren and Xiaohong, 2018; Tao et al., 2018; Dianrong et al., 2020) mentioned the SCV of common peroneal nerve. With the heterogeneity test used, there was considerable heterogeneity between the research (p < 0.10, I2 = 96%). We combined effect size using a random effects model. and we found that the intervention group outperformed the control group in terms of increasing the SCV of the common peroneal nerve, and this difference was statistically significant [MD = 4.16, 95% CI (1.62, 6.70), p = 0.001; Figure 6]. Subgroup analysis showed that there were remarkable differences in intervention time (p < 0.05), intervention method (p < 0.05), and sample size (p < 0.05) among subgroups (Table 3).
In 9 articles (Hongwei et al., 2008; Laibiao et al., 2012; Tsai et al., 2013; Fanrong and Mingkong, 2014; Laibiao et al., 2015; Haibo, 2016; Qiaren and Xiaohong, 2018; Tao et al., 2018; Dianrong et al., 2020), the MCV of the common peroneal nerve was described. According to the heterogeneity test, there was considerable heterogeneity between the papers (p < 0.10, I2 = 94%). The intervention group significantly improved the MCV of the common peroneal nerve compared to the control group, and it was statistically significant that the findings of this investigation, according to the results of the random effect model used to combine the effect size [MD = 4.37, 95% CI (1.82, 6.93), p = 0.0008; Figure 7]. While the intervention time (p = 0.056) was not statistically significant, subgroup analysis revealed that there were significant differences in the intervention method (p < 0.05) and sample size (p < 0.05) in the intervention group (Table 3).
Two articles (Honggang et al., 2018; Haiyan and Yange, 2020) mentioned the SCV of sural nerve. According to the heterogeneity test, there was homogeneity between the studies (p = 0.76, I2 = 0%). The effect size was combined using the fixed effect model, and the findings demonstrated that the intervention group had a greater benefit in improving the SCV of the sural nerve, with the difference being statistically significant [MD = 4.95, 95% CI (3.52, 6.37), p < 0.05; Figure 8A].
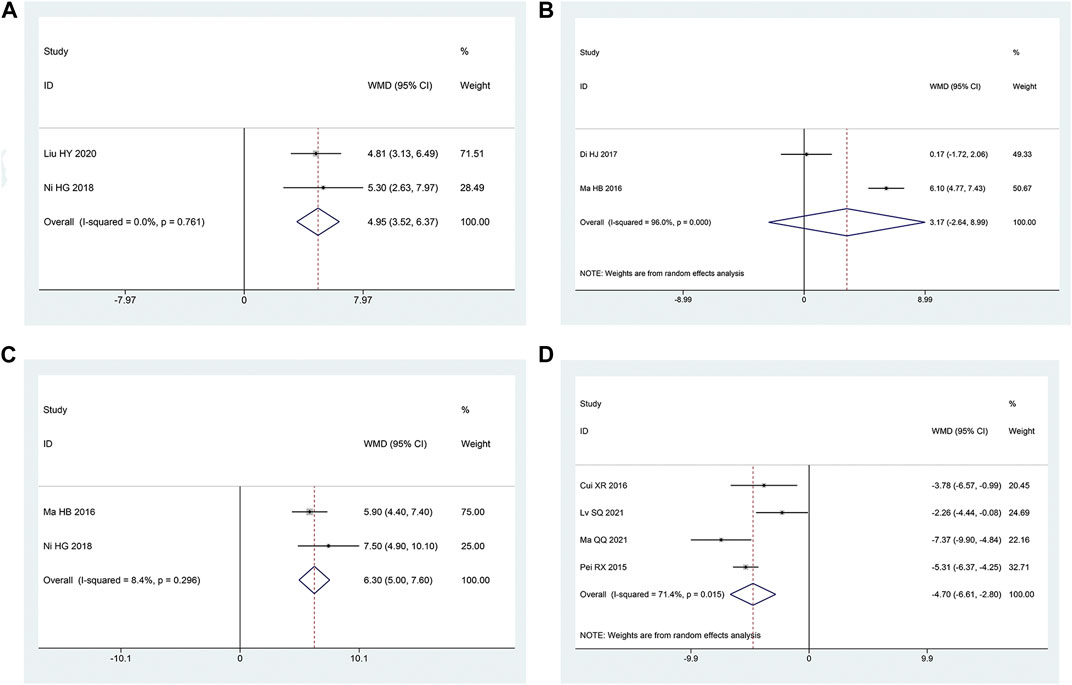
FIGURE 8. Forest plot (A) SCV of Sural Nerve; (B) SCV of Tibial Nerve; (C) MCV of Tibial Nerve; (D) TCM Syndrome Score.
