- 1Department of Pulmonary and Critical Care Medicine, The First Affiliated Hospital with Nanjing Medical University, Nanjing, China
- 2ChosenMed Technology (Beijing) Co, Ltd, Beijing, China
- 3Computer Network Information Center, Chinese Academy of Sciences, Beijing, China
- 4University of the Chinese Academy of Sciences, Beijing, China
- 5Jiangsu Shengze Hospital, Suzhou, China
Insertions in exon 20 represent the third most common type of EGFR mutation following in-frame deletions in exon 19 and the point mutation L858R in exon 21. They are generally associated with primary resistance to EGFR-TKIs. Although mobocertinib and amivantamab were approved for adult patients with non-small cell lung cancer (NSCLC) harboring EGFR exon 20 insertion mutations, the efficacy of these two agents was rather moderate. Therefore, other more potent targeted agents are urgently needed. Here, we report a patient with advanced lung adenocarcinoma harboring an EGFR exon 20 insertion mutation (NM_005228: exon 20: c.2316_2321dup: p.773_774dup). After experiencing platinum-based chemotherapy, this patient received a combination of furmonertinib and anlotinib and achieved lasting stable disease (SD). The treatment was well tolerated, and only mild hand-foot syndrome was reported from the patient. To the best of our knowledge, this case firstly reported the encouraging efficacy of combined furmonertinib and anlotinib in an advanced lung adenocarcinoma patient with an EGFR exon 20 insertion mutation who was previously treated with platinum-based chemotherapy. In addition, we summarize the recent literature on therapies against NSCLC with EGFR exon 20 insertion mutations. This case might provide an alternative approach for clinical oncologists.
Background
Over the past decade, the development of EGFR tyrosine kinase inhibitors (TKIs) has revolutionized the therapy of non-small cell lung cancer (NSCLC) with mutated epidermal growth factor receptor (EGFR) gene (Zhou et al., 2015; Soria et al., 2018). However, patients with NSCLC harboring EGFR exon 20 insertions (EGFR ex20ins) mutations, accounting for approximately 10% of all EGFR-positive NSCLC cases, usually show de novo resistance to the approved EGFR-TKIs (Yasuda et al., 2012).
The incidence of EGFR p.773_774dup is very low, accounting for approximately 2.22% of EGFR ex20ins in China (Shun Xu, 2022). In vitro studies showed that compared with other EGFR ex20ins, EGFR p.773_774dup was the least sensitive to the first-generation or second-generation EGFR-TKIs. Furthermore, a clinical study demonstrated that the overall response rate (ORR) was 0% for the patients with EGFR p.773_774dup mutation, while 17% for the patients with EGFR ex20ins variations when receiving the first-generation EGFR-TKIs (Kobayashi and Mitsudomi, 2016).
