
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 07 October 2022
Sec. Drugs Outcomes Research and Policies
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.996824
This article is part of the Research TopicEmerging Talents in Pharmacology: Drugs Outcomes Research and Policies 2022View all 8 articles
Background: National Centralized Drug Procurement (NCDP), an ongoing government-led policy starting in 2019 in China, aimed at reducing drug costs. During the implementation of NCDP, healthcare professionals (HCPs) still have a certain degree of concern about the policy, which affects the clinical use of related drugs.
Objective: This study aims to assess the level of knowledge, attitude and practice (KAP) of HCPs towards NCDP policy, together with the associated factors that determine their KAP.
Methods: A cross-sectional study was conducted between September and November of 2021 in 30 hospitals in Hubei province in Central China. A self-designed online questionnaire including KAP towards NCDP policy was administered to HCPs. Logistic regression analysis was adopted to identify the factors associated with KAP.
Results: A Total of, 742 HCPs completed the questionnaires. 43.4% of HCPs had good knowledge, 24.7% had a positive attitude, and 23.7% held good practice. Through multivariate logistic regression analysis, HCPs who are males, pharmacists, with senior professional titles and 6–10 years of professional working experience contributed to a higher knowledge level. Pharmacists and HCPs with good knowledge were more likely to have positive attitudes, while HCPs with higher education were less likely to have positive attitudes. Pharmacists, HCPs who had 11–20 years of professional working experience, worked in medium-size urban areas or had good knowledge were more likely to have good practice. Good practice is also associated with the positive attitude towards the efficacy of centralized-purchased medicines and impacts of NCDP policy.
Conclusion: Only a small percentage of HCPs had good KAP towards NCDP policy. Pharmacists showed better KAP than physicians. The positive attitude towards the efficacy of centralized-purchased medicines and impacts of NCDP policy contributed to better practice. High-quality clinical evidence on the therapeutic effects and safety of the centralized-purchased drugs is needed.
The fast rise of pharmaceutical expenditure and the resulting increase in total health expenses have brought a heavy burden to the finances and the daily medical care of the people around the world, accessibility and affordability of medicines have become major issues for healthcare systems globally (Seidman and Atun, 2017; Long et al., 2022; Wen et al., 2022; Zhang et al., 2022). And China is no exception (Ma et al., 2020). The pharmaceutical expenditure for each patient has risen from 36.59 yuan (5.33 dollars) to 467.04 yuan (67.98 dollars) between 1990 and 2008, with an annual growth rate of 15%, even higher than the GDP growth level (He et al., 2018). Curbing inflated drug prices has become the top priority of China’s medical reform. In recent years, from national negotiations to zero-price markups for drugs, to national centralized drug procurement (NCDP), the state has taken numbers of measures to alleviate the burden of medical care. Among them, NCDP policy plays a crucial role in reducing drug prices (Lan et al., 2022).
Actually, the centralized procurement model is not the first of its kind in China. Many countries from low-middle-income to high-income have adopted centralized procurement (or named “pooled procurement”) of medicines to control drug expenses (Dubois et al., 2021; Parmaksiz et al., 2022). For instance, in Denmark and Norway the respective central procurement agencies purchase the medicines for all public hospitals (Vogler et al., 2022). China has implemented NCDP policy by summarizing international experience. The NCDP policy led by government has started since 2019, which requires drug manufacturers to reduce the price of drugs and the government enters into contracts with manufacturers to purchase drugs for a certain amount of use, after which public healthcare institutions are required to enforce that price and amount of use (Chen et al., 2020). In 2019, the State took the lead in conducting a pilot project for centralized drug procurement, including four municipalities and seven sub-provincial cities (referred to as “4 + 7”) (Hua et al., 2021). 25 drugs including 22 generic drugs varieties which has passed consistency evaluation and 3 original research varieties were selected. Compared with the minimum purchase price of the same drug in the pilot cities in 2017, the selected varieties dropped by an average of 52%, and the highest drop was 96% (Yang et al., 2021). From the pilot to nationwide expansion and continuous operation, NCDP policy has been fully implemented across China. The sharp drop in selected drug prices has led to the price linkage of non-selective drugs (Wu et al., 2021), further promoting the price reduction effect, and effectively reduce patients’ medical burden, especially those with chronic and severe illnesses.
However, in the process of implementing NCDP policy, achievements and challenges coexist. Since the use of centralized-purchased drugs is ensured by the policy, the use of non-selected drugs is limited, which restricts physician’s prescription options and affects patients’ adherence. Moreover, due to lack of high-quality efficacy and safety evaluation of the centralized-purchased drugs, physicians still have a certain degree of concern about the policy (Dunne et al., 2014). And the extremely low price of the selected drugs makes patients doubt its quality and efficacy (Yang et al., 2021; Liang et al., 2022). In terms of the supply of selected drugs, there is a risk of supply shortages that affects the treatment of patients due to the drugs are only provided by one company. NCDP policy still needs to be continuously improved in practice.
Currently, there is little information about KAP regarding NCDP policy among HCPs. This may be an unexplored barrier to implement NCDP policy and reduce medical burden. In this study, we investigated KAP and the suggestions of HCPs towards the policy in Hubei province in Central China.
This was a cross-sectional study based on a questionnaire survey. This study was conducted among HCPs in 30 hospitals in Hubei Province in Central China between September and November of 2021.
To determine the sample size for this survey, we utilized the well-known online sample size calculator Raosoft (Raosoft , 2019; Memon et al., 2020). We set the margin of error to 4%, the confidence level at 95% (Marzan et al., 2021). Based on the official figure of 255,995 HCPs working in hospitals in Hubei Province in 2019 (China Government Statistics Information Center, 2020), the required sample size for this survey was 599.
