- 1Department of Pharmacy, Affiliated Hospital of Zunyi Medical University, Zunyi, China
- 2Department of Rheumatology and Immunology, Affiliated Hospital of Zunyi Medical University, Zunyi, China
Azathioprine is clinically used as an immunosuppressant for treating autoimmune diseases. However it has narrow therapeutic indices due to frequent myelosuppression. Polymorphic variants of genes coding for thiopurine S-methyltransferase (TPMT) and nucleoside diphosphate-linked moiety X motif 15 (NUDT15) are critical determinants of AZA intolerance, and the differences in frequencies of the two genetic variants exist among people of different ethnicities. Most reports regarding NUDT15 variant, AZA-induced myelosuppression occurred in patients with inflammatory bowel disease and acute lymphoblastic leukemia. Moreover, detailed clinical characteristics were not frequently reported. Here we present the case of a young Chinese female with the NUDT15 c.415C>T (rs116855232, TT) homozygous variant and wild-type TPMT*2 (rs1800462), TPMT*3B (rs1800460), and TPMT*3C (rs1142345) who received high doses of AZA (2.3 mg/kg/d) for systematic lupus erythematosus and had not been told to undergo routine blood cell counts during AZA ingestion. The patient had suffered from severe AZA-induced myelosuppression and alopecia. Moreover, dynamic changes in blood cell counts and responses to treatment were observed. We also conducted a systematic review of published case reports of patients exclusively with NUDT15 c.415C>T homozygous or heterozygous variants to review the characteristics of dynamic changes in blood cells so as to provide reference information for clinical treatment.
1 Introduction
Azathioprine (AZA) is a prodrug for mercaptopurine (6-MP) that exhibits similar pharmacologic effects to those of other thiopurines (6-MP and thioguanine) and is clinically used as an immunosuppressant for treating severe rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), dermatomyositis, autoimmune chronic active hepatitis, and spontaneous thrombocytopenic purpura. Thiopurines have narrow therapeutic indices owing to frequent myelosuppression commonly characterized by neutropenia and sometimes associated with thrombocytopenia and aplastic anemia (Montgomery et al., 2022).
Upon administration, AZA is first nonenzymatically converted into 6-MP and then enzymatically metabolized into pharmacologically active 6-thioguanine nucleotides (6-TGN) and deoxythioguanosine phosphates. Thiopurine S-methyltransferase (TPMT), which strongly inactivates thiopurine metabolites (Booth et al., 2011), and nucleoside diphosphate-linked moiety X motif 15 (NUDT15), which dephosphorylates thiopurine effector metabolites such as TdGTP and inhibits active metabolite loading onto nucleic acids (Yang et al., 2014), are two major enzymes that work alongside other factors, such as intestinal microbial enzymes (Wang et al., 2022), to influence AZA metabolism. TPMT genetic polymorphisms and NUDT15 variations are therefore critical determinants of thiopurine intolerance (Cargnin et al., 2018). TPMT single nucleotide polymorphisms (SNPs) result in unstable proteins and enhance TPMT degradation (Relling et al., 2019). Variants TPMT*2, TPMT*3A, TPMT*3B, and TPMT*3C are relatively common in Caucasians and Africans, and TPMT*3C is the most frequent variant in Asians, although its frequency is still lower than that of European populations (Kishibe et al., 2018; Su et al., 2020; Chen et al., 2021). NUDT15 is known to have 26 alleles, and NUDT15*2 (p.V18_V19insGV and c.415C>T), *3 (c.415C>T), and *9 (c.50delGAGTCG) are recognized as loss-of-function variants (Tanaka and Saito, 2021). NUDT15 c.415C>T influences protein stability, inhibits or prevents enzyme activity (Valerie et al., 2016), and is relatively common in East Asians and Hispanics but less frequent in Europeans (Moriyama et al., 2016). In Chinese populations, the heterozygote and homozygote frequencies of NUDT15 c.415C>T are 19.95% and 1.97%, respectively (Chen et al., 2021). A report showed that the incidence of severe myelosuppression in patients with homozygote NUDT15 c.415C>T taking 0.85 (0.5–1.09) mg/kg/d AZA was 85.7% (Yang et al., 2014).
