
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol., 13 July 2023
Sec. Drugs Outcomes Research and Policies
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1050412
 Qiong Huang1,2*
Qiong Huang1,2* Minling You1
Minling You1 Weijuan Huang1
Weijuan Huang1 Jian Chen1
Jian Chen1 Qinming Zeng1
Qinming Zeng1 Longfeng Jiang1
Longfeng Jiang1 Xiuben Du3
Xiuben Du3 Xusheng Liu2
Xusheng Liu2 Ming Hong4*
Ming Hong4* Jing Wang1*
Jing Wang1*Background: The comparative benefits and acceptability of HIF-PHIs for treating anemia have not been well researched to date. We sought to compare the effectiveness of 6 HIF-PHIs and 3 ESAs for the treatment of renal anemia patients undergoing dialysis.
Data sources: Cochrane Central Register of Controlled Trials, PubMed, Embase, Cochrane Library, MEDLINE, Web of Science, and clinicaltrials.gov databases.
Results: Twenty-five RCTs (involving 17,204 participants) were included, all of which were designed to achieve target Hb levels by adjusting thee dose of HIF-PHIs. Regarding the efficacy in achieving target Hb levels, no significant differences were found between HIF-PHIs and ESAs in Hb response at the dose-adjusted designed RCTs selected for comparison. Intervention with roxadustat showed a significantly lower risk of RBC transfusion than rhEPO, with an OR and 95% CI of 0.76 (0.56–0.93). Roxadustat and vadadustat had higher risks of increasing the discontinuation rate than ESAs; the former had ORs and 95% CIs of 1.58 (95% CI: 1.21–2.06) for rhEPO, 1.66 (1.16–2.38) for DPO (darbepoetin alfa), and 1.76 (1.70–4.49) for MPG-EPO, and the latter had ORs and 95% CIs of 1.71 (1.09–2.67) for rhEPO, 1.79 (1.29–2.49) for DPO, and 2.97 (1.62–5.46) for MPG-EPO. No differences were observed in the AEs and SAEs among patients who received the studied drugs. Results of a meta-analysis of gastrointestinal disorders among AEs revealed that vadadustat was less effect on causing diarrea than DPO, with an OR of 0.97 (95% CI, 0.9–0.99). Included HIF-PHIs, were proven to be more effective than ESAs in reducing hepcidin levels and increasing TIBC and serum iron level with OR of −0.17 (95% CI, −0.21 to −0.12), OR of 0.79 (95% CI, 0.63–0.95), and OR of 0.39 (95% CI, 0.33–0.45), respectively.
Conclusion: HIF-PHIs and ESAs have their characteristics and advantages in treating anemia undergoing dialysis. With the selected dose-adjusted mode, some HIF-PHIs appeared to be a potential treatment for DD-CKD patients when ompared with rhEPO, due to its effectiveness in decreasing the risk of RBC transfusion rate or regulating iron or lipid metabolism while achieving target Hb levels.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=306511; Identifier: CRD42022306511
Chronic kidney disease (CKD) is a growing worldwide problem. It affects the health of approximately 10% of adults, and in approximately 20 years, it will be the fifth leading cause of human death (Kalantar-Zadeh et al., 2021). As a common complication of CKD, anemia is associated with increased morbidity and mortality in patients (Coresh et al., 2007). As renal function decreases, the prevalence of anemia increases; this prevalence tends to be higher in CKD patients undergoing haemodialysis than in those who do not receive dialysis (Akizawa et al., 2018). Studies have highlighted the shortcomings of this common therapeutic approach to treating anemia in CKD patients, including insensitivity and inconvenience. However, ESA therapy targeting higher haemoglobin levels leads to increased risks for vascular and fatal events compared with therapy targeting lower levels (Palmer et al., 2010). A high dose of ESAs leads to an increased risk of vascular access thrombosis, cardiovascular and cerebrovascular events, and cancer-associated mortality (Szczech et al., 2008; Koulouridis et al., 2013). Therefore, it is meaningful to set the ‘Hb target’ to aim at ESA and to a more individualized approach, thus balancing the risk and benefits of ESA therapy (McMurray et al., 2012).
The mechanisms underlying how cells and tissues monitor and respond to oxygen levels remained unclear until the late 20th century. In 2019, three scientists (Willam Kaelin, Peter Ratcliffe, and Gregg Semenza) shared the Nobel Prize in Medicine or Physiology for discovering the hypoxia-inducible factor (HIF) oxygen-sensing pathway, which influences the erythropoietic response to hypoxia (Hurst, 2016; Fandrey et al., 2019). Cellular responses to hypoxia are regulated by the HIF-prolyl hydroxylase domain (PHD) pathway, which is related to the pathophysiology of multiple diseases, such as ischaemic diseases, anemia, polycythaemia, cancer, and pulmonary arterial hypertension (Semenza, 2011). HIF-PHIs are a new class of drugs that can activate HIF transcription factors and may have broad therapeutic potential in clinical practice (Schödel and Ratcliffe, 2019).
Because they are effective in activating erythropoietin and regulating iron metabolism, roxadustat (FG-4592), daprodustat (GSK-1278863), vadadustat (AKB-6548), enarodustat (JTZ-951), molidustat (BAY 85–3,934) and desidustat (ZYAN1) are approved for the treatment of anemia in CKD. Among these HIF-PHIs, the first four compounds are approved for marketing in Japan, and roxadustat is also approved for clinical usage in China, South Korea, the UK, Europe, and Chile. Desidustat received its first approval in India in March 2022, and enarodustat has progressed into clinical development in the United States and South Korea (Dhillon, 2019; Dhillon, 2020; Markham, 2020; Markham, 2021; Dhillon, 2022; Miao et al., 2022). Recently, FDA approval of Daprodustat for dialysis patients after al least 4 months of treatment in February 2023. Some meta-analyses have found that HIF stabilizers, such as roxadustat, vadadustat and daprodustat, can increase Hb levels and regulate iron metabolism in nondialysis-dependent (NDD) and dialysis-dependent (DD) patients (Fu et al., 2022; Huang et al., 2022; Wang et al., 2022). A more systemic network meta-analysis including all relevant drugs (roxadustat, daprodustat, vadadustat, enarodustat, molidustat, desidustat, ESAs) is necessary to analyse the comparative effects of HIF-PHIs and ESAs for treating renal anemia. One study reported that all HIF-PHIs have the same effect on clinical treatment as ESAs (EPO or DPO) in NDD-CKD patients (Zheng et al., 2020). However, NDD-CKD and DD-CKD should be evaluated separately due to the differences between the trajectories of Hb levels in each condition and in the haemodynamic and metabolic milieus and the different mechanisms of cardiac failure events (Parfrey, 2021). We conducted a systematic review and network meta-analysis (NMA) of RCTs to compare the efficacy in achieving target Hb levels and acceptability of different HIF-PHIs and ESAs for treating anemia in CKD patients undergoing dialysis.
An extensive literature search was performed in PubMed, Cochrane Library, Web of Science, Embase, clinicaltrials.gov databases, and MEDLINE up to 1 June 2022. In addition, the reference lists of all identified publications, drug manufacturers’ websites, and relevant meta-analyses were also mined for further relevant data (search terms and strategies are detailed in Supplementary Table S1). Our a priori inclusion criteria included 1) anaemic patients with DD-CKD treated with HIF-PHD inhibitors or ESAs (rhEPO, DPO, MPG-EPO), 2) intervention of rhEPO means epoetin alfa and beta or both of them, 3) reported efficacy outcomes and tabulated data on discontinuation outcomes, 4) control groups that were treated with ESAs, and 5) all interventions were designed to be dose-adjusted for the purpose of maintaining target Hb levels.
Two authors independently screened citations against the following predefined selection criteria. Eligible papers were RCTs that compared the effects and safety of different anemia treatments in CKD patients undergoing dialysis. All superiority, phase II and III, nonblinded, single-blinded, noninferiority, and double-blinded trials were included. Interventions of interest included 6 HIF-PHIs (roxadustat, vadadustat, daprodustat, enarodustat, molidustat, and desidustat) and 3 ESAs (rhEPO, DPO and MPG-EPO). The exclusion criteria were as follows: 1) participants in the study had primary anemia or anemia resulting from blood loss, cancer, or infectious diseases, 2) only one drug for treating CKD with anemia was studied (for example, comparisons of different doses or dosing frequency of the same drug), 3) participants were <18 years old, 4) outcome data could not be sourced from authors, 5) the control group was a placebo, or 6) ESAs were unknown or EPO and DPO combinations were used.
