
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 05 October 2022
Sec. Drugs Outcomes Research and Policies
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.992713
This article is part of the Research Topic Evidence-Based Drug Treatment of Infectious Diseases - Volume II View all 8 articles
 Athanasios Vassilopoulos1,2
Athanasios Vassilopoulos1,2 Fadi Shehadeh1,2,3
Fadi Shehadeh1,2,3 Gregorio Benitez1,2
Gregorio Benitez1,2 Markos Kalligeros1,2
Markos Kalligeros1,2 Joanne S. Cunha2
Joanne S. Cunha2 Cheston B. Cunha1,2
Cheston B. Cunha1,2 Eleftherios Mylonakis1,2*
Eleftherios Mylonakis1,2*Background: Biologic (bDMARD) and targeted synthetic (tsDMARD) disease-modifying anti-rheumatic drugs have broadened the treatment options and are increasingly used for patients with psoriatic arthritis (PsA). These agents block different pro-inflammatory cytokines or specific intracellular signaling pathways that promote inflammation and can place patients at risk of serious infections. We aimed to review the incidence of opportunistic infections (OIs) in patients with PsA who were treated with these agents.
Methods: We searched PubMed and EMBASE through 14 April 2022 for randomized clinical trials evaluating bDMARD or tsDMARD in the treatment of PsA. Trials were eligible if they compared the effect of a bDMARD or tsDMARD with placebo and provided safety data. We used the Revised Cochrane risk-of-bias tool to assess the risk of bias among trials, and stratified the studies by mechanism of action (MOA) of the agents studied.
Results: We included 47 studies in this analysis. A total of 17,197 patients received at least one dose of an agent of interest. The cumulative incidence of OIs by MOA was as follows: 1) JAK inhibitors: 2.72% (95% CI: 1.05%–5.04%), 2) anti-IL-17: 1.18% (95% CI: 0.60%–1.9%), 3) anti-IL-23: 0.24% (95% CI: 0.04%–0.54%), and 4) anti-TNFs: 0.01% (95% CI: 0.00%–0.21%). Based on their MOA, these agents are known to increase the risk of certain serious infections. The cumulative incidence of herpes zoster infection following treatment with JAK inhibitors (JAKi) was 2.53% (95% CI: 1.03%–4.57%) and the cumulative incidence of opportunistic Candida spp. infections following treatment with anti-IL-17, was 0.97% (95% CI: 0.51%–1.56%).
Conclusion: The overall incidence of OIs among patients with PsA who were treated with biologic and targeted synthetic agents is low. However, careful monitoring is warranted for specific OIs such as herpes zoster infection following JAKi treatment, mucocutaneous candidiasis following anti-IL-17 treatment, and Mycobacterium tuberculosis infection following anti-TNF treatment.
Novel treatment options for psoriatic arthritis (PsA) significantly decrease disease activity, prevent structural damage, and improve patient quality of life (Husni, 2015; Ogdie et al., 2015; Ogdie et al., 2020; Ruyssen-Witrand et al., 2020; Gupta et al., 2021). These treatments include biologic disease-modifying anti-rheumatic drugs (bDMARDs) and the most recently available oral targeted synthetic DMARDs (tsDMARDs) (Ritchlin et al., 2017; Van den Bosch and Coates, 2018; Singh et al., 2019; Ogdie et al., 2020). bDMARDs target pro-inflammatory cytokines such as tumor necrosis factor (TNF), interleukin 12 (IL-12), interleukin 17 (IL-17), and interleukin 23 (IL-23) as well as T cell activation, that are associated with the pathogenesis of PsA (Ritchlin et al., 2017; Veale and Fearon, 2018; Schett et al., 2021). Furthermore, tsDMARDs suppress intracellular signaling pathways that promote inflammation by inhibiting phosphodiesterase 4 (PDE4) or Janus family kinases (JAK) (Haikarainen et al., 2019).
Serious infections, particularly OIs, are a concern in patients with PsA (Minozzi et al., 2016; Ritchlin et al., 2019; Li et al., 2020). Psoriasis and PsA may increase the risk of infections via loss of skin barrier integrity and innate or adaptive immune alterations, while biologic agent use may increase the risk of infections as seen in patients with psoriasis and rheumatoid arthritis (RA) (Bergboer et al., 2012; Singh et al., 2015; Minozzi et al., 2016; Yiu et al., 2016; Subesinghe et al., 2018). Since biologic agents are also used in PsA, they could increase the risk of both serious and OIs.
Given the limited data regarding the risk of OIs in patients with PsA treated with bDMARDs or tsDMARDs, we performed a systematic review and meta-analysis of randomized controlled trials (RCTs) and their extension periods with the aim of estimating the incidence of OIs following treatment with b- and ts-DMARDs with different mechanisms of action (MOAs).
We searched the PubMed and EMBASE databases for RCTs published in English, with last access on 14 April 2022. For our literature search we used the following search term: “psoriatic arthritis” AND “randomized”. An additional manual search of reference lists for eligible studies complemented the initial search. We performed this meta-analysis based on the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement (Page et al., 2021).
We selected RCTs of bDMARD or tsDMARD that compared the effect of a biologic or targeted synthetic agent with placebo and provided safety data. We decided to include in this analysis patients who received concomitant low-dose glucocorticoids, defined as <10 mg/day equivalent to prednisolone, and conventional synthetic DMARDs (csDMARDs) such as methotrexate, leflunomide and sulfasalazine. We excluded studies that randomized patients to two biologic agents with no placebo arm. Moreover, we marked data as unextractable and excluded studies that reported infectious causes of adverse events only with a high-level term and without any further categorization. Lastly, each outcome of interest had to be reported in ≥3 trials and the MOA of the tested treatment regimen had to be present in ≥3 trials.
Two reviewers (AV and GB) independently screened titles and abstracts to determine eligibility. The same reviewers independently retrieved and evaluated the full text of selected articles. They resolved disagreements through discussion and consensus; a third reviewer (FS) independently reviewed unresolved matters.
We independently extracted data regarding patient populations, interventions, outcomes of interest, and quality of data for individual studies. The extracted data included the main characteristics of each study (author and publication year, duration of RCT and extension period), proportion of bDMARD-naïve population, proportion of population with concomitant csDMARD use, proportion of women, number of patients in each treatment arm and placebo arm, and the number of patients that received at least one dose of an agent of interest. For our analysis, we also extracted the number of OIs and their causes during both the entire duration of follow-up and exclusively the placebo-controlled period and the number of herpes zoster and opportunistic Candida spp. infections during the entire duration of follow-up.
For methodological quality, we assessed the risk of bias of RCTs with the Revised Cochrane risk-of-bias tool by evaluating 1) the randomization process, 2) deviations from the intended interventions, 3) amount of missing outcome data, 4) measurement of the outcome, and 5) selection of the reported result (Sterne et al., 2019).
The primary outcome of our analysis was the incidence of all OIs stratified by MOA. We identified OIs based on the recommended definition of OIs for rheumatologic diseases by Winthrop et al. (2015). Secondary outcomes of our study were 1) incidence of herpes zoster infection by MOA, 2) incidence of opportunistic Candida spp. infections following anti-IL-17 treatment, 3) proportion of M. tuberculosis infections in patients with OIs receiving anti-TNFs and 4) the relative risk of OIs stratified by MOA during the placebo-controlled period.
The biologic agents evaluated include anti-TNFs (etanercept, infliximab, golimumab, certolizumab, and adalimumab), anti-IL-17 (ixekizumab, secukinumab, brodalumab, bimekizumab), anti-IL-12/23 (ustekinumab), anti-IL-23 (risankizumab, guselkumab), and T-cell co-stimulation modulators (abatacept, alefacept). The PDE4 inhibitor, apremilast and JAKi (tofacitinib, upadacitinib, filgotinib, deucravacitinib) were the targeted synthetic agents evaluated.
We used Stata v17 software (Stata Corporation, College Station, TX) for data analysis. We stratified by MOA of the agents tested and performed a random effects meta-analysis using the DerSimonian and Laird approach to estimate the cumulative incidence of OIs among patients with PsA during both placebo-controlled and extension periods (DerSimonian and Laird, 1986). In order to stabilize the variances, we used the Freeman Tukey double arcsine transformation (Nyaga et al., 2014). For this meta-analysis, we selected a random effects model due to differences in the proportion of bDMARD-naïve population, the proportion of concomitant csDMARD use, and duration of follow-up periods. Additionally, we conducted a meta-regression analysis to investigate the extent of the differences in study characteristics and their correlation with the heterogeneity between studies (Harbord and Higgins, 2008).
For our secondary analyses, we planned to stratify our data by the most common causes of OIs. We calculated a pooled random-effects estimate using the DerSimonian and Laird approach to estimate the cumulative incidence of our secondary outcomes, as well as the relative risk for OIs during the placebo-controlled period of RCTs (DerSimonian and Laird, 1986). We estimated heterogeneity using the I2 statistic and we used the Egger’s test to explore publication bias and small study effects (Higgins et al., 2003; Peters et al., 2006). For the interpretation of heterogeneity with the I2 statistic we used the approach detailed as follows: I2 values of 25%, 50%, and 75% represent low, moderate, and high heterogeneity, respectively (Higgins et al., 2003). Statistical significance was set at α = 0.05.
Following deduplication between literature search in PubMed and EMBASE, we retrieved 1,066 studies published between 2000 and 2022. One article was added after a manual search of reference lists. After title and abstract screening, we excluded a total of 968 publications and we retrieved 99 publications for full-text detailed evaluation. Subsequently, we excluded 26 publications, resulting in a total of 73 citations eligible for analysis. We retrieved 47 RCTs for this analysis and twenty-six studies reporting extension follow-up data (Figure 1, Supplementary Appendix Table S1).