Herein, we present a case study of a young Chinese female with SLE taking 2.3 mg/kg/d AZA and experiencing severe AZA-induced myelosuppression and alopecia due to a failure to perform AZA metabolism-related genotyping prior to treatment initiation and a lack of routine blood cell counts during AZA administration. The genotype of the patient was revealed to include the NUDT15 c.415C>T(TT) homozygous variant with wild-type TPMT*2, TPMT*3B, and TPMT*3C, as detected when myelosuppression appeared, and these characteristics are described to provide a reference for clinical treatment not only to assess status changes of myelosuppression when it happened but also to remind physicians to perform genotyping before prescribing AZA to determine whether AZA is suitable for patients. Moreover, we present the results of a systematic review of published case reports in order to characterize dynamic changes regarding the severity of AZA-induced leukopenia in patients with NUDT15 c.415C>T homozygous or heterozygous variants alone with the purpose to provide more reference information for clinical treatment of AZA-induced myelosuppression in specific patients with only NUDT15 c.415C>T variants.
2 Case presentation
An 18-year-old female Chinese patient was diagnosed with SLE at the age of 15 years according to the 2012 Systemic Lupus International Collaborating Clinics (SLICC) criteria. The patient presented with clinical manifestations of oral ulcers, cutaneous lupus, nephritis, and both antinuclear (ANA) and antiphospholipid antibodies on 19 August 2018. After 3 years of treatment with hydroxychloroquine (200 mg BID) and prednisone (starting with 30 mg QD with gradual reduction), hydroxychloroquine was discontinued. During the prednisone dose reduction, the attending doctor prescribed her AZA (50 mg bid, 2.32 mg/kg/d) on 19 April 2022, without identifying the presence of AZA metabolism-related genes, likely due to negligence of the physician or lack of knowledge regarding AZA metabolism-related gene testing. On 11 May 2022 (after taking AZA for 22 d), this patient was admitted to a local hospital with a fever (axillary temperature of 38.9 °C), pharyngalgia accompanied by pigmentation of fingertip joint skins, and alopecia. Routine blood tests revealed a white blood cell count of 0.45 × 109 cells/L with normal liver and kidney function. The physician considered the above symptoms adverse reactions to AZA and suggested stopping AZA administration and transferring the patient to the Rheumatic Immunology Department of our hospital. This patient was admitted to our hospital on 13 May 2022 (day 3 after withdrawing AZA). A blood sample was collected to assess AZA metabolism-related genes TPMT and NUDT15 using commercially outsourced next-generation sequencing (Guizhou KingMed Center for Clinical Laboratory, Guiyang city, China) (Table 1) and to determine blood cell counts (Table 1 and Supplementary 1) and liver and kidney function. Liver and kidney function were normal, and antibody testing results were weakly positive for ANAs and negative for antiphospholipid antibodies. No cutaneous lupus was found, and SLE activity was considered stable. The patient exhibited rapid onset of alopecia, and no hair was observed approximately 10 d after discontinuing AZA treatment. During discontinuation, the patient was given folic acid (10 mg TID p.o.) to compete with AZA for hypoxanthine binding; recombinant human granulocyte colony-stimulating factor (150 μg QD∼300 μg BID i.h.); recombinant human thrombopoietin injection (15,000 U QD i.h.) to increase leucocyte and platelet counts; methylprednisolone to reduce inflammation; and meropenem, vancomycin, voriconzole, and famciclovir to treat infections (Figure 1). Despite treatment to promote leukocyte and platelet production, the lowest platelet count of 15 × 109 platelets/L was observed on day 11 (with multiple subcutaneous ecchymosis and negative platelet antibody results), 2 d after the lowest white blood cell count. Platelet recovery began 2 d after that of white blood cells. During treatment, the patient was transferred to the Intensive Care Unit (ICU) with a neutrophil count of 0.00 × 109 cells/L and received supportive care for 10 d. Neutrophil count remained below 0.05 × 109 cells/L for 9 d before the patient was transferred back to the Rheumatic Immunology Department. The patient recovered from myelosuppression gradually, and white blood cell counts were recovered on day 18 after AZA discontinuation (Supplementary 1). The patient was discharged from hospital on 15 June 2022 (day 46), and attending physicians chose hydroxychloroquine (200 mg BID) as the follow-up treatment. The total cost of hospitalization was 83,749.00 Chinese yuan, based on the hospital information system. Informed consent was obtained from the patient for publication of the case report.
