
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol., 30 September 2022
Sec. Drugs Outcomes Research and Policies
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.992376
 Yaowang Lin1†
Yaowang Lin1† Zhigang Cai2†
Zhigang Cai2† Shaohong Dong1
Shaohong Dong1 Huadong Liu1
Huadong Liu1 Xinli Pang1
Xinli Pang1 Qiuling Chen3*
Qiuling Chen3* Jie Yuan1*
Jie Yuan1* Qingshan Geng1,4*
Qingshan Geng1,4*Aimed to evaluate and compare the interactive effects of different antiplatelet or anticoagulation strategies in patients with chronic coronary syndromes (CCS) after percutaneous coronary intervention (PCI). Randomized controlled trials comparing different antiplatelet or anticoagulant strategies in patients with CCS after PCI were included. The primary outcomes were major adverse cardiovascular event (MACE), mortality, ischemic and bleeding events. Compared to aspirin alone, addition of prasugrel or ticagrelor to aspirin resulted in lower risk of myocardial infarction (MI) [odds ratio (OR): 0.38 (95% confidence interval 0.38–0.62); 0.810–0.84 (0.69–0.98)] and any stroke [0.56 (0.42–0.75)] at the expense of increased risk of major bleeding [1.79 (1.34–2.39); 2.08–2.38 (1.56–3.28)], whereas, clopidogrel monotherapy reduced the risk of any stroke, major bleeding, and intracranial bleeding. On subgroup analysis, compared with aspirin alone, addition of prasugrel resulted in lower MACE [0.72 (0.60–0.86)], MI [0.48 (0.38–0.62)], and stent thrombosis [0.29 (0.09–0.91)], whereas, addition of rivaroxaban 2.5 mg resulted in lower risk of MACE [0.72 (0.60–0.87)], cardiac death [0.71 (0.52–0.98)] and any stroke [0.65 (0.45–0.95)], but not reduced MI. Both prasugrel and rivaroxaban 2.5 mg increased major bleeding [1.79 (1.34–2.39); 1.72 (1.33–2.22)]. Clopidogrel monotherapy was associated with lower MACE [0.72 (0.58–0.90)], any stroke [0.42 (0.24–0.73)], and major bleeding [0.62 (0.40–0.96)]. Adding prasugrel or ticagrelor led to a reduced incidence of MI and prasugrel was also found to reduce the risk of MACE and stent thrombosis in CCS patients with low risk of bleeding after PCI. Clopidogrel monotherapy has advantage in reducing MACE, stroke, and major bleeding events in CCS patients at high risk of bleeding after PCI.
Systematic Review Registration: https://clinicaltrials.gov/, PROSPERO Identifier: CRD 42021291050.
Coronary artery disease (CAD) is a pathological process characterized by the formation of atherosclerotic plaques followed by their rupture, ulceration, or erosion (Libby and Theroux, 2005; Asada et al., 2020). Plaque rupture activates platelet aggregation and the coagulation cascade, which leads to acute coronary thrombosis, resulting in acute coronary syndrome (ACS) (Frangogiannis, 2015). Accordingly, antiplatelet and anticoagulant therapy has been recommended as a cornerstone treatment for CAD (Valgimigli et al., 2017).
Maintenance therapy with a single antiplatelet agent is the standard approach for secondary prevention of atherosclerotic cardiovascular events in patients with chronic coronary syndromes (CCS) (Knuuti et al., 2019). Aspirin, a cyclooxygenase pathway inhibitor, which reduces the formation of thromboxane A2 and inhibits platelet aggregation, is predominantly recommended as the standard-of-care monotherapy in patients with CCS (Godley and Hernandez-Vila, 2016). In 2017, the European Society of Cardiology recommended a combination of aspirin and ticagrelor (60 mg twice a day) for CCS patients with risk of ischemia (Ibanez et al., 2017). Similarly, the 2020 European Society of Cardiology update recommended the addition of a second antithrombotic agent (clopidogrel, prasugrel, or low-dose rivaroxaban) to aspirin for extended long-term secondary prevention in patients at high risk of ischemia and low risk of bleeding (Collet et al., 2020).
However, there is no clear consensus on the optimal post-percutaneous coronary intervention (PCI) antithrombotic strategy for CCS patients with respect to either replacement of aspirin with other antiplatelet agents or addition of a P2Y12 inhibitor or a low-dose anticoagulant to aspirin. Therefore, we conducted a network meta-analysis to compare the antithrombotic drugs with aspirin and assess their interactive effect on major adverse cardiovascular events (MACE), mortality, and ischemic and bleeding events in CCS after PCI.
The present study was performed following the Cochrane Collaboration guidelines. Relevant articles published before 30 March 2022 were searched in online biomedical databases (PubMed and Clinical Trials. gov) and Cochrane Central Register (Supplementary Data Sheet S1; Supplementary Tables S1–S3). The keywords included “antiplatelet therapy,” “anticoagulant therapy,” “chronic coronary syndromes,” “stable coronary artery disease (SCAD),” and “randomized control trials (RCTs).” After elimination of duplicates using the EndNote software, two investigators (YL And QC) independently screened the titles and abstracts of the remaining articles using pre-defined criteria.
The inclusion criteria for studies were: 1) study design: randomized controlled trial; 2) study population: patients diagnosed with CCS after PCI; 3) intervention group received oral antiplatelet therapy and/or anticoagulant therapy; patients in the control group received aspirin or placebo in addition to aspirin; 4) outcomes: MACEs, mortality, ischemic events, and bleeding events; 5) language of publication: English.