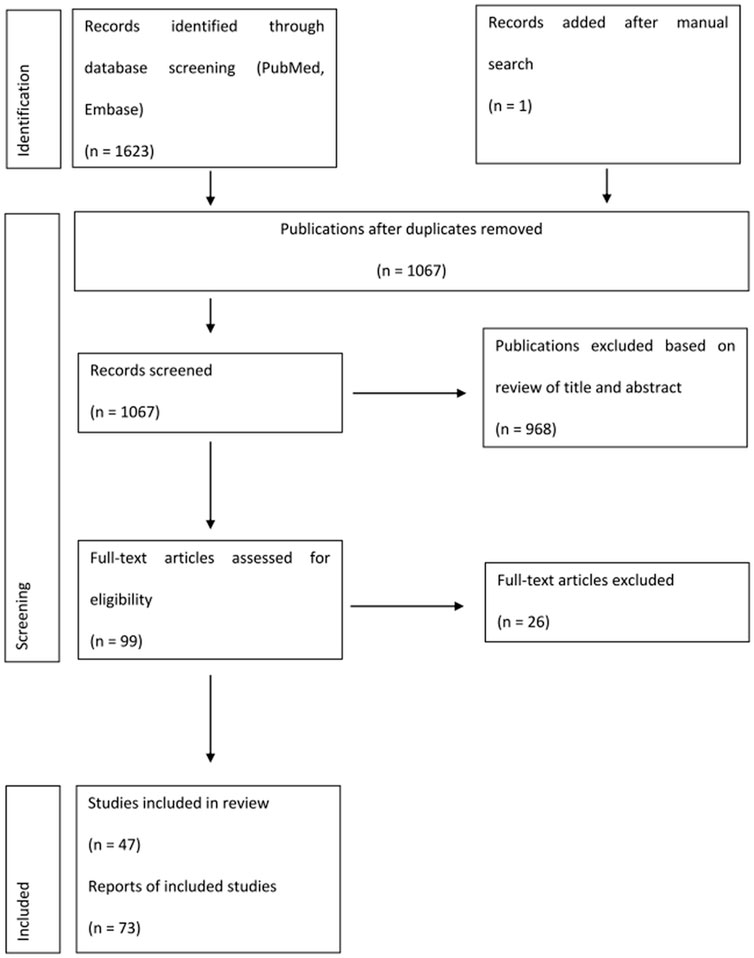
FIGURE 1. Flow diagram for selection of studies included in the systematic review and meta-analysis.
Among the included studies, there were 17 studies evaluating anti-TNFs, 9 studies evaluating anti-IL-17, 6 studies each evaluating JAKi, anti-IL-23, and PDE4 inhibitors, and 3 studies each evaluating anti-IL-12/23 and cytotoxic T lymphocyte-associated antigen-4 Ig (CTLA4-Ig).
Regarding publications with extension periods, there were 11 studies for anti-TNFs, 5 studies for anti-IL-17, 4 studies for PDE4i, 3 studies for anti-IL-23, 2 studies for JAKi, 1 study for alefacept and 1 study for ustekinumab (Gladman et al., 2007; Kavanaugh et al., 2007; Antoni et al., 2008; Mease et al., 2009; Mease and Reich, 2009; Kavanaugh et al., 2012; Kavanaugh et al., 2013; Kavanaugh et al., 2014b; Kavanaugh et al., 2015a; Kavanaugh et al., 2015b; Mease et al., 2015b; Kavanaugh et al., 2017b; Genovese et al., 2018a; van der Heijde et al., 2018; Kavanaugh et al., 2019; Mease et al., 2020c; Husni et al., 2020; van der Heijde et al., 2020; McInnes et al., 2021b; Mease et al., 2021b; McInnes et al., 2021c; Mease et al., 2021c; Orbai et al., 2021; Ritchlin et al., 2021; McInnes et al., 2022; Wells et al., 2022). The baseline characteristics of the studies included are shown in the Supplementary Appendix Table S2.
In total, 11,790 patients were assigned to receive different doses of the tested agents and 6,425 patients were assigned to receive placebo during the placebo-controlled period (range: 12–48 weeks). After taking into consideration the extension periods, a total of 17,197 patients received at least one different dose of an agent for a total follow-up duration ranging from 12 to 268 weeks.
In Figure 2 we present the cumulative incidence of OIs for JAKi, which was 2.72% (95% CI: 1.05%–5.04%) among 2,740 patients receiving at least one dose of a JAKi agent. During the follow-up period (12–56 weeks), the most common OI reported following JAKi treatment was herpes zoster infection, with 130/146 (89%) patients with OIs developing herpes zoster infection.
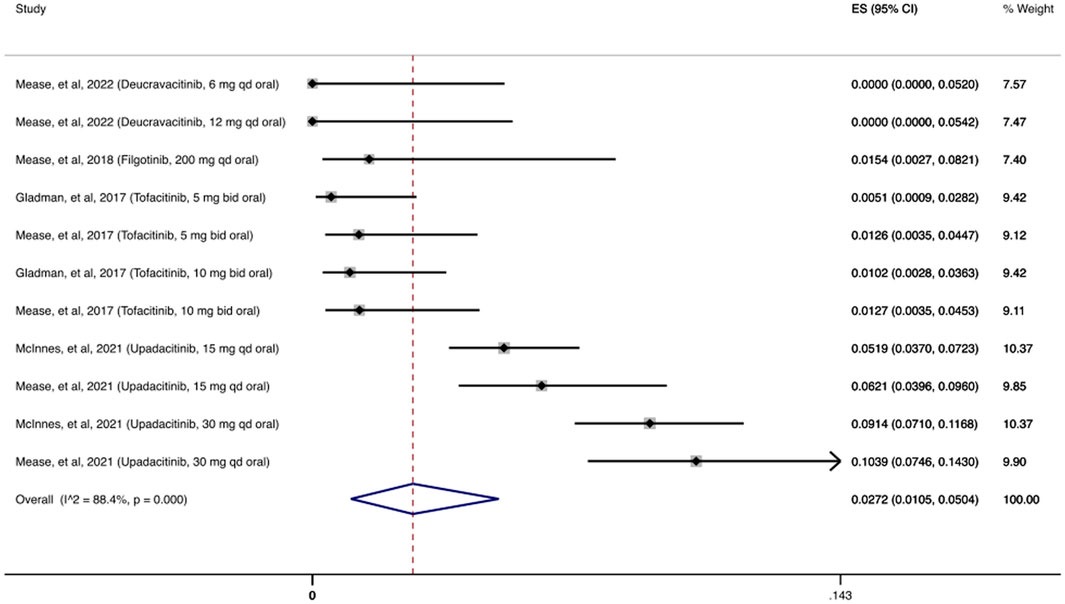
FIGURE 2. Opportunistic infections cumulative incidence for JAK inhibitors during RCTs and their extension periods. Individual and combined estimates of the cumulative incidence of opportunistic infections for patients treated with JAK inhibitors with 95% confidence intervals. ES: Effect Size (Cumulative incidence).
As shown in Figure 3, the cumulative incidence of OIs for anti-IL-17 was 1.18% (95% CI: 0.60%–1.9%) among 4,626 patients receiving at least one dose of an anti-IL-17 agent. The most common OI reported during the follow-up period (12–156 weeks) was due to Candida spp., with 58/67 (87%) patients with OIs developing an opportunistic Candida spp. infection.
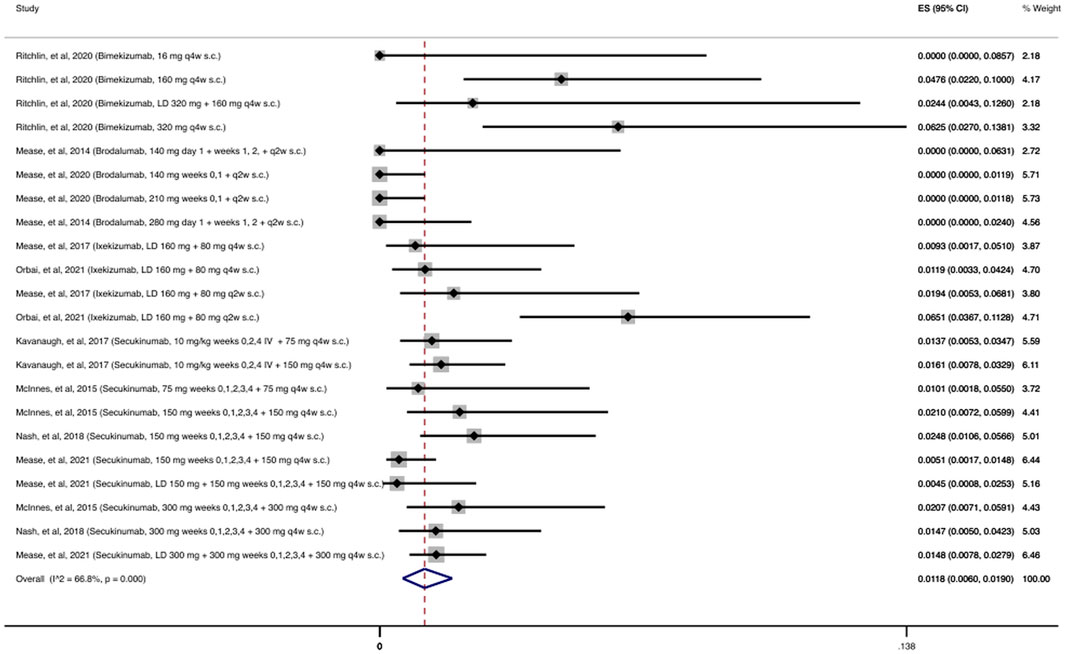
FIGURE 3. Opportunistic infections cumulative incidence for anti-IL-17 during RCTs and their extension periods. Individual and combined estimates of the cumulative incidence of opportunistic infections for patients treated with anti-IL-17 with 95% confidence intervals. ES: Effect Size (Cumulative incidence).