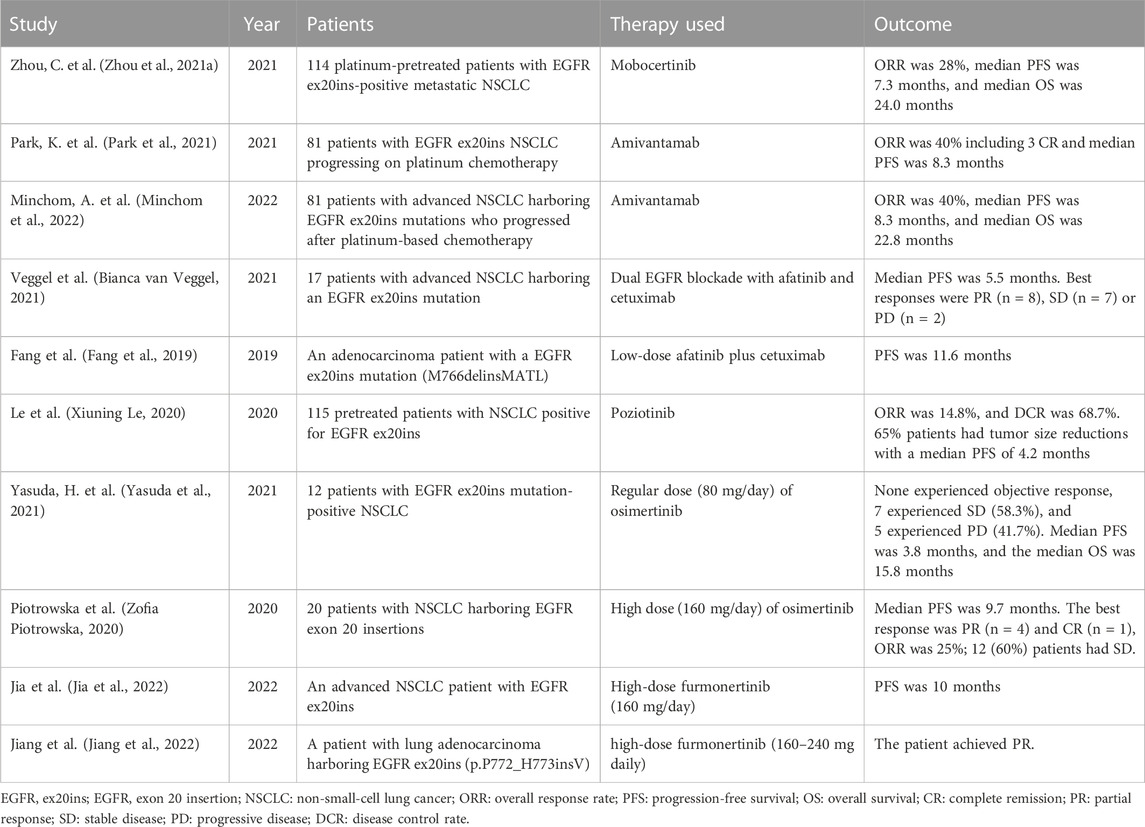
Recently, much progress has been made against NSCLCs with EGFR ex20ins mutations (Meador et al., 2021). For example, the Food and Drug Administration (FDA) of U.S. approved two novel agents (mobocertinib and amivantamab) for adult patients with NSCLC who harbor EGFR ex20ins mutations in 2021. However, the efficacy of these two agents was rather moderate compared with TKIs targeting canonical EGFR mutations (Zhou C. et al., 2021; Park et al., 2021; Minchom et al., 2022). Consequently, other more potent therapeutic strategies are urgently needed.
Here, we report a case of pretreated advanced lung adenocarcinoma with EGFR ex20ins (NM_005228: exon 20:c.2316_2321dup: p.773_774dup) benefiting from combined furmonertinib, a novel third-generation EGFR-TKI, with anlotinib, a novel multi-targeting tyrosine kinase inhibitor, for a long time.
Case presentation
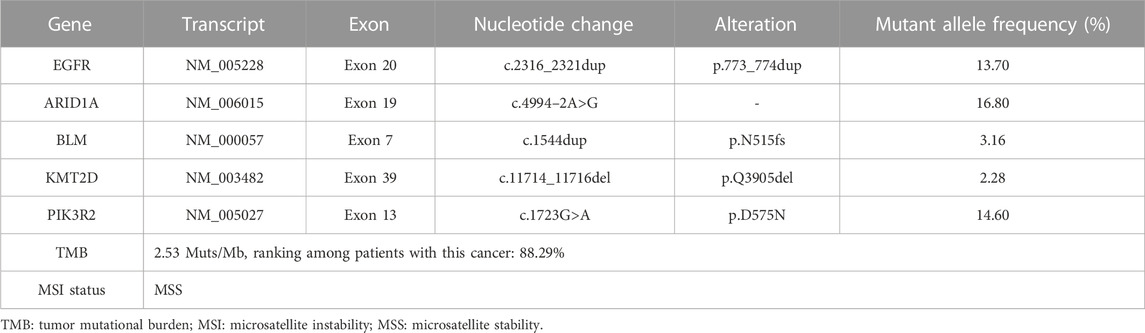
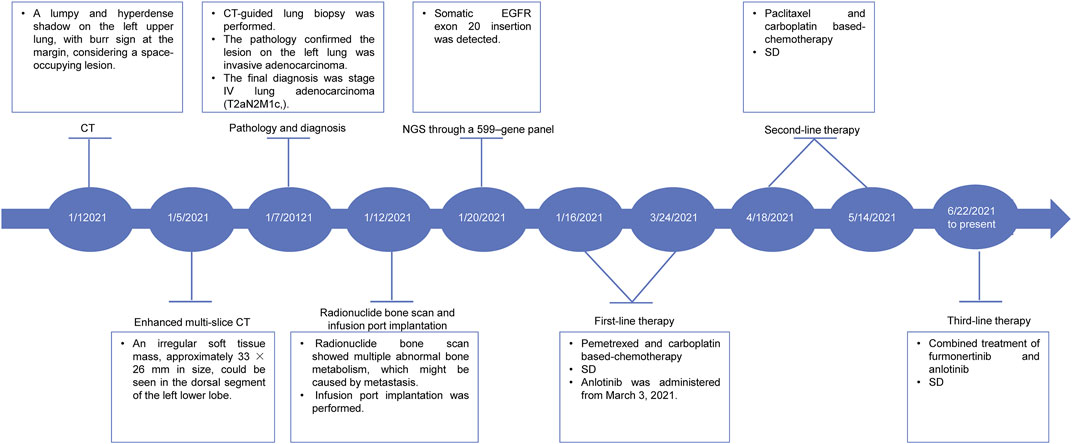
On 01 January 2021, a 68-year-old male was admitted to a local hospital due to chest and back pain on the left side. The patient had a 30-year smoking history (20 cigarettes per day) and quit it 10 years ago. He had no history of drinking. Thoracic computed tomography (CT) demonstrated a lumpy and hyper-density shadow on the left upper lung, considered a space-occupying lesion. The patient was referred to our department on 5 January 2021 for enhanced multi-slice CT scan, which showed multiple bilateral pulmonary nodules. The largest one was approximately 7 mm in diameter. Furthermore, an irregular soft tissue mass, measuring approximately 33 × 26 mm in size, could be seen in the dorsal segment of the left lower lobe. After contraindications were excluded, CT-guided lung biopsy was performed on 7 January 2021. The pathology confirmed that the lesion on the left lung was moderate-differentiated adenocarcinoma (Figure 1A). Immunohistochemistry (IHC) showed positive expression of thyroid transcription factor 1 (TTF1) and Napsin A (Figure 1B). The final diagnosis was stage IV lung adenocarcinoma (T2aN2M1c). On 12 January 2021, a radionuclide bone scan showed multiple abnormal bone metabolism (data not shown), which might be caused by metastasis. After fully communicating with the patient and his family members, the infusion port implantation was performed on 12 January 2021. Meanwhile, paraffin-embedded sections of tumor tissues from the patient were subjected to next-generation sequencing (NGS) through a 599-gene panel (ChosenMed Technology [Beijing] Co. Ltd., Beijing, China). The results showed that the patient harbored a non-frameshift insertion mutation in EGFR (NM_005228: exon 20:c.2316_2321dup: p.773_774dup), with variant allele fraction (VAF) 13.70%. The sequencing reads of EGFR are demonstrated in Figure 1C. The somatic alterations in the patient are shown in Table 1.