The primary outcome was MACE. The secondary outcomes included myocardial infarction (MI), all-cause death, cardiac death, any stroke, major bleeding, fatal bleeding, intracranial bleeding, stent thrombosis, and any revascularization in patients with CCS after PCI.
After independent screening of the titles and abstracts of relevant papers by two authors (YL and QC), the final decision on the inclusion of a study was made by consensus. Next, data were extracted from the full-text articles using standardized tables (including study design, interventions, endpoints, and follow-up data) and then checked independently. Any disagreements between the two authors were resolved by consensus or by consulting a third author (JY).
The risk of bias in the included studies was assessed using the Cochrane Collaboration tool; publication bias was assessed by visual inspection of Begg’s funnel plot; and the indirectness, imprecision, heterogeneity, and inconsistency of the included RCTs were assessed using network meta-analysis (CINeMA) framework (Papakonstantinou et al., 2020).
STATA software, version 14.0 (Stata Corp, United States) was used for statistical analyses. Combined odds ratios (ORs) with 95% confidence intervals (CI) were calculated for the primary and secondary outcomes. Rankogram plotting was performed on the surface under the cumulative ranking (SUCRA) curve to provide a hierarchy of different treatments. Heterogeneity among the studies was assessed using the I2 statistic. In case of significant heterogeneity (I2 > 50%), subgroup analysis was performed to investigate heterogenity. Subgroup analyses were planned on the basis of factors identified a priori as potential sources of heterogeneity. p-values < 0.05 were considered indicative of statistical significance.
Out of the 8,298 articles retrieved on database search, 2,168 duplicate publications and 6,109 articles that did not qualify the inclusion criteria were excluded. In addition, 12 articles were excluded as these were not RCTs, duplicate trials, or no relevant endpoint data were reported. Eventually, nine RCTs (Figure 1) were included in this meta-analysis (Park et al., 2010; Collet et al., 2014; Lee et al., 2014; Mauri et al., 2014; Bonaca et al., 2015; Helft et al., 2016; Connolly et al., 2018; Steg et al., 2019; Koo et al., 2021). Among of these studies, aspirin and clopidogrel were used in five studies (Park et al., 2010; Collet et al., 2014; Lee et al., 2014; Mauri et al., 2014; Helft et al., 2016), aspirin and ticagrelor 90 mg b.i.d. in two studies (Bonaca et al., 2015; Steg et al., 2019), aspirin and rivaroxaban 2.5 mg b.i.d or 5 mg qd were used in one study (Connolly et al., 2018), and single clopidogrel was used in one study (Koo et al., 2021). The major bleeding was defined as TIMI in seven studies (Collet et al., 2014; Lee et al., 2014; Mauri et al., 2014; Helft et al., 2016; Connolly et al., 2018; Steg et al., 2019) and BARC type ≥ 3 in two studiers (Bonaca et al., 2015; Koo et al., 2021). A total of 91,115 patients were randomized to drug intervention group [n = 54,035, aspirin + clopidogrel (n = 5,218), aspirin + clopidogrel (n = 3,263)/prasugrel (n = 1,757), aspirin + ticagrelor 90 mg b.i.d (n = 7,050), aspirin + ticagrelor 60 mg b.i.d (n = 7,045), aspirin + ticagrelor 90 mg or 60 mg b.i.d (n = 9,619), aspirin + rivaroxaban 2.5 mg b.i.d (n = 8,313), rivaroxaban 5 mg (n = 8,250) and clopidogrel monotherapy (n = 2,710)] versus aspirin or aspirin plus placebo group (n = 37,080). The duration of follow-up ranged from 18 to 44 months. All studies were parallel RCTs, among which, four involved double-blinding, while five were open-label studies (Table 1).
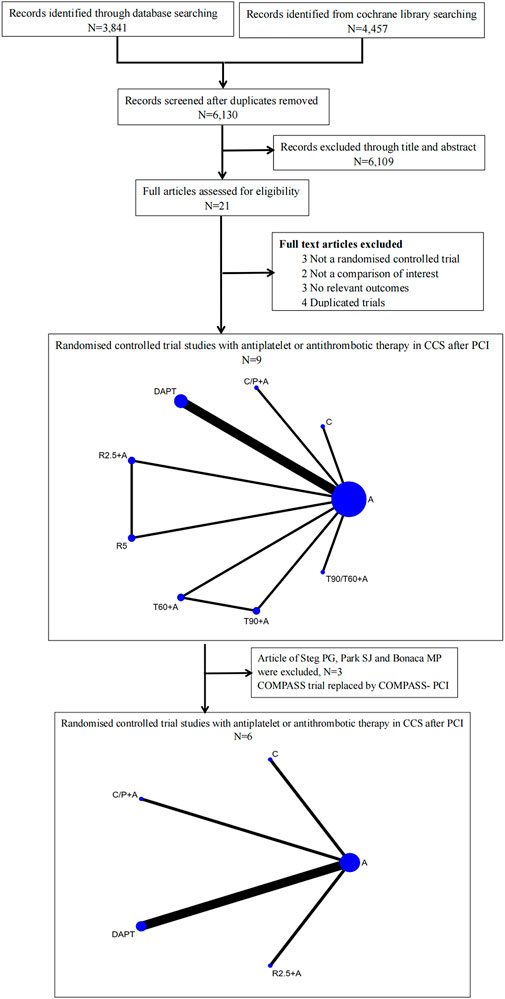
FIGURE 1. Flow diagram illustrating the study selection process and drug strategies in the Network. A, aspirin; DAPT, aspirin + clopidogrel; C/P + A, clopidogrel/prasugrel plus aspirin; T90 + A, ticagrelor 90 mg twice a day plus aspirin; T60, ticagrelor 60 mg twice a day plus aspirin; T90/60 + A, ticagrelor 90 mg/60 mg twice a day plus aspirin; R2.5 + A, rivaroxaban 2.5 mg twice a day plus aspirin; R5, rivaroxaban 5 mg twice a day; C, clopidogrel.