Two articles (Haibo, 2016; Di et al., 2017) reported the SCV of tibial nerve. Following the heterogeneity test, there was significant homogeneity between the papers (p < 0.10, I2 = 96%). The experimental group had advantages in terms of enhancing tibial nerve SCV, but the results of this experiment were not statistically significant, according to the results of the random effect model that was used to combine the effect size [MD = 3.17, 95% CI (-2.64, 8.99), p = 0.28; Figure 8B]. The two studies had a large gap in the intervention time and the intervention method of the intervention group, and the heterogeneity may be related to this.
Two articles (Haibo, 2016; Honggang et al., 2018) were involved with the MCV of tibial nerve. According to the heterogeneity test, there was homogeneity amongst the studies (p = 0.30, I2 = 8%). Hence, we integrate the effect sizes by using the fixed effects model. The results revealed that the experimental group had more advantages than the control group in terms of enhancing the MCV of the tibial nerve, and the findings of this experiment were statistically meaningful. [MD = 6.30, 95% CI (5.00, 7.60), p < 0.05; Figure 8C].
There were 15 articles (Hongwei et al., 2008; Honggang, 2011; Laibiao et al., 2012; Fanrong and Mingkong, 2014; Laibiao et al., 2015; Haibo, 2016; Xiaorui, 2016; Di et al., 2017; Cizhen, 2018; Liting et al., 2018; Qiaren and Xiaohong, 2018; Tao et al., 2018; Ye et al., 2018; Dianrong et al., 2020; Shuquan et al., 2021) involving clinical effective rates. Based on the heterogeneity test, studies were homogeneous (p = 0.698, I2 = 0%). Thus, to combine the effect sizes, we employ a fixed effects model. According to the findings, the intervention group’s clinical effectiveness rate was higher than that of the control group, and this difference was statistically significant [OR = 4.00, 95% CI (2.89, 5.52), p < 0.05; Figure 9].
Four articles (Ruixia et al., 2015; Xiaorui, 2016; Qingqing, 2021; Shuquan et al., 2021) mentioned TCM syndrome scores. The heterogeneity test revealed a significant quantity of heterogeneity among research (p = 0.01, I2 = 71%). Hence, we integrate the effect sizes by using the random effects model. The findings revealed that the experimental group had a bigger benefit over the control group in terms of improving TCM syndromes and it was statistically significant that the difference [ MD = -4.70, 95% CI (-6.61, -2.80), p < 0.05; Figure 8D]. Significant differences between the intervention type subgroups were found in the subgroup analysis. (p = 0.009) (Table 3).
Among the 21 papers included, adverse occurrences were described in 5 pieces of literature (Tsai et al., 2013; Laibiao et al., 2015; Ruixia et al., 2015; Xiaorui, 2016; Qingqing, 2021), covering liver function, renal function, blood routine, urine routine, and digestive system. Four of them (Laibiao et al., 2015; Ruixia et al., 2015; Xiaorui, 2016; Qingqing, 2021) stated that the intervention group’s incidence of negative events was lower compared to the western medicine intervention group. One paper (Tsai et al., 2013) reported that the intervention group had more negative occurrences than the placebo group did. The commonest adverse reactions were stomach discomfort, nausea, dry mouth, etc. However, all adverse reactions were not treated specially, and the symptoms gradually relieved or disappeared. Although the above results suggest that CHM in the treatment of PDN is safe because the sample size is small, more large sample clinical studies are needed to prove the conclusion.
Individual studies were excluded one by one for sensitivity analysis. The findings indicate that after removing the studies, there was no substantial change in the outcomes for any outcome indicators, revealing that the results were stable.
