- 1Department of Anesthesiology, The First Affiliated Hospital of Guangxi Medical University, Nanning, China
- 2Department of Anesthesiology, Hunan Provincial People’s Hospital, The First Affiliated Hospital of Hunan Normal University, Changsha, China
Background: Dexmedetomidine and remifentanil are well known to suppress airway reflex during emergence from anesthesia, but which one is more effective is unclear. We conducted a meta-analysis to compare the effect of dexmedetomidine and remifentanil on reducing the occurrence of coughing.
Methods: We systematically searched PubMed, Embase, and Cochrane Library for randomized controlled trials (published between 1 January 1950, and 30 December 2021; no language restrictions) comparing dexmedetomidine infusion with remifentanil infusion. The primary endpoint was the incidence of moderate to severe coughing during the recovery period. The secondary endpoints were the time of recovery and extubation, and residual sedation. We assessed pooled data by using a random-effects model.
Results: Eight studies with 502 participants were included. The meta-analysis showed no statistically difference between dexmedetomidine and remifentanil in the occurrence of moderate to severe coughing during emergence from anesthesia (OR 1.45,95%CI 0.62–3.38), the extubation time (MD 0.93 min, 95%CI -0.28–2.14), and the residual sedation (OR 2.52, 95%CI 0.92–6.91). Compared with dexmedetomidine, the average recovery time of remifentanil was shorter (MD 3.88 min, 95%CI 1.01–6.75).
Conclusion: Dexmedetomidine and remifentanil infusion had no difference in the occurrence of moderate to severe coughing during emergence from anesthesia.
Clinical Trial Registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42021239710
Introduction
The incidence of cough is 38–74% in patients with tracheal intubation during emergence from general anesthesia. (Estebe et al., 2005; Fagan et al., 2000; Jun et al., 2010) Severe cough in emergence of anesthesia significantly increases intracranial and intraabdominal pressure, which may result in disastrous consequences for patients during postoperative, (Jun et al., 2010) such as intracerebral hemorrhage after craniotomy, (Irwin, 2006) neck hematoma after thyroidectomy, (Harding et al., 2006) and wound dehiscence after abdominal surgery. Emergence cough can further aggravate the airway reflex due to repeated stimulation of the airway by the endotracheal tube, leading to laryngospasm, bronchospasm, pulmonary edema, hypertension, and tachycardia. (Tanoubi et al., 2015) During anesthesia emergence, taking effective measures to suppress peri-extubation cough is a major concern for the anesthesiologist.
Several medications (e.g., lidocaine, dexmedetomidine, opioid agents) have been studied to restrain cough during emergence from general anesthesia. (Choi et al., 2018; Clivio et al., 2019; Liu et al., 2021) However, the use of these medications is limited in clinical application because of related side effects, such as local anesthetic toxicity, delayed recovery time and extubation time, and residual sedation. Remifentanil allows for a faster emergence than other opioid agents due to its short context-sensitive halftime. In comparison with remifentanil, dexmedetomidine has its own advantages to attenuate peri-extubation cough for its respiratory preservation effect (Hsu et al., 2004). Previous studies separately investigated the efficacy of dexmedetomidine and remifentanil for prevention of peri-extubation cough. (Kim et al., 2015; Lee et al., 2009) To the best of our knowledge, relevant studies are limited by single center, small sample sizes and different definitions of coughing incidence. Therefore, we performed a meta-analysis of randomized controlled trials to compare the efficacy and side effects of dexmedetomidine and remifentanil on reducing coughing during emergence from anesthesia.
Materials and methods
Search strategy and selection criteria
This meta-analysis was registered at International Prospective Register of Systematic Reviews (number CRD 42021239710).
We searched related studies published between 1 January 1950, and 30 December 2021, by searching PubMed, Embase, and Cochrane Library. Keywords were related to dexmedetomidine, remifentanil, and randomized controlled trial. The complete search used for PubMed was: ((“Remifentanil" [Mesh]) OR (Ultiva OR “GI 87084B” OR “GI87084B” OR “GI-87084B” OR Remifentanil)) AND ((“Dexmedetomidine" [Mesh]) OR (“MPV-1440″ OR “MPV 1440″ OR “MPV1440” OR Precedex OR “Dexmedetomidine Hydrochloride” OR “Hydrochloride, Dexmedetomidine” OR Dexmedetomidine)) AND (“Randomized controlled trial" [pt] OR “controlled clinical trial" [pt] OR randomized [tiab] OR placebo [tiab] OR “drug therapy" [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]). No search filters were applied. We considered all potentially eligible studies for this review, irrespective of language or the primary outcome.
