- 1Department of Hepatology, Fifth Medical Center of Chinese PLA General Hospital, Beijing, China
- 2307 Clinical Medical College of PLA, Anhui Medical University, Beijing, China
- 3Emergency Department, Seventh Medical Center of Chinese PLA General Hospital, Beijing, China
- 4Chinese PLA Medical School, Beijing, China
Background: Drug-induced liver injury (DILI) is a potentially serious adverse drug reaction. Due to the lack of definite etiology, specific clinical manifestations, and diagnostic methods, its prediction and diagnosis are challenging. Elderly individuals are deemed to be at high risk for DILI due to abnormal pharmacokinetics, aging tissue repair function, comorbidities, and taking multiple drugs. This study aimed to identify the clinical characteristics and explore the risk factors associated with the severity of illness in elderly patients with DILI.
Methods: In the present study, the clinical characteristics at the time of liver biopsy of consecutive patients with biopsy-proven DILI who presented at our hospital from June 2005 to September 2022 were evaluated. Hepatic inflammation and fibrosis were assessed according to the Scheuer scoring system. The presence of autoimmunity was considered if IgG level >1.1 × ULN (1826 mg/dL), or high titer (>1:80) of ANA, or SMA.
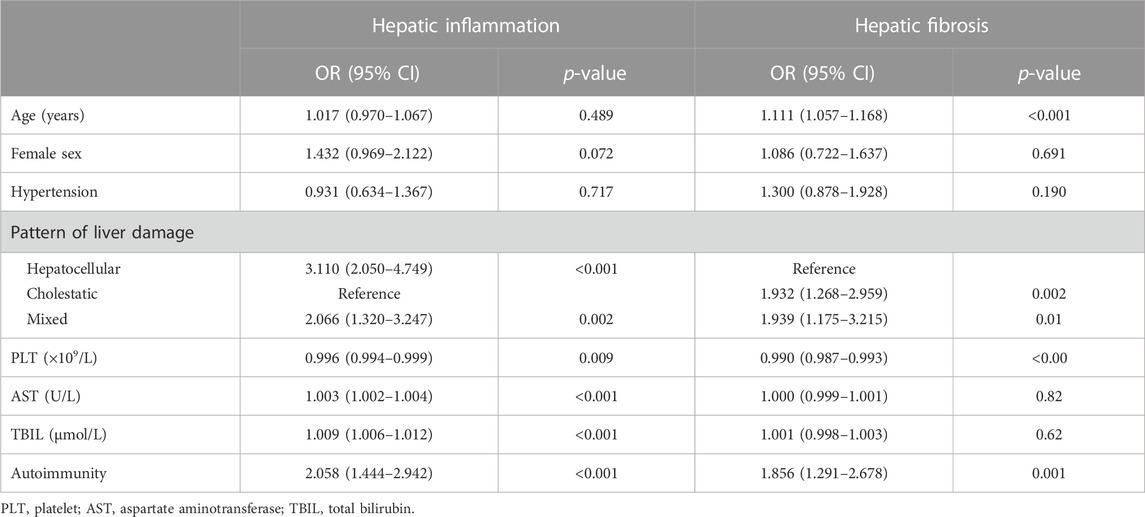
Results: In total, 441 patients were enrolled, and the median age was 63.3 years (IQR, 61.0–66.0); 122 (27.7%), 195 (44.2%), or 124 (28.1%) were classified as having minor, moderate, or severe hepatic inflammation, respectively; and 188 (42.6%), 210 (47.6%) or 43 (9.8%) patients presented minor, significant fibrosis or cirrhosis, respectively. Female sex (73.5%) and the cholestatic pattern (47.6%) were dominant in elderly DILI patients. Autoimmunity existed in 201 patients (45.6%). Comorbidities were not directly associated with the severity of DILI. PLT (OR: 0.994, 95% CI: 0.991–0.997; p < 0.001), AST (OR: 1.001, 95% CI: 1.000–1.003, p = 0.012), TBIL (OR: 1.006, 95% CI: 1.003–1.010, p < 0.001), and autoimmunity (OR: 1.831, 95% CI: 1.258–2.672, p = 0.002) were associated with the degree of hepatic inflammation. Meanwhile, PLT (OR: 0.990, 95% CI: 0.986–0.993, p < 0.001), TBIL (OR: 1.004, 95% CI: 1.000–1.007, p = 0.028), age (OR: 1.123, 95% CI: 1.067–1.183, p < 0.001), and autoimmunity (OR: 1.760, 95% CI: 1.191–2.608, p = 0.005) were associated with the stage of hepatic fibrosis.
Conclusion: This study revealed that the presence of autoimmunity represents a more serious illness state of DILI, deserving more intensive monitoring and progressive treatment.
Introduction
In the case of a reasonable rule out of other causes, drug-induced liver injury (DILI), which remains one of the most challenging diseases faced by hepatologists, is a severe adverse drug reaction caused by hepatotoxic exogenous agents and their metabolites, such as prescriptions, over-the-counter drugs, herbs, and dietary supplements (Teschke et al., 2013; Kleiner et al., 2014; Navarro et al., 2014; EASL-ALEH Clinical Practice Guidelines;Asociacion Latinoamericana para el Estudio del Higado, 2015; Foureau et al., 2015; Bonkovsky et al., 2017; Kullak-Ublick et al., 2017; Andrade et al., 2019b). With the enhancement of people’s health consciousness, herbal and dietary supplements (HDS) or traditional Chinese medicines (TCM) account for an increasing proportion of DILI events worldwide (Wai et al., 2007; Navarro et al., 2014; García-Cortés et al., 2016; Ji et al., 2018). This is especially applicable to body-building and fat-reducing supplements (Grewal and Ahmad, 2019; Santos et al., 2021), exposing people to more uncertain hepatotoxic drugs, which calls for clinicians to have a greater understanding of DILI.
Elderly individuals are at high risk for DILI (Danan and Benichou, 1993; Lucena et al., 2009; Andrade et al., 2019a). First, the distribution and release of drugs are affected by increased body fat in the aged (Lucena et al., 2009). Second, the decrease in liver and kidney function affects the metabolism and excretion of drugs, leading to abnormal pharmacokinetics (Tostmann et al., 2008; Klotz, 2009; Andrade et al., 2019a). At the same time, their tissue-repair capacity is reduced. In addition, the aged have many comorbidities and may require multiple medications, so DILI is complicated by drug-drug interactions and drug-host interactions (Chen et al., 2015; Andrade et al., 2019a). Marcum et al. (2012) reported that people older than 70 take an average of three to seven medications per day. A meta-analysis demonstrated that the prevalence of polypharmacy (using more than five drugs) and potentially inappropriate medications (PIM) in elderly Chinese patients was 48% and 39%, respectively (Tian et al., 2022). Similarly, Koçak et al. (2022) mentioned that the prevalence of PIM among nursing home residents aged ≥60 years was 47.6%.