The funnel plot showed that there was asymmetry in the pain score (Figure 10A), and the symmetry of the clinical effective rate (Figure 10B) and the symmetry of the median nerve SCV (Figure 10 C) were acceptable. Using Begg’s test and Egger’s test, it showed that there was a substantial publication bias in the pain ratings (p = 0.002; p < 0.05), but not in the clinical effective rate (p = 0.216; p = 0.357) or SCV of the median nerve (p = 0.721; p = 0.157).
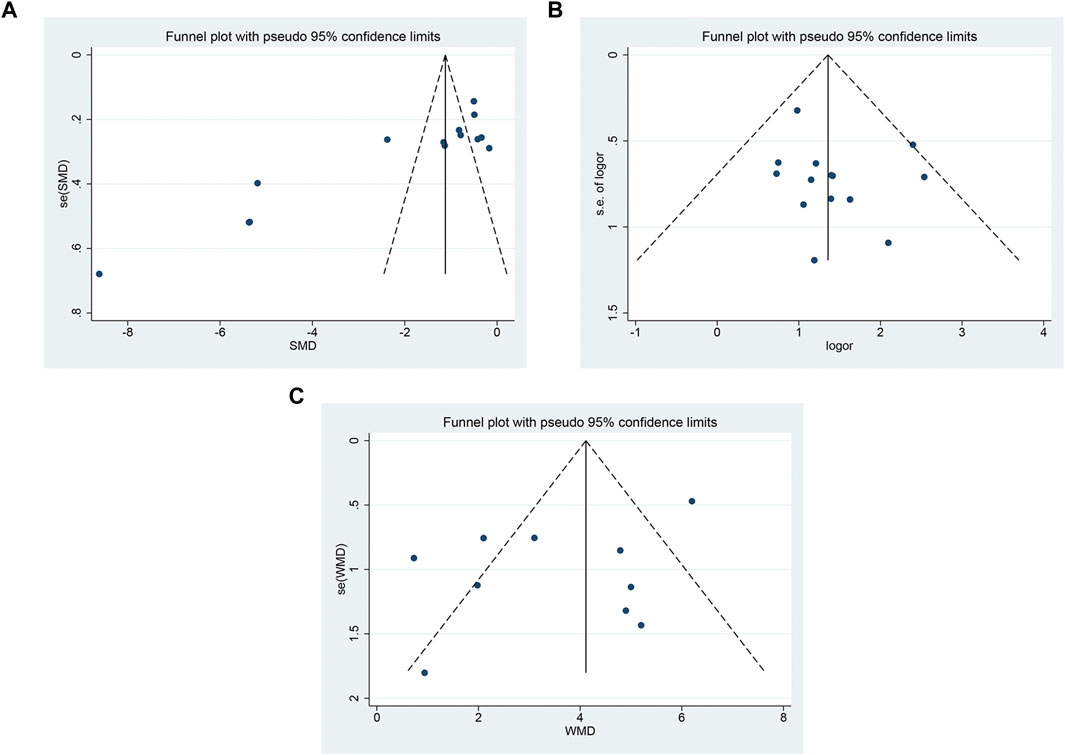
FIGURE 10. The funnel plot (A) pain score; (B) clinical effective rate; (C) SCV symmetry of median nerve.
In this study, a total of 492 publications were found, and 21 articles were used for the meta-analysis. The primary findings of this meta-analysis demonstrated that CHM, whether used as an adjuvant therapy or a stand-alone treatment, enhanced nerve conduction velocity and clinical efficacy during the therapy of PDN and reduced pain scores and TCM syndrome scores. To identify the reasons for heterogeneity, we carried out subgroup analyses based on intervention time, intervention methods, sample size, and so on. The findings of the subgroup analysis demonstrated that one of the reasons of heterogeneity in the SCV of the median nerve, SCV of the common peroneal nerve, and pain score was the intervention time. The sample size is one of the heterogeneous sources of SCV of median nerve, SCV of common peroneal nerve, MCV of common peroneal nerve, and MCV of median nerve. The pain rating scale is also one of the heterogeneous sources of pain scores. The study’s results are steady and dependable, according to sensitivity analysis. According to the publication bias test, there is a risk of bias in this study. In addition, we found that adverse events of CHM treatment are less than conventional western medicine treatment, indicating that Chinese herbal treatment of PDN is safe. Therefore, we provide supporting evidence that CHM is effective and safe in treating PDN.
