
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 01 November 2022
Sec. Inflammation Pharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.998944
This article is part of the Research TopicThe challenges of drug repurposing in diseases related to chronic inflammationView all 6 articles
Atherosclerosis (AS) is the chronic gradual degradation of arteries in combination with inflammation. Currently, the main research focus has been on interactions between inflammatory cells, inflammatory mediators, and immune mechanisms, while some studies have reported natural drugs were exerting a critical role against AS, whereas the usage of natural drugs was always limited by various factors such as poor penetration across biological barriers, low bioavailability, and unclear mechanisms. Herein, we reviewed the potential targets for inflammation against AS, discussed the underlying mechanisms of natural drugs for AS, particularly highlighted the dilemma of current research, and finally, offered perspectives in this field.
Atherosclerosis (AS) is a major medical and social issue, with significant clinical morbidity and mortality manifestations (Libby, 2021). AS is a multifactorial disease characterized by degenerative changes in the aorta wall, followed by lumen occlusion and restricted blood supply to organs and tissues. Subclinical (asymptomatic) AS has the most extensive pathology. It is accepted that many young individuals have atherosclerotic lesions that have developed for decades until the clinical manifestation. In middle age, individuals usually do not have AS clinical manifestations, and in fact, the incidence of atherosclerotic lesions accounts for almost 100% (Insull, 2009). However, current treatment approaches using lipid-lowering statins prevent only 65% of all cardiovascular events (Ridker et al., 2009).
Several theories have been expounded on the cause of AS. Early studies suggested that AS was mostly associated with lipid content, growth factors, and smooth muscle proliferation (Davies, 1994). However, in the past decades, scientists have become increasingly aware of inflammation factors in AS and its associated complications. Critically, inflammation is a trigger, which leads to plaque formation and development, as shown in Figure 1.
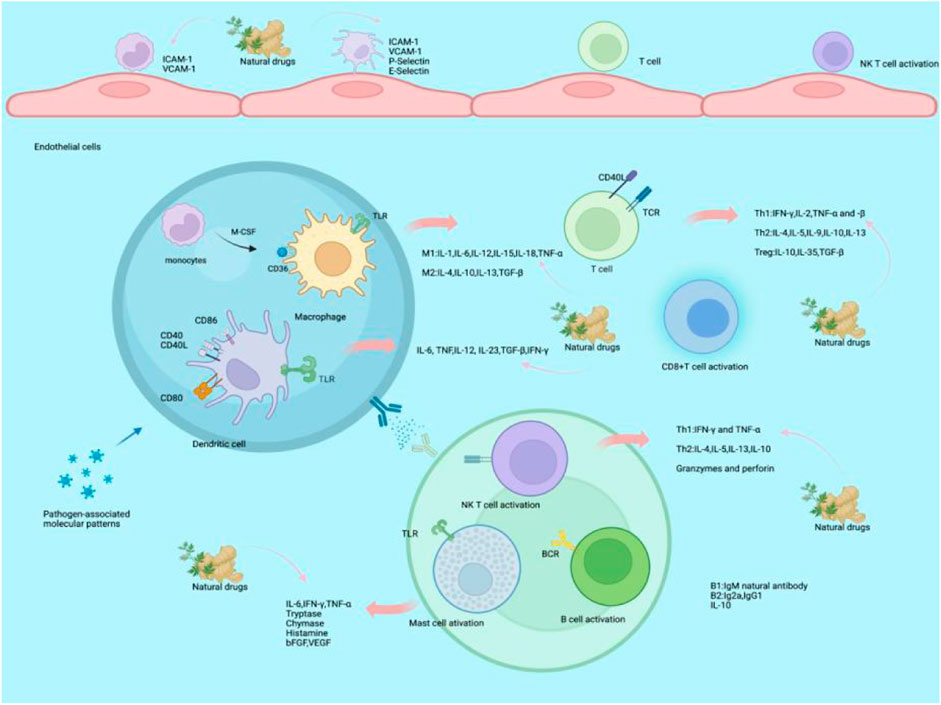
FIGURE 1. Mechanism of natural drugs on atherosclerotic inflammatory factors. At the early stage of AS, monocytes are tethered through the interaction between monocyte P-selectin glycoprotein-1 and endothelial P-selectin. With regard to adhesion and exudation, monocytes express very late antigen 4 (VLA4) and lymphocyte function-associated antigen-1 (LFA-1) to bind endothelial cell ligands, including VCAM-1 and ICAM-1. The monocytes differentiate into macrophages by M-CSF mediators. After activation, monocytes differentiate into two main phenotypes of macrophages: inflammatory M1 and regulatory M2 macrophages. M1 macrophages produce inflammation by secreting pro-inflammatory cytokines after intake of modified LDL and present antigens to T cells through macrophages using pattern-recognition receptors (PRRs), resulting in releasing pro-inflammatory cytokines to activate T cells, including IL-1, IL-6, IL-12, IL-15, IL-18, MIF, and TNF-α. M2 macrophages have anti-inflammatory functions to address plaque inflammation through efferocytosis and the release of Th2 cytokines such as IL-4, IL-10, and IL-13.
In recent years, the multi-target effects of natural drugs have attracted the attention of globe researchers. Studies have found that natural drugs could not only directly participate in the inflammatory process of AS (regulating monocytes, macrophages, and lymphocytes) but also indirectly regulate pro-inflammatory factors, including tumor necrosis factor-α (TNF-α), angiotensin (Ang)-II, interferon-γ (IFN-γ), interleukin-1 (IL-1), IL-6, vascular cellular adhesion molecule-1 (VCAM-1), intracellular adhesion molecule-1 (ICAM-1), and C-reactive protein (CRP). However, the use of natural drugs was always limited by several factors. For instance, repeated administration of natural drugs at high doses is always required and, as a result, may induce a series of side effects. Furthermore, most natural drugs are always characterized by low stability, poor penetration into the diseased site, and limited ability to cross cell membranes.
In this review, we summarized and discussed the regulatory and reparative effects of natural drugs on inflammation processes during AS occurrence and development. We discussed their merits for AS and their underlying mechanisms toward inflammation in AS. Last but not the least, in the course of this study, we found various problems in the research process of natural drugs and also put forward better opinions providing a clearer opinion for future research.
Tetramethylpyrazine (TMP) (2,3,5,6-tetramethylpyrazine) is an active ingredient of traditional Chinese medicine (TCM): Ligusticum chuanxiong has been used to treat AS for decades. It has been reported that TMP can dilate blood vessels, increase blood flow in coronary arteries and other organs, and accelerate microcirculation, which can effectively prevent thrombosis, so it is widely used in various ischemic diseases, including ischemia–reperfusion. In addition, in view of the protective effect of TMP on vascular endothelial cells (VECs), it can be used as a potential developing drug for the treatment of AS (Zhang et al., 2017). The effects of TMP on critical AS components have been intensively investigated. Sun et al. (2018) reported that TMP inhibited AS in ferroportin-1 Tek-Cre mice fed a high-cholesterol diet (HCD) by inhibiting hepcidin, nitric oxide (NO), endothelin-1 (ET-1), reactive oxygen species (ROS), malondialdehyde (MDA), superoxide dismutase (SOD), IL-1, IL-6, and TNF-α. Furthermore, Wu et al. (2012) demonstrated that TMP could improve vascular endothelial dysfunction caused by AS and achieve immunomodulatory effects on endothelial cells by inhibiting ICAM-1 and heat shock protein 60 (HSP60). These results suggested that TMP protects endothelial cells by reducing inflammatory factors and suppressing immune responses, thereby preventing AS.
Berberine (BBR) is an active ingredient extracted from Coptis, a TCM. Recent research reported that BBR exerted good therapeutic effects in AS. Wan et al. (2018) showed that BBR reduced adipsin, lipid, IL-6, and TNF-α serum levels in mice, decreased adipsin, phosphor-p38 (p-p38) mitogen-activated protein kinase (MAPK), and p-c-Jun N-terminal kinase (JNK) protein expression in mice aorta, and reduced adipin distribution in atherosclerotic lesions in apolipoprotein-E knockout Apolipoprotein-E knockout [ApoE (−/−)] mice. BBR reduced blood lipid levels, aortic ROS production, and serum levels of MDA, oxidized low-density lipoprotein (ox-LDL), and IL-6 in ApoE−/− mice, thereby reducing carotid atherosclerotic lesions in mice (Tan et al., 2020). BBR regulated lipid homeostasis and inhibited macrophage foam cell formation by inhibiting activator protein-1 (AP-1) activity and activation of the nuclear factor erythroid 2-related factor 2 (NRF2)/heme oxygenase-1 (HO-1) pathway (Yang et al., 2020). Elevated extracellular matrix metalloproteinase inducer (EMMPRIN) and matrix metalloproteinase-9 (MMP-9) levels in ox-LDL-induced macrophages caused vulnerable plaques via extracellular matrix degradation, while BBR inhibited ox-LDL-induced AMP-activated protein kinase (AMPK)-α and MAPK signaling in macrophages by increasing miR150-5p levels; therefore, P2X7 receptor-mediated (P2X7R) EMMPRIN and MMP-9 expression was inhibited (Lu et al., 2021). In the experiment of the innovative technology BBR-mediated sonodynamic therapy (BBR-SDT) on human leukemia monocytic cell line (THP-1) macrophages, macrophage autophagy was increased after treatment and foam cell autophagy resistance was blocked, inducing cholesterol efflux (Kou et al., 2017). BBR attenuated ox-LDL-induced monocyte adhesion to HUVECs by inhibiting VCAM-1 and ICAM-1 expression, thereby indicating that BBR provided protective roles in early AS stages (Huang et al., 2013). A BBR-embedded nanosystem reduced TNF-α, IL-6, IL-1β, IFN-γ, monocyte chemotactic protein (MCP), and macrophage inflammatory factor (MIP) plasma levels (Ma et al., 2020).
Piperlongumine (PL) is a natural small-molecule alkaloid isolated from Piper longum. PL inhibited the migration and proliferation of vascular cells produced by atherosclerotic lesions by inhibiting platelet-derived growth factor-BB (PDGF-BB) and NF-kB and inhibited the occurrence and development of plaques in AS (Son et al., 2012).