Three authors (Xiuben Du and Minlin You) independently extracted data from the original trial reports using a standardized method and then verified the extraction. Disagreements were resolved via discussion among reviewers. We contacted the corresponding authors and sponsoring pharmaceutical companies of the included trials to request missing data. Data extraction was performed using a self-designed data extraction method (Longfeng Jiang). The main study characteristics included the year of publication, authors, clinical trial number, type of study, population characteristics, sample size, dosage of drugs, treatment duration, control treatment, race, gender, mean age, baseline Hb level, type of replacement therapy, iron supplement, definition of Hb response, and primary and secondary outcomes. We assessed sources of bias using the Cochrane Collaboration’s risk-of-bias tool, which addresses 9 domains (Higgins, 2008). Two authors (Ming Hong and Qinming Zeng) independently completed the assessments, and discrepancies were discussed with one author (Jing Wang) and resolved by consensus. Additionally, the GRADE guidelines were used to assess the quality of evidence contributing to each estimated network (Higgins et al., 2014).
The primary outcome included Hb response associated with interventions. The drug acceptability profile was assessed according to the reported rate of discontinuation, AEs and SAEs.
The secondary outcomes included the main reason for discontinuation, such as AEs, death, refusal of treatment, content withdrawal and kidney transplantation. In addition, the rate of RBC transfusion, iron-related parameters included hepcidin levels, ferritin, transferrin saturation (TSAT), TIBC, serum iron levels, C-reactive protein (CRP) level, low-density lipoprotein (LDL) levels, high-density lipoprotein (HDL) levels, total cholesterol levels, MACE and all-cause mortality.
The risk of bias was assessed per the Cochrane Handbook and PRISMA guidelines for Systematic Reviews of Interventions. Additionally, we analyzed the certainty of evidence contributing to network estimates of the main outcomes with the GRADE framework (Salanti et al., 2014; Brignardello-Petersen et al., 2020).
The results are presented in network plots, in which the node size is proportional to the patient number, while the line thickness between the nodes is proportional to the trial number. The differential contributions of direct comparisons to the network summary effect are presented in contribution plots (Nikolakopoulou et al., 2019). Using random-effects pairwise meta-analysis and network meta-analysis in a frequentist environment, the summary odds ratios (ORs) are also calculated for binary outcomes and standardized mean differences for continuous variables. All comparisons were two-tailed, and a p-value <0.05 denoted statistical significance. The synthesis of study effect sizes is conducted via a random-effects network meta-analysis model. The surface under the cumulative ranking (SUCRA) presents the probability (unit: %) of superior effectiveness for each intervention compared with a theoretical ideal intervention. Thus, interventions were ranked by the SUCRA index.
Heterogeneity was examined by generating forest plots, including summary effects with 95% CIs and 95% prediction intervals for all comparisons. Prediction intervals represent CIs of the approximate predictive distribution of the future trial, considering heterogeneity (Higgins et al., 2009). Via a loop-specific approach, the potential inconsistencies were evaluated between the direct and indirect evidence within the network (Veroniki et al., 2013), and local inconsistencies were also calculated using the node-splitting method (White, 2015). Further, the design-by-treatment model was used to evaluate global inconsistencies of the whole NMA.
Finally, we used comparison-adjusted funnel plots and Egger’s regression to evaluate potentially small study effects and publication bias (Egger et al., 1997). We used meta-regression to assess the impact of study characteristics (effect modifiers) on the results. Subgroup analyses were conducted according to mean age (>60 or ≤60 years), the period of treatment (>24 or ≤24 weeks), Hb baseline level (>10.5 or ≤10.5 g/L), race (US or non US), sample size (>500 or ≤500 participants), follow-up (with or without), the target hemoglobin levels (10–12 or ≥11 g/dl) and dialysis method (hemodialysis or renal replacement therapy with both hemodialysis and peritoneal dialysis). All analyses were conducted using the network and network graph packages of Stata (version 15). The meta-analysis was prospectively registered with the PROSPERO international prospective register of systematic reviews (CRD42022306511) and reported by following the Preferred Reporting Items for Systematic Reviews and Meta-analyses extension for network meta-analyses (Hutton et al., 2015). (Figure 1)
The study founder had no role in the study design, data collection, analysis, interpretation, writing of the report, or the decision to submit.
Twenty-six eligible articles involving 17,204 patients published between 1990 and 2022 were included for pooled analyses (Nissenson et al., 2002; Vanrenterghem et al., 2002; Carrera et al., 2010; Bernieh et al., 2014; Brigandi et al., 2016; Holdstock et al., 2016; Provenzano et al., 2016; Akizawa et al., 2017; Chen et al., 2017; Akizawa et al., 2019; Bailey et al., 2019; Chen et al., 2019; Macdougall et al., 2019; Meadowcroft et al., 2019; Sinha et al., 2019; Akizawa et al., 2020a; Akizawa et al., 2020b; Akizawa et al., 2021a; Nangaku et al., 2021a; Akizawa et al., 2021b; Nangaku et al., 2021b; Charytan et al., 2021; Csiky et al., 2021; Eckardt et al., 2021; Provenzano et al., 2021; Singh et al., 2021). The literature search process is shown in Figure 2. These trials studied 9 different anti-anemia agents, including 6 different HIF-PHIs and 3 commonly used ESAs. There were 27 experiments included in these 25 articles, which contained the following comparisons: HIF-PHIs vs EPO (n = 11), HIF-PHIs vs DPO (n = 9), MPG-EPO vs rhEPO (n = 2), MPG-EPO vs DPO (n = 1), and rhEPO vs DPO (n = 4). Notably, one of the studies reported on two experiments that used different controls: HIF-PHI vs EPO and HIF-PHI vs DPO. One other study reported on two experiments that included incident and prevalent DD-CKD controls. The mean age of the patients ranged from 46.9 to 81 years, and the time of intervention varied from 7 to 104 weeks. The baseline characteristics of the included RCTs are provided in the Supplementary Material (Supplementary Table S2).
We assessed the efficacy and safety of 9 anti-anemia agents for use in anaemic CKD dialysis patients, including six different HIF-PHIs, rhEPO, DPO and MPG-EPO. Figure 3 shows the network of eligible comparisons for Hb response, discontinuation, AEs and SAEs.
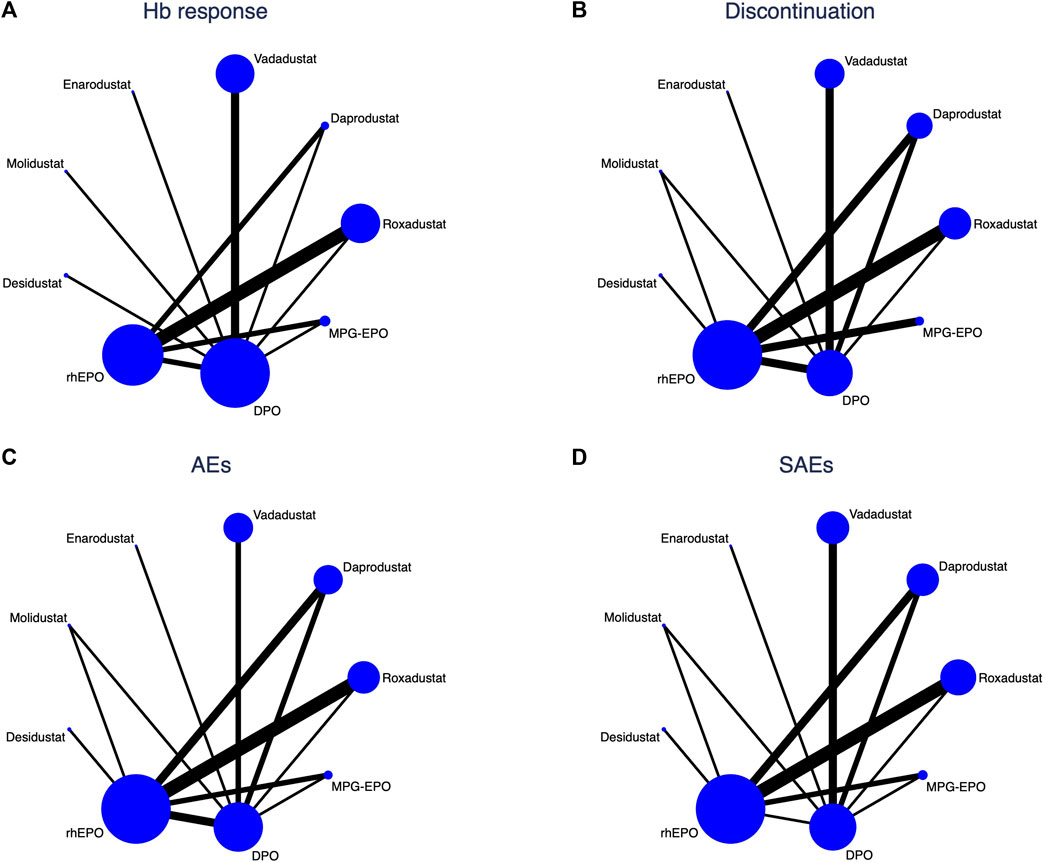
FIGURE 3. Network analysis of eligible comparisons for (A) Hb response (B) Discontinuation, (C) AEs, and (D) SAEs. The circle nodes present the patient numbers assigned to the trials, and the widths of lines denote the number of direct head-to-head comparisons available in contributing trials. When no head-to-head comparison was conducted, there is no line between the two nodes.