A total of 72 Chinese medicines were involved in all formulations. Ranked according to the frequency of Chinese herbal medicines, the top 15 flavors of Chinese medicine frequency distribution are shown in Table 4, of which Astragalus mongholicus Bunge [Fabaceae; Astragali radix], Angelica sinensis (Oliv.) Diels [Apiaceae; Angelicae sinensis radix], and Cnidium monnieri (L.) Cusson [Apiaceae; Chuanxiong rhizoma] frequency rank among the top three, whose frequency is more than 10 times. This conclusion agrees with the findings of Zhang Fuzhi et al. (Fuzhi et al., 2020).
Chemical constituents are the key material basis for revealing the efficacy of CHM (Chuan, 2017). Astragaloside IV, which is the main chemical component of Astragalus, can inhibit Schwann cell apoptosis, regulate nerve growth factor gene expression, increase NA + -K + -ATPase, inhibit erythrocyte aldose reductase activity, thereby inhibiting nerve injury, enhancing the speed of motor nerve conduction and minimizing the buildup of nerve and erythrocyte advanced glycation end products (Yuanyuan et al., 2021). Xie et al. have shown that quercetin, a common flavonoid compound in astragalus, can regulate the expression of intestinal flora and reactive oxygen species in diabetic peripheral neuropathy rats to improve peripheral nerve injury (Xie et al., 2020). Isoeugenol, one of the main components of Angelica Sinensis volatile oil, plays an important role in reducing oxidative stress markers and glutathione levels in the sciatic nerve area (Prasad and Muralidhara, 2013). Additionally capable of removing oxygen free radicals and minimizing peroxide damage are ferulic acid and angelica polysaccharide (Gong et al., 2016). One of the key ingredients of Chuanxiong Rhizoma is ligustrazine, which has the ability to up-regulate the expression of heme oxygenase 1/carbon monoxide and superoxide dismutase and down-regulate the expression of tumor necrosis factor-, nitric oxide synthase/nitric oxide, and malondialdehyde. It is proved that ligustrazine can enhance the body’s antioxidant and anti-inflammatory effects and play a protective role in diabetic pain neuropathy (Fangqin et al., 2021).
Wnt protein is an important signalling molecule. The wnt signalling pathway mediates a variety of biological processes in the body, such as embryogenesis, organogenesis and tumour formation (Sharma et al., 2022). Among them, the wnt signalling pathway is involved in the development of T2DM and its complications by directly influencing the differentiation and proliferation of pancreatic β-cells as well as the secretion and action of insulin (Nie et al., 2021). This provides a direction to explore the mechanism of action of CHM in the treatment of diabetes and its complications. It (Zou et al., 2016; Wang et al., 2020; Zhang et al., 2020; Wu et al., 2022) was found that astragaloside, angelica polysaccharide and chuanxiongzine could all activate or inhibit the wnt signalling pathway in tissues or cells to regulate glucose and lipid metabolism, tissue or organ repair in T2DM patients.
In addition, the traditional Chinese medicine involved in this study is not only effective in the treatment of PDN, but also has a good effect on type 2 diabetes and other complications (such as diabetic heart disease, diabetic retinopathy). Studies have found that ligustrazine can delay the development of DR by inhibiting oxidative stress and retinal ganglion cell apoptosis, down-regulating AGEs content and other ways (Wei, 2021). Astragalus polysaccharide alleviated mitochondrial damage and apoptosis induced by metabolic memory by regulating the miR-182/Bcl-2 axis (Nie et al., 2019). Astragaloside IV improves endothelial dysfunction in thoracic aortas from diabetic rats by reducing oxidative stress and calpain-1 (Gao et al., 2021).
Although there are more and more clinical studies on the treatment of PDN with CHM, there is no systematic evaluation and meta-analysis in this direction. This study is the first study to systematically evaluate CHM for PDN, filling the evidence-based gap in CHM for PDN. The advantages of this meta-analysis mainly include a clear research topic, its selects high-quality RCTs that meet the inclusion criteria and conducted a statistical analysis of this study in strict accordance with the systematic review method. At the same time, we are more cautious about the explanation of the results. We conducted a subgroup analysis to find out the cause of heterogeneity, and we also conducted a sensitivity analysis and publication bias test. This study found that CHM can be recommended for the treatment of PDN, which offers fresh perspectives and ideas for researching PDN.