Panax notoginseng is the dry root and rhizome from Panax notoginseng [panax notoginseng (Burk.) F.H. Chen]. According to reports, the different anatomical sections of Panax notoginseng (roots, stems, leaves, flower buds, and seeds) contain many different saponins, mainly ginsenosides (Rb1\Re\Rg1\Rg2\Rh1), notoginseng saponins (R1–R6), and aescin (VII) (Zhou et al., 2017). In recent years, tablets, capsules, and injections with total Panax notoginseng saponins’ (PNS) as the main components have been formulated. With a market value of billions of Yuan, notoginseng total glycosides tablets, Xuesaitong injections, and Xueshuantong injections have been successfully used to treat cardiovascular diseases (Zhou et al., 2017). Yang et al. (2022) reported that after PNS administration for 8 weeks, nuclear factor-κB (NF-κB) p65, IL-6, IL-1β, TNF-α, and calpain1 protein expression levels in aortic root tissues in ApoE−/− mice were inhibited via NF-κB signaling (Yang et al., 2022). Fan et al. (2012) reported that PNS reduced the size of plaques on the aorta of AS rats by upregulating lipid metabolism by upregulating LXRa; at the same time, the expressions of NF-kB, IL-6, and MCP-1 in blood vessels were also inhibited. Yuan et al. (2011) reported that intraperitoneal injection of PNS could improve AS induced by zymosan A. PNS reduced aortic platelet plaque in model rats and inhibited the formation of foam cells, most integrin families, focal adhesion kinase (FAK) threonine 397 phosphorylation, and NF-kB transcription. Zhang et al. (2008a) discovered that the expression of inflammation-related genes (ICAM-1, VCAM-1, CCL2, CCR2, MMP-2, MMP-9, IL-18, and IL-1β) and vasoactive factor genes [ET-1, ET2, ET3, and coagulation factor III (TF)] was decreased after a 9-week treatment with PNS. Lin et al. reported that PNS inhibited oxidative stress and attenuated the expression of inflammatory cytokines in AS through the advanced glycation end product (RAGE)/MAPK signaling pathway. The authors believed that the reduction of atherosclerotic plaque by PNS mainly depended on reducing inflammation (Dou et al., 2012). In vivo studies showed that PNS inhibited monocyte adhesion on an activated endothelium and the expression of TNF-α-induced endothelial adhesion molecules, such as ICAM-1 and VCAM-1, in a dose-dependent manner. Notably, IL-6 and TNF-α serum levels in all PNS treatment mouse groups were below detection limits and the serum lipid levels were also suppressed (Wan et al., 2009).
Notoginseng saponin R1 (NGR1) is a monomeric component isolated from PNS. Geniposide combined with NGR1 (GN combination) reduced inflammation and apoptosis in AS via the AMPK mechanistic target of the rapamycin (mTOR)/Nrf2 signaling pathway. NOD-, LRR- and pyrin domain containing 3 (NLRP3), caspase-1, IL-1β, and IL-18 expression levels in the aortic tissue of mice were inhibited, while in vitro studies showed that the GN combination inhibited hydrogen peroxide (H2O2)-induced inflammatory responses and HUVEC apoptosis (Liu et al., 2021). Jia et al. showed that NGR1 markedly reduced inflammatory cytokine levels, including IL-2, IL-6, TNF-α, and IFN-γ in ApoE−/− mice (Jia et al., 2014). Zhao et al. (2020a) found that NGR1 mitigated ox-LDL-induced apoptosis, oxidative stress, and inflammatory factor release in HUVECs by modulating the X-inactive specific transcript (XIST)/miR-221-3p/TNF-receptor-associated factor 6 (TRAF6) axis. Zhu et al. (2020) indicated that HUVECs pretreated with 30 µM of NGR1 can reduce the levels of inflammatory cytokines IL-6 and IL-1β induced by ox-LDL. The mechanism could be NGR1 upregulated miR-221-3p expression to inactivate the toll-like receptor 4 (TLR4)/NF-kB pathway. Another study also showed that NGR1 pretreatment at 30 µM enhanced NGR1-induced migration inhibition and MCP-1 and ICAM-1 downregulation by downregulating miR-132, thereby preventing ox-LDL-induced atherosclerotic responses (Fu et al., 2018).
Icariin (ICA) is a flavonoid isolated from the TCM herb Epimedium brevicornum Maxim. ICA commonly exerts multiple effects such as sex hormone regulation and relieving AS and antioxidant activity. Hu et al. (2016) showed that ICA suppressed levels of IL-6, TNF-α, mRNA, and p-p38 MAPK by reducing oxidative stress and inflammation associated with p38 MAPK signaling in rats fed with HCD. Yang et al. (2015) reported that 4 or 20 µM ICA treatment could upregulate the expression of scavenger receptor class B type I (SR-BI) protein and downregulate the expression of CD36 in a dose-dependent manner, respectively, thereby inhibiting the formation of foam cells. ICA also reduced CX3CR1 and CX3CL1 protein levels in artery walls (Wang et al., 2016a). Similarly, 10, 20, and 40 μmol/L ICA limited oxidative damage and monocyte adhesion to HUVECs and reduced the secretion and expression of ICAM-1, VCAM-1, and E-selectin (E-sel) (Hu et al., 2015).
The active components of Cortex Moutan mainly include phenols, monoterpenes, their glycosides, triterpenes, flavonoids, tannins, steroids, etc. Among them, the content of paeonol is relatively high, which is the content determination index of Cortex Moutan. Chen et al. (2014) reported that in their previous research paeono (Pae) was used alone on vascular smooth muscle cells (VSMC) or VECs and the preventive effect of Pae on AS was found. With the deepening of the experiment, it was found that the prevention and treatment mechanism of Pae on AS was due to the inhibition of vascular endothelial growth factor (VEGF) and PDGF-B secretion in VECs and the inhibition of Ras/Raf/extracellular signal-regulated kinase 1/2 (ERK1/2) signaling pathway in VSMCs. Yuan et al. (2016) reported that Pae inhibited monocyte adhesion to ox-LDL-damaged VECs and blocked the activation of phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt)/NF-κB signaling pathway by promoting miR-126 expression. In the meantime, VCAM-1 expression was inhibited. The authors suggested that miR-126 could earn an irreplaceable position in the process of Pae targeting vascular inflammation in the treatment of AS.
Diosgenin is a phytosteroid saponin that comes from a wide range of sources, such as Dioscorea, fenugreek, Smilax, woodsy, and holly, in particular fenugreek and Dioscorea seeds. Diosgenin is also one of the main components in the eight steroidal saponins in Di’ao Xinxuekang preparations. Diosgenin inhibited inhibitor of nuclear factor kappa-B kinase beta (IKKβ)-phosphorylation and downregulated the expression of TNF-α, IL-6, MCP-1, and inducible nitric oxide synthase (iNOS) and protected endothelial function against inflammatory damage (Liu et al., 2012; Chen et al., 2016). Diosgenin also significantly inhibited LPS-induced TNF-α production in macrophage culture supernatants (Okawara et al., 2014). Diosgenin dose-dependently reduced LPS/IFN-γ-induced NF-κB, JNK, and AP-1 activities to suppress macrophage inflammation, but did not affect TNF-α production (Jung et al., 2010). Also, diosgenin dose-dependently inhibited TNF-α-mediated THP-1 monocyte adhesion and ICAM-1 and VCAM-1 mRNA and protein expression and inhibited VSMC adhesion by inhibiting the MAPK/Akt/NF-κB signaling pathway and ROS production (Choi et al., 2010). Another study reported that diosgenin inhibited thrombosis by downregulating the phosphorylation of NF-κB/p65, IKKβ, Akt, the extracellular signal-regulated kinase (ERK), and JNK, and inhibited TNF-α-induced thrombotic activity and tissue factor (TF) expression in monocytes (Yang et al., 2013).
Soy is the main source of phytoestrogen and has long been used as traditional food. A major phytoestrogen subtype includes isoflavones which have been scientifically validated as benefitting several hormone-dependent conditions (Lephart et al., 2002). Register et al. (2005) in a well-established nonhuman primate AS model, showed how specific soy isoflavone doses generated anti-inflammatory effects specific to soluble vascular cell adhesion molecule-1 (sVCAM-1), whereas the effects of conjugated equine estrogens extended to both sVCAM-1 and MCP-1. It was possible that the athero-protective effects of isoflavones and conjugated equine estrogens were mediated, at least in part by the effects on VCAM-1. A previous study reported that soy isoflavones regulated blood lipids in rats with metabolic syndrome and produced anti-atherosclerosis (Davis et al., 2007).
D. moldavica Linn (Labiatae) is used in Uyghur medicine and folk to treat coronary heart diseases and hypertension, including AS. Xing et al. (2013) reported that total flavonoids may inhibit TNF-α-induced proliferation and adhesion molecule expression in VSMCs by inhibiting proliferating cell nuclear antigen (PCNA) expression and NF-κB activation in a dose-dependent manner. Thus, D. moldavica total flavonoids exhibited anti-inflammatory activities, which is why D. moldavica is used for the clinical treatment of AS.
Resveratrol exists in TCM such as Polygonum cuspidatum, Panax notoginseng, mulberry white skin, Magnolia officinalis, Shegan, and plants such as grapes, blueberries, mulberries, and peanuts. The compound is considered a highly effective antioxidant and is bioactive (Biasutto and Zoratti, 2014). Vasamsetti et al. (2016) reported that inhibition of inflammation is the main mechanism by which resveratrol delays the occurrence and development of AS, including inhibition of monocyte differentiation and the production of pro-inflammatory cytokines. Resveratrol could also inhibit the proliferation of VSMCs by promoting the release of Ang-II. The abovementioned mechanisms not only appeared in phorbol myristate acetate (PMA)-induced THP-1 cells but were also fully demonstrated in ApoE−/− mice that had taken resveratrol for 6 weeks. It alleviated the formation of AS plaques and prevented the progression of AS. Chang et al. (2015) reported that resveratrol prevented AS caused by HCD by reducing LDL-C levels and inhibiting atherosclerotic inflammation, as reflected in decreasing the expression of macrophage-specific markers F4/80 and cardiovascular inflammatory marker NF-κB.
Apigenin (API) is a common flavonoid component found in many foods, with high levels found in celery. API is also abundant in fruit, such as verbena, rhizoma, and selaginaceae. Several previous studies reported that API had antioxidant and anti-inflammatory roles. Ren et al. (2018) reported that no matter whether in vivo or in vitro, API had a unique effect in reducing the size of atherosclerotic plaques, and its effective mechanism promotes ATP binding cassette transporter A1 (ABCA1)-mediated cholesterol efflux by inhibiting miR-33 in a time- and dose-dependent manner. At the same time, the phosphorylation of TLR-4, myeloid differentiation primary response protein 88 (MyD88), nuclear factor-κB inhibitor-α (p-IκB-α), and the expression levels of dozens of inflammatory factors such as NF-κB p65 can be reduced by API supplementation, which was achieved by inhibiting the TLR-4/NF-κB signaling pathway. Zeng et al. (2015) reported that API attenuates the inflammation of AS by regulating the apoptosis of macrophages, thereby alleviating the formation of AS, and the mechanism may be to downregulate Plasminogen Activator Inhibitor-2 (PAI-2) by inhibiting the phosphorylation of AKT at Ser473. Zhang et al. (2014) explored the effect and mechanism of API on LPS-induced inflammatory response and found that API can regulate NLRP3 inflammasome assembly, reduce mRNA stability, inhibit macrophage ERK1/2 and NF-κB activation, and finally, LPS-induced levels of IL-6, IL-1β, TNF-α, and other pro-inflammatory cytokines were decreased, indicating that API may slow down the inflammatory response by inhibiting the activation of NLRP3 inflammasome and the production of inflammatory factors.