Regarding Hb response, our network meta-analysis included 20 RCTs involving the administration of 6 HIF-PHIs, rhEPO, DPO and MPG-EPO in 12,861 DD-CKD patients with anemia. No significant differences were found between HIF-PHIs and ESAs in reaching the Hb foreseen target. A meta-regression using restricted maximum likelihood estimators was exploited to assess the potential effects of duration, mean age, baseline Hb levels, race, sample size, follow-up, target haemoglobin levels and dialysis method on the change in Hb levels. The results of this meta-regression revealed that mean age had a significant effect on Hb response (Supplementary Table S4). No incoherence was observed between direct and indirect estimates.
Fifteen trials involving 10,314 participants were RCTs with dose-adjusted design that contributed to the analysis of the use of RBC transfusion as a rescue therapy (Supplementary Figure S1). Intervention with roxadustat showed a significantly lower risk of RBC transfusion than rhEPO, with an OR and 95% CI of 0.72 (0.56–0.93) (Table 1). No differences were found in the RBC transfusion rate between the included HIF-PHIs. Via the same moderating variables of this meta-regression, the results did not show a significant effect on RBC transfusion (Supplementary Table S4). No incoherence was observed between direct and indirect estimates.
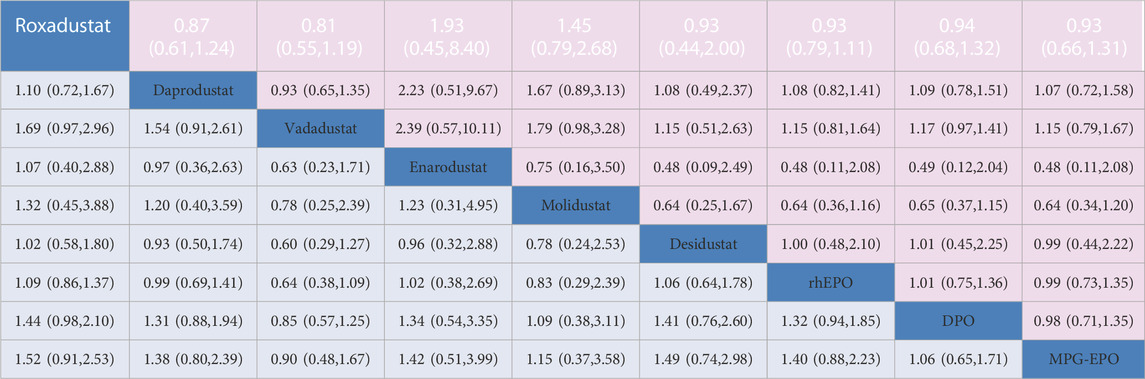
TABLE 1. Network estimates of treatment comparisons for AEs and SAEs. The figure provides a summary of the estimates of odds ratios and 95% confidence intervals. For AEs the odds ratio is for the row treatment compared to the column treatment. For SAEs. the odds ratio is for the column treatment versus the row treatment.
Twenty-four trials (15,794 participants) contributed to the analyses of discontinuation. Roxadustat and vadadustat had higher risks of increasing the discontinuation rate than ESAs; the former had ORs and 95% CIs of 1.58 (95% CI: 1.21–2.06) for rhEPO, 1.66 (1.16–2.38) for DPO, and 1.76 (1.70–4.49) for MPG-EPO, and the latter had ORs and 95% CIs of 1.71 (1.09–2.67) for rhEPO, 1.79 (1.29–2.49) for DPO, and 2.97 (1.62–5.46) for MPG-EPO. Except for enarodustat and desidustat, other HIF-PHIs and rhEPO led to a significantly higher risk of discontinuation than MPG-EPO, with ORs and 95% CIs of 1.76 (1.70–4.49) for roxadustat, 2.13 (1.27–3.58) for daprodustat, 2.97 (1.62–5.46) for vadadustat, 2.92 (1.11–7.68) for molidustat, and 1.74 and (1.15–2.65) for rhEPO (Table 1).
There were five main reasons for discontinuation: adverse events (AEs), death, subjects’ refusal of treatment, conforming to drug withdrawal contents, and kidney transplantation. Twenty trials (14,998 participants) were involved in the analysis of AEs, 13 trials (9,939) involved death, 14 trials (11,897) involved subjects refusing treatment, 19 trials (14.171) involved content withdrawal, and 11 trials (7,972) involved kidney transplantation. Roxadustat was associated with a higher risk of AEs that led to discontinuation than ESAs (rhEPO and DPO), with ORs and 95% CIs of 1.61 (95% CI: 1.19–2.17) for EPO and 2.06 (95% CI: 1.20–3.54) for DPO. Vadadustat had a higher risk of increasing AEs, subjects’ refusal to treatment, conforming to drug withdrawal contents, and rate than DPO, with ORs and 95% CIs of 2.04 (95% CI: 1.34–3.11), 2.22 (1.75–2.80), and 2.97 (1.50–5.88), respectively. No differences were found between the included interventions in the reasons for discontinuation, such as death and kidney transfusion (Supplementary Tables S8-S10). Via the moderating variables of this meta-regression, the results did not show a significant effect on the indicators of acceptability (Supplementary Table S4). No incoherence was observed between direct and indirect estimates (Supplementary Table S3).
Twenty-five trials (16,166 participants) and Twenty-four trials (15,144 participants) contributed to the analyses of AEs and SAEs, respectively. No differences were found in safety between HIF-PHIs, EPO, and DPO as determined by the AEs and SAEs (Table 1).The results did not reveal a significant effect on AEs and SAEs when the moderating variables duration, mean age, baseline Hb levels, race, sample size, follow-up, target haemoglobin levels and dialysis method were used (Supplementary Table S4). No incoherence was observed between direct and indirect estimates. (Supplementary Table S3). Meta-analysis results of HIF-PHIs versus ESAs for gastrointestinal disorders and some other common AEs revealed that vadadustat was superior to DPO in reducing the risk of diarrhea, with an OR of 0.97 (95% CI, 0.95–0.99); I2 = 0%. There was no difference between HIF-PHIs and rhEPO or DPO in increasing the risk of nausea, vomiting, hyperkalemia, and arteriovenous fistula thrombosis (Supplementary Figure S10).
In the context of a dose-adjusted design randomized controlled trial selected for comparation, we used the previously calculated p-scores to rank the efficacy and acceptability of the drugs included in our study. A higher p-score indicated better efficacy or acceptability (Figure 4). Among the included drugs, desidustat ranks highest in the efficiency, with p-scores of 0.791 for Hb response at the doses selected for comparison. Since the top three at low discontinuation rates are all ESAs, they were associated with a higher acceptability when compared with HIF-PHIs (p-score = 0.974 for MPG-EPO, p-score = 0.723 for DPO, and p-score = 0.671 for rhEPO). Vadadustat was associated with the highest safety (p-score = 0.837 in AEs, and p-score = 0.812 in SAEs). Roxadustat ranked last in safety with p-score = 0.239 in AEs.