Study selection and data extraction
We included the studies if they were randomized clinical trials in adults underwent elective surgery under general anesthesia with tracheal intubation. The studies compared dexmedetomidine with remifentanil infusion during emergence from anesthesia to prevent airway response and decrease peri-extubation coughing.
Studies were included if they contained data on the grade or the incidence of cough, or both during emergence from anesthesia. Emergence from anesthesia was defined as from the time of awareness to 5 min after extubation. Coughing severity was classified using the three-point scale described by Minogue et al.: 1 = mild (single) cough, 2 = moderate (≤5 s) cough, and 3 = severe (>5 s) cough. (Minogue et al., 2004)
The exclusion criteria were as follows: dexmedetomidine was not compared with remifentanil; dexmedetomidine and remifentanil were only administered at the beginning of surgery.
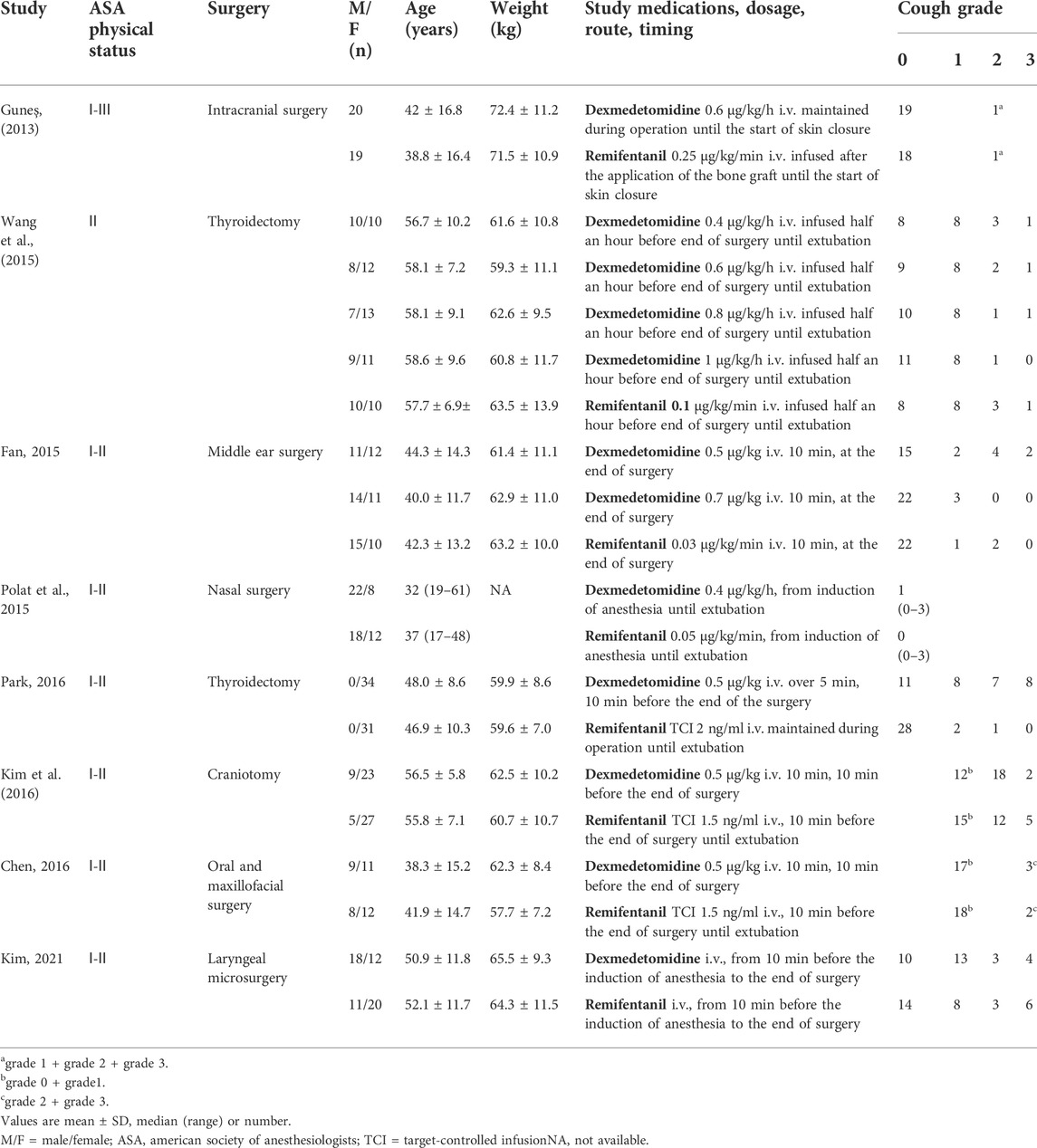
Two investigators, working independently, reviewed the titles and abstracts for potential eligible studies and then retrieved for full text of the studies that met the inclusion criteria. The following data were extracted: authors, study design, randomization, blinding status, total number of participants, age, sex, weight, types of surgery, ASA physical status classification, dose of dexmedetomidine and remifentanil, timing of administration, incidence of cough, recovery time, extubation time, and incidence of residual sedation. Recovery time is from general anesthetics off to recovery. Extubation time is from general anesthetics off to extubation. The residual sedation was defined as no response to verbal commands. We calculated the combined mean ± SD for studies having different dosage groups.