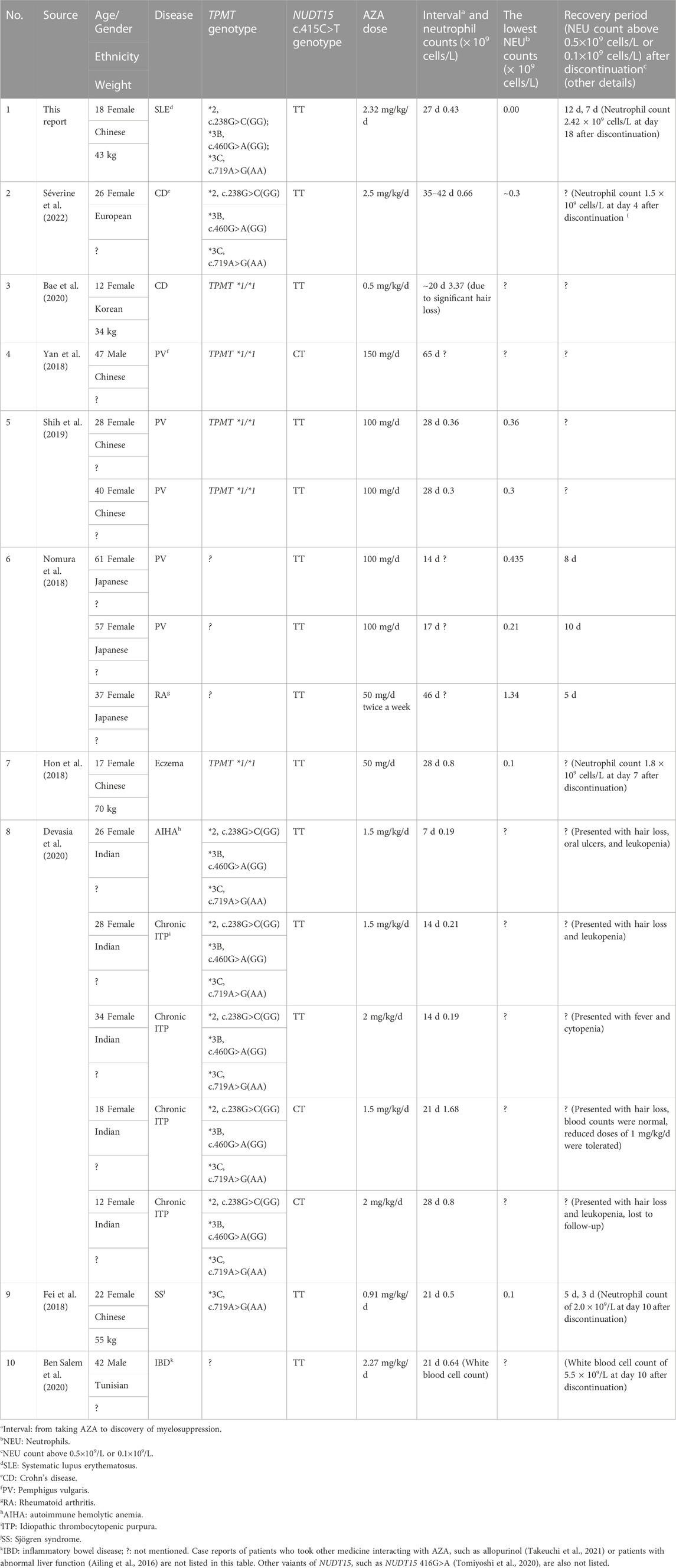
TABLE 1. Reported characteristics of patients with homozygous variant NUDT15 c.415C>T genotypes taking azathioprine (AZA).