FIGURE 1. Pathology, the sequencing reads of EGFR p.773_774dup, dynamic imaging of the space-occupying lesion on the left lung during the treatment. (A) Pathological findings revealed the lesion on the left lung was moderate-differentiated adenocarcinoma. H.E.: hematoxylin-eosin staining. Scale bars represent 250 μm (left) and 100 μm (right), respectively. (B) Immunohistochemistry (IHC) showed positive expression of thyroid transcription factor 1 (TTF1) and Napsin A. Scale bars represent 250 μm (left and right). (C) Visualization of EGFR p.773_774dup mutation using the Integrated Genomics Viewer (IGV) browser. (D) CT images showed that the patient’s condition remained stable after receiving furmonertinib plus anlotinib. CT: computed tomography.
Subsequently, two cycles of pemetrexed (0.8 g on day 1, repeated every 3 weeks) and carboplatin (0.5 g on day 1, repeated every 3 weeks) based-chemotherapy was administered on January 16 and 6 February 2021. During the third-cycle treatment, the lesion was similar to the previous one, and the patient’s condition was assessed as a stable disease (SD). Therefore, the patient continued to receive another two cycles of the abovementioned chemotherapy on March 2 and 24 March 2021. Moreover, anlotinib (12 mg, once a day) was supplemented from 3 March 2021. After four cycles of treatment, the patient’s condition remained stable. Considering that the lesion did not shrink remarkably, the treatment strategy was switched to two cycles of paclitaxel (0.24 g on day 1, repeated every 3 weeks) and carboplatin (0.5 g on day 1, repeated every 3 weeks) based-chemotherapy on April 18 and 14 May 2021. The patient’s condition was accessed as SD on 22 June 2021. However, the patient refused to continue the chemotherapy because of chest pain. Therefore, targeted therapy with the combination of furmonertinib (160 mg, once a day) and anlotinib (12 mg, once a day) was administered from 22 June 2021. The treatment was well tolerated, and only mild hand-foot syndrome was observed (data not shown). According to the latest follow-up on 30 May 2022, the patient’s condition was evaluated as a SD based on thoracic CT (Figure 1D). The patient achieved a long-term SD after receiving furmonertinib and anlotinib. The case timeline is shown as Figure 2.