As shown in Figure 4, 2,215 patients were treated with at least one dose of an anti-IL-23 agent during the follow-up period (24–112 weeks). There were only 8 reported OIs, resulting in a cumulative incidence of 0.24% (95% CI: 0.04%–0.54%).
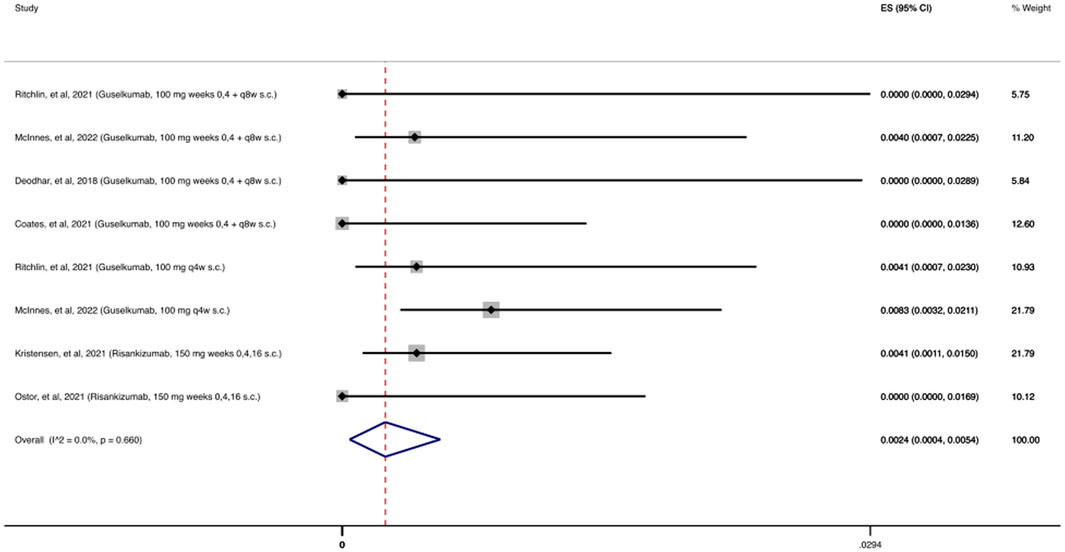
FIGURE 4. Opportunistic infections cumulative incidence for anti-IL-23 during RCTs and their extension periods. Individual and combined estimates of the cumulative incidence of opportunistic infections for patients treated with anti-IL-23 with 95% confidence intervals. ES: Effect Size (Cumulative incidence).
The cumulative incidence of OIs for anti-TNFs, as shown in the Supplementary Appendix Figure S1, was 0.01%, (95% CI: 0.00%–0.21%) among 3,425 patients receiving at least one dose of an anti-TNF agent during the follow-up period (12–268 weeks). More specifically, there were a total of 17 OIs reported, in which 5/17 OIs were due to M. tuberculosis infection. As shown in the Supplementary Appendix Figure S2, we found a 29.24% (95% CI: 29%–71.45%) pooled estimated proportion of M. tuberculosis infection in patients with OIs.
The cumulative incidence of OIs for these MOAs is presented in the Supplementary Appendix Figures S3–S5. Only one Pneumocystis jirovecii infection was reported among patients receiving abatacept and one herpes zoster infection among patients receiving apremilast. No OIs were noted among patients receiving anti-IL-12/23 agents.
We performed a meta-regression analysis for each group of agents based on their MOA and found no association between the cumulative incidence of OIs and the proportion of bDMARD-naïve population, the proportion of concomitant csDMARD use, and duration of follow-up (data not shown).
Besides herpes zoster, Candida spp. and M. tuberculosis infections, other causes of OIs were rarely reported as presented in the Supplementary Appendix Table S3. There were three MOAs under trial for the treatment of PsA that were evaluated by only one study each (Papp et al., 2007; Mease et al., 2016; Mease et al., 2018a; Genovese et al., 2018b). Of note, only two oral candidiasis infections were reported among 239 patients who received at least one dose of ABT-122, an agent targeting both TNF and IL-17A.
Based on the OIs consensus, all herpes zoster infections are adjudicated as OIs (Winthrop et al., 2015). As shown in Figure 5, the cumulative incidence of herpes zoster infection following treatment with JAKi was 2.53% (95% CI: 1.03%–4.57%). In the deucravacitinib study, an investigational agent selectively targeting tyrosine kinase 2 (TYK2 inhibitor), no cases of herpes zoster were reported (Mease et al., 2022). In contrast, upadacitinib studies had the highest number of herpes zoster infections (Mease et al., 2020a; McInnes et al., 2021a; McInnes et al., 2021b; Mease et al., 2021c; Burmester et al., 2022). Across the remaining studies evaluating the other MOAs, the incidence of herpes zoster infection was low with 14 cases among the combined 14,757 patients receiving at least one dose of the agents examined.
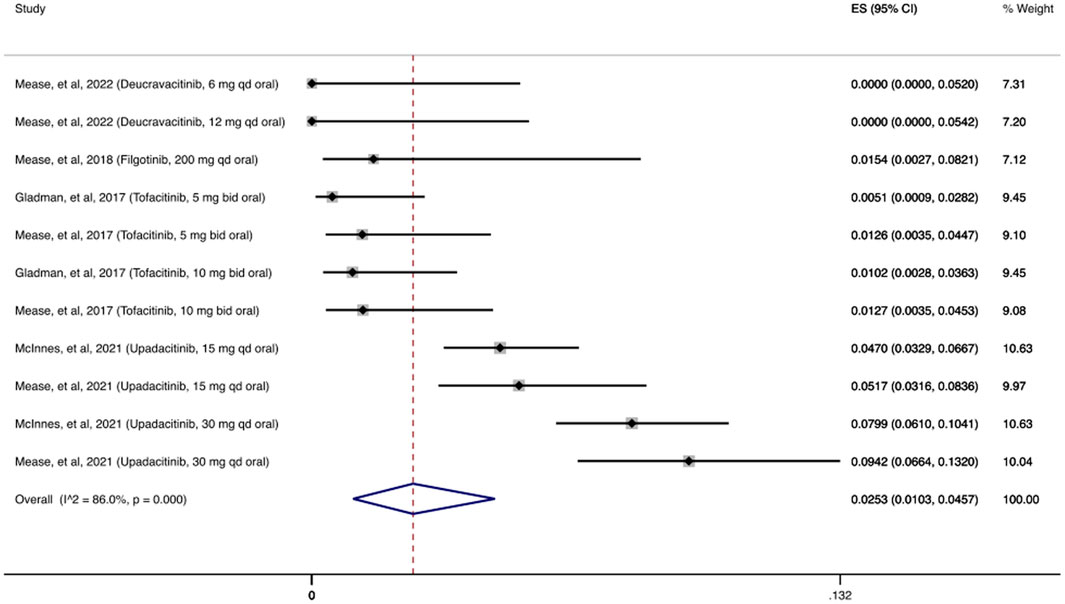
FIGURE 5. Herpes zoster infection cumulative incidence for JAK inhibitors during RCTs and their extension periods. Individual and combined estimates of the incidence of herpes zoster infection for patients treated with JAK inhibitors with 95% cumulative confidence intervals. ES: Effect Size (Cumulative incidence).
As shown in Figure 6, the cumulative incidence of opportunistic Candida spp. infections following treatment with anti-IL-17 was 0.97% (95% CI: 0.51%–1.56%). Most patients had mucocutaneous (oropharyngeal or esophageal) candidiasis. Across the remaining studies evaluating the other MOAs, 11 opportunistic Candida spp. infections were reported among the combined 12,468 patients receiving at least one dose of the agents of interest.
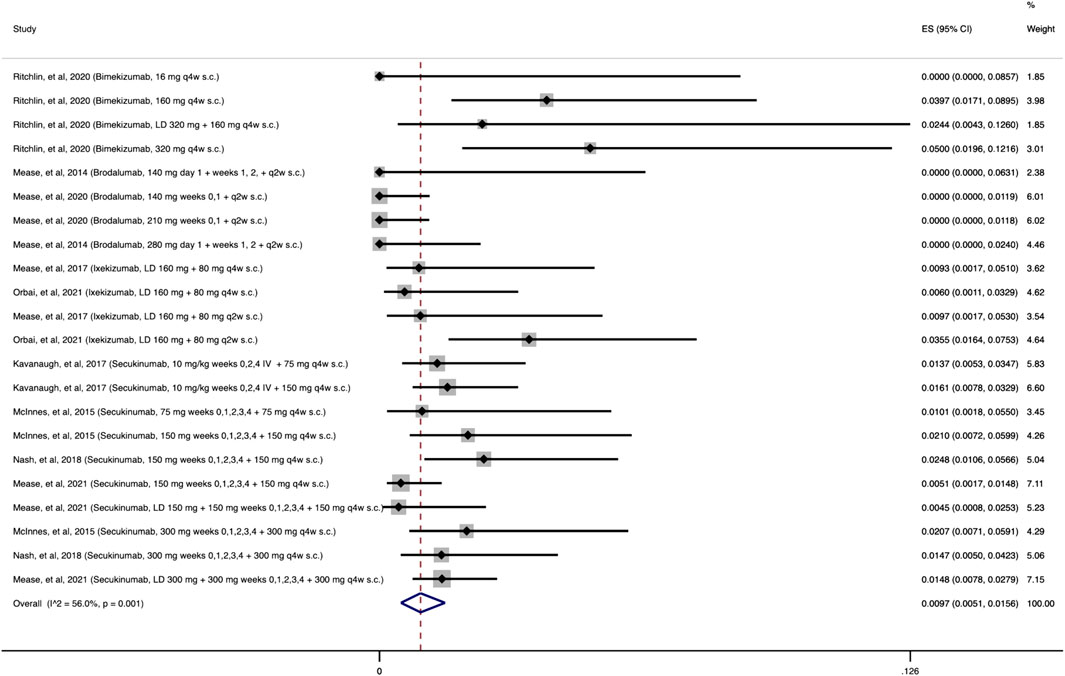
FIGURE 6. Opportunistic Candida spp. infections cumulative incidence for anti-IL-17 during RCTs and their extension periods. Individual and combined estimates of the cumulative incidence of opportunistic Candida spp. infections for patients treated with anti-IL-17 with 95% confidence intervals. ES: Effect Size (Cumulative incidence).