Primary endpoint
The primary endpoint of this meta-analysis was the incidence of moderate to severe coughing during the recovery period from the time of awareness to 5 min after extubation. We analyzed the incidence of moderate to severe coughing as a dichotomous variable and calculated the odds ratio.
Secondary endpoints
We assessed the adverse effects of dexmedetomidine and remifentanil on the following outcomes: recovery time, extubation time, and residual sedation. We analyzed the recovery and extubation time as continuous variables and reported the mean differences. We reported the incidence of residual sedation as a dichotomous variable and calculated the odds ratio. Two independent reviewers assessed the risk for bias using the Cochrane Risk of Bias Tool for Randomized Controlled Trials. (Higgins et al., 2011)
Statistical analysis
We used Review Manager 5.2 for the meta-analysis. For continuous variables, we calculated pooled estimates of the mean differences and 95% confidence interval (CI) by using a random-effects model. For categorical outcomes, we calculated pooled estimates of the odds ratio and 95% CI by using a random-effects model. Because of the limited number (<10) of included studies, we did not evaluate the publication bias. We used Cochran’s Q test and I2 statistics to assess statistical heterogeneity. p > 0.1 and I2 < 50% were indicative of low heterogeneity. For sensitivity analysis, we excluded each study one-by-one from the pooled results to find the source of heterogeneity (Sun et al., 2017) and evaluated the robustness of the outcomes. (Hu et al., 2016)
Results
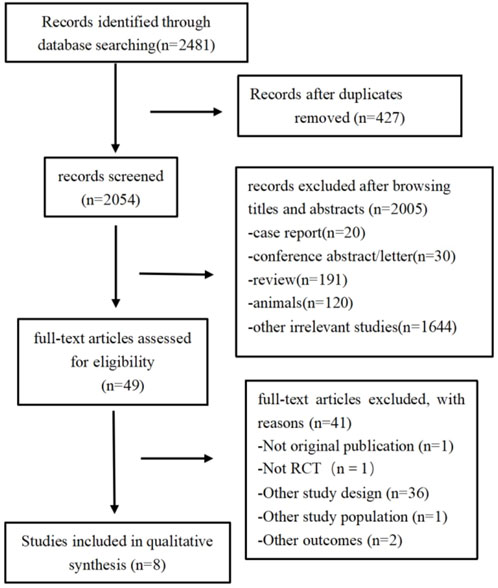
A total of 2,481 citations were retrieved according to the search strategy (PubMed = 277, Embase = 1716, and Cochrane Library = 488). After removing duplicate and ineligible studies, we finally included eight studies (Chen et al., 2016; Güneş et al., 2013; Kim et al., 2021; Kim et al., 2016; Park et al., 2016; Polat et al., 2015; Qing et al., 2015; Wang et al., 2015) (502 participants) for this meta-analysis (Figure 1; Table 1).