FIGURE 1. White blood cell, hemoglobin, and platelet counts for a young Chinese patient with SLE and schedule of treatment. WBC: white blood cells; NEU: neutrophils; LYM: lymphocytes; RBC: red blood cells; HGB: hemoglobin; RIM: rheumatic immunology department. AZA: azathioprine tablet; Rh-GCSF: recombinant human granulocyte colony stimulating factor injection; Methylprednisolone: methylprednisolone sodium succinate for injection; Immunoglobulin: human immunoglobulin for intravenous injection; Folic: folic acid tablets; Piperacillin and sulbactam: piperacillin sodium and sulbactam sodium for injection; Meropenem: meropenem for injection; Azithromycin: azithromycin for injection; Vancomycin: vancomycin for injection; Voriconazole: voriconazole for injection; Famciclovir: famciclovir dispersible tablets.
Three months after patient discharge, follow-up revealed that the patient followed medical advice to repeat routine blood tests at the end of the third week after discharge. Results indicated that white blood cell counts had effectively recovered, and the immunosuppressant tacrolimus was added as an SLE treatment. Two months and 2 weeks after discharge, routine blood tests showed a normal range for white blood cell count (4.86 × 109 cells/L), with a neutrophil proportion of 34%, a little lower than normal. Her hair had grown to 3.5 cm and was sparse but without other discomfort. Notably, because of her hospitalization, she missed a college entrance examination and had to repeat her studies for another year before taking the exam. Her mother quit her job to take care of her, which had an impact on their income. When we reached out to her mother, she complained that the doctor failed to recommend a genotype test before prescribing AZA to her daughter and wished for compensation from the hospital.
3 Literature review
We conducted this review with the goal of providing specific information about neutrophil counts and AZA administration and recovery durations in patients with NUDT15 c.415 C>T only. Therefore, articles describing patients with variants of other sites of NUDT15 or other genes were excluded. In addition, case reports of patients who took medicines interacting with AZA or patients with abnormal liver function are not included in order to reduce the impact of other factors on AZA metabolism. We searched PubMed with retrieval terms “((((“Leukopenia”[Mesh]) OR (((Leukopenias) OR (Leukocytopenia)) OR (Leukocytopenias))) OR (myelosuppression)) AND (((“Azathioprine”[Mesh]) OR (azathioprine)) OR (((Imuran) OR (Immuran)) OR (Imurel)))) AND (NUDT15)” for article published from inception to 31 July 2022 and then performed a secondary search of cited documents for our bibliography retrieval. A total of 67 articles were found. After reading the titles and the full texts, nine reports that included specific neutrophil counts and medication regime-related information were retrieved (Table 1).
4 Results and discussion
A study including 70 Chinese patients with SLE or RA demonstrated that the frequency of TPMT was 1% whereas that of NUDT15 was 14%. Among NUDT15 variants, wild-type, heterozygous, and homozygous NUDT15 c.415C>T genotypes were carried by 51 (72.9%), 18 (25.7%), and 1 (1.4%) patients, respectively (Su et al., 2020), and by 86.9%, 11.5%, and 1.5% of 60 Indian patients, respectively (Shah et al., 2017). Although the homozygote frequency of NUDT15 c.415C>T is lower (1.97%) than that of heterozygote (19.95%) in Chinese populations (Chen et al., 2021), incidence of severe myelosuppression in patients with homozygous NUDT15 c.415C>T taking 0.85 (0.5–1.09) mg/kg/d AZA was high (85.7%) (Yang et al., 2014). We aimed to report the clinical characteristics and response to treatment of a young Chinese female the NUDT15 c.415C>T (TT) homozygous variant and SLE suffering from severe AZA-induced myelosuppression due to failure of the attending physician to perform AZA metabolism-related genotyping before administering AZA and to recommend routine blood cell counts analysis during AZA administration. This case description provides a valuable reference for clinical treatment and serves as a reminder to physicians to perform genotyping before prescribing AZA to patients. We also conducted a systematic review of published case reports to characterize dynamic changes regarding the severity of AZA-induced leukopenia in patients with NUDT15 c.415C>T homozygous or heterozygous variants.