The inclusion criteria were HCPs working at hospitals in Hubei Province at the time the survey conducted. The questionnaire using screeners that automatically excluded any respondents whose filling time was less than 1 min or whose work location was not in Hubei Province.
Three clinical pharmacists developed the KAP questionnaire after conducting extensive literature review and discussion. The content validity of the questionnaire was reviewed by three experts in the field (one expert from pharmacoepidemiology, one expert from health policy, and one expert from cardiology). Pilot questionnaires were administered to 37 HCPs in two hospitals and analyzed for reliability and validity. The content reliability of the scale has a Cronbach’s alpha of 0.781, indicating acceptable internal consistency (Taber, 2017). And Kaiser-Meyer-Olkin and Bartlett’s sphericity test revealed the KMO value was 0.854 (>0.6, p < 0.05), showing excellent validity (Sadowska et al., 2021).
The questionnaire was composed of 18 mandatory single-choice items and one multiple-choice item, and it consisted of five sections: 1) HCPs’ characteristics: profession, education, working experience, etc.; 2) Knowledge part: familiarity with NCDP policy and consistency evaluation of generic drugs, this part was provided on a 5-level Likert scale (1 = “very unfamiliar”, 2 = “less familiar”, 3 = “general”, 4 = “more familiar”, 5 = “very familiar”) ; 3) Attitude part: concerning about the policy impact, this part was provided on a 5-level Likert scale (1 = “strongly disagree”, 2 = “disagree”, 3 = “uncertain”, 4 = “agree”, 5 = “strongly agree”); 4) Practice part: interpretation, guidance for patients, and drug choice, this part was provided on a 5-level Likert scale, the options were assigned in order of 5 to 1 point according to the degree; 5) Suggestions towards NCDP policy: provided in multiple-choice form. The cut-off points for good (or positive) knowledge, attitude or practice was set at ≥ 80% of total points for the individual part (AlRasheed et al., 2021b). Detailed information related to KAP towards NCDP policy were presented in Supplementary Material.
A snowball sampling method was adopted for this study (Leighton et al., 2021). We created an online questionnaire using Questionnaire Star tool. The invitation to complete the questionnaire was distributed through WeChat groups and emails of HCPs. Participants were encouraged to distribute the questionnaire to as many colleagues as possible. The average time to complete the questionnaire was 15 min. Before starting the survey, each participant was asked to review the aim of the survey and to then fill out an electronic informed consent. Participants could withdraw from the survey at any time. We obtained 889 questionnaires by using the snowball method of distribution. 147 questionnaires were excluded based on the exclusion criteria. As a result, 742 questionnaires were evaluated in this study.
Data statistics were applied by using IBM SPSS Statistics 26. Descriptive analysis of frequencies and proportions of KAP levels was performed. The correlation between KAP levels and different demographic characteristics were evaluated through univariate logistic regression. The demographic factors with p < 0.1 in the univariate logistic regression analysis were taken as covariates in the multivariate logistic regression. The multivariate logistic regression method was conducted to identify the associated factors of KAP levels. The statistical significance level was set at p < 0.05.
A total of 742 HCPs were studied. More than half of them were females (56.7%). Most participants were pharmacists (351, 47.3%) and physicians (322, 43.4%). 463 HCPs (62.4%) came from large metropolitan areas, and 570 HCPs (76.8%) worked at tertiary hospitals (Table 1).
62.5% of HCPs agreed that they knew the policy of NCDP, and 47.9% of them agreed that they were familiar with the generic drug consistency evaluation (Supplementary Table S1). Overall, only 43.4% of HCPs got a good score for knowledge (Figure 1).
Although most participants agreed NCDP policy was reasonable and played a significant role in medical reform and reduce patients’ medication costs, merely 11.3% agreed there was no difference in quality or efficacy between brand-name drugs and generic drugs that had passed consistency evaluation, and only 44.7% agreed that the sharp drop in drug prices did not affect drug quality or efficacy. 49.4% of HCPs agreed NCDP policy can reduce patient-doctor disputes (Supplementary Table S2). On the whole, only 24.7% of HCPs had positive attitudes towards NCDP policy (Figure 1).
24.3% of HCPs always or often explain NCDP policy to patients with doubts. Most HCPs (70.9%) always or frequently encourage patients to use centralized procurement medicines. Only 23.7% of HCPs always consider centralized procurement drugs for themselves or their families, while 37.9% sometimes do (Supplementary Table S3). Based on the practice score, only 23.7% of HCPs had good practice (Figure 1).
Amongst the variables involved in the univariate analysis, gender, profession, professional title, professional working experience, and hospital grade have shown a significant association (p < 0.05) (Table 2). In the multivariate logistic analysis, pharmacists (OR = 3.30, 95% CI: 2.28–4.78), HCPs with senior professional titles (OR = 2.38, 95% CI: 1.30–4.33), and 6–10 professional working experience (OR = 1.86, 95% CI: 1.08–3.20) were more likely to have a good knowledge, while female HCPs (OR = 0.59, 95% CI: 0.42–0.82) tended to have poor knowledge.