Quality assessment of the included studies was performed using the Cochrane Collaboration tool in Review Manager 5.3. Each entry had a high risk of selection bias, detection bias, and reporting bias, respectively. Six studies had a high risk of performance bias. The remaining studies had a low risk of bias (Supplementary Data Sheet S2; Supplementary Figure S4). The funnel plot was asymmetrical indicated publication bias with different treatment effects in smaller studies and heterogeneity (I2 = 71.9%; Supplementary Data Sheet S2; Supplementary Figure S5A). Subgroup analyses were conducted to explore by prespecified subgroup analysis (I2 = 0%; Supplementary Data Sheet S2; Supplementary Figure S5B).
No significant difference was observed between all antithrombotic treatment strategies with respect to primary endpoint of MACE. Compared to aspirin alone, adding prasugrel (not clopidogrel monotherapy) or ticagrelor resulted in lower MI [(0.38 (0.38–0.62), p = 0.019, number needed to treat (NNT) = 49; 0.810–0.84 (0.69–0.98), p = 0.033 to 0.040, NNT = 114 to 137] at the expense of increased major bleeding [(1.79 (1.34–2.39), p = 0.027, NNT = 84; 2.08–2.38 (1.56–3.28), p = 0.020 to 0.035; NNT = 95 to 114, Figure 2]. On indirect comparison, adding prasugrel was superior to ticagrelor, low-dose rivaroxaban, and clopidogrel monotherapy; therefore, prasugrel (at a maintenance dose of 10 mg daily in patients weighing >60 kg and a dose of 5 mg daily in patients weighing <60 kg) may be the optimal additional antithrombotic agent in reducing MI (Figure 3) in patients with CCS after PCI. From SUCRA rankogram plots, adding prasugrel was the best treatment strategy for both reducing MACE and MI in CCS after PCI (Supplementary Data Sheet S2; Supplementary Figure S1).
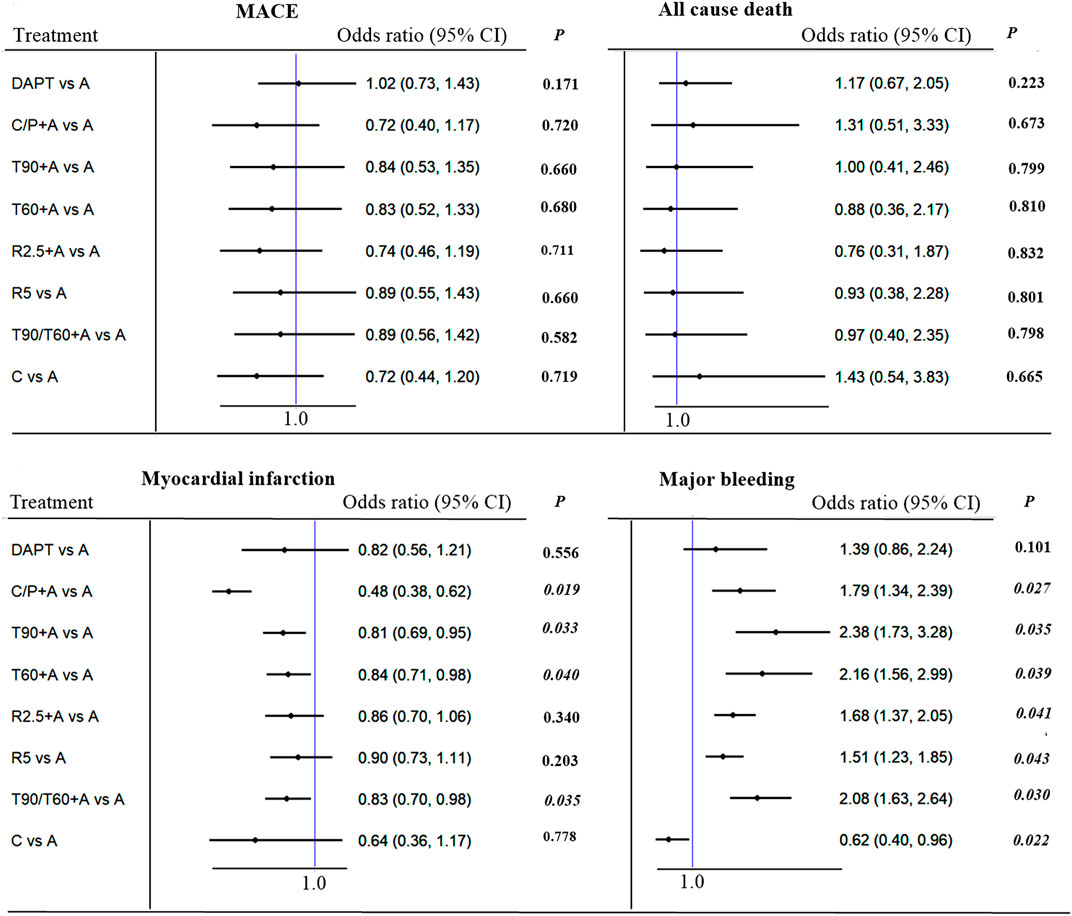
FIGURE 2. MACE (primary outcome), myocardial infarction, all cause death and major bleeding in patients with CCS after PCI: Forest plot (estimates as hazard ratio). Direct comparison from studies.