To the best of our knowledge, this is the first report of a patient with SLE and the homozygote NUDT15 c.415C>T variant who ingested high doses of AZA for 22 d and exhibited severe myelosuppression. The lowest initial neutrophil count was 0.00 × 109 cells/L and neutrophil counts were below 0.05 × 109 cells/L for 9 d during hospitalization, with a recovery period of 18 d after discontinuation of AZA; this is the longest recovery time reported. A report indicated that a European female patient with Crohn’s disease and the homozygous NUDT15 c.415C>T variant ingested a higher dose of AZA (2.5 mg/kg/d) for 35–42 d before withdrawal. However, the lowest neutrophil count in this patient was 0.3 × 109 cells/L and the recovery period was only 4 d after discontinuation of the drug (Séverine et al., 2022). According to one case report (Shih et al., 2019), patients with the same genotype, disease, and ethnicity taking similar doses for a similar period may develop myelosuppression with similar characteristics. Whether the difference in AZA tolerance between the Chinese and European patients was due to disease or ethnicity differences requires further investigation.
Moriyama et al. (Moriyama et al., 2016) reported that multiple heterozygous (c.415C > T; c.416G > A) and homozygous (c.415C > T) NUDT15 variant proteins exhibit similar enzymatic activity in vitro. Otsuka et al. (Otsuka et al., 2019) reported a 57-year-old Japanese man with SLE and multiple heterozygous NUDT15 variants (c.415C > T; c.416G > A) who had ingested a lower dose of AZA (50 mg/d) than the patient in our report and presented with severe AZA-induced leukopenia and neutropenia, which was sustained for 4 weeks (longer than our female Chinese patient). Whether this discrepancy is due to differences in ethnicity or NUDT15 genetic variants remains to be elucidated.
The case of a young Chinese patient described here serves to remind physicians to perform genotyping before prescribing AZA to determine whether AZA is suitable for patients. Starting doses of AZA should be adjusted based on TPMT and NUDT15 genotypes to reduce the incidence of adverse drug reactions (Relling et al., 2019). Patients with NUDT15 heterozygous genotypes received 50 mg AZA daily, while homozygotes were recommended to take alternative drugs (Tanaka and Saito, 2021). If there are not alternative drugs available for patients with homozygous genotype of NUDT15 c.415C > T, we recommend a dose of 0.2 mg/kg/d AZA as the initial dose, as described by Fan et al. (Fan et al., 2019). If NUDT15 genotyping is unavailable, initiation of AZA therapy at a low dose, such as 0.5 mg/kg/day (Yang et al., 2014), is recommended, along with close observation of hair loss and blood cell counts within 2 weeks. This may represent an alternative method for preventing thiopurine-induced early leukopenia in Asian patients. According to Table 1, leukopenia may occur in patients with homozygous NUDT15 c.415C>T variant genotypes within 7–14 d of AZA administration onset. In Asian populations for whom genotyping is unavailable, we recommend weekly monitoring of white cell counts for at least 1 month to ensure early identification of myelosuppression; further testing should be conducted every 2 weeks and then once a month. Moreover, if it is needed, monitoring of 6-TGN target levels may also be a consideration (Kang et al., 2020).
Based on the findings described above, it is inappropriate to prescribe AZA without first testing for the presence of treatment-related genes and then performing routine monitoring of blood cell counts during AZA administration. The failure to perform these steps led to serious myelosuppression in a patient who was hospitalized for more than 1 month, at a total cost of 83,749.00 Chinese yuan, whereas the cost of genetic testing is only 380.00 Chinese yuan. Furthermore, because of her hospitalization, this patient failed to take the annual college entrance examination that is required for every student, setting her back academically. Her parents expressed the intention to claim compensation, which would cause great economic and reputative losses to the hospital. In order to avoid the recurrence of such an event, as clinical pharmacists, we disseminated the information related to this medication (specifically the use of thiopurines) to physicians in our hospital.