Luteolin (3′, 4′, 5, 7-tetrahydroxy flavonoid), as a common natural flavonoid compound in safflower, chrysanthemum, pepper, and other plants, has various biological activities such as curing photoaging (Chen et al., 2012; Lim et al., 2013). Luteolin reduced the expression of inflammatory factors such as ICAM-1, VCAM-1, IL-6, and TNF-α in atherosclerotic ApoE−/− mice fed a high-fat diet and reduced atherosclerotic plaques. However, it showed no effects in ameliorating hyperlipidemia in atherosclerotic ApoE−/− mice. The authors suggested that the mechanism may be related to the enhancement of phosphorylation of signal transducer and activator of transcription 3 (STAT3) (Ding et al., 2019). The 2 μM luteolin pretreatment in LPS-stimulated RAW264.7 cells not only effectively inhibited the occurrence of oxidative stress but also inhibited NLRP3, CARD (ASC), caspase-1, IL-18, and IL-1β inflammatory markers (Zhang et al., 2018). Li et al. (2018) reported that luteolin could inhibit the formation of atherosclerotic plaques by inhibiting the macrophage differentiation marker cluster 68 (CD68), macrophage chemoattractant protein 2 (CCL2), and inflammatory factors. Luteolin also had a therapeutic effect on hyperlipidemia. The mechanism is inseparable from the activation of AMPK-SIRT1 signaling. Jia et al. (2015) emphasized that luteolin inhibited inflammatory markers such as soluble ICAM-1 (sICAM-1) and C-X-C Motif Chemokine Ligand 1 (CXCL1) through the phospho-inhibitor of kappa Bα(IκBα)/NF-κB pathway, thus fully demonstrating the sniping effect of luteolin on vascular inflammation.
Scutellaria baicalensis is a commonly used Chinese herbal medicine used to treat diarrhea, dysentery, hypertension, hemorrhage, insomnia, inflammation, and respiratory tract infections. The main components of S. baicalensis are flavonoids, with baicalin as one of the more important active components. Baicalin displayed anti-tumor, antibacterial, and antioxidant effects and improved AS by reducing IL-1, IL-18, mitochondrial ROS, total ROS, and ICAM-1 and VCAM-1 production by inhibiting the NLRP3 inflammasome (Zhao et al., 2020b). Baicalin also inhibited NF-κB and p38 MAPK signaling pathways in a dose-dependent manner, thereby reducing AS-induced increases in IL-6, TNF-α, and soluble vascular endothelial-cadherin (sVE-cadherin) levels, increasing lipolysis-related protein (peroxisome proliferator-activated receptor-α and CPT-1) expression, reducing adipogenesis-related protein (SREBP-1c and ACS) expression, upregulating SOD, catalase (CAT), glutathione peroxidase (GSH-Px) activity, and downregulating MDA activity (Wu et al., 2018a). Baicalin prevented AS by inhibiting the proliferation and migration of ox-LDL-VSMCs by targeting high mobility group box-1 and upregulating miR-126-5p (Chen et al., 2019). Baicalin also inhibited ox-LDL-induced intracellular lipid accumulation and foam cell formation in THP-1 macrophages via the peroxisome proliferator-activated receptor gamma (PPARγ)/liver X receptor-α (LXRα)/ABCA1/ATP-binding cassette protein G1 (ABCG1) pathway (He et al., 2016).
Kaempferol is a natural edible flavonoid, which is mainly derived from the rhizome of the ginger plant kaempferol, and widely exists in various vegetables and fruits. TNF-α, IL-1β, and MDA levels in atherosclerotic rabbits treated with kaempferol were significantly decreased, serum SOD activity was increased, and the gene and protein expression levels of E-sel, ICAM-1, VCAM-1, and MCP-1 in aortas significantly decreased (Kong et al., 2013). Kaempferol attenuated ox-LDL-induced endothelial cell apoptosis by inhibiting the PI3K/Akt/mTOR pathway and upregulating autophagy (Che et al., 2017). Kaempferol inhibited the TLR4/NF-κB pathway by upregulating the expression of miR-26a, promoted the proliferation of human aortic endothelial cells, and reversed endothelial cell apoptosis caused by ox-LDL (Zhong et al., 2018). Treatment with kaempferol reduced atherosclerotic lesion areas, improved endothelium-dependent vasodilation, and increased maximal diastolic values, while reducing half-maximal effective concentrations, plasma osteopontin (OPN) levels, and aortic OPN and CD44 expression in ApoE−/− mice. Additionally, kaempferol significantly reduced ROS production in the aortas of mice (Xiao et al., 2011). Kaempferol also inhibited macrophage foam cell formation by mediating c-Jun/activator protein-1 (AP-1)-dependent downregulation of CD36 and HO-1-dependent upregulation of SR-BI, ABCA1, and ABCG1 (Li et al., 2013).
Taraxacum officinale (TO), as a medicinal and edible homologous plant, dandelion has been used in traditional edible and medicinal applications in my country for thousands of years. It has multiple pharmacological effects such as antioxidant, immune regulation, hypoglycemic, and tumor inhibition (Schutz et al., 2006). The anti-inflammatory and other effects of TO were revealed by its water extract Taraxacum officinale F. Weber ex Wiggers (TEE) inhibiting NO production and COX-2 expression in vivo and in vitro (Jeon et al., 2008). Kim et al. (2000) concluded that TO leaf extract has a good effect on LPS-induced central nervous system inflammation. Jeon et al. (2017) showed that TO extracts could alleviate LPS-induced inflammatory changes in human umbilical vein endothelial cells by inhibiting the NF-κB pathway, which was manifested by reducing the expression of VCAM-1, MCP-1, and pro-inflammatory cytokines.
Swertiamarin is a unique kind of secoiridoid glycoside isolated from the main bioactive components of the swedia family. Swertiamarin is the active ingredient of a variety of TCMs, and foreign studies have shown that Swertiamarin and its derivatives have anti-diabetic and anti-hyperlipidemic effects (Muhamad Fadzil et al., 2021). Vaidya et al. (2012) reported that Swertiamarin reduced serum glucose, triglyceride, non-esterified free fatty acid, and cholesterol levels and also reduced serum MMP-9 and MMP-3 levels when compared with untreated rats. Swertiamarin seems to be an excavation point worthy of further study for AS caused by diabetes. Vaidya et al. (2009) also reported that the levels of serum glucose, triglycerides, non-esterified free fatty acids, and cholesterol were effectively reduced in the atherosclerotic rats treated with Swertiamarin, and these effects may be attributed to the inhibition of serum MMP-9 and MMP-3 by Swertiamarin.
Gardeniside is a major iridoid glycoside isolated from the dried and ripe fruit of Gardenia jasminoides, a Rubiaceae plant. Gardenia as TCM has medicinal effects of clearing heat, purging fire, cooling blood, anti-inflammatory and analgesic, and protecting the liver and gallbladder. Wang et al. (2016b) reported that baicalin or geniposide monotherapy and combination therapy, inhibited atherosclerotic lesion development in ApoE−/− mice, reduced the level of inflammatory cytokine IL-12, increased WNT Family Member 1 (Wnt1), and decreased dickkopf-related protein-1 (Dkk1) expression; however, only baicalin or geniposide monotherapy could inhibit the expression of NF-κB, which remained to be investigated, but did not prevent the development of geniposide as an inflammatory target in AS.
Andrographolide is the major bioactive component of Andrographis paniculata and has various biological properties, including anti-inflammation, anti-oxidation, and anti-hepatotoxicity. Lu et al. (2014) reported that andrographolide could inhibit the proliferation of VECs, relieve inflammation, activate NADPH oxidase, induce heme oxygenase-1 (HO-1) and glutamate cysteine linkage enzyme (glutamate-cysteine ligase, GCLM) expression, and reduce TNFα-induced ICAM-1 expression by inhibiting PI3K/Akt pathway and inhibit VEC proliferation. Chao et al. (2011) held a similar view and they also found that andrographolide reduced inflammation and endothelial cell dysfunction is inseparable from the inhibition of the IKK/NF-κB signaling pathway. Wu et al. (2018b) shared the same view that andrographolide downregulated the expression of MCP-1 and IL-6 by blocking NF-κB signaling in macrophages. They also complimented that the mechanism by which andrographolide reduces AS is also related to the inhibition of foam cell formation and reduction of oxidative stress. Wu et al. (2018c) reported that considering the low oral bioavailability of andrographolide, a well-targeted andrographolide-loaded micelle was prepared using polyethylene glycol and polypropylene sulfide block copolymer as carriers, which effectively inhibited IL-6 and MCP-1 expression, while reduced oxidative stress in macrophages. Al Batran et al. (2014) reported that andrographolide significantly downregulated IL-1β levels in atherosclerotic rabbit serum. The effect of andrographolide in alleviating atherosclerotic inflammation is not only reflected in the inhibition of NF-κB activation but also closely related to the inhibition of STAT3 activation (Lee et al., 2011).
Artemisia annua L. is an annual medicinal herb and has been used in TCM for a long time. The plant was first thought to have an antipyretic effect by Ge Hong, a medical scientist in the Eastern Jin Dynasty in China. Artemisinin (ART) was successfully extracted from Artemisia annua L. and a new antimalarial drug was generated in the 1970s, in China. Cao et al. (2015) showed that ART could treat AS by inhibiting the proliferation, migration, and inflammation of VSMC, which is shown as the inhibition of PCNA, MMP-2, MMP-9, NO, and prostaglandin E2 (PGE2) by ART. Expectations for in-depth research on ART via the ROS-NF-kB pathway have been raised, too. Jiang et al. (2016) showed that artesunate (a water-soluble hemisuccinate derivative of ART) had an inhibitory effect on the formation of atherosclerotic plaques and a specific inhibitory effect on LPS-induced IL-8 and MCP-1.