In Table 1, we show extracted data from studies specifically during the placebo-controlled period and our results stratified by MOA. Importantly, as shown in Supplementary Appendix Figures S6–S10 we detected no significant difference in the relative risk for OIs between patients treated with anti-TNFs, anti-IL-23, anti-IL-12/23, CTLA4-Ig, or a PDE4 inhibitor and those treated with a placebo. In contrast, patients treated with JAKi and anti-IL-17 agents had a 2.25 (95% CI: 1.16–4.35) and 2.27 (95% CI: 1.03–4.99) higher relative risk of OIs compared with patients treated with placebo, as shown in Supplementary Appendix Figures S11, S12, respectively. Moreover, most of the OIs reported among JAKi-treated patients (Cumulative incidence: 1.10%, 95% CI: 0.53%–1.83%) were due to herpes zoster infection (82.7%), while most of the OIs reported among anti-IL-17-treated patients (Cumulative incidence: 0.26%, 95% CI: 0.01%–0.70%) were due to Candida spp. (93.75%).
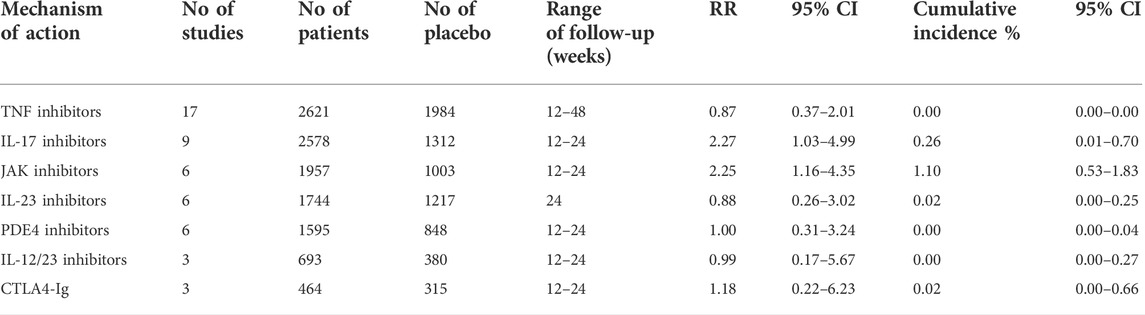
TABLE 1. Opportunistic infections cumulative incidence and relative risk (RR) for bDMARDs, tsDMARDs during placebo-controlled period.
Studies with PDE4 inhibitors, anti-IL-12/23, anti-IL-23 and CTLA4-Ig had low heterogeneity (I2 = 0%, p > 0.05). Anti-TNFs showed moderate heterogeneity (I2 = 31.69%, p < 0.05), while anti-IL-17 and JAKi had high heterogeneity (I2 = 66.8%, p = 0.00 and I2 = 88.4%, p = 0.00, respectively)
We present quality assessment data in the Supplementary Appendix Figure S13. We considered all of the studies to have low risk of bias across all domains evaluated. Also, Egger’s test for publication bias showed no evidence of small-study effects (bias = 0.014, p = 0.67).
New biologic and targeted synthetic disease-modifying agents are becoming increasingly available for the treatment of PsA. Our meta-analysis of almost 17,000 patients treated with different b- or ts-DMARDs across RCTs and their extension periods estimated the cumulative incidence of OIs stratified by MOA. It should be noted that we excluded studies without a placebo arm. Interestingly, we found that the cumulative incidence of OIs was low and that the most common OI differed based on the drug MOA. The randomized nature of the included studies and the lack of statistical heterogeneity in many analyses strengthen our findings, which offer insight about the safety and incidence of OIs in daily clinical practice and highlight the need for careful monitoring of patients treated with these agents for OIs.
In our analysis, the cumulative incidence of OIs was less than 3% across all MOAs examined. Our findings are in line with published OI incidences in real-world studies, which identify the same predicted causes for each MOA, various rates according to the MOA, and minimal risk for severe adverse outcomes (Siegel and Winthrop, 2019; Li et al., 2020). The low incidence may be attributed to the selection of more homogenous populations across RCTs and thorough screening for latent TB and other infections prior to treatment initiation (Ogdie and Coates, 2017). Additionally, short-term follow-up periods and acquired experience for OIs monitoring during therapy may have also influenced the rate of reported OIs. Follow-up periods of placebo-controlled RCTs usually lasted up to 24 weeks. However, most OIs occurred during the extension period and long-term placebo is questionable ethically (Ogdie and Coates, 2017). Therefore, more RCTs with longer follow-up periods and head-to-head comparisons of b- and ts-DMARDs are needed.
Herpes zoster infection was the most common OI among patients treated with JAKi. The cumulative incidence of herpes zoster infection was almost 2.5% in JAKi-treated patients. Age, comorbidities, and the effect of JAKi on T cell function and inhibition of IFN-γ and IL-15 are potential risk factors for varicella zoster virus reactivation (McLornan et al., 2015; Choy, 2019; Sunzini et al., 2020).
Among the 130 herpes zoster infections reported, we observed rare occurrence of disseminated herpes zoster or permanent drug discontinuation due to herpes zoster infection. There were higher rates of herpes zoster infection following treatment with upadacitinib among patients receiving at least one dose of 30 mg qd (8.4%), with this dose not currently approved for treatment of PsA, compared with patients receiving at least one dose of 15 mg qd (4.8%), which is approved for PsA treatment (McInnes et al., 2021b; Mease et al., 2021c). Interestingly, absence of herpes zoster infection in the TYK2 inhibitor study may explain the substantial heterogeneity in the reported outcomes of JAK family inhibitors. TYK2 inhibitors need further studies since the activity of TYK2 plays a major role in the occurrence of psoriatic skin lesions and pathological synovial response (Gao et al., 2016; Mease et al., 2022). Compared with our incidence, the incidence of herpes zoster infection is higher among rheumatoid arthritis (RA) patients treated with tofacitinib and upadacitinib (Cohen et al., 2020; Álvaro-Gracia et al., 2021). The mean incidence rate for herpes zoster infection among patients with RA receiving tofacitinib was 4.1 (95% CI: 3.3–5.2) at a dose of 10 mg qd and 3.3 (95% CI: 2.6–4.3) at a dose of 5 mg qd (Álvaro-Gracia et al., 2021). Higher rates of herpes zoster infection could be explained by the different comorbidities of RA and use of glucocorticoids to treat this disease, while glucocorticoids are sparingly used for PsA (Winthrop et al., 2017).
We found mucocutaneous candidiasis as the most common OI among patients treated with anti-IL-17. The severity of Candida spp. infections was either mild or moderate in patients receiving anti-IL-17 treatment. The incidence of opportunistic Candida spp. infections was 0.97%, which is in concordance with previous research (Saunte et al., 2017). Anti-IL-17 therapies, by either blocking the IL-17 receptor or IL-17A and/or IL-17F homodimers or heterodimers, increase the incidence of Candida spp. infections (Ghoreschi et al., 2021). Patients with inherited deficiencies in the IL-17 pathway (e.g., IL-17RA, IL-17RC, or IL-17F gene mutations) are at higher risk for developing chronic mucocutaneous candidiasis, but do not often develop systemic, disseminated or invasive candidiasis (Puel et al., 2011). Similarly, patients from our included studies developed mucocutaneous candidiasis, which was mostly oropharyngeal. In the majority of cases, discontinuation of anti-IL-17 treatment was not necessary because of monitoring, proper treatment, and adequate treatment response.
Psoriatic arthritis can be effectively treated with anti-TNF agents and disease development, disease severity, and response to anti-TNFs, particularly etanercept and adalimumab, appear to be influenced by several single-nucleotide polymorphisms (Murdaca et al., 2014; Murdaca et al., 2017). Therefore, pharmacogenetic testing of polymorphisms of TNF and TNF receptor, Fc receptors, and IL-17, and HLA gene variants could all be potential predictors of treatment response (Murdaca et al., 2014; Murdaca et al., 2017). The incidence of OIs in anti-TNF treated patients was 0.01%. This comes in contrast to data from a study that assessed the effect of anti-TNF agents on OIs among patients with RA (Kourbeti et al., 2014). Anti-TNF treated patients with RA were more likely to develop an OI (Kourbeti et al., 2014). Nevertheless, in our study, 5 cases of M. tuberculosis infection among well-screened and monitored patients with PsA were reported, emphasizing the need for increased surveillance during anti-TNF treatment.
Regarding study limitations, we could not pinpoint a specific time frame (e.g., week of infection) as when OIs developed. Also, not all published trials followed a universal definition of OIs, such as the consensus suggested by Winthrop et al. (2015). Moreover, we could not assess the risk of OIs in comparison to available treatment options, so further head-to-head studies are needed to determine the risk. Lastly, lengthier study periods are needed to assess the risk of uncommon OIs, especially OIs with prolonged latent periods. Thus, continued evaluation of the incidence of OIs following treatment with b- or ts-DMARDs is encouraged through post-marketing studies and clinical trials with longer follow-up periods.