Risk of bias assessment
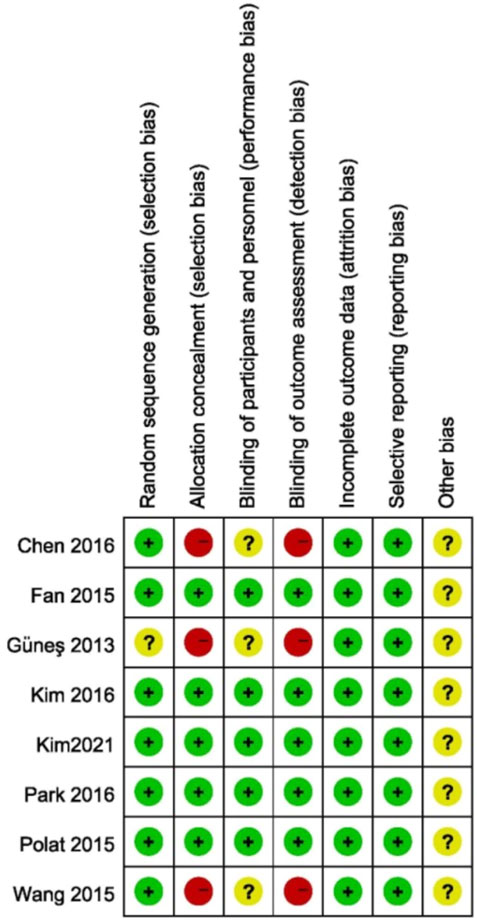
The assessment of risk of bias in the studies is shown in Figure 2. Seven studies described adequate randomization and one study did not describe how to generate random sequences. Three studies did not specify whether the participants and outcome assessors were blinded to the patient’s treatment group.
Incidence of moderate to severe coughing
Six studies (Chen et al., 2016; Kim et al., 2016; Kim et al., 2021; Park et al., 2016; Qing et al., 2015; Wang et al., 2015) comparing dexmedetomidine with remifentanil were included in the pooled analysis to assess the incidence of moderate to severe coughing; one study (Polat et al., 2015) reported grade of coughing using median (range) and one study (Güneş et al., 2013) only reported the incidence of coughing. There was no difference in the occurrence of moderate to severe coughing between two drugs during emergence from anesthesia (p = 0.39), with moderate heterogeneity (I2 = 52%; Figure 3). In the sensitivity analysis, the change of the effects was not significant by excluding each study successively from the analysis.
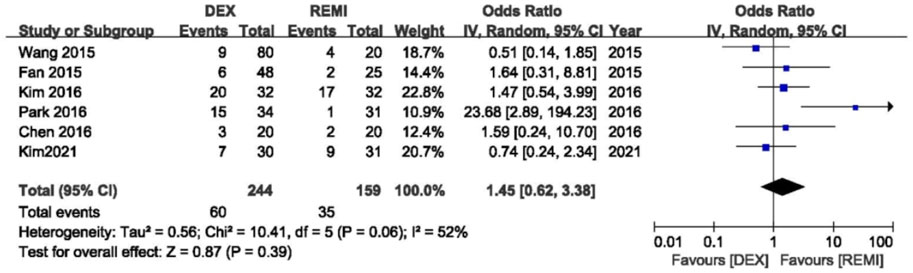
FIGURE 3. Meta-analyses of dexmedetomidine versus remifentanil, comparing incidence of moderate to serve cough.
Adverse events
Six studies (Güneş et al., 2013; Fan et al., 2015; Polat et al., 2015; Wang et al., 2015; Kim et al., 2016; 2021) comparing dexmedetomidine with remifentanil reported recovery time, and the pooled analysis showed that the average recovery time of remifentanil was shorter than dexmedetomidine (p = 0.008), with high heterogeneity (I2 = 95%; Figure 4). In the sensitivity analysis, the advantage of remifentanil still existed even after removing each study from the analysis. When excluding the study of Wang et al., (Wang et al., 2015) the statistical heterogeneity changed from high to moderate (I2 = 35%).
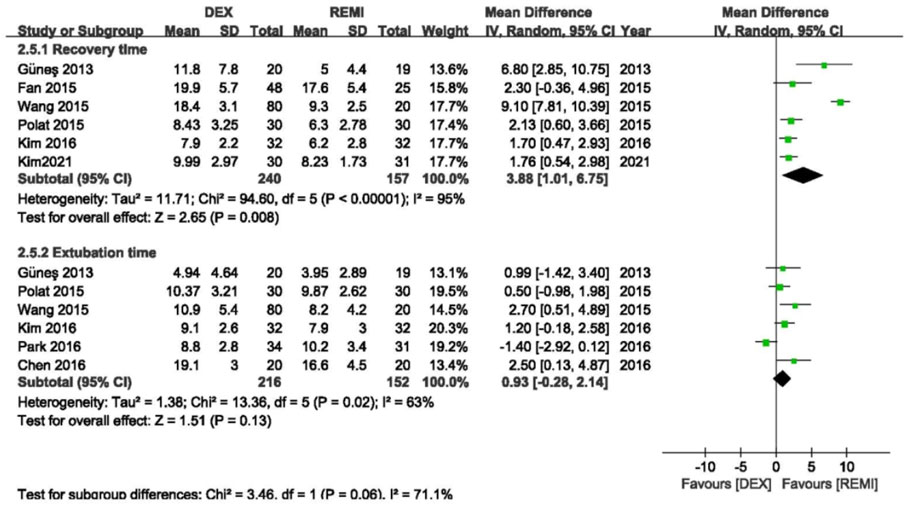
FIGURE 4. Meta-analyses of dexmedetomidine versus remifentanil, comparing the awareness and extubation time.