Salvia miltiorrhiza Bunge is a Lamiaceae plant with dry roots and rhizomes. It was first contained in “Shen Nong’s Materia Medica.” Salvia miltiorrhiza and its components can expand coronary arteries, prevent myocardial ischemia, improve microcirculation, and reduce myocardial oxygen consumption and are widely used in the clinical treatment of cardiovascular diseases. Tanshinone IIA, as the most active lipid-soluble component in Salvia miltiorrhiza, has been widely studied for its various biological activities (Jiang et al., 2019). Tanshinone IIA exhibited certain anti-atherosclerotic effects, protected cells from H2O2 injury (Liu et al., 2014a), suppresses cholesterol accumulation, and affected foam cell formation (Zhang et al., 2011). Stumpf et al. (2013) reported that in the experiment of HUVECs culture, it is found that Salvia miltiorrhiza has a good relieving effect on atherosclerotic inflammation and its inhibition of various adhesion molecules, chemokines, and inflammatory mediators such as platelet P-selectin is sufficient evidence. Li et al. (2015) reported that through the cell co-culture model, it was found that Tanshinone IIA had an obvious curative effect on AS and related inflammatory response and can reduce AS-related inflammatory cytokines, which is closely related to the NF-κB signaling pathway.
Curcumin belongs to the class of diketone compounds, mainly derived from the rhizomes of turmeric plants, in the form of orange-yellow crystalline powder, which can be used as food coloring and seasoning. In China, the rhizome of turmeric has a long history of being used as medicine, and it has good menstrual and pain-relieving effects. Modern research has found that curcumin has pharmacological effects such as anti-cancer, anti-inflammatory, anti-oxidation, prevention of senile dementia, and inhibition of AS, with a low price and low toxicity (Araújo and Leon, 2001; Anand et al., 2007; Goel et al., 2008; Gupta et al., 2013). Plasma and liver cholesterol levels and HMG-CoA (3-hydroxy-3-methylglutaryl-CoA) reductase were inhibited in curcumin-fed LDLR−/− mice for 18 weeks. The level of curcumin prevention and treatment of AS is comparable to that of lovastatin (Shin et al., 2011). Curcumin inhibited the expression of MCP-1, increased the expression of ABCA1 and ASR-BI, and increased the excretion of cholesterol, which all showed its preventive effect on AS and its inflammation. The mechanism may be related to the JNK pathway and NF-κB pathway (Liu et al., 2014b). Hasan et al. (2014) reported that curcumin showed a dose-dependent decrease in mouse body weight, plaque formation, and the expression of IL-6 and other inflammatory factors in a low-to-medium dose range. These modulating effects on AS may be related to the inhibition of adipocyte fatty acid binding protein (aP2) and CD36 expression in macrophages by curcumin.
Magnolol (MAG) is a biphenol compound and the main active ingredient in the TCM Magnolia officinalis. MAG reduced VCAM-1 expression on the cell surface and in the cytoplasm (Chen et al., 2002). In 2017, Lee et al. (2017) synthesized a new MAG nanoparticle formulation, which confirmed MAG inhibitory effects on VCAM-1. MAG could prevent early inflammatory lesions of AS by regulating JNK/p38 and NF-κB signaling pathways (Liang et al., 2014).
Honokiol and MAG are isomers of hydrophobic allyl biphenyl-like structures. Honokiol downregulated TNF-α, IL-6, and IL-1β expression. Additionally, honokiol treatment reduced ROS levels and enhanced SOD activity and also significantly inhibited NO levels, iNOS expression, and the abnormal activation of the NF-κB pathway (Liu et al., 2020). Honokiol significantly inhibited pentraxin 3 (PTX3) overexpression in palm oil-induced HUVECs by inhibiting IKK/IκB/NF-κB signaling pathway. Honokiol also exerted anti-inflammatory effects by significantly inhibiting IL-6, IL-8, and MCP-1 production. It could remind us that PTX3 combined with honokiol may be the crucial inflammation target in treating AS (Qiu et al., 2015). Animal experiments showed that honokiol could inhibit the formation of neointima, the proliferation of smooth muscle cells, and the deposition of extracellular matrix, and its mechanism is closely related to the blocking of NF-κB activation by regulating the ERK signaling pathway (Zhu et al., 2014).
Salvianolic acid B (Sal B) is a highly active ingredient in the water extract of Salvia miltiorrhiza. It has antioxidant, anti-inflammatory, and anti-fibrotic effects and has been studied as a potential drug for cardiovascular treatment for many years (Ho and Hong, 2011). Sal B targeted IFN-γ-induced signaling and STAT1 signaling downstream targets to inhibit the expression of CXC chemokines, IFN-γ-inducible protein 10, MIG, and I-TAC and inhibits IP-10 promoter activity and IP-10 protein secretion (Chen et al., 2011). In vitro studies using TNF-α to induce monocyte adhesion to HUVECs were performed using different genistein concentrations, with MCP-1 and IL-8 levels tested. The results showed that genistein (0.1 μM) significantly inhibited MCP-1 and IL-8 expression when compared with control cells (Lin et al., 2007).
Cinnamomum cassia Presl aqueous extracts were used to study their effects on monocyte differentiation into macrophages and macrophage scavenger receptor activity. In this stage, the aqueous extract of cinnamon can downregulate the expression of scavenger receptor class A (SR-A) and CD36 genes in macrophages, and the inhibition of CD36 expression was concentration-dependent. It was noted that 100 μg/ml cinnamon water extract almost completely blocked macrophage-colony stimulating factor (M-CSF)-induced increases in SR-A protein synthesis (Kang et al., 2014).
Polysaccharide Krestin (PSK) is the main active ingredient in Yunzhi extract, with molecular weights in the 1.3 × 106 range. As a glucan, the PSK contains β-glycosidic bonds and is detected as β (1→3) and β (1→6) glycosides. Polysaccharides extracted from the mycelium and fermentation broth of Yunzhi polysaccharides strongly inhibit cancer cell activity (Ng, 1998). PSK prevented oxidative damage to macrophages by Ox-LDL and the subsequent foamy degeneration of macrophages (Yuan et al., 1996). In view of the oxidative injury caused by Ox-LDL plays an important role in the transformation of macrophages into foam cells and atherogenesis. PSK protected mouse peritoneal macrophages from oxidative injury by upregulating M-CSF gene expression (Pang, 2003). It represented the front-end preventive effect of PSK on AS from an inflammation perspective.
Celastrol is an effective chemical component extracted from TCM Tripterygium wilfordii and other plants and has significant biological activities such as anti-inflammatory and antioxidant (Chen et al., 2018). Allen et al. reported that after Celastrol loaded into poly (ethylene glycol)-b-poly (propylene sulfide) (PEG-b-PPS) micelles, not only has the scope of treatment been expanded but the number of inflammatory cells and plaque area has been greatly reduced and drug safety has also been better guaranteed (Allen et al., 2019). Celastrol reduced the size of atherosclerotic plaques by inhibiting LOX-1 expression and oxidative stress and inhibited various inflammatory factors (Gu et al., 2013). Approximately 100 ng/ml Celastrol reduced inflammation and plaque size by regulating Drp1-mediated mitochondrial fission and fusion, ERK1/2, p38, and NF-κB signaling pathways (Tao et al., 2022).
2,3,4′,5-Tetrahydroxystilbene-2-O-beta-d-glucoside (TSG), an analog of resveratrol, is the main active ingredient in the TCM Polygonum multiflorum (PMRP) and rhubarb. In recent decades, extensive research has shown that TSG has various pharmacological activities such as antioxidant, free radical scavenging, anti-aging, anti-tumor, neuroprotection, etc. It has also been shown that it also plays an important protective role in cardiovascular diseases (Wu et al., 2017). Li et al. showed that PMRP and TSG improved lipid accumulation by reducing the levels of inflammatory factors, glycerol trioleate, and ox-LDL in Apo E−/− mice (Li et al., 2020). TSG could improve the atherosclerotic plaque size and blood lipid level in rats by inhibiting the levels of CRP, IL-6, and other inflammatory factors and the overexpression of MMP-2 and MMP-9 (Zhang et al., 2008b). The high-dose group (120 μg/L) of TSG had the best performance in inhibiting cell adhesion molecules in vivo and also had the same performance in vitro and reduced the plaque area. It is not negligible that 120 μg/L TSG inhibited ICAM-1/VCAM-1 more strongly than 100 μg/L simvastatin (Wang et al., 2013). Finally, TSG pretreatment at 120 μM inhibited the formation of macrophage foam cells in AS, and the mechanism is related to the inhibition of vimentin expression (Yao et al., 2016).
Poria cocos is an important homologous raw material used in medicines and foods. Poria polysaccharide is the main component in Poria sclerotia and has shown great developmental potential in the food, medicine, and healthcare product sectors. PCP inhibited elevated IL-6, TNF-α, NO, LDL, triglycerides, and total cholesterol and also the TLR4/NF-κB pathway activation. PCP also blocked MMP-2 and intercellular adhesion molecule-1 protein expression and interfered with AS in mice (Li et al., 2021). Poria polysaccharides interfered with AS by activating the ERK/Nrf2/HO-1 signaling pathway to exert anti-oxidative stress effects (Zhao et al., 2020c).
Taken together, natural medicines may exert significant anti-atherosclerotic effects by inhibiting inflammation induced by risk factors for AS. Hence, we reviewed the advances in studies from the past ten years on the use of the active components of Chinese herbal medicines to modulate inflammation. We found that 1) current studies show the active ingredients for atherosclerotic mainly in saponins, flavonoids, alkaloids, and terpene; 2) the anti-inflammatory activities of natural drugs mainly focus on TLR4, NF-κB, Nrf2 pathways, and inflammatory factors, such as IL-6, TNF-α, VCAM-1, and MCP-1, as shown in Table 1 and Figure 2; and 3) most previous studies only focused on small molecules derived from plants or herbs for treating AS. Consequently, additional studies could be devoted to investigating potential anti-atherosclerotic macromolecules.