Discussion
To the best of our knowledge, we are the first to describe a case of pretreated advanced lung adenocarcinoma with EGFR ex20ins benefiting from the combined furmonertinib and anlotinib for a long time.
Unlike canonical EGFR mutations, i.e., EGFR 19-del and 21-L858R mutations, patients with EGFR ex20ins are not sensitive to most types of EGFR-TKIs and have shorter overall survival (OS) (Oxnard et al., 2013). The underlying mechanism is that EGFR ex20ins mutation alters the conformation at the kinase active site, limiting the binding of early-generation EGFR-TKIs (Robichaux et al., 2018; Vyse and Huang, 2019). Over the past few years, the standard therapy of EGFR ex20ins has been platinum-based chemotherapy. Recently, two novel agents have been approved for the advanced NSCLC with EGFR ex20ins. One is mobocertinib (TAK-788), which is a targeted inhibitor of EGFR ex20ins (Gonzalvez et al., 2021). The other is amivantamab (JNJ-61186372), which is a bispecific antibody targeting EGFR and MET (Yun et al., 2020). According to previous studies, the median progression-free survival (PFS) with mobocertinib and amivantamab was 7.3 months, 8.3 months, respectively, and the median OS was 24.0, 22.8 months, respectively (Zhou C. et al., 2021; Park et al., 2021; Minchom et al., 2022) (Table 2). Obviously, the efficacy of these two agents is unsatisfactory. Therefore, other treatment strategies are being explored. For example, a study by Veggel et al. demonstrated the combination of afatinib with cetuximab showed moderate efficacy in patients with EGFR ex20ins-positive NSCLC, with a median PFS of 5.3 months (Bianca van Veggel, 2021) (Table 2). In addition, poziotinib is a potent TKI of EGFR and HER2 exon 20 insertion mutants. The investigation (ZENITH20-1) of Le et al. evaluated the efficacy of poziotinib in pretreated patients with NSCLC harboring EGFR ex20ins. The results demonstrated that the ORR was 14.8%, and the disease control rate (DCR) was 68.7%, with a median PFS of 4.2 months (Xiuning Le, 2020) (Table 2).
Meanwhile, scholars also studied the efficacy of third-generation EGFR-TKIs in the patients with NSCLC harboring EGFR ex20ins. They found that a regular dose (80 mg/day) of osimertinib has limited efficacy in these patients, with a median PFS of 3.8 months, and a median OS of 15.8 months (Yasuda et al., 2021). However, high dose (160 mg/day) of osimertinib showed promising clinical activity, with a median PFS of 9.7 months (Zofia Piotrowska, 2020) (Table 2). As a novel third-generation EGFR-TKI, furmonertinib (alflutinib/AST2818) has been approved by the National Medical Products Administration (NMPA) of China for pretreated patients with NSCLC harboring an EGFR T790M mutation. Moreover, preclinical data demonstrated that furmonertinib had an antitumor effect in Ba/F3 cell line expressing EGFR ex20ins, patient-derived xenograft models harboring EGFR ex20ins mutation and treatment-naïve NSCLC patients with EGFR ex20ins (Baohui Han, 2021; Das et al., 2021). In 2022, Jia et al. reported an advanced NSCLC patient with EGFR ex20ins benefited from high-dose furmonertinib (160 mg/day) after progression from mobocertinib, with a PFS of 10 months (Jia et al., 2022) (Table 2). Meanwhile, Jiang et al. also report a patient with lung adenocarcinoma harboring EGFR ex20ins who achieved disease control after receiving high-dose furmonertinib (160–240 mg daily, Table 2) (Jiang et al., 2022). Besides, clinical trials like DZD9008 and CLN-081 against EGFR exon 20 insertion–positive NSCLC are ongoing (James Chih-Hsin Yang, 2021; Zofia Piotrowska, 2021).
The antitumor activity of furmonertinib is similar to osimertinib in patients with advanced NSCLC positive for EGFR T790M mutation. However, the incidence of skin and gastrointestinal disorders with furmonertinib is lower.