DILI with features of autoimmunity represents an important category of hepatotoxicity due to medication exposure, which is a syndrome typically characterized by liver injury accompanied by hypergammaglobulinemia, circulating anti-nuclear antibody (ANA) and/or smooth muscle antibody (SMA) (deLemos et al., 2014). Circulating antibodies targeting intracellular components are also indicative of cellular damage. Furthermore, elderly individuals commonly present a low titer (<1:80) of serum autoantibodies (approximately 25%), making distinguishing between DILI and AIH more complicated (deLemos et al., 2014).
The exclusive diagnosis combined with causality assessment is an essential and arduous task to improve DILI diagnosis (Liu et al., 2021). There is considerable variability in the time to onset, severity, clinical manifestations, laboratory features, findings on liver biopsy, course, and outcome. Moreover, relatively few studies have involved elderly DILI patients with features of autoimmunity, making the database for this group somewhat limited. Thus, in the present study, we aimed to identify the clinical characteristics and explore the risk factors associated with the severity of illness in this special patient population to avoid progressing to poorer clinical outcomes.
Patients and methods
Study design and patients
The present study was a retrospective hospitalization-based cross-sectional study, which was approved by the Ethics Committees of Fifth Medical Center of Chinese PLA General Hospital (No. 2019024D). Written informed consent for liver biopsy was obtained from all patients, whereas patient consent for data collection was waived due to the study design.
The inclusion criteria included the following: 1) admitted from June 2005 to September 2022; 2) age ≥60 years old; 3) met the DILI definition (see definition section); 4) Roussel Uclaf Causality Assessment Method (RUCAM) score >6 points; and 5) the diagnosis of DILI was confirmed by liver biopsy. The exclusion criteria were as follows: 1) those with any other definite etiologies of liver disease (e.g., primary biliary cholangitis, primary sclerosing cholangitis, viral hepatitis, alcoholic or non-alcoholic liver disease, Gilbert syndrome, etc.) according to their relevant guidelines (Lindor et al., 2015; EASL–EASD–EASO Clinical Practice, European Association for the Study of Diabetes EASD, European Association for the Study of Obesity EASO, 2016; Hirschfield et al., 2017; Singal et al., 2018; Wagner et al., 2018; Te and Doucette, 2019); 2) those with severe systemic diseases affecting the liver (heart attack, stroke, kidney, or HIV infection); and 3) those with incomplete important data.
Procedures
The clinical data of the enrolled patients at the time of liver biopsy were retrieved through electronic medical records, such as age, sex, body mass index (BMI), implicated drugs, platelet (PLT), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), alkaline phosphatase (ALP), immunoglobulin G (IgG), anti-nuclear antibody (ANA), smooth muscle antibody (SMA), and anti-mitochondrial antibody (AMA).
Definition
DILI was defined as an adverse hepatic reaction that is unexpected based on the pharmacological action of the drug administered, including one of the following thresholds: 1) ≥5 × ULN elevation in ALT, 2) ≥2 × ULN elevation in ALP (particularly with accompanying elevations in concentrations of GGT in the absence of known bone pathology driving the rise in ALP level), or 3) ≥3 × ULN elevation in ALT and simultaneous elevation of TBIL concentration exceeding 2 × ULN, according to EASL DILI guideline 2019. Three patterns of DILI were categorized by using the R-value ([ALT/upper limit of the normal range (ULN)]/[ALP/ULN]): hepatocellular when ≥5, cholestatic when ≤2 and mixed when 2–5 (Andrade et al., 2019a).
The presence of autoimmunity was considered if IgG level >1.1 × ULN (1826 mg/dL), or high titer (>1:80) of ANA, or SMA (de Boer et al., 2017). The enrolled patients were divided into three groups by age: Group A, ≤65 years (n = 318 [72.1%]); Group B, 65–70 years (n = 92 [20.9%]); and Group C, >70 years (n = 31 [7.0%]).
Histological evaluations
Ultrasound-guided liver biopsy was performed on all patients, and hepatic inflammatory grades and fibrosis stages were evaluated according to the Scheuer scoring system (Scheuer, 1991). To ensure sufficient specimens with at least 12 portal vessels for histological evaluation, a minimum length of 15.0 mm was required for each liver specimen. Subsequently, two liver histopathologists independently evaluated tissue specimens, and when inconsistencies arose, both pathologists re-reviewed the specimens together. The grade of hepatic inflammation was defined as mild (G0-1), moderate (G2), and severe (G3-4). The stage of hepatic fibrosis was defined as mild (S0-1), significant (S2-3), and cirrhosis (S4).
Statistical analysis
Continuous variables were expressed as medians and interquartile ranges (IQR) and compared by using the Kruskal-Wallis test. Categorical variables were presented as numbers (percentage) and compared by Chi-square or Fisher’s exact test. The trend was analyzed by the Cochran-Armitage trend test for the 2 × 3 tables (sex and autoimmunity) and the Jonckheere-Terpstra trend test for the 3 × 3 tables (age, liver damage pattern, medication). Multivariate ordinal polytomous logistic regression was performed to identify the independent risk factors associated with the severity of illness regarding hepatic inflammation or fibrosis. The odds ratio (OR) and 95% confidence interval (CI) were estimated simultaneously. The forest plot was established by using the ggplot2 package of R. A two-tailed p-value of <0.05 was considered statistically significant. All statistical analyses were performed using R software, version 4.2.1 (http://www.r-project.org/).
Results
Clinical characteristics
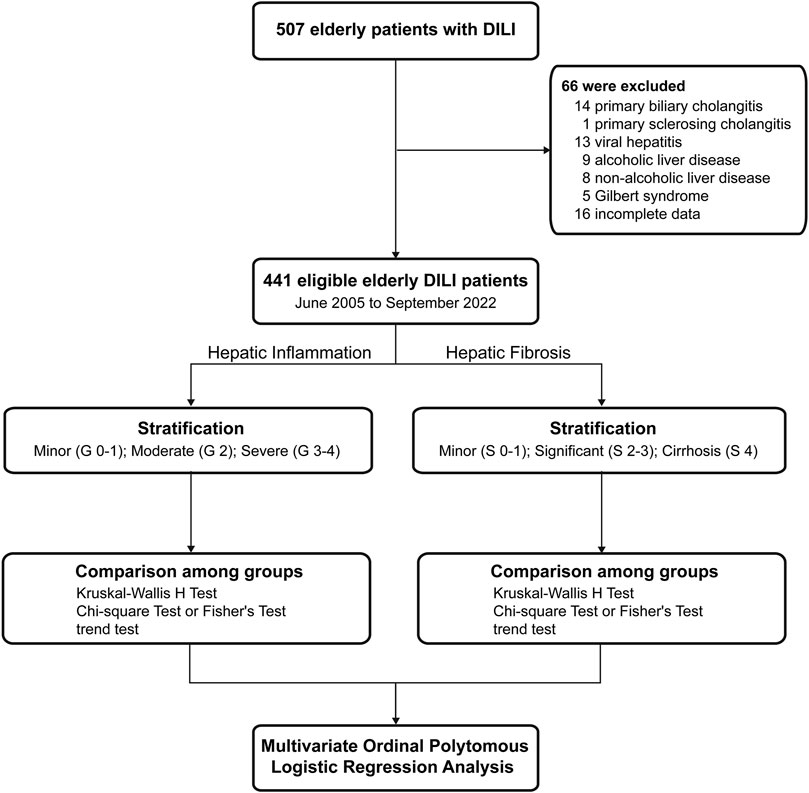
A total of 507 elderly patients with DILI who underwent liver biopsy were screened. Of these patients, 66 patients were excluded. Finally, 441 patients were enrolled in this study and were categorized into three groups according to hepatic inflammation grade or fibrosis stage (Figure 1).