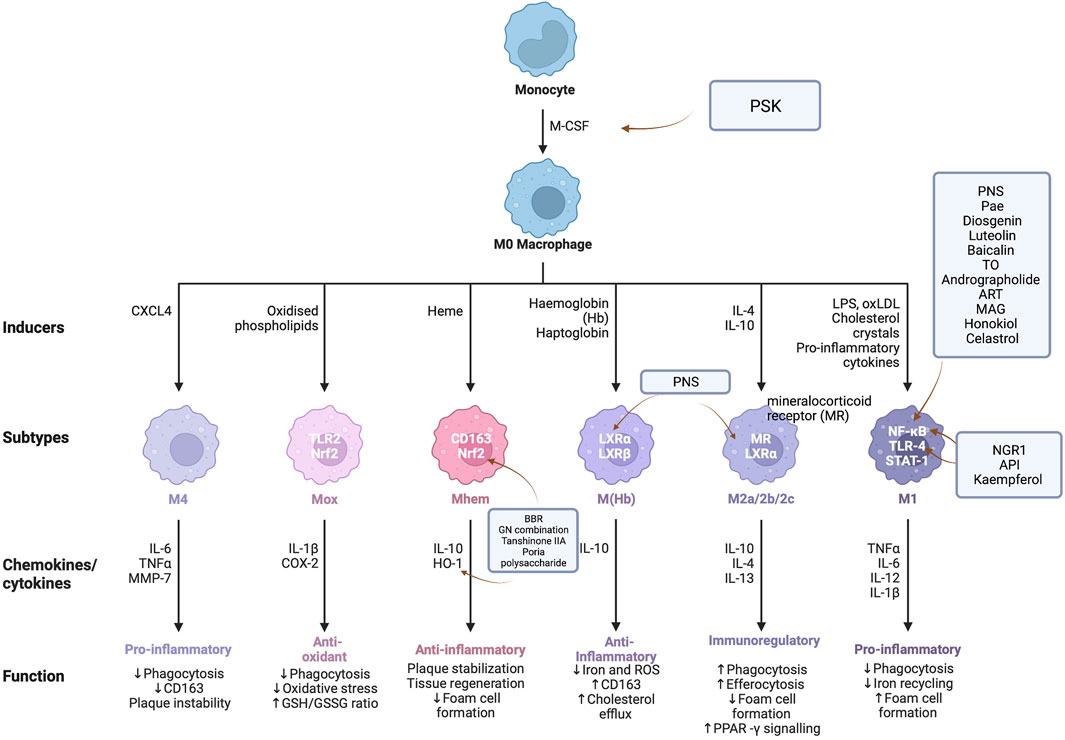
FIGURE 2. Targets/pathway of natural drugs in as inflammation from the perspective of inflammation, the preventive effect of PSK on AS is to upregulate the gene expression of M-CSF. PNS, Pae, diosgenin, luteolin, baicalin, TO, andrographolide, ART, MAG, Honoriol, Celtrol, and other drugs all participate in the inhibition of NF- κB signal pathway for regulating inflammation of atherosclerosis, while NGR1, API, and kaempferol could inhibit the TLR4/NF- κB signal pathway. BBR, GN combination, tanshinone IIA, and Poria cocos polysaccharide could reduce inflammatory damage by activating Nrf2/HO-1 signal pathway.
Although, many studies have been conducted on the ability of natural medicines to inhibit inflammation and most of them have reached a certain depth. However, some problems persist, and many studies should be performed in the future. First, the current research is mostly superficial, based on the results of in vitro or in vivo experiments; therefore, further clinical experiments should be provided conclusive evidence for the anti-atherosclerotic effect of natural drugs. Second, natural medicines have many problems with themselves. For example, as an effective pharmacological component of Ligusticum chuanxiong root, the content of TMP is not high in raw medicine, and even sometimes undetectable in Ligusticum chuanxiong root; ICA may also have problems such as chronic toxicity, subchronic toxicity, reproductive toxicity, and developmental toxicity. Third, compared with the extensive pharmacological effect of natural drugs, the practical application of natural drugs as clinical medicine is relatively small, the biggest problem is the low bioavailability. Therefore, we can adopt the following methods in future research; on one hand, we could optimize the matrix materials to increase the drug loading of new dosage forms and improve the stability of the reparations. On the other hand, we need to strengthen the evaluation of pharmacokinetics and pharmacodynamics of new preparations to improve preclinical research of drugs and promote clinical trials of new preparations.
In conclusion, we hope that this review will provide a reference for understanding the current potential of natural drugs and their pharmacological mechanisms and highlight the advantages of these natural drugs in the prevention and treatment of AS by regulating inflammation.
XS retrieved the literature and wrote the article. XW and DW compiled the table; ZZ made the figures; JL and YL proposed the topics and checked the whole manuscript. All the authors reviewed the manuscript. Each of the authors agrees to be accountable for the content of the work.
This work received support from the National Nature Science Foundation of China (81974566 and 82004277), Shandong Provincial Natural Science Foundation (ZR2020QH309), China Postdoctoral Science Foundation (2021M690099), and Taishan Scholar Post Construction Fund (ts201712042).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Al Batran, R., Al-Bayaty, F., Al-Obaidi, M. M., Hussain, S. F., and Mulok, T. Z. (2014). Evaluation of the effect of andrographolide on atherosclerotic rabbits induced by Porphyromonas gingivalis. Biomed. Res. Int. 2014, 724718. doi:10.1155/2014/724718
Allen, S. D., Liu, Y. G., Kim, T., Bobbala, S., Yi, S., Zhang, X., et al. (2019). Celastrol-loaded PEG-b-PPS nanocarriers as an anti-inflammatory treatment for atherosclerosis. Biomater. Sci. 7 (2), 657–668. doi:10.1039/c8bm01224e
Anand, P., Kunnumakkara, A. B., Newman, R. A., and Aggarwal, B. B. (2007). Bioavailability of curcumin: Problems and promises. Mol. Pharm. 4 (6), 807–818. doi:10.1021/mp700113r
Araújo, C. C., and Leon, L. L. (2001). Biological activities of Curcuma longa L. Mem. Inst. Oswaldo Cruz 96 (5), 723–728. doi:10.1590/s0074-02762001000500026
Biasutto, L., and Zoratti, M. (2014). Prodrugs of quercetin and resveratrol: A strategy under development. Curr. Drug Metab. 15 (1), 77–95. doi:10.2174/1389200214666131211160005
Cao, Q., Jiang, Y., Shi, J., Xu, C., Liu, X., Yang, T., et al. (2015). Artemisinin inhibits the proliferation, migration, and inflammatory reaction induced by tumor necrosis factor-alpha in vascular smooth muscle cells through nuclear factor kappa B pathway. J. Surg. Res. 194 (2), 667–678. doi:10.1016/j.jss.2014.12.013
Chang, G-R., Chen, P-L., Hou, P-H., and Mao, F. C. (2015). Resveratrol protects against diet-induced atherosclerosis by reducing low-density lipoprotein cholesterol and inhibiting inflammation in apolipoprotein E-deficient mice. Iran. J. Basic Med. Sci. 18 (11), 1063–1071.
Chao, C. Y., Lii, C. K., Tsai, I. T., Li, C. C., Liu, K. L., Tsai, C. W., et al. (2011). Andrographolide inhibits ICAM-1 expression and NF-κB activation in TNF-α-treated EA.hy926 cells. J. Agric. Food Chem. 59 (10), 5263–5271. doi:10.1021/jf104003y
Che, J., Liang, B., Zhang, Y., Wang, Y., Tang, J., and Shi, G. (2017). Kaempferol alleviates ox-LDL-induced apoptosis by upregulation of autophagy via inhibiting PI3K/Akt/mTOR pathway in human endothelial cells. Cardiovasc. Pathol. 31, 57–62. doi:10.1016/j.carpath.2017.08.001
Chen, J., Dai, M., and Wang, Y. (2014). Paeonol inhibits proliferation of vascular smooth muscle cells stimulated by high glucose via ras-raf-ERK1/2 signaling pathway in coculture model. Evid. Based. Complement. Altern. Med. 2014, 484269. doi:10.1155/2014/484269
Chen, S. C., Lin, Y. L., Huang, B., Wang, D. L., and Cheng, J. J. (2011). Salvianolic acid B suppresses IFN-gamma-induced JAK/STAT1 activation in endothelial cells. Thromb. Res. 128 (6), 560–564. doi:10.1016/j.thromres.2011.08.032
Chen, S. R., Dai, Y., Zhao, J., Lin, L., Wang, Y., and Wang, Y. (2018). A mechanistic overview of triptolide and Celastrol, natural products from Tripterygium wilfordii hook F. Front. Pharmacol. 9, 104. doi:10.3389/fphar.2018.00104
Chen, Y., Xu, X., Zhang, Y., Liu, K., Huang, F., Liu, B., et al. (2016). Diosgenin regulates adipokine expression in perivascular adipose tissue and ameliorates endothelial dysfunction via regulation of AMPK. J. Steroid Biochem. Mol. Biol. 155 (1), 155–165. doi:10.1016/j.jsbmb.2015.07.005
Chen, Y-H., Lin, S-J., Chen, J-W., Ku, H-H., and Chen, Y-L. (2002). Magnolol attenuates VCAM-1 expression in vitro in TNF-alpha-treated human aortic endothelial cells and in vivo in the aorta of cholesterol-fed rabbits. Br. J. Pharmacol. 135 (1), 37–47. doi:10.1038/sj.bjp.0704458
Chen, Z., Kong, S., Song, F., Li, L., and Jiang, H. (2012). Pharmacokinetic study of luteolin, apigenin, chrysoeriol and diosmetin after oral administration of Flos Chrysanthemi extract in rats. Fitoterapia 83 (8), 1616–1622. doi:10.1016/j.fitote.2012.09.011
Chen, Z., Pan, X., Sheng, Z., Yan, G., Chen, L., and Ma, G. (2019). Baicalin suppresses the proliferation and migration of ox-LDL-VSMCs in atherosclerosis through upregulating miR-126-5p. Biol. Pharm. Bull. 42 (9), 1517–1523. doi:10.1248/bpb.b19-00196
Choi, K. W., Park, H. J., Jung, D. H., Kim, T. W., Park, Y. M., Kim, B. O., et al. (2010). Inhibition of TNF-α-induced adhesion molecule expression by diosgenin in mouse vascular smooth muscle cells via downregulation of the MAPK, Akt and NF-κB signaling pathways. Vasc. Pharmacol. 53 (5-6), 273–280. doi:10.1016/j.vph.2010.09.007
Davies, M. J. (1994). Pathology of arterial thrombosis. Br. Med. Bull. 50 (4), 789–802. doi:10.1093/oxfordjournals.bmb.a072926
Davis, J., Higginbotham, A., O'Connor, T., Moustaid-Moussa, N., Tebbe, A., Kim, Y. C., et al. (2007). Soy protein and isoflavones influence adiposity and development of metabolic syndrome in the obese male ZDF rat. Ann. Nutr. Metab. 51 (1), 42–52. doi:10.1159/000100820
Ding, X., Zheng, L., Yang, B., Wang, X., and Ying, Y. (2019). Luteolin attenuates atherosclerosis via modulating signal transducer and activator of transcription 3-mediated inflammatory response. Drug Des. devel. Ther. 13, 3899–3911. doi:10.2147/DDDT.S207185
Dou, L., Lu, Y., Shen, T., Huang, X., Man, Y., Wang, S., et al. (2012). Panax notogingseng saponins suppress RAGE/MAPK signaling and NF-kappaB activation in apolipoprotein-E-deficient atherosclerosis-prone mice. Cell. Physiol. biochem. 29 (5-6), 875–882. doi:10.1159/000315061
Fan, J. S., Liu, D. N., Huang, G., Xu, Z. Z., Jia, Y., Zhang, H. G., et al. (2012). Panax notoginseng saponins attenuate atherosclerosis via reciprocal regulation of lipid metabolism and inflammation by inducing liver X receptor alpha expression. J. Ethnopharmacol. 142 (3), 732–738. doi:10.1016/j.jep.2012.05.053
Fu, C., Yin, D., Nie, H., and Sun, D. (2018). Notoginsenoside R1 protects HUVEC against oxidized low density lipoprotein (Ox-LDL)-Induced atherogenic response via downregulating miR-132. Cell. Physiol. biochem. 51 (4), 1739–1750. doi:10.1159/000495677
Goel, A., Kunnumakkara, A. B., and Aggarwal, B. B. (2008). Curcumin as "curecumin": From kitchen to clinic. Biochem. Pharmacol. 75 (4), 787–809. doi:10.1016/j.bcp.2007.08.016
Gu, L., Bai, W., Li, S., Zhang, Y., Han, Y., Gu, Y., et al. (2013). Celastrol prevents atherosclerosis via inhibiting LOX-1 and oxidative stress. PLoS One 8 (6), e65477. doi:10.1371/journal.pone.0065477
Gupta, S. C., Patchva, S., and Aggarwal, B. B. (2013). Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 15 (1), 195–218. doi:10.1208/s12248-012-9432-8
Hasan, S. T., Zingg, J. M., Kwan, P., Noble, T., Smith, D., and Meydani, M. (2014). Curcumin modulation of high fat diet-induced atherosclerosis and steatohepatosis in LDL receptor deficient mice. Atherosclerosis 232 (1), 40–51. doi:10.1016/j.atherosclerosis.2013.10.016
He, X. W., Yu, D., Li, W. L., Zheng, Z., Lv, C. L., Li, C., et al. (2016). Anti-atherosclerotic potential of baicalin mediated by promoting cholesterol efflux from macrophages via the PPARγ-LXRα-ABCA1/ABCG1 pathway. Biomed. Pharmacother. 83, 257–264. doi:10.1016/j.biopha.2016.06.046
Ho, J. H., and Hong, C. Y. (2011). Salvianolic acids: Small compounds with multiple mechanisms for cardiovascular protection. J. Biomed. Sci. 18, 30. doi:10.1186/1423-0127-18-30
Hu, Y., Liu, K., Yan, M., Zhang, Y., Wang, Y., and Ren, L. (2015). Effects and mechanisms of icariin on atherosclerosis. Int. J. Clin. Exp. Med. 8 (3), 3585–3589.