Anlotinib is a novel multi-targeting receptor tyrosine kinase (RTK) inhibitor against vascular endothelial growth factor receptor, fibroblast growth factor receptor, platelet-derived growth factor receptors, and c-kit. Previous studies showed anlotinib could inhibit not only tumor angiogenesis, but also tumor cell proliferation (Sebastien Taurin, 2017; Xie et al., 2018). On 8 May 2018, anlotinib was approved as a third-line treatment for patients with advanced NSCLC in China. In addition, anlotinib can remarkably prolong the median PFS in patients with advanced soft tissue sarcoma, medullary thyroid carcinoma and metastatic renal cell carcinoma. Clinical trials have confirmed that anti-angiogenesis agents like bevacizumab and ramucirumab combined with EGFR-TKI could significantly prolong the survival of patients with EGFR-mutant NSCLC (Herbst et al., 2011; Nakagawa et al., 2019; Ninomiya et al., 2019; Zhou Q. et al., 2021). The mechanism might be that anti-angiogenic therapy can transiently restore the effective antitumor immunity, normalize the tumor vessels network, and improve drug delivery and efficacy (Jain et al., 2007; Feng et al., 2018). Compared to bevacizumab and ramucirumab, anlotinib is more convenient, as it is orally administered and it can inhibit more targets. Furthermore, several clinical trials have demonstrated the encouraging efficacy and safety of combined anlotinib with EGFR-TKI for previously untreated, EGFR-mutated advanced NSCLC patients (Dingzhi Huang, 2020; D. Zhong, 2020; Chu et al., 2022). In addition, a clinical trial of anlotinib combined with furmonertinib is ongoing as the first-line treatment in patients with EGFR mutation-positive locally advanced or metastatic NSCLC (NCT04895930). Based on the above, we hypothesized that a combination of anlotinib and furmonertinib could potentially improve the efficacy for EGFR ex20ins-positive advanced NSCLC patients. In addition, the anti-angiogenic activity of anlotinib is stronger than that of three other anti-angiogenesis drugs, including sunitinib, sorafenib, and nintedanib, while it has fewer or milder grade 3 or higher adverse effects (Shen et al., 2018).
Of note, although the patient harbored an ARID1A mutation (c.4994–2A >G) which might be a positive predictor of immune checkpoint Inhibitor (ICI) therapy (Zhu et al., 2021). However, the mutation in the gene EGFR (c.2316_2321dup: p.773_774dup) was supposed to be associated with drug resistance or hyperprogression when receiving ICI therapy (Borghaei et al., 2015; Herbst et al., 2016; Lee et al., 2017; Rittmeyer et al., 2017; Girard et al., 2022). Furthermore, other ICI biomarkers including the low-TMB (2.53 Muts/Mb), MSS status and low PD-L1 expression (22C3: TPS< 1%, CPS< 1) through immunohistochemistry also predict poor clinical outcome of ICI therapy. Therefore, we chose to combine two TKI inhibitors over combination of a TKI with ICI.
To date, this case firstly demonstrates improved efficacy and tolerability of NSCLC patients with EGFR ex20ins when receiving furmonertinib combined with anlotinib. However, the underlying mechanism needs to be clarified in the near future.
Conclusion
To the best of our knowledge, we are the first to report that the combination of furmonertinib and anlotinib is an effective treatment strategy in a patient with EGFR ex20ins-positive advanced lung adenocarcinoma who was previously treated with platinum-based chemotherapy. This case might serve as an alternative approach for clinical oncologists.
Data availability statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the patient for the participation and the publication of clinical details and images. This work was approved by the Ethics Committee of The First Affiliated Hospital with Nanjing Medical University.
Author contributions
Conceptualization: XQ. Attending physician for the patient: XC and WZ. Case identification: MS. Data collection: SC. Manuscript writing: NM. Manuscript revision: BN.
Funding
This work was supported by General Project Fund of Department of Health, Jiangsu Province (No. H2019029).
Acknowledgments
The authors wish to thank the patient for his participation.
Conflict of interest
Authors NM, SC, and BN, were employed by the company ChosenMed Technology (Beijing) Co, Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Baohui Han, C. Z., Wu, L., Yu, X., Li, Q., Liu, F., and Shen, C. (2021). Preclinical and preliminary clinical investigations of Furmomertinib in NSCLC with EGFR exon 20 insertions (20ins). Lugano, Switzerland; ESMO.
Bianca van Veggel, A. J. V. D. W., Paats, M., Hashemi, S. M., Hendriks, L., KarolinaSikorska, D. V. D. B., Kim, M., et al. (2021). Interim results of a phase II single arm trial combining afatinib with cetuximab inpatients with EGFRex20ins positive NSCLC. Virginia, United States; ASCO.