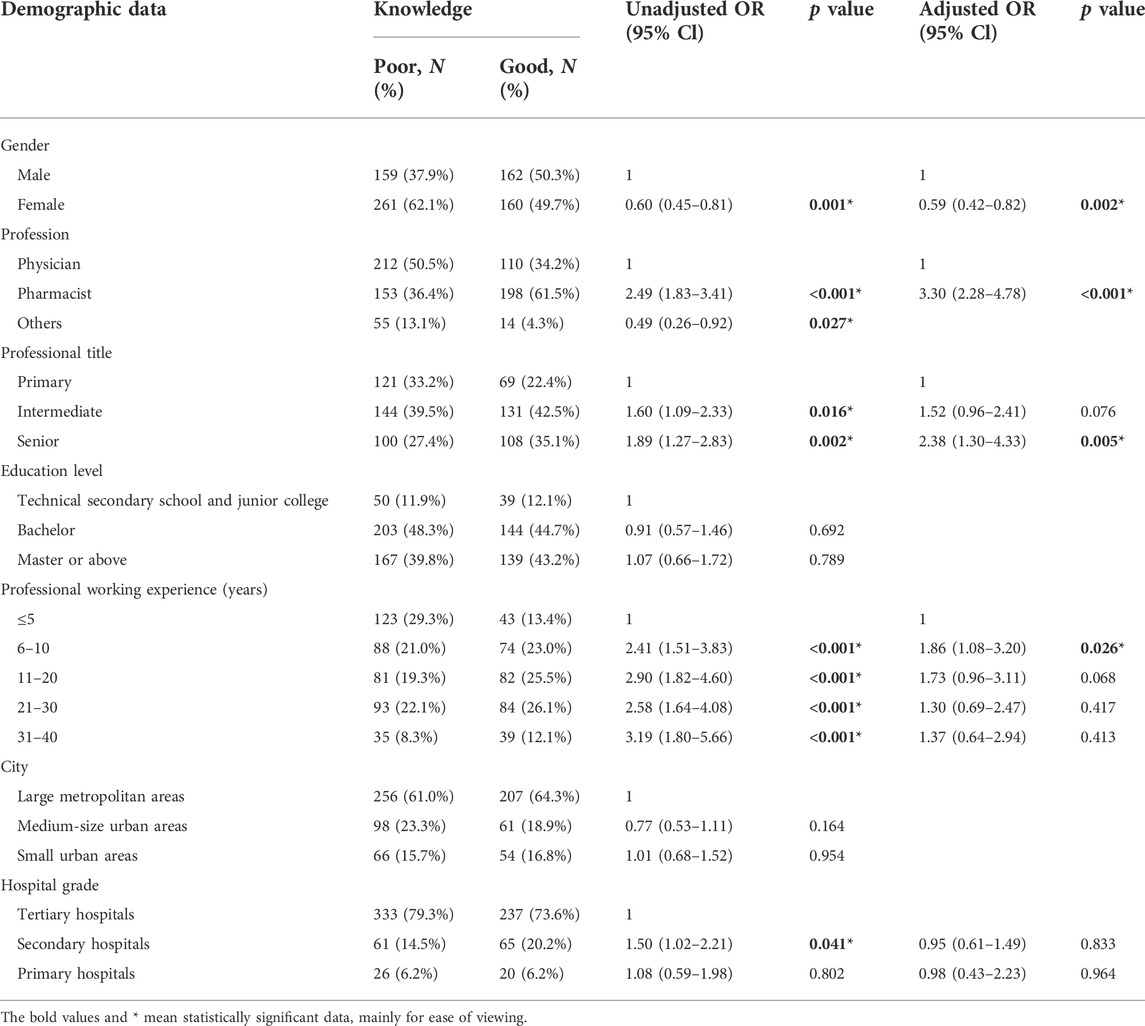
TABLE 2. Univariable and multivariable analysis of associated factors of HCPs’ knowledge towards NCDP policy.
In the univariate analysis, five factors were significantly correlated with a positive attitude level, which were the profession, education level, city, hospital grade and knowledge level (p < 0.05), as exhibited in Table 3. In the multivariate logistic analysis, pharmacists (OR = 1.97, 95% CI: 1.24–3.13) and HCPs who have good knowledge (OR = 5.46, 95% CI: 3.66–8.16) were more likely to have positive attitudes. However, HCPs with bachelor and above educational background were less likely to have positive attitudes (OR = 0.53, 95% CI: 0.30–0.95; OR = 0.37, 95% CI: 0.19–0.74).
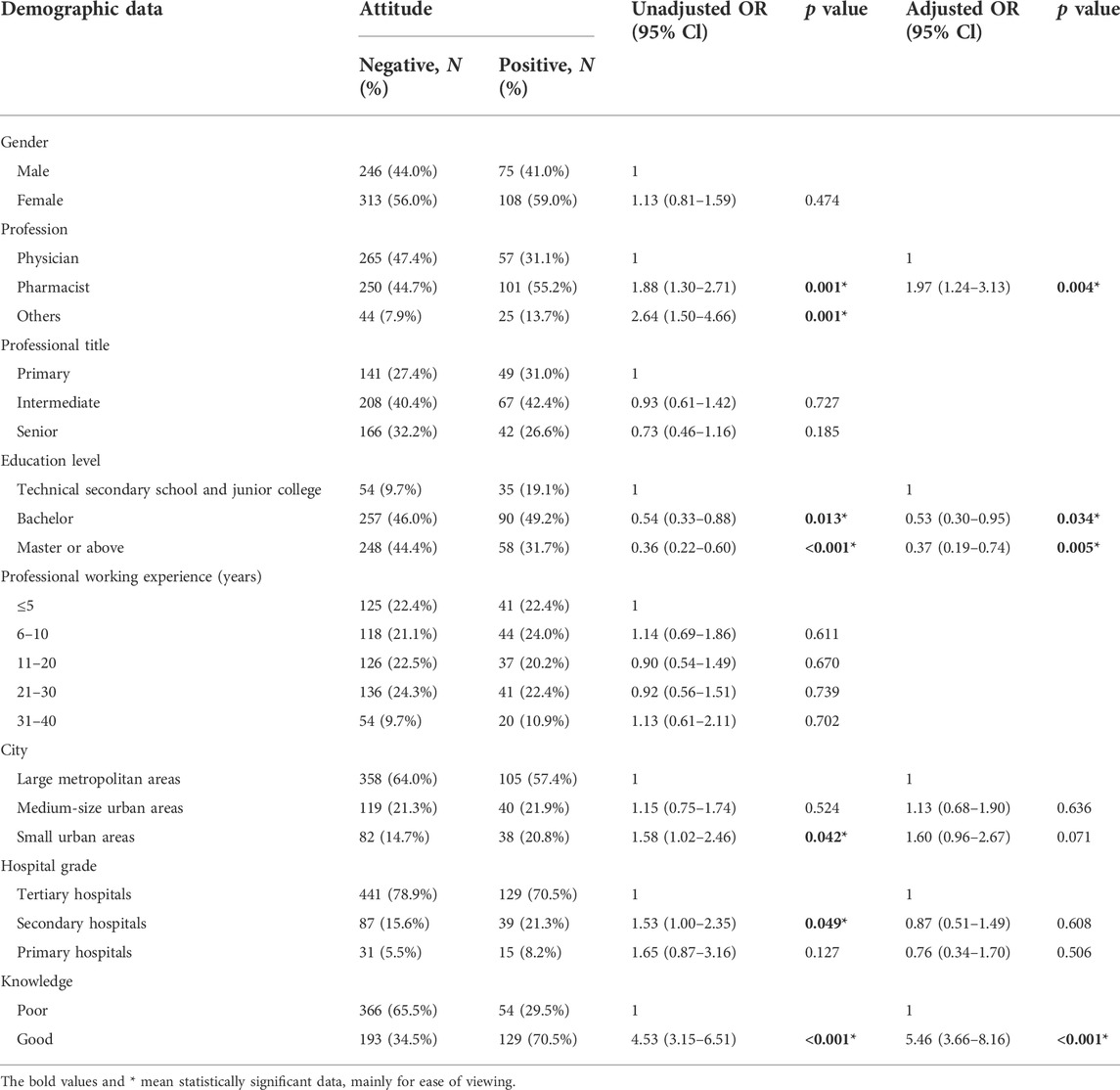
TABLE 3. Univariable and multivariable analysis of associated factors of HCPs’ attitude towards NCDP policy.