This is the largest meta-analysis to date that evaluated the incidence of OIs in patients with PsA. Data from our meta-analysis indicate that both biologic and targeted synthetic DMARDs are safe agents concerning OIs for the treatment of PsA, since the incidence of OIs was found to be low across various different agents. However, due to the increasing use of these biologic agents in clinical settings, it is important to continue thorough monitoring of patients with PsA who are treated with biologic and targeted synthetic DMARDs, particularly for herpes zoster infection in patients treated with JAKi, mucocutaneous candidiasis in patients treated with anti-IL-17, and M. tuberculosis infection in patients treated with anti-TNFs.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
AV, FS, GB, MK, JC, CC, and EM conceptualized and designed the study, and participated in data interpretation. AV and GB participated in data collection and extraction. AV and FS prepared tables and figures and performed the statistical analysis. AV drafted the initial manuscript. FS, GB, MK, JC, CC, and EM reviewed and revised the manuscript. All authors read and approved the final manuscript as submitted and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.992713/full#supplementary-material
CI, confidence intervals; cs, conventional synthetic; CTLA4-Ig, cytotoxic T lymphocyte-associated antigen-4 Ig; DMARD, disease-modifying anti-rheumatic drug; IL, interleukin; JAK, Janus kinase; MOA, mechanism of action; OI, Opportunistic infection; PDE, phosphodiesterase; PsA, psoriatic arthritis; RCT, randomized controlled trial; TNF, tumor necrosis factor; ts, targeted synthetic.
Álvaro-Gracia, J. M., García-Llorente, J. F., Valderrama, M., Gomez, S., and Montoro, M. (2021). Update on the safety profile of tofacitinib in rheumatoid arthritis from clinical trials to real-world studies: A narrative review. Rheumatol. Ther. 8 (1), 17–40. doi:10.1007/s40744-020-00258-9
Antoni, C., Krueger, G. G., de Vlam, K., Birbara, C., Beutler, A., Guzzo, C., et al. (2005). Infliximab improves signs and symptoms of psoriatic arthritis: Results of the IMPACT 2 trial. Ann. Rheum. Dis. 64 (8), 1150–1157. doi:10.1136/ard.2004.032268
Antoni, C. E., Kavanaugh, A., Kirkham, B., Tutuncu, Z., Burmester, G. R., Schneider, U., et al. (2005). Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: Results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT). Arthritis Rheum. 52 (4), 1227–1236. doi:10.1002/art.20967
Antoni, C. E., Kavanaugh, A., van der Heijde, D., Beutler, A., Keenan, G., Zhou, B., et al. (2008). Two-year efficacy and safety of infliximab treatment in patients with active psoriatic arthritis: Findings of the infliximab multinational psoriatic arthritis controlled trial (IMPACT). J. Rheumatol. 35 (5), 869–876.
Bergboer, J. G. M., Zeeuwen, P., and Schalkwijk, J. (2012). Genetics of psoriasis: Evidence for epistatic interaction between skin barrier abnormalities and immune deviation. J. Invest. Dermatol. 132 (10), 2320–2331. doi:10.1038/jid.2012.167
Burmester, G. R., Winthrop, K., Blanco, R., Nash, P., Goupille, P., Azevedo, V. F., et al. (2022). Safety profile of upadacitinib up to 3 Years in psoriatic arthritis: An integrated analysis of two pivotal phase 3 trials. Rheumatol. Ther. 9 (2), 521–539. doi:10.1007/s40744-021-00410-z
Choy, E. H. (2019). Clinical significance of Janus Kinase inhibitor selectivity. Rheumatol. Oxf. 58 (6), 1122–1162. doi:10.1093/rheumatology/kez002
Coates, L. C., Gossec, L., Theander, E., Bergmans, P., Neuhold, M., Karyekar, C. S., et al. (2022). Efficacy and safety of guselkumab in patients with active psoriatic arthritis who are inadequate responders to tumour necrosis factor inhibitors: Results through one year of a phase IIIb, randomised, controlled study (COSMOS). Ann. Rheum. Dis. 81 (3), 359–369. doi:10.1136/annrheumdis-2021-220991
Cohen, S. B., van Vollenhoven, R. F., Winthrop, K. L., Zerbini, C. A. F., Tanaka, Y., Bessette, L., et al. (2020). Safety profile of upadacitinib in rheumatoid arthritis: Integrated analysis from the SELECT phase III clinical programme. Ann. Rheum. Dis. 80 (3), 304–311. doi:10.1136/annrheumdis-2020-218510
Cutolo, M., Myerson, G. E., Fleischmann, R. M., Lioté, F., Díaz-González, F., Van den Bosch, F., et al. (2016). A phase III, randomized, controlled trial of apremilast in patients with psoriatic arthritis: Results of the PALACE 2 trial. J. Rheumatol. 43 (9), 1724–1734. doi:10.3899/jrheum.151376
Deodhar, A., Gottlieb, A. B., Boehncke, W. H., Dong, B., Wang, Y., Zhuang, Y., et al. (2018). Efficacy and safety of guselkumab in patients with active psoriatic arthritis: A randomised, double-blind, placebo-controlled, phase 2 study. Lancet 391 (10136), 2213–2224. doi:10.1016/S0140-6736(18)30952-8
Deodhar, A., Helliwell, P. S., Boehncke, W. H., Kollmeier, A. P., Hsia, E. C., Subramanian, R. A., et al. (2020). Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFα inhibitor treatment (DISCOVER-1): A double-blind, randomised, placebo-controlled phase 3 trial. Lancet 395 (10230), 1115–1125. doi:10.1016/S0140-6736(20)30265-8
DerSimonian, R., and Laird, N. (1986). Meta-analysis in clinical trials. Control. Clin. Trials 7 (3), 177–188. doi:10.1016/0197-2456(86)90046-2
Edwards, C. J., Blanco, F. J., Crowley, J., Birbara, C. A., Jaworski, J., Aelion, J., et al. (2016). Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: A phase III, randomised, controlled trial (PALACE 3). Ann. Rheum. Dis. 75 (6), 1065–1073. doi:10.1136/annrheumdis-2015-207963
Gao, W., McGarry, T., Orr, C., McCormick, J., Veale, D. J., and Fearon, U. (2016). Tofacitinib regulates synovial inflammation in psoriatic arthritis, inhibiting STAT activation and induction of negative feedback inhibitors. Ann. Rheum. Dis. 75 (1), 311–315. doi:10.1136/annrheumdis-2014-207201
Genovese, M. C., Combe, B., Kremer, J. M., Tsai, T. F., Behrens, F., Adams, D. H., et al. (2018). Safety and efficacy of ixekizumab in patients with PsA and previous inadequate response to TNF inhibitors: Week 52 results from SPIRIT-P2. Rheumatol. Oxf. 57 (11), 2001–2011. doi:10.1093/rheumatology/key182
Genovese, M. C., Mease, P. J., Thomson, G. T., Kivitz, A. J., Perdok, R. J., Weinberg, M. A., et al. (2007). Safety and efficacy of adalimumab in treatment of patients with psoriatic arthritis who had failed disease modifying antirheumatic drug therapy. J. Rheumatol. 34 (5), 1040–1050.
Genovese, M. C., Weinblatt, M. E., Mease, P. J., Aelion, J. A., Peloso, P. M., Chen, K., et al. (2018). Dual inhibition of tumour necrosis factor and interleukin-17a with ABT-122: Open-label long-term extension studies in rheumatoid arthritis or psoriatic arthritis. Rheumatol. Oxf. 57 (11), 1972–1981. doi:10.1093/rheumatology/key173
Ghoreschi, K., Balato, A., Enerbäck, C., and Sabat, R. (2021). Therapeutics targeting the IL-23 and IL-17 pathway in psoriasis. Lancet 397 (10275), 754–766. doi:10.1016/s0140-6736(21)00184-7
Gladman, D., Rigby, W., Azevedo, V. F., Behrens, F., Blanco, R., Kaszuba, A., et al. (2017). Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N. Engl. J. Med. 377 (16), 1525–1536. doi:10.1056/NEJMoa1615977
Gladman, D. D., Mease, P. J., Ritchlin, C. T., Choy, E. H., Sharp, J. T., Ory, P. A., et al. (2007). Adalimumab for long-term treatment of psoriatic arthritis: Forty-eight week data from the adalimumab effectiveness in psoriatic arthritis trial. Arthritis Rheum. 56 (2), 476–488. doi:10.1002/art.22379
Gottlieb, A., Menter, A., Mendelsohn, A., Shen, Y. K., Li, S., Guzzo, C., et al. (2009). Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: Randomised, double-blind, placebo-controlled, crossover trial. Lancet 373 (9664), 633–640. doi:10.1016/S0140-6736(09)60140-9
Gupta, S., Syrimi, Z., Hughes, D. M., and Zhao, S. S. (2021). Comorbidities in psoriatic arthritis: A systematic review and meta-analysis. Rheumatol. Int. 41 (2), 275–284. doi:10.1007/s00296-020-04775-2
Harbord, R. M., and Higgins, J. P. T. (2008). Meta-regression in Stata. Stata J. 8 (4), 493–519. doi:10.1177/1536867x0800800403
Haikarainen, T., Raivola, J., and Silvennoinen, O. (2019). Selective JAKinibs: Prospects in inflammatory and autoimmune diseases. BioDrugs 33 (1), 15–32. doi:10.1007/s40259-019-00333-w
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Husni, M. E. (2015). Comorbidities in psoriatic arthritis. Rheum. Dis. Clin. North Am. 41 (4), 677–698. doi:10.1016/j.rdc.2015.07.008