Borghaei, H., Paz-Ares, L., Horn, L., Spigel, D. R., Steins, M., Ready, N. E., et al. (2015). Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. The New England Journal of Medicine. 373 (17), 1627–1639. doi:10.1056/NEJMoa1507643
Chu, T., Zhang, W., Zhang, B., Zhong, R., Zhang, X., Gu, A., et al. (2022). Efficacy and safety of first-line anlotinib-based combinations for advanced non-small cell lung cancer: A three-armed prospective study. Translational Lung Cancer Research. 11 (7), 1394–1404. doi:10.21037/tlcr-22-438
Das, D., Wang, J., and Hong, J. (2021). Next-generation kinase inhibitors targeting specific biomarkers in non-small cell lung cancer (NSCLC): A recent overview. ChemMedChem 16 (16), 2459–2479. doi:10.1002/cmdc.202100166
Dingzhi Huang, D. Z., Zhang, C., Zhang, Y., Shang, Y., and Wang, L. (2020). Study of anlotinib combined with icotinib as the first-line treatment in non-small cell lung cancer (NSCLC) patients harboring activating EGFR mutations (ALTER-L004). Journal of Clinical Oncology. 38 (15). _suppl doi:10.1200/JCO.2020.38.15_suppl.9573
Fang, W., Huang, Y., Gan, J., Shao, Y. W., and Zhang, L. (2019). Durable response of low-dose afatinib plus cetuximab in an adenocarcinoma patient with a novel EGFR exon 20 insertion mutation. Journal of Thoracic Oncology. 14 (10), e220–e221. doi:10.1016/j.jtho.2019.05.023
Feng, P. H., Chen, K. Y., Huang, Y. C., Luo, C. S., Wu, S. M., Chen, T. T., et al. (2018). Bevacizumab reduces S100a9-positive MDSCs linked to intracranial control in patients with EGFR-mutant lung adenocarcinoma. Journal of Thoracic Oncology. 13 (7), 958–967. doi:10.1016/j.jtho.2018.03.032
Girard, N., Minchom, A., Ou, S. I., Gadgeel, S. M., Trigo, J., Viteri, S., et al. (2022). Comparative clinical outcomes between EGFR Ex20ins and wildtype NSCLC treated with immune checkpoint inhibitors. Clinical Lung Cancer 23 (7), 571–577. doi:10.1016/j.cllc.2022.07.007
Gonzalvez, F., Vincent, S., Baker, T. E., Gould, A. E., Li, S., Wardwell, S. D., et al. (2021). Mobocertinib (TAK-788): A targeted inhibitor of EGFR exon 20 insertion mutants in non-small cell lung cancer. Cancer Discovery. 11 (7), 1672–1687. doi:10.1158/2159-8290.CD-20-1683
Herbst, R. S., Ansari, R., Bustin, F., Flynn, P., Hart, L., Otterson, G. A., et al. (2011). Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): A double-blind, placebo-controlled, phase 3 trial. Lancet 377 (9780), 1846–1854. doi:10.1016/S0140-6736(11)60545-X
Herbst, R. S., Baas, P., Kim, D. W., Felip, E., Perez-Gracia, J. L., Han, J. Y., et al. (2016). Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 387 (10027), 1540–1550. doi:10.1016/S0140-6736(15)01281-7
Jain, R. K., Di Tomaso, E., Duda, D. G., Loeffler, J. S., Sorensen, A. G., and Batchelor, T. T. (2007). Angiogenesis in brain tumours. Nature Reviews Neuroscience. 8 (8), 610–622. doi:10.1038/nrn2175
James Chih-Hsin Yang, M. W., Mitchell, P., Fang, J., Nian, W., Chiu, C.-H., Zhou, J., et al. (2021). Preliminary safety and efficacy results from phase 1 studies of DZD9008 in NSCLC patients with EGFR Exon20 insertion mutations. Journal of Clinical Oncology. 39 (15). 9008. _suppl.doi:10.1200/JCO.2021.39.15_suppl.9008
Jia, K., Yang, S., Chen, B., Yu, J., Wu, Y., Li, W., et al. (2022). Advanced lung adenocarcinoma patient with EGFR exon 20 insertion benefits from high-dose furmonertinib for nine months after progression from mobocertinib: A case report. Annals of Translational Medicine. 10 (6), 386. doi:10.21037/atm-22-1167
Jiang, W., Sha, M., and Chen, C. (2022). Successful salvage therapy with a high dose of furmonertinib in a case of lung adenocarcinoma harboring EGFR exon 20 insertion. American Journal of Therapeutics 0 (0), 1–3. doi:10.1097/MJT.0000000000001504
Kobayashi, Y., and Mitsudomi, T. (2016). Not all epidermal growth factor receptor mutations in lung cancer are created equal: Perspectives for individualized treatment strategy. Cancer Science. 107 (9), 1179–1186. doi:10.1111/cas.12996
Lee, C. K., Man, J., Lord, S., Links, M., Gebski, V., Mok, T., et al. (2017). Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer-A meta-analysis. Journal of Thoracic Oncology. 12 (2), 403–407. doi:10.1016/j.jtho.2016.10.007