Six studies (Chen et al., 2016; Güneş et al., 2013; Kim et al., 2016; Park et al., 2016; Polat et al., 2015; Wang et al., 2015) comparing dexmedetomidine with remifentanil reported the extubation time, and the pooled analysis showed that remifentanil did not shorten the extubation time compared with dexmedetomidine (p = 0.13), with high heterogeneity (I2 = 63%; Figure 2). However, the advantage of remifentanil still existed after removing the study of Park et al. (Park et al., 2016) (p = 0.001) and the statistical heterogeneity became insignificant (I2 = 0).
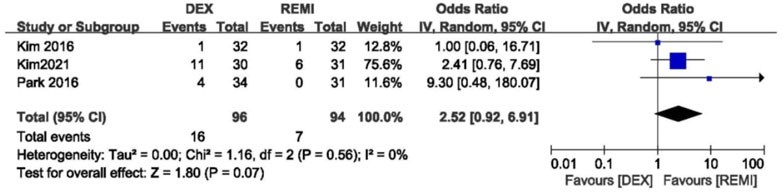
Three studies comparing dexmedetomidine with remifentanil reported the incidence of residual sedation. No difference was observed in the incidence of residual sedation and statistical heterogeneity (p = 0.07; I2 = 0; Figure 5). (Kim et al., 2016; Kim et al., 2021; Park et al., 2016)
Discussion
This meta-analysis not only compared the efficacy of remifentanil and dexmedetomidine in inhibiting emergence cough, but also compared the side effects, which was helpful for doctors to make better clinical decisions. The meta-analysis demonstrated that dexmedetomidine and remifentanil infusion had no difference in the occurrence of moderate to severe coughing during emergence from anesthesia. When remifentanil and dexmedetomidine achieved equal therapeutic effects, there were no differences in extubation time and residual sedation. In addition, the average recovery time of remifentanil was shorter than dexmedetomidine.
Despite there are available studies comparing dexmedetomidine and remifentanil on reducing the occurrence of coughing during emergence from anesthesia, the outcomes are controversial. Part of the reason is that different studies choose different grades of coughing as positive events. Moderate to severe coughing are considered clinically harmful, so the incidence of moderate to severe coughing is the primary endpoint of the present meta-analysis.
The administration of dexmedetomidine and remifentanil may lead to prolong recovery time and extubation time. Remifentanil is an ultra-short-acting opioid and does not rely on the liver for metabolism. The context-sensitive half-time of remifentanil is about 3 min (Battershill and Keating, 2006) A study conducted by Nho et al. reveals that remifentanil does not prolong eye opening time and extubation time compared to placebo. (Nho et al., 2009) Dexmedetomidine is a highly selective α-2 adrenergic receptor agonist that has sympatholytic, sedative, analgesic without respiratory depression. Previous studies have shown that the administration of dexmedetomidine at the end of surgery attenuate emergence cough with a variable impact on recovery time and extubation time. (Aouad et al., 2019; Hu et al., 2019; Kim et al., 2013a; Kim et al., 2013b; Kim et al., 2015) This meta-analysis demonstrated that there was no difference between remifentanil and dexmedetomidine in extubation time, while the average recovery time of remifentanil was shorter than dexmedetomidine. Residual sedation is also one of the adverse effects of remifentanil and dexmedetomidine. Sedation of dexmedetomidine lasts longer than remifentanil. A study conducted by Kim et al. indicates that Modified Observer’s Assessment of Alertness/Sedation is lower in all dexmedetomidine groups than in the control group. (Kim et al., 2013a) Conversely, another study conducted by Aouad et al. indicates that sedation scores are comparable between dexmedetomidine groups and the control group. (Aouad et al., 2019) The current meta-analysis demonstrated that there was no difference between dexmedetomidine and remifentanil in the incidence of residual sedation.