Hu, Y., Sun, B., Liu, K., Yan, M., Zhang, Y., Miao, C., et al. (2016). Icariin attenuates high-cholesterol diet induced atherosclerosis in rats by inhibition of inflammatory response and p38 MAPK signaling pathway. Inflammation 39 (1), 228–236. doi:10.1007/s10753-015-0242-x
Huang, Z., Cai, X., Li, S., Zhou, H., Chu, M., Shan, P., et al. (2013). Berberineattenuated monocyte adhesion to endothelial cells induced by oxidized lowdensity lipoprotein via inhibition of adhesion molecule expression. Mol. Med. Rep. 7 (2), 461–465. doi:10.3892/mmr.2012.1236
Insull, W. (2009). The pathology of atherosclerosis: Plaque development and plaque responses to medical treatment. Am. J. Med. 122 (1), S3–S14. doi:10.1016/j.amjmed.2008.10.013
Jeon, D., Kim, S. J., and Kim, H. S. (2017). Anti-inflammatory evaluation of the methanolic extract of Taraxacum officinale in LPS-stimulated human umbilical vein endothelial cells. BMC Complement. Altern. Med. 17 (1), 508. doi:10.1186/s12906-017-2022-7
Jeon, H. J., Kang, H. J., Jung, H. J., Kang, Y. S., Lim, C. J., Kim, Y. M., et al. (2008). Anti-inflammatory activity of Taraxacum officinale. J. Ethnopharmacol. 115 (1), 82–88. doi:10.1016/j.jep.2007.09.006
Jia, C., Xiong, M., Wang, P., Cui, J., Du, X., Yang, Q., et al. (2014). Notoginsenoside R1 attenuates atherosclerotic lesions in ApoE deficient mouse model. PLoS One 9 (6), e99849. doi:10.1371/journal.pone.0099849
Jia, Z., Nallasamy, P., Liu, D., Shah, H., Li, J. Z., Chitrakar, R., et al. (2015). Luteolin protects against vascular inflammation in mice and TNF-alpha-induced monocyte adhesion to endothelial cells via suppressing IΚBα/NF-κB signaling pathway. J. Nutr. Biochem. 26 (3), 293–302. doi:10.1016/j.jnutbio.2014.11.008
Jiang, W., Cen, Y., Song, Y., Li, P., Qin, R., Liu, C., et al. (2016). Artesunate attenuated progression of atherosclerosis lesion formation alone or combined with rosuvastatin through inhibition of pro-inflammatory cytokines and pro-inflammatory chemokines. Phytomedicine 23 (11), 1259–1266. doi:10.1016/j.phymed.2016.06.004
Jiang, Z., Gao, W., and Huang, L. (2019). Tanshinones, critical pharmacological components in Salvia miltiorrhiza. Front. Pharmacol. 10, 202. doi:10.3389/fphar.2019.00202
Jung, D. H., Park, H. J., Byun, H. E., Park, Y. M., Kim, T. W., Kim, B. O., et al. (2010). Diosgenin inhibits macrophage-derived inflammatory mediators through downregulation of CK2, JNK, NF-kappaB and AP-1 activation. Int. Immunopharmacol. 10 (9), 1047–1054. doi:10.1016/j.intimp.2010.06.004
Kang, H., Park, S-H., Yun, J-M., Nam, T-G., Kim, Y-E., Kim, D-O., et al. (2014). Effect of cinnamon water extract on monocyte-to-macrophage differentiation and scavenger receptor activity. BMC Complement. Altern. Med. 14, 90. [Internet]. doi:10.1186/1472-6882-14-90
Kim, H. M., Shin, H. Y., Lim, K. H., Ryu, S. T., Shin, T. Y., Chae, H. J., et al. (2000). Taraxacum officinale inhibits tumor necrosis factor-αProduction from rat astrocytes. Immunopharmacol. Immunotoxicol. 22 (3), 519–530. doi:10.3109/08923970009026009
Kong, L., Luo, C., Li, X., Zhou, Y., and He, H. (2013). The anti-inflammatory effect of kaempferol on early atherosclerosis in high cholesterol fed rabbits. Lipids Health Dis. 12 (1), 115. doi:10.1186/1476-511X-12-115
Kou, J. Y., Li, Y., Zhong, Z. Y., Jiang, Y. Q., Li, X. S., Han, X. B., et al. (2017). Berberine-sonodynamic therapy induces autophagy and lipid unloading in macrophage. Cell Death Dis. 8 (1), e2558. doi:10.1038/cddis.2016.354
Lee, C. W., Hu, S. C., Yen, F. L., Hsu, L. F., Lee, I. T., Lin, Z. C., et al. (2017). Magnolol nanoparticles exhibit improved water solubility and suppress TNF-alpha-induced VCAM-1 expression in endothelial cells. J. Biomed. Nanotechnol. 13 (3), 255–268. doi:10.1166/jbn.2017.2342
Lee, K. C., Chang, H. H., Chung, Y. H., and Lee, T. Y. (2011). Andrographolide acts as an anti-inflammatory agent in LPS-stimulated RAW264.7 macrophages by inhibiting STAT3-mediated suppression of the NF-κB pathway. J. Ethnopharmacol. 135 (3), 678–684. doi:10.1016/j.jep.2011.03.068
Lephart, E. D., West, T. W., Weber, K. S., Rhees, R. W., Setchell, K. D. R., Adlercreutz, H., et al. (2002). Neurobehavioral effects of dietary soy phytoestrogens. Neurotoxicol. Teratol. 24 (1), 5–16. doi:10.1016/s0892-0362(01)00197-0
Li, F., Zhang, T., He, Y., Gu, W., Yang, X., Zhao, R., et al. (2020). Inflammation inhibition and gut microbiota regulation by TSG to combat atherosclerosis in ApoE(-/-) mice. J. Ethnopharmacol. 247, 112232. doi:10.1016/j.jep.2019.112232
Li, J., Dong, J. Z., Ren, Y. L., Zhu, J. J., Cao, J. N., Zhang, J., et al. (2018). Luteolin decreases atherosclerosis in LDL receptor-deficient mice via a mechanism including decreasing AMPK-SIRT1 signaling in macrophages. Exp. Ther. Med. 16 (3), 2593–2599. doi:10.3892/etm.2018.6499
Li, W., Yu, J., Zhao, J., Xiao, X., Li, W., Zang, L., et al. (2021). Poria cocos polysaccharides reduces high-fat diet-induced arteriosclerosis in ApoE(-/-) mice by inhibiting inflammation. Phytother. Res. 35 (4), 2220–2229. doi:10.1002/ptr.6980
Li, X. Y., Kong, L. X., Li, J., He, H. X., and Zhou, Y. D. (2013). Kaempferol suppresses lipid accumulation in macrophages through the downregulation of cluster of differentiation 36 and the upregulation of scavenger receptor class B type I and ATP-binding cassette transporters A1 and G1. Int. J. Mol. Med. 31 (2), 331–338. doi:10.3892/ijmm.2012.1204
Li, Y., Guo, Y., Chen, Y., Wang, Y., You, Y., Yang, Q., et al. (2015). Establishment of an interleukin-1β-induced inflammation-activated endothelial cell-smooth muscle cell-mononuclear cell co-culture model and evaluation of the anti-inflammatory effects of tanshinone IIA on atherosclerosis. Mol. Med. Rep. 12 (2), 1665–1676. doi:10.3892/mmr.2015.3668
Liang, C. J., Lee, C. W., Sung, H. C., Chen, Y. H., Wang, S. H., Wu, P. J., et al. (2014). Magnolol reduced TNF-α-induced vascular cell adhesion molecule-1 expression in endothelial cells via JNK/p38 and NF-κB signaling pathways. Am. J. Chin. Med. 42 (3), 619–637. doi:10.1142/S0192415X14500402
Libby, P. (2021). The changing landscape of atherosclerosis. Nature 592 (7855), 524–533. doi:10.1038/s41586-021-03392-8
Lim, S. H., Jung, S. K., Byun, S., Lee, E. J., Hwang, J. A., Seo, S. G., et al. (2013). Luteolin suppresses UVB-induced photoageing by targeting JNK1 and p90 RSK2. J. Cell. Mol. Med. 17 (5), 672–680. doi:10.1111/jcmm.12050
Lin, S. J., Lee, I. T., Chen, Y. H., Lin, F. Y., Sheu, L. M., Ku, H. H., et al. (2007). Salvianolic acid B attenuates MMP-2 and MMP-9 expression in vivo in apolipoprotein-E-deficient mouse aorta and in vitro in LPS-treated human aortic smooth muscle cells. J. Cell. Biochem. 100 (2), 372–384. doi:10.1002/jcb.21042
Lin, , Lu, Y., Shen, T., Huang, X., Man, Y., Wang, S., et al. (2012). Panax notogingseng saponins suppress RAGE/MAPK signaling and NF-kappaB activation in apolipoprotein-E-deficient atherosclerosis-prone mice. Cell. Physiol. biochem. 29 (5-6), 875–882.