There are some limitations to this study. In this patient, only NUDT15 c.415C>T; TPMT *2, 238 G>C; TPMT *3B, c.460 G>A; and TPMT *3C, 719 A>G were detected based on their relatively high frequency in China. Full-length TPMT and NUDT15 sequencing was not performed. Moreover, we conducted this review with the goal of providing specific information about neutrophil counts and AZA administration and recovery durations in patients with NUDT15 c.415 C>T only. Therefore, articles describing patients with variants of other sites of NUDT15 or other genes were excluded. Although we conducted a review to identify dynamic changes in myelosuppression induced by AZA in patients with NUDT15 c.415C>T, such dynamic changes in myelosuppression were rarely described. Due to the low frequency of homozygous variant NUDT15 c.415C>T genotype and incidence of severe myelosuppression in patients with homozygous variant NUDT15 c.415C>T genotype, further detailed case reports are required to illustrate the clinical characteristics of patients taking AZA exclusively with the homozygous variant NUDT15 c.415C>T genotype. We believe that our study makes a significant contribution to the literature because it provides references for physicians to treat patients with homozygous variant NUDT15 c.415C>T genotype and suffered from AZA-induced severe myelosuppression. More importantly, our report will remind clinicians of the importance to test the metabolism-related genes prior to prescribing AZA as well as routine blood during patients taking AZA.
Data availability statement
The datasets for this article are not publicly available due to concerns regarding participant/patient anonymity. Requests to access the datasets should be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the ethics committee of Affiliated Hospital of Zunyi Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JG designed the study, searched databases, performed the selection of studies, and analyzed the data; YL contributed to interpreting results. JG and YW contributed to interpreting results and writing the manuscript. All authors reviewed and approved the final manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication. All authors contributed to the article and approved the submitted version.
Funding
This research was supported financially by grants from Science and Technology Support Project of the Department of Science and Technology in Guizhou Province (Qian Ke He support S 2020(2319) and 2019(2829) and the Doctoral Starting Fund of the Affiliated Hospital of Zunyi Medical University (Hospital 2019(06)).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ailing, Z., Jing, Y., Jingli, L., Yun, X., and Xiaojian, Z. (2016). Further evidence that a variant of the gene nudt15 may be an important predictor of azathioprine-induced toxicity in Chinese subjects: A case report. J. Clin. Pharm. Ther. 41, 572–574. doi:10.1111/jcpt.12420
Bae, J., Choe, B., and Kang, B. (2020). Prevention of thiopurine-induced early leukopenia in a Korean pediatric patient with crohn's disease who turned out to possess homozygous mutations in nudt15 r139c. Yeungnam Univ. J. Med. 37, 332–336. doi:10.12701/yujm.2020.00178
Ben Salem, C., Hachana, M., Fathallah, N., Eya, H., and Ksiaa, M. (2020). First case of azathioprine-induced severe hematotoxicity in a tunisian patient with homozygous tt for nudt15 rs116855232. Ann. Pharmacother. 54, 509–510. doi:10.1177/1060028019887921