Husni, M. E., Kavanaugh, A., Murphy, F., Rekalov, D., Harrison, D. D., Kim, L., et al. (2020). Efficacy and safety of intravenous golimumab through one year in patients with active psoriatic arthritis. Arthritis Care Res. 72 (6), 806–813. doi:10.1002/acr.23905
Kavanaugh, A., Gladman, D. D., Edwards, C. J., Schett, G., Guerette, B., Delev, N., et al. (2019). Long-term experience with apremilast in patients with psoriatic arthritis: 5-year results from a PALACE 1-3 pooled analysis. Arthritis Res. Ther. 21 (1), 118. doi:10.1186/s13075-019-1901-3
Kavanaugh, A., Husni, M. E., Harrison, D. D., Kim, L., Lo, K. H., Leu, J. H., et al. (2017). Safety and efficacy of intravenous golimumab in patients with active psoriatic arthritis: Results through week twenty-four of the GO-VIBRANT study. Arthritis Rheumatol. 69 (11), 2151–2161. doi:10.1002/art.40226
Kavanaugh, A., Krueger, G. G., Beutler, A., Guzzo, C., Zhou, B., Dooley, L. T., et al. (2007). Infliximab maintains a high degree of clinical response in patients with active psoriatic arthritis through 1 year of treatment: Results from the IMPACT 2 trial. Ann. Rheum. Dis. 66 (4), 498–505. doi:10.1136/ard.2006.058339
Kavanaugh, A., McInnes, I., Mease, P., Krueger, G. G., Gladman, D., Gomez-Reino, J., et al. (2009). Golimumab, a new human tumor necrosis factor alpha antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: Twenty-four-week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum. 60 (4), 976–986. doi:10.1002/art.24403
Kavanaugh, A., McInnes, I. B., Mease, P., Krueger, G. G., Gladman, D., van der Heijde, D., et al. (2014). Clinical efficacy, radiographic and safety findings through 5 years of subcutaneous golimumab treatment in patients with active psoriatic arthritis: Results from a long-term extension of a randomised, placebo-controlled trial (the GO-REVEAL study). Ann. Rheum. Dis. 73 (9), 1689–1694. doi:10.1136/annrheumdis-2013-204902
Kavanaugh, A., McInnes, I. B., Mease, P. J., Krueger, G. G., Gladman, D. D., van der Heijde, D., et al. (2013). Clinical efficacy, radiographic and safety findings through 2 years of golimumab treatment in patients with active psoriatic arthritis: Results from a long-term extension of the randomised, placebo-controlled GO-REVEAL study. Ann. Rheum. Dis. 72 (11), 1777–1785. doi:10.1136/annrheumdis-2012-202035
Kavanaugh, A., Mease, P. J., Gomez-Reino, J. J., Adebajo, A. O., Wollenhaupt, J., Gladman, D. D., et al. (2015). Longterm (52-week) results of a phase III randomized, controlled trial of apremilast in patients with psoriatic arthritis. J. Rheumatol. 42 (3), 479–488. doi:10.3899/jrheum.140647
Kavanaugh, A., Mease, P. J., Gomez-Reino, J. J., Adebajo, A. O., Wollenhaupt, J., Gladman, D. D., et al. (2014). Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann. Rheum. Dis. 73 (6), 1020–1026. doi:10.1136/annrheumdis-2013-205056
Kavanaugh, A., Mease, P. J., Reimold, A. M., Tahir, H., Rech, J., Hall, S., et al. (2017). Secukinumab for long-term treatment of psoriatic arthritis: A two-year followup from a phase III, randomized, double-blind placebo-controlled study. Arthritis Care Res. 69 (3), 347–355. doi:10.1002/acr.23111
Kavanaugh, A., Puig, L., Gottlieb, A. B., Ritchlin, C., Li, S., Wang, Y., et al. (2015). Maintenance of clinical efficacy and radiographic benefit through two years of ustekinumab therapy in patients with active psoriatic arthritis: Results from a randomized, placebo-controlled phase III trial. Arthritis Care Res. Hob. 67 (12), 1739–1749. doi:10.1002/acr.22645
Kavanaugh, A., van der Heijde, D., McInnes, I. B., Mease, P., Krueger, G. G., Gladman, D. D., et al. (2012). Golimumab in psoriatic arthritis: One-year clinical efficacy, radiographic, and safety results from a phase III, randomized, placebo-controlled trial. Arthritis Rheum. 64 (8), 2504–2517. doi:10.1002/art.34436
Kourbeti, I. S., Ziakas, P. D., and Mylonakis, E. (2014). Biologic therapies in rheumatoid arthritis and the risk of opportunistic infections: A meta-analysis. Clin. Infect. Dis. 58 (12), 1649–1657. doi:10.1093/cid/ciu185
Kristensen, L. E., Keiserman, M., Papp, K., McCasland, L., White, D., Lu, W., et al. (2022). Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 1 trial. Ann. Rheum. Dis. 81 (2), 225–231. doi:10.1136/annrheumdis-2021-221019
Li, X., Andersen, K. M., Chang, H. Y., Curtis, J. R., and Alexander, G. C. (2020). Comparative risk of serious infections among real-world users of biologics for psoriasis or psoriatic arthritis. Ann. Rheum. Dis. 79 (2), 285–291. doi:10.1136/annrheumdis-2019-216102
McInnes, I. B., Anderson, J. K., Magrey, M., Merola, J. F., Liu, Y., Kishimoto, M., et al. (2021). Trial of upadacitinib and adalimumab for psoriatic arthritis. N. Engl. J. Med. 384 (13), 1227–1239. doi:10.1056/NEJMoa2022516
McInnes, I. B., Kato, K., Magrey, M., Merola, J. F., Kishimoto, M., Pacheco-Tena, C., et al. (2021). Upadacitinib in patients with psoriatic arthritis and an inadequate response to non-biological therapy: 56-week data from the phase 3 SELECT-PsA 1 study. RMD Open 7 (3), e001838. doi:10.1136/rmdopen-2021-001838
McInnes, I. B., Kavanaugh, A., Gottlieb, A. B., Puig, L., Rahman, P., Ritchlin, C., et al. (2013). Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet 382 (9894), 780–789. doi:10.1016/S0140-6736(13)60594-2
McInnes, I. B., Mease, P. J., Kirkham, B., Kavanaugh, A., Ritchlin, C. T., Rahman, P., et al. (2015). Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 386 (9999), 1137–1146. doi:10.1016/S0140-6736(15)61134-5
McInnes, I. B., Rahman, P., Gottlieb, A. B., Hsia, E. C., Kollmeier, A. P., Chakravarty, S. D., et al. (2021). Efficacy and safety of guselkumab, an interleukin-23p19-specific monoclonal antibody, through one year in biologic-naive patients with psoriatic arthritis. Arthritis Rheumatol. 73 (4), 604–616. doi:10.1002/art.41553
McInnes, I. B., Rahman, P., Gottlieb, A. B., Hsia, E. C., Kollmeier, A. P., Xu, X. L., et al. (2022). Long-term efficacy and safety of guselkumab, a monoclonal antibody specific to the p19 subunit of interleukin-23, through two years: Results from a phase III, randomized, double-blind, placebo-controlled study conducted in biologic-naive patients with active psoriatic arthritis. Arthritis Rheumatol. 74 (3), 475–485. doi:10.1002/art.42010
McLornan, D. P., Khan, A. A., and Harrison, C. N. (2015). Immunological consequences of JAK inhibition: Friend or foe? Curr. Hematol. Malig. Rep. 10 (4), 370–379. doi:10.1007/s11899-015-0284-z
Mease, P., Coates, L. C., Helliwell, P. S., Stanislavchuk, M., Rychlewska-Hanczewska, A., Dudek, A., et al. (2018). Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active psoriatic arthritis (EQUATOR): Results from a randomised, placebo-controlled, phase 2 trial. Lancet 392 (10162), 2367–2377. doi:10.1016/S0140-6736(18)32483-8
Mease, P., Deodhar, A., Fleischmann, R., Wollenhaupt, J., Gladman, D., Leszczyński, P., et al. (2015). Effect of certolizumab pegol over 96 weeks in patients with psoriatic arthritis with and without prior antitumour necrosis factor exposure. RMD Open 1 (1), e000119. doi:10.1136/rmdopen-2015-000119
Mease, P., Genovese, M. C., Gladstein, G., Kivitz, A. J., Ritchlin, C., Tak, P. P., et al. (2011). Abatacept in the treatment of patients with psoriatic arthritis: Results of a six-month, multicenter, randomized, double-blind, placebo-controlled, phase II trial. Arthritis Rheum. 63 (4), 939–948. doi:10.1002/art.30176
Mease, P., Hall, S., FitzGerald, O., van der Heijde, D., Merola, J. F., Avila-Zapata, F., et al. (2017). Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N. Engl. J. Med. 377 (16), 1537–1550. doi:10.1056/NEJMoa1615975
Mease, P., van der Heijde, D., Landewé, R., Mpofu, S., Rahman, P., Tahir, H., et al. (2018). Secukinumab improves active psoriatic arthritis symptoms and inhibits radiographic progression: Primary results from the randomised, double-blind, phase III FUTURE 5 study. Ann. Rheum. Dis. 77 (6), 890–897. doi:10.1136/annrheumdis-2017-212687
Mease, P. J., Deodhar, A. A., van der Heijde, D., Behrens, F., Kivitz, A. J., Neal, J., et al. (2022). Efficacy and safety of selective TYK2 inhibitor, deucravacitinib, in a phase II trial in psoriatic arthritis. Ann. Rheum. Dis. 81, 815–822. doi:10.1136/annrheumdis-2021-221664
Mease, P. J., Fleischmann, R., Deodhar, A. A., Wollenhaupt, J., Khraishi, M., Kielar, D., et al. (2014). Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a phase 3 double-blind randomised placebo-controlled study (RAPID-PsA). Ann. Rheum. Dis. 73 (1), 48–55. doi:10.1136/annrheumdis-2013-203696