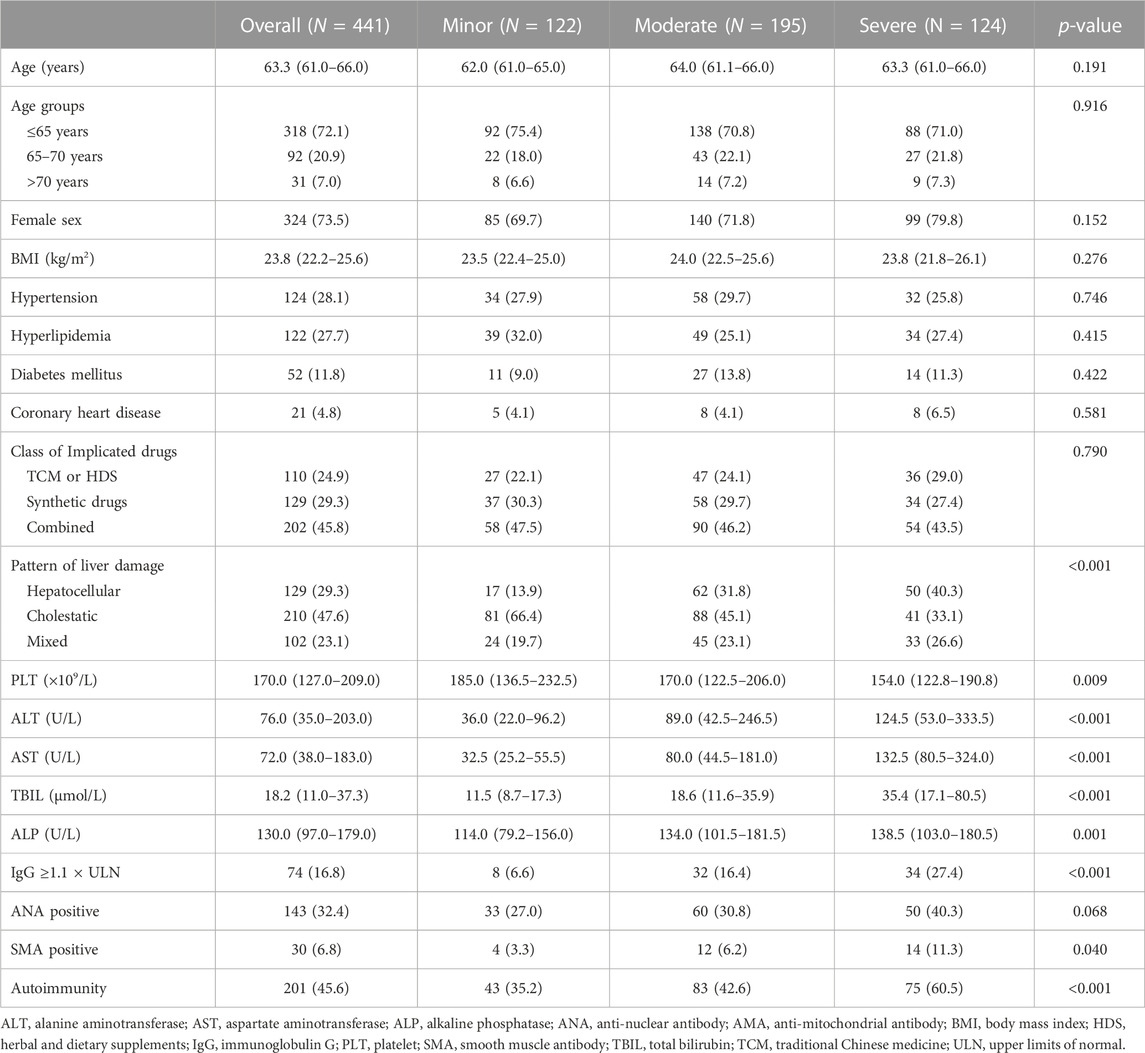
The overall median age was 63.3 years (IQR, 61.0–66.0), and 324 patients (73.5%) were female. Regarding histological characteristics, 122 (27.7%), 195 (44.2%), or 124 (28.1%) patients were classified as having minor, moderate, or severe hepatic inflammation, respectively; 188 (42.6%), 210 (47.6%), or 43 (9.8%) patients presented minor, significant fibrosis, or cirrhosis, respectively. The clinical pattern of liver damage was given priority to cholestatic damage (47.6%), followed by hepatocellular damage (29.3%), and mixed damage (23.1%). The most common comorbidities were hypertension (28.1%), hyperlipidemia (27.7%), diabetes mellitus (11.8%), and coronary heart disease (4.8%). According to the results of autoimmune antibody detection, there were 143 patients (32.4%) with positive ANA, 30 patients (6.8%) with positive SMA, and 74 (16.8%) with ≥1.1 × ULN of IgG. Overall, 201 patients (45.6%) presented the feature of autoimmunity (Table 1).
Furthermore, we investigated the distribution of age stratified by sex. The number of cases was highest among those aged 60–62 years, and the percentage of females was significantly higher than that of males for every 1-year age group (p < 0.05, Figure 2A). The overall percentage of autoimmunity in females was 52.5%, which was significantly higher than that in males (25.6%, p < 0.05, Figure 2B).