Meador, C. B., Sequist, L. V., and Piotrowska, Z. (2021). Targeting EGFR exon 20 insertions in non-small cell lung cancer: Recent advances and clinical updates. Cancer Discovery. 11 (9), 2145–2157. doi:10.1158/2159-8290.CD-21-0226
Minchom, A., Viteri, S., Bazhenova, L., Gadgeel, S. M., Ou, S. I., Trigo, J., et al. (2022). Amivantamab compared with real-world therapies in patients with advanced non-small cell lung cancer harboring EGFR exon 20 insertion mutations who progressed after platinum-based chemotherapy. Lung Cancer 168, 74–82. doi:10.1016/j.lungcan.2022.03.005
Nakagawa, K., Garon, E. B., Seto, T., Nishio, M., Ponce Aix, S., Paz-Ares, L., et al. (2019). Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): A randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet Oncology. 20 (12), 1655–1669. doi:10.1016/S1470-2045(19)30634-5
Ninomiya, T., Ishikawa, N., Inoue, K., Kubo, T., Yasugi, M., Shibayama, T., et al. (2019). Phase 2 study of afatinib alone or combined with bevacizumab in chemonaive patients with advanced non-small-cell lung cancer harboring EGFR mutations: AfaBev-CS study protocol. Clinical Lung Cancer. 20 (2), 134–138. doi:10.1016/j.cllc.2018.10.008
Oxnard, G. R., Lo, P. C., Nishino, M., Dahlberg, S. E., Lindeman, N. I., Butaney, M., et al. (2013). Natural history and molecular characteristics of lung cancers harboring EGFR exon 20 insertions. Journal of Thoracic Oncology. 8 (2), 179–184. doi:10.1097/JTO.0b013e3182779d18
Park, K., Haura, E. B., Leighl, N. B., Mitchell, P., Shu, C. A., Girard, N., et al. (2021). Amivantamab in EGFR exon 20 insertion-mutated non-small-cell lung cancer progressing on platinum chemotherapy: Initial results from the CHRYSALIS phase I study. Journal of Clinical Oncology. 39 (30), 3391–3402. doi:10.1200/JCO.21.00662
Piotrowska, Z., Helena Alexandra, Y., James Chih-Hsin Yang, , Koczywas, M., Smit, E. F., Daniel Shao, W. T., et al. (2021). Safety and activity of CLN-081 (TAS6417) in NSCLC with EGFR Exon 20 insertion mutations (Ins20). Journal of Clinical Oncology. 39 (15). suppl.doi:10.1200/JCO.2021.39.15_suppl.9077
Rittmeyer, A., Barlesi, F., Waterkamp, D., Park, K., Ciardiello, F., Von Pawel, J., et al. (2017). Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 389 (10066), 255–265. doi:10.1016/S0140-6736(16)32517-X
Robichaux, J. P., Elamin, Y. Y., Tan, Z., Carter, B. W., Zhang, S., Liu, S., et al. (2018). Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer. Nature Medicine. 24 (5), 638–646. doi:10.1038/s41591-018-0007-9
Sebastien Taurin, C.-H. Y., Reyes, M., Cho, S., Jarboe, E. A., Werner, T. L., Coombs, D. M., et al. (2017). Abstract 3244: Treatment of endometrial cancer cells with a new small tyrosine kinase inhibitor targeting mutated fibroblast growth factor receptor-2. AACR 77 (13_Supplement), 3244. doi:10.1158/1538-7445.AM2017-3244
Shen, G., Zheng, F., Ren, D., Du, F., Dong, Q., Wang, Z., et al. (2018). Anlotinib: A novel multi-targeting tyrosine kinase inhibitor in clinical development. Journal of Hematology and Oncology. 11 (1), 120. doi:10.1186/s13045-018-0664-7
Shun Xu, Z. F. (2022). The landscape of EGFR exon 20 insertion mutations in Chinese patients with non-small cell lung cancer. AACR. 5838, 82, doi:10.1158/1538-7445.AM2022-5838
Soria, J. C., Ohe, Y., Vansteenkiste, J., Reungwetwattana, T., Chewaskulyong, B., Lee, K. H., et al. (2018). Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. The New England Journal of Medicine. 378 (2), 113–125. doi:10.1056/NEJMoa1713137
Vyse, S., and Huang, P. H. (2019). Targeting EGFR exon 20 insertion mutations in non-small cell lung cancer. Signal Transduction and Targeted Therapy. 4, 5. doi:10.1038/s41392-019-0038-9
Xie, C., Wan, X., Quan, H., Zheng, M., Fu, L., Li, Y., et al. (2018). Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor. Cancer Science. 109 (4), 1207–1219. doi:10.1111/cas.13536
Xiuning Le, J. W. G., Clarke, J. M., Tchekmedyian, N., Piotrowska, Z., and Chu, D. (2020). Poziotinib shows activity and durability of responses in subgroups of previously treated EGFR exon 20 NSCLC patients. Virginia, United States; ASCO.