In the sensitivity analysis, Wang et al.‘s study was the main source of heterogeneity for the pooled analysis of the recovery time. (Wang et al., 2015) Wang et al.‘s study divided four dose groups of dexmedetomidine (0.4, 0.6, 0.8, 1.0 μg/kg/h), which were more high doses compared with other studies. Seo et al.’ s study demonstrated that 0.5 μg/kg dexmedetomidine infusion 30 min before the end of surgery attenuated the hemodynamic responses during emergence without prolonging the extubation time, and more than 0.5 μg/kg of dexmedetomidine significantly prolonged the extubation time. (Seo et al., 2014) For the extubation time, remifentanil was significantly shorter compared with dexmedetomidine with the removal of Park et al.‘s study. (Polat et al., 2015) The reason for the heterogeneity of Park et al.‘s study is probably that 2.0 ng/ml of remifentanil was maintained during emergence until extubation, which was a high dose compared with other studies. Lee et al.‘s study found that the EC95 of effect site concentration of remifentanil to suppress coughing at emergence from anesthesia was 2.14 ng/ml. (Lee et al., 2009) In reviewing the study of Park et al., we decided to include the data from the study. Because the study did not meet the exclusion criteria and was a high quality research.
A limitation of the meta-analysis is that the results may be changed by publication bias. Because the number of included studies was less than 10, publication bias was not evaluated. Second, the study did not compare dexmedetomidine with remifentanil in decreasing the incidence of moderate to severe coughing during emergence from anesthesia. Because there was a lack of related data that these two drugs respectively compared to placebo. Third, the heterogeneity of medication dosage may change the observed effect by attenuating peri-extubation coughing in a dose dependent manner. The optimal dose of remifentanil and dexmedetomidine depends on various factors, such as the administration of other opioids. It is difficult to determine the optimal dose. Güneş et al.‘s study and Chen et al.‘s study respectively use fentanyl and tramadol for analgesia during operation, and there are no differences between dexmedetomidine group and remifentanil group for each study. (Chen et al., 2016; Güneş et al., 2013) However, since increasing the dose of medications may cause more adverse effects, we analyzed both the efficacy and side effects of dexmedetomidine and remifentanil in inhibiting emergence cough. Fourth, the included studies contain tracheal extubation in awake and deeply anesthetized patients, which may cause different airway stimulation. We attempted to eliminate this issue by performing a sensitivity analysis, and the results of the incidence of moderate to severe coughing did not substantially change.
In conclusion, this meta-analysis demonstrated that dexmedetomidine and remifentanil had no differences in the occurrence of moderate to severe coughing, extubation time, and residual sedation during emergence from anesthesia. However, the average recovery time of remifentanil was shorter than dexmedetomidine.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
XF wrote the protocol. XF and HC searched and screened literature, extracted data, evaluated quality, and analyzed data. YX helped analyze the results and the revision process. XF and BP wrote the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Program of Guangxi Key Research and Development (No.AB20159019), the National Key Research and Development Program of China (No. 2018YFC2001905), Key Project of Guangxi Natural Science Foundation (No. 2020GXNSFDA238025).
Acknowledgments
Thanks to Yan Zhu for her help.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aouad, M. T., Zeeni, C., Al Nawwar, R., Siddik-Sayyid, S. M., Barakat, H. B., Elias, S., et al. (2019). Dexmedetomidine for improved quality of emergence from general anesthesia: A dose-finding study. Anesth. Analg. 129 (6), 1504–1511. doi:10.1213/ane.0000000000002763
Battershill, A. J., and Keating, G. M. (2006). Remifentanil : A review of its analgesic and sedative use in the intensive care unit. Drugs 66 (3), 365–385. doi:10.2165/00003495-200666030-00013
Chen, J. W., Lv, X., Zhang, L., and Chen, Z. F. (2016). Effects of remifentanil and dexmedetomidine on recovery profiles after oral and maxillofacial surgery. Shanghai Kou Qiang Yi Xue 25 (1), 101–104.