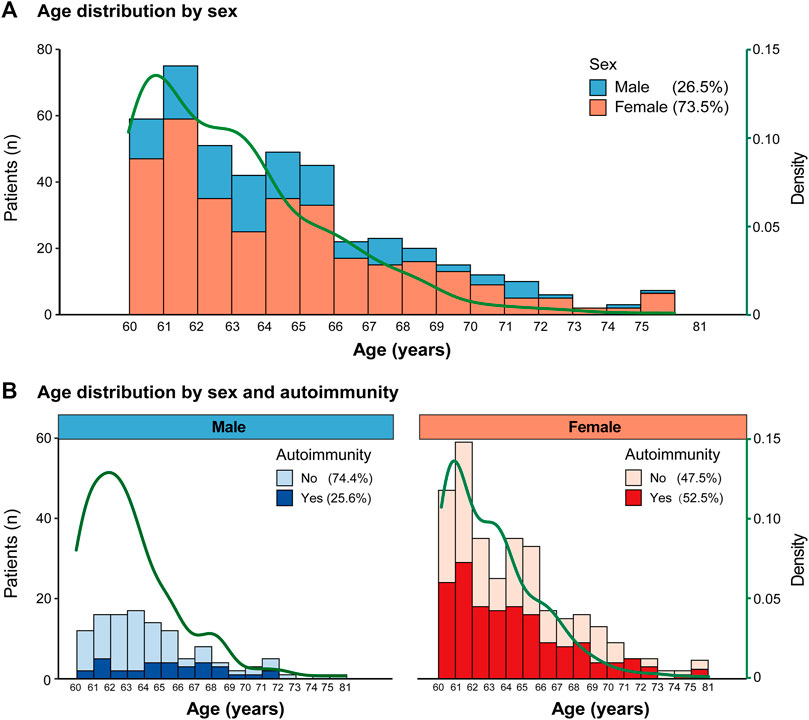
FIGURE 2. Age distribution (A). Age distribution by sex; (B). Age distribution by sex and autoimmunity.
Implicated drugs
In total, 202 (45.8%) patients had taken a combination of synthetic drugs and TCM or HDS, 129 (29.3%) had taken synthetic drugs, and 110 (24.9%) had taken TCM or HDS. The most commonly implicated drugs were cardiovascular drugs (17.0%), followed by herbal and dietary supplements (HDS) (12.9%), gastrointestinal drugs (6.3%), non-steroidal anti-inflammatory drugs (NSAIDs) (5.9%), etc. Meanwhile, there were 16.3% polypharmacy and 21.5% unspecified drugs (Supplementary Figure S1).
Distribution of clinical Parameters by histological assessment
All enrolled patients were categorized into three ordinal groups according to histological assessment and compared. The results showed that patterns of liver damage, PLT, ALT, AST, TBIL, and autoimmunity were significantly different in groups with increasing grades of hepatic inflammation (p < 0.05, Table 1) or in groups with increasing stages of hepatic fibrosis (p < 0.05, Table 2). Furthermore, with the aggravation of hepatic inflammation grade, the proportion of hepatocellular pattern (13.9%, 31.8%, and 40.3%, p for trend = 0.037) and presence of autoimmunity (35.2%, 42.6%, and 60.5%, p for trend <0.001) increased significantly. Similarly, with the progression of hepatic fibrosis stage, the proportion of older age >70 years (2.7%, 9.5%, and 14.0%, p for trend = 0.003), cholestatic pattern (43.6%, 47.6%, and 65.1%, p for trend = 0.009), and presence of autoimmunity (36.7%, 51.0%, and 58.1%, p for trend = 0.002) significantly increased (Figure 3).
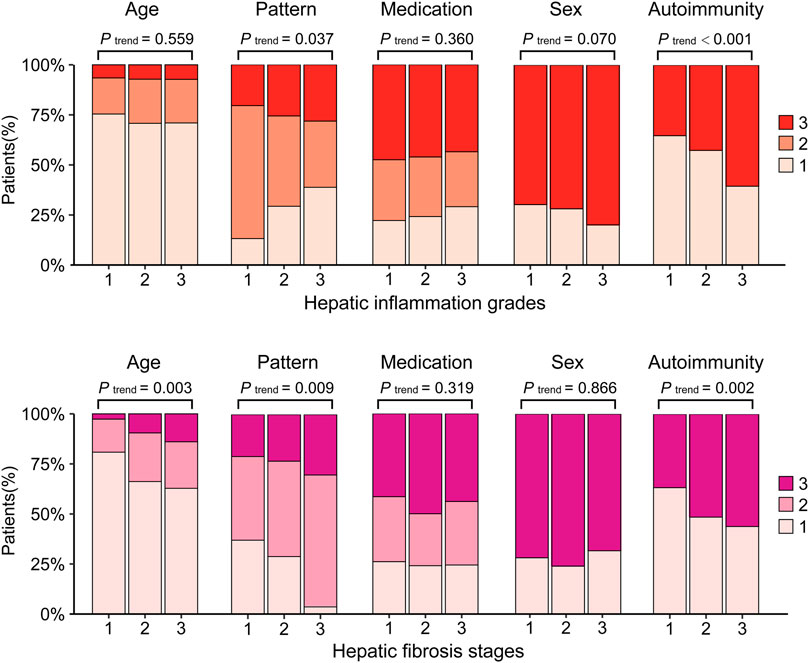
FIGURE 3. The clinical characteristics by histological evaluation.Hepatic inflammation grade: 1: G0-1; 2: G2; 3: G3-4. Hepatic fibrosis stage: 1: S0-1, 2: S2-3, 3: S4. Age, 1: ≤65 years, 2: 65–70 years, 3: >70 years. Pattern, 1: hepatocellular; 2: Cholestatic; 3: Mixed. Medication, 1: Synthetic drugs; 2: Traditional Chinese Medicine or herbal and dietary supplements; 3: Combined. Sex, 1: Male; 3: Female. Autoimmunity: 1: No; 3: Yes.
Multivariate ordinal polytomous logistic regression analysis
After screening by comparison among groups, collinearity analysis, and ordinal univariate analysis, variables with a p-value of <0.1 were included in the multivariate ordinal polytomous logistic regression analysis (Table 3). Ultimately, PLT (OR: 0.994, 95% CI: 0.991–0.997; p < 0.001), AST (OR: 1.001, 95% CI: 1.000–1.003, p = 0.012), TBIL (OR: 1.006, 95% CI: 1.003–1.010, p < 0.001), and autoimmunity (OR: 1.831, 95% CI: 1.258–2.672, p = 0.002) were associated with the degree of hepatic inflammation. Meanwhile, PLT (OR: 0.990, 95% CI: 0.986–0.993, p < 0.001), TBIL (OR: 1.004, 95% CI: 1.000–1.007, p = 0.028), age (OR: 1.123, 95% CI: 1.067–1.183, p < 0.001), and autoimmunity (OR: 1.760, 95% CI: 1.191–2.608, p = 0.005) were associated with the stage of hepatic fibrosis.
Taking the cholestatic damage pattern as a reference, hepatocellular (OR: 1.735, 95% CI: 1.027–2.932, p = 0.038) and mixed (OR: 1.981, 95% CI: 1.249–2.932, p = 0.004) damage were independent risk factors for hepatic inflammation. However, cholestasis (OR: 2.686, 95% CI: 1.563–4.673, p < 0.001) and mixed (OR: 2.738, 95% CI: 1.527–4.959, p = 0.001) were independent risk factors for hepatic fibrosis when referring to the hepatocellular damage pattern. In addition, sex was not associated with either aspect (Figure 4).