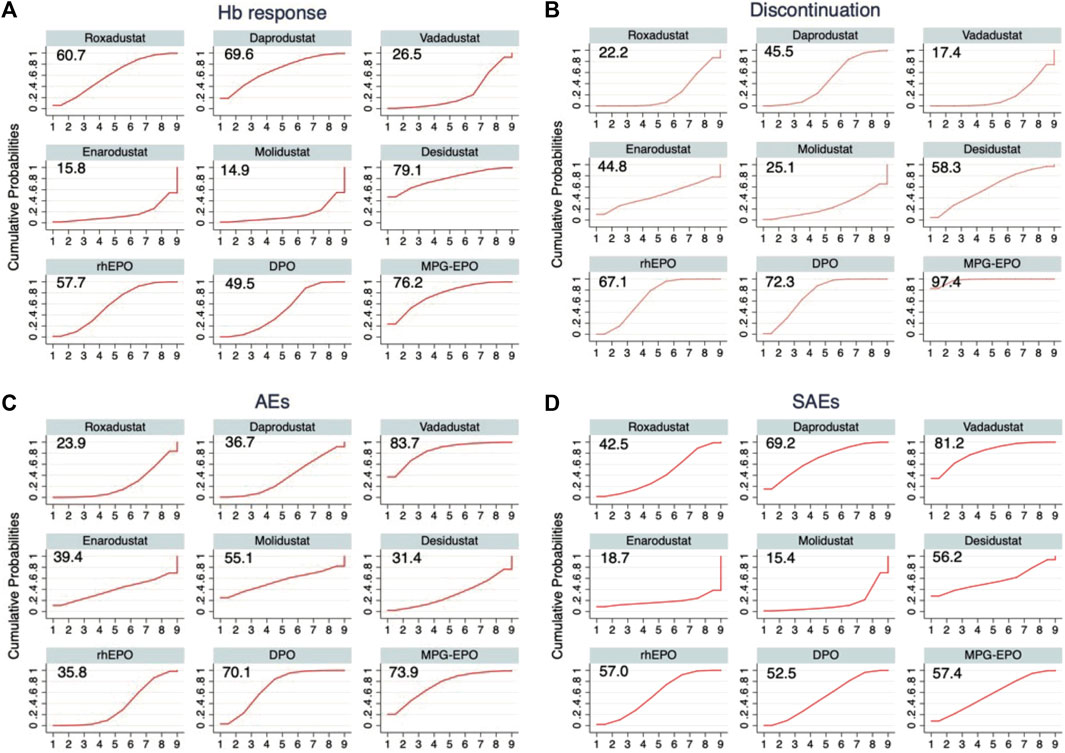
FIGURE 4. Drug ranking curves for (A) Hb response (B) Discontinuation, (C) AEs, and (D) SAEs. The cumulative probabilities of the intervention ranking are denoted by the curves for each outcome from the best to the worst, and the peak indicates the ranking with the highest probability for the corresponding intervention. The surface under the cumulative ranking (SUCRA) value is shown for each treatment and each outcome.
Overall, fourteen of the twenty trials (11,199 participants) reported data on hepcidin levels, 12 trials (11,143 participants) reported data on ferritin levels, 10 trials (7,233 participants) reported TIBC data, 12 trials (11,125 participants) reported TSAT data, and 9 trials (7,096 participants) reported serum iron levels were analysed. All the included HIF-PHIs, were proven to be more effective than ESAs in reducing hepcidin levels, and increasing TIBC and serum iron level with OR of −0.17 (95% CI, −0.21 to −0.12), OR of 0.79 (95% CI, 0.63–0.95), and OR of 0.39 (95% CI, 0.33–0.45), respectively. No differences in the effects on TSAT were found between the included HIF-PHIs and ESAs with OR of 0.04 (95% CI, −0.06–0.14) (Supplementary Table S7). Unlike rhEPO, included HIF-PHIs did not showed differ from DPO in reducing ferritin levels with OR of −0.17 (95% CI, −0.08 to −0.21) (Supplementary Table S7).
• It is well known that patients with the highest CRP levels require substantially higher ESA doses to achieve comparable Hb levels than those with lower CRP levels (Bradbury et al., 2009). While inflammation suppresses the haemoglobin response to ESAs, it does not appear to affect the dose of roxadustat (Chen et al., 2019). A connected network could not be generated to assess CRP levels under the premise that dose-adjusted design RCTs were selected for comparison. Nevertheless, a pairwise meta-analysis revealed that there was no evidence that roxadustat and rhEPO had different effects on decreasing CRP levels (OR, 0.87 [95% CI, 0.67–1.13]; I2 = 69.9%) (Supplementary Figure S8).
A connected network could not be generated to assess lipid metabolism. A pairwise meta-analysis revealed that roxadustat reduced LDL and total cholesterol levels more than rhEPO, with an OR of −0.55 (95% CI, −0.96 to −0.14); I2 = 93.9% and OR, −0.66 (95% CI, −0.86 to −0.14); I2 = 72.5%, respectively (Supplementary Figure S9). There were inadequate data to examine the coherence between estimates derived from direct and indirect evidence regarding LDL levels, HDL levels, and total cholesterol levels (Supplementary Figure S3).
Thirteen trials (13,095 participants) contributed to the analysis of MACEs and twenty-three trials (15,991 participants) contributed to the analysis of all-cause mortality (Supplementary Figure S1). There were no detectable differences in the odds of MACEs and all-cause mortality among the included interventions (Supplementary Figure S9 and Supplementary Figure S12). Via the moderating variables of this meta-regression, the results did not show a significant effect on MACEs and all-cause mortality (Supplementary Table S4).
We found that 25.92% (7/27 items), 59.25% (16/27 items), and 14.81% (4/27 items) of the included studies had an overall low, unclear, and high risk of bias, respectively. The blinding of outcome assessment and concealing procedure after randomization mainly contributed to the unclear risks of bias. The results of the risk of bias assessment of the trials are shown in Supplementary Table S5.
General symmetry was observed in the plots used to assess publication bias across the included studies. The results of Egger’s test indicated no significant asymmetry that might suggest publication bias among the articles included in this NMA (Supplementary Figure S7). In general, inconsistencies concerning either local inconsistency as assessed using the loop-specific approach and the node-splitting method or global inconsistency as determined using the design-by-treatment were not observed in this NMA (Supplementary Figure S3, Supplementary Figure S4, Supplementary Table S4). The GRADE evaluation is presented in Supplementary Table S6, which ranged from moderate to very low in the comparisons.
Several meta-analyses have demonstrated that some HIF-PHIs are significantly superior to placebo in raising haemoglobin levels, and ESAs are superior to placebo in preventing blood transfusion (Palmer et al., 2014; Zheng et al., 2021a; Zheng et al., 2021b). However, most RCTs only showed that some HIF-PHIs were noninferior to some ESAs in the control group with respect to correction and maintenance of haemoglobin concentrations. To date, the specific difference between the various HIF-PHIs and ESAs has not been clarified. Our study is the first network meta-analysis that compared the effectiveness and acceptability of all available HIF-PHIs and ESAs for treating anemia in CKD patients undergoing dialysis. The comparison of results from the RCTs showed some interesting findings. First, all the included RCTs were designed to achieve the target level of Hb by adjusting the dose of the interventions. In this context, there are no significant differences between HIF-PHIs and ESAs in Hb response. Second, roxadustat achieves higher Hb levels than rhEPO and reduces the risk of red blood cell transfusion. An important finding pertains to RBC transfusions, which may result in nonimmunological complications and immunologically mediated transfusion reactions (Klein et al., 2007; McMurray et al., 2012) (Szczech et al., 2008; Palmer et al., 2010). According to the SUCRA analysis, roxadustat was at the top for reducing the risk of RBC transfusions when dose-adjusted design RCTS were selected for comparison. Apparently, treatment with it can reduce the risk of transfusion reactions and preserve the opportunity for future transplantation. Third, with regard to acceptability, although our results showed that in DD-CKD patients, the proportions of AEs and SAEs associated with the different HIF-PHIs were no differ from ESAs with the selected dose-adjusted mode, roxadustat and vadadustat showed a higher risk of increasing the discontinuation rate than ESAs, which ranged from rhEPO and DPO to MPG-EPO. On the other hand, these three ESAs ranked in the top three for reducing the risk of discontinuation, which was higher than all HIF-PHIs. Notably, the convenience of one monthly intravenous injection of MPG-EPO may account for its best performance in acceptability. There are five main reasons for discontinuation. Among them, roxadustat showed a significantly higher risk than rhEPO in terms of increased risk of AEs and subjects’ refusal of treatment. Additionally, there were significant differences between vadadustat and DPO in terms of increased risk of AES, subjects refusing treatment, and meeting required discontinuation, and vadadustat was higher than DPO. Last, with the selected dose-adjusted mode, although roxadustat were not significantly different in increasing the risk of AEs than others, it ranked worst in the AEs, which is one of the safety indicators, among the included interventions. To date, the incidence of most gastrointestinal disorders did not differ significantly between oral HIF-PHIs and SC/IV ESAs, however, surprisingly, oral vadadustat had less effect on causing diarrhea than DPO. Therefore, future research needs to explore the specific AEs and SAEs of HIF-PHIs when compared ESAs, extend this NMA to combine aggregate and individual patient data. Such an analysis will allow the prediction of personalized clinical outcomes, such as discontinuation due to some specific side effects, and the estimate of comparative efficacy at multiple time points. This can provide a more specific reference for clinical practice.