Choi, E. K., Kwon, N., and Park, S. J. (2018). Comparison of the effects of oxycodone versus fentanyl on airway reflex to tracheal extubation and postoperative pain during anesthesia recovery after laparoscopic cholecystectomy: A double-blind, randomized clinical consort study. Medicine 97 (13), e0156. doi:10.1097/md.0000000000010156
Clivio, S., Putzu, A., and Tramèr, M. R. (2019). Intravenous lidocaine for the prevention of cough: Systematic review and meta-analysis of randomized controlled trials. Anesth. Analg. 129 (5), 1249–1255. doi:10.1213/ane.0000000000003699
Estebe, J. P., Gentili, M., Le Corre, P., Dollo, G., Chevanne, F., and Ecoffey, C. (2005). Alkalinization of intracuff lidocaine: Efficacy and safety. Anesth. Analg. 101 (5), 1536–1541. doi:10.1213/01.ane.0000180995.24211.89
Fagan, C., Frizelle, H. P., Laffey, J., Hannon, V., and Carey, M. (2000). The effects of intracuff lidocaine on endotracheal-tube-induced emergence phenomena after general anesthesia. Anesth. Analg. 91 (1), 201–205. doi:10.1097/00000539-200007000-00038
Güneş, Y., Türktan, M., Erman, T., and Özcengiz, D. (2013). Comparison of dexmedetomidine, remifentanil, and esmolol for the control of hypertension during tracheal extubation and emergence from anesthesia after a craniotomy. Neurosurg. Q. 23 (4), 294–298. doi:10.1097/WNQ.0b013e318275e33f
Harding, J., Sebag, F., Sierra, M., Palazzo, F. F., and Henry, J. F. (2006). Thyroid surgery: Postoperative hematoma--prevention and treatment. Langenbecks Arch. Surg. 391 (3), 169–173. doi:10.1007/s00423-006-0028-6
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Hu, J., Zhang, C., Yan, J., Wang, R., Wang, Y., and Xu, M. (2016). Sufentanil and bupivacaine combination versus bupivacaine alone for spinal anesthesia during cesarean delivery: A meta-analysis of randomized trials. PLoS One 11 (3), e0152605. doi:10.1371/journal.pone.0152605
Hu, S., Li, Y., Wang, S., Xu, S., Ju, X., and Ma, L. (2019). Effects of intravenous infusion of lidocaine and dexmedetomidine on inhibiting cough during the tracheal extubation period after thyroid surgery. BMC Anesthesiol. 19 (1), 66. doi:10.1186/s12871-019-0739-1
Hsu, Y. W., Cortinez, L. I., Robertson, K. M., Keifer, J. C., Sum-Ping, S. T., Moretti, E. W., et al. (2004). Dexmedetomidine pharmacodynamics: Part I - Crossover comparison of the respiratory effects of dexmedetomidine and remifentanil in healthy volunteers. Anesthesiology 101 (5), 1066–1076. doi:10.1097/00000542-200411000-00005
Irwin, R. S. (2006). Complications of cough: ACCP evidence-based clinical practice guidelines. Chest 129 (1), 54S–58S. doi:10.1378/chest.129.1_suppl.54S
Jun, N. H., Lee, J. W., Song, J. W., Koh, J. C., Park, W. S., and Shim, Y. H. (2010). Optimal effect-site concentration of remifentanil for preventing cough during emergence from sevoflurane-remifentanil anaesthesia. Anaesthesia 65 (9), 930–935. doi:10.1111/j.1365-2044.2010.06450.x
Kim, D. J., Kim, S. H., So, K. Y., and Jung, K. T. (2015). Effects of dexmedetomidine on smooth emergence from anaesthesia in elderly patients undergoing orthopaedic surgery. BMC Anesthesiol. 15, 139. doi:10.1186/s12871-015-0127-4
Kim, H., Min, K. T., Lee, J. R., Ha, S. H., Lee, W. K., Seo, J. H., et al. (2016). Comparison of dexmedetomidine and remifentanil on airway reflex and hemodynamic changes during recovery after craniotomy. Yonsei Med. J. 57 (4), 980–986. doi:10.3349/ymj.2016.57.4.980
Kim, S. Y., Kim, J. M., Lee, J. H., Song, B. M., and Koo, B. N. (2013). Efficacy of intraoperative dexmedetomidine infusion on emergence agitation and quality of recovery after nasal surgery. Br. J. Anaesth. 111 (2), 222–228. doi:10.1093/bja/aet056
Kim, Y. S., Chang, H. W., Kim, H., Park, J. S., and Won, Y. J. (2021). Comparison of the effects of dexmedetomidine and remifentanil on perioperative hemodynamics and recovery profile of patients undergoing laryngeal microsurgery: A prospective randomized double-blinded study. Med. Baltim. 100 (34), e27045. doi:10.1097/md.0000000000027045