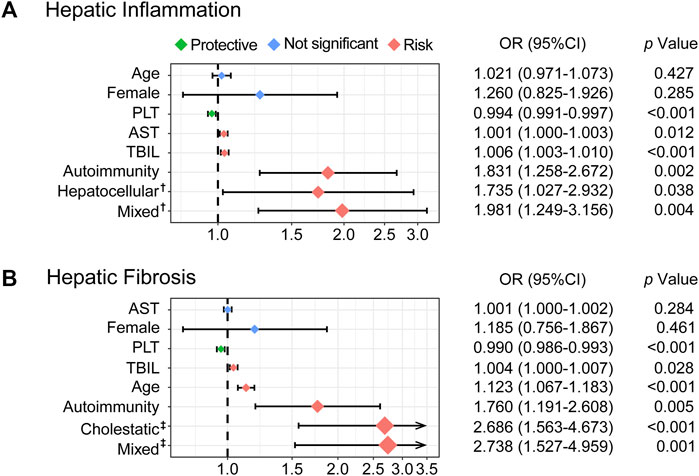
FIGURE 4. Forest plots based on multivariable ordinal polytomous logistic regression analysis. The X-axis was transformed as a log scale (A). The risk factors associated with hepatic inflammation (B). The risk factors associated with hepatic fibrosis. The colored solid diamonds represent the OR and the black lines show 95% CI.OR, odds ratio; CI, confidence interval; AST, aspartate transaminase; TBIL, total bilirubin; PLT, platelet.
Discussion
With the aging of the population, the incidence of elderly DILI is expected to increase (Andrade et al., 2019a). Meanwhile, DILI causes the progression of underlying diseases due to drug withdrawal, a worse quality of life, and an immense economic burden (Stevens and Baker, 2009; Kullak-Ublick et al., 2017). We revealed that the presence of autoimmunity was associated with a higher level of illness severity concerning hepatic inflammation and fibrosis in elderly patients with DILI, providing a reference for the strategy of intensive monitoring and aggressive treatment.
DILI is an invisible killer, and although most cases are improved after drug withdrawal (Aithal et al., 2011; Chen et al., 2011), serious clinical outcomes such as chronic hepatitis, liver failure, cirrhosis, liver transplantation, or even death may occur if long-term drug use is combined with irregular liver function reexamination (Raschi and De Ponti, 2017; Weaver et al., 2020; Wang et al., 2021). In the USA and Europe, DILI accounts for the most cases of acute liver failure (Ostapowicz, 2002; Kullak-Ublick et al., 2017). A prospective study showed that patients aged over 60 years with comorbidities had a higher mortality rate (Chalasani et al., 2015). A retrospective analysis including 595 patients demonstrated that the incidence of chronic hepatitis, liver failure, cirrhosis, and death was 13.4%, 7.9%, 7.6%, and 4.5% in China, respectively (Zhu et al., 2016). The present study showed that 9.8% of patients presented cirrhosis, implying that the adverse outcome of DILI, especially in elderly individuals, might be underestimated and need more attention.
Our study found that the most commonly-used implicated drug in the elderly was cardiovascular drugs, which was different from the ordinary patient population. Previous studies demonstrated antibiotics to be the most commonly implicated agents (Andrade et al., 2019b). This might lie in the dominance of cardiovascular disease as an underlying disease in elderly individuals. Additionally, the elderly population was also found to be exposed to greater usage of over-the-counter drugs and herbal supplements due to the increased consciousness about health in the present study. Moreover, some features in the medication of the elderly should be noted: 1) higher level of polypharmacy usage, 2) more cases of unclear medication duration, 3) longer duration of drug intake until the onset of DILI, and 4) more cases of the cholestatic pattern of liver damage, which should not be neglected before the prescription.
Regarding clinical characteristics, 73.5% of participants were female, and the presence of autoimmunity was found to be more common in specimens from female patients, which is consistent with previous studies (Amacher, 2014; Chalasani et al., 2015) suggesting that females are more susceptible to DILI and autoimmunity. Levels of immunoglobulins and autoantibodies are higher in female, especially, postmenopausal women with DILI and abnormal IgG levels are more likely to progress autoimmune hepatitis (Sakiani et al., 2013; He et al., 2022). This may be attributed to immune function impacted by periodicity of estrogen and progesterone levels (Moulton, 2018). In our study, the elderly female patients were all postmenopausal women with a great potential for autoimmunity. Moreover, sex differences in percentage of body fat, cytochrome P450 isozymes and drug-binding proteins may also explain this phenomenon (Amacher, 2014). The cholestatic (47.6%) pattern was the main pattern of liver damage. Likewise, Lucena et al. (2009) proposed that agedness is positively associated with cholestatic liver injury. This may be explained by the high utilization rate of hyperlipophilic drugs and decreased biliary function (Hunt et al., 2014). Additionally, prolonged tubular excretion and cholangiocyte exposure may be the reason for activating immune responses (Weersink et al., 2021).
There was no statistically significant difference in hepatic inflammation or fibrosis for comorbidities, such as hypertension, hyperlipidemia, diabetes mellitus, or coronary heart disease. A Spanish DILI registry study suggested that hypertension and diabetes may be detrimental to the repair of liver injury, contributing to chronic outcomes (Medina-Caliz et al., 2016). Weersink et al. (2021) showed increasing comorbidity burden (p < 0.001) and polypharmacy (p < 0.001) in elderly patients with DILI, which may explain the increased non-liver-related mortality (p = 0.030). It may be that comorbidities are not direct factors contributing to the illness progression of DILI but make patients forced to take multiple drugs or actively seek informal medical methods such as folk remedies or dietary supplements.
Furthermore, according to the multivariate ordinal polytomous logistic regression analysis, autoimmunity was an independent risk factor for hepatic inflammation (OR: 1.831; 95% CI: 1.258–2.672) or fibrosis (OR: 1.760; 95% CI: 1.191–2.608), promoting illness progression in elderly DILI patients. Moreover, DILI with autoimmunity was not rare, accounting for 45.6% of cases, in the present study. An analysis of the autoimmune features of DILI from the DILI Network prospective study found that the majority of patients (60%–70%) were positive for ANA and SMA, and approximately 40% had elevated IgG serum levels (de Boer et al., 2017). Multidrug compatibility in the elderly can alter the immune and inflammatory response environment (Chen et al., 2015). Damage-associated molecular patterns (DAMPs) released by drug-injured hepatocytes activate innate immunity and lead to sterile inflammation, which further amplifies tissue damage (Mosedale and Watkins, 2017; Gerussi et al., 2021). Once more, inappropriate maturation of dendritic cells due to tissue injury in the elderly may alter the balance between immune function and tolerance, triggering the propensity of autoimmunity (Agrawal et al., 2012; Tajiri and Shimizu, 2013). Despite the application of high-dose glucocorticoids in the follow-up, drug-mediated autoimmune hepatitis mostly occurrs in elderly patients (75% aged >60 years) predisposed to late relapse (Yeong et al., 2016). Thus, our conclusion that autoimmunity promotes the illness progression of elderly DILI is of significant clinical manfulness.