HIF-PHIs regulate iron metabolism through five known mechanisms, some of which require further confirmation. Hepcidin is the main regulator of body iron homeostasis in patients with CKD. Elevated hepcidin levels may interfere with iron mobilization by impairing the mobilization of stored iron and iron absorption from the small intestine due to the degradation of ferroportin (FPN). First, HIF-PHIs can lead to the activation of erythropoiesis, and increased erythropoiesis reduces serum iron concentration because producing new RBCs is associated with a large demand for iron. Second, a mediator named erythroferrone (ERFE) derived from erythroblasts in the bone marrow and spleen can suppress hepcidin production. Thus, HIF-PHIs can downregulate hepcidin levels by reducing serum iron concentrations and promoting the production and secretion of ERFE (Kautz et al., 2014; Ueda and Takasawa, 2017; Hanudel et al., 2018; Hanudel et al., 2021). The excessive expression of hepcidin may result from inflammation and decreased renal clearance (Agarwal, 2021). However, HIF-PHIs have shown anti-inflammatory effects in several disease models, such as sepsis and acute ischaemic injury (Eltzschig et al., 2014; Taylor et al., 2016), which indicated that the HIF system is involved in the processes regulating inflammation and the immune response since hypoxia is a trigger for the inflammatory response and can possibly slow the recovery process (Vanderhaeghen et al., 2020). The benefit of HIF-PHIs in some studies shows that no dose increases are observed for the management of patients with high CRP levels randomized to HIF-PHI at variance with patients treated with ESA who required a higher dose to maintain Hb levels (Nangaku et al., 2021b; Charytan et al., 2021; Eckardt et al., 2021; Provenzano et al., 2021; Fishbane et al., 2022). This study has shown that most of the investigated HIF-PHIs could decrease hepcidin levels while also increasing TIBC levels better than rhEPO with the selected dose-adjusted mode. A few RCTs have reported changes in CRP levels, indicating that more evidence from RCTs in humans is needed to demonstrate whether this anti-inflammatory benefit of HIP-PHIs can directly lead to elevated haemoglobin levels and whether HIP-PHIs can reduce progression to ESRD or the slope of eGFR. HIF-PHIs such as vadadustat and molideustat can improve kidney function in mice with CKD. The improvement of CKD progression may be the fourth method through which HIF-PHIs reduce hepcidin levels (Wheeler and Clinkenbeard, 2019; Ikeda, 2021; Noonan et al., 2021). The final mechanism through which HIF-PHIs modulate iron metabolism involves the improvement of mineral and bone disorders. Under conditions of iron deficiency, the transcription of fibroblast growth factor 23 (FGF-23) is increased. High FGF-23 levels lead to enhanced urinary phosphate wasting and lower 1,25-dihydroxy vitamin D levels, resulting in decreased serum calcium levels, which causes increased parathyroid hormone levels. Parathyroid hormone has direct or indirect effects on erythropoietin release and shortens red blood cell survival (Meytes et al., 1981; Kimata et al., 2005). HIF-PHIs can suppress FGF-23 expression (Noonan et al., 2021). Five studies with roxadustat reported a lower need for iron (Chen et al., 2019; Charytan et al., 2021; Csiky et al., 2021; Provenzano et al., 2021; Fishbane et al., 2022), while other HIF-PHIs seem to have less impact on iron use and iron doses. However, whether this difference can be ascribed directly to a distinctive effect of various HIF-PHIs on iron requirements or indirectly to a different iron need induced by different Hb increases remains unclear. In conclusion, in addition to increasing endogenous erythropoietin production, HIF-PHIs may reduce the need for intravenous IV) iron supplementation by regulating iron metabolism (Haase, 2021). The benefits of regulating iron metabolism suggest that HIF-PHIs are a promising new class of orally administered drugs for treating anemia associated with CKD.
Although roxadustat and daprodustat are superior to epoetin alfa in lowering high LDL cholesterol and total cholesterol levels, which present a major risk factor for cardiovascular diseases among CKD patients (Holdstock et al., 2016; Charytan et al., 2021; Singh et al., 2021), no data from recent studies have shown that the cholesterol-lowering effect of roxadustat was associated with a lower incidence of MACEs, nor was rosuvastatin (Davidson, 2009). Several studies showed that statins are less efficacious in reducing cardiovascular disease risk in patients with CKD, particularly in patients on dialysis therapy (Massy and Zeeuw, 2013). Recent study shows the reason for the less effective of statins motioned above may be due, at least in part, to a higher intracellular cholesterol production by hyperphosphatemia, possibly via a lower membrane LDL receptor expression (Massy et al., 2022). Therefore, more research is needed on the role of HIF-PHIs in regulating lipid metabolism and its benefits in dialysis patients. With respect to all-cause mortality although there was no significant difference between interventions in mortality, roxadustat ranked last in reducing the risk of all-cause mortality. Thus, more large and high-quality studies are needed to confirm the safety profiles of roxadustat.
There appeared to be between-drug differences in the effects on changes in Hb levels and discontinuation. Although roxadustat was associated with higher odds of discontinuation with the selected dose-adjusted mode, it was more effective than rhEPO in reducing the risk of RBC transfusion, which may be related to the higher haemoglobin levels obtained with roxadustat. This advantage will alleviate the imbalance between the supply and demand of its transfusion needs in some special periods and situation. Therefore, it is worthwhile to identify and reduce the side effects that lead to drug withdrawal, as it can improve tolerance while maintaining efficiency. Desidustat was developed in Asian populations. There was no significant difference between desidustat and ESAs in the incidence of discontinuation, and desidustat ranked the highest in Hb reaction and risk of drug discontinuation among HIF-PHIs, showing better efficacy and tolerance. In addition, vadadustat ranked highest in safety and was less effective at causing than DPO. Roxadustat, desidustat and vadadustat may be a potential option based on their effectiveness and acceptability, especially in cases where patients have a personal preference for oral therapy. Regarding the iron parameters, with the selected dose-adjusted mode, included HIF-PHIs were showed superior to ESAs in reducing hepcidin and ferritin levels and improving TIBC and serum iron levels. In addition, the synergistic effect of HIF-PHIs on erythropoietin release and iron/cholesterol metabolism may potentially reduce the need for iron supplementation and lipid-lowering drugs. Therefore, limiting the use of these prescriptions may decrease the occurrence of drug-related side effects, such as frequent gastrointestinal discomfort or even rhabdomyolysis.
Based on direct and indirect evidence, our study provides a preliminary comprehensive ranking of the investigated drugs in terms of their effects on reaching Hb target levels and RBC transfusion, as well as their acceptability based on reported discontinuation; these findings provide a basis for future clinical research. However, our study has some limitations. First, since various HIF-PHI types are currently in phase II or III clinical trials, this study lacked direct head-to-head comparisons between different HIF-PHIs. More indirect comparisons between different agents resulted in reduced accuracy of the results. Second, the number of HIF-PHI RCTs differed for each drug. Thus, the results still need to be further verified by more studies (ClinicalTrials.gov numbers, NCT03400033, NCT03457701, NCT04134026, NCT04899661, etc.). Third, the evidence is limited on the effect of these treatments on changes in VEGF, CRP, lipid metabolism levels and changes in iron requirements in dialysis patients, and studying these parameters allows for a more comprehensive analysis of HIF-PHI characteristics. The efficacy and acceptability indicators of these drugs should be summarized and analysed in more studies.
This network meta-analysis compared all currently available HIF-PHIs for treating anemia in CKD patients undergoing dialysis. HIF-PHIs and ESAs have their own characteristics and advantages. ESAs have better performance than HIF-PHIs in terms of acceptability. With the selected dose-adjusted mode, some HIF-PHIs appeared to be a potential treatment for DD-CKD patients when compared with rhEPO, due to its effectiveness in decreasing the risk of RBC transfusion rate or regulating iron or lipid metabolism while achieving target Hb levels (Table 2).