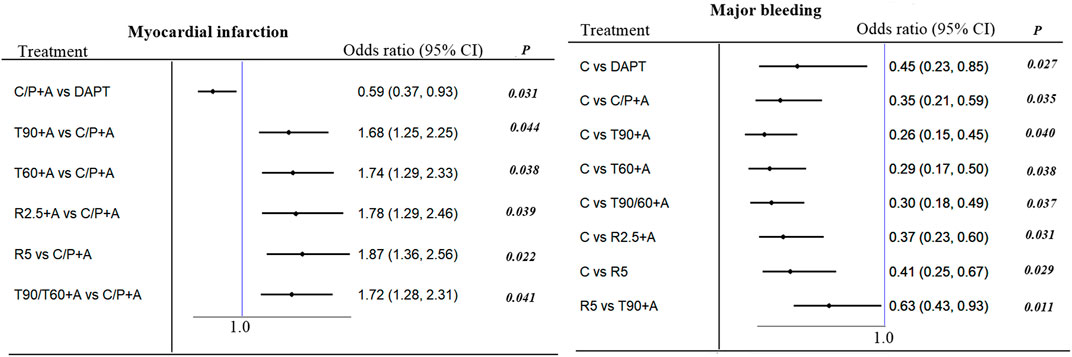
FIGURE 3. MACE, myocardial infarction, all cause death and major bleeding in patients with CCS after PCI: Forest plot (estimates as hazard ratio). Show significant difference between-group from indirect comparison of network.
Clopidogrel monotherapy was predominately associated with a lower risk of any stroke [0.42 (0.24–0.73), p = 0.021, NNT = 109], major bleeding [0.62 (0.40–0.96), p = 0.040, NNT = 139], and intracranial bleeding [0.24 (0.08–0.70), p = 0.029, NNT = 213], whereas, rivaroxaban 2.5 mg plus aspirin reduced the incidence of any stroke [0.56 (0.42–0.75), p = 0.028, NNT = 208]. All the additional antithrombotic agents increased major bleeding except clopidogrel monotherapy in comparison with aspirin. No other significant differences were observed between the various antithrombotic treatment strategies with respect to mortality or fatal bleeding (Figure 2; Supplementary Data Sheet S2; Supplementary Figure S2).
The study by Park SJ (Collet et al., 2014) was excluded because it combined the results of REAL-LATE and ZEST-LATE trials. The two studies by Bonaca MP (Bonaca et al., 2015) and Steg PG (Steg et al., 2019) were excluded as these included patients without PCI. Additionally, the COMPASS trial was replaced by COMPASS-PCI study with all included patients receiving PCI treatment (Bainey et al., 2020). Finally, 6 studies were included in this subgroup analysis with no significant heterogeneity (I2 = 0%). In comparison with aspirin alone, addition of prasugrel to aspirin resulted in a lower risk of MACE [0.72 (0.60–0.86), p = 0.035, NNT = 64], MI [0.48 (0.38–0.62), p = 0.024, NNT = 49], and stent thrombosis [0.29 (0.09–0.91), p = 0.041, NNT = 106] at the expense of major bleeding [1.79 (1.34–2.39), p = 0.027, NNT = 84]. Similarly, addition of rivaroxaban 2.5 mg (twice daily) to aspirin was associated with a lower risk of MACE [0.72 (0.60–0.87), p = 0.037, NNT = 69], cardiac death [0.71 (0.52–0.98), p = 0.043, NNT = 189], and any stroke [0.65 (0.45–0.95), p = 0.040, NNT = 133], and higher risk of major bleeding [1.72 (1.33–2.22), p = 0.029, NNT = 71]. Clopidogrel monotherapy was associated with a lower risk of MACE [0.72 (0.58–090), p = 0.039, NNT = 51], any stroke [0.42 (0.24–0.73), p = 0.019, NNT = 109], and major bleeding [0.62 (0.40–0.96), p = 0.043, NNT = 139] in comparison with aspirin, but with no significant difference with respect to risk of MI. No other differences were found with respect to all-cause death, fatal bleeding, or any revascularization events (Figure 4; Supplementary Data Sheet S2; Supplementary Figure S3).