However, there are a few shortcomings in this work that deserve discussion. First of all, the majority of the included researchers did not use the allocation concealment and blind method, which could cause bias in both selection and implementation. Second, there was significant clinical heterogeneity in the 21 studies with differences in composition, dose, and dosage form of CHM, as well as differences in interventions (type of WM) and duration of intervention in the control group. This would result in a high level of heterogeneity within subgroups when subgroup analyses are performed. However, because of the limited number of studies, we cannot perform relevant subgroup analysis, which in turn affects the accuracy of the results. Third, the duration of the study’s intervention ranged from 4 to 12 weeks, and we could not assess the long-term safety of CHM treatment. Finally, since the randomized controlled trials included in this study are all from China, our study may not be extended to the world. Therefore, a large sample of multi-center studies is needed in the future.
In short, CHM, whether a single treatment or adjuvant therapy, can improve nerve conduction velocity in patients with PDN, reduce pain score and TCM syndrome score, and improve clinical efficiency. These results can guide clinical practice. In addition, CHM is well tolerated and safe in patients with PDN, with a low incidence of adverse events. However, given the study’s heterogeneity and small sample size, bigger multi-center, high-quality RCTs will be required in the future to evaluate the advantages and safety of CHM in the treatment of PDN.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
This experiment was created by MS, DL, ZS, WL, ZL, YX, JIZ, and JUZ. The First drafts of the article were written by MS and DL, and further revisions were contributed to by the other co-authors. The article repair work was completed by MS, YQ, BH, and DL. The final proofreading of the article was completed by MS and BH. The final version of the work was approved by all authors. DL and YQ obtained funding for this study.
This work was supported by the Qilu Geriatric Diseases Chinese and Western Academic School Inheritance Workshop Project (No. 2022-93-1-10), and the General Project of Shandong Natural Science Foundation (ZR2021MH326).
For all the researchers in our working group, the authors want to express much thanks to them. We would also like to thank the Qilu Geriatric Diseases Chinese and Western Academic School Inheritance Workshop and the General Project of Shandong Natural Science Foundation for the financial support. Also thanks Xi Liang and Good, Julia for their language help.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1072991/full#supplementary-material
Chuan, L. (2017). Multi-compound pharmacokinetic research on Chinese herbal medicines: Approach and methodology. China J. Chin. Materia Medica 42 (4), 607–617. doi:10.19540/j.cnki.cjcmm.2017.0016
Cizhen, F. (2018). Effect of liuwei dihuang decoction in treating diabetic painful neuropathy. Psychol. Dr. 24 (11), 152–153.
Di, H., Xiaoqiu, C., Yongxin, H., Kemian, L., and Chao, L. (2017). Clinical study on treating 50 cases of diabetic painful neuropathy in the integrative medicine. Jiangsu J. Traditional Chin. Med. 49 (1), 29–31.
Dianrong, J., Ping, W., Xiaoyan, W., and Yunyun, Z. (2020). Tangbikang in treating 40 cases of diabetic painful neuropathy. West. J. Traditional Chin. Med. 33 (10), 108–110.
Duran, A. M., Beeson, W. L., Firek, A., Cordero-Macintyre, Z., and De Leon, M. (2022). Dietary omega-3 polyunsaturated fatty-acid supplementation upregulates protective cellular pathways in patients with type 2 diabetes exhibiting improvement in painful diabetic neuropathy. Nutrients. 14 (4), 761. doi:10.3390/nu14040761
Fangqin, C., Mingji, H., Li, H., and Yunxia, Z. (2021). Ligustrazine alleviates the tissue injury of the submandibular glands by enhanceing the antioxidant and anti-inflammatory effects in diabetic rats. Chin. J. Cell. Mol. Immunol. 37 (3), 212–218.
Fanrong, L., and Mingkong, C. (2014). Treatment of painful diabetic neuropathy by tongbu shuangtiao. Guangming J. Chin. Med. 29 (9), 1861–1863.