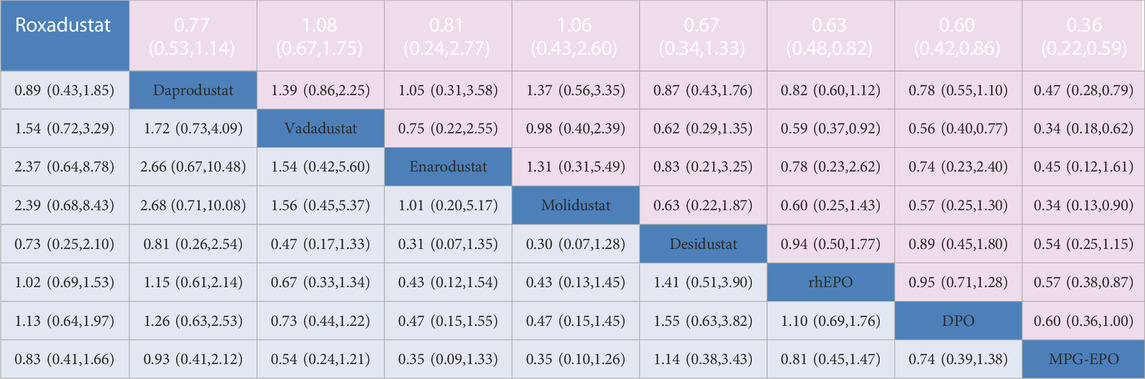
TABLE 2. Network estimates of treatment comparisons for Hb response and discontinuation. The figure provides a summary of the estimates of odds ratios and 95% confidence intervals. For Hb response, the odds ratio is for the row treatment compared to the column treatment. For discontinuation, the odds ratio is for the column treatment versus the row treatment.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
QH, MY, MH, and XD conceived and designed the study. QH, WH, and JC selected the articles and extracted the data. JW, QZ and LJ analysed the data. QH wrote the first draft of the manuscript. JW, MH, XL and XD interpreted the data and contributed to the writing of the final version of the manuscript. All authors contributed to the article and approved the submitted version.
This study was funded by National Natural Science Foundation of China (82004161) and the Foundation of Science, Technology and Innovation Commission of Shenzhen Municipality (JCYJ20180302153701406, KCXFZ20201221173612034).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1050412/full#supplementary-material
Akizawa, T., Macdougall, I. C., Berns, J. S., Bernhardt, T., Staedtler, G., Taguchi, M., et al. (2019). Long-term efficacy and safety of molidustat for anemia in chronic kidney disease: DIALOGUE extension studies. Am. J. Nephrol. 49, 271–280. doi:10.1159/000499111
Agarwal, A. K. (2021). Iron metabolism and management: Focus on chronic kidney disease. Kidney Int. Suppl. 11, 46–58. doi:10.1016/j.kisu.2020.12.003
Akizawa, T., Iwasaki, M., Yamaguchi, Y., Majikawa, Y., and Reusch, M. (2020). Phase 3, randomized, double-blind, active-comparator (darbepoetin alfa) study of oral roxadustat in CKD patients with anemia on hemodialysis in Japan. J. Am. Soc. Nephrol. 31, 1628–1639. doi:10.1681/ASN.2019060623
Akizawa, T., Nangaku, M., Yamaguchi, T., Koretomo, R., Maeda, K., Miyazawa, Y., et al. (2021). A phase 3 study of enarodustat (JTZ-951) in Japanese hemodialysis patients for treatment of anemia in chronic kidney disease: SYMPHONY HD study. Kidney Dis. 7, 494–502. doi:10.1159/000517053
Akizawa, T., Nangaku, M., Yonekawa, T., Okuda, N., Kawamatsu, S., Onoue, T., et al. (2020). Efficacy and safety of daprodustat compared with darbepoetin alfa in Japanese hemodialysis patients with anemia: A randomized, double-blind, phase 3 trial. Clin. J. Am. Soc. Nephrol. 15, 1155–1165. doi:10.2215/CJN.16011219
Akizawa, T., Okumura, H., Alexandre, A. F., Fukushima, A., Kiyabu, G., and Dorey, J. (2018). Burden of anemia in chronic kidney disease patients in Japan: A literature review. Ther. Apher. Dialysis 22, 444–456. doi:10.1111/1744-9987.12712
Akizawa, T., Tsubakihara, Y., Nangaku, M., Endo, Y., Nakajima, H., Kohno, T., et al. (2017). Effects of daprodustat, a novel hypoxia-inducible factor prolyl hydroxylase inhibitor on anemia management in Japanese hemodialysis subjects. Am. J. Nephrol. 45, 127–135. doi:10.1159/000454818
Akizawa, T., Yamada, T., Nobori, K., Matsuda, Y., Hayashi, Y., Hayasaki, T., et al. (2021). Molidustat for Japanese patients with renal anemia receiving dialysis. Kidney Int. Rep. 6, 2604–2616. doi:10.1016/j.ekir.2021.07.015
Bailey, C. K., Caltabiano, S., Cobitz, A. R., Huang, C., Mahar, K. M., and Patel, V. V. (2019). A randomized, 29-day, dose-ranging, efficacy and safety study of daprodustat, administered three times weekly in patients with anemia on hemodialysis. BMC Nephrol. 20, 372–412. doi:10.1186/s12882-019-1547-z
Bernieh, B., Abouchacra, S., Boobes, Y., Al Hakim, M. R., Nagelkerke, N., Chaaban, A., et al. (2014). Comparison between short-and long-acting erythropoiesis-stimulating agents in hemodialysis patients: Target hemoglobin, variability, and outcome. Int. urology Nephrol. 46, 453–459. doi:10.1007/s11255-013-0640-7
Bradbury, B. D., Critchlow, C. W., Weir, M. R., Stewart, R., Krishnan, M., and Hakim, R. H. (2009). Impact of elevated C-reactive protein levels on erythropoiesis-stimulating agent (ESA) dose and responsiveness in hemodialysis patients. Nephrol. Dial. Transplant. 24, 919–925. doi:10.1093/ndt/gfn543
Brigandi, R. A., Johnson, B., Oei, C., Westerman, M., Olbina, G., De Zoysa, J., et al. (2016). A novel hypoxia-inducible factor− prolyl hydroxylase inhibitor (GSK1278863) for anemia in CKD: A 28-day, phase 2A randomized trial. Am. J. Kidney Dis. 67, 861–871. doi:10.1053/j.ajkd.2015.11.021
Brignardello-Petersen, R., Izcovich, A., Rochwerg, B., Florez, I. D., Hazlewood, G., Alhazanni, W., et al. (2020). GRADE approach to drawing conclusions from a network meta-analysis using a partially contextualised framework. BMJ 371, m3907. doi:10.1136/bmj.m3907
Carrera, F., Lok, C. E., de Francisco, A., Locatelli, F., Mann, J. F., Canaud, B., et al. (2010). Maintenance treatment of renal anaemia in haemodialysis patients with methoxy polyethylene glycol-epoetin beta versus darbepoetin alfa administered monthly: A randomized comparative trial. Nephrol. Dial. Transplant. 25, 4009–4017. doi:10.1093/ndt/gfq305
Charytan, C., Manllo-Karim, R., Martin, E. R., Steer, D., Bernardo, M., Dua, S. L., et al. (2021). A randomized trial of roxadustat in anemia of kidney failure: SIERRAS study. Kidney Int. Rep. 6, 1829–1839. doi:10.1016/j.ekir.2021.04.007
Chen, N., Hao, C., Liu, B.-C., Lin, H., Wang, C., Xing, C., et al. (2019). Roxadustat treatment for anemia in patients undergoing long-term dialysis. N. Engl. J. Med. 381, 1011–1022. doi:10.1056/NEJMoa1901713
Chen, N., Qian, J., Chen, J., Yu, X., Mei, C., Hao, C., et al. (2017). Phase 2 studies of oral hypoxia-inducible factor prolyl hydroxylase inhibitor FG-4592 for treatment of anemia in China. Nephrol. Dial. Transplant. 32, 1373–1386. doi:10.1093/ndt/gfx011
Coresh, J., Selvin, E., Stevens, L. A., Manzi, J., Kusek, J. W., Eggers, P., et al. (2007). Prevalence of chronic kidney disease in the United States. Jama 298, 2038–2047. doi:10.1001/jama.298.17.2038
Csiky, B., Schömig, M., Esposito, C., Barratt, J., Reusch, M., Valluri, U., et al. (2021). Roxadustat for the maintenance treatment of anemia in patients with end-stage kidney disease on stable dialysis: A European phase 3, randomized, open-label, active-controlled study (pyrenees). Adv. Ther. 38, 5361–5380. doi:10.1007/s12325-021-01904-6
Davidson, M. H. (2009). AURORA and JUPITER trials: Recognizing the limitations and expanding the benefits of statin treatment. Curr. Cardiol. Rep. 11, 401–403. doi:10.1007/s11886-009-0058-0
Dhillon, S. (2020). Daprodustat: First approval. Drugs 80, 1491–1497. doi:10.1007/s40265-020-01384-y
Dhillon, S. (2022). Drugs, 1207–1212. Desidustat: First approval, 82, 11, doi:10.1007/s40265-022-01744-w