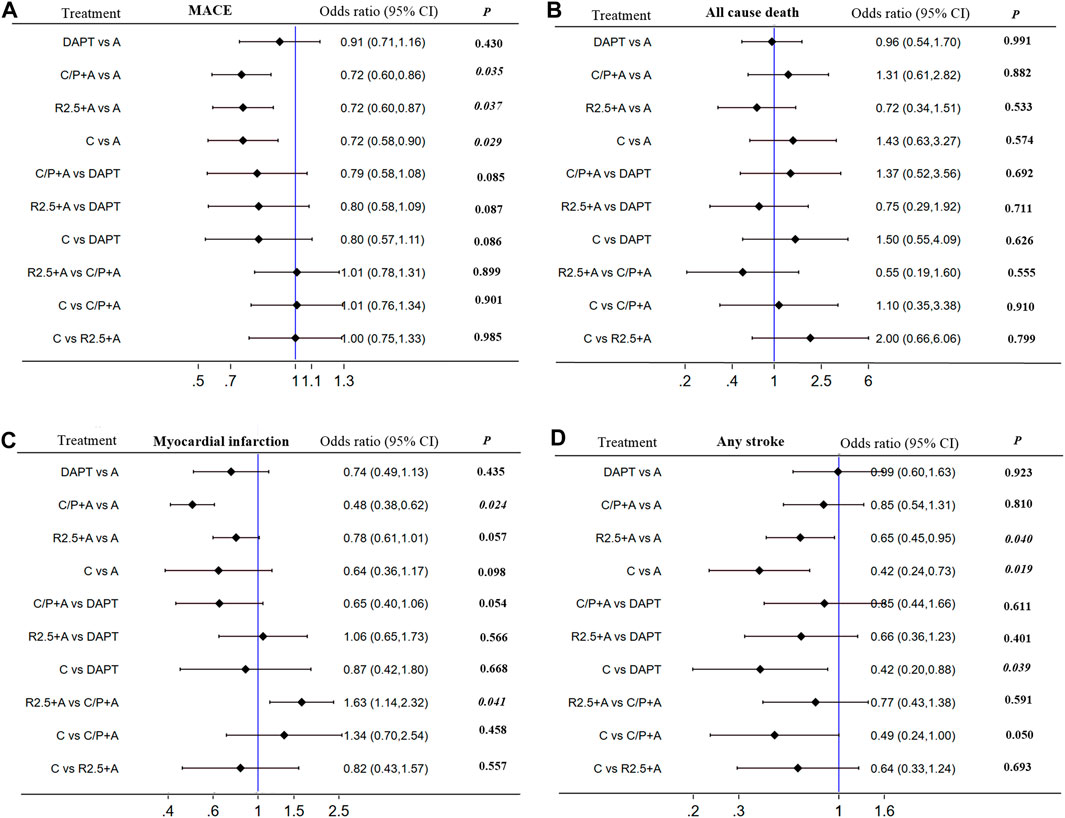
FIGURE 4. Subgroup analyses (I2 = 0%) including direct and indirect comparison. MACE, all cause death, myocardial infarction and any stroke in patientswith CCS after PCI: Forest plot (estimates as hazard ratio)—All trials.
Extended aspirin with clopidogrel after 12 months showed no significant reduction in MACE, mortality, or ischemic events, but led to an increased risk of major bleeding in comparison with aspirin monotherapy in patients with CCS after PCI.
The risk of bias contributions of the included studies are shown in Supplementary Data Sheet S2; Supplementary Figure S6. The heterogeneity, incoherence, and report result of the mixed evidence of included studies were low, while those of the indirect evidence of the included studies were low to moderate by the CINeMA framework study (Supplementary Figures S3–S5).
The main findings of the present network meta-analysis were as follows: 1) adding a P2Y12 inhibitor, either prasugrel (the optimal choice) or ticagrelor was associated with a lower risk of MI; in addition, adding prasugrel was found to reduce the risk of MACE and stent thrombosis at the expense of major bleeding. 2) Clopidogrel monotherapy was found to be superior to aspirin with respect to reducing any stroke, readmission due to acute coronary syndrome (ACS) (no MI), major bleeding, and intracranial bleeding. 3) Extended-term DAPT (aspirin + clopidogrel) was not found to be superior to aspirin monotherapy in CCS after PCI. 4) Addition of low-dose anticoagulant to aspirin reduced the risk of cardiac death and any stroke, but increased the risk of major bleeding and intracranial bleeding.
CCS is defined as a group of clinical syndromes in different evolutionary stages of CAD, excluding situations with ACS (Knuuti et al., 2019). The goal of CCS therapy is to reduce cardiovascular events including MI and mortality, with a focus on reducing acute thrombotic events and the development of ventricular dysfunction. Lifelong antiplatelet therapy with aspirin has been considered as essential for the secondary prevention of MI and cardiovascular disease (CVD) in CCS patients (Knuuti et al., 2019). However, recent trials in the primary prevention setting have shown inconsistent benefits of aspirin in terms of reducing CVD events; in addition, aspirin may be associated with an increased risk of bleeding (McNeil et al., 2018). Therefore, there is no clear consensus on the optimal antithrombotic treatment strategy, including replacement of aspirin with other antiplatelet agents or addition of a P2Y12 inhibitor or a low-dose anticoagulant to aspirin for patients with CCS.
Our network meta-analysis results showed that adding a P2Y12 inhibitor, either prasugrel or ticagrelor, reduced the risk of MI, and adding prasugrel resulted in lower MACE and MI in CCS after PCI. Only the Dual Antiplatelet Therapy (DAPT) study, which included about 35% of patients receiving prasugrel (remaining 65% of patients received clopidogrel), showed a reduced risk of MACE, MI, and stent thrombosis compared with aspirin in CCS patients after PCI (Mauri et al., 2014). However, administration of aspirin in combination with clopidogrel in REAL-LATE and ZEST-LATE trial (Park et al., 2010), ARCTIC-Interruption trial (Collet et al., 2014), DES LATE trial (Lee et al., 2014), OPTIDUAL trial (Helft et al., 2016), and the mono-clopidogrel therapy of HOST-EXAM trial (Koo et al., 2021) showed no significant reduction in MI in comparison with aspirin monotherapy. Adding a P2Y12 inhibitor prasugrel may be the optimal antithrombotic strategy for patients with CCS after PCI. Compared with clopidogrel, prasugrel was shown to have greater antiplatelet efficacy in preventing thrombotic events and was unaffected by drug interactions or CYP2C19 loss-of-function (LOF) variants. Prasugrel was more effective than clopidogrel in reducing rates of ischemic events in patients with ACS after PCI (Wiviott et al., 2007), but not in medically managed patients with ACS (Roe et al., 2012), and was associated with more major bleeding events. In the HOST-REDUCEPOLYTECH-ACS study, Asian ACS patients receiving DAPT with a prasugrel de-escalation strategy (10–5 mg daily) from 1 month after PCI showed a reduced risk of (Kim et al., 2020) composite adverse clinical events [0.70 (0.52–0.92)] up to 1 year, mainly driven by a reduction in bleeding [0.48 (0.32–0.73)] without an increase in ischemia [0.76 (0.40–1.45)]. In Japanese patients with CCS after PCI, low-dose prasugrel (3.75 mg daily) achieved more consistent antiplatelet effects (P2Y12 reaction unit: 133.0 vs. 156.8, p = 0.005 on day 5; 124.3 vs. 158.0, p < 0.001 on day 30) than clopidogrel irrespective of the metabolic phenotype (Akimaru et al., 2022). Prasugrel is the most potent antiplatelet agent that can inhibit acute thrombosis in the coronary arteries; however, the concomitant risk of bleeding should also be considered. Low-dose maintenance of prasugrel may be the optimal antithrombotic strategy for CCS patients after PCI.