Fernandez-Ochoa, A., Cadiz-Gurrea, M. L., Fernandez-Moreno, P., Rojas-Garcia, A., Arraez-Roman, D., and Segura-Carretero, A. (2022). Recent analytical approaches for the study of bioavailability and metabolism of bioactive phenolic compounds. Molecules. 27 (3), 777. doi:10.3390/molecules27030777
Fuzhi, Z., Yadong, L., Lei, L., Wenjing, G., Min, H., Li, K., et al. (2020). Analysis on medication rules of traditional Chinese medicine for treatment of diabetic peripheral neuropathy based on clinical literature. Chin. J. Exp. Traditional Med. Formulae 26 (13), 199–205.
Gao, L. M., Fu, S., Liu, F., Wu, H. B., and Li, W. J. (2021). Astragalus polysaccharide regulates miR-182/bcl-2 Axis to relieve metabolic memory through suppressing mitochondrial damage-mediated apoptosis in retinal pigment epithelial cells. Pharmacology 106 (9-10), 520–533. doi:10.1159/000515901
Glevitzky, I., Glevitzky, M., Pasca, B., Otrisal, P., Bungau, S., et al. (2019). Statistical analysis of the relationship between antioxidant activity and the structure of flavonoid compounds. Rev. Chim. 70 (9), 3103–3107. doi:10.37358/rc.19.9.7497
Gong, A. G., Huang, V. Y., Wang, H. Y., Lin, H. Q., Dong, T. T., and Tsim, K. W. (2016). Ferulic acid orchestrates anti-oxidative properties of danggui buxue tang, an ancient herbal decoction: Elucidation by chemical knock-out approach. PLoS One. 11 (11), e0165486. doi:10.1371/journal.pone.0165486
Haibo, M. (2016). Study on therapeutic mechanism of Yiqi Huoxue Huayu method in treating painful diabetic peripheral neuropathy. Diabetes New World 19 (13), 115–116.
Haiyan, L., and Yange, H. (2020). Effect analysis of huoxue tongbi decoction applied in diabetic painful neuropathy. J. Med. Theory Pract. 33 (9), 1433–1434.
Higgins, J. P., Altman, D. G., Gotzsche, P. C., Juni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Honggang, N. (2011). Effect of jiawei sigu decoction on β-endorphin level in patients with T2DM painful neuropathy. Guiyang University of Chinese Medicine.
Honggang, N., Juan, Y., Xuemei, L., and Li, L. (2018). Effect of jiawei sigu decoction on calcitonin gene - related peptide and neuroelectrophysiology in type 2 diabetic patients with painful neuropathy. Guizhou Med. J. 42 (12), 1495–1496.
Hongwei, L., Guiping, L., Hong, W., and Xuxian, L. (2008). Curative effect observation on treating 43 cases of painful diabetic neuropathy in the integrative medicine. New Chin. Med. 5, 25–26.
Jensen, T. S., Backonja, M. M., Hernandez, J. S., Tesfaye, S., Valensi, P., and Ziegler, D. (2006). New perspectives on the management of diabetic peripheral neuropathic pain. Diab Vasc. Dis. Res. 3 (2), 108–119. doi:10.3132/dvdr.2006.013
Kulkantrakorn, K., and Lorsuwansiri, C. (2013). Sensory profile and its impact on quality of life in patients with painful diabetic polyneuropathy. J. Neurosci. Rural. Pract. 4 (3), 267–270. doi:10.4103/0976-3147.118766
Laibiao, D., Huailin, G., Liye, W., and Huiyu, L. (2012). Clinical observation on 30 cases of diabetic painful neuropathy treated by guilong tongluo capsule. Med. Inf. 25 (10), 105–106.
Laibiao, D., Qing, K., and Huailin, G. (2015). Clinical observation on 40 cases of diabetic painful neuropathy treated with single-flavor chuanwu. World Chin. Med. 10 (12), 1314–1315.
Liting, Z., Huijie, D., Jie, Z., Wenkui, J., and Jianping, Z. (2018). Effect of Jiawei Sigu Decoction on plasma β-endorphin level in type 2 diabetic painful neuropathy. J. Pract. Traditional Chin. Med. 34 (5), 582.