In the univariate analysis, HCPs who are pharmacists, have 6–20 professional working years, work in medium-size urban areas, own good knowledge and attitude tend to hold better practice level (Table 4). After adjustment of covariates, the multivariate model revealed that pharmacists (OR = 2.34, 95% CI: 1.46–3.73) HCPs who have 11–20 professional working experience (OR = 2.37, 95% CI: 1.39–4.06), work in medium-sized urban areas (OR = 2.16, 95% CI: 1.34–3.49), or have good knowledge (OR = 2.98, 95% CI: 2.02–4.39) were more likely to have good practice. Besides, good practice was associated with several attitudes, such as agreement that there is no efficacy difference between original branded drugs and domestically generic drugs that have passed consistency evaluation (OR = 2.08, 95% CI: 1.21–3.56), NCDP policy can effectively reduce the healthcare burden of chronically ill patients (OR = 2.20, 95% CI: 1.19–4.06) and can effectively reduce the patient-doctor disputes (OR = 1.72, 95% CI: 1.16–2.57).
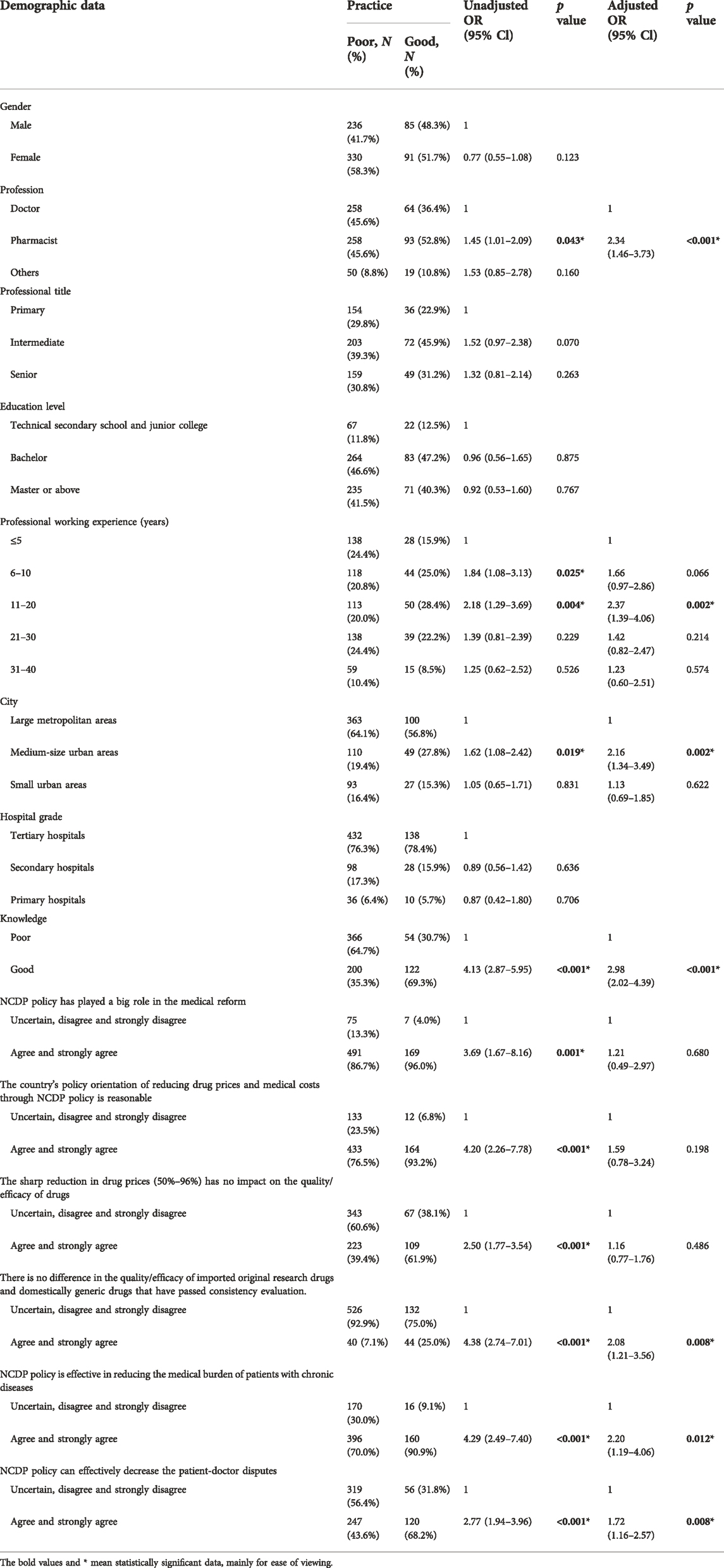
TABLE 4. Univariable and multivariable analysis of associated factors of HCPs’ practice towards NCDP policy.