Therefore, elderly DILI patients with autoimmunity need more frequent follow-up and aggressive treatment. Over the years, several models (Ashby et al., 2021; Li et al., 2021) have been proposed to predict the outcome of DILI. Our previous study found that significant hepatic inflammation (HAI ≥10) was an independent risk factor for biochemical resolution (OR: 21.278, 95% CI: 14.780, 30.632) (Wang et al., 2022), which was consistent with the present result.
The primary treatment for DILI is the discontinuation of suspected drugs, and corticosteroids can improve the condition of DILI with autoimmune features (Chalasani et al., 2021; Björnsson et al., 2022). Nevertheless, clinicians must weigh the risks of infection, osteoporosis, cognitive decline, etc., against the progression of DILI when treating older adults with corticosteroids (Lee et al., 2021).
The strengths of the present study included that: 1) a multilevel ordinal logistic regression analysis was performed to obtain a reliable estimate and standard error, 2) a large sample size of elderly patients was enrolled, which had adequate power to detect the true effect of the independent variables, and 3) a liver biopsy was required to ensure the accurate assessment for hepatic inflammation and fibrosis and exclusion of patients with other etiologies, which is scarce in the current literature.
Although the findings have important clinical implications, several limitations should be noted. First, the cross-sectional study design cannot establish the causal relationship between the severity of DILI (hepatic inflammation or fibrosis) and identified independent variables (e.g., autoimmunity, patterns of injuries, etc.). Second, implicated agents might be misclassified due to the multiple underlying diseases and complexity of drug use. Finally, as this study is a retrospective single-center study, there might be some biases, such as admission rate bias. There were no patients of other races, which might limit the generalizability of this conclusion to broader populations. Further multicenter prospective studies with a larger sample size are warranted to verify the results.
Conclusion
Female sex and the cholestatic liver damage pattern are dominant in the elderly patients with DILI, and comorbidities are not directly associated with the severity of the illness. The presence of autoimmunity represents a more serious illness state of DILI, deserving more intensive monitoring and progressive treatment.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was approved by the Ethics Committees of Fifth Medical Center of Chinese PLA General Hospital (No. 2019024D). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
DJ was responsible for the study design and supervision. Y-TX, J-FW, and X-XN were responsible for manuscript writing and data analysis. DJ, JZ, and Y-MF were responsible for critical revision of the manuscript. All authors were responsible for data acquisition and approval of the final version of the manuscript for submission.
Funding
This work was supported by Beijing Municipal Science and Technology Commission (Z181100001718034) and Beijing Chen Jumei Foundation (2018JM12603003). The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1071709/full#supplementary-material
Abbreviations
ANA, anti-nuclear antibody; AMA, anti-mitochondrial antibody; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; ALP, Alkaline phosphatase; BMI, body mass index; CI, confidence interval; DILI, drug-induced liver injury; EASL, uropean association for the study of the liver; HDS, herbal and dietary supplements; HIV, human immunodeficiency virus; HAI, histological activity index; IgG, immunoglobulin G; IQR, interquartile range; NSAID, non-steroidal anti-inflammatory drug; OR, odds ratio; PLT, platelet; PIM, potentially inappropriate medications; RUCAM, roussel uclaf causality assessment method; SMA, smooth muscle antibody; TBIL, total bilirubin; TCM, traditional Chinese medicine; ULN, upper limit of normal.
References
Agrawal, A., Sridharan, A., Prakash, S., and Agrawal, H. (2012). Dendritic cells and aging: Consequences for autoimmunity. Expert Rev. Clin. Immu 8, 73–80. doi:10.1586/eci.11.77
Aithal, G. P., Watkins, P. B., Andrade, R. J., Larrey, D., Molokhia, M., Takikawa, H., et al. (2011). Case definition and phenotype standardization in drug-induced liver injury. Clin. Pharmacol. Ther. 89, 806–815. doi:10.1038/clpt.2011.58
Amacher, D. E. (2014). Female gender as a susceptibility factor for drug-induced liver injury. Hum. Exp. Toxicol. 33, 928–939. doi:10.1177/0960327113512860
Andrade, R. J., Aithal, G. P., Björnsson, E. S., Kaplowitz, N., Kullak-Ublick, G. A., Larrey, D., et al. (2019a). EASL clinical Practice guidelines: Drug-induced liver injury. J. Hepatol. 70, 1222–1261. doi:10.1016/j.jhep.2019.02.014
Andrade, R. J., Chalasani, N., Björnsson, E. S., Suzuki, A., Kullak-Ublick, G. A., Watkins, P. B., et al. (2019b). Drug-induced liver injury. Nat. Rev. Dis. Prim. 5, 58. doi:10.1038/s41572-019-0105-0
Ashby, K., Zhuang, W., González-Jimenez, A., Alvarez-Alvarez, I., Lucena, M. I., Andrade, R. J., et al. (2021). Elevated bilirubin, alkaline phosphatase at onset, and drug metabolism are associated with prolonged recovery from DILI. J. Hepatol. 75, 333–341. doi:10.1016/j.jhep.2021.03.021
Björnsson, E. S., Vucic, V., Stirnimann, G., and Robles-Díaz, M. (2022). Role of corticosteroids in drug-induced liver injury. A systematic review. Front. Pharmacol. 13, 820724. doi:10.3389/fphar.2022.820724
Bonkovsky, H. L., Kleiner, D. E., Gu, J., Odin, J. A., Russo, M. W., Navarro, V. M., et al. (2017). Clinical presentations and outcomes of bile duct loss caused by drugs and herbal and dietary supplements. Hepatology 65, 1267–1277. doi:10.1002/hep.28967
Chalasani, N., Bonkovsky, H. L., Fontana, R., Lee, W., Stolz, A., Talwalkar, J., et al. (2015). Features and outcomes of 899 patients with drug-induced liver injury: The DILIN prospective study. Gastroenterology 148, 1340–1352.e7. doi:10.1053/j.gastro.2015.03.006