Nayak, B. P., Minaz, N., and Pasha, K. (2021). Molsidomine ameliorates diabetic peripheral neuropathy complications in Wistar rats. Anim. Model Exp. Med. 4 (3), 243–248. doi:10.1002/ame2.12162
Nie, Q., Zhu, L., Zhang, L., Leng, B., and Wang, H. (2019). Astragaloside IV protects against hyperglycemia-induced vascular endothelial dysfunction by inhibiting oxidative stress and Calpain-1 activation. Life Sci. 232, 116662. doi:10.1016/j.lfs.2019.116662
Nie, X., Wei, X., Ma, H., Fan, L., and Chen, W. D. (2021). The complex role of Wnt ligands in type 2 diabetes mellitus and related complications. J. Cell. Mol. Med. 25 (14), 6479–6495. doi:10.1111/jcmm.16663
Page, M. J., Mckenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 134, 103–112. doi:10.1016/j.jclinepi.2021.02.003
Prasad, S. N., and Muralidhara, (2013). Neuroprotective efficacy of eugenol and isoeugenol in acrylamide-induced neuropathy in rats: Behavioral and biochemical evidence. Neurochem. Res. 38 (2), 330–345. doi:10.1007/s11064-012-0924-9
Qiaren, H., and Xiaohong, Z. (2018). Clinical effect of integrated traditional Chinese and Western medicine therapy in treatment of painful diabetic peripheral neuropathy: An analysis of 32 cases. Hunan J. Traditional Chin. Med. 34 (11), 4–6.
Qinghua, W., Bingtao, L., and Jun, T. (2019). Compound traditional Chinese medicine in treatment of diabetes. China J. Chin. Materia Medica 44 (6), 1104–1109. doi:10.19540/j.cnki.cjcmm.20181212.001
Qingqing, M. (2021). To observe the clinical efficacy of jianpi yishen huayu zhitong recipe in the treatment of patients with spleen-kidney deficiency and blood stasis-type painful diabetic peripheral neuropathy. Ningxia Medical University.
Ruixia, P., Xiaolin, B., Guochun, Y., Junke, S., and Shanglin, G. (2015). Treatment of 35 cases of diabetic painful neuropathy by modified chaihu shugan powder. Shandong J. Traditional Chin. Med. 34 (8), 584–586.
Schreiber, A. K., Nones, C. F., Reis, R. C., Chichorro, J. G., and Cunha, J. M. (2015). Diabetic neuropathic pain: Physiopathology and treatment. World J. Diabetes 6 (3), 432–444. doi:10.4239/wjd.v6.i3.432
Sharma, S., Behl, T., Sehgal, A., Singh, S., Sharma, N., Bhatia, S., et al. (2022). Possible role of wnt signaling pathway in diabetic retinopathy. Drug Targets 23 (15), 1372–1380. doi:10.2174/1389450123666220301110140
Shuquan, L., Zhenqiang, W., Huajun, L., Li, L., Xiuhai, S., Yuansong, W., et al. (2021). Effect of wenyang tongluo formula on Hs-crp level and PKC activity in patients with painful diabetic peripheral neuropathy. J. Hebei Traditional Chin. Med. Pharmacol. 36 (5), 5–9.
Sun, Q., Zeng, J., Liu, Y., Chen, J., Zeng, Q. C., Chen, Y. Q., et al. (2020). microRNA-9 and -29a regulate the progression of diabetic peripheral neuropathy via ISL1-mediated sonic hedgehog signaling pathway. Aging (Albany NY) 12 (12), 11446–11465. doi:10.18632/aging.103230
Tao, L., Chen, L., and Yaofu, F. (2018). Observation of 30 cases on modified huangqi guizhi wuwu decoction in treatment of diabetic neuropathy with pain. Acta Chin. Med. 33 (1), 59–61.