Booth, R., Ansari, M., Loit, E., Tricco, A., Weeks, L., Doucette, S., et al. (2011). Assessment of thiopurine s-methyltransferase activity in patients prescribed thiopurines: A systematic review. Ann. Intern. Med. 154, 814–823. doi:10.7326/0003-4819-154-12-201106210-00009
Cargnin, S., Genazzani, A. A., Canonico, P. L., and Terrazzino, S. (2018). Diagnostic accuracy of nudt15 gene variants for thiopurine-induced leukopenia: A systematic review and meta-analysis. Pharmacol. Res. 135, 102–111. doi:10.1016/j.phrs.2018.07.021
Chen, Z. Y., Zhu, Y. H., Zhou, L. Y., Shi, W. Q., Qin, Z., Wu, B., et al. (2021). Association between genetic polymorphisms of metabolic enzymes and azathioprine-induced myelosuppression in 1,419 Chinese patients: A retrospective study. Front. Pharmacol. 12, 672769. doi:10.3389/fphar.2021.672769
Devasia, A., Illangeswaran, R., Raj, I., George, B., and Balasubramanian, P. (2020). Nudt15 polymorphism explains serious toxicity to azathioprine in indian patients with chronic immune thrombocytopenia and autoimmune hemolytic anemia: A case series. Drug metabolism personalized Ther. 35. doi:10.1515/dmpt-2020-0128
Fan, X., Yin, D., Men, R., Xu, H., and Yang, L. (2019). Nudt15 polymorphism confer increased susceptibility to thiopurine-induced leukopenia in patients with autoimmune hepatitis and related cirrhosis. Front. Pharmacol. 10, 346. doi:10.3389/fphar.2019.00346
Fei, X., Shu, Q., Hua, B. Z., Wang, S. Y., Chen, Z. Y., Ge, W. H., et al. (2018). Nudt15 r139c variation increases the risk of azathioprine-induced toxicity in Chinese subjects: Case report and literature review. Med. Baltim. 97, e0301. doi:10.1097/MD.0000000000010301
Hon, K., Chang, M., Chong, S., Yuen, Y., and Tsui, S. (2018). Adverse effects of azathioprine in a child and her mother with eczema. Indian J. Pediatr. 85, 918–919. doi:10.1007/s12098-018-2687-z
Kang, B., Kim, T. J., Choi, J., Baek, S. Y., Ahn, S., Choi, R., et al. (2020). Adjustment of azathioprine dose should be based on a lower 6-tgn target level to avoid leucopenia in nudt15 intermediate metabolisers. Aliment. Pharmacol. Ther. 52, 459–470. doi:10.1111/apt.15810
Kishibe, M., Nozaki, H., Fujii, M., Iinuma, S., Ohtsubo, S., Igawa, S., et al. (2018). Severe thiopurine-induced leukocytopenia and hair loss in Japanese patients with defective nudt15 variant: Retrospective case-control study. J. Dermatol 45, 1160–1165. doi:10.1111/1346-8138.14588
Montgomery, H. D., Agarwal, A. M., and Lim, M. Y. (2022). A case of azathioprine-induced aplastic anemia. Int. J. Lab. Hematol. 44, 1015–1016. doi:10.1111/ijlh.13927
Moriyama, T., Nishii, R., Perez-Andreu, V., Yang, W., Klussmann, F., Zhao, X., et al. (2016). Nudt15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat. Genet. 48, 367–373. doi:10.1038/ng.3508
Nomura, H., Kurihara, Y., Saito, M., Fukushima, A., Shintani, Y., Shiiyama, R., et al. (2018). Azathioprine-induced alopecia and leukopenia associated with nudt15 polymorphisms. J. Eur. Acad. Dermatol Venereol. 32, e386–e389. doi:10.1111/jdv.15028
Otsuka, M., Koga, T., Sumiyoshi, R., Furukawa, K., Okamoto, M., Endo, Y., et al. (2019). Novel multiple heterozygous nudt15 variants cause an azathioprine-induced severe leukopenia in a patient with systemic lupus erythematosus. Clin. Immunol. 200, 64–65. doi:10.1016/j.clim.2019.02.004
Relling, M. V., Schwab, M., Whirl-Carrillo, M., Suarez-Kurtz, G., Pui, C. H., Stein, C. M., et al. (2019). Clinical pharmacogenetics implementation consortium guideline for thiopurine dosing based on tpmt and nudt15 genotypes: 2018 update. Clin. Pharmacol. Ther. 105, 1095–1105. doi:10.1002/cpt.1304