Mease, P. J., Genovese, M. C., Greenwald, M. W., Ritchlin, C. T., Beaulieu, A. D., Deodhar, A., et al. (2014). Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N. Engl. J. Med. 370 (24), 2295–2306. doi:10.1056/NEJMoa1315231
Mease, P. J., Genovese, M. C., Weinblatt, M. E., Peloso, P. M., Chen, K., Othman, A. A., et al. (2018). Phase II study of ABT-122, a tumor necrosis factor- and interleukin-17a-targeted dual variable domain immunoglobulin, in patients with psoriatic arthritis with an inadequate response to methotrexate. Arthritis Rheumatol. 70 (11), 1778–1789. doi:10.1002/art.40579
Mease, P. J., Gladman, D. D., Collier, D. H., Ritchlin, C. T., Helliwell, P. S., Liu, L., et al. (2019). Etanercept and methotrexate as monotherapy or in combination for psoriatic arthritis: Primary results from a randomized, controlled phase III trial. Arthritis Rheumatol. 71 (7), 1112–1124. doi:10.1002/art.40851
Mease, P. J., Gladman, D. D., Gomez-Reino, J. J., Hall, S., Kavanaugh, A., Lespessailles, E., et al. (2020). Long-term safety and tolerability of apremilast versus placebo in psoriatic arthritis: A pooled safety analysis of three phase III, randomized, controlled trials. ACR Open Rheumatol. 2 (8), 459–470. doi:10.1002/acr2.11156
Mease, P. J., Gladman, D. D., and Keystone, E. C. (2006). Alefacept in combination with methotrexate for the treatment of psoriatic arthritis: Results of a randomized, double-blind, placebo-controlled study. Arthritis Rheum. 54 (5), 1638–1645. doi:10.1002/art.21870
Mease, P. J., Gladman, D. D., Ritchlin, C. T., Ruderman, E. M., Steinfeld, S. D., Choy, E. H., et al. (2005). Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: Results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 52 (10), 3279–3289. doi:10.1002/art.21306
Mease, P. J., Goffe, B. S., Metz, J., VanderStoep, A., Finck, B., and Burge, D. J. (2000). Etanercept in the treatment of psoriatic arthritis and psoriasis: A randomised trial. Lancet 356 (9227), 385–390. doi:10.1016/S0140-6736(00)02530-7
Mease, P. J., Gottlieb, A. B., Berman, A., Drescher, E., Xing, J., Wong, R., et al. (2016). The efficacy and safety of clazakizumab, an anti-interleukin-6 monoclonal antibody, in a phase IIb study of adults with active psoriatic arthritis. Arthritis Rheumatol. 68 (9), 2163–2173. doi:10.1002/art.39700
Mease, P. J., Gottlieb, A. B., van der Heijde, D., FitzGerald, O., Johnsen, A., Nys, M., et al. (2017). Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis. Ann. Rheum. Dis. 76 (9), 1550–1558. doi:10.1136/annrheumdis-2016-210724
Mease, P. J., Helliwell, P. S., Hjuler, K. F., Raymond, K., and McInnes, I. (2021). Brodalumab in psoriatic arthritis: Results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann. Rheum. Dis. 80 (2), 185–193. doi:10.1136/annrheumdis-2019-216835
Mease, P. J., Kivitz, A. J., Burch, F. X., Siegel, E. L., Cohen, S. B., Ory, P., et al. (2004). Etanercept treatment of psoriatic arthritis: Safety, efficacy, and effect on disease progression. Arthritis Rheum. 50 (7), 2264–2272. doi:10.1002/art.20335
Mease, P. J., Landewé, R., Rahman, P., Tahir, H., Singhal, A., Boettcher, E., et al. (2021). Secukinumab provides sustained improvement in signs and symptoms and low radiographic progression in patients with psoriatic arthritis: 2-year (end-of-study) results from the FUTURE 5 study. RMD Open 7 (2), e001600. doi:10.1136/rmdopen-2021-001600
Mease, P. J., Lertratanakul, A., Anderson, J. K., Papp, K., Van den Bosch, F., Tsuji, S., et al. (2020). Upadacitinib for psoriatic arthritis refractory to biologics: SELECT-PsA 2. Ann. Rheum. Dis. 80 (3), 312–320. doi:10.1136/annrheumdis-2020-218870
Mease, P. J., Lertratanakul, A., Papp, K. A., van den Bosch, F. E., Tsuji, S., Dokoupilova, E., et al. (2021). Upadacitinib in patients with psoriatic arthritis and inadequate response to biologics: 56-Week data from the randomized controlled phase 3 SELECT-PsA 2 study. Rheumatol. Ther. 8 (2), 903–919. doi:10.1007/s40744-021-00305-z
Mease, P. J., McInnes, I. B., Kirkham, B., Kavanaugh, A., Rahman, P., van der Heijde, D., et al. (2015). Secukinumab inhibition of interleukin-17a in patients with psoriatic arthritis. N. Engl. J. Med. 373 (14), 1329–1339. doi:10.1056/NEJMoa1412679
Mease, P. J., Ory, P., Sharp, J. T., Ritchlin, C. T., Van den Bosch, F., Wellborne, F., et al. (2009). Adalimumab for long-term treatment of psoriatic arthritis: 2-year data from the adalimumab effectiveness in psoriatic arthritis trial (ADEPT). Ann. Rheum. Dis. 68 (5), 702–709. doi:10.1136/ard.2008.092767
Mease, P. J., Rahman, P., Gottlieb, A. B., Kollmeier, A. P., Hsia, E. C., Xu, X. L., et al. (2020). Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): A double-blind, randomised, placebo-controlled phase 3 trial. Lancet 395 (10230), 1126–1136. doi:10.1016/S0140-6736(20)30263-4
Mease, P. J., and Reich, K. (2009). Alefacept with methotrexate for treatment of psoriatic arthritis: Open-label extension of a randomized, double-blind, placebo-controlled study. J. Am. Acad. Dermatol. 60 (3), 402–411. doi:10.1016/j.jaad.2008.09.050
Mease, P. J., van der Heijde, D., Ritchlin, C. T., Okada, M., Cuchacovich, R. S., Shuler, C. L., et al. (2017). Ixekizumab, an interleukin-17a specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: Results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann. Rheum. Dis. 76 (1), 79–87. doi:10.1136/annrheumdis-2016-209709
Minozzi, S., Bonovas, S., Lytras, T., Pecoraro, V., González-Lorenzo, M., Bastiampillai, A. J., et al. (2016). Risk of infections using anti-TNF agents in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: A systematic review and meta-analysis. Expert Opin. Drug Saf. 15 (1), 11–34. doi:10.1080/14740338.2016.1240783
Murdaca, G., Gulli, R., Spanò, F., Lantieri, F., Burlando, M., Parodi, A., et al. (2014). TNF-Α gene polymorphisms: Association with disease susceptibility and response to anti-TNF-α treatment in psoriatic arthritis. J. Invest. Dermatol. 134 (10), 2503–2509. doi:10.1038/jid.2014.123
Murdaca, G., Negrini, S., Magnani, O., Penza, E., Pellecchio, M., and Puppo, F. (2017). Impact of pharmacogenomics upon the therapeutic response to etanercept in psoriasis and psoriatic arthritis. Expert Opin. Drug Saf. 16 (10), 1173–1179. doi:10.1080/14740338.2017.1361404
Nash, P., Kirkham, B., Okada, M., Rahman, P., Combe, B., Burmester, G. R., et al. (2017). Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: Results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet 389 (10086), 2317–2327. doi:10.1016/S0140-6736(17)31429-0
Nash, P., Mease, P. J., McInnes, I. B., Rahman, P., Ritchlin, C. T., Blanco, R., et al. (2018). Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: Results from a randomized, placebo-controlled trial (FUTURE 3). Arthritis Res. Ther. 20 (1), 47. doi:10.1186/s13075-018-1551-x
Nash, P., Ohson, K., Walsh, J., Delev, N., Nguyen, D., Teng, L., et al. (2018). Early and sustained efficacy with apremilast monotherapy in biological-naïve patients with psoriatic arthritis: A phase IIIB, randomised controlled trial (ACTIVE). Ann. Rheum. Dis. 77 (5), 690–698. doi:10.1136/annrheumdis-2017-211568
Nyaga, V. N., Arbyn, M., and Aerts, M. (2014). Metaprop: A Stata command to perform meta-analysis of binomial data. Arch. Public Health 72 (1), 39. doi:10.1186/2049-3258-72-39
Ogdie, A., and Coates, L. (2017). The changing face of clinical trials in psoriatic arthritis. Curr. Rheumatol. Rep. 19 (4), 21. doi:10.1007/s11926-017-0642-z
Ogdie, A., Coates, L. C., and Gladman, D. D. (2020). Treatment guidelines in psoriatic arthritis. Rheumatol. Oxf. 59 (1), i37–i46. doi:10.1093/rheumatology/kez383
Ogdie, A., Schwartzman, S., and Husni, M. E. (2015). Recognizing and managing comorbidities in psoriatic arthritis. Curr. Opin. Rheumatol. 27 (2), 118–126. doi:10.1097/BOR.0000000000000152
Orbai, A. M., Gratacós, J., Turkiewicz, A., Hall, S., Dokoupilova, E., Combe, B., et al. (2021). Efficacy and safety of ixekizumab in patients with psoriatic arthritis and inadequate response to TNF inhibitors: 3-Year follow-up (SPIRIT-P2). Rheumatol. Ther. 8 (1), 199–217. doi:10.1007/s40744-020-00261-0
Östör, A., Van den Bosch, F., Papp, K., Asnal, C., Blanco, R., Aelion, J., et al. (2022). Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 2 trial. Ann. Rheum. Dis. 81 (3), 351–358. doi:10.1136/annrheumdis-2021-221048
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Bmj 372, n71. doi:10.1136/bmj.n71
Papp, K. A., Caro, I., Leung, H. M., Garovoy, M., and Mease, P. J. (2007). Efalizumab for the treatment of psoriatic arthritis. J. Cutan. Med. Surg. 11 (2), 57–66. doi:10.2310/7750.2007.00006