Dhillon, S. (2019). Roxadustat: First global approval. Drugs 79, 563–572. doi:10.1007/s40265-019-01077-1
Eckardt, K.-U., Agarwal, R., Aswad, A., Awad, A., Block, G. A., Bacci, M. R., et al. (2021). Safety and efficacy of vadadustat for anemia in patients undergoing dialysis. N. Engl. J. Med. 384, 1601–1612. doi:10.1056/NEJMoa2025956
Egger, M., Smith, G. D., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. Bmj 315, 629–634. doi:10.1136/bmj.315.7109.629
Eltzschig, H. K., Bratton, D. L., and Colgan, S. P. (2014). Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat. Rev. Drug Discov. 13, 852–869. doi:10.1038/nrd4422
Fandrey, J., Schödel, J., Eckardt, K.-U., Katschinski, D. M., and Wenger, R. H. (2019). Now a Nobel gas: Oxygen. Pflügers Archiv-European J. Physiology 471, 1343–1358. doi:10.1007/s00424-019-02334-8
Fishbane, S., Pollock, C. A., El-Shahawy, M., Escudero, E. T., Rastogi, A., Van, B. P., et al. (2022). Roxadustat versus epoetin alfa for treating anemia in patients with chronic kidney disease on dialysis: Results from the randomized phase 3 ROCKIES study. J. Am. Soc. Nephrol. 33, 850–866. doi:10.1681/ASN.2020111638
Fu, Z., Geng, X., Chi, K., Song, C., Wu, D., Liu, C., et al. (2022). Efficacy and safety of daprodustat vs rhEPO for anemia in patients with chronic kidney disease: A meta-analysis and trial sequential analysis. Front. Pharmacol. 13, 746265. doi:10.3389/fphar.2022.746265
Haase, V. H. (2021). Hypoxia-inducible factor–prolyl hydroxylase inhibitors in the treatment of anemia of chronic kidney disease. Kidney Int. Suppl. 11, 8–25. doi:10.1016/j.kisu.2020.12.002
Hanudel, M. R., Wong, S., Jung, G., Qiao, B., Gabayan, V., Zuk, A., et al. (2021). Amelioration of chronic kidney disease-associated anemia by vadadustat in mice is not dependent on erythroferrone. Kidney Int. 100, 79–89. doi:10.1016/j.kint.2021.03.019
Hanudel, M. R., Rappaport, M., Chua, K., Gabayan, V., Qiao, B., Jung, G., et al. (2018). Levels of the erythropoietin-responsive hormone erythroferrone in mice and humans with chronic kidney disease. Haematologica 103, e141–e142. doi:10.3324/haematol.2017.181743
Higgins, J., Del Giovane, C., Chaimani, A., Caldwell, D., and Salanti, G. (2014). Evaluating the quality of evidence from a network meta-analysis. Value Health 17, e99682. doi:10.1371/journal.pone.0099682
Higgins, J. P. (2008). Cochrane handbook for systematic reviews of interventions. https://training.cochrane.org/handbook.
Higgins, J. P., Thompson, S. G., and Spiegelhalter, D. J. (2009). A re-evaluation of random-effects meta-analysis. J. R. Stat. Soc. Ser. A (Statistics Soc. 172, 137–159. doi:10.1111/j.1467-985X.2008.00552.x
Holdstock, L., Meadowcroft, A. M., Maier, R., Johnson, B. M., Jones, D., Rastogi, A., et al. (2016). Four-week studies of oral hypoxia-inducible factor–prolyl hydroxylase inhibitor GSK1278863 for treatment of anemia. J. Am. Soc. Nephrol. 27, 1234–1244. doi:10.1681/ASN.2014111139
Huang, Q., Liao, Z., Liu, X., Xia, Y., and Wang, J. (2022). Efficacy and safety of vadadustat compared to darbepoetin alfa on anemia in patients with chronic kidney disease: A meta-analysis. Int. Urol. Nephrol. 55, 325–334. doi:10.1007/s11255-022-03316-z
Hurst, J. H. (2016). William Kaelin, peter Ratcliffe, and Gregg Semenza receive the 2016 albert lasker basic medical research award. J. Clin. investigation 126, 3628–3638. doi:10.1172/jci90055
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 162, 777–784. doi:10.7326/M14-2385
Ikeda, Y. (2021). Novel roles of HIF-PHIs in chronic kidney disease: The link between iron metabolism, kidney function, and FGF23. Kidney Int. 100, 14–16. doi:10.1016/j.kint.2021.04.030
Kalantar-Zadeh, K., Jafar, T. H., Nitsch, D., Neuen, B. L., and Perkovic, V. (2021). Chronic kidney disease. Lancet 398, 786–802. doi:10.1016/s0140-6736(21)00519-5
Kautz, L., Jung, G., Valore, E. V., Rivella, S., Nemeth, E., and Ganz, T. (2014). Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat. Genet. 46, 678–684. doi:10.1038/ng.2996
Kimata, N., Akiba, T., Pisoni, R. L., Albert, J. M., Satayathum, S., Cruz, J. M., et al. (2005). Mineral metabolism and haemoglobin concentration among haemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol. Dial. Transplant. 20, 927–935. doi:10.1093/ndt/gfh732
Klein, H. G., Spahn, D. R., and Carson, J. L. (2007). Red blood cell transfusion in clinical practice. Lancet 370, 415–426. doi:10.1016/s0140-6736(07)61197-0
Koulouridis, I., Alfayez, M., Trikalinos, T. A., Balk, E. M., and Jaber, B. L. (2013). Dose of erythropoiesis-stimulating agents and adverse outcomes in CKD: A metaregression analysis. Am. J. Kidney Dis. 61, 44–56. doi:10.1053/j.ajkd.2012.07.014
Macdougall, I. C., Akizawa, T., Berns, J. S., Bernhardt, T., and Krueger, T. (2019). Effects of molidustat in the treatment of anemia in CKD. Clin. J. Am. Soc. Nephrol. 14, 28–39. doi:10.2215/CJN.02510218
Massy, Z., Merkling, T., Wagner, S., Girerd, N., Essig, M., Wanner, C., et al. (2022). Association of serum phosphate with efficacy of statin therapy in hemodialysis patients. Clin. J. Am. Soc. Nephrol. CJASN 17, 546–554. doi:10.2215/CJN.12620921
Massy, Z., and Zeeuw, D. (2013). LDL cholesterol in CKD - to treat or not to treat? Kidney Int. 84, 451–456. doi:10.1038/ki.2013.181
McMurray, J., Parfrey, P., Adamson, J. W., Aljama, P., Berns, J. S., Bohlius, J., et al. (2012). Kidney disease: Improving global outcomes (KDIGO) anemia work group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int. Suppl., 2, 4, 279–335. doi:10.1038/kisup.2012.37
Meadowcroft, A. M., Cizman, B., Holdstock, L., Biswas, N., Johnson, B. M., Jones, D., et al. (2019). Daprodustat for anemia: A 24-week, open-label, randomized controlled trial in participants on hemodialysis. Clin. kidney J. 12, 139–148. doi:10.1093/ckj/sfy014
Meytes, D., Bogin, E., Ma, A., Dukes, P. P., and Massry, S. (1981). Effect of parathyroid hormone on erythropoiesis. J. Clin. Investigation 67, 1263–1269. doi:10.1172/jci110154
Miao, M., Wu, M., Li, Y., Zhang, L., Jin, Q., Fan, J., et al. (2022). Clinical potential of hypoxia inducible factors prolyl hydroxylase inhibitors in treating nonanemic diseases. Front. Pharmacol. 13, 837249. doi:10.3389/fphar.2022.837249
Nangaku, M., Farag, Y. M., DeGoma, E., Luo, W., Vargo, D., and Khawaja, Z. (2021). Vadadustat, an oral hypoxia-inducible factor prolyl hydroxylase inhibitor, for treatment of anemia of chronic kidney disease: Two randomized phase 2 trials in Japanese patients. Nephrol. Dial. Transplant. 36, 1244–1252. doi:10.1093/ndt/gfaa060
Nangaku, M., Kondo, K., Ueta, K., Kokado, Y., Kaneko, G., Matsuda, H., et al. (2021). Efficacy and safety of vadadustat compared with darbepoetin alfa in Japanese anemic patients on hemodialysis: A phase 3, multicenter, randomized, double-blind study. Nephrol. Dial. Transplant. 36, 1731–1741. doi:10.1093/ndt/gfab055