Yasuda, H., Ichihara, E., Sakakibara-Konishi, J., Zenke, Y., Takeuchi, S., Morise, M., et al. (2021). A phase I/II study of osimertinib in EGFR exon 20 insertion mutation-positive non-small cell lung cancer. Lung Cancer 162, 140–146. doi:10.1016/j.lungcan.2021.10.006
Yasuda, H., Kobayashi, S., and Costa, D. B. (2012). EGFR exon 20 insertion mutations in non-small-cell lung cancer: Preclinical data and clinical implications. The Lancet Oncology. 13 (1), e23–e31. doi:10.1016/S1470-2045(11)70129-2
Yun, J., Lee, S. H., Kim, S. Y., Jeong, S. Y., Kim, J. H., Pyo, K. H., et al. (2020). Antitumor activity of amivantamab (JNJ-61186372), an EGFR-MET bispecific antibody, in diverse models of EGFR exon 20 insertion-driven NSCLC. Cancer Discovery. 10 (8), 1194–1209. doi:10.1158/2159-8290.CD-20-0116
Zhong, D., Zhang, C., Zhang, Y., Shang, Y., Wang, L., and Huang, D. (2020). Anlotinib combined with icotinib provides a promising first-line treatment option for EGFR positive NSCLC patients harboring concomitant mutations: Exploratory analysis of the ALTER-L004 study. Annals of Oncology. 31 (S4), 1. doi:10.1016/j.annonc.2020.08.1656
Zhou, C., Ramalingam, S. S., Kim, T. M., Kim, S. W., Yang, J. C., Riely, G. J., et al. (2021a). Treatment outcomes and safety of mobocertinib in platinum-pretreated patients with EGFR exon 20 insertion-positive metastatic non-small cell lung cancer: A phase 1/2 open-label nonrandomized clinical trial. JAMA Oncol. 7 (12), e214761. doi:10.1001/jamaoncol.2021.4761
Zhou, C., Wu, Y. L., Chen, G., Feng, J., Liu, X. Q., Wang, C., et al. (2015). Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Annals of Oncology. 26 (9), 1877–1883. doi:10.1093/annonc/mdv276
Zhou, Q., Xu, C. R., Cheng, Y., Liu, Y. P., Chen, G. Y., Cui, J. W., et al. (2021b). Bevacizumab plus erlotinib in Chinese patients with untreated, EGFR-mutated, advanced NSCLC (ARTEMIS-CTONG1509): A multicenter phase 3 study. Cancer Cell 39 (9), 1279–1291. e1273. doi:10.1016/j.ccell.2021.07.005
Zhu, G., Shi, R., Li, Y., Zhang, Z., Xu, S., Chen, C., et al. (2021). ARID1A, ARID1B, and ARID2 mutations serve as potential biomarkers for immune checkpoint blockade in patients with non-small cell lung cancer. Frontiers in Immunology. 12, 670040. doi:10.3389/fimmu.2021.670040
Keywords: lung adenocarcinoma, EGFR exon 20 insertions, furmonertinib, anlotinib, case report
Citation: Chen X, Zha W, Su M, Meng N, Cao S, Niu B and Qi X (2023) Persistent response of furmonertinib plus anlotinib in a lung adenocarcinoma patient with an EGFR exon 20 insertion mutation: A case report. Front. Pharmacol. 14:1053805. doi: 10.3389/fphar.2023.1053805
Received: 26 September 2022; Accepted: 23 January 2023;
Published: 03 February 2023.
Edited by:
Olivier Feron, Université catholique de Louvain, BelgiumReviewed by:
Aglaia Ntokou, Yale University, United StatesStephanie Dobersch, Fred Hutchinson Cancer Research Center, United States
Copyright © 2023 Chen, Zha, Su, Meng, Cao, Niu and Qi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xu Qi, cWl4dWx5QDE2My5jb20=
†These authors have contributed equally to this work
 Xuesong Chen1†
Xuesong Chen1† Nan Meng
Nan Meng Beifang Niu
Beifang Niu Xu Qi
Xu Qi