Chalasani, N. P., Maddur, H., Russo, M. W., Wong, R. J., and Reddy, K. R.Practice Parameters Committee of the American College of Gastroenterology (2021). Practice parameters committee of the American college of GastroenterologyACG clinical guideline: Diagnosis and management of idiosyncratic drug-induced liver injury. Am. J. Gastroenterol. 116, 878–898. doi:10.14309/ajg.0000000000001259
Chen, M., Suzuki, A., Borlak, J., Andrade, R. J., and Lucena, M. I. (2015). Drug-induced liver injury: Interactions between drug properties and host factors. J. Hepatol. 63, 503–514. doi:10.1016/j.jhep.2015.04.016
Chen, M., Vijay, V., Shi, Q., Liu, Z., Fang, H., and Tong, W. (2011). FDA-approved drug labeling for the study of drug-induced liver injury. Drug Discov. Today 16, 697–703. doi:10.1016/j.drudis.2011.05.007
Danan, G., and Benichou, C. (1993). Causality assessment of adverse reactions to drugs—I. A novel method based on the conclusions of international consensus meetings: Application to drug-induced liver injuries. J. Clin. Epidemiol. 46, 1323–1330. doi:10.1016/0895-4356(93)90101-6
de Boer, Y. S., Kosinski, A. S., Urban, T. J., Zhao, Z., Long, N., Chalasani, N., et al. (2017). Features of autoimmune hepatitis in patients with drug-induced liver injury. Clin. Gastroenterol. Hepatol. 15, 103–112.e2. doi:10.1016/j.cgh.2016.05.043
deLemos, A., Foureau, D., Jacobs, C., Ahrens, W., Russo, M., and Bonkovsky, H. (2014). Drug-induced liver injury with autoimmune features. Semin. Liver Dis. 34, 194–204. doi:10.1055/s-0034-1375959
EASL-ALEH Clinical Practice Guidelines;Asociacion Latinoamericana para el Estudio del Higado (2015). EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J. Hepatol. 63, 237–264. doi:10.1016/j.jhep.2015.04.006
EASL–EASD–EASO Clinical Practice, European Association for the Study of Diabetes EASD, European Association for the Study of Obesity EASO (2016). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 64, 1388–1402. doi:10.1016/j.jhep.2015.11.004
Foureau, D. M., Walling, T. L., Maddukuri, V., Anderson, W., Culbreath, K., Kleiner, D. E., et al. (2015). Comparative analysis of portal hepatic infiltrating leucocytes in acute drug-induced liver injury, idiopathic autoimmune and viral hepatitis. Clin. Exp. Immunol. 180, 40–51. doi:10.1111/cei.12558
García-Cortés, M., Robles-Díaz, M., Ortega-Alonso, A., Medina-Caliz, I., and Andrade, R. (2016). Hepatotoxicity by dietary supplements: A tabular listing and clinical characteristics. Int. J. Mol. Sci. 17, 537. doi:10.3390/ijms17040537
Gerussi, A., Natalini, A., Antonangeli, F., Mancuso, C., Agostinetto, E., Barisani, D., et al. (2021). Immune-mediated drug-induced liver injury: Immunogenetics and experimental models. Int. J. Mol. Sci. 22, 4557. doi:10.3390/ijms22094557
Grewal, P., and Ahmad, J. (2019). Severe liver injury due to herbal and dietary supplements and the role of liver transplantation. World J. Gastroentero 25, 6704–6712. doi:10.3748/wjg.v25.i46.6704
He, T., Ren, L., Gong, M., Guo, Y., Wang, L., Xiao, X., et al. (2022). The progression of chronicity and autoimmune hepatitis in recurrent drug-induced liver injury. Clin. Res. Hepatol. Gas. 46, 102009. doi:10.1016/j.clinre.2022.102009
Hirschfield, G. M., Beuers, U., Corpechot, C., Invernizzi, P., Jones, D., Marzioni, M., et al. (2017). EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J. Hepatol. 67, 145–172. doi:10.1016/j.jhep.2017.03.022
Hunt, C. M., Yuen, N. A., Stirnadel-Farrant, H. A., and Suzuki, A. (2014). Age-related differences in reporting of drug-associated liver injury: Data-mining of WHO Safety Report Database. Regul. Toxicol. Pharmacol. 70, 519–526. doi:10.1016/j.yrtph.2014.09.007
Ji, D., Chen, G., Fu, Y., Wang, C., Chen, J., Shao, Q., et al. (2018). The 10-year chronic liver disease spectrum evolution in China: Experience from the largest tartiary special hospital with 21382 liver biopsy cases. J. Hepatol. 68, S155–S156. doi:10.1016/S0168-8278(18)30523-3
Kleiner, D. E., Chalasani, N. P., Lee, W. M., Fontana, R. J., Bonkovsky, H. L., Watkins, P. B., et al. (2014). Hepatic histological findings in suspected drug-induced liver injury: Systematic evaluation and clinical associations. Hepatology 59, 661–670. doi:10.1002/hep.26709
Klotz, U. (2009). Pharmacokinetics and drug metabolism in the elderly. Drug Metab. Rev. 41, 67–76. doi:10.1080/03602530902722679
Koçak, F. Ö. K., Taşkıran, E., Öztürk, Z. K., and Şahin, S. (2022). Potentially inappropriate medication use among nursing home residents: Medication errors associated with pro re nata medications and the importance of pill burden. Ann. Geriatr. Med. Res. 26, 233–240. doi:10.4235/agmr.22.0096
Kullak-Ublick, G. A., Andrade, R. J., Merz, M., End, P., Benesic, A., Gerbes, A. L., et al. (2017). Drug-induced liver injury: Recent advances in diagnosis and risk assessment. Gut 66, 1154–1164. doi:10.1136/gutjnl-2016-313369
Lee, B. T., Odin, J. A., and Grewal, P. (2021). An approach to drug-induced liver injury from the geriatric perspective. Curr. Gastroenterol. Rep. 23, 6. doi:10.1007/s11894-021-00804-7
Li, Z.-B., Chen, D.-D., He, Q.-J., Li, L., Zhou, G., Fu, Y.-M., et al. (2021). The lac score indicates significant fibrosis in patients with chronic drug-induced liver injury: A large biopsy-based study. Front. Pharmacol. 12, 734090. doi:10.3389/fphar.2021.734090
Lindor, K. D., Kowdley, K. V., and Harrison, E. M.American College of Gastroenterology (2015). ACG clinical guideline: Primary sclerosing cholangitis. Official J. Am. Coll. Gastroenterology | ACG 110, 646–659. doi:10.1038/ajg.2015.112
Liu, W., Zeng, X., Liu, Y., Liu, J., Li, C., Chen, L., et al. (2021). The immunological mechanisms and immune-based biomarkers of drug-induced liver injury. Front. Pharmacol. 12, 723940. doi:10.3389/fphar.2021.723940
Lucena, M. I., Andrade, R. J., Kaplowitz, N., García-Cortes, M., Fernández, M. C., Romero-Gomez, M., et al. (2009). Phenotypic characterization of idiosyncratic drug-induced liver injury: The influence of age and sex. Hepatology 49, 2001–2009. doi:10.1002/hep.22895
Marcum, Z. A., Vande Griend, J. P., and Linnebur, S. A. (2012). FDA drug safety communications: A narrative review and clinical considerations for older adults. Am. J. Geriatric Pharmacother. 10, 264–271. doi:10.1016/j.amjopharm.2012.05.002