Liu, K., Zhao, W., Gao, X., Huang, F., Kou, J., and Liu, B. (2012). Diosgenin ameliorates palmitate-induced endothelial dysfunction and insulin resistance via blocking IKKβ and IRS-1 pathways. Atherosclerosis 223 (2), 350–358. doi:10.1016/j.atherosclerosis.2012.06.012
Liu, T., Li, C., Sun, H., Luo, T., Tan, Y., Tian, D., et al. (2014). Curcumin inhibits monocyte chemoattractant protein-1 expression and enhances cholesterol efflux by suppressing the c-Jun N-terminal kinase pathway in macrophage. Inflamm. Res. 63 (10), 841–850. doi:10.1007/s00011-014-0758-9
Liu, X., Xu, Y., Cheng, S., Zhou, X., Zhou, F., He, P., et al. (2021). Geniposide combined with notoginsenoside R1 attenuates inflammation and apoptosis in atherosclerosis via the AMPK/mTOR/Nrf2 signaling pathway. Front. Pharmacol. 12, 687394. doi:10.3389/fphar.2021.687394
Liu, Y., Cheng, P., and Wu, A-H. (2020). Honokiol inhibits carotid artery atherosclerotic plaque formation by suppressing inflammation and oxidative stress. Aging (Albany NY) 12 (9), 8016–8028. doi:10.18632/aging.103120
Liu, Z., Wang, J., Huang, E., Gao, S., Li, H., Lu, J., et al. (2014). Tanshinone IIA suppresses cholesterol accumulation in human macrophages: Role of heme oxygenase-1. J. Lipid Res. 55 (2), 201–213. doi:10.1194/jlr.M040394
Lu, C. Y., Yang, Y. C., Li, C. C., Liu, K. L., Lii, C. K., and Chen, H. W. (2014). Andrographolide inhibits TNFα-induced ICAM-1 expression via suppression of NADPH oxidase activation and induction of HO-1 and GCLM expression through the PI3K/Akt/Nrf2 and PI3K/Akt/AP-1 pathways in human endothelial cells. Biochem. Pharmacol. 91 (1), 40–50. doi:10.1016/j.bcp.2014.06.024
Lu, L., Huang, J., Xue, X., Wang, T., Huang, Z., and Li, J. (2021). Berberine regulated miR150-5p to inhibit P2X7 receptor, EMMPRIN and MMP-9 expression in oxLDL induced macrophages. Front. Pharmacol. 12, 639558. doi:10.3389/fphar.2021.639558
Ma, X., Zhang, T., Luo, Z., Li, X., Lin, M., Li, R., et al. (2020). Functional nano-vector boost anti-atherosclerosis efficacy of berberine in Apoe ((-/-)) mice. Acta Pharm. Sin. B 10 (9), 1769–1783. doi:10.1016/j.apsb.2020.03.005
Muhamad Fadzil, N. S., Sekar, M., Gan, S. H., Bonam, S. R., Wu, Y. S., Vaijanathappa, J., et al. (2021). Chemistry, Pharmacology and therapeutic potential of swertiamarin - a promising natural lead for new drug discovery and development. Drug Des. devel. Ther. 15, 2721–2746. doi:10.2147/DDDT.S299753
Ng, T. B. (1998). A review of research on the protein-bound polysaccharide (polysaccharopeptide, PSP) from the mushroom Coriolus versicolor (basidiomycetes: Polyporaceae). Gen. Pharmacol. 30 (1), 1–4. doi:10.1016/s0306-3623(97)00076-1
Okawara, M., Hashimoto, F., Todo, H., Sugibayashi, K., and Tokudome, Y. (2014). Effect of liquid crystals with cyclodextrin on the bioavailability of a poorly water-soluble compound, diosgenin, after its oral administration to rats. Int. J. Pharm. 472 (1), 257–261. doi:10.1016/j.ijpharm.2014.06.032
Pang, Z-J. (2003). Effect of polysaccharide krestin on the up-regulation of macrophage colony-stimulating factor gene expression in protecting mouse peritoneal macrophages from oxidative injury. Am. J. Chin. Med. 31 (01), 11–23. doi:10.1142/S0192415X03000813
Qiu, L., Xu, R., Wang, S., Li, S., Sheng, H., Wu, J., et al. (2015). Honokiol ameliorates endothelial dysfunction through suppression of PTX3 expression, a key mediator of IKK/IκB/NF-κB, in atherosclerotic cell model. Exp. Mol. Med. 47, e171. doi:10.1038/emm.2015.37
Register, T. C., Cann, J. A., Kaplan, J. R., Williams, J. K., Adams, M. R., Morgan, T. M., et al. (2005). Effects of soy isoflavones and conjugated equine estrogens on inflammatory markers in atherosclerotic, ovariectomized monkeys. J. Clin. Endocrinol. Metab. 90 (3), 1734–1740. doi:10.1210/jc.2004-0939
Ren, K., Jiang, T., Zhou, H. F., Liang, Y., and Zhao, G. J. (2018). Apigenin retards atherogenesis by promoting ABCA1-mediated cholesterol efflux and suppressing inflammation. Cell. Physiol. biochem. 47 (5), 2170–2184. doi:10.1159/000491528
Ridker, P. M., Danielson, E., Fonseca, F. A. H., Genest, J., Gotto, A. M., Kastelein, J. J. P., et al. (2009). Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: A prospective study of the JUPITER trial. Lancet 373 (9670), 1175–1182. doi:10.1016/S0140-6736(09)60447-5
Schutz, K., Carle, R., and Schieber, A. (2006). Taraxacum--a review on its phytochemical and pharmacological profile. J. Ethnopharmacol. 107 (3), 313–323. doi:10.1016/j.jep.2006.07.021
Shin, S. K., Ha, T. Y., McGregor, R. A., and Choi, M. S. (2011). Long-term curcumin administration protects against atherosclerosis via hepatic regulation of lipoprotein cholesterol metabolism. Mol. Nutr. Food Res. 55 (12), 1829–1840. doi:10.1002/mnfr.201100440
Son, D. J., Kim, S. Y., Han, S. S., Kim, C. W., Kumar, S., Park, B. S., et al. (2012). Piperlongumine inhibits atherosclerotic plaque formation and vascular smooth muscle cell proliferation by suppressing PDGF receptor signaling. Biochem. Biophys. Res. Commun. 427 (2), 349–354. doi:10.1016/j.bbrc.2012.09.061
Stumpf, C., Fan, Q., Hintermann, C., Raaz, D., Kurfurst, I., Losert, S., et al. (2013). Anti-inflammatory effects of danshen on human vascular endothelial cells in culture. Am. J. Chin. Med. 41 (5), 1065–1077. doi:10.1142/S0192415X13500729
Sun, M. Y., Zhang, M., Chen, S. L., Zhang, S. P., Guo, C. Y., Wang, J. S., et al. (2018). The influence of hyperlipidemia on endothelial function of FPN1 tek-cre mice and the intervention effect of tetramethylpyrazine. Cell. Physiol. biochem. 47 (1), 119–128. doi:10.1159/000489754
Tan, W., Wang, Y., Wang, K., Wang, S., Liu, J., Qin, X., et al. (2020). Improvement of endothelial dysfunction of berberine in atherosclerotic mice and mechanism exploring through TMT-based proteomics. Oxid. Med. Cell. Longev. 2020, 8683404. doi:10.1155/2020/8683404
Tao, Z., Xiao, Q., Che, X., Zhang, H., Geng, N., and Shao, Q. (2022). Regulating mitochondrial homeostasis and inhibiting inflammatory responses through Celastrol. Ann. Transl. Med. 10 (7), 400. doi:10.21037/atm-21-7015
Vaidya, H., Giri, S., Jain, M., and Goyal, R. K. (2012). Decrease in serum matrix metalloproteinase-9 and matrix metalloproteinase-3 levels in Zucker fa/fa obese rats after treatment with swertiamarin. Exp. Clin. Cardiol. 17 (1), 12–16.