The first three issues on NCDP policy, according to HCPs, were patients’ doubts about the efficacy of centralized procurement medicines (74.1%), the limited prescribing options of physicians (47.0%), and the unsatisfactory treatment outcomes of the selected drugs (40.6%) (Supplementary Table S4). Another 136 HCPs provided other suggestions, as seen in Table 5.
As the main body of the execution of NCDP policy, behaviors of HCPs are the key to put the policy into effect in medical institutions. It has been reported the increase in knowledge level will change the attitudes, and in turn change the related practices, showing the consistency of KAP (Kasemy et al., 2020; Iradukunda et al., 2021; Rahmah et al., 2021). Thus, this cross-sectional study aims to evaluate KAP level and associated factors of HCPs towards NCDP policy, which is critical to reflect the implementation of the policy in medical institutions and identify potential resistance.
In this study, it showed that 37.5% of HCPs are unclear with NCDP policy, and 52.1% have little knowledge about generic drug consistency evaluation, which is in line with the previous report about poor knowledge of health practitioners in Palestine on generic medicine products (Shraim et al., 2017). Pharmacists have a better understanding about NCDP policy compared to doctors, probably because of pharmacists’ greater exposure and sensitivity to the policy related to drugs. HCPs with senior professional title and longer working years had greater knowledge, which can be explained by their easier access to relevant knowledge and richer work experience.
The positive attitude of HCPs is considered as an impetus to carrying NCDP policy out. This study showed that due to the huge price cut for centralized procurement drugs, most HCPs agree NCDP policy is important, reasonable and useful to release the burden on patients; nearly half believe the policy can ease the doctor-patient relationship. For instance, the monthly cost of entecavir (a drug for treating chronic hepatitis B) for patients was as high as 400–500 yuan before NCDP policy; at present, the cost has been reduced to 49.8 yuan per month (Zhan et al., 2021). The reduction of medication cost also improved patient adherence (Dietze et al., 2020). However, it is worth noting that 88.7% of HCPs do not know or consider there exists big differences in the therapeutic effect between generic and branded drugs. This is consistent with the surveys that quite a few HCPs expressed negative perceptions about the efficacy of generic drugs (Shrank et al., 2011). Since the current consistency evaluation of generic drugs only involves the pharmaceutical characteristics and bioequivalence (Hansen et al., 2017; Chazin et al., 2019), such attitude might be related to lack of high-quality clinical evidence of generic drugs about their comparative safety and effectiveness relative to their brand-name counterparts (Choudhry et al., 2016; Zeng and Song, 2019), especially in the real world. Consequently, 55.2% of HCPs do not know or disagree that the quality of centralized-purchased medicines, mostly generic drugs, will not be affected by price cuts, indicating that they were concerned about the quality and efficacy of centralized-purchased drugs, which may become a major barrier to advancing NCDP policy (Kesselheim et al., 2016; Tang et al., 2019). Pharmacists have more positive attitudes, which can be ascribed to their better knowledge, which is in accordance with the results of other similar researches (AlRasheed et al., 2021a). Notably, HCPs with higher education background have poorer attitudes towards the policy, which may because they are more aware that the curative effect of generic drugs not only depends on consistency evaluation, especially for narrow therapeutic index drugs (Qu et al., 2021), but also should be supported by strong clinical comprehensive evaluation.
Our findings indicate that practice levels among HCPs towards NCDP policy are unsatisfactory. Only a small number of HCPs often or always interpreted the policy for patients, possibly due to their short of understanding and confidence in the quality and efficacy of generic drugs (Dunne et al., 2014). However, when patients refused to use centralized-purchased drugs, the majority of HCPs encouraged patients to accept it, which shows the cooperation of HCPs with the policy. State Council issued official requirements that hospitals should make procurement plans based on no less than 80% of the actual use of the same generic drugs in the previous year, which also put pressure on HCPs to encourage the use of centralized-purchased drugs (China State Council, 2015; Wang et al., 2021). However, quite a few HCPs do not consider centralized-purchased drugs when choosing medicines for themselves and their families, which further suggests their limited confidence in the efficacy of centralized-purchased drugs (Gao et al., 2021). Besides, there is a significant association between HCPs’ characteristics and practice levels. Pharmacists still showed better practice, supported by their better knowledge and attitude towards the policy, which is in keeping with the study that cognition and attitudes affect the behaviors and coping strategies (Zhang et al., 2020). The difference of knowledge level has great significance on HCPs’ practice level, which indicates education intervention is one strategy for better practice (Gidey et al., 2020; Hu et al., 2022). Certain attitudes of HCPs also have a great impact on behavior: HCPs who agree that NCDP policy can reduce the burden of chronic disease patients and alleviate the contradiction between doctors and patients have higher degree of satisfaction to the policy and are more inclined to have better practice. More importantly, HCPs who believe differences in the quality between centralized-purchased medicines and brand-name medicines are more likely to have poorer practices, which shows the necessity of providing clinical evidences for the treatment outcome of the centralized-purchased drugs and thereby enhancing the endogenous motivation of HCPs to use the drugs.