Tesfaye, S., Vileikyte, L., Rayman, G., Sindrup, S. H., Perkins, B. A., Baconja, M., et al. (2011). Painful diabetic peripheral neuropathy: Consensus recommendations on diagnosis, assessment and management. Diabetes Metab. Res. Rev. 27 (7), 629–638. doi:10.1002/dmrr.1225
Tsai, C. I., Li, T. C., Chang, M. H., Lin, S. Y., Lee, I. T., Lee, C. H., et al. (2013). Chinese medicinal formula (mhgwt) for relieving diabetic neuropathic pain: A randomized, double-blind, placebo-controlled trial. Evid. Based Complement. Altern. Med. 2013, 767498. doi:10.1155/2013/767498
Veves, A., Backonja, M., and Malik, R. A. (2008). Painful diabetic neuropathy: Epidemiology, natural history, early diagnosis, and treatment options. Pain Med. 9 (6), 660–674. doi:10.1111/j.1526-4637.2007.00347.x
Wang, E., Wang, L., Ding, R., Zhai, M., Ge, R., Zhou, P., et al. (2020). Astragaloside IV acts through multi-scale mechanisms to effectively reduce diabetic nephropathy. Pharmacol. Res. 157, 104831. doi:10.1016/j.phrs.2020.104831
Wei, Q. (2021). Research progress of Chinese herb monomers in the treatment of diabetic retinopathy. Int. Eye Sci. 21 (8), 1373–1377.
Wu, M., Huang, J., Shi, J., Shi, L., Zeng, Q., and Wang, H. (2022). Ruyi Jinhuang Powder accelerated diabetic ulcer wound healing by regulating Wnt/β-catenin signaling pathway of fibroblasts in Vivo and in Vitro. Vitro. J. Ethnopharmacol. 293, 115321. doi:10.1016/j.jep.2022.115321
Xiaorui, C. (2016). The clinical observation of chaihu shugan powder modificationin in treating diabetic painful neuropathy abstract. Shaanxi University of Chinese Medicine.
Xiaoyu, Z. (2002). Guiding principles for clinical research of new Chinese medicine. Beijing: China Medical Science Press, 236–237.pp
Xie, J., Song, W., Liang, X., Zhang, Q., Shi, Y., Liu, W., et al. (2020). Protective effect of quercetin on streptozotocin-induced diabetic peripheral neuropathy rats through modulating gut microbiota and reactive oxygen species level. Biomed. Pharmacother. 127, 110147. doi:10.1016/j.biopha.2020.110147
Yanli, L., Yichun, Z., Chunhua, H., and Bojian, Z. (2020). Clinical observation on treating painful diabetic neuropathy with the Mudan granules plus epalrestat with mecobalamin. Clin. J. Chin. Med. 12 (32), 71–74.
Ye, G., Xuejian, N., Chunhua, T., and Hongjie, D. I. (2018). Clinical study on buyang huanwu decoction in treating diabetic painful peripheral neuropathy of qi deficiency and blood stasis type. Diabetes New World 21 (3), 161–162.
Yuanyuan, D., Yumei, L., Xiaowen, L., and Chuying, W. (2021). Review of Astragali radix for treating diabetic complications. Chin. J. Comp. Med. 31 (4), 123–128.
Yue, G., Aihua, L., Xiaohui, F., Limin, H., Feiran, H., and Yubo, L. (2019). Safety research in traditional Chinese medicine: Methods, applications, and outlook. Engineering 5 (1), 165–178.
Zhang, Q., Xiao, X., Zheng, J., Li, M., Yu, M., Ping, F., et al. (2020). Qishen yiqi dripping pill protects against diabetic nephropathy by inhibiting the wnt/β-catenin and transforming growth factor-β/smad signaling pathways in rats. Front. Physiol. 11, 613324. doi:10.3389/fphys.2020.613324
Keywords: painful diabetic neuropathy, Chinese herbal medicine, efficacy, safety, systematic review, meta-analysis
Citation: Song M, Huai B, Shi Z, Li W, Xi Y, Liu Z, Zhang J, Zhou J, Qiao Y and Liu D (2023) The efficacy and safety of Chinese herbal medicine in the treatment of painful diabetic neuropathy: A systematic review and meta-analysis. Front. Pharmacol. 14:1072991. doi: 10.3389/fphar.2023.1072991
Received: 18 October 2022; Accepted: 21 February 2023;
Published: 06 March 2023.
Edited by:
Teodorico Castro Ramalho, Universidade Federal de Lavras, BrazilReviewed by:
Simona Gabriela Bungau, University of Oradea, RomaniaCopyright © 2023 Song, Huai, Shi, Li, Xi, Liu, Zhang, Zhou, Qiao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Deshan Liu, bGl1ZGVzaGFuQHNkdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.