Nikolakopoulou, A., Higgins, J. P., Papakonstantinou, T., Chaimani, A., Del Giovane, C., Egger, M., et al. (2019). Assessing confidence in the results of network meta-analysis (CINeMA). BioRxiv, 597047, doi:10.1101/597047
Nissenson, A. R., Swan, S. K., Lindberg, J. S., Soroka, S. D., Beatey, R., Wang, C., et al. (2002). Randomized, controlled trial of darbepoetin alfa for the treatment of anemia in hemodialysis patients. Am. J. kidney Dis. 40, 110–118. doi:10.1053/ajkd.2002.33919
Noonan, M. L., Ni, P., Agoro, R., Sacks, S. A., Swallow, E. A., Wheeler, J. A., et al. (2021). The HIF-PHI BAY 85-3934 (molidustat) improves anemia and is associated with reduced levels of circulating FGF23 in a CKD mouse model. J. Bone Mineral Res. 36, 1117–1130. doi:10.1002/jbmr.4272
Palmer, S. C., Navaneethan, S. D., Craig, J. C., Johnson, D. W., Tonelli, M., Garg, A. X., et al. (2010). Meta-analysis: Erythropoiesis-stimulating agents in patients with chronic kidney disease. Ann. Intern. Med. 153, 23–33. doi:10.7326/0003-4819-153-1-201007060-00252
Palmer, S. C., Saglimbene, V., Mavridis, D., Salanti, G., Craig, J. C., Tonelli, M., et al. (2014). Erythropoiesis-stimulating agents for anaemia in adults with chronic kidney disease: A network meta-analysis. Cochrane Database Syst. Rev. 2014, CD010590. doi:10.1002/14651858.CD010590.pub2
Parfrey, P. (2021). Hypoxia-inducible factor prolyl hydroxylase inhibitors for anemia in CKD. New England Journal of Medicine, 2390–2391. 385, 25, doi:10.1056/NEJMe2117100
Provenzano, R., Besarab, A., Wright, S., Dua, S., Zeig, S., Nguyen, P., et al. (2016). Roxadustat (FG-4592) versus epoetin alfa for anemia in patients receiving maintenance hemodialysis: A phase 2, randomized, 6-to 19-week, open-label, active-comparator, dose-ranging, safety and exploratory efficacy study. Am. J. Kidney Dis. 67, 912–924. doi:10.1053/j.ajkd.2015.12.020
Provenzano, R., Shutov, E., Eremeeva, L., Korneyeva, S., Poole, L., Saha, G., et al. (2021). Roxadustat for anemia in patients with end-stage renal disease incident to dialysis. Nephrol. Dial. Transplant. 36, 1717–1730. doi:10.1093/ndt/gfab051
Salanti, G., Del Giovane, C., Chaimani, A., Caldwell, D. M., and Higgins, J. P. (2014). Evaluating the quality of evidence from a network meta-analysis. PloS one 9, e99682. doi:10.1371/journal.pone.0099682
Schödel, J., and Ratcliffe, P. J. (2019). Mechanisms of hypoxia signalling: New implications for nephrology. Nat. Rev. Nephrol. 15, 641–659. doi:10.1038/s41581-019-0182-z
Semenza, G. L. (2011). Oxygen sensing, homeostasis, and disease. N. Engl. J. Med. 365, 537–547. doi:10.1056/NEJMra1011165
Singh, A. K., Carroll, K., Perkovic, V., Solomon, S., Jha, V., Johansen, K. L., et al. (2021). Daprodustat for the treatment of anemia in patients undergoing dialysis. N. Engl. J. Med. 385, 2325–2335. doi:10.1056/NEJMoa2113379
Sinha, S. D., Bandi, V. K., Bheemareddy, B. R., Thakur, P., Chary, S., Mehta, K., et al. (2019). Efficacy, tolerability and safety of darbepoetin alfa injection for the treatment of anemia associated with chronic kidney disease (CKD) undergoing dialysis: A randomized, phase-III trial. BMC Nephrol. 20, 90–99. doi:10.1186/s12882-019-1209-1
Szczech, L. A., Barnhart, H. X., Inrig, J. K., Reddan, D. N., Sapp, S., Califf, R. M., et al. (2008). Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney Int. 74, 791–798. doi:10.1038/ki.2008.295
Taylor, C. T., Doherty, G., Fallon, P. G., and Cummins, E. P. (2016). Hypoxia-dependent regulation of inflammatory pathways in immune cells. J. Clin. investigation 126, 3716–3724. doi:10.1172/JCI84433
Ueda, N., and Takasawa, K. (2017). Role of hepcidin-25 in chronic kidney disease: Anemia and beyond. Curr. Med. Chem. 24, 1417–1452. doi:10.2174/0929867324666170316120538
Vanderhaeghen, T., Vandewalle, J., and Libert, C. (2020). Hypoxia-inducible factors in metabolic reprogramming during sepsis. FEBS J. 287, 1478–1495. doi:10.1111/febs.15222
Vanrenterghem, Y., Barany, P., Mann, J. F., Kerr, P. G., Wilson, J., Baker, N. F., et al. (2002). Randomized trial of darbepoetin alfa for treatment of renal anemia at a reduced dose frequency compared with rHuEPO in dialysis patients. Kidney Int. 62, 2167–2175. doi:10.1046/j.1523-1755.2002.00657.x
Veroniki, A. A., Vasiliadis, H. S., Higgins, J. P., and Salanti, G. (2013). Evaluation of inconsistency in networks of interventions. Int. J. Epidemiol. 42, 919–345. doi:10.1093/ije/dyt106
Wang, L., Yin, H., Yang, L., Zhang, F., Wang, S., and Liao, D. (2022). The efficacy and safety of roxadustat for anemia in patients with chronic kidney disease: A meta-analysis. Front. Pharmacol. 13, 779694. doi:10.3389/fphar.2022.779694
Wheeler, J. A., and Clinkenbeard, E. L. (2019). Regulation of fibroblast growth factor 23 by iron, EPO, and HIF. Curr. Mol. Biol. Rep. 5, 8–17. doi:10.1007/s40610-019-0110-9
Zheng, Q., Wang, Y., Yang, H., Sun, L., Fu, X., Wei, R., et al. (2021b). Efficacy and safety of daprodustat for anemia therapy in chronic kidney disease patients: A systematic review and meta-analysis. Front. Pharmacol. 11, 573645. doi:10.3389/fphar.2020.573645
Zheng, Q., Yang, H., Fu, X., Huang, Y., Wei, R., Wang, Y., et al. (2021a). The efficacy and safety of roxadustat for anemia in patients with chronic kidney disease: A meta-analysis. Nephrol. Dial. Transplant. 36, 1603–1615. doi:10.1093/ndt/gfaa110
Zheng, Q., Yang, H., Sun, L., Wei, R., Fu, X., Wang, Y., et al. (2020). Efficacy and safety of HIF prolyl-hydroxylase inhibitor vs epoetin and darbepoetin for anemia in chronic kidney disease patients not undergoing dialysis: A network meta-analysis. Pharmacol. Res. 159. doi:105020doi:10.1016/j.phrs.2020.105020
Keywords: HIF-PHIs, ESAS, effectiveness, DD-CKD, anemia, acceptability
Citation: Huang Q, You M, Huang W, Chen J, Zeng Q, Jiang L, Du X, Liu X, Hong M and Wang J (2023) Comparative effectiveness and acceptability of HIF prolyl-hydroxylase inhibitors versus for anemia patients with chronic kidney disease undergoing dialysis: a systematic review and network meta-analysis. Front. Pharmacol. 14:1050412. doi: 10.3389/fphar.2023.1050412
Received: 21 September 2022; Accepted: 19 June 2023;
Published: 13 July 2023.
Edited by:
Francesco Locatelli, Alessandro Manzoni Hospital, ItalyReviewed by:
Ciro Esposito, University of Pavia, ItalyCopyright © 2023 Huang, You, Huang, Chen, Zeng, Jiang, Du, Liu, Hong and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiong Huang, NTMzOTQ3MjhAcXEuY29t; Jing Wang, MTAzNDk1MjI4NkBxcS5jb20=; Ming Hong, MzIyNzM3NDU5N0BxcS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.