Our study is helpful for understanding the KAP of HCPs, exploring the possible barriers towards NCDP policy and improving the policy. First, we found that most HCPs showed relatively poor KAP towards NCDP policy, though it has been implemented for around 2 years. Second, our research showed that the key factor lowering KAP is HCPs’ suspicion on the quality of centralized-purchased drugs. Thus, high-quality research is required to provide enough clinical evidence for the effectiveness and safety of generic medicines, which is critical to enhance HCP’s understanding and confidence for using centralized-purchased medicines. Third, these findings have important implications for completion of procurement volume in the medical institutions as well as the function of NCDP policy in reducing drug prices and increasing drug accessibility. Besides, in the context of the continuous rise in global drug expenditure, it can also provide experience and reference for other countries or regions that need to control drug costs.
Our study has several strengths. First, we collected adequate participant samples with different working status from 30 healthcare institutions. Second, the timing of our investigation is reasonable, when NCDP policy has been implemented for around 2 years, and KAP of HCPs towards it can be fully exposed. Third, to our knowledge, this is the first survey that investigated KAP level of HCPs towards NCDP policy in China.
Our study also has some limitations. First, this study only involves HCPs from tertiary and secondary hospitals in large and medium cities in Central China, therefore the data could not be generalizable to primary hospitals or hospitals in rural areas. Second, the participants mainly include pharmacists and physicians, which could not fully show all types of HCPs’ KAP levels towards the policy.
Only a small percentage of HCPs had good knowledge, attitudes and practice towards NCDP policy. Overall, pharmacists showed better understanding, attitudes and practice than physicians. Good practice was associated with the positive attitudes towards the efficacy of centralized-purchased medicines and impacts of NCDP policy. High-quality clinical evidences on the therapeutic effects and safety of the centralized-purchased drugs is needed.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethical approval for this study was obtained from Ethical Committee of Zhongnan Hospital of Wuhan University. Before starting the survey, each participant was asked to review the aim of the survey and fill out an electronic informed consent. Participants could withdraw from the survey at any time.
HC had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: HC and AH. Acquisition, analysis, or interpretation of data: HC, AH, XW, and YT. Drafting of the manuscript: AH and HC. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: AH, HC, and YT. Administrative, technical, or material support: LL. Supervision: HC.
We greatly appreciate all healthcare professionals in Hubei Province participated in our study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.996824/full#supplementary-material
AlRasheed, M. M., AlShahrani, A. H., AlMuhaini, S. A., AlKofide, H. A., Alhawassi, T. M., Aldemerdash, A., et al. (2021a). Knowledge, attitude, and practice towards COVID-19 among pharmacists: A cross-sectional study. Risk Manag. Healthc. Policy 14, 3079–3090. doi:10.2147/rmhp.s317779
AlRasheed, M. M., AlShahrani, A. H., AlMuhaini, S. A., AlKofide, H. A., Alhawassi, T. M., Aldemerdash, A., et al. (2021b). Knowledge, attitude, and practice towards COVID-19 among pharmacists: A cross-sectional study. Risk Manag. Healthc. Policy 14, 3079–3090. doi:10.2147/rmhp.s317779
Chazin, H. D., Peters, J. R., Catterson, D. M., Osterhout, J. L., Forsyth, L. M., Lee, J. E., et al. (2019). Drug information association pharmacovigilance and risk management strategies 2017: Overview of the generic drug program and surveillance. Ther. Innov. Regul. Sci. 53 (2), 249–253. doi:10.1177/2168479018774557
Chen, L., Yang, Y., Luo, M., Hu, B., Yin, S., and Mao, Z. (2020). The impacts of national centralized drug procurement policy on drug utilization and drug expenditures: The case of shenzhen, China. Int. J. Environ. Res. Public Health 17 (24), E9415. doi:10.3390/ijerph17249415
China Government Statistics Information Center (2021). 2020 China health statistics yearbook. RetrievedAvailable at: http://www.nhc.gov.cn/mohwsbwstjxxzx/tjtjnj/202112/dcd39654d66c4e6abf4d7b1389becd01.shtml December 10, 2021).
China State Council, (2015). Guidance for improving the centralized procurement of drugs in public hospitals. RetrievedAvailable at: http://www.gov.cn/zhengce/content/2015-02/28/content_9502.htm October 10, 2019).