Ticagrelor has the predictable and consistently high level of antiplatelet effect. Compared with clopidogrel, the PLATO study showed that ticagrelor had a greater reduction in ischemic events in aspirin-treated ACS patients, but at the cost of increased risk of non-fatal bleeding (Wallentin et al., 2014). In patients with a history of MI in the preceding 1–3 years, the PEGASUS-TIMI 54 study showed that aspirin combined with ticagrelor (either 90 or 60 mg twice daily) equivalently reduced the 3-year incidence of MI, stroke, or cardiovascular death at the expense of increasing non-fatal bleeding (Bonaca et al., 2015). Similarly, the THEMIS study included CCS patients with diabetes without a history of MI or stroke; in these patients, ticagrelor (two ticagrelor doses) plus aspirin was associated with a lower incidence of MI and stroke, but a higher incidence of major bleeding compared to those who received placebo plus aspirin (Steg et al., 2019). In a recent real-world study, ticagrelor was associated with lower incidence of major adverse cardiovascular and cerebrovascular events without an increase in bleeding events in CCS after PCI in comparison with clopidogrel (Li et al., 2021). From the indirect comparison in our study, prasugrel was found superior to ticagrelor in terms of reducing MI. Ticagrelor may cause dyspnea, which occasionally necessitated switch to a thienopyridine (Storey et al., 2010) and the incidence of MI in the dyspnea group was higher than that in the no dyspnea group (112 (8.7) vs. 393 (5.4), p = 0.008) (Storey et al., 2011). Additionally, ticagrelor is metabolized via CYP3A, and therefore, should not be used with strong CYP3A inhibitors or inducers during maintenance therapy.
In the HOST-EXAM study, clopidogrel monotherapy was associated with a reduced risk of future composite of adverse clinical events (mainly stroke, readmission due to ACS and major bleeding events), but no MI during the 24-month follow-up in patients with CCS after PCI (Koo et al., 2021). We must recognize that the primary endpoint of MACE included major bleeding events, and therefore, may have confounded the statistical difference in MACE. The CAPRIE trial showed that clopidogrel is more effective than aspirin in reducing the combined risk of ischemic stroke, MI, or vascular death in patients with ACS (CAPRIE Steering Committee, 1996). In the past, clopidogrel was considerably more expensive than aspirin. Now with the expiration of patent protection, clopidogrel is considered more cost-effective, especially with the lower risk of bleeding events during long-term treatment for CCS compared to aspirin (Jones et al., 2004). From our network study, only clopidogrel monotherapy was found to reduce bleeding events among all antithrombotic drugs. Avoiding the risk of bleeding associated with antiplatelet de-escalation therapy in CCS after PCI may be more important than preventing stent thrombosis; this is particularly important in the Asian population which has a higher risk of bleeding compared to patients from Western countries. Clopidogrel is limited by poor metabolism of the hepatic cytochrome P450 enzyme CYP2C19 and the LOF variant of the CYP2C19 gene, resulting in a lack of efficacy in some patients. Therefore, carriers of the CYP2C19 LOF allele receiving clopidogrel are at a higher risk of ischemic events compared with non-carriers (Mega et al., 2010). Finally, clopidogrel monotherapy may not be given in ACS patients from the study of STOPDAPT-2 ACS.
In comparison with aspirin alone, dual pathway inhibition with a combination of low-dose rivaroxaban (2.5 mg twice daily) and aspirin reduced the risk of stroke or cardiovascular death, but not MI; this may have been attributable to the increased risk of bleeding events in CCS, regardless of the timing (at least 1 year beyond) of prior PCI or MI in the COPASS-PCI study (Bainey et al., 2020). Similar result was not observed in ACS patients or those with prior PCI less than 1 year ago (Mega et al., 2012). Larger studies are required to substantiate this finding. In addition, the safety of performing PCI without aspirin pre-treatment is unknown.
There are several potential limitations of this meta-analysis. First, the definition of MACE and major bleeding varied among the included studies. The MACEs in the OPTIDUAL and HOST-EXAM studies included major bleeding endpoints. Second, none of the included studies had independently compared prasugrel with aspirin in CCS patients. Large RCTs are required for a more definitive assessment of the efficacy by the results of network meta-analysis. Third, the time of follow-up was different between studies. Finally, no large RCTs comparing ticagrelor (90 mg or 60 mg b.i.d) or prasugrel monotherapy with aspirin have been conducted in CCS patients after PCI.
In CCS patients after PCI, adding prasugrel or ticagrelor led to a reduced incidence of MI and prasugrel was also found to reduce the risk of MACE and stent thrombosis at the expense of major bleeding. Dual pathway inhibition with a combination of low-dose rivaroxaban and aspirin reduced the risk of stroke or cardiovascular death at the cost of increased risk of bleeding. Clopidogrel monotherapy has the advantage of reducing MACE, stroke, and major bleeding events in CCS patients at high risk of bleeding after PCI. Indeed, a clinician-patient shared decision making seems apt when discussing the optimal duration of DAPT combined with the patient’s risk factors of current ischemia and bleeding.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
YL and ZC collected, analyzed and wrote this manuscript. JY, XP, and HL assisted in the conduct of study. QC, SD, and QG were the principal investigator.