Séverine, W., Xavier, K., and Jean-Charles, C. (2022). A rare case of azathioprine-induced leukopenia in an European woman. Acta Clin. Belg. 77, 163–167. doi:10.1080/17843286.2020.1812829
Shah, S. A., Paradkar, M., Desai, D., and Ashavaid, T. F. (2017). Nucleoside diphosphate-linked moiety x-type motif 15 c415t variant as a predictor for thiopurine-induced toxicity in indian patients. J. Gastroenterol. Hepatol. 32, 620–624. doi:10.1111/jgh.13494
Shih, Y. C., Zou, Y. R., Wang, B., Zheng, J., and Pan, M. (2019). Azathioprine-induced myelosuppression in two pemphigus vulgaris patients with homozygous polymorphism of nudt15. J. Dermatol 46, e59–e61. doi:10.1111/1346-8138.14542
Su, S. S., Lin, Y. F., and Zhou, H. (2020). Association of thiopurine s-methyltransferase and nudt15 polymorphisms with azathioprine-induced myelotoxicity in Chinese patients with rheumatological disease. Chin. Med. J. Engl. 133, 1002–1004. doi:10.1097/CM9.0000000000000756
Takeuchi, Y., Noritake, H., Matsumoto, M., Umemura, M., Yamashita, M., Kitsugi, K., et al. (2021). Azathioprine-induced severe myelosuppression accompanied by massive hair loss and painful oral ulcer in an autoimmune hepatitis patient with NUDT15 minor variant: A case report. Clin. case Rep. 9, e04696. doi:10.1002/ccr3.4696
Tanaka, Y., and Saito, Y. (2021). Importance of nudt15 polymorphisms in thiopurine treatments. J. Pers. Med. 11, 778. doi:10.3390/jpm11080778
Tomiyoshi, K., Sato, H., Tominaga, K., Kawata, Y., Okamoto, D., Kakuta, Y., et al. (2020). Rare genotype of his/his in nudt15 codon 139 and thiopurine-associated adverse events in a case of ulcerative colitis. Intern. Med. (Tokyo, Jpn. 59, 1611–1613. doi:10.2169/internalmedicine.4261-19
Valerie, N., Hagenkort, A., Page, B., Masuyer, G., Rehling, D., Carter, M., et al. (2016). Nudt15 hydrolyzes 6-thio-deoxygtp to mediate the anticancer efficacy of 6-thioguanine. Cancer Res. 76, 5501–5511. doi:10.1158/0008-5472.Can-16-0584
Wang, S., Qin, Y., Wen, Q., Xia, Q., Gu, R., Wang, S., et al. (2022). Intestinal microbiota-mediated biotransformations alter the pharmacokinetics of the major metabolites of azathioprine in rats after oral administration. Drug Metab. Pharmacokinet. 45, 100458. doi:10.1016/j.dmpk.2022.100458
Yan, W., Zhou, Y. H., Wang, L., Xiao, J., and Li, W. (2018). Nudt15 polymorphism and severe azathioprine-induced myelosuppression in a Chinese man with pemphigus vulgaris. Br. J. Dermatol 178, e40–e41. doi:10.1111/bjd.15840
Keywords: azathioprine, myelosuppression, the nucleoside diphosphate-linked moiety X motif 15, NUDT15, gene polymorphism
Citation: Gu J, Lin Y and Wang Y (2023) Case report: NUDT15 polymorphism and severe azathioprine-induced myelosuppression in a young Chinese female with systematic lupus erythematosus: a case analysis and literature review. Front. Pharmacol. 14:1001559. doi: 10.3389/fphar.2023.1001559
Received: 09 August 2022; Accepted: 27 April 2023;
Published: 09 May 2023.
Edited by:
Md. Mustafizur Rahman, Khulna University, BangladeshReviewed by:
Amit P. Bhavsar, University of Alberta, CanadaMohammad Safiqul Islam, Noakhali Science and Technology University, Bangladesh
Ramnath Misra, Kalinga Institute of Medical Sciences (KIMS), India
Copyright © 2023 Gu, Lin and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan Gu, anVhbmp1YW4yMDExMjAxMkAxNjMuY29t; Yuhe Wang, d2FuZ3l1aGUyMEAxMjYuY29t
 Juan Gu
Juan Gu Yupei Lin2
Yupei Lin2