Peters, J. L., Sutton, A. J., Jones, D. R., Abrams, K. R., and Rushton, L. (2006). Comparison of two methods to detect publication bias in meta-analysis. Jama 295 (6), 676–680. doi:10.1001/jama.295.6.676
Puel, A., Cypowyj, S., Bustamante, J., Wright, J. F., Liu, L., Lim, H. K., et al. (2011). Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science 332 (6025), 65–68. doi:10.1126/science.1200439
Ritchlin, C., Rahman, P., Kavanaugh, A., McInnes, I. B., Puig, L., Li, S., et al. (2014). Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann. Rheum. Dis. 73 (6), 990–999. doi:10.1136/annrheumdis-2013-204655
Ritchlin, C. T., Colbert, R. A., and Gladman, D. D. (2017). Psoriatic arthritis. N. Engl. J. Med. 376 (10), 957–970. doi:10.1056/NEJMra1505557
Ritchlin, C. T., Helliwell, P. S., Boehncke, W. H., Soriano, E. R., Hsia, E. C., Kollmeier, A. P., et al. (2021). Guselkumab, an inhibitor of the IL-23p19 subunit, provides sustained improvement in signs and symptoms of active psoriatic arthritis: 1 year results of a phase III randomised study of patients who were biologic-naïve or TNFα inhibitor-experienced. RMD Open 7 (1), e001457. doi:10.1136/rmdopen-2020-001457
Ritchlin, C. T., Kavanaugh, A., Merola, J. F., Schett, G., Scher, J. U., Warren, R. B., et al. (2020). Bimekizumab in patients with active psoriatic arthritis: Results from a 48-week, randomised, double-blind, placebo-controlled, dose-ranging phase 2b trial. Lancet 395 (10222), 427–440. doi:10.1016/S0140-6736(19)33161-7
Ritchlin, C. T., Stahle, M., Poulin, Y., Bagel, J., Chakravarty, S. D., Kafka, S., et al. (2019). Serious infections in patients with self-reported psoriatic arthritis from the Psoriasis Longitudinal Assessment and Registry (PSOLAR) treated with biologics. BMC Rheumatol. 3, 52. doi:10.1186/s41927-019-0094-3
Ruyssen-Witrand, A., Perry, R., Watkins, C., Braileanu, G., Kumar, G., Kiri, S., et al. (2020). Efficacy and safety of biologics in psoriatic arthritis: A systematic literature review and network meta-analysis. RMD Open 6 (1), e001117. doi:10.1136/rmdopen-2019-001117
Saunte, D. M., Mrowietz, U., Puig, L., and Zachariae, C. (2017). Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br. J. Dermatol. 177 (1), 47–62. doi:10.1111/bjd.15015
Schett, G., McInnes, I. B., and Neurath, M. F. (2021). Reframing immune-mediated inflammatory diseases through signature cytokine hubs. N. Engl. J. Med. 385 (7), 628–639. doi:10.1056/NEJMra1909094
Schett, G., Wollenhaupt, J., Papp, K., Joos, R., Rodrigues, J. F., Vessey, A. R., et al. (2012). Oral apremilast in the treatment of active psoriatic arthritis: Results of a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 64 (10), 3156–3167. doi:10.1002/art.34627
Siegel, S. A. R., and Winthrop, K. L. (2019). In the real world: Infections associated with biologic and small molecule therapies in psoriatic arthritis and psoriasis. Curr. Rheumatol. Rep. 21 (7), 36. doi:10.1007/s11926-019-0832-y
Singh, J. A., Cameron, C., Noorbaloochi, S., Cullis, T., Tucker, M., Christensen, R., et al. (2015). Risk of serious infection in biological treatment of patients with rheumatoid arthritis: A systematic review and meta-analysis. Lancet 386 (9990), 258–265. doi:10.1016/S0140-6736(14)61704-9
Singh, J. A., Guyatt, G., Ogdie, A., Gladman, D. D., Deal, C., Deodhar, A., et al. (2019). Special article: 2018 American College of rheumatology/national psoriasis foundation guideline for the treatment of psoriatic arthritis. Arthritis Care Res. 71 (1), 2–29. doi:10.1002/acr.23789
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: A revised tool for assessing risk of bias in randomised trials. Bmj 366, l4898. doi:10.1136/bmj.l4898
Subesinghe, S., Rutherford, A. I., Byng-Maddick, R., Leanne Hyrich, K., and Benjamin Galloway, J. (2018). Recurrent serious infections in patients with rheumatoid arthritis-results from the British Society for Rheumatology Biologics Register. Rheumatol. Oxf. 57 (4), 651–655. doi:10.1093/rheumatology/kex469
Sunzini, F., McInnes, I., and Siebert, S. (2020). JAK inhibitors and infections risk: Focus on herpes zoster. Ther. Adv. Musculoskelet. Dis. 12, 1759720X20936059. doi:10.1177/1759720X20936059
Van den Bosch, F., and Coates, L. (2018). Clinical management of psoriatic arthritis. Lancet 391 (10136), 2285–2294. doi:10.1016/S0140-6736(18)30949-8
Van Den Bosch, F., Kruithof, E., Baeten, D., Herssens, A., de Keyser, F., Mielants, H., et al. (2002). Randomized double-blind comparison of chimeric monoclonal antibody to tumor necrosis factor alpha (infliximab) versus placebo in active spondylarthropathy. Arthritis Rheum. 46 (3), 755–765. doi:10.1002/art.511
van der Heijde, D., Deodhar, A., FitzGerald, O., Fleischmann, R., Gladman, D., Gottlieb, A. B., et al. (2018). 4-year results from the RAPID-PsA phase 3 randomised placebo-controlled trial of certolizumab pegol in psoriatic arthritis. RMD Open 4 (1), e000582. doi:10.1136/rmdopen-2017-000582
van der Heijde, D., Mease, P. J., Landewé, R. B. M., Rahman, P., Tahir, H., Singhal, A., et al. (2020). Secukinumab provides sustained low rates of radiographic progression in psoriatic arthritis: 52-week results from a phase 3 study, FUTURE 5. Rheumatol. Oxf. 59 (6), 1325–1334. doi:10.1093/rheumatology/kez420
van Mens Ljj, , de Jong, H. M., Fluri, I., Nurmohamed, M. T., van de Sande, M. G. H., Kok, M., et al. (2019). Achieving remission in psoriatic arthritis by early initiation of TNF inhibition: A double-blind, randomised, placebo-controlled trial of golimumab plus methotrexate versus placebo plus methotrexate. Ann. Rheum. Dis. 78 (5), 610–616. doi:10.1136/annrheumdis-2018-214746
Veale, D. J., and Fearon, U. (2018). The pathogenesis of psoriatic arthritis. Lancet 391 (10136), 2273–2284. doi:10.1016/S0140-6736(18)30830-4
Vieira-Sousa, E., Alves, P., Rodrigues, A. M., Teixeira, F., Tavares-Costa, J., Bernardo, A., et al. (2020). GO-DACT: A phase 3b randomised, double-blind, placebo-controlled trial of GOlimumab plus methotrexate (MTX) versus placebo plus MTX in improving DACTylitis in MTX-naive patients with psoriatic arthritis. Ann. Rheum. Dis. 79 (4), 490–498. doi:10.1136/annrheumdis-2019-216500
Wells, A. F., Edwards, C. J., Kivitz, A. J., Bird, P., Guerette, B., Delev, N., et al. (2022). Apremilast monotherapy for long-term treatment of active psoriatic arthritis in DMARD-naïve patients. Rheumatol. Oxf. 61 (3), 1035–1043. doi:10.1093/rheumatology/keab449
Wells, A. F., Edwards, C. J., Kivitz, A. J., Bird, P., Nguyen, D., Paris, M., et al. (2018). Apremilast monotherapy in DMARD-naive psoriatic arthritis patients: Results of the randomized, placebo-controlled PALACE 4 trial. Rheumatol. Oxf. 57 (7), 1253–1263. doi:10.1093/rheumatology/key032
Winthrop, K. L., Curtis, J. R., Lindsey, S., Tanaka, Y., Yamaoka, K., Valdez, H., et al. (2017). Herpes zoster and tofacitinib: Clinical outcomes and the risk of concomitant therapy. Arthritis Rheumatol. 69 (10), 1960–1968. doi:10.1002/art.40189
Winthrop, K. L., Novosad, S. A., Baddley, J. W., Calabrese, L., Chiller, T., Polgreen, P., et al. (2015). Opportunistic infections and biologic therapies in immune-mediated inflammatory diseases: Consensus recommendations for infection reporting during clinical trials and postmarketing surveillance. Ann. Rheum. Dis. 74 (12), 2107–2116. doi:10.1136/annrheumdis-2015-207841
Yiu, Z. Z. N., Exton, L. S., Jabbar-Lopez, Z., Mohd Mustapa, M. F., Samarasekera, E. J., Burden, A. D., et al. (2016). Risk of serious infections in patients with psoriasis on biologic therapies: A systematic review and meta-analysis. J. Invest. Dermatol. 136 (8), 1584–1591. doi:10.1016/j.jid.2016.03.035
Keywords: psoriatic arthritis, opportunistic infections, BDMARDs, tsDMARDs, JAK inhibitors, herpes zoster, Candida spp
Citation: Vassilopoulos A, Shehadeh F, Benitez G, Kalligeros M, Cunha JS, Cunha CB and Mylonakis E (2022) The incidence of opportunistic infections in patients with psoriatic arthritis treated with biologic and targeted synthetic agents: A systematic review and meta-analysis. Front. Pharmacol. 13:992713. doi: 10.3389/fphar.2022.992713
Received: 12 July 2022; Accepted: 20 September 2022;
Published: 05 October 2022.
Edited by:
Min Yang, Anhui Medical University, ChinaReviewed by:
Carlos Alves, University of Coimbra, PortugalCopyright © 2022 Vassilopoulos, Shehadeh, Benitez, Kalligeros, Cunha, Cunha and Mylonakis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eleftherios Mylonakis, ZW15bG9uYWtpc0BsaWZlc3Bhbi5vcmc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.