Supported by Shenzhen Foundation (JCYJ20210324113614038), Shenzhen Key Medical Discipline Construction Fund (No. SZXK003 and No. SZXK059) and Sanming Project of Medicine in Shenzhen (No. SZSM201412012).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.992376/full#supplementary-material
Akimaru, K., Iwabuchi, M., Ishida, A., Uehara, H., Higa, N., Kakazu, M., et al. (2022). Comparative evaluation of standard maintenance-dose clopidogrel versus low-dose prasugrel in patients with stable coronary artery disease after percutaneous coronary intervention. Int. J. Cardiol. 356, 30–35. doi:10.1016/j.ijcard.2022.02.023
Asada, Y., Yamashita, A., Sato, Y., and Hatakeyama, K. (2020). Pathophysiology of atherothrombosis: Mechanisms of thrombus formation on disrupted atherosclerotic plaques. Pathol. Int. 70 (6), 309–322. doi:10.1111/pin.12921
Bainey, K. R., Welsh, R. C., Connolly, S. J., Marsden, T., Bosch, J., Fox, K. A. A., et al. (2020). Rivaroxaban plus aspirin versus aspirin alone in patients with prior percutaneous coronary intervention (COMPASS-PCI). Circulation 141 (14), 1141–1151. doi:10.1161/CIRCULATIONAHA.119.044598
Bonaca, M. P., Bhatt, D. L., Cohen, M., Steg, P. G., Storey, R. F., Jensen, E. C., et al. (2015). Long-term use of ticagrelor in patients with prior myocardial infarction. N. Engl. J. Med. 372 (19), 1791–1800. doi:10.1056/NEJMoa1500857
CAPRIE Steering Committee (1996). A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet 348 (9038), 1329–1339. doi:10.1016/s0140-6736(96)09457-3
Collet, J-P., Thiele, H., Barbato, E., Barthélémy, O., Bauersachs, J., Bhatt, D. L., et al. (2020). ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 42 (14), 1289–1367.
Collet, J. P., Silvain, J., Barthélémy, O., Rangé, G., Cayla, G., Van Belle, E., et al. (2014). Dual-antiplatelet treatment beyond 1 year after drug-eluting stent implantation (ARCTIC-Interruption): A randomised trial. Lancet 384 (9954), 1577–1585. doi:10.1016/S0140-6736(14)60612-7
Connolly, S. J., Eikelboom, J. W., Bosch, J., Dagenais, G., Dyal, L., Lanas, F., et al. (2018). Rivaroxaban with or without aspirin in patients with stable coronary artery disease: An international, randomised, double-blind, placebo-controlled trial. Lancet 391 (10117), 205–218. doi:10.1016/S0140-6736(17)32458-3
Frangogiannis, N. G. (2015). Pathophysiology of myocardial infarction. Compr. Physiol. 5 (4), 1841–1875. doi:10.1002/cphy.c150006
Godley, R. W., and Hernandez-Vila, E. (2016). Aspirin for primary and secondary prevention of cardiovascular disease. Tex. Heart Inst. J. 43 (4), 318–319. doi:10.14503/THIJ-16-5807
Helft, G., Steg, P. G., Le Feuvre, C., Georges, J. L., Carrie, D., Dreyfus, X., et al. (2016). Stopping or continuing clopidogrel 12 months after drug-eluting stent placement: The OPTIDUAL randomized trial. Eur. Heart J. 37 (4), 365–374. doi:10.1093/eurheartj/ehv481
Ibanez, B., James, S., Agewall, S., Antunes, M. J., Bucciarelli-Ducci, C., Bueno, H., et al. (2017). 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 39 (2), 119–177. doi:10.1093/eurheartj/ehx393
Jones, L., Griffin, S., Palmer, S., Main, C., Orton, V., Sculpher, M., et al. (2004). Clinical effectiveness and cost-effectiveness of clopidogrel and modified-release dipyridamole in the secondary prevention of occlusive vascular events: A systematic review and economic evaluation. Health Technol. Assess. 8 (38), 1. doi:10.3310/hta8380
Kim, H. S., Kang, J., Hwang, D., Han, J. K., Yang, H. M., Kang, H. J., et al. (2020). Prasugrel-based de-escalation of dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome (HOST-REDUCE-POLYTECH-ACS): An open-label, multicentre, non-inferiority randomised trial. Lancet 396 (10257), 1079–1089. doi:10.1016/S0140-6736(20)31791-8
Knuuti, J., Wijns, W., Saraste, A., Capodanno, D., Barbato, E., Funck-Brentano, C., et al. (2019). ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 41 (3), 407–477. doi:10.1093/eurheartj/ehz425
Koo, B. K., Kang, J., Park, K. W., Rhee, T. M., Yang, H. M., Won, K. B., et al. (2021). Aspirin versus clopidogrel for chronic maintenance monotherapy after percutaneous coronary intervention (HOST-EXAM): An investigator-initiated, prospective, randomised, open-label, multicentre trial. Lancet 397 (10293), 2487–2496. doi:10.1016/S0140-6736(21)01063-1
Lee, C. W., Ahn, J. M., Park, D. W., Kang, S. J., Lee, S. W., Kim, Y. H., et al. (2014). Optimal duration of dual antiplatelet therapy after drug-eluting stent implantation: A randomized, controlled trial. Circulation 129 (3), 304–312. doi:10.1161/CIRCULATIONAHA.113.003303