Medina-Caliz, I., Robles-Diaz, M., Garcia-Muñoz, B., Stephens, C., Ortega-Alonso, A., Garcia-Cortes, M., et al. (2016). Definition and risk factors for chronicity following acute idiosyncratic drug-induced liver injury. J. Hepatol. 65, 532–542. doi:10.1016/j.jhep.2016.05.003
Mosedale, M., and Watkins, P. B. (2017). Drug-induced liver injury: Advances in mechanistic understanding that will inform risk management. Clin. Pharmacol. Ther. 101, 469–480. doi:10.1002/cpt.564
Moulton, V. R. (2018). Sex hormones in acquired immunity and autoimmune disease. Front. Immunol. 9, 2279. doi:10.3389/fimmu.2018.02279
Navarro, V. J., Barnhart, H., Bonkovsky, H. L., Davern, T., Fontana, R. J., Grant, L., et al. (2014). Liver injury from herbals and dietary supplements in the U.S. Drug-induced liver injury Network: HEPATOLOGY, vol. XX, No. X, 2014 NAVARRO ET AL. Hepatology 60, 1399–1408. doi:10.1002/hep.27317
Ostapowicz, G., Fontana, R. J., Schiodt, F. V., Larson, A., Davern, T. J., Han, S. H. B., et al. (2002). Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann. Intern Med. 137, 947–954. doi:10.7326/0003-4819-137-12-200212170-00007
Raschi, E., and De Ponti, F. (2017). Drug-induced liver injury: Towards early prediction and risk stratification. World J. Hepatol. 9, 30–37. doi:10.4254/wjh.v9.i1.30
Sakiani, S., Olsen, N. J., and Kovacs, W. J. (2013). Gonadal steroids and humoral immunity. Nat. Rev. Endocrinol. 9, 56–62. doi:10.1038/nrendo.2012.206
Santos, G., Gasca, J., Parana, R., Nunes, V., Schinnoni, M., Medina-Caliz, I., et al. (2021). Profile of herbal and dietary supplements induced liver injury in Latin America: A systematic review of published reports. Phytother. Res. 35, 6–19. doi:10.1002/ptr.6746
Scheuer, P. J. (1991). Classification of chronic viral hepatitis: A need for reassessment. J. Hepatol. 13, 372–374. doi:10.1016/0168-8278(91)90084-O
Singal, A. K., Bataller, R., Ahn, J., Kamath, P. S., and Shah, V. H. (2018). ACG clinical guideline: Alcoholic liver disease. Am. J. Gastroenterol. 113, 175–194. doi:10.1038/ajg.2017.469
Stevens, J. L., and Baker, T. K. (2009). The future of drug safety testing: Expanding the view and narrowing the focus. Drug Discov. Today 14, 162–167. doi:10.1016/j.drudis.2008.11.009
Tajiri, K., and Shimizu, Y. (2013). Liver physiology and liver diseases in the elderly. World J. Gastroenterol. 19, 8459–8467. doi:10.3748/wjg.v19.i46.8459
Te, H., and Doucette, K. (2019). Viral hepatitis: Guidelines by the American society of transplantation infectious disease community of Practice. Clin. Transpl. 33, e13514. doi:10.1111/ctr.13514
Teschke, R., Schwarzenboeck, A., Eickhoff, A., Frenzel, C., Wolff, A., and Schulze, J. (2013). Clinical and causality assessment in herbal hepatotoxicity. Expert Opin. Drug Saf. 12, 339–366. doi:10.1517/14740338.2013.774371
Tian, F., Chen, Z., and Wu, J. (2022). Prevalence of polypharmacy and potentially inappropriate medications use in elderly Chinese patients: A systematic review and meta-analysis. Front. Pharmacol. 13, 862561. doi:10.3389/fphar.2022.862561
Tostmann, A., Boeree, M. J., Aarnoutse, R. E., de Lange, W. C. M., van der Ven, A. J. A. M., and Dekhuijzen, R. (2008). Antituberculosis drug-induced hepatotoxicity: Concise up-to-date review. J. Gastroen Hepatol. 23, 192–202. doi:10.1111/j.1440-1746.2007.05207.x
Wagner, K.-H., Shiels, R. G., Lang, C. A., Seyed Khoei, N., and Bulmer, A. C. (2018). Diagnostic criteria and contributors to Gilbert’s syndrome. Crit. Rev. Cl. Lab. Sci. 55, 129–139. doi:10.1080/10408363.2018.1428526
Wai, C.-T., Tan, B.-H., Chan, C.-L., Sutedja, D. S., Lee, Y.-M., Khor, C., et al. (2007). Drug-induced liver injury at an asian center: A prospective study. Liver Int. 27, 465–474. doi:10.1111/j.1478-3231.2007.01461.x
Wang, C., Deng, Y., Li, P., Zheng, S., Chen, G., Zhou, G., et al. (2022). Prediction of biochemical nonresolution in patients with chronic drug-induced liver injury: A large multicenter study. Hepatology 75, 1373–1385. doi:10.1002/hep.32283
Wang, Q., Huang, A., Wang, J.-B., and Zou, Z. (2021). Chronic drug-induced liver injury: Updates and future challenges. Front. Pharmacol. 12, 627133. doi:10.3389/fphar.2021.627133
Weaver, R. J., Blomme, E. A., Chadwick, A. E., Copple, I. M., Gerets, H. H. J., Goldring, C. E., et al. (2020). Managing the challenge of drug-induced liver injury: A roadmap for the development and deployment of preclinical predictive models. Nat. Rev. Drug Discov. 19, 131–148. doi:10.1038/s41573-019-0048-x
Weersink, R. A., Alvarez-Alvarez, I., Medina-Cáliz, I., Sanabria-Cabrera, J., Robles-Díaz, M., Ortega-Alonso, A., et al. (2021). Clinical characteristics and outcome of drug-induced liver injury in the older patients: From the young-old to the oldest-old. Clin. Pharmacol. Ther. 109, 1147–1158. doi:10.1002/cpt.2108
Yeong, T. T., Lim, K. H. J., Goubet, S., Parnell, N., and Verma, S. (2016). Natural history and outcomes in drug-induced autoimmune hepatitis: Drug-induced autoimmune hepatitis. Hepatol. Res. 46, E79–E88. doi:10.1111/hepr.12532
Keywords: drug-induced liver injury (DILI), elderly, hepatic fibrosis, autoimmunity, liver biopsy
Citation: Xiong Y-T, Wang J-F, Niu X-X, Fu Y-M, Wang K-X, Wang C-Y, Li Q-Q, Wang J-J, Zhao J and Ji D (2023) Autoimmunity associates with severity of illness in elderly patients with drug-induced liver injury. Front. Pharmacol. 14:1071709. doi: 10.3389/fphar.2023.1071709
Received: 16 October 2022; Accepted: 06 February 2023;
Published: 16 February 2023.
Edited by:
Daniela Gabbia, University of Padova, ItalyReviewed by:
Chenghai Liu, Shanghai University of Traditional Chinese Medicine, ChinaYangliu Xia, Dalian University of Technology, China
Copyright © 2023 Xiong, Wang, Niu, Fu, Wang, Wang, Li, Wang, Zhao and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Ji, amlkZzMwMkAxMjYuY29t; Jun Zhao, emhqNjhAMjYzLm5ldA==
†These authors have contributed equally to this work
 Yu-Ting Xiong
Yu-Ting Xiong Jian-Fei Wang3†
Jian-Fei Wang3† Yi-Ming Fu
Yi-Ming Fu Dong Ji
Dong Ji