Vaidya, H., Rajani, M., Sudarsanam, V., Padh, H., and Goyal, R. (2009). Antihyperlipidaemic activity of swertiamarin, a secoiridoid glycoside in poloxamer-407-induced hyperlipidaemic rats. J. Nat. Med. 63 (4), 437–442. doi:10.1007/s11418-009-0350-8
Vasamsetti, S. B., Karnewar, S., Gopoju, R., Gollavilli, P. N., Narra, S. R., Kumar, J. M., et al. (2016). Resveratrol attenuates monocyte-to-macrophage differentiation and associated inflammation via modulation of intracellular GSH homeostasis: Relevance in atherosclerosis. Free Radic. Biol. Med. 96, 392–405. doi:10.1016/j.freeradbiomed.2016.05.003
Wan, J. B., Lee, S. M., Wang, J. D., Wang, N., He, C. W., Wang, Y. T., et al. (2009). Panax notoginseng reduces atherosclerotic lesions in ApoE-deficient mice and inhibits TNF-alpha-induced endothelial adhesion molecule expression and monocyte adhesion. J. Agric. Food Chem. 57 (15), 6692–6697. doi:10.1021/jf900529w
Wan, Q., Liu, Z., Yang, Y., and Cui, X. (2018). Suppressive effects of berberine on atherosclerosis via downregulating visfatin expression and attenuating visfatin-induced endothelial dysfunction. Int. J. Mol. Med. 41 (4), 1939–1948. doi:10.3892/ijmm.2018.3440
Wang, B., Liao, P. P., Liu, L. H., Fang, X., Li, W., and Guan, S. M. (2016). Baicalin and geniposide inhibit the development of atherosclerosis by increasing Wnt1 and inhibiting dickkopf-related protein-1 expression. J. Geriatr. Cardiol. 13 (10), 846–854. doi:10.11909/j.issn.1671-5411.2016.10.013
Wang, Y., Wang, Y. S., Song, S. L., Liang, H., and Ji, A. G. (2016). Icariin inhibits atherosclerosis progress in Apoe null mice by downregulating CX3CR1 in macrophage. Biochem. Biophys. Res. Commun. 470 (4), 845–850. doi:10.1016/j.bbrc.2016.01.118
Wang, Y. Q., Shen, Y., Li, F., Wang, C. H., and Zhang, W. (2013). 2, 3, 4', 5-tetrahydroxystilbene-2-O-beta-D-glucoside suppresses expression of adhesion molecules in aortic wall of dietary atherosclerotic rats and promonocytic U937 cells. Cell biochem. Biophys. 67 (3), 997–1004. doi:10.1007/s12013-013-9595-7
Wu, H. J., Hao, J., Wang, S. Q., Jin, B. L., and Chen, X. B. (2012). Protective effects of ligustrazine on TNF-alpha-induced endothelial dysfunction. Eur. J. Pharmacol. 674 (2-3), 365–369. doi:10.1016/j.ejphar.2011.10.046
Wu, J., Hu, W., Gong, Y., Wang, P., Tong, L., Chen, X., et al. (2017). Current pharmacological developments in 2, 3, 4', 5-tetrahydroxystilbene 2-O-beta-D-glucoside (TSG). Eur. J. Pharmacol. 811, 21–29. doi:10.1016/j.ejphar.2017.05.037
Wu, T., Chen, X., Wang, Y., Xiao, H., Peng, Y., Lin, L., et al. (2018). Aortic plaque-targeted andrographolide delivery with oxidation-sensitive micelle effectively treats atherosclerosis via simultaneous ROS capture and anti-inflammation. Nanomedicine 14 (7), 2215–2226. doi:10.1016/j.nano.2018.06.010
Wu, T., Peng, Y., Yan, S., Li, N., Chen, Y., and Lan, T. (2018). Andrographolide ameliorates atherosclerosis by suppressing pro-inflammation and ROS generation-mediated foam cell formation. Inflammation 41 (5), 1681–1689. doi:10.1007/s10753-018-0812-9
Wu, Y., Wang, F., Fan, L., Zhang, W., Wang, T., Du, Y., et al. (2018). Baicalin alleviates atherosclerosis by relieving oxidative stress and inflammatory responses via inactivating the NF-κB and p38 MAPK signaling pathways. Biomed. Pharmacother. 97, 1673–1679. doi:10.1016/j.biopha.2017.12.024
Xiao, H. B., Lu, X. Y., Sun, Z. L., and Zhang, H. B. (2011). Kaempferol regulates OPN-CD44 pathway to inhibit the atherogenesis of apolipoprotein E deficient mice. Toxicol. Appl. Pharmacol. 257 (3), 405–411. doi:10.1016/j.taap.2011.09.024
Xing, J., Peng, K., Cao, W., Lian, X., Wang, Q., and Wang, X. (2013). Effects of total flavonoids from Dracocephalum moldavica on the proliferation, migration, and adhesion molecule expression of rat vascular smooth muscle cells induced by TNF-α. Pharm. Biol. 51 (1), 74–83. doi:10.3109/13880209.2012.711839
Yang, H., Liu, Z., Hu, X., Liu, X., Gui, L., Cai, Z., et al. (2022). Protective effect of panax notoginseng saponins on apolipoprotein-E-deficient atherosclerosis-prone mice. Curr. Pharm. Des. 28 (8), 671–677. doi:10.2174/1381612828666220128104636
Yang, H., Yan, L., Qian, P., Duan, H., Wu, J., Li, B., et al. (2015). Icariin inhibits foam cell formation by downregulating the expression of CD36 and up-regulating the expression of SR-BI. J. Cell. Biochem. 116 (4), 580–588. doi:10.1002/jcb.25009
Yang, H-P., Yue, L., Jiang, W-W., Liu, Q., Kou, J-P., and Yu, B-Y. (2013). Diosgenin inhibits tumor necrosis factor-induced tissue factor activity and expression in THP-1 cells via downregulation of the NF-κB, Akt, and MAPK signaling pathways. Chin. J. Nat. Med. 11 (6), 608–615. doi:10.1016/S1875-5364(13)60070-9
Yang, X-J., Liu, F., Feng, N., Ding, X-S., Chen, Y., Zhu, S-X., et al. (2020). Berberine attenuates cholesterol accumulation in macrophage foam cells by suppressing AP-1 activity and activation of the Nrf2/HO-1 pathway. J. Cardiovasc. Pharmacol. 75 (1), 45–53. doi:10.1097/FJC.0000000000000769
Yao, W., Huang, L., Sun, Q., Yang, L., Tang, L., Meng, G., et al. (2016). The inhibition of macrophage foam cell formation by tetrahydroxystilbene glucoside is driven by suppressing vimentin cytoskeleton. Biomed. Pharmacother. 83, 1132–1140. doi:10.1016/j.biopha.2016.08.032
Yuan, C., Mei, Z., Shangxi, L., and Yi, L. (1996). PSK protects macrophages from lipoperoxide accumulation and foam cell formation caused by oxidatively modified low-density lipoprotein. Atherosclerosis 124 (2), 171–181. doi:10.1016/0021-9150(96)05835-2
Yuan, X., Chen, J., and Dai, M. (2016). Paeonol promotes microRNA-126 expression to inhibit monocyte adhesion to ox-LDL-injured vascular endothelial cells and block the activation of the PI3K/Akt/NF-κB pathway. Int. J. Mol. Med. 38 (6), 1871–1878. doi:10.3892/ijmm.2016.2778
Yuan, Z., Liao, Y., Tian, G., Li, H., Jia, Y., Zhang, H., et al. (2011). Panax notoginseng saponins inhibit Zymosan A induced atherosclerosis by suppressing integrin expression, FAK activation and NF-κB translocation. J. Ethnopharmacol. 138 (1), 150–155. doi:10.1016/j.jep.2011.08.066
Zeng, P., Liu, B., Wang, Q., Fan, Q., Diao, J. X., Tang, J., et al. (2015). Apigenin attenuates atherogenesis through inducing macrophage apoptosis via inhibition of AKT Ser473 phosphorylation and downregulation of plasminogen activator inhibitor-2. Oxid. Med. Cell. Longev. 2015, 379538. doi:10.1155/2015/379538
Zhang, B-C., Li, Z., Xu, W., Xiang, C-H., and Ma, Y-F. (2018). Luteolin alleviates NLRP3 inflammasome activation and directs macrophage polarization in lipopolysaccharide-stimulated RAW264.7 cells. Am. J. Transl. Res. 10 (1), 265–273.
Zhang, W., Wang, C. H., Li, F., and Zhu, W. Z. (2008). 2, 3, 4', 5-Tetrahydroxystilbene-2-O-beta-D-glucoside suppresses matrix metalloproteinase expression and inflammation in atherosclerotic rats. Clin. Exp. Pharmacol. Physiol. 35 (3), 310–316. doi:10.1111/j.1440-1681.2007.04824.x
Zhang, W. L., Xiao, Y., Liu, J. P., Wu, Z. M., Gu, X., Xu, Y. M., et al. (2011). Structure and remodeling behavior of drug-loaded high density lipoproteins and their atherosclerotic plaque targeting mechanism in foam cell model. Int. J. Pharm. 419 (1-2), 314–321. doi:10.1016/j.ijpharm.2011.07.039
Zhang, X., Wang, G., Gurley, E. C., and Zhou, H. (2014). Flavonoid apigenin inhibits lipopolysaccharide-induced inflammatory response through multiple mechanisms in macrophages. PLoS One 9 (9), e107072. doi:10.1371/journal.pone.0107072
Zhang, Y., Ren, P., Kang, Q., Liu, W., Li, S., Li, P., et al. (2017). Effect of tetramethylpyrazine on atherosclerosis and SCAP/SREBP-1c signaling pathway in ApoE(-/-) mice fed with a high-fat diet. Evid. Based. Complement. Altern. Med. 2017, 3121989. doi:10.1155/2017/3121989
Zhang, Y. G., Zhang, H. G., Zhang, G. Y., Fan, J. S., Li, X. H., Liu, Y. H., et al. (2008). Panax notoginseng saponins attenuate atherosclerosis in rats by regulating the blood lipid profile and an anti-inflammatory action. Clin. Exp. Pharmacol. Physiol. 35 (10), 1238–1244. doi:10.1111/j.1440-1681.2008.04997.x
Zhao, J., Cui, L., Sun, J., Xie, Z., Zhang, L., Ding, Z., et al. (2020). Notoginsenoside R1 alleviates oxidized low-density lipoprotein-induced apoptosis, inflammatory response, and oxidative stress in HUVECS through modulation of XIST/miR-221-3p/TRAF6 axis. Cell. Signal. 76, 109781. doi:10.1016/j.cellsig.2020.109781
Zhao, J., Niu, X., Yu, J., Xiao, X., Li, W., Zang, L., et al. (2020). Poria cocos polysaccharides attenuated ox-LDL-induced inflammation and oxidative stress via ERK activated Nrf2/HO-1 signaling pathway and inhibited foam cell formation in VSMCs. Int. Immunopharmacol. 80, 106173. doi:10.1016/j.intimp.2019.106173
Zhao, J., Wang, Z., Yuan, Z., Lv, S., and Su, Q. (2020). Baicalin ameliorates atherosclerosis by inhibiting NLRP3 inflammasome in apolipoprotein E-deficient mice. Diab. Vasc. Dis. Res. 17 (6), 1. doi:10.1177/1479164120977441
Zhong, X., Zhang, L., Li, Y., Li, P., Li, J., and Cheng, G. (2018). Kaempferol alleviates ox-LDL-induced apoptosis by up-regulation of miR-26a-5p via inhibiting TLR4/NF-κB pathway in human endothelial cells. Biomed. Pharmacother. 108, 1783–1789. doi:10.1016/j.biopha.2018.09.175
Zhou, X., Chen, L. L., Xie, R. F., Lam, W., Zhang, Z. J., Jiang, Z. L., et al. (2017). Chemosynthesis pathway and bioactivities comparison of saponins in radix and flower of Panax notoginseng (Burk.) F.H. Chen. J. Ethnopharmacol. 201, 56–72. doi:10.1016/j.jep.2016.11.008
Zhu, L., Gong, X., Gong, J., Xuan, Y., Fu, T., Ni, S., et al. (2020). Notoginsenoside R1 upregulates miR-221-3p expression to alleviate ox-LDL-induced apoptosis, inflammation, and oxidative stress by inhibiting the TLR4/NF-κB pathway in HUVECs. Braz J. Med. Biol. Res. 53 (6), e9346. doi:10.1590/1414-431x20209346
Keywords: atherosclerosis, inflammation, natural drugs, interleukin, TCM
Citation: Song X, Wang X, Wang D, Zheng Z, Li J and Li Y (2022) Natural drugs targeting inflammation pathways can be used to treat atherosclerosis. Front. Pharmacol. 13:998944. doi: 10.3389/fphar.2022.998944
Received: 20 July 2022; Accepted: 13 October 2022;
Published: 01 November 2022.
Edited by:
Olumayokun Olajide, University of Huddersfield, United KingdomReviewed by:
Mengmeng Zhang, Shaanxi University of Chinese Medicine, ChinaCopyright © 2022 Song, Wang, Wang, Zheng, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Li, MTgyNDAyNTY4MkBxcS5jb20= Yunlun Li, eXVubHVuLmxlZUBob3RtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.