Li, J., Qiu, H., Yan, L., Guo, T., Wang, Y., Li, Y., et al. (2021). Efficacy and safety of ticagrelor and clopidogrel in patients with stable coronary artery disease undergoing percutaneous coronary intervention. J. Atheroscler. Thromb. 28 (8), 873–882. doi:10.5551/jat.57265
Libby, P., and Theroux, P. (2005). Pathophysiology of coronary artery disease. Circulation 111 (25), 3481–3488. doi:10.1161/CIRCULATIONAHA.105.537878
Mauri, L., Kereiakes, D. J., Yeh, R. W., Driscoll-Shempp, P., Cutlip, D. E., Steg, P. G., et al. (2014). Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N. Engl. J. Med. 371 (23), 2155–2166. doi:10.1056/NEJMoa1409312
McNeil, J. J., Wolfe, R., Woods, R. L., Tonkin, A. M., Donnan, G. A., Nelson, M. R., et al. (2018). Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N. Engl. J. Med. 379 (16), 1509–1518. doi:10.1056/NEJMoa1805819
Mega, J. L., Braunwald, E., Wiviott, S. D., Bassand, J. P., Bhatt, D. L., Bode, C., et al. (2012). Rivaroxaban in patients with a recent acute coronary syndrome. N. Engl. J. Med. 366 (1), 9–19. doi:10.1056/NEJMoa1112277
Mega, J. L., Simon, T., Collet, J. P., Anderson, J. L., Antman, E. M., Bliden, K., et al. (2010). Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: A meta-analysis. Jama 304 (16), 1821–1830. doi:10.1001/jama.2010.1543
Papakonstantinou, T., Nikolakopoulou, A., Higgins, J. P. T., Egger, M., and Salanti, G. (2020). CINeMA: Software for semiautomated assessment of the confidence in the results of network meta-analysis. Campbell Syst. Rev. 16 (1), e1080. doi:10.1002/cl2.1080
Park, S. J., Park, D. W., Kim, Y. H., Kang, S. J., Lee, S. W., Lee, C. W., et al. (2010). Duration of dual antiplatelet therapy after implantation of drug-eluting stents. N. Engl. J. Med. 362 (15), 1374–1382. doi:10.1056/NEJMoa1001266
Roe, M. T., Armstrong, P. W., Fox, K. A., White, H. D., Prabhakaran, D., Goodman, S. G., et al. (2012). Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N. Engl. J. Med. 367 (14), 1297–1309. doi:10.1056/NEJMoa1205512
Steg, P. G., Bhatt, D. L., Simon, T., Fox, K., Mehta, S. R., Harrington, R. A., et al. (2019). Ticagrelor in patients with stable coronary disease and diabetes. N. Engl. J. Med. 381 (14), 1309–1320. doi:10.1056/NEJMoa1908077
Storey, R. F., Becker, R. C., Harrington, R. A., Husted, S., James, S. K., Cools, F., et al. (2011). Characterization of dyspnoea in PLATO study patients treated with ticagrelor or clopidogrel and its association with clinical outcomes. Eur. Heart J. 32 (23), 2945–2953. doi:10.1093/eurheartj/ehr231
Storey, R. F., Bliden, K. P., Patil, S. B., Karunakaran, A., Ecob, R., Butler, K., et al. (2010). Incidence of dyspnea and assessment of cardiac and pulmonary function in patients with stable coronary artery disease receiving ticagrelor, clopidogrel, or placebo in the ONSET/OFFSET study. J. Am. Coll. Cardiol. 56 (3), 185–193. doi:10.1016/j.jacc.2010.01.062
Valgimigli, M., Bueno, H., Byrne, R. A., Collet, J-P., Costa, F., Jeppsson, A., et al. (2017). 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 39 (3), 213–260. doi:10.1093/eurheartj/ehx419
Wallentin, L., Lindholm, D., Siegbahn, A., Wernroth, L., Becker, R. C., Cannon, C. P., et al. (2014). Biomarkers in relation to the effects of ticagrelor in comparison with clopidogrel in non-ST-elevation acute coronary syndrome patients managed with or without in-hospital revascularization: A substudy from the prospective randomized platelet inhibition and patient outcomes (PLATO) trial. Circulation 129 (3), 293–303. doi:10.1161/CIRCULATIONAHA.113.004420
Keywords: antiplatelet therapy, chronic coronary syndromes, percutaneous coronary intervention, randomized control trials, geriatric disease, anticoagulant
Citation: Lin Y, Cai Z, Dong S, Liu H, Pang X, Chen Q, Yuan J and Geng Q (2022) Comparative efficacy and safety of antiplatelet or anticoagulant therapy in patients with chronic coronary syndromes after percutaneous coronary intervention: A network meta-analysis of randomized controlled trials. Front. Pharmacol. 13:992376. doi: 10.3389/fphar.2022.992376
Received: 12 July 2022; Accepted: 05 September 2022;
Published: 30 September 2022.
Edited by:
Francesco Pelliccia, Sapienza University of Rome, ItalyReviewed by:
Francesco Pelliccia, Sapienza University of Rome, ItalyCopyright © 2022 Lin, Cai, Dong, Liu, Pang, Chen, Yuan and Geng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiuling Chen, c3pjaGVucWl1bGluZ0B5ZWFoLm5ldA==; Jie Yuan, eG5reXVhbmppZUB5ZWFoLm5ldA==; Qingshan Geng, c3pnZW5ncWluZ3NoYW5AeWVhaC5uZXQ=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.