Choudhry, N. K., Denberg, T. D., and Qaseem, A. (2016). Clinical guidelines committee of American College of, PImproving adherence to therapy and clinical outcomes while containing costs: Opportunities from the greater use of generic medications: Best practice advice from the clinical guidelines committee of the American College of physicians. Ann. Intern. Med. 164 (1), 41–49. doi:10.7326/M14-2427
Dietze, J., Priluck, A., High, R., and Havens, S. (2020). Reasons for the underutilization of generic drugs by us ophthalmologists: A survey. Ophthalmol. Ther. 9 (4), 955–970. doi:10.1007/s40123-020-00292-4
Dubois, P., Lefouili, Y., and Straub, S. (2021). Pooled procurement of drugs in low and middle income countries. Eur. Econ. Rev. 132, 103655. doi:10.1016/j.euroecorev.2021.103655
Dunne, S., Shannon, B., Hannigan, A., Dunne, C., and Cullen, W. (2014). Physician and pharmacist perceptions of generic medicines: What they think and how they differ. Health Policy 116 (2-3), 214–223. doi:10.1016/j.healthpol.2014.03.007
Gao, J. J., Wu, X. F., Xu, Y., Y, L., Zeng, M. G., and Kang, Q. F. (2021). Practice and suggestion of "4+7" volume-based drug procurement policy based on medical personnel questionnaire in fujian province. Strait Pharm. J. 33 (2). doi:10.3969/j.issn.1006-3765.2021.02.095
Gidey, K., Seifu, M., Hailu, B. Y., Asgedom, S. W., and Niriayo, Y. L. (2020). Healthcare professionals knowledge, attitude and practice of adverse drug reactions reporting in Ethiopia: A cross-sectional study. BMJ Open 10, e034553. doi:10.1136/bmjopen-2019-034553
Hansen, R. A., Qian, J., Berg, R., Linneman, J., Seoane-Vazquez, E., Dutcher, S. K., et al. (2017). Comparison of generic-to-brand switchback rates between generic and authorized generic drugs. Pharmacotherapy 37 (4), 429–437. doi:10.1002/phar.1908
He, Y., Dou, G., Huang, Q., Zhang, X., Ye, Y., Qian, M., et al. (2018). Does the leading pharmaceutical reform in China really solve the issue of overly expensive healthcare services? Evidence from an empirical study. PLoS One 13 (1), e0190320. doi:10.1371/journal.pone.0190320
Hu, W., Tao, Y., Lu, Y., Gao, S., Wang, X., Li, W., et al. (2022). Knowledge, attitude and practice of hospital pharmacists in central China towards adverse drug reaction reporting: A multicenter cross-sectional study. Front. Pharmacol. 13, 823944. doi:10.3389/fphar.2022.823944
Hua, Y. F., Lu, J., Bai, B., and Zhao, H. Q. (2021). Can the profitability of medical enterprises Be improved after joining China's centralized drug procurement? A difference-in-difference design. Front. Public Health 9, 809453. doi:10.3389/fpubh.2021.809453
Iradukunda, P. G., Pierre, G., Muhozi, V., Denhere, K., and Dzinamarira, T. (2021). Knowledge, attitude, and practice towards COVID-19 among people living with HIV/AIDS in kigali, Rwanda. J. Community Health 46 (2), 245–250. doi:10.1007/s10900-020-00938-1
Kasemy, Z. A., Bahbah, W. A., Zewain, S. K., Haggag, M. G., Alkalash, S. H., Zahran, E., et al. (2020). Knowledge, attitude and practice toward COVID-19 among Egyptians. J. Epidemiol. Glob. Health 10 (4), 378–385. doi:10.2991/jegh.k.200909.001
Kesselheim, A. S., Gagne, J. J., Eddings, W., Franklin, J. M., Ross, K. M., Fulchino, L. A., et al. (2016). Prevalence and predictors of generic drug skepticism among physicians: Results of a national survey. JAMA Intern. Med. 176 (6), 845–847. doi:10.1001/jamainternmed.2016.1688
Lan, T., Guan, L., Pang, X., Li, X., and Yu, Q. (2022). Impact of the national centralized drug procurement policy (4 + 7 policy) on the drug expenditures of patients treated in outpatient and emergency departments in a large tertiary level-A hospital in China: A single centre, interrupted time series. J. Clin. Pharm. Ther. 47 (1), 104–111. doi:10.1111/jcpt.13551
Leighton, K., Kardong-Edgren, S., Schneidereith, T., and Foisy-Doll, C. (2021). Using social media and snowball sampling as an alternative recruitment strategy for research. Clin. Simul. Nurs. 55, 37–42. doi:10.1016/j.ecns.2021.03.006
Liang, G., Zhao, F., Zhou, Y., Lai, H., and Yuan, Y. (2022). Problems and suggestions on the implementation of drug centralized quantity purchase policy. China J. Pharm. Econ. 17 (3). doi:10.12010/j.issn.1673-5846.2022.03.001
Long, H., Yang, Y., Geng, X., Mao, Z., and Mao, Z. (2022). Changing characteristics of pharmaceutical prices in China under centralized procurement policy: A multi-intervention interrupted time series. Front. Pharmacol. 13, 944540. doi:10.3389/fphar.2022.944540
Ma, Z., Zhao, Z., Sun, S., Li, Y., An, Z., Yan, Y., et al. (2020). Impact of 'chief-pharmacist system' on drug expenditures and rational drug use. Int. J. Clin. Pharm. 42 (1), 167–173. doi:10.1007/s11096-019-00954-9
Marzan, M., Islam, D. Z., Lugova, H., Krishnapillai, A., Haque, M., and Islam, S. (2021). Knowledge, attitudes, and practices of antimicrobial uses and resistance among public university students in Bangladesh. Infect. Drug Resist. 14, 519–533. doi:10.2147/IDR.S289964
Memon, M. A., Ting, H., Cheah, J.-H., Thurasamy, R., Chuah, F., and Cham, T. H. (2020). Sample size for survey research: Review and recommendations. J. Appl. Struct. Equ. Model. 4 (2), i–xx. doi:10.47263/jasem.4(2)01
Parmaksiz, K., Pisani, E., Bal, R., and Kok, M. O. (2022). A systematic review of pooled procurement of medicines and vaccines: Identifying elements of success. Glob. Health 18 (1), 59. doi:10.1186/s12992-022-00847-z
Qu, J., Zuo, W., Wang, S., Du, L., Liu, X., Gao, Y., et al. (2021). Knowledge, perceptions and practices of pharmacists regarding generic substitution in China: A cross-sectional study. BMJ Open 11 (10), e051277. doi:10.1136/bmjopen-2021-051277
Rahmah, P. A., Khairani, A. F., Atik, N., Arisanti, N., and Fatimah, S. N. (2021). Correlation of knowledge, attitude, and practice toward probiotics for the digestive system Among health science students. J. Multidiscip. Healthc. 14, 1135–1144. doi:10.2147/JMDH.S305670
Raosoft, I. (2019). Raosoft sample size calculator. Retrieved 10 September, 2019Available at: http://www.raosoft.com/samplesize.html.