Kim, Y. S., Kim, Y. I., Seo, K. H., and Kang, H. R. (2013). Optimal dose of prophylactic dexmedetomidine for preventing postoperative shivering. Int. J. Med. Sci. 10 (10), 1327–1332. doi:10.7150/ijms.6531
Lee, B., Lee, J. R., and Na, S. (2009). Targeting smooth emergence: The effect site concentration of remifentanil for preventing cough during emergence during propofol-remifentanil anaesthesia for thyroid surgery. Br. J. Anaesth. 102 (6), 775–778. doi:10.1093/bja/aep090
Liu, Y., Ai, D., and Wang, X. (2021). Efficacy of perioperative intravenous dexmedetomidine administration for the prevention of postoperative sore throat: A meta-analysis. J. Int. Med. Res. 49 (5), 3000605211017686. doi:10.1177/03000605211017686
Minogue, S. C., Ralph, J., and Lampa, M. J. (2004). Laryngotracheal topicalization with lidocaine before intubation decreases the incidence of coughing on emergence from general anesthesia. Anesth. Analg. 99 (4), 1253–1257. doi:10.1213/01.ane.0000132779.27085.52
Nho, J. S., Lee, S. Y., Kang, J. M., Kim, M. C., Choi, Y. K., Shin, O. Y., et al. (2009). Effects of maintaining a remifentanil infusion on the recovery profiles during emergence from anaesthesia and tracheal extubation. Br. J. Anaesth. 103 (6), 817–821. doi:10.1093/bja/aep307
Park, J. S., Kim, K. J., Lee, J. H., Jeong, W. Y., and Lee, J. R. (2016). A randomized comparison of remifentanil target-controlled infusion versus dexmedetomidine single-dose administration: A better method for smooth recovery from general sevoflurane anesthesia. Am. J. Ther. 23 (3), e690–e696. doi:10.1097/01.mjt.0000433939.84373.2d
Polat, R., Peker, K., Baran, I., Bumin Aydın, G., Gülöksüz Ç, T., Dönmez, A., et al. (2015). Comparison between dexmedetomidine and remifentanil infusion in emergence agitation during recovery after nasal surgery: A randomized double-blind trial. Anaesthesist 64 (10), 740–746. doi:10.1007/s00101-015-0077-8
Qing, F., Chunbo, H., Min, Y., and Xia, S. (2015). Dexmedetomidine for tracheal extubation in deeply anesthetized adult patients after otologic surgery: A comparison with remifentanil. BMC Anesthesiol. 15 (1), 106. doi:10.1186/s12871-015-0088-7
Seo, K. H., Kim, Y. I., and Kim, Y. S. (2014). Optimal dose of dexmedetomidine for attenuating cardiovascular response during emergence in patients undergoing total laparoscopic hysterectomy. J. Int. Med. Res. 42 (5), 1139–1149. doi:10.1177/0300060514531925
Sun, S., Wang, J., Bao, N., Chen, Y., and Wang, J. (2017). Comparison of dexmedetomidine and fentanyl as local anesthetic adjuvants in spinal anesthesia: A systematic review and meta-analysis of randomized controlled trials. Drug Des. devel. Ther. 11, 3413–3424. doi:10.2147/dddt.s146092
Tanoubi, I., Sun, J. N., Drolet, P., Fortier, L. P., and Donati, F. (2015). Replacing a double-lumen tube with a single-lumen tube or a laryngeal mask airway device to reduce coughing at emergence after thoracic surgery: A randomized controlled single-blind trial. Can. J. Anaesth. 62 (9), 988–995. doi:10.1007/s12630-015-0403-2
Keywords: dexmedetomidine, remifentanil, emergence coughing, extubation, general anaesthesia
Citation: Fan X, Cai H, Pan B and Xie Y (2022) Comparison of dexmedetomidine and remifentanil on reducing coughing during emergence from anesthesia with tracheal intubation: A meta-analysis. Front. Pharmacol. 13:993239. doi: 10.3389/fphar.2022.993239
Received: 20 July 2022; Accepted: 14 September 2022;
Published: 30 September 2022.
Edited by:
Somchai Amornyotin, Mahidol University, ThailandReviewed by:
Marco Echeverria-Villalobos, The Ohio State University, United StatesAlberto A. Uribe, The Ohio State University, United States
Copyright © 2022 Fan, Cai, Pan and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yubo Xie, eHliZG9jdG9yQDE2My5jb20=
 Xing Fan
Xing Fan Hai Cai1
Hai Cai1 Yubo Xie
Yubo Xie