Sadowska, A., Nowak, M., and Czarkowska-Paczek, B. (2021). Assessment of the reliability of the polish language version of the FATCOD-B scale among nursing students. J. Cancer Educ. 36 (3), 561–566. doi:10.1007/s13187-019-01665-5
Seidman, G., and Atun, R. (2017). Do changes to supply chains and procurement processes yield cost savings and improve availability of pharmaceuticals, vaccines or health products? A systematic review of evidence from low-income and middle-income countries. BMJ Glob. Health 2 (2), e000243. doi:10.1136/bmjgh-2016-000243
Shraim, N. Y., Al Taha, T. A., Qawasmeh, R. F., Jarrar, H. N., Shtaya, M. A. N., Shayeb, L. A., et al. (2017). Knowledge, attitudes and practices of community pharmacists on generic medicines in Palestine: A cross-sectional study. BMC Health Serv. Res. 17 (1), 847. doi:10.1186/s12913-017-2813-z
Shrank, W. H., Liberman, J. N., Fischer, M. A., Girdish, C., Brennan, T. A., and Choudhry, N. K. (2011). Physician perceptions about generic drugs. Ann. Pharmacother. 45 (1), 31–38. doi:10.1345/aph.1P389
Taber, K. S. (2017). The use of Cronbach’s alpha when developing and reporting research instruments in science education. Res. Sci. Educ. 48 (6), 1273–1296. doi:10.1007/s11165-016-9602-2
Tang, Y. Y., Chen, J., and Li, X. (2019). Utilization analysis of the original and generic drugs for hypertension and diabetes in a tertiary public hospital in jiangsu province. China Pharm. 30 (21), 2890–2893. doi:10.6039/j.issn.1001-0408.2019.21.03
Vogler, S., Habimana, K., and Haasis, M. A. (2022). Purchasing medicines for the public sector: Evaluation of the performance of centralised procurement in Portugal. Int. J. Health Plann. Manage. 37 (4), 2007–2031. doi:10.1002/hpm.3444
Wang, N., Yang, Y., Xu, L., Mao, Z., and Cui, D. (2021). Influence of Chinese national centralized drug procurement on the price of policy-related drugs: An interrupted time series analysis. BMC Public Health 21 (1), 1883. doi:10.1186/s12889-021-11882-7
Wen, X., Wang, Z., Xu, L., Luo, J., Geng, X., Chen, X., et al. (2022). The impacts of the “4+7” pilot policy on the volume, expenditures, and daily cost of serotonin-specific reuptake inhibitors (SSRIs) antidepressants: A quasi-experimental study. Front. Pharmacol. 13, 829660. doi:10.3389/fphar.2022.829660
Wu, H., Li, X., Chen, J., Su, Y., and Pang, J. (2021). Impact of volume-based procurement on the utilization of original and generic drugs in northern jiangsu. Pharm. Clin. Res. 29 (3), 237–240. doi:10.13664/j.cnki.pcr.2021.03.020
Yang, Y., Chen, L., Ke, X., Mao, Z., and Zheng, B. (2021). The impacts of Chinese drug volume-based procurement policy on the use of policy-related antibiotic drugs in shenzhen, 2018-2019: An interrupted time-series analysis. BMC Health Serv. Res. 21 (1), 668. doi:10.1186/s12913-021-06698-5
Zeng, J. F., and Song, J. C. (2019). Revisted on quality and efficacy consistency evaluation of generic drugs. Her. Med. 38 (7), 884–887. doi:10.3870/j.issn.1004-0781.2019.07.011
Zhan, Q., Ge, J., and Huang, Y. (2021). Investigation on the application of entecavir under "4+7" volume-based procurement policy. Pharm. Care Res. 21 (2), 152–155. doi:10.5428/pcar20210216
Zhang, M., Zhou, M., Tang, F., Wang, Y., Nie, H., Zhang, L., et al. (2020). Knowledge, attitude, and practice regarding COVID-19 among healthcare workers in henan, China. J. Hosp. Infect. 105 (2), 183–187. doi:10.1016/j.jhin.2020.04.012
Zhang, Y., Ren, Y., Zheng, Q., Tan, J., Yao, M., Huang, Y., et al. (2022). The impact of national centralized drug procurement on health expenditures for lung cancer inpatients: A difference-in-differences analysis in a large tertiary hospital in China. Front. Public Health 10, 956823. doi:10.3389/fpubh.2022.956823
Keywords: knowledge, attitude, practice, National Centralized Drug Procurement policy, healthcare professionals
Citation: Huang A, Wang X, Tao Y, Lin L and Cheng H (2022) Healthcare professionals’ knowledge, attitude and practice towards National Centralized Drug Procurement policy in central China: A cross-sectional study. Front. Pharmacol. 13:996824. doi: 10.3389/fphar.2022.996824
Received: 18 July 2022; Accepted: 15 September 2022;
Published: 07 October 2022.
Edited by:
Joseph O. Fadare, Ekiti State University, NigeriaReviewed by:
Anlu Zhang, Huazhong Agricultural University, ChinaCopyright © 2022 Huang, Wang, Tao, Lin and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Cheng, Y2hlbmdob25nQHpuaG9zcGl0YWwuY24=; Likai Lin, bGlubGlrYWlfMTk2M0AxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.